Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02408659 2005-11-09
SYSTEMS FOR CONTROLLING A DRYING
CYCLE IN A DRYING APPARATUS
Field of the Invention
The present invention relates to systems for controlling a drying cycle in a
drying
apparatus by monitoring the lipophilic fluid vapor concentration. The systems
utilize a
gas sensor capable of sensing the concentration of lipophilic fluid vapor in
the drying
apparatus drum or a combination of sensorsJcondition detectors, at least one
of which is
capable of sensing the concentration of lipophilic fluid vapor in the drying
appatah~s
drum.
~acl~ro,~n~ of the Imrention
Conventional laundering techniques for the cleaning and treatment of fabric
articles such as garm~ts have long involved both traditional aqueous-based
washing and
a technique commonly referred to as "dry cleaning". Traditional aqueous based
washing
techniques have involved immersion of the fabric articles in a solution of
water and
detergent or soap products, followed by rinsing and drying. However, such
conventional
immersion cleaning techniques have pmven unsatisfactory on a wide range of
fabric
articles that require special handling and/or cleaning methods due to fabric
content,
construction, etcetera, which may be unsuitable for immersion in water.
Accordingly, "dry cleaning" has been developed. Dry cleaning typically
involves
the use of non-aqueous, lipophilic fluids as the solvent or solution for
cleaning. While
the absence of water permits the cleaning of fabrics without the potential
disastrous side
CA 02408659 2005-11-09
effects water may cause, these lipophilic fluids do not perform well on
hydrophilic and/or
combination sails.
As a result, new methods have been developed wherein a lipophilic fluid is
emulsified with water in order to better perform on these hydrophilic and/or
combination
soils. Along with this development, however, have arisen new problems. First,
many
lipophilic fluids have established "safe" exposureJinhalation limits. Second,
the drying of
items cleaned with the lipophilic fluid cannot be done automatically with the
use of only
a humidity sensor. Third, energy savings could be appreciated if the items to
be dried are
exposed to heat and tumbling only for time needed to achieve drying. This
would also
reduce heat and tumbling damage to the items. Lastly, because these lipophilic
fluids
possess far greater fouling capabilities than water alone as well as potential
flash point
problems, sensors typically used in clothes dryers may not be suitable for use
in lipophilic
fluid clothes dryers.
U.S. Patent No. 6,122,480 discloses a system for determining drying
time in a clothes dryer that utilizes a humidity sensor. However,
the present invention is directed to drying lipophilic fluid from fabrics -
not water.
Further, U.S. Patent No. 4,111,034 is directed to an apparatus for
monitoring the solvent content of air in association with a dry-cleaning
plant. The present invention is directed to detecting levels of several
lipophilic fluids,
some of which tend to foul sensors, in many environments, including the home.
Accordingly, the need remains for a system that controls a drying cycle in a
drying apparatus by utilizing a gas sensor capable of sensing the
concentration of
lipophilic fluid vapor in the drying apparatus drum or a combination of
sensors/condition
detectors, at least one of which is capable of sensing the concentration of
lipophilic fluid
vapor in the drying apparatus drum.
Summax~of the Invention
The present invention provides control over a drying cycle in a drying
apparatus
that utilizes a gas sensor capable of sensing the concentration of lipophilic
fluid vapor in
the drying apparatus drum or a combination of sensors/condition detectors, at
least one of
2
CA 02408659 2005-11-09
which is capable of sensing the concentration of lipophilic fluid vapor in the
drying apparatus
drum.
In a first embodiment, the present invention provides a system for controlling
a drying
cycle in a drying apparatus, wherein said drying apparatus comprises a
lipophilic fluid vapor, a
gas sensor capable of sensing the concentration of lipophilic fluid vapor
present in said drying
apparatus and transmitting a signal representative of the lipophilic fluid
vapor concentration
such that said drying cycle is controlled.
In a second embodiment, the present invention provides a system for
controlling a
drying cycle in a drying apparatus comprising, a condition detector and a gas
sensor wherein
said condition detector is capable of activating said gas sensor and said gas
sensor is capable of
generating a signal representative of the lipophilic fluid vapor concentration
in the dryer such
that the drying cycle is controlled.
In a third embodiment, the present invention provides a method for treating
fabrics in
need of treatment comprising placing said fabrics in a drying apparatus
comprising a gas
sensor capable of sensing lipophilic fluid vapor concentration within said
drying apparatus and
transmitting a signal representative of said lipophilic fluid vapor
concentration, contacting said
fabrics with a lipophilic fluid, and operating said drying apparatus such that
the drying cycle is
controlled by said gas sensor.
In one particular embodiment there is provided a system for controlling a
drying cycle
in a drying apparatus comprising: a first means for sensing the concentration
of a lipophilic
fluid vapor present in the drying apparatus; at least one second means for
sensing a condition
in the drying apparatus; and a signal processor operatively connected to said
first and second
means; wherein said signal process is configured to compare a first signal
from said first
means against a first threshold value and a second signal from said second
means against a
second threshold value.
3
CA 02408659 2005-11-09
These and other aspects, features and advantages will become apparent to those
of
ordinary skill in the art from the following detailed description and the
appended claims. All
percentages, ratios and proportions herein are by weight, unless otherwise
specified. All
temperatures are in degrees Celsius (°C) unless otherwise specified.
All measurements are in
SI units unless otherwise specified.
Brief Description of The Drawings
FIG. 1 is a box diagram of a drying time determining system in accordance with
one of
the embodiments of the present invention.
FIG. 2 shows annotations used in the procedure algorithms.
3a
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
FIG. 3 is a signal handling procedure 70 including a gas sensor.
FIG. 4 is a signal handling procedure 130 that includes a gas sensor and
condition
sensors.
FIG. 5 is another signal handling procedure 170 that includes the gas sensor,
and the
condition sensors.
Detailed Description of the Invention
Definitions
The term "lipophilic fluid" used herein is intended to encompass any non-
aqueous
fluid or vapor capable of removing sebum, as qualified by the test described
below.
The term "fabrics" and "fabric" used herein is intended to mean any article
that is
customarily cleaned in a water-based laundry process or in a solvent-based dry
cleaning
process. As such the teen encompasses bulk fabrics and fibers, as well as
finished
articles of clothing, linens, drapery, and clothing accessories. The term also
encompasses
other items made in whole or in part of fabric, such as tote bags, furniture
covers,
tarpaulins and the like.
The term "condition detector" used herein is intended to mean any detector
and/or
sensor capable of quantitatively andlor qualitatively measuring some
scientific quality.
These qualities may include, and are not limited to, time, temperature, fluid
flow, and
torque. Further, the measure may be of a physical quality within the drying
apparatus(e.g., torque on the spinning drum or inlet air temperature), a
physical quality of
the fabric load to be dried (e.g., mass or time exposed to drying conditions),
a physical
quality measured on the outside of the drying apparatus (e.g., condensed fluid
flow or
outlet air temperature), or any combination thereof.
The term "drying apparatus" used herein is intended to mean any apparatus
capable of removing fluid from fabrics. The removal means can be temperature
change,
gas circulation, light introduction, tumbling, agitation, and combinations of
any of these
means. The term includes an apparatus capable of "dual mode" functions. A
"dual
4
CA 02408659 2005-11-09
mode" apparatus is one capable of both washing and drying fabrics within the
same
drum. These apparati are commercially available.
The term "safe" used herein is intended to mean the established exposure level
for
any type of human contact. Specifically, lipophilic fluids suitable for use
with the present
invention may have established exposure level classifications like contact or
absorption,
inhalation, and ingestion. For lipophilic fluids with established contact
and/or inhalation
maximum levels, one of the objects of the present invention is to dry fabrics
within a
drying operation comprising lipophilic fluid vapor to a level at or below the
least of the
maximum exposure levels for a particular lipophilic fluid. Safe exposure
limits for
various lipophilic fluids can be found in: American Conference of Governmental
Industrial Hygienists (ACGIH), "Threshold Limit Values (TLVs~ for Chemical
Substances and Physical Agents" Second Printing,1995, ISBN 1-88217-11-9 and
National Research Council, "Spacecraft Maximum Allowable Concentrations for
Selected Airborne Contaminants," Volume 4, Chapter B7, Page 151-173, Published
by
National Academy Press, 2000, ISBN 0-309-06795-2 .
Lipophilic Fluid
In general, lipophilic fluid can be fully liquid at ambient temperature and
pressure, can be an easily melted solid, e.g., one which becomes liquid at
temperatures in
the range from about 0 deg. C to about 60 deg. C, or can comprise a mixture of
liquid and
vapor phases at ambient temperatures and pressures, e.g., at 25 deg. C and 1
atm.
pressure. Thus, the essential lipohilic fluid is not a compressible gas such
as carbon
dioxide. It is preferred that the lipophilic fluid herein be nonflammable or
have relatively
high flash points and/or low VOC characteristics, these terms having their
conventional
meanings as used in the dry cleaning industry, to equal or, preferably, exceed
the
characteristics of known conventional dry cleaning fluids.
Suitable lipophilic fluids herein readily flow and are non-viscous. In
general, the
lipophilic fluids herein are required to be fluids capable of at least
partially dissolving
sebum (e.g. body soil) as defined in the test hereinafter. Mixtures of
lipophilic fluid are
also suitable, and provided that the requirements of the test are met, the
lipophilic fluid
5
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
can include any fraction of dry-cleaning solvents, especially newer types
including non-
fluorinated solvents, or perfluorinated amines. Some perfluorinated amines
such as
perfluorotributylamines while unsuitable for use as lipohilic fluid may be
present as one
of many possible adjuncts present in the lipohilic fluid. Other suitable
lipohilic fluids
include diol solvent systems e.g., higher diols such as C6- or C8- or higher
diols;
organosilicone solvents including both cyclic and acyclic types, and the like;
and
mixtures thereof.
A preferred group of nonaqueous liquids suitable for incorporation as the
major
component of the lipophilic fluid includes low-volatility non-fluorinated
organics,
silicones, especially those other than amino-functional silicones, and
mixtures thereof.
Low volatility nonfluorinated organics include for example OLEAN~ and other
polyol
esters, or certain relatively nonvolatile biodegradable mid-chain branched
petroleum
fractions. Suitable silicones for use as a major component, e.g., more than
50%, of the
lipophilic fluid include cyclopentasiloxane, sometimes termed "DS", or linear
analogs
having approximately similar volatility, optionally complemented by other
compatible
silicones. Suitable silicones are well known in the literature, see, for
example, Kirk
Othmer's Encyclopedia of Chemical Technology, and are available from a number
of
commercial sources, including General Electric, Toshiba Silicone, Bayer, and
Dow
Corning. Other suitable fluids are commercially available from Procter &
Gamble or
from Dow Chemical and other suppliers. For example one suitable silicone is SF-
1528
available from GE silicone fluids. Notably, SF-1528 fluid is 90%
cyclopentasiloxane
(DS).
Any non-aqueous fluid that is both capable of meeting known requirements for a
dry-cleaning fluid (e.g., flash point etc.) and is capable of at least
partially dissolving
sebum, as indicated by the test method described below, is suitable as a
lipophilic fluid
herein. The ability of a particular material to remove sebum can be measured
by any
known technique. As a general guideline, perfluorobutylamine (Fluorinert FC-
43~) on its
own (with or without adjuncts) is a reference material that, by definition, is
unsuitable as
the lipophilic fluid herein (it is essentially a non-solvent) while DS
dissolves sebum.
The following is the method for investigating and qualifying other materials,
e.g.,
other low-viscosity, free-flowing silicones, for use as the lipophilic fluid.
The method
6
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
uses commercially available Crisco ~ canola oil, oleic acid (95% pure,
available from
Sigma Aldrich Co.) and squalene (99% pure, available from J.T. Baker) as model
soils
for sebum. The test materials should be substantially anhydrous and free from
any added
adjuncts, or other materials during evaluation.
Prepare three vials. Place 1.0 g of canola oil in the first; in a second vial
place 1.0
g of the oleic acid (95%), and in a third and final vial place l.Og of the
squalene (99.9%).
To each vial add 1 g of the fluid to be tested for lipophilicity. Separately
mix at room
temperature and pressure each vial containing the lipophilic soil and the
fluid to be tested
for 20 seconds on a standard vortex mixer at maximum setting. Place vials on
the bench
and allow settling for 15 minutes at room temperature and pressure. If, upon
standing, a
single phase is formed in any of the vials containing lipophilic soils, then
the fluid
qualifies as suitable for use as a "lipophilic fluid" in accordance with the
invention.
However, if two or more separate layers are formed in all three vials, then
the amount of
fluid dissolved in the test fluid will need to be further determined before
rejecting or
accepting the fluid as qualified.
In such a case, with a syringe, carefully extract a 200 microliter sample from
each
layer in each vial. The syringe-extracted layer samples are placed in GC
autosampler
vials and subjected to conventional GC analysis after determining the
retention time of
calibration samples of each of the three models soils and the fluid being
tested. If more
than 1% of the test fluid by GC, preferably greater, is found to be present in
any one of
the layers which consists of the oleic acid, canola oil or squalene layer,
then the test fluid
is also qualified for use as a lipophilic fluid. If needed, the method can be
further
calibrated using heptacosafluorotributylamine, i.e., Fluorinert FC-43 (fail)
and
cyclopentasiloxane (pass).
A suitable GC is a Hewlett Packard Gas Chromatograph HP5~90 Series II
equipped with a split/splitless injector and FID. A suitable column used in
determining
the amount of lipophilic fluid present is a J&W Scientific capillary column DB-
1HT, 30
meter, 0.25mm id, O.lum film thickness cat# 1221131. The GC is suitably
operated
under the following conditions:
Carrier Gas: Hydrogen
Column Head Pressure: 9 psi
7
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
Flows: Column Flow @ ~1.5 ml/min.
Split Vent @ 250-500 ml/min.
Septum Purge @ 1 ml/min.
Injection: HP 7673 Autosampler, 10 ul syringe, lul injection
Injector Temperature: 350 °C
Detector Temperature: 380 °C
Oven Temperature Program: initial 60 °C, hold 1 min.
rate 25 °C/min.
final 380 °C hold 30 min.
Preferred lipophilic fluids suitable for use herein can further be qualified
for use
on the basis of having an excellent garment care profile. Garment care profile
testing is
well known in the art and involves testing a fluid to be qualified using a
wide range of
garment or fabric article components, including fabrics, threads and elastics
used in
seams, etc., and a range of buttons. Preferred lipophilic fluids for use
herein have an
excellent garment care profile, for example they have a good shrinkage or
fabric
puckering profile and do not appreciably damage plastic buttons.
For purposes of garment care testing or other qualification, e.g.,
flammability, a
lipophilic fluid can be present in a mixture, e.g., with water, at
approximately the ratio to
be used in the final lipophilic fluid that will come into contact with fabric
articles. Certain
materials that remove sebum and which otherwise qualify for use as lipophilic
fluids, for
example, ethyl lactate can be quite objectionable due to its tendency to
dissolve buttons.
If such a material is to be used in the lipophilic fluid, it will be
formulated with water
and/or other solvents such that the overall mix is not substantially damaging
to buttons.
Other lipophilic fluids, DS for example, meet the garment care requirements
commendably. Some suitable lipophilic fluids may be found in granted U.S.
Patent Nos.
5,865,852; 5,942,007; 6,042,617; 6,042,618; 6,056,789; 6,059,845; and
6,063,135.
Lipophilic solvents can include linear and cyclic polysiloxanes, hydrocarbons
and
chlorinated hydrocarbons. More preferred are the linear and cyclic
polysiloxanes and
hydrocarbons of the glycol ether, acetate ester, lactate ester families.
Preferred lipophilic
solvents include cyclic siloxanes having a boiling point at 760 mm Hg. of
below about
8
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
250°C. Specifically preferred cyclic siloxanes for use in, this
invention are
octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, and
dodecamethylcyclohexasiloxane. Preferably, the cyclic siloxane comprises
decamethylcyclopentasiloxane (D5, pentamer) and is substantially free of
octamethylcyclotetrasiloxane (tetramer) and dodecamethylcyclohexasiloxane
(hexamer).
However, it should be understood that useful cyclic siloxane mixtures might
contain, in addition to the preferred cyclic siloxanes, minor amounts of other
cyclic
siloxanes including octamethylcyclotetrasiloxane and
hexamethylcyclotrisiloxane or
higher cyclics such as tetradecamethylcycloheptasiloxane. Generally the amount
of these
other cyclic siloxanes in useful cyclic siloxane mixtures will be less than
about 10 percent
based on the total weight of the mixture. The industry standard for cyclic
siloxane
mixtures is that such mixtures comprise less than about 1% by weight of the
mixture of
octamethylcyclotetrasiloxane.
Accordingly, the lipophilic fluid of the present invention preferably
comprises
more than about 50%, more preferably more than about 75%, even more preferably
at
least about 90%, most preferably at least about 95% by weight of the
lipophilic fluid of
decamethylcyclopentasiloxane. Alternatively, the lipophilic fluid may comprise
siloxanes which are a mixture of cyclic siloxanes having more than about 50%,
preferably
more than about 75%, more preferably at least about 90%, most preferably at
least about
95% up to about 100% by weight of the mixture of decamethylcyclopentasiloxane
and
. less than about 10%, preferably less than about 5%, more preferably less
than about 2%,
even more preferably less than about 1%, most preferably less than about 0.5%
to about
0% by weight of the mixture of octamethylcyclotetrasiloxane and/or
dodecamethylcyclohexasiloxane.
Adjunct Ingredients
Adjunct materials can vary widely and can be used at widely ranging levels.
For
example, detersive enzymes such as proteases, amylases, cellulases, lipases
and the like
as well as bleach catalysts including the macrocyclic types having manganese
or similar
transition metals all useful in laundry and cleaning products can be used
herein at typical
or atypical levels. Adjunct materials that are catalytic, for example enzymes,
can be used
9
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
in "forward" or "reverse" modes, a discovery independently useful from the
present
invention. For example, a lipolase or other hydrolase may be used, optionally
in the
presence of alcohols as adjuncts, to convert fatty acids to esters, thereby
increasing their
solubility in the lipophilic fluid. This is a "reverse" operation, in contrast
with the normal
use of this hydrolase in water to convert a less water-soluble fatty ester to
a more water-
soluble material. In any event, any adjunct ingredient must be suitable for
use in
combination with the lipophilic fluid.
The compositions may comprise emulsifiers. Emulsifiers are well known in the
chemical art. Essentially, an emulsifier acts to bring two or more insoluble
or semi
soluble phases together to create a stable or semi-stable emulsion. It is
preferred in the
claimed invention that the emulsifier serves a dual purpose wherein it is
capable of acting
not only as an emulsifier but also as a treatment performance booster. For
example, the
emulsifier may also act as a surfactant thereby boosting cleaning performance.
Both
ordinary emulsifiers and emulsifier/surfactants are commercially available.
Some suitable cleaning additives include, but are not limited to, builders,
surfactants, enzymes, bleach activators, bleach catalysts, bleach boosters,
bleaches,
alkalinity sources, antibacterial agents, colorants, perfumes, pro-perfumes,
finishing aids,
lime soap dispersants, composition malodor control agents, odor neutralizers,
polymeric
dye transfer inhibiting agents, crystal growth inhibitors, photobleaches,
heavy metal ion
sequestrants, anti-tarnishing agents, anti-microbial agents, anti-oxidants,
anti-redeposition
agents, electrolytes, pH modifiers, thickeners, abrasives, divalent or
trivalent ions, metal
ion salts, enzyme stabilizers, corrosion inhibitors, diamines or polyamines
and/or their
alkoxylates, suds stabilizing polymers, solvents, process aids, fabric
softening agents,
optical brighteners, hydrotropes, suds or foam suppressors, suds or foam
boosters, fabric
softeners, antistatic agents, dye fixatives, dye abrasion inhibitors, anti-
crocking agents,
wrinkle reduction agents, wrinkle resistance agents, soil release polymers,
soil repellency
agents, sunscreen agents, anti-fade agents, and mixtures thereof.
The term "surfactant" conventionally refers to materials that are surface-
active
either in the water, the lipophilic fluid, or the mixture of the two. Some
illustrative
surfactants include nonionic, cationic and silicone surfactants as used in
conventional
aqueous detergent systems. Suitable nonionic surfactants include, but are not
limited to:
CA 02408659 2005-11-09
a) Polyethylene oxide condensates of nonyl phenol and myristyl alcohol, such
as
in US 4685930 Kasprzak; and
b) fatty alcohol ethoxylates, R-(OCH2CH2),OH a=1 to 100, .typically 12-40, R--
hydrocarbon residue 8 to 20 C atoms, typically linear alkyl. Examples
S polyoxyethylene lauryl ether, with 4 or 23 oxyethylene groups;
polyoxyethylene cetyl ether with 2, 10 or 20 oxyethylene groups;
polyoxyethylene stearyl ether, with 2, 10, 20, 21 or 100 oxyethylene groups;
polyoxyethylene (2), (10) oleyl ether, with 2 or 10 oxyethylene groups.
TM
Commercially available examples include, but are not limited to: ALFONIC,
TM TM TM TM TM
BRIJ, GENAPOL, NEODOL, SURFONIC, TRYCOL. See also US 6013683
Hill et al.,.
Suitable cationic surfactants include, but are not limited to,
dialkyldimethylammonium
salts having the formula:
R'R"I~'(CH3)aX'
Where each R'R" is independently selected from the group consisting of 12-30 C
atoms
or derived from tallow, coconut oil or soy, X=CI or Br, Examples include:
didodecyldimethylammonium bromide (DDAB), dihexadecyldimethyl ammonium
chloride, dihexadecyldimethyl ammonium bromide, dioctadecyldimethyl ammonium
chloride, dieicosyldimethyl ammonium chloride, didocosyldimethyl ammonium
chloride,
dicoconutdimethyl ammonium chloride, ditallowdimethyl ammonium bromide (DTAB).
TM TM
Commercially available examples include, but are not limited to: ADOGEN,
ARQUAD,
TM TM
TOMAH , VARIQUAT. See also US 6013683 Hill et al., .
Suitable silicone surfactants include, but are not limited to the
polyalkyleneoxide
polysiloxanes having a dimethyl polysiloxane hydrophobic moiety and one or
more
hydrophilic polyalkylene side chains and have the general formula:
R'--(CH3)2'SiO--((CH3~'S1O]a--~(CH3)(R')S1OJ6--SI(CH3)~
wherein a + b are from about 1 to about 50, preferably from about 3 to about
30 , more
preferably from about 10 to about 25, and each R' is the same or different and
is selected
from the group consisting of methyl and a poly(ethyleneoxidelpropyleneoxide)
copolymer
group having the general formula:
11
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
-(CH2)n O(C2 H4 O)c (C3 H6 O)d R2
with at least one R' being a poly(ethyleneoxidelpropyleneoxide) copolymer
group, and
wherein n is 3 or 4, preferably 3; total c (for all polyalkyleneoxy side
groups) has a value
of from 1 to about 100, preferably from about 6 to about 100; total d is from
0 to about
14, preferably from 0 to about 3; and more preferably d is 0; total c+d has a
value of from
about 5 to about 150, preferably from about 9 to about 100 and each R2 is the
same or
different and is selected from the group consisting of hydrogen, an alkyl
having 1 to 4
carbon atoms, and an acetyl group, preferably hydrogen and methyl group.
Examples of
these surfactants may be found in U.S. Patent No.'s 5,705,562 and 5,707,613,
both to
Hill.
Examples of this type of surfactants are the Silwet~ surfactants which are
available CIA Witco, OSi Division, Danbury, Connecticut. Representative Silwet
surfactants are as follows.
Name Average MW Average a+b Average total c
L-7608 600 1 9
L-7607 1,000 2 17
L-77 600 1 9
L-7605 6,000 20 99
L-7604 4,000 21 53
L-7600 4,000 11 68
L-7657 5,000 20 76
L-7602 3,000 20 29
The molecular weight of the polyalkyleneoxy group (Rl) is less than or equal
to
about 10,000. Preferably, the molecular weight of the polyalkyleneoxy group is
less than or
equal to about 8,000, and most preferably ranges from about 300 to about
5,000. Thus, the
values of c and d can be those numbers which provide molecular weights within
these
ranges. However, the number of ethyleneoxy units (-C2H40) in the polyether
chain (Rl)
must be sufficient to render the polyalkyleneoxide polysiloxane water
dispersible or water
soluble. If propyleneoxy groups are present in the polyalkylenoxy chain, they
can be
distributed randomly in the chain or exist as blocks. Preferred Silwet
surfactants are L-
12
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
7600, L-7602, L-7604, L-7605, L-7657, and mixtures thereof. Besides surface
activity,
polyalkyleneoxide polysiloxane surfactants can also provide other benefits,
such as
antistatic benefits, and softness to fabrics.
The preparation of polyalkyleneoxide polysiloxanes is well known in the art.
Polyalkyleneoxide polysiloxanes of the present invention can be prepared
according to
the procedure set forth in U.S. Pat. No. 3,299,112. Another suitable silicone
surfactant is
SF-1488, which is commercially available from GE silicone fluids.
These and other surfactants suitable for use in combination with the
lipophilic fluid
as adjuncts are well knovcm in the art, being described in more detail in Kirk
Othmer's
Encyclopedia of Chemical Technology, 3rd Ed., Vol. 22, pp. 360-379,
"Surfactants and
Detersive Systems." Further suitable nonionic detergent surfactants are
generally
disclosed in U.S. Patent 3,929,678, Laughlin et al., issued December 30, 1975,
at column
13, line 14 through column 16, line 6.
The adjunct may also be an antistatic agent. Any suitable well-known
antistatic
agents used in laundering and dry cleaning art are suitable for use in the
methods and
compositions of the present invention. Especially suitable as antistatic
agents are the
subset of fabric softeners which are known to provide antistatic benefits. For
example
those fabric softeners which have a fatty acyl group which has an iodine value
of above
20, such as N,N-di(tallowoyl-oxy-ethyl)-N,N-dimethyl ammonium methylsulfate.
However, it is to be understood that the term "antistatic agent" is not to be
limited only to
this subset of fabric softeners and includes all antistatic agents.
Although the methods and/or compositions utilized in present invention will be
described in detail, it should be understood, and one skilled in the art will
recognize, that
any compositions, processes, and/or apparati capable of carrying out the
invention could
be used.
Sensors
Many sensors can operate with the present invention. Listed below are
nonlimiting examples of suitable sensors for use in the present invention. The
list is not
intended to be complete or exhaustive. A list of sensor types and their
manufacturers can
13
CA 02408659 2005-11-09
be found in the trade publication Sensors, September 2000, Volume I7, Number
9, pages
27 to 40.
Reactive Sensors
Reactive sensors generate a signal by measuring some aspect of a chemical
reaction of the analyte. The analyte may be destroyed in the process. The
smallest and
cheapest sensors tend to be reactive types.
Electrochemical Sensors -- A more precise term for this type is the "porous
electrode amperometric gas sensor". It is also called a fuel cell sensor. It
will respond to
gases that can be electrolytically reduced or oxidized on a metallic catalyst
such as
platinum or gold. Typical gases measured are Oz, CO, NOz, NO, and HZS , and
organic
vapors such as alcohols, aldehydes, or ketones. Typical sensitivities are in
the 3-30 PPM
range, but some proprietary sensors are capable of detecting as little as 2
PPB of gases
such as ozone, NOz , H2S or arsine.
Solid State Semiconductor Sensors -- This sensor typically consists of a bead
of
tin oxide formed around two fine coils of platinum wire. When the bead is
heated using
one of the coils, the analyzed gas will oxidize on the bead surface, changing
the electrical
conductivity as measured between the heated and unheated coils. Nearly all
oxidizable
gases can be detected on the SSS sensor. Selectivity can be improved slightly
by
changing the operating temperature and by doping the tin oxide with various
elements.
Combustible Gas Sensor -- The electrical resistance of most metals will
increase
with temperature. The combustible gas sensor consists of little more than a
coil of
platinum wire that is electrically heated. When gases combust on the surface,
some of the
heat of combustion is transferred to the wire coil. The increase in coil
temperature is
reflected as an increase in electrical resistance.
Flame Ionization Detector (F1D) -- The F1D works by burning the analyte gas in
a
hydrogen flame. In this environment, organic compounds produce positive ions,
which
are collected at a cylindrical electrode above the flame. A very small current
will be
generated between the collector and the metal flame jet. The FI17 is very
sensitive and
linear over many orders of magnitude. Because of the needs fox hydrogen and a
mechanically stable environment for the flame, the resulting instruments are
complex, but
14
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
some companies such as Foxboro have succeeded in making reliable FID
instruments.
FIDs are nearly nonselective among organic compounds, but they do limit their
responses
to organic compounds only.
Chemiluminescence -- Certain chemical reactions generate light, which can be
measured with great sensitivity. The most common application of
chemiluminescence in
gas detection is the measurement of nitric oxide by reaction with ozone;
measurements of
NO can be made down to the parts per trillion.
Physical P~ope~ty Sei2so~s
Physical property sensors generally leave the analyte gas undisturbed, and
measure some property such as absorption of light or thermal conductivity.
Nondispersive Infrared (NDIR) -- These are the simplest of the spectroscopic
sensors. The key components are an infrared source, a light tube, an
interference
(wavelength) filter, and an infrared detector. The gas is pumped or diffuses
into the light
tube, and the electronics measures the absorption of the characteristic
wavelength of
light. NDIR sensors are most often used for measuring carbon dioxide. The best
of these
have sensitivities of 25-50 PPM.
Spectroscopic Sensors -- These use conventional means to generate
monochromatic light in the ultraviolet or infrared and to measure its
absorption by a gas.
An ultraviolet spectrometer, for example, is the 'gold standard' method for
measuring
ozone. Specific organic compounds can sometimes be individually measured by
measuring absorption of infrared light at one or more wavelengths.
Photoacoustic Sensors -- If a short pulse of infrared light is passed through
an
absorbing gas, the absorbed light energy becomes heat. The sudden expansion of
the gas
generates a pressure, or acoustic, wave, which can be measured with a
microphone.
These are a variation of infrared spectroscopic sensors, with an important
twist: the PA
sensor measures the light absorbed by the sample. This is in contrast to
conventional
spectroscopy, which measures the light not absorbed. Since photometric error
is
eliminated, very sensitive detection is possible. These sensors only became
practical in
recent years, when digital signal processing (DSP) chips became available to
distinguish
signal from background noise.
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
Sorption Sensors
Many types of sensors depend on the physical or chemical sorption of the
analyte
into a coating on the sensing surface. Depending on the device, the sorption
phenomenon
may be detected by measurement of mass, refractive index, color change,
electrical
resistance, etc.
Fiber-Optic -- A thin glass or plastic fiber is coated with a thin layer of a
compound that will absorb the analyte. When light is passed through the fiber
and
reflects from its inside surface, some of the light energy extends beyond the
surface of the
fiber. This effect is known as the evanescent wave, and its influence is
usually no more
than a few nanometers. A simple surface coating may absorb organic gases,
changing its
refractive index. The amount of light reflected inside the fiber is changed;
this is detected
by a receiver at the other end of the fiber from the light source. Other
surface coatings
may react with the analyte gas and change color, which will affect the
spectrum of the
reflected light.
Microbalances -- The simplest form of this sensor uses a quartz crystal that
is
electronically made to vibrate at its natural frequency. The crystal is coated
with a
material that absorbs the analyte gas. The mass of the coating increases and
slows down
the natural rate of vibration of the crystal. The resulting frequency shifts
can be
measured electronically with great sensitivity. The basic microbalance has
been
elaborated into more sophisticated devices such as SAW (surface acoustic wave)
and
resonator devices, which are more sensitive than the simple bulk crystal. This
class of
sensors is sometimes referred to as gravimetric.
Conductive Polymer -- Certain polymers, such as polyanilines and
polythiophenes, are electrically conductive. The conductivity changes when the
polymers
absorb certain gases. The polymers can also be "tuned" to certain compounds by
carrying
out the polymerization in the presence of the analyte.
Elastomer Chemiresistor Sensors -- These measure the very slight physical
expansion of a film of an elastomeric material that occurs when it absorbs a
gas. The
elastomer, silicone rubber, for example, contains electically conductive
particles such as
carbon. The concentration of particles is adjusted so that there are
relatively few
16
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
conducting paths through the elastomer. Slight expansion of the elastomer
causes some
of these paths to be broken, and the electrical resistance rapidly increases.
Reactive-Gate Semiconductor Devices -- Most active semiconductor devices,
such as MOSFET transistors, use voltages directly applied to the gate to
control the flow
of charge carriers. Chemically sensitive devices, however, use a chemical
interaction to
change the transconductance.
System
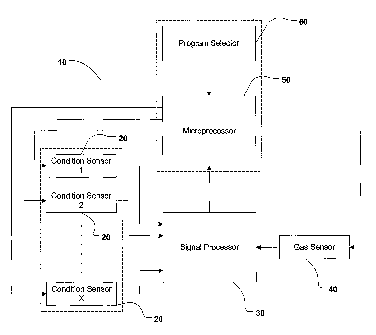
Figure 1 is a box diagram of a drying time determining system 10 in accordance
with one of the embodiments of the present invention. System 10 can include
one or
more condition sensors 20, a signal processor 30, a gas sensor (or solvent
vapor detector)
40, a fuzzy logic control system 50, and a program selector 60. Items in
dotted lines are
optional.
In one aspect of the present invention, the drying time determining system 10
comprises a gas sensor 40 and a signal processor 30. In operation, the gas
sensor 40
measures a concentration of lipophilic fluid vapor within a drying apparatus.
Once the
gas sensor 40 measures a "safe" lipophilic fluid vapor concentration, the gas
sensor 40
sends a signal to the signal processor 30 which causes the drying apparatus to
cease
operating and subsequently permits a user of the drying apparatus to open the
drying
apparatus and gain access to the internal chamber of the drying apparahts.
In another aspect of the present invention, the system 60 comprises a gas
sensor
40, a signal processor 30 and one or more condition sensors 20 and optionally,
but
preferably, a fuzzy logic control system 50 and a program selector 60.
The condition sensor 20 could be a humidity sensor, a mass load sensor, a
temperature sensor, a timer, etcetera. The condition sensor 20 is electrically
coupled and
can transmit a signal to a signal processor 30. Signal processor 30 is adapted
to trigger
the gas sensor 40 once a predetermined set point for the condition sensor 20
is reached.
The gas sensor 40 then starts tracking the solvent vapor concentration and
transmits its
readings back to the signal processor 30. The signal processor 30 can also be
coupled to
fuzzy logic control system 50. Fuzzy logic control system 50 utilizes the
signal coming
from the signal processor 30 and the signal coming from the program selector
60 to
17
CA 02408659 2005-11-09
estimate the remaining drying time for a particular load, of clothes. The
program selector
60 can be activated by the user and may reflect parameters such as the type of
garments
(e.g. silk, cotton, wool, etcetera) to be cleaned. These signals can then be
incorporated into
a programmed or programmable algorithm of the fuzzy logic control system 50 to
S determine remaining drying time.
Figure 2 illustrates the annotations used in the procedure algorithms of the
remaining Figures. The shape identified by reference numeral 1 A represents a
predefined
process step (a microprocessor with program selector). The shape identified by
reference
numeral 1 B represents a sensor output step (current or voltage produced in
response to a
detected condition). The shape identified by reference numeral 1 C represents
a decision
making step (signal processor). In the figures and description that follows
the
abbreviations are defined as noted:
S - gas sensor output signal,
CX - condition sensor output signal,
F - flash point value related to a combination of S and CX,
TX - threshold value for condition sensors, and
V - threshold value for gas sensor.
Figure 3 schematically illustrates a gas sensor signal handling procedure 70
for use
with the drying time determining system of the present invention. The
procedure 70 is
executed once a drying cycle of the drying apparatus begins (step 80),
preferably by input
from a user and through a microprocessor (fuzzy logic controller). When the
drying cycle
begins, a gas sensor 90 is activated (step 95), preferably by a
microprocessor. The gas
sensor 90 generates a signal S that represents vapor concentration present in
the drying
apparatus (step 90). A signal processor 100 monitors the gas sensor signal S.
The signal
processor 100 compares the value of S to a preset constant threshold value V
that
represents a vapor concentration equal or below the safe exposure limit (step
100). As long
as the vapor concentration in the drying apparatus is greater than the safe
exposure limit,
18
CA 02408659 2005-11-09
the drying cycle continues (step 110). When the relation of S and V indicates
that the vapor
concentration in the drying apparatus is equal or below the safe exposure
limit, the signal
processor 100 sends a trigger signal to the microprocessor to end drying cycle
(step 120).
Figure 4 schematically illustrates an alternative embodiment of a condition
sensor
signal handling procedure 130 for use with the drying time determining system
of the
present invention. The procedure 130 handles condition sensor signals produced
by one or
more condition sensors 140 and then executes previously described gas sensor
signal
handling procedure 70, as illustrated on Figure 3. The purpose of the
procedure 130 is to
prevent the gas sensor fouling due to excessive concentration of vapor. The
procedure 130
is executed once a drying cycle begins, preferably by input from a user and
through a
microprocessor (fuzzy logic controller) (step 80). When the drying cycle
begins, one or
more condition sensors 140 named Al, A2. . .AX are activated (step 145),
preferably by a
microprocessor (step 120). The condition sensors 140 generate a set of signals
Cl,
C2. . . CX that represent a set of physical conditions such as time,
temperature, pressure
18a
CA 02408659 2005-11-09
etc. A signal processor 150 monitors the condition sensors signals and
compares the
values of C1, C2...CX to corresponding preset constant threshold values Tl,
TZ...TX
that represent the set of predetermined physical conditions indicating the
safe operation
of the gas sensor 90 in Figure 3. As long as the relation of the predetermined
combinations of C1 and T1, C2 and T2,....CX and TX is not met, the drying
cycle
continues (step 160) . When the above set of relations is achieved, the signal
processor 150
sends a trigger signal activating the gas sensor signal handling procedure 70
of Figure 3,
preferably via a microprocessor.
Figure 5 schematically illustrates yet another embodiment of a combined gas
sensor signal handling procedure 170 for use with the drying time determining
system of
the present invention. The procedure 170 is designed to: (1) determine the end
of drying
(procedure 70); (2) optionally, prevent gas sensor fouling (procedure 130);
and (3)
optionally, prevent reaching a flash point condition whenever a
combustible/flamrnable
lipophilic fluid is used (procedure 180). The procedure 70 is executed, as
described in
detail in Figure 4, once a drying cycle of the drying apparatus begins (step
80), preferably
by input from a user and preferably through a microprocessor (fuzzy Logic
controller).
After the start of the drying cycle (step 80), the procedure 130 is executed
to determine
the physical conditions that will not cause gas sensor fouling. Once the
appropriate
physical conditions are met, the gas sensor signal handling procedure 70, as
described in
detail in Figure 3, is activated. The procedure 70 is similar to the procedure
70 in Figure
3 except that the step 110 is preferably replaced with the flash point
procedure 180. The
procedure 180 is executed as long as the step 100 indicates the vapor
concentration is
above the safe exposure limit. After the signal processor 100 acquires the gas
sensor
signal S, compares it to the threshold value V, and determines that the vapor
concentration in the drying apparatus is above the safe exposure Limit, then
the flash point
procedure 180 is executed. In the flash point procedure 180, the signal
processor 190
forms a set of sensor signals that includes the gas sensor output S, and one
or more
condition sensors signals C1, C2 ...CX. The condition sensors signals are
selected to
provide physical conditions that are suited to determine the flash point of
the vapor.
In the preferred embodiment, the condition sensor is a temperature sensor.
Since
the flash point of the vapor is characterized by the amount of vapor and the
temperature
19
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
of the vapor, the combination of the gas sensor and the temperature sensor
signals can
indicate when the vapor on the drying apparatus is close to reaching the flash
point.
The signal processor 190 compares the set of the gas senor and the condition
sensors to
the corresponding set of threshold values V, T1, T2 ...TX. The threshold
values form a
"code value" representing the conditions that are of indication that the
drying apparatus is
approaching the flash point. If the "code value" is not met, the drying cycle
continues
(step 200). Once the "code value" is reached, the signal processor 190 sends a
trigger
signal to the microprocessor to start a cooling cycle (step 210), since
reduction of
temperature would move the vapor condition from the flash point. The
microprocessor
may have a program to perform cooling for some period of time.
One of the systems of the present invention will be capable of automatically
ending a drying cycle in a drying apparatus. It will include a lipophilic
fluid vapor, and a
gas sensor. The gas sensor should be capable of sensing the concentration of
lipophilic
fluid vapor present in the drying apparatus and transmitting a signal
representative of the
lipophilic fluid vapor concentration.
The signal transmitted from the gas sensor will trigger the drying cycle to
end.
This could occur at a lipophilic fluid vapor concentration of less than about
40 ppm, more
preferably at less than about 20 ppm, even more preferably at less than about
15 ppm, and
most preferably at less than about 10 ppm. However, one of the objects of the
present
invention is to automatically end a drying cycle once a "safe" level, if
established for the
particular lipophilic fluid, is reached. The "safe" level for any lipophilic
fluid will be the
lowest established exposure level for any type of human exposure. For
instance, the
established vapor inhalation "safe" limit is 10 ppm for
decamethylcyclopentasiloxane, a
preferred lipophilic fluid. Further, if decamethylcyclopentasiloxane is the
lipophilic fluid
utilized, vapor concentrations should be at or below 10 ppm before humans
should come
in contact with the fabrics. Therefore, a gas or vapor sensor used in a drying
apparatus
should trigger the drying cycle to end when the decamethylcyclopentasiloxane
vapor
concentration is at or below 10 ppm.
There are many gas sensors that would be suitable for use with the present
invention. The gas sensors can be, but are not limited to, reactive sensors,
physical
property sensors, sorption sensors, and combination sensors utilizing at least
two of the
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
above sensing mechanisms. More specifically, the gas sensors can be, but are
not limited
to, electrochemical sensors, solid state semiconductor sensors, combustible
gas sensors,
flame ionization detectors, chemiluminescence sensors, nondispersive infrared
sensors,
spectroscopic sensors, photoacoustic sensors, fiber-optic sensors,
microbalance sensors,
conductive polymer sensors, elastomer chemiresistor sensors, reactive-gate
semiconductor sensors, and combination sensors utilizing at least two of the
above sensor
types.
A preferred lipophilic fluid that generates the lipophilic fluid vapor to be
sensed
by the gas sensor is a linear siloxane, a cyclic siloxane, or a mixture of the
two. More
specifically, the siloxane can be octamethylcyclotetrasiloxane,
decamethylcyclopentasiloxane, dodecamethylcyclohexasiloxane, or a mixture of
at least
two. Preferably, decamethylcyclopentasiloxane is the primary lipophilic fluid;
and more
preferably, the lipophilic fluid is substantially free of
octamethylcyclotetrasiloxane.
The second system of the present invention will also be capable of
automatically
ending a drying cycle in a drying apparatus. This system will include a
lipophilic fluid
vapor to be sensed, a condition detector, and a gas sensor. The condition
detector will be
capable of activating the gas sensor and the gas sensor will, in turn,
generate a signal
representative of the lipophilic fluid vapor concentration in the dryer.
Once a predetermined lipophilic vapor concentration is reached and a signal
representative of that vapor concentration is transmitted, the drying cycle
will
automatically end. The signal transmitted from the gas sensor will trigger the
drying
cycle to end. This could occur at a lipophilic fluid vapor concentration of
less than about
40 ppm, more preferably at less than about 20 ppm, even more preferably at
less than
about 15 ppm, and most preferably at less than about 10 ppm. However, one of
the
objects of the present invention is to automatically end a drying cycle once a
"safe" level,
if established for the particular lipophilic fluid, is reached. As discussed
above, the
"safe" level for any lipophilic fluid will be the lowest established exposure
level for any
type of human exposure.
Fouling can be a problem for gas sensors, particularly when siloxane or
silicone-
based lipophilic fluids are utilized. Siloxanes have a particular tendency to
leave a sticky
or greasy residue on surfaces. This can occur as a result of lipophilic vapor
condensing
21
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
on a surface, or as a result of lipophilic fluid or vapor coming in contact
with a hot
surface. Many gas sensors have surfaces that operate at high temperatures and
are
therefore prone to fouling, particularly in a lipophilic fluid or vapor
environment.
As such, the second system adds a condition detector to minimize or eliminate
the
fouling of the gas sensors. It does this by minimizing the total time a gas
sensor is
exposed to the lipophilic fluid vapor. A physical condition other than
lipophilic fluid
vapor is measured until the lipophilic fluid vapor concentration is lower. Non-
limiting
examples of physical conditions that can be measured include time, fabric load
mass,
temperature, lipophilic fluid flow from said drying apparatus, drying
apparatus drum
torque, inlet drying air temperature, outlet drying air temperature, humidity,
and
combinations of physical conditions.
For example, when a drying cycle is begun, a sensor that detects condensed
lipophilic fluid flow from the dryer is automatically turned-on. This physical
condition
sensor measures condensed lipophilic fluid flow until it is reduced to a
predetermined
flow rate. Once the lower condensed lipophilic fluid flow rate is detected,
the gas sensor
is automatically turned-on. Because the gas sensor is "saved" until lipophilic
fluid vapor
concentrations are lower, the amount of fouling that occurs on the sensor is
minimized or
eliminated.
As in the first system, many gas sensors would be suitable for use with the
present
invention. They include, but are not limited to, reactive sensors, physical
property
sensors, sorption sensors, and combination sensors utilizing at least two of
the above
sensing mechanisms. More specifically, the gas sensors can be, but are not
limited to,
electrochemical sensors, solid state semiconductor sensors, combustible gas
sensors,
flame ionization detectors, chemiluminescence sensors, nondispersive infrared
sensors,
spectroscopic sensors, photoacoustic sensors, fiber-optic sensors,
microbalance sensors,
conductive polymer sensors, elastomer chemiresistor sensors, reactive-gate
semiconductor sensors, and combination sensors utilizing at least two of the
above sensor
types.
Also as in the first system, the preferred lipophilic fluid to be detected is
a linear
siloxane, a cyclic siloxane, or a mixture of the two. More specifically, the
siloxane can
be octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane,
22
CA 02408659 2005-11-09
dodecamethylcyclohexasiloxane, or a mixture of at least two. Preferably,
decamethylcyclopentasiloxane is the primary lipophilic fluid; and more
preferably, the
lipophilic fluid is substantially free of octamethylcyclotetrasiloxane.
It will be understood that the systems of the present invention may be
combined
with other fabric treatments. For example, prior to the application of the
lipophilic fluid,
the fabric articles may be subjected to a particulate removal method. The
systems of the present
invention can then be used to help dry the clothes automatically.
The systems of the present invention may be used in a service, such as a dry
cleaning service, diaper service, uniform cleaning service, or commercial
business, such
as a Laundromat, dry cleaner, linen service which is part of a hotel,
restaurant, convention
center, airport, cruise ship, port facility, casino, or may be used in the
home.
The systems of the present invention may be performed in an apparatus that is
a
modified existing apparatus and is retrofitted in such a manner as to conduct
the process
of the present invention in addition to related processes.
The systems of the present invention may also be performed in an apparatus,
which is not a modified existing apparatus but is one specifically built in
such a manner
so as to conduct the present invention or may be added to another apparatus as
part of a
lipophilic fluid processing system. This would include all the associated
plumbing, such
as connection to a chemical and water supply, and sewerage for waste wash
fluids.
The systems of the present invention may be used in an apparatus, which is not
a
modified existing apparatus but is one specifically built in such a manner so
as to conduct
the present invention and related processes.
The systems of the present invention may also be performed in an apparatus
capable of "dual mode" functions. A "dual mode" apparatus is one capable of
both
washing and drying fabrics within the same drum. These apparati are
commercially
available, particularly in Europe.
An apparatus used to carry out the present invention will typically contain
some
type of control system. These include electrical systems, such as, the so-
called smart
control systems, as well as more traditional electro-mechanical systems. The
control
23
CA 02408659 2002-11-12
WO 01/94686 PCT/USO1/18265
systems would enable the user to select the size of the fabric load to be
cleaned, the type
of soiling, the extent of the soiling, the time for the cleaning cycle.
Alternatively, the
user could use pre-set cleaning and/or refreshing cycles, or the apparatus
could control
the length of the cycle, based on any number of ascertainable parameters. This
would be
especially true for electrical control systems. For example, when the
collection rate of
lipophilic fluid reaches a steady rate the apparatus could turn its self off
after a fixed
period of time, or initiate another process for the lipophilic fluid.
In the case of electrical control systems, one option is to make the control
device a
so-called "smart device". This could mean including, but not limited to, self
diagnostic
system, load type and cycle selection, linking the machine to the Internet and
allowing for
the consumer to start the apparatus remotely, be informed when the apparatus
has cleaned
a fabric article, or for the supplier to remotely diagnose problems if the
apparatus should
break down. Furthermore, if the system of the present invention is only a part
of a
cleaning system, the so called "smart system" could be communicating with the
other
cleaning devices which would be used to complete the remainder of the cleaning
process,
such as a washing machine, and a dryer.
24