Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
IMPROVEMENT OF NOISE BEHAVIOR OF NON-ASBESTOS FRICTION
MATERIALS THROUGH USE OF FLUOROPOLYMERS
BACKGROUND OF THE INVENTION
1. Technical Field
The present invention relates generally to friction materials, and
more particularly, to friction materials containing at least one fluoropolymer
for
use in friction linings for applications such as, but not limited to brake
disk
pads.
2. Discussion
Friction materials, such as those typically employed in brake
linings, are usually comprised of either asbestos fibers, mixtures of asbestos
fibers and other heat resistant inorganic or organic fibers, asbestos-free
mixtures of heat resistant inorganic or organic fibers, or metal powders such
as iron powder, copper powder, steel powder or mixtures thereof, in
combination with an organic monomeric or polymeric binder system (e.g.,
phenolic or cresylic resin). Because asbestos has been alleged to be the
cause of certain health problems and is no longer environmentally acceptable,
most modern friction materials are made without asbestos. Thus, most current
friction materials are made from synthetic and steel fibers, and iron,
ceramic,
and metallic powders.
A typical friction material formulation may optionally contain one
or more of the following components: thermosetting resinous binders (e.g.,
phenolic resins such as phenol-formaldehyde resins, epoxies, and the like)
present in conventional amounts; reinforcing fibers (e.g., aramid, steel,
acrylic,
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
and the like) present in conventional amounts; metal powders (e.g., iron,
copper, brass, zinc, aluminum, antimony, and the like) present in conventional
amounts; solid lubricants (e.g., molybdenum disulfide, graphite, coke, stannic
sulfide, antimony trisulfide, and the like) present in conventional amounts;
abrasives (e.g., tin oxide, magnesia, silica, iron oxide, alumina, rutile, and
the
like) present in conventional amounts; organic fillers (e.g., rubber
particles,
cashew nut shell particles, nitrite rubber particles, and the like) present in
conventional amounts; and inorganic fillers (e.g., barytes, gypsum, mica, and
the like) present in conventional amounts. Other materials may be added as
well, as is known in the art.
In practice, friction materials are used in automotive brakes to
slow (i.e., decelerate) and stop vehicles, by substantially converting kinetic
energy into heat. In operation, the formed friction materials run against the
cast iron mating surfaces of brake rotors or drums, depending on the
configuration of the brake system. Friction materials are typically comprised
of thermoset composites containing several different materials, as previously
described. The important end-use performance aspects of friction materials
which are of interest to automotive manufacturers are the friction behavior,
wear life, and noise levels. Unsatisfactory noise and vibration behavior of
brake systems is an important source of customer complaints and
dissatisfaction. Customer dissatisfaction results in large warranty costs to
automotive manufacturers.
During use, the physiochemical changes at the interface between
the friction material and the cast iron rotors/drums of the brake system
govern
2
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
the performance behavior of the friction couple. When friction materials run
against the cast iron surfaces in a friction couple, transfer films (also
referred
to as a glaze) with complex chemical compositions are formed on the cast iron
and friction material surfaces. The transfer films generated during brake use
are thermal reaction products of the cast iron and the chemical ingredients
present in the friction material.
Transfer film formation is a dynamic process, i.e., the film is
continuously generated and destroyed during brake use. The chemical and
physical characteristics of the transfer film govern brake performance. The
formation of a thin and uniform transfer film is desirable in brake operation.
Conversely, uneven transfer films are thought to produce undesirable levels
of noise and/or vibration in brake systems, especially during braking
operations.
Therefore, there exists a need for a friction material that is
capable of continuously generating a thin and uniform transfer film, and which
can be universally incorporated in a variety of compositions for the
preparation
of friction materials, especially in asbestos-free, semi-metallic, and/or low-
metallic friction materials, and above all, offers low-noise and low-vibration
levels during braking operations.
3
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
SUMMARY OF THE INVENTION
In accordance with one embodiment of the present invention, a
friction material matrix comprises at least one fluoropolymer and at least one
binder system.
In accordance with another embodiment of the present invention,
a friction material matrix comprises at least one fluoropolymer present in an
amount up to about 2 weight percent based on the total weight of the friction
material matrix, and at least one binder system.
In accordance with still another embodiment of the present
invention, a friction material matrix comprises at least one fluoropolymer
present in an amount from about 0.1 to about 5 weight percent based on the
total weight of the friction material matrix, wherein the fluoropolymer is
selected from the group consisting of fluorinated ethylene propylene,
polyvinylidene fluoride, polytetrafluoroethylene, and combinations thereof,
and
at least one binder system.
A more complete appreciation of the present invention and its
scope can be obtained from the following brief description of the drawings,
detailed description of the invention, and the appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
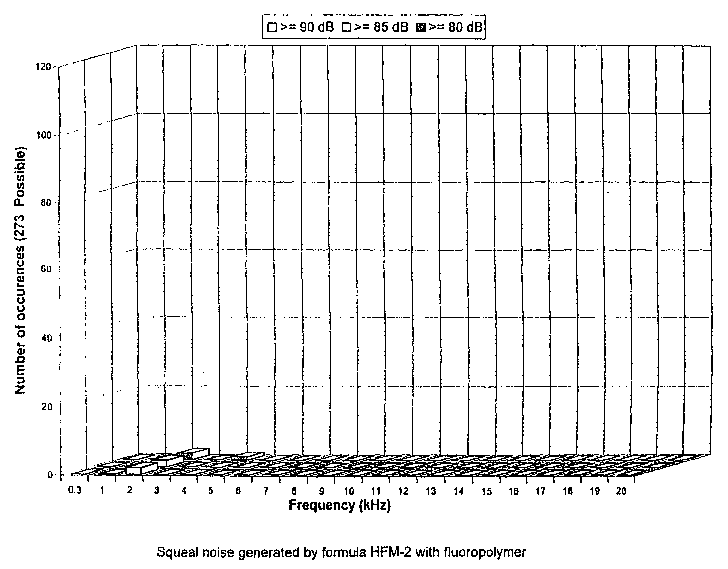
Figure 1 is a graphical illustration of the results of a dynamometer
test of the noise behavior characteristics of a pair of brake pads having a
brake lining formulation containing no fluoropolymer material; and
Figure 2 is a graphical illustration of the results of a dynamometer
4
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
test of the noise behavior characteristics of a pair of brake pads having a
brake lining formulation containing at least one fluoropolymer material, in
accordance with one embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The present invention proposes the use of fluoropolymers in
friction materials to reduce or prevent unacceptable levels of brake noise
during routine operation of the automobile's brake system.
As that term is used herein, "friction material matrix" means at
least one binder system (e.g., phenolic resin), and optionally, additives such
as, but not limited to, reinforcing fibers, metal powders, abrasives,
lubricants,
organic fillers, inorganic fillers, and the like.
As that term is used herein, "fluoropolymer" means at least one
polymeric material containing fluorine.
Fluoropolymers are composed basically of linear polymers in
which some of or all the hydrogen atoms are replaced with fluorine, and they
are characterized by relatively high crystallinity and molecular weight. As a
class, fluoropolymers rank among the best of the plastics in chemical
resistance and elevated-temperature performance. Their maximum service
temperature ranges up to about 500°F (260°C). They also have
excellent
frictional properties and cannot be wet by many liquids. Their dielectric
strength is high and is relatively insensitive to temperature and power
frequency. Mechanical properties, including tensile creep and fatigue
strength,
are only fair, although impact strength is relatively high.
5
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
There are three major classes of fluoropolymers. In order of
decreasing fluorine replacement of hydrogen, they are fluorocarbons,
chlorotrifluoroethylene, and fluorohydrocarbons.
There are two fluorocarbon types of note: polytetrafluoroethylene
(PTFE) and fluorinated ethylene propylene (FEP)
PTFE, a perfluorinated straight-chain high polymer having the
chemical formula (CF2CF2)n, is made by polymerizing the tetrafluoroethylene
(TFE) monomer. The white-to-translucent solid polymer has an extremely high
molecular weight, i.e., in the 106-10' range, and, consequently, has a
viscosity
of about 10 GPa~s (10" P) at 380°C. Its high thermal stability results
from the
strong carbon-fluorine bond and characterizes PTFE as a very useful high
temperature polymer. Its heat resistance, chemical inertness, electrical
insulation properties, and its low coefficient of friction in a very wide
temperature range make PTFE the most outstanding plastic in the industry.
-l5 PTFE is readily commercially available from Honeywell, Inc.
(under the tradename HALON), Daikin Kogyo (under the tradename
POLYFLON), DuPont (under the tradename TEFLON), Hoechst (under the
tradename HOSTAFLON), and ICI (under the tradename FLUON).
One particular grade of TEFLON that is particularly preferred in
the practice of the present invention is available under the tradename TEFLON
PTFE K-10 (Du Pont). TEFLON PTFE K-10 is a free-flowing white powder
having an average particle size of 560 micrometers, a bulk density of 570 g/L,
a standard specific gravity of 2.16, a melting range of 320-340 C (608-644 F),
is insoluble in all common solvents, and is stable to all common reagents at
6
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
ordinary temperatures, and reacts with alkali metals and fluorine or reactive
agents yielding fluorine.
FEP is a semi-crystalline perl:luorinated polymer closely related
to PTFE being a copolymer of tetrafluoroethylene and hexafluoropropylene.
lts properties are similar, but generally a little inferior to, those of PTFE;
but,
FEP has the great practical advantage of being melt-processable, albeit at
greater expense.
Compared to PTFE, FEP has similarly excellent chemical
resistance and electrical properties (up to very high frequencies) and good
weathering resistance. FEP has better radiation resistance and much higher
impact strength than PTFE, but has lower maximum use and heat deflection
temperatures and is even a little less stiff and strong.
FEP is readily commercially available from DuPont (under the
tradename TEFLON FEP) and Hoechst (under the tradename HOSTAFLON
FEP).
The filuorohydrocarbons are of two kinds: polyvinylidene fluoride
(PVF2, or PVDF) and polyvinyl fluoride (PVF). While similar to the other
fluoropolymers, fluorohydrocarbons have somewhat lower heat resistance and
considerably higher tensile and compressive strength.
PVDF is the polymer of 1,1-difluoroethjrlene. PVDF is a semi-
crystalline polymer containing 59.4% fluorine. The symmetrical arrangement
of the hydrogen and fluorine atoms in the chain contributes to the unique
polarity which influences the polymer's dielectric properties and solubility.
PVDF is readily commercially available from Pennwalt Corp.,
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
(under the tradename KYNAR).
Accordingly, fluoropolymers such as, but not limited to PTFE,
FEP, and PVDF can be used for the purposes of this invention.
Fluoropolymers, in either a powder or dispersion form, can be incorporated, in
varying amounts, info the friction material during a wet or dry mixing
process.
In accordance with a preferred embodiment of the present
invention, the fluoropolymer material is present in the friction material
matrix
in an amount of from about 0.1 to about 5 weight percent, based on the total
weight of the friction material matrix. In accordance with a highly preferred
embodiment of the present invention, the fluoropolymer material is present in
the friction material matrix in an amount of up to about 2 weight percent,
based
on the total weight of the friction material matrix.
By way of a non-limiting example, a typical formulation of a
friction material matrix containing at least one fluoropolymer material, in
accordance with one embodiment of the present invention, is presented in
Example l, below:
EXAMPLE I
INGREDIENT VOLUME PERCENT
Aramid Fiber 6
Phenolic Resin 17
A umrna 1.5
Rubber Powder 7.5
-
opper Fiber 5
Graphite 7
Coke 4.3
Barytes 14
Calcium Hydroxide 4
Antimony Sulfide 3
ermrculite 7
s
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
Molybdenum Disulfide 3
Cashew Friction Dust 17
Carbon Fiber
Fluoropolymer 1.7
By way of a non-limiting example, a typical formulation of a
friction material matrix containing at least one fluoropolymer material, in
accordance with another embodiment of the present invention, is presented in
Example II, below:
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
EXAMPLE II
INGREDIENT VOLUME PERCENT
Aramid Fiber 3
Steel Fiber 33
iron Powder
Graphite 29
Coke
Phenolic Resin 1$
Magnesium xide
Fluoropolymer
The friction materials containing at least one fluorapolymer
material of the present invention reduce the tendency for brakes to generate
noise (as well as vibration) by ensuring, for example, that a thin and uniform
transfer film is continuously being generated at the friction couple between
the
brake pad and the rotor or drum.
In order to evaluate the noise behavior characteristics of brake
pads having a friction lining formulation containing at least one
fluoropolymer
material at levels in accordance with the present invention, a comparison test
was performed. A first pair of brake pads having a friction lining formulation
containing no fluoropolymer material (designated HFM-1 ) was subjected to a
dynamometer test in order to evaluate the total noise produced at various
frequencies (see Figure 1 ). A second pair of brake pads having a friction
lining formulation containing at least one fluoropoiymer material present at
levels in accordance with the present invention, i.e., from about 0.1 to about
5 weight percent, was also subjected to a dynamometer test in order to
evaluate the total noise produced at various frequencies (see Figure 2).
As can be determined from comparing Figure 1 with Figure 2, the
to
CA 02413249 2002-12-16
WO 01/98682 PCT/USO1/19398
tots( noise of the HFM-1 formulation, especially at 5000 Hz, was significantly
higher than the HFM-2 formulation, thus indicating that brake pads containing
at least one fluoropolymer material in accordance with the present invention
will produce less noise, especially less high frequency noise (i.e., squeal).
Aiso noteworthy was the fact that the HFM-2 formulation did not produce any
noise whatsoever above the 4000 Hz frequency.
The foregoing description is considered illustrative only of the
principles of the invention. Furthermore, because numerous modifications and
changes will readily occur to those skilled in the art, it is not desired to
limit the
invention to the exact construction and process shown as described above.
Accordingly, all suitable modifications and equivalents that may be resorted
to
that fall within the scope of the invention as defined by the claims that
follow.
Z1