Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
BUTYL POLYMER COMPOSITION HAVING IMPROVED
COLD FLOW PROPERTIES
In one of its aspects, the present invention relates to a butyl polymer
composition having
improved cold flow properties. In another of its aspects, the present
invention relates to a process
for producing a butyl rubber vulcanizate having a desirable balance of
properties, including
improved air impermeability.
Butyl polymers have been known and commercially available for many years. They
possess
a variety of inherently satisfactory properties as elastomers which has
enabled them to find utility
in many commercial areas. Among their satisfactory inherent properties are
their impermeability
to air, high damping of low frequency vibrations and good resistance to aging,
heat, acids, bases,
ozone and other chemicals, after vulcanization. These properties render butyl
polymers well suited
for use in a variety of applications including articles requiring low or
reduced permeability to air.
Non-limiting examples of such applications include tire inner tubes, tire
curing bladders and various
air bladders.
Halogenated butyl polymers have also been known and commercially available for
many
years. In addition to possessing the satisfactory inherent properties of butyl
polymers described
above, halogenated butyl polymers also possess cure compatibility with more
highly unsaturated
rubbers and good adhesion to such other rubbers after vulcanization, which
renders them well suited
for use in pneumatic tire imzer liners.
Despite the advances made in the art to date, there is still room for
improvement.
One of the properties of butyl rubber which is commercially important is
resistance to cold
flow. Specifically, users of butyl rubber typically will calendar the polymer
into continuous very
long sheets. In some cases, the polymer may be premixed with one or more of a
filler (e.g., carbon
black), a plasticizer, a tackifier, an extender oil and the like - such a
composition may be
characterized as a vulcanizable composition.
Once the calendar sheets are produced, it is quite common to reversibly fold
or "wig-wag"
the sheets on a pallet such that the height of the folded sheet is a number of
feet. If the vulcanizable
composition is susceptible to cold flow during shipping or storage, the weight
of the folded polymer
sheet causes the surface coating of the sheet (particularly at the bottom of
the pile) to break, allowing
adjacent fresh surfaces to contact, which leads to sticking of the sheets.
This problem is exacerbated
during long transportation or storage times, and/or high ambient temperatures.
By the time the
-1-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
vulcaiuzable composition is ready for further processing it is more difficult
to remove as a single
sheet compared to the manner in which it was placed on the pallet.
While this can be alleviated, to some extent, by putting less polymer on a
pallet, this
increases the number of pallets required to transport or store a given amount
of vulcanizable
composition, thereby increasing costs.
United States Patent 4,754,793 (Mohammed) teaches butyl elastomer compositions
having
reduced permeability to gases. Specifically, there is taught a rubber
composition comprising a butyl
polymer, an a-methylstyrene homopolymer, carbon black in a curing system, and
optionally, a
hydrocarbon extender oil. Mohammed fails to teach or suggest any effect on
cold flow properties
of the butyl polymer conferred by the addition of the a-methylstyrene
homopolymer.
Further, to the knowledge of the inventors, a-methylstyrene homopolymer is not
available
in quantities commercially significant to be used as an additive in butyl
polymer compositions. The
principal reason may be attributed to a toughening of enviromnent laws which
had the effect of
making it impractical to produce large quantities of the material fox use as
an additive as
contemplated by Mohammed. Accordingly, while the teachings of Mohammed are
useful, the
commercial significance thereof is restricted somewhat by the unavailability
of significant
commercial quantities of a-methylstyrene homopolymer.
It would be desirable to have a butyl polymer composition having improved cold
flow
properties and a desirable balance of other properties, such as air
impermeability, green strength and
processability.
It is the obj ect of the present invention to provide a novel butyl polymer
composition having
improved cold flow properties.
It is another obj ection of the present invention to provide a novel process
for producing a
butyl polyner vulcanizate.
Accordingly, in one of its aspects, the present invention provides a butyl
polymer
composition having improved cold flow properties, the composition comprising a
butyl polymer and
a styrene resin, the styrene resin comprising a first copolymer of a-
methylstyrene and another
styrenic monomer.
Thus, it has been discovered that the use of a styrene resin which is a
copolymer of a-
rnethylstyrene and another styrenic monomer has advantageous results when used
as an additive in
a butyl polymer composition. Specifically, it has been unexpectedly and
surprisingly discovered
that the use of such an additive results in the following improvements to the
butyl rubber
composition and/or a vulcanizate produced therefrom:
-2-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
~ increase in green strength of butyl polymer;
~ reduction in cold flow of the butyl polymer composition;
~ decrease in the tendency of the butyl polymer composition to adhere to
metal; and
~ desirable mechanical properties in the vulcanizate produced from the butyl
polymer
composition.
These advantages are seen without significantly compromising other important
properties of the
butyl polymer composition and vulcanizates produced therefrom.
Embodiments of the present invention will be described with reference to the
accompanying
drawings in which:
Figures 1-14 illustrate various physical properties of polymer compositions
and of
vulcanizates derived therefrom produced in the Examples set out below.
As mentioned hereinabove, an aspect of the present invention relates to a
butyl polymer
composition.
One component of the present butyl polymer composition is a butyl polymer.
The terms "butyl polymer", "butyl rubber" and "butyl rubber polymer" are used
interchangeably throughout this specification and are intended to mean a
polymer prepared by
reacting a major portion of an isoolefin monomer with a minor portion of a
multiolefin monomer.
Preferably, the butyl polymer comprises a copolymer of a C4 to C8 monoolefin
monomer and
a C4 to C14 multiolefin monomer.
The preferred C4 to C8 monoolefin comprises an isomonoolefm. Non-limiting
examples of
useful monoolefin monomers may be selected from the group comprising
isobutylene, 2-methyl-1-
butene, 3-methyl-1-butene, 2-methyl-2-butene, 4-methyl-1-pentene and mixtures
thereof.
The preferred C4 to C1~ multiolefm comprises a C4 to C,o conjugated diolefin.
Non-limiting
examples of useful conjugated diolefins may be selected from the group
comprising isoprene,
butadiene, 2,4-dimethylbutadiene, piperyline, 3-methyl-1,3-pentadiene, 2,4-
hexadiene, 2-neopentyl-
1,3-butadiene, 2-methyl-1,5-hexadiene, 2,5-dimethyl-2,4-hexadiene, 2-methyl-
1,4-pentadiene, 2-
methyl-1,6-heptadiene, cyclopentadiene, methylcyclopentadiene, cyclohexadiene,
1-vinyl-
cyclohexadiene and mixtures thereof.
The butyl polymer may be derived from a mixture comprising from about 70 to
about 99.5
parts by weight of the C4 to C$ monoolefin monomer and from about 30 to about
0.5 parts by weight
of the Cø to C14 multiolefin monomer. More preferably, the butyl polymer is
derived from a mixture
-3-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
comprising from about 80 to about 99.5 parts by weight of the C4 to C$
monoolefin monomer and
from about 20 to about 0.5 parts by weight of the C4 to C1ø multiolefm
monomer.
The most preferred butyl polymer for use in the present butyl polymer
composition is
derived from a mixture comprising from about 97 to about 99.5 parts by weight
of isobutylene and
S from about 3 to about O.S parts by weight of isoprene.
The manner of producing the butyl polymer useful herein is not particularly
restricted and
is within the purview of a person skilled in the art.
Those of skill in the art will recognize that it is possible to include an
optional third
monomer to produce a butyl terpolymer. For example, it is possible to include
a styrenic monomer
in the monomer mixture, preferably in an amount up to about 1 S percent by
weight of the monomer
mixture. The preferred styrenic monomer may be selected from the group
comprising p
methylstyrene, styrene, a-methylstyrene, p-chlorostyrene, p-methoxystyrene,
indene, indene
derivatives and mixtures thereof. The most preferred styrenic monomer may be
selected from the
group comprising styrene, p-methylstyrene and mixtures thereof. Other suitable
copolymerizable
1S termonomers will be apparent to those of skill in the art.
A particularly preferred class of butyl polymers for use in the present butyl
polymer
composition is the class of halogenated butyl polymers, particularly
chlorinated butyl polymers and
brominated butyl polymers.
Preferably, the halogenated butyl polymer comprises a halogen in the amount of
from about
0.1 to about 8% by weight of the polymer. More preferably, the halogenated
butyl polymer
comprises a halogen in the amount of from about O.S to about 4% by weight of
the polymer. Most
preferably, the halogenated butyl polymer comprises a halogen in the amount of
from about 1.5 to
about 3.0% by weight of the polymer.
The halogenated butyl polymer may be produced by halogenating a previously
produced
2S butyl polyner in a conventional manner. See, for example, United States
patent 5,886,106. Thus,
the halogenated butyl rubber may be produced either by treating finely divided
butyl rubber with a
halogenating agent, such as chlorine or bromine, preferably bromine, or by
producing brominated
butyl rubber by the intensive mixing, in a mixing apparatus, of brominating
agents such as N-
bromosuccinimide with a previously made butyl rubber. Alternatively, the
halogenated butyl rubber
may be produced by treating a solution or a dispersion in a suitable organic
solvent of a previously
made butyl rubber with corresponding brominating agents. See, for more detail,
Ullmann's
Encyclopedia of Industrial Chemistry (Fifth, Completely Revised Edition,
Volume A23; Editors
-4-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
Elvers et al.). The amount of halogenation during this procedure may be
controlled so that the final
polymer has the preferred amounts of halogen described hereinabove.
Another component of the present butyl polymer composition is a styrene resin
comprising
a first copolymer of a-methylstyrene and another styrenic monomer.
Preferably, the other styrenic monomer is selected from the group comprising
styrene, vinyl
styrene, p-methylstyrene (vinyl toluene), p-chlorostyrene, p-methoxystyrene,
indene, indene
derivatives and mixtures thereof. The most preferred styrenic monomer may be
selected from the
group comprising styrene, p-methylstyrene and mixtures thereof. Copolymers of
a-methylstyrene
and such styrenic monomers are known and thus are within the purview of a
person skilled in the
art.
A copolymer of a-methylstyrene and styrene useful in the present invention is
commercially
available from Hercules Inc. under the tradename Kristalex~ 1120.
A copolymer of a-methylstyrene and vinyl toluene useful in the present is
commercially
available from Hercules Inc. under the tradename Piccotex~ 120.
Preferably, the styrene resin is present in an amount of up to about 25 parts
by weight per
hundred parts by weight butyl polymer. More preferably, the styrene resin is
present in an amount
in the range of from about 5 to about 20 parts by weight per hundred parts by
weight butyl polymer.
Even more preferably, the styrene resin is present in an amount in the range
of from about 5 to
about 15 parts by weight per hundred parts by weight butyl polymer. Most
preferably, the styrene
resin is present in an amount in the range of from about 10 to about 15 parts
by weight per hundred
parts by weight butyl polymer.
Preferably, the present butyl polymer composition further comprises a filler.
The nature of
the filler is not particularly restricted, and the choice of suitable fillers
is within the purview of a
person skilled in the art. Non-limiting examples of suitable fillers include
carbon black (e.g., FEF,
MT, GPF and SRF), clays, titanium dioxide, silica fillers (with or without
unsaturated silanes),
calcium carbonate, talc (magnesium silicate) and the like. The amount of
filler is conventional.
Preferably, the filler is present in an amount in the range of from about 20
to about 200 parts by
weight per hundred parts by weight of the polymer. More preferably, the filler
is present in an
amount in the range of from about 20 to about 100 parts by weight per hundred
parts by weight of
the polymer. Most preferably, the filler is present in an amount in the range
of from about 40 to
about ~0 parts by weight per hundred parts by weight of the polymer.
The present butyl polymer composition may further comprise a hydrocarbon
extender oil.
The use of hydrocarbon extender oils, is well known in the art. Suitable
hydrocarbon extender oils
-5-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
include the paraffinic or naphthenic extender oils, preferably paraffinic.
Also known in the art is that
the use of such extender oils in butyl rubber vulcanizates generally causes
the air permeability of
such vulcanizates to increase. Thus, if used in the present butyl pol~nner
composition, such
hydrocarbon extender oils should be present in minor amounts. Preferably, the
oils) is present in
an amount of from 0 to about 7, preferably from 0 to about 4, parts by weight
per hundred parts by
weight butyl polymer in the composition.
The present butyl polymer composition may fixrther comprise a curing system.
The choice
of curing system suitable for use is not particularly restricted and is within
the purview of a person
skilled in the art. A typical curing system comprises: (i) a metal oxide, (ii)
elemental sulfur and (iii)
at least one sulfur-based accelerator. The use of metal oxides as a component
in the curing system
is well known in the art. A suitable metal oxide is zinc oxide, which is
typically used in the amount
of from about 1 to about 10, preferably from about 2 to about 5, parts by
weight per hundred parts
by weight butyl polymer in the composition. Elemental sulphur, comprising
component (ii) of the
preferred curing system is typically used in amounts of from about 0.2 to
about 2 parts by weight,
per hundred parts by weight butyl polymer in the composition. Suitable sulfur-
based accelerators
(component (iii) of the preferred curing system) are typically used in amounts
of from about 0.5 to
about 3 parts by weight, per hundred parts by weight butyl polymer in the
composition. Non-
limiting examples of useful sulfur-based accelerators may be selected from the
thiuram sulfides such
as tetramethyl thiuram disulfide (TMTD), the thiocarbamates such as zinc
dimethyl dithiocarbamate
(ZDC) and the thiazyl and benzothiazyl compounds such as mercaptobenzothiazyl
disulfide
(MBTS). Preferably, the sulphur based accelerator is mercaptobenzothiazyl
disulfide.
Stabilizers, anti-oxidants and tackifiers may also be added in the usual way
and in the normal
amounts for compounding butyl-type rubbery polymers.
The procedure of mixing the various components of this invention is not
specifically
restricted. In one embodiment, all of the ingredients of the rubber
composition described above may
be mechanically mixed at an initial temperature of not more than about
80°C in an internal mixer
and then vulcanized in a conventional manner. In another embodiment, the butyl
polymer and
styrene resin may be solution blended, the blend recovered from solution prior
to being mechanically
mixed with the remaining ingredients and then vulcanized in a conventional
manner. Thus, a blend
of abutyl polymer and styrene resin is suitably achieved using solutions of
the polymers in mutually
compatible hydrocarbon liquid solvents. Such a blend may be recovered from
solution by standard
recovery techniques of solvent removal and dryuig, followed by the compounding
and vulcanization
-6-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
procedure described above. In yet another embodiment, the styrene resin may be
added, in bulk, to
the butyl polymer during a final stage of the manufacturing process of the
latter.
Generally, it is preferred to carry out the compounding procedure in two
stages.
In the first stage the polymers may be mixed with conventional compounding
ingredients
such as one or more of carbon blaclc, hydrocarbon extender oil, taclcifiers,
stabilizers, processing aids
and anti-oxidants. This results in the production of a vulcanizable
composition which can be
transported, stored and /or vulcanized.
In the second stage of the compounding procedure, vulcanization is carried
out. Specifically,
one or more curatives are preferably added to the vulcanizable composition
prepared in the first stage
of compound. Practically, this can be carried out on a rubber mill or in an
internal mixer operated
at a temperature normally not in excess of about 60°C. The curative may
be selected from the group
comprising elemental sulphur, accelerators, zinc oxide and mixtures thereof.
After addition of the curative(s), the vulcanizable composition may be cured
in a
conventional manner, for example, by heating for from about 5 to about 60
minutes at temperatures
of from about 150°C to about 200°C to form elastomeric
vulcanizates.
Embodiments of the present invention will be illustrated with reference to the
following
Examples, which should not be use to construe or limit the scope of the
present invention.
In the Examples, the following materials were used:
BB 2030: a brominated butyl rubber having a halogen content of about 2.0% by
weight,
commercially available from Bayer Inc. under the tradename POLYSAR~ Bromobutyl
2030;
N-660: carbon black commercially available from Sterling-V;
Sunpar 2280: paraffinic oil commercially available from Sun Refining &
Marketing Co.;
HCR #1: a-methylstyrene-styrene copolymer (Mw=2950; Tg=56°C; softening
point=120°C)
commercially available from Hercules Inc. under the tradename Kristalex~ 1120;
HCR #2: a-methylstyrene-vinyl toluene copolymer (Mw=3800; Tg=68°C;
softening
point=118°C) commercially available from Hercules Inc. under the
tradename Piccotex~ 120;
Pentalyn "A": a pentaerythritol ester of rosin, used as a tackifier;
Stearic acid: used as an activator/processing aid;
MBTS: mercaptobenzothiazyl disulfide, used as an accelerator, commercially
available
under the tradename VulcacitTM DM/C;
sulfur: curative; and
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
ZnO: zinc oxide, used as a vulcanization agent, commercially available under
the tradename
I~adoxTM 920.
Unless otherwise stated in the Examples, the amounts of ingredients are
expressed in parts
by weight.
EXAMPLES 1-3
The recipes for the bromobutyl rubber compositions used in these Examples is
set out in
Table 1. As will be appreciated by those of skill in the art, Example 1
contains no styrene resin and,
thus, this Example is provided for comparative purposes only. In Examples 2
and 3, the extender
oil used in Example 1 has been substituted for by the specified styrene
polymers.
All ingredients except the sulfur and the zinc oxide were mixed in a Banbury
mixer for 3
minutes. The reaction mixture was cooled, the temperature of the cooliizg
water being approximately
30°C. Thereafter, the mixture was transferred to a cool 10" x 20" mill.
The sulfur and zinc oxide
were added and the resulting mixture was milled until the additives were
incorporated. At this point,
the following unvulcanized properties of the mixture were measured and the
results illustrated in the
Figures specified:
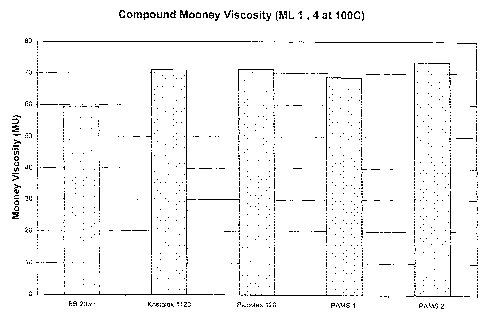
Compound Mooney viscosity (ML 1, 4 @ 100°C) - Figure 1;
Green Strength @ 23°C - Figure 2; and
Cure Rate - Figure 3.
Thereafter, the vulcanizable mixture was cured at 166°C for a period of
30 minutes. The following
properties of the vulcanizate were measured and the results illustrated in the
Figures specified:
Stress/Strain (Die C, test @ 23°C) - Figure 4;
Hardness (Shore A) - Figure 5;
Stress/Strain (hot oven aging : 168 hr. @ 120°C) - Figure 6;
Permeability to air (test @ 65.5°C) - Figure 7; and
Rubber Adhesion test - Figure 8.
_g_
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
With reference to Figure 1, it will be seen that there is a slight increase in
the Mooney
viscosity when the styrene polymer is added to the butyl polymer composition.
This is within
acceptable limits.
With reference to Figure 2, it will be seen that the green strength of the
butyl polymer
composition of Examples 2 and 3 is much better than that of Example 1 - in
effect this is the same
as an improvement in the cold flow properties of the butyl polymer
composition.
With reference to Figure 3, it will be seen that the cure rate (time to 90%
cure) is
approximately the same for the butyl compositions of Examples 2 and 3 compaxed
to that of
Example 1.
With reference to Figure 4, it will be seen that the stress/strain property of
the butyl polymer
composition of Examples 2 and 3 is were increased slightly compared to that of
Example 1.
With reference to Figure 5, it will be seen that the hardness of vulcanizates
made from the
butyl polymer compositions of Examples 2 and 3 is increased compared to that
of Example 1.
With reference to Figure 6, it will be seen that the hot air aging properties
of the vulcanizates
produced in Examples 2 and 3 is similar to that in Example 1 (i.e., there is
no apparent degradation
of this property as a result of adding styrene polymer to the butyl polymer
composition).
With reference to Figure 7, it will be seen that the permeability to air
decreased in the
vulcauzates of Examples 2 and 3 when compared to the vulcanizates of Example 1
(i.e., the use of
the styrene polymer additive in the present butyl polymer composition
increases in the air
impermeability properties of the resulting vulcanizates).
With reference to Figure 8, it will be seen that the adhesion properties of
vulcanizates of
Examples 2 and 3 is increased when compared to the vulcanizate of Example 1
(as measured by the
adhesion of an inner liner to the carcass of a tire).
EXAMPLES 4-14
The recipes for the bromobutyl rubber compositions used in these Examples are
set out in
Table 2. As will be appreciated by those of skill in the art, Example 4
contains no styrene resin and
thus, this Example is provided for comparative purposes only. Examples 5-14
contain various
combinations of styrene resin and extender oil.
The methodology used to mix the recipes was the same as that reported for
Examples 1-3.
The following unvulcanized properties of the mixture were measured and the
results
illustrated in the Figures specified:
-9-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
Compound Mooney viscosity (ML l, 4 @ 100°C) - Figure 9;
Compound Mooney scorch (125°C) - Figure 10; and
Green Strength @ 23°C - Figure 11.
The following properties of the vulcanizate were measured and the results
illustrated in the Figures
specified:
Stress/Strain (Die C, test @ 23°C) - Figure 12,;
Hardness (Shore A) - Figure 13; and
Permeability to air (test @ 65.5°C) - Figure 14.
With reference to Figure 9, it will be seen that the combination of
hydrocarbon extender oil
and styrene polymer is useful in mediating the compound Mooney viscosity of
the butyl rubber
composition between the case where there is no styrene polymer additive and
the case where there
is no hydrocarbon extender oil additive.
A similar trend can be seen in Figure 10 with reference to compounding Mooney
scorch.
With reference to Figure 11, it will be seen that an increase to 30-50% green
strength is
possible using 2-15 parts styrene polymer with 0-2 parts extender oil - this
translates into a
significant improvement in the cold flow properties of the butyl rubber
composition.
With reference to Figure 12, it will be seen that there is no significant
degradation in the
stress/strain profile of vulcanizates produced in Examples 5-14 compared with
the vulcanizates
produced in Example 4.
With reference to Figure 13, it will be seen that the addition of the styrene
polymer in
Examples 5-14 did not deleteriously affect the hardness of the resulting
vulcanizates compared to
the vulcanizate produced in Example 4.
With reference to Figure 14, it will be seen that the permeability to air
decreased in the
vulcanizates of Examples 5-14 compared to the vulcanizate of Example 4 - i.e.,
the use of the styrene
polymer additive in the present butyl polymer composition increases the air
impermeability
properties of the resulting vulcanizates.
In conclusion, it can be clearly seen that a butyl polymer of the present
invention has certain
improved properties (such as green strength and cold flow) compared to
compositions of the art, with
no deleterious effects on other important properties (such as permeability to
air).
-10-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
While this invention has been described with reference to illustrative
embodiments and
examples, the description is not intended to be construed in a limiting sense.
Thus, various
modifications of the illustrative embodiments, as well as other embodiments of
the invention will
be apparent to persons skilled in the art upon reference to this description.
It is therefore
contemplated that the appended claims will cover any such modifications or
embodiments.
All publications, patents and patent applications referred to herein are
incorporated by
reference in their entirety to the same extent as if each individual
publication, patent or patent
application was specifically and individually indicated to be incorporated by
reference in its entirety.
Table 1
Ingredient Example Example Example
1 2 3
BB 2030 100 100 100
Sunpar 2280 7 - _
Kristalex~ - 10 -
1120
Piccotex~ - - 10
120
N-660 60 60 60
Pentalyn "A" 4 4 4
Stearic Acid 1 1 , 1
MBTS 1.3 1.3 1.3
Sulfur 0.5 0.5 0.5
Zn0 3 3 3
-11-
CA 02417266 2003-O1-24
WO 02/10277 PCT/CA01/01064
o ~ ~n o ~r --' c-~~n r:
o ~0
0
.--.
o N N ~ Wit--- r~ ~n r~
a
,.,. - O
.,-.
p N ~n y7-.-. M '~ c~
N r.. ...~O
N O ~ 'd'~-' c~1~n c'~
O
N N ~ d '-' ty n M
O ~" ~ a
.r
O N ~n b wt .-. ~.r~t~'~M
O ...'~O
~-.O
k
W
' N ~ 'V'-~ ch ~'7c~
"." .--~O
' vo d- .-~ in c'7
~
.r, O
Q ' a ~ ~r .-~ M V~ M
e
...-
O v ~ ~ ~ M ~ fn
-.
~r "' O
N
M H M
.r
O
N
r. ,.r
O
D N
.
0.~:n ~ z c i ~ r~ ~
r
12
SUBSTITUTE SHEET (RULE 26)