Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
1
APPARATUS AND METHOD FOR MULTIPLE CHANNEL HIGH
THROUGHPUT PURIFICATION
TECHNICAL FIELD
The present invention is directed to apparatus and methods, usable in,
s as an example, sample purification, and more particularly, to apparatus and
methods usable in, as an example, high throughput purification of samples from
a
chemical library.
BACKGROUND OF THE INVENTION
The relationship between structure and functions of molecules is a
to fundamental issue in the study of biological and other chemistry-based
systems.
Structure-function relationships are important in understanding, for example,
the
function of enzymes, cellular communication, cellular control and feedback
mechanisms. Certain macromolecules are known to interact and bind to other
molecules having a specific 3-dimensional spatial and electronic distribution.
Any
is macromolecule having such specificity can be considered a receptor, whether
the
macromolecule is an enzyme, a protein, a glycoprotein, and antibody, or an
oglionucleotide sequence of DNA, RNA, or the like. The various molecules which
bind to receptors are known as ligands.
A common way to generate ligands is to synthesize molecules in a
2o stepwise fashion in a liquid phase or on solid phase resins. Since the
introduction
of liquid phase and solid phase synthesis methods for peptides,
oglionucleotides,
and small organic molecules, new methods of employing liquid or solid phase
strategies have been developed that are capable of generating thousands, and
in
some cases even millions of individual compounds using automated or manual
2s techniques. A collection of compounds is generally referred to as a
chemical
library. In the pharmaceutical industry, chemical libraries of compounds are
typically formatted into 96-well microtiter plates. This 96-well formatting
has
essentially become a standard and it allows for convenient methods for
screening
these compounds to identify novel ligands for biological receptors.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
Recently developed synthesis techniques are capable of generating
large chemical libraries in a relatively short period of time as compared to
previous
synthesis techniques. As an example, automated synthesis techniques for sample
generation allows for the generation of up to 4,000 compounds per week. The
s samples, which contain the compounds, however, typically include 20% - 60%
impurities in addition to the desired compound. When samples having these
impurities are screened against selected targets, such as a novel ligand or
biological
receptors, the impurities can produce erroneous screening results. As a
result,
samples that receive a positive result from initial screening must be further
analyzed
to and screened to verify the accuracy of the initial screening result. This
verification
process requires that additional samples be available. The verification
process also
increases the cost and time required to accurately verify that the targeted
compound
has been located.
Samples can be purified in an effort to achieve an 85% purity or
~s better. Screening of the purified samples provides more accurate and
meaningful
biological results. Conventional purification techniques, however, are very
slow
and expensive. As an example, conventional purification techniques using high
pressure liquid chromatography (HPLC) take approximately 30 minutes to purify
each sample. Therefore, purification of the 4,000 samples generated in one
week
2o would take at least 2,000 hours (i.e. 83.3 days or 2.77 months).
Conventional purification techniques, such as HPLC, also require
large volumes of solvents and result in large volumes of waste solvent.
Disposal of
the solvents, particularly halogenated solvents, must be carefully controlled
for
legal and environmental reasons, so the disposal process can be laborious and
very
2s costly. Disposal of non-halogenated solvents is less rigorous. Accordingly,
when
halogenated and non-halogenated solvents are used, the waste solvents are
separated. The separation process of large volumes of solvents, however, can
be a
difficult process to perform efficiently and inexpensively. Accordingly,
purification of large chemical libraries can be economically prohibitive.
Therefore,
so there is a need for a faster and more economical manner of purifying
samples of
large chemical libraries.
Supercritical fluid chromatography (SFC) provides faster purification
techniques than HPLC. SFC utilizes a multiphase flow stream that includes a
gas,
such as carbon dioxide, in a supercritical state, a carrier solvent and a
selected
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
3
sample. The flow stream passes through a chromatography column, and is then
analyzed in an effort to locate target compounds. SFC is beneficial because
the
solvent and sample are earned by the gas and the amount of solvent needed
during
a purification run is substantially less than the volume used in HPLC. Also,
the
s amount of waste solvent at the end of a run is substantially less, so less
waste
solvent needs to be handled. SFC, however, requires pressure and temperature
regulation that is difficult to control accurately and reliably long term.
Purification systems have been developed to provide multiple
channels to increase the volume of samples purified by the system. The samples
in
io the multiple channels are analyzed in an effort to detect target compounds.
Improved efficiency can be achieved by using multiple channel high-speed
purification systems that provide high-speed sampling from the channels to a
mass
spectrometer or other selected analyzer. These high-speed multiple channel
systems, however, have developed complex and cumbersome techniques for taking
is high-speed samplings from multiple channels and tracking the positions of
the
samples within the multiple channels from which the high-speed samplings were
taken.
There are many different configurations of the purification
instruments. They typically share commonality in the concept wherein that
samples
2o are delivered to a chromatography instrument where compounds are separated
in
time, and a fraction collector collects the target compound. In order for
these
instruments to maintain the high throughput process, the instruments must be
able
to handle large sample numbers, as well as large samples in terms of mass
weight
and solvent volume. Tradition would specify the use of a semiprep or prep
scale
2s chromatography system for a typical milligram synthesis. While this is
achievable,
it has a low feasibility in a high throughput environment because several
issues
become apparent in such practice: large solvent usage, generation of large
amounts
of solvent waste, expensive large-bore columns, and relatively large
collection
volumes of target compounds. If the proper flow rate or column size is not
used,
so sufficient chromatographic purity will not be achieved.
A variety of column configurations have been developed in an effort
to improve the chromatographic results. U.S. Patent No. 4,554,071 discloses a
pre-
column for high pressure pre-concentration of material to be chromatographed
when the substances are provided in trace amounts. The pre-column is a vessel-
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
4
shaped body that narrows internally at both ends and that is packed with a
selected
carrier material. The pre-column is connectable to a conventional
chromatography
column. Liquid sample is added at high pressure into the narrowed top end, and
the
selected components are absorbed by the carrier material. The non-absorbed
fluid
s is drained from the pre-column through a separate outlet tube not connected
to the
chromatography column. The concentrated material is eluted with a solvent or
solvent mixture and the concentrated sample and solvent are then loaded into
the
chromatographic column. This concentration process and subsequent separation
process through the column can utilize a large amount of solvent to achieve a
to desired separation of the sample.
U.S. Patent No. 4,719,011 discloses a modular, high pressure liquid
chromatography column. The column includes segments with flanged sections that
can be combined to increase or decrease the column length. Segments having
different inner diameters can also be combined to provide an inner diameter
is deemed necessary to provide the type of chromatography for the mobile phase
being treated. Accordingly, the same modular components are usable in
different
combinations for different chromatographic runs. The mass sample and solvent
volume, however, dictate the diameter and length of the column to be
constructed
with these modular segments.
2o Columns used for high throughput processes must be able to handle
large sample numbers and large samples in terms of mass weight and solvent
volume. Conventional chromatography for large samples typically uses large-
bore
columns and large volumes of solvent. If the proper flow rate or column size
is not
used, the desired chromatographic purity will not be achieved. As a result,
2s chromatography of large samples results in large solvent usage, generation
of large
amounts of solvent waste, increased expense of replacing large-bore columns,
and
relatively large collection volumes of target compounds. Accordingly, there is
a
need for a chromatographic column for high throughput purification systems
that
overcomes drawbacks experienced by the prior art.
3o Further drawbacks experienced with high throughput purification
techniques include durability of components to accommodate the high pressures,
high volumes, or high flow rates of samples through the purification system.
The
purification system requires extreme accuracy and very high tolerances to
avoid
cross-contamination and to ensure purified compounds. The system components,
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
thus, must be sufficiently durable to accept the aggressive environment while
still
providing the accurate results required. If the components are not
sufficiently
durable and they break or require repair too quickly, the purification system
must
be taken out of service to replace or repair the components.
s Conventional SFC systems expose its components to extremely
hostile environments at high pressures. The high pressures must be accurately
controlled and maintained, typically by pressure regulators. The extremely
erosive
nature of the environment, however, can ruin valuing components in the
regulator.
As a result, manufacturers have made the valuing components out of very hard
and
to erosion-resistant materials. In high pressure environments, however, the
hard
materials are brittle, fragile, and susceptible to breaking or cracking. The
valuing
components are also exposed to cold temperatures due to the high pressure gas.
As
a result, the valuing components can ice up, which can compromise the accuracy
of
the pressure regulation.
is The pressure regulators of the high pressure systems must also be able
to move the valuing components very quickly and accurately for acceptable
pressure control. The electromagnetic control mechanisms have been used for
moving the valuing components. Such mechanisms axe typically large and have
unswept dead volumes that can retain portions of the samples passing through
the
2o system. This upswept dead volume can result in cross-contamination between
samples by sample carry-over or sample tailing. These mechanisms for
controlling
the valuing mechanisms also experience difficulties in controlling the speed
and
velocity of the moving components so as to avoid accidental damage to the
mechanisms. Accordingly, there is a need for pressure regulating devices
usable in
2s highly erosive, high pressure environments that achieve sufficient
accuracy, control
and durability.
A further drawback experienced in conventional purification
processes of large chemical libraries includes sample management during the
purification process. As an example, the chemical libraries are typically
maintained
3o in sets of 96-well microtiter plates, wherein each well includes a separate
sample.
Each sample is carefully tracked by its "well address" within the microtiter
plate.
When a sample or portion of a sample is removed for purification from a
selected
well of a microtiter plate, the purified sample is typically collected in a
separate
container, processed, and eventually returned to a receiving well in a similar
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
6
microtiter plate. That receiving well preferably has a corresponding well
address in
the microtiter plate so as to maintain the accuracy of the library records
regarding
sample location in the respective microtiter plate.
Conventional purification processes typically require the reformatting
s of a purified sample because the large collected volumes of fluid (e.g., the
solvent
that contains the purified sample) is greater than the volume of a receiving
well in a
conventional microtiter plate. The large collected volumes must be reduced to
a
volume that fits into the microtiter plate's well. The reduced volume of fluid
containing the purified sample is also tracked and deposited into the
appropriate
to well of the receiving microtiter plate that correctly maps to the well
location from
which the sample was taken at the start of the purification run. Such
reformatting
of purified samples into the receiving microtiter plate increases the time
requirements and cost of the purification processes. Therefore, there is a
need for a
purification process that allows for quick and economical purification of
samples
is that result in purified samples being collected directly to microtiter
plates mapped
directly to the original plate.
SUMMARY OF THE INVENTION
The present invention is directed to apparatus and methods, usable for
multiple channel high throughput purification of samples from a chemical
library
2o that overcome drawbacks experienced in the prior art. In an illustrated
embodiment
utilizing a apparatus in accordance with the present invention, the method of
multiple channel high throughput purification simultaneously purifies a
plurality of
samples, such as four samples, from a chemical library.
The purification process includes simultaneously purifying by
2s supercritical fluid chromatography (SFC) all four samples in four channels
of a
purification system. The method includes passing a first sample along a SFC
flow
path of the first channel, separating the first sample into sample portions,
spacing
the sample portions apart from each other along at least a portion of the
first fluid
path. The pressure of the supercritical fluid in the flow stream is regulated
with a
so back pressure regulator and a pressure relief valve in accordance with an
embodiment of the present invention. The method also includes moving the
separated sample portions along the fluid path, and detecting at least one
sample
portion flowing along the fluid path. The method further includes diverting a
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
7
sampling away from the sample portion, directing the sampling to an analyzer
while
the remainder of the sample portion continues along the fluid path, analyzing
the
sampling with the analyzer, and determining if the one sample portion has
selected
sample characteristics. The method also includes collecting the one sample
portion
s in a first receptacle, such as a well of a first microtiter plate, only if
the sample
portion has the selected sample characteristics. If the sample portion does
not have
the selected sample characteristics, the sample portion is collected in a
second
receptacle, such as a corresponding well in a second microtiter plate.
The multiple channel high throughput purification method of this
to illustrated embodiment further includes purifying a second sample along a
second
channel substantially simultaneously with the purification of the first
sample.
Purifying the second sample includes passing the second sample along a second
flow path of the second channel, separating the second sample into sample
portions,
and spacing the sample portions apart from each other along at least a portion
of the
is second fluid path with a pressure regulating assembly in accordance with an
embodiment of the present invention. The method also includes moving the
separated sample portions along the second fluid path, and detecting at least
one of
the sample portions flowing along the second fluid path. The method includes
regulating the second sample's pressure along the flow path with a pressure
2o regulating assembly in accordance with an embodiment of the present
invention.
The method further includes taking a sampling from the one sample portion and
directing the sampling to the same analyzer used for the first channel. The
remainder of the sample portion continues to flow along the second fluid path.
The method also includes analyzing the second sampling with the
2s analyzer, wherein the first and second samplings are analyzed separately in
accordance with a selected analysis priority protocol. The analysis of the
second
sampling determines if the sample portion has selected sample characteristics.
The
method further includes collecting the sample portion in a separate
receptacle, such
as a separate well in the first microtiter plate identified above, only if the
sample
3o portion has the second selected sample characteristics. If the sample
portion does
not have the selected sample characteristics, the sample portion is collected
in
another receptacle, such as a separate well in the second microtiter plate
identified
above.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
g
In one embodiment of the invention, the method of high throughput
purification includes purifying third and fourth samples along corresponding
third
and fourth channels in a manner similar to the purification discussed above
regarding the first and second samples. In this embodiment, the same analyzer
is
s used to analyze samplings from all four samples. The samplings are all
analyzed
separately and in accordance with the selected analysis priority protocol.
One aspect of the invention provides a high throughput liquid
chromatography column assembly configured to receive a selected injection of a
sample for flow therethrough at a selected flow rate to achieve
chromatographic
to separation of the sample. The sample has a selected mass weight and fluid
volume.
The assembly includes a loading column with a loading chamber therein having a
first inner diameter and a first length. The loading chamber is sized to
retain a
selected volume of a solid phase packing material onto which the sample is
loaded
and spatially distributed within the loading chamber. The volume of the
loading
is chamber is sufficient to fully load the sample therein, but the length of
the loading
chamber is insufficient to achieve the selected chromatographic separation of
the
sample as the sample passes through the packing material.
A separation column with a separation chamber is positioned to
receive the sample from the loading column. The separation chamber has a
2o diameter smaller than the loading column's diameter, and a length greater
than the
loading column's length. The separation chamber retains a solid phase packing
material therein, and the separation chamber's length is sufficient to achieve
the
selected chromatographic separation as the sample passes through the packing
material at the selected flow rate. The separation column's inner diameter is
such
2s that the separation chamber has a volume over the same length as the
loading
column's length that is insufficient to act as a loading area for the entire
selected
sample.
Another aspect of the invention is directed to a pressure regulating
assembly usable in one embodiment in a multiple channel high throughput
so purification system for substantially simultaneously purifying a plurality
of samples
from a chemical library. In one illustrated embodiment, the system includes a
controller and a sample analyzer coupled to the controller, wherein the
analyzer is
configured to determine whether the samplings have selected sample
characteristics. First, second, third, and fourth purification channels are
coupled to
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
9
the sample analyzer. The first purification channel includes a separation
device
positioned to receive a sample flow and to separate a first sample into sample
portions so the sample portions are spaced apart from each other in the sample
flow. A detector is positioned to receive the sample flow from the separation
s device and to detect at least one sample portion within the first sample. An
adjustable back pressure regulator assembly receives the flow stream from the
detector and controls the pressure of the flow stream within the first channel
in
accordance with an embodiment of the present invention.
In one embodiment of the invention, a pressure regulator assembly is
provided for use in a high throughput fluid system having a fluid channel for
carrying a fluid flow therethrough. The pressure regulator assembly includes
an
inlet line and an outlet line connectable to the fluid channel. A regulator
body has a
regulator inlet and outlet with the regulator inlet being connected to the
inlet line
and the regulator outlet being connected to the outlet line. The regulator
body has a
is chamber therein in fluid communication with the regulator inlet and outlet.
A
nozzle is in fluid communication with the regulator inlet. The nozzle has a
nozzle
outlet adjacent to the chamber. A stem is axially aligned with the nozzle
outlet.
The stem has one end forming a regulating surface and another end forming a
mounting portion. The regulating surface is positioned adjacent to the nozzle
outlet
2o and being positioned to restrict the fluid flow through the chamber to the
regulator
outlet.
The pressure regulator assembly in this embodiment also includes a
mounting rod attached to the stem's mounting portion. The mounting rod and
stem
are axially moveable in the regulator body relative to the nozzle outlet. An
2s adjustment member is connected to the mounting rod and is axially moveable
to
adjust the position of the stem relative to the nozzle outlet. The adjustment
mechanism has a dual concentric thread arrangement with first and second
threads
thereon. The first threads engage the mounting rod and are configured to move
the
mounting rod and stem as a unit in a first direction and at a first rate
relative to the
so nozzle outlet. The second threads are configured to move the adjustment
member,
the mounting rod, and the stem as a unit in a second direction and at a second
rate
relative to the nozzle outlet. The second direction being opposite the first
direction,
and the first rate being different than the second rate, to provide an
attenuated
movement of the stem's regulating surface relative to the nozzle outlet to
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
1~
selectively adjust a pressure of the fluid flow in the chamber. A drive
mechanism is
connected to the adjustment member and positioned to rotate the adjustment
member for axial adjustment of the stem relative to the nozzle outlet for
pressure
control of the fluid flow. The pressure regulator assembly provides for highly
s accurate pressure control with virtually no dead volume that could result in
cross-
contamination between samples.
A microsampling device is positioned to receive the sample flow from
the back pressure regulator and is moveable between open and closed positions
k
while allowing a substantially continuous flow stream to pass through the
device.
1o In the closed position, the microsampling device blocks the flow stream
from
passing to the analyzer and allows the flow stream to continue to flow through
the
device. In the closed position, the microsampling device also allows a
substantially
continuous flow of earner fluid to pass therethrough to the analyzer. In the
open
position, the microsampling device directs a sampling of at least the one
sample
is portion to the analyzer for analysis, while a remainder of the one sample
portion in
the sample flow moves substantially uninterrupted through the microsampling
device.
A pressure relief valve assembly, which in an embodiment is similar
to the back pressure regulator, receives the remainder sample flow from the
2o microsampling device and maintains a selected pressure in the sample flow
downstream of the microsampling device. A flow directing valve is in fluid
communication with the first flow path and is positioned to receive the sample
flow
downstream of the pressure relief valves. The flow directing valve is moveable
to a
first position to direct the one sample portion in one direction if the
analyzer has
2s determined that the one sample portion has the selected sample
characteristics. The
flow directing valve is moveable to a second position to direct the one sample
portion in another direction if the analyzer has determined that the one
sample
portion does not have the selected sample characteristics. A first receptacle,
such
as a well of a microtiter plate, is positioned to receive the one sample
portion from
so the flow directing device when the flow directing device is in the first
position
because the sample portion has the selected characteristics. A second
receptacle,
such as a well in a second microtiter plate, is positioned to receive the one
sample
portion when the flow directing device is in the second position because the
sample
portion does not have the selected characteristics.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
11
The second purification channel of the purification system includes a
separation device positioned to receive a second sample flow and to separate a
second sampXe into sample portions. A separate detector is coupled to the
separation device and is positioned to receive the second sample from the
s separation device. The detector is configured to detect at least one of the
sample
portions within the sample flow. A microsampling device is positioned to
receive
the sample flow from the detector and is moveable between open and closed
positions. When the microsampling device is in the closed position, the
microsampling device allows the second sample flow to pass therethrough and
to blocks the flow from passing to the analyzer. In the open position, the
microsampling device directs a sampling of the one sample portion to the
analyzer
for analysis, while the remainder of the sample portion continues along the
second
flow path substantially uninterrupted.
A back pressure regulator and a pressure relief valve receive the
is second sample flow upstream and downstream, respectively, of the
microsampling
device to selectively control the pressure of the second sample flow along the
second purification channel. A flow directing valve is in fluid communication
with
the second flow path and is positioned to receive the sample flow
therethrough.
The flow directing valve is moveable to a first position to direct the one
sample
2o portion in one direction if the analyzer has determined the sample portion
has the
selected sample characteristic. The flow directing valve is moveable to a
second
position to direct the one sample portion in another direction if the analyzer
has
determined that the sample portion does not have the selected sample
characteristics. A waste receptacle receives the remainder of the flow that
does not
2s include the sample portion.
A receptacle, such as a separate well in the first microtiter plate, is
positioned to receive the sample portion from the flow directing device when
the
flow directing device is in the first position because the sample portion has
the
selected characteristics. Another receptacle, such as a separate well in the
second
so microtiter plate, is positioned to receive the sample portion when the flow
directing
device is in the second position because the sample portion does not have the
selected characteristics.
In one embodiment of the invention, the purification system includes
third and fourth purification channels that purify third and fourth samples
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
1~
substantially simultaneous with the purification of the first and second
samples.
Each of the third and fourth purification channels are coupled to the same
analyzer
and direct the sample portions to receptacles, such as wells in the first and
second
microtiter plates, discussed above.
s In one embodiment, the system includes a controller and a sample
analyzer coupled to the controller, wherein the analyzer is configured to
determine
whether the samplings have selected sample characteristics. First, second,
third,
and fourth purification channels are coupled to the sample analyzer. The first
purification channel includes a separation device positioned to receive a
sample
to flow and to separate a first sample into sample portions so the sample
portions are
spaced apart from each other in the sample flow. A detector is positioned to
receive the sample flow from the separation device and to detect at least one
sample
portion within the first sample. An adjustable backpressure regulator receives
the
flow stream from the detector and controls the pressure of the flow stream
within
is the first channel.
Another aspect of the invention provides the microsampling device
that includes a body with a sample flow inlet, a sample flow outlet, and a
sample
passageway therebetween. The sample flow inlet and outlet are positionable for
fluid communication with the sample flow path of the fluid system. The body
has a
2o carrier flow inlet and a carrier flow outlet positioned for fluid
communication with
the carrier fluid flow path of the high throughput fluid system. The carrier
flow
inlet and carrier flow outlet are axially misaligned. A stem is movably
disposed in
the body and is in fluid communication with the sample passageway.
The stem is moveable in the body between first and second positions.
2s The stem has a fluid bypass that fluidly interconnects the carrier flow
inlet and
outlet when the stem is in the first position to allow a selected carrier
fluid to flow
through the valve body. The stem blocks the sample flow in the sample
passageway from flowing to the carrier flow outlet when in the first position.
The
fluid bypass is in fluid communication with the sample passageway and the
carrier
so flow outlet when in the second position to allow a selected sampling of the
sample
flow to flow to the carrier flow outlet. One or more actuators are coupled to
the
stem and is activatable to move the stem between the first and second
positions.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
13
Another aspect of the invention includes an automated fraction
collection assembly that retains the microtiter plates in a fixed position and
dispenses the sample portions into the selected wells in the microtiter
plates. The
fraction collection assembly includes a dispensing needle through which the
sample
s portion is dispensed into disposable expansion chambers and then into the
microtiter plate. The dispensing needle is mounted on a dispensing head
adapted to
extend into a disposable expansion chamber into which the sample portion is
condensed and then dispensed into the microtiter plate.
The dispensing head is movable from a pickup station, where the
~o expansion chambers are picked up. The expansion chambers are delivered to
the
pickup station from a dispenser assembly. The dispensing head picks up the
expansion chambers and moves to a collection position over the microtiter
plates,
where the sample portions are dispensed into the selected well of the
microtiter
plate. The dispensing head is also movable from the dispensing position to a
is chamber drop-off position, where the expansion chambers are released into a
waste
receptacle, so the dispensing needles are exposed. The dispensing head is
further
movable to a wash position at a wash station on the fraction collection
assembly,
where the dispensing needles are washed to avoid cross-contamination between
samples.
2o In one aspect of the invention, the automated fraction collector
assembly includes a dispensing head movable relative to a frame along three
axes
of movement. The dispensing head is adapted to deposit the portion of the
selected
sample in a receiving well of a receiving container, wherein the receiving
well has a
one-to-one corresponding location relative to the supply well from which the
2s sample was taken.
One embodiment includes a chambered delivery assembly sized to
contain a plurality of expansion chambers and that has a delivery member
positioned to deliver the expansion chambers to the pickup station. The
chamber
delivery system has a chamber storage portion with a plurality of the
expansion
3o chambers therein. A dispensing drum is rotatably mounted adjacent to the
chamber
storage portion and positioned to receive expansion chambers from the chamber
storage portion. An engagement member is movably positioned adjacent to the
drum to engage the expansion chamber on the dispensing drum to direct the
expansion chamber to the pickup station. The fraction collection assembly of
an
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
14
embodiment also includes a rinse station that provides a "fluid squeegee"
rinsing
process for rinsing the dispensing needles of the dispensing head.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic view of one portion of a multiple channel high
s throughput purification system with pressure regulator assemblies in
accordance
with an embodiment of the present invention.
Figure 2 is a schematic view of another portion of the multiple
channel high throughput purification system of Figure 1.
Figure 3 is a schematic view of the multiple channel high throughput
to purification system of Figures 1 and 2, wherein the system has four
channels.
Figure 4 shows a side elevation view of a two-piece column of the
purification system of Figure 3.
Figure 5 shows a cross-sectional view of the two-piece column taken
substantially along line 5-5 of Figure 4.
is
Figure 6A shows a side elevation view of a one-piece
chromatography column in accordance with an alternate embodiment of the
invention.
Figure 6B shows a cross-sectional view of the one-piece column
2o taken substantially along line 6B-6B of Figure 6A.
Figure 7A is a cross-sectional view of a chromatography column in
accordance with an alternate embodiment of the invention.
Figure 7B is a cross-sectional view of another alternate embodiment
of a chromatography column according to the invention.
2s Figure 7C is a cross-sectional view of another alternative embodiment
of a chromatography column according to the invention.
Figure 7D is a cross-sectional view of another alternate embodiment
of a chromatography column according to the invention.
3o Figures 8A-C show results of three chromatographic runs showing the
improvement over prior art.
Figure 9 is an enlarged exploded isometric view of a back pressure
regulator assembly from the purification system of Figure 3.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
Figure 10 is an enlarged exploded isometric view of a back pressure
regulator module from the assembly of Figure 9.
Figure 11 is an enlarged isometric view of a regulator/motor assembly
of the back pressure regulator module of Figure 10.
5 Figure 12A is an enlarged cross-sectional view of the regulator
assembly taken substantially along line 12-12 of Figure 11.
Figure 12B is an enlarged cross-sectional view of an alternate nozzle
in the regulator assembly of Figure 12A in accordance with an alternate
embodiment.
to Figure 13 is an enlarged isometric view of a microsample valve
assembly from the purification system of Figure 3.
Figure 14A is an isometric view of a microsample valve from the
assembly of Figure 13.
Figure 14B is an enlarged, exploded isometric view of a microsample
is valve from the assembly of Figure 13.
Figure 15A is a plan view of a valve body of the microsample valve
of Figure 14.
Figure 15B is a cross-sectional view of the valve body taken
substantially along line 15B-15B of Figure 14.
2o Figure 16 is an enlarged cross-sectional view taken substantially
along line 16-16 of Figure 14, the microsample valve being shown in a non-
sampling position.
Figure 17 is an enlarged cross-sectional view taken substantially
along line 17-17 of Figure 14, the microsample valve being shown in a sampling
position.
Figure 18 is an enlarged cross-sectional view of a dispensing head
and an expansion chamber from the purifcation system of Figure 3, the
dispensing
head being shown in a dispensing position.
Figure 19 is an isometric view of an automated fraction collection
so assembly of the purification system of Figure 3 in accordance with one
embodiment
of the invention, the fraction collection assembly shown in a chamber pickup
position.
Figure 20 is an isometric view of the fraction collection assembly of
Figure 19 shown in a collection position.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
16
Figure 21 is an enlarged partially exploded front isometric view of a
dispenser assembly and hopper of the fraction collection assembly of Figure 19
shown removed from the frame for purposes of clarity.
Figure 22 is an enlarged partially exploded rear isometric view of the
s drum assembly of Figure 21.
Figure 23 is an enlarged isometric view of a chamber feed and brake
assembly of the drum assembly of Figure 22.
Figure 24 is an enlarged partial isometric view of the distribution
assembly of Figure 21 with a right alignment guide shown in a forward
dispensing
to position and a left alignment guide shown in a retracted position.
Figure 25 is a schematic view of a supplying microtiter plate with
wells , for containing unpurified samples and two receiving microtiter plates
for a
target and reaction by-products that receive purified portions of the sample
in a
well-to-well mapping process.
is ~ Figure 26 is an isometric view of the fraction collection assembly of
Figure 19 shown in a chamber drop-off position.
Figure 27 is an isometric view of the fraction collection assembly of
Figure 19 shown in a rinse position.
Figure 28 is an enlarged cross-sectional view taken substantially
2o along lines 28-28 of Figure 26 showing a rinse station of the fraction
collection
assembly.
DETAILED DESCRIPTION OF THE INVENTION
The structure and function of exemplary embodiments of the present
invention can best be understood by reference to the drawings. The same
reference
2s numbers may appear in multiple figures. The reference numbers refer to the
same
or corresponding structure in those figures.
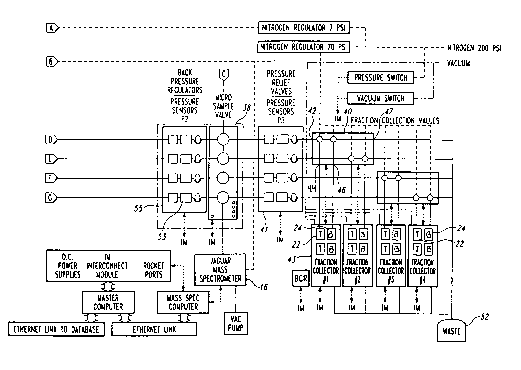
A multiple channel high throughput purification system 10 having a
back pressure regulator assembly 55 and a pressure relief valve assembly in
accordance with an illustrated embodiment is shown in Figures 1-3, and
so components of the system are shown in Figures 4-22. The illustrated
purification
system 10 is configured to simultaneously purify four samples 12 from a
chemical
library, wherein each sample is purified along a respective purification
channel 14
in the system. Purification in the illustrated embodiment is achieved by
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
17
chromatography, and more particularly by supercritical fluid chromatography
(SFC), discussed in greater detail below.
Each channel 14 receives a selected sample from a supplying
microtiter plate 20. Each channel 14 is coupled to a common analyzer, such as
a
s mass spectrometer 16 that analyzes selected portions of the samples in
accordance
with a predetermined analysis priority protocol. In one embodiment, the
analyzer
includes a plurality of compound identification devices. In the illustrated
embodiment, each supplying microtiter plate 20 includes a bar code or other
selected symbology or tracking mechanism that provides information specific to
to that supplying microtiter plate. The purification system 10 includes a bar
code
reader 15 or the like that identifies the specific supplying microtiter plates
20 used
for each purification run.
The components of each channel 14, including the mass spectrometer
16 and the bar code reader 15, are coupled to a computer controller 18 that
is monitors and controls operation of the components during a purification
run. The
mass spectrometer 16 is also connected to a computer 17 that can provide a
user
with additional control or monitoring capabilities during a purification run.
After each sample 12 is analyzed by the mass spectrometer 16, a
substantially purified sample portion is distributed directly into a
corresponding
2o well of a receiving microtiter plate 22 (Figure 2) or another selected
sample
collector. The other portions of the sample detected by the detector, known as
reaction by-products, are distributed directly into a corresponding well in a
second
microtiter plate 24, also illustrated in Figure 2. Accordingly, the four
samples 12
are drawn from the supplying microtiter plate 20, purified, and each sample is
2s deposited directly into a corresponding well location in two receiving
microtiter
plates 22 and 24, one containing the purified target compound and the other
containing the reaction by-products. In one embodiment, the four samples are
drawn from the supplying microtiter plate sequentially by the same drawing
needle
assembly. In an alternate embodiment, the four samples are drawn substantially
so simultaneously by a drawing assembly having four drawing needles.
The receiving microtiter plates 22 and 24 have bar codes or the like
on them, and a bar code reader 25 (Figure 2) is provided adjacent to the
receiving
microtiter plates. The second bar code reader 25 is also coupled to the
computer
controller 18 (Figure 1) to identify and track the samples deposited into the
selected
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
18
wells of each microtiter plate. The purified target compounds in the
microtiter
plates 22 and 24 can then be screened in a selected manner in an effort to
locate a
specific target compound.
The microtiter plates 22 are securely retained in an automated fraction
s collection assembly 23 coupled to the computer controller 18 (Figure 1). The
fraction collection assembly 23 directs selected sample portions of either
purified
taxget components or purified reaction by-products to selected wells of the
microtiter plates 22 or 24. The fraction collection assembly 23 is automated
and
configured to pick up, clean, disposable or reusable expansion chambers in
which
~o vaporous sample portions are condensed and then delivered to the microtiter
plates
22 or 24. The fraction collection assembly 23 includes a wash station in which
sample dispensing needles are washed after a sample portion is delivered to
the
respective microtiter plate and before the next set of clean expansion
chambers are
picked up for delivery of the next sample portions.
is In the purification process of the illustrated embodiment, selected
supplying microtiter plates 20 are identified by the bar code reader IS and
positioned on an autosampler 21 (Figure 1). In one embodiment, the autosampler
21 is a Gilson 215 autosampler, manufactured by Gilson, Inc. of Middleton,
Wisconsin. As best seen in the schematic diagram of Figure 3, each sample is
2o drawn by the autosampler 21 from a selected well of a supplying microtiter
plate 20
and is fed into a sample flow path 30 of a respective one of the four channels
14.
The four samples 12 are substantially simultaneously introduced into the
respective
purification channels 14. Although the illustrated embodiment substantially
simultaneously purifies four samples 12, other numbers of samples can be
2s simultaneously purified with a system in accordance with the present
invention.
As best seen in Figure 3, the sample 12 is combined with carbon
dioxide from a C02 source 29 and a modifier solvent from a solvent source 33
to
form a carrier flow that flows through the respective channel 14 at a selected
flow
rate. The carbon dioxide flows through a heat exchanger 36 is chilled with a
so recalculating cooling bath 35 and is pumped via a C02 pump 37 to a mixer
39. The
flow of CO~ is also passed through a pulse damper to mininuze any pulsation
caused by the pump 37. The modifier solvent flows through a solvent pump 41
into
the mixer 39 where the solvent is mixed with the carbon dioxide. The carbon
dioxide and solvent mixture then flows to a sample injection valve 43, where
the
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
19
sample 12 is received from the autosampler 21 and is combined with the carrier
flow to form the sample flow 31.
The sample flow 31 is passed through a heat exchanger 45 at which
time the fluid becomes supercritical, and then a separation media, such as an
SFC
s column 32, that spatially separates the sample components within the sample
flow
31. Accordingly, each sample component is spaced apart from the other
components and separated in time as the sample flow exits the SFC column 32
and
moves through the purification channel 14.
In one embodiment of the invention, the column 32 is a two-piece
to column, as illustrated in Figures 4 and 5, for use in supercritical fluid
chromatography. As best seen in Figure 4, the components of the column 32
include an upper dilution body 400 that defines that a dilution chamber 408
therein.
The top portion of the dilution body 400 is connected to an inlet tube 410
through
which the sample flow 31 passes and moves into the column 32. The upper
is dilution body 400 is connected to a loading body 402 and securely retained
in place
by a top end cap 401. The dilution chamber body 400 is compressed downwardly
by the top end cap 401 that screws externally onto the threads of the loading
body
402. In an alternate embodiment for use in liquid chromatography, the dilution
chamber is not needed, so the column 32 does not include the dilution body
2o attached to the loading body.
The dilution chamber body 400, the top end cap 401, and the loading
body 402 of the illustrated embodiment are made from an inert material, such
as
stainless steel. In alternate embodiments, other inert materials can be used
for
construction of the column's components. A separation body 403 at its upper
end
2s is attached to the lower portion of the loading body 402. The lower end of
the
separation body 403 is securely connected to a bottom end cap 404 that
connects to
an outlet tube 412, through which the separated sample flow 31 exits the
column
32.
As best seen in a cross-sectional view of Figure 5, the sample flow 31
3o enters the column 32 at a top-threaded port 505 to which an inlet tube 410
is sealed
by an external ferrule that seats onto the top ferrule sealing point 506 in
the
threaded port. The sample flow is directed radially from the inlet tube 410
into the
upper dilution chamber 408 by means of an inverted top funnel portion 507. The
top funnel portion 507 is substantially conical in geometry and it defines the
top of
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
the dilution chamber 408. The main body of the dilution chamber 408 is
substantially cylindrical, although it can be constructed with other geometric
shapes
in alternate embodiments. The bottom of the dilution chamber 408 has an
inverted
bottom funnel portion 509 that flares radially outwardly from the dilution
s chamber's main body. Accordingly, the bottom funnel portion 509 flares to a
lower
opening having a greater diameter than the dilution chamber's main body. The
lower opening of the bottom funnel portion 509 is positioned over a top frit
510
located below the dilution chamber 408.
The dilution chamber's entire volume is void of stationary phase
io material. Dilution of the sample in the sample flow takes place in the
dilution
chamber 408 as the sample flow moves downwardly through the main body to the
bottom funnel portion 509, where the sample flow passes through the top frit
510.
The top frit 510 distributes the sample over a column bed 512 in a loading
region
520 directly below the top frit 510. Sealing of the dilution chamber 408 is
achieved
is at the top frit 510 where the dilution chamber body 400 fits internally
into the
loading body 402.
The loading body 402 has a loading region 520 below the top frit 510
and a transition region 522 below the loading region. The loading and
transition
regions 520 and 522 in the loading body 402 are filled with a stationary phase
2o material, such as cyano, that defines a column bed 512 in the column 32. In
alternate embodiments, other stationary phase materials can be used to form
the
column bed S 12. The loading region 520 has an inner diameter approximately
two
or more times greater than the inner diameter of the separation region 524,
and a
length of approximately one-half or less than the length of the separation
region. In
2s the loading region 520, the sample flow traverses downwardly through the
column
bed S 12 into the transition region 522, which has a conical shape as defined
by the
loading body 402. The transition region 522 directs the sample flow into the
separation region 524 of the column bed 512.
The loading region 520 is wide but short, so the sample is distributed
so over the wider area of the column bed 512. Accordingly, the sample is
spatially
distributed across a larger horizontal plane, thus separating it from the non
compatible loading solvent. The column bed 512 has selected absorptive
characteristics. The length of the loading region 520 and the depth of the
column
bed 512 provides a minimum vertical absorptive profile that allows sufficient
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
21
absorption of the sample onto the stationary phase material forming the column
bed. The loading region's length, however, is insufficient for the selected
chromatographic separation of the sample for its given mass load and the
solvent
volume.
s Because the loading chamber or region 520 is for loading of the
sample rather than for chromatographic separation, the loading chamber does
not
control the required flow rate for such separation. Instead, the flow rate is
determined by the diameter of the separation region 524. Once the sample has
been
properly loaded into the loading chamber 520, the elution gradient process of
the
to flow will elute the sample and pass it directly to the separation column.
The
separation region 524 has a smaller diameter than the loading region's
diameter,
and this smaller diameter controls the flow rate for the sample for its given
sample
mass and volume. This smaller diameter allows the sample flow to be run at a
lower rate, thereby lowering solvent consumption and solvent waste
generation.The
Is top of the separation body 403 is threadably attached to the bottom of the
loading
body 402 by a threaded connection and is sealed by an adjoitling frit 511
sandwiched therebetween. The separation body 403 of the illustrated embodiment
is made of stainless steel and is shaped so the interior chamber containing
the
separation region 524 of the column bed 512 has a tapered cylindrical geometry
2o with a wider upper end and a nar~~ower lower end. The interior chamber of
separation region 524 of the column bed 512 is filled with the stationary
phase
material. The sample flow travels downwardly through the column bed 512 in the
separation region 524 past a bottom frit 513 and onto a bottom fluid funnel
514
formed in the bottom end cap 404. The bottom of the separation region 524 is
2s sealed by the bottom end cap 404 screwed externally onto the separation
body 403.
The bottom frit 513 is sandwiched between the bottom end cap 404 and the
separation body 403. The bottom fluid funnel 514 is conical and directs the
fluid
into a bottom threaded port 516 for~.ned in the bottom end cap 404 to which
the
outlet tube 412 can be screwed. The outlet tube 412, when screwed into the
outlet
so port 516, is sealed against the bottom end cap 404 at a bottom ferrule
sealing point
515 by use of an external fei~-ule.
In an alternate embodiment illustrated iui Figure 6A, the column 32 is
a "one-piece" column. In view of the similarities between the two embodiments,
components that are the same between the two embodiments are identified in
flee
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
22
figures by the same reference numbers for purposes of clarity. The one-piece
column is substantially the same as the two-piece column discussed above, with
the
exception that the loading body 602 'and the separation body 603 are
integrally
formed from a single stainless steel unit to define a One-Piece Loading and
s Separation (OPLAS) body 617. Accordingly, the upper frit 511 used in the two-
piece column is not needed and thus omitted.
As best seen in the cross-sectional view of Figure 6B, the dilution
chamber body, 400 fits internally into the OPLAS body 617 and is secured by
the
top end cap 401 that screws externally onto the OPLAS body. The lower end of
the
to OPLAS body 617 screws internally into the bottom end cap 404. Accordingly,
the
loading region 520 formed in the OPLAS body 517 has a diameter approximately
two or more times greater than the inner diameter of the separation region
524, and
a length of approximately one-half or less than the length of the separation
region.
In an alternate embodiment illustrated in Figure 7A, the column 32 is
~s similar to the "two-piece" column discussed above and shown in Figures 4
and 5.
The dilution chamber body 400 has a lower end 407 that sits on top of the
loading
body 402. The dilution chamber body 400 has. the same outer diameter as the
outer
diameter of the loading body 402. The ~ top frit 510 is sandwiched between the
lower end 407 of the dilution chamber body 400 and the top of the loading body
20 402.
In the illustrated embodiment, a support frit 704 is positioned above
the top frit 510 and immediately below the bottom funnel portion 509 of the
dilution chamber 408. When the dilution chamber 408 is filled with an inert
material, such as plastic or stainless steel beads, the support frit 704
retains the inert
2s media within the dilution chamber 408. The support frit 704 also provides
support
to the top frit 510 to prevent it from bulging.
In another alternate embodiment illustrated in Figure 7B, the column
32 is similar to the "one-piece" column discussed above and shown in Figures
6A
and 6B. The dilution chamber body 400, however, is integrally connected to the
3o top end cap 401 that threadably engages the loading body 402. The dilution
chamber body 400 and top end cap 401 are positioned to sandwich the top frit
510
against the top of the loading body 402. This embodiment also includes the
support
frit 704, as discussed above, positioned to retain any inert media when used
within
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
the dilution chamber 408 and to supp rt the top frit 510 against bulging. The
loading body 402 is integrally connected to the separation body 403.
The separation body 403 of the alternate embodiments illustrated in
Figures 7A and 7B are shown with a generally conical-shaped separation region
s 524. In alternate embodiments, the separation. region 524 can have a
cylindrical
shape with a constant cross-sectional area along its length.
In another embodiment of the present invention shown in Figure 7C,
the column 32 is a staged column assembly having a dilution column 740, a
loading
column 742, and a separation column 744 spaced apart from each other. The
to dilution, loading and separation columns 740, 742, and 744 are connected in
series
by sections of small-bore tubing 746. The dilution column 740 has a dilution
chamber body 750 with a top port 752 that receives the inlet tube 410. The
inlet
port 752 is in fluid communication with a dilution chamber 754 within the
dilution
body 750, so the sample flows from the inlet tube 410, through the top port
752,
is and into the dilution chamber 754. The dilution chamber 754 can be empty,
or in
alternate embodiments, can contain inert media, such as plastic or stainless
steel
beads. The beads facilitate dilution of the sample flow as it enters the
dilution
chamber 754. The bottom of the dilution chamber body 750 has an outlet port
756
in fluid communication with the dilution chamber 754. The outlet port 756 is
2o connected to an upper section 758 of the small bore tubing 746 to direct
the sample
flow out of the dilution column 740. In the illustrated embodiment, the small
bore
tubing 746 is HPLC tubing having an inner diameter of approximately 0.010
inches,
although other tubing can be used.
The upper section 758 of the tubing 746 is connected to an inlet port
2s 760 in the loading body 762 of the loading column 742. The inlet port 760
is in
fluid communication with a loading chamber 764 formed within the loading body
762. The loading body 762 is formed by an upper section 765 and a lower
section
766 securely held together in axial alignment by a threaded top cap 767. The
upper
section 765 has the inlet port 760 and the lower section 766 has an outlet
port 768
so both in fluid communication with the loading chamber 764. The top cap 767
extends over the upper section 765 and internal threads 769 on the top cap
screw
onto external threads 770 on the lower section 766. A locking ring 771 snaps
onto
the loading body's upper section 765 over the top cap 767 to lock the top cap
in
place on the upper section.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
24
The loading chamber 764 contains a selected stationary phase
material, such as cyano, or other selected material, that defines a column bed
772.
In the illustrated embodiment, the column bed 772 is contained in a guard
column
cartridge 773 having a shell portion 775 that encases the column bed. Frits
774 are
s contained in the guard column cartridge 773 on the top and bottom of the
column
bed 772. The.frits 774 are positioned so the sample passes through them as the
sample flows through the loading chamber 764 to the outlet port 768. In
alternate
embodiments, the loading column 742 does not use the guard column cartridge
773.
The column bed 772 is packed directly into the loading chamber 764 and the
frits
l0 774 are positioned on the top and bottom of the column bed.
The loading chamber 764 has a volume defined by the diameter and
the length that contains a selected volume of the packing material to provide
a
vertical absorption profile that allows the full sample to be loaded into the
loading
column 742. The loading chamber's length, however, is insufficient to
Is chromatographically separate the sample. As a result, the loading chamber
764 can
receive large samples and spatially distribute the sample across a larger
horizontal
plane so as to separate the sample from noncompatible loading solvent.
The outlet port 768 of the loading body 762 is connected to a lower
section 776 of the small bore tubing 746 that carries the sample flow away
from
20 loading column 742. The tubing's lower section 776 is connected to an inlet
port
778 in a filter 780. The filter 780 is connected to an inlet port 781 in a
separation
body of the separation column ?44. In an alternative embodiment, the filter
780 is
not used, so the tubing's lower section 776 is connected directly to the
separation
body's inlet port 778.
2s The separation body 782 has an elongated separation chamber 784 in
fluid communication with the inlet port 781 to receive the sample flow. The
separation chamber 784 contains a selected separation media forming the column
bed 787 through which the sample flow travels and wherein the sample's
components are chromatographically separated. The separation chamber 784 has a
3o diameter that is approximately 1/2 or less than the diameter of loading
chamber
764, and a length that is two or more times the loading chamber's length. The
sample flow rate is determined by the diameter of the separation chamber 784.
The
separation chamber 784 of the illustrated embodiment has a cylindrical shape
with a
substantially continuous cross-sectional area along it length. Alternate
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
embodiments can have a separation chamber 784 that tapers to a smaller
diameter at
its bottom end.
The bottom end of the separation body 782 is connected to a bottom
end cap 786 with an outlet port 788 therein in fluid connection with the
separation
s chamber 784. The outlet port 788 is connected to the outlet tube 412 so as
to
receive the separated sample flow exiting the separation column 782.
The staged column assembly utilizes the benefit of the large diameter
loading column 742 that can handle increased solvent loading, and the smaller
diameter separation cohunn 744 that allows for the desired high-volume
throughput
to while achieving the selected chromatographic separation. Accordingly, a
large bore
cohunn is not needed to achieve the desired separation results.
hl another alternate embodiment illush~ated in Figure 7D, the column
32 is a staged column assembly similar to the embodiment discussed above and
illustrated in Figure 7C, except the assembly does not have a dilution chamber
~s spaced apart from the loading column. The dilution chamber 790 is provided
in the
loading column 742. The loading column 742 is connected at its inlet port 760
to
the inlet tube 410. The loading column 742 contains an annular spacer 792
sandwiched bct<veen the loading body's upper section 765 and the top of the
guard
column carhidge 773. The annular spacer 792 has an open center area 794 in
fluid
2o colnmmication with the inlet port 760 and the guard column cartridge 773
with the
column bed 772 therein. The spacer's open center area 794 defines the dilution
chamber 790 that receives the sample flow before the sample flow is loaded
onto
the column bed 772. Accordingly, the dilution chamber 790 and loading chamber
764 are integrally connected in the same stage of the stage column assembly.
In the
2s illustrated embodiment, the dilution chamber 790 is empty so as to form a
void
above the loading chamber 764. Iri an alternate embodiment, inert beads or
other
material can be contained in the dilution chamber 790.
In this alteanate embodiment, the loading chamber 764 contains
selected stationary phase material fomning the column bed 772 within the guard
3o column caz-tridge 773, as discussed above, and the frits 774 sandwich the
column
bed therebetween. hi an alternate embodiment, the guard column cartridge 773
is
not used and the column bed 772 and frits 774 are packed directly in the
loading
chamber 764.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
26
The loading body's lower. ~ section 766 has the outlet port 768 as
discussed above connected ,to, a segment of the ~ small bore HPLC tubing 746
receives the sample flow from the loading chamber 764. The small bore tubing
746
is connected to the inlet port 778 of the filter 780 as discussed above in
connection
s with the embodiment illustrated in Figure 7C.
Figures 8A-C show gr~.phical.results from three chromotographic runs
showing improvement over the prior art provided by the column 32 in accordance
with the present invention. All three chroinotographic runs were injected with
the
same mass loading of a three-compound mixture and run under the same
to chromotographic conditions. Run 200 (Figure 8A) shows the separation
results
using a single prior art column injected with a small volume solvent mixture.
Run
201 (Figure 8B) shows the separation results using the same prior art single
column
as in run 200, wherein the prior art coltainn was injected with a large volume
solvent mixture. Run 202 (Figure 8C) shows the separation results using a two-
part
is column 32 in accordance with an embodiment of the present invention as
discussed
above. Run 202 was injected with the same large volume solvent mixture as
i-un 201
The first portions of the column 32 (e.g., the loading and transition
portions) have a larger inner diameter than the column's second portion (the
2o separation region) and a shorter length than the column's second portion.
Accordingly, the column 32 in accordance with the present invention can handle
large volume solvent mixtures with multiple compounds and provide highly
accurate separation and detection of the different compounds, such as by use
of a
mass spectrometer or the like. This accuracy in conjunction with corresponding
2s speed for handling large volume solvent mixtures with multiple compounds
provides a faster and more efficient processing capability.
Referring again to Figure 3, the sample flow 31 exits the SFC column
32, flows through another heat exchanger 47; and flows to a detector 34. The
detector 34 is adapted to detect the different components or peaks in the
sample
so flow 31 that have been separated from each other by the SFC column 32. In
the
illustrated embodiment, the detectors 34 are ultraviolet light (UV) detectors.
While
UV detectors are used in the illustrated embodiment, other detectors can be
used,
such as infrared (IR) detectors or any other suitable detector capable of
identifying
a peak within the sample flow 31.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
27
Each detector 34 is coupled to.the common computer controller 18.
When the detector 34 identifies a peak, the detector provides a signal to the
computer controller 18 indicating the peak. Because the sample flow rate is
known
in each channel 14, the computer controller 18 can calculate the location of
each
s peak within each channel 14 as the sample flow 31 moves through the channel.
As
an example, when two peaks are detected in the same sample flow 31, the
computer
controller 18 calculates and monitors where those peaks are within the channel
14.
The computer controller 18 also calculates where the peaks are relative to
each
other during the entire purification process.
to ~ As the sample flow 31 moves through the purification channel, it is in
a vaporous state. After the sample flow 31 exits the detector 34, additional
solvent,
referred to as makeup solvent 49, is added to the sample flow as needed to
increase
the volume of liquid in the sample flow to facilitate transport of the sample
to the
fraction collector assembly (discussed below). The makeup solvent 49 is pumped
is from a solvent container by solvent pumps 51 into the respective
purification
channel 14. The solvent container and the solvent pumps 51 are each coupled to
the computer controller 18 so the computer controller can monitor the solvent
volumes used and can control the solvent pumps as necessary for the selected
purification run. The computer controller 18 also monitors the amount of
makeup
2o solvent 49 needed within the purification channel during a run, so it can
detect if a
potential problem arises, and can provide an alarm or other warning to an
operator
of the system.
After any of the makeup solvent 49 is added to the sample flow 3I,
the sample flow passes through a back pressure regulator module 53 in a back
2s pressure regulator assembly 55. The back pressure regulator module 53
detects and
controls the back pressure within the channel 14 to maintain the desired
pressure
within the channel.
As best seen in Figure 9, the back pressure regulator assembly 55
includes a housing 900 that removably retains four back pressure regulator
modules
so 53, one for each purification channel 14. The assembly 55 also includes a
communication panel 902 to which the back pressure regulator modules 53 attach
for communication to and from the computer controller 18 (Figure 3). The
modules
53 plug into the housing 900 and onto the communication panel 902.
Accordingly,
if a new or substitute module 53 is needed in the purification system, it can
be
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
28-
installed quickly and easily upon unplugging one module and plugging in the
replacement module.
As best seen in Figure 10, the pressure regulator module 53 includes a
housing 1002 that contains and protects a regulator assembly 1004. The
regulator
s assembly 1004 controls the back pressure in the sample flow as it moves
through
the respective purification channel 14. The regulator assembly 1004 is
electrically
connected to a stepper motor controller 1006 which activates and adjusts the
regulator assembly as needed during a purification run. The stepper motor
controller 1006 is connected to a printed circuit board 1008 which also
attaches to
the housing 1002. The printed circuit board 1008 includes a plurality of
connectors
1010 that releasably plug into the communication panel 902 (Figure 9) of the
regulator assembly. Accordingly, communication to and from the computer
controller 18 is provided to the pressure. regulator module 53 through the
printed
circuit board and to the regulator assembly 1004 via the stepper motor
controller
is 1006.
The pressure regulator module 53 also includes a front faceplate 1012
that mounts to the housing 1002. The front faceplate 1012 has an inlet port
1014
into which the tubing of the purification channel extends so as to allow the
sample
flow 3.1 to pass into the pressure regulator module 53. The sample flow passes
2o through a pressure sensor 1013, which is also coupled to the printed
circuit board
1008, so as to identify the sample flow's pressure. After the sample flow 31
enters
the regulator assembly 1004 and the sample flow's pressure is modified as
needed,
as discussed in greater detail below, the sample flow exits the pressure
regulator
module 53 through an outlet port 1018 on the front faceplate 1012.
2s As best seen in Figures 11 and 12, the regulator assembly 1004
includes a stepper motor 1100 having wiring 1102 that connects to the stepper
motor controller 1006 (Figure 10). The stepper motor 1100 is connected to a
motor
mount 1104 that interconnects the stepper motor to a back pressure regulator
1106.
The back pressure regulator 1106 is securely retained to the stepper motor
1100 by
so a plurality of mounting screws 1108 that extend through the motor mount
1104 and
screw into the housing of the stepper motor 1100.
The regulator assembly 1004 also includes a heater 1110 adapted to
heat the sample flow 31 within the purification channel's tubing so as to
prevent
formation of ice crystals or the Iike that may occur as a result of pressure
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
29
differentials occurring across the pressure regulator. The heater 1110
includes a
heat transfer body 1112 that extends over the back pressure regulator 1106 and
a
heater band 1114 clamped onto the heat transfer body by a band clamp 1116. The
heater band 1114 is coupled to the computer controller 18 to allow the heater
band
s to regulate its temperature to provide different heating configurations to
the back
pressure regulator during a purification run. The heat transfer body 1112
includes a
temperature sensor 1118 that monitors the temperature of the heat transfer
body
during the purification run. The temperature sensor 1118 is coupled to the
computer controller 18 (Figure 3) so the computer controller can regulate the
heat
to provided from the heater band 1114 as needed during operation of the
regulator
assembly 1004. The heater 1110 is controlled to prevent formation of ice or
crystals in the pressure regulator 1106.
As best seen in Figure 12A, the regulator 1106 has a flow filter 1250
that receives the purification tube 1201 carrying the sample flow 31. The flow
is filter 1250 includes a frit 1252 or other filtering member positioned in
the path of
sample flow 31. The sample flow 31 passes through the frit 1252, and the frit
filters out any particulates in the sample flow before the flow progresses
through
the regulator 1106. The flow filter 1250 has a connector end 1254 that extends
into
and is securely received by an inlet port 1200 that receives the filtered
sample flow
20 31. In an alternate embodiment, the flow filter 1250 is not used, so the
purification
tube entering the regulator 1106 extends directly into the inlet port 1200.
The inlet port 1200 has an inlet channel 1202 that communicates with
a nozzle 1204 positioned below the inlet port. The nozzle 1204 in the
illustrated
embodiment is a ceramic component having a diamond coating so as to provide an
2s extremely hard, erosion-resistant, and durable nozzle within the regulator.
The
nozzle 1204 is exposed to very harsh conditions, including caustic solvents
and
pressures of approximately 2000 psi or greater. The inlet port 1200 is
threadably
connected to the nozzle retainer 1205 so the inlet port is easily removable to
provide access to the nozzle 1204 if replacement of a nozzle is necessary.
3o The nozzle 1204 includes an inlet channel 1211 extending
therethrough that communicates with a very small chamber that receives the
sample
flow 31 from the nozzle's inlet channel. The lower end of the inlet channel
1211
forms a nozzle orifice through which the sample flow passes. A stem 1208
positioned below , the nozzle 1204 extends through a seal 1210, into the small
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
chamber 1206, and terminates immediately adjacent to the nozzle orifice at the
lower end of the inlet channel 1211. The stem 1208 is moveable relative to the
nozzle orifice so as to adjustably close the flow path through the regulator
1206. In
the illustrated embodiment, the stem 1208 is a sapphire stem. In alternate
s embodiments, the stem 1208 can be made of other very hard, erosion-
resistant,
materials, such as diamond, ruby or the like. The stem 1208 is moveable
relative to
the nozzle 1204 to adjust the opening size so as to regulate the pressure of
the
sample flow 31.
As best seen in Figure 12B, the nozzle 1204 in an alternate
to embodiment has a nozzle body 1281 and a nozzle insert 1280 retained in a
cavity 1282 formed in the nozzle body's lower end 1283 facing the stem 1208
(Figure 12A). The nozzle insert 1280 of the illustrated embodiment is retained
in
the cavity by a rolled crimp formed in the lower end of the nozzle body 1281.
The
nozzle insert 1280 in another embodiment can be retained in other suitable
ways to
1s securely hold the insert in place in the nozzle's cavity.
The nozzle insert 1280 is made of a very hard, erosion-resistant
material such as sapphire, ruby, diamond or other suitable material that
exhibits
sufficient hardness and erosion resistance. In one embodiment, the nozzle
body 1281 is made of a ceramic component, and the nozzle insert 1280 is
sapphire.
2o The nozzle insert has an aperture 1284 aligned with the inlet channel 1211
in the
nozzle body. The lower end of the aperture 1284 forms the nozzle orifice
through
which the sample flow passes.
The sample flow 31 moves from the nozzle 1204 through the orifice
and into an outlet channel 1212 that is in fluid communication with the small
2s chamber 1206. The outlet channel 1212 extends through an outlet port 1214
that
receives the exit tube 1201 therein so as to carry the sample flow 31 out of
the
regulator 1106. The exit tube 1201 extends from the outlet port 1214 and wraps
around the heat transfer body 1112 approximately two times so the exit tube is
heated, thereby preventing the formation of ice crystals within the
purification tube
3o and condensation on the outside of the exit tube. The purification tube
1201 then
extends from the heat transfer body 1112 away from the regulator assembly and
to
the outlet port 1018 on the regulator module's faceplate 1012 (Figure 10) as
discussed above.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
31
In the illustrated embodiment, the stem 1208 is a sapphire stem
having hardness and erosion-resistance characteristics suitable for use in the
high
pressure and harsh environment within the regulator assembly 1004. The
sapphire
stem 1208 is connected at its lower end to a rod 1218 movably positioned
within a
s holding member 1220 having a threaded lower end. The holding member 1220
contains a biasing member 1222, such as Bellville washers, wave washers, or
the
like, that bias the rod 1218 and the stem 1208 toward the nozzle 1204. When
the
stem 1208 directly engages the nozzle 1204 and additional force is exerted on
the
stem, the biasing member 1222 will be compressed so as to avoid damaging the
to sapphire stem 1208 or the nozzle 1204 during operation. The biasing member
1222, however, has a sufficient spring stiffiiess so it is not compressed
during
normal pressures of the sample flow within the tubing of the purification
channel
14 during a purification run.
Adjustment of the regulator assembly 1106 is provided by dual
is concentric screws that move the stem 1208 relative to the nozzle 1204. As
best
seen in Figure 12, the holding member 1220 is threaded into internal threads
1230
formed in a shaft 1224 of an adjustment screw 1226. In the illustrated
embodiment,
the internal threads 1230 have a pitch of 28 threads per inch (tpi). The
adjustment
screw's shaft 1224 also has external threads 1232 that screw into a threaded
2o aperture in the regulator body 1106. In the illustrated embodiment, the
external
threads 1232 have a pitch of 27 tpi. Accordingly, the external threads 1232 of
the
adjustment screw 1226 have a thread pitch different than the pitch value of
the
internal threads 1230. The internal and external threads 1230 and 1232 are
both
right-handed pitch threads oriented in opposing directions so as to form the
dual
as concentric adjustment screw configuration for attenuated movement of the
stem
1208 relative to the nozzle 1204 for each turn of the adjustment screw.
The adjustment screw 1226 has an internal driving spline 1234 that
securely engages a drive spline 1236 on the stepper motor 1100. The drive
spline
1236 is press fit into the internal driving spline 1234. When the stepper
motor 1100
3o is activated by the computer controller 18 (not shown), the driving spline
1236
rotates, thereby rotating the adjustment screw 1226. As the adjustment screw
1226
rotates one revolution, the dual concentric screw configuration counteracts
the
range of motion of the holding member 1228, and thus the stem 1208. As an
example, if the stepper motor 1100 rotates the adjustment screw one full
revolution,
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
32
the holding member 1220 moves only one pitch value because of the pitch
differentiation between the internal and external threads 1230 and 1232.
In one embodiment, one revolution of the adjustment screw along the
external threads 1232 would move the adjustment screw 1226 and the holding
s member 1220 approximately 0.0373 inches. The internal threads 1230, however,
move in the opposite direction approximately 0.03571 inches, resulting in a
net
movement of approximately 0.0013 inches. Accordingly, the dual concentric
screw
configuration within the regulator 1106 provides for extremely accurate and
fine
adjustments of the stem 1208 relative to the nozzle 1204 to closely control
pressure
to regulation within the sample flow 31 as it passes through the back pressure
regulator assembly 1004.
The back pressure regulator 1004 is formed with a minimum amount
of dead volume and unswept volume within the purification channel extending
therethrough to prevent or minimize the risk of cross-contamination between
is purification runs for different samples. The back pressure regulator
assembly is
constructed with extremely durable components that will withstand the harsh
environments experienced during the purification run at very high pressures,
while
providing sufficient safety characteristics to avoid damaging the back
pressure
regulator in the event of pressure spikes or the like.
2o In one embodiment, the stepper motor includes a rotational stop 1238
that prevents travel of the drive spline 1236 and, thus, rotation of the
adjustment
screw 1226 past a selected position relative to the regulator. The travel stop
1238 is
positioned to block the stepper motor from driving the adjustment screw 1226
too
far after the stem 1208 has engaged the nozzle 1204, thereby preventing the
dual
2s concentric threads from binding as a result of overdriving by the stepper
motor.
The illustrated embodiment of the purification system utilizes the
regulator assembly with the dual concentric screw configuration controlled by
the
computer controller 18. In alternate embodiments, the pressure regulator
assembly
53 can be a stand alone regulator with selected control mechanisms.
so As best seen in Figure 3, the sample flow 31 travels from the pressure
regulator assembly 55 to the microsample valve 38. The microsample valve 38 is
operatively connected to the computer controller 18 and is activated by the
computer controller when a peak in the sample flow 31 is moving past the
microsample valve. Upon activation, the microsample valve 38 diverts a
sampling
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
33
from the sample flow 31 and directs it to the mass spectrometer 16 for
analysis.
The remaining portion of the sample flow 3.1 continues along the flow path of
the
respective channel 14 substantially uninterrupted. Each microsample valve 38
is
activated so the sampling contains a selected portion of just the peak. The
mass
s spectrometer.16 analyzes the sampling and determines whether the peak is a
target
compound or not.
As the four sample flows 31 moves simultaneously through the
respective channels 14 and through the detectors 34, the peaks from the four
channels will likely occur at separate times during the sample runs.
Accordingly,
io the mass spectrometer 16 usually receives the samplings from the four
channels
with some time between the samplings. In some cases, however, two or more
detectors 34 may detect a peak in its sample flow at the same time or at
overlapping
times during the sample run. The computer controller 18 is programmed with an
analysis priority protocol that controls the activation sequence of the
microsample
is valve 38 when peaks in the different channels I4 occur at the same time or
overlapping times. Accordingly, the priority protocol controls the timing of
when
the samplings of the peaks are diverted to the mass spectrometer 16, so each
peak
can be analyzed separately by the same analyzer. In one embodiment, when a
peak
from separate channels 14 are detected simultaneously, the computer controller
18
2o activates the microsample valves 38 at different times so samplings of the
respective peaks are sequentially directed to the mass spectrometer 16.
Activation
of each microsample valve 38 can be controlled by revising the computer
controller's analysis priority protocol to provide sequential sampling.
As best seen in Figure 13, the four microsample valves 38 are part of
2s a microsample valve assembly 1300 that has four valve modules 1302. Each
valve
module 1302 contains a microsample valve 38 for its respective purification
channel 14. The valve modules 1302 are removably received by a housing 1304
and plug into connectors coupled to a communication panel 1306. The
communication panel 1306 is, in turn, coupled to the computer controller 18
(not
so shown), so the computer controller can control the activation of each
microsample
valve 3 8.
As best seen in Figures I4A and 14B, each valve module 1302
includes a faceplate 1400 and opposing side plates 1402 that securely engage
the
microsample valve 38. The faceplate 1400 has an inlet port 1404 and an outlet
port
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
34 .
1406 that receive the purification channel's tubing and direct the sample flow
into
and out of the valve module 38.
The microsample valve 38 includes a valve body 1408 positioned
between a pair of electromagnetic solenoids 1410. The solenoids 1410 are
s activatable by the computer controller 18 (not shown) to control activation
of the
microsample valve, as discussed in detail below. The solenoids 1410 are each
sandwiched between the valve body 1408 and. outer mounting plates 1414, and
mounting screws 1416 secure the outer mounting plates to the valve body.
to As best seen in Figures 15A-17, the valve body 1408 has a sample
inlet port 1502, a sample outlet port 1504 (Figures 15A and 15B), a solvent
inlet
port 1506, and a flow outlet port 1508. The solvent inlet port 1506 is axially
misaligned with the flow outlet port 1508. The flow outlet port 1508 is in
fluid
communication with the mass spectrometer 16; so fluid exiting the microsample
is valve 38 through the flow outlet port is carried to the mass spectrometer
16
(Figure 3). The microsample valve 38 has a stem 1510 slidably disposed within
an
interior chamber 1512 in the valve body 1408. The stem 1510 slidably extends
through the valve body 1408 and is connected at opposite ends to the
electromagnetic solenoids 1410. The solenoids 1410 control the stem's axial
2o position within the valve body 1408. The solenoids 1410 are connected to
the
computer controller 18 (Figure 3), so the computer controller can control or
adjust
the stem's axial position. Upper and lower seals 1514 are positioned within
the
valve body 1408 adjacent to the solenoids 1410, and a center plastic sleeve
1516
extends between the upper and lower seals. The stem 1510 extends through the
2s upper and lower seals 1514 and the plastic sleeve 1516 such that a fluid-
tight seal is
formed therebetween. In the illustrated embodiment, the stem 1510 is press fit
into
the plastic sleeve 1516, thereby preventing dead space around the stem.
As best seen in Figures 16 and 17, the stem 1510 has a through hole
1518 in fluid communication with the flow outlet port 1508 and to the mass
so spectrometer 16. The stem 1510 also has an axial groove 1520 on the outflow
side
of the valve body 1408 and in fluid communication with the flow outlet port
1508.
The axial groove 1520 extends upwardly from the through hole 1518, along the
stem's surface, and is sized to direct the fluid flow upwardly from the
through hole
along the groove between the stem's surface and the center plastic sleeve
1516.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
The through hole 1518 is shaped and sized to allow either a flow of Garner
solvent
or a sampling of a peak from the sample flow to pass toward the mass
spectrometer
16.
Referring now between Figures 3, 15 and 16, the solvent inlet port
s 1506 (Figures 15 and 16) is connected to a carrier solvent line 1602 that
connects to
a carrier solvent source 1604 (Figure 3) and a carrier solvent pump 1606. The
Garner solvent pump 1606 is also coupled to the computer controller 18 that
controls the flow of carrier solvent to the microsample valves 38. A
substantially
continuous flow of carrier solvent is provided to the microsample valves 38
during
to a purification run. In the illustrated embodiment, the carrier solvent line
1602
connects to all four microsample valves 38 in series, so the carrier solvent
will flow
through all of the microsample valves and to the mass spectrometer 16.
Accordingly, the carrier solvent enters the first microsample valve 38 through
the
solvent inlet port 1506 (Figures 15 and 16), exits through the flow outlet
port 1508
is (Figure 16), back into the carrier solvent line 1602, and flows into the
next
microsample valve through its solvent inlet port. The flow continues through
each
microsample valve 38 and then to the mass spectrometer 16.
The microsample valve 38 in each purification channel 14 also has a
continuous flow of the sample flow 31 passing through it. The sample flow 31
2o enters the microsample valve 38 through the sample inlet port 1502 (Figures
15 and
16), through a sample line 1522 extending through the valve body 1408
immediately adjacent to the stem 1510, and out through the sample outlet port
1504. Accordingly, the sample flow 31 in the illustrated embodiment is
transverse
to the flow of the Garner solvent.
2s When the microsample valve 38 is in a lowered normal position,
shown in Figure 16, the through hole 1518 is below and out of communication
with
the sample flow 31. The stem 1510 blocks the sample flow 31 from passing
through the flow outlet port 1508 to the mass spectrometer 16 (Figure 3). When
the
stem 1510 is in the lowered position, a continuous flow of carrier solvent
passes
so into the valve body 1408 through the solvent inlet port 1506, through the
through
hole 1518, up the axial groove 1520, and out of the valve body 1408 through
the
flow outlet port 1508 toward the mass spectrometer 16.
During normal use, when a peak has not been identified, the-
microsample valve 38 remains in this lowered normal position, so only the
carrier
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
36
solvent flows through the microsample valves to the mass spectrometer 16. When
the detector 34 (Figure 3) detects a peak in the sample flow 31 and the
computer
controller 18 activates the microsample valve 38, the solenoids 1410
immediately
move the stem 1510 axially from the lowered position to a raised sampling
position,
s shown in Figure 17. In this raised sampling position, the through hole 1518
in the
stem 1510 is in fluid communication with the sample line 1522 through which
the
sample flow 31 travels between the sample inlet and outlet ports 1502 and
1504.
Accordingly, the flow of carrier solvent is temporarily interrupted and a
small
sampling of the peak traveling through the sample line 1522 is diverted from
the
to sample line, through the through hole 1518 to the flow outlet port 1508,
and into
the carrier line at the location where the Garner solvent flow was
interrupted. The
sampling then flows to the mass spectrometer 16 (Figure 3) for analysis.
As the peak is moving past the through hole 1518 at a selected time,
as determined by the computer controller 18, the stem 1510 is switched back to
the
is lowered position (Figure 16). The solenoids 1410 are activated, thereby
immediately moving the stem 1510 axially to the lowered position, so the only
part
of the sample flow 31 received by the mass spectrometer 16 for analysis is the
sampling of the peak. When the stem 1510 is returned to the lowered position,
the
flow of the carrier solvent to the mass spectrometer 16 is resumed. Therefore,
the
2o mass spectrometer 16 receives a continuous flow of fluid, and the samplings
are
effectively inserted as segments of that continuous flow when the microsample
valve 38 is activated.
The axial movement of the stem 1510 between the lowered position
and the raised sampling position allows for an extremely fast switching
between
2s positions, thereby providing for small yet highly accurate samplings of the
selected
portion of the sample flow. In the illustrated embodiment, the microsample
valve
28 is configured to be switched from the normal lowered position, to the
raised
sampling position and back to the normal lowered position within a time period
of
approximately 15 to 100 milliseconds, inclusive. In one embodiment the time
so period is less than 20 milliseconds, so as to divert sample volumes as
small as
approximately 2 pico liters or less to the mass spectrometer 16. In an
alternate
embodiment, the microsample valve 28 is configured to be moveable from the
normal lowered position, to the raised sampling position and back to the
normal
lowered position in one second or less. This extremely fast switching also
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
37
minimizes the chance of cross-contamination within the valve body between
samplings of a plurality of peaks within the sample flow.
The microsample valve 38 is designed and constructed so the flow
paths through the valve body 1408 and the stem 1510 provide virtually no dead
s space or upswept volumes that could cause cross-contamination between
different
samples flowing through the microsample valve. Accordingly, the microsample
valve 38 allows for very accurate results in the purification process. The
microsample valve 3 8 is also configured to quickly take the small sample
portions
from the sample flow, thereby minimizing the pressure drop in the sample flow
to across the microsample valve 38. In the illustrated embodiment, the
pressure drop
across the microsample valve is less than approximately SO psi.
As best illustrated in Figure 3, the sample flow 31 in each channel 14
moves from the microsample valve 38 to a pressure relief valve assembly 41
that
controls the pressure within the flow downstream of the microsample valve. In
the
Is illustrated embodiment, the pressure relief valve assembly 41 has the same
construction as the back pressure regulator assembly 55 discussed above,
except
that the heaters are not provided on the back pressure regulator valve. In
alternate
embodiments, the heaters can be used if needed as a result of ice formation or
larger pressure drops experienced in the system. In other alternate
embodiments,
20 other back pressure regulators can be used, provided they are durable
enough and
provide sufficient pressure control for the purification valve.
The use of the pressure relief valve 41 allows the flow volume to the
analyzer to be very small because of either use of a small bore capillary to
the
analyzer or an active back-pressure regulator. Accordingly, the pressure
2s differential is reduced and the flow volume to the mass spectrometer 16 is
reduced.
The sample flow 31 exits the pressure relief valve assembly 41 and
flows to two flow directing valves, referred to as a fraction collection valve
assemblies 40 with first and second collection valves 40a and 40b for each
channel.
Each fraction collection valve assembly 40 has, for each channel, one inlet
port 42,
so two outlet ports 44 and 46 for collection, and a waste port 47. The inlet
port 42 is
coupled to both of the first and second collection valves 40a and 40b, and
each
outlet port 44 and 46 is connected to a respective one of the first or second
collection valves. Each of the first and second collection valves 40a and 40b
are
also operatively coupled to the computer controller 18. When a portion of the
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
38
sample flow 31 containing a peak enters the fraction collection valve assembly
40
through the inlet port 42, as identified by the computer controller 18, the
computer
controller activates the first or second fraction collection valve 40a and 40b
to
control whether the peak in the sample flow is directed out of the first
outlet port 44
s or the second outlet port 46.
If the mass spectrometer 16 determines that the peak is the target
compound, the computer controller 18 activates the first collection valve 40a,
so the
collection valve moves to a first position. In this position, the sample
portion
containing the peak is directed out of the first collection valve 40 through
the first
to outlet valve 44. The sample portion is directed to a fraction collector
assembly 43
and is collected directly into a predetermined location in a selected well of
the first
receiving microtiter plate 22.
When a portion of a sample flow containing a peak passes through the
fraction collection valve assembly 40, and that peak is a reaction by-product
rather
is than the target compound, the second collection valve 40b is switched to
direct a
portion of the sample flow through the second outlet port 46. This portion of
the
sample flow 31 exits the second outlet port 46, passes through the fraction
collection assembly 43 and is collected directly into a selected well of the
second
receiving microtiter plate 24. When a portion of the sample flow 31 passes
through
2o the fraction collection valve and that portion does not contain any peaks,
the sample
flow passes through the waste outlet 47 and is carried to a waste receptacle
52.
The purification system 10 of the exemplary embodiment allows the
purified samples to be automatically dispensed into selected wells 2024 of the
receiving microtiter plate 22 or 24. Each purified portion of the sample is
2s dispensed into a well 2024 having the same relative location in the
receiving
microtiter plate 22 or 24 as the well in the supplying microtiter plate 20
from which
the sample was initially drawn to begin the purification run. As an example,
referring to Figure 25, the supplying microtiter plate 20 and each receiving
microtiter plate 22 and 24 have a rectangular array of ninety-six wells 2024.
Each
so well 2024 has a well address defined by its position relative to the rows
(A-H) and
columns (1-12) the array of wells. Accordingly, the well address of the well
2024
in the upper left corner of each plate as shown in Figure 25 has an address of
A1,
and the well in the lower right corner has an address of H12.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
39
Information about each sample in each well 2024 of the supplying
microtiter plate 20 is known prior to the purification run. When the sample
from,
as an example, well A1 is drawn out of the supplying microtiter plate 20 and
run
through the purification system 10, the purified portion of the sample
containing the
s target compound is deposited directly into the corresponding well A1 of the
target
receiving microtiter plate 22. The purified reaction by-products from that
same
sample are deposited directly into well A1 of the by-product receiving
microtiter
plate 24. Therefore, the purified target compound is deposited directly into a
well
having a one-to-one corresponding well address as the original sample well.
to Similarly, the reaction by-products are deposited directly into a well
having a
corresponding one-to-one well address and the second receiving microtiter
plate.
This one-to-one mapping of wells 2024 and direct depositing of the
target compounds into a selected well of a receiving microtiter plate 22 or 24
allows
for easy tracking of information regarding the samples, the purified targets,
and the
is purified reaction by-products. The one-to-one mapping and direct depositing
avoids fiu-ther processing and formatting before the purified target compounds
are
put into microtiter plates. Accordingly, the efficiency of the purification
process is
increased and the time and cost requirements are decreased. In addition,
receiving
microtiter plate 22 or 24 is labeled with, as an example, a bar code so
information
2o about the purified components in each receiving microtiter plate is easy to
track and
maintain.
This purification system 10 of the illustrated embodiment results in
the collection of purified compounds having an 85% purity or better. It is
preferred, of course, to provide samples having purity as close to 100% pure
as
2s possible. Upon collection of the purified target compounds in the receiving
microtiter plate 22, these purified target compounds are ready for a screening
process or other selected process.
As best seen in Figures 19 and 20, the fraction collector assembly 43
includes a frame 2000 and expansion chamber dispensing assembly 2001 at one
end
so of the frame. A docking station 2002 is supported at the other end of the
frame and
is positioned to removably receive the receiving microtiter plates 22 and 24.
The
docking station 2002 includes an array of , indicators coupled to the computer
controller and positioned to prompt the operator where to place the receiving
microtiter plates 22 or 24 on the docking station. In an alternate embodiment,
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
sensors are positioned to detect the location of each receiving microtiter
plate 22 or
24 when it is placed on the docking station 2002. The fraction collector
assembly
43 also includes a dispensing head 2004 that travels along rails 2005, 2006
and
2007 mounted to the frame 2000 for movement along three axes of movement (X,
s Y and Z) relative to the frame between several operating positions.
Accordingly,
the dispensing head 2004 can move forelaft in the Z-axis along one rail 2006,
left/right in the X-axis along another rail 2007, and up/down in the Y-axis
along the
third rail 2005. This 3-axis movement allows for accurate positioning of the
dispensing head 2004 during the fraction collection process, as discussed
below.
to As seen in Figure 21, the dispensing assembly 2001 includes a
housing 2102 formed by a back wall 2104, left and right sidewalls 2106 and
2108.
The right sidewall 2108 is a straight vertical wall and the left sidewall 2106
is
contoured with a middle angled support portion 2112. Accordingly, the back
wall
2104 and the left and right sidewalk 2106 and 2108 define an asymmetric
receiving
is area 2113. The asymmetric receiving area 2113 removably retains an
asymmetric
hopper 2008 that contains clean disposable or reusable expansion chambers
2010.
When the hopper 2008 is in the receiving area 2113, a lower left panel 2016 of
the
hopper is positioned on the left sidewall's angled support panel 2012.
Accordingly,
the hopper 2008 has a corresponding asymmetric shape as the receiving area
2113.
2o The hopper 2008 of the illustrated embodiment is an asymmetric bin
formed by a plurality of perforated panels 2114. The perforated panels 2114 of
the
illustrated embodiment are stainless steel panels, although other materials
can be
used. The hopper's perforated panel 2114 facing the housing's forward wall
2110
has smaller perforations than those perforations in the panels facing the
housing's
2s left and right sidewalls 2106 and 2108 and the rear wall 2104. The smaller
perforations in the hopper's front wall are smaller than the tip of the
expansion
chamber 2010 so the expansion chambers can not extend through the
perforations.
The larger perforations are larger than the tip of the expansion chambers 2010
but
smaller than the open rear ends of the expansion chambers. The expansion
3o chambers 2010 are, thus, installed in the hopper 2008 with the tips facing
forwardly
toward the panel with the smaller perforations. Accordingly, the smaller
perforations in the hopper's front wall provide directional orientation for
installation of the expansion chambers 2010. This directional orientation
assures
easy identification and proper alignment of the expansion chambers 2010 within
the
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
41
hopper 2008. The asymmetric configuration of the hopper 2008 also provides for
easy alignment and accuracy of installation of the hopper within the housing
2102
for proper set up of the dispensing assembly 2001 prior to a purification run.
The hopper 2008 has an open top 2118 through which the expansion
s chambers 2010 can be loaded. The bottom of the hopper 2008 has a dispensing
aperture 2120 through which the expansion chambers 2110 are removed during a
dispensing operation, as discussed below. A removable top cover 2122 is
attachable to the hopper 2008 to cover the open top 2118, and a bottom cover
2124
is slideably attachable to the hopper to close the dispensing aperture 2120.
In one
to embodiment, the top cover 2122 is not installed on the hopper 2008 when the
hopper is installed in the housing. The bottom cover 2124 has slide portions
2126
that slideably receive rails 2128 on the hopper 2008 adjacent to the
dispensing
aperture 2120 so as to retain the bottom cover in a closed position on the
hopper
2008.
Is In the illustrated embodiment, expansion chambers 2010 can be
loaded into the hopper 2008 when the bottom cover 2124 is covering the
dispensing
aperture 2120. The top cover 2122 can then be attached to close the top
opening
2118 so as to fully enclose the expansion chambers 2010 within the hopper
2008.
If the expansion chambers 2010 contained within the hopper 2008 are not clean
or
2o need processing prior to use in the purification run, the hopper with its
top and
bottom covers 2122 and 2124 can be loaded as a unit into a washing device so
as to
thoroughly clean the expansion chambers 2120~in preparation for a purification
run.
The~hopper 2008 containing the clean expansion chambers 2010 can then be
loaded
as a unit directly into the dispensing assembly 2001. The bottom cover 2124 is
2s then removed so the clean expansion chambers 2010 can be dispensed during
the
purification process.
When the hopper 2008 and expansion chambers 2010 are positioned
in the housing's receiving area 2113, the dispensing aperture 2120 is directly
above
a dispensing drum assembly 2130. As best seen in Figures 21 and 22, the drum
so assembly 2130 includes a horizontally oriented drum 2202 rotatably
contained
within a drum guide 2204. The drum guide 2204 has separate left, right and
bottom
guide portions 2206, 2208 and 2210, respectively. The drum 2202 has a
plurality
of channels 2212 formed along the drum's outer surface parallel with the
drum's
longitudinal axis. The channels 2212 are ~arcuate channels shaped to removably
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
42
receive the expansion chambers 2010 dispensed from the hopper 2008 (Figure
21).
In the illustrated embodiment, the drum 2202 has ten channels 2212 formed
around
it's periphery, although a drum with greater or fewer channels can be used as
needed for, as an example, if different size expansion chambers 2010 are to be
s used.
The drum guide's left and right guide portions 2206 and 2208 have
upper edges spaced apart from each other so as to provide an upper opening in
the
drum guide 2204 for access to the channels 2212 in the drum 2202. The
expansion
chambers 2010 are dispensed from the hopper 2008 (Figure 21) into the drum's
io channels 2212 that are adjacent to the upper opening in the drum guide. The
drum
guide 2204 extends around the remaining portion of the drum 2202 so as to
retain
the expansion chambers 2010 within the respective channels 2212 as the drum
rotates within the drum guide. Accordingly, the expansion chambers 2010 are
loaded into the drum 2202 from the top side, and the drum rotates within the
drum
is guide 2204 to position empty channels 2212 adjacent to the drum guide's
opening to
receive another clean expansion chamber.
The drum 2202 is mounted on a drive shaft 2214 that rotatably
mounts at it's rear end to a bearing 2216 retained in the rear wall 2104 of
the
housing 2102. A forward portion 2218 of the drive shaft 2214 is rotatably
2o supported in a bearing 2220 in a front mounting plate 2222 to which the
housing's
front wall 2110 is connected. Accordingly, the drum 2202 is suspended
horizontally for rotation relative to the hopper 2008.
As best seen in Figure 23, the drum 2202 has a hub index 2224
securely mounted to the drum's front end. The forward portion 2218 of the
drive
2s shaft 2214 extends through the hub index 2224. The hub index 2224 has an
elongated slot 2228 that securely receives an index pin 2228 mounted to the
drive
shaft's forward portion 2218. Accordingly, rotational forced from the drive
shaft
2214 are transmitted to the drum 2202 via the index pin 2228 and the hub index
2224 for simultaneous rotation of the drum.
so The drive shaft 2214 is rotatably driven by a drum actuator 2234
securely mounted to the front mounting plate 2222 (Figure 22). The drum
actuator
2234 has a shaft 2232 that extends into a keyhole 2230 in the drive shaft's
forward
portion 2218. In the illustrated embodiment, the keyhole 2230 has a non-
circular
cross-sectional shape, such as a square or a hexagonal shape, that receives
the
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
43
similarly shaped shaft 2232 of the drum actuator 2234. The drum actuator 2234
is
coupled to and controlled by the purification system's computer controller 18
so as
to accurately control rotation of the drum 2202 for selected loading and
dispensing
of the expansion chambers 2010.
s As best seen in Figures 22 and 23, a drum brake 2240 is connected to
the back end portion of the drum 2202. The brake 2240 includes a break hub
2242
securely mounted to the housing's back wall 2104 (Figure 22). The brake hub
2242
extends into a cylindrical break recess 2244 formed in the drum's back end
portion.
As best seen in Figure 23, the brake hub 2242 has an enlarged channel 2246
that
to slidably receives a pair of brake pads 2248. The brake pads 2248 are biased
radially outwardly by a pair of springs 2249 to frictionally engage the drum
2202
within the brake recess 2244. The springs 2249 are selected to provide
sufficient
biasing force for frictional engagement between the brake pads 2248 and drum
2202 to allow for rotation of the drum 2202 when the drum actuator 2234 is
Is activated. The frictional engagement, however, is sufficient to quickly
stop rotation
of the drum 2202 when rotation of the drum actuator 2234 stops, thereby
preventing
drum-overdrift relative to the hopper's dispensing aperture 2120 (Figure 21).
Accurately controlling drum position and preventing drum-overdrift allows for
accurate alignment of the drum's channels 2212 relative to the hopper 2008 for
fast
2o and accurate positioning of the expansion chambers 2010 into the channels.
After an expansion chamber 2010 has been loaded into a selected
channel 2212 in the drum 2202, the drum actuator 2234 rotates the drum to move
the loaded expansion chamber into a dispensing position. As seen in Figure 21,
dispenser brackets 2250 are slidably positioned adjacent to the left and right
sides
2s of the drum 2202. Each dispenser bracket 2250 is positioned to push the
expansion
chamber 2010 axially out of its respective channel 2212 and, thereby
dispensing the
expansion chamber from the drum 2202. Each dispenser bracket 2250 engages the
expansion chamber 2010 with a generally horizontally oriented dispenser tab
2252.
The dispenser tab 2252 is positioned to slide through a raceway 2254 formed in
the
so respective left or right side of the drum guide 2204. In the illustrated
embodiment,
the drum guide 2204 has a left raceway 2254 formed by a space between the left
guide portion 2206 and the bottom guide portion 2210. A right raceway 2256 is
formed by a space provided between the right guide portion 2208 and the bottom
guide portion 2210.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
44
The dispenser tabs 2252 are sized to extend through the respective
left or right raceway 2254 or 2256 and partially into the channel 2212
adjacent to
that raceway. The dispenser tabs 2252 engage the large open end of the
expansion
chamber 2010 contained in the channel 2212 positioned adjacent to the
respective
s left or right raceway 2254 or 2256. When the dispensing assembly is ready to
dispense an expansion chamber 2010, the dispenser bracket 2250 is moved
forwardly so the dispenser tab 2252 slides axially along the raceway 2254 or
2256
and through the channel 2212, thereby pushing the expansion chamber 2010
axially
out of the channel. In the illustrated embodiment, the dispenser brackets 2250
can
to be moved simultaneously or independently to dispense two expansion chambers
2010 from the drum assembly 2130 as needed during the selected purification
run.
As best seen in Figures 21 and 24, each of the left and right dispenser
brackets 2250 are slideably mounted on rails 2160 for movement between a
rearward position and a forward position. The right dispenser bracket 2250 is
is shown is Figure 21 in the forward position, and the left dispenser bracket
is shown
in the rearward position. Each dispenser bracket 2250 is movable linearly
along the
rail 2160 by an actuator coupled to the computer controller 18 of the
purification
system 10. Accordingly, the computer controller 18 controls the timing for
movement of the dispenser brackets 2250 along the respective rails 2160,
thereby
2o controlling the dispensing of the expansion chambers 2010. The actuators
for each
of the left and right dispenser brackets 2250 are independently controlled so
the
dispenser brackets can be moved simultaneously or at separate times for
dispensing
of the expansion chambers 2010.
As each dispenser bracket 2250 moves from the rearward position
2s toward the forward position, the dispenser tab 2252 slides the expansion
chamber
2010 forwardly along the drum's channel 2212. The expansion chamber 2010
slides tip first through an aperture 2260 in the front mounting plate 2222 and
through a respective left or right alignment mount 2262. Each alignment mount
2262 is coaxially aligned with the channel 2212 from which the expansion
chamber
so 2010 is dispensed.
Once the expansion chamber 2010 has been pushed out of its channel
2212 in the drum 2202, the dispenser bracket 2250 is returned to its rearward
position. The drum actuator 2234 rotates the drum 2202 to move another clean
expansion chamber 2010 into alignment with the respective left or right
raceway
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
2254. In the illustrated embodiment, the dispensing assembly 2001 can dispense
two expansion chambers 2010 simultaneously from the drum 2202. Accordingly,
the drum actuator 2234 is indexed to move the drum 2202 two positions relative
to
the raceways 2254 and 2256 and the dispenser brackets 2250 upon each
activation
s of the actuator. This two position movement results in a timing and pattern
that
always provides an expansion chamber in the channel 2212 in alignment with
both
dispenser brackets 2250. While the illustrated embodiment provides indexing of
the drum by two positions, other indexing configurations can be used by
controlling
the drum actuator 2234 for movement of the drum 2202.
As best seen in Figures 21 and 24, the dispensing assembly includes
left and right chamber guides 2402 pivotally mounted adjacent to the alignment
mounts 2262 on the front mounting plate ~ 2222. The chamber guides 2402 are
pivotally movable between a forward, dispensing position, as shown in Figure
24,
and a rearward, stowed position, as shown with the left chamber guide in
Figure 21.
is Each chamber guide 2402 has a guide channel 2404 adapted to receive the
expansion chamber 2010 as the expansion chamber is pushed through an alignment
aperture 2406 in the alignment mount 2262. The upper portion 2408 of the guide
channel 2404 has a convex shape and it is positioned at its top end below the
alignment aperture 2406 in the alignment mount 2262. The guide channel's upper
2o portion 2408 is integrally connected at its bottom end to a straight slide
portion
2410. Accordingly, when the expansion chamber 2010 is pushed through the
alignment aperture 2406, it slides over the convex upper portion 2408 of the
guide
channel 2404 and down the straight slide portion 2410. When the chamber guide
2402 is in the forward, dispensing position, the straight slide portion 2410
is aimed
2s to direct the expansion chamber 2010 to slide into the pickup station 2012,
so the
expansion chamber is held in a vertical orientation with its tip pointing
downwardly.
The chamber guide 2402 is moved from the rearward, stowed position
to the forward, dispensing position by a displacement pin 2414 projecting
inwardly
so from the respective left or right dispenser bracket 2250. As the dispenser
bracket
2250 moves from the rearward position to the forward position, as shown in
Figure 24, the displacement pin 2414 engages the back side of the chamber
guide
2402 and pivots the chamber guide forwardly to the forward, dispensing
position.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/1s469
46
The dispenser guide 2402 is biased by a spring toward the rearward stowed
position.
In the illustrated embodiment, the displacement pin 2414 is
positioned along an elongated slot 2416 in the dispenser bracket 2250 to
provide
s adjustability for the displacement pin's position relative to the chamber
guide 2402.
Such adjustment is provided to allow for accurate positioning of the chamber
guide
2402 to properly aim the straight slide portion 2410 when the chamber guide is
in
the forward, dispensing position, so the expansion chambers 2010 consistently
land
in the pickup station 2012.
to As the dispenser bracket 2250 and displacement pin 2414 are moving
forwardly, the dispenser tab 2252 is simultaneously pushing the expansion
chamber
2010 forwardly. The alignment mounts 2406 are positioned to hold the expansion
chambers 2010 substantially horizontal as they are pushed through the
alignment
apertures 2406 until the expansion chamber's open top end 2020 is pushed
through
is the alignment aperture 2406. Once the expansion chamber 2010 moves fully
out of
the alignment aperture 2406, the expansion chamber drops into the guide
channel
2404 and slides along the channel and into the pickup station 2012. When the
dispenser bracket 2250 and displacement pin 2414 returns to the rearward
position,
the alignment guide 2402 also returns to the rearward, stowed position spaced
apart
2o from the pickup station 2012 and the dispensed expansion chamber 2010.
As best seen in Figure 19, the pickup stations 2012 holds the
expansion chambers 2010 in a substantially vertical orientation with the open
top
end 2020 of the expansion chamber facing upwardly. Each pickup station 2012
has
a cylindrical housing 1902 with a cylindrical aperture 1904 that removably
receives
2s the expansion chambers 2010 from the respective left or right chamber guide
2402.
The cylindrical housing 1902 has a biasing member 1906, such as a spring, in
the
cylindrical aperture 1904 so as to support the tip end of expansion chamber
2010
when loaded into the pickup station 2012. The biasing member 1906 allows the
expansion chamber 2010 to move axially within the pickup station 2012 if a
3o downward force is exerted on the expansion chamber 2010. Accordingly, if
the
expansion chamber 2010 is axially misaligned with the dispensing head 2004 as
the
dispensing head attempts to pick up the expansion chamber, the biasing member
1906 absorbs some of the force and protects the misaligned expansion chamber
2010 from being damaged.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
47
In one embodiment, the pickup station 2012 has optical sensors in the
housing's cylindrical aperture 1904 and coupled to the system's computer
controller 18. The optical sensors detect whether an expansion chamber has
been
properly dispensed into the pickup station 2012. If the optical sensors do not
s properly detect an expansion chamber 2010 as the dispensing head 2004 begins
its
pickup process, a signal is provided to the system's computer controller and
the
computer controller stops the pickup motion and generates an error message.
The
dispensing head 2004 is movable along the rails 2005, 2006 and 2007 to a
position
over the pickup station 2012 and movable downwardly to pickup the expansion
to chamber. As the dispensing head 2004 moves downwardly, dispensing needles
2014 on the dispensing head 2004 extend into the expansion chambers 2010
through the chamber's open top end 2020. In the exemplary embodiment, the
dispensing head 2004 is positioned so the dispensing needles 2014 are
initially
coaxially aligned with the expansion chambers 2010 in the pickup station 2012.
As
is the dispensing head 2004 is moved downwardly so the dispensing needles 2014
extend into the expansion chambers 2010, the dispensing head slightly moves
along
the X-axis or Z-axis, thereby axially misaligning the dispensing needles
within the
expansion chambers. This axial misalignment of the dispensing needles 2014
within the expansion chambers 2010, as discussed below, facilitates sample
2o collection through the expansion chambers.
When the dispensing head 2004 moves to the lowered position, the
dispensing head extends over the open top end 2020 of the expansion chambers
2010. The dispensing head 2004 grasps the expansion chamber 2010 around the
open top end 2020, and lifts it out of the pick-up station 2012. As best seen
in
2s Figure 20, the dispensing head 2004 moves along the rails 2005, 2006, and
2007,
and moves the expansion chambers 2010 from the pickup station 2012 to a
dispensing position over selected wells 2024 in the receiving microtiter
plates 22
and 24. The dispensing head 2004 is coupled to the computer controller 18 that
controls the positioning of the expansion chambers 2010 over the wells 2024 so
as
3o to correspond to the well locations from which the sample was originally
taken in
the one-to-one well correspondence, as discussed above. The dispensing head
2004
moves the expansion chambers 2010 downwardly so as to extend at least
partially
into the selected wells 2024. Once the expansion chamber 2010 is lowered, the
sample portion containing either the target or the sample by-product is
deposited
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
48
from the dispensing needle 2014, into the expansion chamber 2010, and into the
selected well 2024 in the microtiter plate 22 or 24.
As best seen in Figure 18, the dispensing head 2004 of the illustrated
embodiment releasably holds two expansion chambers 2010 in tubular holding
s members 2011. A pneumatic gripping assembly 2015 is connected to each
tubular
holding member 2011 in a position to releasably engage the expansion chambers
2010. The gripping assembly 2015 includes a pair of grippers 2017 connected to
pneumatic cylinders 2019. The pneumatic cylinders 2019 move the grippers 2017
relative to the tubular holding member 2011 between holding and released
to positions. In the holding position, each gripper 2017 presses the expansion
chamber 2010 against the tubular holding member 2011, so the expansion chamber
is frictionally held in the tubular holding member. In the released position,
each
gripper 2017 is positioned to allow the respective expansion chamber 2010 to
freely
move into or out of the tubular holding member 2011.
is The expansion chamber 2010 is a tubular member having the open
top end 2020 that is releasably engaged by the gripping assembly 2015 of the
dispensing head 2004, and a tapered, open bottom end 2022. The open bottom end
2022 is positionable partially within a selected well 2024 of the microtiter
plate 22
or 24. The expansion chamber's open top end 2020 is positioned so the
dispensing
2o needle 2014 extends therethrough into the expansion chamber's interior area
2028.
The dispensing needle 2014 is positioned adjacent to the expansion chamber's
sidewall with the needle axially misaligned with the expansion chamber. The
distal
end 2013 of the dispensing needle 2014 is angled so as to point toward the
respective expansion chamber's sidewall.
2s Each dispensing needle 2014 receives the sample portions through its
open top end 1820 that connects to an outlet port 1822 in a coupler 1824. The
coupler 1824 has, on its top end, a sample inlet port 1826 coaxially aligned
with the
outlet port 1822. Accordingly, the coupler 1824 directs the sample portion
containing the target or reaction by-product into the dispensing needle 2014
for
so delivery into the expansion chamber 2010.
The coupler 1824 of the illustrated embodiment also has a secondary
inlet port 1828 in fluid communication with the coupler's outlet port 1820.
The
secondary inlet port 1828 is connected to a small-bore, high pressure line
1830
carrying liquid carbon dioxide, nitrogen, or other selected chilled liquid or
gas. The
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
49
coupler 1824, thus, can selectively direct a flow of the pressurized liquid or
gas into
the dispensing needle 2014.
In one embodiment, the fraction collection assembly 23 is configured
to direct a flow of high pressure liquid carbon dioxide gas through the
coupler 1824
s and the dispensing needle 2014 before the sample portion is directed through
the
needle. This flow of high pressure liquid carbon dioxide against sidewalls of
the
expansion chamber 2010 chills the sidewalk to facilitate collection of the
sample
portion. As the sample portion is dispensed from the dispensing needle 2014
into
the interior area 2028 of the expansion chamber 2010, the sample portion is in
an
io atomized state. The atomized sample portion enters the expansion chamber
2010
through the needle's angled distal end 2013, and the distal end direct the
flow
toward the expansion chamber's sidewall. The atomized sample portion condenses
on the expansion chamber's chilled sidewalls as a liquid, and is directed so
the
condensed liquid moves along the sidewalls in a downwardly spiral direction.
is The condensed, non-atomized liquid sample portion flows out of the
open expansion chamber's bottom end 2022 into the selected well 2024 in the
microtiter plate 22 or 24. As the atomized sample portion is being dispensed
into
the expansion chamber 2010, the CO~ vapor exits the expansion chamber through
its open top end 2020. In the illustrated embodiment, a vacuum is drawn within
the
2o expansion chamber to draw the C02 vapors out and away from the expansion
chamber's open top end 2020, thereby avoiding cross-contamination between
channels. After a sample portion has been passed through the dispensing
needle, a
puff of carbon dioxide or other gas can be passed through the dispensing
needle to
ensure that there is no residual fluid left in the needle.
2s As the sample portion is condensed in the expansion chamber 2010,
some of the liquid sample portion may remain in the bottom of the expansion
chamber because of a capillary action at the narrow open bottom end 2022. At
this
point, the fraction collection valve dispenses a selected solvent into the
expansion
chamber to rinse it out and carry any remaining sample into the microtiter
plate 22
30 or 24. After the sample portion has been fully dispensed, the dispensing
head 2004
can provide a puff of carbon dioxide or other gas into the expansion chamber
2010.
The gas forces the remaining liquid sample out of the expansion chamber 2010
and
into the well 2024.
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
As best seen in Figure 26, after the sample has been dispensed into
the microtiter plate 22 or 24, the dispensing head 2004 moves to a chamber
drop-
off position so the expansion chambers 2010 are positioned past the edge of
the
frame 2000. The gripping assembly 2015 of the dispensing head 2004 moves to
the
s released position and the expansion chambers 2010 drop into a suitable waste
receptacle. In one embodiment, the expansion chambers 2010 are thrown away. In
an alternate embodiment, the expansion chambers 2010 are recycled so as to be
reusable. In another embodiment, the used expansion chambers 2010 are
collected
in receiving hopper substantially identical to the hopper 2008 in the chamber
to dispensing assembly 2001 discussed above. The receiving hopper with the
used
expansion chambers 2010 can be taken as a unit and placed into a washing
assembly that cleans the expansion chambers. The receiving hopper and clean
expansion chambers 2010 can then be loaded directly into the housing 2102 of
the
dispensing assembly 2001. Accordingly, use of the receiving hopper can save a
is significant amount of time and manpower in preparing the expansion chambers
for
use in the fraction collection assembly 23.
After the dispensing head 2004 drops off the expansion chambers, the
dispensing head moves to a needle rinse position, illustrated in Figure 22. In
this
needle rinse position, the dispensing head 2004 is positioned over a pair of
rinse
2o stations 2030. As seen in Figure 28, each rinse station 2030 includes a
substantially
cylindrical body 2802 mounted at its bottom end to the frame 2000 of the
fraction
collection assembly 23. The body 2802 has an elongated aperture 2804 extending
vertically along the body's longitudinal axis. An inner wash tube 2803 is
positioned within the elongated aperture 2804. The inner wash tube 2806 has an
2s outer diameter smaller than the aperture's inner diameter, such that an
annular
passageway 2806 is formed between the wash tube and the body.
An outer wash tube 2812 is concentrically disposed around the inner
wash tube 2803. The outer wash tube 2812 has an inner diameter greater than
the
i_n_n_er wash tube's outer diameter. Accordingly, the annular solvent
passageway
so 2806 extends between the inner and outer wash tubes 2803 and 2812. The
inner
and outer wash tubes 2803 and 2812 are held in the concentric orientation by a
top
cap 2814 that provides a top closure to the solvent passageway 2806.
The bottom portion of the body 2802 has a solvent inlet port 2816
coupled to a solvent source and in fluid communication with the solvent
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
51
passageway 2806. A selected solvent or other cleaning fluid is directed
through the
solvent inlet 2816 and into the solvent passageway 2806. An O-ring seal 2818
is
positioned in the bottom portion of the body 2802 and around the inner wash
tube
2803 so as to provide a bottom closure to the solvent passageway 2806. The
s solvent enters the solvent passageway 2806 and flows upwardly through the
passage. The upper end portion of the inner wash tube 2803 has a plurality of
holes
2820 that communicate with the solvent passageway 2806. The solvent flowing
through the solvent passageway is forced through the holes 2820 into the
interior
area 2822 of the inner wash tube 2803. The holes 2820 are sized to direct jets
of
to the solvent radially inwardly from the periphery of the interior area 2822.
When the dispensing needle 2014 is lowered into its respective rinse
station 2030, the dispensing needle is positioned within the inner wash tube's
interior area 2822. The computer controller 18 activates the flow of solvent
from
the solvent source, and solvent flows into the annular solvent passageway 2806
and
is through the holes 2820 into the interior area 2822. The jets of cleaning
solvent
clean or rinse the dispensing needle 2014. In the exemplary embodiment, the
cleaning solvent is dispensed through the holes 2820 when the dispensing
needle
2014 is moved upwardly out of the inner wash tube 2803. As the dispensing
needle
2014 moves upwardly, the jets of cleaning solvent hitting the dispensing
needle act
2o as a "fluid squeegee," thereby cleaning the dispensing needle from its top
or middle
portion to its tip as the dispensing is withdrawn from the inner wash tube
2803.
The cleaning solvent that flows into the inner wash tube's interior
area 2822 flows downwardly through the interior area and exits the inner wash
tube
through an open bottom end 2824. The open bottom end 2824 is coupled to a
2s waste line that carries the used cleaning solvent to a selected receptacle
for
containing the waste solvent.
After the dispensing needles 2014 are lifted out of the wash stations
2030. The dispensing head 2004 is moved back to the expansion chamber pickup
position, illustrated in Figure 19. New, clean expansion chambers 2010 that
have
so been delivered to the pickup stations 2012 are then picked up by the
dispensing
head 2004 for dispensing other sample portions into the respective receiving
microtiter plates 22 and 24.
The high throughput purification system 10 of the illustrative
embodiment allows for relatively fast sample purification as compared to
CA 02408410 2002-10-30
WO 01/86283 PCT/USO1/15469
52
conventional purification processes. A purification run of a selected sample
can be
accomplished in approximately 6-8 minutes or faster. Therefore, purification
of
samples contained in a 96 well microtiter plate will take approximately 144-
192
minutes. Purification of 4,000 samples generated in a week using sample
s generation techniques, discussed above, will only take in the range of 250-
330.3
hours, as opposed to the 2,000 hours required to purify the 4,000 samples,
using
conventional purification techniques. Therefore, the high throughput
purification
system in accordance with the present invention allows for a significant
increased
speed of purification. This system also provides for collecting the purified
samples
to directly into a microtiter plate in wells having a location address
corresponding to
the location address of the well in the microtiter plate from which the
samples were
originally drawn. Thus, the purified compounds are ready to be screened or
otherwise processed. The result is a significantly increased capacity for
purification that allows for a less expensive purification process.
is From the foregoing it will be appreciated that, although specific
embodiments of the invention have been described herein for purposes of
illustration, various modifications may be made without deviating from the
spirit
and scope of the invention. Accordingly, the invention is not limited except
as by
the appended claims.