Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
SUBSTITUTED PHENYL FARNESYLTRANSFERASE INHIBITORS
Technical Field
The instant invention provides substituted phenyl compounds which inhibit
farnesyltransferase, methods for making the compounds, pharmaceutical
compositions
l0 containing the compounds, and methods of treatment using the compounds.
Background of The Invention
Ras oncogenes are the most frequently identified activated oncogenes in human
tumors, and transformed protein Ras is involved in the proliferation of cancer
cells. The Ras
15 must be farnesylated by farnesyl pyrophosphate before this proliferation
can occur, and
farnesylation of Ras by farnesyl pyrophosphate is effected by protein
farnesyltransferase.
Inhibition of protein farnesyltransferase, and thereby farnesylation of the
Ras protein, blocks
the ability of transformed cells to proliferate.
Activation of Ras and related proteins which are farnesylated also partially
mediates
2o smooth muscle cell proliferation (Circulatiofa, I-3: 88 (1993)). Inhibition
of protein isoprenyl
transferases, and thereby farnesylation of the Ras protein, also aids in the
prevention of
intimal hyperplasia associated with restenosis and atherosclerosis, a
condition which
compromises the success of angioplasty and surgical bypass for obstructive
vascular lesions.
Because of this pivotal role played by farnesyltransferase in tumor formation
and
25 metastasis, compounds such as those reported in WO 97/36897, WO 97/36881,
WO 97/36875, WO 97/36901, WO 99/17777, WO 99/18096, WO 99/20609,
WO 99/27928, WO 99/27933, WO 99/27929, WO 99/28313, and WO 99/28314 have been
the subject of current research.
However, there is still an ongoing need for farnesyltransferase inhibitors
with
3o modified or improved profiles of activity.
-1-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Summary of The Invention
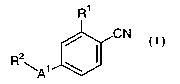
In its principle embodiment, therefore, the instant invention discloses
compounds of
formula (I)
R1
CN
2
R \A1 \
(I)~
or pharmaceutically acceptable salts thereof, wherein
A1 is Ll-Ml-L~ or alkylene, wherein the alkylene can be optionally substituted
with
one, two, or three substituents independently selected from the group
consisting of amino,
hydroxyl, oxo, and -Q1-Q2-R3;
with the proviso that when A1 is methylene, the methylene is substituted;
Ll and L2 are independently absent or alkylene, wherein the alkylenes defining
L1 and
L2 can be optionally substituted with one or two substituents independently
selected from the
group consisting of alkyl, cycloalkyl, cycloalkylalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, and oxo;
with the proviso that at least one of L1 or L2 is present;
Ml is selected from the group consisting of O, N(R4), N(RS)S02, SOZN(R~),
N(RS)C(O), C(O)N(RS), OC(O), C(O)O, C(O), N(RS)C(O)O,
OC(O)N(RS), OC(O)O, N(R5)C(O)N(R5), and S(O)t, wherein t is zero, one, or two;
wherein, for the groups defining Ml, the left ends are attached to Ll and the
right ends are
2o attached to L2;
Q1 is absent or selected from the group consisting of O, N(R4), N(RS)C(O),
N(R5)502, and S(O)t;
Q2 is absent or selected from the group consisting of alkylene, alkenylene,
and
alkynylene;
Rl is selected from the group consisting of halo, cycloalkyl, aryl, and
heteroaryl;
R~ is a heteroaryl selected from the group consisting of imidazolyl,
pyrazolyl,
pyrrolyl, thienyl, triazolyl, pyridyl, and thiazolyl;
R3 is selected from the group consisting of alkyl, cycloalkyl, aryl,
heteroaryl, and
heterocycloalkyl;
R4 is selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl,
allcanoyl, alkylsulfonyl, a nitrogen protecting group, aminosulfonyl, aryl,
arylalkyl, aryloyl,
arylsulfonyl, cycloalkyl, cycloalkylalkyl, cycloalkyloyl, cycloalkylsulfonyl,
heteroaryl,
heteroarylalkyl, heteroaryloyl, heteroarylsulfonyl, heterocycloalkyl,
heterocycloalkylalkyl,
heterocycloalkyloyl, and heterocycloalkylsulfonyl; and
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
R5 is selected from the group consisting of hydrogen, alkyl, aryl, arylalkyl,
cycloalkyl, cycloalkylalkyl, heteroaryl, heteroarylalkyl, heterocycloalkyl,
and
heterocycloalkylalkyl.
In another embodiment, the instant invention discloses compounds of formula
(II)
RB R1
CN
X /
RA
(u)~
or a pharmaceutically acceptable salt thereof, wherein
RA is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, and a nitrogen protecting group;
l0 RB is absent or selected from the group consisting of optionally
substituted alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy;
W is C(H)=C(H), X is N, and Y and Z are C(H); or
W is C(H)=N or N=C(H), wherein each group is drawn with its left end attached
to X
and its right end attached to the carbon substituted with L2; and X, Y and Z
are C(H); or .
W is N or S, one of X, Y, or Z is C(H), and the remainder are
C(H) or N;
with the proviso that RA is present when and only when W is N.
In a preferred embodiment of compounds of formula (II) are compounds wherein
M1 is O;
Ll is optionally substituted alkylene;
L2 is optionally substituted alkylene;
W and Y are N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
4-(((4-cyanophenyl)(1-methyl=1H-imidazol-5-yl)methoxy)methyl)-2-(1-naphthyl)-
benzonitrile,
4-((2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethoxy)methyl)-2-(1-
naphthyl)benzonitrile,
4-((1-(1-methyl-1H-imidazol-5-yl)-3-phenylpropoxy)methyl)-2-(1-naphthyl)-
benzonitrile,
4-(((1-methyl-1H-imidazol-5-yl)(phenyl)methoxy)methyl)-2-(1-naphthyl)-
benzonitrile, and
-3-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((1-(1-methyl-1H-imidazol-5-yl)-2-phenylethoxy)methyl)-2-(1-naphthyl)-
benzonitrile.
In another preferred embodiment of compounds of formula (II) are compounds
wherein
Ml is O;
Ll is optionally substituted alkylene;
L2 is optionally substituted alkylene;
W is N=C(H); and
X, Y, and Z are C(H).
1o Compounds which support this embodiment include, but are not limited to,
Example 516,
Example 517,
Example 518,
Example 519, and
Example 521.
In another preferred embodiment of compounds of formula (II) are compounds
wherein
Ml is O;
Ll is optionally substituted alkylene;
2o L2 is optionally substituted alkylene;
W is S;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
Example 775,
Example 776,
Example 777,
Example 778, and
Example 780.
In another preferred embodiment of compounds of formula (II) are compounds
wherein
Ml is N(R4);
W is N;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
-q._
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-((benzyl((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2'-methyl( 1,1'-
biphenyl)-2-carbonitrile,
4-(((4-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methy1)-2-(1-
naphthyl)benzonitrile,
4-(((4-chlorobenzyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-( 1-
naphthyl)benzonitrile,
4-((((1-methyl-lI~i-imidazol-5-yl)methyl)(4-(trifluoromethoxy)benzyl)amino)-
methyl)-2-(1-naphthyl)benzonitrile,
4-(((4-cyanobenzyl) ( 1 H-imidazol-5-ylmethyl) amino)methyl)-2-( 1-naphthyl)-
to benzonitrile,
5-(((2-cyclohexylethyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2'-
methyl ( 1,1'-biphenyl)-2-carbonitrile,
4-(((2-cyclohexylethyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-(1-
naphthyl)benzonitrile,
15 4-(((cyclohexylmethyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((4-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methy1)-2-(8-
quinolinyl)benzonitrile,
4-(((3,4-dichlorobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-(1-
20 naphthyl)benzonitrile,
4-cyano-N-(4-cyanobenzyl)-N-((1-methyl-1H-imidazol-5-yl)methyl)-3-(1-
naphthyl)benzamide,
4-((((1-methyl-1H-imidazol-5-yl)methyl)(4-
(trifluoromethyl)benzyl)amino)methyl)-2-
(1-naphthyl)benzonitrile,
25 4-(((4-cyano-3-(1-naphthyl)benzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-
methyl)benzoic acid,
N-(4-(((4-cyano-3-(1-naphthyl)benzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-
methyl)phenyl)acetamide,
4-((((1-methyl-1H-imidazol-5-yl)methyl)(4-(methylsulfonyl)benzyl)amino)methyl)-
2-
3o (1-naphthyl)benzonitrile,
4-cyano-N-(4-cyano-3-(1-naphthyl)benzyl)-N-((1-methyl-1H-imidazo1-5-y1)-
methyl)benzamide,
3,4-dichloro-N-(4-cyano-3-(1-naphthyl)benzyl)-N-((1-methyl-1H-imidazol-5-yl)-
methyl)benzamide,
35 4-chloro-N-(4-cyano-3-(1-naphthyl)benzyl)-3-fluoro-N-((1-methyl-1H-imidazol-
5-
yl)methyl)benzamide,
-5-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5,6-dichloro-N-(4-cyano-3-( 1-naphthyl)benzyl)-N-(( 1-methyl-1H-imidazol-5-yl)-
methyl)nicotinamide,
4-cyano-N-(4-cyanobenzyl)-N-(( 1-methyl-1H-imidazol-5-yl)methyl)-3-(8-
quinolinyl)-benzamide,
4-(((2-hydroxy-5-(trifluoromethoxy)benzyl)((1-methyl-1H-imidazol-5-yl)methyl)-
amino)methyl)-2-( 1-naphthyl)benzonitrile,
methyl 6-(((4-cyano-3-(1-naphthyl)benzyl)((1-methyl-1H-imidazol-5-yl)methyl)-
amino)-methyl)nicotinate,
ethyl 4-((4-cyano-3-(1-naphthyl)benzyl)((1-methyl-1H-imidazol-5-yl)methyl)-
to amino)-1-piperidinecarboxylate,
2'-methyl-5~((( 1-methyl-1H-imidazol-5-yl)methyl)amino)( 1,1'-biphenyl)-2-
carbonitrile,
5-(benzyl((1-methyl-1H-imidazol-5-yl)methyl)amino)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile,
15 4-(methyl((1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-
naphthyl)benzonitrile,
4-(allyl(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-
naphthyl)benzonitrile,
5-((4-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-~'-methyl(1,1'-
biphenyl)-2-carbonitrile,
4-(((1-methyl-1H-imidazol-5-yl)methyl)(3-phenylpropyl)amino)-2-(1-
2o naphthyl)benzonitrile,
4-((4-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-naphthyl)-
benzonitrile,
4-(benzyl((1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-naphthyl)benzonitrile,
4-(hexyl((1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-naphthyl)benzonitrile,
25 4-(((1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-naphthyl)benzonitrile,
N-(4-cyano-3-(1-naphthyl)phenyl)-N-((1-methyl-1H-imidazol-5-yl)methyl)-
benzamide,
N-(6-cyano-2'-methyl(1,1'-biphenyl)-3-yl)-N-((1-methyl-1H-inaidazol-5-
yl)methyl)-
benzamide,
30 5-((3-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
4-((((1-methyl-1H-imidazol-5-yl)(phenyl)methyl)amino)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((1-(1-methyl-1H-imidazol-5-yl)-2-phenylethyl)amino)methyl)-2-(1-naphthyl)-
35 benzonitrile,
4-(((1-(1-methyl-1H-imidazol-5-yl)-3-phenylpropyl)amino)rnethyl)-2-(1-
naphthyl)-
benzonitrile,
-6-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethyl)amino)methyl)-2-(1-
naphthyl)benzonitrile,
4-((3-chlorobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-naphthyl)-
benzonitrile,
4-(benzyl ( 1 H-imidazol-5-ylmethyl)amino)-2-( 1-naphthyl)benzonitrile,
4-((3-cyanobenzyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-naphthyl)-
benzonitrile,
N-(4-cyano-3-( 1-naphthyl)phenyl)-N-(( 1-methyl-1 H-imidazol-5-
yl)methyl)benzene-
sulfonamide,
to methyl4-((4-cyano((1-methyl-1H-imidazol-5-yl)methyl)-3-(1-naphthyl)anilino)-
methyl)benzoate,
4-((4-cyano((1-methyl-1H-imidazol-5-yl)methyl)-3-(1-naphthyl)anilino)methyl)-
benzoic acid,
5-(benzyl( 1 H-imidazol-5-ylmethyl)amino)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile,
15 methyl3-((4-cyano((1-methyl-1H-imidazol-5-yl)methyl)-3-(1-naphthyl)anilino)-
methyl)benzoate,
4-(((4-cyanophenyl)( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-naphthyl)-
benzonitrile, and
6-(((4-cyano-3-(1-naphthyl)benzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)-
20 methyl)nicotinonitrile.
In another preferred embodiment of compounds of formula (II) are compounds
wherein
Ml is N(R4);
W is N=C(H); and
25 X, Y and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
2'-methyl-5-((3-pyridinylamino)methyl)(1,1'-biphenyl)-2-carbonitrile,
5-((benzyl(3-pyridinylmethyl)amino)methyl)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile,
2'-methyl-5-((3-pyridinylmethyl)amino)( 1,1'-biphenyl)-2-carbonitrile,
30 5-(benzyl(3-pyridinylmethyl)amino)-2'-methyl(1,1'-biphenyl)-2-carbonitrile,
Example 322,
Example 328,
Example 329,
Example 363,
35 Example 364,
Example 365,
Example 390,
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 450,
Example 467,
Example 468,
Example 469,
Example 470,
Example 471,
Example 472,
Example 473,
Example 474,
i0 Example 475,
Example 482,
Example 483,
Example 484,
Example 485,
Example 490,
Example 491,
Example 492,
Example 493,
Example 494,
2o Example 495,
Example 496,
Example 497,
Example 498,
Example 499,
Example 500,
Example 520,
Example 522,
Example 523,
Example 524,
3o Example 527,
Example 528,
Example 529,
Example 530,
Example 531,
Example 532,
Example 548,
and
Example 549.
_g_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
In another preferred embodiment of compounds of formula (II) are compounds
wherein .
Ml is N(R4);
W is S;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
Example 578,
Example 580,
l0 Example 586,
Example 587,
Example 620,
Example 621,
Example 622,
Example 646,
Example 706,
Example 723,
Example 724,
Example 725,
2o Example 726,
Example 727,
Example 728,
Example 729;
Example 730,
Example 731,
Example 738,
Example 739,
Example 740,
Example 741,
3o Example 753,
Example 754,
Example 755,
Example 756,
Example 757,
Example 758,
Example 759,
Example 779,
-9-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 781,
Example 782,
Example 783,
Example 786,
Example 787,
Example 788,
Example 788,
Example 789,
Example 790,
to Example 791,
Example 807, and
Example 808.
In another embodiment, the instant invention discloses compounds of formula
(III)
RB Ri
~~~Z / CN
X
v \
W
A/
OH
(III),
or a pharmaceutically acceptable salt thereof, wherein
RA is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, and a nitrogen protecting group;
RB is absent or selected from the group consisting of optionally substituted
alkyl,
2o allcoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, penluoroallcyl, and
perfluoroalkoxy; and
W is C(H)=C(H), X is N, and Y and Z are C(H); or
W is C(H)=N or N=C(H), wherein each group is drawn with its left end attached
to X
and its right end attached to the carbon substituted with L2; and X, Y and Z
are C(H); or
W is N or S, one of X, Y, or Z is C(H), and the remainder are
C(H) or N;
with the proviso that RA is present when and only when W is N.
In a preferred embodiment of compounds of formula (III) are compounds wherein
Wish;
3o Y is N; and
X and Z are C(I~.
A compound which supports this embodiment includes, but is not limited to,
-10-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(l, l'-biphenyl)-2-
carbonitrile.
In another preferred embodiment of compounds of formula (III) are compounds
wherein
W is S;
Y is N; and
X and Z are C(H).
A compound which supports this embodiment includes, but is not limited to,
5-(hydroxyl1,3-thiazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile.
In another preferred embodiment of compounds of formula (III) are compounds
wherein
W is N=C(H); and
X, Y, and Z are C(H).
A compound which supports this embodiment includes, but is not limited to,
5-(hydroxyl3-pyridinyl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile,
In another embodiment, the instant invention discloses compounds of formula
(IV)
RB Ri
CN
w
A/ 1
2.Q
Q
Rs
or a pharmaceutically acceptable salt thereof, wherein
Q2 is absent or alkylene;
RA is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, and a nitrogen protecting group;
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy; and
W is C(H)=C(H), X is N, and Y and Z are C(H); or
W is C(H)=N or N=C(H), wherein each group is drawn with its left end attached
to X
and its right end attached to the carbon substituted with L2; and X, Y and Z
are C(H); or
W is N or S, one of X, Y, or Z is C(H), and the remainder are
3o C(H) or N;
with the proviso that RA is present when and only when W is N.
-11-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
In a preferred embodiment of compounds of formula (IV) are compounds wherein
Ql.
1S ~;
Wish;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl) (phenoxy)methyl) ( 1,1'-biphenyl)-2-
carbonitrile,
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methoxy( 1,1'-biphenyl)-2-
l0 carbonitrile,
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-3'-phenyl(1,1'-biphenyl)-2-
carbonitrile,
(2-(9-anthryl)-4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile,
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-isopropyl(1,1'-biphenyl)-2-
15 carbonitrile,
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1,2-dihydro-5-acenaphth-
ylenyl)benzonitrile,
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-chloro(1,1'-biphenyl)-2-
carbonitrile,
20 5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile,
4-((cyclohexylmethoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(8-quinolinyl)-
25 benzonitrile,
4-(((4-cyanobenzyl)oxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-(4-quinolinyl)-
benzonitrile,
4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(5-quinolinyl)-
benzonitrile,
30 4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(5-
isoquinolinyl)-
benzonitrile,
4-(((4-cyanobenzyl) oxy) ( 1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile,
4-((1-methyl-1H-imidazol-5-yl)((4-nitrobenzyl)oxy)methyl)-2-(1-naphthyl)-
benzonitrile,
35 4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-
iodobenzonitrile,
4-(((3-chloro-4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
-12-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((4-cyano-3-iodobenzyl)oxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)-
benzonitrile,
methyl 4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)-
methyl)benzoate,
4-((1-methyl-1H-imidazol-5-yl)((4-(trifluoromethyl)benzyl)oxy)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((4-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile,
4-((1-methyl-1H-imidazol-5-yl)((4-(trifluoromethoxy)benzyl)oxy)methyl)-2-(1-
naphthyl)benzonitrile,
4-(( 1-methyl-1H-imidazol-5-yl)((3-(trifluoromethyl)benzyl)oxy)methyl)-2-( 1-
naphthyl)benzonitrile,
4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-
benzoic acid,
4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-
N,N-dimethylbenzamide,
4-(((2,4-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-( 1-naphthyl)-
benzonitrile,
4-(( 1-methyl-1 H-imidazol-5-yl)((4-(methylsulfonyl)benzyl)oxy)methyl)-2-( 1-
2o naphthyl)benzonitrile,
4-(((2,6-dichloro-4-pyridinyl)methoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((3-bromo-4-cyanobenzyl)oxy)( 1-methyl-1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile,
6-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-
nicotinonitrile,
4-(((4-cyano-3-fluorobenzyl)oxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile,
5-((benzyloxy) ( 1-methyl-1 H-imidazol-5-yl)methyl) ( 1,1'-biphenyl)-2-c
arbonitrile,
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile,
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(3-thienyl)benzonitrile,
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-3'-methyl( 1,1'-biphenyl)-2-
carbonitrile,
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(2-naphthyl)benzonitrile,
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-4'-methyl(1,1'-biphenyl)-2-
carbonitrile,
-13-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-phenyl(1,1'-biphenyl)-2-
carbonitrile,
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2',5'-dimethyl(1,1'-
biphenyl)-2-
carbonitrile,
4-(((4-cyanobenzyl)oxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-( 1-naphthyl)-
benzonitrile,
4-(((2-methoxy-5-nitrobenzyl)oxy)( 1-methyl-1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)-benzonitrile,
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-ethyl(1,1'-biphenyl)-2-
i0 carbonitrile,
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2',3'-dimethyl(l, l'-
biphenyl)-2-
carbonitrile,
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-cyclohexylbenzonitrile,
4-((benzyloxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-(5,6,7, 8-tetrahydro-1
15 naphthalenyl)benzonitrile,
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(2-methyl-1-naphthyl)-
benzonitrile,
2-(1-anthryl)-4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile,
4-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2-(4-
isoquinolinyl)benzonitrile,
20 4-((benzyloxy)(1-(ethoxymethyl)-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile,
4-(((4-cyanobenzyl)oxy)(1-(ethoxymethyl)-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
5-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-phenyl(1,1'-
25 biphenyl)-2-carbonitrile,
4-(((4-cyanobenzyl)oxy)( 1-(3-hydroxypropyl)-1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile,
4-(((4-fluoro-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-
benzonitrile,
30 5-(((3-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((4-(tert-butyl)benzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((4-cyanobenzyl)oxy) ( 1-methyl-1 H-imidazol-5-yl )methyl)-2'-methyl( 1,1'-
35 biphenyl)-2-carbonitrile,
5-(((3-iodobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-
2-carbonitrile,
-14-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(((4-fluorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(l, l'-
biphenyl)-2-carbonitrile,
5-(((4-bromobenzyl)oxy) ( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( 1,1'-
biphenyl)-2-carbonitrile,
5-(((3-chlorobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
(2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((4-nitrobenzyl)oxy)methyl)( 1,1'-
biphenyl)-2-carbonitrile,
5-(((2-methoxy-5-nitrobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl-
(1,1'-biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((3-
(trifluoromethyl)benzyl)oxy)methyl)-
( 1,1'-biphenyl)-2-carbonitrile,
5-(((2,6-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( l,
l'-
biphenyl)-2-carbonitrile,
5-(((3,4-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-c arbonitrile,
5-(((2-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
(2'-methyl-5-(((4-methylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)(1,1'-
2o biphenyl)-2-carbonitrile,
(2'-methyl-5-(((3-methylbenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)(1,1'-
biphenyl)-2-carbonitrile,
5-(((2,5-difluorobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(
1,1'-
biphenyl)-2-carbonitrile,
methyl4-(((6-cyano-2'-methyl(1,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-
yl)methoxy)-methyl)benzoate,
5-(((3,5-difluorobenzyl)oxy) ( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(
1,1'-
biphenyl)-2-carbonitrile,
5-(((~-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(l, l'-
3o biphenyl)-2-carbonitrile,
5-(((4-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((3-methoxybenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( 1,1'-
biphenyl)-2-c arbonitrile,
(2'-methyl-5-(((2-methylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)(1,1'-
biphenyl)-2-carbonitrile,
-15-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(((3-fluorobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((2,6-dichloro-4-pyridinyl)methoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl( l, l'-biphenyl)-2-carbonitrile,
5-(((2-fluorobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( 1,1'-
biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((4-(trifluoromethyl)benzyl)oxy)-
methyl) ( 1,1'-biphenyl)-2.-carbonitrile,
5-(((3,5-dimethylbenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-~'-methyl(
1,1'-
biphenyl)-2-carbonitrile,
5-(((4-fluoro-2-(trifluoromethyl)benzyl)oxy)( 1-methyl-1H-imidazol-5-
yl)methyl)-2'-
methyl-( 1,1'-biphenyl)-2-carbonitrile,
(2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((2-nitrobenzyl)oxy)methyl)(1,1'-
biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((3-(trifluoromethoxy)benzyl)oxy)-
methyl)( 1,1'-biphenyl)-2-carbonitrile,
4-(((6-cyano-2'-methyl( l,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-
yl)methoxy)-
methyl)-6-methylisophthalonitrile,
5-(((2'-cyano( 1,1'-biphenyl)-4-yl)methoxy)( 1-methyl-1H-imidazol-5-yl)methyl)-
2'-
2o methyl(1,1'-biphenyl)-2-carbonitrile,
methyl 3-(((6-cyano-2'-methyl(1,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-
yl)methoxy)-methyl)benzoate,
5-(((3,4-difluorobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((3,4,5-trimethoxybenzyl)oxy)methyl)-
( 1,1'-biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)(8-quinolinylmethoxy)methyl)( 1,1'-
biphenyl)-2-carbonitrile,
5-(((3,5-dimethoxybenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1=
3o biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((4-(methylsulfonyl)benzyl)oxy)-
methyl)( l, l'-biphenyl)-2-carbonitrile,
5-(((6-chloro-1, 3-benzodioxol-5-yl)methoxy) ( 1-methyl-1 H-imi dazol-5-
yl)methyl)-2'-
methyl( 1,1'-biphenyl)-2-carbonitrile,
5-(((4-isopropylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(l,l'-
biphenyl)-2-carbonitrile,
-16-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(((3,4-dimethylbenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((4-(benzyloxy)benzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(
1,1'-
biphenyl)-2-carbonitrile,
5-(((6-fluoro-4H-1,3-benzod.Toxin-8-yl)methoxy)(1-methyl-IH-imidazol-5-
yl)methyl)-2'-methyl( 1,1'-biphenyl)-2-c arbonitrile,
5-(((2,4-dichlorobenzyl)oxy)(1-methyl-IH-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((3,5-dimethylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1=
to biphenyl)-2-carbonitrile,
5-(((5-(tert-butyl)-I,2,4-oxadiazol-3-yl)methoxy)( 1-methyl-IH-imidazol-5-
yl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile,
5-(((4-iodobenzyl)oxy)( I-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-
2-carbonitrile,
15 5-(((1,1'-biphenyl)-4-ylmethoxy)(1-methyl-IH-imidazol-5-yl)methyl)-2'-
methyl(1,1
biphenyl)-2-carbonitrile,
5-(((2-(4-chlorophenyl)-1,3-thiazol-4-yl)methoxy)(1-methyl-1H-imidazol-5-yl)-
methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile,
5-(((5-(2-methoxyphenyl)-1,2,4-oxadiazol-3-yl)methoxy) ( 1-methyl-1 H-imidazol-
5-
2o yl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile,
5-(((4-chloro-2-nitrobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'-
biphenyl)-2-carbonitrile,
methyl 5-(((6-cyano-2'-methyl( 2,1'-biphenyl)-3-yl) ( 1-methyl-IH-imidazol-5-
yl)-
methoxy)methyl)-2-furoate,
25 2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((5-(4-(trifluoromethyl)phenyl)-
1,2,4-
oxadiazol-3-yl)methoxy)methyl)(1,1=biphenyl)-2-carbonitrile,
methyl 8-(((6-cyano-2 =methyl(l,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)-4H-1,3-benzodioxine-6-carboxylate,
(2'-methyl-5-((1-methyl-1H-inudazol-5-yl)((6-nitro-4H-1,3-benzodioxin-8-
3o yl)methoxy)methyl)(1,1'-biphenyl)-2-carbonitrile,
2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((5-(3-(trifluorornethyl)phenyl)-1,2,4-
oxadiazol-3-yl)methoxy)methyl) ( 1,1'-biphenyl)-2-carbonitrile,
5-(((5-acetyl-2-methoxybenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'-biphenyl)-2-carbonitrile,
35 2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((5-phenyl-1,2,4-oxadiazol-3-yl)-
methoxy)methyl)( l, l'-biphenyl)-2-carbonitrile,
-17-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-({{5-(4-methoxyphenyl)-1,2,4-oxadiazol-3-yl)methoxy){1-methyl-IH-imidazol-5-
yl)methyl)-2'-methyl(1,1=biphenyl)-2-carbonitrile,
5-(((5-(3-methoxyphenyl)-1,2,4-oxadiazol-3-yl)methoxy)(1-methyl-1H-imidazol-5-
yl)methyl)-2'-methyl( l, l'-biphenyl)-2-carbonitrile,
2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((2-(4-(trifluoromethyl)phenyl)-1,3-
thiazol-
4-yl)methoxy)methyl)(1,1'-biphenyl)-2-carbonitrile,
2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((5-methyl-3-
isoxazolyl)methoxy)methyl)-
(1, I'-biphenyl)-2-carbonitrile,
(2'-methyl-5-{{1-methyl-1H-imidazol-5-yl)({2-methyl-1-naphthyl)methoxy)methyl)-
to (1,1'-biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((2,3,5,6-
tetramethylbenzyl)oxy)methyl)-
(1, I'-biphenyl)-2-carbonitrile,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((4-(trifluoromethoxy)benzyl)oxy)-
methyl)(1,1'-biphenyl)-2-carbonitrile,
15 5-{((5,6-dichloro-3-pyridinyl)methoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'-biphenyl)-2-carbonitrile,
5-(((3-chloro-5-(trifluoromethyl)-2-pyridinyl)methoxy)(1-methyl-1H-imidazol-5-
yl)-
methyl)-2'-methyl( 1,1'-biphenyl)-2-carbonitrile,
2'-methyl-5-((1-methyl-1H-imidazol-5-yl){2-naphthylmethoxy)methyl)(1,1'-
2o biphenyl}-2-carbonitrile,
5-(((3-bromobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(l, l'-
biphenyl)-2-carbonitrile,
5-(((2-bromobenzyl)oxy)( 1-methyl-IH-irnidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
25 5-(((2,6-difluorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl}methyl)-2'-
methyl(l,l'-
biphenyl)-2-carbonitrile,
5-(((2-fluoro-4-(trifluoromethyl)benzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-
2'-
methyl(1,1'-biphenyl)-2-carbonitrile,
4-{{(6-cyano-2'-methyl{l,1'-biphenyl)-3-yl)(I-methyl-1H-imidazol-5-yl)methoxy)-
3o methyl)-benzamide,
4-(((6-cyano-2''-methyl(1,1'-biphenyl)-3-yl)(I-methyl-1H-imidazol-5-
yl)methoxy)-
methyl)-N-methylbenzamide,
4-(((6-cyano-2'-methyl( 1,1'-biphenyl)-3-yl)(1-methyl-IH-imidazol-5-
yl)methoxy)-
methyl)-N,N-dimethylbenzamide,
35 5-{((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-formyl(1,1'-
biphenyl)-2-carbonitrile,
-18-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-(trifluoromethyl)-
(1,1'-biphenyl)-2-carbonitrile,
2',4'-dichloro-5-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)( 1,1
biphenyl)-2-carbonitrile,
2-(1-benzothien-2-yl)-4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-
yl)methyl)-
benzonitrile,
5-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-(hydroxymethyl)-
( 1,1'-biphenyl)-2-carbonitrile,
2'-cyano-5'-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methy1)(1,1'-
1o biphenyl)-2-carboxylic acid,
4-(((3,4-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(8-
quinolinyl)-
benzonitrile,
4-(((3-fluoro-4-(trifluoromethyl)benzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-
2-
(8-quinolinyl)benzonitrile,
15 4-(((4-fluoro-3-(trifluoromethyl)benzyl)oxy)(1-methyl-1H-imidazol-5-
yl)methyl)-2-
(8-quinolinyl)benzonitrile,
4-(((4-cyano-3-(8-quinolinyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-
benzoic acid,
6-(((4-cyano-3-(8-quinolinyl)phenyl)( 1-methyl-1H-imidazol-5-
yl)methoxy)methyl)-
20 nicotinamide,
6-(((4-cyano-3-(8-quinolinyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-
nicotinic acid,
4-(((3-chloro-5-(trifluoromethyl)-2-pyridinyl)methoxy)(1-methyl-1H-imidazol-5-
yl)methyl)-2-(8-quinolinyl)benzonitrile,
25 6-(((4-cyano-3-(8-quinolinyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)-
nicotinonitrile,
5-(((3,4-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-(trifluoro-
methyl)(1,1'-biphenyl)-2-carbonitrile,
5-(((3-fluoro-4-(trifluoromethyl)benzyl)oxy)( 1-methyl-1H-imidazol-5-
yl)methyl)-2'-
30 (trifluoromethyl)(1,1'-biphenyl)-2-carbonitrile,
5-(((4-fluoro-3 -(trifluoromethyl)benzyl)oxy) ( 1-methyl-1 H-imidazol-5-
yl)methyl)-2'-
(trifluoromethyl)(l, l'-biphenyl)-2-carbonitrile,
6-(((6-cyano-2'-(trifluoromethyl)(1,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-
yl)-
methoxy)methyl)nicotinonitrile,
35 4-(((3-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile,
4-(((4-bromobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
-19-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
benzonitrile,
4-(((6-cyano-2'-methyl( 1,1'-biphenyl)-3-yl) ( 1-methyl-1 H-imidazol-5-
yl)methoxy)-
methyl)-benzoic acid,
4-((1-methyl-1H-imidazol-5-yl)((3-chlorobenzyl)oxy)methyl)-2-(1-naphthyl)-
benzonitrile,
5-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-2-
pyridinecarbonitrile,
4-((1-methyl-1H-imidazol-5-yl)((4-azidobenzyl)oxy)methyl)-2-(1-naphthyl)-
benzonitrile,
l0 methyl6-(((6-cyano-2'-(trifluoromethyl)(1,1'-biphenyl)-3-yl)(1-methyl-1H-
imidazol-
5-yl)methoxy)methyl)nicotinate,
5-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2',3'-dimethyl(
1,1'-
biphenyl)-2-carbonitrile,
2',3'-dichloro-5-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)( 1,1'-
biphenyl)-2-carbonitrile,
6-(((2',3'-dichloro-6-cyano(1,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-
yl)methoxy)-methyl)nicotinonitrile,
6-(((6-cyano-2',3'-dimethyl(1,1'-biphenyl)-3-yl)(1-methyl-1H-irnidazol-5-yl)-
methoxy)methyl)nicotinonitrile, and
4-((4-cyanophenoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Q~ is O;
W is N=C(H); and
X, Y, and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
6-(((4-cyano-3-(1-naphthyl)phenyl)(3-pyridinyl)methoxy)methyl)nicotinonitrile,
Example 296,
Example 297,
Example 29~,
Example 299,
Example 300,
Example 301,
Example 302,
Example 303,
Example 306,
-20-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 310,
Example 311,
Example 331,
Example 332,
Example 344,
Example 345,
Example 346,
Example 348,
Example 349,
to Example 350,
Example 351,
Example 352,
Example 353,
Example 354,
Example 355,
Example 357,
Example 358,
Example 359,
Example 360,
2o Example 362,
Example 366,
Example 368,
Example 369,
Example 370,
Example 371,
Example 372,
Example 373,
Example 374,
Example 375,
3o Example 376,
Example 377,
Example 378,
Example 379,
Example 380,
Example 381,
Example 382,
Example 383,
-21-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 384,
Example 389,
Example 391,
Example 392,
Example 393,
Example 394,
Example 395,
Example 396,
Example 397,
l0 Example 398,
Example 399,
Example 400,
Example 401,
Example 402,
Example 403,
Example 404,
Example 405,
Example 406,
Example 407,
2o Example 408,
Example 409,
Example 410,
Example 411,
Example 412,
Example 413,
Example 414,
Example 415,
Example 416,
Example 417,
3o Example 418,
Example 419,
Example 420,
Example 421,
Example 422,
Example 423,
Example 424,
Example 425,
-22-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 426,
Example 427,
Example 428,
Example 429,
Example 430,
Example 431,
Example 432,
Example 433,
Example 434,
l0 Example 435,
Example 436,
Example 437,
Example 43
8,
Example 439,
Example 440,
Example 441,
Example 442,
Example 443,
Example 444,
2o Example 445,
Example 446,
Example 447,
Example 448,
Example 449,
Example 451,
Example 453,
Example 454,
Example 455,
Example 456,
Example 457,
Example 458,
Example 459,
Example 460,
Example 461,
Example 462,
Example 463,
Example 464,
-23-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 465,
Example 466,
Example 476,
Example 477,
Example 478,
Example 479,
Example 480,
Example 481,
Example 503,
Example 504,
Example 505,
Example 506,
Example 507,
Example 508,
i5 Example 509,
Example 510,
Example 511,
Example 512,
Example 513,
2o Example 514,
Example 525,
Example 526,
Example 533,
Example 534,
25 Example 535,
Example 537,
Example 538,
Example 539,
Example 540,
3o Example 541,
Example 542, and
Example 547.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
35 Ql is O;
W is S;
Y is N; and
-24-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
X, and Z are C(I~.
Compounds which support this embodiment include, but are not limited to,
5-((benzyloxy)(1,3-thiazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile,
4-(((4-cyanobenzyl)oxy)(1,3-thiazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile,
6-(((4-cyano-3-(1-naphthyl)phenyl)(1,3-thiazol-5-
yl)methoxy)methyl)nicotinonitrile,
Example 552,
Example 553,
Example 554,
Example 555,
1o Example 556,
Example 557,
Example 558,
Example 559,
Example 563,
Example 567,
Example 568,
Example 589,
Example 590,
Example 602,
2o Example 603,
Example 604,
Example 606,
Example 607,
Example 608,
2s Example 609,
Example 610,
Example 611,
Example 612,
Example 613,
30 Example 615,
Example 616,
Example 617,
Example 618,
Example 619,
35 Example 623,
Example 625,
Example 626,
-25-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 627,
Example 628,
Example 629,
Example 630,
Example 631,
Example 632,
Example 633,
Example 634,
Example 635,
l0 Example 636,
Example 637,
Example 638,
Example 639,
Example 640,
Example 645,
Example 647,
Example 648,
Example 649,
Example 650,
2o Example 651,
Example 652,
Example 653,
Example 654,
Example 655,
Example 656,
Example 657,
Example 658,
Example 659,
Example 660,
3o Example 661,
Example 662,
Example 663,
Example 664,
Example 665,
Example 666,
Example 667,
Example 668,
-26-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 669,
Example 670,
Example 671,
Example 672,
Example 673,
Example 674,
Example 675,
Example 676,
Example 677,
to Example 678,
Example 679,
Example 680,
Example 681,
Example 682,
Example 683,
Example 684,
Example 685,
Example 686,
Example 687,
2o Example 688,
Example 689,
Example 690,
Example 691,
Example 692,
Example 693,
Example 694,
Example 695,
Example 696,
Example 697,
3o Example 698,
Example 699,
Example 700,
Example 701,
Example 702,
Example 703,
Example 704,
Example 705,
_27_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 707,
Example 709,
Example 710,
Example 711,
Example 712,
Example 713,
Example 714,
Example 715,
Example 716,
l0 Example 717,
Example 718,
Example 719,
Example 720,
Example 721,
Example 722,
Example 732,
Example 733,
Example 734,
Example 735,
Example 736,
Example 737,
Example 762,
Example 763,
Example 764,
Example 765,
Example 766,
Example 767,
Example 768,
Example 769,
Example 770,
Example 771,
Example 772,
Example 773,
Example 784,
Example 785,
Example 792,
Example 793,
_28_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 794,
Example 796,
Example 797,
Example 798,
Example 799,
Example 800,
Example 801, and
Example 806.
In another preferred embodiment of compounds of formula (1V) are compounds
to wherein
Ql is N(R4);
W is N;
Y is N; and
X and Z are C(H).
15 Compounds which support this embodiment include, but are not limited to,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((4-nitrobenzyl)amino)methyl)(1,1'-
biphenyl)-2-carbonitrile,
4-(((4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile,
20 5-(((1-benzoyl-4-piperidinyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl-
(1,1'-biphenyl)-2-carbonitrile,
4-((1-methyl-1H-imidazol-5-yl)((4-(methylsulfonyl)benzyl)amino)methyl)-2-(1-
naphthyl)benzonitrile,
5-((benzylamino)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
25 carbonitrile,
5-(((cyclohexylmethyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(((4-cyanobenzyl)amino)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( 1,1'-
biphenyl)-2-carbonitrile,
30 5-((((6-cyano-2'-methyl(l,1'-biphenyl)-3-yl)methyl)amino)(1-methyl-1H-
imidazol-5-
yl)methyl)-2'-methyl( 1,1'-biphenyl)-2-carbonitrile,
5-((ethyl(4-nitrobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(
1,1'-
biphenyl)-2-carbonitrile,
5-(((4-cyanobenzyl)(ethyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl-
35 (1,1'-biphenyl)-2-carbonitrile,
4-(((4-cyanobenzyl)(methyl)amino)(1-methyl-1H-imidazol-5-yl)methy1)-2-(1-
naphthyl)benzonitrile,
-29-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((butyl(4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)-
benzonitrile,
4-((1-methyl-1H-imidazol-5-yl)(phenethylamino)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((3-bromo-4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methy1)-2-(1-
naphthyl)benzonitrile,
4-(((3-chloro-4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
4-(((1-(4-cyanophenyl)ethyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
l0 4-(((4-cyano-3-iodobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
methyl 4-((((4-cyano-3-( 1-naphthyl)phenyl)( 1-methyl-1 H-imidazol-5-
yl)methyl)-
amino)methyl)benzoate,
4-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)amino)-
15 methyl)benzoic acid,
4-(((4-chlorobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile,
4-(((3,4-dichlorobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)-
benzonitrile,
20 4-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)amino)-
methyl)-N-methylbenzamide,
ethyl 4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)-
amino)-1-piperidinecarboxylate,
6-((((4-cyano-3-( 1-naphthyl)phenyl)( 1-methyl-1 H-imidazol-5-yl)methyl)amino)-
25 methyl)nicotinonitrile,
methyl 6-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)-
amino)methyl)nicotinate,
N-(4-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)amino)-
methyl)phenyl)acetamide,
3o benzyl4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)-
amino)-1-piperidinecarboxylate,
4-((( 1-benzyl-4-piperidinyl) amino) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-(
1-
naphthyl)benzonitrile,
tent-butyl 4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methyl)-
35 amino)-1-piperidinecarboxylate,
4-((( 1-benzoyl-4-piperidinyl) amino) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2-(
1-
naphthyl)benzonitrile,
-30-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)amino)-
methyl)benzamide,
4-((1-methyl-1H-imidazol-5-yl)(((1-methyl-2-oxo-1,2-dihydro-4-
pyridinyl)methyl)-
amino)methyl)-2-( 1-naphthyl)benzonitrile,
4-((4-cyanoanilino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile,
and
4-((3-cyanoanilino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is N(R4);
W is N=C(H);
X, Y, and Z are C(H).
Compounds which support this embodiment include, but are not Limited to,
Example 304,
Example 305,
Example 308,
Example 309,
Example 312,
Example 313,
Example 314,
Example 315,
Example 316,
Example 317,
Example 318,
Example 319,
Example 320,
Example 321,
Example 323,
Example 324,
Example 325,
Example 326,
. Example 327,
Example 330,
Example 333,
Example 334,
Example 335,
Example 336,
-31-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 337,
Example 338,
Example 339,
Example 340,
Example 341,
Example 342,
Example 343,
Example 452,
Example 544, and
1o Example 545.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is N(R4);
W is S;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
Example 561,
Example 562,
2o Example 565,
Example 566,
Example 569,
Example 570,
Example 571,
Example 572,
Example 573,
Example 574,
Example 575,
Example 576,
Example 577,
Example 579,
Example 581,
Example 582,
Example 583,
Example 584,
Example 585,
Example 588,
-32-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 591,
Example 592,
Example 593,
Example 594,
Example 595,
Example 596,
Example 597,
Example 598,
Example 599,
to Example 600,
Example 601,
Example 708,
Example 747,
Example 748,
Example 749,
Example 750,
Example 751,
Example 752,
Example 803, and
Example 804.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is S(O)t, wherein t is zero, one, or two;
Wish;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
4-(((4-cyanobenzyl)sulfanyl)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile,
and
4-(((4-cyanobenzyl)sulfonyl)(1-methyl-1H-irnidazol-5-yl)methyl)-2-(1-naphthyl)-
benzonitrile.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Q1 is S(O)t, wherein t is zero, one, or two;
W is N=C(H); and
X, Y, and Z are C(H).
-33-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Compounds which support this embodiment include, but are not limited to,
Example 347, and
Example 356.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is S(O)t, wherein t is zero, one, or two;
WisS;
Y is N; and
X and Z are C(H).
1o Compounds which support this embodiment include, but are not limited to,
Example 605, and
Example 614.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Q1 is N(R5)S02;
W is N;
Y is N; and
X and Z are C(H).
A compound which supports this embodiment includes, but is not limited to,
2o 4-cyano-N-((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)-
benzenesulfonamide.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is N(R5)SO~;
W is N=C(H); and
X, Y, and Z are C(H).
A compound which supports this embodiment includes, but is not limited to,
Example 543. q'
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is N(R5)502;
W is S;
Y is N; and
X and Z are C(H).
A compound which supports this embodiment includes, but is not limited to,
Example 802.
-34-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Q1 is absent;
W is N;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)(3-oxo-4-(3-(trifluoromethoxy)phenyl)-
1-
piperazinyl)methyl)(1,1'-biphenyl)-2-carbonitri1e, and
to tert-butyll-((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methyl)-4-
piperidinylcarbamate.
In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is absent;
W is N=C(H); and
X, Y, and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
Example 307, and
Example 546.
2o In another preferred embodiment of compounds of formula (IV) are compounds
wherein
Ql is absent;
WisS;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
Example 564, and
Example 805.
In another embodiment, the instant invention discloses compounds of formula
(V)
Ra Ri
CN
W \
RA/ Q2 OH
3o Ra
(V),
-35-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
or a pharmaceutically acceptable salt thereof, wherein
RA is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, and a nitrogen protecting group;
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy; and
W is C(H)=C(H), X is N, and Y and Z are C(H); or
W is C(H)=N or N=C(H), wherein each group is drawn with its left end attached
to X
and its right end attached to the carbon substituted with L2; and X, Y and Z
are C(H); or
W is N or S, one of X, Y, or Z is C(H), and the remainder are
C(H) or N;
with the proviso that RA is present when and only when W is N.
In a preferred embodiment of compounds of formula (V) are compounds wherein
W is N;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
5-(1-hydroxy-1-(1-methyl-1H-imidazol-5-yl)-3-phenyl-~-propynyl)-2'-methyl(1,1'-
biphenyl)-2-carbonitrile,
5-(1-hydroxy-1-(1-methyl-1H-imidazol-5-yl)-3-phenylpropyl)-2'-methyl(l,l'-
biphenyl)-2-carbonitrile, and
4-(1-hydroxy-1-(1-methyl-1H-imidazol-5-yl)-3-phenyl-2-propynyl)-2-(1-
naphthyl)benzonitrile.
In another preferred embodiment of compounds of formula (V) are compounds
wherein
W is N=C(H); and
X, Y, and Z are C(H). '
Compounds which support this embodiment include, but are not limited to,
Example 385,
3o Example 386, and
Example 387.
In another preferred embodiment of compounds of formula (V) are compounds
wherein
W is S;
Y is N; and
X and Z are C(H).
Compounds which support this embodiment include, but are not limited to,
-36-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 641,
Example 642, and
Example 643.
In another embodiment, the instant invention discloses compounds of formula
(VI)
RA1 RB Rj
W~z~'~' / CN
X\\/,~ M1
Y
(VI),
or pharmaceutically acceptable salts thereof, wherein
W' is N or S; and
one of X', Y', or Z' is C(H), and the remainder are C(H) or N;
RA~ is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, hydroxyl, and a nitrogen protecting group; and
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy;
with the proviso that RA~ is present when and only when W' is N.
In another embodiment, the instant invention discloses compounds of formula
(VII)
R1
W~Z~ / CN
X \Y~ I
H
(Vn)
or pharmaceutically acceptable salts thereof, wherein
W' is N or S; and
one of X', Y', or Z' is C(H), and the remainder are C(H) or N;
RA~ is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, hydroxyl, and a nitrogen protecting group; and
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, allcoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perFluoroalkyl, and
perfluoroalkoxy;
with the proviso that RA~ is present when and only when W' is N.
In another embodiment, the instant invention discloses compounds of
formula (VIII)
-37-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Ra~ Re, R1
il V jZ' /
X~\\ ~~
Y \,
H2~a
R3
or pharmaceutically acceptable salts thereof, wherein
a is zero to six;
W' is N or S; and
one of X', Y', or Z' is C(H), and the remainder are C(H) or N;
RA is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, hydroxyl, and a nitrogen protecting group; and
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perFluoroalkoxy;
with the proviso that RA~ is present when and only when W' is N.
In a preferred embodiment of compounds of formula (VIII) are compounds wherein
Ql is O;
W' is N;
Y' is N; and
X' and Z' are C(H).
A compound which supports this embodiment includes, but is not limited to,
4-(((4-cyanobenzyl)oxy)(1-trityl-1H-imidazol-4-yl)methyl)-2-(1-
naphthyl)benzonitrile.
In another preferred embodiment of compounds of formula (VIII) are compounds
wherein
Ql is O;
W' is S; and
X', Y', and Z' are C(H).
Compounds which support this embodiment include, but are not limited to,
5-((benzyloxy)(3-thienyl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile, and
6-(((4-cyano-3-( 1-naphthyl)phenyl) (3-thienyl)methoxy)methyl)nicotinonitrile.
In another embodiment, the instant invention discloses compounds of formula
(IX)
-3 ~-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
RA~ RB R1
i1 V jZ' /
X~\Y,' \ \ ,
2 OOH
R3
or pharmaceutically acceptable salts thereof, wherein
W' is N or S; and
one of X', Y', or Z' is C(H), and the remainder are C(H) or N;
RA~ is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, hydroxyl, and a nitrogen protecting group; and
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
1o carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy;
with the proviso that RA~ is present when and only when W' is N.
In another embodiment, the instant invention discloses compounds of formula
(X)
R1
Y~X / CN
I M~
RB ~N~~ ~~i \
b
(X),
15 or pharmaceutically acceptable salts thereof, wherein
b is two to six;
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy; and
2o one of X and Y is C(H) and the other is C(H) or N.
In another embodiment, the instant invention discloses compounds of formula
(XI)
R1
X~N
Y~
RB
-39-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
or pharmaceutically acceptable salts thereof, wherein
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy; and
one of X and Y is C(H) and the other is C(H) or N.
In another embodiment, the instant invention discloses compounds of formula
(XII)
Ri
N ~ c ~ \
CN
B/
R ~~H2)a
R3
to or pharmaceutically acceptable salts thereof, wherein
a is zero to six;
c is zero to two;
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
15 carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroallcyl, and
perfluoroalkoxy; and
one of X and Y is C(H) and the other is C(H) or N.
In a preferred embodiment of compounds of formula (XII) are compounds wherein
c is zero;
X is. C(H);
2o Y is N; and
Ql.
is O.
A compound which supports this embodiment includes, but is not limited to,
5-(1-(benzyloxy)-2-( 1H-imidazol-1-yl)ethyl)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile.
In another embodiment, the instant invention discloses compounds of
25 formula (XIII)
R1 .
N
Y
R3
-40-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
or pharmaceutically acceptable salts thereof, wherein
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy; and
one of X and Y is C(H) and the other is C(H) or N.
In another embodiment, the instant invention discloses compounds of formula
(XIV)
R1
R
CN
W \
RA O
(XIV),
or a pharmaceutically acceptable salt thereof, wherein
RA is absent or selected from the group consisting of hydrogen, optionally
substituted
alkyl, alkoxycarbonyl, and a nitrogen protecting group;
RB is absent or selected from the group consisting of optionally substituted
alkyl,
alkoxy, alkanoyl, alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino,
aminosulfonyl, azido,
carboxamido, carboxyl, cyano, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy; and
W is C(H)=C(H), X is N, and Y and Z are C(H); or
W is C(H)=N or N=C(H), wherein each group is drawn with its left end attached
to X
and its right end attached to the carbon substituted with L2; and X, Y and Z
are C(H); or
W is N or S, one of X, Y, or Z is C(H), and the remainder are
C(H) or N;
2o with the proviso that RA is present when and only when W is N.
In a preferred embodiment of compounds of formula (XIV) are compounds wherein
W is N;
Y is N; and
X and Z are C(H).
. Compounds which support this embodiment include, but are not limited to,
(2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)carbonyl)(1,1'-biphenyl)-2-
carbonitrile,
4-((1-methyl-1H-imidazol-5-yl)carbonyl)-2-(8-quinolinyl)benzonitrile, and
5-(( 1-methyl-1 H-imidazol-5-yl)c arbonyl)-2'-(trifluoromethyl) ( l , l'-
biphen y1)-2-
carbonitrile.
3o In another preferred embodiment of compounds of formula (XIV) are compounds
wherein
W is N=C(H); and
X, Y, and Z are C(H).
-41-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Compounds which support this embodiment are
Example 367,
Example 501, and
Example 502.
In another preferred embodiment of compounds of formula (XIV) are compounds
wherein
W is S; and
X, Y, and Z are C(H).
Compounds which support this embodiment are
l0 Example 624,
Example 760, and
Example 761.
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
15 compound of formula (I).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (II).
In another embodiment, the instant invention discloses a method for inhibiting
20 farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (III).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (IV).
25 In another embodiment, the instant invention discloses a method for
inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (V).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
30 compound of formula (VI).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (VII).
In another embodiment, the instant invention discloses a method for inhibiting
35 farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (VIII).
-42-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (IX).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (X).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (XI).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (XII).
In another embodiment, the instant invention discloses a method for inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (XIII).
In another embodiment, the instant invention discloses a method of inhibiting
farnesyltransferase comprising administering a pharmaceutically acceptable
amount of a
compound of formula (XIV).
In another embodiment, the instant invention discloses a method for treating
cancer in
2o a mammal in recognized need of such treatment comprising administering to
the mammal a
pharmaceutically acceptable amount of a compound of formula (I).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (II).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (III).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
3o pharmaceutically acceptable amount of a compound of formula (IV).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (V).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (VI).
-43-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (VII).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (VIII).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (IX).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (X).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (XI).
In another embodiment, the instant invention discloses a method for treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (XII).
In another embodiment, the instant invention discloses a method for treating
cancer in
2o a mammal in recognized need of such treatment comprising administering to
the mammal a
pharmaceutically acceptable amount of a compound of formula (XIII).
In another embodiment, the instant invention discloses a method fox treating
cancer in
a mammal in recognized need of such treatment comprising administering to the
mammal a
pharmaceutically acceptable amount of a compound of formula (XIV).
In another embodiment, the instant invention discloses a compound of formula
(I) in
combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(II) in
combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(III) in
3o combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(IV) in
combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(V) in
combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(VI) in
combination with a pharmaceutically acceptable carrier.
-44-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
In another embodiment, the instant invention discloses a compound of formula
(VII)
in combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(VIII)
in combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(IX) in
combination with a pharmaceutically acceptable Garner.
In another embodiment, the instant invention discloses a compound of formula
(X) in
combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(XI) in
1o combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(XII)
in combination with a pharmaceutically acceptable carrier.
In another embodiment, the instant invention discloses a compound of formula
(XIII)
in combination with a pharmaceutically acceptable carrier.
15 In another embodiment, the instant invention discloses a compound of
formula (XIV)
in combination with a pharmaceutically acceptable carrier:
Detailed Description of The Invention
The instant invention provides substituted phenyl farnesyltransferase
inhibitors. As
2o used in the specification, the following terms have the meanings indicated.
The term "alkanoyl," as used herein, refers to an alkyl group, as defined
herein, or a
substituted alkyl group, as defined herein, attached to the parent molecular
group through a
carbonyl, as defined herein.
The term "alkoxy," as used herein, refers to an alkyl group, as defined
herein, or a
25 substituted alkyl group, as defined herein, attached to the parent
molecular group through an
oxygen atom.
The term "alkoxycarbonyl," as used herein, refers to an ester group; e.g., an
alkoxy
group as defined herein, attached to the parent molecular group through a
carbonyl, as
defined herein.
30 The term "alkenyl," as used herein, refers to a monovalent straight or
branched chain
hydrocarbon radical having from tvo to six carbons and at least one carbon-
carbon double
bond.
The term "alkenylene," as used herein, refers to a divalent straight or
branched chain
hydrocarbon radical having from two to six carbons and at least one carbon-
carbon double
35 bond.
The term "alkyl," as used herein, refers to a saturated, monovalent straight
or
branched chain hydrocarbon having from one to six carbons.
-45-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The term "alkylene," as used herein, refers to a divalent straight or branched
chain
saturated hydrocarbon diradical having from one to six carbons.
The term "alkylsulfonyl," as used herein, refers to an alkyl group, as defined
herein,
or a substituted alkyl group, as defined herein, attached to the parent
molecular group through
a sulfonyl group, as defined herein.
The term "alkynyl," as used herein, refers to a monovalent straight or
branched chain
hydrocarbon group having from two to six carbons and at least one carbon-
carbon triple
bond.
The term "alkynylene," as used herein, refers to a divalent straight or
branched chain
to hydrocarbon group having from two to six carbons and at least one carbon-
carbon triple
bond.
The term "amino," as used herein, refers to -NH2 or derivatives thereof formed
by
independent replacement of one or both hydrogen atoms thereon with a
substituent or
substituents independently selected from the group consisting of alkyl,
alkenyl, alkynyl, aryl,
i5 arylalkyl, cycloalkyl, cycloalkylalkyl, heteroaryl, heteroarylalkyl, and an
amino protecting
group.
The term "aminosulfonyl," as used herein, refers to an amino group, as defined
herein,
attached to the parent molecular group through a sulfonyl group, as defined
herein.
The terms "amino protecting group," or "nitrogen protecting group," as used
herein,
20 refer to selectively introducible and removable groups which protect amino
groups against
undesirable side reactions during synthetic procedures. Examples of amino
protecting groups
include methoxycarbonyl, ethoxycarbonyl, trichloroethoxycarbonyl,
benzyloxycarbonyl
(Cbz), chloroacetyl, trifluoroacetyl, phenylacetyl, formyl, acetyl, benzoyl,
tert-
butoxycarbonyl (Boc), para-methoxybenzyloxycarbonyl, isopropoxycarbonyl,
phthaloyl,
25 succinyl, benzyl, diphenylmethyl, triphenylmethyl (trityl),
methanesulfonyl,
para-toluenesulfonyl, trimethylsilyl, triethylsilyl, triphenylsilyl, and the
like. Preferred
nitrogen protecting groups of the instant invention are benzyloxycarbonyl
(Cbz), formyl,
acetyl, methoxycarbonyl, ethoxycarbonyl, benzoyl, tent-butoxycarbonyl (Boc),
and
triphenylmethyl (trityl).
30 The term "aryl," as used herein, refers to groups containing at least one
aromatic,
carbocyclic ring. Aryl groups of the instant invention are exemplified by
phenyl, naphthyl,
dihydronaphthyl, tetrahydronaphthyl, indanyl, indenyl, anthracenyl,
acenaphthylenyl,
dihydroacenaphthylenyl, and the like. The aryl groups of the instant invention
can be
optionally substituted with one, two, three, four, or five radicals
independently selected from
35 the group consisting of optionally substituted alkyl, alkenyl, alkynyl,
alkoxy, alkanoyl,
alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino, aminosulfonyl, azido,
carboxamido,
carboxyl, cyano, halo, hydroxyalkyl, hydroxyl, nitro, perfluoroalkyl,
perfluoroalkoxy, oxo,
-46-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
thioalkoxy, phenyl, heteroaryl selected from the group consisting of furanyl,
thienyl, pyrrolyl,
oxazolyl, thiazolyl, imidazolyl, isoxazolyl, isothiazolyl, oxadiazolyl,
oxadiazolyl, triazolyl,
thiadiazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, and triazinyl, and
heterocycloalkyl
selected from the group consisting of tetrahydrofuranyl, piperidinyl,
piperazinyl,
morpholinyl, and thiomorpholinyl. The phenyl, the heteroaryl, and the
heterocycloalkyl
groups optionally substituting the aryl groups of the instant invention are
attached to the aryl
groups through either a covalent bond, an alkyl group, an oxygen atom, or a
carbonyl group,
as defined herein. The phenyl, the heteroaryl, and the heterocycloalkyl groups
optionally
substituting the aryl groups of the instant invention can also be further
substituted with one,
two, or three substituents independently selected from the group consisting of
alkyl, alkoxy,
carboxyl, azido, carboxaldehyde, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy.
The term "arylalkyl," as used herein, refers to an aryl group, as defined
herein,
attached to the parent molecular group through an alkyl group, as defined
herein.
The term "arylsulfonyl," as used herein, refers to an aryl group, as defined
herein,
attached to the parent molecular group through a sulfonyl group, as defined
herein.
The term "aryloyl," as used herein, refers to an aryl group, as defined
herein, attached
to the parent molecular group through a carbonyl group, as defined herein.
The term "azido," as used herein, refers to -N3.
The term "carbonyl," as used herein, refers to -C(O)-.
The term "carboxamido," as used herein, refers to an amide; e.g., an amino
group
attached to the parent molecular group through a carbonyl group, as defined
herein.
The term "carboxyl," as used herein, refers to -C02H or a derivative thereof
formed
by replacement of the hydrogen atom thereon by a carboxyl protecting group.
The term "carboxyl protecting group," as used herein, refers to selectively
introducible and removable groups which protect carboxyl groups against
undesirable side
reactions during synthetic procedures and includes all conventional carboxyl
protecting
groups. Examples of carboxyl groups include methyl, ethyl, n-propyl,
isopropyl, 1,1-
dimethylpropyl, n-butyl, tert-butyl, phenyl, naphthyl, benzyl, diphenylmethyl,
triphenylmethyl (trityl), para-nitrobenzyl, para-methoxybenzyl, acetylmethyl,
benzoylmethyl,
3o para-nitrobenzoylmethyl, para-bromobenzoylmethyl, 2-tetrahydropyranyl 2-
tetrahydrofuranyl, 2,2,2-trichloroethyl cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
methoxymethyl, methoxyethoxymethyl, arylalkoxyalkyl benzyloxymethyl 1,1-
dimethyl-2-
propenyl, 3-methyl-3-butenyl, allyl, and the like. Preferred carboxyl
protecting groups of the
instant invention are alkyl and arylalkyl.
The term "cyano," as used herein, refers to -CN.
The term "cycloalkyl," as used herein, refers to a monovalent saturated cyclic
hydrocarbon group of three to seven carbons. The cycloalkyl groups of the
instant invention
-47-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
can be optionally substituted with one, two, three, or four substituents
independently selected
from the group consisting of alkyl, amino, alkoxy, alkoxycarbonyl,
carboxaldehyde,
carboxyl, halo, hydroxyl, phenyl, heteroaryl, heterocycloalkyl, and oxo.
The phenyl, the heteroaryl, and the heterocycloalkyl groups optionally
substituting the
cycloalkyl groups of the instant invention can also be further substituted
with one, two, or
three substituents independently selected from the group consisting of alkyl,
alkoxy,
carboxyl, azido, carboxaldehyde, halo, hydroxyl, perfluoroalkyl, and
perfluoroalkoxy.
The term "cycloalkylalkyl," as used herein, refers to a cycloalkyl group, as
defined
herein, attached to the parent molecular group through an alkyl group, as
defined herein.
The term "cycloalkyloyl," as used herein, refers to a cycloalkyl group, as
defined
herein, attached to the parent molecular group through a carbonyl group, as
defined herein.
The term "cycloalkylsulfonyl," as used herein, refers to a cycloalkyl group,
as defined
herein, attached to the parent molecular group through a sulfonyl group, as
defined herein.
The terms "halo" or "halide," as used herein, refer to F, Cl, Br, or I.
The term "heteroaryl," as used herein, refers to cyclic, aromatic five- and
six-
membered groups, wherein at least one atom is selected from the group
consisting of
nitrogen, oxygen, and sulfur, and the remaining atoms are carbon. The five-
membered rings
have two double bonds, and the six-membered rings have three double bonds.
Heteroaryls of
the instant invention are exemplified by furanyl, thienyl, pyrrolyl, oxazolyl,
thiazolyl,
imidazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, oxadiazolyl, triazolyl,
thiadiazolyl, pyridyl,
pyridazinyl, pyrimidinyl, pyrazinyl, pyrazolyl, triazinyl, and the like. The
heteroaryl groups
of the instant invention are connected to the parent molecular group through a
carbon atom in
the ring or, as exemplified by imidazole and pyrazolyl, through either a
carbon atom or
nitrogen atom in the ring. The heteroaryl groups of the instant invention can
be optionally
substituted with one, two, or three radicals independently selected from the
group consisting
of optionally substituted alkyl, alleenyl, alkynyl, alkoxy, alkanoyl,
alkanoyloxy,
alkoxycarbonyl, alkylsulfonyl, amino, asninosulfonyl, azido, carboxamido,
carboxyl, cyano,
halo, hydroxyalkyl, hydroxyl, nitra, perfluoroalkyl, perfluoroalkoxy, oxo,
thioalkoxy, a
nitrogen protecting group, phenyl, and a heterocycloalkyl selected from the
group consisting
of tetrahydrofuranyl, piperidinyl, piperazinyl, morpholinyl, and
thiomorpholinyl. The phenyl
and the heterocycloalkyl groups optionally substituting the heteroaryl groups
of the instant
invention are attached to the heteroaryl through either a covalent bond, an
alkyl group, an
oxygen, or a carbonyl group, as defined herein. The phenyl and the
heterocycloalkyl groups
optionally substituting the heteroaryl groups of the instant invention can
also be further
substituted with one, two, or three substituents independently selected from
the group
consisting of alkyl, alkoxy, carboxyl, azido, carboxaldehyde, halo, hydroxyl,
perfluoroalkyl,
and perfluoroalkoxy. The heteroaryl groups of the instant invention can also
be fused to a
-48-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
phenyl ring, in which case the heteroaryl group can be connected to the parent
molecular
group through either the heteroaryl part or the phenyl part of the fused ring
system.
Heteroaryl groups of this type are exemplified by quinolinyl, isoquinolinyl,
benzodioxolyl,
benzodioxinyl, and the like.
The term "heteroarylalkyl," as used herein, refers to a heteroaryl group, as
defined
herein, attached to the parent molecular group through an alkyl group, as
defined herein.
The term "heteroaryloyl," as used herein, refers to a heteroaxyl group, as
defined
herein, attached to the parent molecular group through a carbonyl group, as
defined herein.
The term "heteroarylsulfonyl," as used herein, refers to a heteroaryl group,
as defined
i0 herein, attached to the parent molecular group through a sulfonyl group, as
defined herein.
The term "heterocycloalkyl," as used herein, refers to cyclic, non-aromatic,
four-,
five-, six-, or seven-membered groups containing at least one atom selected
from the group
consisting of oxygen, nitrogen, and sulfur. The four-membered rings have zero
double
bonds, the five-membered rings have zero or one double bonds, and the six- and
seven-
membered rings have zero, one, or two double bonds. Heterocycloalkyl groups of
the instant
invention are exemplified by dihydropyridinyl, imidazolinyl, morpholinyl,
piperazinyl,
pyrrolidinyl, pyrazolidinyl, tetrahydropyridinyl, piperidinyl,
thiomorpholinyl, 1,3-dioxolanyl,
1,4-dioxanyl, 1,3-dioxanyl. , The heterocycloalkyl groups of the instant
invention can be
attached through a carbon atom or nitrogen atom in the ring. The
heterocyalkalkyls of the
instant invention can be optionally substituted one, two, or three
substituents independently
selected from the group consisting of optionally substituted alkyl, alkoxy,
alkanoyl,
alkanoyloxy, alkoxycarbonyl, alkylsulfonyl, amino, aminosulfonyl, azido,
carboxamido,
carboxyl, cyano, halo, hydroxyalkyl, hydroxyl, a nitrogen protecting group,
perfJuoroalkyl,
perfluoroalkoxy, oxo, phenyl, and heteroaryl selected from the group
consisting of furanyl,
thienyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, isoxazolyl, isothiazolyl,
oxadiazolyl,
oxadiazolyl, triazolyl, thiadiazolyl, pyridyl, pyridazinyl, pyrimidinyl,
pyrazinyl, pyrazolyl,
and triazinyl. The phenyl and the heteroaryl groups optionally substituting
the
heterocycloalkyl groups of the instant invention can be attached through a
covalent bond, an
alkyl group, an oxygen atom, or a carbonyl group. The phenyl and the
heteroaryl groups
optionally substituting the heterocycloalkyl groups of the instant invention
can also be further
substituted with one, two, or three substituents independently selected from
the group
consisting of alkyl, alkoxy, carboxyl, azido, carboxaldehyde, halo, hydroxyl,
perfluoroalkyl,
and perfluoroalkoxy. The term "heterocycloalkyl" also includes bicyclic groups
in which the
heterocycloalkyl ring is fused to a phenyl group, in which case the
heterocycloalkyl group
can be connected to the parent molecular group through either the
heterocycloalkyl part or
the phenyl part of the fused ring system. Heterocycloalkyl groups of this type
are
-49-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
exemplified by 1,3-benzodioxanyl, 1,3-benzodioxolyl, 2,4-dihydro-2H-1,4-
benzoxazinyl, and
the like.
The term "heterocycloalkylalkyl," as used herein, refers to a hetexocycloalkyl
group,
as defined herein, attached to the parent molecular group through an alkyl
group, as defined
herein.
The term "heterocycloalkyloyl," as used herein, refers to a heterocycloalkyl
group, as
defined herein, attached to the parent molecular group through a carbonyl
group, as defined
herein.
The term "heterocycloalkylsulfonyl," as used herein, refers to a
heterocycloalkyl
group, as defined herein, attached to the parent molecular group through a
sulfonyl group, as
defined herein.
The term "hydroxyalkyl," as used herein, refers to a hydroxyl group attached
to the
parent molecular group through an alkyl group, as defined herein.
The term "hydroxyl," as used herein, refers to -OH or a derivative thereof
formed by
replacement of the hydrogen atom thereon with a hydroxyl protecting group.
The term "hydroxyl protecting group," as used herein, refers to selectively
introducible and removable groups which protect hydroxyl groups against
undesirable side
reactions during synthetic procedures. Examples of hydroxyl protecting groups
include
benzyloxycarbonyl, 4-nitrobenzyloxycarbonyl, 4-bromobenzyloxycarbonyl,
4-methoxybenzyloxycarbonyl, methoxycarbonyl, tent-butoxycarbonyl,
isopropoxycarbonyl,
diphenylmethoxycarbonyl, 2,2,2-trichloroethoxycarbonyl, 2-
(trimethylsilyl)ethoxycarbonyl,
2-furfuryloxycarbonyl, allyloxycarbonyl, acetyl, formyl, chloroacetyl,
trifluoroacetyl,
methoxyacetyl, phenoxyacetyl, benzoyl, methyl, tart-butyl, 2,2,2-
trichloroethyl, 2-
trimethylsilylethyl, 1,1-dimethyl-2-propenyl, 3- methyl-3-butenyl, allyl,
benzyl, para-
methoxybenzyldiphenylmethyl, triphenylmethyl (trityl), tetrahydrofuryl
methoxymethyl,
methylthiomethyl, benzyloxymethyl, 2,2,2-trichloroethoxymethyl, 2-
(trimethylsilyl)ethoxymethyl, methanesulfonyl, para-toluenesulfonyl,
trimethylsilyl,
triethylsilyl, triisopropylsilyl, and the like. Preferred hydroxyl protecting
groups for the
instant invention are acetyl, benzyl (Bn), benzoyl (Bz), and tert-butyl.
The term "oxo," as used herein, refers to a group formed by the replacement of
two
hydrogen atoms on the same carbon atom with a single oxygen atom.
The term "perFluoroalkoxy," as used herein, refers to a perfluoroalkyl group
attached
to the parent group through an oxygen atom.
The term "perfluoroalkyl," as used herein, refers to an alkyl group in which
all of the
hydrogen atoms have been replaced with fluoride atoms.
The compounds of the instant invention can exist as pharmaceutically
acceptable
salts. The term "pharmaceutically acceptable salt," as used herein, refers to
salts or
-50-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
zwitterionic forms of the compounds of the instant invention which are water
or oil-soluble or
dispersible, which are suitable for treatment of diseases without undue
toxicity, irritation, and
allergic response, which are commensurate with a reasonable benefit/risk
ratio, and which are
effective for their intended use. The salts can be prepared during the final
isolation and
purification of the compounds or separately by reacting an amino group with a
suitable acid.
Representative acid addition salts include acetate, adipate, alginate,
citrate, aspartate,
benzoate, benzenesulfonate, bisulfate, butyrate, camphorate, camphorsufonate,
digluconate,
glycerophosphate, hemisulfate, heptanoate, hexanoate, formate, fumarate,
hydrochloride,
hydrobromide, hydroiodide, 2-hydroxyethansulfonate (isethionate), lactate,
maleate,
mesitylenesulfonate, methanesulfonate, naphthylenesulfonate, nicotinate, 2-
naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-
phenylpropionate, picrate,
pivalate, propionate, succinate, tartrate, thiocyanate, trichloroacetic,
trifluoroacetic,
phosphate, glutamate, bicarbonate, pare-toluenesulfonate, and undecanoate.
Also, amino
groups in the compounds of the instant invention can be quaternized with as
methyl, ethyl,
propyl, and butyl chlorides, bromides and iodides; dimethyl, diethyl, dibutyl,
and diamyl
sulfates; decyl, lauryl, myristyl, and stearyl chlorides, bromides, and
iodides; benzyl and
phenethyl bromides. Examples of acids which can be employed to form
pharmaceutically
acceptable acid addition salts include inorganic acids such as hydrochloric,
hydrobromic,
sulphuric, and phosphoric and organic acids such as oxalic, malefic, succinic,
and citric.
2o Basic addition salts can be prepared during the final isolation and
purification of the
compounds by reacting a carboxyl group with a suitable base such as the
hydroxide,
carbonate, or bicarbonate of a metal cation or with ammonia or an organic
primary,
secondary or tertiary amine. Pharmaceutically acceptable salts canons based on
lithium,
sodium, potassium, calcium, magnesium, and aluminum and nontoxic quaternary
ammonia
and amine canons such as ammonium, tetramethylammonium, tetraethylammonium,
methylamine, dimethylamine, trimethylannine, triethylamine, diethylamine,
ethylamine,
tributlyamine, pyridine, N,N-dimethylaniline, N-methylpiperidine, N-
methylmorpholine,
dicyclohexylamine, procaine, dibenzylamine, N,N-dibenzylphenethylamine, 1-
ephenamine,
and N,N'-dibenzylethylenediamine. Other representative organic amines useful
for the
3o formation of base addition salts include ethylenediamine, ethanolamine,
diethanolamine,
piperidine, and piperazine.
The compounds of the instant invention can also exist as pharmaceutically
acceptable
prodrugs. The term "pharmaceutically acceptable prodrug," as used herein,
refers to those
prodrugs of the compounds of the present invention which are, within the scope
of sound
medical judgment, suitable for use in contact with the tissues of humans and
lower animals
with undue toxicity, irritation, allergic response, and the like, commensurate
with a
-51-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
reasonable benefit/risk ratio, and effective for their intended use, as well
as the zwitterionic
forms, where possible, of the compounds of the instant invention.
The term "prodrug," as used herein, represents compounds which are rapidly
transformed ifa vivo to parent compounds of formulas (I)-(XIII), for example,
by hydrolysis in
blood.
The term "substituted alkyl," as used herein, refers to an alkyl group
substituted with
one, two, or three substituents independently selected from the group
consisting of alkoxy,
alkanoyloxy, alkoxycarbonyl, alkoxy, alkoxyalkoxy, amino, carboxaldehyde,
cycloalkyl,
cyano, halo, hydroxyl, oxo, phenyl, heterocycloalkyl, and heteroaryl.
to The term "sulfonyl," as used herein, refers to -SOZ-.
Asymmetric centers exist in the compounds of the instant invention. The
instant
invention contemplates stereoisomers and mixtures thereof. Individual
stereoisomers of
compounds are prepared by synthesis from starting materials containing the
chiral centers or
by preparation of mixtures of enantiomeric products followed by separation
such as
15 conversion to a mixture of diastereomers followed by separation or
recrystallization,
chromatographic techniques, or direct separation of the enantiomers on chiral
chromatographic columns. Starting compounds of particular stereochemistry are
either
commercially available or are made by the methods described below and resolved
by
techniques well-known in the art.
20 Tautomers can exist in the compounds of the instant invention. The instant
invention
contemplates tautomers due to proton shifts from one atom to another atom of
the same
molecule generating two distinct compounds which are in equilibrium with each
other.
The term "tautomer" as used herein refers to a proton shift from one atom of a
molecule to another atom of the same molecule to provide two or more
structurally distinct
25 compounds which are in equilibrium with each other.
According to methods of treatment, the compounds of the instant invention can
be
useful for the prevention of metastases from the tumors described above either
when used
alone or in combination with radiotherapy and/or other chemotherapeutic
treatments
conventionally administered to patients for treating cancer. When using the
compounds of
3o the instant invention for chemotherapy, the specific therapeutically
effective dose level for
any particular patient will depend upon factors such as the disorder being
treated and the
severity of the disorder; the activity of the particular compound used; the
specific
composition employed; the age, body weight, general health, sex, and diet of
the patient; the
time of administration; the route of administration; the rate of excretion of
the compound
35 employed; the duration of treatment; and drugs used in combination with or
coincidently with
the compound used. For example, when used in the treatment of solid tumors,
compounds of
the instant invention can be administered with chemotherapeutic agents such as
alpha
-52-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
inteferon, COMP (cyclophosphamide, vincristine, methotrexate, and prednisone),
etoposide,
mBACOD (methortrexate, bleomycin, doxorubicin, cyclophosphannide, vincristine,
and
dexamethasone), PRO-MACE/MOPP (prednisone, methotrexate (wlleucovin rescue),
doxorubicin, cyclophosphamide, taxol, etoposide/mechlorethamine, vincristine,
prednisone,
and procarbazine), vincristine, vinblastine, angioinhibins, TNP-470, pentosan
polysulfate,
platelet factor 4, angiostatin, LM-609, SU-101, CM-101, Techgalan,
thalidomide, SP-PG, and
the like. For example, a tumor may be treated conventionally with surgery,
radiation or
chemotherapy and a compound of the instant invention with subsequent compound
adminsteration of the compound to extend the dormancy of micrometastases and
to stabilize
to and inhibit the growth of any residual primary tumor.
The compounds of the instant invention can be administered orally,
parenterally,
osmotically (nasal sprays), rectally, vaginally, or topically in unit dosage
formulations
containing carriers, adjuvants, diluents, vehicles, or combinations thereof.
The term
"parenteral" includes infusion as well as subcutaneous, intravenous,
intramuscular, and
15 intrasternal injection.
Parenterally adminstered aqueous or oleaginous suspensions of the compounds of
the
instant invention can be formulated with dispersing, wetting, or suspending
agents. The
injectable preparation can also be an injectable solution or suspension in a
diluent or solvent.
Among the acceptable diluents or solvents employed are water, saline, Ringer's
solution,
2o buffers, dilute acids or bases, dilute annino acid solutions,
monoglycerides, diglycerides, fatty
acids such as oleic acid, and fixed oils such as monoglycerides or
diglycerides.
The chernotherapeutic effect of parenterally administered compounds can be
prolonged by slowing their absorption. One way to slow the absorption of a
particular
compound is adminstering injectable depot forms comprising suspensions of
crystalline,
25 amorphous, or otherwise water-insoluble forms of the compound. The rate of
absorption of
the compound is dependent on its rate of dissolution which is, in turn,
dependent on its
physical state. Another way to slow absorption of a particular compound is
administering
injectable depot forms comprising the compound as an oleaginous solution or
suspension.
Yet another way to slow absorption of a particular compound is administering
injectable
3o depot forms comprising microcapsule matrices of the compound trapped within
liposomes,
microemulsions, or biodegradable polymers such as polylactide-polyglycolide,
polyorthoesters or polyanhydrides. Depending on the ratio of drug to polymer
and the
composition of the polymer, the rate of drug release can be controlled.
Transdermal patches also provide controlled delivery of the compounds. The
rata of
35 absorption can be slowed by using rate controlling membranes or by trapping
the compound
within a polymer matrix or gel. Conversely, absorption enhancers can be used
to increase
absorption.
-53-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Solid dosage forms for oral administration include capsules, tablets, pills,
powders,
and granules. In these solid dosage forms, the active compound can optionally
comprise
diluents such as sucrose, lactose, starch, talc, silicic acid, aluminum
hydroxide, calcium
silicates, polyamide powder, tableting lubricants, and tableting aids such as
magnesium
stearate or microcrystalline cellulose. Capsules, tablets and pills can also
comprise buffering
agents; and tablets and pills can be prepared with enteric coatings or other
release-
controlling coatings. Powders and sprays can also contain excipients such as
talc, silicic
acid, aluminum hydroxide, calcium silicate, polyamide powder, or mixtures
thereof. Sprays
can additionally contain customary propellants such as
chlorofluorohydrocarbons or
1o substitutes therefor.
Liquid dosage forms for oral administration include emulsions, microemulsions,
solutions, suspensions, syrups, and elixirs comprising inert diluents such as
water. These
compositions can also comprise adjuvants such as wetting, emulsifying,
suspending,
sweetening, flavoring, and perfuming agents.
15 Topical dosage forms include ointments, pastes, creams, lotions, gels,
powders,
solutions, sprays, inhalants, and transdermal patches. The compound is mixed
under sterile
conditions with a carrier and any needed preservatives or buffers. These
dosage forms can
also include excipients such as animal and vegetable fats, oils, waxes,
paraffins, starch,
tragacanth, cellulose derivatives, polyethylene glycols, silicones,
bentonites, silicic acid, talc
2o and zinc oxide, or mixtures thereof. Suppositories for rectal or vaginal
administration can be
prepared by mixing the compounds of the instant invention with a suitable
nonirritating
excipient such as cocoa butter or polyethylene glycol, each of which is solid
at ordinary
temperature but fluid in the rectum or vagina. Ophthalmic formulations
comprising eye
drops, eye ointments, powders, and solutions are also contemplated as being
within the scope
25 of the instant invention.
The total daily dose of the compounds of the instant invention administered to
a host
in single or divided doses can be in amounts from about 0.1 to about 200 mg/kg
body weight
or preferably from about 0.25 to about 100 mg/kg body weight. Single dose
compositions
can contain these amounts or submultiples thereof to make up the daily dose.
Determination of Biological Activitx
Farnesyltransferase Inhibition
Farnesyltransferase (FTase) or geranylgeranyltransferase I (GGTase I)
fractions were
isolated from bovine brains and purified by a series of methods which separate
FTase from
GGTase I and GGTase I from GGTase II. The methods involved a partial
purification of all
three enzymes by precipitation from a beef brain homogenate with 30% to 50%
saturated
-54-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
(NH4)2S04 followed by chromatography on DEAE Sepharose. A Hydrophobic
Interaction
Chromatography (HIC) media, Fractogel-Phenyl (EM Industries) was used to
separate FTase
from GGTase; and chromatography of each enzyme on MonoQ (Pharmacia) resulted
in
further purification of the enzymes. The catalytic purity of each enzyme was
assayed
separately with substrate acceptor proteins specific for that enzyme.
After quickly freezing in liquid nitrogen, the various prenyl transferases
were stored at
-80 °C.
Bovine FTase was assayed at 37 °C for 30 minutes in a volume of 100 ~L
containing
44 mM HEPES, pH 7.4, 26 mM MgCl2, 4.4 mM DTT, 18 mM KCI, 0.009% Triton X-100,
l0 256 nM [3H]-farnesyl pyrophosphate, triammonium salt ([3H]-FPP, 759
GBqlmmol, New
England Nuclear), 100 nM biotin-K-ras peptide (American Peptide Company), and
FTase
(12.5 ~,g/mL total protein). Reactions are initiated by the addition of FTase
and stopped by
the addition of 75 ~,L of a 1.43 mg/mL suspension of streptavidin SPA
(Scintillation
Proximity Assay) beads (Amersham) in 0.2M sodium phosphate, pH 4, containing
1.5M
15 MgCl2, 0.5% BSA and 0.05% sodium azide. The quenched reactions stood for 1
hour before
analysis in a Packard TopCount scintillation counter. Purified compounds were
dissolved in
100% ethanol and diluted 10-fold into the assay. The percent inhibition of the
compounds of
the instant invention at 10 6 M was then measured.
The percent inhibition of representative compounds of the instant invention
are shown
2o in Table 1.
-55-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Table 1
Inhibitory Potencies of Representative Compounds
Example % Inhibition Example % Inhibition
at at
10 6 M 10 6 M
4 88 142 94
91 143 97
6 61 144 96
7 92 145 97
8 100 146 93
9 64 147 96
94 148 95
11 94 149 93
12 100 150 93
13 100 151 94
14 100 152 89
100 153 92
16 100 154 95
17 100 155 92
I8 I00 156 92
19 100 157 97
23 I00 158 95
24 100 159 91
100 160 96
26 100 161 96
27 100 162 94
28 100 163 84
29 100 164 99
~ 100 168 96
31 100 169 70
32 93 170 85
33 100 171 75
34 100 172 85
100 173 80
36 100 175 75
37 100 177 77
-56-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
38 100 178 90
39 100 179 93
40 100 180 95
41 100 181 93
42 100 182 89
43 100 183 90
44 100 184 95
45 100 I85 85
46 100 186 85
47 100 187 93
48 100 188 92
49 62 189 95
50 100 190 90
51 100 191 99
52 100 193 91
53 100 194 97
54 100 195 98
55 100 196 90
56 100 197 93
57 100 198 94
58 100 199 89
59 100 200 99
60 96 201 99
61 100 202 92
62 100 203 86
63 100 204 96
64 100 205 90
65 100 206 88
66 100 207 91
67 100 208 95
68 100 209 93
69 100 210 48
70 100 211 30
71 100 212 10
-57-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
72 100 213 41
73 100 214 20
I
74 100 215 29
75 100 216 27
76 100 217 71
77 100 225 50
80 12 226 100
81 14 227 100
82 87 228 100
85 46 229 100
86 86 230 100
87 89 231 100
90 90 232 100
91 94 233 100
92 53 234 100
93 58 235 100
100 83 236 93
105 82 237 100
109 100 240 95
110 96 241 98
111 100 242 98
112 100 243 82
113 90 244 90
114 99 245 90
115 89 246 90
116 99 247 95
117 90 248 98
118 90 249 98
119 93 250 99
120 90 251 99
121 96 256 94
122 94 260 90
123 97 262 100
-58-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
124 ~ 90 263 100
125 97 264 100
126 92 265 100
127 93 266 100
128 94 267 100
129 93 268 100
130 97 269 100
131 95 270 100
132 94 271 100
133 97 273 100
134 93 274 100
135 94 275 100
136 95 276 100
137 96 277 95
138 92 278 99
139 97 279 99
140 94 280 98
141 95 281 98
289 75
Representative compounds of the instant invention were also tested for
cardiovascular
liability (see Journal of Cardiovascular Pharmacology, 607-618: 37 (2001 )).
Example 291
was shown to possess an improved electrophysiological profile.
As shown by the data in Table 1, the compounds of the instant invention,
including
but not limited to those specified in the examples, are useful for the
treatment of diseased
caused or exascerbated by farnesyltransferase. As farnesyltransferase
inhibitors, these
compounds are useful in the treatment of both primary and metastatic solid
tumors and
carcinomas of the breast; colon; rectum; lung; oropharynx; hypopharynx;
esophagus;
stomach; pancreas; liver; gallbladder; bile ducts; small intestine; urinary
tract (kidney,
bladder, and urothelium); female genital tract (cervix, uterus, and ovaries);
male genital tract
(prostate, seminal vesicles, and testes); endocrine glands (thyroid, adrenal,
and pituitary);
skin (hemangiomas, melanomas, and sarcomas); tumors of the brain, nerves, and
eyes;
meninges (astrocytomas, gliomas, glioblastomas, retinoblastomas, neuromas,
neuroblastomas, and meningiomas); solid tumors arising from hematopoietic
malignancies
(leukemias and chloromas); plasmacytomas; plaques; tumors of mycosis
fungoides;
cutaneous T-cell lymphoma/leukemia; lymphomas including Hodgkin's and non-
Hodgkin's
-59-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
lymphomas; prophylaxis of autoimmune diseases (rheumatoid, immune and
degenerative
arthritis); ocular diseases (diabetic retinopathy, retinopathy of prematurity,
corneal graft
rejection, retrolental fibroplasia, neovascular glaucoma, rubeosis, retinal
neovascularization
due to macular degeneration, and hypoxia); skin diseases (psoriasis,
hemagiomas and
capillary proliferation within atherosclerotic plaques).
Synthetic Methods
The compounds and processes of the instant invention will be better understood
in
connection with the following synthetic schemes which illustrate methods by
which the
compounds can be prepared. The compounds of the instant invention can be
prepared by a
variety of synthetic routes. Representative procedures are shown below in
Schemes 1-19.
The rou s a b c A1 Ll LZ Ml Qi Q2 Ra Rb Rl R2 R3 R4. Rs W W' X X' Y Y' Z
g p > > > > > > > > > > > > > > > > > > > > > >
and Z' are defined above, and the groups Mlp, Qlp, and Q2p are defined below.
It will be
readily apparent to one of ordinary skill in the art that the compounds can be
synthesized by
substitution of the appropriate reactants and agents in the syntheses shown
below. It will also
be apparent to one skilled in the art that the selective protection and
deprotection steps, as
well as the order of the steps themselves, can be carried out in varying
order, depending on
the nature of a b c A1 Ll L2 Ml Ql Q2 Ra Rb R1 R2 R3 R4 RS W W' X X' Y Y' Z
> > > > > > > > > > > > > > > > > > > > > >
and Z' to successfully complete the syntheses of compounds of the instant
invention.
2o Abbreviations which have been used in the descriptions of the schemes and
the
examples that follow are: OAc for acetate; PyBop for benzotriazol-1-yl-oxy-
tris-
(pyrrolidino)phosphoniumhexafluorophosphate; DMAP for 4-(N,N-
dimethylamino)pyridine;
DME for dimethoxyethane; DMF for N,N-dimethylformamide; DMSO for
dimethylsulfoxide; EDC for 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide
hydrochloride; HOBt for 1-hydroxybenzotriazole hydrate; HPLC for high pressure
liquid
chromatography; LDA for lithium diisopropylamide; MTBE for methyl tert-butyl
ether; TEA
for triethylamine; TFA for trifluoroacetic acid; and THF for tetrahydrofuran.
-60-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 1
Re RB
~Yll Z . ~Y~Z
p ~ X,W ~L2~OH
RA H Ra
(1)
R1
CN
1
M ~ L1 \
(3)
R1
RB
CN
.W L2 ~O~ L1 \
RA
formula (II)
As shown in Scheme l, compounds of formula (1) can be converted to compounds
of
formula (2), wherein L2 is optionally substituted alkylene, by treatment of
the former with an
organometallic nucleophile in a solvent such as THF, dioxane, MTBE, or diethyl
ether.
Representative organometallic nucleophiles include Crrignard reagents,
organolithium
reagents, organozinc reagents, and organocadmium reagents. The reaction
temperature is
about -78 °C to about 35 °C and depends on the method chosen.
Reaction times are typically
about 0.5 to about 4 hours. Compounds of formula (1) can be converted to
compounds of
formula (2), wherein L2 is alkylene, by treatment of the former with a
reducing agent in a
solvent such as THF, dioxane, or diethyl ether. Representative reducing agents
include
LiAlH4 and NaBH4. The reaction temperature is about -78 °C to about 35
°C and depends on
the method chosen. Reaction times are typically about 0.5 to about 4 hours.
Compounds of
formula (2) can be converted to compounds of formula (II) by treatment of the
former with
compounds of formula (3), wherein Mlp is an Ml precursor such as halo, in the
presence of
silver(I) oxide in a solvent such as dichloromethane, carbon tetrachloride, or
chloroform. The
reaction temperature is about 20 °C to about 40 °C and depends
on the method chosen.
Reaction times are typically about 6 to about 48 hours.
-61-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 2
R1
R1
R4-NH2 / CN
H (~) R4 I
HN~L1
(4) (6) RB
,Y' Z
X ~ O
1N
RA H
(1) R1
RB
CN
,Y.~ Z Ra. I
X.W~~2.N~~1
IA
R formula (II)
As shown in Scheme 2, compounds of formula (4) can be converted to compounds
of
formula (6) by treatment of the former with compounds of formula (5) in the
presence of a
reducing agent such as sodium triacetoxyborohydride, sodium cyanoborohydride,
sodium
borohydride, or borane-pyridine in a solvent such as 1,~-dichloroethane,
dichloromethane,
chloroform, or carbon tetrachloride. The reaction temperature is about 0
°C to about
40 °C and depends on the method chosen. Reaction times are typically
about 6 to about 24
hours. Compounds of formula (6) can be converted to compounds of formula (II)
by
condensation of the former with compounds of formula (1) as described in
Scheme 1.
Scheme 3
Rs Ri Re Ri
~Y/Z ~y ,Z / CN
X' 1
wJ + H X ~ ~I
w
RA
R OH
(4) formula (III)
As shown in Scheme 3, compounds of formula (7) can be converted to compounds
of
formula (III) by sequential treatment of the former with a base such as tert-
butyllithium, n-
butyllithium, and lithium hexamethyldisilazide and compounds of formula (4) in
a solvent
-62-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
RB Ri
such as THF, MTBE, or diethyl ether. The reaction temperature is about -78
°C to about 0 °C
and depends on the method chosen. Reaction times are typically about 0.5 to
about 2 hours.
Compounds of formula (III) can be oxidized to compounds of formula (ITIa) by
treatment of
the same with an oxidizing agent such as manganese dioxide, potassium
permanganate,
potassium dichromate, or Jones reagent in a solvent such as dioxane, acetone,
THF, or
dichloromethane. The reaction temperature is about 0 °C to about 100
°C and depends on the
method chosen. Reaction times are typically about 0.5 to about 12 hours.
Cnhama d
RB Ri
x Yr~z / CN 2 Q1 p
Q
w \ + I
3
RA OH
formula (III) (8)
CN
x I \
w
RA O
Q2
R3
formula (IV)
As shown in Scheme 4, compounds of formula (III) can be treated with compounds
of
formula (8), wherein Qlp is a Ql precursor such as halo, under the conditions
described in
Scheme 1 to provide compounds of formula (IV).
-63-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 5
Rm-t ~ B R1
R
x Y~Z ~ CN ~Y~Z / CN
inn ~. I x, 1 ~
w
~A
R O RA NH2
formula (XIV) (9)
O
2~H
O
R3
(10)
RB R1
~y~Z / CN
x I ~ I
w
RA 2,NR4
Q
R3
formula (IV)
As shown in Scheme 5, compounds of formula (XIV) can be converted to compounds
of formula (9) by sequential treatment of the former with a chlorinating agent
such as SOCl2,
PPh3/CC14, PCl$, or PPh3lNCS and ammonium hydroxide in a solvent such as
dichloromethane, carbon tetrachloride, or chloroform. The reaction temperature
is about -10
°C to about 25 °C and depends on the method chosen. Reaction
times are typically about 1 to
about 12. hours. Conversion of compounds of formula (9) to compounds of
formula (IV) can
be accomplished by the methods described in Scheme ~.
l0
-64-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 6
Re Ri Re Ri
CN
CN X Y~ Z
/I
iN w w w
RA OH RA CI
formula (III) (11)
,SH
Q2
R3
(12)
RB R1
,Y/ _Z /
w
~A
R _S(O)t
R3
formula (IV)
As shown in Scheme 6, compounds of formula (III) can be converted to compounds
of formula (11) by 'treatment of the former with the chlorinating agent in a
solvent such as
dichloromethane, carbon tetrachloride, or chloroform. The reaction temperature
is about -10
°C to about 25 °C and depends on the method chosen. Reaction
times are typically about 1 to
about 12 hours. Conversion of compounds of formula (11) to compounds of
formula (IV),
wherein t is 0, can be accomplished by treatment of the former with compounds
of formula
(12) in the presence of a base such as triethylamine, diisopropylethylamine,
or pyridine in a
l0 solvent such as dichloromethane, carbon tetrachloride, or chloroform. The
reaction
temperature is about 20 °C to about 35 °C and depends on the
method chosen. Reaction .
times are typically about 12 to about 24 hours. Conversion of compounds of
formula (IV),
wherein t is 0, to compounds of formula (IV), wherein, t is 1 or 2, can be
accomplished by
treatment of the former with an oxidizing agent such as m-CPBA, hydrogen
peroxide, NaI04,
and NaOCI in a solvent such as dichloromethane, carbon tetrachloride, and
chloroform. The
reaction temperature is about 20 °C to about 40 °C and depends
on the method chosen.
Reaction times are typically about 12 to about 72 hours.
-65-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 7
RB R1
CN O
X Y~Z / ~S
~O CI
RA N H2 R3
(g) (13)
RB R1
X y~Z / CN
V11
RA NH
O SAO
Q2
~3
R
formula (IV)
As shown in Scheme 7, compounds of formula (9) can be converted to compounds
of
formula (IV) by treatment of the former with compounds of formula (13) in the
presence of a
base such as DMAP, triethylamine, diisopropylethylamine, pyridine, or mixtures
thereof in a
solvent such as dichloromethane, chloroform, or carbon tetrachloride. The
reaction
temperature is about 20 °C to about 40 °C and depends on the
method chosen. Reaction
times are typically about 6 hours to about 24 hours.
to Scheme 8
RB R1 RB Ri
~Y~z / CN ~y~Z / CN
X1N/ I ~ I + hi-R3 X / I \
RA CI RA Rs
(11) (14)
formula (IV)
As shown in Scheme 8, compounds of formula (11) can be converted to compounds
of formula (IV) by treatment of the former with compounds of formula (14),
wherein R3 is an
alcohol, thiol, or a primary or secondary amine, in the presence of a base
such as
-66-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
diisopropylethylamine, pyridine, or triethylamine in a solvent~such as
dichloromethane,
carbon tetrachloride, or chloroform. The reaction temperature is about 30
°C to about 100 °C
and depends on the method chosen. Reaction times are typically about 1 to
about 12 hours.
Scheme 9
Rs Ri Re R1
y ~ ,. CN ~Y~z ~. CN
W + Rs W ~ a
RA O RA Q2 OH
R3
formula (XIV) (15)
formula (V)
As shown in Scheme 9, compounds of formula (15), wherein Q2p is an alkynyl Q2
precursor, can be treated sequentially with a base such as tent-butyllithium,
n-butyllithium,
LDA, or lithium hexamethyldisilazide and compounds of formula (XIV) to provide
to compounds of formula (V), wherein Q2 is alkynylene, in a solvent such as
THF, MTBE,
dioxane, or diethyl ether. The reaction temperature is about -78 °C to
about 25 °C and
depends on the method chosen. Reaction times are typically about 0.5 to about
24 hours.
Compounds of formula (V), wherein Q2 is alkynyl, can be intraconverted to
compounds of
formula (V), wherein Q2 is alkylene or alkenylene, by hydrogenation in the
presence of
15 palladium catalysts such as Pd/BaSO4, Pd/CaC03, and PdIC in a solvent such
as methanol,
ethanol, or isopropanol. The reaction temperature is about 25 °C to
about 40 °C and depends
on the method chosen. Reaction times are typically about 2 to about 32 hours.
-67-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme l n
Ra, RB Ra~ Re
a ' W
X~ ~
X ~Y, 1 ~ ~ ~Y,~L2.OH
H
(16) (17) Ri
CN
1
M ~ L1 \
(3)
R1
RA, RB
CN
Xw ,~ .Ow \
Y L2 L1
formula (VI)
As shown in Scheme 10, compounds of formula (16) can be converted to compounds
of formula (17) and subsequently to compounds of formula (VI) by the methods
described in
Scheme 1.
-68-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 11
R1
R
N CN
H + R-NH2 - -'- HN4 \
. L1
(5)
RA RB
'
W' Z'
X~sY~ ~ O
(I6) H
Ra Rs R1
' CN
W j~Z' R~.
Y ~2- .~i
formula (VI)
As shown in Scheme I1, compounds of formula (4) can be converted to compounds
of formula (6) and subsequently to compounds of formula (VI) by the methods
described in
Scheme 2.
R1
Scheme 12
RA, RB N RA, RB R1
W
~~/Z' ~--I , iN ~I
x°,, , J + X,Y,
Y
(18) (4) formula (V1I)
As shown in Scheme 12, compounds of formula (18) can be converted to compounds
of formula (VII) by the methods described in Scheme 3.
-69-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 13
1
Ra° RB R1 RA~ RB R
,
W° Z, / CN ~;Nj~z~ /
X~ ---~. X ~~ I I
~\ I \ I Y, \
Y' ~ ~
Q1
OH (CH2)a
formula (IIIa) Rs
formula (VIII)
As shown in Scheme 13, compounds of formula (IIIa) can be converted to
compounds
of formula (VIII) by the methods described in Schemes 4 through 8.
Scheme 14
RA' RB R' RA, RB H .
~W~Z, / CN W~Z, / CN
XvY>/ I \ ~ + Q2p -.~ X~\Y~/ I \ I
R3
O Q2 OH
R3
formula (VII) (15)
formula (IX)
As shown in Scheme 14, compounds of formula (VII) can be converted to
compounds
of formula (IX) by treatment with compounds of formula (15) under the
conditions described
in Scheme 9.
-70-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 15
Ra Re
Y~X O Y~X
+ V ---> ~ N OH
(19) (20) (21) Ri
CN
i
M ~Li \
(3)
Ri
RB
CN
Y X I
~N O~L.i \
formula (X)
As shown in Scheme 15, compounds of formula (19) can be reacted with oxirane
to
provide compounds of formula (21), wherein b is 2, which can be converted to
compounds of
formula (X) by treatment with compounds of formula (3) under the conditions
described in
Scheme 1.
-71-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 16
RB RB
Y~X Y~X H
OH ~ ~N O
b c
(21 ) (22)
R1
CN
R4
HN~~i \
(6)
R1
R
CN
Y~.X R4
~N N~Li \
formula (X)
As shown in Scheme 16, compounds of formula (21) can be converted to compounds
of formula (22) by treatment with an oxidizing agent such as Dess-Martin
periodinane,
MnO2, PCC, and K2Cr2O7 in a solvent such as these reactions include
dichloromethane,
chloroform, and carbon tetrachloride. The reaction temperature is about 0
°C to about 35 °C
and depends on the method chosen. Reaction times are typically about 0.5 to
about 12 hours.
Compounds of formula (22) can be condensed with compounds of formula (6) to
provide
compounds of formula (X) using the conditions described in Scheme 2.
_72_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 17
R~ R1
CN N
H
O
(4) (23)
RB
Y~X
~NH
Re (19)
Y1LX OH
N ~ R1
~ /
CN
formula (XI)
As shown in Scheme 17, compounds of formula (4) can be converted to compounds
of formula (23) by treatment of the former with a sulfonium ylide such as
trimethylsulfonium
iodide in the presence of a base such as potassium hydroxide or sodium
hydroxide in a
solvent such as DMSO, DMF, or mixtures thereof. The reaction temperature is
about 25 °C
to about 80 °C and depends on the method chosen. Reaction times are
typically about 1 to
about 6 hours. Compounds of formula (23) can be converted to compounds of
formula
compounds of formula (XI) by treatment of the former with catalytic base such
as DMAP,
1o pyridine, or diisopropylethylamine and compounds of formula (19) in
solvents such as
methanol, ethanol, or isopropanol. The reaction temperature is about 35
°C to about 100 °C
and depends on the method chosen. Reaction times are typically about 2 to
about 24 hours.
Scheme 1 R
RB
RB Rs R1
Y~X OH ~ 2 CN
\ R1 Y~X Q /
-----.~ ~N
CN ~c
formula (XI) formula (XII)
As shown in Scheme 18, compounds of formula (XI) can be converted to compounds
of formula (XII) using the conditions described in Schemes 4 through 8.
-73-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Scheme 19
RB RB R3
Y~X O Y~X Q2 OH
~N \ Ri Q2 ~N \ Ri
'I' 13
CN R ~ CN
formula (XI) (15) formula (XIII)
As shown in Scheme 19, compounds of formula (XI) can be converted to compounds
of formula (XIII) by treatment of the former with compounds of formula (15)
under the
conditions described in Scheme 9.
The instant invention will now be described in connection with other
particularly
preferred embodiments of Schemes 1-19, which are not intended to limit its
scope. On the
contrary, the instant invention covers all alternatives, modifications, and
equivalents which
are included within the scope of the claims. Thus, the following examples will
illustrate an
especially preferred practice of the instant nvention, it being understood
that the examples are
for the purposes of illustration of certain preferred embodiments and are
presented to provide
what is believed to be the most useful and readily understood description of
its procedures
and conceptual aspects.
It will be evident to one skilled in the art that the instant invention is not
limited to the
forgoing examples, and that it can be embodied in other specific forms without
departing
from the essential attributes thereof. Thus, it is desired that the examples
be considered as
illustrative and not restrictive, reference being made to the claims, and that
all changes which
come within the meaning and range of equivalency of the claims be embraced
therein.
Example 1
5-((benzylox'r)(6-fluoro-2'meth~(1,1'-biphen l~~)methyl)-1-methyl-1H-imidazole
hydrochloride
Example 1A
6-fluoro-2'-methyl( 1,1'-biphenyl)-3-carbaldehyde
A mixture of 3-bromo-4-fluorobenzaldehyde (1.1 g, 5.9 mmol),
2-methylphenylboronic acid (9.05 mg, 6.6 mmol), palladium(II) acetate (23 mg,
6.6 mmol),
2M Na2C03 (14 mL), and triphenylphosphine (102 mg, 0.39 mmol) in toluene (13
mL) was
heated to 100 °C for 90 minutes with vigorous stirring, and cooled to
room temperature to
-74-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
provide two separate layers. The organic layer was concentrated, and the
concentrate was
purified by flash column chromatography on silica gel with 95:5/hexanes:ethyl
acetate to
provide the desired product.
MS (DCI/NH3) m/z 214 (M+H)+ and 232 (M+NHq.)~;
1H NMR (300 MHz, CDCl3) 810.0 (s, 1H), 8.95 (m, 1H), 8.83 (dd, 1H), 7.40-7.15
(m, 5H),
2.2 (s~ 3H).
Example 1B
(6-fluoro-2'-meth~(1,1'-biphen lY)-3-yl)(1-methyl-1H-imidazol-5-yl)methanol
A solution of Example 87F (471.3 mg, 2.4 mmol) in THF (5 mL) at -75
°C was
treated with 1.7M tert-butyllithium in pentane (1.7 mL, 2.88 mmol), stirred
for 15 minutes,
treated with Example 1A (514 mg, 2.4 mmol) in THF (5 mL), stirred for 1 hour,
warmed to 0
°C for 20 minutes, treated sequentially with methanol (3 mL) and 1M
tetrabutylammonium
fluoride in THF (2.4 mL, 2.4 mmol), warmed to room temperature, stirred for 18
hours,
poured into water (50 mL), and extracted with ethyl acetate. The extract was
washed
sequentially with saturated NaHC03, water, and brine, dried (Na2SOq.),
filtered, and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 96.5:2.5:1 to 89:10:1 ethyl acetate/methanol/ concentrated ammonium
hydroxide to
provide the desired product.
2o MS (DCIlNH3) m!z 297 (M+H)~;
1H NMR (300 MHz, CD30D) ~ 7.6 (s, 1H), 7.45 (m, 1H), 7.35-7.10 (m, 5H), 6.55
(s, 1H),
5.90 (s, 1H), 3.65 (s, 3H), 2.15 (s, 3H), 1.90 (s; 1H).
Example 1C
5-((benz loxy)(6-fluoro-2'methyl(1,1'-biphen l~yl)methyl)-1-methyl-1H-
imidazole
hydrochloride
A solution of Example 1B (133 mg, 0.45 mmol) in DMF (1 mL) at -3 °C was
treated
with a 60% oily sodium hydride (28 mg, 0.68 mmol), stirred for 1 hour, treated
with
(bromomethyl)benzene (60 p.L, 0.5 mmol), stirred for 18 hours at room
temperature, treated
with water, and extracted with ethyl acetate. The extract was washed with
brine, dried
(Na2S04), filtered, and concentrated. The concentrate was purified by
preparative HPLC
with 4:1/CH3CN:0.1% aqueous TFA to 0.1% aqueous TFA. The appropriate fractions
were
combined and concentrated. The concentrate was treated with saturated NaHC03,
and the
resulting solution was extracted with ethyl acetate. The extract was dried
(Na~S04), filtered,
and concentrated. The concentrate was dissolved in 4M HCl in dioxane (2 mL),
and the
resulting solution was stirred for 2 hours and concentrated. This concentrate
was dissolved in
water and lyophilized to provide the desired product.
-75-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (ESI(+)) m/z 387 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.1 (s, 1H), 7.6-7.2 (m, 12H), 5.95 (s, 1H), 4.55
(q, 2H),
3.75 (s, 3H), 2.15 (s, 3H);
Anal. calcd for Ca5H24CIFN20~1.25H20: C, 67.41; H, 6.00; N, 6.29. Found: C,
67.48; H,
s 5.85; N 6.13.
Example 2
benzyl (2'-methyl(1,1'-biphen ly_)-3-yl)(1-methyl-1H-imidazol-5-yl)methyl
ether,
l~drochloride
Example 2A
2'-methyl(1,1'-biphenyl)-3-carbaldehyde
The desired product was prepared by substituting 3-bromobenzaldehyde for
3-bromo-4-fluorobenzaldehyde in Example 1A.
is MS (DCI/NH3) m/z 214 (M+NH4)+;
1H NMR (300 MHz, CDC13) 810.1 (s, 1H), 7.85 (m, 2H), 7.6 (m, 2H), 7.35-7.2
(rn, 4H),
2.25 (s, 3H).
Example 2B
(2'-methyl( l,1'-biphenyl)-3-yl)(1-meth-1H-irnidazol-5-yl)methanol
The desired product was prepared by substituting Example 2A for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 279 (M+I~~;
1H NMR (300 MHz, CD30D) 8 7.6 (s, 1H), 7.5-7.1 (m, 7H), 6.55 (s, 1H), 5.95 (s,
1H), 3.7 (s,
3H), 2.2 (s, 3H).
Example 2C
benzyl (2'-methyl(1,1'-biphenyl)-3-yl)(1-methyl-1H-imidazol-5-yl)methyl ether
l~drochloride
The desired product was prepared by substituting Example 2B for Example 1B in
Example 1C, and purified by flash column chromatography on silica gel with
95:5:0.1/ethyl
acetate:methanol:concentrated ammonium hydroxide. The appropriate fractions
were
concentrated, and the concentrate was dissolved in 4M HCl in dioxane (1.5 mL),
stirred for 3
hours, and concentrated. The concentrate was treated with water and
lyophilized to provide
the desired product.
MS (ESI(+)) m/z 369 (M+H)+;
-76-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 9.1 (s, 1H), 7.8-7.2 (m, 13H), 5.95 (s, 1H), 4.6
(q, 2H),
3.75 (s, 3H), 2.2 (s, 3H);
Anal. calcd for C2~HZSC1N20~ 1.35H20: C, 69.95; H, 6.50; N, 6.53. Found: C,
69.89, H,
6.23; N, 6.78.
Example 3
5-((benzyloxy)(6-chloro-2'-methyl(1,1'-biphen~)-3- 1)~yl)-1-meth~lH-imidazole
hydrochloride
to Example 3A
4-chloro-3-iodobenzoic acid
A solution of 3-amino-4-chlorobenzoic acid (8.6 g, 50 mmol) in 2:1
3M HCl/acetone (150 mL) at -3 °C was treated dropwise with sodium
nitrite (3.8 g, 55 mmol)
in water (30 mL), stirred for 30 minutes, treated with potassium iodide (14.5
g, 87.5 mmol) in
water (50 mL), stirred for 15 minutes at 0 °C and at room temperature
for 2 hours, and treated
with water (500 mL) and excess NaHC03 to provide a solid. The solid was
collected by
filtration and recrystalized from 20% methanol/water to provide the desired
product.
MS (DCI/NH3) m/z 282 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 813.35 (br s, 1H), 8.4 (d, 1H), 7.95 (dd, 1H), 7.7
(d, 1H).
Example 3B
4-chloro-3-iodo-N-methoxy-N-methylbenzamide
A mixture of Example 3A (2.82 g, 10 mmol), EDC (2.1I g, 11 mmol), HOBt (1.68
g,
11 mmol), and N,O-dimethylhydroxylamine hydrochloride (1.26 g, 13 mmol) in DMF
(30
mL) was stirred until all of the reagents dissolved, treated with
triethylamine (2.54 mL, 18
mmol), stirred for 3 days at room temperature, treated with 1:1/ethyl
acetate:water, stirred for
1 hour, poured into water, and extracted with ethyl acetate. The extract was
washed
sequentially with 2M Na2C03, water, and brine, dried (Na2S04), filtered, and
concentrated.
The concentrate was purified by flash column chromatography on silica gel with
3:1/hexanes:ethyl acetate to provide the desired product.
MS (DCI/NH3) m/z 343 (M+NH4)+;
IH NMR (300 MHz, CDC13) ~ 8.2 (d, 1H), 7.65 (dd, 1H), 7.5 (s, 1H), 3.55 (s,
3H), 3.35 (s,
3H).
Example 3C
6-chloro-N-methoxy-N 2'-dimethyl~l 1'-biphenyl)-3-carboxamide
-77-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A mixture of Example 3B (2.61 g, 8.02 mmol), 2-methylphenylboronic acid (1.20
g,
8.82 mmol), (1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium (II) (196
mg, 0.24
mmol), CsF (2.44 g, 16.14 mmol), and DME (40 mL) was heated to reflux for 18
hours,
cooled to room temperature, treated with diethyl ether, filtered through
diatomaceous earth
(Celite~), and concentrated. The concentrate was purified by flash column
chromatography
on silica gel with 7:3/hexanes:ethyl acetate to provide the desired product.
MS (DCI/NH3) m/z 307 (M+NH4)~;
1H NMR (300 MHz, CDC13) 8 7.7-7.1 (m, 7H), 3.55 (s, 3H), 3.35 (s, 3H), 2.1 (s,
3H).
to Example 3D
6-chloro-2'-methyl(1,1'-biphenyl)-3-carbaldehyde
A solution of Example 3C (1.167 g, 4 mmol) in THF (10 mL) at -10 °C was
treated
dropwise with 1M lithium aluminum hydride in THF (4.4 mL, 3.3 mmol), stirred
for 2 hours,
treated sequentially with THFlwater (1 mL:0.17 mL), 4M NaOH (0.17 mL), and
water (0.5
mL), warmed to room temperature, and extracted with 1:1/ethyl acetate:hexanes.
The extract
was dried (Na2S04), filtered through a pad of silica gel, and concentrated to
provide material
of sufficient purity for subsequent use without further purification.
MS (DCI/NH3) m/z 230 (M+H)+;
1H NMR (300 MHz, CDC13) 810.0 (s, 1H), 7.9-7.1 (m, 7H), 2.1 (s, 3H).
Example 3E
(6-chloro-2'-methyl(1,1'-biphen l~~)(1-methyl-1H-imidazol-5-yl)methanol
The desired product was prepared by substituting Example 3D for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 313 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.5-7.0 (m, 7H), 6.7 (s, 1H), 5.9 (s, 1H), 3.6 (d,
3H), 2.1 (d,
3H).
Example 3F
5-((benzyloxy)(6-chloro-2'-methyl(1,1'-biphen 1~-3-yl)methyl)-1-methyl-1H-
imidazole
hydrochloride
The desired product was prepared by substituting Example 3E for Example 1B in
Example 1C.
MS (ESI(+)) m/z 403 (M+H)~;
1H NMR (300 MHz, DMSO-d6) b 9.1 (s, 1H), 7.7 (dd, 1H), 7.5 (dt, 1H), 7.4-7.1
(m, 10H),
5.95 (s, 1H), 4.6 (q, 2H), 3.75 (d, 3H), 2.1 (s, 3H);
_7g_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Anal. calcd for CZSH24C12N20~ 1.05H20: C, 65.52; H, 5.74; N, 6.11. Found: C,
65.49; H,
5.77; N, 6.18.
Example 4
2'-methyl-5-((1-methyl-1H-imidazol-5-~phenoxy)methyl)(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
Example 4A
S-(hydroxy( 1-methyl-1 H-imidazol-5-yl)methyl)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile
to The desired product was prepared by substituting Example 86I for Example 1A
in
Example 1B. .
MS (DCI/NH3) m/z 304 (M+H)+;
1H NMR (300 MHz, CD30D) ~ 7.85 (dd, 1H), 7.6-7.1 (m, 6H), 6.55 (s, 1H), 6.0
(s, 1H), 3.7
(s, 3H).
Example 4B
2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)(phenoxy)methyl)( 1,1'-biphenyl)-2-
carbonitrile
A solution of Example 4A (106 mg, 0.35 mmol), phenol (38.5 mg, 0.35 mmol), and
triphenylphosphine (139.2 mg, 0.525 mmol) in THF (2 mL) was treated with
diethyl
azodicarboxylate (90 ~L, 0.525 mmol), stirred for 24 hours, poured into water,
and extracted
with ethyl acetate. The extract was washed with brine, dried (NaZS04),
filtered, and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 96:5:2.5:1/ethyl acetate:methanol:concentrated ammonium hydroxide to
provide the
desired product.
MS (ESI(+)) m/z 380 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.8-6.9 (m, 12H), 6.7 (s, 1H), 6.35 (s, 1H), 3.6 (s,
3H).
Example 4C
2'-methyl-5-(( 1-methyl-1 H-imidazol-5-~Lphenoxy)methyl) ( 1,1'-biphenyl)-2-
carbonitrile
3o hydrochloride
A solution of Example 4B (82 mg ) in 4M HCl in dioxane (2 mL) was stirred for
2
hours and concentrated. The concentrate was treated with water (2 mL) and
lyophilized. The
product was purified by HPLC with continuous 20% to 100%:0.1%TFA/water: CH3CN.
The
appropriate fractions were combined, adjusted to pH 7-8 with NaHC03, and
extracted with
ethyl acetate. The extract was dried (NaS04), filtered, and concentrated. The
concentrate was
dissolved in 4M HCl in dioxane (0.5 mL), stirred for 3 hours, and
concentrated. The
concentrate was treated with water and lyophilized to provide the desired
product.
-79-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (ESI(+)) m/z 380 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 9.I (s, 1H), 8.05 (d, 2H), 7.75-6.95 (m, I3H), 3.8
(s, 3H),
3.55 (s, 1H), 2.0 (s, 3H);
Anal. calcd for C25H22C1N30~2.6H20: C, 64.76; H, 5.93; N, 9.06. Found: C,
64.90; H, 5.40;
N, 7.60.
Example 5
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)meth~-2'-methoxy( 1,1'-biphenyl)-2-
carbonitrile
hydrochloride
Example 5A
ethyl 6-cyano-2'-methoxy( 1,1'-biphenyl)-3-carboxylate
The desired product was prepared by substituting ethyl 3-bromo-4-cyanabenzoate
and
2-methoxyphenylboronic acid fox 3-bromo-4-fluorobenzaldehyde and
2-methylphenylboronic acid, respectively, in Example 1A.
MS (DCI/NH3) m/z 299 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 8.1 (m, 2H), 7.8 (d, 1H), 7.45 (m, 1H), 7.25 (dd,
1H), 7.05 (m,
2H), 4.4 (q, 2H), 3.8 (s, 3H), 1.4 (t, 3H).
Example 5B
5-(h~~ ethyl)-2'-methoxy(l,l'-biphenyl)-2-carbonitrile
A solution of Example 5A (389 mg, I.38 mmol) in THF (3 mL) was treated
sequentially with calcium chloride (312 mg, 2.76 mmol), absolute ethanol (4
mL), and
sodium borohydride (209 mg, 5.52 mmol), stirred for 48 hours, treated with
water (1 mL) and
2M HCl (2 mL) to break up any solid, and extracted with diethyl ether. The
extract was
washed with brine, dried (Na2S0~), filtered, and concentrated. The product was
purified by
flash column chromatography on silica gel with 1:1 ethyllacetate:hexanes to
provide the
desired product.
MS (DCI/NH3) m/z 357 (M+NH4)+;
1H NMR (300 MHz, CD30D) 8 7.75 (d, 1H), 7.45 (m, 3H), 7.25 (dd, 1H), 7.15-7.0
(m, 2H),
4.7 (s, 2H), 3.8 (s, 3H).
Exam 1p a 5C
5-formyl-2'-rnethoxy( I ,1'-biphenyl)-2-c arbonitrile
A solution of oxalyl chloride (0.25 mL, 2.76 mmol) in dichloromethane (2 mL)
at
-78 °C was treated with DMSO (0.4 mL, 5.52 mmol), stirred for 20
minutes, treated with
Example 5B (331 mg, 1.38 mmol) in dichloromethane (3 mL), stirred for 3 hours
at
_g0_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
-78 °C, treated with triethylamine (0.77 mL, 5.52 mmol), warmed to room
temperature,
poured into diethyl ether (20 mL), washed sequentially with water, saturated
NaHC03, water,
and brine, dried (Na2S04), filtered, and concentrated to provide material of
sufficient purity
for subsequent use without further purification.
MS (DCI/NH3) m/z 255 (M+NH4)+;
1H NMR (300 MHz, CDCl3) ~ 10.1 (s, 1H), 7.95 (m, 3H), 7.45 (dt, 1H), 7.3 (dd,
1H), 7.15-
7.0 (m, 2H), 3.85 (s, 3H).
Example 5D
l0 5-(h day(1-methyl-1H-imidazol-5-yl)methyl)-2'-method(1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 5C for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 320 (M+H)+;
1H NMR (300 MHz, CD30D) 8 7.8 (d, 1H), 7.7-7.4 (m, 3H), 7.3-6.9 (m, 3H), 6.6
(s, 1H), 6.0
15 (s, 1H), 3.8 (s, 3H), 3.7 (s, 3H).
Example 5E
5-((benzyloxy) ( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methoxy( 1,1'-biphenyl)-
2-carbonitrile
hydrochloride
2o A mixture of Example 5D (113 mg, 0.35 mmol), silver(I) oxide (91 mg, 0.39
mmol),
(bromomethyl)benzene (0.05 mL, 0.42 mmol), and dichloromethane (15 mL) was
stirred for
36 hours in darkness, filtered through a pad of diatomaceous earth (Celite~),
and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 95:5:1/ethyl acetate:methanol:concentrated ammonium hydroxide. The
appropriate
25 fractions were concentrated, and the concentrate was dissolved in 4M HCl in
dioxane (1 mL),
stirred for 3 hours, and concentrated. The concentrate was treated with water
and lyophilized
to provide the desired product.
MS (ESI(+)) m/z 410 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.0 (s, 1H), 8.0-7.0 (m, 12H), 6.0 (s, 1H), 4.55
(q, 2H),
30 3.75 (s, 3H), 3.65 (s, 3H).
Example 6
5-((benz~y)(1-methyl-1H-imidazol-5-yl)meth l~phenyl)(l,l'-biphenyl)-2-
carbonitrile
hydrochloride
Example 6A
3-~dihydroxyboryl)-1,1'-biphenyl
-81-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of 1.7M tent-butyllithium in pentane (12.6 mL, 21.5 mmol) in
diethyl ether
(65 mL) at -78 °C was treated with 3-bromo-1,1'-biphenyl (2 g, 8.6
nnmol) in diethyl ether
(20 mL), stirred for 1 hour, treated with triisopropylborate (5 mL, 21.5
mmol), warmed to
room temperature over 1 hour, poured into 2M NaOH (200 mL), stirred for 15
minutes,
cooled, adjusted to pH 1 with concentrated HCl, and extracted with diethyl
ether and ethyl
acetate. The extract was washed with brine, dried (Na2SO4), filtered, and
concentrated to
provide material of sufficient purity for subsequent use without further
purification.
MS (DCI/NH3) m/z 198 (M+H)+.
to Example 6B
eth~yano-3'-(phenyl) ( 1,1'-biphen~)-3-carboxyl ate
'The desired product was prepared by substituting 3-bromo-4-cyanoethylbenzoate
and
Example 6A for 3-bromo-4-fluorobenzaldehyde and 2-methoxyphenylboronic acid,
respectively, in Example 1A, and purified by flash column chromatography on
silica gel with
15 95:5/hexanes:ethyl acetate.
MS (DCI/NH3) m/z 345 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 8.25 (d, 1H), 7.9-7.3 (m, 10H), 4.45 (q, 2H), 1.4
(t, 3H).
Example 6C
2o 5-(hydroxymethyl)-3'-(phenxl (1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 6B for Example 5A in
Example 5B. t
MS (DCI/NH3) m/z 303 (M+NH4)+;
1H NMR (300 MHz, CD30D) 8 8.1-7.3 (m, 12H), 4.8 (s, 2H).
Example 6D
5-formyl-3'-(phenyl)( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 6C for Example 5B in
Example 5C, and purified by flash column chromatography on silica gel with
7:3/hexanes:ethyl acetate.
MS (DCI/NH3) m/z 301 (M+NH4)+;
1H NMR (300 MHz, CDCl3) S 10.15 (s, 1H), 8.1-7.35 (m, 12H).
Example 6E
5-(h dery(1-methyl-1H-imidazol-5-yl)methyl~phenyl)(1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 6D for Example 1A in
Example 1B.
_82_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDC13) S 7.8-7.3 (m, 12H), 6.75 (s, 1H), 6.0 (s, 1H), 3.6 (s,
3H), 3.4-3.0
(br s, 1H).
Example 6F
5-((benzyloxy)(1-methyl-1H-imidazol-5-y~meth l~)-3'-(phenyl)(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 6E for Example 5D in
Example 5E.
MS (ESI(+)) m/z 456 (M+H)+;
l0 1H NMR (300 MHz, DMSO-d6) 8 9.1 (s, 1H), 8.1-7.3 (m, 12H), 6.1 (s, 1H), 4.6
(q, 2H), 3.8
(s, 3H);
Anal. calcd for C31H26C1N3O~2.3H2O: C, 69.56; H, 5.80; N, 7.85. Found: C,
69,43; H, 5.50;
N, 8.32.
Example 7
(2-(9-anthryl)-4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile h
d~loride
Example 7A
9-anthrylboronic acid
2o The desired product was prepared by substituting 9-bromoanthracene for
3-bromo-l,l'-biphenyl in Example 6A, and purified by flash column
chromatography on
silica gel with 9:1/hexanes:ethyl acetate to 7:3/hexanes:ethyl acetate.
MS (DCI/NH3) m/z 268 (M+2NH4)+.
Example 7B
ethyl3-(9-anthr l~yanobenzoate
The desired product was prepared by substituting ethyl 3-bromo-4-cyanobenzoate
and
Example 7A for 3-bromo-4-fluorobenzaldehyde and 2-methoxyphenylboronic acid,
respectively, in Example 1A, and purified by flash column chromatography on
silica gel with
9:1/hexanes:ethyl acetate.
MS (DCI/NH3) mlz 369 (M+NH4)+;
1H NMR (300 MHz, CDC13) S 8.6 (s, 1H), 8.35 (dd, 1H), 8.25-7.95 (m, 3H), 7.85-
7.3 (m,
6H), 4.4 (q, 2H), 1.4 (t, 3H).
Example 7C
2-(9-anthr 1y )-4-(hydrox.~yl)benzonitrile
-83-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 7B for Example 5A in
Example 5B.
MS (DCI/NH3) m/z 327 (M+NH4)+;
1H NMR (300 MHz, CD30D) 8 8.65 (s, 1H), 8.17 (s, 1H), 8.12 (s, 1H), 8.0 (d,
1H), 7.8-7.6
(m, 2H), 7.6-7.4 (m, 6H), 4.8 (s, 2H).
Example 7D
2-(9-anthryl)-4-formylbenzonitrile
The desired product was prepared by substituting Example 7C for Example 5B in
1o Example 5C, and purified by flash column chromatography on silica gel with
7:3/hexanes:ethyl acetate.
MS (DCI/NH3) m/z 325 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 810.2 (s, 1H), 8.15 (s, 1H), 8.3-7.8 (m, 5H), 7.6-7.35
(m, 6H).
Example 7E
2-(9-anthryl)-4-(hydroxy(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
The desired product was prepared by substituting Example 7D for Example 1A in
Example 1B and purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:methanol:concentrated ammonium hydroxide.
2o MS (DCI/NH3) m/z 390 (M+H)+.
Example 7F
2-(9-anthrvl)-4-((benzvloxv)(1-methyl-1H-imidazol-5-vl)methvllbenzonitrile
hydrochloride
The desired product was prepared by substituting Example 7E and
dichloromethane
for Example 1B and DMF, respectively, in Example 1C.
MS (ESI(+)) m/z 480 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.8 (s, 1H), 8.3-8.2 (m, 2H), 7.9 (dd, 1H), 7.7-
7.3 (m,
14H), 6.1 (s, 1H), 4.65 (q, 2H), 3.8 (s, 3H).
3o Example 8
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methxl)-2'-isopropyl( 1,1'-bi~henyl)-
2-carbonitrile
hydrochloride
Example 8A
2-isopro~ylphenylboronic acid
A mixture of magnesium (720 mg, 30 mmol) and diethyl ether (15 mL) was treated
with a small aliquot of 1-bromo-2-isopropylbenzene, stirred for 30 minutes,
treated dropwise
-84-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
with 2-bromoisopropylbenzene (4.978 g, 25 rnmol) in diethyl ether (10 mL),
stirred at reflux
for 1 hour, cooled to room temperature, added to a solution of
triisopropylborate (6.4 mL,
27.5 mmol) in diethyl ether (15 mL ) at -78 °C, warmed to room
temperature, treated with
4M NaOH (10 mL), stirred for 10 minutes, poured into water, washed with
diethyl ether,
adjusted to pH 1 with concentrated HCl, and extracted with diethyl ether. The
extract was
dried (Na2S0~), filtered, and concentrated to provide material of sufficient
purity for
subsequent use without further purification.
MS (DCI/NH3) m/z 182 (M+NH4)+.
to Example 8B
eth~yano-2'-isopropyl(1,1'-biphenyl)-3-carboxvlate
The desired product was prepared by substituting ethyl 3-bromo-4-cyanobenzoate
and
Example 8A for 3-bromo-4-fluorobenzaldehyde and 2-methoxyphenylboronic acid,
respectively, in Example 1A. and purified by flash column chromatography on
silica gel with
9:1/hexanes:ethyl acetate.
MS (DCI/NH3) m/z 311 (M+NH4)~;
1H NMR (300 MHz, CDCl3) 8 8.1 (dd, 1H), 8.05 (d, 1H), 7.8 (d, 1H), 7.48 (s,
1H), 7.42 (s,
1H), 7.25 (m, 1H), 7.15 (d, 1H), 4.4 (q, 2H), 2.7 (sept., 1H), 1.4 (t, 3H),
1.25 (dd, 6H).
2o Exam 1p a 8C
5-(hydroxymethyl)-2'-isopropyl( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 8B for Example 5A in
Example 5B.
MS (DCI/NH3) mlz 269 (M+NH4)~;
1H NMR (300 MHz, CD30D) s 7.8 (d,lH), 7.6-7.35 (m, 4H), 7.25 (dt, 1H), 7.1 (d,
1H), 4.7
(s, 2H), 2.7 (sept., 1H), 1.15 (dd, 6H).
Example 8D
5-form 1-2'-isopropyl(1,1'-biphe~l)-2-carbonitrile
The desired product was prepared by substituting Example 8C for Example 5B in
3o Example 5C.
MS (DCI/NH3) m/z 267 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 810.1 (s, 1H), 8.0-7.8 (m, 3H), 7.48 (s, 1H), 7.45 (s,
1H), 7.3
(m, 1H), 7.15 (d, 1H), 2.7 (sept., 1H), 1.2 (dd, 6H).
Exam 1p e~8E
5-(hydroxy(1-methyl-1H-imidazol-5-yl)methyl)-2'-iso~ropyl(1 1'-biphenyl)-2-
carbonitrile
-85-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 8D for Example 1A in
Example 1B, and purified by flash column chromatography on silica gel with
95:5:llethyl
acetate:methanol:concentrated ammonium hydroxide.
MS (DCI/NH3) m/z 332 (M+H)+;
1H NMR (300 MHz, CDC13) ~ 7.8-6.9 (m, 7H), 6.75 (s, 1H), 6.0 (s, 1H), 3.6 (s,
3H), 2.7
(sept., 1H), 1.1 (dd, 6H).
Example 8F
5-((benzvloxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-isopropyl-1,~ 1' biphenyl)-2-
carbonitrile
1o hydrochloride
The desired product was prepared by substituting Example 8E for Example 5D in
Example 5E, and purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:methanol:concentrated ammonium hydroxide.
MS (ESI(+)) m/z 422 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.1 (s, 1H), 8.05 (dd, 1H), 7.7-7.1 (m, 11H), 6.1
(s, 1H),
4.6 (q, 2H), 3.75 (s, 3H), 2.65 (sept., 1H), 1.05 (dd, 6H);
Anal. calcd for C~8H2sC1N30~0.85H20: C, 71.05; H, 6.32; N, 8.88. Found: C,
71.15; H,
6.36; N, 8.01.
Example 9
4-((benzyloxy)(1-methyl-1H-imidazol-5- 1)y meth)-2-(1,2-dihydro-5
acenapht~lenyl)benzonitrile hydrochloride
Example 9A
1,2-dihydro-5-acenaphthylenylboronic acid
The desired product was prepared by substituting
5-bromo-1,2-dihydroacenaphthylene for 3-bromo-1,1'-biphenyl in Example 6A.
MS (DCI/NH3) m/z 216 (M+NH4)+;
1H NMR (300 MHz, CD30D) 8 7.55 (dd, 2H), 7.45 (t, 1H), 7.25 (dd, 2H), 3.4 (s,
4H).
Example 9B
eth~yano-3-( 1,2-dihydro-5-acenaphth ,~lenyl)benzoate
The desired product was prepared by substituting ethyl 3-bromo-4-cyanobenzoate
and
Example 9A for 3-bromo-4-fluorobenzaldehyde and 2-methoxyphenylboronic acid,
respectively, in Example 1A, and purified by flash column chromatography on
silica gel with
3:1/hexanes:ethyl acetate.
MS (DCI/NH3) mlz 345 (M+NH4)+;
-86-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDC13) 8 8.25 (d, 1H), 8.15 (dd, 1H), 7.9 (d, 1H), 7.5-7.25
(m, 5H), 4.4
(q, 2H), 3.5 (s, 4H), 1.4 (t, 3H).
Example 9C
2-(1,2-dihydro-5-acenaphth~yl)-4-(hydroxymethyl)benzonitrile
The desired product was prepared by substituting Example 9B for Example 5A in
Example 5B.
MS (DCI/NH3) m/z 303 (M+NHq.)+;
1H NMR (300 MHz, CD30D) 8 7.6 (t, 2H), 7.45-7.25 (m, 5H), 7.35 (d, 1H), 4.75
(s, 2H),
3.45 (s, 4H).
Example 9D
2-( 1,2-dihydro-5-acenaphthylenyl)-4-formylbenzonitrile
The desired product was prepared by substituting Example 9C for Example 5B in
Example 5C, and purified by flash column chromatography on silica gel with
7:3/hexanes:ethyl acetate.
MS (DCI/NH3) m/z 301 (M+NH4.)+;
1H NMR (300 MHz, CDC13) 810.15 (s, 1H), 8.1 (d, 1H), 8.0 (t, 2H), 7.5-7.25 (m,
5H), 3.45
(s, 4H).
Example 9E
2-(1,2-dihydro-5-acenaphth~rlen~ -4-(hydroxy(1-meth~rl-1H-imidazol-5
y~methyl)benzonitrile
The desired product was prepared by substituting Example 9D fox Example 1A in
Example 1B, and purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:methanol:concentrated ammonium hydroxide.
MS (DCI/NH3) m/z 366 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.85 (d, 1H), 7.65 (s, 1H), 7.55 (m,1H), 7.5-7.3 (m,
5H), 6.75
(s, 1H), 6.0 (s, 1H), 3.7 (s, 1H), 3.6 (s, 3H), 3.45 (s, 4H).
Example 9F
4-((benzyloxy)(1-methyl-1H-imidazol-5- 1)~ methyl)-2 X1,2-dihydro-5
acenaphthylenyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 9E for Example 5D in
Example 5E, and purified by flash column chromatography on silica gel with
95:5:llethyl
acetate:methanol:concentrated ammonium hydroxide.
MS (ESI(+)) m/z 456 (M+H)+;
_g7_
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 9.I (s, 1H), 8.2-7.2 (m, 13H), 6.1 (s, 1H), 4.6
(q, 2H), 3.8
(s, 3H), 3.5 (s, 4H);
Anal. calcd for C31H26C1N30~ 1.5H20: C, 71.61; H, 5.64; N, 8.08. Found: C,
71.62; H, 5.35;
N, 8.26.
Example 10
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-chloro( 1,1'-biphenyl)-2-
carbonitrile
hydrochloride
Example 10A
2'-chloro-6-(methox carbonyl)(1,1'-biphenyl)-3-carboxylic acid
The desired product was prepared by substituting dimethyl 2-iodoterephthalate
and 2-
chlorophenylboronic acid and for Example 3B and 2-methylphenylboronic acid,
respectively,
in Example 3C to provide material of sufficient purity for use without further
purification.
Example lOB
2'-chloro-6-(methoxycarbonyl)(1,1'-biphenyl)-3-carboxylic acid
A solution of Example 10A in THF (100 mL) was treated with 1M LiOH (33 mL),
stirred for 4 days, concentrated, treated with water, and adjusted to pH 1
with 4M HCl to
precipitate a first crop of desired product. This first crop was
recrystallized from 1:1
ethanol/water and filtered. The filtrate was concentrated to remove the
ethanol and extracted
with ethyl acetate. The extract was washed water and brine, dried (Na2S04),
filtered, and
concentrated to provide a second crop material of sufficient purity for
subsequent use without
further purification.
1H NMR (300 MHz, CDCI3) 8 8.2 (dd, 1H), 8.1 (d, 1H), 8.05 (d, IH), 7.45 (m,
1H), 7.35 (m,
2H), 7.25 (m, 1H), 3.7 (s, 3H).
Example 10C
methyl2'-chloro-5-(h drox ri~yl)(1,1'-biphenyl)-2-carboxylate
3o A solution of Example lOB (6.29 g, 21.64 mmol) in THF (30 mL) at 0
°C was treated
with lOM borane~dimethylsulfide in THF (4.4 mL, 43.28 mmol), stirred for 24
hours, treated
with additional borane~dimethylsulfide (2 mL), stirred for 24 hours, treated
dropwise with
4:1/THF:water (25 mL), stirred for 1 hour, and treated with 3M HCl (50 mL) to
form two
separate layers. The layers were separated, and the aqueous layer was
extracted with ethyl
acetate. The extract was washed sequentially with 2M Na2C03, water, and brine,
dried
(Na2SOq.), filtered, and concentrated. The product was purified by flash
column
_88-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
chromatography on silica gel with 3:1 to 3:2/hexanes:ethyl acetate to provide
the desired
product.
MS (DCI/NH3) m/z 294 (M+NH4.)+.
Exam 1p a 10D
methyl 2'-chloro-5-formyl( 1,1'-biphen~)-2-c arboxylate
The desired product was prepared by substituting Example lOC for Example 5B in
Example 5C, and purified by flash column chromatography on silica gel with
75:25/hexanes:ethyl acetate.
1o MS (DCI/NH3) m/z 292 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) S 10.1 (s, 1H), 8.15 (dd, 1H), 8.0 (dd, 2H), 7.8 (d,
1H), 7.5 (m,
1H), 7.35 (m, 1H), 3.7 (s, 3H).
Example 10E
methyl2'-chloro-5-(hydroxy(1-methyl-1H-imidazol-5~ 1)y methyl)(1,1'-biphenyl)-
2-
carbo~late
The desired product was prepared by substituting Example lOD for Example 1A in
Example 1B, and purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:methanol:concentrated ammonium hydroxide.
2o MS (DCI/NH3) m/z 356 (M+H)+;
1H NMR (300 MHz, CD30D) S 8.0 (dd, 1H), 7.6-6.9 (m, 6H), 6.6 (s, 1H), 6.0 (s,
1H), 3.65
(s, 3H), 3.6 (s, 3H).
Exam 1p a lOF
methyl5-((benzyloxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-chloro(1 1'-biphen 1
carboxylate
The desired product was prepared by substituting Example 10E for Example 5D in
Example 5E, and purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:methanol:concentrated ammonium hydroxide. ,
3o MS (DCI/NH3) m/z 447 (M+H)+;
1H NMR (300 MHz, CDC13) ~ 8.05 (dd, 1H), 7.6-7.1 (m, 11H), 6.9 (s, 1H), 5.6
(s, 1H), 4.55
(s, 2H), 3.65 (s, 3H), 3.45 (s, 3H).
Example 10G
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-chloro(11'-biphenyl)-2-
carboxylic
acid
-89-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 10F (835 mg, 1.87 mmol) in methanol (10 mL) was treated
with 4M NaOH, heated to reflux for 4 hours, cooled, concentrated, poured into
0.5M H3P04
in diethyl ether, and extracted with 4:10/chloroform:isopropyl alcohol. The
extract was dried
(Na2S04), filtered, and concentrated to provide material of sufficient purity
for subsequent
use without further purification.
MS (DCI/NH3) m/z 433 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.1 (s, 1H), 7.7-7.2 (m, 11H), 6.85 (s, 1H), 5.6 (s,
1H), 4.55 (s,
2H), 3.45 (s, 3H).
l0 Example lOH
5-((benzyloxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-chloro(1,1'-biphenyl)-2-
carboxamide
A slurry of Example lOG (794 mg, 1.83 mmol), EDC (385 mg, 2.01 mmol), and
HOBt (271 mg, 2.01 mmol) in DMF (4 mL) was stirred until a clear solution
resulted, treated
with concentrated ammonium hydroxide (0.62 mL, 9.15 mmol), stirred for 24
hours, treated
15 with ethyl acetate, washed sequentially with 0.5M H3P04, saturated NaHC03,
and brine,
dried (Na2SOq.), filtered, and concentrated to provide the desired product of
sufficient purity
for subsequent use without further purification.
1H NMR (300 MHz, CD30D) S 7.95 (s, 1H), 7.8-7.2 (m, 11H), 6.65 (s, 1H), 5.75
(s, 1H),
4.55 (s, 2H), 3.55 (s, 3H), 3.0 (s, 1H), 2.85 (s, 1H).
Example 10I
5-((benzyloxy)(1-methyl-1H-imidazol-5-)methyl)-2'-chloro( 1,1'-biphenyl)-2-
carbonitrile
hydrochloride
A solution of Example lOH (519 mg, 1.20 mmol) in THF (2.5 mL) at 0 °C
was treated
with triethylamine (1 mL, 7.08 mmol), stirred for 10 minutes, treated with
trifluoroacetic
anhydride (0.5 mL, 3.60 mmol), stirred for 40 minutes, warmed to room
temperature, stirred
for 1 hour, poured onto ice, treated with concentrated ammonium hydroxide /THF
until a
clear solution formed, poured into water, and extracted with diethyl ether.
The extract was
washed with brine, and the washes were back-extracted with diethyl ether. The
extract was
3o dried (Na2S04), filtered, and concentrated. The product was purified by
flash column
chromatography on silica gel with 95:5:1/ethyl acetate:methanol:concentrated
ammonium
hydroxide. The appropriate fractions were concentrated, and the concentrate
was dissolved in
4M HCl in dioxane (1 mL), stirred for 3 hours, and concentrated. The
concentrate was
treated with water and lyophilized to provide the desired product.
MS (ESI(+)) m/z 414 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.2 (s, 1H), 8.1 (d 1H), 7.8-7.45 (m, 5H), 7.4-7.2
(m, 6H),
6.1 (s, 1H), 4.6 (q, 2H), 3.75 (s, 3H);
-90-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Anal. calcd for C25H21C12N30~0.7H20~0.35TFA: C, 61.38; H, 4.56; N, 8.36.
Found: C,
61.47; H, 4.62; N, 8.09.
Example 11
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 4A for Example 1B in
Example 1C.
MS (ESI(+)) m/z 394 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.05 (s, 1H), 8.05 ( d, 1H), 7.7 (dd, 1H), (d,1H),
7.4-7.2
(m, 1H), 6.05 (s, 1H), 4.6 (q, 2H), 3.75 (s, 3H), 2.15 (s, 3H);
Anal. calcd for C26H24C1N3O~O.75H2O: C, 70.42; H, 5.80; N, 9.48. Found: C,
70.44; H,
5.86; N, 8.90.
Example 12
(2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((4-nitrobenzyl)amino)methyl)( 1,1'-
biphenyl)-2
carbonitrile
Example 12A
5-(amino(1-methyl-1H-imidazol-5-vl)methyl)-2'-methyl(l.l'-binhenvll-2-
carbonitrile
A suspension of Example 4A (0.3 g, 1.0 mmol) in dichloromethane (3 mL) was
cooled to 0 °C, treated with a solution of thionyl chloride (240 mg,
2.0 mmol) in
dichloromethane (2 mL), stirred 30 minutes, warmed to room temperature,
stirred 4 hours,
cooled to 0 °C, treated with concentrated ammonium hydroxide (5 mL),
warmed to room
temperature, stirred for 16 hours, and concentrated. The concentrate was
treated with ethyl
acetate, washed with water and brine, dried (Na2S04), filtered, and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
9:lldichloromethane:methanol to provide the desired product.
Example 12B
(2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((4-nitrobenzyl)amino)methyl)( 1,1'-
biphenyl)-2
carbonitrile
A solution of Example 12A (100 mg, 0.33 mmol) in 1,2-dichloroethane (2 mL) was
treated with 4-nitrobenzaldehyde (94 mg, 0.62 mmol) and acetic acid (150 mg,
2.5 mmol),
stirred for 30 minutes, treated with sodium triacetoxyborohydride (265 mg,
1.25 mmol), and
stirred for 16 hours. The mixture was diluted with ethyl acetate, washed
sequentially with
saturated NaHC03, water, and brine, dried (MgS04), filtered, and concentrated.
The
-91-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
concentrate was treated with dichloromethane (5 mL) and a solution of 4M HCl
in dioxane (1
mL), stirred for 30 minutes, and concentrated. The concentrate was treated
with ethyl
acetate, washed sequentially with saturated NaHC03, water, and brine, dried
(MgS04),
filtered, and concentrated. The concentrate was purified by flash column
chromatography on
silica gel with dichloromethane then 98:2/dichloromethane: methanol to provide
the desired
product.
MS (ESI(+)) m/z 438 (M+H)+;
1H NMR (300 MHz, CDC13) S 8.20 (d, 2H), 7.78 (d, 1H), 7.50-7.29 (m, 8H), 7.20-
7.17 (m,
1H), 6.88 (s, 1H), 4.95 (s, 1H), 3.89 (abq, 2H), 3.53 (s, 3H), 2.I7 (s, 3H),
2.05 (s, 1H).
to
Example 13
4-(((4-cyanobenzyl~amino)( 1-methyl-1H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile
dihydrochloride
15 Exam 1p a 13A
4-amino 1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile
A suspension of Example 89D (0.5 g, 1.48 mmol) in dichloromethane (10 mL) at
0 °C was treated with thionyl chloride (0.65 mL, 8.85 mmol), stirred
for 30 minutes, warmed
to room temperature, stirred for 1.5 hours, and concentrated. The concentrate
was treated
2o with dichloromethane (3 mL), and the resulting solution was added to a
solution of
concentrated ammonium hydroxide (10 mL) at 0 °C. This solution was
stirred for 30
minutes, warmed to room temperature, stirred for 2 hours, and concentrated.
The concentrate
was treated with ethyl acetate, washed with water and brine, dried (Na2SO4),
filtered, and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
25 with 9:1/dichloromethane:methanol to provide the desired product.
MS (ESI(+)) m/z 339 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.95 (dd, 2H), 7.84 (d, 1H), 7.57-7.42 (m, 8H), 6.86
(d, 1H),
5.32 (d, 1H), 3.59 (d, 3H).
30 Example 13B
4-(((4-cvanobenzvl)amino)(1-methyl-1H-imidazol-5-vl)methyl)-2-(1-
nanhthvl)benzonitrile
dihydrochloride
The desired product was prepared by substituting 4-formylbenzonitrile and
Example
13A for 4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
35 concentrate was dissolved in dichloromethane, treated with 1M HCl in
diethyl ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 454 (M+H)+;
-92-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (400 MHz, CDC13) 8 7.96-7.93 (m, 2H), 7.83 (d, 1H), 7.60-7.38 (m, 12H),
6.89-
6.88 (m, 1H), 4.96 (d, 1H), 3.90-3.80 (m, 2H), 3.53 (d, 3H).
Example 14
4-((c clohexylmethoxy)(1-methyl-1H-imidazol-5- 1)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
A suspension of Example 89D (68 mg, 0.2 mmol) in dichloromethane (4 mL) at
0 °C was treated with thionyl chloride (48 mg, 0.4 mmol), stirred for
30 minutes, warmed to
room temperature, stirred for 1.5 hours, treated with cyclohexylmethanol and
N,N-
to diisopropylethylamine, warmed to 35 °C, stirred for 16 hours, and
concentrated. The
concentrate was treated with ethyl acetate, washed with water and brine, dried
(MgS04),
filtered, and concentrated. The concentrate was purified by flash column
chromatography on
silica gel with dichloromethane then 98:2/dichloromethane:methanol, treated
with
dichloromethane and 1M HCl in diethyl ether, and concentrated to provide the
desired
product.
MS (ESI(+)) m/z 436 (M+H)+;
1H NMR (500 MHz, CDC13) 8 7.98-7.91 (m, 2H), 7.84-7.82 (m, 1H), 7.60-7.40 (m,
8H), 6.81
(d, 1H),'5.50 (d, 1H), 3.52 (d, 3H), 3.29 (d, 2H), 1.77-1.63 (m, 6H), 1.27-
1.10 (m, 3H), 0.99-
0.92 (m, 2H).
Example 15
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)(3-oxo-4-(3-(trifluoromethoxy)phenyl)-
1
piperazinyl)methyl)(1,1'-biphenyl)-2-carbonitri1e dihydrochloride
Example 15A
tert-butyl 2-oxoethylcarbamate
A solution of tent-butyl allylcarbamate (5.0 g, 31 mmol) in dichloromethane
(200 mL)
and methanol (25 mL) at -78 °C was treated with ozone until green,
treated with zinc (4.0 g,
61.0 mmol) and acetic acid (4 mL), stirred for 16 hours, filtered through a
pad of silica gel,
and concentrated to provide the desired product of sufficient purity for
subsequent use
without further purification.
Example 15B
tert-butyl 2-(3-(trifluoromethoxy) anilino)ethylcarbamate
A solution of 3-(trifluoromethoxy)aniline (4.45 g, 25 mmol) in 1,2-
dichloroethane
(100 mL) was treated with Example 15A (4.0 g, 25 mmol) and acetic acid (9.0 g,
150 mmol),
stirred for 30 minutes, treated with sodium triacetoxyborohydride (15.9 g, 75
mmol), stirred
-93-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
for 16 hours, and concentrated. The residue was treated with ethyl acetate,
washed
sequentially with saturated NaHCO3, water, and brine, dried (MgS04), filtered,
and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 4:1/hexanes:ethyl acetate to provide the desired product.
1H NMR (300 MHz, CDCl3) 8 7.14 (t, 1H), 6.55-6.48 (m, 2H), 6.40 (s, 1H), 4.78
(s, 1IT),
4.31 (s, 1H), 3.42-3.36 (m, 2H), 3.28-3.22 (m, 2IT), 1.45 (s, 9H).
Exam 1p a 15C
tert-butyl 2-( (chloroacet,~l)-3-(trifluoromethox'r)anilino)ethylcarbamate
1o A solution of Example 15B (1.5 g, 4.68 mmol) in ethyl acetate (20 mL) at 0
°C was
treated with chloroacetyl chloride (0.38 mL, 5.6 mmol) and saturated NaHC03
(20 mL), and
stirred for 2 hours to provide two layers. The layers were separated, and the
aqueous layer
was extracted with ethyl acetate. The extract was dried (MgS04), filtered, and
concentrated
to provide the desired product of sufficient purity for subsequent use without
further
15 purification.
MS (ESI(+)) m/z 397 (M+H)~;
1H NMR (300 MHz, CDCl3) 8 7.51 (t, 1H), 7.31-7.28 (m, 2H), 7.20 (s, 1H), 4.88-
4.87 (m,
1H), 3.87-3.81 (m, 4H), 3.39-3.32 (m, 2H),1.41 (s, 9H).
2o Example 15D
tert-butyl3-oxo-4-(3-(trifluoromethox~phen l~piperazinecarbox
A solution of Example 15C (1.7 g, 4.4 mmol) in DMF (10 mL) at 0 °C was
treated
with cesium carbonate (1.4 g, 4.3 mmol), warmed to room temperature, stirred
for 16 hours,
treated with ethyl acetate, washed with water and brine, dried (MgS04),
filtered, and
25 concentrated. The concentrate was purified by flash column chromatography
on silica gel
with 10:1 to 2:llhexanes:ethyl acetate to provide the desired product.
1H NMR (300 MHz, CDCl3) 8 7.44 (t, 1H), 7.28-7.13 (m, 3H), 4.84-4.74 (m, 4H),
4.27 (s,
2H), 1.50 (s, 9H).
3o Exam 1p a 15E
1-(3-(trifluoromethox~phenyl)-2-piperazinone hydrochloride
A solution of Example 15D (1.2 g, 3.3 mmol) in ethyl acetate (10 mL) at room
temperature was treated with 1M HCl in diethyl ether (20 mL), stirred for 30
minutes, and
concentrated to provide 0.98 g of the desired product of sufficient purity for
subsequent use
35 without further purification.
Example 15F
-94-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)(3-oxo-4-(3-(trifluoromethoxy)phenyl)-
1
~iperazinyl)methyl)(1,1'-biphenyl)-2-carbonitri1e dihydrochloride
A suspension of Example 4A (30 mg, 0.1 mrnol) in dichloromethane (2 mL) at
0 °C was treated with thionyl chloride (0.15 mL, 2.05 mmol), stirred
for 30 minutes, warmed
to room temperature, stirred for 3.5 hours, and concentrated. The concentrate
was treated
with a solution of Example 15E (35 mg, 0.12 mmol) in acetonitrile (2 mL) and
N,N-
diisopropylethylamine(100 ~.L, 0.57 mmol), warmed to 80 °C, stirred for
3 hours, diluted
with ethyl acetate, washed sequentially with saturated NaHC03, water, and
brine, dried
(MgS04), filtered, and concentrated. The concentrate was purified by flash
column
to chromatography on silica gel with dichloromethane then
95:5/dichloromethane:methanol.
The appropriate fractions were dissolved in dichloromethane, treated with 1M
HCl in diethyl
ether, and concentrated to provide the desired product.
MS (ESI(+)) m/z 546 (M+H)+;
1H NMR (400 MHz, CDCl3) ~ 7.76 (d, 1H), 7.54 (dd, 1H), 7.47 (d, 1H), 7.44-7.12
(m, 9H),
7.08 (s, 1H), 4.73 (s, 1H), 3.76-3.67 (m, 2H), 3.62 (s, 3H), 3.33 (q, 2H),
2.97-2.82 (m, 2H),
2.16 (d, 3H).
Example 16
5-((( 1-benzovl-4-piperidinyl)amino)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl( 1,1'-
2o biphenyl)-2-carbonitrile dihydrochloride
The desired product was prepared by substituting 1-benzoyl-4-piperidinone for
4-
nitrobenzaldehyde in Example 12B. The purified concentrate was dissolved in
dichloromethane, treated with 1M HCl in diethyl ether, and concentrated to
provide the
desired product.
MS (ESI(+)) m/z 490 (M+H)+;
1H NMR (400 MHz, CDCl3) 8 7.73 (d, 1H), 7.43 (dd, 1H), 7.41-7.24 (m, 10H),
7.18 (d, 1H),
6.72 (s, 1H), 5.11 (s, 1H), 4.51 (s, 1H), 3.76-3.70 (m, 1H), 3.57 (s, 3H),
2.95 (s, 2H), 2.75-
2.70 (m, 1H), 2.17 (s, 3H), 1.99-1.65 (m, 3H), 1.50-1.26 (m, 2H).
Example 17
4-(( 1-methyl-1H-imidazol-5-yl)((4-(methylsulfonyl)benzyl)arnino)meth~~ 2-( 1
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting 4-(methylsulfonyl)-
benzaldehyde
for 4-nitrobenzaldehyde in Example 12B. The purified concentrate was dissolved
in
dichloromethane, treated with 1M HCl in diethyl ether, and concentrated to
provide the
desired product.
MS (ESI(+)) m/z 507 (M+H)+;
-95-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (400 MHz, CDC13) 8 7.94 (dd, 2H), 7.88-7.82 (m, 3H), 7.59-7.38 (m,
10H), 6.88 (d,
1H), 4.97 (s,1H), 3:92-3.83 (m, 2H), 3.54 (d, 3H), 3.01 (s, 3H), 2.25 (s, 1H).
Example 18
4-(((4-cyanobenz 1~)oxy)(1-methyl-1H-imidazol-5- 1)methyl)-2-($-
quinolinyl)benzonitrile
dih_ydrochloride
The desired product was prepared by substituting 8-quinolinylboronic acid for
Example 43B in Example 43C.
MS (ESI(+)) mlz 456 (M+H)+;
l0 1H NMR (400 MHz, CDC13) 8 8.85-8.83 (m, 1H), 8.22 (dd, 1H), 7.93 (dd, 1H)
7.83 (d, 1H),
7.75 (dd, 1H), 7.66-7.61 (m, 4H), 7.57-7.54 (m, 1H), 7.46-7.42 (m, 4H), 7.01
(s, 1H), 5.70 (s,
1H), 4.67-4.60 (m, 2H), 3.50 (s, 3H).
Example 19
4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)y methyl)-2-(4-
quinolinyl)benzonitrile
dihydrochloride
Exam 1p a 19A
4-iodoquinoline
2o A solution of 4-chloroquinoline (5.0 g, 30.56 mmol) in 2-butanone (40 mL)
at room
temperature was treated with sodium iodide (23 g, 153 mmol) and 47°lo
hydriodic acid (20
mL), heated to reflux for 8 hours, cooled to room temperature, adjusted to pH
7 with
saturated NaHC03, and extracted with ethyl acetate. The extract was
concentrated, and the
concentrate was purified by flash column chromatography on silica gel with
4:llhexanes:ethyl acetate to provide the desired product.
MS (ESI(+)) mlz 256 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.46 (d, 1H), 8.06-8.00 (m, 3H), 7.79-7.73 (m, 1H),
7.66-7.61
(m, 1H).
3o Example 19B
4-quinolinylboronic acid
The desired product was prepared by substituting Example 19A for Example 43A
in
Example 43B.
1H NMR (300 MHz, DMSO-d6) ~ 8.86 (d, 1H), 8.75 (s, 1H), 8.25 (dd, 1H), 8.25
(dd, 1H),
8.00 (dd, 1H), 7.76-7.70 (m, 1H), 7.62-7.56 (m, 1H).
Example 19C
-96-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(4-
guinolinyl)benzonitrile
dil~drochloride
The desired product was prepared by substituting Example 19B for Example 43B
in
Example 43C. The purified concentrate was dissolved in dichloromethane,
treated with 1M
HCl in diethyl ether, and concentrated to provide the desired product.
MS (ESI(+)) mlz 456 (M+H)+;
1H NMR (400 MHz, CDC13) 8 9.03 (dd, 1H), 8.23 (d, 1H), 7.90 (d, 1H), 7.80-7.75
(m, 1H),
7.67-7.32 (m, 10H), 6.98 (d, 1H), 5.70 (d, 1H), 4.63 (abq, 2H), 3.46 (s, 3H).
Example 20
5-((5-(h~ymethyl)-1H-imidazol-1-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
Example 20A
5-(bromomethyl)-2'-methyl( 1,1'-biphe~l)-2-carbonitrile
The desired product was prepared by substituting Example 86H for Example 61A
in
Example 61B.
1H NMR (300 MHz, CDC13) S 7.72 (d, 1H), 7.47 (dd, 1H), 7.40 (d, 1H), 7.39-7.26
(m, 3H),
7.22-7.18 (m, 1H), 4.50 (s, 2H), 2.20 (s, 3H).
Example 20B
t 1-trill-1H-imidazol-4-xl)methanol
A solution of 1H-imidazol-5-ylmethanol hydrochloride (1.37 g, 10.2 mmol) and
triethylamine (3.55 mL, 25.5 mmol) in DMF (7 mL) at room temperature was
treated with a
solution of triphenylmethyl chloride (3.0 g, 10.7 mmol) in DMF (14 mL),
stirred for 3 days,
poured into ice water, and filtered. The filter cake was washed with ice water
and dried in a
vacuum oven for 16 hours to provide the desired product of sufficient purity
for subsequent
use without further purification.
1H NMR (300 MHz, CDCl3) 8 7.45-7.34 (m, 10H), 7.11-7.07 (m, 6H), 6.72-6.71 (m,
1H),
4.86 (t, 1H), 4.33 (d, 2H).
Example 20C
(1-trityl-1H-imidazol-4- 1)~ methyl acetate
A solution of Example 20B (3.5 g, 10.3 mmol) in pyridine (20 mL) at room
temperature was treated with acetic anhydride (2.0 mL, 21.2 mmol), stirred for
2 days,
cooled, treated with ethyl acetate, washed sequentially with saturated NaHC03,
water, and
brine, dried (MgSO~.), filtered, and concentrated to provide the desired
product of sufficient
purity for subsequent use without further purification.
-97-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDC13) 8 7.42 (d, 1H), 7.35-7.31 (m, 9H), 7.15-7.10 (m, 6H),
6.88-6.87
(m, 1H), 5.01 (s, 2H), 2.07 (s, 3H).
Example 20D
(1-((6-cyano-2'-methyl(1,1'-biphen'rl)-3-yl)methyl)-1H-imidazol-5-yl)methyl
acetate
hydrobromide
A solution of Example 20C (2.39 g, 6.25 mmol) in ethyl acetate (15 mL) at 60
°C was
treated with Example 20A (1.79 g, 6.25 mmol), stirred for 16 hours, cooled to
room
temperature, and filtered. The filtrate was reheated to 60 °C, stirred
for 16 hours, cooled to
room temperature, and filtered a second time. The combined solids were
dissolved in
methanol (20 mL), heated to 60 °C, stirred for 6 hours, cooled to room
temperature, filtered,
and washed with hexanes to provide the desired product of sufficient purity
for subsequent
use without further purification.
Example 20E
5-((5-(hvdroxvmethvl)-1H-imidazol-1-vl)methvl)-2'-methyl( 1,1'-binhenvl)-2-
carbonitrile
A solution of Example 20D in 3:1 THF/water at 0 °C was treated with
lithium
hydroxide monohydrate (840 mg, 19.1 mmol), stirred for 2 hours, and extracted
with ethyl
acetate. The extract was concentrated, and the concentrate was purified by
flash column
2o chromatography on silica gel with 9:1/dichloromethane:methanol to provide
the desired
product.
MS (ESI(+)) m/z 304 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.72 (d, 1H), 7.53 (s, 1H), 7.37-7.09 (rn, 6H), 7.01
(s, 1H),
5.34 (s, 2H), 4.52 (s, 2H), 2.14 (s, 3H).
Example 21
2'-methyl-5-((5-((3-oxo-4-(3-(trifluoromethoxy)phen~)-1-piperazinyl)methyl)-1H-
imidazol
1-yl)methyl)(1,1'-biphenyl)-2-carbonitri1e dihydrochloride
3o Example 21A
5-((5-formyl-1 H-imidazol-1-yl)methyl)-2'-meth( 1,1'-biphenyl)-2-c arbonitrile
The desired product was prepared by substituting Example 20E for Example 37A
in
Example 37B.
MS (ESI(+)) m/z 302 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 9.76-9.75 (m, 1H), 7.88 (s, 1H), 7.79 (s, 1H), 7.72
(d, 1H),
7.38-7.12 (m, 6H), 5.61 (s, 2H), 2.13 (s, 3H).
-98-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 21B
2'-methyl-5-((5-((3-oxo-4-(3-(trifluoromethoxy)phen l~~perazin 1)~eth~)-1H-
imidazol
1-yl)meths)(1,1'-biphenyl)-2-carbonitrile dihydrochloride
The desired product was prepared by substituting Example 21A and Example 15E
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) mlz 546 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.74 (d, 1H), 7.60 (s, 1H), 7.44-7.11 (m, 9H), 7.06
(s, 2H),
l0 5.38 (s, 2H), 3.52 (dd, 2H), 3.44 (s, 2H), 3.28 (s, 2H), 2.74 (dd, 2H),
2.13 (s, 3H).
Example 22
2'-methyl-5-((5-(((1-methyl-2-oxo-1,2-dihydro-6-quinoli ~l)amino)meth~)-1H-
imidazol-1
yl)methyl)(1,1'-biphenyl)-2-carbonitrile dihydrochloride
Example 22A
6-amino-1-methyl-2(lH~quinolinone
A solution of 1-methyl-6-vitro-2(1H)-quinolinone in ethanol (5 mL) at room
temperature was treated with Pd/C (10 rng), stirred under 1 atmosphere of
hydrogen gas for
16 hours, filtered through a pad of diatomaceous earth (Celite~), and
concentrated to provide
the desired product of sufficient purity for subsequent use without further
purification.
Example 22B
2'-methyl-5-((5-((( 1-methyl-2-oxo-1,2-dihydro-6-guinolinyl)amino~methyl)-1H-
imidazol-1-
1)y meths)(1,1'-biphenyl)-2-carbonitrile dihydrochloride
The desired product was prepared by substituting Example 21A and Example 22A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
3o MS (ESI(+)) m/z 460 (M+H)+;
1H NMR (300 MHz, CDC13) ~ 7.74-7.69 (m, 1H), 7.62-7.55 (m, 1H), 7.47 (d, 1H),
7.36-7.00
(m, 9H), 6.79 (dd, 1H), 6.67-6.65 (m, 1H), 5.35-5.29 (m, 2H), 4.18 (d, 2H),
3.67 (s, 3H),
3.62-3.56 (m, 1H), 2.10 (m, 3H).
Example 23
5-((benzylamino)(1-methyl-1H-imidazol-5=yl)meth~)-2'-methyl(1 1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting benzaldehyde for
-99-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-nitrobenzaldehyde in Example 12B.
MS (ESI(+)) m/z 393 (M+H)+;
1H NMR (300 MHz, CDC13) b 7.75 (d, 1H), 7.46 (dd, 1H), 7.39-7.26 (m, 10H),
7.19 (d, 1H),
6.82 (s, 1H), 4.93 (s, 1H), 3.76 (abq, 2H), 3.52 (s, 3H), 2.18 (s, 3H).
Example 24
5-(((c'rclohexylmethxl)amino)( 1-methyl-1H-imidazol-5-yl)meths)-2'-methyl(1,1'-
biphenyl)
2-carbonitrile
The desired product was prepared by substituting cyclohexylcarboxaldehyde for
l0 4-nitrobenzaldehyde in Example 128.
MS (ESI(+)) m/z 399 (M+H)+;
1H NMR (300 MHz, CDC13) b 7.77-7.70 (m, 2H), 7.47 (d, 1H), 7.38-7.27 (m, 4H),
7.20 (d,
1H), 6.88 (s, IH), 4.9I (s, 1H), 3.65 (s, 3H), 2.47-2.36 (m, 2H), 2.I7 (s,
3H), 1.80-1.62 (m,
4H), 1.52-1.38 (m, 1H), 1.30-1.05 (m, 4H), 1.00-0.82 (m, 2H).
Example 25
5-(((4-cyanobenzyl)amino)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( l, l'-
biphenyl)-2
carbonitrile
The desired product was prepared by substituting 4-formylbenzonitrile for
4-nitrobenzaldehyde in Example I2B.
MS (ESI(+)) mlz 418 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.77 (d, 1H), 7.63 (d, 2H), 7.47-7.28 (m, 8H), 7.19
(d, 1H),
6.86 (s, 1H), 4.93 (s, 1H), 3.84 (abq, 2H), 3.52 (s, 3H), 2.17 (s, 3H), 2.00
(s, 1H).
Example 26
5-((((6-cyano-2'-methyl(1,1'-biphenyl)-3-yl)methyl)amino)(I-methyl-1H-imidazol-
5
yl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 86I for 4-
nitrobenzaldehyde in Example 12B.
3o MS (ESI(+)) m/z 508 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.77-7.70 (m, 2H), 7.48-7.24 (m, 11H), 7.16 (dd,
2H), 6.85 (s,
1H), 4.97 (s, 1H), 3.92-3.82 (m, 2H), 3.54 (s, 3H), 2.16 (s, 3H), 2.14 (s,
3H), 2.02 (s, 1H).
Example 27
5-((ethyl(4-nitrobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methxl)-2'-
methyl(1,1'-biphen~-
2-carbonitrile dihydrochloride
-100-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The free base of the desired product was obtained as a byproduct in Example
12B.
The purified concentrate was dissolved in dichloromethane, treated with 1M HCl
in diethyl
ether, and concentrated to provide the desired product.
MS (ESI(+)) miz 466 (M+H)+;
1H NMR (400 MHz, CDC13) S 8.17 (d, 2H), 7.74 (d, 1H), 7.46-7.43 (m, 4H), 7.38-
7.26 (m,
4H), 7.17 (m, lI-~, 6.99 (s, 1H), 5.06 (s, 1H), 3.80 (abq, 2H), 3.47 (s, 3H),
2.77-2.68 (m, 1H),
2.64-2.55 (m, 1H), 2.16 (s, 3H), 1.08 (t, 3H).
Example 28
l0 5-(((4-cyanobenzyl)(ethyl)amino)(1-methyl-1H-imidazol-5- 1)~methyl)-2'-
methyl(1,1'-
biphenyl)-2-carbonitrile dihydrochloride
The free base of the desired product was obtained as a byproduct in Example
25. The
purified concentrate was dissolved in dichloromethane, treated with 1M HCl in
diethyl ether,
and concentrated to provide the desired product.
MS (ESI(+)) m/z 446 (M+H)+;
1H NMR (400 MHz, CDC13) 8 8.17 (d, 2H), 7.74 (d, 1H), 7.46-7.27 (m, 8H), 7.17
(d, 1H),
6.99 (s, 1H), 5.06 (s, 1H), 3.80 (abq, 2H), 3.47 (s, 3H), 2.77-2.68 (m, 1H),
2.64-2.55 (m, 1H),
2.16 (s, 3H), 1.08 (t, 3H).
2o Example 29
4-(((4-cyanobenzyl)(methyl)amino)(1-meth~rl-1H-imidazol-5-yl methyl -) 2(1
naphthyl)benzonitrile
A solution of Example 13B (42 mg, 0.09 mmol) in formic acid (5 mL) was treated
with 37% aqueous formaldehyde(3 mL), heated to 95 °C for 4 hours,
cooled to room
temperature, and extracted with ethyl acetate. The extract was washed
sequentially with
saturated NaHC03, water, and brine, dried (MgSO~.), filtered, and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
dichloromethane
then 98:2/dichloromethane:methanol to provide the desired product.
MS (ESI(+)) m/z 468 (M+H)~;
1H NMR (300 MHz, CDCl3) 8 7.97 (d, 1H), 7.96-7.93 (m, 1H), 7.85 (d, 1H), 7.66-
7.35 (m,
12H), 7.05 (d, 1H), 4.89 (d, 1H), 3.66 (d, 2H), 3.62 (d, 3H), 2.20 (d, 3H).
Example 30
4-((butyl(4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
na h~thyl)benzonitrile dihydrochloride
The desired product was prepared by substituting butyraldehyde and Example 13B
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
-101-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 510 (M+H)+;
1H NMR (400 MHz, CDC13) 8 7.98-7.93 (m, 2H), 7.82 (dd, 1H), 7.61-7.35 (m,
12H), 7.02 (d,
1H), 5.06 (s, 1H), 3.85-3.70 (m, 2H), 3.44 (d, 3H), 2.69-2.62 (m, 1H), 2.58-
2.50 (m, 1H),
1.50-1.41 (m, 2H), 1.26-1.16 (m, 2H), 0.80 (t, 3H).
Example 31
4 ~(1-metal-IH-imidazol-5-~)(phenethYlamino)methyl)-2-(1-na~ht~~benzonitrile
31-A
and
4-(((2-hydroxy-2-phen 1y eth 1~(2-phenylethyl)amino)(1-methyl-1H-imidazol-5-
yl)methyl)-2-(1-naphthyl)benzonitrile
31-B
The desired product was prepared by substituting a 9:1 mixture of
phenylacetaldehyde/styrene oxide and Example 13A for 4-nitrobenzaldehyde and
Example
12A, respectively, in Example 12B to provide a 9:1 mixture of Example 31-A and
Example
31-B.
31-A: MS (ESI(+)) m/z 443 (M+H)+;
1H NMR (400 MHz, CDC13) S 7.94 (dd, 2H), 7.78, (d, 1H), 7.58-7.08 (m, 13H),
6.74 (d, 1H),
4.93 (d, IH), 3.47 (d, 3H), 2.90-2.86 (m, 2H), 2.82-2.78 (m, 2H);
31-B: MS (ESI(+)) m/z 563 (M+H)+;
1H NMR (400 MHz, CDCl3) 8 6.69-6.66 (m, 1H), 4.86 (dd, 1H), 4.20-4.12 (m, 1H),
3.38 (d,
3H), 3.23-3.14 (m, 1H), 3.09-3.02 (m, 2H), 2.73-2.69 (m, 1H), 2.69-2.51 (m,
2H).
Example 32
5-((benz~l-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2'-methyl(1,1'-biphen
1
carbonitrile dihydrochloride
Example 32A
( 1-methyl-2-sulfanyl-1H-imidazol-5-yl)methanol
A solution of 1,3-dihydroxyacetone dimer (25 g, 0.28 mol) in n-butanol (115
mL) at
room temperature was treated with acetic acid (56 mL), potassium thiocyanafe
(80.75 g, 0.83
mol) and methylamine hydrochloride (41.15 g. 0.61 mol), stirred at room
temperature for 3
days, treated with a 1:l mixture of diethyl ether:hexanes (I00 mL), washed
with water, and
-102-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
concentrated to provide the desired product of sufficient purity for
subsequent use without
further purification.
1H NMR (400 MHz, DMSO-d6) 8 12.0 (s, 1H), 6.81 (s,1H), 5.21 (t, 1H), 4.32 (d,
2H), 3.45
(s, 3H).
Example 32B
(1-methyl-1H-imidazol-5-yl)methanol
A solution of Example 32A (50 g, 0.35 mol) in ethanol (500 mL) was treated
with
Raney~ nickel (500 g), heated to reflux fox 1 hour, cooled to room
temperature, filtered, and
1o concentrated to provide the desired product of sufficient purity for
subsequent use without
further purification.
MS (DCI/NH3) m/z 113 (M+H)+;
1H NMR (400 MHz, DMSO-d6) b 7.50 (s, 1H), 6.75 (s, 1H), 5.01 (s, 1H), 4.41 (s,
2H), 3.59
(s, 3H).
EXamble 32C
1-methyl-1H-imidazole-5-carbaldehyde
A solution of Example 32B (2.3 g, 20 mmol) in dioxane (100 mL), was treated
with
manganese dioxide (17.3 g, 200 mmol), heated to reflux for 16 hours, cooled to
room
2o temperature, filtered through a pad of diatomaceous earth (Celite~), and
concentrated to
provide the desired product of sufficient purity for subsequent use without
further
purification.
1H NMR (300 MHz, CDCl3) 8 9.77 (d, 1H), 7.79 (s, 1H), 7.62 (s, 1H), 3.95 (d,
3H).
Exam 1p a 32D
5-((benzylamino)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 86I and benzylamine
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B.
1H NMR (300 MHz, CDCl3) 8 7.75-7.68 (m, 2H), 7.47-7.14 (m, 10H), 3.90 (s, 2H),
3.83 (s,
2H), 2.19 (s, 3H), 1.65, (s, 1H).
Example 32E
5-((benz~ (( 1-methyl-1 H-imidazol-5-yl)methyl)amino)meth~l)-2'-methyl( 1,1'-
biphenyl)-2
carbonitrile dihydrochloride
The desired product was prepared by substituting Example 32C and Example 32D
for
nitrobenzaldehyde and Example 12A, respectively, in Example 12B.
MS (ESI(+)) m/z 407 (M+H)+;
-103-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDCl3) S 7.69 (d, 1H), 7.40-7.27 (m, 11H), 7.18 (d, 1H), 6.97
(s, 1H),
3.63 (s, 2H), 3.57 (s, 2H), 3.54 (s, ZH), 3.47 (s, 3H), 2.18 (s, 3H).
Example 33
4-(((3-bromo-4-cyanobenzyl)amino)(1-methyl-1H-irnidazol-5-yl)meth,~l)-2-(1
na~hthyl)benzonitrile dihxdrochloride
Example 33A
4-amino-3-bromobenzaldeh,Yde
to A solution of 4-aminobenzaldehyde (3.0 g, 25 mmol) in methanol (50 mL),
acetone
(100 mL), and water (30 mL) was treated with p-toluenesulfonic acid
monohydrate (1.0 g,
5.26 mmol), heated to reflux for 8 hours, cooled to 0 °C, treated with
N-bromosuccinimide
(4.4 g, 25 mmol), stirred for 30 minutes, and concentrated. The concentrate
was treated with
ethyl acetate, washed sequentially with saturated Na2C03, water, and brine,
dried (Na2S04),
15 filtered, and concentrated to provide the desired product of sufficient
purity for subsequent
use without further purification.
1H NMR (300 MHz, CDC13) 8 9.72 (s, 1H), 7.95 (d, 1H), 7.64 (dd,1H), 6.80 (d,
1H), 4.72 (s,
2H).
2o Exam 1p a 33B
2-bromo-4-form_ylbenzonitrile
A solution of Example 33A (1.0 g, 5 mmol) in acetone (5 mL) at 0 °C was
added to
4.5M HCl (8 mL). The mixture was treated with 40% sodium nitrite (1 mL),
warmed to
room temperature, and stirred for 1 hour. The mixture was added to a
0°C solution of
25 copper(I) cyanide (0.45 g, 5 mmol) and potassium cyanide (0.65 g, 10 mmol)
in water (20
mL) and toluene (50 mL), warmed to room temperature, stirred for 16 hours, and
concentrated. The concentrate was extracted with ethyl acetate, washed
sequentially with
saturated Na2C03, water, and brine, dried (Na2S04), filtered, and concentrated
to provide the
desired product of sufficient purity for subsequent use without further
purification.
30 1H NMR (300 MHz, CDC13) b 10.04 (s, 1H), 8.18 (d, 1H), 7.93 (dd, 1H), 7.85
(d, 1H).
Example 33C
4-(((3-bromo-4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5- 1)y methyl)-2-(1
naphthyl)benzonitrile dih_ydrochloride
35 The desired product was prepared by substituting Example 33B and Example
13A for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
concentrate was
purified by flash column chromatography on silica gel with dichloromethane
then
-104-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
98:2/dichloromethane:methanol. The concentrate was dissolved in
dichloromethane, treated
with 1M HCl in diethyl ether, and concentrated to provide the desired product.
MS (ESI(+)) m/z 534 (M+2H)+;
1H NMR (300 MHz, CDC13) 8 7.97 (t, 2H), 7.87 (d, 1H), 7.67 (s, 1H), 7.61-7.43
(m, 9H),
7.36 (d, 1H), 6.91 (d, 1H), 4.98 (s, 1H), 3.91-3.82 (m, 2H), 3.56 (d, 3H).
Exam 1p a 34
4-(((4-c~nobenzyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-( 1
naphthyl)benzonitrile
Example 34A
4-(aminomethyl)benzonitrile
A solution of 4-(bromomethyl)benzonitrile (27.5 g, 0.14 mol) in DMF (125 mL)
was
treated with potassium phthalimide (27.8 g, 0.15 mol), heated to 130 °C
for 2.5 hours, cooled
to room temperature, poured over ice, filtered, rinsed with water and a 1:1
mixture of
hexanes:diethyl ether, and dried for 16 hours in a vacuum oven at 50
°C. The compound was
treated with ethanol (200 mL) and 35% aqueous hydrazine (8 mL), heated to
reflux for 3
hours, cooled to room temperature, filtered, and concentrated. The concentrate
was purified
by vacuum distillation to provide the desired product.
1H NMR (300 MHz, CDC13) 8 7.63 (d, 2H), 7.45 (d, 2H), 3.96 (s, 2H), 1.48 (s,
2H).
Example 34B
4-(((4-cyanobenzyl)amino)methyl)-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89C and Example 34A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B.
MS (ESI(+)) m/z 374 (M+H)+.
Example 34C
4-(((4-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methy1)-2-(1
3o naphthyl)benzonitrile
The desired product was prepared by substituting Example 32C and Example 34B
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B.
1H NMR (300 MHz, CDCl3) 8 7.92-7.88 (m, 2H), 7.74-7.71 (m, 1H), 7.54-7.29 (m,
12H),
6.90 (s, 1H), 3.68-3.39 (m, 6H), 3.37 (s, 3H).
Exam 1p a 35
-105-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((3-chloro-4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5- 1)methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
Example 35A
2-chloro-4-iodobenzonitrile
The desired product was prepared by substituting 4-amino-2-chlorobenzonitrile
for 5-
aminoquinoline in Example 43A.
1H NMR (300 MHz, CDC13) 8 7.92 (d, 1H), 7.74 (dd, 1H), 7.36 (d, 1H).
to Example 35B
methyl 3-chloro-4-cyanobenzoate
A solution of Example 35A (39.9 g, 0.15 mol) in methanol (1 L) was treated
with
(1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium(II) complex with
dichloromethane
(1:1) (1.25 g, 1.53 mmol) and triethylamine (24 mL), heated to 97 °C
under 500 psi CO
15 pressure for 20 hours, cooled to room temperature, filtered, and
concentrated to provide the
desired product of sufficient purity for subsequent use without further
purification.
tH NMR (300 MHz, CDC13) 8 8.17 (d, 1H), 8.02 (dd, 1H), 7.77 (d, 1H), 3.97 (s,
3H).
Example 35C
2o 2-chloro-4-(hydrox~~benzonitrile
The desired product was prepared by substituting Example 35B for Example 5A in
Example 5B.
Example 35D
25 2-chloro-4-formylbenzonitrile
A solution of Example 35C (5.49 g, 32.76 mmol) in dichloromethane (100 mL) at
room temperature was treated with Dess-Martin periodinane (25 g, 58.9 mmol),
stirred for 20
minutes, treated with saturated NaHC03 and saturated Na~,S203, stirred for 5
minutes,
concentrated, and extracted with diethyl ether. The extracts were washed with
brine, dried
30 (Na2S04), filtered, and concentrated to provide the desired product.
MS (DCI(+)) m/z 183 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 810.06 (s, 1H), 8.03-8.02 (m, 1H), 7.88 (d, 2H).
Exam 1p a 35E
35 4-(((3-chloro-4-cyanobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
na~hthyl)benzonitrile dihydrochloride
-106-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 35D and Example 13A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 488 (M+H)+;
1H NMR (400 MHz, CDC13) ~ 7.96 (t, 2H), 7.86 (d, 1H) 7.62-7.42 (m, 10H), 7.31
(d, 1H),
6.91 (d, 1H), 4.98 (s, 1H), 3.90 -3.81 (m, 2H), 3.55 (d, 3H).
Example 36
l0 4-(((1-(4-cyano~henyl)ethyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting 4-acetylbenzonitrile and
Example
13A for 4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
15 concentrated to provide the desired product.
MS (ESI(+)) m/z 468 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.98-7.35 (m, 15H), 7.00-6.72 (m, 1H), 4.81-4.58 (m,
1H),
3.93-3.70 (m, 1H), 3.56-3.47 (m, 3H), 1.41-1.36 (m, 3H).
2o Example 37
4-(((4-cyano-3-iodobenzyl)amino)(1-methyl-1H-imidazol-5- 1)~ methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
Example 37A
25 4-(h~droxymethyl)-2-iodobenzonitrile
A suspension of Example 63A (296 mg, 1.0 mmol) in water (10 mL) was treated
with
diatomaceous earth (Celite~) (296 mg), heated to reflux for 2 hours, cooled to
room
temperature, and filtered. The filtrate was extracted with ethyl acetate,
dried (MgS04),
filtered, and concentrated to provide the desired product of sufficient purity
for subsequent
3o use without further purification.
MS (DCI/NH3) m/z 277 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) 8 7.96 (s, 1H), 7.60 (d, 1H), 7.44 (dt, 1H), 4.75 (d,
2H), 1.86 (t,
1H).
35 Example 37B
4-formyl-2-iodobenzonitrile
-107-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 37A (70 mg, 0.27 mmol) in DMSO (2 mL) and triethylamine
(190 ~.L, 1.35 mmol) at room temperature was treated with small portions of
pyridine sulfur
trioxide (107 mg, 0.68 mmol), stirred for 16 hours, treated with ethyl
acetate, washed
sequentially with 1M HCI, water, and brine, dried (Na2S04), and filtered. The
filtrate was
treated with activated charcoal, stirred fox 45 minutes, filtered through a
pad of diatomaceous
earth (Celite0) with 9:1/dichloromethane:methanol, and concentrated to provide
the desired
product of sufficient purity for subsequent use without further purification.
MS (DCI/NH3) m/z 257 (M)~;
1H NMR (300 MHz, CDCl3) 810.01 (s, 1H), 8.41 (s, 1H), 7.96 (d, 1H), 7.80 (d,
1H).
Example 37C
4-(((4-cyano-3-iodobenzyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
A solution of 13A (32 mg, 009 mmol) and molecular sieves (100 mg) in 1,2-
dichloroethane (2 mL) at room temperature was treated with Example 37B (34 mg,
0.57
mmol) and acetic acid, stirred for 30 minutes, treated with sodium
triacetoxyborohydride (60
mg, 0.28 mmol), and stirred for 16 hours. The mixture was treated with ethyl
acetate, washed
sequentially with saturated NaHC03, water, and brine, dried (MgSOq.),
filtered, and
concentrated. The concentrate was dissolved in dichloromethane (5 mL), treated
with 4M
2o HCl in dioxane (1 mL), stirred for 30 minutes, and concentrated. The
concentrate was
dissolved in ethyl acetate, washed sequentially with saturated NaHC03, water,
and brine,
dried (MgS04), filtered, and concentrated. The concentrate was purified by
flash column
chromatography on silica gel with dichloromethane then
98:2/dichloromethane:methanol.
The concentrate was dissolved in dichloromethane, treated with 1M HCl in
diethyl ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 580 (M+H)+;
1H NMR (400 MHz, CDC13) b 7.87 (t, 2H), 7.81-7.76 (m, 2H) 7.52-7.29 (m, 10H),
6.81 (d,
1H), 4.87 (s, 1H), 3.78-3.69 (m, 2H), 3.47 (d, 3H), 1.97 (s, 1H).
Example 38
methyl 4-((((4-cyano-3-( 1-naphthyl)phenyl)( 1-methyl-1H-imidazol-5
1)~~rl)amino)methyl)benzoate
The desired product was prepared by substituting methyl 4-formylbenzoate for
Example 37B in Example 37C. The concentrate was purified by flash column
chromatography on silica gel with dichloromethane then
98:2/dichloromethane:methanol to
provide the desired product.
MS (ESI(+)) m/z 487 (M+H)+;
-108-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDCl3) 8 8.01-7.89 (m, 5H), 7.62-7.32 (m, 10H), 7.10-7.00 (m,
1H),
5.00-4.93 (m, 1H), 3.92 (s, 3H), 3.90-3.64 (m, 2H), 3.75-3.60 (m, 3H).
Example 39
lithium 4-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methyl)amino)methyl)benzoate
A solution of Example 38 (55 mg, 0.113 mmol) in methanol (2 mL) and water (0.5
mL) at room temperature was treated with lithium hydroxide monohydrate (4.7
mg, 0.112
mmol), stirred for 16 hours, treated with a second portion of lithium
hydroxide monohydrate
to (2.4 mg, 0.057 mmol), stirred for 8 hours, and concentrated. The
concentrate was treated
with THF (1 mL) and water (1.0 mL), stirred for 16 hours, treated with a third
portion of
lithium hydroxide monohydrate (3.0 mg, 0.07 mmol), stirred for 16 hours, and
concentrated.
The concentrate was dissolved in water (3 mL), washed with diethyl ether, and
lyophilized to
provide the desired product.
MS (ESI(+)) m/z 473 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.08-8.01 (m, 3H), 7.76-7.42 (m, 10H), 7.16 (d, 2H),
6.53 (d,
1H), 4.98 (s, 1H), 3.72-3.60 (m, 2H), 3.54 (d, 3H).
Example 40
4-~((4-chlorobenzyl)((1-methyl-1H-imidazol-5- 1)~~rl)amino)methyl)-2-(1-
naphthyl)benzonitrile dihydrochloride
Exam lie 40A
4-~((4-chlorobenzy)amino)methyl)-2-( 1-na~hthyl)benzonitrile
The desired product was prepared by substituting Example 89C and (4-
chlorophenyl)methylamine for 4-nitrobenzaldehyde and Example 12A,
respectively, in
Example 12B.
MS (ESI(+)) m/z 383 (M+H)+;
1H NMR (400 MHz, CDCl3) ~ 7.94 (dd, 2H), 7.79 (d, 1H), 7.58-7.42 (m, 8H), 7.30-
7.24 (m,
3H), 3.91 (s, 2H), 3.81 (s, 2H).
Example 40B
4-(((4-chlorobenzyl)((1-methyl-1H-imidazol-5- 1y )methyl)amino)methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 32C and Example 40A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
-109-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 479 (M+2H)+;
1H NMR (300 MHz, CDCl3) 8 7.96 (dd, 2H), 7.80-7.77 (m, 1H), 7.58-7.19 (m,
12H), 7.10-
7.00 (m, 1H), 3.70-3.48 (m, 6H), 1.90 (m, 3H).
Example 41
4-((((1-methyl-1H-imidazol-5- 1)~yl)(4-(trifluoromethoxy)benzyl)amino)methyl)-
2-(1
naphthyl)benzonitrile dihydrochloride
to
Example 41A
4-(((4-trifluoromethoxybenzyl)amino)methyl)-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89C and 4-
trifluoromethoxybenzylamine for 4-nitrobenzaldehyde and Example 12A,
respectively, in
15 Example 12B.
MS (ESI(+)) m/z 433 (M+H)+;
1H NMR (400 MHz, CDC13) 8 7.94 (dd, 2H), 7.82-7.78 (m, 1H), 7.58-7.34 (m, 9H),
7.16 (d,
2H), 3.93 (s, 2H), 3.84 (s, 2H).
20 Example 41B
4-((((1-methyl-1H-imidazol-5- 1)~ methyl)(4-(trifluoromethox
)b~enz_yl)amino)methyl)-2-(1
naphth~l)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 32C and Example 41A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
25 concentrate was dissolved in dichloromethane, treated with 1M HCl in
diethyl ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 527 (M+H)+;
1H NMR (400 MHz, CDC13) 8 7.97-7.94 (m, 2H), 7.77 (d, 1H), 7.60-7.28 (m, 10H),
7.14 (d,
2H), 6.96 (s, 1H), 3.74-3.49 (m, 6H), 3.43 (s, 3H).
Example 42
4-(((4-chlorobenzvl)amino)( 1-methyl-1H-imidazol-5-vl)methvl)-2-( 1-
nanhthvllbenzonitrile
The desired product was prepared by substituting 4-chlorobenzaldehyde and
Example
13A for 4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B.
MS (ESI(+)) m/z 463 (M+H)+;
1H NMR (400 MHz, CDCl3) 8 7.97-7.92 (m, 2H), 7.84 (d, 1H), 7.60-7.39 (m, 8H),
7.29-7.20
(m, 4H), 6.85 (d, 1H), 4.94 (d, 1H), 3.82-3.70 (m, 2H), 3.43 (d, 3H).
-110-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 43
4-(((4-cyanobenzyl)axy)(1-methyl-1H-imidazol-5- l~yl)-2-(5-
quinolinyl)benzonitrile
dihydrochloride
Example 43A
5-iodoguinaline
A solution of 5-aminoquinoline (5.5 g, 38.1 mmol) in 3M HCl (100 mL) at 0
°C was
treated dropwise with a solution of sodium nitrite (3.65 g, 52.9 mmol) in
water (25 rnL}, then
with a solution of potassium iodide (13.0 g, 78.3 mmol) in water (25 mL) with
periodic
treatment with acetone to prevent foaming. The reaction was warmed to room
temperature,
stirred for 16 hours, treated with saturated sodium thiosulfate, and extracted
with ethyl
acetate. The extract was dried (Na~,S04), filtered, and concentrated. The
concentrate was
purified by flash column chromatography on silica gel with 8:1 to
4:1/hexanes:ethyl acetate
to provide the desired product.
1H NMR (400 MHz, CDC13) S 8.89 (d, 1H), 8.39 (d, 1H), 8.14-8.10 (m, 2H), 7.51-
7.41 (m,
2H).
Example 43B
5-auinolin~lboronic acid
A solution of 1.6M n-butyllithium in diethyl ether (15.6 mL, 25 mmol) in
diethyl
ether (40mL) at -78 °C was treated with a solution of Example 43A (2.55
g, 10 mmol) in
diethyl ether (30 mL), stirred for 40 minutes, treated with a solution of
tributyl borate (6.9 g,
17.4 mmol) in diethyl ether (10 mL), warmed to room temperature, and stirred
for 16 hours.
The mixture was cooled to 0 °C, and adjusted to pH 2 with 1M HCI. The
aqueous layer was
cooled to 0 °C, adjusted to pH 7 with saturated NaHC03, and the
resulting precipitate was
filtered and dried to provide the desired product of sufficient purity for
subsequent use
without further purification.
1H NMR (300 MHz, DMSO-d6) 8 8.88-8.82 (m, 1H), 8.46 (s, 1H), 8.04-8.00 (dd,
1H), 7.88
(dd, 1H), 7.?2 (dd, 1H), 7.51 (dd, 1H).
Example 43C
4-(((4-cyanobenz~)oxy (1-methyl-1H-irnidazol-5- 1)~meth 1)-~ 2-(5-
duinolinyl)benzonitrile
dih_ydrochloride
A solution of Example 60C (45 mg, 0.1 mmol) in toluene (1 mL) and ethanol (1
mL)
was treated with Example 43B (35 mg, 0.2 mmol), 2M Na2C03 (0.15 mL, 0.3 mmol),
lithium
chloride (13 mg, 0.3 mmol), and Pd(PPh3)4 (5.8 mg, 0.005 mmol), heated to
reflux for 16
-111-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
hours, and cooled to room temperature. The mixture was treated with ethyl
acetate, washed
with water and brine, dried (Na2SOq.), filtered, and concentrated. The
concentrate was
purified by flash column chromatography on silica gel with dichloromethane
then 99:1 to
90: l0ldichloromethane/methanol. The concentrate was dissolved in
dichloromethane, treated
with 1M HCl in diethyl ether, and concentrated to provide the desired product.
MS (ESI(+)) m/z 456 (M+H)+;
1H NMR (400 MHz, CDC13) 8 8.98-8.97 (m, 1H), 8.24 (d, 1H), 7.90-7.78 (m, 3H),
7.66-7.40
(m, 9H), 6.98-6.97 (m, 1H), 5.70-5.69 (m, 1H), 4.69-4.58 (m, 2H), 3.49-3.44
(m, 3H).
to Example 44
4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(5-
isoquinolinyl)benzonitrile
Exam 1p a 44A
5-iodoisoduinoline
The desired product was prepared by substituting 5-aminoisoquinoline for 5-
aminoquinoline in Example 43A.
1H NMR (300 MHz, CDCl3) 8 9.15 (s, 1H), 8.64 (d, 1H), 8.28 (d, 1H), 7.99 (d,
1H), 7.85 (d,
1H), 7.37 (t, 1H).
2o Example 44B
5-isoquinolinylboronic acid
The desired product was prepared by substituting Example 44A for Example 43A
in
Example 43B.
Example 44C
4-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2-(5-
isoquinolinyl)benzonitrile
The desired product was prepared by substituting Example 44B for Example 43B
in
Example 43C. The concentrate was purified by flash column chromatography on
silica gel
with dichloromethane then 99:1 to 90:10/dichloromethane:methanol to provide
the desired
product.
MS (ESI(+)) m/z 456 (M+H)+;
1H NMR (400 MHz, CDC13) 8 9.36 (s, 1H), 8.50 (dd, 1H), 8.12-8.08 (m, 1H), 7.89
(d, 1H),
7.73-7.41 (m, 9H), 7.27 (dd, 1H), 6.98 (d, 1H), 5.70 (d, 1H), 5.69-4.59 (m,
2H), 3.47 (s, 3H).
Exam 1p a 45
4-(((4-cyanobenzyl) ( 1 H-imidazol-5~lmethyl) amino)methyl)-2-( 1-
na~hthyl)benzonitrile
dihydrochloride
-112-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 34B (25 mg, 0.067 mmol) in 1,2-dichloroethane (1 mL) at
room temperature was treated with 1H-imidazole-5-carbaldehyde (9.6 mg, 0.1
rnmol) and
acetic acid (2 mL, 35 mmol), stirred for 30 minutes, treated with sodium
triacetoxyborohydride (140 mg, 0.66 mmol), stirred for 72 hours, treated with
additional 1H-
imidazole-5-carbaldehyde (20 mg, 0.21 mmol), and stirred for 2 days. The
mixture was
treated with ethyl acetate, washed sequentially with saturated NaHC03, water,
and brine,
dried (MgS04), filtered, and concentrated. The concentrate was dissolved in
dichloroniethane (5 mL), treated with 4M HCl in dioxane (1 mL}, stirred for 30
minutes, and
concentrated. The concentrate was dissolved in ethyl acetate, washed
sequentially with
to saturated NaHC03, water, and brine, dried (MgSO~.), filtered, and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
dichloromethane
then 98:2 to 95:5/dichloromethane/methanol. The concentrate was dissolved in
dichloromethane, treated with 1M HCl in diethyl ether, and concentrated to
provide the
desired product.
MS (ESI(+)) m/z 454 (M+H)+;
1H NMR (400 MHz, CDC13) 8 7.92 (dd, 2H), 7.75 (d, 1H), 7.64 (s, 1H), 7.57-7.32
(m, 12H),
6.89 (s, 1H), 3.79-3.61 (m, 6H).
Example 46
4-(((4-cyanobenzyl)oxy)(1H-imidazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile
hydrochloride
A solution of Example 49C (50 mg, 0.07 mmol) in 80% aqueous acetic acid (5
mL),
at room temperature was stirred for 16 hours and concentrated. The concentrate
was purified
by flash column chromatography on silica gel with 9:lldichloromethane:methanol
to provide
the desired product.
MS (ESI(+)) mlz 441 (M+H)+;
1H NMR (500 MHz, CDCl3) 8 7.94-7.91 (m, 2H), 7.82 (dd,1H), 7.68-7.36 (m,13H),
6.93 (d,
1H), 5.66 (d, 1H), 4.68 (abq, 2H).
Example 47
4-(((3,4-dichlorobenzyl)amino)( 1-methyl-1H-imidazol-5-yl)methxl)-2-( 1-
naphthyl)benzonitrile
The desired product was prepared by substituting 3,4-dichlorobenzaldehyde and
Example 13A for 4-nitrobenzaldehyde and Example 12A, respectively, in Example
12B.
MS (ESI(+)) m/z 497 (M)~;
~H NMR (300 MHz, CDC13) 8 7.98-7.93 (m, 2H), 7.86 (d, 1H); 7.61-7.36 (m, 10H),
7.12 (d,
1H), 6.87 (s, 1H), 4.94 (s, 1H), 3.82-3.69 (m, 2H), 3.56 (d, 3H).
-113-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p e~48
4-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methyl)amino)methyl)-N
methylbenzamide dih~drochloride
A solution of Example 39 (20 mg, 0.04 mmol) in DMF (1 mL) at room temperature
was treated with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
(12 mg, 0.06
mmol), 1-hydroxybenzotriazole (8.5 mg, 0.06 mmol), methylamine hydrochloride
(28.4 mg,
0.42 mmol), and 4-methylmorpholine (46 ~,L, 0.42 mmol), stirred for 16 hours,
treated with
ethyl acetate, washed sequentially with saturated NaHC03, water, and brine,
dried (MgS04),
filtered and concentrated. The concentrate was purified by flash column
chromatography on
to silica gel with dichloromethane then 98:2 to 95:5/dichloromethane:methanol.
The
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 486 (M+H)+;
1H NMR (400 MHz, CDCl3) b 7.96-7.92 (m, 2H), 7.83 (d, 1H), 7.71 (d, 2H), 7.59-
7.33 (m,
10H), 6.86 (d, 1H), 6.14 (m, 1H), 4.94 (d, 1H), 3.89-3.67 (m, 2H), 3.53 (d,
3H), 3.00 (d, 3H).
Example 49
4-(((4-cyanobenz 1~)oxy)(1-trityl-1H-imidazol-4-~)methyl)-2-(1-
naphthyl)benzonitrile
2o Exam 1p a 49A
4-iodo-1-trityl-1H-imidazole
A suspension of 4-iodoimidazole (3.38 g, 17.4 mmol) and triphenylmethyl
chloride
(5.56 g, 19.9 mmol) in DMF (15 mL) at 0 °C was treated with
triethylamine (1.5 mL, 10.8
mmol), warmed to room temperature, stirred for 16 hours, poured into ice
water, filtered, and
dried in a vacuum oven at 50 °C to provide the desired product of
sufficient purity fox
subsequent use without further purification.
Example 49B
4-(hydroxyl1-trityl-1H-imidazol-4-yl)methyl)-2-(1-naphthXl)benzonitrile
3o A solution of Example 49A (873 mg, 2.0 mmol) in dichloromethane (8 mL) at
room
temperature was treated with 3M ethyl magnesium bromide in diethyl ether (0.73
mL, 2.2
mmol), stirred for 30 minutes, and cooled to -20 °C. The mixture was
treated with a solution
of Example 89C (514 mg, 2 mmol) in dichloromethane (2 mL), warmed to room
temperature,
stirred for 16 hours, treated with saturated ammonium chloride, and
concentrated. The
concentrate was extracted with ethyl acetate, the extracts were washed with
water and brine,
dried (MgSO~.), filtered, and concentrated to provide the desired product.
-114-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDCl3) 8 7.94 (dd, 2H), 7.79 (dd, 1H), 7.68-7.23 (m, 17H),
7.08-7.05
(m, 6H), 6.64-6.62 (m, 1H), 5.87 (d, 1H).
Example 49C
4-(((4-cyanobenz 1~)oxy)(1-trityl-1H-imidazol-4-)methyl)-2-(1-naphth
l)benzonitrile
A solution of Example 49B (113 mg, 0.2 mmol) in dichloromethane (1 mL) at room
temperature was treated with 4-(bromomethyl)benzonitrile (50 mg, 0.25 mmol)
and silver (I)
oxide (140 mg, 0.6 mmol), and stirred for 72 hours. The mixture was purified
by flash
column chromatography on silica gel with 6:1 to 4:1/hexanes:ethyl acetate to
provide the
1o desired product.
MS (ESI(+)) m/z 382 (M)+;
1H NMR (400 MHz, CDC13) S 7.94 (dd, 2H), 7.80 (dd, 1H), 7.65 (dq, 1H), 7.58-
7.23 (m,
20H), 7.09-7.06 (m, 6H), 6.73 (dd, 1H), 5.56 (s, 1H), 4.69-4.60 (m, 2H).
Exam 1p a 50
ethyl4-(((4-cyano-3-(1-naphth~phenyl)(1-methyl-1H-imidazol-5- 1)~methxl)amino)-
1
piperidinecarboxylate dihydrochloride
The desired product was prepared by substituting ethyl 4-oxo-1-
piperidinecarboxylate
and Example 13A for 4-nitrobenzaldehyde and Example 12A, respectively, in
Example 12B.
2o The purified concentrate was dissolved in dichloromethane, treated with 1M
HCl in diethyl
ether, and concentrated to provide the desired product.
MS (ESI(+)) m/z 494 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.96 (dd, 2H), 7.85 (d, 1H), 7.61-7.42 (m, 8H), 6.74
(d, 1H),
5.15 (s, 1H), 4.16-4.00 (m, 2H), 4.11 (q, 2H), 3.62 (d, 3H), 2.87-2.78 (m,
2H), 2.70-2.61 (m,
1H), 1.97-1.73 (m, 2H), 1.40-1.20 (m, 5H).
Exam, 1p a 51
6-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5
yl)methyl)amino~methyl)nicotinonitrile trih~drochloride
Exam In a 51A
6-f orm~nicotinonitrile
A solution of 6-methylnicotinonitrile (590 mg, 5.0 mmol) in dioxane (10 mL)
and
water (0.5mL) was treated with selenium dioxide (555 mg, 5.0 mmol), heated to
reflux for 16
hours, cooled to room temperature, and concentrated. The concentrate was
purified by flash
column chromatography on silica gel with hexanes then 9:1/hexanes:ethyl
acetate to provide
the desired product.
-115-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDCl3) 810.13-10.12 (m, 1H), 9.06-9.05 (m, 1H), 8.19-8.16 (m,
1H),
8.09-8.05 (m, 1H).
Example5lB
6-((((4-cyano-3-(1-naphth~phenyl)(1-methyl-1H-imidazol-5
1)v methyl)amino)methyl)nicotinonitrile trihydrochloride
The desired product was prepared by substituting Example 51A and Example 13A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
concentrate was
purified by flash column chromatography on silica gel with dichloromethane
then 99:1 to
98:2/dichloromethane:methanol. The purified concentrate was dissolved in
dichloromethane,
treated with 1M HCl in diethyl ether, and concentrated to provide the desired
product.
MS (ESI(+)) m/z 455 (M+H)+;
1H NMR (300 MHz, CDC13) 8 8.80 (s, 1H), 7.97-7.82 (m, 4H), 7.59-7.36 (m, 9H),
6.94 (s,
1H), 5.07 (s, 1H), 3.99 (s, 2H), 3.56 (d, 3H), 2.67 (s, 1H).
Example 52
methyl 6-((((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5
1)y methyl)amino)methyl)nicotinate trihydrochloride
2o Exam lm~ a 52A
methyl 6-(hydrox ny ~eth~rl)nicotinate
The desired product was prepared by substituting dimethyl 2,5-
pyridinedicarboxylate
for Example 5A in Example 5B.
Example 52B
methyl 6-formylnicotinate
The desired product was prepared by substituting Example 52A for Example 37A
in
Example 37B.
3o Example 52C
meths 6-.((((4-~ano-3-(1-naphthyl)phe~l)(1-methyl-1H-imidazol-5
1)~ methyl)amino)methyl)nicotinate trih~drochloride
The desired product was prepared by substituting Example 52B and Example 13A
for
4-nitrobenzaldehyde and Example 12A, respectively, in Example 12B. The
purified
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 488 (M+H)+;
-116-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDC13) 8 9.14 (s, 1H), 8.23 (dd, 1H), 7.97-7.92 (m, 2H), 7.83
(d, 1H),
7.61-7.40 (m, 7H), 7.32-7.24 (m, 2H), 6.93 (s, 1H), 5.05 (s, 1H), 3.98-3.92
(m, 5H), 3.56 (s,
3H), 2.71 (s, 1H).
Exam 1p a 53
N-(4-((((4-cyano-3-(1-naphth~phen~)(1-methyl-1H-imidazol-5
yl)methyl)amino)methxl)phenyl)acetamide di~drochloride
The desired product was prepared by substituting N-(4-formylphenyl)acetamide
and
Example 13A for 4-nitrobenzaldehyde and Example 12A, respectively, in Example
12B. The
to purified concentrate was dissolved in dichloromethane, treated with 1M HCl
in diethyl ether,
and concentrated to provide the desired product.
MS (ESI(+)) mlz 486 (M+H)+;
1H NMR (400 MHz, CDCl3) 8 7.93 (dd, 2H), 7.82 (d, 1H), 7.58-7.38 (m, 10H),
7.21 (d, ZH),
6.84 (d, 1H), 4.94 (s, 1H), 3.79-3.67 (m, 2H), 3.52 (d, 3H), 2.13 (s, 3H).
Example 54
benzyl 4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
Xl)methyl)amino)-1
piperidinecarboxylate dihydrochloride
The desired product was prepared by substituting benzyl-4-oxo-1-
2o piperidinecarboxylate and Example 13A for 4-nitrobenzaldehyde and Example
12A,
respectively, in Example 12B. The concentrate was dissolved in
dichloromethane, treated
with 1M HCl in diethyl ether, and concentrated to provide the desired product.
MS (ESI(+)) m/z 556 (M+H)~;
1H NMR (400 MHz, CDC13) 8 7.94 (dd, 2H), 7.83 (d, 1H), 7.59-7.28 (m, 13H),
6.74 (d, 1H),
5.13-5.11 (m, 3H), 4.07 (m, 2H), 3.60 (d, 3H); 2.90-2.83 (m, 2H), 2.69-2.61
(m, 1H), 1.95-
1.72 (m, 2H), 1.36-1.30 (m, 2H).
Example 55
4-~(( 1-benz~-4-piperidin~l)amino) ( 1-methyl-1 H-imidazol-5=yl)meth~l)-2-( 1-
3o naphthyl)benzonitrile trih~drochloride
The desired product was prepared by substituting 1-benzyl-4-piperidinone and
Example 13A for 4-nitrobenzaldehyde and Example 12A, respectively, in Example
12B. The
concentrate was dissolved in dichloromethane, treated with 1M HCl in diethyl
ether, and
concentrated to provide the desired product.
MS (ESI(+)) m/z 512 (M+H)+;
-117-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (400 MHz, CDCl3) 8 7.96-7.91 (m, 2H), 7.82 (d, 1H), 7.59-7.22 (m, 13H),
6.71 (d,
1H), 5.12 (s, 1H), 3.60 (d, 3H), 3.51-3.45 (m, 2H), 2.82-2.74 (m, 2H), 2.52-
2.43 (m, 1H),
2.05-1.43 (m, 6H).
Example 56
tert-butyl 4- ((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5
yl)meth~)amino)-1
piperidinecarbox late
A solution of Example 13A (20 mg, 0.06 mmol) in 1,2-dichloroethane (1 mL) at
room
temperature was treated with tert-butyl 4-oxo-1-piperidinecarboxylate (11.8
mg, 0.06 mmol)
to and acetic acid (21 mg, 0.35 mmol), stirred for 30 minutes, treated with
sodium
triacetoxyborohydride (37.5 mg, 0.18 mmol), stirred for 16 hours, treated with
ethyl acetate,
washed sequentially with saturated NaHC03, water, and brine, dried (MgS04),
filtered, and
concentrated. The concentratewas dissolved in methanol (3 mL), heated to 60
°C for 1 hour,
cooled to room temperature, and concentrated. The concentrate was purified by
flash column
15 chromatography on silica gel with dichloromethane then 99:1 to
97:3/dichloromethane:methanol to provide the desired product.
MS (ESI(+)) m/z 522 (M+H)+;
1H NMR (500 MHz, CDC13) 8 7.96-7.92 (m, 2H), 7.83 (d, 1H), 7.59-7.39 (m, 8H),
6.71 (d,
1H), 5.14 (s, 1H), 3.99-3.98 (m, 2H), 3.61 (d, 3H), 2.80-2.75 (m, 2H), 2.67-
2.60 (m, 1H),
2o 1.88-1.60 (m, 3H), 1.45 (s, 9H), 1.33-1.25 (m, 2H).
Example 57
4-(t(1-benzoyl-4-piperidinyl)amino)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
25 The desired product was prepared by substituting 1-benzoyl-4-piperidinone
for tert-
butyl 4-oxo-1-piperidinecarboxylate in Example 56.
MS (ESI(+)) m/z 526 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 7.98-7.93 (m, 2H), 7.85 (d, 1H), 7.61-7.36 (m, 13H),
6.76 (d,
1H), 5.15 (s, 1H), 4.56 (s, 1H), 3.73-3.59 (m, 1H), 3.62 (d, 3H), 2.95-2.72
(m, 3H), 2.10-0.80
30 (m, 5H).
Example 58
4-((((4-cyano-3-(1-naphthyl)phenyl)(1-metal-1H-imidazol-5
yl)methyl)amino)met~l)benzamide dihydrochloride
35 A solution of Example 39 (20 mg, 0.04 mmol) in DMF (0.5 mL) at room
temperature
was treated sequentially with PyBOP (33 mg, 0.06 mmol), 0.5M ammonia in
dioxane (1 mL,
0.5 mmol), and HOBt, stirred for 16 hours, treated with ammonia, stirred for
16 hours, treated
-118-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
with ethyl acetate, washed sequentially with saturated NaHC03, water, and
brine, dried
(MgS04), filtered, and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with dichloromethane then 95:5 to
90:10/dichloromethane:methanol. The concentrate was dissolved in
dichloromethane, treated
with 1M HCl in diethyl ether, and concentrated to provide the desired product.
MS (ESI(+)) m/z 472 (M+H)+;
1H NMR (500 MHz, CDC13) 8 8.39 (d, 1H), 8.00-7.96 (m, 3H), 7.82 (dd, 2H), 7.75-
7.72 (m,
1H), 7.68-7.42 (m, 8H), 7.11 (s, 1H), 5.17 (d, 1H), 3.92-3.82 (m, 2H), 3.77
(d,~3H).
to Example 59
4-((1-methyl-1H-imidazol-5-'rl)((4-nitrobenzyl)ox~r)methyl)-2-(1-
na~hthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 89D and 4-nitrobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCT/NH3) m/z 475 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.09 (s, 1H), 8.22-8.02 (m, 6H), 7.79 (m, 1H),
7.69-7.43
(m, 9H), 6.19 (s, 1H), 4.8 (m, 2H), 3.79 (d, 3H).
Example 60
4-(((4-cyanobenz 1y )oxy)(1-meth 1-~midazol-5- 1)~yl)-2-iodobenzonitrile
hydrochloride
Example 60A
4-(hydroxymethyl)-2-iodobenzonitrile
The desired product was prepared by substituting Example 93C for Example 5A in
Example 5B.
Exam 1p a 60B
4-formyl-2-iodobenzonitrile
3o The desired product was prepared by substituting Example 60A for Example 5B
in
Example 5C.
Example 60C
4-(hydroxy(1-meth~rl-1H-imidazol-5 1y )methyl)-2-iodobenzonitrile
The desired product was prepared by substituting Example 60B for Example 1A in
Example 1B.
-119-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 8.04 (s, 1H), 7.85 (d, 1H), 7.58 (m, 2H), 6.39 (s,
1H), 6.22
(d, 1H), 5.88 (d, 1H), 3.55 (s, 3H).
Example 60D
4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-iodobenzonitrile
hydrochloride
The desired product was prepared by substituting Example 60C and 4-cyanobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively, in Example SE.
MS (DCI/NH3) m/z 455 (M+H)+;
l0 1H NMR (300 MHz, CDCl3) 8 9.1 (s, 1H), 8.0 (m, 1H), 7.7 (m, 4H), 7.5 (m,
3H), 5.68 (br s,
1H), 4.63 (m, 2H), 3.8 (br s, 3H).
Example 61
4-(((3-chloro-4-cyanobenzxl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2-(1
naphth~)benzonitrile hydrochloride
Example 61A
2-chloro-4-(hydroxymeth~)benzonitrile
The desired product was prepared by substituting Example 35B for Example 5A in
2o Example 5B.
MS (DCI/NH3) m/z 185 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 7.67 (d, 1H), 7.57 (s, 1H), 7.37 (d, 1H), 4.69 (d,
2H), 1.90 (t,
1H).
Example 61B
4-(bromometl~l)-2-chlorobenzonitrile
A solution of Example 61A (0.22 g, 1.31 mmol) and Liar (0.13 g, 1.44 mmol) in
DMF (2 mL) at 0 °C was treated with PBr3 (0.38 g, 1.39 mmol), stirred
for 30 minutes,
treated with water, and extracted with diethyl ether. The extract was washed
with water and
3o brine, dried (Na2S04), filtered, and concentrated to provide the desired
product of sufficient
purity to be used in subsequent steps without further purification.
1H NMR (300 MHz, CDC13) 8 7.67 (d, 1H), 7.57 (d, 1H), 7.40 (dd, 1H), 4.44 (s,
2H).
Exam 1p a 61C
4-(((3-chloro-4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methvl)-2-( 1-
naphthyl)benzonitrile hydrochloride
-120-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 89D and Example 61B
for
Example SD and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCItNH3) m/z 489 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.08 (m, 3H), 7.94 (m, 1H), 7.6 (m, 11H), 6.62 (m,
1H),
5.98 (s, 1H), 4.65 (m, 2H), 4.05 (s, 3H).
Example 62 .
4-(((4-cyanobenzyl)sulfanyl)(1-methyl-1H-imidazol-5- 1)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
to
Example 62A
4-(sulfanylmethyl)benzonitrile
A mixture of 4-cyanobenzyl bromide (10 g, 50 mmol) and thiourea (9.8 g, 100
mmol)
in ethanol (70 mL) was refluxed for 1 hour, cooled, and concentrated. The
concentrate was
15 washed with ethyl acetate, treated with 1.6M NaOH (100 mL), stirred for 22
hours, adjusted
to pH 4 with concentrated HCI, and extracted with diethyl ether. The extract
was washed
with water and brine, dried (Na2S04), filtered, and concentrated to provide
the desired
product of sufficient purity to be used in subsequent steps without further
purification.
20 Example 62B
4-(chloro(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile
A solution of Example 89D (100 mg, 0.29 mmol) in dichloromethane (10 mL) at
0 °C was treated with thianyl chloride (70 mg, 0.59 mmol), stirred for
15 minutes, warmed to
room temperature, stirred for 2 hours, and concentrated to provide the desired
product of
25 sufficient purity to be used in subsequent steps without further
purification.
Example 62C
4-(((4-cvanobenzvl)sulfanvl)(1-methyl-1H-imidazol-5-vl)methyl)-2-(1-
naphthvl)benzonitrile
hydrochloride
3o A solution of Example 62B in dichloromethane (5 mL) at room temperature was
treated with Example 62A (53 mg, 0.35 mmol) and diisopropylethylarnine (5 mL,
0.71
mmol), stirred for 18 hours, and concentrated. The concentrate was purified by
flash column
chromatography on silica gel with 98:2/chloroform:methanol, treated with 1M
HCl in diethyl
ether, and filtered to provide the desired product.
35 MS (DCI/NH3) mlz 471 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.3 (br s, 1H), 7.9 (m, 4H), 7.5 (m, 13H), 4.9 (br
s, 2H),
4.2 (br s, 1H), 3.8 (br s, 3H).
-121-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 63
4-(((4-cyano-3-iodobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)methxl)-2-(1
naphthyl)benzonitrile hydrochloride
Example 63A
2-iodo-4-methylbenzonitrile
The desired product was prepared by substituting 2-iodo-4-methylaniline for
Example
87A in Examples 87B and 87C.
l0
Example 63B
4-(bromometh'rl)-2-iodobenzonitrile
A mixture of Example 63A (11.6 g, 47.2 mmol), N-bromosuccinimide (9.2 g, 51.9
mmol), and benzoyl peroxide (57 mg, 0.24 mmol) in carbon tetrachloride (150
mL) was
15 heated to reflux for 18 hours, cooled to room temperature, washed with
water and brine, dried
(Na2S04), filtered, and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with 9:1/hexanes:ethyl acetate to provide the
desired product.
MS (DCI/NH3) m/z 339 and 341 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 8 7.95 (d, 1H), 7.59 (d, 1H), 7.48 (dd, 1H), 4.40 (s,
2H).
Example 63C
4-(((4-cyano-3-iodobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and Example 63B
for
Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 581 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.05 (s, 1H), 8.1 (m, 4H), 7.8 (m, 2H), 7.6 (m,
9H), 6.13
(s, 1H), 4.7 (m, 2H), 3.79 (d, 3H).
3o Example 64
methyl 4-((~4-cyano-3-(1-naphth~phenyl)(1-methyl-1H-imidazol-5
yl)methoxy)methyl)benzoate hydrochloride
The desired product was prepared by substituting Example 89D and 4
(bromomethyl)-benzoate for Example 5D and (bromomethyl)benzene, respectively,
in
Example 5E.
MS (DCI/NH3) m/z 488 (M+H)+;
-122-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 9.02 (s, 1H), 8.17 (m, 1H), 8.09 (m, 2H), 7.93 (m,
2H),
7.79 (m, 1H), 7.55 (m, 9H), 6.12 (s, 1H), 4.7 (m, 2H), 3.85 (s, 3H), 3.79 (d,
3H).
Example 65
4-((1-methyl-1H-imidazol-5-yl)((4-(trifluorometh~)benz l~y)methyl)-2-(1-
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and
4-(trifluoromethyl)benzyl bromide for Example 5D and (bromomethyl)benzene,
respectively,
in Example 5E.
to MS (DCI/NH3) m/z 498 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.09 (m, 1H), 8.17 (m, 1H), 8.08 (m, 2H), 7.79 (d,
1H), 7.6
(m, 12H), 6.18 (s, 1H), 4.75 (m, 2H), 3.80 (d, 3H).
Example 66
4-(((4-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)~xl)-2-(1-
naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 89D and
4-chlorobenzyl bromide for Example 5D and (bromomethyl)benzene, respectively,
in
Example 5E.
2o MS (DCI/NH3) m/z 464 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.03 (s, 1H), 8.17 (m, 1H), 8.09 (m, 2H), 7.78 (m,
2H),
7.62 (m, 5H), 7.51 (m, 2H), 7.41 (m, 4H), 6.10 (s, 1H), 4.6 (m, 2H), 3.78 (d,
3H).
Example 67
4-((1-methyl-1H-imidazol-5-yl)((4-(trifluoromethoxy)benzyl)oxy)methyl)-2-(1-
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and
4-(trifluoromethoxy)benzyl bromide for Example 5D and (bromomethyl)benzene,
respectively, in Example 5E.
3o MS (DCI/NH3) m/z 514 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.94 (s, 1H), 8.16 (m, 1H), 8.09 (m, 2H), 7.78 (m,
1H),
7.61 (m, 4H), 7.50 (m, 5H), 7.35 (m, 3H), 6.11 (s, 1H), 4.65 (m, 2H), 3.77 (d,
3H).
Example 68
4-(( 1-methyl-1 H-imidazol-5-yl) ((3-(trifluoromethyl)benzyl)oxy~methyl)-2-( 1-
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and
-123-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
3-(trifluoromethyl)benzyl bromide for Example 5D and (bromomethyl)benzene,
respectively,
in Example 5E.
MS (DCI/NH3) m/z 498 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 8.98 (s, 1H), 8.17 (m, 1H), 8.09 (m, 2H), 7.79 (m,
1H), 7.6
(m, 11H), 7.40 (m, 1H), 6.13 (s, 1H), 4.7 (m, 2H), 3.78 (d, 3H).
Example 69
lithium 4-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzoate
1o A solution of Example 64 (98 mg, 0.20 mmol) in methanol (2 mL) at room
temperature was treated with 1M LiOH (0.21 mL, 0.21 mmol), stirred for 48
hours, and
concentrated. The concentrate was treated with water, washed with diethyl
ether, and
lyophilized to provide the desired product of sufficient purity to be used in
subsequent steps
without further purification.
MS (DCI/NH3) m/z 474 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 8.09 (m, 3H), 7.78 (m, 3H), 7.6 (m, 7H), 7.20 (m,
2H),
6.55 (d, 1H), 5.89 (d, 1H), 4.51 (m, 2H), 3.54 (d, 3H).
Example 70
4-(((4-cyano-3-(1-naphth~phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-N,N-
dimethylbenzamide hydrochloride
A solution of Example 69 (50 mg, 0.10 mmol) and oxalyl chloride (0.10 mmol) in
dichloromethane (2 mL) was treated with DMF (1 drop), stirred for 1 hour, and
concentrated.
The concentrate was treated with ethyl acetate, washed with water and brine,
dried (Na2S04),
filtered, and concentrated. The concentrate was purified by flash column
chromatography on
silica gel with 98:2/chloroform:methanol, treated with HCI, and concentrated
to provide the
desired product.
MS (DCI/NH3) m/z 501 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 8.61 (m, 1H), 8.1 (m, 3H), 7.78 (m, 1H), 7.5 (m,
11H),
3o 7.17 (m, 1H), 6.08 (m, 1H), 4.65 (m, 2H), 3.71 (d, 3H), 2.97 (s, 3H), 2.88
(s, 3H).
Example 71
4 -(((4-cyanobenzyl)sulfonyl)( 1-methyl-1H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile
hydrochloride
A solution of Example 62C (31 mg, 0.07 mmol) in dichloromethane (2 mL) at room
temperature was treated with 70% m-CPBA (100 mg), stirred for 48 hours,
treated with ethyl
acetate, washed with saturated NaHC03 and brine, dried (Na2SOq.), filtered,
and
-124-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 98:2/chloroform:methanol. The appropriate fractions were treated with HCl
and
concentrated to provide the desired product.
MS (DCI/NH3) m/z 503 (M+H)+.
Example 72
4-(((2,4-dichlorobenz l~y)(1-methyl-1H-imidazol-5- 1)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
to Example 72A
2,4-dichloro-1-(iodomethyl)benzene
A solution of 2,4-dichlorobenzyl chloride (65 mg, 0.33 mmol) and NaI (0.5 g,
3.3
mmol) in acetone (5 mL) was heated to 50 °C, stirred for 18 hours, and
concentrated. The
concentrate was treated with dichloromethane (1.5 mL) and filtered to provide
the desired
product of sufficient purity for use in subsequent steps without further
purification.
Example 72B
4-(((2,4-dichlorobenzvl)oxv)(1-methyl-1H-imidazol-5-vl)methyl)-2-(1-
nanhthvl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 89D and Example 72A
for
Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 498 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.09 (s, 1H), 8.16 (m, 1H), 8.09 (m, 2H), 7.78 (m,
1H),
7.55 (m, 11H), 6.18 (s, 1H), 4.7 (m, 2H), 3.79 (d, 3H).
Example 73
4-((1-methyl-1H-imidazol-5-yl)((4-(methylsulfon 1)benz l~y)methyl)-2-(1
naphthyl)benzonitrile hydrochloride
3o Example 73A
1-(iodomethyl)-4-(methylsulfon 1 benzene
The desired product was prepared by substituting 4-(methylsulfonyl)benzyl
chloride
for 2,4-dichlorobenzyl chloride in Example 72A.
MS (DCI/NH3) m/z 314 (M+H)+.
Example 73B
-125-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((1-methyl-1H-imidazol-5-yl)((4-(methylsulfonyl)benzyl)ox )y methyl)-2-(1
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and Example 73A
for
Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 508 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.01 (m, 1H), 8.17 (m, 1H), 8.09 (m, 2H), 7.90 (m,
4H),
7.80 (m, 1H), 7.6 (m, 8H), 6.16 (s, 1H), 4.75 (m, 2H), 3.79 (d, 3H), 3.20 (s,
3H).
Example 74
l0 4-(((2,6-dichloro-4-p~ridinyl)methoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-
(1-
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 89D and
4-(bromomethyl)-2,6-dichloropyridine for Example 5D and (bromomethyl)benzene~
respectively, in Example 5E.
MS (DCI/NH3) m/z 499 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.04 (s, 1H), 8.17 (m, 1H), 8.08 (m, 2H), 7.81
(m,1H),
7.58 (m, 11H), 6.16 (m, 1H), 4.7 (m, 2H), 3.79 (d, 3H).
Example 75
4-(((3-bromo-4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile hydrochloride
Example 75A
2-bromo-4-(h~ymethyl)benzonitrile
The desired product was prepared by substituting Example 87C for Example 5A in
Example 5B.
MS (DCI/NH3) m/z 229 and 231 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) b 7.74 (s, 1H), 7.65 (m, 1H), 7.41 (d, 4.30 (s, 2H),
1.89 (br s,
1H).
Exam 1p a 75B
2-bromo-4-(bromomethyl)benzonitrile
The desired product was prepared by substituting Example 75A for Example 61A
in
Example 61B.
MS (DCI/NH3) m/z 293 (M+NH4.)+;
1H NMR (300 MHz, CDCl3) 8 7.73 (m, 1H), 7.64 (d, 1H), 7.44 (dd, 1H), 4.42 (s,
2H).
-126-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 75C
4-(((3-bromo-4-cyanobenzy~oxy) ( 1-methyl-1H-imidazol-5-yl)methyl)-2-( 1
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and Example 75B
for
Example SD and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCIlNH3) miz 533 and 535 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.07 (s, 1H), 8.17 (m, 1H), 8.09 (m, 2H), 7.91 (m,
2H),
7.80 (m, 1H), 7.55 (m, 9H), 6.15 (s, 1H), 4.73 (m, 2H), 3.79 (d, 3H).
to Example 76
6-(( 4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5
yl)methox )~yl)nicotinonitrile dihydrochloride
Example 76A
15 6-(bromomethyl)nicotinonitrile
The desired product was prepared by substituting 6-methylnicotinonitrile for
Example
63A in Example 63B.
MS (DCIiNH3) m/z 197 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.86 (s, 1H), 7.99 (dd, 1H), 7.60 (d, 1H), 4.58 (s,
2H).
Example 76B
6-(((4-cyano-3-( 1-naphthyl)phenxl) ( 1-methyl-1 H-imidazol-5
yl)methox )~yl)nicotinonitrile dihydrochloride
The desired product was prepared by substituting Example 89D and Example 76A
for
Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 456 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 9.12 (s, 1H), 8.98 (m, 1H), 8.32 (m, 1H), 8.11 (m,
3H),
7.80 (m, 1H), 7.6 (m, 10H), 6.23 (m, 1H), 4.72 (m, 2H), 3.81 (d, 3H).
3o Exam 1p e77
4~~(4-cyano-3-fluorobenz 1)oxv )(1-methyl-1H-imidazol-5-yl)methyl)-2-(1
naphthyl)benzonitrile I~drochloride
Exam 1p a 77A
2-fluoro-4-methylbenzonitrile
The desired product was prepared by substituting 2-fluoro-4-methylaniline for
Example 87A in Examples 87B and 87C.
-127-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (DCI/NH3) m/z 153 (M+NH~.}+;
1H NMR (300 MHz, CDC13} 8 7.50 (m, 1H), 7.16 (m, 2H), 2.44 (s, 3H).
Example 77B
4-(bromomethXl)-2-fluorobenzonitrile
The desired product was prepared by substituting Example 77A for Example 63A
in
Example 63B.
Example 77C
4-(((4-cyano-3-fluorobenz l~~r)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile hydrochloride
The desired product Was prepared by substituting Example 89D and Example 77B
for
Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) mlz 473 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.88 (br s, 1H), 8.16 (m, 1H), 8.08 (m, 2H}, 7.92
(m, 1H),
7.79 (m, 1H), 7.55 (m, 10H), 6.12 (m, 1H), 4.75 (m, 2H), 3.75 (d, 3H).
Example 78
5-((benzyloxy)(1-metal-1H-1,2,4-triazol-5-yl methyl-2'-methyl~l,l'-biphenyl)-2
carbonitrile hydrochloride
Example 78A
5-(hvdroxv( 1-methvl-1H-1.2.4-triazol-5-vllmethvll-2'-methyl( 1.1'-binhenvll-2-
carbonitrile
A solution of 1-methyl-1H-1,2,4-triazole (68 mg, 0.82 mmol) in THF (3 mL) at
-78 °C was treated with n-butyllithium (2.5M, 0.33 mL, 0.82 mmol),
stirred for 1 hour,
treated with a solution of 86I (150 mg, 0.68 mmol) in THF (2 mL), stirred for
16 hours while
warming to room temperature, and treated with 5.5M ammonium chloride to
provide two
layers. The aqueous layer was adjusted to a pH greater than 7 with sodium
bicarbonate and
extracted with dichloromethane. The extract was dried (MgS04), filtered, and
concentrated.
3o The concentrate was purified by flash column chromatography on silica gel
with 3:1/ethyl
acetate:hexanes to provide the desired product.
MS (ESI(+}) m/z 305 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.88 (s, 1H), 7.77 (d, 1H), 7.52 (m, 1H), 7.42 (s,
1H), 7.38-
7.25 (m, 3H), 7.17 (d, 1H), 6.26 (s, 1H), 3.81 (s, 3H), 2.15 (s, 3H).
Example 78B
-128-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-((benz~oxy)(1-methyl-1H-1,2,4-triazol-5-yl)methyl)-2'-methvl(1,1'-bi~hen l
carbonitrile hydrochloride
A solution of 78A (126 mg, 0.41 mmol) in dichloromethane (8 mL) at room
temperature was treated with silver(I) oxide (115 mg, 0.5 mmol) and benzyl
bromide (59 [~L,
0.5 mmol), stirred for 48 hours in darkness, filtered, and concentrated. The
concentrate was
purified by flash column chromatography on silica gel with 60:40/hexanes:ethyl
acetate. The
appropriate fractions were dissolved in acetonitrile, treated with 1M HCI, and
lyopholized to
provide the desired product.
MS (ESI(+)) m/z 395 (M+H)+;
1o 1H NMR (300 MHz, DMSO-d6) S 8.01 (d, 1H), 7.91 (s, 1H), 7.66 (dd, 1H), 7.50
(s, 1H),
7.42-7.28 (m, 8H), 7.24 (m, 1H), 6.14 (s, 1H), 4.57 (s, 2H), 3.84 (s, 3H),
2.11 (s, 3H);
Anal. calcd for C25H22N40~HC1~0.5 H20: C, 68.25; H, 5.50; N, 12.73; Cl, 8.06.
Found: C,
67.9; H, 5.61; N, 12.90; Cl, 8.34.
Exam 1p a 79
5-((benzyloxy)(1-meth~pyrazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
Example 79A
5-(hvdroxv(1-methyl-1H-nvrazol-5-vl)methyl)-2'-methyl(1.1'-binhenvll-2-
carbonitrile
The desired product was prepared by substituting 1-methyl-1H-pyrazole for 1-
methyl-
1H-1,2,4-triazole in Example 78A.
MS (ESI(+)) m/z 304 (M+H)+;
1H NMR (300 MHz, CDCl3) ~ 7.78 (d, 1H), 7.50 (d, 1H), 7.40 (m, 2H), 7.38-7.25
(m, 3H),
7.19 (d, 1H), 6.05 (d, 1H), 6.04 (s, 1H), 3.84 (s, 3H), 2.17 (s, 3H).
Example 79B
5-((benzyloxy)(1-methyl-1H-pyrazol-5-yl)meths)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
3o The desired product was prepared by substituting Example 79A for Example
78A in
Example 78B.
MS (DCI/NH3) m/z 394 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.01 (d, 1H), 7.64 (dd, 1H), 7.46 (d, 1H), 7.42-
7.27 (m,
5H), 6.00 (d, 1H), 5.98 (s, 1H), 4.54 (q, 2H), 3.75 (s, 3H), 2.11 (s, 3H);
Anal. calcd for C26Ha3N3O-HCl: C, 72.63; H, 5.63; N, 9.77. Found: C, 72.61; H,
5.64; N,
9.62.
-129-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 80
5-((benzYloxy)(3-thienyl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
Example 80A
5-(hydroxy(3-thien 1)~yl)-2'-meths(1,1'-biphenxl)-2-carbonitrile
A solution of 3-bromothiophene (70 ~,L, 0.75 mmol) in hexanes (3 mL) at -40
°C was
treated with n-butyllithium (2.5M, 0.33 mL, 0.82 mmol), stirred for 20
minutes, added to a
solution of 86I (150 mg, 0.68 mmol) in THF (3 mL) at -78 °C, stirred
for 16 hours while
warming to room temperature, treated with 5.5M ammonium chloride, and
extracted with
to dichloromethane. The extract was dried (MgS04), filtered, and concentrated.
The
concentrate was purified by flash column chromatography on silica gel with 9:1
to
4:1/hexanes:ethyl acetate to provide the desired product.
MS (ESI(+)) mlz 323 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 7.66 (d, 1H), 7.44 (dd, 1H), 7.36 (s, 1H), 7.29-7.11
(m, 6H),
6.92 (d, 1H), 5.91 (s, 1H), 2.10 (s, 3H).
Example 80B
5-((benz~y)(3-thienvl)methyl -2'-methxl(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 80A for Example 78A
in
2o Example 788.
1H NMR (300 MHz, CDC13) 8 7.72 (d, 1H), 7.49 (dd, 1H), 7.40-7.12 (m, 12H),
6.98 (m, 1H),
5.56 (s, 1H), 4.55 (m, 2H), 2.16 (s, 3H);
HRMS (FAB) calcd m/z for C26H22NO5: 396.1422 (M+H)+. Found: 396.1419.
Exam 1p a 81
5-((benzyloxy)(1-methyl-1H-1,2,3-triazol-5-yl)methyl)-2'-methyl(1,1'-biphen l
carbonitrile hydrochloride
Example 81A
5-(hydroxy(1-methyl-1H-1,2,3-triazol-5-yl)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting 1-methyl-1H-1,2,3-triazole
for 1-
methyl-1H-1,2,4-triazole in Example 78A.
MS (ESI(+)) m/z 305 (M+H)+.
Example 81B
5-((benz~y) ( 1-meth~rl-1H-1,2,3-tri azol-5-yl)methyl)-2'-methyl( 1,1'-
biphenyl)-2-
carbonitrile hydrochloride
-130-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 81A for Example 78A
in
Example 78B.
MS (ESI(+)) m/z 395 (M+H)+;
1H NMR (300 MHz, CD30D) 8 7.93 (d, 1H), 7.72 (s, 1H), 7.64 (dd, 1H), 7.51 (d,
1H), 7.37-
7.29 (m, 8H), 7.22 (m, 1H), 6.02 (s, 1H), 4.64 (s, 2H), 4.04 (s, 3H), 2.16 (s,
3H);
Anal. calcd for C25H22N40'HCI: C, 69.68; H, 5.38; N, 13.00. Found: C, 70.01;
H, 5.37; N,
13.08.
Example 82
5-(((2-cyclohexylethyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2'-
meth 1(
biphenyl)-2-carbonitrile hydrochloride
Example 82A
5-(((2-cyclohexylethyl)amino)methxl)-2'-methyl( 1,1'-biphenyl)-2-carbonitrile
A solution of 86I and 2-(cyclohexyl)ethylamine (153 mg, 1.21 mmol) in
dichloromethane (15 mL) at room temperature was treated with acetic acid (3
drops), stirred
for 1 hour, treated with sodium triacetoxyborohydride (384 mg, 1.81 mmol),
stirred for three
hours, treated with ethyl acetate, washed with saturated NaHC03, dried
(MgS04), filtered,
and concentrated. The concentrate was purified by flash column chromatography
on silica
2o gel with 0.2°1o concentrated ammonium hydroxide/ethyl acetate to
provide the desired
product.
MS (ESI(+)) m/z 333 (M+H)+;
1H NMR (300 MHz, CDC13) ~ 7.70 (d, 1H), 7.46 (m, 1H), 7.35 (m, 1H), 7.31 (m,
2H), 7.26
(m, 1H), 7.20 (d, 1H), 3.89 (s, 2H), 2.66 (dd, 2H), 2.19 (s, 3H), 1.67 (m,
5H), 1.43 (m, 2H),
1.32-1.10 (m, 4H), 0.95-0.83 (m, 2H).
Example 82B
5-(((2-cyclohexylethyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2'-
meth 1(
biphenyl)-2-carbonitrile hydrochloride
The free base of the desired product was prepared by substituting Example 82A
and
Example 32C for 2-(cyclohexyl)ethylamine and Example 86I, respectively, in
Example 82A.
The purified concentrate was dissolved in acetonitrile, treated with 1M HCI,
and lyopholized
to provide the desired product.
MS (ESI(+)) m/z 427 (M+H)+;
1H NMR (300 MHz, CD30D) 8 9.04 (s, 1H), 7.96 (m, 2H), 7.84 (m, 1H), 7.74 (m,
1H), 7.37
(m, 2H), 7.35-7.28 (m, 1H), 7.22 (m, 1H), 4.56 (br s, 4H), 3.94 (s, 3H), 3.18
(m, 2H), 2.19 (s,
3H), 1.73-1.64 (m, 7H), 1.31-1.14 (m, 4H), 1.01-0.89 (m, 2H);
-131-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Anal. calcd for CZ8H34Nq.~3.0I HCh0.48 H20: C, 61.71; H, 7.02; N, 10.28.
Found: C, 61.77;
H, 7.02; N, 9.91.
Example 83
4-(((2-cyclohexylethyl)((1-methyl-1H-imidazol-5- 1)~yl)amino)methyl)-2-(1-
naphthyl)benzonitrile dihydrochloride
Example 83A
4-(((2-cyclohexylethyl)amino)meth~)-2-( 1-naphth~rl)benzonitrile
to The desired product was prepared by substituting Example 89C for Example
86I in
Example 82A.
MS (ESI(+)) m/z 369 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.96 (d, 1H), 7.93 (d, 1H), 7.79 (d, 1H), 7.59-7.41
(m, 7H),
3.92 (s, 2H), 2.66 (t, 2H), 1.67 (m, 5H), 1.51 (s, 1H), 1.40 (m, 2H), 1.35-
1.06 (m, 4H), 0.96
0.83 (m, 2H).
Example 83B
4-(((2-cyclohexylethyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-(1
nanhthyl)benzonitrile dihydrochloride
2o The desired product was prepared by substituting Example 83A for Example
82A in
Example 82B.
MS (ESI(+)) m/z 463 (M+H)+;
1H NMR (300 MHz, CD30D) ~ 9.02 (s, 1H), 8.02 (m, 3H), 7.94 (m, 2H), 7.84 (m,
1H), 7.62
(m, 1H), 7.56 (m, 1H), 7.49 (m, 3H), 4.55 (br s, 4H), 3.95 (s, 3H), 3.18 (br
s, 2H), 1.66 (m,
7H), 1.29-0.91 (m, 6H);
Anal. calcd for C37H34N4~2.18 HChl.58 H20: C, 65.26; H, 6.95; N, 9.82. Found:
C, 65.30;
H, 6.95; N, 9.56.
Example 84
4-(((cyclohexylmethyl)((1-methyl-1H-imidazol-5-yl)methxl)amino)methyl)-2-(1-
naphthyl)benzonitrile dihydrochloride
Example 84A
4-(((c~clohex l~methyl)amino)methyl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting cyclohexylmethylamine for
2-(cyclohexyl)ethylamine in Example 83A.
-132-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 84B
4-(((c~clohex l~yl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 84A for Example 83A
in
Example 83B.
1H NMR (300 MHz, CD30D) b 9.01 (s, 1H), 8.09-7.75 (m, 6H), 7.64-7.47 (m, 5H),
4.5 (br s,
4H), 3.96 (s, 3H), 3.0 (br s, 2H), 1.85-1.68 (m, 6H), 1.29-1.13 (m, 3H), 0.92-
0.88 (m, 2H);
HRMS (FAB) calcd m/z for C3pHggNq.: 449.2705 (M+H)+. Found: 449.2715;
Anal. calcd for C3oH32N4'2.31 HC1~2.02 H20: C, 63.30; H, 6.79; N, 9.84. Found:
C, 63.28;
1o H, 6.79; N, 9.94.
Example 85
N-(4-cyano-3-(1-naphtha)benzyl)-N-(2-cyclohexylethyl)-2-(1H-imidazol-1-
yl)acetamide
15 Example 85A
2-chloro-N-(4-cyano-3-(-1-naphthyl)benzyl)-N-(2-cyclohexylethyl)acetamide
A solution of Example 83A (103 mg, 0.28 mmol) in dichloromethane (3 mL) and
pyridine (0.05 mL) at 0 °-C was treated with chloroacetic anhydride (53
mg, 0.31 mmol),
stirred for 1 hour, poured into 1M NaHS04, and extracted with dichloromethane.
The extract
20 was dried (MgS04), filtered, and concentrated to provide the desired
product of sufficient
purity for subsequent use without further purification.
Example 85B
N-(4-cyano-3-(1-naphth~)benzvl)-N-(2-cyclohex l~yl)-2-(1H-imidazol-1-
l~acetamide
25 A solution of Example 85A in DMSO (3 mL) at room temperature was treated
with
imidazole (57 mg, 0.83 mmol), stirred for 2 hours, heated to 50 °C, and
stirred for 16 hours.
The mixture was treated with saturated NaHC03, extracted with dichloromethane,
dried
(MgS04), filtered, and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with 98.8:1:0.2/ethyl
acetate:methanol:concentrated ammonium
3o hydroxide to provide the desired product.
MS (ESI(+)) m/z 477 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.09-7.99 (m, 3H), 7.68-7.41 (m, 8H), 7.03 (m,
1H), 6.84
(s, 1H), 5.06 (m, 2H), 4.80-4.68 (m, 2H), 3.40-3.25 (m, 2H), 2.54 (s, 2H),
1.63-0.83 (rn,
11H).
Example 86
5-(hydroxy(1-methyl-1H-imidazol-5-yl)meth~l)-2'-methyl(1,1'-bi henyl)-2-
carbonitrile
-133-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 86A
dimethyl2'-methyl(1,1'-biphenyl)-2,5-dicarbox late
The desired product was prepared by substituting 2-methylphenylboronic acid
for 2-
chlorophenylboronic acid in Example 10A.
Example 86B
6-(methoxycarbonyl)-2'-methyl(1,1'-biphenyl)-3-carbolic acid
The desired product was prepared by substituting Example 86A for Example 10A
in
to Example 10B.
Exam 1p a 86C
meth l~~~yl)-2'-methyl(1 1'-biphenyl)-2-carboxylate
The desired product was prepared by substituting Example 86B for Example lOB
in
15 Example 10C.
MS (DCIlNH3) m/z 257 (M+H)t;
IH NMR (300 MHz, CDCl3) 8 7.98 (d, 1H), 7.43 (dd, 1H), 7.28-7.16 (m, 4H), 7.07
(br d,
1H), 4.77 (s, 2H), 3.62 (s, 3H), 2.05 (s, 3H), 1.78 (br s, 1H).
20 Example 86D
4-(hydroxymethyl)-2-(1-na~hthyl)benzonitrile
A solution of Example 86C (6.0 g, 23.4 mmol) in dichloromethane (25 mL) at
room
temperature was treated with chloromethyl ethyl ether (4.4 mL, 4.5 g, 47 mmol)
and
diisopropylethyl amine (8.3 mL, 6.1 g, 47 mmol), stirred for 1.5 hours,
treated with water and
25 diethyl ether, and extracted with diethyl ether. The extract was washed
with brine, dried
(Na2S04), filtered, and concentrated to provide the desired product of
sufficient purity for
subsequent use without further purification.
MS (DCM3) m/z 315 (M+H)+;
~H NMR (300 MHz, CDCl3) 8 7.98 (d, 1H), 7.40 (dd, 1H), 7.28-7.16 (m, 4H), 7.07
(br d,
3o 1H), 4.78 (s, 2H), 4.67 (s, 2H), 3.65 (q, 2H), 3.60 (s, 3H), 2.05 (s, 3H),
1.21 (t, 3H).
Example 86E
5-((ethoxymethox )m~eth_yl)-2'-methyl,l'-biphenyl)-2-carboxylic acid
The desired product was prepared by substituting Example 86D for Example lOF
in
35 Example 10G.
Example 86F
-134-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-((ethoxymethox )methyl)-2'-methyl(1,1'-biphenvl~2-carboxamide
The desired product was prepared by substituting Example 86E for Example lOG
in
Example 10H.
MS (DCI/NH3) mlz 300 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.50 (d, 1H), 7.37 (dd, 1H),7.31 (br s, 1H), 7.25-
7.08 (m,
6H), 4.72 (s, 2H), 4.59 (s, 2H), 3.55 (q, 2H), 2.05 (s, 3H), 1.21 (t, 3H).
Example 86G
5-(hydroxymethyl)-2'-methyl 1,1'-biphenyl)-2-c arboxamide
to A solution of Example 86F (0.37 g, 1.2 mmol) in methanol (5 mL) at room
temperature was treated with concentrated HCl (0.1 mL), stirred for 16 hours,
and
concentrated. The concentrate was treated with toluene, concentrated, and
dried under
vacuum with PROS to provide the desired product of sufficient purity for
subsequent use
without further purification.
Example 86H
~hydroxymethyl -2'-methXl(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 86G for Example lOH
in
Example 10I.
2o MS (DCM3) mlz 241 (M+NH4.)~;
1H NMR (300 MHz, CDCl3) b 7.73 (d, 1H), 7.45 (m, 1H), 7.37 (m, 1H), 7.30 (m,
2H), 7.25
(m, 1H), 7.18 (br d, 1H), 4.80 (br d, 2H), 2.20 (s, 3H), 1.93 (br t, 1H).
Example 86I
5-formyl-2'-methyl( 1,1'-biphenyl)-2-c arbanitrile
The desired product was prepared by substituting Example 86H for Example 5B in
Example SC.
MS (DCI/NH3) mlz 239 (M+NH~.)+;
1H NMR (300 MHz, CDCl3) 8 10.12 (s, 1H), 7.95 (m, 2H), 7.89 (s, 1H), 7.42-7.30
(m, 3H),
7.22 (br d, 1H), 2.24 (s, 3H).
Exam 1p a 86J
5-(hydroxy(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 86I for Example 1A in
Example 1B.
MS (DCTlNH3) m/z 304 (M+H)~;
-135-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) ~ 7.95 (d, 1H), 7.60 (br d, 1H), 7.55 (s, 1H), 7.44
(br s, 1H),
7.38 (m, 2H), 7.30 (m, lI~, 7.22 (br d, 1H), 6.42 (s, 1H), 6.18 (d, 1H), 5.94
(d, 1H), 3.58 (s,
3H), 2.13 (br s, 3H).
Anal. calcd for C19H17N30~0.20 H20: C, 74.34; H, 5.71; N, 13.69. Found: C,
74.26; H, 5.74;
N, 13.68.
Exam 1p a 87
5-((benz,~y)(1-meths-1H-imidazol-5-,1)~ methyl)(1,1'-biphenyl)-2-carbonitrile
hydrochloride
Example 87A
ethyl 4-amino-3-bromobenzoate
A solution of ethyl 4-aminobenzoate (5.5 g, 33 mmol) in dichloromethane (48
mL), at
-12 °C, was treated with pyridine (5.5 mL, 5.4 g, 68 mmol) and a
solution of bromine (1.75
mL, 5.4 g, 34 mmol) in dichloromethane (15 mL), warmed to room temperature,
stirred for
16 hours, and treated with diethyl ether and 0.5M H3POq. to provide two
layers. The organic
layer was washed with brine, dried (Na2S04), filtered, and concentrated. The
concentrate
was purified by flash column chromatography on silica gel with
85:15/hexanes:ethyl acetate
to provide the desired product.
MS (DCIlNH3) mIz 244 and 246 (M+H)+, 261 and 263 (M+NH4)+;
1H NMR (300 MHz, CDCl3) ~ 8.11 (d, 1H), 7.80 (dd, 1H), 6.72 (d, 1H), 4.50 (br
s, 2H), 4.33
(q, 2H), 1.38 (t, 3H).
Example 87B
2-bromo-4-(ethoxycarbonyl)benzenediazonium tetrafluoroborate
A solution of Example 87A (1.2 g, 4.8 mmol) in dichloromethane (10 mL) at
-8 °C, was treated with a -8 °C solution of BF3~OEt2 (0.9 mL,
1.0 g, 7.3 mmol) and
tert-butyl nitrite (0.7 mL, 0.6 g, 5.9 mmol) and warmed to room temperature.
The mixture
was treated with hexanes and the resulting solid was removed by filtration to
provide the
3o desired product.
Example 87C
ethyl 3-bromo-4-cyanobenzoate
A solution of copper(I) cyanide (520 mg, 5.8 mmol) and sodium cyanide (710 mg,
14.5 mmol) in water (3.5 mL) at 5 ° C was treated with toluene (1.5 mL)
and Example 87B,
stirred for 30 minutes, warmed to room temperature, heated to 60 °C for
25 minutes, cooled
to room temperature, and treated with water and ethyl acetate. The organic
layer was washed
-136-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
with brine, dried (Na2S04), filtered, and concentrated. The concentrate was
treated with
hexanes (22 mL), heated to 60 °C, decanted, cooled to room temperature,
cooled to 4 °C for
16 hours, and filtered to provide the desired product.
MS (DCI/NH3) m/z 271 and 273 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 8.33 (d, 1H), 8.07 (dd, 1H), 7.74 (d, 1H), 4.43 (q,
2H), 1.42 (t,
3H).
Example 87D
eth.Yl 6-cyano( 1,1'-biphenyl)-3-carbox~rlate
The desired product was prepared by substituting Example 87C and phenylboronic
acid for 3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid,
respectively, in
Example 1A.
MS (DCI/NH3) m/z 269 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 8.19 (d, 1H), 8.10 (dd, 1H), 7.84 (d, 1H), 7.59 (m,
2H), 7.50
(m, 3H), 4.43 (q, 2H), 1.42 (t, 3H).
Example 87E
5-(~droxymethyl)( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 87D for Example 5A in
2o Example 5B.
MS (DCI/NH3) m/z 227 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 7.77 (d, 1H), 7.50 (m, 7H), 4.82 (d, 2H), 1.91 (t,
1H).
Example 87F
1-meth-2-(triethylsilyl)-1H-imidazole
A solution of 1-methylimidazole (23 mL, 23.7 g, 288 mmol) in THF (700 mL) at
-73 °C was treated dropwise with 2.5M n-butyllithium in hexanes (125
mL, 312 mmol),
warmed to 0 °C, stirred for 30 minutes, cooled to -73 °C,
treated with chlorotriethylsilane (50
g, 330 mmol), warmed to room temperature, stirred for 16 hours, and
concentrated. The
concentrate was treated with ethyl acetate and water, the organic layer was
washed with
brine, dried (Na2S04), filtered, and concentrated. The concentrate was
purified by vacuum
distillation (0.5-0.6 mmHg, 98-100 °C) with a 6 inch Vigeraux column to
provide the desired
product.
1H NMR (300 MHz, CDC13) S 7.19 (s, 1H), 6.96 (s, 1H), 3.75 (s, 3H), 1.00 (m,
9H), 0.93 (m,
6H).
Example 87G
-137-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-formyl(1,1'-biphen~)-2-carbonitrile
The desired product was prepared by substituting Example 87E fox Example 5B in
Example 5C.
MS (DCI/NH3) m/z 225 (M+NH~.)+;
1H NMR (300 MHz, CDCl3) ~ 10.12 (s, 1H), 8.02 (m, 1H), 7.95 (m, 2H), 7.60 (m,
2H), 7.54
(m, 3H).
Example 87H
5-(h~droxy( 1-methyl-1H-imidazol-5-'rl)methyl)( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 87G for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 290 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.95 (d, 1H), 7.63 (s, 1H), 7.55 (s, 1H), 7.55 (m,
7H)~ 6.46
(s, 1H), 6.18 (d, 1H), 5.95 (d, 1H), 3.58 (s, 3H).
Example 87I
5-((benzyloxy)(1-methyl-1H-imidazol-5- 1)~ methxl)(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 87H for Example 2B in
2o Example 2C.
MS (APCI(+)) mlz 380 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.06 (d, 1H), 7.69 (m, 2H), 7.60 (m,
2H),
7.55 (m, 3H), 7.35 (m, 6H), 6.10 (s, 1H), 4.65 (dd, 1H), 4.55 (dd, 1H), 3.78
(s, 3H).
Example 88
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)carbonyl)( 1,1'-biphenyl)-2-
carbonitrile
hydrochloride
A solution of Example 86F (50 mg, 0.16 mmol) in dioxane (2 mL), at 85
°C was
treated with Mn02 (105 mg, 1.2 mmol), stirred for 1 hour, cooled to room
temperature,
3o filtered through diatomaceous earth (Celite~), and concentrated. The
concentrate was
purified by flash column chromatography on silica gel with chloroform then
98:2/chloroform:methanol to provide the desired product.
MS (DCI/NH3) m/z 302 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.93 (br s, 1H), 8.17 (d, 1H), 8.08 (br s, 1H),
8.00 (dd,
1H), 7.82 (d, 1H), 7.40 (m, 2H), 7.35 (m, 2H), 4.03 (s, 3H), 2.19 (s, 3H).
Exam 1p a 89
-138-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile
hydrochloride
Example 89A
ethyl 4-cyano-3-(1-naphthyl)benzoate
The desired product was prepared by substituting Example 87C and
1-naphthylboronic acid for 3-bromo-4-fluorobenzaldehyde and 2-
methylphenylboronic acid,
respectively, in Example 1A.
MS (DCI/NH3) m/z 319 (M+NHq.)+;
1H NMR (300 MHz, CDC13) S 8.20 (m, 2H), 7.96 (dd, 2H), 7.90 (d, 1H), 7.55 (m,
2H), 7.47
(m, 3H), 4.43 (q, 2H), 1.39 (t, 3H).
Example 89B
4-(~drox,methyl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89A for Example 5A in
Example 5B.
MS (DCI/NH3) m/z 277 (M+NH4.)+;
1H NMR (300 MHz, CDC13) 8 7.94 (m, 2H), 7.82 (d, 1H), 7.50 (m, 7H), 4.87 (s,
2H).
Exam 1p a 89C
2o 4-formyl-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89B for Example 5B in
Example 5C.
MS (DCIlNH3) mlz 275 (M+NHq.)+;
1H NMR (300 MHz, CDC13) S 10.15 (s, 1H), 8.02 (m, 5H), 7.62-7.46 (m, SIB.
Example 89D
4-(h d~y(1-methyl-1H-imidazol-5- 1)~yl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89C for Example 1A in
Example 1B.
1H NMR (300 MHz, CDC13) b 7.95 (m, 2H), 7.84 (d, 1H), 7.62-7.38 (envelope,
8H), 6.74
and 6.72 (both s, total 1H), 6.02 (s, 1H), 3.61 and 3.59 (both s, total 3H).
Example 89E
4-((benz~y)(1-methyl-1H-imidazol-5- 1)~yl)-2-(1-naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 89D for Example 5D in
Example 5E.
MS (APCI(+)) m/z 430 (M+H)+;
-139-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.16 (m, 1H), 8.09 (m, 2H), 7.79 (d,
1H),
7.64 (m, 4H), 7.51 (m, 2H), 7.44 (s, 1H), 7.35 (m, 5H), 6.11 (s, 1H), 4.64 (m,
2H), 3.80 and
3.78 (both s, total 3H);
Anal. calcd for C~9H2q.C1N30~0.95 H20: C, 72.10; H, 5.40; N, 8.70. Found: C,
72.17; H,
5.43; N, 8.70.
Example 90
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(3-thienyl)benzonitrile
hydrochloride
to Example 90A
ethyl 4-cyano-3-(3-thienXl)benzoate
The desired product was prepared by substituting Example 87C and 3-
thienylboronic
acid for 3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid,
respectively, in
Example 1A.
MS (DCI/NH3) m/z 275 (M+NH4)+;
1H NMR (300 MHz, CDCl3) ~ 8.23 (m, 1H), 8.03 (dd, 1H), 7.80 (d, 1H), 7.73 (m,
1H), 7.47
(m, 3H), 7.46 (m, 2H), 4.43 (q, 2H), 1.42 (t, 3H).
Example 90B
4-(hydroxymethyl)-2-(3-thienyl)benzonitrile
The desired product was prepared by substituting Example 90A for Example 5A in
Example 5B.
MS (DGI/NH3) m/z 233 (M+NH4)+.
Example 90C
4-formyl-2-(3-thienyl)benzonitrile
The desired product was prepared by substituting Example 90B for Example 5B in
Example 5C.
MS (DCI/NH3) m/z 231 (M+NH4)+.
Example 90D
4-(hydroxyl 1-methyl-1H-imidazol-5-yl)methyl)-2-(3-thienyl)benzonitrile
The desired product was prepared by substituting Example 90C for Example 1A in
Example 1B.
MS (APCI(+)) m/z 296 (M+H)+.
Example 90E
-140-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((benz loxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(3-thien~)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 90D for Example 5D in
Example 5E.
MS (APCI(+)) m/z 386 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.02 (d, 1H), 7.95 (m, 1H), 7.77 (m,
2H),
7.62 (dd, 1H), 7.48 (dd, 1H), 7.37 (m, 6H), 6.04 (s, 1H), 4.58 (dd, 2H), 3.78
(s, 3H).
Example 91
5-((benzyloxy~(1-methyl-1H-imidazol-5-yl)methyl)-3'-methyl(1,1'-bi henyl)-2-
carbonitrile
to hydrochloride
Example 91A
ethyl 6-cyano-3'-methyl( 1,1'-biphenyl)-3-carboxylate
The desired product was prepared by substituting Example 87C and 3-
methylphenylboronic acid for 3-bromo-4-fluorobenzaldehyde and 2-
methylphenylboronic
acid, respectively, in Exaranple 1A.
MS (APCI(+)) m/z 283 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) 8 8.18 (d, 1H), 8.08 (dd, 1H), 7.82 (d, 1H), 7.40 (m,
3H), 7.30
(m, 1H), 4.43 (q, 2H), 2.45 (s, 3H), 1.42 (t, 3H).
Example 91B
5-(h~ymethyl)-3'-methyl( 1,1'-biphenyl)-2-c arbonitrile
The desired product was prepared by substituting Example 91A for Example 5A in
Example 5B.
MS (DCI/NH3) mlz 241 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) b 7.75 (d, 1H), 7.51 (s, 1H), 7.43 (d, 1H), 7.37 (m,
3H), 7.27
(m, 1H), 4.82 (s, 2H), 2.44 (s, 3H).
Example 91C
5-formyl-3'-methyl( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 91B for Example 5B in
Example 5C.
MS (DCI/NH3) mlz 239 (M+NH4)+;
1H NMR (300 MHz, CDC13) 810.12 (s, 1H), 8.00 (m, 1H), 7.92 (m, 2H), 7.40 (m,
3H), 7.30
(m, 1H), 2.46 (s, 3H).
Example 91D
-141-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(hydroxy(1-methyl-1H-imidazol-5- 1)~ methyl)-3'-methyl(1,1'-bi henyl)-2-
carbonitril
The desired product was prepared by substituting Example 91C for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 304 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.94 (d, 1H), 7.62 (s, 1H), 7.57 (m, 2H), 7.42 (m,
1H),
7.38 (m, 2H), 7.30 (m, 1H), 6.45 (s, 1H), 6.20 (d, 1H), 5.95 (d, 1H), 3.58 (s,
3H), 2.40 (s,
3H).
Exam 1p a 91E
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-3'-methyl(1,1'-biphen~)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 91D for Example 5D in
Example 5E.
MS (APCI(+)) m/z 394 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.07 (m, 1H), 7.68 (m, 2H), 7.38 (m,
10H),
6.09 (s, 1H), 4.60 (dd, 2H), 3.78 (s, 3H), 2.40 (m, 3H);
Anal. calcd for C26H~3C1N3O~H2O: C, 69.71; H, 5.85; N, 9.38. Found: C, 69.75;
H, 5.70; N,
9.38.
2o Example 92
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(2-naphthyl)benzonitrile
hydrochloride
Exam 1p a 92A
ethyl 4-cyano-3-(2-naphthxl)benzoate
The desired product was prepared by substituting Example 87C and 2-
naphthylboronic acid for 3-bromo-4-fluorobenzaldehyde and 2-
methylphenylboronic acid,
respectively, in Example 1A.
MS (DCI/NH3) m/z 319 (M+NH4)+;
1H NMR (300 MHz, CDCl3) b 8.30 (d, 1H), 8.13 (dd, 1H), 8.07 (d, 1H), 8.00 (d,
1H), 7.95
(m, 2H), 7.90 (d, 1H), 7.70 (dd, 1H), 7.58 (m, 2H), 4.44 (q, 2H), 1.43 (t,
3H).
Exam 1p a 92B
4-(hydroxymethyl)-2-(2-naphthyl)benzonitrile
The desired product was prepared by substituting Example 92A for Example 5A in
Example 5B.
MS (DCIlNH3) m/z 277 (M+NH4)+;
-142-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDCl3) 8 8.05 (d, 1H), 7.96 (d, 1H), 7.90 (m, 2H), 7.80 (d,
1H), 7.67
(dd, 1H), 7.63 (s, 1H), 7.53 (m, 2H), 7.47 (dd, 1H), 4.85 (d, 2H), 1.88 (t,
1H).
Example 92C
4-formyl-2-(2-naphthyl)benzonitrile
The desired product was prepared by substituting Example 92B fox Example 5B in
Example 5C.
MS (DCI/NH3) m/z 275 (M+NHq.)~;
tH NMR (300 MHz, CDC13) 810.18 (s, 1H), 8.14 (s, 1H), 8.09 (s, 1H), 8.00 (m,
3H), 7.94
(m, 2H), 7.70 (dd, 1H), 7.59 (m, 2H).
Example 92D
4-(h droxy~l-methyl-1H-imidazol-5-yl)methyl)-2-(2-naphthyl)benzonitri1e
The desired product was prepared by substituting Example 92C for Example 1A in
Example 1B.
MS (DCIlNH3) m1z 340 (M+H)+;
1H NMR (300 MHz, CDC13) 8 8.13 (s, 1H), 8.08 (d, 1H), 8.02 (m, 2H), 7.98 (d,
1H), 7.77 (s,
1H), 7.70 (dd, 1H), 7.60 (m, 3H), 7.57 (s, 1H), 6.48 (s, 1H), 6.21 (d, 1H),
5.98 (d, 1H), 3.60
(s, 3H).
Example 92E
4-((benzyloxy)(1-methyl-1H-imidazol-5- 1)~ methyl)-2-(2-naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 92D for Example 5D in
Example 5E.
MS (APCI(+)) m/z 430 (M+H)~;
1H NMR (300 MHz, DMSO-d6) 8 9.12 (s, 1H), 8.15 (m, 2H), 8.11 (m, 2H), 8.03 (m,
2H),
7.81 (s, 1H), 7.73 (m, 2H), 7.62 (m, 2H), 7.38 (m, 5H), 6.12 (s, 1H), 4.62
(dd, 2H), 3.80 (s,
3H);
Anal. calcd for C29H24C1N30~1.00 H20: C, 71.87; H, 5.41; N,8.68. Found: C,
71.87; H,
5.39; N, 8.65.
Example 93
5-((benzyloxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-4'-methyl( l , l'-
bip_henyl)-2-carbonitrile
hydrochloride
Example 93A
ethyl 4-amino-3-iodobenzoate
-143-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of ethyl 4-aminobenzoate (6.0 g, 36 mmol) in dichloromethane (110
mL)
and methanol (65 mL) at room temperature was treated with calcium carbonate
(10.8 g, 108
mmol) and benzyltrimethylammonium dichloroiodate (25 g, 72 mmol), stirred for
16 hours,
and filtered. The filtrate was washed with 5% NaHS03, dried (Na2S04),
filtered, and
concentrated to provide a solid. The solid was recrystallized from ethanol and
water, treated
with diethyl ether, stirred for 30 minutes, and filtered. The filtrate was
concentrated to
provide the desired product.
MS (DCI/NH3) mlz 292 (M+H)+ and 309 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 8.33 (d, 1H), 7.82 (dd, 1H), 6.70 (d, 1H), 4.51 (br
s, 2H), 4.42
(q, 2H), 1.38 (t, 3H).
Example 93B
4-(ethoxycarbonyl)-2-iodobenzenediazanium tetrafluoroborate
The desired product was prepared by substituting Example 93A for Example 87A
in
Example 87B.
Exam 1p a 93C
ethyl 4-cyano-3-iodobenzoate
The desired product was prepared by substituting Example 93B for Example 87B
in
2o Example 87C.
1H NMR (300 MHz, CDC13) 8 8.56 (d, 1H), 8.10 (dd, 1H), 6.70 (d, 1H), 4.42 (q,
2H), 1.41 (t,
3H).
Example 93D
methyl 4-amino-3-iodobenzoate
The desired product was prepared by substituting methyl-4-aminobenzoate for
ethyl-
4-aminobenzoate in Example 93A.
Example 93E
3o 2-iodo-4-(methoxycarbonyl)benzenediazonium tetrafluoroborate
The desired product was prepared by substituting Example 93D for Example 93A
in
Example 93B.
Example 93F
ethyl 4-cyano-3-iodobenzoate
The desired product was prepared by substituting Example 93E for Example 93B
in
Example 93C.
-144-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 93G
methyl 6-cyano-4'-methyl( 1,1'-biphenyl)-3-carboxylate
The desired product was prepared by substituting Example 93F and
4-methylphenylboronic for 3-bromo-4-fluorobenzaldehyde and 2-
methylphenylboronic acid
in Example 1A.
MS (DCI/NH3) m/z 269 (M+NH4.)+;
1H NMR (300 MHz, CDCl3) 8 8.18 (d, 1H), 8.06 (dd, 1H), 7.82 (d, 1H), 7.49 (d,
2H), 7.32
(d, 2H), 3.98 (s, 3H), 2.4 (s, 3H).
l0
Example 93H
5-(h~droxymethyl)-4'-methyl ( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 93G for Example 5A in
Example 5B.
MS (DCI/NH3) mlz 241 (M+NH4.)+;
1H NMR (300 MHz, CDCl3) ~ 7.75 (d, 1H), 7.51 (s, 1H), 7.49 (d, 2H), 7.43 (d,
1H), 7.32 (d,
2H), 4.82 (s, 2H), 2.44 (s, 3H).
Example 93I
5-formyl-4'-methyl(1,1'-biphenxl)-2-carbonitrile
The desired product was prepared by substituting Example 93H for Example 5B in
Example 5C.
MS (DCI/NH3) m/z 239 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 10.12 (s, 1H), 8.00 (s, 1H), 7.92 (s, 2H), 7.49 (d,
2H), 7.33 (d,
2H), 2.44 (s, 3H).
Example 93J
5-Lhydro~(1-rneth~-1H-imidazol-5-yl)methyl)-4'-methyl(1,1'-biphen~)-2-
carbonitrile
The desired product was prepared by substituting Example 93I for Example 1A in
3o Example 1B.
MS (DCI/NH3) m/z 304 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 7.93 (d, 1H), 7.62 (s, 1H), 7.55 (m, 3H), 7.46 (m,
2H),
7.35 (m, 2H), 6.45 (s, 1H), 6.20 (d, 1H), 5.95 (d, 1H), 3.58 (s, 3H), 2.40 (s,
3H).
Example 93I~
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-4'-methyl(1,1'-biphen~)-2-
carbonitrile
hydrochloride
-145-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 93J for Example 5D in
Example 5E.
MS (APCI(+)) mlz 394 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.07 (m, 1H), 7.65 (m, 2H), 7.50 (d,
2H),
7.38 (m, 8H), 6.09 (s, 1H), 4.60 (dd, 2H), 3.78 (s, 3H), 2.40 (m, 3H);
Anal. calcd for C26H23C1N3O~O.8O H2O: C, 70.28; H, 5.81; N, 9.46. Found: C,
70.21; H,
5.82; N, 9.46.
Example 94
5-((benz~rlox~)(1-methyl-1H-imidazol-5-'rl)meth 1y )2'-phenyl(1,1'-biphenyl)-2-
caxbonitrile
hydrochloride
Example 94A
2-(dihydroxyboryl)-1,1'-biphenyl
The desired product was prepared by substituting 2-bromobiphenyl for 3-bromo-
1,1'-
biphenyl in Example 6A.
MS (DCI/NH3) m/z 216 (M+NHq,)+.
Example 94B
2o ethyl 6-cyano-2'-phenyl( 1,1'-biphenyl)-3-c arboxylate
The desired product was prepared by substituting Example 93C and Example 94A
for
3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid, respectively, in
Example
1 A.
MS (DCI/NH3) m/z 345 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 7.95 (m, 2H), 7.62 (m, 1H), 7.55-7.40 (m, 4H), 7.20
(m, 2H),
7.10 (m, 2H), 4.35 (q, 2H), 1.38 (t, 3H).
Example 94C
5-(hydroxyl)-2'-phenyl( 1,1'-biphenyl)-2-carbonitrile
3o The desired product was prepared by substituting Example 94B for Example 5A
in
Example 5B.
MS (DCI/NH3) m/z 303 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 7.57 (d, 1H), 7.50 (m, 2H), 7.43 (m, 2H), 7.30 (m,
1H), 7.19
(m, 3H), 7.10 (m, 3H), 4.63 (s, 2H).
Exam 1p a 94D
5-form-2'-phenyl( 1,1'-biphenyl)-2-carbonitrile
-146-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 94C for Example 5B in
Example 5C.
MS (DCI/NH3) mlz 301 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 9.92 (s, 1H), 7.82 (m, 1H), 7.73 (m, 2H), 7.55-7.40
(m, 4H),
7.20 and 7.10 (both m, total 5H).
' Example 94E
5-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)-2'-phenxl(1 1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 94D for Example 1A in
to Example 1B.
MS (DCI/NH3) m/z 366 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.76 (d, 1H), 7.60-7.40 (m, 5H), 7.40-6.90
(envelope, 7H),
6.30-6.05 (envelope, 2H), 5.80 (d, 1H).
Exam 1p a 94F
5-((benz~y)(1-methyl-1H-imidazol-5-~rl)methyl)-2'-phen~l(1 1'-biphenyl)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 94E for Example 5D in
Example 5E.
2o MS (APCI(+)) m/z 456 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.05 (s, 1H), 7.96 (d, 1H), 7.55 (m, 5H), 7.40-
6.90
(envelope, 12H), 5.87 (s, 1H), 4.38 (dd, 2H), 3.50 (s, 3H);
Anal. calcd for C31H26C1N30~1.00 H20: C, 73.00; H, 5.53; N, 8.24. Found: C,
72.97; H,
5.54; N, 8.37.
Example 95
5-((benzyloxy)(1-methyl-1H-imidazol-5- 1)y methyl)-2' 5'-dimethyl(1 1'-biphen
l
carbonitrile hydrochloride
Example 95A
2,5-dimethylphenylboronic acid
The desired product was prepared by substituting 2-bromo-p-xylene for 3-bromo-
1,1'-
biphenyl in Example 6A.
MS (DCI/NH3) mlz 168 (M+NHq.)+.
Example 95B
ethyl 6-cyano-2',5'-dimethyl(1 1'-biphenyl)-3-carboxylate
-147-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 93C and Example 95A
for
3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid, respectively, do
Example
1A.
MS (DCI/NH3) m/z 297 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 8.10 (dd, 1H), 8.05 (d, 1H), 7.82 (d, 1H), 7.20 (m,
2H), 7.02
(s, 1H), 4.41 (q, 2H), 2.39 (s, 3H), 2.16 (s, 3H), 1.41 (t, 3H).
Example 95C
~~ymethyl)-2',5'-dimethyl( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 95B for Example 5A in
Example 5B.
MS (DCI/NH3) m/z 255 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 8 7.72 (d, 1H), 7.45 (m, 1H), 7.36 (s, 1H), 7.17 (m,
2H), 7.00 (s,
1H), 4.80 (s, 2H), 2.35 (s, 3H), 2.13 (s, 3H).
Example 95D
5-formyl-2',5'-dimethyl( l , l'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 95C for Example 5B in
Example 5C.
MS (DCI/NH3) m/z 253 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 10.11 (s, 1H), 7.95 (dd, 1H), 7.91 (d, 1H), 7.86 (m,
1H), 7.20
(m, 2H), 7.00 (s, 1H), 2.38 (s, 3H), 2.14 (s, 3H).
Example 95E
5-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)-2',5'-dimethyl(1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 95D for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 318 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.93 (d, 1H), 7.58 (m, 2H), 7.42 (br s, 1H), 7.20
(m, 2H),
7.02 (br s, 1H), 6.40 (s, 1H), 6.18 (d, 1H), 5.93 (d, 1H), 3.58 (s, 3H), 2.32
(s, 3H), 2.09 (s,
3H).
Example 95F
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2',5'-dimethyl(1,1'-biphenyl)-
2
carbonitrile hydrochloride
The desired product was prepared by substituting Example 95E for Example 5D in
Example 5E.
-148-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (APCI(+)) m/z 408 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.09 (s, 1H), 8.04 (d, 1H), 7.67 (dd, 1H), 7.49
(d, 1H),7.38
(m, 6H), 7.22 (m, 2H), 7.07 (br s, 1H), 6.07 (s, 1H), 4.60 (dd, 2H), 3.76 (s,
3H), 2.32 (s, 3H),
2.09 (s, 3H);
Anal. calcd for C27H26C1N3O~O.7O H2O: C, 71.03; H, 6.05; N, 9.20. Found: C,
71.03; H,
6.20; N, 9.26.
Example 96
4-(((4-cyanobenzyl)oxy)( 1-methyl-1 H-imidazol-5-)methyl)-2-( 1-
naphthyl)benzonitrile
to hydrochloride
The desired product was prepared by substituting Example 89D and 4-cyanobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively in Example 5E.
MS (APCI(+)) m/z 455 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 9.02 (s, 1H), 8.15 (dd, 1H), 8.09 (m, 2H), 7.80
(m, 3H),
i5 7.65 (m, 2H), 7.58 (m, 5H), 7.45 (m, 2H), 6.15 (s, 1H), 4.73 (m, 2H), 3.79
and 3.77 (both s,
total 3H);
Anal. calcd for C3~H23C1N40' 1.OO HZO: C, 70.79; H, 4.95; N, 11.01. Found: C,
70.99; H,
4.99; N, 10.93.
20 Example 97
4-(((2-methoxy-5-nitrobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(1
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 89D and 2-methoxy-5-
nitrobenzyl bromide for Example 5D and (bromomethyl)benzene, respectively, in
Example
25 5E.
MS (APCI(+)) miz 505 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.08 (s, 1H), 8.25 (m, 2H), 8.15 (dd, 1H), 8.09
(m, 2H),
7.76 (d, 1H), 7.70-7.50 (m, 5H), 7.46 (d, 1H), 7.40 (d, 1H), 7.20 (m, 1H),
6.18 and 6.17 (both
s, total 1H), 4.70 (m, 2H), 3.82 and 3.80 (both s, total 6H);
30 Anal. calcd for C3oH25C1N404~0.85 H20: C, 64.77; H, 4.84; N, 10.07. Found:
C, 64.74; H,
4.81; N, 10.01.
Example 98
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methxl)-2'-ethyl( 1,1'-biphenyl)-2-
carbonitrile
35 hydrochloride
Example 98A
-149-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
2-eth l~phenylboronic acid
The desired product was prepared by substituting 2-bromoethylbenzene for 3-
bromo-
1,1'-biphenyl in Example 6A.
MS (DCI/NH3) m/z 168 (M+NH4)+.
Example 98B
ethyl 6-cyano-2'-ethyl(1,1'-biphenyl)-3-carbox
The desired product was prepared by substituting Example 93C and Example 98A
for
3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid, respectively, in
Example
to 1A.
MS (DCI/NH3) m/z 297 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) 8 8.12 (d, 1H), 8.06 (s, 1H), 7.82 (d, 1H), 7.40 (m,
2H), 7.30
(m, 1H), 7.17 (d, 1H), 4.40 (q, 2H), 2.50 (m, 2H), 1.40 (t, 3H), 1.19 (t, 3H).
Exam 1p a 98C
2'-eth 1-~ydroxymethyl)(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 98B for Example 5A in
Example 5B.
MS (DCI/NH3) m/z 255 (M+NH~.)+;
1H NMR (300 MHz, CDC13) 8 7.72 (d, 1H), 7.46 (m,1H), 7.38 (m, 3H), 7.17 (m,
2H), 4.82
(s, 2H), 2.50 (m, 2H), 1.09 (t, 3H).
Example 98D
2'-ethyl-5-formyl( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 98C for Example 5B in
Example 5C.
MS (DCI/NH3) m/z 253 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) ~ 10.11 (s, 1H), 7.97 (dd, 1H), 7.92 (d, 1H), 7.89 (m,
1H), 7.40
(m, 2H), 7.30 (m, 1H), 7.18 (d, 1H), 2.50 (m, 2H), 1.10 (t, 3H).
Example 98E
2'-eth 1-~ydroxy(1-methyl-1H-imidazol-5- 1)~yl)(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 98D for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 318 (M+H)+;
-150-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 7.93 (d, 1H), 7.57 (m, 2H), 7.40 (m, 3H), 7.30 (m,
1H),
7.20 (m, 1H), 6.40 (m, 1H), 6.19 (m, 1H), 5.93 (d, 1H), 3.55 (s, 3H), 2.40 (m,
2H), 1.00 (m,
3H).
Example 98F
5-((benzyloxy)( 1-methyl-1H-imidazol-5-yl)methxl)-2'-ethyl( 1,1'-biphenyl)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 98E for Example 5D in
Example 5E.
1o MS (APCI(+)) m/z 408 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.12 (s, 1H), 8.07 (d, 1H), 7.70 (d, 1H), 7.51 (s,
1H), 7.40
(m, 3H), 7.35 (m, 7H), 6.09 (s, 1H), 4.60 (m, 2H), 3.76 (s, 3H), 2.40 (m, 2H),
1.00 (m, 3H);
Anal. calcd for C27H26C1N3O~O.9O H2O: C, 70.47; H, 6.09; N, 9.130. Found: C,
70.68; H,
5.85; N, 9.24.
Example 99
5-((benzyloxy) ( 1-methyl-1 H-imidazol-5-yl)methyl)-2', 3'-dimethyl ( 1,1'-
biphe~l)-2
carbonitrile hydrochloride
2o Example 99A
2,3-dimeth~phenylboronic acid
The desired product was prepared by substituting 3-bromoOxylene for 3-bromo-
1,1'-
biphenyl in Example 6A.
MS (DCI/NH3) m/z 168 (M+NH4)+.
Exam 1p a 99B
methyl 6-cyano-2', 3'-dimethyl( 1,1'-biphenyl)-3-carbox
The desired product was prepared by substituting Example 93F and Example 99A
for
3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid, respectively, in
Example
1A.
MS (DCI/NH3) m/z 283 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 8 8.10 (dd, 1H), 8.04 (m, 1H), 7.30 (d, 1H), 7.27 (d,
1H), 7.20
(dd, 1H), 7.04 (d, 1H), 3.96 (s, 3H), 2.09 (s, 3H).
Exam 1p a 99C
5-(h~ymethyl)-2',3'-dimethyl( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 99B for Example 5A in
-151-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 5B.
MS (DCI/NH3) m/z 255 (M+NH4);
1H NMR (300 MHz, CDCl3) 8 7.72 (d, 1H), 7.45 (m, 1H), 7.36 (s, 1H), 7.23 (d,
1H), 7.17
(dd, 1H), 7.03 (d, 1H), 4.81 (d, 2H), 2.35 (s, 3H), 2.09 (s, 3H), 1.85 (t,
1H).
Example 99D
5-formyl-2',3'-dimethyl( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting Example 99C for Example 5B in
Example 5C.
1o MS (DCI/NH3) m/z 253 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 10.11 (s, 1H), 7.95 (dd, 1H), 7.91 (d, 1H), 7.87 (s,
1H), 7.27
(d, 1H), 7.20 (d, 1H), 7.05 (d, 1H), 2.38 (s, 3H), 2.10 (s, 3H).
Example 99E
5-(hey(1-methyl-1H-imidazol-5-yl)methyl)-2',3'-dimethyl(l,l'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 99D for Example 1A in
Example 1B.
MS (DCI/NH3) m/z 318 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.93 (dd, 1H), 7.56 (m, 2H), 7.40 (d, 1H), 7.27
(d, 1H),
7.20 (m, 1H), 7.06 (m, 1H), 6.41 and 6.40 (both s, total 1H), 6.18 (m, 1H),
5.93 (d, 1H), 3.58
and 3.56 (both s, total 3H), 2.32 and 2.30 (both s, total 3H), 2.03 and 1.97
(both s, total 3H).
Example 99F
5-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2',3'-dimethyl(1,1'-biphenyl)-
2
carbonitrile hydrochloride
The desired product was prepared by substituting Example 99E for Example 5D in
Example 5E.
MS (APCI(+)) 408 m/z (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.09 (s, 1H), 8.04 (m, 1H), 7.67 (m, 1H), 7.48 (s,
1H), 7.37
(m, 7H), 7.20 (m, 1H), 7.14 and 7.05 (both d, total 1H), 6.07 (s, 1H), 4.60
(m, 2H), 3.76 (s,
3H), 2.32 and 2.30 (both s, total 3H), 2.03 and 1.97 (both s, total 3H);
Anal. calcd for C27H26C1N30~0.90 H20: C, 70.47; H, 6.09; N, 9.13. Found: C,
70.54; H,
5.88; N, 8.86.
Exam In a 100
4-((benz~y)(1-methyl-1H-imidazol-5- 1)~ l~yclohexylbenzonitrile hydrochloride
-152-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 100A
methyl 4-cyano-3-cyclohexylbenzoate
A mixture of 93F (400 mg, 1.4 mmol) and Pd(PPh3)4 (247 mg, 0.2 mmol) was
treated
with 0.33M cyclohexylzinc bromide in THF (5.5 mL, 1.8 mmol), heated to reflux,
stirred for
1 hour, cooled to room temperature, treated with water, diethyl ether, and 2M
HCl (3 drops),
washed with brine, dried (Na2S04), filtered, and concentrated. The concentrate
was purified
by flash column chromatography on silica gel with 95:5/hexanes:ethyl acetate
to provide the
desired product.
MS (DCI/NH3) m/z 261 (M+NH4)+;
l0 1H NMR (300 MHz, CDC13) 8 8.04 (d, 1H), 7.92 (dd, 1H), 7.69 (d, 1H), 3.96
(s, 3H), 3.01
(m, 1H), 1.90 (m, 4H), 1.80 (m, 1H), 1.50 (m, 4H), 1.30 (m, 1H).
Example 100B
2-cyclohex 1-~~ymethyl)benzonitrile
The desired product was prepared by substituting Example 100A for Example 5A
in
Example 5B.
MS (DCI/NH3) m/z 233 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 7.60 (d, 1H), 7.36 (s, 1H), 7.27 (m, 1H), 4.77 (d,
2H), 3.00
(m, 1H), 1.90 (m, 5H), 1.80 (m, 1H), 1.50 (m, 4H), 1.30 (m, 1H).
Example 100C
2-cyclohexyl-4-formylbenzonitrile
The desired product was prepared by substituting Example 1008 for Example 5B
in
Example 5C.
MS (DCI/NH3) m/z 231 (M+NH4)+;
1H NMR (300 MHz, CDC13) 810.08 (s, 1H), 7.88 (s, 1H), 7.78 (s, 2H), 3.00 (m,
1H), 1.90
(m, 4H), 1.80 (m, 1H), 1.50 (m, 4H), 1.30 (m, 1H).
Example 100D
2-cyclohexyl-4-(hydroxyl 1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
The desired product was prepared by substituting Example 100C for Example 1A
in
Example 1B.
MS (DCI/NH3) m/z 296 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.75 (d, 1H), 7.55 (m, 2H), 7.39 (d, 1H), 6.33 (s,
1H), 6.11
(d, 1H), 5.87 (d, 1H), 3.58 (s, 3H), 2.86 (m, 1H), 1.80 (m, 5H), 1.40 (m, 5H).
Example 100E
-153-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((benz~~(1-methyl-1H-imidazol-5-yl methyl)-2-cyclohexylbenzonitrile
hydrochloride
The desired product was prepared by substituting Example 100D for Example 5D
in
Example 5E.
MS (APCI(+)) m/z 386 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 7.90 (d, 1H), 7.59 (s, 1H), 7.48
(dd, 1H), 7.37
(m, 5H), 7.27 (s, 1H), 6.00 (s, 1H), 4.62 (d, 1H), 4.46 (d, 1H), 3.76 (s, 3H),
2.90 (m, 1H),
1.85 (m, 4H), 1.74 (m, 1H), 1.45 (m, 4H), 1.25 (m, 1H);
Anal. calcd for C25H2gC1N30~0.65 H20: C, 69.24; H, 6.81; N, 9.69. Found: C,
69.29; H,
6.79; N, 9.79._
Example 101
4-((benz~y)(1-methyl-1H-imidazol-5- 1)~ methyl)-2-(5,6,7,8-tetrah d
naphthalenyl)benzonitrile hydrochloride
Example lOlA
5-bromo-1,2,3,4-tetrah d~phthalene
A solution of copper(II) bromide (10.4 g, 46.7 mmol) and tert-butyl nitrite
(7.0 mL,
6.1 g, 58.5 mmol) in acetonitrile(150 mL) at 65 °C, was treated
dropwise with a solution of 1-
amino-5,6,7,8-tetrahydronaphthalene (6.1 mL, 6.5 g, 44 mmol) in acetonitrile
(10 mL),
stirred for 10 minutes, cooled to room temperature, treated with 3M HCI, and
extracted with
diethyl ether. The extract was washed with 3M HCl and brine, dried (Na2S04),
filtered, and
concentrated. The concentrate was distilled under vacuum (0.3 mm Hg, 77-86
°C) and
purified by flash column chromatography on silica gel with hexanes to provide
the desired
product.
1H NMR (300 MHz, CDC13) 8 7.38 (d, 1H), 7.01 (d, 1H), 6.95 (dd, 1H), 2.75 (m,
4H), 1.80
(m, 4H).
Example lOlB
5,6,7,8-tetrahydro-1-naphthalen~lboronic acid
The desired product was prepared by substituting Example lOlA for 3-bromo-1,1'-
biphenyl in Example 6A.
MS (DCI/NH3) m/z 194 (M+NH4)+.
Example lOlC
ethyl 4-cyano-3-(5,6,7,8-tetrahydro-1-naphthalenyl)benzoate
The desired product was prepared by substituting Example 93C and Example lOlB
for 3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid, respectively,
in
-154-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 1A.
MS (DCI/NH3) m/z 323 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 8 8.10 (dd, 1H), 8.03 (d, 1H), 7.80 (d, 1H), 7.20 (m,
2H), 7.00
(m, 1H), 4.41 (q, 2H), 2.86 (m, 2H), 2.42 (m, 2H), 1.80 (m, 4H), 1.40 (t, 3H).
Example 101D
4-(hydroxymethyl)-2-(5,6,7,8-tetrahydro-1-naphthalenyl)benzonitrile
The desired product was prepared by substituting Example 101C for Example 5A
in
Example 5B.
1o MS (DCI/NH3) m/z 281 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) b 7.73 (d, 1H), 7.44 (d, 1H), 7.38 (s, 1H), 7.16 (m,
2H), 7.00
(m, 1H), 4.81 (d, 2H), 2.88 (m, 2H), 2.45 (m, 2H), 1.80 (m, 5H).
Example lOlE
4-formyl-2-(5,6,7,8-tetrahydro-1-naphthalenyl)benzonitrile
The desired product was prepared by substituting Example lOlD for Example 5B
in
Example 5C.
MS (DCI/NH3) mlz 279 (M+NH4)+;
1H NMR (300 MHz, CDC13) 810.11 (s, 1H), 7.95 (dd, 1H), 7.91 (d, 1H), 7.87 (s,
1H), 7.20
(m, 2H), 7.00 (m, 1H), 2.88 (m, 2H), 2.45 (m, 2H), 1.80 (m, 4H).
Example 101F
4-(hydroxy(1-methyl-1H-imidazol-5- 1)~ methyl)-2-(5,6,7,8-tetrahydro-1
naphthalenyl)benzonitrile
The desired product was prepared by substituting Example 101E for Example 1A
in
Example 1B.
MS (DCI/NH3) m/z 344 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.90 (dd, 1H), 7.55 (m, 2H), 7.40 (d, 1H), 7.20
(m, 2H),
7.00 (m, 1H), 6.42 and 6.38 (both s, total 1H), 6.14 (m, 1H), 5.92 (d, 1H),
3.57 and 3.55 (both
3o s, total 3H), 2.80 (m, 2H), 2.35 (m, 2H), 1.70 (m, 4H).
Example lOlG
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)meths)-2-(5,6,7,8-tetrahydro-1
naphthalenyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example lOlF for Example 5D
in
Example 5E.
MS (APCI(+)) mlz 434 (M+H)+;
-155-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-dd) 8 9.10 (s, 1H), 8.05 (dd, 1H), 7.65 (dd, 1H), 7.48
(s, 1H),
7.37 (m, 6H), 7.20 (m, 2H), 7.10 and 7.00 (both m, total 1H), 6.06 (s, 1H),
4.60 (m, 2H), 3.76
and 3.74 (both s, total 3H), 2.81 (m, 2H), 2.37 (m, 2H), 1.70(m, 4H);
Anal. calcd for C29H28C1N30~1.20 HBO: C, 70.85; H, 6.23; N, 8.55. Found: C,
70.90; H,
6.17; N, 8.55.
Example 102
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(2-methyl-1-
naphthyl)benzonitrile
hydrochloride
l0
Example 102A
2-meth.1-~phthylboronic acid
A slurry of Rieke~ magnesium (0.5 g, 21 mmol) in THF (10 mL) at room
temperature was treated with 1-bromo-2-methylnaphthalene (3.0 mL, 4.2 g, 19
mmol), stirred
for 30 minutes, treated with a solution of trimethyl borate (10 mL, 9.1 g, 88
mmol) in diethyl
ether (20 mL), stirred for 1 hour, treated sequentially with NaOH and
concentrated HCI, and
extracted with ethyl acetate. The extract was dried (Na2S04), filtered, and
concentrated. The
concentrate was triturated with hexanes, and purified by flash column
chromatography on
silica gel with 4:1/hexanes:ethyl acetate to provide the desired product.
2o MS (DCI/NH3) m/z 218 (M+NH4)~.
Example 102B
ethyl 4-cyano-3-(2-methyl-1-naphth~)benzoate
The desired product was prepared by the method described in Synlett., 1992,
page 207
using Examples 93C and 102A.
MS (DCI/NH3) m/z 333 (M+NH4)+.
Example 102C
4-(h d~ymethyl)-2-(methyl-1-naphthyl)benzonitrile
3o The desired product was prepared by substituting Example 102B for Example
5A in
Example 5B.
Example 102D
4-formyl-2-(2-methyl-1-naphthyl)benzonitrile
A solution of Example 102C (45 mg, 0.16 mmol) in dichloromethane (1.7 mL) at
room temperature was treated with Dess-Martin periodinane (87 mg, 0.2 mmol),
stirred for
minutes, washed with saturated. NaHC03, dried (Na~S04), filtered, and
concentrated. The
-156-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
concentrate was purified by flash column chromatography on silica gel with
88:121hexanes:ethyl acetate to provide the desired product.
MS (DCI/NH3) m/z 289 (M+NH4)+.
Example 102E
4-(hey(1-methyl-1H-imidazol-5-yl)meths)-2-(2-methyl-1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 102D for Example 1A
in
Example 1B.
MS (DCI/NH3) m/z 354 (M+H)+.
Example 102F
4-((benz~y)(1-methyl-1H-imidazol-5-yl)methXl)-2-(2-meth 1-~phthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 102E for example 5D
in
Example 5E.
MS (APCI(+)) m/z 444 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.09 (s, 1H), 8.20 (d, 1H), 7.98 (d, 2H), 7.80 (m,
1H), 7.50
(m, 3H), 7.36 (m, 7H), 7.20 and 7.13 (both d, total 1H), 6.10 (s, 1H), 4.62
(m, 2H), 3.76 and
3.74 (both s, total 3H), 2.22 and 2.15 (both s, total 3H);
Anal. calcd for C3oH26C1N30~1.50 H20: C, 71.07; H, 5.76; N, 8.29. Found: C,
71.09; H,
5.57; N, 8.35.
Example 103
2-(1-anthryl)-4-((benz~y)(1-methyl-1H-imidazol-5- 1)~ methyl)benzonitrile
h'rdrochloride
Example 103A
1-iodoanthracene
A solution of 1-aminoanthracene (5.0 g, 26 mmol) in acetone (500 mL) was
treated
with 2M HCl (50 mL), cooled to 3 °C, treated dropwise with a solution
of sodium nitrite (2.0
3o g, 29 mmol) in water (25 mL), stirred for 1 hour, treated with urea (10.6
g, 10 mmol) and a
solution of KI (7.5 g, 45 mmol) in water (25 mL), stirred forl5 minutes,
warmed to room
temperature, stirred for 16 hours, heated to 60 °C, stirred for 20
minutes, cooled to room
temperature, and treated with 2M Na2S03 to provide a precipitate. The
precipitate was
collected by filtration and dried under vacuum with P205. The filtrate was
partially
concentrated and extracted with diethyl ether. The extract was dried (Na2S04),
filtered,
concentrated, and combined with the precipitate. The mixture was purified by
flash column
chromatography on silica gel with hexanes to provide the desired product.
-157-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (DCI/NH3) m/z 305 (M+H)+.
Example 103B
1-anthrylboronic acid
The desired product was prepared by substituting Example 103A for 3-bromo-1,1'-
biphenyl in Example 6A.
MS (DCI/NH3) m/z 240 (M+NHq.)+.
Example 103C
1o ethyl3-(1-anthr l~yanobenzoate
The desired product was prepared by substituting Example 93C and Example 103B
for 3-bromo-4-fluorobenzaldehyde and 2-methylphenylboronic acid, respectively,
in
Example 1A.
MS (DCIlNH3) mlz 369 (M+NHq.)+;
~H NMR (300 MHz, CDC13) 8 8.54 (s, 1H), 8.37 (m, 2H), 8.14 (d, 1H), 8.04 (d,
1H), 7.96
(m, 2H), 7.83 (d, 1H), 7.56 (m, 1H), 7.45 (m, 3H), 4.43 and 4.42 (both q,
total 2H), 1.39 (t,
3H).
Example 103D
2-(1-anthryl)-4-(hydroxymethyl)benzonitrile
The desired product was prepared by substituting Example 103C for Example 5A
in
Example 5B.
MS (DCI/NH3) m/z 327 (M+NH4.)+;
1H NMR (300 MHz, CDC13) S 8.51 (s, 1H), 8.10 (d, 1H), 8.03 (m, 2H), 7.85 (m,
2H), 7.60
(m, 2H), 7.55 (m, 2H), 7.45 (m, 2H), 4.89 (s, 2H).
Example 103E
2-(1-anthryl)-4-formylbenzonitrile
The desired product was prepared by substituting Example 103D for Example 102C
in Example 102D.
MS (DCI/NH3) m/z 325 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) 810.18 (s, 1H), 8.55 (s, 1H), 8.18-7.97 (m, 5H), 7.83
(d, 1H),
7.57 (m, 1H), 7.47 (m, 3H).
Example 103F
2-(1-anthryl)-4-(hydroxy(1-methyl-1H-imidazol-5-yl)methXl)benzonitrile
The desired product was prepared by substituting Example 103E for Example 1A
in
-158-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 1B.
MS (DCI/NH3) m/z 390 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.72 (d, 1H), 8.24 (d, 1H), 8.10 (m, 3H), 7.95 (m,
1H),
7.73 and 7.63 (both m, total 3H), 7.52 (m, 4H), 6.57 (s, 1H), 6.22 (m, 1H),
6.03 (d, 1H), 3.54
and 3.52 (both s, total 3H).
Example 1036
2-(1-anthryl)-4-((benz~y)(1-methyl-1H-imidazol-5- 1)~yl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 103F for Example 5D
in
Example 5E.
MS (APCI(+)) m/z 480 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.09 (s, 1H), 8.73 (s, 1H), 8.23 and 8.15 (both m,
total
4H), 8.02 and 7.92 (both d, total 1H), 7.83 (m 1H), 7.73 (m, 1H), 7.64 and
7.53 (both m, total
5H), 7.37 and 7.30 (both m, total 5H), 6.14 (s, 1H), 4.65 (m, 2H), 3.61 and
3.59 (both s, total
3H);
Anal. calcd for C33HzsC1N30~1.30 H20: C, 73.47; H, 5.34; N, 7.79. Found: C,
73.53; H,
5.47; N, 7.79
Example 104
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)-2-(4-
isoquinolinyl)benzonitrile
dihydrochloride
Example 104A
4-(diethylbor.1)~quinoline
The desired product was prepared by the method described in Heterocycles 1984,
Vo1.22, p.2471.
MS (DCI/NH3) m/z 198 (M+H)+.
Example 104B
methyl 4-cyano-3-(4-isoquinolinxl)benzoate
The desired product was prepared by substituting Example 93G, Example 104A,
and
DMF for Example 3B, 2-methylphenylboronic acid, and DME, respectively, in
Example 3C.
MS (DCI/NH3) m/z 289 (M+H)+;
1H NMR (300 MHz, CDC13) 8 9.39 (br s, 1H), 8.53 (br s, 1H), 8.26 (m, 1H), 8.22
(s, 1H),
8.12 (m, 1H), 7.96 (d, 1H), 7.71 (m, 2H), 7.52 (m, 1H), 3.98 (s, 3H).
Example 104C
-159-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(hydrox methyl)-2-(4-isoquinolinxl)benzonitrile
The desired product was prepared by substituting Example 104B for Example 5A
in
Example 5B, and by adjusting the aqueous layer to pH >7 with saturated NaHC03
prior to
extraction with diethyl ether.
MS (DCI/NH3) mlz 261 (M+H)+.
Example 104D
4-formyl-2-(4-isoquinolinyl)benzonitrile
The desired product was prepared by substituting Example 104C for Example 102C
in
l0 Example 102D.
MS (DCIlNH3) mlz 259 (M+H)~.
Example 104E
4-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)-2-(4-isoguinolinyl)benzonitrile
The desired product was prepared by substituting Example 104D for Example 1A
in
Example 1B.
MS (DCIlNH3) m/z 341 (M+H)~".
Example 104F
4-((benz~y)(1-methyl-1H-imidazol-5-yl)methyl)-2-(4-isoquinolinyl)benzonitrile
dihydrochloride
The desired product was prepared by substituting Example 104E for Example 5D
in
Example 5E.
MS (APCI(+)) 431 m/z (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.17 and 9.13 (both s, total 1H), 9.14 (s, 1H),
8.18 and
8.10 (both s, total 1H), 8.40 (m, 1H), 8.23 (m 1H), 7.90 (m, 3H), 7.70 (m,
2H), 7.46 (s, 1H),
7.37 (m, 5H), 6.13 (s, 1H), 4.63 (m, 2H), 3.81 and 3.79 (both s, total 3H).
Example 105
4-((benz loxy)(1-(ethoxymethyl)-1H-imidazol-5-yl)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
Example 105A
1-(etho~methyl)-1H-imidazole
A solution of imidazole (13 g, 191 mmol) in THF (200 mL) at room temperature
was
treated with small portions of 60% NaH (7.6 g, 190 mmol), stirred for 30
minutes, treated
with THF (100 mL) and chloromethyl ethyl ether (17.5 mL, 17.8 g, 189 mmol),
and stirred
-160-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
for 16 hours, filtered through a pad of diatomaceous earth (Celite~) and
concentrated. The
concentrate was purified by vacuum distillation (5-5.5 mmHg, 96-98 °C)
to provide the
desired product.
1H NMR (300 MHz, CDC13) 8 7.62 (s, 1H), 7.11 (s, 1H), 7.06 (s, 1H), 5.30 (s,
2H), 3.45 (q,
2H), 1.19 (t, 3H).
Example 105B
1-(ethoxymethyl)-2-(triethylsilyl)-1H-imidazole
The desired product was prepared substituting Example 105A for
l0 1-methylimidazole in Example 87F.
1H NMR (300 MHz, CDC13) 8 7.22 (s, 1H), 7.12 (s, 1H), 5.31 (s, 2H), 3.45 (q,
2H), 1.19 (t,
3H), 0.95 (m, 15H).
Example 105C
4-((1-(ethox~yl)-1H-imidazol-5-yl)(hydroxy)methyl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 105B and Example 89C
for Example 87F and Example 1A, respectively, in Example 1B.
MS (DCI/NH3) m/z 384 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.06 (m, 3H), 7.77 (s, 1H), 7.70-7.40 (m, 7H),
6.51 and
6.50 (both s, total 1H), 6.28 (d, 1H), 6.00 (d, 1H), 5.40 (m, 2H), 3.35 (m,
2H), 1.08 and 0.93
(both m, total 3H).
Example 105D
4-((benzyloxy) ( 1-(ethoxymethyl)-1 H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 105C for Example 5D
in
Example 5E.
MS (APCI(+)) m/z 474 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.11 (br s, 1H), 8.10 (m, 3H), 7.75 (d, 1H), 7.70-
7.45 (m,
7H), 7,34 (m, 5H), 6.07 (s, 1H), 5.55 (m, 2H), 4.60 (m, 2H), 3.35 (m, 2H),
0.94 (m, 3H);
Anal. calcd for C31H28C1N3O2~0.75 H2O: C, 71.12; H, 5.68; N, 8.03. Found: C,
71.16; H,
5.69; N, 8.08.
Example 106
4-(((4-cyanobenzyl)oxy)(1-(ethox r~~)-1H-imidazol-5- 1)methyl)-2-(1-
nanhthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 105C and
-161-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-cyanobenzyl bromide for Example 5D and (bromomethyl)benzene, respectively,
in
Example 5E.
MS (APCI(+)) mlz 499 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.10 (br s, 1H), 8.10 (m, 3H), 7.86 (m, 1H), 7.79
(m, 2H),
7.75-7.45 (m, 9H), 6.10 (s, 1H), 5.55 (m, 2H), 4.67 (m, 2H), 3.35 (rn, 2H),
0.94 (m, 3H);
Anal. calcd for C32H27C1N402~0.90 H20: C, 69.72; H, 5.27; N, 10.16. Found: C,
69.78; H,
5.28; N, 10.01.
Example 107
l0 4 ~((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methxl)- ,
2'-phenyl(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting Example 94E and 4-cyanobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (APCI(+)) miz 481 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 8.95 (br s, 1H), 7.95 (d, 1H), 7.82 (d, 2H), 7.50
(m, 7H),
7.30-6.90 (br m, 7H), 5.90 (s, 1H), 4.48 (m, 2H), 3.50 (s, 3H);
Anal. calcd for C32H25C1N40~1.30 H20: C, 71.12; H, 5.15; N, 10.37. Found: C,
71.13; H,
4.90; N, 10.35.
2o Example 108
4-(((4-cyanobenzyl oxy)(1-(3-hydroxypra ~)-1H-imidazol-5-yl)methyl)-2 ~1
naphthyl)benzonitrile ~drachloride
Example 108A
1-~3-((tert-butyl(dimethyl)silyl)oxy~propyl)-1H-imidazole
The desired product was prepared by substituting (3-chloropropoxy)-tert-
butyldimethylsilane for chloromethyl ethyl ether in Example 105A, and purified
by flash
column chromatography on silica gel with ethyl actate then 98:2:1/ethyl
acetate:ethanol:concentrated ammonium hydroxide.
3o MS (DCI/NH3) m/z 241 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.47 (s, 1H), 7.05 (s, 1H), 6.91 (s, 1H), 4.07 (t,
2H), 3.56 (t,
2H), 1.94 (m, 2H), 0.91 (s, 9H), 0.05 (s, 6H).
Example 108B
1-(3-((tent-butyl(dimethxl)silyl)oxy)propel)-2-(triethylsilyl)-1H-imidazole
The desired product was prepared by substituting Example 108A for imidazole in
Example 87F.
-162-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 108C
4-((1-(3-((tert-butyl(dimethyl)silyl)oxy)propyl)-1H-imidazol-5-y1)(h day
methyl)-2-(1
naphthyl)benzonitrile
The desired product was prepared by substituting Example 89C and Example 108B
for Example 1A and Example 87F, respectively, and by eliminating TBAF in
Example 1B.
MS (APCI(+)) m/z 498 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.06 (m, 3H), 7.71-7.37 (m, 8H), 6.51 and 6.52
(both s,
total 1H), 6.25 (m, 1H), 5.97 (d, 1H), 4.00 (m, 2H), 3.52 (m, 2H), 1.82 (m,
2H), 0.83 and 0.81
(both s, total 9H), 0.05 (m, 6H).
Example 108D
4- 1- 3-((tert-butyl(dimethyl)silyl)oxy)prop)-1H-imidazol-5-yl)((4
cyanobenzyl)oxy)meths)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 108C and
4-cyanobenzyl bromide for Example 5D and (bromomethyl)benzene, respectively,
in
Example 5E.
MS (APCI(+)) m/z 613 (M+H)+.
2o Example 108E
4-(((4-cyanobenz lay)(1-(3-hydroxypropyl)-1H-imidazol-5-yl)methyl)-2-(1
naphthyl)benzonitrile hydrochloride
A solution of Example 1080 (32 mg, 0.05 mmol) in THF (0.25 mL) at room
temperature was treated with 1M tetrabutylammonium fluoride in 95:5/THF:water
(0.1 mL),
stirred for 2.5 hours, and treated with half-saturated NH4Cl and ethyl acetate
to provide two
layers. The organic layer was washed with brine, dried (Na2SO4), filtered, and
concentrated.
The concentrate was purified by flash column chromatography using 97:3:1/ to
96:4:1/ethyl
acetate:ethanol:concentrated ammonium hydroxide. The appropriate fractions
were
concentrated and converted to the HCl salt to provide the desired product.
3o MS (APCI(+)) m/z 499 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.15 (m, 1H), 8.08 (m, 2H), 7.70 (m,
3H),
7.70-7.40 (m, 9H), 6.15 (s, 1H), 4.73 (m, 2H), 4.20 (m, 2H), 3.50 (m, 2H),
1.86 (m, 2H).
Example 109
5-(1-hydroxy-1-(1-methyl-1H-imidazol-5-xl)-3-phenyl-2-propynyl)-2'-methyl(1,1'-
biphenyl)-
2-carbonitrile
-163-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 109A
5-(1-oxo-1-(1-methyl-1H-imidazol-5-yl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
A solution of Example 4A (400 mg, 1.32 mrnol) in dioxane (8 mL) at 45
°C was
treated with manganese dioxide (400 mg, 4.6 mmol), refluxed for 5 hours,
cooled to room
temperature, filtered through a pad of diatomaceous earth (Celite~), and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
10:0.6:0.1/ethyl
acetate:methanol:concentrated ammonium hydroxide to provide the desired
product.
MS (DCI/NH3) m/z 302 (M+H)+ and 319 (M+NH4.)+;
1H NMR (300 MHz, CD30D) 8 8.02-7.95 (m, 3H), 7.80 (d, 1H), 7.61 (d, 1H), 7.42-
7.25 (m,
4H), 4.03 (s, 3H), 2.21 (s, 3H).
Example 109B
5-(1-hydroxy-1-(1-methyl-1H-imidazol-5-yl)-3-phenXl-2-prop~yl)-2'-methyl(1 1'-
b~henyl)
2-carbonitrile
i5 A solution of phenylacetylene (37 ~.L, 0.34 mmol) in THF (1 mL) at -78
°C was
treated with 1.5M tart-butyllithium in pentane (0.27 mL, 0.34 mmol), stirred
for 1 hour,
treated with Example 109A (50 mg, 0.17 mmol) in THF (1 mL), stirred for 16
hours while
warming to room temperature, treated with water, and extracted with ethyl
acetate. The
extract was dried (MgS04), filtered, and concentrated. The concentrate was
purified by flash
2o column chromatography on silica gel with 10:0.6:0.1/ethyl
acetate:methanol:concentrated
ammonium hydroxide to provide the desired product.
MS (DCI/NH3) m/z 404 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.81-7.68 (m, 3H), 7.46-7.21 (m, 10H), 6.96 (br s,
1H), 3.60
(s, 3H), 2.17 (s,~ 3H).
Example 110
5-(1-hydroxy-1-(1-methyl-1H-imidazol-5-yl)-3- henylpropyl)-2'-methvl(1,1'-
biphenyl)-2
carbonitrile
A mixture of Example 109B (25 mg, 0.062 mmol), palladium on barium sulfate (20
3o mg), and potassium hydroxide (20 mg) in methanol (2 mL) was stirred under
hydrogen (1
atm) for 16 hours, filtered through a pad of diatomaceous earth (Celite~), and
concentrated.
The concentrate was purified by flash column chromatography on silica gel with
10:0.6:0.1/ethyl acetate:methanol:concentrated ammonium hydroxide to provide
the desired
product.
MS (APCI(+)) m/z 408 (M+H)+;
1H NMR (300 MHz, CDC13) S 7.74 (d, 1H), 7.48-7.11 (m, 13H), 3.30 (s, 3H), 2.84
(m, 1H),
2.58-2.52 (m, 2H), 2.35 (m, 1H), 2.13 (s, 3H).
-164-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 111
4-( 1-hydrox~-1-( 1-methyl-1H-imidazol-5-yl)-3-phenyl-2-propynxl)-2-(1
naphthyl)benzonitrile
Example 111A
4-( 1-oxo-1-( 1-methyl-1H-imidazol-5-yl))-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89D for Example 4A in
Example 109A.
1o MS (DCI/NH3) m/z 338 (M+H)+ and 355 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) 8 8.00-7.95 (m, 4H), 7.77-7.46 (m, 8H), 4.13 (s, 3H).
Example 111B
4-(1-hydroxy-1-(1-methyl-1H-imidazol-5- l~)-3-phen~propynyl)-2-(1
naphthyl)benzonitrile
The desired product was prepared by substituting Example 111A for Example 109A
in Example 109B.
MS (DCI/NH3) m/z 440 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.98-7.80 (m, 3H), 7.71-7.26 (m, 13H), 6.98 (d, 1H),
3.64 (d,
3H).
Example 112
4-(((4-fluoro-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzonitrile para-toluenesulfonic acid salt
Example 112A
4-fluoro-3-(1-naphthyl)benzaldehyde
The desired product was prepared by substituting 2-naphthylboronic acid and
tetrakis(triphenylphosphine)palladium(0) for 2-methylphenylboronic acid and
palladium(II)
acetate, respectively in Example 1A.
MS (DCI/NH3) m/z 250 (M+H)+;
1H NMR (300 MHz, CDC13) 810.04 (s, 1H), 8.06-7.9 (m, 4H), 7.59-7.32 (m, 6H).
Example 112B
(4-fluoro-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5=yl)methano1
-165-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 112A for Example 1A
in
Example 1B and chromatographed on silica gel with 10:0.6:0.1iethyl
acetate:methanol:concentrated ammonium hydroxide to provide the desired
product.
MS (DCI/NH3) m/z 333 (M+H)+;
1H NMR (300 MHz, CD30D) 8 7.93 (d, 2H), 7.59-7.39 (m, 7H), 7.28 (dd, 1H), 7.00
(dt, 1H),
6.61 (s, 1H), 5.96 (s, 1H), 3.69 (s, 3H).
Example 112C
4-(((4-fluoro-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5
to yl)methox ),~methyl)benzonitrile pare-toluenesulfonic acid salt
The desired product was prepared by substituting Example 112B and
4-(bromomethyl)benzonitrile for Example 5D and (bromomethyl)benzene,
respectively, in
Example 5E, and chromatographed on silica gel with 10:0.6:0.1/ethyl
acetate:methanol:concentrated ammonium hydroxide. The appropriate fractions
were
15 concentrated, and the free base was dissolved in ethanol, treated with pare-
toluenesulfonic
acid, and concentrated to provide the desired product.
MS (DCI/NH3) m/z 448 (M+H)+;
1H NMR (300 MHz, CD30D) S 8.89 (s, 1H), 7.94 (d, 2H), 7.70-7.62 (m, 4H), 7.60-
7.46 (m,
6H), 7.39 (t, 2H), 7.27 (m, 1H), 7.17 (d, 2H), 5.95 (s, 1H), 4.77 (s, 2H),
4.73 (m, 1H), 3.86 (s,
20 3H), 2.33 (s, 3H);
Anal. calcd. for C29H22N3F0~(CH3)C6HøSO3H~H2O: C, 67.80; H, 5.06; N, 6.59.
Found: C,
67.97; H, 5.09; N, 6.47.
Example 113
25 5-(((3-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
A suspension of silver(I) oxide (45 mg, 0.196 mmol) in dichloromethane(2 mL)
at
room temperature was treated with Example 86J (20 mg, 0.066 mmol) and
3-(bromomethyl)benzonitrile (15 mg, 0.076 mmol), and stirred for 16 hours,
treated with
30 methanol (2 mL), centrifuged, decanted, and concentrated. The concentrate
was dissolved in
1:1lDMSO:methanol (1 mL) and purified by preparative HPLC. The concentrate was
dissolved in dichloromethane (1 mL), treated with 1M HCl in diethyl ether (1
mL), and
concentrated to provide the desired product.
MS (APCI(+)) mlz 419 (M+H)+;
35 MS (APCI(-)) m/z 453 (M+Cl) ;
-166-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (500 MHz, DMSO-d6) 8 9.04 (s, 1H), 8.05 (d, 1H), 7.85 (s, 1H), 7.79
(dt, 1H), 7.71
(dt, 1H), 7.68 (dd, 1H), 7.57 (t, 1H), 7.52 (d, 1H), 7.42 (s, 1H), 7.40-7.37
(m, 2H), 7.32 (m,
1H), 7.26 (br s, 1H), 6.09 (s, 1H), 4.71 (d, 1H), 4.61 (d, 1H), 3.75 (s, 3H),
2.12 (s, 3H).
Example 114
4-(((4-cyanobenzyl)((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-(8
quinolinyl)benzonitrile
Example 114A
to 4-formyl-2-(8-quinolinyl)benzonitrile
The desired product can be prepared by substituting Example 200A and
8-quinolinylboronic acid for 3-bromo-4-fluorobenzaldehyde and 2-
methylphenylboronic
acid, respectively in Example 1A.
Example 114B
' 4-(((4-cyanobenzyl)amino)methyl)-2-(8-guinolinyl)benzonitrile
The desired product can be prepared by substituting Example 114A for Example
89C
in Example 34B.
Example 114C
4-(((4-cyanobenzyl)((1-methyl-1H-imidazol-5- 1)~yl)amino)methyl)-2-(8-
quinolinyl)benzonitrile
The desired product can be prepared by substituting Example 114B fox Example
34B
in Example 34C.
Example 115
5-(((4-(tert-butvl)benzvl)oxv) ( 1-methyl-1H-imidazol-5-vl)methvll-2'-methyl(
1.1'-binhenvll-
2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-tert-
butylbenzene fox 3-(bromomethyl)benzonitrile in Example 113.
3o MS (APCI(+)) m/z 450 (M+H)+;
MS (APCI(-)) m/z 484 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.04 (d, 1H), 7.65 (dd, 1H), 7.50
(d, 1H),
7.40-7.26 (m, 5H), 7.36 (d, 2H), 7.28 (d, 2H), 6.06 (s, 1H), 4.61 (d, 1H),
4.51 (d, 1H), 2.13 (s,
3H), 1.26 (s, 9H).
Example 116
-167-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
5-(((4-cyanobenz l~y)(1-methyl-1H-imidazol-5- 1)methyl)-2'-methyl(1,1'-
biphen~)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 4-(bromomethyl)benzonitrile
for 3-
(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 419 (M+H)+;
MS (APCI(-)) m/z 453 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.07 (s, 1H), 8.05 (d, 1H), 7.82 (d, 2H), 7.68
(dd, 1H),
7.57 (d, 2H), 7.52 (d, 1H), 7.44 (s, 1H), 7.42-7.25 (m, 4H), 6.11 (s, 1H),
4.75 (d, 1H), 4.65 (d,
1H), 3.75 (s, 3H), 2.12 (s, 3H).
to
Example 117
5-(((3-iodobenz lay)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-biphen 1
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-3-iodobenzene
for
3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 520 (M+H)+;
MS (APCI(-)) m/z 554 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 9.12 (s, 1H), 8.05 (d, 1H), 7.73 (t, 1H), 7.69-
7.66 (m, 2H),
7.51 (d, 1H), 7.42 (s, 1H), 7.40-7.26 (m, 5H), 7.16 (t, 1H), 6.08 (s, 1H),
4.63 (d, 1H), 4.53 (d,
1H), 3.76 (s, 3H), 2.13 (s, 3H).
Exam 1p a 118
5-(((4-fluorobenzyl)oxy)( 1-methyl-1H-imidazol-5-.1)~yl)-2'-methyl( 1,1'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-
fluorobenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 412 (M+H)+;
MS (APCI(-)) m/z 446 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.10 (s, 1H), 8.05 (d, 1H), 7.67 (dd, 1H), 7.50
(d, 1H),
7.43-7.26 (m, 7H), 7.18 (t, 2H), 6.06 (s, 1H), 4.63 (d, 1H), 4.54 (d, 1H),
3.75 (s, 3H), 2.13 (s,
3H).
Example 119
5-(((4-bromobenzyl)oxy) ( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl( 1 1'-
biphen~2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-bromo-4-
(bromomethyl)benzene
for 3-(bromomethyl)benzonitrile in Example 113.
-168-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (APCI(+)) m/z 474 (M+H)+;
MS (APCI(-)) mlz 508 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) b 9.05 (s, 1H), 8.05 (d, 1H), 7.66 (dd, 1H), 7.55
(d, 2H),
7.50 (d, 1H), 7.41-7.26 (m, 5H), 7.33 (d, 2H), 6.06 (s, 1H), 4.62 (d, 1H),
4.52 (d, 1H), 3.74 (s,
3H), 2.12 (s, 3H).
Example 120
5-(((3-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1,1'-
biphenyl)-2
carbonitrile hydrochloride
1o The desired product was prepared by substituting 1-(bromomethyl)-3-
chlorobenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 428 (M+H)+;
MS (APCI(-)) m/z 462 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.13 (s, 1H), 8.05 (d, 1H), 7.68 (dd, 1H), 7.52
(d, 1H),
7.43 (br s, 2H), 7.40-7.26 (m, 7H), 6.10 (s, 1H), 4.67 (d, 1H), 4.56 (d, 1H),
3.76 (s, 3H), 2.13
(s, 3H).
Example 121
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((4-nitrobenzyl)oxy)methyl)(1,1'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-
nitrobenzene for
3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) mlz 439 (M+H)+;
MS (APCI(-)) m/z 473 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.12 (s, 1H), 8.21 (d, 2H), 8.06 (d, 1H), 7.70
(dd, 1H),
7.66 (d, 2H), 7.53 (d, 1H), 7.47 (s, 1H), 7.42-7.25 (m, 4H), 6.15 (s, 1H),
4.82 (d, 1H), 4.71 (d,
1H), 3.77 (s, 3H), 2.12 (s, 3H).
Example 122
5-(((2-methoxy-5-nitrobenz lay)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'-
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 2-(bromomethyl)-1-methoxy-4-
nitrobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 469 (M+H)+;
MS (APCI(-)) m/z 503 (M+Cl) ;
-169-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (500 MHz, DMSO-d6) 8 9.11 (s, 1H), 8.26-8.24 (m, 2H), 8.06 (d, 1H),
7.66 (dd,
1H), 7.55 (d, 1H), 7.42-7.20 (m, 6H), 6.14 (s, 1H), 4.72 (d, 1H), 4.63 (d,
1H), 3.86 (s, 3H),
3.79 (s, 3H), 2.13 (s, 3H).
Example 123
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((3-(trifluoromethyl)benz l~y)meth 1)
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-3-
(trifluoromethyl)benzene for 3-(bromomethyl)benzonitrile in Example 113.
l0 MS (APCI(+)) m/z 462 (M+H)+;
MS (APCI(-)) m/z 496 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.93 (s, 1H), 8.05 (d, 1H), 7.70-7.66 (m, 4H),
7.60 (t, 1H),
7.51 (d, 1H), 7.42-7.25 (m, 5H), 6.09 (s, 1H), 4.76 (d, 1H), 4.66 (d, 1H),
3.74 (s, 3H), 2.12 (s,
3H).
Example 124
5-(((2,6-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1,1'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 2-(bromomethyl)-1,3-
dichlorobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 462 (M+H)+;
MS (APCI(-)) m/z 496 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) 8 9.00 (s, 1H), 8.06 (d,1H), 7.69 (dd, 1H), 7.56 (br
s, 1H),
7.50 (d, 1H), 7.49-7.25 (m, 7H), 6.14 (s, 1H), 4.85 (d, 1H), 4.73 (d, 1H),
3.81 (s, 3H), 2.14 (s,
3H).
' Example 125
5-(((3,4-dichlorobenz l~y)(1-methyl-1H-imidazol-5- 1)~ methyl)-2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
3o The desired product was prepared by substituting 4-(bromomethyl)-1,2-
dichlorobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 462 (M+H)+;
MS (APCI(-)) m/z 469 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.77 (s, 1H), 8.05 (d, 1H), 7.65 (dd, 1H), 7.62-
7.60 (m,
3H), 7.48 (d, 1H), 7.42-7.25 (m, 5H), 6.03 (s, 1H), 4.64 (d, 1H), 4.55 (d,
1H), 3.70 (s, 3H),
2.12 (s, 3H).
-170-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 126
5-(((2-cyanobenzyl)oxy)(1-meth 1-y 1H-imidazol-5- 1)methyl)-2'-methyl(1,1'-
biphenyl~2
carbonitrile hydrochloride
The desired product was prepared by substituting 2-(bromomethyl)benzonitrile
for 3-
(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 419 (M+H)+;
MS (APCI(-)) mlz 453 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.87 (s, 1H), 8.04 (d, 1H), 7.85 (dd, 1H), 7.72
(td, 1H),
7.68-7.65 (m, 2H), 7.55 (td, 1H), 7.52 (d, 1H), 7.42-7.25 (m, 5H), 6.13 (s,
1H), 4.81 (d, 1H),
l0 4.71 (d, 1H), 3.72 (s, 3H), 2.12 (s, 3H).
Example 127
(2'-methyl-5-(((4-methylbenzyl)oxy) ( 1-methyl-1 H-imidazol-5-yl)rnethyl) (
1,1'-biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-
methylbenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 408 (M+H)+;
MS (APCI(-)) m/z 442 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) ~ 8.87 (s, 1H), 8.04 (d, 1H), 7.65 (dd, 1H), 7.48
(d, 1H),
7.40-7.20 (m, 5H), 7.23 (d, 2H), 7.16 (d, 2H), 6.00 (s, 1H), 4.57 (d, 1H),
4.49 (d, 1H), 3.71 (s,
3H), 2.29 (s, 3H), 2.12 (s, 3H).
Exarn 1p a 128
(2'-methyl-5-(((3-methylbenzyl)oxy)(1-methyl-1H-imidazol-5- 1)y-methyl)(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-3-
methylbenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 408 (M+H)+;
MS (APCI(-)) mlz 442 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 8.91 (s, 1H), 8.10 (d, 1H), 7.72 (dd, 1H), 7.55
(d, 1H),
7.45-7.17 (m, 9H), 6.07 (s, 1H), 4.64 (d, 1H), 4.55 (d, 1H), 3.77 (s, 3H),
2.34 (s, 3H), 2.18 (s,
3H).
Example 129
5-(((2,5-difluorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl -2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
-171-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting 2-(bromomethyl)-1,4-
difluorobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 430 (M+H)+;
MS (APCI(-)) m/z 464 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.83 (s, 1H), 8.04 (d, 1H), 7.65 (dd, 1H), 7.50
(d, 1H),
7.40-7.21 (m, 8H), 6.07 (s, 1H), 4.67 (d, 1H), 4.59 (d, 1H), 3.72 (s, 3H),
2.12 (s, 3H).
Example 130
methyl 4-(((6-cyano-2'-methyl(1,1'-biphenyl)-3-~)( 1-methyl-1H-imidazol-5-
l0 yl)methoxy)methyl)benzoate hydrochloride
The desired product was prepared by substituting methyl
4-(bromomethyl)benzoate for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 452 (M+H)+;
MS (APCI(-)) m/z 486 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.78 (s, 1H), 8.04 (d, 1H), 7.94 (d, 2H), 7.67
(dd, 1H),
7.51 (d, 2H), 7.50 (d, 1H), 7.41-7.28 (m, 4H), 7.25 (s, 1H), 6.06 (s, 1H),
4.73 (d, 1H), 4.63 (d,
1H), 3.85 (s, 3H), 3.71 (s, 3H), 2.12 (s, 3H).
Example 131
5-(((3,5-difluorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-3,5-
difluorobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 430 (M+H)+;
MS (APCI(-)) m/z 464 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.04 (s, 1H), 8.18 (d, 1H), 7.81 (dd, 1H), 7.65
(d, 1H),
7.54-7.22 (m, 8H), 6.20 (s, 1H), 4.81 (d, 1H), 4.71 (d, 1H), 3.86 (s, 3H),
2.26 (s, 3H).
Example 132
5-(((2-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5-~)methyl)-2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-2-
chlorobenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 428 (M+H)+;
MS (APCI(-)) m/z 462 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) S 8.90 (s, 1H), 8.15 (d, 1H), 7.78 (dd, 1H), 7.67-
7.34 (m,
10H), 6.21 (s, 1H), 4.83 (d, 1H), 4.73 (d, 1H), 3.83 (s, 3H), 2.23 (s, 3H).
-172-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 133
5-(((4-chlorobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)y methyl)-2'-methyl(l,l'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-
chlorobenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 428 (M+H)+;
MS (APCI(-)) m/z 462 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.78 (s, 1H), 8.04 (d, 1H), 7.65 (dd, 1H), 7.48
(d, 1H),
l0 7.44-7.22 (m, 9H), 6.02 (s, 1H), 4.62 (d, 1H), 4.53 (d, 1H), 3.70 (s, 3H),
2.12 (s, 3H).
Example 134
5-(((3-methoxybenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphen 1
carbonitrile hydrochloride
15 The desired product was prepared by substituting 1-(bromomethyl)-3-
methoxybenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 424 (M+H)+;
MS (APCI(-)) m/z 458 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.03 (s, 1H), 8.05 (d, 1H), 7.67 (dd, 1H), 7.51
(d, 1H),
20 7.40-7.22 (m~ 5H), 6.94-6.85 (m, 4H), 6.05 (s, 1H), 4.61 (d, 1H), 4.52 (d,
1H), 3.75 (s, 3H),
3.72 (s, 3H), 2.13 (s, 3H).
Exam 1p a 135
(2'-methyl-5-(((2-methylbenz 1y )ox~r)(1-methyl-1H-imidazol-5- 1)~yl)(1,1'-
biphen 1y )2-
25 carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-2-
methylbenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 408 (M+H)+;
MS (APCI(-)) mlz 442 (M+Cl) ;
3o 1H NMR (500 MHz, DMSO-d6) 8 8.98 (s, 1H), 8.05 (d, 1H), 7.66 (dd, 1H), 7.51
(d, 1H),
7.42-7.15 (m, 9H), 6.06 (s, 1H), 4.64 (d, 1H), 4.56 (d,1H), 3.71 (s, 3H), 2.24
(s, 3H), 2.12 (s,
3H).
Example 136
35 5-(((3-fluorobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1 1'-
biphenyl)-2-
carbonitrile hydrochloride
-173-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting 1-(bromomethyl)-3-
fluorobenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 412 (M+H)+;
MS (APCI(-)) m/z 446 (M+Cl) ; '
1H NMR (500 MHz, DMSO-d6) 8 8.89 (s, 1H), 8.05 (d, 1H), 7.67 (dd, 1H), 7.51
(d, 1H),
7.42-7.12 (m, 9H), 6.05 (s, 1H), 4.66 (d, 1H), 4.56 (d, 1H), 3.72 (s, 3H),
2.12 (s, 3H).
Example 137
5-(((2,6-dichloro-4-pyridinyl)methoxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl( 1,1'
1o biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 4-(bromomethyl)-2,6-
dichloropyridine for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 463 (M+H)+;
MS (APCI(-)) m/z 497 (M+Cl) ;
15 1H NMR (500 MHz, DMSO-d6) 8 8.94 (s, 1H), 8.05 (d, 1H), 7.69 (dd, 1H), 7.57-
7.54 (m,
3H), 7.42-7.25 (m, 5H), 6.10 (s, 1H), 4.74 (d, 1H), 4.63 (d, 1H), 3.73 (s,
3H), 2.12 (s, 3H).
Example 138
5-(((2-fluorobenz l~y)(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1,1'-biphenyl)-
2-
2o carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-2-
fluorobenzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 412 (M+H)+;
MS (APCI(-)) m/z 446 (M+Cl) ;
25 1H NMR (500 MHz, DMSO-d6) S 8.85 (s, 1H), 8.04 (d, 1H), 7.71-7.49 (m, 9H),
7.65 (dd,
1H), 7.50 (d, 1H), 6.06 (s, 1H), 4.68 (d, 1H), 4.60 (d, 1H), 3.72 (s, 3H),
2.13 (s, 3H).
Example 139
(2'-meth;rl-5-((1-methyl-1H-imidazol-5-yl)((4-
(trifluoromethyl)benzyl)oxy~methyl)(1,1'
3o biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-
(trifluoromethyl)benzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 462 (M+H)+;
3s MS (APCI(-)) m/z 496 (M+Cl) ;
-174-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (500 MHz, DMSO-d6) 8 8.88 (s, 1H), 8.05 (d, 1H), 7.72 (d, 2H), 7.67
(dd, 1H),
7.59 (d, 2H), 7.51 (d, 1H), 7.41-7.25 (m, 5H), 6.08 (s, 1H), 4.75 (d, 1H),
4.64 (d, 1H), 3.73 (s,
3H), 2.12 (s, 3H).
Example 140
5-(((3,5-dimeth l~yl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-methyl(1,1'-biphen
2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-3,5-
dimethylbenzene for 3-(bromomethyl)benzonitrile in Example 113.
1o MS (APCI(+)) m/z 422 (M+H)+;
MS (APCI(-)) mlz 456 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.86 (s, 1H), 8.05 (d, 1H), 7.65 (dd, 1H), 7.49
(d, 1H),
7.41-7.25 (m, 5H), 6.93 (s, 3H), 6.00 (s, 1H), 4.55 (d, 1H), 4.45 (d, 1H),
3.72 (s, 3H), 2.24 (s,
6H), 2.13 (s, 3H).
Example 141
5-(((4-fluoro-2-(trifluoromethyl)benzyl)oxy)( 1-methyl-1H-imidazol-5-
yl)methyl)-2'
methyl(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-fluoro-2-
(trifluoromethyl)benzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 480 (M+H)+;
MS (APCI(-)) m/z 514 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 8.73 (s, 1H), 7.86 (d, 1H), 7.59-7.42 (m, 1H),
7.47-7.28
(m, 4H), 7.22-7.03 (m, 5H), 5.97 (s, 1H), 4.59 (d, 1H), 4.47 (d, 1H), 3.53 (s,
3H), 1.91 (s,
3H).
Example 142
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((2-nitrobenzyl)oxy)methyl)( 1,1'-
biphenyl)-2
carbonitrile hydrochloride
3o The desired product was prepared by substituting 1-(bromomethyl)-2-
nitrobenzene for
3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 439 (M+H)+;
MS (APCI(-)) m/z 473 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.12 (dd, 1H), 8.10 (d, 1H), 8.01 (s, 1H), 7.86
(dd, 1H),
7.83 (td, 1H), 7.71 (dd, 1H), 7.67 (td, 1H), 7.57 (d, 1H), 7.48 (s, 1H), 7.46-
7.31 (m, 4H), 6.22
(s, 1H), 4.95 (d, 1H), 4.07 (d, 1H), 3.76 (s, 3H), 2.18 (s, 3H).
-175-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 143
(2'-methyl-5-((1-methyl-1H-imidazol-5 yl)((3-(trifluoromethoxy)benzyl)ox.
)~meth,1)
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-3-
(trifluoromethoxy)benzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 478 (M+H)+;
MS (APCI(-)) m/z 512 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.99 (s, 1H), 8.05 (d, 1H), 7.67 (dd, 1H), 7.52
(d, 1H),
7.50 (t, 1H), 7.41-7.25 (m, 8H), 6.08 (s, 1H), 4.71 (d, 1H), 4.61 (d, 1H),
3.74 (s, 3H), 2.12 (s,
3H).
Exam 1p a 144
4-(((6-cyano-2'-methyl(1,1'-biphenyl)-3-yl)( 1-methyl-1H-imidazol-5-
yl)methoxy)methyl)-6
methylisophthalonitrile hydrochloride
The desired product was prepared by substituting 4-(bromomethyl)-6-
methylisophthalonitrile for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 458 (M+H)+;
MS (APCI(-)) m/z 492 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.89 (s, 1H), 8.40 (s, 1H), 8.05 (d, 1H), 7.75 (s,
1H), 7.67
2o (dd, 1H), 7.52 (d, 1H), 7.41-7.25 (m, 5H), 6.16 (s, 1H), 4.86 (d, 1H), 4.74
(d, 1H), 3.72 (s,
3H), 2.55 (s, 3H), 2.12 (s, 3H).
Example 145
5-(((2'-cyano ( 1,1'-biphenyl)-4-yl)methoxy) ( 1-methyl-1 H-imidazol-5-
yl)methyl)-2'-
methyl(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 4'-(bromomethyl)(1,1'-
biphenyl)-2-
carbonitrile for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 495 (M+H)+;
MS (APCI(-)) m/z 529 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) ~ 8.99 (s, 1H), 8.07 (d, 1H), 7.95 (dd, 1H), 7.80
(td, 1H),
7.72 (dd, 1H), 7.62-7.53 (m, 7H), 7.41-7.25 (m, 5H), 6.12 (s, 1H), 4.73 (d,
1H), 4.64 (d, 1H),
3.77 (s, 3H), 2.14 (s, 3H).
Example 146
methyl3-(((6-cyano-2'-methyl(1 1'-biphen l~yl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzoate hydrochloride
-176-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting methyl 3-
(bromomethyl)benzoate
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 452 (M+H)+;
MS (APCI(-)) m/z 486 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.99 (s, 1H), 8.09 (d, 1H), 7.96-7.94 (m, 2H),
7.71-7.69
(m, 2H), 7.58-7.50 (m, 2H), 7.41-7.25 (m, 5H), 6.12 (s, 1H), 4.78 (d, 1H),
4.68 (d, 1H), 3.89
(s, 3H), 3.78 (s, 3H), 2.17 (s, 3H).
Example 147
l0 5-(((3,4-difluorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'-biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 4-(bromomethyl)-1,2-
difluorobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 430 (M+H)+;
15 MS (APCI(-)) m/z 464 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.95 (s, 1H), 8.05 (d, 1H), 7.67 (dd, 1H), 7.50
(d, 1H),
7.48-7.22 (m, 8H), 6.05 (s, 1H), 4.62 (d, 1H), 4.54 (d, 1H), 3.73 (s, 3H),
2.12 (s, 3H).
Example 148
20 (2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((3,4,5-
trimethoxybenzyl)oxy)methyl)(1,1'-
biphenyl)-2-carbonitrile hydrochloride
A solution of 5-(chloromethyl)-1,2,3-trimethoxybenzene (20 mg, 0.092 mmol) in
acetone (3 mL) at 60 °C was treated with KI (166 mg, lmmol), stirred
for 16 hours,
centrifuged, decanted, and concentrated. The concentrate was dissolved in
dichloromethane
25 (2 mL), treated with Example 86J (20 mg, 0.066 mmol) and silver(I) oxide
(140 mg, 0.604
mmol), stirred for 16 hours, treated with methanol, centrifuged, decanted, and
concentrated.
The concentrate was treated with 1:1/:methanol/DMSO, purified by preparative
HPLC,
dissolved in dichloromethane, treated with 1M HCl in diethyl ether, and
concentrated to
provide the desired product.
3o MS (APCI(+)) m/z 484 (M+H)+;
MS (APCI(-)) m/z 518 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.94 (s, 1H), 8.04 (d, 1H), 7.65 (dd, 1H), 7.51
(d, 1H),
7.41-7.25 (m, 5H), 6.64 (s, 2H), 6.02 (s, 1H), 4.56 (d, 1H), 4.49 (d, 1H),
3.74 (s, 3H), 3.72 (s,
6H), 3.63 (s, 3H), 2.13 (s, 3H).
Example 149
-177-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)(8-quinolinylmethox )y methyl)(1,I'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 8-(chloromethyl)quinoline for
5-
(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 445 (M+H)+;
MS (APCI(-)) m/z 479 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.38 (d, 1H), 8.04 (dd, 1H), 7.98 (d, 1H), 7.93
(d, 1H),
7.76 (td, 1H), 7.71 (br d, 1H), 7.64 (d, 1H), 7.61 (t, 1H), 7.56 (br s, 1H),
7.41-7.22 (m, 6H),
6.31 (br s, 1H), 4.90 (br d, 1H), 4.82 (br d, 1H), 3.76 (br s, 3H), 2.11 (s,
3H).
to
Example 150
5-(((3,5-dimethox~yl oxy)(1-methyl-1H-imidazol-5-yl)rnethyl)-2'-methyl(1,1'
biphen~)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(chloromethyl)-3,5-
15 dimethoxybenzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example
148.
MS (APCI(+)) m/z 454 (M+H)+;
MS (APCI(-)) m/z 488 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.94 (br s, 1H), 8.04 (d, 1H), 7.65 (d, 1H), 7.50
(s, 1H),
7.39-7.25 (m, 5H), 6.51-6.40 (m, 3H), 6.02 (br s, 1H), 4.56 (d, 1H), 4.47 (d,
1H), 3.73 (s,
20 3H), 3.70 (s, 6H), 2.12 (s, 3H).
Example 151
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((4-(methylsulfonyl)benz 1~ )~ 1)~
(11'
bi henyl)-2-carbonitrile hydrochloride
25 The desired product was prepared by substituting 1-(chloromethyl)-4-
(methylsulfonyl)benzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 472 (M+H)~';
MS (APCI(-)) m/z 506 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 9.09 (s, 1H), 8.06 (d, 1H), 7.91 (d, 2H), 7.68
(dd, 1H),
3o 7.64 (d, 2H), 7.53 (d, 1H), 7.45 (s, 1H), 7.41-7.25 (m, 4H), 6.13 (s, 1H),
4.78 (d, 1H), 4.67 (d,
1H), 3.76 (s, 3H), 3.19 (s, 3H), 2.13 (br s, 3H).
Example 152
5-(((6-chloro-1,3-benzodioxol-5-yl)methoxy)(1-methyl-1H-imidazol-5-yl)meth, l
35 methyl(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 5-chloro-6-(chloromethyl)-1,3-
benzodioxole for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
-178-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (APCI(+)) m/z 472 (M+H)~;
MS (APCI(-)) m/z 506 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.85 (br s, 1H), 8.04 (d, 1H), 7.67 (br d, 1H),
7.50 (br s,
1H), 7.41-7.25 (m, 5H), 7.10 (s, 1H), 7.08 (s, 1H), 6.06 (s, 3H), 4.58 (d,
1H), 4.51 (d, 1H),
3.72 (s, 3H), 2.12 (s, 3H).
Example 153
5-(((4-isopropylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1 1'-
biphenyl)-2
carbonitrile hydrochloride
1o The desired product was prepared by substituting 1-(chloromethyl)-4-
isopropylbenzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 436 (M+H)~;
MS (APCI(-)) m/z 470 (M+Cl)~;
1H NMR (500 MHz, DMSO-d6) 8 9.03 (s, 1H), 8.04 (d, 1H), 7.65 (br d, 1H), 7.50
(br s, 1H),
7.41-7.25 (m, 5H), 7.27 (d, 2H), 7.21 (d, 2H), 6.05 (s, 1H), 4.59 (d, 1H),
4.50 (d, 1H), 3.74 (s,
3H), 2.87 (heplet, 1H), 2.13 (s, 3H), 1.18 (d, 6H).
Example 154
5-(((3,4-dimethylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1 1'-
biphenyl)
2-carbonitrile hydrochloride
The desired product was prepared by substituting 4-(chloromethyl)-1,2-
dimethylbenzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 422 (M+H)+;
MS (APCI(-)) m/z 456 (M+Cl)y;
1H NMR (500 MHz, DMSO-d6) 8 8.60 (br s, 1H), 8.03 (d~ 1H), 7.63 (d, 1H), 7.46
(s, 1H),
7.41-7.25 (m, 5H), 7.15-7.02 (m, 3H), 6.00 (br s, 1H), 4.52 (d, 1H), 4.44 (d,
1H), 3.66 (s,
3H), 2.19 (s, 3H), 2.18 (s, 3H), 2.12 (s, 3H).
Example 155
5-(((4-(benz~y)benz lay)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1 1'-
biphenyl)-
2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(benzyloxy)-4-
(chloromethyl)benzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example
148.
MS (APCI(+)) m/z 500 (M+H)+;
MS (APCI(-)) m/z 534 (M+Cl) ;
-179-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
IH NMR (500 MHz, DMSO-d6) ~ 8.93 (br s, 1H), 8.04 (d, 1H), 7.64 (dd, 1H), 7.48
(d, 1H),
7.41-7.24 (m, 10H), 7.28 (d, 2H), 6.98 (d, 2H), 6.00 (s, 1H), 5.10 (s, 2H),
4.54 (d, 1H), 4.46
(d, IH), 3.71 (s, 3H), 2.12 (s, 3H).
Example 156
~~(6-fluoro-4H-1,3-benzodioxin-8-yl)methoxy~(1-methyl-1H-imidazol-5-yl)methyl)-
2'
methyl(1,1=biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 8-(chloromethyl)-6-fluoro-4H-
I,3-
benzodioxine for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
1o MS (APCI(+)) m/z 470 (M+H)+;
MS (APCI(-)) m/z 504 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.95 (br s, 1H), 8.05 (d, 1H), 7.66 (d, 1H), 7.53
(s, 1H),
7.41-7.25 (m, 5H), 7.20 (dd, 1H), 6.95 ( dd, 1H), 6.08 (br s, 1H), 5.17 (d,
1H), 5.15 (d, 1H),
4.85 (s, 2H), 4.58 (d, 1H), 4.49 (d, 1H), 3.75 (s, 3H), 2.12 (s, 3H).
I5
Example 157
5-(((2,4-dichlorobenzvl)oxv) ( 1-methyl-1 H-imidazol-5-vl)methvl)-2'-methyl(
1,1'-binhenvll-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 2,4-dichloro-1-
20 (chloromethyl)benzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 462 (M+H)+;
MS (APCI(-)) m/z 496 (M+Cl)-;
IH NMR (500 MHz, DMSO-d6) 8 8.84 (br s, 1H), 8.04 (d, 1H), 7.65 (dd, IH), 7.62
(d, 1H),
7.57 (d, 1H), 7.50 (d, 1H), 7.44 (dd, 1H), 7.4I-7.25 (m, 5H), 6.10 (s, 1H),
4.70 (d, 1H), 4.60
25 (d, 1H), 3.72 (s, 3H), 2.12 (s, 3H).
Example 158
5-(((3,5-dimethylbenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1 1'-
bi~hen~)
2-carbonitrile hydrochloride
3o The desired product was prepared by substituting 1,3-dichloro-5-
(chloromethyl)benzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example
148.
MS (APCI(+)) m/z 462 (M+H)+;
MS (APCI(-)) m/z 496 (M+Cl) ;
IH NMR (500 MHz, DMSO-d6) 8 8.45 (br s, 1H), 8.02 (d, 1H), 7.64 (d, 1H), 7.53
(t, 1H),
35 7.47 (s, 1H), 7.41 (d, 2H), 7.39-7.25 (m, 5H), 6.02 (br s, 1H), 4.64 (d,
1H), 4.54 (d, 1H), 3.65
(s, 3H), 2.12 (s, 3H).
-180-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 159
5-(((5 ~tert-butyl)-1,2,4-oxadiazol-3=yl~methoxy)(1-methyl-1H-imidazol-
5yl)methyl)-2'
metl~l~l,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 5-tert-butyl-3-(chloromethyl)-
1,2,4-
oxadiazole for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 442 (M+H)~;
MS (APCI(-)) m/z 476 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) 8 8.00 (d, 1H), 7.59 (br d, 1H), 7.48 (br s, 1H),
7.41-7.25 (m,
4H), 7.24 (s, 1H), 7.14 (s, 1H), 7.03 (s, 1H), 4.68 (s, 2H), 3.63 (s, 3H),
2.13 (s, 3H),1.34 (s,
9H).
Example 160
5-(((4-iodobenz lay)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-4-iodobenzene
for
3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 520 (M+H)+;
MS (APCI(-)) m/z 554 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.78 (br s, 1H), 8.03 (d, 1H), 7.71 (d, 2H), 7.65
(dd, 1H),
7.47 (d, 1H), 7.41-7.25 (m, 5H), 7.17 (d, 2H), 6.01 (s, 1H), 4.58 (d, 1H),
4.49 (d, 1H), 3:70 (s,
3H), 2.12 (s, 3H).
Exam 1p a 161
5-(((1,1'-biphenyl)-4-ylmethox ) 1-methyl-1H-imidazol-5- 1)~yl)-2'-meth 1(y
11'-
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 4-(chlororriethyl)-1,1'-
biphenyl for
5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 470 (M+H)+;
MS (APCI(-)) m/z 504 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.99 (s, 1H), 8.06 (d, 1H), 7.69 (dd, 1H), 7.66
(s, 2H), 7.64
(s, 2H), 7.52 (d, 1H), 7.49-7.25 (m, 10H}, 6.09 (s, 1H), 4.69 (d, 1H}, 4.59
(d, 1H), 3.76 (s,
3H), 2.13 (s, 3H).
Example 162
5-(((2-(4-chlorophenyl)-1,3-thiazol-4=yl)methoxy~(1-methyl-1H-imidazol-5-'rl
methyl)-2'-
methyl(1,1'-biphenyl)-2-carbonitrile hydrochloride
-181-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting 4-(chloromethyl)-2-(4-
chlorophenyl)-1,3-thiazole for 5-(chloromethyl)-1,2,3-trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 511 (M+H)~;
MS (APCI(-)) m/z 545 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.12 (s, 1H), 8.04 (d, 1H), 7.89 (d, 2H), 7.78 (s,
1H), 7.67
(dd, 1H), 7.58 (d, 1H), 7.55 (d, 2H), 7.41-7.25 (m, 5H), 6.17 (s, 1H), 4.78
(d, 1H), 4.73 (d,
1H), 3.81 (s, 3H), 2.12 (s, 3H).
Exam 1p a 163 .
5-(((5-(2-methox~rphenyl)-1,2,4-oxadiazol-3-yl)methoxy)(1-methyl-1H-imidazol-5
1)methyl)-2'-methyl(1,1'-biphen~rl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-(2-
methoxyphenyl)-1,2,4-oxadiazole for 5-(chloromethyl)-1,2,3-trimethoxybenzene
in Example
148.
MS (APCI(+)) m/z 492 (M+H)+;
MS (APCI(-)) m/z 526 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) b 8.01 (dd, 1H), 7.90 (dd, 1H), 7.67 (td, 1H), 7.63
(d, 1H),
7.51 (br s, 1H), 7.41-7.25 (m, 6H), 7.30 (d, 1H), 7.14 (td, 1H), 6.08 (br s,
1H), 4.78 (br s,
2H), 3.68 (br s, 3H), 2.11 (s, 3H).
Example 164
~-(((4-chloro-2-nitrobenzyl)oxy)( 1-methyl-1H-irnidazol-5-yl)methyl)-2'-
metl~l( l , l'
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 4-chloro-1-(chloromethyl)-2-
nitrobenzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 473 (M+H)~;
MS (APCI(-)) m/z 509 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.89 (br s, 1H), 8.15 (d, 1H), 8.04 (d, 1H), 7.85
(dd, 1H),
7.81 (d, 1H), 7.63 (d, 1H), 7.49 (s, 1H), 7.41-7.25 (m, 5H), 6.17 (s, 1H),
4.97 (d, 1H), 4.85 (d,
1H), 3.69 (s, 3H), 2.11 (s, 3H).
Example 165
methyl 5-(((6-cyano-2'-methyl(1,1'-biphenxl)-3-yl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)-2-furoate ~drochloride
The desired product was prepared by substituting methyl 5-(chloromethyl)-2-
furoate
for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 442 (M+H)+;
-182-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (APCI(-)) m/z 476 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) ~ 8.88 (br s, 1H), 8.03 (d, 1H), 7.61 (dd, 1H), 7.50
(d, 1H),
7.46 (s, 1H), 7.41-7.25 (m, 5H), 7.23 (d, 1H), 6.66 (d, 1H), 6.04 (s, 1H),
4.67 (s, 2H), 3.79 (s,
3H), 3.72 (s, 3H), 2.13 (s, 3H).
Example 166
2'-methyl-5-(( 1-methyl-1 H-imidazol-5-yl)((5-(4-(trifluorometh~phenyl)-1,2,4-
oxadiazol-3
yl)methoxy)methyl)(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-(4-
(trifluoromethyl)phenyl)-1,2,4-oxadiazole for 5-(chloromethyl)-1,2,3-
trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 530 (M+H)+;
MS (APCI(-)) m/z 564 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) & 8.98 (br s, 1H), 8.27 (d, 2H), 8.06 (d, 1H), 8.01
(d, 2H),
7.66 (dd, 1H), 7.57 (s, 1H), 7.41-7.25 (m, 5H), 6.22 (s, 1H), 4.87 (d, 1H),
4.70 (d, 1H), 3.81
(s, 3H), 2.13 (s, 3H).
Example 167
methyl8-(((6-cyano-2'-methyl(1,1'-biphen l~)-3-yl)(1-methyl-1H-imidazol-5
2o yl)methox'r)methyl)-4H-1,3-benzodioxine-6-carboxylate hydrochloride
The desired product was prepared by substituting methyl 8-(chloromethyl)-4H-
1,3-
benzodioxine-6-carboxylate for 5-(chloromethyl)-1,2,3-trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 510 (M+H)+;
MS (APCI(-)) m/z 544 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.93 (br s, 1H), 8.05 (d, 1H), 7.82 (d, 1H), 7.69
(d, 1H),
7.64 (d, 1H), 7.53 (s, 1H), 7.41-7.25 (m, 5H), 6.08 (s, 1H), 5.28 (d, 1H),
5.26 (d, 1H), 4.92 (s,
3H), 4.65 (d, 1H), 4.57 (d, 1H), 3.80 (s, 3H), 3.76 (s, 3H), 2.13 (s, 3H).
Example 168
(2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((6-nitro-4H-1,3-benzodioxin-8-
~rl)methox )methyl)(l,l'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 8-(chloromethyl)-6-nitro-4H-
1,3-
benzodioxine for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 497 (M+H)+;
MS (APCI(-)) m/z 531 (M+Cl)-;
-183-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (500 MHz, DMSO-d6) S 9.01 (s, 1H), 8.15 (d, 1H), 8.07 (d, 1H), 8.06 (d,
1H), 7.66
(dd, 1H), 7.54 (d, 1H), 7.41-7.25 (m, 5H), 6.13 (s, 1H), 5.35 (d, 1H), 5.33
(d, 1H), 4.98 (s,
2H), 4.70 (d, 1H), 4.61 (d, 1H), 3.77 (s, 3H), 2.13 (s, 3H).
Example 169
2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((5-(3-(trifluoromethyl)phenyl)-1,2,4-
oxadiazol-3
yl)methoxy)methyl)(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-(3-
(trifluoromethyl)phenyl)-1,2,4-oxadiazole for 5-(chloromethyl)-1,2,3-
trimethoxybenzene in
l0 Example 148.
MS (APCI(+)) m/z 530 (M+H)~;
MS (APCI(-)) m/z 564 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 9.05 (s, 1H), 8.36 (d, 1H), 8.30 (s, 1H), 8.12 (d,
1H), 8.05
(d, 1H), 7.91 (t, 1H), 7.67 (dd, 1H), 7.56 (d, 1H), 7.41-7.25 (m, 5H), 6.23
(s, 1H), 4.92 (d,
1H), 4.87 (d, 1H), 3.83 (s, 3H), 2.12 (s, 3H).
Example 170
5-(((5-acetyl-2-methox~benz,1~)oxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(3-(chloromethyl)-4-
methoxyphenyl)ethanone for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example
148.
MS (APCI(+)) m/z 466 (M+H)+;
MS (APCI(-)) mlz 500 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.96 (s, 1H), 8.05 (d, 1H), 7.97 (d, 1H), 7.93 (s,
1H), 7.65
(d, 1H), 7.54 (s, 1H), 7.41-7.25 (m, 5H), 7.11(dd, 1H), 6.08 (s, 1H), 4.66 (d,
1H), 4.59 (d,
1H), 3.80 (s, 3H), 3.76 (s, 3H), 2.50 (s, 3H), 2.13 (s, 3H).
Example 171
2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((5-phenyl-1,2,4-oxadiazol-3
3o yl)methoxy)methyl)(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-phenyl-
1,2,4-
oxadiazole for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 462 (M+H)+;
MS (APCI(-)) m/z 497 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) 8 8.95 (s, 1H), 8.07-8.04 (m, 3H), 7.73 (tt, 1H),
7.67-7.63
(m, 3H), 7.58 (d, 1H), 7.41-7.25 (m, 5H), 6.20 (s, 1H), 4.88 (d, 1H), 4.84 (d,
1H), 3.81 (s,
3H), 2.13 (s, 3H).
-184-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 172
5-(((5-(4-methoxyphenyl)-1,2,4-oxadiazol-3-~)methoxy)(1-methyl-1H-imidazol-5
yl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-(4-
methoxyphenyl)-1,2,4-oxadiazole for 5-(chloromethyl)-1,2,3-trimethoxybenzene
in Example
148.
MS (APCI(+)) m/z 492 (M+H)+;
MS (APCI(-)) m/z 526 (M+Cl) ;
1o 1H NMR (500 MHz, DMSO-d6) 8 9.00 (s, 1H), 8.05 (d, 1H), 8.00 (d, 2H), 7.66
(dd, 1H),
7.57 (d, 1H), 7.41-7.25 (m, 5H), 7.16 (d, 2H), 6.20 (s, 1H), 4.85 (d, 1H),
4.81 (d, 1H), 3.88 (s,
3H), 3.82 (s, 3H), 2.13 (s, 3H).
Example 173
5-(((5-(3-methoxyphenyl)-1,2,4-oxadiazol-3-yl)methoxy)(1-methyl-1H-imidazol-5-
1 methyl)-2'-meth'rl(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-(3-
methoxyphenyl)-1,2,4-oxadiazole for 5-(chloromethyl)-1,2,3-trimethoxybenzene
in Example
148.
2o MS (APCI(+)) m/z 492 (M+H)+;
MS (APCI(-)) m/z 526 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.07 (s, 1H), 8.05 (d, 1H), 7.68-7.63 (m, 2ITj,
7.58-7.53
(m, 3H), 7.41-7.25 (m, 6H), 6.22 (s, 1H), 4.89 (d, 1H), 4.86 (d, 1H), 3.86 (s,
3H), 3.83 (s,
3H), 2.13 (s, 3H).
Example 175
2'-methyl-5-((1-methyl-1H-imidazol-5-yl)((2-(4-(trifluoromethxl)phenyl)-1,3-
thiazol-4
yl)methox )~yl)(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 4-(chloromethyl)-2-(4-
(trifluoromethyl)phenyl)-1,3-thiazole for 5-(chloromethyl)-1,2,3-
trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 545 (M+H)+;
MS (APCI(-)) m/z 579 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 9.03 (s, 1H), 8.09 (d, 2H), 8.04 (d, 1H), 7.87 (s,
1H), 7.84
(d, 2H), 7.67 (dd, 1H), 7.57 (d, 1H), 7.41-7.25 (m, 5H), 6.16 (s, 1H), 4.81
(d, 1H), 4.76 (d,
1H), 3.80 (s, 3H), 2.12 (s, 3H).
-185-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 176
4-((1-methyl-1H-imidazol-5-yl)(((1-methyl-2-oxo-1,2-dihydro-4
pyridin~)methyl)amino)methyl)-2-( 1-naphthyl)benzonitrile
Example 176A
4-(methox. c~n~l)-1-meth~~yridinium iodide
A solution of 4-carbomethoxypyridine (5.6 g, 40 mmol) in toluene (20 mL) at
40 °C was treated dropwise with methyl iodide (2.5 mL, 5.7 g, 40 mmol),
cooled to room
temperature, stirred for 1.5 hours, heated to 80 °C, stirred for 1
hour, treated with toluene (30
to mL), and filtered to provide a solid was of sufficient purity for
subsequent use without further
purification.
Example 1768
1-methyl-2-oxo-1,2-dihydro-4-pyridinecarboxylic acid
15 A solution of Example 176A (4.0 g, 18 mmol) in water (20 mL) at room
temperature
was treated alternatively, at 45-minute intervals, with 2 mL and 3 mL portions
of I~3Fe(CN)6
(9.6 g, 29 mmol) in water (16 mL) at 50 °C and NaOH (3.5 g, 87 mrnol)
in water (6 mL) at
room temperature. After the fourth addition (of the NaOH solution), the
mixture was treated
four times with 3 mL of K3Fe(CN)6 solution at 45 minute intervals, heated to
55 °C, stirred
20 for 1 hour, cooled to room temperature, adjusted to pH 3 with NaOH, and
filtered to provide
the desired product of sufficient purity for subsequent use without further
purification.
MS (DCI/NH3) m/z 154 (M+H)+ and 171 (M+NH4)+;
1H NMR (300 MHz, CD30D) 8 7.73 (d, 1H), 7.10 (d, 1H), 6.79 (dd, 1H), 3.60 (s,
3~I).
25 Example 176C
4-(hydroxymethvl)-1-methyl-2(1H)-~yridinone
A solution of Example 176B (612 mg, 4.0 mmol) in THF (40 mL), at -8
°C, was
treated with isobutylchloroformate (0.57 mL, 0.60 g, 4.4 mmol) and N-
methylmorpholine
(0.48 mL, 0.44 g, 4.4 mmol), stirred for 1 hour, treated with sodium
borohydride (930 mg,
30 24.6 mmol) and MeOH (12 mL), stirred for 2 hours, treated with concentrated
HCl (2 mL),
filtered, and concentrated. The concentrate was purified by flash column
chromatography on
silica gel with 85:15/hexanes:ethyl acetate to provide the desired product.
MS (DCI/NH3) m/z 140 (M+H)+ and 157 (M+NHq.)+;
1H NMR (300 MHz, CDCl3) 8 7.23 (d, 1H), 6.57 (d, 1H), 6.18 (dd, 1H), 4.53 (s,
2H), 3.53 (s,
35 3H), 2.97 (br s, 1H).
Example 176D
-186-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1-methyl-2-oxo-1,2-dihydro-4-p~rridinecarbaldeh
The desired product was prepared by substituting Example 176C for Example 102C
in
Example 102D.
MS (DCI/NH3) m/z 138 (M+H)+ and 155 (M+NH4)+;
1H NMR (300 MHz, CDC13) 8 9.89 (s, 1H), 7.42 (d, 1H), 7.00 (d, 1H), 6.56 (dd,
1H), 3.60 (s,
3H).
Example 176E
4-(( 1-methyl-1H-imidazol-5-~((( 1-methyl-2-oxo-1,2-dihydro-4-
~yridinKl)methyl)amino)methyl)-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 13A and Example 176D
for
Example 12A and 4-nitrobenzaldehyde, respectively, in Example 12B.
MS (APCI(+)) m/z 460 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.95 (m 2H), 7.84 (d, 1H), 7.54 (m, 4H), 7.45 (m,
4H), 7.21
(d, 1H), 6.89 (d, 1H), 6.52 (s, 1H), 6.12 (dd, 1H), 5.00 (d, 1H), 3.62 (d,
2H), 3.47 and 3.48
(both s, total 3H), 3.53 (s, 3H);
Anal. calcd for C~9H27C12N50~0.65 H20: C, 64.01; H, 5.24; N, 12.87. Found: C,
64.11; H,
5.60; N, 12.50.
2o Example 177
2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((5-methyl-3-
isoxazolyl)methoxy)methyl)( 1,1'-
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-5-
methylisoxazole
for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 399 (M+H)+;
MS (APCI(-)) m/z 433 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) ~ 9.01 (s, 1H), 8.05 (d, 1H), 7.64 (dd, 1H), 7.49
(d, 1H),
7.41-7.25 (m, 5H), 6.31 (s, 1H), 6.08 (s, 1H), 4.65 (d, 1H), 4.59 (d, 1H),
3.74 (s, 3H), 2.38 (s,
3H), 2.13 (s, 3H).
Example 178
(2'-meth~~l-methyl-1H-imidazol-5-yl)((2-meth 1-~phthyl)methoxy)methyl)(1,1'
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(chloromethyl)-2-
methylnaphthalene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example 148.
MS (APCI(+)) m/z 458 (M+H)+;
MS (APCI(-)) m/z 492 (M+Cl)-;
-187-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (500 MHz, DMSO-d6) 8 8.96 (s, 1H), 8.06 (d, 1H), 8.05 (d, 1H), 7.88
(dd, 1H),
7.82 (d, 1H), 7.67 (dd, 1H), 7.54 (s, 1H), 7.49-7.22 (m, 8H), 6.20 (s, 1H),
5.12 (d, 1H), 4.99
(d, 1H), 3.68 (s, 3H), 2.43 (s, 3H), 2.13 (s, 3H).
Example 179
(2'-methyl-5-(( 1-methyl-1H-imidazol-5-yl)((2,3,5,6-
tetramethylbenzyl)oxy)methyl)(1,1'-
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-(chloromethyl)-1,2,4,5-
tetramethylbenzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example
148.
1o MS (APCI(+)) m/z 450 (M+H)+;
MS (APCI(-)) m/z 484 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) 8 8.98 (s, 1H), 8.06 (d, 1H), 7.67 (dd, 1H), 7.51
(s, 1H),
7.41-7.25 (m, 5H), 6.94 (s, 1H), 6.08 (s, 1H), 4.67 (d, 1H), 4.55 (d, 1H),
3.71 (s, 3H), 2.15 (s,
6H), 2.13 (s, 3H), 2.10 (s, 6H).
Example 180
(2'-methyl-5-( ( 1-methyl-1 H-imidazol-5-yl) ( (4-
(trifluoromethoxy)benzyl)oxy)methyl) ( 1,1'
biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(chloromethyl)-4-
(trifluoromethoxy)benzene for 5-(chloromethyl)-1,2,3-trimethoxybenzene in
Example I48.
MS (APCI(+)) m/z 478 (M+H)~;
MS (APCI(-)) m/z 512 (M+Cl)-;
tH NMR (500 MHz, DMSO-d6) 8 8.99 (s, 1H), 8.04 (d, 1H), 7.67 (dd, 1H), 7.51
(s, 1H), 7.50
(d, 2H), 7.41-7.25 (m, 5H), 7.34 (d, 2H), 6.08 (s, 1H), 4.68 (d, 1H), 4.58 (d,
1H), 3.74 (s, 3H),
2.12 (s, 3H).
Example 181
5-(((5,6-dichloro-3-p~ridinyl)methoxy)(1-methyl-1H-imidazol-5-yl)methyl)-2'-
methyl(1,1'
biphenyl)-2-carbonitrile hydrochloride
3o The desired product was prepared by substituting 2,3-dichloro-5-
(chloromethyl)pyridine for 5-(chloromethyl)-1,2,3-trimethoxybenzene in Example
148.
MS (APCI(+)) m/z 463 (M+H)~;
MS (APCI(-)) m/z 497 (M+Cl)~;
1H NMR (500 MHz, DMSO-d6) 8 9.01 (s, 1H), 8.41 (d, 1H), 8.16 (d, 1H), 8.04 (d,
1H), 7.67
(dd, 1H), 7.52 (d, 1H), 7.41-7.25 (m, 5H), 6.10 (s, 1H), 4.72 (d, 1H), 4.63
(d, 1H), 3.74 (s,
3H), 2.12 (s, 3H).
-188-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 182
5-(((3-chloro-5-(trifluoromethyl)-2-pyridinyl)methoxy)(1-methyl-1H-imidazol-5-
yl)methxl)
2'-methyl(l,l'-biphenxl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 3-chloro-2-(chloromethyl)-5-
(trifluoromethyl)pyridine for 5-(chloromethyl)-1,2,3-trimethoxybenzene in
Example 148.
MS (APCI(+)) m/z 497 (M+H)~;
MS (APCI(-)) m/z 531 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) ~ 9.01 (s, 1H), 8.92 (d, 1H), 8.48 (d, 1H), 8.03 (d,
1H), 7.65
(dd, 1H), 7.52 (d, 1H), 7.41-7.25 (m, 5H), 6.18 (s, 1H), 4.92 (d, 1H), 4.85
(d, 1H), 3.80 (s,
l0 3H), 2.13 (s, 3H).
Example 183
2'-methyl-5-((1-methyl-1H-imidazol-5-yl)(2-naphthylmethoxy)methyl)(1,1'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 2-(bromomethyl)naphthalene
for 3-
(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 444 (M+H)+;
MS (APCI(-)) m/z 480 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 8.95 (s, 1H), 8.05 (d, 1H), 7.92-7.87 (m, 4H),
7.71 (dd,
1H), 7.53-7.51 (m, 4H), 7.41-7.25 (m, 5H), 6.10 (s, 1H), 4.72 (d, 1H), 4.68
(d, 1H), 3.76 (s,
3H), 2.12 (s, 3H).
Example 184
5-(((3-bromobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-bromo-3-
(bromomethyl)benzene
for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 474 (M+H)+;
MS (APCI(-)) m/z 508 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) S 8.94 (s, 1H), 8.05 (d, 1H), 7.66 (dd, 1H), 7.55
(t, 1H), 7.50
(d, 1H), 7.41-7.25 (m, 8H), 6.06 (s, 1H), 4.65 (d, 1H), 4.55 (d, 1H), 3.73 (s,
3H), 2.13 (s, 3H).
Example 185
5-(((2-bromobenzyl)oxy) ( 1-methyl-1H-imidazol-5-.1)~yl)-2'-methyl ( 1,1'-
biphenyl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 1-bromo-2-
(bromomethyl)benzene
for 3-(bromomethyl)benzonitrile in Example 113.
-189-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (APCI(+)) m/z 473 (M+H)+;
MS (APCI(-)) m/z 508 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.97 (s, 1H), 8.04 (d, 1H), 7.68 (dd, 1H), 7.62
(dd, 1H),
7.55 (dd, 1H), 7.54 (d, 1H), 7.41-7.25 (m, 7H), 6.13 (s, 1H), 4.70 (d; 1H),
4.60 (d, 1H), 3.75
(s, 3H), 2.12 (s, 3H).
Example 186
5-(((2;6-difluorobenz lay)(1-methyl-1H-imidazol-5-yl)methyl)-2'-methyl(1,1'-
biphenyl)-2-
carbonitrile hydrochloride
1o The desired product was prepared by substituting 2-(bromomethyl)-1,3-
difluorobenzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) mlz 430 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 8.99 (s, 1H), 8.06 (d, 1H), 7.64 (dd, 1H), 7.50
(d, 1H),
7.48-7.15 (m, 7H), 7.12 (t, 1H), 6.10 (s, 1H), 4.70 (d, 1H), 4.61 (d, 1H),
3.77 (s, 3H), 2.14 (s,
3H).
Example 187
5-(((2-fluoro-4-(trifluoromethyl)benz lay)(1-methyl-1H-imidazol-5-yl)methxl)-
2'
methyl(l,l'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 1-(bromomethyl)-2-fluoro-4-
(trifluoromethyl)benzene for 3-(bromomethyl)benzonitrile in Example 113.
MS (APCI(+)) m/z 480 (M+H)+;
MS (APCI(-)) m/z 514 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.99 (s, 1H), 8.04 (d, 1H), 7.74-7.65 (m, 3H),
7.60 (d, 1H),
7.51 (d, 1H), 7.41-7.25 (m, 5H), 6.13 (s, 1H), 4.79 (d, 1H), 4.69 (d, 1H),
3.75 (s, 3H), 2.12 (s,
3H).
Example 188
~6-cyano-2'-methyl(l , l'-biphenyl)-3-yl)( 1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzamide
A solution of Example 272 (12 mg, 0.028 mmol) in dichloromethane (1 mL) at
room
temperature was treated with PyBop (17.5 mg, 0.033 mmol, 1.2 eq) and 2M
ammonia in
methanol (100 ~,L), stirred for 16 hours, and concentrated. The concentrate
was dissolved in
1:1/DMSO:methanol (1 mL) and purified by preparative HPLC to provide the
desired
product.
MS (APCI(+)) m/z 437 (M+H)+;
MS (APCI(-)) m/z 471 (M+Cl) ;
-190-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (500 MHz, DMSO-d6) 8 8.55 (br s, 1H), 8.04 (d, 1H), 7.93 (br s, 1H),
7.85 (d, 2H),
7.66 (dd, 1H), 7.49 (d, 1H), 7.42 (d, 2H), 7.41-7.25 (m, 5H), 7.11 (br s, 1H),
6.01 (s, 1H),
4.66 (d, 1H), 4.58 (d, 1H), 3.67 (s, 3H), 2.12 (s, 3H).
Example 189
4-(((6-cvano-2'-methyl( 1,1'-binhenvl)-3-vl) ( 1-methyl-1H-imidazol-5-
vl)methoxv)methvl)-N-
methylbenzamide
A solution of Example 272 (12 mg, 0.028 mmol) in dichloromethane (1 mL) at
room
temperature was treated with PyBop (17.5 mg, 0.033 mmol, 1.2 eq) and 2M
methylamine in
methanol (100 ~,L), stirred for 16 hours, and concentrated. The concentrate
was dissolved in
1:1/DMSO:methanol (1 mL) and purified by preparative HPLC to provide the
desired
product.
MS (APCI(+)) m/z 451 (M+H)+;
MS (APCI(-)) m/z 485 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) ~ 8.97 (s, 1H), 8.39 (q, 1H), 8.05 (d, 1H), 7.81 (d,
2H), 7.67
(dd, 1H), 7.51 (d, 1H), 7.43 (d, 2H), 7.41-7.25 (m, 5H), 6.06 (s, 1H), 4.68
(d, 1H), 4.59 (d,
1H), 3.73 (s, 3H), 2.78 (d, 3H), 2.12 (s, 3H).
Example 190
4-(((6-cvano-2'-methyl(1,1'-binhenvl)-3-vl)(1-methyl-1H-imidazol-5-
vl)methoxv)methvl)-
N,N-dimethylbenzamide
A solution of Example 272 (12 mg, 0.028 mmol) in dichloromethane (1 mL) at
room
temperature was treated with PyBop (17.5 mg, 0.033 mmol, 1.2 eq) and 2M
dimethylamine
in THF (100 ~.L), stirred for 16 hours, and concentrated. The concentrate was
dissolved in
1:1/DMSO:methanol (1 mL) and purified by preparative HPLC to provide the
desired
product.
MS (APCI(+)) mlz 465 (M+H)+;
MS (APCI(-)) m/z 499 (M+Cl)-;
1H NMR (500 MHz, DMSO-d6) 8 8.92 (s, 1H), 8.05 (d, 1H), 7.67 (dd, 1H), 7.51
(d, 1H),
7.41-7.25 (m, 9H), 6.06 (s, 1H), 4.68 (d, 1H), 4.58 (d, 1H), 3.74 (s, 3H),
2.97 (s, 3H), 2.88 (s,
3H), 2.13 (s, 3H).
Example 191
4-cyano-N-(4-cyanobenzyl)-N-((1-methyl-1H-imidazol-5-yl meth,)-3-(1
naphthyl)benzamide
-191-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 191A
4-((((1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)benzonitrile
The desired product was prepared by substituting 34A for 192C in Example 192D.
MS (APCI(+)) m/z 227 (M+H)+;
1H NMR (500 MHz, CDC13) 8 7.61 (d, 1H), 7.44 (d, 1H), 7.41 (s, 1H), 6.91 (s,
1H), 3.86 (s,
2H), 3.76 (s, 2H), 3.66 (s, 3H).
Example 191B
l0 4-carboxy-2-(I-naphthyl)benzonitrile
A solution of Example 89A (0.20 g, 0.70 mmol) in THF (5.0 mL) and water (2.0
mL)
at room temperature was treated with lithium hydroxide (0.040 g, 1.67 mmol),
stirred for 2
hours, and concentrated. The concentrate was dissolved in water (10 mL) and
adjusted to pH
3 with 10°7o HCl to provide a precipitate. The precipitate was filtered
and washed with cold
water to provide the desired product of sufficient purity for subsequent use
without further
purification.
MS (APCI(+)) m/z 291 (M+NH4)+;
1H NMR (500 MHz, DMSO-d6) S 8.18 (s, 2H), 8.11 (d, 1H), 8.08 (d, 1H), 8.02 (s,
1H), 7.69-
7.51 (m, 4H), 7.45 (d, 1H).
Example 191 C
4-cyano-N-(4-cyanobenzyl)-N-((1-methyl-1H-imidazol-5-yl)methyl)-3-(1
naphthyl)benzamide
The desired product was prepared by substituting Example 191A and Example 191B
for Example 192D and 4-cyanobenzoic acid, respectively, in Example 196.
MS (APCI(+)) m/z 482 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 8.61 (s, 1H), 8.06-8.01 (m, 3H), 7.74 (d, 1H),
7.70 (d, 2H),
7.62-7.59 (m, 2H), 7.55 (t, 1H), 7.44-7.37 (m, 5H), 7.29 (d, 1H), 4.75-4.69
(br s, 4H), 3.70 (s,
3H).
Example 192
4-((((1-methyl-1H-imidazol-5-yl)methyl)(4-trifluoromethylbenzyl)amino)methxl)-
2-( 1
naphthyl)benzonitrile dihydrochloride
Example 192A
4~romomethyl)-2-( 1-naphthyl)benzonitrile
-192-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 89B (1.90 g, 7.34 mmol) in dioxane (35 mL) at room
temperature was treated with N-bromosuccinimide (1.44 g, 8.09 mmol) and
triphenylphosphine (2.12 g, 8.08 mmol), heated to 80 °C for 10 minutes,
cooled to room
temperature, and concentrated. The concentrate was treated with ethyl acetate
(100 mL),
washed with brine, dried (MgS04), filtered, and concentrated. The concentrate
was purified
by flash column chromatography on silica gel with 4:1/hexanes:ethyl acetate to
provide the
desired product.
MS (DCI/NH3) m/z 339, 340, 341 and 342 (M+NH4)+;
1H NMR (500 MHz, CDC13) ~ 7.92-7.8 (m, 2H), 7.83-7.80 (m, 1H), 7.60-7.44 (m,
7H), 4.53
(s, 2H).
Example 192B
4-(azidomethyl)-2-(1-naphthyl)benzonitrile
A solution of Example 192A (1.71 g, 5.31 mmol) in DMF (25 mL) at room
~15 temperature was treated with sodium azide (3.46 g, 53.1 mmol) and sodium
iodide (80 mg,
0.53 mmol), stirred for 10 minutes, treated with ethyl acetate (100 mL),
washed with brine
(100 mL), dried (MgS04), filtered, and concentrated to provide the desired
product of
sufficient purity for subsequent use without further purification.
MS (DCI/NH3) m/z 302 (M+NH4)+;
1H NMR (500 MHz, CDCl3) 8 7.96 (d, 1H), 7.94 (d, 1H), 7.85 (d, 1H), 7.59-7.44
(m, 7H),
4.51 (s, 2H).
Example 192C
4-(aminomethyl)-2-(1-naphthyl)benzonitrile hydrochloride
A solution of Example 192B in THF (20 mL) at room temperature was treated with
triphenylphosphine (1.39 g, 5.31 mmol), stirred for 30 minutes, treated with
water (5 mL),
heated to 60 °C for 30 minutes, and concentrated. The concentrate was
treated with ethyl
acetate (100 mL) and extracted with 2M HCl (100 mL). The aqueous extract was
adjusted to
pH 12 with sodium carbonate and extracted with diethyl ether (100 mL). The
extract was
dried (MgS04), filtered, and treated with 1M HCl in diethyl ether (10 mL) to
provide a solid.
The solid was collected by filtration and washed with diethyl ether to provide
the desired
product of sufficient purity for subsequent use without further purification.
MS (DCI/NH3) m/z 259 (M+H)+ and 276 (M+NH4)+;
1H NMR (500 MHz, DMSO-d6) 8 8.46 (br s, 2H), 8.13-8.07 (m, 3H), 7.79 (d, 1H),
7.75 (s,
1H), 7.70-7.52 (m, 4H), 7.50 (s, 1H), 4.22 (s, 2H).
Example 192D
-193-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((( 1-methyl-1H-imidazol-5-yl)methyl)amino)methyl)-2-( 1-
naphtl~l)benzonitrile
di)~drochloride
A solution of Example 252A (0.68 g, 2.93 mmol) and Example 192C (0.82 g, 2.78
mmol) in 5% acetic acid/DMF (25 mL) at room temperature was treated with 4A
molecular
sieves, stirred for 1 hour, treated with sodium cyanoborohydride (0.26 g, 4.17
mmol), stirred
for 16 hours, treated with ethyl acetate (100 mL), washed with saturated
sodium carbonate
and brine, dried (MgS04), filtered, and concentrated. The concentrate was
treated with
1:1/methanol:lM HCl (100 mL), stirred for 16 hours, and concentrated. The
concentrate was
adjusted to pH 12 with sodium carbonate and extracted with ethyl acetate. The
extract was
dried (MgS04), filtered, and concentrated to provide the desired product of
sufficient purity
for subsequent use without further purification.
MS (APCI(+)) m/z 353 (M+H)+;
1H NMR (500 MHz, CDC13) b 7.96-7.92 (m, 2H), 7.79 (d, 1H), 7.73 (s, 1H), 7.58-
7.42 (m,
7H), 7.03 (s, 1H), 3.96 (s, 2H), 3.85 (s, 2H), 3.71 (s, 3H).
Example 192E
4-~(((1-methyl-1H-imidazol-5-yl)methyl)(4-
(trifluoromethyl)benzyl)amino)methyl)-2-(1
naphthyl)benzonitrile dihydrochloride
A solution of Example 192D in 5% acetic acid/DMF (1.0 mL) at room temperature
2o was treated with 4-(trifluoromethyl)benzaldehyde (35 mg, 2.0 mmol) and
anhydrous Na2S04,
stirred for 2 hours, treated with sodium cyanoborohydride (13 mg, 2.0 mmol),
stirred for 16
hours, treated with ethyl acetate (1.0 mL), washed with saturated sodium
carbonate and brine,
filtered through a Chem Elut~ CE1000M tube (Alltech, Northbrook, IL), and
concentrated.
The concentrate was treated with l:l/methanol:2M HCl (1.0 mL), stirred for 16
hours, and
concentrated. The concentrate was adjusted to pH 12 with sodium carbonate and
extracted
with ethyl acetate. The extract was dried (MgS04), filtered, and concentrated.
The
concentrate was purified by preparative HPLC, and the appropriate fractions
were treated
with dichloromethane (0.5 mL) and 1M HCl in diethyl ether (0.5 mL) and
concentrated to
provide the desired product.
MS (ESI(+)) m/z 511 and 512 (M+H)+;
1H NMR (400 MHz, DMSO-d6) 8 8.94 (s, 1H).8.08 (d, 1H), 8.06 (d, 1H), 7.94 (d,
1H), 7.67-
7.46 (m, 9H), 7.50 (s, 1H), 7.46 (dd, 1H), 7.39 (d, 1H), 3.84-3.74 (m, 6H),
3.72 (s, 3H).
Example 193
4-(((4-cyano-3-(1-naphthyl)benzyl)((1-methyl-1H-imidazol-5-
yl)methyl)amino)methyl)benzoic acid dihydrochloride
-194-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting 4-formylbenzoic acid for 4-
(trifluoromethyl)benzaldehyde in Example 192E.
MS (ESI(+)) m/z 487 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 8.94 (s, 1H), 8.08 (d, 1H), 8.06 (d, 1H), 7.95
(d,1H), 7.85
(d, 2H), 7.66-7.58 (m, 3H), 7.53 (s, 1H), 7.50-7.46 (m, 3H), 7.43 (d, 2H),
7.39 (dd, 1H), 3.82-
3.70 (m, 6H), 3.68 (s, 3H).
Example 194
N-(4-(((4-cyano-3-(1-naphth 1)~yl)((1-methyl-1H-imidazol-5
yl)methyl)amino)methyl)phenyl)acetamide dihydrochloride
to The desired product was prepared by substituting N-(4-
formylphenyl)acetamide for 4-
(trifluoromethyl)benzaldehyde in Example 192E.
MS (ESI(+)) m/z 500 (M+H)+;
tH NMR (500 MHz, DMSO-d6) 8 9.93 (s, 1H), 8.94 (s, 1H), 8.08 (d, 1H), 8.06 (d,
1H), 7.95
(d, 1H), 7.66-7.47 (m, 9H), 7.40 (d, 1H), 7.23 (d, 2H), 3.78-3.57 (m, 6H),
3.68 (s, 3H).
Example 195
4-(((( 1-methyl-1H-imidazol-5-yl)methyl)(4-
(methylsulfon.1)benzyl)amino)methyl)-2-( 1
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting 4-
(methylsulfonyl)benzaldehyde for
4-(trifluoromethyl)benzaldehyde in Example 192E.
MS (ESI(+)) m/z 521 (M+H)+;
tH NMR (500 MHz, DMSO-d6) ~ 8.80 (s, 1H), 8.09 (d, 1H), 8.06 (d, 1H), 7.95 (d,
1H), 7.83
(d, 2H), 7.67-7.47 (m, 9H), 7.40 (d, 1H), 3.84-3.76 (m, 6H), 3.69 (s, 3H),
3.17 (s, 3H).
Example 196
4-cyano-N-(4-cyano-3-(1-naphthyl)benzxl)-N-((1-methyl-1H-imidazol-5
yl)methyl)benzamide
A solution of Example 192D (35 mg, 0.10 mmol) in dichloromethane (0.5 mL) at
room temperature was treated with a solution of 4-cyanobenzoic acid (15 mg,
1.0 mmol),
3o PyBop (47 mg, 0.10 mmol), and N,N-diisopropylethylamine (39 mg, 0.30 mmol)
in
dichloromethane (0.5 mL), stirred for 72 hours, washed with brine, filtered
through a Chem
Elut~ CE1000M tube, and concentrated. The concentrate was purified by
preparative HPLC
(CH3CN/O.OlOM NH40Ac) to provide the desired product.
MS (APCI(+)) m/z 482 (M+H)~;
1H NMR (500 MHz, DMSO-d6) $ 8.03 (d, 1H), 8.02 (d, 1H), 7.89 (d, 1H), 7.83 (d,
2H), 7.62
(t, 1H), 7.59-7.55 (m, 3H), 7.51 (dt, 1H), 7.46-7.42 (m, 3H), 7.38 (d, 1H),
7.27 (s, 1H), 6.80
(s, 1H), 4.65 (s, 4H), 3.43 (s, 3H).
-195-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 197
3,4-dichloro-N-(4-cyano-3-(1-naphthyl)benzyl)-N-((1-methyl-1H-imidazol-5
yl)methyl)benzamide
The desired product was prepared by substituting 3,4-dichlorobenzoic acid for
4-
cyanobenzoic acid in Example 196.
MS (APCI(+)) m/z 525, 526, 527 and 528 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 8.03 (d, 1H), 8.02 (d, 1H), 7.89 (d, 1H), 7.64-
7.60 (m, 3H),
7.57 (dt, 1H), 7.51 (dt, 1H), 7.45-7.44 (m, 3H)~ 7.41-7.37 (m, 2H), 7.28 (s,
1H), 6.80 (s, 1H),
4.66 (s, 4H), 3.44 (s, 3H).
Example 198
4-chloro-N-(4-cyano-3-( 1-naphthyl)benzyl)-3-fluoro-N-(( 1-methyl-1 H-imidazol-
5
yl)methyl)benzamide
The desired product was prepared by substituting 4-chloro-3-fluorobenzoic
acid, for
4-cyanobenzoic acid in Example 196.
MS (APCI(+)) m/z 509, 510, 511 and 512 (M+H)+;
1H NMR (500 MHz, DMSO-d6) b 8.03 (d, 1H), 8.02 (d, 1H), 7.89 (d, 1H), 7.63-
7.55 (m, 3H),
7.50 (dt, 1H), 7.46-7.42 (m, 4H), 7.39 (d, 1H), 7.28 (s, 1H), 7.25 (dd, 1H),
6.80 (s, 1H), 4.66
(s, 2H), 4.65 (s, 2H), 3.44 (s, 3H).
Example 199
5,6-dichloro-N-(4-cyano-3-( 1-naphthyl)benzyl)-N-(( 1-methyl-1 H-imidazol-5
yl)methyl)nicotinamide
The desired product was prepared by substituting 5,6-dichloronicotinic acid
for 4-
cyanobenzoic acid in Example 196.
MS (APCI(+)) m/z 526, 527, 528 and 529 (M+H)+;
1H NMR (500 MHz, DMSO-d6) b 8.42 (d, 1H), 8.14 (d, 1H), 8.03 (d, 1H), 8.02 (d,
1H), 7.89
(d, 1H), 7.62 (dt, 1H), 7.57 (dt, 1H), 7.49 (dt, 1H), 7.47-7.44 (m, 3H), 7.39
(d, 1H), 7.31 (s,
1H), 6.82 (s, 1H), 4.70 (s, 4H), 3.45 (s, 3H).
Example 200
5-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-formyl( l, l'-
biphenyl)-2
carbonitrile ~drochloride
Example 200A
2-bromo-4-formylbenzonitrile
-196-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of compound 87C (5.1 g, 20.0 mmol) in dichloromethane (150 mL) at
-100 °C was treated dropwise with 1M DIBAL-H in toluene (26.0 mL, 26.0
mmol), stirred
for 30 minutes, treated with methanol (20 mL), stirred for 10 minutes, treated
with saturated
potassium sodium tartrate, warmed to room temperature, extracted with ethyl
acetate, dried
(MgS04), filtered, and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with 4:1/hexanes:ethyl acetate to provide the
desired product.
1H NMR (300 MHz, CDC13) 810.04 (s, 1H), 8.17 (d, 1H), 7.93 (dd, 1H), 7.86 (d,
1H).
Example 200B
2-bromo-4-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
A solution of Example 87F (2.59 g, 13.2 mmol) in THF (40 mL) at-78 °C
was treated
dropwise with 1.7M tent-butyllithium in pentane (7.06 mL, 12.0 mmol), stirred
for 30
minutes, treated with a solution of Example 200A (2.1 g, 10.0 mmol) in THF (10
mL), stirred
for~1 hour, treated with methanol (10 mL), stirred for 20 minutes, treated
with saturated
ammonium chloride (100 mL), warmed to room temperature, and extracted with
ethyl
acetate. The extract was dried (MgSOq.), filtered, and concentrated. The
concentrate was
purified by flash column chromatography on silica gel with 92:5:3/ethyl
acetate:methanol:
triethylamine to provide the desired product.
MS (APCI(+)) mlz 292 and 294 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.94 (d, 1H), 7.86 (s, 1H), 7.57 (dd, 2H), 6.41 (s,
1H), 6.25 (d,
1H), 5.91 (d, 1H), 3.56 (s, 3H).
Example 200C
2-bromo-4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
A solution of Example 200B (2.48 g, 8.5 mmol) and 4-cyanobenzyl bromide (2.50
g,
12.8 mmol) in dichloromethane (60 mL) at room temperature was treated with
silver(I) oxide
(7.8 g, 34 mmol), stirred for 16 hours in darkness, filtered through a pad of
diatomaceous
earth (Celite~) with methanol and concentrated. The concentrate was purified
by flash
column chromatography on silica gel with 92:5:3/ethyl
acetate:methanolariethylamine to
provide the desired product.
MS (APCI(+)) m/z 407 and 409 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.76 (s, 1H), 7.72-7.67 (m, 4H), 7.45-7.41 (m, 3H),
7.03 (br s,
1H), 5.61 (s, 1H), 4.65 (d, 1H), 4.57 (d, 1H), 3.44 (s, 3H).
Example 200D
5-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2'-formyl( 1,1'-
biphen~)-2
carbonitrile hydrochloride
-197-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 200C (30 mg, 0.074 mmol) and 2-formylphenylboronic acid
(13 mg, 0.085 mmol) in n-propanol (0.5 mL) was treated with Pd(OAc)2 (1.5 mg),
triphenylphosphine (4.5 mg), 2.0M Na2C03 (0.044 mL), and water (0.25 mL),
heated to 100
°C, stirred for 3 hours, and extracted with ethyl acetate. The extract
was concentrated and the
concentrate was purified by preparative HPLC to provide the desired product.
MS (APCI(+)) m/z 433 (M+H)~;
1H NMR (300 MHz, DMSO-d6) 8 9.87 (d, 1H), 9.08 (s, 1H), 8.10-8.03 (m, 2H),
7.86-7.75
(m, 3H), 7.75-7.73 (m, 2H), 7.60-7.50 (m, 4H), 7.41 (br s, 1H), 6.12 (s, 1H),
4.75 (d, 1H),
4.66 (d, 1H), 3.76 (s, 3H).
to
Example 201
5-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5- 1)~yl)-2'-
(trifluoromethyl)(1,1'
bi henyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting 2-
trifluoromethylphenylboronic acid
15 for 2-formylphenylboronic acid in Example 200D.
MS (APCI(+)) m/z 473 (M+H)~;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.07 and 9.05 (2s, 1H each), 8.08 (t,
1H), 7.92
(t, 1H), 7.84-7.82 (m, 2H), 7.77-7.40 (m, 2H), 7.60-7.50 (m, 4H), 7.38 (d,
1H), 6.13 (s, 1H),
4.74 (dd, 1H), 4.63 and 4.60 (2d, 1H each), 3.72 and 3.70 (2s, 3H each).
Example 202
2' 4'-dichloro-5-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methxl)(1,1'-
biphen~)-2
carbonitrile hydrochloride
The desired product was prepared by substituting with 2,4-
dichlorophenylboronic acid
for 2-formylphenylboronic acid in Example 200D.
MS (APCI(+)) m/z 473 (M+H)+;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.05 (s, 1H), 8.09 (d, 1H), 7.85-7.75
(m, 3H),
7.74 (dd, 1H), 7.65-7.45 (m, 5H), 7.41 (s, 1H), 6.13 (s, 1H), 4.75 (d, 1H),
4.65 and 4.61 (2d,
1H each), 3.73 (s, 3H).
Example 203
2-(1-benzothien-2-yl)-4-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5
yl methyl)benzonitrile hydrochloride
The desired product was prepared by substituting benzothiophene-2-boronic acid
for
2-formylphenylboronic acid in Example 200D.
MS (APCI(+)) m/z 461 (M+H)+;
-198-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) ~ 9.06 (s, 1H), 8.12 (d, 1H), 8.07 (t, 1H), 8.00 (d,
1H), 7.96
(s, 1H), 7.87 (s, 1H), 7.85 (d, 2H), 7.70 (d, 1H), 7.60 (d, 2H), 7.49-7.45 (m,
2H), 7.43 (s, 1H),
6.16 (s, 1H), 4.78 (d, 1H), 4.66 (d, 1H), 3.74 (s, 3H).
Example 204
5-(((4-cyanobenzyl)oxy (1-methyl-1H-imidazol-5~ 1)y methyl)-2'-
(hydroxymethyl)(1,1'-
biphenyl)-2-carbonitrile
A solution of Example 200D (55 mg) in THF (1 mL) at room temperature was
treated
with a solution of CaCl2 (30 mg) in ethanol (1 mL) and NaBH4 (19 mg), stirred
for 3 hours,
l0 and filtered. The filtrate was purified by preparative HPLC to provide the
desired product.
MS (DCI/NH3) m/z 435 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.00 (d, 2H), 7.83 (d, 3H), 7.60-7.20 (m, 8H),
6.59 (s, 1H),
5.91 (s, 2H), 4.65 (d, 1H), 4.57 (d, 1H), 3.74 (s, 3H).
Example 205
2'-cyano-5'-(((4-cyanobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)methy1)( 1,1'-
biphenyl)-2-
carboxylic acid
A solution of Example 200D (50 mg) in acetone (2 mL) at room temperature was
titrated with 2M Cr03 in concentrated H2S04 (Jones'reagent) until the orange
endpoint,
2o stirred for 16 hours, and concentrated. The concentrate was purified by
preparative HPLC
and lyophilized to provide the desired product.
MS (DCI/NH3) m/z 449 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.88-7.79 (m, 5H), 7.60-7.27 (m, 8H), 6.16 (s,
1H), 4.61
(d, 1H), 4.55 (d, 1H), 3.74 (s, 3H).
Example 206
4-cyano-N-(4-cyanobenzyl)-N-((1-methyl-1H-imidazol-5- 1)y methyl)-3-(8
quinolinyl)benzamide
3o Example 206A
3-bromo-4-cyanobenzoic acid
A solution of Example 87C (150 mg) in methanol (3 mL) and water (1 mL) was
treated with LiOH (80 mg) and stirred for 2 hours. The solution was adjusted
to pH 2 with
1M HCI, then extracted with ethyl acetate. The extract was dried (MgS04),
filtered, and
concentrated to provide the desired product of sufficient purity for
subsequent use without
further purification.
MS (DCI/NH3) m/z 243 and 245 (M+NH4)+;
-199-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, CDCl3) S 8.40 (d, 1H), 8.13 (dd, 1H), 7.79 (d, IH).
Example 206B
3-bromo-4-cyano-N-(4-cyanobenzyl)-N-((1-methyl-1H-imidazol-5- 1)~yl)benzamide
A solution of Example 206A (27 mg) and Example 191A (25 mg) in dichloromethane
(1 mL) at room temperature was treated with diisopropylethylamine (63 mL) and
bromotris(pyrrolidino)phosphonium hexafluorophosphate (53.5 mg) and stirred
for 16 hours.
The mixture was purified by preparative HPLC and lyophilized to provide the
desired
product.
MS (APCI(+)) m/z 434 and 436 (M+H)+.
Example 206C
4-cyano-N-(4-cyanobenzyl)-N-((1-methyl-1H-imidazol-5-yl)methyl)-3-(8
Quinolinyl)benzamide
A solution of Example 206B (10 mg) and 8-quinolinylboronic acid (8.0 mg) in
n-propanol (0.8 mL) and water (0.4 mL) was treated with Pd(OAc)2 (1.0 mg),
triphenylphosphine (3.0 mg), and 2M Na2C03 (15 mL), heated to 90 °C,
and stirred for 2
hours. The mixture was purified by preparative HPLC and lyophilized to provide
the desired
product.
2o MS (APCI(-)) m/z 517 (M+Cl)-; r,
IH NMR (300 MHz, DMSO-d6, at 90 °C) ~ 8.92 (s, 1H), 8.79 (dd, 1H), 8.45
(dd, 1H), 8.12
(dd, 1H), 7.96 (d, 1H), 7.71-7.39 (m, 10H), 4.77 (s, 2H), 4.74 (s, 2H), 3.74
(s, 3H).
Example 210
5-(1-(benzyloxy)-2-(1H-imidazol-1-yl)ethyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
Example 210A
2'-methyl-5-(2-oxiranyl)( 1,1'-biphenyl)-2-carbonitrile
A solution of Example 86I (0.5 g, 2.26 mmol) in acetonitrile/water (30:1) was
treated
with trimethylsulfonium iodide (0.48 g, 2.32 mmol) and potassium hydroxide
(0.226 g, 4.52
mmol), heated to 60 °C, stirred for 4 hours, filtered, and
concentrated. The concentrate was
purified by flash column chromatography on silica gel with 9:1/hexanes:ethyl
acetate to
provide the desired product.
Example 2108
5-( 1-hydroxy-2-( 1H-imidazol-1-yl)ethyl)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile
-200-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 210A (0.39 g, 1.65 mmol) in ethanol (15 mL) was treated
with
imidazole (0.12I g, 1.82 mmol) and catalytic pyridine, heated to reflux for 12
hours, and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 98:2/dichloromethane:methanol to provide the desired product.
Example 210C
5-(1-(benzyloxy)-2-(IH-imidazol-1-yl)ethyl)-2'-methyl(I 1'-biphenyl)-2-
carbonitrile
hydrochloride
The free base of the desired product was prepared by substituting Example 210B
for
1o Example 5D in Example 5E. The purified concentrate was treated with 1M HCl
in diethyl
ether and concentrated to provide the desired product.
MS (ESI(+)) m/z 394 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.0 (s, 1H), 8.05 (d, 1H), 7.63 (s, 2H), 7.43 (s,
IH), 7.4-7.2
(m, 9H), 7.2-7.1 (m, 2H), 5.1-5.0 (m, 1H), 4.6-4.5 (m, 2H), 4.49 (d, 1H), 4.43
(m, 1H), 2.15
(s, 3H).
Example 211
5-(hydrox~p~yl)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting 3-bromopyridine for Example
87F
in Example 1B.
MS (ESI(+)) m/z 301 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.64 (d, 1H), 8.45 (dd, 1H), 7.90 (d, 1H), 7,77
(dd, 1H),
7.61 (dd, 1H), 7.52 (s, 1H), 7.40-7.25 (m, 4H), 7.21 (d, 1H), 6.35 (d, 1H),
5.93 (d, 1H), 2.08
(s, 3H);
Anal. calcd for C2oH16N2O-O.2 H2O: C, 79.03; H, 5.44; N, 9.22. Found: C,
79.15; H, 5.55;
N, 8.99.
Example 212
2'-methyl-5-((3-pyridinylamino)methyl)( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting 3-aminopyridine for
picolylamine in
Example 215A.
MS (ESI(+)) m/z 300 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.97 (d, 1H), 7.91 (d, 1H), 7.76 (dd, 1H), 7.43
(s, 1H),
7.40-7.25 (m, 3H), 7.20 (d, 1H), 7.04 (dd, 1H), 6.9-6.8 (m, 1H), 6.64 (t, 1H),
4.45 (d, 2H),
2.04 (s, 3H);
Anal. calcd for CzoH17N3-0.3 H20): C, 78.82; H, 5.82; N, 13.79. Found: C,
79.19; H, 5.96;
N, 13.41.
-201-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 213
5-((benzyloxy) ( 1, 3-thiazol-5-yl)methyl)-2'-methyl( 1,1'-biphenyl)-2-
caxbonitrile
hydrochloride
The desired product was prepared by substituting Example 214 for Example 5D in
Example 5E.
MS (ESI(+)) m/z 397 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.11 (s, 1H), 8.0-7.9 (m, 2H), 7.66 (dd, 1H), 7.50
(s, 1H),
7.5-7.2 (m, 9H), 6.15 (s, 1H), 4.57 (s, 2H), 2.09 (s, 3H).
to
Example 214
5-(hydroxyl 1,3-thiazol-5-yl)methyl)-2'-methyl( l , l .'-biphenyl)-2-c
arbonitrile
The desired product was prepared by substituting 2-trimethylsilylthiazole for
Example
87F in Example 1B.
MS (ESI(+)) m/z 307 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.02 (s, 1H), 7.94 (d, 1H), 7.79 (s, 1H), 7.63
(dd, 1H), 7.50
(s, 1H), 7.40-7.25 (m, 3H), 7.22 (d, 1H), 6.69 (d, 1H), 6.23 (d, 1H), 2.10 (s,
3H);
Anal. calcd for C18H14N2S0-0.2 H2O: C, 69.74; H, 4.68; N, 9.04. Found: C,
69.78; H, 4.79;
N, 8.82.
Example 215
5-((benz l~pyridin lmethyl)amino)methyl)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
hydrochloride
Example 215A
5-((3-pyridinylmethyl)amino)methyl-2'-methyl( 1,1'-biphenyl)-2-carbonitrile
A solution of Example 86I (0.2 g, 0.9mmol) in 1,2-dichloroethane (10 mL) at
room
temperature was treated with picolylamine (0.12 g, l.Ommo1), acetic acid
(3.6mmo1), and
sodium (triacetoxy)borohydride, stirred for 16 hours, treated with saturated
NaHC03, and
extracted with ethyl acetate. The extract was washed with water and brine,
dried (MgS04),
filtered, and concentrated. The concentrate was purified by flash column
chromatography on
silica gel with 97:3/dichloromethane:methanol to provide the desired product.
Example 215B
5-((benzyl(3-p~ l~yl)amino)methyl)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
hydrochloride
-202-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The free base of the desired product was prepared by substituting benzaldehyde
and
Example 215A for Example 86I and picolylamine, respectively, in Example 215A.
The
purified concentrate was treated with 1M HCl in diethyl ether and concentrated
to provide the
desired product.
MS (ESI(+)) m/z 404 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.9 (br s, 1H), 8.80 (d, 1H), 8.57 (d, 1H), 7.90
(d, 2H), 7.4-
7.3 (m, 8H), 7.17 (d, 1H), 3.7-3.5 (m, 6H), 2.10 (s, 3H).
Example 216
2'-methyl-5-((3-pyridinylmethyl)amino) ( 1,1'-biphenyl)-2-carbonitrile
The desired product was prepared by substituting 3-pyridinecarboxaldehyde and
Example 225B for Example 86I and picolylamine, respectively, in Example 215A.
MS (ESI(-)) m/z 298 (M-H) ;
MS (ESI(+)) m/z 300 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.58 (s, 1H),'8.47 (d, 1H), 7.73 (d, 1H), 7.52 (d,
1H), 7.4-
7.2 (m, 5H); 7:12 (d, 1H), 6.61 (d, 1H), 6.5 (s, 1H), 4.43 (d, 2H), 2.05 (s,
3H).
Example 217
5-(benzyl(3-p ~rridinylmethyl)amino)-2'-methyl(1,1'-biphenyl)-2-carbonitrile
A solution of Example 216 (206 mg, 0.69 mmol) in THF at 0 °C was
treated dropwise
with 1M potassium tent-butoxide in THF (750 ~uL, 0.75 mmol), stirred for 30
minutes, treated
with benzyl bromide (132 mg, 0:75 mmol), warmed to room temperature, stirred
for 16
hours, treated with water, and extracted with ethyl acetate. The extract was
washed with
brine, dried (MgS04), filtered, and concentrated. The concentrate was purified
by flash
column chromatography on silica gel with 98:2/dichloromethane:methanol to
provide the
desired product.
MS (ESI(+)) m/z 390 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.5-8. 4 (m, 2H), 7.65-7.55 (m, 2H), 7.4-7.2 (m,
8H), 7.08
(d, 1H), 6.83 (d, 1H), 6.55 (s, 1H), 4.9-4.8 (br m, 4H), 2.89 (s, 3H).
Example 218
4-((benz~y)(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile hydrochloride
Example 218A
4-(hydroxy(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
The desired product was prepared by substituting 4-cyanobenzaldehyde for
Example
1A in Example 1B.
-203-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 218B
4-((benzyloxy)(1-methyl-1H-imidazol-5-yl)methyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 218A for Example 210B
in Example 210C.
MS (ESI(+)) mlz 304 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.15 (s, 1H), 7.97 (d, 2H), 7.67 (d, 2H), 7.4-7.3
(m, 6H),
6.05 (s, 1H), 4.55 (m, 2H), 3.74 (s, 3H);
Anal. calcd for C19H17N3O-O.8 H2O: C, 64.42; H, 5.58; N, 11.86. Found: C,
64.44; H, 5.62;
to N, 11.01.
Example 219
4-(((1-methyl-1H-imidazol-5-yl~(phenyl)methoxy)methyl)benzonitri1e
hydrochloride
Example 219A
(1-methyl-1H-imidazol-5-yl)(phen~l)methanol
The desired product was prepared by substituting benzaldehyde for Example 1A
in
Example 1B.
2o Example 219B
4-(((1-methyl-1H-imidazol-5-y_l~(phenyl)methoxy)methyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 219A and 4-
cyanobenzyl
bromide for Example 210B and benzyl bromide, respectively, in Example 210C.
MS (ESI(+)) m/z 304 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.12 (d, 1H), 7.84 (d, 2H), 7.57 (d, 2H), 7.5-7.4
(m, 5H),
7.34 (s, 1H), 5.95 (s, 1H), 4.63 (m, 2H), 3.74 (s, 3H);
Anal. calcd for C19Hi7N30-1.0 H20: C, 63.77; H, 5.63; N, 11.74. Found: C,
63.99; H, 5.60;
N, 10.68.
Example 220
5-(1-(benzyloxy)-2-(1-methyl-1H-imidazol-2-yl)ethyl)-2'-methyl(1,1'-biphen l
carbonitrile hydrochloride
Example 220A
5-(hydroxyl1-rnethyl-1H-imidazol-5-yl)meths)-2'-meths(1,1'-biphen~2-
carbonitrile
The desired product was prepared by substituting 1,2-dimethylimidazole for
Example
87F in Example 1B.
-204-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 220B
5-(1-(benzyloxy)-2-( 1-methyl-1H-imidazol-2-yl)ethyl)-2'-methyl( 1,1'-
biphenyl)-2
carbonitrile hydrochloride
The desired product was prepared by substituting Example 220A for Example 210B
in Example 210C.
MS (ESI(+)) m/z 408 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.05 (d, 1H), 7.7-7.1 (m, 14H), 5.05-4.95 (m, 1H),
4.38
(m, 2H), 3.71 (s, 3H), 2.13 (s, 3H), 14.43 (br s, 1H);
to Anal. calcd for C27H26N30C1-1.25 HaO: C, 69.51; H, 6.15; N, 9.00. Found: C,
69.61; H,
5.96; N, 8.23.
Example 221
5-( benz~y)(1-methyl-1H-imidazol-2- 1)~yl)-2'-methyl(l,l'-biphenyl)-2-
carbonitrile
hydrochloride
Example 221A
5-(hydroxyl1-methyl-1H-imidazol-2-yl)methyl)-2'-methyl(l, l'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting 1-methylimidazole for Example
87F
2o in Example 1B.
Example 221B
5-((benzyloxy)( 1-methyl-1H-imidazol-2-yl)methyl)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile
hydrochloride
The desired product was prepared by substituting Example 221A for Example 210B
in Example 210C.
MS (ESI(+)) mlz 394 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.06 (d, 1H), 7.7-7.5 (m, 4H), 7.4-7.2 (m, 9H),
6.41 (s,
1H), 4.69 (s, 2H), 3.77 (s, 3H), 2.11 (s, 3H).
Example 222
5-(1H-imidazol-1-ylmethyl)-2'-methyl(1,1'-biphenyl)-2-carbonitri1e
hydrochloride
A suspension of Example 20A (200 mg, 0.7 mmol) in DMF (5 mL) was treated with
imidazole (57 mg, 0.84 mmol) and K2C03 (193 mg, 1.4 mmol), heated to 50
°C, stirred for 2
hours, treated with ethyl acetate, washed with brine, dried (MgS04), filtered,
and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
-205-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
with 95:5/dichloromethane:methanol, treated with 1M HCl in diethyl ether, and
concentrated
to provide the desired product.
MS (ESI(+)) m/z 274 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.35 (s, 1H), 8.02 (d, 1H), 7.86 (t, 1H), 7.73 (t,
1H), 7.65-
7.55 (m, 2H), 7.5-7.3 (m, 3H), 7.23 (d, 1H), 5.59 (s, 2H), 2.11 (s, 3H);
Anal. calcd for C18H16N3C1-0.9 H20: C, 66.32; H, 5.50; N, 12.89. Found: C,
66.45; H, 5.67;
N, 11.74.
Example 223
4-(((1-methyl-1H-imidazol-5-yl)(3-(1-naphthyl)phenyl)methox )~yl)benzonitrile
The desired product was prepared by substituting 4-(bromomethyl)benzonitrile
for
benzyl bromide in Example 224C.
MS (ESI(+)) m/z 430 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.0-7.9 (m, 2H), 7.9-7.7 (m, 3H), 7.6-7.4 (m,
11H), 6.61
(s, 1H), 5.87 (s, 1H), 4.65 (m, 2H), 3.57 (s, 3H);
Anal. calcd for C29H23N30-0.25 H2O: C, 80.25; H, 5.45; N, 9.68. Found: C,
80.02; H, 5.56;
N, 9.56.
Example 224
benzyl (1-methyl-1H-imidazol-5-yl)(3-(1-naphth~phenyl)methyl ether
hydrochloride
Example 224A
3-( 1-naphthyl)benzaldehyde
The desired product was prepared by substituting 3-bromobenzaldehyde and 1-
naphthylboronic acid for 3-bromo-4-fluorobenzaldehyde and 2-
methylphenylboronic acid,
respectively, in Example 1A.
Example 224B
(1-methyl-1H-imidazol-5-~)(3-(1-naphthyl)phenyl)methanol
3o The desired product was prepared by substituting Example 224A for Example 1
in
Example 1B.
Example 224C
benzyl (1-methyl-1H-imidazol-5-yl)(3-(1-naphthyl)phen l~et~l ether
h'~drochloride
The desired product was prepared by substituting Example 224B for Example 5D
in
Example 5E.
MS (ESI(+)) m/z 405 (M+H)+;
-206-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) ~ 8.00 (d, 1H), 7.97 (d, 1H), 7.79 (d, 1H), 7.6-7.4
(m, 9H),
7.40-7.25 (m, 5H), 6.56 (s, 1H), 5.81 (s, 1H), 4.54 (m, 2H), 3.56 (s, 3H);
Anal. calcd for CZ8H24N2O-O.S HZO: C, 81:15; H, 5.89; N, 6.56. Found: C,
81.32; H, 6.09;
N, 6.77.
Example 225
2'-methyl-5-(((1-methyl-1H-imidazol-5-yl)methyl)amino)(1,1'-bi henyl)-2-
carbonitrile
Example 225A
l0 6-cyano-2'-methyl(1,1'-biphenyl)-3-carboxylic acid
A solution of Example 86H (2.0 g, 8.9 mmol) in acetone (25 mL) at 0 °C
was titrated
with Jones'reagent, stirred for 30 minutes, treated with iso-propanol and
concentrated to 1/3
its original volume treated with water (200 mL) while stirring vigorously,
then filtered and
dried in a vacuum oven to provide the desired product.
Example 225B
3-((tent-butoxycarbonyl)amino-6-cyano-2'-methyl-1,1'-biphen~
A solution of Example 225A (2.16 g, 9.11 mmol) in tent-butanol (30 mL) was
treated
with diphenylphosphoryl azide (1.96 rnL, 9.11 mmol) and triethylamine (1.3 mL,
9.11
mmol), heated to reflux, stirred for 21 hours, cooled to room temperature, and
concentrated.
The concentrate was treated with ethyl acetate (50 mL), washed sequentially
with water, 5%
citric acid , water, 5% NaHC03, and brine, dried (MgS04), filtered, and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
85:15/hexanes:ethyl acetate, to provide the desired product.
Example 225C
5-amino-2'-methyl( l , l'-biphenyl)-2-carbonitrile
A solution of Example 225B in dichloromethane (5 mL) was treated with
trifluoroacetic acid (5 mL), stirred for 45 minutes, and concentrated under a
nitrogen
3o atmosphere. The concentrate was treated with ethyl acetate, washed with
saturated NaHC03
and brine; dried (MgS04), filtered, and concentrated. The concentrate was
purified by flash
column chromatography on silica gel with 60:40/hexanes:ethyl acetate to
provide the desired
product.
Example 225D
1-metal-2-triethylsilylimidazole-5-carboxaldehyde
-207-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
A solution of Example 87F (1 g, 5.10 mmol) in THF (20 mL) at -78 °C was
treated
dropwise with 1.7M tert-butyllithium in hexanes (3 mL, 5.10 mmol), stirred for
10 minutes,
treated slowly with N-formylmorpholine, stirred for 1 hour, treated with
saturated NaHC03
and extracted with ethyl acetate. The extract was washed with brine, dried
(MgS04), filtered,
and concentrated to provide the desired product of sufficient purity for
subsequent use
without further purification.
Example 225E
2'-methyl-5-(~( 1-methyl-1H-imidazol-5-yl)methyl)amino)( 1,1'-biphenyl)-2-
carbonitrile
1o A solution of Example 225C (100 mg, 0.48 mmol) in 1,2-dichloroethane (5 mL)
at
room temperature was treated with Example 225D (215 mg, 0.96 mmol),
(triacetoxy)borohydride (283 mg, 1.33 mmol), and acetic acid (136 ~,L, 2.38
mmol), stirred
for 16 hours, treated with saturated NaHC03 and extracted with ethyl acetate.
The extract
was washed with saturated NaHC03 and brine, dried (MgS04), filtered, and
concentrated.
15 The concentrate was purified by flash column chromatography on silica gel
with
95:5/dichloromethane:methanol to provide the desired product.
MS (ESI(+)) m/z 303 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 7.6-7.5 (m, 2H), 7.4-7.2 (m, 3H), 7.16 (d, 1H),
7.06 (t,
1H), 6.85 (d, 1H), 6.76 (dd, 1H), 6.58 (d, 1H), 4.32 (d, 2H), 3.60 (s, 3H),
2.13 (s, 3H);
20 Anal. calcd for C19H1gN4-0.75 H20: C, 72.24; H, 6.22; N, 17.73. Found: C,
72.50; H, 5.97;
N, 17.17.
Example 226
5-(benzyl(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
25 A solution of Example 225E (100 mg, 0.33 mmol) in THF (2 mL) at room
temperature was treated dropwise with 1M potassium tert-butoxide in THF (500
~.L, 0.50
mmol) and benzyl bromide (50 mL, 0.42 mmol), sealed in a screw-cap vial,
heated to
50 °C, stirred for 3 hours, cooled to room temperature, treated with
ethyl acetate, washed
with water and brine, dried (MgSO4), filtered, and concentrated. The
concentrate was
30 purified by flash column chromatography on silica gel with
96:4/dichloromethane:methanol
to provide the desired product.
MS (ESI(+)) m/z 393 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.60 (d, 1H), 7.55 (d, 1H), 7.40-7.15 (m, 8H),
7.11 (d, 1H),
6.89 (dd, 1H), 6.7-6.6 (m, 2H), 4.9-4.7 (m, 4H), 3.56 (s, 3H), 1.94 (s, 3H);
35 Anal. calcd for C~6H24N4-0.25 HaO: C, 78.65; H, 6.22; N, 14.11. Found: C,
78.71; H, 6.24;
N, 13.88.
-208-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 227
4-(methyl((1-methyl-1H-imidazol-5-xl)methyl)amino)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting methyl iodide for benzyl
bromide in
Example 232.
MS (ESI(+)) m/z 353 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.02 (d, 2H), 7.72 (d, 1H), 7.6-7.4 (m, 6H), 7.01
(dd, 1H),
6.88 (d, 1H), 6.66 (s, 1H), 4.67 (m, 2H), 3.54 (s, 3H), 3.02 (s, 3H);
Anal. calcd for C~3H2oN4-1.0 H20: C, 74.57; H, 5.98; N, 15.12. Found: C,
74.55; H, 5.85; N,
13.83.
to
Example 228
4-(allyl(( 1-methyl-1 H-imidazol-5-yl)meth'rl)amino)-2-( 1-
naphthyl)benzonitrile
The desired product was prepared by substituting allyl bromide for benzyl
bromide in
Example 232.
MS (ESI(+)) m/z 379 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 8.01 (d, 2H), 7.70 (d, 1H), 7.6-7.4 (m, 6H), 7.00
(dd, 1H),
6.85 (d, 1H), 6.69 (s, 1H), 5.85-5.75 (m, 1H), 5.2-5.1 (m, 2H), 4.66 (m, 2H),
4.07 (dd, 2H),
3.54 (s, 3H);
Anal. calcd for C25H22Na.-O.S HZO: C, 77.49; H, 5.98; N, 14.45. Found: C,
77.50; H, 6.00; N,
2o 14.14.
Example 229
5-((4-cyanobenz'rl)((1-methyl-1H-imidazol-5- 1)~meth~rl)amino)-2'-methyl(1,1'-
biphen 1y )2
carbonitrile
The desired product was prepared by substituting 4-(bromomethyl)benzonitrile
for
benzyl bromide in Example 226.
MS (ESI(+)) m/z 418 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.81 (d, 2H), 7.63 (d, 1H), 7.55 (s, 1H), 7.38 (d,
2H), 7.35-
7.20 (m, 3H), 7.12 (d, 1H), 6.88 (dd, 1H), 6.7-6.6 (m, 2H), 4.82 (br s, 4H),
3.55 (s, 3H), 1.94
3o (s, 3H);
Anal. calcd for C27Hz3N5-0.4 H20: C, 76.36; H, 5.65; N, 16.49. Found: C,
76.40; H, 5.58; N,
16.17.
Example 230
4-(((1-methyl-1H-imidazol-5- 1)meth l~phen~propyl)amino)-2-(1-
naphthyl)benzonitrile
The desired product was prepared by substituting 1-bromo-3-phenylpropane for
benzyl bromide in Example 232.
-209-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (ESI(+)) m/z 457 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.02 (d, 2H), 7.7-7.4 (m, 7H), 7.2-7.0 (m, 5H),
6.94 (dd,
1H), 6.72 (d, 1H), 6.61 (s, 1H), 4.64 (m, 2H), 3.52 (s, 3H), 3.5-3.3 (m, 2H),
2.6-2.5 (m, 2H),
1.75-1.90 (m, 2H).
Exam 1p a 231
4-((4-cyanobenzyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-
naphthyl)benzonitrile
The desired product was prepared by substituting 4-(bromomethyl)benzonitrile
for
benzyl bromide in Example 232.
to MS (ESI(+)) m/z 454 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.99 (d, 2H), 7.81 (d, 2H), 7.72 (d, 1H), 7.6-7.5
(m, 3H),
7.5-7.3 (m, 5H), 6.99 (dd, 1H), 6.80 (d, 1H), 6.71 (s, 1H), 5.0-4.7 (m, 4H),
3.54 (s, 3H);
Anal. calcd for C3oH23N5-0.75 H2O: C, 77.14; H, 5.28; N, 14.99. Found: C,
77.32; H, 5.31;
N, 14.66.
Example 232
4-(benzyl(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-
naphthyl)benzonitrile
The desired product was prepared by substituting Example 234 for Example 225E
in
Example 226.
2o MS (ESI(+)) m/z 429 (M+H)t;
1H NMR (300 MHz, DMSO-d6) 8 8.00 (d, 2H), 7.70 (d, 1H), 7.6-7.2 (m, 11H), 7.00
(dd, 1H),
6.84 (d, 1H), 6.71 (s, 1H), 4.83 (m, 2H), 4.74 (m, 2H), 3.54 (s, 3H);
Anal. calcd for C29H2a.N4-0.5 H2O: C, 79.60; H, 5.75; N, 12.80. Found: C,
79.80; H, 5.79; N,
12.68.
Example 233
4-(hexyl(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting hexyl iodide for benzyl
bromide in
Example 232.
3o MS (ESI(+)) m/z 423 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.01 (d, 2H), 7.7-7.4 (m, 7H), 6.96 (dd, 1H), 6.81
(d, 1H),
6.64 (s, 1H), 4.63 (m, 2H), 3.53 (s, 3H), 3.5-3.3 (m, 2H), 1.6-1.4 (m, 2H),
1.3-1.2 (m, 6H),
0.9-0.7 (m, 3H);
HRMS calcd m/z for C28H31N4: 423.2549 (M+H)+. Found: 423.2551.
Example 234
4-(((1-methyl-1H-imidazol-5 yl)methyl)amino)-2-(1-naphthyl)benzonitrile
-210-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 234A
tent-butyl 4-cyano-3-(1-naphth~phenylcarbamate
The desired product was prepared by substituting Example 191B for Example 225A
in Example 2258.
Example 234B
4-amino-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 234A for Example 225B
l0 in Example 225C.
Example 234C
4-((( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 234B for Example 225C
in
Example 225E.
MS (ESI(+)) m/z 339 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.01 (d, 2H), 7.7-7.4 (m, 6H), 7.12 (t, 1H), 6.9-
6.8 (m,
2H), 6.74 (d, 1H), 4.34 (d, 2H), 3.60 (s, 3H);
Anal. calcd for C2oH18Nq.02-1.25 H20: C, 73.21; H, 5.72; N, 15.52. Found: C,
73.07; H,
5.43; N, 14.84.
Example 235
N- 4-cyano-3-(1-naphthyl)phenyl)-N-((1-methyl-1H-imidazol-5-
yl)methyl)benzamide
The desired product was prepared by substituting benzoyl chloride for benzyl
bromide
in Example 232.
MS (ESI(+)) m/z 443 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.00 (dd, 2H), 7.95 (d, 1H), 7.6-7.3 (m, 10H),
7.24 (d, 1H),
7.13 (d, 1H), 6.72 (s, 1H), 6.64 (d, 1H), 5.24 (s, 2H), 3.59 (s, 3H);
Anal. calcd for C29H22Na.0-0.75 HaO: C, 76.38; H, 5.19; N, 12.28. Found: C,
76.58; H, 5.23;
3o N, 12.08.
Exam 1p a 236
N-(6-cyano-2'-methyl(1,1'-biphen l~yl)-N-((1-methyl-1H-imidazol-5
yl)methyl)benzamide
The desired product was prepared by substituting benzoyl chloride for benzyl
bromide
in Example 226.
MS (ESI(+)) m/z 407 (M+H)+;
-211-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) 8 7.82 (d, 1H), 7.51 (s, 1H), 7.4-7.2 (m, 9H), 7.05
(d, 1H),
6.92 (d, 1H), 6.68 (s, 1H), 5.21 (s, 2H), 3.58 (s, 3H), 1.73 (s, 3H);
Anal. calcd for C2oH18N402-0.5 H20: C, 75.16; H, 5.57; N, 13.48. Found: C,
75.40; H, 5.63;
N, 13.40.
Example 237
5-((3-cyanobenzyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2'-methyl( 1,1'-
biphenyl)-2-
carbonitrile
The desired product was prepared by substituting 3-(bromomethyl)benzonitrile
for
1o benzyl bromide in Example 226.
MS (ESI(+)) mlz 418 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 7.8-7.5 (m, 6H), 7.35-7.20 (m, 3H), 7.12 (d, 1H),
6.90 (dd,
1H), 6.7-6.6 (m, 2H), 4.9-4.7 (m, 4H), 3.85 (s, 3H), 1.94 (s, 3H);
Anal. calcd for C27H23N5-0.75 HBO: C, 75.23; H, 5.72; N, 16.24. Found: C,
75.38; H, 5.56;
N, 16.33.
Example 238
~l-methyl-1H-imidazol-5-yl)carbons)-2-(8-quinolinyl)benzonitrile
2o Example 238A
2-bromo-4-((1-methyl-1H-imidazol-5-yl)carbonyl)benzonitrile
A solution of Example 200B (250 mg) in dichloromethane(5.0 mL) at room
temperature was treated with silver(I) oxide (0.79 g), stirred for 16 hours,
filtered through a
pad of diatomaceous earth (Celite~), and concentrated. The concentrate was
purified by
flash column chromatography on silica gel with 95:5/ethyl acetate:methanol to
provide the
desired product.
MS (APCI(+)) m/z 290 and 292 (M+H)+.
Example 238B
4-~1-methyl-1H-imidazol-5-yl)carbons)-2-(8-quinolinyl)benzonitri1e
The desired product was prepared by substituting Example 238A and
8-quinolinylboronic acid for Example 200C and 2-formylphenylboronic acid,
respectively, in
Example 200D.
MS (APCI(+)) m/z 338 (M+H)+.
Example 240
-212-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((3,4-dichlorobenzyl)oxy)(1-methyl-1H-imidazol-5-yl)meth~)-2-(8
quinolinyl)benzonitrile dihydrochloride
Example 240A
2-bromo-4-(((3,4-dichlorobenz l~y)(1-methyl-1H-imidazol-5-
1)~methyl)benzonitrile
The desired product was prepared by substituting 3, 4-dichlorobenzyl bromide
for 4-
cyanobenzyl bromide in Example 200C.
MS (APCI(+)) m/z 450 and 452 (M+H)+
to Example 240B
4-(((3,4-dichlorobenz l~y)(1-methyl-1H-imidazol-5- 1)~, methyl)-2-(8
guinolinyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 240A for Example 238A
in Example 238B.
15 MS (APCI(-)) m/z 533, 535, and 537 (M+3s~37C1)-;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.13 and 9.11 (2s, 1H each), 8.96 (d,
1H), 8.54
and 8.51 (2d, 1H each), 8.16 and 8.07 (2d, 1H each), 7.94-7.36 (m, 8H), 7.09
(d, 1H), 6.77
(br s, 0.5IT), 6.36 (dd, 0.5H), 6.24 and 6.11 (2s, 1H each), 4.75-4.57 (m,
2H), 3.82 and 3.80
(2s, 3H each).
Example 241
4-(((3-fluoro-4-(trifluoromethyl)benzyl)oxy)(1-rnet~l-1H-imidazol-5-yl)methyl)-
2-(8
quinolinyl)benzonitrile dihydrochloride
Example 241A
2-bromo-4-(((3-fluoro-4-(trifluorometh,~l benzyl)oxy)(1-methyl-1H-imidazol-5
yl)methyl)benzonitrile
The desired product was prepared by substituting 4-trifluoromethyl-3-fluoro-
benzyl
bromide for 4-cyanobenzyl bromide in Example 200C.
3o MS (APCI(+)) m/z 468 and 470 (M+H)+.
Example 241B
4-(((3-fluoro-4-(trifluoromethyl)benzyl)oxy)(1-methyl-1H-imidazol-5- 1)~ethyl)-
2-(8
quinolinyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 241A and 8-
quinolinylboronic acid for Example 200C and 2-formylphenylboronic acid,
respectively, in
Example 200D.
-213-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (APCI(+)) m/z 517 (M+H)+;
MS (APCI(-)) m/z 551 (M+Cl) ;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.13 and 9.11 (2s, 1H each), 8.96 and
8.94 (2d,
1H each), 8.54 and 8.51 (2d, 1H each), 8.16 and 8.07 (2d, 1H each), 7.96-7.38
(m, 8H), 7.09
(d, 1H), 6.77 (br s, 0.5H), 6.36 (dd, 0.5H), 6.25 and 6.12 (2s, 1H each), 4.75-
4.57 (m, 2H),
3.82 and 3.80 (2s, 3H each).
Example 242
4-(((4-fluoro-3-(trifluorometh 1)~~ oxy)(1-metl~l-1H-imidazol-5yl)methyl)-2-(8-
1o quinolinyl)benzonitrile dihydrochloride
Example 242A
2-bromo-4-(((4-fluoro-3-(trifluoromethyl)benzyl)oxy)(1-methyl-1H-imidazol-5
1)y methyl)benzonitrile
The desired product was prepared by substituting 3-trifluoromethyl-4-fluoro-
benzyl
bromide for 4-cyanobenzyl bromide in Example 200C.
MS (APCI(+)) m/z 468 and 470 (M+H)+.
Example 242B
4-(((4-fluoro-3-(trifluoromethvl)benzvl)oxv)(1-methvl-1H-imidazol-5-vl)methvl)-
2-(8
quinolinyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 242A and 8-
quinolinylboronic acid for Example 200C and 2-formylphenylboronic acid,
respectively, in
Example 200D.
MS (APCI(+)) mlz 517 (M+H)+;
MS (APCI(-)) m/z 551 (M+Cl) ;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.16 and 9.11 (2s, 1H each), 8.96 and
8.94 (2d,
1H each), 8.56 and 8.51 (2d, 1H each), 8.16 and 8.07 (2d, 1H each), 7.95-7.20
(m, 8H), 7.09
(m, 1H), 6.79 (br s, 0.5H), 6.35 (dd, 0.5H), 6.28 and 6.15 (2s, 1H each), 4.82-
4.64 (m, 2H),
3.83 and 3.81 (2s, 3H each).
Example 243
4-(((4-cyano-3-(8-quinolin~phenyl)( 1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzoic
acid dihydrochloride
Example 243A
methyl 4-(((3-bromo-4-cyanophenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzoate
-214-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting methyl 4-
(bromomethyl)benzoate
for 4-cyanobenzyl bromide in Example 200C.
MS (APCI(+)) m/z 440 and 442 (M+H)+.
Example 243B
4~~(4-cyano-3-(8-quinolinyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)benzoic
acid dihydrochloride
The desired product was prepared by substituting Example 243A and 8-
quinolinylboronic acid for Example 200C and 2-formylphenylboronic acid,
respectively, in
to Example 200D.
MS (APCI(+)) m/z 475 (M+H)+;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.12 and 9.10 (2s, 1H each), 9.00-8.85
(m, 1H),
8.51 and 8.49 (2d, 1H each), 8.32 (d, 1H), 8.11 (d, 1H), 7.97-7.48 (m, 8H),
7.09 (m, 1H), 6.80
(br s, 0.5H), 6.38 (dd, 0.5H), 6.26 and 6.13 (2s, 1H each), 4.82-4.65 (m, 2H),
3.81 and 3.80
15 (2s, 3H each).
Example 244
6-(((4-cyano-3-(8-quinolin~phenyl)( 1-methyl-1H-imidazol-5
yl)methoxy)methyl)nicotinamide trihydrochloride
Example 244A
6-(((3-bromo-4-cyanophenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)nicotinonitrile
The desired product was prepared by substituting 6-bromomethyl nicotinonitrile
for 4-
cyanobenzyl bromide in Example 200C.
MS (APCI(+)) m/z 408 and 410 (M+H)+
Example 244B
6-(((4-cyano-3-(8-eluinoli~I)phenyl)(I-methyl-1H-imidazol-5
yl)methoxy)methyl)nicotinamide trihydrochloride
The desired product was prepared by substituting Example 244A and
8-quinolinylboronic acid for Example 200C and 2-formylphenylboronic acid,
respectively, in
Example 200D.
MS (APCI(+)) m/z 475 (M+H)+;
MS (APCI(-)) m/z 509 (M+Cl)-;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.12 (2s, 1H), 9.02-8.74 (m, 2H), 8.59
and 8.50
(2d, 1H each), 8.29-7.48 (m, 10H), 6.38 and 6.35 (2s, 1H each), 4.88-4.70 (m,
2H), 3.86 and
3.83 (2s, 3H each).
-215-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 245
6-(((4-c ado-3-(8-quinolin~phenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)nicotinic
acid trihydrochloride
Example 245A
methyl 6-(((3-bromo-4-cyanophenyl)(1-methyl-1H-imidazol-5-
yl)methoxy)methyl)nicotinate
The desired product was prepared by substituting methyl 6-bromomethyl-
nicotinate
for 4-cyanobenzyl bromide in Example 200C.
to MS (APCI(+)) m/z 441 (M+H)+.
Example 245B
6-(((4-cyano-3-(8-quinolinyl)phenyl)( 1-methyl-1H-imidazol-5-
yl)methoxy)methyl)nicotinic
acid trihydrochloride
The desired product was prepared by substituting Example 245A and
8-quinolinylboronic acid for Example 200C and 2-formylphenylboronic acid,
respectively, in
Example 200D.
MS (APCI(+)) m/z 476 (M+H)+;
MS (APCI(-)) m/z 510 (M+Cl) ;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.12 and 9.11 (2s, 1H each), 9.04-8.88
(m, 2H),
8.58 and 8.50 (2d, 1H each), 8.31 and 8.25 (2d, 1H each), 8.15 and 8.06 (2d,
1H each), 7.98-
7.88 (m, 1H), 7.78-7.48 (m, 5H), 7.13-7.08 (m, 1H), 6.35 (s, 1H), 4.91-4.74
(m, 2H), 3.85
and 3.83 (2s, 3H each).
Example 247
6-(((4-cyano-3-(8-quinolinyl)phenyl)(1-methyl-1H-imidazol-5=
yl)methoxy)methyl)nicotinonitrile trihydrochloride
A solution of Example 244A (34 mg, 0.084 mmol) and 8-quinolinylboronic acid
(23
mg, 0.13 mmol) in 1,2-dimethoxyethane (1.5 mL) was treated with cesium
fluoride (32 mg,
0.2 mmol) and Pd(Ph3P)4 (4 mg), purged with argon, heated to 100 °C,
stirred for 16 hours,
and filtered. The filtrate was purified by HPLC on a C1g reverse phase column
with
acetonitrile/10 mM ammonium acetate, concentrated, lyophilized, dissolved in
dichloromethane, treated with 1M HCl in diethyl ether, and concentrated to
provide the
desired product.
MS (ESI(+)) m/z 457 (M+H)+ and 489 (M+Na)+;
-216-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) b 9.09 (s, 1H), 8.97 (dd, 1H), 8.87 (dd, 1H), 8.50
(dd, 1H),
8.31 (dd, 1H), 8.16 (dd, 1H), 8.06 (d, 1H), 7.87 (dd, 1H), 7.79-7.70 (m, 4H),
7.62 (dd, 1H),
7.56 (s, 1H), 6.22 (s, 1H), 4.80 (q, 2H), 3.80 (s, 3H).
Example 248
5-(((3,4-dichlorobenzvl)oxv)(1-methyl-1H-imidazol-5-vl)methyl)-2'-
(trifluoromethvl)(1.1'-
biphenyl)-2-carbonitrile ~drochloride
The desired product was prepared by substituting Example 240A and
2-(trifluoromethyl)phenylboronic acid for Example 200C and 2-
formylphenylboronic acid,
respectively, in Example 200D.
MS (APCI(-)) m/z 550, 552, and 554 (M+35/37C1j ;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 8.82 (2s, 1H), 8.08 (dd, 1H), 7.92 (t,
1H), 7.88-
7.80 (m, 1H), 7.76-7.67 (m, 2H), 7.63-7.50 (m, 4H), 7.40-7.25 (m, 2H), 6.07
(s, 1H), 4.66-
4.49 (m, 2H), 3.69 and 3.68 (2s, 3H each)
Example 249
5-(((3-fluoro-4-(trifluoromethyl)benz 1~)oxy)(1-methyl-1H-imidazol-5-
yl)methyl)-2'
(trifluoromethyl)(1,1'-biphenyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting Example 241A and
2-(trifluoromethyl)phenylboronic acid for Example 2000 and 2-
formylphenylboronic acid,
respectively, in Example 200D.
MS (APCI(-)) m/z 568 (M+Cl)';
MS (APCI(+)) m/z 534 (M+H)~;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.03 arid 9.01 (2s, 1H each), 8.08 (dd,
1H), 7.92
(t, 1H), 7.84-7.71 (m, 4H), 7.60-7.49 (m, 3H), 7.40-7.36 (m, 2H), 6.14 (s,
1H), 4.76 (d, 1H),
4.67 and 4.60 (2d, 1H each), 3.72 and 3.70 (2s, 3H each).
Example 250
5-(((4-fluoro-3-(trifluoromethyl)benzyl)oxy)(1-methyl-1H-imidazol-5- 1)~ 1~-2'-
(trifluoromethyl)(1,1'-bi henyl)-2-carbonitrile hydrochloride
The desired product was prepared by substituting Example 242A and
2-(trifluoromethyl)phenylboronic acid for Example 200C and 2-
formylphenylboronic acid,
respectively, in Example 200D.
MS (APCI(-)) m/z 568 (M+Cl)';
MS (APCI(+)) m/z 534 (M+H)+;
-217-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.03 and 9.01 (2s, 1H each), 8.08 (dd,
1H),
7.94-7.68 (m, 6H), 7.60-7.49 (m, 3H), 7.34 (d, 1H), 6.11 (s, 1H), 4.78-4.60
(m, 2H), 3.72 and
3.70 (2s, 3H each).
Exam 1p a 251
6-(((6-cyano-2'-(trifluoromethyl)( 1,1'-biphenyl)-3-yl)( 1-methyl-1H-imidazol-
5
yl)methoxy)methyl)nicotinonitrile dihydrochloride
The desired product was prepared by substituting Example 244A and
2-(trifluoromethyl)phenylboronic acid for Example 200C and 2-
formylphenylboronic acid,
to respectively, in Example 200D.
MS (APCI(-)) m/z 508 (M+Cl) ;
MS (APCI(+)) m/z 474 (M+H)+;
1H NMR (300 MHz, DMSO-d6) (rotamers) S 9.09 and 908 (2s, 1H each), 8.97 (dd,
1H)~
8.34-8.31 (m, 1H), 8.08 (dd, 1H), 7.92 (t, 1H), 7.84-7.68 (m, 4H), 7.60-7.59
(m, 1H), 7.55-
15 7.52 (m, 1H), 7.43 (d, 1H), 6.21 (s, 1H), 4.84-4.68 (m, 2H), 3.74 and 3.72
(2s, 3H each).
Example 252
4-(2-((4-cyanobenzyl)oxy)-2-(1-methyl-1H-imidazol-5-yl)ethyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
Example 252A
1-methyl-2-(trieth~silyl)-IH-imidazole-5-carbaldehyde
A solution of Example 87F (10 g, 51 mmol) in THF (150 mL) at -74 °C,
was treated
dropwise with 1.7M tart-butyllithium in pentane (32 mL, 54 mmol), stirred for
20 minutes,
treated with 4-formylmorpholine (5.5 mL, 6.3 g, 5.5 mmol), stirred for 1 hour,
warmed to
room temperature, and treated with ethyl acetate and water. The organic layer
was washed
with brine, dried (Na2S04), filtered, and concentrated to provide the desired
product of
sufficient purity for subsequent use without further purification.
MS (DCI/NH3) mlz 225 (M+H)+;
1H NMR (300 MHz, CDC13) 8 9.76 (s, 1H), 7.89 (s, 1H), 4.00 (s, 3H), 1.00 (m,
15H).
Example 252B
4-~romometh~)-2-( 1-naphthxl)benzonitrile
A solution of Example 89B (1.3 g, 5.0 mmol) in DMF (10 mL) at 0 °C was
treated
with Liar (0.44 g, 5.1 mmol) and PBr3 (0.47 mL, 1.35 g, 5.0 mmol), warmed to
room
temperature, poured over ice and extracted with diethyl ether. The extract was
washed with
water and brine, dried (Na2S04), filtered, and concentrated to provide the
desired product of
-218-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
sufficient purity for subsequent use without further purification.
1H NMR (300 MHz, CDCl3) 8 7.96 (m, 3H), 7.82 (m, 1H), 7.55 (m, 7H), 4.56 (s,
2H).
Example 252C
S 4-(2-hydroxy-2-(1-methyl-1H-imidazol-5-yl)ethyl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by the method described in J. Org. ClZem.
1988,
Vo1.53, page 5789 using Example 252A and Example 252B, then purified by flash
column
chromatography on silica gel with 95:4:1 to 90:9:1/ethyl
acetate:ethanol:concentrated
ammonium hydroxide.
1o MS (DCI/NH3) m/z 354 (M+H)+.
Example 252D
4-(2-((4-cvanobenzvl)oxv)-2-(1-methyl-1H-imidazol-5-vllethvl)-2-(1-
nanhthvllbenzonitrile
hydrochloride
15 The desired product was prepared by substituting Example 252C and 4-
cyanobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.-
MS (APCI(+)) m/z 469 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.09 (s, 1H), 8.06 (m, 2H), 8.00 (d, 1H), 7.75-
7.22
(envelope, 12H), 5.20 (m 1H), 4.62 (m, 1H), 4.50 (m, 1H), 3.88 (s, 3H), 3.50
(m, 2H);
20 Anal. calcd for C31H2sC1N40~3.00 H20: C, 66.60; H, 5.59; N, 10.02. Found:
C, 66.19; H,
5.46; N, 10.50.
Example 253
4-(((4-cyanophenyl)( 1-methyl-1 H-imidazol-5-yl)methoxy)methyl)-2-( 1-
naphthyl)benzonitrile
25 hydrochloride
Example 253A
4-(hydroxyl 1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
Example 252A and 4-cyanophenylzinc iodide were processed as described in J.
Org.
30 Chem.1988, Vo1.53, page 5789, treated with NaBH4, and purified to provide
the desired
product.
MS (DCI/NH3) m/z 214 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.84 (m, 2H), 7.60 (s, 1H), 7.55 (m, 2H), 6.38 (s,
1H), 6.18
(d, 1H), 5.90 (d, 1H), 3.57 (s, 3H).
Example 253B
-219-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)methoxy)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 253A and Example 252B
for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (APCI(+)) m/z 455 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.07 (d, 1H), 8.06 (m, 3H), 7.93 (m, 2H), 7.66 (m,
4H),
7.60 (m, 2H), 7.50 (m, 2H), 7.43 (d, 1H), 7.35 (s, 1H), 6.12 (s, 1H), 4.82,
(m, 1H), 4,68 (m,
1H), 3.75 and 3.74 (both s, total 3H);
Anal. calcd for C3pH23C1Nq.O~1.75 H2O: C, 68.96; H, 5.11; N, 10.72. Found: C,
68.65; H,
4.92; N, 11.17.
Example 254
4-(~2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethox'r)meth~)-2(1
naphthyl)benzonitrile hydrochloride
Example 254A
4-(2-h day-2-(1-methyl-1H-imidazol-5-yl)ethyl)benzonitrile
Example 252A and 4-cyanobenzylzinc bromide were processed as described in J.
Org. Chem. 1988, Vo1.53, page 5789, treated with NaBH4, and purified to
provide the desired
product.
MS (DCIlNH3) m/z 228 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.73 (m, 2H), 7.59 (m, 2H), 7.55 (s, 1H), 6.80 (s,
1H), 5.30
(d, 1H), 4.81(m, 1H), 3.60 (s, 3H), 3.15 (m, 2H).
Example 254B
4-((2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethox )~yl)-2-(1
naphthyl)benzonitrile hydrochloride
The desired product was prepared by substituting Example 254A and Example 252B
for Example 5D and (bromomethyl)benzene, respectively, in Example 5E, and by
substituting
4:1 dichloromethane/DMF for dichloromethane. -
MS (APCI(+)) m/z 469 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.10 (s, 1H), 8.07 (m, 2H), 7.96 (d, 1H), 7.88 and
7.78
(both m, total 1H), 7.70-7.35 (m, 11H), 5.13 and 5.00 (both m, total 1H), 4.67
(m, 1H), 4.55
(m, 1H), 3.86 (s, 3H), 3.30 (m, 2H).
Example 255
-220-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-((1-(1-metal-1H-imidazol-5- l~phenylpropoxy)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
Example 255A
1-(1-methyl-1H-imidazol-5-yl)-3-phenyl-1-propanol
The desired product was prepared by substituting phenethylmagnesium chloride
for
phenylmagnesium bromide in Example 256A.
MS (DCI/NH3) m/z 217 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.43 (s, 1H), 7.30 (m, 2H), 7.20 (m, 3H), 6.94 (s,
1H), 4.63 (t,
l0 1H), 3.69 (s, 3H), 2.80 (m, 2H), 2.23 (m, 2H).
Example 255B
4-(( 1-( 1-methyl-1H-imidazol-5-girl)-3-phen~propoxy)methyl)-2-( 1-
naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 255A and Example 252B
for Example 5D and (bromomethylbenzene), respectively, in Example 5E. -
MS (APCI(+)) m/z 458 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.05 (s, 1H), 8.08 (m, 3H), 7.75 (m, 1H), 7.65 (m,
3H),
7.54 (m, 3H), 7.43 (m, 1H), 7.20 (m, 5H), 4.80 (m, 1H), 4.60 (m, 2H), 3.82 and
3.80 (both s,
total 3H), 2.70 (m, 2H), 2.30 (m, 1H), 2.13 (m, 1H);
Anal. calcd for C31H2gC1N30~2.40 H2O: C, 69.30; H, 6.15; N, 7.83. Found: C,
69.15; H,
5.59; N, 7.83.
Example 256
4-(((1-methyl-1H-imidazol-5-yl)(phenyl)methoxy)methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
Example 256A
(1-methyl-1H-imidazol-5-yl)(phenyl)methanol
3o A solution of Example 252A (1.2 g, 5.4 mmol) in THF (11 mL) at -10
°C, was treated
with phenylmagnesium bromide (3.M, 1.8 mL, 5.4 mmol), stirred for 1 hour,
treated with
methanol, warmed to room temperature, stirred for 16 hours, concentrated, and
treated with
ethyl acetate and water. The organic layer was washed with brine, dried
(Na2S04), filtered,
and concentrated to provide the desired product of sufficient purity for
subsequent use
without further purification.
MS (DCI/NH3) m/z 189 (M+H)+;
-221-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) b 7.51 (s, 1H), 7.38 (m, 4H), 7.29 (m, 1H), 6.38 (s,
1H), 5.91
(d, 1H), 5.77 (d, 1H),°3.55 (s, 3H).
Example 256B
4-(((1-methyl-1H-imidazol-5-yl)( henyl)methox )~yl)-2-(1-naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 256A and Example 252B
for Example 5A and (bromomethyl)benzene, respectively, in Example 5E, and by
substituting
4:1/dichloromethane:DMF for dichloromethane.-
l0 MS (APCI(+)) m/z 430 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.07 (s, 1H), 8.05 (m, 3H), 7.65 (m, 5H), 7.50 (m,
7H),
7.30 (s, 1H), 5.99 (s, 1H), 4.80 (m, 1H), 4.66 (m, 1H), 3.75 and 3.74 (both s,
total 3H);
Anal. calcd for C29H24C1N3O~1.70 H2O: C, 70.14; H, 5.56; N, 8.46. Found: C,
70.14; H,
5.49; N, 8.49.
Example 257
(((1-methyl-1H-imidazol-5-yl)(phenyl)methyl)amino)methyl)-2-(1-
naphthyl)benzonitrile
dihydrochloride
2o Example 257A
(1-methyl-1H-imidazol-5;~phenyl)methanamine hydrochloride
The desired product was prepared by substituting Example 256A for Example 89D
in
Example 13A.
MS (DCI/NH3) m/z 188 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.45 (s, 1H), 7.35 (m, 5H), 6.55 (s, 1H), 5.09 (s,
1H), 3.50
(s, 3H), 2.26 (br s, 2H).
Example 257B
(((1-methXl-1H-imidazol-5-yl)(phen 1)~ methyl)amino)methyl)-2-(1-
naphthyl)benzonitrile
3o dihydrochloride
Example 89C and Example 257A were processed as described in Example 12B,
substituting dichloromethane for 1,2-dichloroethane. The mixture was treated
with methanol
and stirred for 4 hours prior to treatment with ethyl acetate to provide the
desired product.
MS (APCI(+)) m/z 429 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.05 (s, 1H), 8.05 (m, 3H), 7.82, 7.72, and 7.60
(envelope,
6H), 7.45 (m, 7H), 5.75 (br s, 1H), 4.15 (br m, 2H), 3.81 and 3.79 (both s,
total 3H);
-222-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Anal. calcd for C29H26C1zN4~1.65 H20: C, 65.57; H, 5.56; N, 10.55. Found: C,
65.61; H,
5.54; N, 10.49.
Example 258
4-((1-(1-methyl-1H-imidazol-5- l~phen lei )y methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
Example 258A
1-(1-methyl-1H-imidazol-5- l~phenylethanol
The desired product was prepared by substituting benzylmagnesium chloride for
phenylmagnesium bromide in Example 256A.
MS (DCI/NH3) m/z 203 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.46 (s, 1H), 7.24 (m, 5H), 6.80 (s, 1H), 5.23 (d,
1H), 4.77
(m, 1H), 3.55 (s, 3H), 3.05 (m, 2H).
Example 258B
4-((I-(1-methyl-1H-imidazol-5- I~phenylethox )~ methyl)-2-(1-
naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 257A and Example 252B
for Example 5D and (bromomethyl)benzene, respectively, in Example 5E, and
substituting
4:1 dichloromethane/DMF for dichloromethane.-
MS (APCI(+)) m/z 444 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.05 (s, 1H), 8.09 (m, 2H), 7.95 (d, 1H), 7.70-
7.00
(envelope, 13H), 5.07 (m, 1H), 4.70 (m, 1H), 4.55 (m, 1H), 3.80 (m, 3H), 3.12
(m, 2H);
Anal. calcd for C3pH26C1N3O~2.7O H2O: C, 68.16; H, 5.99; N, 7.95. Found: C,
68.14; H,
5.89; N, 7.99.
Example 259
4-(((1-(1-methyl-1H-imidazol-5- 1,~2-phenylethyl)amino)methyl)-2-(1-
naphtl~l)benzonitrile
dihydrochloride
Example 259A
1-(-1-methyl-1H-imidazol-5-xl)-2-phenylethanediazonium chloride
A solution of Example 258A (0.4 g, 2.0 mmol) in dichloromethane at 0 °C
was treated
with thionyl chloride, stirred for 30 minutes, warmed to room temperature,
stirred for 1.5
hours, and concentrated. The concentrate'was treated with DMF (5 mL) and
sodium azide
(0.54 g, 8.2 mmol), heated to 55 °C, stirred for 3.5 hours, and treated
with ethyl acetate and
-223-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
0.5M NaHC03. The organic layer was washed with brine, dried (Na2S04),
filtered, and
concentrated to provide the desired product of sufficient purity for
subsequent reaction
without further purification.
Example 259B
1-(1-methyl-1H-imidazol-5-yl)-2-phenylethylamine
A solution of Example 259A in THF (5 mL) was treated with triphenylphosphine
(0.75 g, 2.8 mmol), heated to reflux, stirred for 1 hour, cooled to room
temperature, treated
with water (0.5 mL), stirred for 16 hours, concentrated, and treated with 2M
HCl and ethyl
acetate. The aqueous layer was adjusted to pH 10 with 2M Na~C03 and extracted
with
3:1/chloroform:isopropanol. The extract was dried (Na2S04), filtered, and
concentrated to
provide the desired product of sufficient purity for subsequent use without
further
purification.
MS (DCI/NH3) mlz 202 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 7.42 (s, 1H), 7.24 (m, 2H), 7.18 (m, 3H), 6.78 (s,
1H), 4.05
(m, 1H), 3.52 (s, 3H), 2.94 (m, 2H).
Example 2590
4~,(( 1-( 1-methyl-1H-imidazol-5-yl)-2-phenylethyl)amino)methyl)-2-( 1-
naphthyl)benzonitrile
2o dihydrochloride
The desired product was prepared by substituting Example 259A for Example 257A
in Example 257B.
MS (APCI(+)) m/z 443 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.95 (s, 1H), 8.20 (s, 1H), 8.10 (m, 3H), 7.95 (br
d, 1H),
7.86 (br d, 1H), 7.66 (m, 1H), 7.60 (m, 1H), 7.53 (m, 3H), 7.25 (m, 3H), 7.14
(m, 2H), 4.91
(br s, 1H), 4.40 (br m, 2H), 3.80 (br m, 1H), 3.59 (s, 3H), 3.30 (m, 1H);
Anal. calcd for C3oH28C12N4~ 1.40 H20: C, 66.64; H, 5.74; N, 10.36. Found: C,
66.92; H,
5.83; N, 9.92.
3o Example 260
4~((1=(1-methyl-1H-imidazol-5-.l~phen l~propyl)amino)methyl)-2-(1-
naphthyl)benzonitrile dihydrochloride
Example 260A
I-(1-methyl-1H-imidazol-S- 1y )3-phenyl~ropyldiazonium chloride
The desired product was prepared by substituting Example 255A for Example 258A
in Example 259A.
-224-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 260B
1-(1-methyl-1H-imidazol-5-, l~phen~~p~rlamine
The desired product was prepared by substituting Example 260A for Example 259A
in Example 259B.
MS (DCI/NH3) m/z 216 (M+H)+.
Examp1e260C
4-~(( 1-( 1-methyl-1H-imidazol-5-yl)-3-phen~propyl)amino)methyl)-2-( 1
naphthyl)benzonitrile dihydrochloride
The desired product was prepared by substituting Example 260B for Example 257A
in Example 257B.
MS (APCI(+)) mlz 457 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.05 (br s, 1H), 8.10 (m, 2H), 8.00 (br s, 1H),
7.90 (br m,
1H), 7.81 (s, 1H), 7.60 (m, 4H), 7.50 (m, 2H), 7.25 (m, 2H), 7.16 (m, 3H),
4.50, 4.35, and
4.20 (envelope, 3H), 3.79 and 3.75 (both s, total 3H), 2.60 (m, 2H), 2.40 and
2.20 (both br m,
total 2H).
Example 261
~((2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethyl)amino)methyl)-2-(1-
naphthyl)benzonitrile ditrifluoroacetic acid salt
Example 261A
2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethanediazonium chloride
The desired product was prepared by substituting Example 254A for Example 258A
in Example 259A.
Example 261B
4-(2-amino-2-(1-methyl-1H-imidazol-5- l~yl)benzonitrile
3o The desired product was prepared by substituting Example 261A for Example
259A
in Example 259B.
MS (DCI/NH3) m/z 227 (M+H)+.
Example 261C
4-(((2-(4-cyanophenyl)-1-(1-methyl-1H-imidazol-5-yl)ethyl)amino)methyl)-2-(1-
naphthyl)benzonitrile ditrifluoroacetic acid
-225-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 261B for Example 257A
in Example 257B, and purified by preparative HPLC with 0-70% acetonitrile/0.1%
trifluoroacetic acid.
MS (APCI(+)) m/z 468 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 8.86 (s, 1H), 8.10 (m, 3H), 7.77-7.56 (envelope,
7H), 7.55-
7.35 (envelope, 5H), 4.60 (br s, 1H), 4.13 (br s, 2H), 3.25 (br m, 2H);
Anal. calcd for C35H27F6N5O4~ 1.70 H2O: C, 57.89; H, 4.22; N, 9.64. Found: C,
57.85; H,
4.11; N, 9.71.
to Example 262
4-(((3-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-xl)methyl)-2-(1-
naphthyl)benzonitrile
The desired product was prepared by substituting Example 89D and
3-(bromoethyl)benzonitrile for Example 1B and (bromomethyl)benzene,
respectively, in
Example 1C.
MS (ESI(+)) m/z 455 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 9.15 (s, 1H), 8.15 (dd, 1H), 8.1 (t, 2H), 7.9-7.45
(m, 11H),
6.15 (s, 1H), 4.7 (q, 2H), 3.8 (d, 3H), 3.6 (s, 1H);
Anal. calcd for C3pH23C1N4O~ 1.6H20: C, 69.32; H, 5.08; N, 10.78. Found: C,
69.40; H,
5.16; N, 10.21.
Example 263
4-(((4-bromobenzvl)oxv) ( 1-methyl-1 H-imidazol-5-vl)methvl)-2-( 1-
nanhthvl)benzonitrile
The desired product was prepared by substituting Example 89D and
1-bromo-4-(bromomethyl)benzene for Example 1B and (bromomethyl)benzene,
respectively,
in Example 1C.
MS (ESI(+)) m/z 508 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.1 (s, 1H), 8.15 (dd, 1H), 8.05 (t, 2H), 7.8 (d,
1H), 7.7-7.4
(m, 8H), 7.35 (dd, 2H), 6.6 (s, 1H), 4.6 (q, 2H), 3.8 (d, 3H);
Anal. calcd for C29Hz3BrC1N30~0.9H20: C, 62.08; H, 4.46; N, 7.49. Found: C,
62.13; H,
4.50; N, 7.43.
Example 264
4-((3-chlorobenzyl)((1-methyl-1H-irnidazol-5-yl)methyl)amino)-2-(1-
naphthyl)benzonitrile
The desired product was prepared by substituting 3-(bromomethyl)-1-
chlorobenzene
for benzyl bromide in Example 232.
MS (ESI(+)) m/z 463 (M+H)+;
-226-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) S 7.99 (d, 2H), 7.72 (d, IH), 7.6-7.3 (m, 8H), 7.25-
7.20 (m,
1H), 7.2-7.1 (m, 1H), 7.00 (dd, 1H), 6.83 (d, 1H), 6.70 (s, 1H), 4.84 (m, 2H),
4.75 (m, 2H),
3.55 (s, 3H);
Anal. calcd for C29H23N4C1-O.5 H2O: C, 73.79; H, 5.12; N, 11.87. Found: C,
73.74; H, 5.03;
N, 11.72.
Example 265
4-(benzyl( 1H-imidazol-5-ylmethyl)amino)-2-( 1-naphthXl)benzonitrile
l0 Example 265A
4-((1H-imidazol-5-ylmethyl)amino)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 270A for Example 234B
in Example 234C.
Example 265B
4-(benzyl( 1 H-imidazol-5-ylmethyl)amino)-2-( 1-naphthxl)benzonitrile
The desired product was prepared by substituting Example 265A for Example 234C
in Example 234D.
MS (ESI(+)) m/z 415 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 811.96 (br s, 1H), 7.98 (d, 2H), 7.7-7.5 (m, 4H),
7.5-7.2 (m,
8H), 7.1-7.0 (m, 2H), 6.83 (d, 1H), 4.9-4.7 (m, 2H), 4.59 (m, 2H);
Anal. calcd for C28H22Nq.-0.75 H2O: C, 78.57; H, 5.53; N, 13.08. Found: C,
78.33; H, 5.21;
N, 12.93.
Example 266
4-((3-c~ranobenzyl)(( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-
naphthyl)benzonitrile
The desired product was prepared by substituting 3-(bromomethyl)benzonitrile
for
benzyl bromide in Example 232.
MS (ESI(+)) m/z 454 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 7.99 (d, 2H), 7.8-7.3 (m, 11H), 7.01 (dd, 1H, 1H),
6.82 (d,
IH), 6.71 (s, 1H), 5.0-4.7 (m, 4H), 3.54 (s, 3H);
Anal. calcd for C3oH23N5-1.0 H20: C, 76.41; H, 5.34; N, 14.85. Found: C,
76.47; H, 5.14; N,
I4.46.
Example 267
N-(4-cyano-3-(1-naphthyl)phenyl)-N-((1-methyl-1H-imidazol-5
yl methyl)benzenesulfonamide
-227-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting benzenesulfonyl chloride for
benzyl
bromide in Example 232.
MS (ESI(+)) m/z 479 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.1-8.0 (m, 2H), 7.97 (d, 1H), 7.80-7.45 (m, 10H),
7.38
(dd, 1H), 7.27 (d, 1H), 7.09 (d, 1H), 6.59 (s, 1H), 4.93 (m, 2H), 3.66 (s,
3H);
Anal. calcd for C~gH22N4O2S-0.75 H2O: C, 68.34; H, 4.81; N, 11.38. Found: C,
68.30; H,
4.73; N, 10.93.
Example 268
1o methyl 4-((4-cyano((1-methyl-1H-imidazol-5-yl)methyl)-3-(1-
naphthyl)anilino)methyl)benzoate
The desired product was prepared by substituting methyl 4-(bromomethyl)-
benzoate
for benzyl bromide in Example 232.
MS (ESI(+)) m/z 487 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 7.98 (d, 2H), 7.92 (d, 2H), 7.70 (d, 1H), 7.6-7.5
(m, 3H),
7.40 (dd, 1H), 7.4-7.3 (m, 4H), 7.00 (dd, 1H), 6.81 (d, 1H), 6.72 (s, 1H), 5.0-
4.7 (m, 4H),
3.85 (s, 3H), 3.54 (s, 3H);
Anal. calcd for C~oH18N402-1.0 H20: C, 73.79; H, 5.59; N, 11.10. Found: C,
73.87; H, 5.40;
N, 10.60.
Example 269
4-((4-cvano((1-methyl-1H-imidazol-5-vl)methyl)-3-(1-
nanhthvl)anilino)methvl)benzoic acid
The desired product was prepared by substituting Example 268 for Example lOF
in
Example 10G.
MS (ESI(+)) m/z 473 (M+H)~;
1H NMR (300 MHz, DMSO-d6) 8 12.90 (br s, 1H), 7.97 (dd, 2H), 7.90 (d, 2H),
7.71 (d, 1H),
7.6-7.3 (m, 8H), 6.82 (d, 1H), 6.73 (s, 1H), 5.0-4.7 (m, 4H), 3.55 (s, 3H);
HRMS calcd mlz for C3pH25N4O2: 473.1978 (M+H)+. Found: 473.1984.
Example 270
5-(benzyl( 1 H-imidazol-5-ylmethyl)amino)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile
Example 270A
hen, l~yl)imidazole-4-carboxaldehyde
The desired product was prepared as described in J. Med. Chem., 1996, Vo1.39,
page
353.
-228-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 270B
2'-methyl-5-(((1-trityl-1H-imidazol-4-yl)methyl)amino)( 1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 270Afor Example 225D
in
Example 225E.
Example 270C
5-(benzyl((1-trityl-1H-imidazol-4- 1)~yl)amino)-2'-methyl(1,1'-biphenyl)-2-
carbonitrile
The desired product was prepared by substituting Example 270B for Example 225E
in
Example 226.
Example 270D
5-(benzyl( 1H-imidazol-5-ylmethyl)amino)-2'-methyl( 1,1'-biphenyl)-2-
carbonitrile
A solution of Example 270C (380 mg, 0.61 mmol) in dichloromethane (10 mL) at
room temperature was treated with trifluoroacetic acid (3 mL) and
triethylsilane (1.5 mL),
stirred for 2 hours, and concentrated under a nitrogen atmosphere. The
concentrate was
treated with ethyl acetate, washed with saturated NaHC03 and brine; dried
(MgS04), filtered,
and concentrated. The concentrate was purified by flash column chromatography
on silica
gel with 95:5/dichloromethane:methanol to provide the desired product.
MS (ESI(+)) m/z 379 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 811.90 (br s, 1H), 7.6-7.5 (m, 2H), 7.4-7.1 (m, 9H),
7.1-7.0
(m, 2H), 6.95 (dd, 1H), 6.64 (d, 1H), 4.78 (br s, 2H), 4.57 (br s, 2H), 1.96
(s, 3H);
Anal. calcd for C25H22N4-O~S HZO: C, 77.49; H, 5.98; N, 14.45. Found: C,
77.20; H, 6.08; N,
13.96
Example 271
methyl 3-((4-cyano((1-methyl-1H-imidazol-5-yl)methyl)-3-(1-
naphthyl)anilino)methyl)benzoate
The desired product was prepared by substituting methyl 3-
(bromomethyl)benzoate
for benzyl bromide in Example 232.
3o MS (ESI(+)) m/z 487 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.0-7.9 (m, 2H), 7.9-7.8 (m, 1H), 7.77 (s, 1H),
7.71 (d,
1H), 7.60-7.45 (m, 5H), 7.41 (dd, 1H), 7.31 (d, 2H), 7.03 (dd, 1H), 6.85 (d,
1H), 6.72 (s, 1H),
5.0-4.7 (m, 4H), 3.83 (s, 3H), 3.56 (s, 3H);
Anal. calcd for C31H26N402-0.75 HaO: C, 74.45; H, 5.54; N, 11.20. Found: C,
74.58; H,
5.31; N, 10.83.
Example 272
-229-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
4-(((6-c~ano-2'-methyl( 1,1'-biphen~)-3-yl~( 1-methyl-1H-imidazol-5
yl)methoxy)methyl)benzoic acid
A solution of Example 130 in THF (10 mL) and water (5 mL) at room temperature
was treated with LiOH (100 mg ), stirred for 16 hours, adjusted to pH 7 with
saturated
ammonium chloride (20 mL) and extracted with ethyl acetate. The extract was
dried
(NaZS04), filtered, and concentrated to provide the desired product of
sufficient purity for
subsequent use without further purification.
MS (APCI(+)) m/z 438 (M+H)+;
MS (APCI(-)) m/z 472 (M+Cl) ;
1H NMR (500 MHz, DMSO-d6) 8 8.95 (br s, 1H), 8.06 (d, 1H), 7.93 (d, 2H), 7.68
(dd, 1H),
7.52 (s, 1H), 7.48 (d, 2H), 7.41-7.25 (m, 5H), 6.08 (s, 1H), 4.66 (d, 1H),
4.64 (d, 1H), 3.74 (s,
3H), 2.13 (s, 3H).
Example 273
4-((1-methyl-1H-imidazol-5-vl)((3-chlorobenzvlloxvlmethvll-2-(1-
nanhthvllbenzonitrile
~drochloride
The desired product was prepared by substituting Example 89D and 3-
chlorobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 464 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 9.05 (s, 1H), 8.17 (m, 1H), 8.09 (m, 2H), 7.78 (m,
1H), 7.5
(m, 11H), 7.40 (m, 1H), 6.12 (s, 1H), 4.65 (m, 2H), 3.79 (d, 3H).
Example 274
5-(((4-cyano-3-(1-naphth~phenyl)(1-methyl-1H-imidazol-5-yl)methoxy)meth l~)-2
pyridinecarbonitrile dihydrochloride
Example 274A
methyl 6-cyanonicotinate
A solution of 6-cyanonicotinic acid (5 g) in methanol (100 mL) was titrated to
a
3o yellow endpoint with 2M trimethylsilyldiazomethane in hexanes and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
3:1/hexanes:ethyl acetate to provide the desired product.
Example 274B
5-(hydroxymethxl)-2-pyridinecarbonitrile
The desired product was prepared by substituting Example 274A for Example 5A
in
Example 5B.
-230-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
MS (DCI/NH3) m/z 136 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.71 (m, 1H), 7.88 (m, 1H), 7.71 (m, 1H), 4.86 (d,
2H).
Example 274C
5 -(bromometh, l~~yridinecarbonitrile
The desired product was prepared by substituting Example 274B for Example 61A
in
Example 61B.
MS (DCI/NH3) m/z 197 and 199 (M+H)+;
1H NMR (300 MHz, CDC13) 8 8.73 (d, 1H), 7.89 (dd, 1H), 7.70 (d, 1H), 4.50 (s,
2H).
Example 274D
5-(((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methox )Y methyl)-
2
pyridinecarbonitrile dihydrochloride
The desired product was prepared by substituting Example 89D and Example 274C
for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 456 (M+H)+;
1H NMR (300 MHz, CDC13) 8 8.69 (s, 1H), 7.99 (t, 2H), 7.89 (d, 1H), 7.79 (m,
2H), 7.68 (m,
1H), 7.5 (m, 7H), 7.08 (d, 1H), 5.75 (d, 1H), 4.70 (s, 2H), 3.55 (d, 3H).
2o Example 275
5-(((4-cyanobenzyl)oxy)( 1-methyl-1 H-imidazol-5-yl)methyl)-3-( 1-naphthyl)-2
p~rridinecarbonitrile dihydrochloride
Example 275A
methyl 5,6-dichloronicotinate
A solution of 5,6-dichloronicotinic acid (19.2 g, 100 mmol) in methanol (150
mL) at
0 °C was treated with thionyl chloride (10.9 mL, 150 mmol), warmed to
room temperature
over 18 hours, treated with ethyl acetate, washed sequentially with half-
saturated NaHC03,
water, and brine, dried (Na2S04), filtered, and concentrated to provide the
desired product of
3o sufficient purity for subsequent use without further purification.
Example 275B
methyl 5-chloro-6-cyanonicotinate
A mixture of Example 275A (2.0 g, 10 mmol), potassium iodide (830 mg, 5 mmol),
K2C03 (6.91 g, 50 mmol), and potassium cyanide (3.26 g, 50 mmol) in DMSO (20
mL) at 80
°C was stirred for 6 hours, cooled, treated with ethyl acetate, washed
with water and brine,
-231-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
and concentrated. The concentrate was purified by flash column chromatography
on silica
gel with 3:1/hexanes:ethyl acetate to provide the desired product.
Example 275C
meth. 6-cyano-5-(1-naphthyl)nicotinate
A mixture of Example 275B (800 mg, 4 mmol), 1-naphthaleneboronic acid (1.5 g.,
8.7
mmol), palladium(II) acetate (17 mg, 0.08 mmol), 2-dimethylamino-2'-
dicyclohexylphosphino-biphenyl (44 mg, 0.11 mmol), and CsF (2 g, 13 mmol) in
dioxane (20
mL) at room temperature was stirred for 48 hours, treated with ethyl acetate,
washed with
water and brine, dried (Na2S04), filtered, and concentrated. The concentrate
was purified by
flash column chromatography on silica gel with 9:1/hexanes:ethyl acetate to
provide the
desired product.
Example 275D
5-(h~ymethyl)-3-( 1-naphthyl)-2-pyridinecarbonitrile
The desired product was prepared by substituting Example 275C for Example 5A
in
Example 5B.
Example 275E
5-formyl-3-(1-naphth 1~)-2-pyridinecarbonitrile
The desired product was prepared by substituting Example 275D for Example 35C
in
Example 35D.
1H NMR (300 MHz, CDC13) 810.28 (s, 1H), 9.25 (d, 1H), 8.36 (d, 1H), 8.02 (m,
2H), 7.57
(m, 4H), 7.42 (m, 1H), 4.50 (s, 2H).
Example 275F
5-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)-3-(1-naphth 1~)-2-
~~rridinecarbonitrile
The desired product was prepared by substituting Example 275E for Example 1A
in
Example 1B.
MS (DCM3) m/z 341 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.81 (dd, 1H), 7.98 (m, 3H), 7.5 (m, 4H), 6.78 (s,
1H), 6.11 (s,
1H), 4.10 (m, 2H), 3.65 (d, 3H).
Example 2756
~~(4-cyanobenz 1)oxy)(1-methyl-1H-imidazol-5-y1)methyl)-3-(1-naphthxl)-2-
pyridinecarbonitrile dihydrochloride
-232-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 275F and 4-
cyanobenzyl
bromide for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 456 (M+H)+;
1H NMR (300 MHz, CDCl3) b 8.82 (dd, 1H), 7.98 (m, 3H), 7.5 (m, 4H), 7.09 (d,
1H), 5.80
(s, 1H), 4.66 (m, 2I~, 3.54 (s, 3H).
Example 276
4-((1-methyl-1H-imidazol-5-yl)((4-azidobenzyl)oxy)methyl)-2-(1-
naphthyn)benzonitrile
~drochloride
to
Example 276A
4-(hydroxymethyn)benzenediazonium tetraflu0roborate
The desired product was prepared by substituting 4-aminobenzyl alcohol for
Example
87A in Example 87B.
Example 276B
(4-azidophenyl)methanon
The desired product was prepared by substituting Example 276A and sodium azide
for Example 87B and CuCN/NaCN, respectively, in Example 87C.
1H NMR (300 MHz, CDC13) ~ 7.45 (d, 2H), 7.02 (d, 2H), 4.69 (s, 2H).
Examine 276C
1-azido-4~bromometh~~l)benzene
The desired product was prepaxed by substituting Example 276B for Example 61A
in
Example 618.
Example 276D
4-(( 1-methyl-1H-imidazol-5-yl)((4-azidobenzyl)oxy)methyl)-2-( 1-
naphthyl)benzonitrile
hydrochloride
The desired product was prepared by substituting Example 89D and Example 276C
for Example 5D and (bromomethyl)benzene, respectively, in Example 5E.
MS (DCI/NH3) m/z 471 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.96 (m, 2H), 7.87 (d, 1H), 7.70 (s, 1H), 7.50 (m,
7H), 7.29
(m, 2H), 6.99 (m, 3H), 5.63 (s, 1H), 4.54 (s, 2H), 3.52 (d, 3H).
Example 277
-233-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
methyl 6-(((6-cyano-2'-(trifluoromethyl)( l , l'-biphenyl)-3-yl)( 1-methyl-1H-
imidazol-5-
yl)methoxy)methyl)nicotinate dihydrochloride
The desired product was prepared by substituting Example 245A and 2-
(trifluoromethyl)phenylboronic acid for Example 200C and 2-formylphenylboronic
acid,
respectively, in Example 200D.
MS (APCI(-)) m/z 541 (M+Cl) ;
MS (APCI(+)) mlz 507 (M+H)+;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.13 and 9.12 (2s, 1H each), 9.02 (dd,
1H),
8.32-8.28 (m, 1H), 8.09 (dd, 1H), 7.92 (t, 1H), 7.83-7.51 (m, 6H), 7.60-7.49
(m, 1H), 7.41
to (dd, 1H), 6.22 (s, 1H), 4.82 (d, 1H), 4.72 (dd, 1H), 3.76 and 3.74 (2s, 3H
each).
Example 278
5-(((4-cyanobenzyl)oxy)( 1-methyl-1H-imidazol-5-yl)methyl)-2',3'-dimethyl(1,1'-
biphen~)-2
carbonitrile hydrochloride
The desired product was prepared by substituting 2,3-dimethylphenylboronic
acid for
2-formylphenylboronic acid in Example 200D.
MS (APCI(-)) m/z 467 (M+Cl) ;
MS (APCI(+)) m/z 433 (M+H)+;
1H NMR (300 MHz, DMSO-d6) (rotamers) 8 8.99 (s, 1H), 8.04 (dd, 1H), 7.82 (d,
2H), 7.68-
7.66 (m, 1H), 7.57 (d, 2H), 7.47 (d, 1H), 7.40 (d, 1H), 7.28 (d, 1H), 7.21 (q,
1H), 7.13-7.02
(m, 1H), 6.09 (s, 1H), 4.76-4.62 (m, 2H), 3.73 (s, 3H),.2.32 (d, 3H), 2.03 and
1.97 (2s, 3H
each).
Exam 1p a 279
2',3'-dichloro-5-(((4-cvanobenzvl)oxv)(1-methyl-1H-imidazol-5-vl)methvll(1.1'-
binhenvl)-2-
carbonitrile hydrochloride
The desired product was prepared by substituting 2,3-dichlorophenylboronic
acid for
2-formylphenylboronic acid in Example 200D.
MS (APCI(-)) m/z 507 and 509 (M+Cl)-;
MS (APCI(+)) m/z 473 and 475 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 8.99 (s, 1H), 8.04 (dd, 1H), 7.82 (d, 2H), 7.68-
7.66 (m,
1H), 7.57 (d, 2H), 7.47 (d, 1H), 7.39 (d, 1H), 7.28 (d, 1H), 7.21 (q, 1H),
7.13-7.02 (m, 1H),
6.09 (s, 1H), 4.76-4.62 (m, 2H), 3.73 (s, 3H).
Example 280
6-(((2',3'-dichloro-6-cyano(1,1'-biphen 1~~)(1-methyl-1H-imidazol-5
yl)methoxy)methyl)nicotinonitrile dihydrochloride
-234-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
The desired product was prepared by substituting Example 244A and
2,3-dichlorophenylboronic acid for Example 200C and 2-formylphenylboronic
acid,
respectively, in Example 200D.
MS (ESI(+)) m/z 474 and 476 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 9.08 (s, 1H), 8.98 (dd, 1H), 8.33 (dd, 1H), 8.10
(d, 1H),
7.82-7.46 (m, 7H), 6.21 (s, 1H), 4.85-4.71 (m, 2H), 3.76 (s, 3H).
Example 281
6-(((6-cyano-2',3'-dimethyl( 1,1'-biphenyl)-3-yl)( 1-methyl-1H-imidazol-5-
l0 yl)methoxy)methyl)nicotinonitrile dihydrochloride
The desired product was prepared by substituting Example 244A and
2,3-dimethylphenylboronic acid for Example 200C and 2-formylphenylboronic
acid,
respectively, in Example 200D.
MS (ESI(+)) m/z 434 (M+H)+ and 456 (M+Na)+;
15 1H NMR (300 MHz, DMSO-d6) (rotamers) 8 9.11 (s, 1H), 8.33 (dd, 1H), 8.04
(d, 1H), 7.72-
7.50 (m, 6H), 7.29 (d, 1H), 7.21 (dd, 1H), 7.12 (d, 1H), 6.19 (s, 1H), 4.85-
4.70 (m, 2H), 3.78
(s, 3H), 2.32 (d, 3H), 2.03 and 1.97 (2s, 3H each).
Example 282
20 4-cyano-N-((4-cyano-3-( 1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-
yl)methyl)benzenesulfonamide
A solution of Example 13A (50 mg, 0.148 mmol) in dichloromethane (1 mL) at
room
temperature was treated with 4-cyanobenzenesulfonyl chloride (35 mg, 0.174
mmol),
triethylamine (150 ~.L), and catalytic DMAP, stirred for 14 hours, and
concentrated. The
25 concentrate was purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:ethanol:concentrated ammonium hydroxide to provide the desired
product.
MS (DCI/NH3) rn/z 504 (M+H)+;
1H NMR (300 MHz, DMSO-d6) ~ 9.13 (br s, 1H), 8.06 (m, 2H), 7.89 (m, 3H), 7.72-
7.45 (m,
9H), 7.22-7.10 (m, 1H), 6.31-6.26 (two s,1H), 5.96 (br. 1H), 3.63-3.60 (two s,
3H);
Example 283
4-((4-cyanoanilino)( 1-methyl-1H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile
Example 283A
4-(h day(1-methyl-1H-imidazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile
A solution of Example 89D (1.13 g, 3.33 mmol) in dichloromethane (20 mL) at
-235-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
0 °C was treated dropwise with thionyl chloride (1.4 mL, 19.2 mmol),
warmed to room
temperature, stirred for 2 hours, concentrated, treated with toluene, and
concentrated to
provide the desired product of sufficient purity for subsequent use without
further
purification.
Example 283B
4-((4-cyanoanilino)(1-methyl-1H-imidazol-5- 1)~ methyl)-2-(1-
naphthyl)benzonitrile
A solution of Example 283A (100 mg, 0.280 mmol) in DMF (2 mL) at room
temperature was treated with 4-cyanoaniline (165 mg, 1.40 mmol) and
diisopropylethylamine
to (100 ~,L, 0.574 mmol), stirred for 72 hours, treated with ethyl acetate,
washed with water and
brine, dried (MgS04), filtered, and concentrated. The concentrate was purified
by flash
column chromatography on silica gel with 95:5/dichloromethane:methanol to
provide the
desired product.
MS (DCI/NH3) m/z 440 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 8.08 (m, 3H), 7.5-7.20 (m, 10H), 6.78 (m, 2H),
6.40-6.36
(two s, 1H), 6.15-6:11 (two s, 1H), 3.61-3.59 (two s, 3H);
Example 284
4-((3-cyanoanilino)(1-methyl-1H-imidazol-5- 1)~yl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting 3-cyanoaniline for 4-
cyanoaniline in
Example 283B.
MS (DCI/NH3) m/z 440 (M+H)+;
1H NMR (300 MHz, CDC13) 8 7.90 (m, 3H), 7.52 (m, 7H), 7.25 (m, 2H), 7.07 (m.
1H), 6.80
(m, 2H), 6.62 (m, 1H), 5.68 (m, 1H), 4.72 (m, 1H), 3.69-3.67 (two s, 3H);
Example 285
tent-butyl 1-((4-cyano-3-(1-naphthyl)phenyl)(1-methyl-1H-imidazol-5-yl)methyl)-
4-
piperidinylcarbamate
A solution of Example 283A (30 mg, 0.0838 mmol) in DMF (1 mL) at room
3o temperature was treated with tert-butyl 4-piperidinylcarbamate (90 mg,
0.449 mmol) and
diisopropylethylamine (80 ~uL, 0.46 mmol), stirred for 72 hours, heated to 60
°C, stirred for
16 hours, treated with ethyl acetate, washed with water and brine, dried
(MgS04), filtered,
and concentrated. The concentrate was purified by flash column chromatography
on silica
gel with 95:5/dichloromethane:methanol to provide the desired product.
MS (ESI(+)) m/z 522 (M+H)+;
-236-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
1H NMR (300 MHz, DMSO-d6) ~ 8.05 (m, 1H), 7.94 (s, 1H), 7.50 (m, 7H), 6.89 (m,
1H),
6.75 (m, 1H), 4.02 (m, 2H), 3.60-3.50 (m, 3H), 3.31 (s, 3H), 3.05 (m, 1H),
2.70 (m, 2H), 1.72
(m, 2H), 1.38 (s, 9H).
Exam 1p a 286
4-((4-cyanophenoxy) ( 1-methyl-1H-imidazol-5-yl)methyl)-2-( 1-
naphthyl)benzonitrile
A solution of Example 89D in THF (2 mL) at room temperature was treated with
DEAD (60 ~.L, 0.38 mrnol), 4-hydroxybenzonitrile (42 mg, 0.353 mmol), and
triphenylphosphine (93 mg, 0.355 mmol), stirred for 16 hours, treated with
diethyl ether,
to washed with 1M NaOH, water, and brine, dried (MgS04), filtered, and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
95:5:1/ethyl
acetate:ethanol:concentrated ammonium hydroxide to provide the desired
product.
MS (DCI/NH3) m/z 441 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 8.08 (m, 3H), 7.80-7.15 (m, 11H), 7.07(s, 1H),
6.63-6.60
(two s, 1H), 3.62-3.60 (two s, 3H);
Example 287
~((4-cyanophen~)( 1-methyl-1H-imidazol-5-yl)methyl)amino)-2-( 1-
naphthyl)benzonitrile
Example 287A
2-( 1-naphthyl)-4-nitrobenzonitrile
A solution of 1-naphthylboronic acid (2.58 g, 15.0 mmol) in toluene (20 mL)
and
dioxane (20 mL) was treated with 2-chloro-4-nitrobenzonitrile (1.83 g, 10.0
mmol), trans-
dichloro(bis(tricyclohexylphosphino))palladium (370 mg, 0.50 mmol), and 2M
Na2C03 (20
mL), purged with nitrogen, heated to reflux, stirred for 19 hours, treated
with ethyl acetate,
washed with water and brine, dried (MgS04), filtered, and concentrated. The
concentrate
was triturated with ethyl acetate/hexanes to provide the desired product.
MS (DCI/NH3) m/z 292 (M+NH4)+;
1H NMR (300 MHz, CDCl3) 8 8.41(m, 2H), 8.00 (m, 3H), 7.53(m, 5H).
Example 287B
4-amino-2-( 1-naphthyl)benzonitrile
A suspension of Example 287A (500 mg, 1.82 mmol) in ethanol (7 mL) at room
temperature was treated with concentrated HCI (2 mL) and a solution of
SnC12.2H~0 (I.25 g,
5.54 mmol) in ethanol (4 mL), stirred for 3 hours, and concentrated. The
concentrate was
treated with diethyl ether and 30% NaOH. The aqueous layer was extracted with
diethyl
ether, and the extract was washed sequentially with 1M NaOH, water, and brine,
dried
-237-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
(MgS04), filtered, and concentrated. The concentrate was triturated with
hexanes to provide
the desired product.
MS (DCI/NH3) m/z 262 (M+NHq.)+;
1H NMR (300 MHz, CDC13) 8 7.91 (m, 1H), 7.55 (m, 8H), 6.70 (m, 2H), 4.2 (br s,
2H).
Example 287C
4-(hydroxyl1-methyl-1H-imidazol-5-yl)methyl)benzonitrile
A solution of Example 87F (3.35 g, 17.08 mmol) in THF (50 mL) at -78
°C was
treated dropwise with 1.5M tert-butyllithium in pentane (11.4 mL, 17.1 mmol),
stirred for 30
to minutes, treated with a solution of 4-cyanobenzaldehyde (2.04 g, 15.56
mmol) in THF (10
mL) at -78 °C, stirred for 1 hour, treated with methanol (4 mL), warmed
to room temperature,
stirred for 1 hour, treated with 1M HCl (40 mL), stirred for 1.5 hours,
adjusted to pH 12 with
30°lo NaOH, and extracted with ethyl acetate. The extract was washed
with brine, dried
(MgS04), filtered, and concentrated. The concentrate was triturated with
4:1/hexanes:ethyl
acetate to provide the desired product.
MS (DCI/NH3) m/z 214 (M+H)+ and 231 (M+NH4)+;
1H NMR (300 MHz, CDC13) ~ 7.67 (d, 2H), 7.52 (d, 2H), 7.40 (s, 1H), 6.67 (s,
1H), 5.95 (s,
1H), 3.53 (s, 3H).
Example 287D
4-(chloro(1-methyl-1H-imidazol-5- 1)methyl)benzonitrile
A solution of Example 286 in dichloromethane (40 mL) at 0 °C was
treated with
thionyl chloride (2.8 mL, 38.4 mmol), warmed to room temperature, stirred for
4 hours, and
concentrated. The concentrate was treated with toluene and concentrated to
provide the
desired product of sufficient purity for subsequent use without further
purification.
Example 287E
4-(((4-c~nophenyl)(1-methyl-1H-imidazol-5-yl)methyl)amino)-2-(1-
naphthyl)benzonitrile
A solution of Example 287D (143 mg, 0.534 mmol) in DMF (4 mL) at room
3o temperature was treated with Example 287B (130 mg, 0.532 mmol) and
diisopropylethylamine (470 ~L, 2.70 mmol), stirred for 72 hours, treated with
ethyl acetate,
washed with water and brine, dried (MgS04), filtered, and concentrated. The
concentrate
was purified by flash column chromatography. on silica gel with
95:5/dichloromethane:methanol to provide the desired product.
MS (DCI/NH3) m/z 440 (M+H)+;
1H NMR (300 MHz, CDCl3) S 7.90-7.50 (m, 11H), 6.60 (m, 3H), 5.66 (m, 1H), 5.05
(m, 1H),
3.68-3.62 (two s, 3H).
-238-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 288
6-(((4-cyano-3-( 1-naphthyl)benzyl)(( 1-methyl-1H-imidazol-5
yl)methyl)amino)methyl)nicotinonitrile
A solution of Example 192D (35 mg, 0.10 mmol) in 5% acetic acid/DME (1.0 mL)
at
room temperature was treated with 6-formylnicotinonitrile (30 mg, 3.0 mmol)
and 41~
molecular sieves, stirred for 1 hour, treated with sodium
triacetoxyborohydride (40 mg, 2.0
mmol), stirred for 16 hours, treated with ethyl acetate (1.0 mL), washed with
saturated
sodium bicarbonate and brine, filtered through a Chem Elut~ CE1000M tube
(Alltech,
Northbrook, IL), and concentrated. The concentrate was purified by preparative
HPLC
(CH3CN/O.OlOM NH40Ac) to provide the desired product.
MS (APCI(+)) m/z 469 (M+H)+;
1H NMR (300 MHz, DMSO-d6) S 9.78 (s, 1H), 8.88 (s, 1H), 8.17 (dd, 1H), 8.07
(d, 1H), 8.05
(d, 1H), 7.93 (d, 1H), 7.66-7.45 (m, 8H), 7.39 (d, 1H), 3.83 (s, 2H), 3.80 (s,
2H), 3.69 (s, 2H),
3.50 (s, 3H).
Example 289
6-(((4-cyano-3-(1-naphthyl)phen~)(3-thienyl)methoxy)methyl)nicotinonitrile
Example 289A
4-(hydroxyl3-thienyl)methyl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting Example 89C for Example 86I
in
Example 80A.
MS (DCI/NH3) rn/z 359 (M+NH4)+;
1H NMR (500 MHz, CDC13) 8 7.92 (m, 2H), 7.80 (dd, 1H), 7.60-7.41 (m, 7H), 7.31
(m; 1H),
7.23 (m, 1H), 7.01 (dd, 1H), 5.98 (d, 1H), 2.42 (d, 1H).
Example 289B
6-(((4-cyano-3-( 1-naphth~phenyl)(3-thienyl)methoxy)methyl)nicotinonitrile
Example 289A (360 mg, 1.06 mmol) and Example 76A (416 mg, 2.11 mmol) were
3o dissolved in THF (5 mL). The solution was purged with nitrogen and cooled
to -5 °C.
Sodium hydride (30 mg, 1.25 mmol) was added and the reaction was stirred for
1.5 hours.
Aqueous ammonium chloride was added and the mixture was partitioned between
ethyl
acetate and water. The organic phase was dried (MgS04), filtered, and
concentrated.
Chromatography of the residue on silica gel with 4:1 hexanes:ethyl acetate
provided the
desired product.
MS (DCI/NH3) m/z 458 (M+H)+;
-239-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
~H NMR (300 MHz, CDC13) 8 8.79 (dd, 1H), 7.94 (m, 3H), 7.83 (m, IH), 7.65-7.44
(m, 8H),
7.35 (m, 1H), 7.30 (m, 1H), 7.02 (dd, 1H), 5.72 (s, 1H), 4.75 (d, 2H).
Example 290
4-(((4-cyanobenz~)oxy)( 1,3-thiazol-5-, l)methXl)-2-( 1-naphthyl)benzonitrile
Example 290A
4-(hydroxyl1,3-thiazol-5-yl)methyl)-2-(1-naphthyl)benzonitrile
The desired product was prepared by substituting 2-triethylsilylthiazole and
Example
l0 89C for Example 87F and Example 1A, respectively, in Example 1B.
MS (ESI(+)) m/z 343 (M+H)+;
1H NMR (500 MHz, DMSO-d6) 8 9.11 (s, 1H), 8.03 (m, 3H), 7.87 (d, 1H), 7.73 (d,
1H), 7.63
(m, 2H), 7.57 (m, 1H), 7.50 (m, 2H), 7.41 (m, 1H), 6.28 (s, 1H).
Example 290B
4-(((4-cyanobenzyl)oxy)( 1,3-thiazol-5-yl)methyl)-2-( I-naphthyl)benzonitrile
The desired product was prepared by substituting Example 290A for Example 289A
and 4-cyanobenzyl bromide for example 76A in Example 289.
MS (ESI(+)) m/z 458 (M+H)+;
1H NMR (500 MHz, CH30D) 8 9.69 (s, 1H), 8.14 (d, 1H), 7.98 (m, 3H), 7.79 (m,
1H), 7.68
(m, 3H), 7.60-7.51 (m, 4H), 7.49-7.41 (m, 3H), 6.20 (d, 1H), 4.78 (m, 2H);
Anal. Calcd. for C29H19N30S~HCl: C, 70.51; H, 4.08; N, 8.51. Found: C, 70.42;
H, 4.20; N,
8.61.
Example 291
6-(((4-cyano-3-( 1-naphthyl)phenyl)( 1,3-thiazol-5-
yl)methoxy)methyl)nicotinonitrile
The desired product was prepared by substituting Example 290A for Example 289A
in Example 289 and was converted to the trifluoroacetic acid salt.
MS (ESI(+)) m/z 459 (M+I~+;
1H NMR (500 MHz, DMSO-d6) ~ 9.14 (s, 1H), 8.97 (d, 1H), 8.32 (m, 1H), 8.08 (m,
3H),
7.98 (d, 1H), 7.80 (dd, 1H), 7.70-7.37 (m, 7H), 6.33 (s, 1H), 4.77 (m, 2H);
Anal. Calcd. for C2gH1gN4OS~O.95 C2HF3O2: C, 63.35; H, 3.37; N, 9.88. Found:
C, 63.34;
H, 3.22; N, 9.87.
Example 292
6-(((4-cyano-3-(1-naphthyl)phenyl)(3-pyridinyl)methox )y
meth~l)nicotinonitrile
-240-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 292A
4-(hydroxy(3-pyridinyl)methyl)-2-( 1-naphth~)benzonitrile
A solution of 3-iodopyridine (588 mg, 2.87 mmol) in THF (10 rnL) at-50
°C was
slowly treated with 0.8M isopropyl magnesium bromide (3.6 mL, 2.88 mmol). The
mixture
was stirred at <-25 °C for approximately 1 hour, treated with Example
89C (491 mg, 1.91
mmol), and stirred overnight while warming to room temperature. The reaction
was
quenched with aqueous NH4Cl and extracted with ethyl acetate. The combined
extracts were
dried (MgS04), filtered, and concentrated. Chromatography of the residue on
silica gel
eluting with 70 to 80°Io ethyl acetate/hexanes provided the desired
product. .
1o MS (ESI(+)) rn/z 337 (M+H)+;
1H NMR (300 MHz, CDCl3) 8 8.58 (s, 1H), 8.49 (d, 1H), 7.93 (m, 2H), 7.80 (dd,
1H), 7.70
(ddd, 1H), 7.59-7.44 (m, 4H), 7.41 (m, 3H), 7.28 (dd, 1H), 5.94 (s, 1H).
Example 292B
6-(((4-cyano-3-(1-naphth~)phen 1y )(3-pyridinyl)methoxy)methyl)nicotinonitrile
trifluoroacetate salt
The desired product was prepared by substituting Example 292A for Example 289A
in Example 289B and converting the product to the trifluoroacetate salt.
MS (ESI(+)) m/z 453 (M+H)+;
1H NMR (500 MHz, CDCl3) 8 8.98 (t, 1H), 8.93 (s, 1H), 8.71 (d, 1H), 8.34 (ddd,
1H), 8.25
(m, 1H), 8.07 (m, 3H), 7.86-7.77 (m, 3H), 7.70 (dd, 1H), 7.67-7.37 (m, 5H),
6.11 (d, 1H),
4.84-4.76 (m, 2H).
Anal. Calcd. for C3oH2oN40' 1.49 C2HF302~0.25 H20: C, 63.19; H, 3.54; N, 8.94.
Found:
C, 63.17; H, 3.49; N, 9.04.
Following the schemes and the examples described above, the following
compounds
can be prepared:
-241-
Example 296
Example 297
Example 298
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 299 Example 305
Example 300 Example 306
Example 301 Example 304
-242-
Example 302
Example 307
Example 303
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 311 Example 314
Example 309 Example 312
-243-
Example 313
Example 308
Example 315
Example 310
Example 316
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 317 Example 323
Example 318 Example 324
EXample 319 Example 325
-244-
Example 320
Example 321
Example 322
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
/
02CH3 Example 329
Example 326 Example 332
Example 327 Example 333
\
/ /
cN
i ~
~N
/
I
Example 328 Example 331
Example 334
-245-
Example 330
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
\
/ /
CN
/
\ \
HN
N O
Example 335 Example 341
N ~~N O
\ I /
N \
Example 336 Example 342
/ ~N
02CH3
Example 337 H2N
Example 343
-246-
Example 338
Example 339
Example 340
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 344 Example 347 Example 350
Example 348 Example 351
Example 349 Example 352
-247-
Exam 1p a 345
Example 346
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 353 Example 359
Example 354 Example 360
Example 357
Exam 1p a 355 Example 358
-248-
Example 356
Example 362
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 367
Exam 1p a 371
N
Exam 1p a 364 Example 368
-249-
Example 363
Example 369
Example 372
Example 370
Example 365
Example 373
Example 366
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 374 Example 377 Example 380
Example 378 Example 381
Example 379 Exam 1p a 382
-250-
Example 375
Exam 1p a 376
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam In a 383 Example 386 Example 390
Example 384 Example 391
Exam 1p a 385
Exam 1p a 392
-251-
Exam 1p a 387
Example 389
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 393 Example 396 Example 399
Example 394 Example 397
Example 398 exam 1p a 4U1
Example 395
-252-
Exam l~ a 400
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 402 Exam 1p a 405 Exam 1p a 408
Example 403 Example 406 Example 409
Example 404
Example 407 Example 410
-253-
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 411 Example 414
Example 415 Example 418
Example 416 Example 419
-254-
Example 412
Example 417
Example 413
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 420 Example 423
example 4~5 Example 428
-255-
Example 421
Example 424
Example 426
Example 427
Example 422
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 429 Example 435
Example 430 Example 436
-256-
Example 431
Example 432
Example 437
Example 433
Example 434
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam In a 438
Example 442
Exam 1p a 439
Example 443 Example 446
-257-
Exam 1p a 440
Example 441
Example 445
Example 444
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 450 Example 453
-258-
Example 447
Example 451
Example 454
Example 452
Example 455
Example 448
Example 449
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 456 Example 459
Exam 1p a 460 example 4b:i
Example 458 Example 464
-259-
Example 457
Example 461
Example 462
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
/ ~ \
\ /
N \ ~ \ CN
N ~ /
F3
Example 468 Example 471
H
Example 465
\ \
N O N
N
\ I \
O2H N
Example 469 Example 472
\
N Example 473
Example 470
N
Example 467
-260-
Example 466
Example 474
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 475
Example 481
I\
~ N
\ I / CN
N \I
\ C
I~
N
Example 482
-2.61-
Example 478
Example 483
Example 476
Example 477
Example 479
Example 480
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
\
N \ /
~1 \ CN
\ ~ N ~ /
Example 491
Example 495
Example 485 Exam 1p a 496
Example 486 Example 497
-262-
Example 484
Example 492
Exam 1p a 498
Exam 1p a 493
Example 490
Example 494
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
\ CN
/
\ N
Example 499
Example 503 Exam 1p a 506
\ CN
I. /
\ N
\ CN
/
Example 500
Example 501
Example 502
EXample 504
Example 507
-263-
Example 505
Example 508
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 509 Example 512
Example 510 Example 513
Example 511 Example 514
-264-
Example 515
EXample 516
Exam 1e~517
Example 51 ~
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 526
Example 523 Example 527
Example 524 Example 528
Example 519
Example 525
Example 529
Example 520
-265-
Exam 1p a 530
Example 521
Example 522
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
/ /
CN
/
N
N
/ 02H
Exam 1p a 531
Example 538
Example 535
Example 539
Example 536
Example 540
Example 537
-266-
Exam 1p a 532
Example 533
Exam 1p a 534
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 544 Example 547
Example 541 \
CN
H
Example 545 N
Example 54~
O%b \ / N C~C~ Example 549
Exam 1p a 543 Example 546
-267-
Exam 1p a 542
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 552
Example 553 Example 556 Example 559
Example 554 Example 557
Example 558
Example 555
Example 562
-268-
Example 561
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 563 Example 569
Example 564
Example 567
Example 565 Example 571
-269-
Example 566
Exam 1p a 570
Example 56~
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 572 ' r%x~ple 575
Exam lie 576
Example 573 - N
N
N
Example 580
-270-
Example 574
Example 577
Example 578
Example 579
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
~I
\ ..
~02CH3 Example 587
Example 581 Example 584
~I
\
N Example 588
Example 582 Example 585
I \ \
N
CN
N \I
~I
I
Example 586 Example 589
-271-
Example 583
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
/ /
CN
HN
N p
Example 593
/ ~N
02CH3
Example 595
H Example 598
Example 592
-272-
Example 590
Example 596
Example 594
Example 591
Example 597
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 602 Exam Ip a 605
~~N O
~I
Example 603
Example 600
H2N Example 604
Example 601
Example 607
-273-
Example 599
Example 606
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 608 Example 614
Exam 1p a 612
Example 609 Exam 1p a 615
Example 610 Exam 1p a 613 Exam 1p a 616
-274-
Example 611
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
N I /
I
/ CN
N \I
Example 624
Example 620
Example 625
Example 621
-275-
Example 617
Example 626
Example 627
Example 622
Example 6I8
Example 623
Example 619
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 628 Exam 1p a 631
Example 629 Example 632
Example 630 Example 633
-276-
Example 634
Example 635
Example 636
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 637 Example 643
Example 638
Example 645
N
N
N
Example 639 Exam 1p a 646
-277-
Exam 1p a 640
Exam 1p a 641
Example 642
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 650 Example 653
Example 647
Example 654
Exam 1p a 651
Example 652 Exam 1p a 655
Example 649
-278-
Example 648
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
~xampte b5N Example 662
Example 657 Example 660
Example 663
Example 658 Example 661 Example 664
-279-
Example 656
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 665 Example 671
Example 666 Example 672
-280-
Example 673
Example 667
Example 668
Example 669
Example 670
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 676 Example 682
-281-
Example 674
Example 677
Example 680
Example 675
Example 681
Exam 1p a 678
Example 679
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 683 example 6~6 Example 689
Example 684 Example 687
Example 688 Exam 1p a 691
-282-
Example 685
Example 690
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
example 6y6 Example 699
Example 693
~i
Example 694
vy .
Example 700
Example 697
-283-
Example 692
Example 695
Example 698
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 701 Example 704 Example 707
Example 702 Example 708
Example 703 Example 706
-284-
Exam 1p a 705
Example 709
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Exam 1p a 710 Example 716
Example 711 Example 717
-285-
Example 712
Example 713
Example 718
Example 714
Example 715
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 72I
Example 726
-286-
Example 719
Example 722
Example 725
Example 727
Example 720
Example 723
Exam 1~ a 724
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 732 Exam 1p a 735
Example 733 Example 736
Exam 1p a 737
Example 734
-287-
Example 728
Exam 1p a 729
Example 730
Example 73I
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
N
N
Exam 1p a 747
N ~ I Example 751
Example 738 \
\ \
\ CN
N ~ \ CN
Exam 1p a 748
\ \
Exam 1p a 739 ~ ~ Example 752
CN
\ I ~ \ \
~N
NHS I \/
Example 749 ~ \ CN
N \ N
\
N
Example 753
~O ~
Example 750
N
~~N
Example 741 .
-288-
Example 740
Example 754
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
\
\ CN
/
N!~N
-SJ CN
Example 755 ~ /
L \ \ Example 759
/ /
CN Example 763
/
N~N
N
Example 756
Example 760
Example 757 Example 761
-289-
Example 758
Example 764
Example 762
Example 765
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 769 Example 772
Example 766
Example 770 Example 773
Example 767
-290-
Example 768
Example 771
Exam 1p a 774
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 779 Exam 1p a 783
-291-
Example 775
Example 784
Example 780
Example 781
Example 785
Example 776
Example 782
Example 777
Example 778
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 786
/
CN
/
N~N
-S ~ CC2CHg
Example 791
-292-
Example 794
Example 787
Example 792
Exam 1p a 788
Example 795
Example 789
Example 793
Example 796
Example 790
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
Example 797 Exam 1p a 800
Example 798 Example 801
Example 802 Example 805
-293-
Example 799
Example 803
Exam 1p a 804
HN
O'/ O
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
N \ \
\ CN
N i
~N
N
Example 808
-294-
Example 806
Example 807
CA 02407093 2002-10-22
WO 01/81316 PCT/USO1/13678
It will be evident to one skilled in the art that the invention is not limited
to the
foregoing illustrative examples, and that it can be embodied in other specific
forms without
departing from the essential attributes thereof. It is therefore desired that
the examples be
considered in all respects as illustrative and not restrictive, reference
being made to the
appended claims, and all changes which come within the meaning and range of
equivalency
of the claims are therefore intended to be embraced within.
-295-