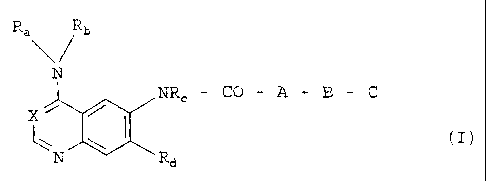Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02403152 2002-09-13
72984fft.203
Boehringer Ingelheim Pharma KG Case 5/1289
D-55216 Ingelheim/Rhein Foreign filing text
Bicyclic heterocycles, pharmaceutical compositions containing
these compounds, their use and processes for preparing them
The present invention relates to bicyclic heterocycles of
general formula
Rab
N
NRC - CO - A - B - C
X~
Zll~ I , cI>
N Rd
the tautomers, the stereoisomers and the salts thereof,
particularly the physiologically acceptable salts thereof with
inorganic or organic acids or bases which have valuable
pharmacological properties, particularly an inhibitory effect
on signal transduction mediated by tyrosine kinases, the use
thereof for treating diseases, particularly tumoral diseases,
diseases of the lungs and respiratory tract, and the
preparation thereof.
In the above general formula I
X denotes a methyne group substituted by a cyano group or a
nitrogen atom,
Ra denotes a hydrogen atom or a C1_4-alkyl group,
Rb denotes a phenyl, benzyl or 1-phenylethyl group wherein the
phenyl nucleus in each case is substituted by the groups R1 to
R3, whilst
CA 02403152 2002-09-13
- 2 -
R1 and R2, which may be identical or different, in each
case denote a hydrogen, fluorine, chlorine, bromine or
iodine atom,
a C1_4-alkyl, hydroxy, Cl_4-alkoxy, C3_6-cycloalkyl,
C4_6-cycloalkoxy, C2_5-alkenyl or C2_5-alkynyl group,
an aryl, aryloxy, arylmethyl or arylmethoxy group,
a C3_5-alkenyloxy or C3_5-alkynyloxy group, whilst the
unsaturated moiety may not be linked to the oxygen atom,
a C1_4-alkylsulphenyl, Cl_,-alkylsulphinyl,
C1_4-alkylsulphonyl, C7._4-alkylsulphonyloxy,
trifluoromethylsulphenyl, trifluoromethylsulphinyl or
trifluoromethylsulphonyl group,
a methyl or methoxy group substituted by 1 to 3 fluorine
atoms,
an ethyl or ethoxy group substituted by 1 to 5 fluorine
atoms,
a cyano or nitro group or an amino group optionally
substituted by one or two C1_4-alkyl groups, whilst the
substituents may be identical or different, or
R. together with R2, if they are bound to adjacent carbon
atoms, denote a -CH=CH-CH=CH-, -CH=CH-NH- or -CH=N-NH-
group and
R3 denotes a hydrogen, fluorine, chlorine or bromine atom,
a Cl_,-alkyl, trifluoromethyl or C1_4-alkoxy group,
R,, denotes a hydrogen atom or a C1_4-alkyl group,
CA 02403152 2002-09-13
- 3 -
Ra denotes a hydrogen atom, a C1_6-alkoxy, C4_,-cycloalkoxy or
C3_,-cycloalkyl-C1_4-alkoxy group,
a C2_6-alkoxy group, which is substituted from position 2 by a
hydroxy, C1_9-alkoxy, Cq_,-cycloalkoxy, C3_,-cycloalkyl-
C1_3-alkoxy, di- (Cl_4-alkyl) -amino, pyrrolidino, piperidino,
morpholino, piperazino or 4-(C1_4-alkyl)-piperazino group,
whilst the abovementioned cyclic imino groups may be
substituted by one or two C1_2-alkyl groups,
a tetrahydrofuran-3-yloxy, tetrahydropyran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuranylmethoxy or
tetrahydropyranylmethoxy group,
A denotes a 1,1- or 1,2-vinylene group optionally substituted
by a methyl or trifluoromethyl group or by two methyl groups,
a 1,3-butadien-l,4-ylene group optionally substituted by a
methyl or trifluoromethyl group or by two methyl groups, or an
ethynylene group,
B denotes a C1_6-alkylene group wherein one or two hydrogen
atoms may be replaced by fluorine atoms, or, if B is bound to
a carbon atom of group C, it may also denote a bond,
C denotes a pyrrolidino group wherein the two hydrogen atoms
in the 2 position are replaced by a group D, wherein
D denotes a -CHZ-O-CO-CH2-, -CH2CH2-O-CO-, -CH2-O-CO-CHZCHZ-,
-CHZCH2-O-CO-CHZ- or -CH2CH2CH2-O-CO- bridge optionally
substituted by one or two C1_2-alkyl groups,
a pyrrolidino group wherein the two hydrogen atoms in the 3
position are replaced by a group E, wherein
E denotes an -O-CO-CHZCHZ-, -CH2-O-CO-CH2-, -CH2CH2-O-CO-,
-O-CO-CH2CH2CH2- , -CH2-0-CO-CH2CH2- , -CHzCHz-O-CO-CHz-,
M
CA 02403152 2002-09-13
- 4 -
-CH2CHZCH2-O-CO-, -O-CO-CH2-NR4-CH2-, -CH2-O-CO-CH2-NR4-,
-O-CO-CH2-O-CH2- or -CH2-O-CO-CH2-O- bridge optionally
substituted by one or two C1_z-alkyl groups,
whilst R4 denotes a hydrogen atom or a C1_4-alkyl group,
a piperidino or hexahydroazepino group wherein the two
hydrogen atoms in the 2 position are replaced by a group D,
whilst D is as hereinbefore defined,
a piperidino or hexahydroazepino group wherein the two
hydrogen atoms in the 3 position or in the 4 position are
replaced by a group E, whilst E is as hereinbefore defined,
a piperazino or 4-(Cz_4-alkyl)-piperazino group wherein the two
hydrogen atoms in the 2 position or in the 3 position of the
piperazino ring are replaced by a group D, where D is as
hereinbefore defined,
a pyrrolidino or piperidino group wherein two vicinal hydrogen
atoms are replaced by an -O-CO-CHz-, -CH2-O-CO-, -O-CO-CH2CH2-,
-CH2-O-CO-CH2-, -CH2CH2-O-CO-, -O-CO-CH2-NR4- or -O-CO-CHz-O-
bridge optionally substituted by one or two C1_2-alkyl groups,
whilst R4 is as hereinbefore defined and the heteroatoms of the
abovementioned bridges are not bound at the 2 or 5 position of
the pyrrolidine ring and are not bound at the 2 or 6 position
of the piperidino ring,
a piperazino or 4-(C1_4-alkyl)-piperazino group wherein a
hydrogen atom in the 2 position together with a hydrogen atom
in the 3 position of the piperazino ring are replaced by a
-CHz-O-CO-CHZ- or -CH2CHZ-0-CO- bridge optionally substituted by
one or two Cl_2-alkyl groups,
a piperazino group wherein a hydrogen atom in the 3 position
together with the hydrogen atom in the 4 position are replaced
by a-CO-O-CHZCH2- or -CH2-O-CO-CH2- bridge optionally
CA 02403152 2002-09-13
- 5 -
substituted by one or two C1_z-alkyl groups, whilst in each case
the left-hand end of the abovementioned bridges is bound to
the 3 position of the piperazino ring,
apyrrolidino, piperidino or hexahydroazepino group substitu-
ted by the group R5, wherein
R. denotes a 2-oxo-tetrahydrofuranyl, 2-oxo-tetrahydro-
pyranyl, 2-oxo-l,4-dioxanyl or 2-oxo-4-(C1_4-alkyl)-
morpholinyl group optionally substituted by one or two
C1_2-alkyl groups,
a pyrrolidino group substituted in the 3 position by a 2-oxo-
morpholino group, whilst the 2-oxo-morpholino group may be
substituted by one or two C1_2-alkyl groups,
a piperidino or hexahydroazepino group substituted in the 3 or
4 position by a 2-oxo-morpholino group, whilst the 2-oxo-
morpholino group may be substituted by one or two Cl_Z-alkyl
groups,
a 4- (C1_4-alkyl) -piperazino or 4- (C1_9-alkyl) -homopiperazino
group substituted at a cyclic carbon atom by RS , wherein RS is
as hereinbefore defined,
a piperazino or homopiperazino group substituted in the 4
position by the group R6, wherein
R6 denotes a 2-oxo-tetrahydrofuran-3-yl, 2-oxo-tetrahydro-
furan-4-yl, 2-oxo-tetrahydropyran-3-yl, 2-oxo-tetrahydro-
pyran-4-yl or 2-oxo-tetrahydropyran-5-yl group optionally
substituted by one or two C1_z-alkyl groups,
a pyrrolidino group substituted in the 3 position by a(R4NR6),
R60, R6S, R6SO or R6S02 group, whilst RQ and R6 are as
hereinbefore defined,
CA 02403152 2002-09-13
- 6 -
a piperidino or hexahydroazepino group substituted in the 3 or
4 position by an (RqNR6) , R60, R6S, R6SO or R6S02 group wherein R4
and R6 are as hereinbefore defined,
a pyrrolidino,piperidino or hexahydroazepino group substi-
tuted by a R5-C1_4-alkyl, (RqNR6) -Cl_4-a1ky1, R60-Cl_4-alkyl,
R6S-C1_4-alkyl, R6SO-C1_4-alkyl, R6SOz-C1_4-alkyl or R4NR6-CO group
wherein R4 to R. are as hereinbefore defined,
a pyrrolidino group substituted in the 3 position by a
RS-CO-NR4, RS-C1_4-alkylene-CONR4, (R4NR6) -Cl_4-alkylene-CONR4,
R60-C1_4-alkylene-CONR4, R6S-C1_4-alkylene-CONR9, R6SO-Cl_4-al-
kylene-CONR4, R6SOz-C1_4-alkylene-CONR4, 2-oxo-morpholino-
C1_4-alkylene-CONR4, RS-Cl_4-alkylene-Y or C2_4-alkyl-Y group,
whilst the C2_4-alkyl moiety of the C2_4-alkyl-Y group is
substituted in each case from position 2 by a(R4NR6) , R60, R6S,
R6SO or R6S02 group and the 2-oxo-morpholino moiety may be
substituted by one or two C1_z-alkyl groups, wherein
R4 to R6 are as hereinbefore defined and
Y denotes an oxygen or sulphur atom, an imino,
N-(C1_4-alkyl)-imino, sulphinyl or suiphonyl group,
a piperidino or hexahydroazepino group substituted in the 3 or
4 position by a RS-CO-NR4, RS-C1_4-alkylene-CONR4, (R4NR6) -C1_4-a1-
kylene-CONR4, R6O-C1_4-alkylene-CONR4, R6S-Cl_4-alkylene-CONR4,
R6SO-C1_4-alkylene-CONR4, R6SOz-C1_4-alkylene-CONR4, 2-oxo-mor-
pholino-C1_4-alkylene-CONR4, RS-Cl_4-alkylene-Y or C2_4-alkyl-Y
group wherein Y is as hereinbefore defined, the 2-oxo-morpho-
lino moiety may be substituted by one or two C1_Z-alkyl groups
and the C2_4-alkyl moiety of the C2_4-alkyl-Y group is
substituted in each case from position 2 by a (R4NR6), R60, R6S,
R6SO or R6SO2 group, whilst R4 to R6 are as hereinbefore defined,
a 4- (C1_4-alkyl) -piperazino or 4- (C1_4-alkyl) -homopiperazino
group substituted at a cyclic carbon atom by an R5-C1_4-alkyl,
CA 02403152 2002-09-13
- 7 -
(R4NR6) -C1_4-alkyl, R60-C1_4-alkyl, R6S-C1_4-alkyl, R6SO-C1_4-alkyl,
R6S02-C1_4-alkyl or RQNR6-CO group, wherein R4 to R. are as
hereinbefore defined,
a piperazino or homopiperazino group substituted in the 4
position by an RS-Cl_4-alkyl, RS-CO, RS-C1_4-alkylene-CO,
(R4NR6) -C1_4-alkylene-CO, R60-C1_4-alkylene-CO, R6S-C1_9-alkylene-
CO, R6S0-C1_4-alkylene-CO or R6S02-C1_4-alkylene-CO group wherein
R4 to R. are as hereinbefore defined,
a piperazino or homopiperazino group substituted in the 4
position by a C2_4-alkyl group, wherein the C2_4-alkyl group is
substituted in each case from position 2 by an (R4NR6) , R60,
R6S, R6SO or R6S02 group, whilst R4 and R6 are as hereinbefore
defined,
a pyrrolidino, piperidino or hexahydroazepino group
substituted by a 2-oxo-morpholino-C1_4-alkyl group, wherein the
2-oxo-morpholino moiety may be substituted by one or two
C1_2-alkyl groups,
a pyrrolidino group substituted in the 3 position by a
C2_4-alkyl-Y group, wherein Y is as hereinbefore defined and the
C2_4-alkyl moiety of the C2_4-alkyl-Y group is substituted in
each case from position 2 by a 2-oxo-morpholino group
optionally substituted by one or two C1_2-alkyl groups,
a piperidino or hexahydroazepino group substituted in the 3 or
4 position by a C2_4-alkyl-Y group wherein Y is as hereinbefore
defined and the C2_4-alkyl moiety of the C2_4-alkyl-Y group is
substituted in each case from position 2 by a 2-oxo-morpholino
group optionally substituted by one or two C1_z-alkyl groups,
a 4- (C1_4-alkyl) -piperazino or 4- (C1_4-alkyl) -homopiperazino
group substituted at a cyclic carbon atom by a 2-oxo-
morpholino-C1_4-alkyl group, wherein the 2-oxo-morpholino moiety
may be substituted by one or two C1_2-alkyl groups,
CA 02403152 2002-09-13
- 8 -
a piperazino or homopiperazino group substituted in the 4
position by a 2-oxo-morpholino-C1_4-alkylene-CO group, wherein
the 2-oxo-morpholino moiety may be substituted by one or two
C1_2-alkyl groups,
a piperazino or homopiperazino group substituted in the 4
position by a C2_9-alkyl group, wherein the C2_4-alkyl moiety is
substituted in each case from position 2 by a 2-oxo-
morpholino group optionally substituted by one or two C1_2-alkyl
groups,
a pyrrolidinyl or piperidinyl group substituted in the 1
position by the group R6, by an RS-C1_4-alkyl, RS-CO, RS-C1_4-
alkylene-CO, (R4NR6) -C1_4-alky1ene-CO, R6O-C1_4-alkylene-CO,
R6S-C1_4-alkylene-CO, R6SO-C1_4-alkylene-CO, R6S02-C1_4-alkylene-CO
or 2-oxo-morpholino-C1_4-alkylene-CO group wherein R4 to R6 are
as hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two C1_z-alkyl groups,
a pyrrolidinyl or piperidinyl group substituted in the 1
position by a C2_4-alkyl group wherein the C2_4-alkyl moiety is
substituted in each case from position 2 by an (RQNR6) , R60,
R6S, R6SO, R6SO2 or 2-oxo-morpholino group, whilst R4 and R. are
as hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two C1_2-alkyl groups,
a pyrrolidin-3-yl-NR4, piperidin-3-yl-NRq or piperidin-4-yl-NRq
group substituted in each case at the cyclic nitrogen atom by
the group R6, by an R5-C1_4-alkyl, RS-CO, R5-C1_4-alkylene-CO,
(R4NR6) -C1_4-alkylene-CO, R60-C1_4-alkylene-CO, R6S-Cl_4-alkylene-
CO, R6SO-C1_4-alkylene-CO, R6SO2-Cl_4-alkylene-CO or 2-oxo-
morpholino-C1_4-alkylene-CO group, wherein R4 to R6 are as
hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two C1_2-alkyl groups,
CA 02403152 2002-09-13
- 9 -
a pyrrolidin-3-yl-NR4, piperidin-3-yl-NR4 or piperidin-4-yl-NR4
group substituted in each case at the cyclic nitrogen atom by
a C2_4-alkyl group, wherein the C2_4-alkyl moiety is substituted
in each case from position 2 by an (R4NR6) , R60, R6S, R6SO, R6S02
or 2-oxo-morpholino group, whilst R4 and R6 are as hereinbefore
defined and the 2-oxo-morpholino moiety may be substituted by
one or two C1_2-alkyl groups,
a RS-C1_4-alkylene-NR4 group wherein R4 and R5 are as
hereinbefore defined, or
a C2_4-alkyl-NR4-group, wherein the C2_4-alkyl moiety is
substituted in each case from position 2 by an (R4NR6), R60,
R6S, R6SO, R6S02 or 2-oxo-morpholino group, whilst R4 and R6 are
as hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two C1_2-alkyl groups, while
by the abovementioned aryl moieties is meant a phenyl group
which may in each case be mono- or disubstituted by R', while
the substituents may be identical or different and
R' denotes a fluorine, chlorine, bromine or iodine atom, a
C1_2-alkyl, trifluoromethyl or C1_2-alkoxy group or
two groups R', if they are bound to adjacent carbon atoms,
together denote a C3_4-alkylene, methylenedioxy or 1,3-butadien-
1,4-ylene group.
Preferred compounds of the above general formula I are those
wherein
X denotes a nitrogen atom,
Ra denotes a hydrogen atom,
CA 02403152 2002-09-13
- 10 -
Rb denotes a phenyl, benzyl or 1-phenylethyl group wherein the
phenyl nucleus is substituted in each case by the groups R,to
R3, whilst
R1 and Rz, which may be identical or different, in each
case denote a methyl group or a hydrogen, fluorine,
chlorine or bromine atom and
R3 denotes a hydrogen atom,
R, denotes a hydrogen atom,
Rd denotes a hydrogen atom, a methoxy, ethoxy, C4_6-cycloalkoxy,
C3_6-cycloalkylmethoxy, 2-methoxy-ethoxy, 2-(cyclobutyloxy)-
ethoxy, 2-(cyclopentyloxy)-ethoxy, 2-(cyclohexyloxy)-ethoxy,
2-(cyclopropylmethoxy)-ethoxy, tetrahydrofuran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuran-2-ylmethoxy,
tetrahydrofuran-3-ylmethoxy, tetrahydropyran-2-ylmethoxy or
tetrahydropyran-4-ylmethoxy group,
A denotes a 1,2-vinylene group,
B denotes a methylene or ethylene group or, if B is bound to a
carbon atom of group C, it may also denote a bond,
C denotes a pyrrolidino group wherein the two hydrogen atoms
in the 3 position are replaced by a group E, wherein
E denotes a -O-CO-CH2CHZ-, -CH2-O-CO-CH2-, -CHZCHz-O-CO-,
-CH2CH2-0-CO-CH2-, -O-CO-CH2-NR4-CH2-, -CH2-O-CO-CH2-NR4-,
-O-CO-CH2-O-CH2- or -CH2-O-CO-CHZ-O- bridge optionally
substituted by one or two methyl groups,
where R4 denotes a methyl or ethyl group,
CA 02403152 2002-09-13
- 11 -
a piperidino group wherein the two hydrogen atoms in the 3
position or in the 4 position are replaced by a group E, where
E is as hereinbefore defined,
a pyrrolidino or piperidino group wherein two vicinal hydrogen
atoms are replaced by an -O-CO-CH2-1 -CH2-O-CO-, -O-CO-CHz-NRQ-
or -O-CO-CHz-O- bridge optionally substituted by one or two
methyl groups, whilst R4 is as hereinbefore defined and the
heteroatoms of the abovementioned bridges are not bound at the
2 or 5 position of the pyrrolidine ring and are not bound at
the 2 or 6 position of the piperidino ring,
a piperazino group wherein a hydrogen atom in the 3 position
together with the hydrogen atom in the 4 position are replaced
by a-CO-O-CHZCH2- or -CH2-O-CO-CH2- bridge optionally
substituted by one or two methyl groups, whilst in each case
the left-hand end of the abovementioned bridges is bound to
the 3 position of the piperazino ring,
a pyrrolidino or piperidino group substituted by the group R5,
wherein
R5 denotes a 2-oxo-tetrahydrofuranyl, 2-oxo-l,4-dioxanyl or
2-oxo-4-(C1_4-alkyl)-morpholinyl group optionally
substituted by one or two methyl groups,
a pyrrolidino group substituted in the 3 position by a 2-oxo-
morpholino group, whilst the 2-oxo-morpholino group may be
substituted by one or two methyl groups,
a piperidino group substituted in the 3 or 4 position by a
2-oxo-morpholino group, whilst the 2-oxo-morpholino group may
be substituted by one or two methyl groups,
a piperazino group substituted in the 4 position by the group
R6, wherein
CA 02403152 2002-09-13
- 12 -
R6 denotes a 2-oxo-tetrahydrofuran-3-yl or 2-oxo-
tetrahydro-furan-4-yl group optionally substituted by one
or.two methyl groups,
a pyrrolidino group substituted in the 3 position by an
(R4NR6) , R60, R6S, R6SO or R6S02 group, whilst R4 and R6 are as
hereinbefore defined,
a piperidino group substituted in the 3 or 4 position by an
(R4NR6) , R60, R6S, R6SO or R6SOZ group, whilst R4 and R6 are as
hereinbefore defined,
a pyrrolidino or piperidino group substituted by an
(R4NR6) -C1_2-alkyl, HNR6-CO or R4NR6-CO group, whilst R4 and R6 are
as hereinbefore defined,
a pyrrolidino group substituted in the 3 position by an
RS-CO-NH or R5-CO-NR4 group, whilst R4 and RS are as hereinbefore
defined,
a piperidino group substituted in the 3 or 4 position by an
R5-CO-NH or RS-CO-NR4 group, whilst R4 and R. are as hereinbefore
defined,
a piperazino group substituted in the 4 position by an
RS-C1_z-alkyl, RS-CO, RS-C1_2-alkylene-CO, (R4NR6) -C1_2-alkylene-CO,
R60-C1_z-alkylene-CO, R6S-Cl_2-alkylene-CO, R6SO-C1_z-alkylene-CO
or R6S02-C1_2-alkylene-CO group wherein R4 to R. are as
hereinbefore defined,
a piperazino group substituted in the 4 position by a C2_3-alkyl
group wherein the C2_j-alkyl group is substituted in each case
from position 2 by an (R4NR6) , R60, R6S, R6SO or R6S0Z group,
where R4 and R6 are as hereinbefore defined,
CA 02403152 2002-09-13
- 13 -
a pyrrolidino or piperidino group substituted by a 2-oxo-
morpholino-C1_2-alkyl group wherein the 2-oxo-morpholino moiety
may be.substituted by one or two methyl groups,
a piperazino group substituted in the 4 position by a 2-oxo-
morpholino-C1_2-alkylene-CO group wherein the 2-oxo-morpholino
moiety may be substituted by one or two methyl groups,
a piperazino group substituted in the 4 position by a C2_3-alkyl
group wherein the C2_3-alkyl moiety is substituted in each case
from position 2 by a 2-oxo-morpholino group optionally
substituted by one or two methyl groups,
a piperidinyl group substituted in the 1 position by the group
R6, by an RS-C1_z-alkyl, R5-CO, R5-C1_z-alkylene-CO, (R4NR6) -C1_2-
alkylene-CO, R60-C1_z-alkylene-CO, R6S-C1_2-alkylene-CO, R6SO-C1_Z-
alkylene-CO, R6S02-C1_2-alkylene-CO or 2-oxo-morpholino-Cl_2-
alkylene-CO group, whilst R4 to R. are as hereinbefore defined
and the 2-oxo-morpholino moiety may be substituted by one or
two methyl groups,
a piperidinyl group substituted in the 1 position by a
C2_3-alkyl group wherein the C2_3-alkyl moiety is substituted in
each case from position 2 by an (R4NR6) , R60, R6S, R6SO, R6S02 or
2-oxo-morpholino group, whilst RQ and R6 are as hereinbefore
defined and the 2-oxo-morpholino moiety may be substituted by
one or two methyl groups,
a pyrrolidin-3-yl-NR4, piperidin-3-yl-NR4 or piperidin-4-yl-NR4
group substituted in each case at the cyclic nitrogen atom by
the group R6, by an R5-Cl_z-alkyl, RS-CO, RS-C1_2-alkylene-CO,
(R4NR6) -C1_2-alkylene-CO, R60-C1_2-alkylene-CO, R6S-C1_2-alkylene-
CO, R6SO-C1_2-alkylene-CO, R6S02-C1_z-alkylene-CO or 2-oxo-mor-
pholino-C1_2-alkylene-CO group, wherein RQ to R. are as
hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two methyl groups, or
CA 02403152 2002-09-13
- 14 -
a pyrrolidin-3-yl-NR4, piperidin-3-yl-NR4 or piperidin-4-yl-NR4
group substituted in each case at the cyclic nitrogen atom by
a C2_3-alkyl group, wherein the C2_3-alkyl moiety is substituted
in each case from position 2 by an (R4NR6) , R60, R6S, R6S0, R6S02
or 2-oxo-morpholino group, whilst R4 and R. are as hereinbefore
defined and the 2-oxo-morpholino moiety may be substituted by
one or two methyl groups,
the tautomers, the stereoisomers and the salts thereof.
Particularly preferred compounds of general formula I are
those wherein
X denotes a nitrogen atom,
Ra denotes a hydrogen atom,
Rb denotes a phenyl, benzyl or 1-phenylethyl group wherein the
phenyl nucleus is substituted in each case by the groups R1 to
R3, whilst
R1 and R2, which may be identical or different, each denote
a methyl group or a hydrogen, fluorine, chlorine or bromine
atom and
R3 denotes a hydrogen atom,
Rr denotes a hydrogen atom,
Rd denotes a hydrogen atom, a methoxy, ethoxy, C4_6-cycloalkoxy,
C3_6-cycloalkylmethoxy, 2-methoxy-ethoxy, 2-(cyclobutyloxy)-
ethoxy, 2-(cyclopentyloxy)-ethoxy, 2-(cyclohexyloxy)-ethoxy,
2-(cyclopropylmethoxy)-ethoxy, tetrahydrofuran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuran-2-ylmethoxy,
tetrahydrofuran-3-ylmethoxy, tetrahydropyran-2-ylmethoxy or
tetrahydropyran-4-ylmethoxy group,
CA 02403152 2002-09-13
- 15 -
A denotes a 1,2-vinylene group,
B denotes a methylene or ethylene group or, if B is bound to a
carbon atom of group C, it may also denote a bond,
C denotes a piperidino group wherein the two hydrogen atoms in
the 4 position are replaced by a-CHz-O-CO-CHz-, -CH2CH2-0-CO-,
-CH2CH2-0-CO-CH2-, -O-CO-CH2-NCH3-CH2- or -O-CO-CHZ-O-CHz- bridge,
a piperazino group wherein a hydrogen atom in the 3 position
together with the hydrogen atom in the 4 position are repiaced
by a-CO-O-CHz-CH2- or -CH2-O-CO-CH2- bridge, whilst in each
case the left-hand end of the above bridges is bound to the 3
position of the piperazino ring,
a piperidino group substituted by a 2-oxo-tetrahydrofuranyl
group,
a piperidino group which is substituted in the 4 position by a
2-oxo-morpholino or 2-oxo-morpholinomethyl group, whilst the
2-oxo-morpholino moiety may in each case be substituted by one
or two methyl groups,
a piperazino group which is substituted in the 4 position by a
2-oxo-tetrahydrofuran-3-yl or 2-oxo-tetrahydrofuran-4-yl
group,
a piperidino group which is substituted in the 4 position by a
CH3NR6 or R6S group, whilst
R. denotes a 2-oxo-tetrahydrofuran-3-yl or 2-oxo-
tetrahydro-furan-4-yl group,
a piperazino group which is substituted in the 4 position by a
2-oxo-tetrahydrofuranylmethyl or 2-oxo-tetrahydrofuranyl-
carbonyl group,
CA 02403152 2002-09-13
- 16 -
a piperazino group which is substituted in the 4 position by a
straight-chained C2_3-alkyl group, whilst the C2_3-alkyl moiety
is terminally substituted in each case by a 2-oxo-tetrahydro-
furan-3-ylsulphenyl group,
a piperidin-4-yl group which is substituted in the 1 position
by a 2-oxo-tetrahydrofuran-3-yl or 2-oxo-tetrahydrofuran-4-yl
group, or
a piperidin-4-yl-NCH3 group which is substituted at the cyclic
nitrogen atom by a 2-oxo-tetrahydrofuran-3-yl, 2-oxo-tetra-
hydrofuran-4-yl or 2-oxo-tetrahydrofuranylcarbonyl group,
the tautomers, the stereoisomers and the salts thereof.
Most especially preferred compounds of the above general
formula I are those wherein
X denotes a nitrogen atom,
Ra denotes a hydrogen atom,
Rb denotes a 1-phenylethyl group or a phenyl group substituted
by the groups Rl to R3, wherein
R1 and Rz, which may be identical or different, each denote
a methyl group or a hydrogen, fluorine, chlorine or bromine
atom and
R3 denotes a hydrogen atom,
R, denotes a hydrogen atom,
Rd denotes a hydrogen atom, a methoxy, ethoxy, 2-methoxy-
ethoxy, cyclobutyloxy, cyclopentyloxy, cyclopropylmethoxy,
cyclobutylmethoxy, tetrahydrofuran-3-yloxy, tetrahydropyran-
4-yloxy or tetrahydrofuran-2-ylmethoxy group,
CA 02403152 2002-09-13
- 17 -
A denotes a 1,2-vinylene group,
B denotes a methylene group or, if B is bound to a carbon atom
of group C, it may also denote a bond,
C denotes a piperidino group in which the two hydrogen atoms
are replaced in the 4 position by a-CH2-O-CO-CHz-,
-CH2CH2-O-CO-, -CH2CH2-O-CO-CH2-, -O-CO-CH2-NCH3-CH2- or
-O-CO-CH2-O-CH2- bridge,
a piperazino group wherein a hydrogen atom in the 3 position
together with the hydrogen atom in the 4 position are replaced
by a-CO-O-CH2-CHz- or -CHz-O-CO-CHz- bridge, while in each case
the left-hand end of the abovementioxied bridges is bound to
the 3 position of the piperazino ring,
a piperidino group substituted by a 2-oxo-tetrahydrofuranyl
group,
a piperidino group which is substituted in the 4 position by a
2-oxo-morpholino or 2-oxo-morpholinomethyl group, while the
2-oxo-morpholino moiety may in each case be substituted by one
or two methyl groups,
a piperazino group which is substituted in the 4 position by a
2-oxo-tetrahydrofuran-3-yl or 2-oxo-tetrahydrofuran-4-yl
group,
a piperidino group which is substituted in the 4 position by a
CH3NR6 or R6S group, where
R6 denotes a 2-oxo-tetrahydrofuran-3-yl or 2-oxo-tetra-
hydrofuran-4-yl group,
CA 02403152 2002-09-13
- 18 -
a piperazino group which is substituted in the 4 position by a
2-oxo-tetrahydrofuranylmethyl or 2-oxo-tetrahydro-
furanylcarbonyl group,
a piperazino group which is substituted in the 4 position by a
[2-(2-oxo-tetrahydrofuran-3-ylsulphenyl)ethyl] group,
a piperidin-4-yl group which is substituted in the 1 position
by a 2-oxo-tetrahydrofuran-3-yl or 2-oxo-tetrahydrofuran-4-yl
group, or
a piperidin-4-yl-NCH3group which is substituted at the cyclic
nitrogen atom by a 2-oxo-tetrahydrofuran-3-yl, 2-oxo-
tetrahydrofuran-4-yl or 2-oxo-tetrahydrofuranylcarbonyl group,
the tautomers, the stereoisomers and the salts thereof.
The following are mentioned as most particularly preferred
compounds of general formula I:
(a) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
tetrahydrofuran-3-yl)-piperazin-1-yl]-1-oxo-2-buten-l-
yl}arnino)-7-cyclopropylmethoxy-quinazoline,
(b) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
tetrahydrofuran-4-yl)-piperazin-1-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline,
(c) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (4-{2- [ (2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-ethyl}-piperazin-1-yl)-1-oxo-
2-buten-1-yl]amino}-7-cyclopropylmethoxy-quinazoline,
(d) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-1-yl]amino}-
7-cyclopropylmethoxy-quinazoline,
CA 02403152 2002-09-13
- 19 -
(e) (S)-4-[(3-chloro-4-fluoro-phenyl)amino]-6-[(4-{4-[(5-oxo-
tetrahydrofuran-2-yl)carbonyl]-piperazin-1-yl}-1-oxo-2-buten-
1-yl)amino]-7-cyclopropylmethoxy-quinazoline,
(f) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- { [4- (1-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-yl]amino}-
7-cyclopropylmethoxy-quinazoline and
(g) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4- {4- [ (2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-piperidin-1-yl}-l-oxo-2-buten-
1-yl)amino]-7-cyclopropylmethoxy-quinazoline
and the salts thereof.
The compounds of general formula I may be prepared, for
example, by the following methods:
a. reacting a compound of general formula
Ra Rb
N R
/__c
X N ~ -~ \H
I' \ ~ , (II)
N Rd
wherein
Ra to Rd and X are as hereinbefore defined,
with a compound of general formula
HO-CO - A - B - C , (III)
wherein
A to C are as hereinbefore defined, or with the reactive
derivatives thereof.
CA 02403152 2002-09-13
- 20 -
The reaction is optionally carried out in a solvent or mixture
of solvents such as methylene chloride, dimethylformamide,
benzene, toluene, chlorobenzene, tetrahydrofuran or dioxane,
optionally in the presence of a dehydrating agent, e.g. in the
presence of isobutyl chloroformate, thionyl chloride, trimethyl
chlorosilane, phosphorus trichloride, phosphorus pentoxide,
N,N'-dicyclohexylcarbodiimide,
N,N'-dicyclohexylcarbodiimide/N-hydroxysuccinimide,
N,N'-carbonyldiimidazole, triphenylphosphine/carbon
tetrachloride or O-(benzotriazol-l-yl)-
N,N,N',N'-tetramethyluronium-tetrafluoroborate or with a
corresponding reactive derivative such as a corresponding
ester, acid halide or anhydride, optionally with the addition
of an inorganic or organic base, preferably with the addition
of an organic base such as triethylamine, N-ethyl-
diisopropylamine or 4-dimethylamino-pyridine, conveniently at
temperatures between 0 and 150 C, preferably at temperatures
between 0 and 80 C.
b. reacting a compound of general formula
Ra Rb
N
NRC - CO - B - Z1
X
(IV)
N Rd
optionally formed in the reaction mixture, wherein
Ra to Rd , A, B and X are as hereinbefore defined and
Z1 denotes an exchangeable group such as a halogen atom or a
substituted sulphinyl or sulphonyl group, e.g. a chlorine or
bromine atom, a methylsulphinyl, propylsulphinyl, phenyl-
sulphinyl, benzylsulphinyl, methylsulphonyl, propylsulphonyl,
phenylsulphonyl or benzylsulphonyl group,
with a compound of general formula
CA 02403152 2002-09-13
- 21 -
H - G , (V)
wherein
G denotes one of the groups mentioned for C hereinbefore which
is linked to the group B via a nitrogen atom.
However, G may also denote a group which can be converted by
lactonisation into one of the groups mentioned for C
hereinbefore, which are linked to the group B via a nitrogen
atom (e.g. a correspondingly substituted gamma- or delta-
hydroxycarboxylic acid ester group).
The reaction is conveniently carried out in a solvent such as
isopropanol, butanol, tetrahydrofuran, dioxane, toluene,
chlorobenzene, dimethylformamide, dimethylsulphoxide,
methylene chloride, ethyleneglycol monomethylether,
ethyleneglycol diethylether or sulpholane, optionally in the
presence of an inorganic or tertiary organic base, e.g. sodium
carbonate or potassium hydroxide, a tertiary organic base,
e.g. triethylamine, or in the presence of N-ethyl-diisopropyl-
amine (Hunig base), whilst these organic bases may
simultaneously serve as the solvent, and optionally in the
presence of a reaction accelerator such as an alkali metal
halide at temperatures between -20 and 150 C, but preferably
at temperatures between -10 and 100 C. The reaction may,
however, also be carried out without a solvent or in an excess
of the compound of general formula V used.
If G denotes a group which can be converted by lactonisation
into one of the groups mentioned for C hereinbefore which are
linked to the group B via a nitrogen atom, cyclisation to form
the corresponding lactone may optionally follow. The
cyclisation to form the corresponding lactone is optionally
carried out in a solvent such as acetonirile, methylene
chloride, tetrahydrofuran, dioxane or toluene in the presence
of an acid such as hydrochloric acid, p-toluenesulphonic acid
CA 02403152 2002-09-13
- 22 -
or trifluoroacetic acid at temperatures of between -10 and
120 C.
c. In order to prepare a compound of general formula I wherein
C denotes one of the groups mentioned for C hereinbefore which
contains an imino or HNRq group substituted by R6 or by an
RS-C1_4-alkyl group wherein R4 to R6 are as hereinbefore defined:
reacting a compound of general formula
Ra Rb
N
NRC - CO - A - B - C
X
, (VI)
N Rd
wherein
Ra to Rd, A and B are as hereinbefore defined and
C' denotes one of the groups mentioned for C hereinbefore
which contains a corresponding unsubstituted imino or HNRQ
group, where R4 is as hereinbefore defined,
with a compound of general formula
Z2 - U , (VI I )
wherein
U denotes the group R6 or a RS-C1_4-alkyl group, where RS and R6
are as hereinbefore defined, and
Z2 denotes an exchangeable group such as a halogen atom or a
substituted sulphonyloxy group, e.g. a chlorine or bromine
atom, a methylsulphonyloxy, propylsulphonyloxy,
phenylsulphonyloxy or benzylsulphonyloxy group, or
Z. together with an adjacent hydrogen atom denotes another
carbon-carbon bond which is attached to a carbonyl group.
CA 02403152 2002-09-13
- 23 -
The reaction is conveniently carried out in a solvent such as
methanol, ethanol, isopropanol or acetonitrile and optionally
in the.presence of a base such as triethylamine, N-ethyl-
diisopropylamine or 2-dimethylamino-pyridine at temperatures
between 0 and 100 C, but preferably at the boiling temperature
of the reaction mixture.
If in a compound of general formula VII Z2 denotes an
exchangeable group, the reaction is preferably performed in a
solvent or mixture of solvents such as methylene chloride,
dimethylformamide, dimethylsulphoxide, sulpholane, benzene,
toluene, chlorobenzene, tetrahydrofuran,
benzene/tetrahydrofuran or dioxane, conveniently in the
presence of a tertiary organic base such as triethylamine or
N-ethyl-diisopropylamine (Hunig base), whilst these organic
bases may simultaneously serve as the solvent, or in the
presence of an inorganic base such as sodium carbonate,
potassium carbonate or sodium hydroxide solution, conveniently
at temperatures between -20 and 200 C, preferably at
temperatures between 0 and 150 C, or
if in a compound of general formula VII Z2 together with an
adjacent hydrogen atom denotes another carbon-carbon bond
attached to a carbonyl group, the reaction is preferably
carried out in a solvent such as methanol, ethanol,
isopropanol or acetonitrile at temperatures between 0 and
100 C, but preferably at temperatures between 20 C and the
boiling temperature of the reaction mixture.
d. In order to prepare a compound of general formula I wherein
C denotes one of the groups mentioned for C hereinbefore which
contains an imino or HNR4 group substituted by an RSCO,
RS-C1_4-alkylene-CO, (R4NR6) -C1_4-alkylene-CO, R60-C1_4-alkylene-CO,
R6S-C1_4-alkylene-CO, R6SO-C1_4-alkylene-CO, R6S02-C1_4-alkylene-CO
or 2-oxo-morpholino-C1_4-alkylene-CO group, where R4 to R6 are as
hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two C1_z-alkyl groups:
CA 02403152 2002-09-13
- 24 -
reacting a compound of general formula
Ra Rb
N
NRC - CO - A - B - C'
X~
~ \ I , (VI)
N Rd
wherein
Ra to Rd, A and B are as hereinbefore defined and
C' denotes one of the groups mentioned for C hereinbefore
which contains a corresponding unsubstituted imino or HNR4
group, where R4 is as hereinbefore defined,
with a compound of general formula
HO-CO - W , (VI I I )
wherein
W denotes the group RS or a RS-C1_4-alkyl, (R4NR6) -C1_4-alkyl,
R6O-C1_4-alkyl, R6S-Cl_Q-alkyl, R6SO-C1_4-alkyl, R6SO2-C1_4-alkyl or
2-oxo-morpholino-C1_,-alkyl group, wherein R4 to R6 are as
hereinbefore defined and the 2-oxo-morpholino moiety may be
substituted by one or two C1_2-alkyl groups.
The reaction is optionally carried out in a solvent or mixture
of solvents such as methylene chloride, dimethylformamide,
benzene, toluene, chlorobenzene, tetrahydrofuran or dioxane,
optionally in the presence of a dehydrating agent, e.g. in the
presence of isobutyl chloroformate, thionyl chloride, trimethyl
chlorosilane, phosphorus trichloride, phosphorus pentoxide,
N,N'-dicyclohexylcarbodiimide,
N,N'-dicyclohexylcarbodiimide/N-hydroxysuccinimide,
N,N'-carbonyldiimidazole, triphenylphosphine/carbon
tetrachloride or O-(benzotriazol-l-yl)-
CA 02403152 2002-09-13
- 25 -
N,N,N',N'-tetramethyluronium-tetrafluoroborate or with a
corresponding reactive derivative such as a corresponding
ester,.acid halide or anhydride, optionally with the addition
of an inorganic or organic base, preferably with the addition
of an organic base such as triethylamine, N-ethyl-
diisopropylamine or 4-dimethylamino-pyridine, conveniently at
temperatures between 0 and 150 C, preferably at temperatures
between 0 and 80 C.
In the reactions described hereinbefore, any reactive groups
present such as hydroxy, carboxy, amino, alkylamino or imino
groups may be protected during the reaction by conventional
protecting groups which are cleaved again after the reaction.
For example, a protecting group for a hydroxy group may be a
trimethylsilyl, acetyl, benzoyl, methyl, ethyl, tert.butyl,
trityl, benzyl or tetrahydropyranyl group,
protecting groups for a carboxy group may be a trimethylsilyl,
methyl, ethyl, tert.butyl, benzyl or tetrahydropyranyl group,
and
protecting groups for an amino, alkylamino or imino group may
be a formyl, acetyl, trifluoroacetyl, ethoxycarbonyl,
tert.butoxycarbonyl, benzyloxycarbonyl, benzyl, methoxybenzyl
or 2,4-dimethoxybenzyl group and additionally, for the amino
group, a phthalyl group.
Any protecting group used is optionally subsequently cleaved
for example by hydrolysis in an aqueous solvent, e.g. in water,
isopropanol/water, acetic acid/water, tetrahydrofuran/water or
dioxane/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in
the presence of an alkali metal base such as sodium hydroxide
or potassium hydroxide or aprotically, e.g. in the presence of
CA 02403152 2002-09-13
- 26 -
iodotrimethylsilane, at temperatures between 0 and 1200C,
preferably at temperatures between 10 and 100 C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is
cleaved, for example hydrogenolytically, e.g. with hydrogen in
the presence of a catalyst such as palladium/charcoal in a
suitable solvent such as methanol, ethanol, ethyl acetate or
glacial acetic acid, optionally with the addition of an acid
such as hydrochloric acid at temperatures between 0 and 100 C,
but preferably at room temperatures between 20 and 60 C, and at
a hydrogen pressure of 1 to 7 bar, but preferably 3 to 5 bar. A
2,4-dimethoxybenzyl group, however, is preferably cleaved in
trifluoroacetic acid in the presence of anisole.
A tert.butyl or tert.butyloxycarbonyl group is preferably
cleaved by treating with an acid such as trifluoroacetic acid
or hydrochloric acid or by treating with iodotrimethylsilane
optionally using a solvent such as methylene chloride, dioxane,
methanol or diethyl ether.
A trifluoroacetyl group is preferably cleaved by treating with
an acid such as hydrochloric acid, optionally in the presence
of a solvent such as acetic acid at temperatures between 50 and
120 C or by treating with sodium hydroxide solution optionally
in the presence of a solvent such as tetrahydrofuran at
temperatures between 0 and 50 C.
A phthalyl group is preferably cleaved in the presence of
hydrazine or a primary amine such as methylamine, ethylamine or
n-butylamine in a solvent such as methanol, ethanol,
isopropanol, toluene/water or dioxane at temperatures between
20 and 50 C.
Moreover, the compounds of general formula I obtained may be
resolved into their enantiomers and/or diastereomers, as
mentioned hereinbefore. Thus, for example, cis/trans mixtures
may be resolved into their cis and trans isomers, and
CA 02403152 2002-09-13
- 27 -
compounds with at least one optically active carbon atom may
be separated into their enantiomers.
Thus, for example, the cis/trans mixtures may be resolved by
chromatography into the cis and trans isomers thereof, the
compounds of general formula I obtained which occur as
racemates may be separated by methods known per se (cf.
Allinger N. L. and Eliel E. L. in "Topics in Stereochemistry",
Vol. 6, Wiley Interscience, 1971) into their optical antipodes
and compounds of general formula I with at least 2 asymmetric
carbon atoms may be resolved into their diastereomers on the
basis of their physical-chemical differences using methods
known per se, e.g. by chromatography and/or fractional
crystallisation, and, if these compounds are obtained in
racemic form, they may subsequently be resolved into the
enantiomers as mentioned above.
The enantiomers are preferably separated by column separation
on chiral phases or by recrystallisation from an optically
active solvent or by reacting with an optically active
substance which forms salts or derivatives such as e.g. esters
or amides with the racemic compound, particularly acids and the
activated derivatives or alcohols thereof, and separating the
diastereomeric mixture of salts or derivatives thus obtained,
e.g. on the basis of their differences in solubility, whilst
the free antipodes may be released from the pure diastereomeric
salts or derivatives by the action of suitable agents.
Optically active acids in common use are e.g. the D- and
L-forms of tartaric acid or dibenzoyltartaric acid, di-
o-tolyltartaric acid, malic acid, mandelic acid,
camphorsulphonic acid, glutamic acid, aspartic acid or quinic
acid. An optically active alcohol may be for example (+) or
(-)-menthol and an optically active acyl group in amides, for
example, may be a (+)-or (-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I obtained may be
converted into the salts thereof, particularly for
CA 02403152 2002-09-13
- 28 -
pharmaceutical use into the physiologically acceptable salts
with inorganic or organic acids. Acids which may be used for
this purpose include for example hydrochloric acid,
hydrobromic acid, sulphuric acid, phosphoric acid, fumaric
acid, succinic acid, lactic acid, citric acid, tartaric acid
or maleic acid.
The compounds of general formulae II to VIII used as starting
materials are known from the literature in some cases or may be
obtained by methods known from the literature (cf. Examples I
to XV).
As already mentioned hereinbefore, the compounds of general
formula I according to the invention and the physiologically
acceptable salts thereof have valuable pharmacological
properties, particularly an inhibiting effect on signal
transduction mediated by the Epidermal Growth Factor receptor
(EGF-R), whilst this may be achieved for example by inhibiting
ligand bonding, receptor dimerisation or tyrosine kinase
itself. It is also possible that the transmission of signals
to components located further along is blocked.
The biological properties of the new compounds were
investigated as follows:
The inhibition of the EGF-R-mediated signal transmission can
be demonstrated e.g. with cells which express human EGF-R and
whose survival and proliferation depend on stimulation by EGF
or TGF-alpha. A cell line of murine origiil dependent on
interleukin-3-(IL-3) which was genetically modified to express
functional human EGF-R was used here. The proliferation of
these cells known as F/L-HERc can therefore be stimulated
either by murine IL-3 or by EGF (cf. von Ruden, T. et al. in
EMBO J. 2, 2749-2756 (1988) and Pierce, J. H. et al. in
Science 239, 628-631 (1988)).
CA 02403152 2002-09-13
- 29 -
The starting material used for the F/L-HERc cells was the cell
line FDC-P1, the production of which has been described by
Dexter; T. M. et al. in J. Exp. Med. 152, 1036-1047 (1980).
Alternatively, however, other growth-factor-dependent cells
may also be used (cf. for example Pierce, J. H. et al. in
Science 239, 628-631 (1988), Shibuya, H. et al. in Cell ZQ,
57-67 (1992) and Alexander, W. S. et al. in EMBO J. 1Q, 3683-
3691 (1991)). For expressing the human EGF-R cDNA (cf.
Ullrich, A. et al. in Nature 309, 418-425 (1984)) recombinant
retroviruses were used as described by von Ruden, T. et al.,
EMBO J. 2, 2749-2756 (1988), except that the retroviral vector
LXSN (cf. Miller, A. D. et al. in BioTechniques .Z, 980-990
(1989)) was used for the expression of the EGF-R cDNA and the
line GP+E86 (cf. Markowitz, D. et al. in J. Virol. jjZ, 1120-
1124 (1988)) was used as the packaging cell.
The test was performed as follows:
F/L-HERc cells were cultivated in RPMI/1640 medium
(BioWhittaker), supplemented with 10 % foetal calf serum (FCS,
Boehringer Mannheim), 2 mM glutamine (BioWhittaker), standard
antibiotics and 20 ng/ml of human EGF (Promega), at 37 C and
5% CO2. In order to investigate the inhibitory activity of the
compounds according to the invention, 1.5 x 104 cells per well
were cultivated in triplicate in 96-well dishes in the above
medium (200 l), the cell proliferation being stimulated with
either EGF (20 ng/ml) or murine IL-3. The IL-3 used was
obtained from culture supernatants of the cell line X63/0 mIL-
3 (cf. Karasuyama, H. et al. in Eur. J. Immunol. 1$, 97-104
(1988)). The compounds according to the invention were
dissolved in 100% dimethylsulphoxide (DMSO) and added to the
cultures in various dilutions, the maximum DMSO concentration
being 1%. The cultures were incubated for 48 hours at 37 C.
In order to determine the inhibitory activity of the compounds
according to the invention the relative cell number was
measured in O.D. units using the Cell Titer 96TM AQueous Non-
CA 02403152 2002-09-13
- 30 -
Radioactive Cell Proliferation Assay (Promega). The relative
cell number was calculated as a percentage of the control
(F/LHERc cells without inhibitor) and the concentration of
active substance which inhibits the proliferation of the cells
by 50% (IC50) was derived therefrom. The following results
were obtained:
Compound Inhibition of
(Example No.) EGF-dependent
proliferation
IC50 [nM]
1 0.05
2 0.60
4 3
4(3) 10
The compounds of general formula I according to the invention
thus inhibit the signal transduction by tyrosine kinases, as
demonstrated by the example of the human EGF receptor, and are
therefore useful for treating pathophysiological processes
caused by hyperfunction of tyrosine kinases. These are e.g.
benign or malignant tumours, particularly tumours of
epithelial and neuroepithelial origin, metastasisation and the
abnormal proliferation of vascular endothelial cells
(neoangiogenesis).
The compounds according to the invention are also useful for
preventing and treating diseases of the airways and lungs
which are accompanied by increased or altered production of
mucus caused by stimulation of tyrosine kinases, e.g. in
inflammatory diseases of the airways such as chronic
bronchitis, chronic obstructive bronchitis, asthma,
bronchiectasias, allergic or non-allergic rhinitis or
sinusitis, cystic fibrosis, al-antitrypsin deficiency, or
coughs, pulmonary emphysema, pulmonary fibrosis and
hyperreactive airways.
CA 02403152 2002-09-13
- 31 -
The compounds are also suitable for treating diseases of the
gastrointestinal tract and bile duct and gall bladder which
are associated with disrupted activity of the tyrosine
kinases, such as may be found e.g. in chronic inflammatory
changes such as cholecystitis, Crohn's disease, ulcerative
colitis, and ulcers in the gastrointestinal tract or such as
may occur in diseases of the gastrointestinal tract which are
associated with increased secretions, such as Menetrier's
disease, secreting adenomas and protein loss syndrome,
also for treating nasal polyps and polyps of the
gastrointestinal tract of various origins, such as for example
villous or adenomatous polyps of the large bowel, but also
polyps in familial polyposis coli, intestinal polyps in
Gardner's syndrome, polyps throughout the entire
gastrointestinal tract in Peutz-Jeghers Syndrome, inflammatory
pseudopolyps, juvenile polyps, colitis cystica profunda and
pneumatosis cystoides intestinales.
Moreover, the compounds of general formula I and the
physiologically acceptable salts thereof may be used to treat
kidney diseases, particularly cystic changes as in cystic
kidneys, for treating renal cysts which may be idiopathic in
origin or which occur in syndromes such as e.g. tubercular
sclerosis, in von-Hippel-Lindau Syndrome, in nephronophthisis
and spongy kidney and other diseases caused by abnormal
functioning of tyrosine kinases such as e.g. epidermal hyper-
proliferation (psoriasis), inflammatory processes, diseases of
the immune system, hyperproliferation of haematopoietic cells,
etc.
By reason of their biological properties the compounds
according to the invention may be used on their own or in
conjunction with other pharmacologically active compounds, for
example in tumour therapy, in monotherapy or in conjunction
with other anti-tumour therapeutic agents, for example in
CA 02403152 2002-09-13
- 32 -
combination with topoisomerase inhibitors (e.g. etoposide),
mitosis inhibitors (e.g. vinblastin), compounds which interact
with nucleic acids (e.g. cis-platin, cyclophosphamide,
adriamycin), hormone antagonists (e.g. tamoxifen), inhibitors
of metabolic processes (e.g. 5-FU etc.), cytokines (e.g. inter-
ferons), antibodies, etc. For treating respiratory tract
diseases, these compounds may be used on their own or in
conjunction with other therapeutic agents for the airways, such
as substances with a secretolytic, broncholytic and/or anti-
inflammatory activity. For treating diseases in the region of
the gastrointestinal tract, these compounds may also be
administered on their own or in conjunction with substances
having an effect on motility or secretion or with anti-
inflammatory substances. These combinations may be administered
either simultaneously or sequentially.
These compounds may be administered either on their own or in
conjunction with other active substances by intravenous,
subcutaneous, intramuscular, intrarectal, intraperitoneal or
intranasal route, by inhalation or transdermally or orally,
whilst aerosol formulations are particularly suitable for
inhalation.
For pharmaceutical use the compounds according to the invention
are generally used for warm-blooded vertebrates, particularly
humans, in doses of 0.01-100 mg/kg of body weight, preferably
0.1-15 mg/kg. For administration they are formulated with one
or more conventional inert carriers and/or diluents, e.g. with
corn starch, lactose, glucose, microcrystalline cellulose,
magnesium stearate, polyvinylpyrrolidone, citric acid, tartaric
acid, water, water/ethanol, water/glycerol, water/sorbitol,
water/polyethyleneglycol, propyleneglycol, stearylalcohol,
carboxymethylcellulose or fatty substances such as hard fat or
suitable mixtures thereof in conventional galenic preparations
such as plain or coated tablets, capsules, powders,
suspensions, solutions, sprays or suppositories.
CA 02403152 2002-09-13
- 33 -
The following Examples are intended to illustrate the present
invention without restricting it:
PrPpara i on of t-.h _s ar _ i ng comnoLnda :
F'xa~p 1~)_P_ I
4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (piperazin-l-yl) -
I -oxo- amino}-7-Gyclopropylm _ -hoxy-CL,ina .o1 inP
ml of trifluoroacetic acid are added dropwise to 1.80 g of
4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (tert . -
butyloxycarbonyl)-piperazin-l-yl]-1-oxo-2-buten-1-yl}amino)-
7-cyclopropylmethoxy-quinazoline in 10 ml methylene chloride
whilst cooling with an ice bath. After one hour the ice bath
is removed and the reaction mixture is stirred overnight at
ambient temperature. Then the mixture is evaporated to
dryness, the flask residue is divided between 150 ml methylene
chloride/methanol (9:1) and 100 ml of 1N sodium hydroxide
solution. The aqueous phase is extracted twice more with the
mixture of solvents, the combined organic phases are dried
over magnesium sulphate and concentrated by evaporation. The
brownish crude product reacted further without any more
purification.
Yield: 1.32 g (88 % of theory),
Rf value: 0.60 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:1)
mass spectrum (ESI+) : m/z = 511, 513 [M+H] +
The following compounds are obtained analogously to Example I:
(1) N-{2-[(2-oxo-tetrahydrofuran-3-yl)sulphanyl]-ethyl}-
piperazine x 2 trifluoroacetic acid
(The reaction mixture is concentrated by evaporation without
aqueous working up.)
Rf value: 0.68 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:1)
CA 02403152 2002-09-13
- 34 -
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(4-methylamino-
piperidin-1-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclopropyl-
methoxy-quinazoline
Rf value: 0.50 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:1)
mass spectrum (ESI-) : m/z = 537, 539 [M-H] -
(3) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [3- (piperidin-4-
yl)-1-oxo-2-propen-1-yl]amino)-7-cyclopropylmethoxy-
quinazoline
Rf value: 0.50 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:1)
mass spectrum (ESI-) : m/z = 494, 496 [M-H] -
(4) Perhydro-pyrazino [2, 1-c] [1, 4] oxazin-3-one x 2 trifluoro-
acetic acid
(The reaction mixture is concentrated by evaporation without
aqueous working up.)
mass spectrum (ESI') : m/z = 157 [M+H] +
(5) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [N- (piperidin-
4-yl)-N-methyl-amino]-1-oxo-2-buten-l-yl}amino)-7-cyclopropyl-
methoxy-quinazoline
mass spectrum (ESI-) : m/z = 537, 539 [M-H]
(6) Perhydro-pyrazino[2,1-c][1,4]oxazin-l-one x 2 trifluoro-
acetic acid
(The reaction mixture is concentrated by evaporation without
aqueous working up.)
mass spectrum (ESI+) : m/z = 157 [M+H]'
(7) 4-[(2-oxo-tetrahydrofuran-3-yl)sulphanyl]-piperidine
x 1 trifluoroacetic acid
(The reaction mixture is concentrated by evaporation without
aqueous working up.)
CA 02403152 2002-09-13
- 35 -
Rf value: 0.57 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:1)
mass spectrum (ESI+) : m/z = 202 [M+H] +
(8) 4-[(2-oxo-6,6-dimethyl-morpholin-4-yl)methyl]-piperidine
x trifluoroacetic acid
(The starting material, 1-(tert.butyloxycarbonyl)-4-
[N-(ethoxycarbonylmethyl)-N-((2-hydroxy-2-methyl-propyl)-
aminomethyl]-piperidine, is obtained by reacting
1-(tert.butyloxycarbonyl)-4-(methanesulphonyloxymethyl)-
piperidine with N-(ethoxycarbonylmethyl)-2-hydroxy-2-methyl-
propylamine)
mass spectrum (ESI+) : m/z = 227 [M+H] +
Examnle II
4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( (4- [4- (tert.butyloxy-
carbonyl)-piperazin-1-yl]-1-oxo-2-buten-1-yl}amino)-7-
cyclonropylmethoxy-auinazoline
4.7 ml of oxalyl chloride are added dropwise at ambient
temperature to a solution of 4.51 g of 4-bromo-crotonic acid
in 100 ml of inethylene chloride. After the addition of one
drop of N,N-dimethylformamide the reaction mixture is stirred
for about another 45 minutes at ambient temperature until the
development of gas has ceased. Then the solvent is distilled
off from the resulting acid chloride in vacuo. In the meantime
7.00 g of 6-amino-4-[(3-chloro-4-fluoro-phenyl)amino]-7-
cyclopropylmethoxy-quinazoline and 10.2 ml of
diisopropylethylamine in 250 ml of tetrahydrofuran are cooled
to 0 C in an ice bath. The crude acid chloride is taken up in
20 ml of methylene chloride and added dropwise within 5
minutes whilst cooling with an ice bath. The reaction mixture
is stirred for 45 minutes at 0 C and for one hour at ambient
temperature, then a suspension of 18.17 g of tert.butyl
piperazine-l-carboxylate in 5 ml of N,N-dimethylformamide is
added. After another 48 hours stirring at ambient temperature
the solvent is distilled off in vacuo and the residue is
CA 02403152 2002-09-13
- 36 -
divided between water and ethyl acetate. The aqueous phase is
extracted with ethyl acetate, the combined organic extracts
are washed with saturated sodium chloride solution, dried over
magnesium sulphate and concentrated by evaporation. The crude
product is purified by chromatography through a silica gel
column with ethyl acetate/methanol (15:1 to 9:1).
Yield: 5.2 g (44 % of theory),
Rf value: 0.42 (silica gel, methylene chloride/methanol = 9:1)
mass spectrum (ESI-) : m/z = 609, 611 [M-H] -
The following compounds are obtained analogously to Example
II:
(1) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6- [ (4-{4- [N- (tert.-
butyloxycarbonyl)-N-methylamino]-piperidin-1-yl}-1-oxo-
2-buten-l-yl)amino]-7-cyclopropylmethoxy-quinazoline
Rf value: 0.35 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI") : m/z = 637, 639 [M-H] -
(2) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{N- [1- (tert. -
butyloxycarbonyl)-piperidine-4-yl]-N-methyl-amino}-1-oxo-2-
buten-l-yl)amino]-7-cyclopropylmethoxy-quinazoline
R. value: 0.39 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI-) : m/z = 637, 639 [M-H] -
Exam= l e TTT
6-amino-4-[(3-chloro-4-fluoro-phenyl)amino]-7-cyclopropyl-
methoxy-auinazol;ne
30.00 g of 4-[(3-chloro-4-fluoro-phenyl)amino]-7-
cyclopropylmethoxy-6-nitro-quinazoline are suspended in a
mixture of 900 ml of ethanol, 120 ml of glacial acetic acid
and 300 ml of water. The suspension is refluxed, producing a
clear solution. Then 17.24 g of iron powder are carefully
added batchwise, whilst the reaction mixture effervesces each
time. About 15 minutes after it has all been added, a
precipitate is formed. The reaction mixture is stirred for a
CA 02403152 2002-09-13
- 37 -
further 15 minutes and then evaporated to dryness in vacuo.
The residue in the flask is taken up in 1000 ml of methylene
chloride/methanol (9:1) and combined with 30 ml of 33% aqueous
ammonia solution. The iron slurry is filtered off and washed
with methylene chloride : methanol (9:1). The brown filtrate
is filtered through a silica gel pack and evaporated to
dryness. The residue in the flask is stirred with 60 ml of
diethylether, suction filtered and dried in the air.
Yield: 22.60 g (82 % of theory),
melting point: 208 C
mass spectrum (ESI+) : m/z = 359, 361 [MtH] +
Examr~l P TV
- z - -
4-[(3-chloro-4-fluoro-phenyl)amino]-7-cyclopropylmethoxy-6-
ni .ro-crn uinaznl ine
66.66 g of potassium tert. butoxide are added batchwise to
47.07 ml of cyclopropylmethanol in 500 ml of N,N-
dimethylformamide whilst cooling with an ice bath, while the
temperature is not allowed to exceed 12 C. The reaction
mixture is stirred for another 30 minutes in the cooling bath,
then 50.00 g of 4-[(3-chloro-4-fluoro-phenyl)amino]-7-fluoro-
6-nitro-quinazoline are added batchwise, whereupon the
reaction mixture turns dark red and the temperature rises to
not more than 15 C. Then the cooling bath is removed and the
reaction mixture is stirred for another hour at ambient
temperature. To work it up the reaction mixture is poured onto
4000 ml of water and neutralised with about 210 ml of
2N hydrochloric acid. The precipitate formed is suction
filtered, washed with water and dried at 40 C.
Yield: 60.47 g of crude product,
Rf value: 0.60 (silica gel, cyclohexane/ethyl acetate = 1:1)
mass spectrum (ESI-) : m/z = 387, 389 [M-H]
CA 02403152 2002-09-13
- 38 -
Fxamnl. V
1-(tert.butyloxycarbonyl)-4-{2-[(2-oxo-tetrahydrofuran-3-yl)-
sulj)hanyy1] -ethy]1 piperazine
4.02 g of 1-(tert.butyloxycarbonyl)-
4-[2-(methanesulphonyloxy)-ethyl]-piperazine dissolved in
methylene chloride are added to a methanolic solution of 1.40
g of sodium-2-oxo-tetrahydrofuran-3-thiolate (prepared by
treating 3-[(methylcarbonyl)sulphanyl]-tetrahydrofuran-2-one
with sodium methoxide in methanol). Then 10 ml of N,N-
dimethylformamide are added and the reaction mixture is
stirred for 2.5 hours at 50 C. For working up 100 ml of ethyl
acetate are added. The organic phase is separated off, washed
with water and sodium chloride solution, dried over magnesium
sulphate and concentrated by evaporation. The red oily crude
product is reacted without any further purification.
Yield: 3.50 g (96 % of theory)
Rf value: 0.48 (silica gel, methylene chloride/methanol = 9:1)
mass spectrum (ESI-) : m/z = 329 [M-H] -
Example VI
3- (Ipiperidin-4-yl 1 -dihydro-fLran-2-one
3.60 g of 3-(l-benzyl-piperidin-4-ylidene)-dihydro-furan-2-one
are dissolved in 40 ml of methanol, combined with 360 mg of
palladium on active charcoal (10%) and hydrogenated at ambient
temperature. After the calculated amount of hydrogen has been
taken up, the catalyst is filtered off and the filtrate is
concentrated by evaporation in vacuo. A colourless oil
remains, which is reacted without any further purification.
Rf value: 0.16 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (EI ) : m/z = 169 [M] +
The following compounds are obtained analogously to Example
VI:
CA 02403152 2002-09-13
- 39 -
(1) 4-[N-(tert.Butyloxycarbonylmethyl)-N-(2-hydroxyethyl)-
amino]-piperidine
Starting material: compound of Example XIV (1)
Rf value: 0.34 (silica gel, methylene chloride/methanol/conc.
aqueous ammonia = 4:1:0.1)
(2) (R) -4 - [N- ( tert . Butyloxycarbonylmethyl ) -N- ( 2 -
hydroxypropyl)-amino]-piperidine
Starting material: Compound of Example XIV (2)
Mass spectrum (ESI+) : m/z = 273 [M+H] +
(3) 4-Hydroxy-4-[N-methyl-N-(ethoxycarbonylmethyl)-
aminomethyl]-piperidine
Starting material: Compound of Example XVI
Rf value: 0.38 (silica gel, methylene chloride/methanol/conc.
aqueous ammonia = 4:1:0.1)
Fxamnle VIT
3- (l -benzyl-piperidin-4-yl id n .) -dihydro-furan-2-one
24.00 g of diethyl (2-oxo-tetrahydrofuran-3-yl)-phosphonate
are slowly added dropwise to 4.40 g of sodium hydride in 25 ml
of toluene. Then 1-benzyl-9-piperidine-4-one is added and the
reaction mixture is refluxed for 3 hours. After cooling to
ambient temperature the supernatant solution is decanted off,
diluted with toluene and washed with water and saturated
sodium chloride solution. The organic phase is dried over
magnesium sulphate, concentrated by evaporation and
chromatographed over a silica gel column with ethyl
acetate/methanol (95:5) as eluant.
Yield: 14.43 g (52 % of theory),
Rf value: 0.64 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI-) : m/z = 256 [M-H] -
CA 02403152 2002-09-13
- 40 -
Fxamnle VTTI
z- --
4-[(3-chloro-4-fluoro-phenyl)amino]-6-({3-[1-
(tert.butyloxycarbonyl)-piperidin-4-yl]-1-oxo-2-propen-l-
yl}amino)-7- .yclon_ropylmethoxy-aFi-nazoline
1.61 g of 4-[(3-chloro-4-fluoro-phenyl)amino]-6-
({[(diethoxyphosphoryl)methyl]carbonyl}amino)-7-
cyclopropylmethoxy-quinazoline are added to 127 mg of
anhydrous lithium chloride in 20 ml of absolute
tetrahydrofuran under argon. The mixture is stirred for
15 minutes at ambient temperature, then cooled to 0 C in an
ice bath and 0.45 ml of 1,8-diazabicyclo[5.4.0]undec-7-ene are
added. After another 30 minutes at 0 C, 690 mg of 4-formyl-l-
(tert.butoxycarbonyl)-piperidine are added. The reaction
mixture is left overnight to come up to ambient temperature.
For working up, the solvent is eliminated in vacuo and the
residue in the flask is taken up with ethyl acetate/methanol
(9:1). The solution is washed with water and saturated sodium
chloride solution, dried over magnesium sulphate and
concentrated by evaporation. The oily, brown crude product is
purified by chromatography through a silica gel column with
ethyl acetate as eluant.
Yield: 1.30 g (73 % of theory),
Rf value: 0.80 (silica gel, ethyl acetate)
mass spectrum (EI): m/z = 595, 597 [M]+
F.xaml e TX
- z - -
4-[(3-chloro-4-fluoro-phenyl)amino]-6-({[(diethoxyphosphoryl)-
mP hyyl l carbonyl} ami no) - 7-.ycl onropyl mPt-hnxy-cl ,; na ol ; nP
2.77 ml of triethylamine, 3.43 g of diethoxyphosphoryl-acetic
acid and 5.62 g of benzotriazol-i-yl-N-tetramethyl-uronium-
tetrafluoroborate are added successively to 5.00 g of 6-amino-
4-[(3-chloro-4-fluoro-phenyl)amino]-7-cyclopropylmethoxy-
quinazoline in 25 ml of N,N-dimethylformamide. The reaction
mixture is stirred for one hour at ambient temperature. Then
250 ml of water are added and the mixture is extracted with
CA 02403152 2002-09-13
- 41 -
250 ml of ethyl acetate/methanol (10:1). The organic phase is
washed with water and saturated sodium chloride solution,
dried over magnesium sulphate and concentrated by evaporation.
The residue in the flask is crystallised by stirring with
diethylether.
Yield: 7.00 g (94 % of theory),
melting point: 186 C
mass spectrum (ESI-) : m/z = 535, 537 [M-H]
Fxam1j2 X
8-(tert.butyloxycarbonyl)-perhydro-pyrazino[2,1-cl[1,4]oxazin-
';-one
2.00 g of 1-(tert.butyloxycarbonyl)-4-
[(ethoxycarbonyl)methyl]-3-hydroxymethyl-piperazine in 2.5 ml
of acetonitrile are combined with 500 mg of p-toluenesulphonic
acid monohydrate. The reaction mixture is refluxed for 3 hours
until the reaction is complete. Then the solvent is distilled
off in vacuo. The crude product is further reacted without
additional purification.
Rf value: 0.80 (silica gel, ethyl acetate/methanol = 9:1)
The following compound is obtained analogously to Example X:
(1) 8-(tert.butyloxycarbonyl)-perhydro-pyrazino[2,1-c][1,4]-
oxazin-l-one
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
mass spectrum (ESI') : m/z = 257 [M+H] +
Examnle XI
1-(tert.butyloxycarbonyl)-4-[(ethoxycarbonyl)methyl]-3-
hydroxymethyl-piperazine and 8- (tert-butyloxycarbonyl)-
nerhydro-pyyrazino f2, 1-cl fl, 41 oxazin-3-one
3.90 ml of ethyl bromoacetate are added to 5.80 g of 1-
(tert.butyloxycarbonyl)-3-hydroxymethyl-piperazine and 4.50 ml
of triethylamine in 60 ml of acetonitrile. The reaction
CA 02403152 2002-09-13
- 42 -
mixture is refluxed overnight, during which time two products
are formed, according to thin layer chromatography. For
working up the reaction mixture is concentrated by evaporation
in vacuo and the residue is divided between ethyl acetate and
water. The organic phase is dried over magnesium sulphate,
concentrated by evaporation and chromatographed over a silica
gel column with ethyl acetate/methanol (97:3). The following
two products are obtained as yellowish oils:
8-(tert.butyloxycarbonyl)-perhydro-pyrazino[2,1-c][1,4]oxazin-
3-one
Yield: 3.43 g (50 % of theory),
Rf value: 0.80 (silica gel, ethyl acetate/methanol = 9:1)
1-(tert.butyloxycarbonyl)-4-[(ethoxycarbonyl)methyl]-3-
hydroxymethyl-piperazine
Yield: 2.08 g (26 % of theory),
Rf value: 0.58 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI+) : m/z = 303 [M+H]'
The following compounds are obtained analogously to Example
XI:
(1) 8-(tert.butyloxycarbonyl)-perhydro-pyrazino[2,1-c][1,4]-
oxazin-l-one
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
mass spectrum (ESI+) : m/z = 257 [M+H]'
(2) 1-(tert.butyloxycarbonyl)-3-(ethoxycarbonyl)-4-(2-hydroxy-
ethyl)-piperazine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
mass spectrum (EI) : m/z = 302 [M] +
CA 02403152 2002-09-13
- 43 -
F=xamnl e XTT
1- ( r _ . h uty1 oxycarbonyl ) -3-hydroxynPr_hyl -pi n re_ a .; np
A solution of 8.00 g of 1-(tert.butyloxycarbonyl)-3-
ethoxycarbonyl-piperazine in 10 ml of tetrahydrofuran is added
dropwise to a suspension of 900 mg of lithium borohydride in
20 ml of tetrahydrofuran and then the mixture is refluxed for
3 hours. For working up the reaction mixture is concentrated
by evaporation, adjusted to pH 4 with 10% aqueous citric acid
solution and stirred for about 40 minutes whilst cooling with
an ice bath. Then the mixture is made alkaline with
concentrated sodium hydroxide solution and left to stand
overnight. The next morning it is extracted with tert-
butylmethylether. The organic phase is dried over magnesium
sulphate and concentrated by evaporation. A clear oil is left,
which slowly crystallises.
Yield: 5.80 g (87 % of theory),
Rf value: 0.28 (silica gel, ethyl acetate/methanol = 4:1)
mass spectrum (ESI+) : m/z = 217 [M+H]'
F.xa=l e XTTT
1- (tert_bLtyloxycarbonyl)-3-ethoxycarbonyl-pip r in
21.80 g of di-tert.butyl pyrocarbonate are added to 15.80 g of
2-ethoxycarbonyl-piperazine in 400 ml of ethanol whilst
cooling with an ice bath. The reaction mixture is stirred for
another 3 hours at 0 C. Then it is concentrated by evaporation
and the residue is divided between ethyl acetate and water.
The organic phase is dried over magnesium sulphate,
concentrated by evaporation and purified by chromatography
through a silica gel column with ethyl acetate/methanol (95:5)
as eluant.
Yield: 24.30 g(94 % of theory),
Rf value: 0.40 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI+) : m/z = 281 [M+Na] '
CA 02403152 2002-09-13
- 44 -
F.xamnl e XTV
1- t-ert-. . bõtyl oxycarl-~onyyl -4 -methyl ami no-pip ri di n
25.50 g of methylamine hydrochloride are added to 15.00 g of
1-(tert.butyloxycarbonyl)-4-piperidinone and 31.20 g of sodium
acetate in 300 ml of tetrahydrofuran. Then 19.00 g of sodium
triacetoxyborohydride are added batchwise. The reaction
mixture is stirred overnight at ambient temperature and the
next day concentrated by evaporation. The residue is divided
between 5% sodium hydrogen carbonate solution and methylene
chloride. The aqueous phase is adjusted to about pH 11 with 4
N hydrochloric acid and extracted with ethyl acetate. The
organic phase is dried over magnesium sulphate and
concentrated by evaporation, leaving a colourless oil.
Yield: 12.74 g (79 0 of theory),
Rf value: 0.22 (silica gel, ethyl acetate/methanol/concentrated
aqueous ammonia solution = 90:10:1)
mass spectrum (ESI+) : m/z = 215 [M+H] +
The following compounds are obtained analogously to Example
XIV:
(1) 1-Benzyloxycarbonyl-4-[N-(tert.-butyloxycarbonylmethyl)-N-
(2-hydroxyethyl)-amino]-piperidine
Mass spectrum (ESI+) : m/z = 393 [M+H]'
Rf-value: 0.50 (silica gel, cyclohexane/ethyl acetate = 9:1)
(2) (R)-l-Benzyloxycarbonyl-4-[N-(tert.-
butyloxycarbonylmethyl)-N-(2-hydroxypropyl)-amino]-piperidine
Mass spectrum (ESI+) : m/z = 407 [M+H] +
Rf-value: 0.56 (silica gel, cyclohexane/ethyl acetate = 9:1)
CA 02403152 2002-09-13
- 45 -
Fxamnlg. XV
1-(tert.butyloxycarbonyl)-4-[(2-oxo-tetrahydrofuran-3-yl)-
sullphanyy11 -pilDeridine
2.73 g of potassium tert. butoxide are slowly added to 5.28 g
of 1-(tert.butyloxycarbonyl)-4-mercapto-piperidine in 20 ml of
N,N-dimethylformamide whilst cooling with an ice bath. The
mixture is stirred for another 30 minutes whilst cooling with
an ice bath, then a solution of 2.02 ml of 3-bromo-dihydro-
furan-2-one in 20 ml of N,N-dimethylformamide is added
dropwise. After the addition has finished, the reaction
mixture is stirred for two hours at ambient temperature. Then
it is neutralised with glacial acetic acid and concentrated by
evaporation. The residue is divided between ethyl acetate and
water. The organic phase is dried over magnesium sulphate and
concentrated by evaporation. 7.60 g of a brownish-orange oil
remain, which is reacted without further purification.
Rf value: 0.32 (silica gel, ethyl acetate/cyclohexane = 2:3)
mass spectrum (ESI-) : m/z = 300 [M-H] -
Examlp1 e XVI
1-Benzyloxycarbonyl-4-hydroxy-4-[N-methyl-N-
( ethoxy arbnyl m-thyl )-am; nom ..hyl ]-pi T eri dinP
A mixture of 2.7 g of sarcosine ethyl ester and 3.09 g of 6-
benzyloxycarbonyl-l-oxa-6-aza-spiro[2.5]octane in 20 ml of
ethanol is heated to 90 C for 6 hours in a bomb tube. After
cooling overnight the mixture is evaporated down and the
residue is purified by chromatography over a silica gel column
with cyclohexane/ethyl acetate (1:1).
Yield: 1.8 g (25% of theory)
Mass spectrum (ESI+) : m/z = 365 [M+H]'
Rf-value: 0.35 (silica gel, cyclohexane/ethyl acetate= 1:1)
CA 02403152 2002-09-13
- 46 -
Prei arat-i nn of the final coirit7o 7~dy
Rxamnl e l
4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
tetrahydrofuran-3-yl)-piperazin-1-yl]-1-oxo-2-buten-l-
yl lami no) -7-c~rclo}~rop~rlmethoxyy~>>inazoline
a
0.42 ml of triethylamine are added to 608 mg of 4-[(3-chloro-
4-fluoro-phenyl)amino]-6-{[4-(piperazin-1-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclopropylmethoxy-quinazoline in 3.0 ml of
tetrahydrofuran. The mixture is cooled in the ice bath and a
solution of 215 mg of 3-bromo-dihydro-furan-2-one in 1.0 ml of
tetrahydrofuran is added dropwise and the mixture is stirred
for one hour with cooling. Then the ice bath is removed and
the mixture is stirred for about 48 hours at ambient
temperature. The reaction mixture is chromatographed directly,
without further processing, over a silica gel column, with
methylene chloride/methanol (95:5 to 90:10) as eluant. The
foamy crude product is crystallised by trituration with a
little diethylether.
Yield: 393 mg (56 % of theory),
melting point: 130-131 C
mass spectrum (ESI-) : m/z = 593, 595 [M-H] -
The following compounds are obtained analogously to Example 1:
(1) (R)-4-[(3-chloro-4-fluoro-phenyl)amino]-6-[(4-{4-[(5-oxo-
tetrahydrofuran-2-yl)methyl]-piperazin-1-yl}-1-oxo-2-buten-
1-yl)amino]-7-cyclopropylmethoxy-quinazoline
(The reaction is carried out with (R)-5-mesyloxymethyl-2-oxo-
tetrahydrofuran without a solvent)
melting point: 145-150 C
mass spectrum (ESI-) : m/z = 607, 609 [M-H] -
(2) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [N- (2-oxo-
tetrahydrofuran-3-yl)-N-methylamino]-piperidin-1-yl}-1-oxo-
2-buten-1-yl)amino]-7-cyclopropylmethoxy-quinazoline
CA 02403152 2002-09-13
- 47 -
Rf value: 0.22 (silica gel, ethyl acetate/methanol = 7:3)
mass spectrum (ESI-) : m/z = 621, 623 [M-H]
(3) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6- [ (4-{N- [l- (2-oxo-
tetrahydrofuran-3-yl)-piperidin-4-yl]-N-methyl-amino}-1-oxo-
2-buten-1-yl)amino]-7-cyclopropylmethoxy-quinazoline
R. value: 0.73 (aluminium oxide, ethyl acetate/methanol = 9:1)
mass spectrum (ESI-) : m/z = 621, 623 [M-H]
Example 2
4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
tetrahydrofuran-4-yl)-piperazin-l-yl]-1-oxo-2-buten-l-
yl}amino) -7- .ycl_ot~_rop~,rlm ho y-cluinazol inP
78 l of 2(5H)-furanone are added to 500 mg of 4-[(3-chloro-4-
fluoro-phenyl)amino]-6-{[4-(piperazin-1-yl)-l-oxo-2-buten-l-
yl]amino}-7-cyclopropylmethoxy-quinazoline in 2 ml of
methanol. The reaction mixture is stirred for about 48 hours
at ambient temperature and then evaporated to dryness in
vacuo. The residue in the flask is purified by chromatography
through a silica gel column with methylene chloride/methanol
(95:5 to 90:10) as eluant. The product obtained is
recrystallised from ethyl acetate.
Yield: 170 mg (29 % of theory),
Rf value: 0.43 (silica gel, methylene chloride/methanol = 9:1)
mass spectrum (ESI-) : m/z = 593, 595 [M-H] -
The following compound is obtained analogously to Example 2:
(1) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {3- [1- (2-oxo-
tetrahydrofuran-4-yl)-piperidin-4-yl]-1-oxo-2-propen-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
melting point: 138 C
mass spectrum (ESI-) : m/z = 578, 580 [M-H] -
CA 02403152 2002-09-13
- 48 -
Rxam 1~P 3
(S) -4-.[ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [ (5-oxo-
tetrahydrofuran-2-yl)carbonyl]-piperazin-l-yl}-1-oxo-2-buten-
1-y] 1 ami nol -7-cycl onropylmethoxy-Q,i na .ol i n
130 mg of (S)-5-oxo-tetrahydrofuran-2-carboxylic acid, 0.21 ml
of triethylamine and 321 mg of benzotriazol-1-yl-N-
tetramethyl-uronium-tetrafluoroborate are added to 500 mg of
4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (piperazin-1-yl) -i-
oxo-2-buten-1-yl]amino}-7-cyclopropylmethoxy-quinazoline in 3
ml of N,N-dimethylformamide. The reaction mixture is left to
stand for about 48 hours at ambient temperature. Then 20 ml of
water are added, whereupon a greasy precipitate is formed.
This is suction filtered, washed with water and purified by
chromatography through a silica gel column with methylene
chloride/methanol (95:5 to 90:10) as eluant.
Yield: 330 mg (54 % of theory),
melting point: 155-157 C
mass spectrum (ESI") : m/z = 621, 623 [M-H]
The following compound is obtained analogously to Example 3:
(1) 4- [ (3-Chloro-4-fluoro-phenyl)amino] -6-{ [4- (N-{1- [ ( (S) -5-
oxo-tetrahydrofuran-2-yl)carbonyl]-piperidin-4-yl}-N-methyl-
amino)-1-oxo-2-buten-l-yl]amino}-7-cyclopropylmethoxy-
quinazoline
Rf-value: 0.36 (silica gel, ethyl acetate/methanol/concentrated
aqueous ammonia solution = 9:1:0.2)
Mass spectrum (EI): m/z = 650, 652 [M]'
F,xam= 1c- 4
4- [ (3-chloro-4-fluoro-phenyl)amino] -6-{ [4- (4-{2- [ (2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-ethyl}-piperazin-1-yl)-1-oxo-
2-buten-1-yyl 1 amino}-7-cyclopYolpylmethoxy-auina .ol in .
0.67 ml of oxalyl chloride are added dropwise at ambient
temperature to a solution of 644 mg g of 4-bromo-crotonic acid
CA 02403152 2002-09-13
- 49 -
in 15 ml of inethylenechloride. After the addition of one drop
of N,N-dimethylformamide the reaction mixture is stirred for
about another hour at ambient temperature, until the
development of gas has ceased. Then the solvent is distilled
off in vacuo from the acid chloride formed. In the meantime
1.00 g of 6-amino-4-[(3-chloro-4-fluoro-phenyl)amino]-7-
cyclopropylmethoxy-quinazoline and 8.0 ml of
diisopropylethylamine in 30 ml of tetrahydrofuran are cooled
to 0 C in an ice bath. The crude acid chloride is taken up in
ml of methylene chloride and added dropwise within 5
minutes whilst cooling with an ice bath. The reaction mixture
is stirred for another hour at 0 C and for two hours at
ambient temperature. Then a suspension of 4.35 N-{2-[(2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-ethyl}-piperazine in 5 ml of
methylene chloride is added and the whole is stirred for
another 48 hours at ambient temperature. The reaction mixture
is concentrated by evaporation in vacuo and the residue in the
flask is chromatographed through a silica gel column with
ethyl acetate/methanol (95:5 to 90:10) as eluant.
Yield: 20 mg (1 % of theory),
Rf value: 0.32 (silica gel, methylene chloride/methanol = 9:1)
mass spectrum (ESI-) : m/z = 653, 655 [M-H] -
The following compounds are obtained analogously to Example 4:
(1) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
tetrahydrofuran-3-yl)-piperidin-l-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
Rf value: 0.17 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI-) : m/z = 592, 594 [M-H] -
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-2-oxa-
8-aza-spiro[4.5]dec-8-yl)-l-oxo-2-buten-l-yl]amino}-
7-cyclopropylmethoxy-quinazoline
(The 2-oxa-8-aza-spiro [4 . 5] decan-3-one used is obtained from
8-benzyl-2-oxa-8-aza-spiro[4.5]decan-3-one by hydrogenolytic
cleaving of the benzyl group)
CA 02403152 2002-09-13
- 50 -
Rf value: 0.37 (silica gel, methylene chloride/methanol = 9:1)
mass spectrum (EI): m/z = 579, 581 [M]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-perhydro-
pyrazino [2, 1-c] [1, 4] oxazin-8-yl) -l-oxo-2-buten-l-yl] amino} -
7-cyclopropylmethoxy-quinazoline
Rf value: 0.19 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI") : m/z = 579, 581 [M-H] "
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(1-oxo-2-oxa-
8-aza-spiro[4.5]dec-8-yl)-1-oxo-2-buten-1-yl]amino}-7-
cyclopropylmethoxy-quinazoline
Rf value: 0.48 (silica gel, methylene chloride/methanol = 9:1)
mass spectrum (EI) : m/z = 579, 581 [M]'
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(1-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-yl]amino}-
7-cyclopropylmethoxy-quinazoline
Rf value: 0.21 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI") : m/z = 579, 581 [M-H] "
(6) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [ (2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-piperidin-1-yl}-1-oxo-2-buten-
1-yl)amino]-7-cyclopropylmethoxy-quinazoline
Rf value: 0.23 (silica gel, ethyl acetate/methanol = 9:1)
mass spectrum (ESI-) : m/z = 624, 626 [M-H] "
(7) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4- {4- [ (2-oxo-6, 6-
dimethyl-morpholin-4-yl)methyl]-piperidin-l-yl}-1-oxo-2-buten-
1-yl)amino]-7-cyclopropylmethoxy-quinazoline
Rf value: 0.48 (silica gel, ethyl acetate/methanol/conc.
aqueous ammonia = 9:1:0.2)
mass spectrum (ESI-) : m/z = 649, 651 [M-H] -
(8) 4- ( (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
morpholin-4-yl)-piperidin-1-yl]-l-oxo-2-buten-l-yl}amino)-7-
cyclopropyl-methoxy-quinazoline
CA 02403152 2002-09-13
- 51 -
prepared by lactonising the intermediate product (4-[(3-
chloro-4-fluoro-phenyl) -amino] -6- ( {4- [4- [N- (tert. -
butyloxycarbonylmethyl)-N-(2-hydroxyethyl)-amino]-piperidin-l-
yl]-1-oxo-2-buten-1-yl}amino)-7-cyclopropylmethoxy-
quinazoline, melting point 114-117 C (with foaming), Rf-value:
0.46 (silica gel, methylene chloride/methanol = 9:1)) in the
presence of 5 equivalents of trifluoracetic acid in
acetonitrile under reflux
melting point: from 140 C (with foaming)
mass spectrum (ESI') : m/z = 609, 611 [M+H] +
Rf value: 0.26 (silica gel, ethyl acetate/methanol = 4:1)
(9) (R) -4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (6-
methyl-2-oxo-morpholin-4-yl)-piperidin-l-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
prepared by lactonising the intermediate product ((R)-4-[(3-
chloro-4 - f luoro-phenyl ) amino] - 6 - ( { 4 - [4 - [N- ( tert . -
butyloxycarbonylmethyl)-N-(2-hydroxypropyl)-amino]piperidin-l-
y]-1-oxo-2-buten-1-yl}amino)-7-cyclopropylmethoxy-quinazoline,
melting point: from 115 C (with foaming); Rf-value: 0.53
(silica gel, methylene chloride/methanol 9:1)) in the presence
of 5 equivalents of trifluoroacetic acid in acetonitrile under
ref lux
melting point: from 126 C (with foaming)
mass spectrum (ESI+) : m/z = 623, 625 [M+H] '
Rf value: 0.29 (silica gel, ethyl acetate/methanol 4:1)
(10) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( [4- (4-methyl-2-
oxo-l-oxa-4,9-diaza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclopropylmethoxy-quinazoline
melting point: 125-130 C (with foaming)
mass spectrum (ESI+) : m/z = 609, 611 [M+H] +
Rf value: 0.46 (silica gel, methylene chloride/methanol = 9:1)
(11) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6-{ [4- (2-oxo-3-oxa-9-
aza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-l-yl]amino}-7-
cyclopropylmethoxy-quinazoline
CA 02403152 2002-09-13
- 52 -
mass spectrum (ESI-) : m/z = 592, 594 [M+H]
Rf value: 0.24 (silica gel, methylene chloride/methanol 9:1)
The starting material 2-oxo-3-oxa-9-aza-spiro[5.5]undecane is
prepared as follows:
(a) 4,4-bis-(2-hydroxyethyl)-piperidine prepared by catalytic
hydrogenation of 1-benzyl-4,4-bis-(2-hydroxyethyl)-piperidine
in ethanol in the presence of palladium on activated carbon
(10% Pd)
mass spectrum (ESI+) : m/z = 174 [M+H]'
(b) 1-benzyloxycarbonyl-4,4-bis-(2-hydroxyethyl)-piperidine
prepared by reaction of 4,4-bis-(2-hydroxyethyl)-piperidine
with benzyl chloroformate in tetrahydrofuran in the presence
of triethylamine
Rf value: 0.32 (silica gel, ethyl acetate/methanol = 9:1)
(c) 9-benzyloxycarbonyl-2-oxo-3-oxa-9-aza-spiro[5.5] undecane
prepared by reaction of 1-benzyloxycarbonyl-4,4-bis-(2-
hydroxyethyl)-piperidine with 4-methylmorpholine-N-oxide in
methylene chloride/acetonitrile in the presence of
tetrapropylammonium-perruthenate and pulverised molecular
sieve
mass spectrum (ESI-) : m/z = 302 [M+H] -
(d) 2-oxo-3-oxa-9-aza-spiro[5.5]undecane prepared by catalytic
hydrogenation of 9-benzyloxycarbonyl-2-oxo-3-oxa-9-aza-
spiro[5.5]undecane in ethanol in the presence of palladium on
activated carbon (10% Pd) (the produkt must be reacted further
immediately after careful evaporation)
mass spectrum (ESI+) : m/z 170 [M+H]'
The following compounds may also be obtained analogously to
the preceding Examples and other methods known from the
literature:
CA 02403152 2002-09-13
- 53 -
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(7-oxo-6-oxa-
2,9-diaza-spiro[4.5]dec-2-yl)-1-oxo-2-buten-1-yl]amino}-
7-cyclppropylmethoxy-quinazoline
(2) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- { [4- (7-oxo-6, 9-
dioxa-2-aza-spiro[4.5]dec-2-yl)-1-oxo-2-buten-1-yl]amino}-7-
cyclopropylmethoxy-quinazoline
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-2-oxa-
7-aza-spiro[4.4]non-7-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclo-
propylmethoxy-quinazoline
(4) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6-{ [4- (2-oxo-1,4-
dioxa-9-aza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]amino}-7-
cyclopropylmethoxy-quinazoline
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(2-oxo-4-methyl-
1-oxa-4,9-diaza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]-
amino}-7-cyclopropylmethoxy-quinazoline
(6) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(2-oxo-i-oxa-
9-aza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclo-
propylmethoxy-quinazoline
(7) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (3-oxo-1, 4-
dioxa-9-aza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]amino}-7-
cyclopropylmethoxy-quinazoline
(8) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(4-oxo-perhydro-
furo[3,4-b]pyrrol-l-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclo-
propylmethoxy-quinazoline
(9) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (6-oxo-perhydro-
furo[3.4-b]pyrrol-l-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclo-
propylmethoxy-quinazoline
CA 02403152 2002-09-13
- 54 -
(10) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6-{ [4- (2-oxo-
perhydro-furo[2,3-c]pyrrol-5-yl)-1-oxo-2-buten-1-yl]amino)-7-
cyclopropylmethoxy-quinazoline
(11) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- { [4- (2-oxo-4-
methyl-perhydro-pyrrolo-[3,4-b][1.4]oxazin-6-yl)-1-oxo-2-
buten-1-yl]amino}-7-cyclopropylmethoxy-quinazoline
(12) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (2-oxo-
perhydro-[1.4]dioxino[2,3-c]pyrrol-6-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclopropylmethoxy-quinazoline
(13) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
tetrahydrofuran-4-yl)-piperidin-1-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
(14) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-4-
methyl-morpholin-3-yl)-piperidin-1-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
(15) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-({4-[4-(2-oxo-4-
methyl-morpholin-6-yl)-piperidin-1-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
(16) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [3- (2-oxo-6, 6-
dimethyl-morpholin-4-yl)-pyrrolidin-1-yl]-1-oxo-2-buten-1-yl}-
amino)-7-cyclopropylmethoxy-quinazoline
(17) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-6, 6-
dimethyl-morpholin-4-yl)-piperidin-1-yl]-1-oxo-2-buten-l-
yl}amino)-7-cyclopropylmethoxy-quinazoline
(18) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [ (2-oxo-
tetrahydrofuran-3-yl)oxy]-piperidin-1-yl}-1-oxo-2-buten-l-
yl)amino]-7-cyclopropylmethoxy-quinazoline
CA 02403152 2002-09-13
- 55 -
(19) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6-{ [4- (4-{2- [ (2-oxo-
tetrahydrofuran-3-yl)oxy]ethyl}-piperazin-1-yl)-1-oxo-2-buten-
1-yl]amino}-7-cyclopropylmethoxy-quinazoline
(20) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (4-{ [ (2-oxo-
tetrahydrofuran-3-yl)oxy]acetyl}-piperazin-1-yl)-i-oxo-2-
buten-1-yl]amino}-7-cyclopropylmethoxy-quinazoline
(21) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (4-{ [ (2-oxo-
tetrahydrofuran-3-yl)sulphanyl]acetyl}-piperazin-1-yl)-1-oxo-
2-buten-1-yl]amino}-7-cyclopropylmethoxy-quinazoline
(22) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [2- (2-oxo-
6,6-dimethyl-morpholin-4-yl)ethyl]-piperazin-1-yl}-1-oxo-
2-buten-1-yl)amino]-7-cyclopropylmethoxy-quinazoline
(23) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [ (2-oxo-
6,6-dimethyl-morpholin-4-yl)acetyl]-piperazin-1-yl}-1-oxo-
2-buten-1-yl)amino]-7-cyclopropylmethoxy-quinazoline
(24) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [ (2-oxo-
6,6-dimethyl-morpholin-4-yl)methyl]-piperidin-1-yl}-1-oxo-
2-buten-1-yl)amino]-7-cyclopropylmethoxy-quinazoline
(25) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (N-{1- [ (5-oxo-
tetrahydrofuran-2-yl)carbonyl]-piperidin-4-yl}-N-methyl-
amino)-1-oxo-2-buten-1-yl]amino}-7-cyclopropylmethoxy-
quinazoline
(26) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4- {4- [ (5-oxo-
tetrahydrofuran-2-yl)carbonylamino]-piperidin-1-yl}-1-oxo-
2-buten-1-yl)amino]-7-cyclopropylmethoxy-quinazoline
(27) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- { [4- (4- {N- [ (5-oxo-
tetrahydrofuran-2-yl)carbonyl]-N-methyl-amino}-piperidin-
1-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclopropylmethoxy-
quinazoline
CA 02403152 2002-09-13
- 56 -
(28) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclobutylmethoxy-quinazoline
(29) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclobutyloxy-quinazoline
(30) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclopentyloxy-quinazoline
(31) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl]amino}-7-[(tetrahydrofuran-3-yl) oxy]-quinazoline
(32) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl]amino}-7-cyclohexyloxy-quinazoline
(33) 4- [ (3-chloro-4-fluoro-phenyl)amino] -6-{ [4- (3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl]amino}-7-[(tetrahydropyran-4-yl)oxy]-quinazoline
(34) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl] amino} -7- [2- (cyclobutyloxy) ethoxy] -quinazoline
(35) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (3-oxo-
perhydro-pyrazino-[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-l-
yl] amino} -7- [2- (cyclopropylmethoxy) ethoxy] -quinazoline
(36) (R) -4- [ (1-phenylethyl) amino] -6-{ [4- (3-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-1-yl]amino}-
quinazoline
CA 02403152 2002-09-13
- 57 -
(37) (R) -4- [ (1-Phenylethyl)amino] -6-{ [4- (3-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-1-yl]amino}-7-
methoxy-quinazoline
(38) (R) -4- [ (1-phenylethyl)amino] -6-{ [4- (3-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-1-yl]amino}-7-
ethoxy-quinazoline
(39) (R) -4- [ (1-phenylethyl) amino] -6- { [4- (3-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-1-yl]amino}-
7- [2- (methoxy) ethoxy] -quinazoline
(40) (R) -4- [ (1-phenylethyl) amino] -6- { [4- (3-oxo-perhydro-
pyrazino[2,1-c][1,4]oxazin-8-yl)-1-oxo-2-buten-1-yl]amino}-
7- [3- (methoxy)propyloxy] -quinazoline
(41) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
morpholin-4-yl)-piperidin-1-yl]-1-oxo-2-buten-1-yl}amino)-7-
methoxy-quinazoline
(42) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (2-oxo-
morpholin-4-yl)-piperidin-1-yl]-1-oxo-2-buten-1-yl}amino)-7-
cyclopropylmethoxy-quinazoline
(43) 4- [ (R) - (1-phenyl-ethyl) amino] -6- ( {4- [4- (2-oxo-morpholin-
4-yl)-piperidin-1-yl]-1-oxo-2-buten-1-yl}amino)-quinazoline
(44) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (6-methyl-
2-oxo-morpholin-4-yl)-piperidin-l-yl]-1-oxo-2-buten-1-yl}-
amino)-7-methoxy-quinazoline
(45) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- ( {4- [4- (6-methyl-
2-oxo-morpholin-4-yl)-piperidin-1-yl]-1-oxo-2-buten-1-yl}-
amino)-7-cyclopropylmethoxy-quinazoline
CA 02403152 2002-09-13
- 58 -
(46) 4- [ (R) - (1-phenyl-ethyl) amino] -6- ( {4- [4- (6-methyl-2-oxo-
morpholin-4-yl)-piperidin-1-yl]-1-oxo-2-buten-1-yl}amino)-
quinazoline
(47) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (2-oxo-3-oxa-
9-aza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]amino}-
7-methoxy-quinazoline
(48) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6-{ [4- (2-oxo-3-oxa-
9-aza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]amino}-7-cyclo-
propylmethoxy-quinazoline
(49) 4- [ (R) - (1-phenyl-ethyl) amino] -6-{ [4- (2-oxo-3-oxa-9-aza-
spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]amino}-quinazoline
(50) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-(4-methyl-2-
oxo-l-oxa-4,9-diaza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-l-
yl]amino}-7-methoxy-quinazoline
(51) 4- [ (R) - (1-Phenyl-ethyl)amino] -6-{ [4- (4-methyl-2-oxo-
1-oxa-4,9-diaza-spiro[5.5]undec-9-yl)-1-oxo-2-buten-1-yl]-
amino}-quinazoline
(52) 4- [ (3-chloro-4-fluoro-phenyl) amino] -6- [ (4-{4- [ (2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-piperidin-1-yl}-1-oxo-2-buten-
1-yl) amino] -7- [ (tetrahydrofuran-2-yl)methoxy] -quinazoline
(53) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[(4-{4-[(2-oxo-
tetrahydrofuran-3-yl)sulphanyl]-piperidin-1-yl}-1-oxo-2-buten-
1-yl)amino]-7-[(tetrahydropyran-4-yl)methoxy]-quinazoline
CA 02403152 2002-09-13
- 59 -
Exam in e 5
rQated tabl ets contai ni ng 75 mg of a__ i v ibs anc,P
1 tablet core contains:
active substance 75.0 mg
calcium phosphate 93.0 mg
corn starch 35.5 mg
polyvinylpyrrolidone 10.0 mg
hydroxypropylmethylcellulose 15.0 mg
magnesium stearate 1.5
230.0 mg
Prenaration=
The active substance is mixed with calcium phosphate, corn
starch, polyvinylpyrrolidone, hydroxypropylmethylcellulose and
half the specified amount of magnesium stearate. Blanks 13 mm
in diameter are produced in a tablet-making machine and these
are then rubbed through a screen with a mesh size of 1.5 mm
using a suitable machine and mixed with the rest of the
magnesium stearate. This granulate is compressed in a tablet-
making machine to form tablets of the desired shape.
Weight of core: 230 mg
die: 9 mm, convex
The tablet cores thus produced are coated with a film
consisting essentially of hydroxypropylmethylcellulose. The
finished film-coated tablets are polished with beeswax.
Weight of coated tablet: 245 mg.
Exam in e 6
Tablets containing 100 mg of a iy subs a P
Composition:
1 tablet contains:
active substance 100.0 mg
lactose 80.0 mg
corn starch 34.0 mg
CA 02403152 2002-09-13
- 60 -
polyvinylpyrrolidone 4.0 mg
magnesium stearate 2.0
220.0 mg
M . .hod of r xparation:
The active substance, lactose and starch are mixed together and
uniformly moistened with an aqueous solution of the
polyvinylpyrrolidone. After the moist composition has been
screened (2.0 mm mesh size) and dried in a rack-type drier at
50 C it is screened again (1.5 mm mesh size) and the lubricant
is added. The finished mixture is compressed to form tablets.
Weight of tablet: 220 mg
Diameter: 10 mm, biplanar, facetted on both sides
and notched on one side.
Exa=le 7
Tablets containing 150 mg of active Gi an
Composition:
1 tablet contains:
active substance 50.0 mg
powdered lactose 89.0 mg
corn starch 40.0 mg
colloidal silica 10.0 mg
polyvinylpyrrolidone 10.0 mg
magnesium stearate 1.0 mg
300.0 mg
PrPr~ara i on =
The active substance mixed with lactose, corn starch and silica
is moistened with a 20% aqueous polyvinylpyrrolidone solution
and passed through a screen with a mesh size of 1.5 mm. The
granules, dried at 45 C, are passed through the same screen
again and mixed with the specified amount of magnesium
stearate. Tablets are pressed from the mixture.
CA 02403152 2002-09-13
- 61 -
Weight of tablet: 300 mg
die: 10 mm, flat
Examnle 8
Hard gel ati_ne capsules contai ni ng 150 mg of active ubs an _
1 capsule contains:
active substance 50.0 mg
corn starch (dried approx. 80.0 mg
lactose (powdered) approx. 87.0 mg
magnesium stearate 3,L-Mg
approx. 420.0 mg
Prenaration-
The active substance is mixed with the excipients, passed
through a screen with a mesh size of 0.75 mm and homogeneously
mixed using a suitable apparatus. The finished mixture is
packed into size 1 hard gelatine capsules.
Capsule filling: approx. 320 mg
Capsule shell: size 1 hard gelatine capsule.
Exam ~~~P--9
SuIppogi ori -, containing l50 mg of active suhs an P
1 suppository contains:
active substance 150.0 mg
polyethyleneglycol 1500 550.0 mg
polyethyleneglycol 6000 460.0 mg
polyoxyethylene sorbitan monostearate 840.0 mg
2,000.0 mg
CA 02403152 2002-09-13
- 62 -
Pre]aarati on=
After the suppository mass has been melted the active substance
is homogeneously distributed therein and the melt is poured
into chilled moulds.
Example 10
Suspension containing 0 mg of active substance
100 ml of suspension contain:
active substance 1.00 g
carboxymethylcellulose-Na-salt 0.10 g
methyl p-hydroxybenzoate 0.05 g
propyl p-hydroxybenzoate 0.01 g
glucose 10.00 g
glycerol 5.00 g
70% sorbitol solution 20.00 g
flavouring 0.30 g
dist. water ad 100 ml
Prgnaration :
The distilled water is heated to 70 C. The methyl and propyl
p-hydroxybenzoates together with the glycerol and sodium salt
of carboxymethylcellulose are dissolved therein with stirring.
The solution is cooled to ambient temperature and the active
substance is added and homogeneously dispersed therein with
stirring. After the sugar, the sorbitol solution and the
flavouring have been added and dissolved, the suspension is
evacuated with stirring to eliminate air.
ml of suspension contain 50 mg of active substance.
CA 02403152 2002-09-13
- 63 -
F.xam= 1 e 11
Amrjo u1 s on _aininQ 10 mQ a_ iv -sii an
Composition:
active substance 10.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 2.0 ml
Prenaration:
The active substance is dissolved in the necessary amount of
0.01 N HC1, made isotonic with common salt, filtered sterile
and transferred into 2 ml ampoules.
Example 12
Ampoules containing 50 mg of active sLbstance
Composition:
active substance 50.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 10.0 ml
Preparation:
The active substance is dissolved in the necessary amount of
0.01 N HC1, made isotonic with common salt, filtered sterile
and transferred into 10 ml ampoules.
Examrle 13
Carpsu 1 es for powder i nhal a i on containing S m!a of a i v
1 capsule contains:
CA 02403152 2002-09-13
- 64 -
active substance 5.0 mg
lactose for inhalation 15.0 ma
20.0 mg
Preparation:
The active substance is mixed with lactose for inhalation. The
mixture is packed into capsules in a capsule-making machine
(weight of the empty capsule approx. 50 mg).
weight of capsule: 70.0 mg
size of capsule = 3
Rxample 14
Solution for inhalation for h nd-h d n_hiilis.rs o aining
2. S mg active sLi-~stance
1 spray contains:
active substance 2.500 mg
benzalkonium chloride 0.001 mg
1N hydrochloric acid q.s.
ethanol/water (50/50) ad 15.000 mg
Preparation:
The active substance and benzalkonium chloride are dissolved
in ethanol/water (50/50). The pH of the solution is adjusted
with iN hydrochloric acid. The resulting solution is filtered
and transferred into suitable containers for use in hand-held
nebulisers (cartridges).
Contents of the container: 4.5 g