Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02415388 2009-11-27
70577-117
Flexibly Mounted High-Temperature Fuel Cell
The present invention relates to a high-temperature fuel cell.
A fuel cell incorporates a cathode, an electrolyte, and an anode. An oxidizing
agent, e.g., air, is routed to the cathode, and fuel, e.g. hydrogen, is routed
to the anode.
There are various types of fuel cells; these include the SOFC described in DE
44 30 958 C1, and the PEM fuel cell that is described in DE 195 31 852 C1.
The SOFC is also referred to as a high-temperature fuel cell since its
operating temperature can reach 1000 C. Oxygen ions form on the cathode
of a high-temperature fuel cell in the presence of the oxidizing agent. The
oxygen ions diffuse through the electrolyte and recombine with the hydrogen
that originates from the fuel to form water on the anode side. Electrons are
liberated during this recombination, and electrical energy is generated
thereby.
1
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
As a rule, a plurality of fuel cells are connected together electrically and
mechanically by connecting elements-also referred to as inter-connectors-
in order to generate large amounts of electrical power. One example of a
connecting element is the bipolar plate. Fuel cells that the stacked one
above the other and electrically connected in series are formed using bipolar
plates. This arrangement is referred to as a fuel-cell stack. The fuel-cell
stacks comprise the interconnectors and the electrode-electrolyte units.
In addition to their electrical and mechanical characteristics,
interconnectors
incorporate gas-distribution structures. In the case of the bipolar plate,
these are realized in the form of bars with electrode contact that separate
the gas channels used to supply the electrodes from each other (DE 44 10
711 Cl). Gas-distribution structures ensure that the operating medium is
distributed evenly into the electrode spaces (the spaces in which the
electrodes are located).
The following problems can arise with fuel cells and fuel-cell stacks:
metal bipolar plates with a high aluminum content form A1203 covering
layers that can act as an undesirable electrical insulator;
2
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
- thermal stresses that are accompanied by relative movement of the
individual components generally occur during cyclical temperature
loads; these result from the various expansion behaviours or the
different coefficients of expansion associated with the materials that
are used during operation;
in the prior art, glass bonds that are of low elasticity are used to seal
the individual components of a fuel cell. Because of this, as a result of
thermal stresses, there is a risk that cracks will form and there will be
a loss of adhesion.
In this regard, in the prior art there is still no adequate compatibility
between the comparatively high coefficients of expansion of, for example,
the metallic bipolar plates and the electrode materials known at this time,
the coefficients of expansion of which are comparatively small. On the one
hand, thermal stresses can occur between electrodes and interconnectors,
and these can result in damage within the fuel cells. On the other hand, this
also applies to the glass bonds that are intended to ensure the integrity of
the fuel cells, and that are frequently used in fuel cells.
3
CA 02415388 2009-01-20
70577-117
For this reason, it is the objective of some embodiments to present a fuel
cell in which long-term stable mechanical-electrical contact with the cathodes
and anodes by the interconnectors is ensured. Problems that are based on
thermal stresses, e.g., inadequate sealing, are to be precluded.
This objective can be achieved by a high-temperature fuel cell. This
comprises an anode, an electrolyte, and a cathode, as well
as a cathode interconnector and an anode interconnector, there being at
least one elastic medium arranged between the anode and the anode
interconnector to absorb relative movements. Because of this, thermal
stresses on the anode side that result from the different expansion
behaviours of the individual components are balanced out.
It is, advantageous that the cathode interconnector has a projecting surface
that contacts the cathode. The effect of this projecting surface is
that the cathode interconnector has an encircling edge, the height of which is
smaller in cross section that it is on the cathode contact surface. The result
of this is that there is a certain freedom of movement for the electrodes on
the cathode side. The expansion behaviour of the materials, and in
4
CA 02415388 2009-01-20
70577-117
particular thermal stresses, can then be evened out better on the anode side
and the cathode side.
It is particularly advantageous that there be an additional elastic medium
between the cathode interconnector and the electrolyte, this being, in
particular, a corrugated, perforated plate. Because of this, the
electrodes and the electrolyte are freely suspended and particularly great
freedom of movement is achieved for the electrodes. This means that the
bending of the individual components that frequently occurs during operation
is prevented. The perforated plate does not have to be corrugated overall,
but can have a flat edge in order that it can be better stabilized by other
components of the fuel cell.
The cathode can be of a smaller area than the anode. Because of
this, on the surfaces that are not covered by the cathode the perforated
plate can contact the electrolyte through its troughs so as to form a gas
tight
contact. This means that the cathode is protected against
pressure and damage. Because of the gas-tight contact, it is possible to
dispense with the glass ceramics that are used in the prior art in order to
seal the gaps between the fuel cell and the interconnectors that separate the
CA 02415388 2009-01-20
70577-117
cathode space from the anode space. Because of their different expansion
behaviours, these joints are particularly critical, and entail the above-
discussed disadvantages with respect to the formation of cracks. The
elasticity of the perforated plate can be varied with respect to its
thickness,
the angle of slope of the corrugations, and the number of corrugations. The
profile of the corrugations is compressed between of the cathode
interconnector and the electrolyte after assembly of the high-temperature
fuel cell, so that ultimately pressure is exerted on the anode. This brings
about the desired sealing effect between the cathode space and the anode
space. The perforated plate can be of a high-temperature alloy, in particular
an iron-chromium-aluminum alloy, e.g., Aluchrom YHf (Material No.
1.4767), or a nickel based alloy, e.g., Nicrofer 6025 HT (Material No.
2.4633). What is important is that the material be highly creep resistant and
sufficiently elastic at high temperatures.
The cathode interconnector can be connected to the anode interconnector
through a frame, so as to be electrically isolated from it. The
frame fulfills the function of an insulating connecting element and a spacer
for the interconnectors. When the high-temperature fuel cell is pressed
together, the maximal force that can be exerted on the perforated plate on
6
CA 02415388 2009-01-20
70577-117
the cathode side and the additional elastic medium on the anode side is
limited by the frame.
The frame can be connected to the perforated plate. To this end,
the perforated plate should have a flat edge. The connection can be
established by way of a soldering or welding process. Since, because of the
projecting surface, the height of the cathode interconnector is smaller in
cross section at its edge than at the cathode contact surface, on the edge in
question, the cathode interconnector can contact the corrugated plate all
around its flat edge. This results in mechanical stabilization of the
perforated plate.
A glass ceramic layer can be arranged between the troughs of the perforated
plate and the electrolyte. This layer serves to increase the
tightness of the seal between the troughs and the electrolyte, and to create
sealed and gas-tight electrode chambers.
It is preferred that the frame be of an iron-based alloy that contains
aluminum. Such a frame can be annealed above 10000C while air
is being supplied to it. After this process, the surface of the frame has an
7
CA 02415388 2009-01-20
70577-117
electrically insulating aluminum oxide layer. The surface of the
frame can thus very simply be made electrically insulating. A medium for
insulating the frame can also be arranged between the frame and the anode
interconnector. A glass ceramic layer can be used as such a medium.
Since the frame and the interconnector exhibit very
similar expansion behaviour at high temperatures, this location for a seal
with a glass ceramic is less critical. However, it is also possible to use a
layer of mica as a medium for insulating the frame electrically.
This can be applied using a suitable paste technique. Because of its
stratified structure, such a mica layer is sufficiently elastic, and this
avoids
the formation of cracks and a loss of adhesion during operation. If such an
additional medium is used, one is not restricted to iron-based alloys that
contain aluminum when selecting the material for the frame.
An electrically insulating medium can also be arranged between the frame
and the anode. The electrically insulating medium is so arranged
that it ensures electrical insulation between the frame and the anode and
prevents short circuits. As a rule, the space between the frame and the
anode is small once the high-temperature fuel cell has been assembled; this
insulating medium may be necessary for this reason. The anode can have a
8
CA 02415388 2009-01-20
70577-117
glass-ceramic layer as the medium for electrical insulation. This
can be applied to the sides that face the frame. Glass ceramics can be
applied in a simple manner. However, insulation between the anode and
the frame can also be ensured by the electrolyte. To this end, the anode
must be coated with the electrolyte as far as the side that is opposite the
cathode. Electrical insulation is ensured because of the material
of the electrolyte that consists, for example, of yttrium-stabilized ZrO2.
According to an embodiment of the application, at least one elastic medium for
absorbing relative
movement can be arranged between the anode interconnector and the
anode. This can be a corrugated, elastic foil that incorporates openings. The
material can consist of a high-temperature alloy, in
particular an iron-chromium-aluminum alloy, e.g., Aluchrom YHf, or a
nickel-based alloy such as Nicrofer 6025 HT. It is important that the
material be highly creep resistant and sufficiently elastic at high
temperatures. In addition, the foil incorporates openings. These openings
can be punched out of the foil before it is shaped. The openings serve to
supply the anode with fuel. If, for example, the anode is formed as a bipolar
plate, the fuel flows out of gas channels, through the openings in the foil,
and onto the anode.
9
CA 02415388 2009-01-20
70577-117
In one further embodiment of the present invention, provision is made such
that the foil has nickel-aluminum alloys on both sides, at least in part. To
this end, the elastic foil is joined on both sides to foils that contain
99% nickel. Because of the high temperature resistance of the nickel, the
nickel-aluminum alloys ensure that the foil will possess good electrical
conductivity, which will remain stable over a long period, on its surface.
It is advantageous that the anode interconnector contain aluminum. This
results in additional possibilities for ensuring the electrical
conductivity between the anode interconnector and the elastic medium or
the anode. The anode interconnector can also contain nickel-aluminum
alloys, at least in part. To this end, at least one foil that contains
nickel must be connected to the contact surfaces of the anode interconnector
for the anode by the formation of alloy. This can either be the
shaped foil that has the nickel-aluminum alloys, the troughs of which are
connected to the anode interconnector by the formation of alloy,
or other nickel foils can be connected with the anode interconnector by the
formation of alloy. The anode interconnector thus contains nickel aluminum
alloys, at least in part, on the contact surfaces to the corrugated foil.
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
The nickel-aluminum alloys can, for example, be nickel aluminides (e.g.,
NiAI, NiAI2, Ni3AI). Generally speaking, when used as contact layers in high-
temperature fuel cells, such nickel-aluminum alloys offer the following
advantages:
Nickel-aluminum alloys act as diffusion barriers for alloy components of
the steels used for interconnectors and other components of the fuel
cell and avoid the formation of low-conductivity corrosion products
(e.g., aluminum oxide) on boundary surfaces, for example, between
the anode interconnector and the nickel coating of the foil.
Nickel-aluminum alloys are resistant to high temperatures (e.g., the
melting point of NiAI is 1638 C).
Nickel-aluminum alloys are electrically conductive to a sufficient
degree.
Low material and machining costs.
The formation of insulating aluminum oxide layers is avoided because of the
properties of the nickel-aluminum alloys. From this it follows that a
reduction of contact resistance or a high level of conductivity on the anode
11
CA 02415388 2009-01-20
70577-117
side is achieved by the nickel aluminum alloys, and this leads to contact,
particularly in the fuel-cell stack, that is stable over a long period.
It has been shown that hot-pressing methods at temperatures of up to
1150 C are particularly well-suited for producing nickel-aluminum alloys that
are stable for a long period. Welding methods carried out in an atmosphere
of protective gas as well as, to some extent, plasma spraying methods can
also be used.
A further possibility for producing nickel-aluminum alloys is to use a
galvanic
nickel plating process. The nickel plated surface is subsequently annealed in
a vacuum with the formation of nickel-aluminum alloys, preferably at
1150 C.
An elastic nickel grid can be arranged between the anode and the anode
ihterconnector. The nickel grid not only evens out relative
movements between the anode and the interconnector; it also offers the
.added advantage that it ensures electrical contact with the anode evenly
through the grid points of the grid itself and thus balances out the above-
described disadvantageous manufacturing tolerances. It is also possible to
12
CA 02415388 2009-01-20
70577-117
have both elastic media, which is to say the nickel grid and the corrugated
foil, arranged on the anode side.
Finally a cathode contact layer can be arranged between the cathode
interconnector and the cathode. This can lie flush on the
cathode. During the joining process, this layer can serve, on the one hand,
to balance out tolerances,.and on the other can serve as a diffusion barrier
for chromium that is vapourizing out of the cathode interconnector.
A fuel cell stack comprises at least two such high-temperature fuel cells.
This achieves higher power outputs.
The present invention will be described in greater detail below on the basis
of two embodiments shown in the drawings appended hereto. These
drawings show the following:
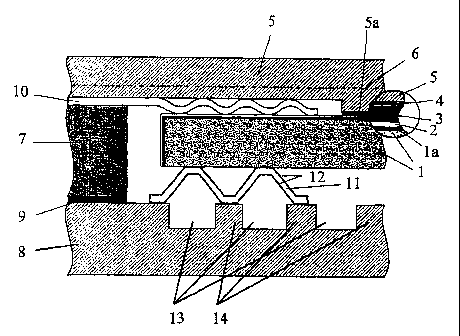
Figure 1: A cross-section through a high-temperature fuel cell with a
corrugated foil that. absorbs relative movements;
Figure 2: A cross-section through a high-temperature fuel cell with a nickel
grid to absorb relative movements.
13
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
On the right-hand side of both the drawings there is a circular cross-
sectional enlargement that is provided to show more clearly those
components that are located close together.
In Figure 1, the anode 1 consists of NiO and 8YSZ-stabilized Zr02, It is 1500
pm thick, so that the anode is configured as an anode substrate and
performs a supporting function. The anode function layer la is 5 pm thick
and is of the same material as the anode 1. The anode function layer la is
of a lower porosity than the anode 1 so as to ensure an even coating with
the electrolyte 2 of 8 YSZ. The anode 1 is completely coated with the
electrolyte 2 down as far as the lower base surface. This coating is at least
5
pm thick. The electrolyte 2 is of a sufficiently low electrical conductivity
to
isolate the anode 1 from its adjacent components in the fuel cell as far as
the anode interconnector 8. The area of the cathode 3 is smaller than the
anode 1, and is arranged on the electrolyte 2. In its standard form, this
consists of La0,66 Sr03 Mn03 and its layer thickness is 40 pm; it consists,
for
example, of LaCoO3 and it is 75 pm thick; it is shown only in the section
14
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
enlargement. Amongst other things, manufacturing tolerances that originate
during the production of bipolar plates or electrode-electrolyte units can be
evened out by the cathode contact layer 4, so that low-conductivity contact
points between the cathode 3 and the cathode interconnector 5 are avoided.
The cathode contact layer 4 is contacted through a projecting surface 5a of
the cathode interconnector 5. Because of the projecting surface 5a, the
cathode interconnector 5 has a circumferential edge, the height of which-in
cross section-is smaller than on the aforementioned projecting surface 5a.
A steel, Material No. 1.4742, can be used as the material for this. The
cathode interconnector 5 is in the form of a bipolar plate. The gas channels
are indicated by the dashed-dotted line 6. The flow of gas runs in the
horizontal plane, for example, from left to right. On the edge in question,
the cathode interconnector is connected to an anode interconnector through
a frame 7 so as to be electrically insulating. The frame 7 can be of an iron-
based alloy. It is connected through an electrically insulating layer 9 to the
anode interconnector 8. On the cathode side, the frame 7 is connected to a
perforated plate 10 which is flat on the edges and which possesses elastic
properties and which, for the remainder, is corrugated; the troughs of this
corrugated plate 10 contact the electrolyte 2 all round on the surfaces that
are not covered by the cathode 3, so as to be gas tight. The crests of the
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
perforated plate 10 contact the cathode interconnector 5 on its edge which,
as discussed above, is of a lesser height in cross section than the projecting
surface 5a, which contacts the cathode contact layer 4. The perforated plate
is of Nicrofer , and is 100 pm thick. However, the thickness can vary
between 50 and 300 pm. All in all, the perforated plate 10 has four troughs
to provide a gas-type contact, although more or fewer corrugations can be
used. The gas-tight electrode chambers are formed thereby. On the contact
surface with the anode interconnector 8, the frame 7 has an electrically
insulating medium 9, e.g., of glass ceramic or mica. The anode
interconnector 8 is also in the form of a bipolar plate, and incorporates gas
channels 13 that are separated from each other by the bars 14. The
operating media for the anode and cathode are thus supplied in a cross-flow
pattern. A parallel gas feed (direct or counter-flow pattern) can also be
used. A more homogenous temperature distribution across the fuel cell can
be anticipated if a parallel gas feed is used. The bars 14 contact the anode.
Between the anode interconnector 8 and the anode 1 there is an elastic
medium 11 that is in the form of a corrugated elastic foil that incorporates
openings. This absorbs relative movements between the anode and the
anode interconnector, and serves to balance out the expansion of the
individual components that is brought about by thermal stresses. The foil 11
16
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
can be of Aluchrom YHf or Nicrofer 6025HT, and can be, for example, 100
pm thick, although this can vary between 50 and 300 pm. The openings
serve to supply the anode 1 with fuel. The fuel flows out of the gas channels
13 in the anode interconnector 8, through the openings in the foil 11, to the
anode 1. The foil contains nickel-aluminum alloys 12 on both sides to reduce
contact resistance. The anode-side foil 11, the cathode-side perforated plate
10, and the projecting contact surface 5a of the cathode interconnector 5
thus ensure equalization of the v.a. thermal stresses that occur during
recycling.
Figure 2 differs from Figure 1 with respect to the electrical insulation of
the
frame 17, the material of the frame itself, as well as the elastic medium 21
that is arranged between the anode interconnector 8 and the anode 1, so as
to absorb relative movements.
In Figure 2, the frame 17 consists of an iron-based alloy that contains
aluminum, e.g., Aluchrom YHf. It is connected to the anode interconnector
8 and has an electrically insulating layer 19 of aluminum oxide on its upper
surface. The proportion of 5% aluminum in the frame is sufficient to form
this electrically insulating covering layer 19 of aluminum oxide by annealing
17
CA 02415388 2003-01-08
WO 02/05368 PCT/DE01/02459
at 1000 C during the addition of air. The insulating covering layer 19
completely covers the surface of the frame 17, and is for this reason shown
as an enclosing layer. In Figure 2, frame 17 is sufficiently high that
additional elastic medium for absorbing relative movements can be arranged
between of the anode interconnector 8 and the anode 1. In Figure 2, this is
an elastic nickel grid 21 that is 250 pm thick and has a mesh size of 200 pm.
The diameter of the wire is 125 pm. The fuel flows out of the gas channels
13 of the anode interconnector 8, through the mesh of the nickel grid 21, to
the anode 1.
The embodiments that are shown in Figure 1 and Figure 2 can be combined
with each other without restriction. Thus, in Figure 1, there can be a frame
17 that contains aluminum, as is described with respect to Figure 2. On the
other hand, in Figure 2, a frame 7 such as the one described in Figure 1 can
be used .
18