Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
Description
FLEXIBLE GRAPHITE ARTICLE AND FUEL CELL ELECTRODE
WITH ENHANCED ELECTRICAL AND THERMAL CONDUCTIVITY
Field of the Invention
The present invention relates to an article useful in an electrode assembly
for an
electroclzemical fuel cell. The inventive assembly includes an article formed
of flexible
graphite sheet that is fluid permeable and has enhanced isotropy with respect
to thermal and
electrical conductivity.
Background of the Invention
Graphites are made up of layer planes of hexagonal arrays or networks of
carbon
atoms. These layer planes of hexagonally arranged carbon atoms are
substantially flat and are
oriented or ordered so as to be substantially parallel and equidistant to one
another. The
substantially flat, parallel equidistant sheets or layers of carbon atoms,
usually referred to as
basal planes, are linked or bonded together and groups thereof are arranged in
crystallites.
Highly ordered graphites consist of crystallites of considerable size: the
crystallites being
highly aligned or oriented with respect to each other and having well ordered
carbon layers.
In other words, highly ordered graphites have a high degree of preferred
crystallite
orientation. It should be noted that graphites possess anisotropic structures
and thus exhibit
or possess many properties that are highly directional, especially thermal and
electrical
conductivity and fluid diffusion. Briefly, graphites may be characterized as
laminated
structures of carbon, that is, structures consisting of superposed layers or
laminae of carbon
atoms joined together by weak van der Waals forces. In considering the
graphite structure,
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
two axes or directions are usually noted, to wit, the "c" axis or direction
and the "a" axes or
directions. For simplicity, the "c" axis or direction may be considered as the
direction
perpendicular to the carbon layers. The "a" axes or directions may be
considered as the
directions parallel to the carbon layers or the directions perpendicular to
the "c" direction.
The natural graphites suitable for manufacturing flexible graphite possess a
very high degree
of orientation.
As noted above, the bonding forces holding the parallel layers of carbon atoms
together are only weak van der Waals forces. Graphites can be treated so that
the spacing
between the superposed carboii layers or laminae can be appreciably opened up
so as to
provide a marked expansion in the direction perpendicular to the layers, that
is, in the "c"
direction and thus form an expanded or intumesced graphite structure in which
the laminar
character of the carbon layers is substantially retained.
Natural graphite flake which has been greatly expanded and more particularly
expanded so as to have a final thickness or "c" direction dimension which is
at least about 80
or more times the original "c" direction dimension can be formed without the
use of a binder
into cohesive or integrated flexible graphite sheets of expanded graphite,
e.g. webs, papers,
strips, tapes, or the like. The formation of graphite particles which have
been expanded to
have a final thickness or "c" dimension which is at least about 80 times the
original "c"
direction dimension into integrated flexible sheets by compression, without
the use of any
binding material is believed to be possible due to the excellent inechanical
interlocking, or
cohesion which is achieved between the voluminously expanded graphite
particles.
In addition to flexibility, the sheet material, as noted above, has also been
found to
possess a high degree of anisotropy with respect to thermal and electrical
conductivity and
fluid diffusion, comparable to the natural graphite starting material due to
orientation of the
expanded graphite particles substantially parallel to the opposed faces of the
sheet resulting
from very high compression, e.g. roll pressing. Sheet material thus produced
has excellent
flexibility, good strength and a very high degree of orientation.
-2-
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
Briefly, the process of producing flexible, binderless anisotropic graphite
sheet
material, such as web, paper, strip, tape, foil, mat, or the like, comprises
compressing or
compacting under a predetermined load and in the absence of a binder, expanded
graphite
particles which have a "c" direction dimension which is at least about 80
times that of the
original particles so as to form a substantially flat, flexible, integrated
graphite sheet. The
expanded graphite particles, which generally are worm-like or vermiform in
appearance, once
compressed, will maintain the compression set and alignment with the opposed
major
surfaces of the sheet. The density and thickness of the sheet material can be
varied by
controlling the degree of compression. The density of the sheet material can
be within the
range of from about 5 pounds per cubic foot to about 125 pounds per cubic
foot. The flexible
graphite sheet material exhibits an appreciable degree of anisotropy due to
the alignment of
graphite particles parallel to the major opposed, parallel surfaces of the
sheet, with the degree
of anisotropy increasing upon roll pressing of the sheet material to increased
density. In roll
pressed anisotropic sheet material, the thickness, i.e. the direction
perpendicular to the
opposed, parallel sheet surfaces comprises the "c" direction and the
directions ranging along
the length and width, i.e. along or parallel to the opposed, major surfaces
comprises the "a"
directions and the tliermal, electrical and fluid diffusion properties of the
sheet are very
different, by orders of magnitude, for the "c" and "a" directions.
This very considerable difference in properties, known as anisotropy, which is
directionally dependent, can be disadvantageous in some applications. For
example, in
gasket applications where flexible graphite sheet is used as the gasket
material and in use is
held tightly between metal surfaces, the diffusion of fluid, e.g. gases or
liquids, occurs more
readily parallel to and between the major surfaces of the flexible graphite
sheet. It would, in
most instances, provide for greater gasket performance, if the resistance to
fluid flow parallel
to the major surfaces of the graphite sheet ("a" direction) were increased,
even at the expense
of reduced resistance to fluid diffusion flow transverse to the major faces of
the graphite
sheet ("c" direction). With respect to electrical properties, the resistivity
of anisotropic
flexible graphite sheet is high in the direction transverse to the major
surfaces ("c" direction)
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
of the flexible graphite sheet, and very substantially less in the direction
parallel to and
between the major faces of the flexible graphite sheet ("a" direction). In
applications such as
fluid flow field plates for fuel cells and seals for fuel cells, it would be
of advantage if the
electrical resistance transverse to the major surfaces of the flexible
graphite sheet ("c"
direction) were decreased, even at the expense of an increase in electrical
resistivity in the
direction parallel to the major faces of the flexible graphite sheet ("a"
direction).
With respect to thermal properties, the thermal conductivity of a flexible
graphite
sheet in a direction parallel to the upper and lower surfaces of the flexible
graphite sheet is
relatively high, while it is relatively very low in the "c" direction
transverse to the upper and
lower surfaces.
The foregoing situations are accommodated by the present invention.
Summary of the Invention
In accordance with the present invention, a meinbrane electrode assembly for
an
electro-chemical fuel cell is provided, comprising a pair of electrodes and an
ion exchange
membrane positioned between the electrodes, at least one of the electrodes
being formed of a
sheet of a compressed mass of expanded graphite particles having a plurality
of transverse
fluid channels passing through the sheet between first and second opposed
surfaces of the
sheet, one of the opposed surfaces abutting the ion exchange membrane.
Advantageously,
the transverse fluid channels are formed by mechanically impacting an opposed
surface of the
sheet to displace graphite within the sheet at predetermined locations. The
transverse fluid
channels are adjacently positioned and separated by walls of compressed
expanded graphite
at least some of which permit interconnection between adjacent channels (such
as by having
grooves therein) to enable fluid flow therebetween.
Brief Description of the Drawings
Figure 1 is a plan view of a transversely permeable sheet of flexible graphite
having
interconnected transverse channels in accordance with the present invention;
-4-
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
Figure 1(A) shows a flat-ended protrusion element used in making the channels
in the
perforated sheet of Figure 1;
Figure 2 is a side elevation view in section of the sheet of Figure 1;
Figures 2(A), (B), (C) sliow various suitable flat-ended configurations for
transverse
interconnected channels in accordance with the present invention;
Figures 3, 3(A), 3(B) show a mechanism for making the article of Figure 1;
Figures 3(C), 3(D) show enlarged perspective views of portions of transversely
permeable flexible graphite sheet in accordance with the present invention;
Figure 3(E) is a photograph of a portion of transversely permeable flexible
graphite
sheet corresponding to Figure 3(C);
Figure 4 shows an enlarged sketch of an elevation view of the oriented
expanded
graphite particles of flexible graphite sheet material;
Figure 5 is a sketch of an enlarged elevation view of an article formed of
flexible
graphite sheet in accordance with the present invention;
Figure 5, 6, 7 and 7(A) show a fluid pernzeable electrode assembly which
includes a
transversely permeable article in accordance with the present invention; and
Figure 8 is a photograph at 100X (original magnification) corresponding to a
portion
of the side elevation view sketch of Figure 5.
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2008-02-19
Detailed Description of the Invention
Graphite is a crystalline form of carbon comprising atoms covalently bonded in
flat layered planes with weaker bonds between the planes. By treating
particles of
graphite, such as natural graphite flake, with an intercalant of, for
instance, a solution
of sulfuric and nitric acid, the crystal structure of the graphite reacts to
form a
compound of graphite and the intercalant. The treated particles of graphite
are
hereafter referred to as "particles of intercalated graphite". Upon exposure
to high
temperature, the particles of intercalated graphite expand in dimension as
much as
about 80 or more times its original volume in an accordion-like fashion in the
"c"
direction, i.e., in the direction perpendicular to the crystalline planes of
the graphite.
The exfoliated graphite particles are veriniform in appearance, and are
therefore
commonly referred to as worms. The worms may be compressed together into
flexible
sheets that, unlike the original graphite flakes, can be formed and cut into
various
shapes and provided with small transverse openings by deforming mechanical
impact.
A common method for manufacturing graphite sheet, e.g., foil from flexible
graphite is described by Shane et al. in U.S. Pat. No. 3,404,061. In the
typical practice
of the Shane et al. method, natural graphite flakes are intercalated by
dispersing the
flakes in a solution containing an oxidizing agent of, for example, a mixture
of nitric
and sulfuric acid. The intercalation solution contains oxidizing and other
intercalating
agents known in the art. Examples include those containing oxidizing agents
and
oxidizing mixtures, such as solutions containing nitric acid, potassium
chlorate,
chromic acid, potassium pennanganate, potassium chromate, potassium
dichromate,
perchloric acid, and the like, or mixtures, such as for example, concentrated
nitric acid
and chlorate, chromic acid. and phosphoric acid, sulfuric acid and nitric
acid, or
mixtures of a strong organic acid, such as trifluoroacetic acid, and a strong
oxidizing
agent soluble in the organic acid.
In a preferred embodiment, the intercalating agent is a solution of a mixture
of
sulfuric acid, or sulfuric acid and phosphoric acid, and an oxidizing agent, i
e., nitric
acid,
-6-
CA 02405303 2008-02-19
perchloric acid, chromic acid, potassium permanganate, hydrogen peroxide,
iodic or
periodic acids, or the like. Although less preferred, the intercalation
sohitions may
contain metal halides such as ferric chloride, and ferric chloride mixed with
sulfuric
acid, or a halide, such as bromine as a solution of bromine and sulfuric acid
or bromine
in an organic solvent.
After the flakes are intercalated, any excess solution is drained from the
flakes
and the flakes are water-washed. The quantity of intercalation solution
retained on the
flakes after draining may range from 20 to 150 parts of solution by weight per
100
parts by weight of graphite flakes (pph) and more typically about 50 to 120
pph.
Alternatively, the quantity of the intercalation solution may be limited to
between 10
to 50 parts of solution per hundred parts of graphite by weight (pph) which
permits the
washing step to be eliminated as taught and described in U.S. Pat. No. 4,
895,713. The
thus treated particles of graphite are sometimes refer-red to as "particles of
intercalated
graphite". Upon exposure to high temperature, e.g. up to about 700 C to 1000 C
and
higher, the particles of intercalated graphite expand as much as about 80 to
1000 or
more times its original volume in an accordion-like fashion in the c-
direction, i.e., in
the direction perpendicular to the crystalline planes of the constituent
graphite
particles. The expanded (or exfoliated) graphite particles are vermiform in
appearance,
and are therefore commonly referred to as worms. The worms may be compressed
together into flexible sheets that, unlike the original graphite flakes, can
be formed and
cut into various shapes and provided with small transverse openings by
deforming
mechanical impact as hereinafter described.
Flexible graphite sheet and foil are coherent, with good handling strength,
and
are suitably compressed, such as by roll-pressing, to a thickness of 0.003 to
0.15 inch
and a density of 0.1 to 1.5 grams per cubic centimeter. From about 1.5-30% by
weight
of ceramic additives, can be blended with the intercalated graphite flakes as
described
in U.S. Patent 5,902,762 to provide enhanced resin impregnation in the final
flexible
graphite product. The additives include ceramic fiber particles having a
length of 0.15
to 1.5 millimeters. The width of the particles is suitably from 0.04 to 0.004
mm. The
ceramic fiber particles are non-reactive and non-adhering to
-7-
CA 02405303 2008-02-19
graphite and are stable at tempera.tures. up to 2000 F, preferably 2500 F.
Suitable ceramic
fiber particles are formed of macerated quartz glass fibers, carbon and
graphite fibers,
zirconia, boron nitcide, silicon carbide and magnesia fibers, naturally
occuming mineral fibers
such as calcium metasilicate fibers, calcium a.luminum silicate fibers,
aluminum oxide fibers
and the like.
With reference to Figure 1 and Figure 2, a compressed mass of expanded
graphite
particles, in the form of a f lexible graphite sheet is shown at 10. The f
lexible graphite sheet
is provided with channels 20, which are preferably smooth-sided as indicated
at 67 in
.Fignres 5 and 8, and which pass between the parallel, opposed surfaces 30, 40
of flexible
graphite sheet 10, and are separated by walls 3 of compressed expandable
graphite. The
walls 3 are advantageously provided with grooves 5, havin.g a'depth of 1/10 to
1/3 the depth
of the channels in accordance with the present invention. - The channeLs L 20
preferabiy have
openings 50 on one of the opposed suifaces 30 which aze larger than tlie
openings 60 in #he
other opposed. surface 40. The channels 20 can have different configurations
as s:hoown at 20'
- 20"' in Figures 2(A), 2(B), 2(C) which are formed using fiat-ended
protrusion elements of
different shapes as shown at 75, 175, 275, 375 in Figares 1(A) and 2(A), 2(B),
2(C), 2(D),
suitably formed of metal, e.g: steel, and integral with and extending from the
pressing roller
70 of the impacting device shown in Figure 3. The smooth flat-ends of the
channel-Ãorming
protrusion elements 75,175, 275, 375, shown at 77,177, 277, 377, and the
smooth #lat ends
of the groove-forming protrusion elements 675, 775, 875, 975 shown at 677,
777, 877, 977,
and the smooth bearing surface 73, of roller 70, and the smooth bearing
surface 78 of t+oller
72 (or altematively f lat metal plate 79), ensure deform.a.tion and
displacement of graphite
within the flexible graphite sheet, preferably such that there are no rough or
ragged edges or
debris resalting from the channel-forming impact. The groove-fomaing
prottusion elements
675, 775, 875, 975 also result in deformation and displacement of graphite
within the flexible
graphite sheet. Preferred channel-forming protrusion elements 77 have
decreasing cross-
section in the direction away from the pressing roller 70 to provide larger
channel openings
on the side of the sheet which is initially impacted. The development of
smooth,
-8-
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
unobstructed surfaces 63 surrounding channel openings 60, enables the free
flow of fluid into
and through smooth-sided (at 67) channels 20.
In a preferred embodiment, openings at one of the opposed surfaces are larger
than the
channel openings in the other opposed surface, e.g. from I to 200 times
greater in area, and
result from the use of protrusion elements having converging sides such as
shown at 76, 276,
376. The transverse channels 20 are formed in the flexible graphite sheet 10
at a plurality of
pre-determined locations by mechanical impact at the predetermined locations
in sheet 10
using a mechanism such as shown in Figure 3 comprising a pair of steel rollers
70, 72 with
one of the rollers having truncated, i.e. flat-ended, prism-shaped protrasions
75 which impact
surface 30 of flexible graphite sheet 10 to displace graphite and penetrate
sheet 10 to fonn
open chamlels 20. In the present invention, the channel-forming protrusions 75
are bridged
by groove-forming protrusions 675 which form interconnecting grooves 5 between
channels
20 in a row of aligned channels concurrently with formation of channels 20
which is
illustrated in the sketch of Figure 3(C) and the photograph of Figure 3(E).
Additionally,
groove-forming protrusion elements 675' can be included as shown in Figures
3(A), 3(B) to
form interconnecting grooves 5' in a parallel row of transverse channels 20 as
shown in
Figure 3(D). In practice, both rollers 70, 72 can be provided with "out-of-
register"
protrusions, and a flat metal plate indicated at 79, can be used in place of
smooth-surfaced
roller 72. Figure 4 is an enlarged sketch of a sheet of flexible graphite 110
that shows a
typical prior art orientation of compressed expanded graphite particles 80
substantially
parallel to the opposed surfaces 130, 140. This orientation of the expanded
graphite particles
80 results in anisotropic properties in flexible graphite sheets; i.e. the
electrical conductivity
and thermal conductivity of the sheet is substantially lower in the direction
transverse to
opposed surfaces 130, 140 ("c " direction) than in the direction ("a"
direction) parallel to
opposed surfaces 130, 140. In the course of impacting flexible graphite sheet
10 to form
channels 20, as illustrated in Figure 3, graphite is displaced within flexible
graphite sheet 10
by flat-ended (at 77) channel-forming protrusions 75 to push aside graphite as
it travels to
and bears against smooth surface 73 of roller 70 to disrupt and deform the
parallel orientation
of expanded graphite particles 80 as shown at 800 in Figure 5. Groove forming
protrusions
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2008-02-19
675 concurrently deform the parallel orientation of expanded graphite
particles. This
region of 800, adjacent channels 20 and grooves 5, shows disruption of the
parallel
orientation into an oblique, non-parallel orientation is optically observable
at
magnifications of 100X and higher. In effect the displaced graphite is being
"die-
molded" by the sides 76 of adjacent protrusions 75 and the smooth surface 73
of
roller 70 as illustrated in Figure 5. This reduces the anisotropy in flexible
graphite
sheet 10 and thus increases the electrical and thermal conductivity of sheet
10 in the
direction transverse to the opposed surfaces 30, 40. A similar effect is
achieved with
frusto-conical and parallel-sided peg-shaped flat-ended protrusions 275 and
175. The
perforated gas permeable flexible graphite sheet 10 of Figure l can be used as
an
electrode in an electrochemical fuel cel1500 shown schematically in Figures 6,
7 and
7(A).
Figure 6, Figure 7 and Figure 7(A) show, schematically, the basic elements of
an electrochemical Fuel Cell, more con-iplete details of which are disclosed
in U.S.
Patents 4,988,583 and 5,300,370 and PCT WO 95/16287 (15 June 1995).
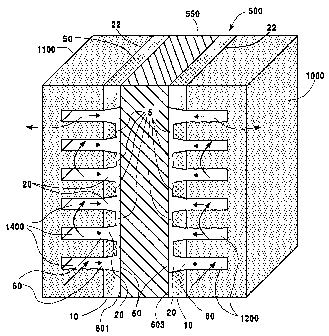
With reference to Figure 6, Figure 7 and Figure 7(A), the Fuel Cell indicated
generally at 500, comprises electrolyte in the fonn of a plastic e.g. a solid
polymer
ion exchange membrane 550 catalyst coated at surfaces 601, 603, e.g. coated
with
platinum 600 as shown in Figure 7(A); perforated flexible graphite sheet
electrodes
in accordance with the present invention; and flow field plates 1000, 1100
which
respectively abut electrodes 10. Pressurized fuel is circulated through
grooves 1400
of fuel flow field pate 1100 and pressurized oxidant is circulated through
grooves
1200. In operation, the fuel flow field plate 1100 becomes an anode, and the
oxidant
flow field plate 1000 becomes a cathode with the result that an electric
potential, i.e.
voltage is developed between the fuel flow field plate 1000 and the oxidant
flow field
plate 1100. The above described electrochemical fiiel cell is combined with
others in
a fuel cell stack to provide the desired level of electric power as described
in the
above-noted U.S. Patent 5,300,370.
-10-
CA 02405303 2008-02-19
The operation of Fuel Cell 500 requires that the electrodes 10 be porous to
the
ftiel and oxidant fluids, e. g. hydrogen and oxygen, to pei-init these
components to
readily pass froin the grooves 1400, 1200 through electrodes 10 to contact the
catalyst 600, as shown in Figure 7(A), and enable protons derived from
hydrogen to
migrate through ion exchange membrane 550. In the electrode 10 of the present
invention, channels 20 are positioned to adjacently cover grooves 1400, 1200
of the
flow field plates so that the presstuized gas from the grooves passes through
the
smaller openings 60 of channels 20 and exits the larger openings 50 of
chaimels 20.
In the event of a blockage in a channel 20, fluid from adjacent channels can
flow
through grooves 5 so that gas- catalyst contact adjacent the blocked channel
is
maintained. The initial velocity of the gas at the smaller openings 60 is
higher than
the gas flow at the larger openings 50 with the result that the gas is slowed
down
when it contacts the catalyst 600 and the residence time of gas-catalyst
contact is
increased and the area of gas exposure at the membrane 550 is maximized. This
feature, together with the increased electrical conductivity of the flexible
graphite
electrode of the present invention enables more efficient fuel cell operation.
Figure 8 is a photograph (original magnification 100X) of a body of flexible
graphite corresponding to a portion of the sketch of Figure 5.
The articles of Figures 1 and 5 and the material shown in the photograph
( l OOX) of Figure 8 can be shown to have increased thermal and electrical
conductivity in the direction transverse to opposed parallel, planar surfaces
30, 40 as
compared to the thermal and electrical conductivity in the direction
transverse to
surfaces 130,140 of the material of Figure 4 in which particles of expanded
natural
graphite unaligned with the opposed planar surfaces are not optically
detectable.
A sample of a sheet of flexible graphite 0.01 inch thick having a density of
0.3 grams/cc, representative of Figure 4, was mechanically impacted by a
device
similar to that of Figure 3 to provide channels of different size in the
flexible graphite
sheet. The transverse
-11-
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
("c" direction) electrical resistance of the sheet material samples was
measured and the
results are shown in the table below.
Also, the transverse gas permeability of channeled flexible graphite sheet
samples, in
accordance with the present invention, was measured, using a Gurley Model 4118
for Gas
Permeability Measurement.
Samples of channeled flexible graphite sheet in accordance with the present
invention
were placed at the bottom opening (3/8 in. diam.) of a vertical cylinder (3
inch diameter
cross-section). The cylinder was filled with 300 cc of air and a weighted
piston (5 oz.) was
set in place at the top of the cylinder. The rate of gas flow through the
channeled samples
was measured as a function of the time of descent of the piston and the
results are shown in
the table below.
Flexible Graphite Sheet
(0.01 inch thick; density = 0.3 grns/cc)
1600 channels per 250 channels per
No Channels square inch - 0.020 square inch - 0.020
inch wide at top; inch wide at top;
0.005 inch wide at 0.007 inch wide at
bottom bottom
Transverse Electrical 80 8 0.3
Resistance (micro
ohms)
Diffusion Rate - - 8 seconds 30 seconds
Seconds
In the present invention, for a flexible graphite sheet having a thickness of
.003 inch
to .015 inch adjacent the channels and a density of 0.5 to 1.5 grams per cubic
centimeter, the
preferred channel density is from 1000 to 3000 channels per square inch and
the preferred
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02405303 2002-10-09
WO 01/78179 PCT/US00/09508
channel size is a channel in which the ratio of the area of larger channel
opening to the
smaller is from 50:1 to 150:1.
In the practice of the present invention, the flexible graphite sheet can, at
times, be
advantageously treated with resin and the absorbed resin, after curing,
enhances the moisture
resistance and handling strength, i.e. stiffiiess of the flexible graphite
sheet. Suitable resin
content is preferably 20 to 30% by weight, suitably up 60% by weight.
The article of the present invention can be used as electrical and thermal
coupling
elements for integrated circuits in computer applications, as conformal
electrical coiitact pads
and as electrically energized grids in de-icing equipment.
The above description is intended to enable the person skilled in the art to
practice the
invention. It is not intended to detail all of the possible variations and
modifications which
will become apparent to the skilled worker upon reading the description. It is
intended,
however, that all such modifications and variations be included within the
scope of the
invention which is defined by the following claims. The claims are intended to
cover the
indicated elements and steps in any arrangement or sequence which is effective
to meet the
objectives intended for the invention, unless the context specifically
indicates the contrary.
-13-
SUBSTITUTE SHEET (RULE 26)