Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02411786 2007-01-11
1
TITLE OF INVENTION
A process for the preparation of polypeptides from N-carboxyanhydrides of
amino acids.
FIELD OF INVENTION
The present invention refers to a new process for the synthesis of polypeptide
1 and novel
intermediates useful in the synthesis thereof.
BACKGROUND OF THE INVENTION
The present invention refers to a new process for the synthesis of polypeptide
1,
comprising the following amino acid units in the structure, namely: L-alanine,
L-glutamic acid,
L-lysine and L-tyrosine randomly arranged in the polypeptide 1; of which
Glatiramer Acetate is
a representative example.
Glatiramer Acetate is a synthetic polypeptide analog of myelin basic protein
(MBP),
which is a natural component of the myelin sheath. It is also defined in the
Physicians' Desk
Reference, 56"' Edition 2002 as consisting of acetate salts of synthetic
polypeptides, containing
four naturally occurring amino acids: namely, L-glutamic acid, L-alanine, L-
tyrosine and Lr
lysine with an average molar fraction of 0.141, 0.427, 0.095 and 0.338
respectively. The average
molecular weight is 4,700-11,000 daltons.
Interest in Glatiramer Acetate as an imxnunotherapy agent for multiple
sclerosis stems
from the 1950's observations that myelin components such as MBP prevent or
arrest
experimental allergic encephalomyelitis, a disease resembling multiple
sclerosis (US 3,849,550).
Recently, it has been shown that Glatiramer Acetate is a novel, safe and
effective treatment for
patients with the exacerbating-remitting form of multiple sclerosis and it is
the active ingredient
of CopaxoneTM, a medicanment used for the treatment of multiple sclerosis.
The process for the preparation of Glatiramer Acetate has been described in US
patents
6,048,898; 5,800,808; 5,981,589 and 3,849,550. They all employ as starting
materials four N-
carboxyanhydrides derived from alanine, 7-benzyl glutamate, Nc-trifluoroacetyl
lysine and
tyrosine. These monomers of N-carboxyanhydrides were prepared as described in
the literature
by the phosgene method shown in Scheme 1.
CA 02411786 2002-11-13
2
Scheme 1
R
H R H
o C C12~- HM, 0
H2U~-~
COOH THF ~0
0
R= CH3, COOCH2C6H5, NHCOCF3, C6H40H
The process for the synthesis of Glatiramer Acetate is based on the
polymerization of N-
carboxyanhydrides of alanine 2, y-benzyl glutamate 3, NE-trifluoroacetyl
lysine 7 and tyrosine 5,
in anhydrous and cancer suspect solvent dioxane at room temperature for 24
hours using
diethylamine as initiator (Scheme 2).
Scheme 2
CO2CH2C6H5 NHCOCF3 C 6Ha0H
CH3 (CH02 H(CH2)4
H CH2
0 ~0 ~0 0
HN
HNHN` ~
~ "C 0
0 Z 0 3 Et2NH 0 7 0 5
dioxane
protected copolymer 8
The deblocking of 7-benzyl groups (first deprotection) is effected by stirring
the protected
copolymer 8 in hydrobromic acid/acetic acid at room temperature for 17 hours.
These conditions
also facilitate the cleavage of the polypeptide thereby furnishing the
intermediate 9.
The next step in the prior art literature process is the removal of NE-
trifluoroacetyl groups
(second deprotection) of intermediate 9 by treatment with 1 M piperidine. In
the final steps,
Glatiramer Acetate is obtained by purification of intermediate 10 through
dialysis, followed by
treatment with acetic acid to form the acetate salt and by another
purification by dialysis against
water (Scheme 3).
CA 02411786 2002-11-13
3
Thus, the prior art literature procedure involves the polymerization of four N-
carboxyanhydrides, two deprotection steps of intermediates 8 and 9 and two
purification steps
(Step 3 and 5 in Scheme 3) and one acetate salt formation step (Step 4 in
Scheme 3).
Scheme 3
NHCOCF3
1) HBr/acetic acid ~ 2) Piperidine
protected copolymer 8 poiymer intermediate 10
CO~i I 3) dialysis against
water to pH=8
intermediate 9 4) dialysis against
0.3% acetic acid to pH=6
5) dialysis against
water
Glatiramer Acetate
Glatiramer acetate with the required average molecular weight (4.7 to 11 kDa)
can be
obtained either by chromatography of intermediate 10 containing high molecular
weight species
and collecting the fractions without the undesired species or by partial acid
or enzymatic
hydrolysis to remove the high molecular weight species with subsequent
purification by dialysis
or ultrafiltration. Further methods to obtain Glatiramer Acetate having the
required average
molecular weight are based on the preparation of the desired species while the
amino acids are
still protected, followed by deprotection.
SUMMARY OF THE INVENTION
An object of the present invention is to provide a new and improved process
for the
synthesis of polypeptide 1. This process provides a three-step procedure for
the synthesis of
polypeptide 1 relative to the prior art.
More specifically, the present invention is directed to a new process for the
preparation of
a polypeptide designated in the present invention as 1 comprising the
following amino acid units
in the structure, namely: L-alanine, L-glutamic acid, L-lysine and L-tyrosine
randomly arranged
in the polypeptide 1, or a pharmaceutically acceptable salt thereof wherein
said process,
comprises the steps of:
CA 02411786 2002-11-13
4
(a) polymerization of a mixture of the N-carboxyanhydrides of L-alanine, L-
tyrosine, a
protected L-glutamate, and a protected L-lysine, to obtain protected copolymer
6 or a salt
thereof;
(b) deprotection of the protected copolymer 6 or a salt thereof to afford
polypeptide 1 or a
pharmaceutically acceptable salt thereof in one single step;
(c) separation and purification of the polypeptide 1 or a pharmaceutically
acceptable salt
thereof.
The advantages of the current process are the result of (i) the novel choice
of side chain
protection on the glutamic acid and lysine moieties and (ii) the utilization
of acetic acid as
solvent for the deprotection step thereby permitting the isolation of
polypeptide 1 as an acetate
salt directly from the reaction mixture without any additional procedures.
This results in
advantages such as simplicity and minimized number of steps, a cost-effective
process and an
optimization of the productivity by carrying out more than one synthetic
transformation in one
step.
The new and improved process, according to the present invention, is also
based on the
polymerization of four N-carboxyanhydrides to prepare a new protected
copolymer 6 but the
deprotection is achieved in one step due to the selection of the protecting
groups in one instance,
benzylic and carbobenzyloxy groups present on the glutamic acid and lysine
units of the
protected copolymer 6. The second step in the process is the deprotection of
protected
copolymer 6 said deprotection step is selected from the group consisting of
(i) catalytic
hydrogenation under hydrogen pressure and (ii) catalytic transfer
hydrogenation (CTH)
preferably in acetic acid. More preferably, the catalysts are selected from
the group consisting of
PdIC, Pd(OH)2, and the like. In a preferred embodiment, the polypeptide 1 (as
an acetate salt) is
isolated directly from the reaction mixture after a single dialysis step.
According to one aspect of the invention, there is provided a process for the
preparation
of a polypeptide designated in the present invention as polypeptide 1,
comprising the following
amino acid units in the structure, namely: L-aianine, L-glutamic acid, L-
lysine and L-tyrosine
CA 02411786 2002-11-13
randomly arranged in the polypeptide 1, or a pharmaceutically acceptable salt
thereof wherein
said process comprises the steps of:
(a) polymerization of a mixture of the N-carboxyanhydrides of L-alanine, L-
tyrosine,
protected L-glutamate, and protected L-lysine, to obtain protected copolymer 6
or a salt
5 thereof;
(b) deprotection of the protected copolymer 6 or a salt thereof to afford
polypeptide 1 or a
pharmaceutically acceptable salt thereof in substantially a single step;
(c) separation and purification of the polypeptide 1 or a pharmaceutically
acceptable salt
thereof,.preferably, said polymerization is carried out at a temperature
ranging between
about 0 to about $0 C, and preferably said process further comprises a
solvent. Said
solvent is preferably selected from the group consisting of DMF, DMSO, CH2Cl2,
dioxane or mixtures thereof.
In another aspect of the invention, said polymerization is carried out in the
presence of an
initiator, preferably said initiator comprises at least one of the following:
diethylamine,
triethylamine and diisopropylamine.
According to yet another embodiment of the invention, there is provided a
process of
manufacturing Glatiramer Acetate comprising a single step deprotection of a
protected
copolymer 6, said protected copolyrner 6 comprising a mixture of L-alanine, L-
tyrosine, a
protected L-glutamate and a protected L-lysine, protected by at least one
protecting group,
preferably said at least one protecting group is selected from a substituted
or unsubstituted y-
benzyl group or a substituted or unsubstituted N-benzyloxycarbonyl group or
an aryl group,
preferably said substituted y-benzyl group or NE-benzyloxycarbonyl group is
substituted with at
least one of the following: Br, Cl, NO2, OCH3.
Preferably, the deprotection step is selected from the group consisting of
(i) catalytic transfer hydrogenation; and
(ii) catalytic hydrogenation under hydrogen pressure.
CA 02411786 2002-11-13
6
In another aspect of the invention, said separation and purification of the
polypeptide 1 is
carried out in a single step, preferably said single step involves a single
dialysis against water.
Preferably, said deprotection step is carried out in acetic acid, and
preferably, said
deprotection step is carried out at a temperature in the range of about 50 to
about 80 C, and
preferably, said deprotection step is carried out in the presence of a
catalyst, preferably said
catalyst is selected from Pd/C and Pd(OH)2, and preferably, carried out at a
pressure in the range
of about 40 to about 100 psi.
Preferably, said catalytic transfer hydrogenation is carried out in the
presence of acetic
acid, and preferably, at a temperature in the range of about 50 to about 80 C,
and preferably
carried out under hydrogen pressure of about 40 to about 100 psi.
According to another aspect of the invention, said process further comprises
at least one
reagent selected from the group consisting of formic acid, sodium formate,
trialkyl ammonium
formates, hydrazine, 1,3-cyclohexadiene, 1,4-cyclohexadiene; cyclohexene, and
ammonium
formate or mixtures thereof.
According to another aspect of the invention, the process of manufacturing
Glatiramer
Acetate further comprises subsequent separation and purification of Glatiramer
Acetate,
preferably said separation and purification of the Glatiramer Acetate is
carried out in a single
step, preferably said single step involves a single dialysis against water.
According to yet another aspect of the invention, the polypeptide 1 has an
average
molecular weight between 4,700 and 11,000 Da.
According to yet another aspect of the invention, said Glatiramer Acetate has
an average
molecular weight between 4,700 and 11,000 Da.
According to yet another aspect of the invention, there is provided a
protected L-lysine is
a protected, substituted NE-Benzyloxycarbonyl L-lysine of formula 11:
CA 02411786 2002-11-13
7
NHC02CH2X
H
0
HW
0~
11
where X= C6H5, C6H4Br, C6H4CI, C6H4NO2
C6H4OCH3, aryl.
According to yet another aspect of the invention, there is provided a
protected,
substituted y-benzyl L-glutamate of formula 12:
C02CH2X
H(~~
-0
0~-
12
where X CA, C6H4Br, C6H4CI, C6H4NO2
C6H4OCH3, aryl.
According to yet another aspect of the invention, there is provided a
substituted y-benzyl
L-glutamate of formula 12:
CA 02411786 2002-11-13
8
C02CH2X
0
H~'
12
where X C6H5, C6H4Br, C6H4CI, C6H4NO2
C6H4OCH3, aryl.
According to yet another aspect of the invention, there is provided a
substituted NE-
Benzyloxycarbonyl L-lysine of formula 11:
NHCOaCH2X
0
HN
11
where X= C6H5, C6H4Br, C6H4CI, C6H4NO2
C6H40CH3, aryl.
According to yet another aspect of the invention, there is provided a
substituted y-benzyl
L-glutamate of formula 12:
CA 02411786 2002-11-13
9
C02CH2X
0
HM'
12
where X- C6H5, C6H4Br, C6H4CI, C6H4NO2
C6H4OCH3, aryl.
According to yet another aspect of the invention, there is provided a
substituted NE-
Benzyloxycarbonyl L-lysine of formula 11:
NHC02CH2X
H
0
HI4'
0
11
where X= C6H5, C6H4Br, C6H4CI, C6H4NO2
C6H4OCH3, aryl.
According to yet another aspect: of the invention, there is provided a
protected copolymer
6 comprising a mixture of amino acids selected from the group consisting of L-
alanine, L-
tyrosine, L-glutamate and L-lysine, wherein said L-glutamate and L-lysine are
protected by at
least one protecting group, preferably said at least one protecting group is
selected from a
substituted or unsubstituted y-benzyl group or a substituted or unsubstituted
N-
benzyloxycarbonyl group, or an aryl group, preferably said protected L-
glutamate is as depicted
below:
CA 02411786 2002-11-13
C02CHzX
0
HN
12
where X C6H5, C6H4Br, C6H4Cf, C6H4NO2
C6H4OCH3, aryl.
and protected L-lysine is as depicted below:
NHC02CH2X
i0
HN'
~
0
11
where X= C6H5, C6H4Br, C6H4CI, CAN02
C6H4OCH3, aryl.
Further and other objects of the invention will become apparent to a person
reading the
5 following.
DESCRIPTION OF THE INVENTION
In one embodiment of the present invention, polypeptide 1, comprising the
following
amino acid units in the structure, namely: L-alanine, L-glutamic acid, L-
lysine and L-tyrosine
randomly arranged in the polypeptide 1, is prepared by the polymerization of
the N-
10 carboxyanhydrides of L-alanine, tyrosirie, y-benzyl glutamate and NE-
benzyloxycarbonyl lysine,
CA 02411786 2002-11-13
11
in various solvents. The four N-carboxyanhydrides are prepared starting from
the corresponding
commercially available benzyloxycarbonyl (Cbz) amino acids by using literature
procedures.
In a preferred embodiment of the process according to the present invention,
benzyl and
benzyloxycarbonyl are preferably selected as a combination of protecting
groups on glutamic
acid and lysine, respectively, due to the facile cleavage of both by
hydrogenation under hydrogen
pressure or by catalytic transfer hydrogenation. This represents an elegant
and simple procedure
that can be executed without special equipment and resulting unexpectedly in a
high yield in one
instance a 70% yield, and facile performance thereof.
According to another preferred embodiment, the benzyl and benzyloxycarbonyl
groups
were substituted with at least one of the following: Br, Cl, NO2, OCH3, aryl.
In the present application, the term "room temperature" should be understood
to mean a
temperature ranging from about 20 Cto about 26 C.
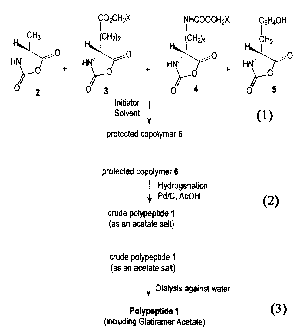
The polymerization reaction of the four N-carboxyanhydrides may preferably be
carried
out in a solvent selected from the group comprising DMF, DMSO, CH2C12, dioxane
or mixtures
of these solvents, in one instance DMSO/DMF, DMF/CH2Cl2, dioxane/DMSO at
temperatures
ranging from about 0 C to about 80 C. Preferably, the polymerization is
carried out in the
presence of an initiator which is selected from the group comprising:
diethylamine,
triethylamine and diisopropylamine (Scheme 4). In one instance, protected
copolymer 6 was
precipitated directly from the reaction mixture by addition of water.
CA 02411786 2002-11-13
12
Scheme 4
i C02CH2X i NHCOOCH2X IC6H4OH
CH3 (CH2)2 H (CH2)4 H CH2
0 0 0 0
Hw HN HW HM
+ + +
2 3 4 5
Initiator
Solvent
Protected Copolymer 6
where X = C6H5, C6H48r; C6H4CI, C6H4N02, C6H40CH3, aryl.
After polymerization, according to a preferred embodiment of the present
invention, the
deprotection step comprising the single-step removal of the y-benzyl and NE-
benzyloxycarbonyl
protecting groups present on protected copolymer 6 is carried out either by
catalytic
hydrogenation under high pressure (about 40 to about 100 psi) preferably at
temperatures of
about 50 to about 80' C and more preferably in the presence of acetic acid, or
by catalytic
transfer hydrogenation (CTH), preferably in acetic acid and more preferably
the catalysts are
selected from the group consisting of Pd/C,. Pd(OH)2, and the like, and also
preferably at
temperatures ranging from about 50 C to about 80 C (Scheme 5).
Scheme 5
protected copolymer 6
Hydrogenation
Pd/C, AcOH
crude polypep6de I
(as an acetate salt)
Preferably, the catalytic transfer hydrogenation incorporates various
reagents, comprising
1,4-cyclohexadiene, cyclohexene, axnrnonium formate, formic acid, sodium
formate, hydrazine,
1,3-cyclohexadiene, and trialkylanunonium formates, or mixtures thereof.
Catalytic transfer
hydrogenation reagents such as these and others are well known in the prior
art, and a selection
can be made from these well-known reagents.
CA 02411786 2002-11-13
13
In one embodiment of the present invention, the resulting polypeptide 1 is
isolated
directly as an acetate salt after purification of the crude polypeptide 1 by a
single dialysis step
against water until the average molecular weight reaches the required value
(Scheme 6).
Scheme 6
¾nide polypeptide I
(as an acetate salt)
Dialysis against water
Polypeptide 1
(including Glatiramer Acetate)
In a preferred embodiment of the present invention, the average molecular
weight of the
polypeptide 1 is in the range of 4,700-11,000 Da. This value is representative
for polypeptide 1
as well as Glatiramer acetate. The average molecular weight of the polypeptide
was determined
by Gel Permeation Chromatography.
In another preferred embodiment of the present invention, the polypeptide 1 is
Glatiramer
acetate and its preparation is performed as previously described in the
present disclosure.
The following examples are purely illustrative of the invention and are not to
be
considered to limit the scope of the invention in any manner.
Example 1
Preparation of protected copolymer 6
Alanine N-carboxyanhydride (10.08 g, 87.6 mmol), y-Benzyl glutamate N-
carboxyanhydride (7.04 g, 26.7 mmol), NE-Benzyloxycarbonyl lysine N-
carboxyanhydride (19.2
g, 62.7 mmol) and tyrosine N-carboxyanhydride (3.68 g, 17.7 mmol) were
dissolved in
dimethylformamide (160 mL) and treated with 0.9% wt. Et3N. The reaction
mixture was stirred
for 24 hours at room temperature and under nitrogen and then poured into water
(320 mL) and
stirred for 6 hours. The product (protected copolymer 6: 27.39 g, 68%) was
filtered, washed with
water and dried.
CA 02411786 2007-01-11
14
Example 2
Preparation of protected copolymer 6
Alanine N-carboxyanhydride (2.52 g, 2.19 rnmol), y-Benzyl glutamate N-
carboxyanhydride (1.76 g, 6.6 mmol), Ne-Benzyloxycarbonyl lysine N-
carboxyanhydride (4.8 g,
15.6 nvnol) and tyrosine N-carboxyanhydride (0.92 g, 4.4 mmol) were dissolved
in
dimethylforrnamide (35.8 mL) and dichloromethane (15.4 ml) and treated with
0.9% wt. Et2NH.
The reaction mixture was stirred for 24 hours at room temperature and under
nitrogen and then
the dichioromethane was evaporated. The suspension was then poured into water
(102 mL) and
stirred for 6 hours. The product (protected copolymer 6: 7.35 g, 74%) was
filtered, washed with
water and dried.
Example 3
Preparation of polypeptide 1 by catalytic transfer hydrogenation
Protected Copolymer 6(2.00 g) was dissolved in 40 mL of acetic acid by heating
at 80 C
under nitrogen_ To the yellow solution was added 0.6 g Pd/C (30% wt.) and
cyclohexene (5 mL)
and then the reaction mixture was stirred at 80 C under nitrogen for 4 hours.
The reaction was
filtered through Celite and the cake was washed with 4 mL of hot acetic acid.
After
evaporation of the filtrate with 32 mL toluene, a beige solid was obtained
(polypeptide 1, 1.4 g,
70%). The material obtained was dissolved in 28 ml of water, filtered through
2 g of Celite and
the clear solution was dialyzed against water in a dialysis bag for 24 h. Upon
completion of the
dialysis, the solution from the bag was evaporated to dryness by co-
evaporation with toluene to
yield polypeptide 1 as an off white solid.
Example 4
Preparation of polypeptide 1 by catalytic transfer hydrogenation
Protected copolymer 6 (5.00 g) was dissolved in 100 mL of acetic acid by
heating at 80 C
under nitrogen. To the yellow solution was added 1.5 g Pd/C (30% wt.) and 1,4-
cyclohexadiene
(7.4 mL) and then the reaction mixture was stirred at 80 C under nitrogen
for 48 hours. The
reaction was filtered through Celite and the cake washed with 20 mL of hot
acetic acid. After
evaporation of the filtrate with 32 mL toluene, a beige solid was obtained
(polypeptide 1, 2.8 g,
CA 02411786 2007-01-11
56%). The material obtained was dissolved in 28 ml of water, filtered through
2 g of Celite and
the clear solution was dialyzed against water in a dialysis bag for 24 h. Upon
completion of the
dialysis, the solution from the bag was evaporated to dryness by co-
evaporation with toluene to
yield polypeptide 1 as an off white solid.
5 Example 5
Preparation of polypeptide 1 by catalytic transfer hydrogenation
Protected copolymer 6 (1.00 g) was dissolved in 20 mL of acetic acid by
heating at 80 C
under nitrogen. To the yellow solution was added 0.3 g Pd/C (30% wt.) and 1,4-
cyclohexadiene
(2.5 mL) and then the reaction mixture was stirred at 60 C under nitrogen for
4 hours. The
10 reaction was filtered through Celite and the cake washed with 10 mL of hot
acetic acid. After
evaporation of the filtrate with 20 mL toluene, a beige solid was obtained
(polypeptide 1, 0.54 g,
54%).
The material obtained by catalytic transfer hydrogenation may be purified by
dialysis as
previously described in Example 3.
15 Example 6
Preparation of polypeptide 1 by catalytic transfer hydrogenation
Protected copolymer 6 (0.5 g) was dissolved in 10 mL of acetic acid by heating
at 80 C
under nitrogen. To the yellow solution was added 0.15 g PdJC (30% wt.) and
ammonium formate
(0.4 g) and then the reaction mixture was stirred at 70 C under nitrogen for
24 hours. The
reaction was filtered through Celite and the cake washed with 10 mL of hot
acetic acid. After
evaporation of the filtrate with 20 mL toluene, the polypeptide 1 was obtained
as a beige solid.
The material obtained by catalytic transfer hydrogenation may be purified by
dialysis as
previously described in Example 3.
Example 7
Preparation of polypeptide I by hydrogenation
Protected copolymer 6 (2.00 g) was dissolved in 40 mL acetic acid by heating
at 80 C
under nitrogen. The yellow solution was added 0.6 g Pd/C (30% wt.) and a
hydrogen pressure of
CA 02411786 2007-01-11
16
80 psi was applied to the reaction mixture. After 10 h of stirring at 80 C and
80 psi, the reaction
was filtered through CeliteV and the cake washed with 4 mL of hot acetic acid.
After co-
evaporation of the filtrate with 32 mL toluene, a beige solid was obtained
(polypeptide 1, 1.4 g,
70%).
The material obtained by high pressure hydrogenation may be purified by
dialysis as
previously described in Example 3.
Example 8
Preparation of polypeptide 1 by hydrogenation
Protected copolymer 6 (1.00 g) was dissolved in 20 mL acetic acid by heating
at 80 C
1o under nitrogen. To the yellow solution was added 0.3 g Pd/C (30% wt.) and a
hydrogen pressure
of 60 psi was applied to the reaction mixture. After 10 h of stirring at 80 C
and 60 psi, the
reaction was filtered through Celite and the cake washed with 10 mL of hot
acetic acid. After
co-evaporation of the filtrate with 20 mL toluene, a beige solid was obtained
(polypeptide 1, 0.6
g, 60%).
The material obtained by high pressure hydrogenation may be purified by
dialysis as
previously described in Example 3.