Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
PYRIDO[2,3-d]PYRIMIDINE-2,7-DIAMINE KINASE INHIBITORS
FIELD OF THE INVENTION
This invention relates to pyrido[2,3-d]pyrimidine-2,7-diamines that inhibit
cyclin-dependent serine/threonine kinases and growth factor-mediated tyrosine
kinase enzymes, and as such are useful to treat cell proliferation diseases
and
disorders.
BACKGROUND OF THE INVENTION
Summarv of the Related Art
Cell cvcle kinases are naturallv occurrina enzvmes involved in regulation
of the cell cycle (Meijer L.. "Chemical Inhibitors of Cyclin-Dependent
Kinases,"
Progress in Cell Cycle Research, 1995;1:351-363). Typical enzymes include
serine/threonine kinases such as the cyclin-dependent kinases (cdks) cdkl,
cdk2,
cdk4, cdk5. cdk6, as well as tyrosine kinases involved in cell cycle
regulation.
Increased activity or temporally abnormal activation or regulation of these
kinases
has been shown to result in development of human tumors and other
proliferative
disorders. Compounds that inhibit cdks. either by blocking the interaction
between
a cvclin and its kinase partner. or by binding to and inactivating the kinase,
cause
inhibition of cell proliferation. and are thus useful for treating tumors or
other
abnormally proliferating cells.
Several compounds that inhibit cdks have demonstrated preclinical
antitumor activity. For example. flavopiridol is a flavonoid that has been
shown to
be a potent inhibitor of several types of breast and lung cancer cells (Kaur
et al.,
J. 1Vatl. Cancer Inst., 1992:84:1736-1740; Int. J. Oncol., 1996;9:1143-1168).
The
compound has been shown to inhibit cdk2 and cdk4. Olomoucine
[2-(hvdroxyethvlamino)-6-benzylamine-9-methvlpurine] is a potent inhibitor of
cdk2 and cdk5 (Veselv et al.. Eur. J. Biochem., 1994;224:771-786), and has
been
shown to inhibit proliferation of approximately 60 different human tumor cell
lines used bv the National Cancer Institute (NCI) to screen for new cancer
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-~-
therapies (Abraham et al., BioloD, of the Cell. 1995;83:105-120). More
recently.
the purvalanol class of cdk inhibitors has emerged as more potent derivatives
of
olomoucine (Gray N.S. et al.. Science. 1998:281:533-538).
Tvrosine kinases are essential for the propagation of growth factor signal
transduction leading to cell cvcle progression. cellular proliferation,
differentiation, and migration. Tyrosine kinases include cell surface growth
factor
receptor tyrosine kinases such as FGFr and PDGFr, as well as nonreceptor
tvrosine kinases. including c-Src and lck. Inhibition of these enzymes has
been
demonstrated to cause antitumor and antiangiogenesis activity (Hambv et al.,
Pharmacol. Tlier.. 1999; 82(2-3):169-193 ).
Several pyrido[2.3-d]pyrimidines that inhibit cdks and gromh factor-
mediated kinase enzymes are known (WO 98/33798). US Patent Nos. 5.733,913
and 5,733,914 describe 6-aryl-pyrido[2.3-d]pvrimidines.
Despite the progress that has been made, the search continues for low
molecular weight compounds that are orally bioavailabie and useful for
treating a
wide variety of human tumors and other proliferative disorders. including
restenosis, angiogenesis. diabetic retinopathy. psoriasis. surgical adhesions,
macular degeneration. and atherosclerosis. The present invention provides such
compounds, their pharmaceutical formulations. and their use in treating
proliferative disorders.
SUMMARY OF THE INVENTION
This invention provides novel pyrido[2.3-d]pyrimidine-2.7-diamine
compounds which function as inhibitors of cell cycle regulatory kinases such
as
the cyclin dependent kinases as well as the growth factor-mediated tyrosine
kinases. Thus, these compounds are useful to treat cell proliferative
disorders such
as atherosclerosis and restenosis: cancer: angiogenesis: viral infections
including
DNA viruses such as herpes and RNA viruses such as HIV: fungal infections;
type 1 diabetes. diabetic neuropathy and retinopathy: multiple sclerosis;
glomerulonephritis: neurodegenerative diseases including Alzheimer's disease;
autoimmune diseases such as psoriasis. rheumatoid arthritis. and lupus; organ
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
,
-~-
transplant rejection and host versus graft disease; gout; polycvstic kidnev
disease:
and inflammation including inflammatory bowel disease.
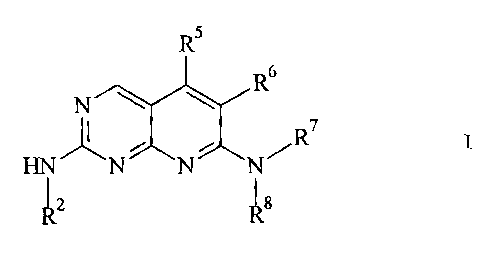
Accordingly, the present invention provides pyrido[2,3-d]pyrimidine
having the generic structure of Formula I
R5
R6
r; XIN XN > HNN R7
R2 Rg
wherein:
R2, R7, R13, R14 and R15 are independentlv hvdro(yen, or
lower alkyl, lower alkenyl, or lower alkynyl, each of which is optionally
substituted with up to 5 groups independently selected from
halogen, cyano, nitro, -R9, -NR9R10, -OR9. -(CH2)nCO-)R9,
-(CH-))nSO2R11, -(CH,))nRl1, -COR9, -CONR9R10, -S03R9,
-SO2NR9RIO, -SO-)R9, -SR9. -PO;R9R10, -POR9R10,
-PO(NR9R10)2, -NR9COR10, -NR9CO,~ R10, -NR9CONR9R10,
-NR9SO-)R10, or
a heterocvcle optionally substituted with up to 3 groups
independentlv selected from -R9, -NR9R10, -OR9.
-NR9COR10, -COR10, -(CH-))nSO-)RI l, -(CH-))nRl1, or
-(CH2)nRlI optionally substituted with up to 5 groups independently
selected from halogen, cyano, nitro, -R9, -NR9R 10, -OR9,
-(CH-))nCO-)R9, -(CH-))nSO-)R11, -(CH2)nR11. -COR9.
-CONR9R10, -SO-R9, -SO,)NR9R10. -SO-)R9. -SR9,
-PO;R9R10, -POR9R10, -PO(NR9R10)2. -NR9COR10.
-NR9CO-)R10. -NR9CONR9R10, -NR9SO-)RIO. or
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-4-
a heterocvcle optionally substituted with up to 3 groups
independentlv selected from -R9. -NR9R10, -OR9.
-NR9COR10, -COR10. -(CH2)nSO--)RI 1, -(CH-))nR11;
R5 is haloizen. cvano. nitro. -R9, -NR9R 10, or -OR9:
R6 is halogen. cvano. nitro, -R9, -NR9R10, -OR9. -C02R9. -COR9,
-CONR9R10. -NR9COR10, -SO-)NR9R10, -SO2R9. -S03R9, -SR9.
-P03R9R10. -POR9R10. -PO(NR9R10)2, or
lower alkenyl or lower alkvnyl optionally substituted w=ith -R9;
R8 is H. -CO2R1'. -COR13, -CONR13RI4. -CSNR13R14, -C(NR1')NR14R15
-S03R1', -SOiRl', -SO-)NR13R14 -P03R13R14, -POR13R14
-PO(NR13R14),,;
R9 and R10 are independently hydrogen, or
lower alkvl, optionally substituted with up to 3 groups selected from the
aroup consistina of halogen. amino, mono- or dialkylamino,
hvdroxv. lower alkoxy, phenvl or substituted phenvl,
or when taken toQether with the nitrogen to which they are attached, R9
and R10 form a rinR having from 3-7 members, up to four of which
0
may be selected from 0. S. and NR20, where R20 is
hvdrocen, loxver alkyl. or -CO lo,~ver alkvl:
Rl I is a heteroaryl or a heterocyclic group:
Rl' is a cvcloalkvl, a heterocvclic. an arvl, or a heteroarvl group;
nis0, 1,2,or3:
and the pharmaceuticallv acceptable salts, esters, amides, and prodrugs
thereof.
The present invention also provides a composition that comprises a
compound of Formula I together with a pharmaceuticallv acceptable carrier,
diluent, or excipient.
The present invention also provides methods for inhibiting cyclin-
dependent kinase and grow-th factor-mediated kinase enzymes.
CA 02397961 2002-07-22
WO 01/55147 PCT/1B01/00069
-J-
The present invention also provides a method of treating subjects suffering
from diseases caused by cellular proliferation. The method comprises
inhibiting
proliferation of tumorigenic cells of epithelial origin and vascular smooth
muscle
proliferation, and/or cellular migration by administering a therapeutically
effective
amount of a compound of Formula I to a subject in need of treatment.
The present invention also provides a method of treating subjects sufferina
from diseases caused bv DNA tumor viruses, such as herpes viruses comprising
administering a compound of Formula I.
DETAILED DESCRIPTION OF THE INVENTION
The compounds encompassed by the instant invention are those described
bv the general Formula I set forth above, and the pharmaceutically acceptable
salts, esters. amides, and prodrugs thereof.
In addition to the compounds of Formula I. the invention provides
preferred compounds of Formula II:
R5
R6
~,T a~" ~ / R
7
HIv' N N N
I II
R17 R18 R
R16
wherein R5. R6. R7. and R8 are as defined above for Formula I;
R16. R17. and R18 are as defined above for the (CH-))nRl2 substituents, and
preferably are independently hydrogen. halogen. amino. mono- or
dialkvlamino. hvdroxv, lower alkyl. lower alkoxv, cvano, nitro, carboxy,
carboxvalkvl. aminocarbonyl, mono- or dialkylaminocarbonyl,
alkvlcarbonyl. -SO;R9, -SO~NR9R10, -SO-)R9, -SR9. -PO;R9RI0,
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-6-
-POR9R10, -PO(NR9R10)'), -NR9COR10, -NR9CO~R10.
-NR9CONR9RI0. -NR9SO,)R10; or
R16 is a carbocyclic group containing from 3-7 members, up to ? of which
members are heteroatoms selected from oxygen and nitrogen, wherein the
carbocyclic group is unsubstituted or substituted with 1, ?. or 3 groups as
defined above, but preferably are independently selected from the group
consisting of halogen, hvdroxv, lower alkvl, trifluoromethyl, lower alkoxy.
amino, mono- or dialkvlamino, aryl, heteroaryl, arvlalkvl, heteroarylalkyl,
heteroarvlsulfonyl, heteroarvlsulfonylalkvl, heterocyclvlalkyl,
heterocvclvlsulfonvl. or heterocvclvlsulfonvlalkvl.
Preferred compounds of Formula Il are where R5 is hydrogen or lower
alkyl; R6 is hydrogen, lower alkyl, cvano or halogen; R17 and R18 are
independently hydrogen, halogen, amino, mono- or dialkylamino, hydroxy, lower
alkyl, lower alkoxy, aminocarbonyl, mono- or dialkylaminocarbonyl,
-SO2NR9R10 or -NR9COR10; and R16 is optionally substituted N-piperidine,
N-piperazine, or N-pyrrolidine, for instance where the rina substituents are
selected from -R9, -NR9R10, -OR9. NR9COR10. and COR10.
In addition, the present invention also provides preferred compounds of
Formula III:
R5
R6
N
~
R~1
HN N N N III
1 R2 IIN~O
R19
wherein R2. R5, and R6 are as defined above for Formula I; and
R 19 is hydrogen, or
lower alkyl, lower alkenyl. or lower alkynyl, each of which is optionally
substituted with up to 5 groups independentlN, selected from
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-7-
halogen, amino, mono- or dialkylamino, hydroxy, lower alkoxy.
cyano, nitro, carboxy, carboxyalkyl. aminocarbonyl. mono- or
dialkvlaminocarbonyl, lower alkvlcarbonvl, -SO3R9,
-S02NR9R10, -S02R9. -SR9, -PO;R9R10, -POR9R10.
-PO(NR9R10)2, -NR9COR10, -NR9C02R10. -NR9CONR9R10,
-NR9SO2R10, where R9 and RIO are as defined above, or
aryl. heteroaryl, arvlalkyl, heteroarylalkyl. cycloalkyl or cvcloalkyl-alkvl,
where each aryl, heteroarvl or cvcloalkyl group is optionally
substituted with up to 5 groups independently selected from
halogen, amino, mono- or dialkvlamino, hvdroxy, lower alkoxv.
cvano, nitro, carboxy, carboxvalkyl, aminocarbonyl, mono- or
dialkylaminocarbonyl, alkylcarbonyl, -S03R9, -SO2NR9R10,
-S02R9, -SR9. -P03R9R10, -POR9R10, -PO(NR9RIO)2,
-NR9COR10, -NR9C02R10, -NR9CONR9R10, -NR9SO2RI0, or
a(CH2)n-carbocyclic group containing from 3-7 members, up to 2 of
which members are heteroatoms selected from oxygen and
nitrogen, wherein the carbocyclic group is unsubstituted or
substituted with 1. 2. or 3 groups independently selected from the
group consisting of halogen, hvdroxy, lower alkyl. trifluoromethvl,
lower alkoxv, amino. mono- or dialkylamino, arvl, heteroarvl,
arylalkyl, heteroarvlalkyl, heteroarvlsulfonyl,
heteroarylsulfonvlalkvl, heterocyclvlalkyl, heterocyclylsulfonyl, or
heterocvclvlsulfonvlalkvl; and
R21 is hvdrogen, lower alkyl. or lower alkyl substituted with phenyl or
substituted phenvl.
Preferred compounds of Formula III are where R5 is hydrogen or lower
alkvl. R6 is hvdrogen or halogen. Rl- is optionallv substituted phenyl; R21 is
hydrogen or methyl: and R 19 is optionally substituted lower alkyl,
cycloalkyl, or
(CH-))n-carbocvclic.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-8-
An especially preferred group of pvrido[2.3-d]pyrimidines have
Formula IV:
R5
R6
lT ~ \
~ R7
..1
I-I?'' N N IiT IV
C=0
R17
18 FfN
R
R19
R16
wherein R5, R6. R16 R17 R18 R19 and R21 are as defined above. Preferred
compounds of Formula IV are those wherein R21 is hvdrogen or methvl.
Another especially preferred group of invention compounds have
Formula V:
R')
R6
1"T
N
~
R_1
HN N N N V
R1 ~ R18 C=O
T
N R16
19
R
wherein R5, R6, R 16. R 17. R I S. R 19. and R21 are as defined above.
Preferred
compounds of Formula V are those wherein R21 is hydrogen or methyl.
The most preferred invention compounds have Formula VI
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-9-
R5
I R6
N
HNI Nr N N-H or alkvl
C=0
R17 R18 NH VI
I
alkvl or cvcloalkvl
N
R2-,
R?2 '
H or alkanovl
wherein R5. R6. R 17, and R 18 are as defined above, and R2 2 and R23
independently are hydrogen or alkyl.
By "alkvl," "lower alkyl," and "(C I-C I 0)-alkyl" in the present invention is
meant straight or branched chain alkyl groups having 1 to 10 carbon atoms,
preferably CI-C6 alkyl. Typically alkvl groups include methyl, ethyl. n-
propyl,
isopropyl. n-butyl. sec-butyl, tert-butvl. pentyl, 2-pentyl, isopentvl.
neopentyl,
hexyl. 2-hexyl, 3-hexyl, decyl, octvl, and 3-methvlpentyl. These groups may be
substituted. for instance with halo. C I -C - alkyl. amino, alkvlamino,
dialkylamino,
hvdroxy. alkoxy. and the like. Examples include chloromethyl, 2-amino-ethyl,
and
3-dimethvl-aminopropyl.
Bv "alkenyl." "lo,~ver alkenyl." and (C-)-C I O)-alkenyl is meant straight or
branched chain alkyl groups having I to 10 carbon atoms and having 1 or
2 nonadjacent double bonds. Examples of alkenvls include, but are not limited
to,
3-butenyl and I -methyl-3-pentenyl.
Bv "alkynyl," "lower alkynyl." and (C-)-C I 0)-alkynyl is meant straight or
branched chain alkvl groups having I to 10 carbon atoms and having a triple
bond. Typical alkynvl groups include 2-propynyl and 1.1-dimethvl-3-butynyl.
Substituted alkenvl and alkvnyl groups include 4.4-dibromo-2-pentenyl and
3-amino-5 -hexvnvl.
Bv "alkoxy.- "lower alkoxy." or "(C I-C I 0)-alkoxy" in the present
invention is meant straight or branched chain alkoxy groups having 1 to 10
carbon
atoms. such as. for example. methoxy. ethoxy. propoxy, isopropoxy. n-butoxy,
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-10-
sec-butoxy, tert-butoxy, pentoxy. 2-pentyl. isopentoxy, neopentoxy. hexoxy.
2-hexoxy, 3-hexoxv. and 3-methylpentoxy.
The term "alkanoyl" means an alkyl group bonded through a carbonyl
moiety. Examples include acetyl and pentanoyl. "Aminoalkanovl- means the alkyl
group is substituted with an amino group. Examples include aminoacetyl and
3-aminohexanovl. "Alkylaminoalkanovl" means an aminoalkanoyl group wherein
the amine is substituted with a C 1-C 10 alkyl group, and includes
methylaminoacetyl and 4-(isobutylamino)-octanoyl. "Dialkvlaminoalkanoyl"
means an N.N-di-substituted aminoalkanoyl group such as
diisopropylaminoacetyl.
By halogen in the present invention is meant fluorine, bromine, chlorine,
and iodine.
The term "aryl" means an unsubstituted aromatic carbocvclic group having
a single ring (e.g., phenyl), multiple rings (e.g., biphenyl), or multiple
condensed
rings in which at least one is aromatic (e.(Y., 1,2,3,4-tetrahvdronaphthyl,
naphthyl,
anthryl, or phenanthryl). The term "substituted aryl'' means an aryl
substituted by
1 to 4 substituents selected from alkyl. 0-alkyl and S-alkyl. -OH, -SH, -CN,
halo,
1.3-dioxolanvl, -CF;, -NO2, -NH2. -NHCH;, -N(CH3)2, -NHCO-alkyl,
-(CH2)mCO2H, -(CH2)mCO2-alkyl, -(CH2)mSO;H. -NH alkyl, -N(alkyl)2,
-(CH2)mPO3H2, -(CH2)mPO3(alkyl)2, -(CH2)mSO2NH2, and -(CH2)mSO2NH-
alkyl. wherein alkyl is defined as above and m is 0. 1. 2, or 3. Some examples
of
substituted aryl groups are methylphenyl. isopropoxyphenvl, chlorophenyl,
2-bromo-3-trifluoromethvl-4-nitro-5-aminophenvl. 4-bromobiphenyl, 3-
acetamidonaphthyl, 3-dimethylaminoanthryl. 3.4-dimethoxyphenanthryl, and 2,8-
dibromobiphenylen-l-vl.
By "heteroaryl" is meant one or more aromatic ring svstems of 5-, 6-, or
7-members containing at least I and up to 4 heteroatoms selected from
nitrogen,
oxygen, or sulfur. Such heteroaryl groups include, for example, thienyl,
furanyl,
thiazolyl, imidazolvl. (is)oxazolyl. tetrazolyl, pyridyl, pyrazinyl,
pyrimidinyl,
pyrazolyl, (iso)quinolinyl, napthyridinyl, phthalimidyl, benzimidazolyl,
benzoxazolyl. A"substituted heteroaryl" group can be substituted with 1, 2, 3,
or 4
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-11-
of the groups mentioned above for "substituted aryl." such as 2.3.4.6-
tetrachloropyridvl and 2-methoxy-3-trifluoromethvlthien-4-yl.
The term "heterocvclic group" means a non-aromatic ring having 5-. 6-. or
7-ring atoms, from I to 4 of which are selected from nitrogen. oxygen. or
sulfur.
Examples of heterocyclic groups include morpholino, piperidino. piperazino.
pvrrolidinyl. and tetrahydrothienyl. Such groups can be substituted with the
same
groups described above for substituted heteroaryl.
A"carbocyclic group" or "cycloalkyl" is a nonaromatic cyclic ring or
fused rings having from 3- to 7-ring carbon members. Examples include
cyclopropyl, cvclobutyl, and cycloheptyl. These rin--s may be substituted
,vith one
or more of the substituent groups mentioned above for arvl, for example alkvl,
halo, amino, hvdroxy, and alkoxy. Typical substituted carbocvclic groups
include
2-chlorocyclopropyl, 2.3-diethoxycyclopentyl, and 2.2,4,4-
tetrafluorocvclohexyl.
The carbocyclic group may contain 1 or 2 heteroatoms selected from oxygen,
sulfur, and nitrogen, and such ring systems are referred to as "heterocyclyl"
or
"heterocyclic". Examples include piperidyl, piperazinyl, pyrrolidinyl,
pyranyl,
tetrahydrofuranyl, and dioxanyl. These heterocyclyl groups may be substituted
with up to 4 of the substituent groups mentioned for aryl to give groups such
as
3,5-dimethylpiperazin-l-yl, 3.3-diethylpiperazin-l-yl, 3,3,4,4-
tetramethvlpvrrol-
idinvl. 3-chloro-2-dioxanyl, and 3.5-dihydroxymorpholino. These can also bear
a
keto aroup, for instance, 3-ketopiperidyl.
The term "cancer" includes. but is not limited to, the following tumor
types: breast, ovary, cervix, prostate. testis. esophagus, glioblastoma,
neuroblastoma, stomach. skin, keratoacanthoma, lung, epidermoid carcinoma,
large cell carcinoma, adenocarcinoma, bone, colon. adenoma, pancreas. thvroid,
follicular carcinoma, undifferentiated carcinoma, papillary carcinoma,
seminoma,
melanoma, sarcoma, bladder carcinoma, liver carcinoma and biliary passages,
kidney carcinoma, mveloid disorders, lvmphoid disorders. Hodgkin's. hairy cell
carcinoma, cancer of the buccal cavity and pharynx (oral), lip, tongue. mouth,
pharynx, small intestine. colon, rectum, large intestine, brain and central
nervous
svstem; and leukemia.
The compounds of Formulas I to VI can exist as pharmaceutically
acceptable salts. esters. amides. and prodrugs. The term "pharmaceutically
CA 02397961 2004-12-29
65920-142
-12-
acceptable salts. esters. amides. and prodrugs- as used herein refers to those
carboxvlate salts. amino acid addition salts, esters. amides. and prodrugs of
the
compounds of the present invention which are. within the scope of sound
medical
judgment. suitable for use in contact with the tissues of patients -t=ithout
undue
~ toxicity. in:itation. allergic response, and the like, commensurate with a
reasonable
benefiv'risk ratio. and effective for their intended use, as well as the
nN=itterionic
forms. -;,here possible. of the compounds of the invention. The term "salts"
refers
to the relatively nontoxic, inorganic, and organic acid addition salts and
base salts
of compounds of the above formulas. These salts can be prepared in situ durina
the final isolation and purification of the compounds. or by separately
reacting the
purified compound in its free base form, for example. with a suitable organic
or
inorganic acid. and isolating the salt thus formed. Representative salts
include the
hydrobromide, hydrochloride, sulfate, bisulfate. nitrate, acetate. oxalate,
valerate,
oleate, palmitate, stearate. laurate. borate. benzoate, lactate, phdsphate.
tosylate,
citrate, maleate, fumarate. succinate, tartrate. naphthylate mesylate,
glucoheptonate, lactobionate, and laurylsulphonate salts, and the like. When
the
compound of the above formulas has one or more acidic groups. it can form a
salt
by reaction with a base. These salts may include cations based on the alkali
and
alkaline earth metals. such as sodium. lithium, potassium. calcium. magnesium,
and the like. as well as inorganic bases such as amnionium. quaternary
ammonium. and other amine cations includine. tetrameth~Ylammonium.
tetraethylammonium. methylamine, dimethylamine. trimethylamine.
triethylamine. ethylamine, and the like. Pharmaceutically acceptable salts are
well-I:nown to those skilled in the art of medicinal chemistry. (See, for
example,
Berge S.M. et al.. "Pharmaceutical Salts." J. Pharm. Sci.. 1977:66:1-19).
Examples of pharmaceutically acceptable. nontoxic esters of the
compounds of this invention include C 1-C6 alkvl esters. wherein the alkvl
group
is a straicht or branched hydrocarbon. substituted or unsubstituted. Esters
also
include C5-C7 cvcloalkvl esters. as well as arylalkyl esters such as benzvl
and
triphetn=lmethyl. C I-C4 Alkyl esters are preferred. such as methyl. ethvl.
2?.%trichloroeth~~l, and tert-but~=l. Esters of the compounds of the present
CA 02397961 2004-12-29
65920-142
-13-
invention may be prepared according to conventional methods, for example by
reaction of an acid with an alcohol.
Examples of pharmaceutically acceptable amides of the compounds of this
invention include amides derived from ammonia. primary C I-C6 alkNl amines and
secondary C I-C6 dialkyl amines. wherein the alkyl groups are straiaht or
branched. In the case of secondary amines. the amine may also be in the form
of a
i- or 6-membered heterocycle containing I nitrogen atom. Amides derived from
ammonia. C 1-C; alkyl primarv amines. and C I -C-) dialkvl secondary amines
are
preferred. Amides of the compounds of the invention may be prepared according
to conventional methods well-known to the medicinal chemists.
The term "prodrug" refers to compounds that are rapidly transformed
in vivo to yield the parent compound of the above formulae, for example, by
hvdrolysis in blood or stomach fluids. A thorough discussion of prodrugs is
provided bx= Higuchi T. and Stella V.. "Pro-drugs as Novel Delivery Systems."
Vol.
14 of the A.C.S. Symposium Series. and in Biorei-ersible Carriers in Drug
Design. ed. Edward B. Roche. American Pharmaceutical Association and
Perzamon Press. 1987.
Representative compounds of the invention are sho%vn below in Table I.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-14-
Table I
NI NI f F
i i
HN NH H7~; NH HN N N H
HN' p FfNp
\ I \ I \ I
ci
N ~ N
.~ .~
N
H H H
2 3
N N \ \ N \ \
I i i
HNN NH ~ I N NH HN N NH
. O HN p HN O
I I
\ I \ ~ \ ci
N N
H p~\ p~
6
\ \ F
N \ \ ?~ \ \
HN~ NH H.N)NH HN N N NH
HN O HN
HN O " k
O
\ I \ 1
\ /(\
N
N CN,)
C) N
.
~ ~
H
O p~/\
7 g 9
CA 02397961 2002-07-22
WO 01/55147 PCT/IBOl/00069
-15-
Table 1 (cont'd)
F
Tr \ \ N \ \
I r \ \
HN NH HN NH
HN N N
14N O HN O
CI I / I HN ---O
N N
H H N
11 H 12
,
F
N N r
~ i i I i i
11N~ NH HN Iv' NH HN NH
HN 'O HNO H'''O
\ \ I \
C1
N N N
' N
13 14 1-5
N N N
r N NI-{ HN N N NH IINN NH
14N0 HNO / HNO
\
6 \ I \
CI
N) N OH N) OH
C
, H
~ N
H
O 16 17 18
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-16-
Table 1 (cont'd)
F N
N \ \ N \ \
HN N N NH
NH I--I-NI N N NH
HN O
HN O tIN O
\ \ I
OH
N OH OH
.~
N
H 19 H 20 O 21
NI N F
I N \ \
ITN Nr~ N H FIN~i i N H ~ i
HN N NH
HN O HN O
( I / I HN O
Cl
N OH CN) OH
C ~ N OH
~
N N
22 23 O 24
F
N \ \ \ \ N \ \
i ~
HNN N H HN N/ N/ H 1iNNN H
HN O HN O HNO
cl
N
N
C)
~
N N N
H 25 H 26 H 17
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-17-
Table 1 (cont'd)
INH N Nr N~ i ~~~~ T HN N N NH
HNT'N NH HN---O HNO
H-'; p
ci
N CN)
CN~ N N,
H 28 O i9 O~ 30
N F N \
HN N NH HN Ni N~
NH
HN N N H
HN O [ rn; p
I I I ~ HN O
CN) ~N N
~
~ N
p 31 O 32 33
N
II / ~ N \ \ ~
J N
HN\N N NH HN NH HN N N H
N N ~
HN ~ O / HN O 11~1 \ / I HN O
N N
34 35 36 OH
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-18-
Table I (cont'd)
Nr F N F
! \ \ N\ F
,~
HT~ N N ~ HN N N NH HN N N ~
HN O ~ O HNI O
N N N 37 38 39
F
N r N
,~
Ht''N N/ NH HNN N NH
~ HN N N NH ~
~
HN O O HN O
N N N
OH
40 41 42
N~ I \
NI N
~
HN N Ni NH
HN N N ~ HN N N ~
\ I HN O H1v O CN J O
N
N N
OH CH,
43 44 ~N) N H = 3 HC1
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-19-
~
N N~ \
~ I
HN N N NH2 HI~T N N I~T-CH,
C=0
I
NH
CN) _(N),.
N CH, CH0
C 0+
0
CO.,Et
NT N \ \ ~
HN \N N IT-CH~CH, HN N N j'TH2
C=0 \
NH
= 3 HCl
1~T N
N N
H IC-O+
0
OCH0
0 CHNH'?
~T ~ \ NHCCH ~ ~ SO,CH,
~
TJ~T N NH ~Iv T I
~ N HN N NH
CHICH2 NH~ ~ -O CI ~ =S
CH, NH-1
Br
C=0
I
N
CHI CH,
CA 02397961 2002-07-22
WO 01/55147 PCT/IBOI/00069
-20-
NO-, CN O
11
~T SCH3 N i -OCH;
OCH.3
HN N N NH H N N ~'T NH ,
N i =0 CH3 SO~CH2OCHI
~
N = TFA
N
H
Representative compounds of the present invention, ,vhich are
encompassed bv Formula I. include, but are not limited to. the compounds in
Table 1 and their pharmaceutically acceptable acid or base addition salts,
ester or
amide analoas, and prodrugs thereof.
The compounds of the present invention can exist in unsolvated forms as
well as solvated forms. includin2 hvdrated forms. In general. the solvated
forms,
including hydrated forms, are equivalent to unsolvated forms and are intended
to
be encompassed within the scope of the present invention.
Some of the compounds of Formula I have one or more chiral centers, and
can thus exist as individual stereoisomers and mixtures thereof. Other
compounds
can exist in more than one aeometric form. This invention includes all optical
and
tzeometric isomers and forms. and mixtures thereof. Racemic mixtures of
invention compounds are readily resolved into individual isomers by routine
methods such as chromatography. fractional crvstallization. and classical
resolution using opticallv active acids and salts. The individual isomers can
also
be prepared by chiral svnthesis. including chiral hvdrogenations and the like
using
commerciallv available chiral catalysts.
The compounds of the present invention are useful for treatins-i cancer (for
example. leukemia. and cancer of the lung. breast, prostate. and skin such as
melanoma) and other proliferative diseases, including. but not limited to,
psoriasis. HSV. HIV, restenosis. and atherosclerosis. To utilize a compound of
the
present invention to treat cancer, a patient having cancer is administered a
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-21-
therapeutically effective amount of a pharmaceutically acceptable composition
comprising an invention compound.
A further embodiment of this invention is a method of treating patients
sufferina from diseases caused by vascular smooth muscle cell proliferation.
Compounds within the scope of the present invention effectively inhibit
vascular
smooth muscle cell proliferation and migration. The method entails inhibiting
vascular smooth muscle proliferation, and/or migration by administering an
effective amount of a compound of Formulas I to VI to a subject in need of
treatment. "Subject" and "patient". as used herein, is a mammal such as a
human,
but also includes horses, cattle, sheep. and companion animals such as do;s
and
cats.
The compounds of the present invention can be formulated and
administered in a wide variety of oral and parenteral dosage forms, including
transdermal and rectal administration. It will be recognized to those skilled
in the
art that the following dosage forms may comprise as the active component,
either
a compound of Formula I or a corresponding pharmaceutically acceptable salt or
solvate of a compound of Formula I.
A further embodiment of this invention is a pharmaceutical composition
comprising a compound of Formulas I to VI together with a pharmaceutically
acceptable carrier. diluent. or excipient therefor. For preparing
pharmaceutical
compositions with the compounds of the present invention.
pharmaceuticallyacceptable carriers can be either a solid or liquid. Solid
form preparations include
powders, tablets, pills, capsules. cachets. suppositories. and dispensable
granules.
A solid carrier can be one or more substances which may also act as diluents,
flavoring agents, binders, preservatives. tablet disintegrating agents, or an
encapsulating material.
In powders. the carrier is a finely divided solid such as talc or starch which
is in a mixture with the finely divided active component. In tablets, the
active
component is mixed with the carrier having the necessarv binding properties in
suitable proportions and compacted in the shape and size desired.
The formulations of this invention preferably contain from about 5% to
about 70% or more of the active compound. Suitable carriers include ma(inesium
carbonate. magnesium stearate, talc. suLar. lactose. pectin. dextrin. starch,
gelatin.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-22-
tragacanth, methvlcellulose, sodium carboxvmethvlcellulose. a low melting wax,
cocoa butter, and the like. A preferred form for oral use are capsules. which
include the formulation of the active compound with encapsulating material as
a
carrier providing a capsule in which the active component with or without
other
carriers. is surrounded by a carrier, which is thus in association with it.
Similarly.
cachets and lozenges are included. Tablets, powders, capsules, pills. cachets.
and
lozenges can be used as solid dosage forms suitable for oral administration.
For preparing suppositories. a low melting wax, such as a mixture of fatty
acid glycerides or cocoa butter. is first melted, and the active component is
dispersed homogeneously therein. as by stirring. The molten homoaenous mixture
is then poured into convenient size molds. allowed to cool, and thereby to
solidify.
Liquid form preparations include solutions, suspensions, and emulsions
such as water or water/propylene glycol solutions. For parenteral injection,
liquid
preparations can be formulated in solution in aqueous polyethylene glycol
solution, isotonic saline, 5% aqueous glucose, and the like. Aqueous solutions
suitable for oral use can be prepared by dissolving the active component in
water
and adding suitable colorants. flavors, stabilizing and thickening agents as
desired.
Aqueous suspensions suitable for oral use can be made by dispersing the finely
divided active component in water and mixina with a viscous material. such as
natural or synthetic gums. resins, methvlcellulose. sodium
carboxvmethylcellulose. or other well-known suspending agents.
Also included are solid form preparations that are intended to be
converted, shortly before use. to liquid form preparations for oral
administration.
Such liquid forms include solutions, suspensions, and emulsions. These
preparations may contain, in addition to the active component. colorants,
flavors,
stabilizers. buffers, artificial and natural sweeteners, dispersants,
thickeners,
solubilizing aaents, and the like. Vvaxes, polymers, microparticles, and the
like can
be utilized to prepare sustained-release dosage forms. Also, osmotic pumps can
be
employed to deliver the active compound uniformly over a prolonged period.
The pharmaceutical preparations of the invention are preferably in unit
dosage form. In such form. the preparation is subdivided into unit doses
containing appropriate quantities of the active component. The unit dosage
form
can be a packaged preparation. the package containing discrete quantities of
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-23-
preparation, such as packeted tablets, capsules, and powders in vials or
ampules.
Also. the unit dosage form can be a capsule. tablet, cachet. or lozenge
itself. or it
can be the appropriate number of any of these in packaged form.
The therapeutically effective dose of a compound of Formula I will
generally be from about 1 to about 100 mg/kg of body weight per day. Typical
adult doses will be about 50 to about 800 mg per day. The quantity of active
component in a unit dose preparation may be varied or adjusted from about 0.1
to
about 500 ma, preferably about 0.5 to 100 mg according to the particular
application and the potencv of the active component. The composition can, if
desired, also contain other compatible therapeutic agents. A subject in need
of
treatment with a compound of Formula I is administered a dosage of about 1 to
about 500 mg per day. either singly or in multiple doses over a 24-hour
period.
The compounds of the present invention are capable of binding to and
inhibiting the activity of proteins having the ability to phosphorvlate other
proteins, such as cdks, PDGFr. FGFr, c-Src, and EGFr. Cdks form complexes with
cyclins, and these complexes phosphorylate key proteins allowing cells to
proceed
through the cell cycle (Meijer L., Progress in Cell Cvcle Research,
1995:1:35 1-363). The compounds of this invention inhibit this phosphorylation
and therefore can be used as anti-proliferative agents for the treatment of
cancer
and/or restenosis and other proliferative diseases.
Because of their inhibitory activitv against cdks and other kinases, the
compounds of the present invention are also useful research tools for studving
the
mechanism of action of those kinases, both in vitro and in vivo.
The preparation and use of the compounds of this invention are further
described in the followinQ detailed example. The examples are intended to
illustrate particular embodiments of the invention, and are not intended to
limit the
scope of the specification or the claims in anv way. The invention compounds
are
prepared by svnthetic methodologies well-known to those skilled in the art of
or2anic chemistnT, and utilize commercially available starting materials and
reaaents.
It may be desirable during the synthesis of an invention compound to
derivatize reactive functional groups in the molecule undergoing reaction so
as to
avoid unwanted side reactions. Functional groups such as hydroxy, amino, and
CA 02397961 2002-07-22
WO 01/55147 PCT/1B01/00069
-24-
acid groups typically are protected with suitable groups that can be readily
removed when desired. Use of common protecting groups is described fullv by
Green and Wuts in Protective Groups in Organic Svnthesis, John Wiley and Sons.
New York. Ne~~- York (?"d Edition. 1991). Typical hvdroxv protecting groups
include ether forming groups such as benzvl, and aryl groups such as
tert-butoxvcarbonyl (Boc). formvl, and acetv1. Amino protecting groups include
benzvl, arvl such as acetvl, and trialkvlsilvl groups. Carboxylic acid groups
typically are protected by conversion to an ester that can be easilv
hydrolyzed, for
example. trichloroethyl, tert-butyl. benzyl, and the like.
As noted above. some of the invention compounds have one or more chiral
centers. and thus can exist as individual optical isomers and geometric
isomers,
and mixtures thereof. Compound 106, for example. has two asvmmetric centers,
and has the cis configuration. This invention includes all such geometric
isomers,
enantiomers and RS racemates. as well as the individual R or S isomers of
chiral
compounds. All individual isomers and mixtures thereof are included in this
invention. Individual isomers are readily prepared by a chiral synthesis, or
by
conventional resolution techniques well-known to those skilled in the art.
An illustration of the preparation of compounds of the present invention is
shown in Schemes 1-4. The synthesis of Compound 1(Example 15) is depicted in
Scheme 1: however, it should be recognized that the general scheme is
applicable
to all of the invention compounds. Each step shown in the Schemes is further
illustrated in the detailed examples that follow.
In Scheme 1. a 2-methvlthio-4-halo-5 -alkoxvcarbonvlpvrimidine is reacted
with ammonium hydroxide to give the correspondino 4-amino derivative. The
ester is reduced by reaction by reaction with LiAI H4 to give the
5-hvdroxvmethvl analog. which in turn is oxidized to a 5-formyl derivative.
The
5-formvl group is converted to an unsaturated (acrvlate) group, which is
cvclized
to form a pvrido[2.3-d]pvrimidine. The pvridopvrimidine is converted to a key
intermediate. namelv 2-methvlsulfanyl-pvrido[2.3-d]pvrimidine-7-vlamine, which
is readilv oxidized to 2ive a?-methvlsulfinvl analog. The 2-methvlsulfinyl
group
is easily displaced bv reaction with an amine R-)NH-) to provide the invention
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
_2 5_
compounds of Formula I. The 7-amino group on the pyridopyrimidine ring is
readily converted to a urea by reaction with an isocyanate such as R19 N=C=O.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-26-
Scheme 1
O O
N O~\ a N b N OH
o
SN CI SN NH2 S N NH-7
I I ~
0 0
c N H d N\ O/\ e
~ ---~ ~ ---~
--~ i
i NH-,
N
I NH
N \ \ 1;
~ f
S,~ O S-N N C1 S N NH,
H
N
HN NH2
,
N \ \ i / j
i NH-,
CN)
N
I
Boc
N N
HN N 'H -~ HN IN H
HN11~1O HN'I-~IO
\ I \ I
(ND CN)
N N
I H
Boc
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-27-
The reactants shoAn in Scheme I have the following meanings:
(a) NH3; (b) LAH; (c) Mn02; (d) Ph3PCHCO2Et; (e) DBU: (f) POC13; (g) NH-:
(h) ( )-Trans-2-(phenylsulfonyl)-3-phenyloxaziridine; (i) 4-(4-Boc-
piperidine)aniline; (j) NaH, t-Butvlisocyanate; (k) HC1
Scheme 2 illustrates an alternative synthesis of pyridopyrimidine having a
urea functionality at the 7-position. Whereas such ureas were prepared in
Scheme I by reaction of an isocyanate with a 7-amino-pyridopyrimidine,
Scheme 2 utilizes carbonvldiimidazole to provide an intermediate imidazolide.
The imidazolide readilv reacts with an amine R19NH2 to give the correspondina
urea. Scheme 2 illustrates this process by showing the synthesis of Compound
51,
and is more fully described in Example 3 2.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-28-
Scheme 2
N \ \ ~i \ \
H1~ / N H, a 1 IT~ H b_c
NN O
\ \ ~
CN) N
N
Boc Boc
r
HN H
HN O
N
H 51
Conditions: (a) NaH. CarbonvIdiimidazole: (b) Cvclopentvlamine; (c) HCI
Compounds of Formula 1 may also be prepared according to Scheme 3,
~ wherein the svnthesis of compound 4 (Example 45) is depicted. 4-Amino-2-
methanesulfanyl-pyrimidine-5 -carboxaldehy de is reacted with methyl magnesium
bromide to 2ive the corresponding -~-(2-h},droxveth),l)-pyrimidine. The
alcohol is
oxidized to give the methyl ketone analoii. The methyl ketone is reacted with
diethvl cyanomethvl phosphonate and cvclized to a5-methvl-7-amino-
pyridopvrimidine. Further reaction as in Schemes I or 2 gives invention
compounds such as compound 4.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-29-
3
Scheme
O
?''I \ H a N OH b N O
S NH,~
NH-2 S~'J~N NH-2
~ ~ I I
N
asin HN ' ' NH
c N\ Scheme I or 2_
HN' O
i N NH2
CN)
N
H 4
Conditions: (a) MeMgBr; (b) MnO-): (c) (EtO)2P(O)CH-)CN
Compounds of Formula I may also be prepared according to Scheme 4,
~ -,,vherein the svnthesis of compound 12 (Example 40) is depicted. In this
scheme,
the ?-methvlthio group of a pyrimidine is first oxidized to the corresponding
methvlsulfinyl analog. The methylsulfinyl group is displaced by reaction with
an
amine R2NH-). The 5-carboxaldehvde is then derivatized as in Scheme 1 and
cvclized to give the corresponding 2-(R2NH) substituted 7-amino
pyridopvrimidine. The 7-amino uroup is arvlated or otherwise derivatized as
illustrated in Schemes 1-3.
CA 02397961 2004-12-29
65920-142
-30-
Scheme 4
1\ ~ O
N O a N O b IfN NH,
I --~ --~
S NH, O"NH,
Boc
N N i i
c HN NH, Scheme I or ? HN H
r
HN O
N
H 12
Boc
Conditions: (a) ( )-Trans-2-(phenylsulfonyl)-3-phenyloxaziridine: (b) 4-(4-Boc-
piperidine)-aniline: (c) (EtO)2P(O)CH-)CN
Any of the invention compounds of Formulas 1-VI mav be prepared
according to Schemes 1-4. wherein the synthesis of compounds 1. 51. 4. and 12.
respectively, are illustrated. Those having skill in the art of organic
chemistry will
recognize that the startina materials may be varied. and additional steps may
be
employed to produce compounds encompassed bv the present invention, as
demonstrated by the following specific examples.
CA 02397961 2004-12-29
65920-142
-30a-
Examples of compounds falling within the scope of
the present invention include:
1-tert-Butyl-3-(2-phenylamino-pyrido[2,3-d]pyrimidin-7-yl)-
urea;
1-tert-Butyl-3-[2-(4-fluoro-3-methyl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(4-Chloro-phenyl)-3-[2-(4-fluoro-3-methyl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Isopropyl-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-tert-Butyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-Cyclohexyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-Cyclopentyl-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimdin-7-yl]-urea;
1-Cyclohexyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-6-fluoro-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-Cyclopentyl-3-[5-methyl-2-(4-piperazin-l-yl-phenylamino)-
pyrido[2,3-d]pyrimdin-7-yl]-urea;
1-Cyclohexyl-3-[6-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-3-[6-bromo-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-3-[6-cyano-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
CA 02397961 2004-12-29
65920-142
-30b-
1-Cyclohexyl-3-[6-chloro-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-3-[6-fluoro-5-methyl-2-(4-piperazin-1-yl-
phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-3-[6-bromo-5-methyl-2-(4-piperazin-1-yl-
phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-3-[6-chloro-5-methyl-2-(4-piperazin-1-yl-
phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Isopropyl-3-[5-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Ethyl-3-[5-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-[5-Methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Methyl-3-[5-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-l-methyl-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(4-Hydroxy-cyclohexyl)-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(4-Amino-cyclohexyl)-3-[2-(4-piperazin-l-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(2-Dimethylamino-ethyl)-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(3-Morpholino-4-yl-propyl)-3-[2-(4-piperazin-1-yl-
phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea;
CA 02397961 2004-12-29
65920-142
-30c-
3-Cyclohexyl-l-methyl-l-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
N,N-Dimethyl-N'-[5-methyl-2-[[4-(1-piperazinyl)phenyl]-
amino]-pyrido[2,3-d]pyrimidin-7-yl]-sulfamide;
1-Cyclohexyl-3-[5-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-thiourea;
N-[2-(4-Piperazin-1-yl-phenylamino)-pyrido[2,3-d]pyrimidin-
7-yl]-acetamide;
4-[7-(3-Cyclohexyl-ureido)-pyrido[2,3-d]pyrimidin-2-ylamino]-
benzenesulfonamide;
1-Cyclohexyl-3-{2-[4-(1-piperazin-1-yl-methanoyl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-Cyclohexyl-3-[2-(4-fluoro-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(2-{4-[4-(2-Amino-4-methyl-pentanoyl)-piperazin-1-yl]-
phenylamino}-pyrido[2,3-d]pyrimidin-7-yl)-3-cyclohexyl-urea;
1-(2-{4-[4-(2-Amino-3-methyl-butanoyl)-piperazin-1-yl]-
phenylamino}-pyrido[2,3-d]pyrimidin-7-yl)-3-cyclohexyl-urea;
4-{4-[7-(3-tert-Butyl-ureido)-pyrido[2,3-d]pyrimidin-2-
ylamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl
ester;
4-{4-[7-(3-Cyclohexyl-ureido)-pyrido[2,3-d]pyrimidin-2-
ylamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl
ester;
1-(3-Hydroxy-propyl)-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
CA 02397961 2004-12-29
65920-142
-30d-
1-((S)-1-Hydroxymethyl-3-methyl-butyl)-3-[2-(4-piperazin-l-
yl-phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea;
4-Methyl-piperazine-l-carboxylic acid [2-(4-piperazin-1-yl-
phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-amide;
Morpholine-4-carboxylic acid [2-(4-piperazin-1-yl-
phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-amide;
3-[2-(4-Piperazin-1-yl-phenylamino)-pyrido[2,3-d]pyrimidin-
7-yl]-1,1-dipropyl urea;
Piperazine-l-carboxylic acid[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-amide;
1-((R)-1-Hydroxymethyl-2-methyl-propyl)-3-[2-(4-piperazin-l-
yl-phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea;
1,1-Bis-(2-hydroxy-ethyl)-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-[6-Bromo-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butyl-urea;
1-[6-Bromo-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-3-methyl-urea;
1-{6-Bromo-2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-3-tert-butyl-urea;
1-{6-Bromo-2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-3-cyclohexyl-urea;
1-[2-(4-Fluoro-phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-3-
(3-morpholin-4-yl-propyl)-urea;
1-[2-(4-Fluoro-phenylamino)-pyrido[2,3-d]pyrimidin-7-yl]-3-
(2-hydroxy-ethyl)-urea;
CA 02397961 2004-12-29
65920-142
-30e-
1-(2-Amino-ethyl)-3-[2-(4-fluoro-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-(2-Dimethylamino-ethyl)-3-[2-(4-fluoro-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-Cyclohexyl-3-{2-[4-(3,3-dimethyl-piperazin-l-yl)-
phenylamino]-6-fluoro-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-tert-Butyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-6-fluoro-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-{2-[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-
pyrido[2,3-d]pyrimidin-7-yl}-3-(3-morpholin-4-yl-propyl)-
urea;
1-tert-Butyl-3-{6-chloro-2-[4-(cis-3,5-dimethyl-piperazin-l-
yl)-phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-urea;
3-Cyclohexyl-l-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-1-methyl-urea;
3-Cyclohexyl-l-ethyl-l-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
3-tert-Butyl-l-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-l-ethyl-urea;
1-[5-Methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-3-propyl-urea;
7-(3-tert-Butyl-ureido)-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidine-6-carboxylic acid ethyl ester;
1-[6-Fluoro-5-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-3-isopropyl-urea;
1-Cyclohexyl-3-{2-[4-(3,3-dimethyl-piperazin-1-yl)-
phenylamino]-pyrido[2,3-d]pyrimidin-7-yl}-urea;
CA 02397961 2004-12-29
65920-142
-30f-
1-Cyclohexyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-6-methyl-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-tert-Butyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-6-methyl-pyrido[2,3-d]pyrimidin-7-yl}-urea;
1-tert-Butyl-3-[6-methyl-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea;
1-{2-[4-(cis-3,5-Dimethyl-piperazin-1-yl)-phenylamino]-6-
methyl-pyrido[2,3-d]pyrimidin-7-yl}-3-isopropyl-urea;
1-Cyclopropyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-6-methyl-pyrido[2,3-d]pyrimidin-7-yl}-urea; and
1-tert-Butyl-3-{2-[4-(cis-3,5-dimethyl-piperazin-1-yl)-
phenylamino]-6-ethyl-pyrido[2,3-d]pyrimidin-7-yl}-urea.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-31-
The invention is illustrated further by the following detailed examples
which are not to be construed as limiting the invention in scope or spirit to
the
specific procedures described in them. The starting materials and various
intermediates may be obtained from commercial sources, prepared from
commercially available organic compounds, or prepared using well-known
synthetic methods.
EXAMPLE 1
4-Amino-2-methanesulfanylp,rimidine-5-carboxy/ic acid ethYl ester
To a room temperature solution of 4-chloro-2-methanesulfanvl-
pyrimidine-5-carboxvlic acid ethyl ester (15.0 g, 65 mmol) in 200 mL of
tetrahvdrofuran is added 25 mL of triethylamine followed bv 35 mL of aqueous
ammonium hydroxide. After stirring at room temperature for 1.5 hours, an
additional 30 mL of aqueous ammonium hydroxide is added. and stirring is
continued for 1 hour. The reaction mixture is concentrated in vacuo and
partitioned between ethyl acetate and saturated aqueous sodium bicarbonate.
The
organic laver is washed with brine. dried over magnesium sulfate, filtered,
and
concentrated in vacuo. Ethyl acetate and hexane are added, and the resultant
solid
is collected by filtration to provide 10.84 g (79%) of 4-amino-2-
methanesulfanyl-
pyrimidine-5-carboxvlic acid ethvl ester.
EXAMPLE 2
(4-Anzino-2-methanesulfanti7! pyrimidiur-5-t,!)-methano!
A solution of 4-amino-2-methanesulfanvl-pyrimidine-5-carboxvlic acid
ethyl ester (13.36 g, 63 mmol) in 250 mL of tetrahvdrofuran is added dropwise
to
a room temperature suspension of lithium aluminum hydride (3.82 g. 100 mmol)
in 250 mL of tetrahvdrofuran. After 30 minutes. the reaction is cooled to 0 C,
and
isopropyl alcohol is added until bubbling diminishes. The reaction is quenched
with 15 mL of water. 15 mL of 15% NaOH, and 50 mL of water. and the mixture
is stirred for 1 hour. The white precipitate is removed by filtration and
washed
with ethyl acetate. The filtrate is concentrated in vacuo and 3:1 hexane:ethyl
acetate is added. The solids are collected, washed with 3: ] hexane:ethyl
acetate,
followed bv hexane. The solid is dissolved in ethyl acetate, and the solution
is
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-32-
dried over magnesium sulfate. Filtration followed by concentration in vacuo
gives
8.14 g (76%) of (4-amino-2-methanesulfanvl-pvrimidin-5-vl)-methanol.
Analysis calculated for C6H9N3OS:
C. 42.09: H. 5.30; N. 24.54.
Found: C. 42.31: H. 5.24; N, 24.27.
EXAMPLE 3
4-Afnino-2-methanesulfany,lp=rimidine-S-carboxaldeh_yde
To (4-amino-2-methanesulfanyl-pvrimidin-5-vl)-methanol (8.14 g.
48 mmol) in 1 L of chloroform is added manEianese oxide (33.13 ~. 381 mmol).
The suspension is stirred at rooni temperature overnight then filtered through
celite and Nvashed with 300 mL of chloroform. The filtrate is concentrated
in vacuo to give 8.14 g (quantitative yield) of 4-amino-2-methanesulfanvl-
pvrimidine-5-carboxaldehvde, mp 185-187 C. Literature mp = 183-184 C, JOC,
1958:23:1738.
Analvsis calculated for C6H7N3OS:
C. 42.59; H. 4.17: N. 24.83.
Found: C. 42.84: H, 4.21: N. 24.73.
EXAMPLE 4
Ethy13-(4 Amiizo-2-methanesulfaiiylp,rirnidin-5-y1)acrvlate
To a room temperature solution of 4-amino-2-methanesulfanvl-pvrimidine-
5-carboxaldevde (4.08 g. 24.14 mmol) in 100 mL of tetrahydrofuran is added
(carbethoxvmethylene) triphenvlphosphorane (10.80 31 mmol). The reaction
mixture is heated at reflux for 3 hours then stirred at room temperature
overnight.
The reaction mixture is concentrated in vacuo, and the residue is purified by
flash
chromatography. eluting with 1:1 ethyl acetate:hexane. to provide 4.30 g (75%)
of
ethyl 3-(4-amino-2-methanesulfanv l-pvrimidin-5-vl )acrvlate: mp softens at
108 C.
Analvsis calculated for C 1 pH 1;N ;O~S:
C. 50.19: H. 5.48: N. 17.56.
Found: C. 50.22: H. 5.45: N. 17.24.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-3J-
EXAMPLE 5
2-Methanesulfanhl-8H-pvrido[2,3-dJpvrimidin- 7-one
To a room temperature solution of ethyl 3-(4-amino-2-methanesulfanvl
pvrimidin-5-vl)acrvlate (368 mg, 1.53 mmol) in 3 mL of N.N-
diisopropylethylamine is added 380 L of 1,8-diazabicvclo[5.4.0]undec-7-ene.
The reaction mixture is heated at reflux for 3 hours then cooled to room
temperature and concentrated. The residue is purified by flash chromatography
eluting v6th ethvl acetate. The fractions containinci the product are
partiallv
concentrated in vacuo, and the solids are removed bv filtration to provide 134
ma
(45%) of 2-methanesulfanvl-BH-pyrido[2.3-d]pvrimidin-7-one. mp 269-271 C.
Analvsis calculated for C8H7N3OS:
C. 49.73. H. 3.65 : N. 21.75.
Found: C. 49.67: H. 3.46: N. 21.49.
EXAMPLE 6
7-C/rloro-2-ntethvlsulfanyl pvrido[2,3-dJpvrintidine
A suspension of 1.0 cy (5.2 mmol) of 2-methvlsulfanvl-8H-
pvrido[2.3-d]pyrimidin-7-one (Example 5) in 10 mL of phosphorus oxvchloride is
heated under reflux for 1 hour. The resultina solution is cooled and
concentrated
to 2ive a solid. which is triturated with cold water and filtered to aive 1.05
g of
crude product. Recrvstallization from acetonitrile gives 0.76 g(69 ro) of the
product. mp 201-203 C.
MS (APCI) M+1: Calcd 212.0; Found 212Ø
Anal. Calcd for C8H6C1 I S I N3:
C. 45.3 9: H. 2.86: N. 19.85.
Found: C. 45.53: H. 2.90: N. 19.74.
EXAMPLE 7
2-Meth hlsttlfan t,lp,rido[2,3-d]pti=rimiditt- 7-vlantine
A suspension of 2.95 g(13.9 mmol) of 7-chloro-2-methvlsulfanvl-
pvrido[2.3-d]pvrimidine (Example 6) in 200 mL of isopropanol saturated with
ammonia is sealed and heated at 40 C for 65 hours. The suspension is
resaturated
CA 02397961 2002-07-22
WO 01/55147 PCT/1B01/00069
-34-
with ammonia and heated for another 18 hours at 40 C. The solid is collected
bv
filtration and triturated with water to give 1.98 g (74.2%) of the product,
mp >250 C.
MS (APCI) M+l : Calcd 193.1: Found 193Ø
Anal. Calcd for C8H8N4S I:
C. 49.98; H. 4.19: N. 29.14.
Found: C, 50.14: H. 4.22: N, 29.04.
EXAMPLE 8
2-Nfetltanesuljrnti,! pti,rido[2,3-dJpt'rintidin-7-ylantine
A suspension of 10.63 g (55.3 mmol) of 2-methylsulfanvl-
pvrido[2,3-d]pvrimidin-7-ylamine (Example 7) in 300 mL of dichloromethane and
300 mL of methanol is treated with 18.06 g (69.1 mmol) of ( )-trans-2-
(phenvlsulfonyl)-3-phenyloxaziri dine and stirred overnight. The suspension is
filtered to remove a small amount of solid. concentrated to approximately 25
mL,
and diluted with ethvl acetate. The solid is collected by filtration to give
9.27 g
(80.5%) of the product. mp 180 C (dec).
MS (APCI) M+1: Calcd 209.0; Found 209.1.
EXAMPLE 9
N2-Plienv! pti,rido[2,3-dJpt'rintidine-2, 7-diantine
A suspension of 0.44 - (2.1 mmol) of 2-methanesulfinvl-pvrido[2.3-
d]pyrimidin-7-ylamine (Example 8) and 0.39 mL (4.2 mmol) of aniline in 2 mL of
dimethylsulfoxide is heated at 100 C overnight. The resulting solution is
cooled
and poured into water. Ethyl acetate is added to the suspension. and the solid
is
collected by filtration. The solid is purified by flash chromatography.
eluting with
gradient of 0% to 20% methanol/dichloromethane durino 30 minutes to give
0.14 g(29%) of the product. mp 255-260 C.
MS (APCI) M+1: Calcd 238.1: Found 238.1.
Anal. Calcd for CI - HI IN5=0.18 H20:
C. 64.92: H, 4.76: N. 29.12.
Found: C. 65.26: H. 4.75: N. 28.76.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-35-
EXAMPLE 10
1-tert-Butv1-3-(2 phenvlamino pyrido[2,3-dJpvrimidin-7 yl)-urea
To a solution of 0.1022 g (0.431 mmol) of N2-phenyl-pvrido[2.3-
d]pyrimidine-2,7-diamine (Example 9) in 2 mL of dimethvlformamide. cooled in
an ice bath, is added 0.019 g (0.47 mmol) of 60% sodium hydride. The resulting
solution, cooled in an ice bath. is then treated with 0.054 mL (0.47 mmol) of
tert-butyl isocyanate. The solution is stirred cold for 15 minutes. then at
room
temperature for 1 hour. The solution is poured into ice-water to give a solid
which
is collected bv filtration and washed with hexane to give 0.0849 g (57.8%) of
the
product (compound 45), mp 227 C (dec).
MS (APCI) M+1: Calcd 337.2; Found 337.1.
Anal. Calcd for C 1 8H2ON6O1 =0.27 H20:
C, 63.35; H. 6.07; N, 24.63.
Found: C. 63.73: H. 5.82: N. 24.20.
EXAMPLE 11
4-(4-Nitrophenyl) piperazine-l-carboxvlic acid tert-butvl ester
A suspension of 7.5 g(36 mmol) of 1-(4-nitrophenvl)-piperazine and
6.94 mL (40 mmol) of ethyl-diisopropyl-amine in 75 mL of dichloromethane is
treated with 8.69 g (40 mmol) of di-tert-butyl dicarbonate and stirred at room
temperature overnight. The resulting solution is washed with saturated aqueous
sodium bicarbonate, then with water, dried (magnesium sulfate), and
concentrated.
The resulting material is purified by flash chromatography eluting with a
gradient
of 10% to 30% ethyl acetate/hexane during 10 minutes to give 8.62 g (77.5%) of
the product, mp 136-140 C.
MS (APCI) M+l: Calcd 308.2: Found 308.2.
EXAMPLE 12
4-(4-Antino p/renvl) piperazine-l-carboxylic acid tert-butyl ester
To a suspension of 1.46 g (4.8 mmol) of 4-(4-nitrophenvl)-piperazine-
1-carboxvlic acid tert-butvl ester (Example 11) and I g of Raney Nickel in 50
mL
of tetrahvdrofuran is added hvdroaen to an initial pressure of 54.5 psi. The
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-36-
reaction is shaken for 14 hours and then filtered. The filtrate is
concentrated to
give 1.29 g (97%) of the product as a solid.
MS (APCI) M+1: Calcd 278.2; Found 278.2.
Anal. Calcd for C15H23N302:
C. 64.96: H. 8.36: N. 15.15.
Found: C. 65.22: H. 8.58: N, 14.58.
EXAMPLE 13
4-14-(7-Amino pvrido[2,3-dJp1'rimidin-2-i,lamino) pheiit'IJ-piperazine-
1-carboxi=lic acid tert-butyl ester
Bv substitutin~ 4-(4-amino-phen),l)-piperazine-I-carboxvlic acid tert-butyl
ester (Example 12) for aniline in Example 9. 0.0744 g(36.0%) of the product is
obtained. mp 219-220 C.
MS (APCI) M +1: Calcd 422.2; Found 422.2.
Anal. Calcd for C22H-27N7O2-0.5 H20:
C. 61.38: H, 6.56: N. 22.77.
Found: C. 61.34: H, 6.30: N. 22.47.
EXAMPLE 14
4-{4 -17-(3-tert-Butyl-ureido) pti,rido/2,3-d]phrintidin-2-ti'laminoJ-phenv!}-
piperazine-1-carbo.zylic acid tert-butl,/ ester
Bv substitutina 4-[4-(7-amino-pyrido[2.3-d]pyrimidin-2-ylamino)-
phenyl]-piperazine-l-carboxvlic acid tert-butyl ester (Example 13) for N2-
phenyl-
pyrido[2.3-d]pyrimidine-2.7-diamine in Example 10, 0.3354 g12 (67.9%) of the
product (compound 79) is obtained, mp 225 C (dec).
MS (APCI) M+l: Calcd 521.3; Found 521.2.
Anal. Calcd for C.27H36N803:
C. 62.29: H. 6.97: N. 21.52.
Found: C. 62.33: H. 6.81: N. 21.43.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-3 7-
EXAMPLE 15
1-tert-Butvl-3-[2-(4 piperazin-1 yl-phenylannino) pyrido[2,3-dJpt'rin:idin-7-
t'1J-
urea
To a suspension of 0.100 g(0.192 mmol) of 4-{4-[7-(3-tert-butvl-ureido)-
pvrido[2.3-d]pyrimidin-2-v lamino]-pheny l}-piperazine-l-carboxy lic acid tert-
butvl ester (Example 14) in 2 mL of methanol is added 2 mL of 4 M hydrogen
chloride/dioxane to give a solution. The suspension is stirred at room
temperature
overnisiht, then diluted with diethyl ether. The material is collected by
filtration to
give 0.0941 - (93.4%) of the product (compound 1). mp 21 5 C (dec).
MS (APCI) M+l: Calcd 421.2; Found 421.1.
Anal. Calcd for C-)H-)gNgO1 -2.10 HC1. 1.51 H-)O:
C. 50.40: H. 6.37: N. 21.37; Cl (total). 14.20.
Found: C. 50.40: H. 6.18: N. 21.03: Cl (total), 14.33.
EXAMPLE 16
4-{4-[7-3-Cvclohe.till-ureido) pt'rido[2,3-dJpt~rimidin-2-ylan:irnoJ-phenyl}-
piperazine-1-carboxylic acid tert-butyl ester
BN, substitutin2 cvclohexvl isoc-vanate for tert-butvl isocvanate in
Example 14. 0.1463 g (70.4%) of the product (compound 80) is obtained,
mp 241 C (dec).
MS (APCI) M-1: Calcd 547.3: Found -547.4.
Anal. Calcd for C-)9H38NgO;-0.28 H-)O:
C. 63. I 3: H. 7.04: N. 20. 31.
Found: C. 63.14: H. 6.81: N. 20.2 5.
EXAMPLE 17
1-C=clohe~:1,l-3-[2-(4 piperazhr-1-~=I-phent.lantino) phrido[2,3-dJpt~rin:idin-
7-ylJ-urea
Bv substituting 4-,4-[7-(3-c%,clohexvl-ureido)-ptirido[2.3-d]pyrimidin-
2-vlamino]-phen,,l }-piperazine- l-carboxvlic acid tert-butyl ester (Example
16) for
4-{4-[7-(3-tert-butvl-ureido)-p~'rido[2.3-d]pvrimidin-2-vlamino]-phenvl}-
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-38-
piperazine-l-carboxylic acid tert-butyl ester in Example 15. 0.0871 g (81.4%)
of
the product (compound 9) is obtained, mp 200 C (dee).
MS (APCI) M+1: Calcd 447.3; Found 447.3.
Anal. Calcd for C24H30N8O 1=2.55 HC1=2.82 H2O:
C. 48.83; H. 6.52; N, 18.98; Cl (total), 15.31.
Found: C. 48.83: H, 6.18: N. 18.89; Cl (total). 15.37.
EXAMPLE 18
N2-(4-Fl uoro-3-meth ti,l-ph en yl) prido[2,3-d]phrimidine-2, 7-diamine
Bv substitutinR 4-fluoro-3-methvlaniline for aniline in Example 9,
0.2025 g(392%) of the product is obtained as a solid.
MS (APCI) M + 1: Calcd 270.1; Found 270Ø
EXAMPLE 19
1-tert-Butvl-3-[2-(4 Jluoro-3-methl~l-phenylamino) pvrido[2,3-dJpyrimidin-
7 y1]-urea
By substituting N2-(4-fluoro-3-methvl-phenvl)-pyrido[2.3-d]pyrimidine-
2.7-diamine (Example 18) for N2-phenvl-pvrido[2.3-d]pvrimidine-2.7-diamine in
Example 10, 0.0656 g (47.9%) of the product (compound 46) is obtained,
mp 230 C (dec).
MS (APCI) M+1: Calcd 369.2: Found 369.1.
Anal. Calcd for C I qH-) 1 F 1 N601:
C. 61.94: H. 5.75: N. 22.81.
Found: C. 61.82: H. 5.73: N. 22.75.
EXAMPLE 20
1-(4-Chloro phent'1)-3-[2-(4 fluoro-3-methl,l pheiiti,lan:irno)-
phrido[2,3-dJpvr[ntidin-7-vIJ-ttrea
Bv substituting 4-chlorophenvl isocvanate for tertiarv-butvl isocyanate in
Example 19, 0.050 g (37%) of the product (compound 47) is obtained,
mp >250 C.
MS (APCI) M+1: Calcd 423.1: Found 423.1.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-39-
Anal. Calcd for C,? IH16FIClIN601=0.23 H,)O:
C. 59.07; H. 3.89: N, 19.68.
Found: C. 59.09: H. 3.97: N. 19.65.
EXAMPLE 21
1-{2-[4-(4 Acetyl piperazin-1 yl) phenylaminoJ-pyrido[2,3-dJpyritnidin-
7-y1}-3-tert-butyl-urea
To a suspension of 0.145 g (0.277 mmol) of 1-tert-butyl-3-[2-(4-piperazin-
1-yl-phenylamino)-pyrido[2.3-d]pyrimidin-7-vl]-urea (Example 15) in 5 mL of
dichloromethane is added 0.19 mL (l.l l mmol) of ethyl-diisopropyl-amine. The
suspension is cooled in an ice bath and treated with 0.024 mL (0.33 mmol) of
acetyl chloride. The suspension is stirred at room temperature overnight, then
filtered. The solid is washed with dichloromethane. The filtrate and washinas
are
combined, washed with water, dried (magnesium sulfate), and concentrated. The
material is purified by flash chromatography eluting with a gradient of 0% to
5%
methanol/dichloromethane during 30 minutes to give 0.0674 g (51.8%) of the
product (compound 5). mp 206-208 C (dec).
MS (APCI) M+1: Calcd 463.3: Found 463.3.
Anal. Calcd for C24H30N8O-)=0.40 H-)O:
C. 61.36: H. 6.61: N. 23.85.
Found: C. 61.38: H. 6.37; N. 23.98.
EXAMPLE 22
4-{4-[7-(3-Isopropyl-ureido)-pyridol2,3-dJpyrimidiir-2 ylaminoJ-phenylJ-
piperazine-l-carbo.xylic acid tert-butti=l ester
By substituting isopropyl isocyanate for tert-butyl isocvanate in
Example 14. 0.909 g(69.910) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 507.3: Found 507.4.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-40-
EXAMPLE 23
1-Isopropvl-3-[2-(4 piperazin-1-v1 plrenvlamino) pvrido[2,3-dJpvrimidiir-7-vIJ-
urea
By substituting 4-{4-[7-(3-isopropyl-ureido)-pyrido[2.3-d]pyrimidin-
2-vlamino]-phenyl}-piperazine-l-carboxylic acid tert-butvl ester (Example 22)
for
4-{4-[7-( -' 3 -tert-butyl-ureido)-pyrido[2,3-d]pyrimidin-2-vlamino]-phenyl }-
piperazine- l -carboxylic acid tert-butyl ester in Example 15. 0.0287 g(27.9
/o) of
the product (compound 48) is obtained, mp 190 C (dec).
MS (APCI) M+1: Calcd 407.2; Found 407.1.
Anal. Calcd for C-) 1 H26NgO1 =2.05 TFA-0.84 H-)O:
C. 46. 00: H. 4.57: N. 17.10.
Found: C. 46.00; H. 4.65: N. 17.09.
EXAMPLE 24
Cis-3,5-dimethvl-l-(4-nitro plrenv!) piperazine
A suspension of 6.74 g (47.8 mmol) of 4-fluoro-nitro-benzene and 10.91 g
(95.5 mmol) of cis-2,6-dimethyl-piperazine is heated at 45 C for 1 hour. The
reaction mixture is cooled and shaken with dichloromethane and water. The
organic laver is dried (maanesium sulfate) and concentrated to give 11.62 g
(>100 /0) of the product as a solid.
EXAMPLE 25
Cis-2,6-dinrethyl-4-(4-nitro phent,!) piperazine-l-carbo.rti=lic acid tert-
butyl ester
By substitutinp cis-3.5-dimethvl-1-(4-nitro-phenyl)-piperazine
(Example 24) for 1-(4-nitrophenyl)-piperazine in Example 11. 14.87 g (92.8%)
of
the product as a solid is obtained.
EXAMPLE 26
4-(4-Amino pheir1,/)-cis-2,6-dimethi,/-piperazine-l-carboxl,/ic acid tert-
butyl
ester
Bv substitutin2 cis-2.6-dimethvl-4-(4-nitro-phenyl )-piperazine-
1-carboxvlic acid tert-but~~l ester (Example 25) for 4-(4-nitro-phenyl)-
piperazine-
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-41-
1-carboxvlic acid tert-butyl ester in Example 12. 5.03 g (64.7%) of the
product as
a solid is obtained.
EXAMPLE 27
4-[4-(7-Aminop,rido[2,3-d]pyrimidin-2ylamino) pheiiti,lJ-cis-2,6-dimetht'!-
piperazine-1-carboxylic acid tert-butvl ester
Bv substituting 4-(4-amino-phenyl)-cis-2.6-dimethyl-piperazine-
1-carboxvlic acid tert-butvl ester (Example 26) for aniline in Example 9.
0.6463 g
(59.8%) of the product is obtained, mp 245 C (dec).
MS (APCI) M+1: Calcd 450.3; Found 450.3.
EXAMPLE 28
4-{4-[7-(3-tert-Butyl-ureido) pvrido[2,3-d]pvrimidin-2 ylaminoJ phenti,!}-cis-
2,6-dimethy! piperazine-l-carboxylic acid tert-bcityl ester
Bv substituting 4-[4-(7-amino-pyrido[2,3-d]pyrimidin-2-ylamino)-
phenyl]-cis-2.6-dimethyl-piperazine-1-carboxylic acid tert-butyl ester
(Example 27) for N2-phenyl-pyrido[2.3-d]pvrimidine-2,7-diamine in Example 10,
0.1828 g (74.9%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 549.3: Found 549.4.
EXAMPLE 29
1-tert-Bufil-3-{2-[4-(cis-3,5-dimetht,! piperazin-1 y1) phenylaminoJ-
pyrido[2,3-d1pyrimidin-7yl}-urea
Bv substituting 4-{4-[7-(3-tert-butyl-ureido)-pyrido[2.3-d]pyrimidin-
2-ylamino]-phenvl}-cis-2.6-dimethyl-piperazine-l-carboxylic acid tert-butvl
ester
(Example 28) for 4-{4-[7-(3-tert-butvl-ureido)-pvrido[2.3-djpvrimidin-
2-vlamino]-phenvl}-piperazine-l-carboxvlic acid tert-butyl ester in Example 15
is
obtained 0.0910 g(92.9 io) of the product (compound 49). mp 245 C (dec).
MS (APCI) M+1: Calcd 449.3; Found 449.2.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-42-
EXAMPLE 30
4-{4-[7-(3-Cyclohe.xf,l-ureido) pyrido[2,3-d]pyrimidin-2 -ylan:inoJ-phenI'l}-
cis-
2,6-dimethvl piperazine-l-carboxvlic acid tert-butt,l ester
Bv substitutin2 cvclohexvl isocyanate for tert-butyl isocvanate in
Example 28. 0.1156 g (60.8%) of the product is obtained as a solid.
MS (APCI) M+l: Calcd 575.3; Found 575.3.
EXAMPLE 31
1-Ct'clohe_ry1-3-[2-[4-(cis-3,5-dimethyl piperazin-l.t'1) pheirrla-nino]-
phrido[2,3-dJpyrinnidin- 7-yl}-urea
By substituting 4-{4-[7-(3-cvclohexvl-ureido)-pyrido[2.3-d]pvrimidin-
2-vlamino]-phenyl }-cis-2.6-dimethyl-piperazine-l-carboxvlic acid tert-butvl
ester
(Example 30) for 4-{4-[7-(3-tert-butyl-ureido)-pyrido[2,3-d]pvrimidin-2-
vlamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl ester in Example 15,
0.1022 g of the product is obtained (compound 50), mp 228 C (dec).
MS (APCI) M+1: Calcd 475.2; Found 475.2.
EXAMPLE 32
4-{4-[7-(3-Cyclopentyl-ureido) pvrido[2,3-dJpyrimidin-2 ylaminoJ-phenl~lJ-
piperazine-1-carboxylic acid tert-butyl ester
To a solution of 0.150 g(0.'l 6 mmol) of 4-[4-(7-amino-pyrido[2.3-
d]pvrimidin-2-vlamino)-phenvl]-piperazine-l-carboxvlic acid tert-butvl ester
(Example 13) in 2 mL of dimethvlformamide, cooled in an ice bath, is added
0.022 g (0.54 mmol) of 60% sodium hydride. The cooled solution is stirred for
15 minutes, then treated with 0.088 g (0.54 mmol) of carbonvldiimidazole. The
cooled solution is stirred for another 30 minutes. then treated with 0.071 mL
(0.72 mmol) of cvclopentvlamine. The resulting solution is stirred at room
temperature for 1 hour. then added to cold water. The solid is collected by
filtration to give a first crop of material. The aqueous filtrate is then
extracted with
dichloromethane. and the extracts are dried (magnesium sulfate) and
concentrated
to give a second crop of material. The 2 crops are combined and purified by
flash
chromatography. eluting with a gradient of 0% to 5% methanol/dichloromethane
durinu 30 minutes to give 0.1159 g(60.4%) of the product as a solid.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-43-
MS (APCI) M+1: Calcd 533.3: Found 533.4.
EXAMPLE 33
I-Cclopentvl-3-[2-(4 piperazin-1 yl phenvlamino)p'rido[2,3-dlpti,rimidin-
7 y1J-urea
By substitutinp- 4-{4-[7-(3-cyclopentvl-ureido)-pyrido[2.3-d]p,,rimidin-
2-vlamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl ester (Example 32)
for
4- { 4-[7-(3-tert-butvl-ureido )-pvrido [2,3 -d]pyrimidin-2-ylamino]-phenyl } -
piperazine-1-carboxylic acid tert-butyl ester in Example 15, 0.0937 g (80.8%)
of
the product (compound 5 1) is obtained, mp 210-213 C (dec).
MS (APCI) M+1: Calcd 433.2; Found 433.2.
Anal. Calc for C23H-?8Ng01-2.49 HCl=1.65 H2O=0.1 dioxane:
C, 50.02: H. 6.21: N. 19.94: Cl (total), 15.71.
Found: C, 49.89; H. 5.81: N. 19.74; Cl (total). 14.74.
EXAMPLE 34
1-(4 Amino-2-methylsulfanti,l pvrimidin-5 y1)-ethanol
To a suspension of 5.0 R(29 mmol) 4-amino-2-methylsulfanvl-pvrimidine-
5-carboxaldehvde (Example 3) in 150 mL of tetrahvdrofuran, cooled by an
ice bath. is added during 20 minutes. 23.2 mL of a 3.0 M methvlmaQnesium
bromide solution in diethvl ether (69.4 mmol). After 1 hour at 0 C, another
23.2 mL of the 3.0 M methvlmaLynesium bromide solution is added. and the
suspension is allowed to come to room temperature and stirred overniaht. The
reaction is quenched with 100 mL of saturated aqueous ammonium chloride. and
partitioned between water and ethvl acetate. The organic laver is dried
(mainesium sulfate) and concentrated to give 5.24 g(96%) of the product,
mp 140-142 C.
MS (APCI) M+1: Calcd 186.1: Found 185.9.
EXAMPLE 35
1-(4-Amino-2-medtvlsulfanvl pyrintidin-S yl)-ethanone
By substitutini! 1-(4-amino-2-methvlsulfanvl-pvrimidin-5-till)-ethanol
(Example 34) for (4-amino-2-meth~,lsulfanvl-pyrimidin-5-vl )-methanol in
CA 02397961 2002-07-22
WO 01/55147 PCT/IBOl/00069
-44-
Example 3 and conducting the reaction at 80 C in toluene. 3.74 g(72%) of the
product as a solid is obtained.
MS (APCI) M+1: Calcd 184.0; Found 183.9.
EXAMPLE 36
1-(4 Amino-2-methanesulfinylprimidin-5 }~l)-ethanone
Bv substituting 1-(4-amino-2-methvlsulfanyl-p~,rimidin-5-vl)-ethanone
(Example 35) for 2-methylsulfanyl-pyrido[2.3-d]pyrimidin-7-vlamine in
Example 8, 9.57 c, (88%) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 200: Found 200.
EXAMPLE 37
4-[4-(5 Acetyl-4-aminoprimidin-2 ylamino) pheyryl]piperazine-l-carboxylic
acid tert-butyl ester
Bv substituting 1-(4-amino-2-methanesulfinyl-pyrimidin-5-N,1)-ethanone
(Example 36) for 2-methanesulfinyl-p~-rido[2.3-d]pyrimidin-7-~,lamine in
Example 13, 4.04 g(65 /o) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 413; Found 413.
EXAMPLE 38
4-[4-(7-Amino-5-methyl pt'rido[2,3-dJpvrimidin-2-1,lami-to) phenti=lJ-
piperazine-
1-carboxi.,lic acid tert-butvl ester
To a suspension of 0.58 g (14.6 mmol) of 60% sodium hydride in 10 mL of
tetrahydrofuran. at 0 C. is added dropxvise 2.58 g (14.56 mmol) of diethyl
(cyanomethyl) phosphonate. The reaction mixture is stirred at 0 C for 5
minutes,
then at room temperature for 20 minutes. The mixture is then cooled to 0 C and
treated with 2 a(4.85 mmol) of 4-[4-(5-acetvl-4-amino-p~'rimidin-2-ylamino)-
phen},l]-piperazine-l-carboxN,lic acid tert-butvl ester (Example 37). The
mixture is
stirred at room temperature overnight, and then treated with water and
saturated
aqueous ammonium chloride. The resulting solid is collected by filtration and
washed ,vith ether to give 1.069 p- (80%) of the product.
MS (APCI) M+l: Calcd 436: Found 436.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBOl/00069
-45-
EXAMPLE 39
4-{4-[7-(3-Cvclohexyl-ureido)-5-meNr_yl pyrido[2,3-dJpt,rin:idirr-2-
t,latrrinoJ-
phenyl} piperazine-l-carboxvlic acid tert-butvl ester
Bv substituting 4-[4-(7-amino-5-methyl-p_yri do [2.3-d]pvrimidin-2-
ylamino)-phenyl]-piperazine-l-carboxvlic acid tert-butyl ester (Example 38)
for
4-[4-(7-amino-pvrido[2,3-d]pvrimidin-2-vlamino)-phenyl]-piperazine-l-
carboxylic acid tert-butl ester in Example 16. 0.199 2 (42%) of the product as
a
solid is obtained.
MS (APCI) M+1: Calcd 561; Found 561.
EXAMPLE 40
1-Cclohek-vl-3-[5-methyl-2-(4 piperazirt-1 yl phenvlamino)-
pyrido[2,3-dJpyrimidin-7 y1J-urea
By substituting 4-{4-[7-(3-cyclohexyl-ureido)-5-methyl-pyrido[2.3-
d]pyrimidine-2-vlamino]-phenyl; piperazine-l-carboxylic acid tert-butvl ester
(Example 39) for 4-{4-[7-()-tert-butyl-ureido)-pyrido[2,3-d]pyrimidin-2-
vlamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl ester in Example 15,
the
product (compound 12) as a solid is obtained. mp 238 C (dec).
MS (APCI) M+1: Calcd 461: Found 461.
EXAMPLE 41
5-Met/r_yl-2-met/tylsulfant'1 pt,rido[2,3-dJpvrimidin-7-ylamine
Bv substituting 1-(4-amino-2-methylsulfanvl-pyrimidin-5-vl)-ethanone
(Example 35) for 4-[4-(5-acetvl-4-amino-pyrimidin-2-vlamino)-phenyl]-
piperazine-l-carboxylic acid tert-butyl ester in Example 38, 0.97 g (85%) of
the
product as a solid is obtained.
MS (APCI) M+l: Calcd 207: Found 207.
EXAMPLE 42
2-Methanesuljn1-l-5-methyl pi,rido[2,3-dJpl,rimidin-7-vlanrine
By substituting 5-meth\-l-2-methylsulfanyl-pvrido[2.3-d]pvrimidin-7-
vlamine (Exaniple 41) for 2-meth~'lsulfanyl-pyrido[2.3-d]pyrimidin-7-ylamine
in
Example S. 0.85 -, (83%) of the product as a solid is obtained.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-46-
MS (APCI) M+1: Calcd 223; Found 223.
EXAMPLE 43
4-[4-(7-Amino-5-meth yl-p yrido[2, 3-dJpyrimidin-2-ylamino)-ph eir ylJ-
piperazin e-
1-carboxvlic acid tert-buh~l ester
By substituting 2-methanesulfinvl-5-methyl-pvrido[2.3-d]pvrimidin-7-
vlamine (Example 42) for 2-methanesulfinyl-pyrido[2.3-d]pyrimidin-7-ylamine in
Example 13, 0.33 g (20%) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 436: Found 436.
EXAMPLE 44
4-[4-[7-(3-tert-Butyl-ureido)-5-met/ryl pyrido[2,3-dJpyrimidiii-2-ylamino]-
phenyl} piperazine-l-carbox},lic acid tert-butyl ester
By substituting 4-[4-(7-amino-5-methyl-pvrido[2,3-d]pvrimidin-2-
ylamino)-phenyl]-piperazine-l-carboxylic acid tert-butyl ester (Example 43)
for
N2-phenyl-pyrido[2.3-d]pyrimidine-2,7-diamine in Example 10. 0.17 g (45%) of
the product as a solid is obtained.
MS (APCI) M+1: Calcd 535; Found 535.
EXAMPLE 45
1-tert-Butvl-3-[5-methyl-2-(4 piperazin-1 },! phenylamino)-
pyrido[2,3-dJpyrimidin- 7 .ylJ-urea
By substituting 4-{4-[7-(3-tert-butyl-ureido)-5-methyl-pyrido[2,3-
d]pyrimidin-2-vlamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl ester
(Example 44) for 4-{4-[7-(3-tert-but-,,l-ureido)-pvrido[2,3-d]pyrimidin-2-
ylamino]-phenyl}-piperazine-1-carboxvlic acid tert-butyl ester in Example 15,
0.070 g (72%) of the product (compound 4) as a solid is obtained. mp 230-232 C
(dec).
MS (APCI) M+l: Calcd 435: Found 435.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-47-
EXAMPLE 46
6-FI uoro-2-meth_ylsulfanyl-8H-pvrido[2,3-d]pyrimidin-7-one
A solution of 1.74 g (10.33 mmol) of (diethoxv-phosphoryl)-fluoro-acetic
acid ethvl ester in 20 mL of tetrahydrofuran is cooled to -78 C and treated
dropwise with 12.9 mL (20.65 mmol) of a 1.6 M solution of n-butvl lithium in
hexanes. After stirring for 30 minutes at -78 C, the solution is treated with
1.74 g
(10.33 mmol) of 4-amino-2-methylsulfanyl-pyrimidine-5-carboxaldehyde
(Example 3). allowed to warm to room temperature. and stirred overnight. The
reaction is treated with saturated aqueous ammonium chloride, then water. The
solid is collected by filtration and washed with diethyl ether to give 2.01
g(92%)
of the product.
MS (APCI) M+1: Calcd 212: Found 212.
EXAMPLE 47
7-Chloro-6-[luoro-2-methtylsulfanyl pyrido[2,3-dJpyrimidine
By substituting 6-fluoro-2-methylsulfanyl-8H-pyrido[2,3-d]pyrimidin-
7-one (Example 46) for 2-methylsulfanyl-8H-pyrido[2,3-d]pyrimidin-7-one in
Example 6 is obtained 1.86 g(85%) the product as a solid.
MS (APCI) M-'-1: Calcd 230. 232; Found 230. 2.3 )2.
EXAMPLE 48
6-Fluoro-2-metlrvlsulfanti,l pti,rido[2,3-dJpyrintidine-7-vlanune
By substituting 7-chloro-6-fluoro-2-methylsulfanyl-pyrido[2,3-
d]pyrimidine (Example 47) for 7-chloro-2-methvlsulfanyl-pyrido[2.3-
d]pyrimidine in Example 7 is obtained 0.29 g (90%) of the product as a solid.
MS (APCI) M+1: Calcd 211: Found 211.
EXAMPLE 49
6-Fluoro-2-methanesulfin1!l pvrido[2,3-dJpy,rimidin-7 ylarnine
By substituting 6-fluoro-2-methvlsulfanyl-pyrido[2.3-d]pyrimidin-7-
ylamine (Example 48) for 2-methylsulfanyl-pyrido[2,3-d]pyrimidin-7-ylamine in
Example 8. 0.26 g (95%) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 227: Found 227.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-48-
EXAMPLE 50
4-[4-(7-Amino-6 fluoroprido[2,3-dJpvrintidin-2-l'lamino) phen1,1J-cis-
2,6-dimethy!-piperazine-l-carboxvlic acid tert-butvl ester
BN, substitutinE 6-fluoro-2-methanesulfinvl-pvrido[2.3-d]pvrimidin-7-
ylamine (Example 49) for 2-methanesulfinyl-pyrido[2. 3 -d]pvrimidin-7-vlamine
in
Example 27. 0.040 g (63%) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 468; Found 468.
EXAMPLE 51
4-{4-[7-(3-Ct,clohex},1-ureido)-6 fTuoro pl,rido[2,3-dJpi,ritnidin-2
_ti,laminoJ-
phenti,lJ-cis-2,6-dimeth1,1piperaziire-l-carbox-i=lic acid tert-buh'l ester
By substituting 4-[4-(7-amino-6-fluoro-pvrido[2,3-d]pvrimidin-2-
vlamino)-phenvl]-cis-2.6-dimethyl-piperazine-l-carboxvlic acid tert-butyl
ester
(Example 50) for 4-[4-(7-amino-pyrido[2.3-d]pvrimidin-2-vlamino)-phenyl]-
piperazine-l-carboxylic acid tert-butyl ester in Example 16. 0.10 g (74%) of
the
product as a solid is obtained.
MS (APCI) M+1: Calcd 593: Found 593.
EXAMPLE 52
1-Cyclohexvl-3-{2-[4-(cis-3,5-dimetht,l piperaziir-1-1,1) pheirt'laminoJ-6-
Jluoro-
pyrido[2,3-dJpl!rimidin-7-ylJ-urea
Bv substituting 4-}4-[7-(3-cyclohex~,l-ureido)-6-fluoro-pyrido[2.3-
d]pvrimidin-2-vlamino]-phenvl}-cis-2.6-dimethvl-piperazine-l-carboxylic acid
tert-butvl ester (Example 51) for 4-}4-[7-(3-tert-butvl-ureido)-pvrido[2.3-
d]pyrimidin-2-vlamino]-phenyl }-piperazine- l -carboxylic acid tert-butyl
ester in
Example 15, 0.060 g(75%) of the product (compound 52) as a solid is obtained,
mp 227-229 C.
MS (APCI) M+l : Calcd 493: Found 493.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-49-
EXAMPLE 53
4-{4-[7-(3-Cyclopenh,l-ureido)-S-methylpyrido[2,3-dJpyrimidin-2 ylaminoJ-
phenyl} piperazine-l-carboxylic acid tert-butyl ester
Bv substituting 4-[4-(7-amino-5-methyl-pvrido[2.3-d]pvrimidin-2-
vlamino)-phenvl]-piperazine- l -carboxylic acid tert-butvl ester (Example 43 )
for
4-[4-(7-amino-pvrido[2.3-d]pvrimidin-2-vlamino)-phenvl]-piperazine-l-
carboxvlic acid tert-butvl ester in Example 32. 0.18 g (55%) of the product as
a
solid is obtained.
N1S (APCI) M+l : Calcd 547; Found 547.
EXAMPLE 54
1-Ct'clopenh~l-3-[5-ntethyl-2-(4 piperazin-1 yl phenvlantino)-
pyrido[2,3-dJpyrimidin-7 y!J-urea
Bv substituting 4-{4-[7-(3-cyclopentvl-ureido)-5-methyl-pvrido[2,3-
d]pvrimidin-2-vlamino]-phenyl}-piperazine-l-carboxylic acid tert-butyl ester
(Example 53) for 4-{4-[7-()-tert-butyl-ureido)-pvrido[2.3-d]pvrimidin-2-
vlamino]-phenvl}-piperazine-l-carboxvlic acid tert-butvl ester in Example 15,
0.08 g(70%) of the product (compound 53) is obtained. mp 234 C (dec).
MS (APCI) M+1: Calcd 447: Found 447.
EXAMPLE 55
4-(4-{7-[3-(3-Hydrozy-propy!)-ureidoJ-pyrido[2,3-dJpyrimidin-2-t'lantino}-
pheny!) piperazine-l-carboxt,lic acid tert-bufil ester
Bv substituting 3-amino-l-propanol for cvclopentvlamine. and sodium
tertiarv butoxide for sodium h%,dride in Example 32. 0.1295 g (52.2%) of the
product as a solid is obtained.
MS (APCI) M+1: Calcd 523.3: Found 523.2.
EXAMPLE 56
1-(3-Hydro_rti propt,l)-3-[2-(4 piperazin-1-ylphen_ylantino) pyrido
[2,3-d1pyrintidin- 7-y!/-urea
B%, substituting the product of Example 55 in Example 15. 0.1077 g of the
product (compound 81) as a solid is obtained. mp 183 C (dec).
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-50-
MS (APCI) M+1: Calcd 423.2; Found 423. l.
EXAMPLE 57
4-{4-[7-(3-Cclohexy1-3-n:ethyl-ureido) pvrido[2,3-dJpyrifnidin-2-vlaminoJ-
phenyl]piperazine-l-carbo.xylic acid tert-buhl ester
B}, substitutina N-methylc'Iclohexylamine for 3-amino-l-propanol in
Example 55. 0.1932 g(72.7%) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 561.3; Found 561.2.
EXAMPLE 58
1-Cvclohexvl-l-metht,l-3-[2-(4 piperazin-1-v1 phenylamino) pt=rido
[2,3-dJpyrintidin-7-YI]-urea
By substituting the product of Example 57 in Example 15. 0.1645 g of the
product (compound 65) as a solid is obtained, mp 177 C (dec).
MS (APCI) M+l : Calcd 461.3: Found 461.2.
EXAMPLE 59
4-(4-{7-[3-((S)-1-Hvdrotvmetlrl,1-3-n:ethvl-buryl)-ureidoJ pvrido
[2,3-d]pvrimidin-2-vlamino} phen_vl) piperazine-l-carbo.x-i~lic acid tert-
buh'l
ester
Bv substituting (S)-(+)-leucinol for 3-amino-l-propanol in Example 55,
0.1048 g(39.1 ro) of the product as a solid is obtained.
MS (APCI) M+1: Calcd 565.3: Found 565.3.
EXAMPLE 60
1-((S)-1-Hvdroxymet/zyl-3-meNtvl-butyl)-3-[2-(4 piperazin-1-v1 phenvlamino)-
pvrido[2,3-dJpvrimidin-7-vlJ-urea
By substituting the product of Example 59 in Example 15. 0.0802 g of the
product (compound 83) as a solid is obtained, mp 185 C (dec).
MS (APCI) M+1: Calcd 465.3; Found 465.2.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-51-
EXAMPLE 61
4-[4-(7-{[]-(4-Mediyl piperazin-1 yl)-methanoilIJ-amino} pvrido[2,3-
dJpyrimidin-2-vlamino) phenylJ-piperazine-l-carboxylic acid tert-buttI ester
Bv substitutinQ N-methylpiperazine for 3-amino-l-propanol in
Example 55. the product as a solid is obtained.
MS (APCI) M+l: Calcd 548.3; Found 548.3.
EXAMPLE 62
4-Methyl piperazine-l-carboxvlic acid [2-(4 piperazin-1 yl phenvlamino)-
pj,rido[2,3-dJpvrimidin-7-ylJ-arnide
By substituting the product of Example 61 in Example 15, 0.1194 g of the
product (compound 84) as a solid is obtained, mp 200 C (dec).
MS (APCI) M+l,: Calcd 448.3; Found 448.2.
EXAMPLE 63
4-(4-{7-[(1-Morpl:olin-4 .ti!l-nrethanoyl)-an:inoJ-pyrido[2,3-dJpyrintidin-2-
ylamino} phenyl) piperazine-l-carboxvlic acid tert-butyl ester
By substituting morpholine for 3-amino-l-propanol in Example 55. the
product as a solid is obtained.
MS (APCI) M+1: Calcd 535.3: Found 535.2.
EXAMPLE 64
Morpholine-4-carboxylic acid [2-(4 piperazin-1 .vl p/ten_vlantino) pvridu[2,3-
dJpvrinzidin-7 y1J-amide
Bv substituting the product of Example 63 in Example 15, 0.1132 g of the
product (compound 85) as a solid is obtained. mp 190 C (dec).
MS (APCI) M+1: Calcd 435.2; Found 435.2.
EXAMPLE 65
4-{4-[7-(3,3-Dipropvl-ureido)-pvrido[2,3-dJpvrimidin-2 yla-ninoJ-phenvl}-
piperazine-1-carbo.zylic acid tert-butyl ester
B}, substituting dipropylamine for 3-amino-l-propanol in Example 55, the
product as a solid is obtained.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-52-
MS (APCI) M+1: Calcd 549.3: Found 549.3.
EXAMPLE 66
3-[2-(4-Piperazin-1-ti,1 phenvlamino) pvrido[2,3-dJpti,rimidin-7-y1]-1,1-
dipropyl
urea
By substitutina the product of Example 65 in Example 15, 0.1278 g of the
product (compound 86) as a solid is obtained, mp 190 C (dec).
MS (APCI) M+1: Calcd 449.3; Found 4492
.
EXAMPLE 67
4-[4-(7-{[]-(4-Boc piperazin-1-i,1)-nzetltanoti,lJ-amino} pyrido[2,3-
d]pti,rimidin-2-
Vlamino) phenvlJ piperazine-l-carboxylic acid tert-bufil ester
Bv substituting Boc-piperazine for 3-amino-l-propanol in Example 55. the
product as a solid is obtained.
MS (APCI) M+1: Calcd 634.3; Found 634.3.
EXAMPLE 68
Piperazine-1-carboxy,lic acid [2-(4 piperazirn-1 yl pl:enti,lanrino)p'rido[2,3-
dJpyrimidin-7 yl]-anzide
Bv substituting the product of Example 67 in Example 15. 0.0342 g of the
product (compound 87) as a solid is obtained. mp 220 C (dec).
MS (APCI) M+1: Calcd 434.2: Found 434.2.
EXAMPLE 69
4-(4-{7-[3-((R)-1-Hhdroxvmethy,l-2-met/rv! propyl)-ureidoJ-pyrido[2,3-
dJpyrimidin-2-ylamino} phenyl) piperazine-l-carbatl,lic acid tert-butyl ester
By substituting (R)-valinol for 3-amino-l-propanol in Example 55, the
product as a solid is obtained.
MS (APCI) M+l: Calcd 551.3: Found 551.3.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-53-
EXAMPLE 70
1-((R)-1-Hydroxvmethyl-2-methyl propyl)-3-[2-(4 piperazin-1 yl phenvlamino)-
pvrido[2,3-dlpyrimidin- 7-y1]-urea
Bv substituting the product of Example 69 in Example 15. 0.0639 g of the
product (compound 88) as a solid is obtained. mp 200 C (dec).
MS (APCI) M+1: Calcd 451.3; Found 451.2.
EXAMPLE 71
4-(4-{7-[3,3-Bis-(2-hydro.x-t,-ethyl)-ureido]-pyrido[2,3-dJpyrinridin-2-ylamin
o}-
pheiTt,1) piperazine-l-carboxylic acid tert-butyl ester
Bv substituting diethanolamine for 3-amino-1-propanol in Example 55, the
product as a solid is obtained.
MS (APCI) M+1: Calcd 553.3: Found 553.2.
EXAMPLE 72
1,1-Bis-(2-lrydroxy-eNryl)-3-[2-(4 piperazin-1 yl phenylantino) pyrido[2,3-
dJpyriniidin-7 y1J-urea
By substituting the product of Example 71 in Example 15. 0.0916 g of the
product (compound 89) as a solid is obtained. mp 185 C (dec).
MS (APCI) M+1: Calcd 453.2: Found 453?.
EXAMPLE 73
6-Bromo-2-n:ethylsulfan1,1-8H-pt=rido[2,3-dJpvrimidin-7-one
To 5.00 g (25.9 mmol) of 2-methanesulfanyl-BH-pyrido[2.3-d]pyrimidin-
7-one (Example 5) in 130 mL of DMF is added 5.00 g (28.1 mmol) of
N-bromosuccinimide. The resulting suspension is stirred at room temperature
overniaht and concentrated. The solid is triturated with hot water. then
washed
with isopropanol to give 5.59 ~(79.4 0) of the product as a solid. mp 266-270
C.
EXAMPLE 74
6-Bromo-7-chloro-2-methylsulfanyl pyrido[2,3-dJpvrimidine
By substituting the product of Example 73 in Example 6. 2.73 g (97.2%)
of the product is obtained as a solid.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-54-
MS (APCI) M+1: Calcd 289.9: Found 289.8.
EXAMPLE 75
6-Bronto-2-meth yls ulfan yl p'rid o[2, 3-dJprintid in- 7-,yl amin e
By substituting the product of Example 74 in Example 7. 2.09 ~(82.9%)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 271.0: Found 270.8.
EXAMPLE 76
6-Bronto-2-ntethanesulfinyl-pti,rido[2,3-dJpyrintidin- 7-14amine
By substituting the product of Example 75 in Example S. 1.81 g (81.9%)
of the product is obtained as a solid, mp 245 C (dec).
MS (APCI) M+1: Calcd 287.0; Found 286.8.
EXAMPLE 77
4-[4-7-Antino-6-bromo pvrido[2,3-dJpyrimidin-2 ylantino) phenylJ-piperazine-
I-carboxylic acid tert-butyl ester
By substituting the product of Example 76 in Example 13, 1.40 g (44.4%)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 500.1; Found 500Ø
EXAMPLE 78
4-{4-[6-Bromo-7-(3-cyclohe.zyl-ureido) pt,rido[2,3-dJpt'rimidin-2 ylaminoJ-
phenyl} piperazine-l-carboxylic acid tert-butti=l ester
By substituting the product of Example 77 in Example 16, 0.1160 g
(46.4%) of the product is obtained as a solid.
MS (APCI) M+l: Calcd 625.2: Found 625.1.
EXAMPLE 79
1-[6-Bronto-2-(4 piperazin-1-y1 pltenylantino)prido[2,3-dJpllrintidin-7-ylJ-3-
cyclolte.rhl-ttrea
Bv substituting the product of Example 78 in Example 15. 0.0886 g
(77.0%) of the product (compound 55) is obtained as a solid. mp 195 C (dec).
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-55-
MS (APCI) M+l: Calcd 525.2: Found 525.1.
Anal. Calcd for C24H29BrINgO1-1.64 H20-1.83 HCI:
C. 46.37: H. 5.53: N, 18.02; Cl. 10.44.
Found: C. 46.53: H. 5.34: N. 17.73: Cl. 10.15.
EXAMPLE 80
4-{4-[6-Bromo-7-(3-tert-butvl-ureido) pyrido[2,3-dJpyrimidin-2ylamino]-
phenvl} piperazine-l-carbo.xylic acid tert-butvl ester
BN, substituting the product of Example 77 in Example 10, 0.2571 g
(42.9 ro) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 599.2; Found 599.2.
EXAMPLE 81
1-[6-Bromo-2-(4 piperazin-1 yl phenylamino) pvrido[2,3-d]pyrintidin-7 y1J-3-
tert-bu4,l-urea
BN, substituting the product of Example 80 in Example 15, 0.0481 g of the
product (compound 91) is obtained as a solid.
MS (APCI) M+1: Calcd 499.2: Found 499Ø
EXAMPLE 82
4-{4-[6-Bromo-7-(3-methyl-ureido) pvrido[2,3-dJpvrin:idin-2 ylaminoJ-phenyl}-
piperazine-1-carboaylic acid tert-butyl ester
BN, substituting the product of Example 77 and methylamine in
Example 32, 0.170 g(29.9 ro) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 557.2: Found 557.1.
EXAMPLE 83
1-[6-Bromo-2-(4 piperaznl-I-v1 phenvlamino) pvrido[2,3-dJpy,rimiditr-7 y1J-3-
inethyl-urea
BN, substitutina the product of Example 82 in Example 15, 0.0963 g (69%)
of the product (compound 93) is obtained as a solid.
MS (APCI) M+1: Calcd 457.1: Found 457.1.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-56-
Anal. Calcd for C 19H~ I BrI N8O 1=3 HC1=3 H2O:
C. 36.76: H. 4.87; N. 18.05; Cl. 17.13; H20. 8.71.
Found: C, 36.49: H. 4.35; N. 17.52; Cl. 15.79: H-)O, 8.12.
EXAMPLE 84
4-[4-(7 Antino-6-brofno pvrido[2,3-dJpvrin:idin-2-vlamino) phen}'lJ-cis-2,6-
dimet/ryl piperazine-l-carbo.aylic acid tert-butt,l ester
Bv substituting the product of Example 76 in Example 27. 2.10 g(63.1 %)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 528.2; Found 528.2.
EXAMPLE 85
4-{4-[6-Bromo-7-(3-tert-butyl-ureido) pvrido[2,3-dJpvrimidin-2 ylaminoJ-
pheiryl}-cis-2,6-dimethvl piperazlne-l-carboxvlic acid tert-butvl ester
By substituting the product of Example 84 in Example 10. 0.1725 g
(72.6%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 627.2: Found 627?.
EXAMPLE 86
1-{6-Bronro-2-[4-(cis-3,5-dimethvl pipera<,in-1-v1) phe-rvlanrinoJ-pvrido[2,3-
dJpvrimidin-7-y1}-3-tert-butvl-urea
By substituting the product of Example 85 in Example 15. 0.1593 g
(96.0%) of the product (compound 94) is obtained as a solid. mp 202 C (dec).
MS (APCI) M+1: Calcd 527.2; Found 527.2.
Anal. Calcd for C-24H~ I BrIN8O1 -2.55 HC1= 1.70 H-) O:
C. 44.28: H. 5.71 N. 17.21: Cl. 13.89.
Found: C. 44.28; H. 5.72: N. 17.09: Cl. 12.49.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-57-
EXAMPLE 87
4-{4-[6-Bronto-7-(3-cvc/ohexyl-ureido) pvrido[2,3-dJpvrintidin-2ylantinoJ-
phenvl}-cis-2,6-dimeth_vl-piperazine-l-carboxvlic acid tert-bufil ester
Bv substituting the product of Example 84 in Example 16. 0.1750 c,
(70.7%) of the product is obtained as a solid.
MS (APCI) M+l: Calcd 653.3; Found 653.3.
EXAMPLE 88
1-[6-Bronto-2-[4-(cis-3,5-dimetht'! piperazin-1-v1) phenvlaminoJ-pvrido[2,3-
d]pvrintidin-7 y!}-3-cl!clohexti,!-urea
By substituting the product of Example 87 in Example 15. 0.1614 g
(95.4%) of the product (compound 95) is obtained as a solid. mp 198 C (dec).
MS (APCI) M+1: Calcd 553.2: Found 553?.
Anal. Calcd for C26H33N8O1Brl =2.76 HCl=2.02 H2O:
C. 45.22: H. 5.81: N. 16.23: Cl. 14.17.
Found: C, 45.23: H. 5.82; N. 16.08: Cl, 13.53.
EXAMPLE 89
N2-(4-Fluoro phenyl) pvrido[2,3-dJpvrintidine-2,7-diantine
Bv substitutinQ 4-fluoroaniline in Example 9. 1.1529 Q (45?%) of the
product is obtained as a solid. mp 245-248 C.
MS (APCI) M+1: Calcd 256.1; Found 255.9.
EXAMPLE 90
1-[2-(4-Flttoro pltenvlantino) phrido[2,3-dJpvrimidut-7-I,1/-3-(3-ntorpholin-4
y!-
propv!)-urea
Bv substitutinQ the product of Example 89 and 3-morpholin-4-vl-
propvlamine in Example 32. 0.1465 R(58.6 ro) of the product (compound 96) is
obtained as a solid. mp 253-256 C.
MS (APCI) M+l: Calcd 426.2: Found 426.1.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-58-
Anal. Calcd for C-) 1 H24F I N702:
C. 59.2 S. H. 5.69; N. 23.04.
Found: C. 59.18: H. 5.66: N. 23.04.
EXAMPLE 91
1-[2-(4-Fluoro phenylantino) pyrido[2,3-dJpyrintidin-7-y1J-3-(2-ltydroiy-
ethyl)-
urea
Bv substituting the product of Example 89 and 2-hydroxy-ethylamine in
Example 32. 0.0811 a(40.3 ro) of the product (compound 97) is obtained as a
solid, mp 238-240 C.
MS (APCI) M+1: Calcd 343.1: Found 343.1.
Anal. Calcd for CI6H15F1N602:
C. 56.14; H. 4.42; N. 24.55.
Found: C. 55.82: H. 4.52: N. 24.15.
EXAMPLE 92
1-(2 Antino-ethyl)-3-[2-(4 Jluoro phenylantino) pyrido[2,3-dJpyrintidin-7 y!J-
urea
By substituting the product of Example 89 and ethylenediamine in
Example 32. 0.1000 g(49.3 io) of the product (compound 98) is obtained as a
solid, mp 217-220 C.
MS (APCI) M+1: Calcd 342.1; Found 342Ø
Anal. Calcd for C16H16FIN7O1=0.2 H-)O:
C, 55.71; H. 4.79; N. 28.42.
Found: C. 55.72: H. 4.57: N. 28.07.
EXAMPLE 93
1-(2-Dimethylantino-eNtt'I)-3-[2-(4 fluoro-phenylafnino) pyrido[2,3-
dlpyrintidin-7-_y1J-urea
By substituting the product of Example 89 and 2-dimethylamino-
ethvlamine in Example 32. 0.0778 ~(35.8 o) of the product (compound 99) is
obtained as a solid. mp 251-255 C.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-59-
MS (APCI) M+1: Calcd 370.2; Found 370Ø
Anal. Calcd for C I 8H20F I N701:
C, 58.53; H, 5.46; N, 26.54.
Found: C. 58.39: H. 5.51; N. 26.26.
EXAMPLE 94
3,3-Dintethyl-l-(4-nitro pheny!) piperazine
B~, substituting 2,2-dimethyl-piperazine in Example 24, 29.43 g(88.4 /0)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 236; Found 236.
EXAMPLE 95
2-2-Dintethyl-4-(4-nitro phenyl) piperazine-l-carboxylic acid tert-butvl ester
By substituting the product of Example 94 in Example 11, 38 g (93%) of
the product is obtained as a solid.
MS (APCI) M+1: Calcd 336: Found 336.
EXAMPLE 96
4-(4 Amino phe-{ti,!)-2,2-dimethy! piperazine-1-carboxvlic acid tert-butvl
ester
By substituting the product of Example 95 in Example 12. 27 g (78%) of
the product is obtained as a solid.
MS (APCI) M+l: Calcd 306: Found 306.
EXAMPLE 97
4-[4-(7-A min o-6Jluoro pvrido[2,3-d]pvrintidin-2 ylamino) phenylJ-2,2-
dimetlty!-piperazine-l-carboxvlic acid tert-butvl ester
By substituting the product of Example 96 in Example 50, 0.4346 g
(59.0%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 468.2: Found 468.3.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-60-
EXAMPLE 98
4-{4-[7-(3-Cyclohexyl-ureido)-6 fluoro pyrido[2,3-dJpyrimidin-2 ylanrinoJ-
phenyl}-2,2-dimethyl piperazine-l-carbozylic acid tert-buh'l ester
Bv substituting the product of Example 97 in Example 16, 0.170 g(31.2%)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 593.2; Found 593.4.
EXAMPLE 99
1-Cyclohezyl-3-{2-[4-(3,3-dimethy! piperazin-1-y!) phenylaminoJ-6 Jluoro-
pyrido[2,3-dJpyrimidin-7-yl]-urea
Bv substituting the product of Example 98 in Example 15. 0.040 g of the
product (compound 100) is obtained as a solid.
MS (APCI) M+1: Calcd 493.3; Found 493.2.
EXAMPLE 100
4-[4-(7 Amino-6-fluoro pyrido[2,3-dJpi,rimidin-2 ylamino) pheirylJ-piperazine-
1-carboxt-'lic acid tert-butvl ester
By substituting the product of Example 12 in Example 50. 0.2017 g
(29.7%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 440.2; Found 440.2.
EXAMPLE 101
4-{4-[7-(3-Ct'clohexy!-ureido)-6 fluoro pyrido[2,3-dJpyrimidin-2 .ylaminoJ-
p/:eny!} piperazine-l-carboxylic acid tert-butyl ester
Bv substituting the product of Example 100 in Example 16. 0.2036 g
(78.6%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 565.3: Found 565.3.
EXAMPLE 102
1-Cyclohexyl-3-[6 fluoro-2-(4 piperazin-1-y! phenylamirno) pyrido[2,3-
dJpyrimidin-7-_ylJ-urea
Bv substituting the product of Example 101 in Example 15. 0.1084 g
(96.0 io) of the product (compound 11) is obtained as a solid.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-61-
MS (APCI) M+1: Calcd 465.2: Found 465.2.
Anal. Calcd for C24H29F I N8O 1=2.75 HC1=3.5 H2O:
C. 45.91; H. 5.10; N, 17.85; Cl. 15.53; H2O, 10.04.
Found: C. 46.20; H, 5.86: N, 17.45: Cl. 15.22: H-)O. 8.97.
~ EXAMPLE 103
4-{4-[7-(3-tert-Butvl-ureido)-6 fluoro pvrido[2,3-dJpvrin:idin-2-vlaaunoJ-
phenvl}-cis-2,6-dimetlr-vl piperazine-l-carbo.zylic acid tert-but1I ester
Bv substituting the product of Example 50 in Example 10. 0.070 g(17.9%)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 567.3; Found 567.3.
EXAMPLE 104
1-tert-Butvl-3-{2-[4-(cis-3,5-dimetht,l piperazin-1 yl) phenvlaminoJ-6 JTuoro-
pvrido[2,3-d]pi'rimidin-7-t'IJ-urea
By substituting the product of Example 103 in Example 15. 0.0585 g of
1~ the product (compound 102) is obtained as a solid.
MS (APCI) M+1: Calcd 467.3: Found 467.3.
EXAMPLE 105
1-[4-(4-Nitro phenyl) piperazin},IJ-ethanone
To a solution of 5.0 2 (24.1 mmol) of 1-(4-nitro-phenyl)-piperazine in
100 mL of dichloromethane ,vas added 5.04 mL (28.9 mmol) of diisopropyl-
ethvlamine. The solution is cooled in an ice-bath. treated ,~vith 1.89 mL
(26.5 mmol) of acetyl chloride. and stirred at room temperature overnight. The
reaction is -,vashed successivelv with water, 0.5 M HC1. saturated sodium
hvdroaen carbonate. and brine, and dried over magnesium sulfate. and
concentrated to give 5.91 2 (98.5%) of the product as a solid.
MS (APCI) M+1: Calcd 250.1: Found 250Ø
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-62-
EXAMPLE 106
1-[4-(4 Amino phenvl) piperazin-1 y1J-ethanone
By substituting the product of Example 105 in Example 12. 4.35 g (84.1%)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 220.1; Found 220.1.
EXAMPLE 107
1-{4-[4-(7 Aminop,rido[2,3-dJpvrimidin-2 ylamino) phenv1J-piperazifi-1 yl}-
etfhanone
By substituting the product of Example 106 in Example 9, 0.1829 g
(50.1 %) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 364.2; Found 364.2.
Anal. Calcd for C 19H2I N7O 1= 1.0 H20:
C, 59.46; H. 6.11; N. 25.55.
Found: C, 59.51; H. 6.03; N. 25.28.
EXAMPLE 108
1-{2-[4-(4 Acetv! piperazin-1 yl) phe-rvlaminoJ-pvrido[2,3-dJpvrin:idin-7-vl}-
3-
(3-morpholin-4 yl propvl)-urea
By substituting the product of Example 107 and 3-morpholin-4-yl-
propylamine in Example 32. 0.0338 g (22.6%) of the product (compound 103) is
obtained as a solid, mp 222-225 C (dec).
MS (APCI) M+1: Calcd 534.3; Found 534.2.
Anal. Calcd for C27H35N9O3=0.5 H2O:
C. 59.76; H. 6.69: N. 23.25.
Found: C, 59.74; H. 6.53; N. 23.35.
EXAMPLE 109
6-Chloro-2-metlivlsulfanvl-8H-pvrido[2,3-dJpvrimidin-7-one
By substituting N-chlorosuccinimide in Example 74, 0.3700 g(31.4%) of
the product is obtained as a solid, mp 264-266 C (dec).
MS (APCI) M+1: Calcd 228.0: Found 227.9.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-63-
EXAMPLE 110
6, 7-Dichloro-2-methvlsulfanyl pyrido[2,3-d]pyrintidine
By substituting the product of Example 109 in Example 6, 0.6534 g
(86.5%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 246.0; Found 245.8.
EXAMPLE 111
6-Chloro-2-methvlsulfanyl pyrido[2,3-dJpyrimidin-7-ylantine
BN, substituting the product of Example 110 in Example 7. 0.38 g (63%) of
the product is obtained as a solid.
MS (APCI) M+1: Calcd 227.0: Found 226.9.
EXAMPLE 112
6-Chloro-2-methanesulfinyl pyrido[2,3-dJpyrintidin-7-ylantine
By substitutina the product of Example 111 in Example 8, 0.2328 g
(57.1%) of the product is obtained as a solid, mp 260-262 C.
MS (APCI) M+l : Calcd 243.0; Found 242.9.
EXAMPLE 113
4-[4-(7-Amino-6-chloro pyrido[2,3-dJpyrintidin-2-ylantino) phenylJ-cis-2,6-
dinteNtyl piperazine-l-carboxylic acid tert-butyl ester
By substitutin~ the product of Example 112 in Example 27, 0.22 g(49%)
of the product is obtained as a solid.
MS (APCI) M+1: Calcd 484.2; Found 484.2.
EXAMPLE 114
4-[4-[7-(3-tert-Butyl-ureido)-6-chloro pyrido[2,3-dJpt'rimidin-2 ylaminoJ-
phenyl}-cis-2,6-dintethyl piperazine-l-carboxti,lic acid tert-butyl ester
By substituting the product of Example 113 in Example 10. 0.0995 g
(39.2%) of the product is obtained as a solid.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-64-
EXAMPLE 115
1-tert-Butvl-3-{6-cltloro-2-[4-(cis-3,5-dimethvl piperazin-1-v1) phenvlaminoJ-
pvrido[2,3-dJpvrimidin- 7-yl}-urea
By substituting the product of Example 114 in Example 15. 0.0995 g of the
product (compound 104) is obtained as a solid, mp 205 C (dec).
MS (APCI) M+1: Calcd 483.2: Found 483.2.
EXAMPLE 116
AIethyl-(2-methylsulfan1,1pvrido[2,3-dJpyrin:idin-7-yl)-an:ine
By substituting methvlamine in Example 7. 1.46 g(30.0%) of the product
is obtained as a solid.
MS (APCI) M+l: Calcd 207.1: Found 206.9.
EXAMPLE 117
(2-Methanesulfinyl p,rido[2,3-dJpvrimidin-7-y1)-methyl-amine
By substituting the product of Example 116 in Example 8. 1.31 g(83.4%)
of the product is obtained as a solid. mp 185 C.
MS (APCI) M+l : Calcd 223.1: Found 223Ø
EXAMPLE 118
4-[4-(7-Meth_ylamino pvrido[2,3-dJpvrinzidin-2 ylamino) phenv/J-piperazine-l-
carboxvlic acid tert-buhl ester
By substituting the product of Example 117 in Example 13. 0.4934 g
(62.9%) of the product is obtained as a solid.
MS (APCI) M+1: Calcd 436.2: Found 436.2.
EXAMPLE 119
4-{4-[7-(3-Cvclohexvl-l-methi=l-ureido) pvrido[2,3-dJpyrimidin-2ylJ phenyl}-
piperazine-1-carboxylic acid tert-butvl ester
Bv substituting the product of Example 118 in Example 16. and using
acetonitrile as solvent and no base. 0.8535 g (78.8%) of the product is
obtained as
a solid.
MS (APCI) M+l: Calcd 561.3: Found 561.3.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-65-
EXAMPLE 120
3-Cvclohexyl-l-methyl-l-[2-(4 piperazin-1 yl phenvlamino) pt,rido[2,3-
dJpyrimidin-7-ylJ-urea
Bv substituting the product of Example 119 in Example 15. 0.2548 ~
(36.0%) of the product (compound 70) is obtained as a solid. mp 169-175 C.
MS (APCI) M+1: Calcd 461.3; Found 461.2.
Anal. Calcd for C25H32N801-0.25 H2O:
C. 64.56: H, 7.04: N. 24.09.
Found: C. 64.57; H. 7.01: N. 23.98.
EXAMPLE 121
3-Cvclohexvl-l-{2-[4-(cis-3,5-dimethyl piperazin-1 yl) phent'laminoJ-
pyrido[2,3-
dJpyrimidin-7-yl]-1-nzethvl-urea
Using the general procedure by which Example 120 is synthesized,
0.1366 g(95.6%) of the product (compound 106) is obtained as a solid,
mp 170 C (dec).
MS (APCI) M+1: Calcd 489.3; Found 489.3.
Anal. Calcd for C27H36N8O1 =3.32 H-)0-2.69 HC1:
C. 50.16; H. 7.07; N. 17.33; Cl, 14.75.
Found: C. 50.36: H, 6.98: N. 16.97: Cl. 15.07.
EXAMPLE 122
3-Cvclohe_tvl-l-ethvl-l-[2-(4 piperazin-1 yl p/renvlamino) pvrido[2,3-dJ
pvrimidin-7 ylJ-urea
Using the general procedure by which Example 120 is svnthesized, 0.118 g
(94%) of the product (compound 107) is obtained as a solid.
MS (APCI) M+l : Calcd 475.3: Found 475.3.
Anal. Calcd for C26H;4?vgOl =3.0 HCl=0.3 diethylether:
C. 53.89: H. 6.65; N. 18.48.
Found: C. 53.75: H. 6.96: N. 18.57.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-66-
EXAMPLE 123
3-tert-Butvl-l-{2-[4-(cis-3,5-dimethvl piperazin-1 yl) phenvlan:inoJ-
pl,rido[2,3-
d]pyrimidin-7 yl}-1-ethvl-urea
Using the general procedure by which Example 15 is synthesized, 0.022 g
(56%) of the product (compound 108) is obtained as a solid.
MS (APCI) M+1: Calcd 477.3; Found 477.3.
EXAMPLE 124
1-Met/tl,l-3-[5-methv1-2-(4 piperazin-1-1,1 phen_vlantino)p,rido[2,3-
dJpyrinridin-
7ylJ-urea
Using the general procedure by which Example 40 is synthesized, the
product (compound 64) is obtained as a solid, mp 204-206 C (dec).
MS (APCI) M+1: Calcd 393; Found 393.
EXAMPLE 125
1-Et/tyl-3-[S-n:ethyl-2-(4 piperazin-I yl phenvlamino) pyrido[2,3-dJpvrimidin-
7-ylj-urea
Using the general procedure by which Example 40 is synthesized, the
product (compound 28) is obtained as a solid. mp 220-222 C.
MS (APCI) M+1: Calcd 407: Found 407.
EXAMPLE 126
1-[S-Methyl-2-(4 piperazin-1 y! phenvlamino) pvrido[2,3-dJpvrimidin-7 y1J-
3 propyl-urea
Using the general procedure by which Example 40 is synthesized, the
product (compound 111) is obtained as a solid, mp 223-225 C.
MS (APCI) M+1: Calcd 421: Found 421.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-67-
EXAMPLE 127
11;N-Dimethyl-N=[S-metltyl-2-[[4-(1 piperazinyl)phenylJ-ami oJ-pyrido[2,3-
dJpyrimidin-7-_yl-sulfamide
Using the general procedure by which Example 40 is svnthesized. but
usina dimethyl sulfamyl chloride rather than cyclohexylisocvanate, the product
(compound 71) is obtained as a solid. mp 228-230 C (dec).
MS (APCI) M+1: Calcd 443: Found 443.
EXAMPLE 128
7-Antino-2-ntethylsulfan_yl pyrido[2,3-dJpt'rintidine-6-carbo.x~,lic acid
et/tyl ester
To a solution of 4-amino-2-methanesulfanyl-pyrimidine-5-carboxaldehvde
(Example 3) in 10 mL of tetrahydrofuran is added 0.126 mL (1.18 mmol) of ethyl
cyanoacetate. The solution is cooled to -10 C, and treated with 2.36 mL
(2.36 mmol) of titanium tetrachloride. To the solution is slowly added 0.52 mL
(4.72 mmol) of N-methyl morpholine. The reaction is warmed to room
temperature over 2 hours. and partitioned between ethvl acetate and saturated
aqueous ammonium chloride. The organic laver is concentrated to give a solid,
which is triturated with ether to give 0.30 g (96%) of the product as a solid.
MS (APCI) M+1: Calcd 265.1; Found 264.9.
EXAMPLE 129
7-Antitzo-2-chloro pyrido[2,3-dJpyrimidine-6-carbozylic acid ethyl ester
To a suspension of the product of Example 128 in 50 mL of chloroform is
slowly added sulfuryl chloride. followed by 2 drops of ethanol. The reaction
is
stirred at room temperature for 16 hours. poured into ether, and the solid
collected
to give 0.50 g (98%) of the product.
MS (APCI) M+1: Calcd 253.1: Found 253.1.
EXAMPLE 130
7-Antino-2-[4-(4-tert-butoxycarbonyl piperazin-1-y1) phenylantinoJ-pyrido[2,3-
dJpt,rintidine-6-carboxylic acid etliyl ester
A solution of the product of Example 12 and the product of Example 129
in dioxane is heated under reflux for 1.5 hours. The reaction is poured into
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-68-
hexane/ethyl acetate (1:1). and the solid is collected. Flash chromatography
using
dichloromethane as eluant gave 0.08 g (16%) of the product as a solid.
MS (APCI) M+1: Calcd 494.2; Found 494.1.
EXAMPLE 131
2-[4-(4-tert-Butoxtilcarbonyl piperazin-1 yl) phenl,lantinoJ-7-(3-tert-butl,l-
ureido) pvridol2,3-dJpt,rintidine-6-carboxylic acid ethyl ester
By substituting the product of Example 130 in Example 10. 0.05 g (48%)
of the product as a solid is obtained.
EXAMPLE 132
7-(3-tert-Butvl-ureido)-2-(4 piperazin-1 yl phenylamino) pt'rido[2,3-
dJpyrintidine-6-carboxvlic acid ethyl ester
By substitutina the product of Example 131 in Example 15. 0.036 g of the
product (compound 113) as a solid is obtained, mp >300 C.
EXAMPLE 133
]-[6-Fluoro-S-methyl-2-(4 piperazin-1-t'1 phen_ylantino) pt'rido(2,3-
dJpj=rintidin-
7-y1J-3-isopropvl-urea
Using the general procedure bv which Example 52 is svnthesized, but
using 1-(4-amino-2-methvlsulfan~,l-pyrimidin-5-vl)-ethanone (Example 35), 4-(4-
amino-phenyl)-piperazine-l-carboxylic acid tert-butvl ester (Example 12). and
isopropyl isocvanate as reagents. the product (compound 114) is obtained as a
solid, mp 208 C (dec).
MS (APCI) M+1: Calcd 439.2: Found 439.3.
EXAMPLE 134
1-Cti,clohex_y1-3-{2-14-(3,3-dintethti=1 piperazin-1 yl) pheni'/aminoJ-
pt,rido}2,3-
dJpyrintidin-7-y1}-urea
Using the general procedure by which Example 17 is svnthesized, but
using 4-(4-amino-phenyl)-2.2-dimethyl-piperazine-l-carboxylic acid tert-butyl
ester (Example 96). 0.95 g (100%) of the product (compound 115) is obtained as
a solid.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-69-
MS (APCI) M+1: Calcd 475.6; Found 475.3.
Anal. Calcd for C26H34N8O1 =3 HC1-1 H-)O:
C. 51.96: H. 6.37; N. 18.64: Cl. 17.69: H,~O, 2.99.
Found: C. 52.00: H. 6.41: N. 18.53: Cl. 16.51: H-)O. 3.06.
EXAMPLE 135
6-Methyl-2-nret/t_ylsttlfanylprido[2,3-dJpyrimidin-7-ylamine
To a suspension of 2.18 g (54 mmol) of 60% oil dispersed sodium hvdride
in 300 mL of tetrahvdrofuran. cooled to 10 C, is added 10.2 ~(5 3.4 mmol) of
(1-cvano-l-methvl-methvl)-phosphonic acid diethvl ester (Svnthesis. 1975:516).
To the cooled suspension is added 4.30 g(25.4 mmol) of 4-amino-2-
methanesulfanyl-pvrimidine-5-carboxaldehvde (Example 3). and the reaction is
stirred at room temperature for 22 hours. The resulting solution is
concentrated
and filtered to give a solid. which is washed ,vith tetrahvdrofuran. dissolved
in
1N citric acid. and re-precipitated by adjusting the pH to 8 with 50% sodium
hvdroxide. The solid is collected by filtration to give 1.1 g(21%) of the
product,
mp 268-270 C.
MS (APCI) M+1: Caled 207.3: Found 207Ø
EXAMPLE 136
1-Cy,clohexy1-3-{2-[4-(cis-3,5-dimeNry,l piperazin-1-y,l) phenti,laminoJ-6-
met/ryl-
pyrido[2,3-dJpyrimidin-7-171]-urea
Using the general procedure by which Example 31 is synthesized, but
usina the product of Example 135 as the starting material, 0.14 g(42 ro) of
the
product (compound 116) is obtained as a solid.
MS (APCI) M+1: Calcd 589.6: Found 589.3.
Anal. Calcd for C7H36N8O1=2.5 HC1.1.5 H-)O:
C. 53.80: H. 6.73: N. 18.01: Cl. 14.11: H-)O. 4.06.
Found: C. 53.44: H. 6.89: N. 18.46: Cl. 14.60; H-)O. 4.48.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-70-
EXAMPLE 13 7
1-tert-Butvl-3-{2 -14-(cis-3,5-dimethyl piperazin-1 yl) phenylaminoJ-6-ntethyl-
pyrido[2,3-dJpyrimidin-7 yl}-urea
Using the general procedure by which Example 29 is synthesized. but
using the product of Example 135 as the starting material. 0.26 g (89%) of the
product (compound 117) is obtained as a solid.
MS (APCI) M+1: Calcd 463.6; Found 463.3.
Anal. Calcd for C25H36N8O1 =2.4 HC1=1.75 H-)O:
C. 51.62; H. 6.91; N, 19.26; Cl. 14.63; H-)O. 5.42.
Found: C. 51.23; H. 6.55; N. 18.92; Cl, 14.73; H2O. 5.10.
EXAMPLE 138
1-tert-Butt,1-3-[6-ntethyl-2-(4 piperazin-1-y1) p/tenylamino) pyrido[2,3-
dJpyrintidin-7 y1J-urea
Using the general procedure by which Example 15 is synthesized, but
using the product of Example 135 as the starting material, 1.02 g (100%) of
the
product (compound 118) is obtained as a solid.
MS (APCI) M+1: Calcd 435.3; Found 435.3.
Anal. Calcd for C-)3H30N8OI=5 HC1=1.75 H-)O:
C. 42.60; H. 5.98; N. 17.28; Cl. 27.34; H-)O, 4.86.
Found: C. 42.03; H. 6.04; N. 16.81: Cl. 22.95: H-) O. 4.72.
EXAMPLE 139
1-{2-[4-(cis-3,5-Dimethvl piperazin-1 yl) phenvlaminoJ-6-methyl pvrido[2,3-
dJpyrimidin-7 yl}-3-isopropvl-urea
Using the general procedure by which Example 33 is synthesized, but
using the product of Example 135 as the starting material. along with 4-(4-
amino-
phenvl)-cis-2.6-dimethvl-piperazine-l-carboxvlic acid tert-butyl ester and
isopropylamine as reagents, 0.130 g(100%) of the product (compound 119) is
obtained as a solid.
MS (APCI) M+1: Calcd 449.3; Found 449.3.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-71-
Anal. Calcd for C24H;2N801 =3 HC1= 1.75 H,)O:
C. 48.90; H, 6.58; N. 19.01; Cl. 16.04; H-)O. 5.35.
Found: C. 49.03; H, 6.63; N. 18.70; Cl. 16.03; H20. 5.19.
EXAMPLE 140
1-Cl,clopropyl-3-{2-[4-(cis-3,5-dimeN:yl piperazin-1 yl) phenylaminoJ-6-methvl-
pvrido[2,3-dJpvrimidin- 7-yl}-urea
Using the general procedure bv which Example 33 is synthesized, but
usin~ the product of Example 135 as the starting material, along with 4-(4-
amino-
phenyl)-cis-2,6-dimethyl-piperazine-l-carboxylic acid tert-butyl ester and
cyclopropylamine as reagents, 0.099 g (100%) of the product (compound 120) is
obtained as a solid.
MS (APCI) M+1: Calcd 447.3; Found 447.3.
Anal. Calcd for C4H30N801:
C. 49.83; H. 6.19; N. 19.37; C1. 18.39; H20, 3.89.
Found: C, 49.76; H, 6.23; N, 18.92; Cl. 15.66; H20, 3.06.
EXAMPLE 141
1-tert-Butyl-3-{2-[4-(cis-3,5-dimetlryl piperazin-1 yl) phenylaminoJ-6-ethvl-
pvrido[2,3-dJpyrimidin-7 yl]-urea
Using the general procedure by which Example 137 is synthesized, but
using (1-cyano-propyl)-phosphonic acid diethyl ester as starting material,
0.34 g
(95%) of the product (compound 121) is obtained as a solid.
MS (APCI) M+1: Calcd 477.3: Found 477.3.
Anal. Calcd for C6H26N801 =2.5 HC1=1 H20:
C, 53.26: H, 7.05; N, 19.11: Cl. 15.18; H20. 3.07.
Found: C. 53.63; H. 7.31: N. 18.46: Cl. 15.32: H2O, 3.48.
EXAMPLE 142
The following compounds are prepared essentially according to the
procedures described in Examples 1-141 and shown in Schemes 1-4:
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-72-
(a) 1-tert-Butyl-3-[2-(3-chloro-4-piperazin-l-yl-phenylamino)-
pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 2):
(b) 1-tert-Butyl-3-[6-fluoro-2-(4-piperazin-l-vl-phenylamino)-
pyrido[2.3-d]pyrimidin-7-yl]- urea (compound 3):
(c) 1-{2-[4-(4-Acetyl-piperazin-1-yl)-3-chloro-phenylamino]-
pyrido[2.3-d]pyrimidin-7-yl}-3-tert-butyl-urea (compound 6):
(d) 1-{2-[4-(4-Acetyl-piperazin-l-y1)-phenylamino]-6-fluoro-
pyrido[2.3-d]pyrimidin-7-yl } -3-tert-butyl-urea (compound 7);
(e) 1-{2-[4-(4-Acetyl-piperazin-l-yl)-phenylamino]-5-methyl-
pyrido[2.3-d]pyrimidin-7-yl}-3-tert-butvl-urea (compound 8):
(f) 1-[2-(3-Chloro-4-piperazin-l-vl-phenylamino)-
pyrido[2.3-d]pyrimidin-7-yl]-3-cvclohexyl-urea (compound 10);
(g) 1-{2-[4-(4-Acetyl-piperazin-l-yl)-phenylamino]-
pyrido[2.3-d]pyrimidin-7-yl}-3-cyclohexyl-urea (compound 13);
(h) 1-{2-[4-(4-Acetvl-piperazin-1-yl)-3-chloro-phenylamino]-
pyrido[2.3-d]pyrimidin-7-yl}-3-cyclohexyl-urea (compound 14):
(i) 1-{2-[4-(4-Acetvl-piperazin-l-yl)-phenylamino]-6-fluoro-
pyrido[2.3-d]pyrimidin-7-yl}-3-cyclohexvl-urea (compound 15);
(j) 1-{2-[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-5-methyl-
pyrido[2.3-d]pyrimidin-7-yl}-3-cyclohexyl-urea (compound 16):
(k) 1-(2-Hydroxv-ethyl)-3-[2-(4-piperazin-l-_yl-phem-lamino)-
pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 17);
(1) 1-[2-(3-Chloro-4-piperazin-l-vl-phenylamino)-
pyrido[2.3-d]pyrimidin-7-yl]-3-(2-hydroxy-ethyl)-urea (compound 18);
(m) 1-[6-Fluoro-2-(4-piperazin-l-vl)-phenylamino)-
pyrido[2.3-d]pyrimidin-7-yl]-3-(2-hydroxy-ethyl)-urea (compound 19):
(n) 1-(2-Hydroxy-ethyl)-3-[5-methyl-2-(4-piperazin-l-yl-
phenylamino)-pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 20):
(o) 1-{2-[4-(4-Acetyl-piperazin-l-vl)-phenylamino]-
pvrido[2.3-d]pyrimidin-7-yl}-3-(2-hydroxy-ethyl)-urea (compound 21):
(p) 1-{2-[4-(4-Acetyl-piperazin-l-yl)-3-chloro-phenylamino]-
pyrido[2.3-d]pyrimidin-7-yl}-3-(2-hydroxy-ethvl)-urea (compound 22):
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-73-
(q) I-{2-[4-(4-Acetyl-piperazin-l-yl)-phenylamino]-6-fluoro-
pyrido[2.3-d]pyrimidin-7-vl}-3-(2-hydroxy-ethyl)-urea (compound 23):
(r) 1-{2-[4-(4-Acetyl- piperazin-l-yl)-phenylamino]-5 -methvl-
pyrido[2.3-d]pyrimidin-7-vl}-3-(2-hydroxv-ethvl)-urea (compound 24):
(s) 1-Ethyl-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido[2.3-d]pyrimidin-7-~-1]-urea (compound 25);
(t) 1-[2-(3-Chloro-4-piperazin-l-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-11]-3-ethyl-urea (compound 26):
(u) 1-Ethvl-3-[6-fluoro-2-(4-piperazin-1-yl-phenylamino)-
pyrido[2.3-d]pvrimidin-7-vlJ-urea (compound 27);
(N-) 1-{2-[4-(4-Acet),l-piperazin-l-yl)-phem,lamino]-
pyrido[2.3-d]pyrimidin-7-~=1}-3-ethyl-urea (compound 29);
(w) 1-{2-[4-(4-Acetyl-piperazin-l-yl)-3-chloro-phenylamino]-
pyrido[2.3-d]pyrimidin-7-yl}-3-ethyl-urea (compound 30):
(x) 1-{2-[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-6-fluoro-
pvrido[2.3-d]pvrimidin-7-~,l}-3-ethyl-urea (compound 31):
(y) 1- { 2-[4-(4-Acetyl-piperazin-l-yl)-phenylamino]-5-methyl-
pvrido[2.3-d]pvrimidin-7-y1}-3-ethvl-urea (compound 32):
(z) 1-tert-Buty l-3-[2-(pyridin-4-ylamino)-pyrido[2.3-d]pyrimidin-
7-vl]-urea (compound 33):
(aa) 1-Cyclohexyl-3-[2-(pyridin-4-vlamino)-pyrido[2.3-d]pyrimidin-
7-yl]-urea (compound 34):
(bb) I-Ethy1-3-[2-(pyridin-4-ylamino)-pvrido[2,3-d]pyrimidin-
7-yl]-urea (compound 35):
(cc) 1-(Hydroxy-ethvl)-3-[2-(pvridin-4-vlamino)-
pyrido[2.3-d]pyrimidin-7-1-1]-urea (compound 36):
(dd) 1-tert-But~i-3-[6-fluoro-2-(pvridin-4-vlamino)-
pyrido[2.3-d]pvrimidin-7- y1]-urea (compound 37):
(ee) 1-Cyclohexyl-3-[6-fluoro-2-(pyridin-4-vlamino)-
pyrido[2,3-d]pyrimidin-7-~'1]-urea (compound 38);
(ff) 1-Ethy1-3-[6-fluoro-2-(pyridin-4-vlamino)-
pyrido[2.3-d]pvrimidin-7-y1]- urea (compound 39):
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-74-
(gg) 1-[6-Fluoro-2-(pyridin-4-ylamino)-pyrido[2.3-d]pyrimidin-7-vl]-
3-(2-hydroxv-ethyl)-urea (compound 40);
(hh) 1-tert-Butyl-3-[5-methyl-2-(pyridin-4-ylamino)-
pyrido[2.3-d]pyrimidin-7- ylj-urea (compound 41);
(ii) 1-Cyclohexyl-3-[5-methyl-2-(pyridin-4-ylamino)-
pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 42);
(j j ) 1-Ethyl-3-[5-methyl-2-(pyridin-4-ylamino)-
pyrido[2,3-d]pyrimidin-7-yl]- urea (compound 43);
(kk) 1-(2-Hydroxy-ethyl)-3-[5-methvl-2-(pyridin-4-vlamino)-
pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 44);
(11) 1-Cyclohexyl-3-[6-methyl-2-(4-piperazin-l-vI-phenylamino)-
pyrido [2.3-d]pyrimidin-7-yl]-urea (compound 54);
(mm) 1-Cyclohexyl-3-[6-cyano-2-(4-piperazin-1-yl-phenylamino)-pyrido
[2,3-d]pyrimidin-7-yl]-urea (compound 56);
(nn) 1-Cyclohexyl-3-[6-chloro-2-(4-piperazin-l-yl-phenylamino)-
pyrido [2.3-d]pyrimidin-7-yl]-urea (compound 57);
(oo) 1-Cyclohexyl-3-[6-fluoro-5-methyl-2-(4-piperazin-1-yl-
phenylamino)-pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 58);
(pp) 1-Cyclohexyl-3-[6-bromo-5-methvl-2-(4-piperazin-1-vl-
phenylamino)-pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 59):
(qq) 1-Cyclohexyl-3- [6-chloro-5-methvl-2-(4-piperazin-1-yl-
phenylamino)-pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 60);
(rr) I -Isopropyl-3-[5-methvl-2-(4-piperazin-l-yl-phenylamino)-
pyrido[2,3-d]pyrimidin-7-yl]-urea (compound 61):
(ss) 1-[5-Methyl-2-(4-piperazin-l-yl-phenylamino)-
pyrido[2.3-d]pyrimidin-7-yl]-urea (compound 63):
(tt) 1-(4-Hydroxy-cyclohexyl )-3-[2-(4-piperazin-l-yl-phenylamino)-
pyrido [2.3-d]pyrimidin-7-vl]-urea (compound 66);
(uu) 1-(4-Amino-cvclohexyl)-3-[2-(4-piperazin-l-yl-phenylamino)-
pyrido [2.3-d]pyrimidin-7-vl]-urea (compound 67):
(vv) l -(2-Dimethvlamino-ethyl)-3-[2-(4-piperazin-1-yl-phenylamino)-
pyrido [2.3-d]pyrimidin-7-vl]-urea (compound 68):
CA 02397961 2004-12-29
65920-142
-75- 4
(ww) 1-(3-Morpholino-4-yl-propvl)-3-[2-(4-piperazin-l-vl-
phenvlamino)-pyrido [2.3-d]pyrimidin-7-yl]-urda (compound 69):
(xx) I -Cyclohexyl-3-[5-methyl-2-(4-piperazin- I -yi-phenylamino)-
pyrido [2.3-d]pyrimidin-7-yl]-thiourea (compound 72):
(yy) N-[2-(4-Piperazin-l-vl-phenylamino)-pyrido[2.3-d]pyrimidin-.
7-vl]-acetamide (compound 73);
(zz) 4-[7-(3-Cyclohexyl-ureido)-pyrido[2,3-d]pyrimidin-2-vlamino]-
benzenesulfonamide (compound 74);
(aaa) 1-Cyclohexyl-3- { 2-[4-(1-piperazin-l-yl-methanovl)-phenylamino]-
pvrido[2,3-d]pyrimidin-7-yl}-urea (compound 75):
(bbb) 1-Cyclohexyl-3-[2-(4-fluoro-phenylamino)-
pyrido[2,3-d]pyrimidin-7-vl]-urea (compound 76):
(ccc) 1-(2-{4-[4-(2-Amino-4-methyl-pentanoyl)-piperazin-1-yl]-
phenylamino}-pyrido[2.3-d]pyrimidin-7-yl)-3-cvclohexyl-urea (bompound 77);
and
(ddd) 1-(2-{4-[4-(2-Amino-)-methyl-butanoyl)-piperazin-1-yl]-
phenylamino}-pyrido[2,3-d]pyrimidin-7-vl)-3-cyclohexyl-urea (compound 78).
EXAMPLE 143
Biological AssRt-
As noted above. the compounds of this invention are potent inhibitors of
cdks. and accordinaly, are useful in treatinsz and preventing atherosclerosis
and
other cell proliferative disorders like cancer that are mediated by such cdk
enzymes. The compounds exhibit excellent inhibitotv activitv against a number
of
cdk enzvmes, including cdkcdkl/cyclinB. cdk2/cyclinA. cdk2/cvclinE, and
cdk4/cvclinD. when evaluated in standard assays routinelv utilized by those
skilled in the art to measure cdk inhibitorv activities. Typical assays are
carried
out as follows.
Ct-clin Dependent 1-inase 4(cdk4) Assa}-
Enzvme assays for IC50 determinations and kinetic evaluation are
performed in 96-well filter plates (Millipore MADVN6550). The total volume is
0.1 mL containine a final concentration of 20 mM TRIS
* Trade-mark
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-76-
(tris[hydroxymethyl]aminomethane). at pH 7.4. 50 mM NaCI. 1 mM
dithiothreitol. 10 mM MgCI~. 25 M ATP containing 0.25 Ci of [~2P]ATP, 20 ng
of cdk4. 1 g of retinoblastoma. and appropriate dilutions of a compound of
the
present invention. All components except the ATP are added to the wells. and
the
plate is placed on a plate mixer for 2 minutes. The reaction is started by
adding
['2P]ATP, and the plate is incubated at 25 C for 15 minutes. The reaction is
terminated bv addition of 0.1 mL of 20% trichloroacetic acid (TCA). The plate
is
kept at 4 C for at least 1 hour to allo,.v the substrate to precipitate. The
'%vells are
then ~vvashed 5 times xvith 0.2 mL of 10% TCA and 32P incorporation is
determined with a beta plate counter (Wallac Inc.. Gaithersburg, MD).
Cvclin Dependent liinase 1 and 2 Assavs (cdk1/chclinB, cdk2/cti'clinA,
cdk2/cyclinE)
Enzyme assays for IC50 determinations and kinetic evaluation are
performed in a 96-well filter plate (Millipore MADVN6550) in a total volume of
0.1 mL of 20 mM TRIS (tris[hydroxymethyl]aminomethane), at pH 7.4, 50 mM
NaCl. 1 mM dithiothreitol, 10 mM MgC12, 12 mM ATP containina 0.25 Ci
of p3 2P]ATP. 20 ng of enzyme (either cdkl/B. cdk2/A, or cdk2E). 1 g
retinoblastoma, and appropriate dilutions of the particular invention
compound.
All components except the ATP are added to the wells. and the plate is placed
on a
plate mixer for 2 minutes. The reaction is begun by addition of ['2P]ATP, and
the
plate is incubated at 25 C for 15 minutes. The reaction is terminated by
addition
of 0.1 mL of 20% TCA. The plate is kept at 4 C for at least 1 hour to allow
the
substrate to precipitate. The rells are then washed 5 times with 0.2 mL of
10%
TCA and'2P incorporation determined with a beta plate counter ( 'allac Inc.,
Gaithersburg. MD).
Ct'clin Dependent Kinase 5/p25 Proline-directed Protein hinaseAssa},
Source of enzvme: recombinant baculovirus-infected insect cell
sf'9-expressed recombinant cdk5-p25 complex.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-77-
Purpose: To evaluate the ability of test agents to inhibit cdk5/p25
phosphorylation of Histone H I.
Method: Baculovirus-insect cell His-tagged cdk5/Glu-tagged p25 (or
GST-p25) enzvme complex is diluted to a concentration of 50 na/20 L in
Enzyme Dilution Buffer (EDB - 50 mM Tris-HCI [pH 8.0], 10 mM NaC1, 10 mM
MaC12. and 1 mM DTT). A 20 L sample of test agent (diluted in EDB) is then
combined -,vith 20 L of the of the final cdk5/p25 enzyme preparation and
allowed
to stand for 5 minutes at room temperature. Twentv-five microliters of
substrate
solution containinEi 115 L/mL Histone H1, 30 M ATP (vanadate-free), and
30 Ci/mL y->;P ATP (Amersham) in EDB is then added to the test agent/enzyme
preparation and shaken at 30 C for 45 minutes. A 50 L sample of the final
preparation is added to 100 mL of 150 mM phosphoric acid on ice for 30 minutes
to facilitate precipitation. The precipitate is then filtered through a 96-
well
phosphocellulose filter plate and subsequently rinsed 3 times with 75 mM
phosphoric acid. Each well then receives 20 L of scintillation cocktail, and
the
plates are counted for beta emissions using a Trilux Counter (33P filter
protocol).
Test samples are compared to Control (no test aaent present: as 0% inhibition)
and
Baseline level (no enzyme, no test agent: as 100 /o inhibition) beta emissions
to
determine the percent inhibition of Histone H 1 phosphorvlation.
The results of the foreQoing assays for representative invention compounds
are presented in Table 2 below. The invention compounds exhibit IC50 values
ranging from 0.027 N1 to >5 M against cdk 1/B, from 0.010 M to >5 M
against cdk2/A, from 0.020 to >5 M against cdk2/E, and from 0.004 to >5 M
against cdk4/D. The most potent compound overall is compound 9, which
exhibits IC50 values of 0.027 M. 0.0 10 M. 0.020 M, 0.005 M, against
cdkl/B, cdk2A. cdk2E. and cdk4D, respectivelv.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-78-
TABLE 2. Inhibition of Cdks: IC50 ( M)
Compound Cdkl/B Cdk2/A Cd1:2/E Cdk4D
1 0.219 0.060 0.130 0.006
4 >5 >5 >5 1.5
0.463 0.130 0.130 0.037
9 0.027 0.010 0.020 0.005
11 0.159 0.092 0.125 0.011
12 >5 >5 >5 2.100
28 >5 >5 >5 >5
45 0.552 0.054 0.110 0.045
46 0.075 0.300 >5
47 >5 >5 >5 >5
48 0.257 0.113 0.098 0.018
49 0.911 0.528 0.475 0.050
50 0.069 0.022 0.035 0.007
51 0.053 0.024 0.030 0.004
52 0.472 0.213) 0.126 0.027
53 >5 >5 >5 >5
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-79-
TABLE 2. Inhibition of Cdks; IC50 ( M) (cont)
Compound Cdkl/B Cdk2/A Cdk2/E Cdk4D
55 >5 >5 >5 0.300
64 >5 >5 >5 >5
65 >5 >5 >5 >5
70 1.448 0.697 0.530 0.017
71 >5 >5 >5 >5
79 >5 1.066 >5 >5
80 0.461 0.092 0.230 0.460
81 2.610 1.560 3.250 0.500
g3 0.399 0.305 0.315 0.055
84 >5 >5 >5 >5
85 >5 >5 >5 >5
86 >5 >5 >5 >5
87 >5 >5 >5 >5
88 0.418 0.043 0.055 0.025
89 >5 >5 >5 >5
91 >5 >5 >5 0.070
93 >5 >5 >5 >5
94 >5 0.101
95 >5 0.310
96 6.365 1.108 1.550 >5
97 0.862 0.278 0.345 >5
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-80-
TABLE 2. Inhibition of Cdks: IC50 ( M) (cont)
Compound Cdkl/B Cdk2/A Cdl?/E Cdk4D
98 0.442 0.157 0.140 1.050
99 1.810 1.012 0.410 >5
100 0.265 0.153 0.415 0.035
102 3.130 3.590 4.500 0.165
103 >5 >5 >5 >5
104 >5 >5 >5 0.185
106 0.350 0.440
107 1.728 1.950 1.650 0.019
108 2.425 2.035 3.050 0.067
111 >5 >5 >5 >5
113 >5 >5 >5 3.000
114 >5 >5 >5 >5
115 0.094 0.022 0.051 0.007
116 >5 >5 3.750 0.313
117 >5 >5 4.000 0.076
118 >5 >5 3.800 0.079
119 >5 >5 >5 1.600
120 >5 >5 >5 1.900
121 >5 >5 >5 0.092
The compounds of this invention also are inhibitors of the growth factor
receptor tyrosine kinase enzymes. FGFr and PDGFr. and of the nonreceptor
tvrosine kinase enzvme. c-Src. Several of the invention compounds have been
CA 02397961 2004-12-29
65920-142
-81-
evaluated via standard assays that measure their ability to inhibit tNrosine
kinase
enzymes. These assays are carried out as follows:
PDGFand FGF Receptor Tvrosine hinaseAssqtis
Full-length cDNAs for the mouse PDGF-0 and human FGF-1 (fle)
receptor tyrosine kinases are obtained from J. Escobedo and prepared as
described
in.J. Biol. Chem.. 1991:262:1482-1487. PCR primers are designed to amplify a
fraament of DNA that codes for the intracellular tvrosine kinase domain. The
fraRment is inserted into a baculovirus vector. cotransfected =ith AcNINPV
DNA.
and the recombinant virus isolated. SF9 insect cells are infected with the
virus to
overexpress the protein, and the cell lysate is used for the assay. Assays are
performed in 96-well plates (100 L/incubation/well), and conditions are
optimized to measure the incorporation of 32P from y32P-ATP into aQlutamate-
tyrosine co-polymer substrate. Briefly, to each well is added 82.5t L of
incubation
buffer containing 25 mM Hepes (pH 7.0). 150 mM NaCI. 0.1% Triton .X-100,
0.2 mM PMSF 0.2 mM Na3VO:}. 10 mM MnCI-). and 750 g/mL of Poly (4:1)
glutamate-tyrosine followed by 2.5 L of inhibitor and 5 L of enzyme lysate
(7.5 g/ L FGF-TK or 6.0 g/ L PDGF-TK) to initiate the reaction. Following a
10-minute incubation at 25 C. 10 mL of y3'P-ATP (0.4 Ci plus 50 M ATP) is
added to each well. and samples are incubated for an additional 10 minutes at
25 C. The reaction is terminated by the.addition of-100 L of 30%
trichloroacetic
acid (TCA) cdntaining 20 mM sodium pvrophosphate and precipitation of
material onto glass fiber mats (Wallac). Filters are washed 3 times with 15%
TCA
containing 100 mM sodium pyrophosphate. and the radioactivity retained on the
filters counted in a Wallac 1250 Betaplate reader. Nonspecific activity is
defined
as radioactivitv retained on the filters following incubation of samples with
buffer
alone (no enzyme). Specific enzymatic activity (enzyme plus buffer) is defined
as
total activity minus nonspecific activity. The % inhibition at 50 M is
determined.
and for the more potent compounds the concentration of the compound that
inhibited specific activitv by 50% (IC50) is determined based on the
inhibition
curve.
* Trade-mark
CA 02397961 2002-07-22
WO 01/55147 PCT/1B01/00069
-82-
C-Src kinaseAssaY
C-Src kinase is purified from baculovirus infected insect cell lysates using
an antipeptide monoclonal antibody directed against the N-terminal amino acids
(amino acids 2-17) of c-Src. The antibody. covalentlv linked to 0.65 um latex
beads. is added to a suspension of insect cell lysis buffer comprised of 150
mM
NaCI. 50 mM Tris pH 7.5, 1 mM DTT, 1% NP-40. 2 mM EGTA. 1 mM sodium
vanadate. 1 mM PMSF, 1 ug/mL each of leupeptin. pepstatin, and aprotinin.
Insect
cell lysate containing c-Src protein is incubated with these beads for 3 to 4
hours
at 4 C ,vith rotation. At the end of the lvsate incubation, the beads are
rinsed
3 times in lvsis buffer. resuspended in lysis buffer containina 10% glycerol,
and
frozen. These latex beads are thawed, rinsed 3 times in assay buffer (40 mM
Tris.
pH 7.5, 5 mM MgC12), and suspended in the same buffer. In a Millipore 96-well
plate with a 0.65 um polyvinylidine membrane bottom are added the reaction
components: 10 uL c-Src beads. 10 uL of 2.5 mg/mL poly GluTyr substrate, 5 uM
ATP containing 0.2 uCi labeled 32P-ATP. 5 uL DMSO containing inhibitors or as
a solvent control. and buffer to make the final volume 125 uL. The reaction is
started at room temperature by addition of the ATP and quenched 10 minutes
later
by the addition of 125 uL of 30% TCA. 0.1 M sodium pyrophosphate for
5 minutes on ice. The plate is then filtered and the wells washed with two 250-
mL
aliquots of 15% TCA. 0.1 M pyrophosphate. The filters are then punched.
counted
in a liquid scintillation counter. and the data examined for inhibitory
activity in
comparison to a knovN,n inhibitor such as erbstatin. The method is also
described in
J. Med. Chem., 1994:37:598-609.
The tyrosine kinase inhibitorv activity for representative invention
compounds evaluated in the foregoin' assays is P-iven in Table 3
.
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-83-
TABLE 3. Inhibition of Tvrosine Kinases; % Inhibition at 50 N1
(IC50 [ M] in parenthesis if determined)
Compound PDGFr FGFr
1 94.4 (0.593) 93.7
9 89.8
11 (0.131) (0.284)
45 21.9 67.4
46 17.5 19.5
47 10.5
55 (0.033) (0.151)
70 (0.536) (1.15)
80 18.6
117 (0.081) (0.061)
As noted above, the invention also provides pharmaceutical compositions
comprising an invention compound admixed with a carrier, diluent, or
excipient.
The following examples illustrate typical compositions provided by this
invention.
EXAMPLE 144
A pharmaceutical compositions in the form of hard gelatin capsules for
oral administration is prepared using the folloxving inaredients:
Quantity (mg/capsule)
Active compound 250
Starch powder 200
Maiinesium stearate 10
Total 460
The above ingredients are mixed and filled into hard gelatin capsules in
460 mg quantities. A typical active ingredient is 1-isobutyl-3-[2-{(2-chloro-4-
piperazin-l-yl)-phenylamino; -pyrido[2.3-d]pyrimidin-7-yl]-urea. The
composition is administered from 2 to 4 times a day for treatment of
postsurgical
restenosis.
CA 02397961 2002-07-22
WO 01/55147 PCT/1B01/00069
-84-
EXAMPLE 144a
Compositions for Oral Suspension
Ingredient Amount
1-Isopropvl-3-[5-methvl-6-bromo-2-(3-ethylp~,ridin-4- 500 mg
ylamino}-pvrido-[2.3-d]pyrimidin-7-yl]-urea
Sorbitol solution (70% NF) 40 mL
Sodium benzoate 150 ma
Saccharin 10 mg
Chem flavor 50 mg
Distilled water q.s. ad 100 mL
The sorbitol solution is added to 40 mL of distilled water. and the
pvridopyrimidine is suspended therein. The saccharin. sodium benzoate. and
flavorina are added and dissolved. The volume is adjusted to 100 mL with
distilled water. Each milliliter of syrup contains 5 mg of active ingredient.
EXAMPLE 144b
Tablets Each Containina 60 ma of Active Ingredient
Active inaredient 60 m2
Starch -1 ~ mg
Microcrvstalline cellulose mg
Polvvinylpyrrolidone (as 10% solution in ~vater) 4 mg
Sodium carboxymethvl starch 4.5 mg
Magnesium stearate 0.5 mg
Talc 1.0 mg
Total 1 -50 mg
The active in7redient. starch. and cellulose are passed through a No. 45
mesh US sieve and mixed thorouahlv. The solution of polyvinylpyrrolidone is
mixed with the resultant powders and then passed throuvh a No. 14 mesh US
sieve. The granules are dried at 50 C to 60 C and passed through a No. 18 mesh
US sieve. The sodium carboxvmethvl starch. maUnesium stearate. and talc,
CA 02397961 2002-07-22
WO 01/55147 PCT/IB01/00069
-85-
previously passed through a No. 60 mesh US sieve, are then added to the
granules
which, after mixing, are compressed on a tablet machine to yield tablets each
weighing 150 mg.
A typical active ingredient utilized in the above preparation is the
compound of Example 40 (Compound 12). This composition is well-suited to
treatment of diabetic retinopathy.
EXAMPLE 144c
A parenteral composition suitable for administration by injection is
prepared by dissolving 100 mg of compound 77 in 250 mL of 0.9% aqueous
sodium chloride solution and adjusting the pH of the solution to about 7Ø
This
formulation is 'ell-suited for the treatment of breast cancer.
EXAMPLE 144d
Preparation for Suppositories
A mixture of 500 mg of 1-n-butyl-3-[2-(4-piperazin-l-yl-phenylamino)-
pvrido[2.3-d]p~,rimidin-7-vl]-urea and 1500 mg of theobroma oil are blended to
uniformitv at 60 C. The mixture is cooled to 24 C in tapered molds. Each
suppositon, will weigh about 2 g and can be administered from I to 2 times
each
day for treatment of viral infections such as herpes and HIV.
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-86-
EXAMPLE 144e
Topical Preparation
Inaredient Amount (mg)
1-Cycloheavl-3- { [2-(4-morpholin-l-yl-phenvlamino)]-5,6- 20
difluoro-pyrido[2,3-d]pyrimidin-7-yl } -urea
Propylene glycol 100
White Petrolatum 500
Cetearvl alcohol 50
Glvicervl stearate 100
PEG 100 stearate 100
Ceteth-20 50
Monobasic sodium phosphate 80
Total 1000
The invention compound is blended to uniformitv with the other
ingredients to make a thick suspension. The suspension is applied evenly to an
adhesive backed polymeric film and cut into a 2-inch square. The patch is
applied
to the skin of a patient suffering from psoriasis.
EXAMPLE 144f
Slow Release Preparation
Five hundred milligrams of 7-acetamido-6-bromo-2-[4-(2-
diethylaminoethoay)-phenylamino]pyrido[2.3-d]pyrimidine hydrochloride was
placed in an osmotic pump tablet and administered orally to a subject for
treatment and prevention of restenosis.
The invention and the manner and process of making and using it are now
described in such full. clear. concise and exact terms as to enable any person
skilled in the art to which it pertains to make and use the same. It is to be
understood that the foregoiny describes preferred embodiments of the present
invention, and that modifications may be made therein without departing from
the
spirit or scope of the present invention as set forth in the claims. To
particularly
CA 02397961 2002-07-22
WO 01/55147 PCT/IBO1/00069
-87-
point out and distinctly claim the subject matter regarded as the invention,
the
following claims conclude this specification.