Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02418689 2003-02-11
1
TITLE OF THE INVENTION
GOLD AND SILVER RECOVERY FROM POLYMETALLIC
SULFIDES BY TREATMENT WITH HALOGENS
FIELD OF THE INVENTION
[0001] The present invention relates to gold and silver recovery from
polymetallic sulfides by treatment with halogens.
BACKGROUND OF THE INVENTION
[0002) The use of chemical agents, particularly halides, for the
recovery of gold and silver is quite ancient. It was noted very early that the
adjunction of sodium chloride to mercury improved the performances of the
amalgamation process. This discovery translated into the Patio or ~azo
processes, which were implemented on an empirical basis from the early
1600's in Central and South America more than 150 years before the discovery
of elemental chlorine by Scheele in 1774. The Patio method involved the
digestion of a finely divided gold ore with mercury and sodium chloride, in
the
presence of air and moisture over a three month period. The values were then
collected by further leaching with mercury, followed by amalgam distillation
(T.
Egleston, The IVletallurgy of Silver, Gold and Mercury in the United States,
Vol.
1, p. 261, John Wiley, 1887).
CA 02418689 2003-02-11
2
[0003] Later, in the late 1700s, chloridizing roasting followed by
barrel amalgamation was developed in Central Europe as an improved method
for gaining access to precious metals from sulfide ores. This process called
upon a high temperature treatment of the goldlsilver ores in the presence of
sodium chloride, air and steam, in order to transform the precious metal
sulfides into their corresponding chlorides. The gold and silver was then
recovered either by amalgamation or cementation on pure copper (T. Varley et
al, IJ.S. Bureau of Mines, Bulletin N° 211, 1923). However, it was
discovered
that the high temperature chloridizing of gold or silver ores resulted in very
important losses of values by volatilization. In some cases these losses
reached 80 °!o or more of the precious metal content (S.B. Christy,
Transaction
of the American Institute of Mining Engineering, Vol. 17, p. 3, 1888).
[0004] It appeared that the presence of pyrites or iron sulfides
contributed significantly to the volatilization of gold and silver during the
high
temperature chtoridization with NaCI (S. Croasdale, The Engineering and Mining
Journal, August 29, 1903, p. 312). It was finally established that the
mechanism
explaining these losses involves the formation of a mixed chloride of gold and
iron
(AuCl3 ~ FeCt3), which is highly volatile at the chloridization temperatures
(J. A.
Eisele et al. U.S. Bureau of Mines, Report N° 7489).
[0005] Elemental chlorine dissolved in water, as introduced by Plattner
around 1850, constituted an alternative to high temperature chloridization.
CA 02418689 2003-02-11
3
However, this process was characterized by low efficiency in addition to
collecting
the gold by amalgamation.
[0006) The general characteristics of the various processes involving
chlorine, either as elemental chlorine or as chlorides, either at ambient
temperatures or at high temperatures, were not attractive. The yields obtained
with these processes were generally iow (often below h0 %) and the values were
collected as amalgams or as cemented products on copper or iron. In addition,
complex procedures were involved in order to obtain the precious metals in a
pure form. The environmental impacts of such operations, where large amounts
of sulfur are disposed with the tailings, would have been completely
unacceptable
by current standards.
[0007) The advent of cyanide extraction in 1916, tem~inated the
extraction of gold by various forms of chloridation. The cyanide process calls
upon the action of a cyanide salt such as sodium cyanide on gold in the
presence
of oxygen, to give a soluble gold salt (Eq. I):
2 Au + 4 NaCN + 1l2 02 + HZO -~ 2 NajAu(CN)2] + 2 Na~H
[0008) The gold can then be recovered from the cyanide complex by
the action of excess zinc (Eq. II):
2 Na[Au(CN)2) + Zn,~cegs, --~ Na2[Zn(CN)~, + 2 Au
[0009) Under the best circumstances, gold recovery can be as high as
CA 02418689 2003-02-11
4
98 %. This process calls for a contact time of one to three days at near
ambient
temperature in the presence of air.
[0010 In some instances the cyanide process performs very poorly.
Ores refractory to cyanide extraction can be grouped under the general term of
polymetailic ores. In such ores, one fnds small amounts of base metals such as
copper or zinc, typically 0.1 % Cu or 0.3% Zn. Such srnall amounts qualify the
ore
as of very low grade for the production of copper or zinc. If such a
polymetallic ore
body contains some gold (for example, 4 gTT Au or Ag or a mixture of both},
the
cyanide extraction process does not perfiorm well. The poor performance is due
to
the base metals, either copper or zinc, (as well as silver), having a much
stronger
ability to form complexes with cyanide than gold. In fact, this inherent
property is
used to recover gold from a pregnant solution by zinc treatment following
cyanide
extraction (see Eq. Il). The base metals will consume all the cyanide present
and
the gold extraction will only begin after all the available base metals, as
well as
silver, have been dissolved. Because of the excessive consumption of
relatively
costly cyanide, this process for recovering gold is uneconomical.
[0011] There exist other types of gold ores refractory to cyanide
extraction, namely arsenopyrites and carbonated ores. With certain types of
arsenopyrites, the gold exists as a solid solution in the crystal lattice. In
order to
gain access to gold, the structure of the crystal lattice (sulfide} must be
completely
broken up.
CA 02418689 2003-02-11
[0012] In order to break up the crystal latkice, and to gain access to
gold contained therein, a reagent tike cyanide cannot be used and a more
aggressive approach is required. Vllith carbonated gold ores, the carbon
carried
by the ore acts as an adsorbent for the cyanide complexed gold, and will cause
it
to reprecipitate it in situ. Another approach is thus required in order to
extract the
gold content of these types of ores.
[0013] Polymetallic ores constitute complex mixtures of sulfides. The
tailings discarded as a result of gold and silver extraction using the cyanide
process, as well as by other methods, still contain very substantial amounts
of
sulfur.. This sulfur is prone to bio-oxidation (Thiobacilius fer~-ooxidans),
and the
resulting drainage is quite acidic and toxic due to its metallic content.
[0014] The spent cyanide solutions, kept in ,large ponds following gold
recovery, represents a substantial environmental hazard and has recently
created
major disasters in Guyana and Central Europe, thus restricting the use of the
cyanide process in many areas.
[0015] In the last twenty years, chloridation has been reconsidered as
a process for extracting base metals such as copper, nickel or silver. The
lntec
base Metal process (J. Moyes and F. Houllis, Chloride Metallurgy 2002, Vol.
II, p.
577, Canadian Institute of Mining, Metallurgy and Petroleum) constitutes a
typical
example. This process calls for the digestion at 85°C, over a period
ranging from
12 to 14 hours, of the sulfides of copper or zinc in a concentrated brine
solution
CA 02418689 2003-02-11
6
(250 g/l NaCI) comprising a cupric mixed halide (BrCl2)Cu prepared
electrolytically. The mixture is aerated and the copper is collected as
cuprous
chloride. The cuprous chloride is decomposed at the cathode to elemental
copper
by electrolysis upon regeneration of the mixed halide of copper (Eq. III):
2 CuFeS2 + 5 BrCl2 -+ 2 Cu+~ + ~ Fe+3 + 4 S° + 5 Br + 10 CI'
[0016] The above described chloridation process was reported as also
extracting gold, if present. However, the requirement of recycling copper so
as to
have the cupric/cuprous system needed to oxidize iron and sulfur, makes this
approach very cumbersome when the main concern is gold recovery rather than
copper recovery. Further, the electroiytical oxidation of sulfur via the
cupric salt,
which is regenerated by electrolysis, is a very costly process rendering the
treatment of a gold ore not having a very large gold content uneconomical.
Finally, the presence of elemental sulfur in the tailings is a potential
source of acid
drainage.
[0017] Another chloridation process called Platsol, was reported as
being very efficient for the recovery of base and precious metals from sulfide
ores
(C.J. Ferron et al, Chloride MetaNurgy 2002, Vol. I, p. 11, Canadian Institute
of
Mining, Metallurgy and Petroleum). This process involves a pressure oxidation
in
the presence of oxygen and sulfuric acid, in an autoclave at a temperature
above
200°C. The implementation of such a technique is very capital-
incentive, calling
for titanium autoclaves and a source of pure oxygen. The operation of this
CA 02418689 2003-02-11
equipment is also prone to problems due to scaling ofi the reactor,
complicating
heat transfer. The sulfur resulting from the operation is in an innocuous
form, i.e.
a hydrated iron sulfate (jarosite). The high capital and operating costs
render this
approach unattractive for polymetailic sulfides having a modest gold content.
(00'18] Other techniques such as the Plint process (fbid, Vol. I, p. 29)
or, the Ito process (Ibid, Vol. I, p. 69), are techniques used for the
recovery of
gold and silver from sulfides, by oxidation with ferric chloride in
concentrated
brine. The ferrous chloride is re-oxidized to ferric chloride by chlorine
alone or
by exposure to air and hydrochloric acid (Eq. IV):
2 PbS ~ Ag2S ~ 3 Sb2S3 + 24 FeCl3 -~ 24 FeCl2 + 2 PbCiz + 2 AgCI + 6 SbCl3 +
12 S°
[0019] In these processes, sulfur is again oxidized electrochemically
via the oxidation of ferrous chloride by chlorine or HCI. As explained
previously,
such an approach is costly for the recovery of gold or silver from sulfide
ores,
because of the electrochemistry involved. Elemental sulfur is again discarded
with
the tailings, generating a potential source of acid drainage.
[0020] There thus remains a need for an improved process for the
recovery of gold and silver from polymetailic ores.
[0021 ] The present invention seeks to meet these and other needs.
[0021] The present invention refers to a number of documents, the
content of which is herein incorporated by reference in their entirety.
CA 02418689 2003-02-11
SUMMARY OF THE 9NVENT10N
[0022] The present invention relates to a process for the recovery of
gold and silver from polymetallic ores, characterized by Bow operational and
cost investments.
[0023] The present invention relates to a process for the recovery of
gold and silver from polymetallic ores, characterized by being carried out at
atmospheric pressure and at low temperatures prior to leaching.
[0024] The present invention also relates to a process for the
recovery of gold and silver from polymetailic ores, characterized by producing
tailings devoid of elemental sulfur, sulfides, or soluble sulfates and by fast
reaction rates allowing for high rates of treatment.
[0025] In addition, the present invention relates to a process for the
recovery of precious metals such as gold and silver, as well as base metals
such
as copper, nickel, cobalt, zinc, tin and lead, in addition to relating to the
safe
removal of sulfur, arsenic and mercury as well as to the disposal of iron,
chromium, aluminum and titanium in an inert and insoluble form.
[0026] Further scope and applicability will become apparent from the
detailed description given hereinafter. It should be understood however, that
this
detailed description, while indicating preferred embodiments of the invention,
is
given by way of illustration only, since various changes and modifications
within
CA 02418689 2003-02-11
the spirit and scope will become apparent to those skilled in the art.
DRIEF DESCRIPTION OF TFiE DRAU111NGS
[0027] In the appended drawings:
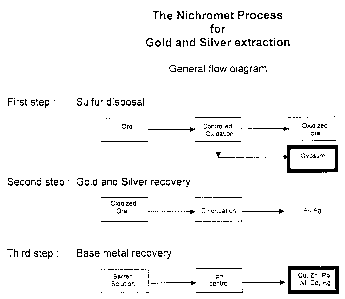
[0028] Figure 'I is a block diagram illustrating the various steps of
the process of the present invention;
[0029) Figure 2 is a block diagram illustrating the various steps of
the sulfur removal aspect of the process of the present, invention;
[0030] Figure 3 is a block diagram illustrating the various steps of
the gold and silver recovery aspect of the process of the present invention;
and
[0031] Figure 4 is a block diagram illustrating the various steps of
the base metal recovery aspect of the process of the present invention; and
[0032] Figure 5 is a schematic illustration of an electrolytic cell used
in the process of the present invention.
DETAILED DESCRIPTION OE THE INVENTION
[0033] In a broad sense, the present invention relates to a new
process for the recovery of precious metals such as gold and silver, as well
as
base metals such as copper, nickel, cobalt, zinc, tin, mercury, and lead. The
present invention also relates to the safe removal of sulfur, arsenic and
mercury
as well as to the disposal of iron, chromium, aiuminurr~ and titanium in an
inert
and insoluble form. This is achieved at considerably lower cost than with the
CA 02418689 2003-02-11
current chloridation or cyanide processes, by avoiding sulfur oxidation by
electrochemical means. The process of the present invention is very time
efficient, of the order of a few hours, and is carried out at atmospheric
pressure
and at near ambient temperatures. The process allows for the separation of the
precious metals as well as the base metals from the common metals, while
recycling the reagents and releasing only inert waste materials into the
environment.
[0034) Gold and silver, along with base metals such as copper, zinc,
lead, tin, nickel, cobalt and mercury can be recovered from polymetallic
sulfides in
yields well above 90 % by the process of 'the present invention comprising the
following steps:
(0035] (a) Oxidizing the polymetallic ore using lean air having about
10% 02, at a temperature ranging from about 400 to about 600°C reducing
the
sulfur content of the ore to about 0.5 % S (as sulfide) or less. 'The
resulting S02 is
fixed by calcium carbonate as calcium sulfite, which auto-oxidizes to calcium
sulfate dihydrate (gypsum). This results in the elimination of sulfur in a
manner
compatible with environmental regulations.
(0036) (b) slurring the sulfur free ore with a near-saturated (275 to
300 g/l} aqueous solution of sodium chloride (brine), and adding a solution of
chlorine/HCl/hypochlorous acid such that the precious metals and the base
metals are chlorinated and dissolved in the strongly complexing brine milieu.
The
CA 02418689 2003-02-11
11
chloridation reaction is significantly accelerated by the presence of a
catalytic
amount, less than one percent of the halides present in the brine, of bromide
ion.
The chlorinelHCl/hypochlorous acid solution, containing a catalytic amount of
bromine, is generated by circulating a portion of the brine solution used to
slurry
the oxidized ore through the anodic compartment of an electrolytic cell, at a
rate
sufficient to dissolve the chlorine in the brine solution. Following the
slurring
operation, the ore is maintained in suspension in the acidic halogenated brine
at a
temperature ranging from about 35-45°C by slow stirring, without
aeration, for a
period of 2-3 hours for most ores, and up to 5 hours for exceptionally
refractory
ores. After separating the barren solid followed by washing with brine, the
combined filtrate and rinsings are circulated over activated carbon for gold
and
silver recovery.
[0037] ~c) treating the soiution deprived of precious metals with a
sodium hydroxide solution raising the pH to about 3 to 4.. The sodium
hydroxide
required to achieve this partial neutralization is produced by circulating the
initial
brine solution through the cathodic compartment of the electrolytic cell. The
caustic sodium hydroxide solution is generated concomitantly at the cathode,
in a
stoEChiometric ratio, with the chlorinelhydrochloric acid/hypochlorous acid
solution
produced at the anode of the electrolytic cell. Raising the pH to about 3-4
induces
the precipitation of iron, aluminum, chromium and titanium as insoluble oxides
of
these metals, in various hydrated forms. These oxides are filtered and washed
with brine. Raising the pH of the resulting filtrate to values above 8,
induces the
CA 02418689 2003-02-11
12
precipitation ofi the base metals such as copper, zinc, Bead, tin, nickel and
cobalt
as a base metal concentrate.
[0038] Any arsenic, often present in significant amounts in polymetallic
sulfides, is eliminated along with the sterile solids after the digestion as
ferric
arsenate, an insoluble and inert arsenic salt. Mercury, if present, is largely
recovered with the flue dusts after oxidation, and any remaining traces of
this
metal are Iixiviated by the chlorinated brine, and recovered as an amalgam
upon
the removal of the base metals.
[0039] The brine solution, following the removal of the metals, is
recirculated for further leaching. The sterile solids are rinsed with water
and the
rinsings concentrated by evaporation, using waste heat from the sulfide
oxidation
step. The concentrated rinsings, along with the brine solution, are then
recycled
so as to prevent salt losses or salt release into the environment.
Sulfur removal tFir~ure 1)
[0040] The gold and/or silver containing ore, additionally comprising
small amounts (0.1-2.0%) of base metals such as Cu, Zn, Pb, Sn, Ni, and Cu, is
a
sulfide or complex sulfide. The ore may further incorporate one or more other
common metals such as iron, aluminum, titanium, chromium, as well as elements
such as arsenic, antimony or bismuth. Mercury is occasionally also present in
the
ore.
CA 02418689 2003-02-11
13
[0041 The ore is reduced to a particle size of less than about 140
mesh by standard methods known in the art. The sulfur content of the ore,
which
can be as high as 15 %, is reduced to about 0.5% or less (as sulfides) by
controlled oxidation in a reactor or kiln (2). The reactor or kiln provides
for a
control of the oxygen content in the reaction chamber. A relatively low
oxidation
temperature, typically ranging from about 400 to about 600°C, is very
advantageous since it prevents any sintering of the material and generates a
solid
product having a large surface area and having good reactivity. This treatment
is
much preferred to standard roasting where temperatures as high as
1200°C have
beeri observed. Such high reaction temperatures induce much sintering and
volatilization. Standard roasting involves the free burying of the sulfides in
the
presence of excess air.
[0042] The contr~I of the low oxidation temperatures is achieved by
recycling part of the lean air back to the reactor. This allows for the oxygen
content in the reactor to be maintained at values not exceeding 10°/~
02. It is
important to prevent any sodium chloride present in the ore from being
oxidized. It
is well known that sodium chloride contaminations as low as 0.01 percent, can
induce significant volatilization of gold and silver.
[0043] The gas stream from the oxidation reactor is cooled in a settling
chamber, allowing for the collection of volatile oxides or products generated
during the oxidative treatment such as arsenic oxide, traces of zinc oxide,
and
metallic mercury if present in the starting ore. Dusts carried mechanically
from the
CA 02418689 2003-02-11
14
fines in the reactor are also collected in the settling chamber. The amount of
solids collected is generally small, less than one percent of the weight of
the ore
treated. The solids thus collected can be recovered arid used for recuperation
of
values such as As203 or mercury, or they can be safely disposed of in sealed
containers. The gas at the exit of the settling chamber, essentially composed
of
SO2 and lean air, is partly redirected back to the oxidation reactor for
oxygen level
control, and partly directed to a S02 scrubbing unit. The SO2 is adsorbed
using a
finely divided limestone slurry (200 mesh), allowing for the transformation of
essentially all of the S02 (about 98%) into calcium sulfite, which auto-
oxidizes to
calcium sulfate dehydrate or gypsum. Gypsum is a very stable and inert product
representing a definitive solution for the safe disposal of sulfur. It can be
used as
a building material in the production of Portland cement or as land fill. The
water,
following the dewatering of the gypsum, is recirculated back to a water thank.
Since gypsum is a dehydrate, there is a net consumption of water in the
scrubbing
process. The gases freed of SO2, are vented through a flue duct.
[0044] In the first step of the process therefore, the ore was made
more reactive towards leaching, and essentially ail of the sulfur initially
present
has been disposed of in a safe and environmentally compatible manner. The
present approach constitutes an economically attractive alternative to the
presently available methods. The cost of electrochemically oxidizing 1 % of
sulfur
in one metric ton of sulfide ore is BUS 4.71 per unit percent of S2- per ton
with a
KWh at $US 0.09 per kilowatt and with an efficiency of 80 °/~. The
cost of
CA 02418689 2003-02-11
oxidizing the sulfide content of an ore containing 10°l0 S2- to
elemental sulfur,
using an electrochemically-produced reagent such as chlorine, would be in the
best case scenario $US 47.10 per ton of ore for power only. The controlled
oxidation of the sulfur content using lean air can be done at 10 % or less of
that
cost; and transforms the sulfur into a safe and environmentally disposable
form.
The electrochemical oxidation process leaves elemental sulfur in the tailings
generating a potential source of acid drainage.
Goldlsilver recovery (Figure 2)
[0045] The recovery of gold and silver from the oxidized ore is
achieved by leaching with a reagent derived from elemental halogens. The ..
halogens (Br2, CI2) have significantly different behaviors towards gold.
Bromine
can readily dissolve gold at room temperature, even in the absence of water
~Kruss and Schmidt, Berichte der Deutschen Chemichen Gesellschaft, 20, 2634,
1887). Gold, on the other hand, is inert to dry chlorine at room temperature,
and
the attack of this gas on gold requires the presence of water and slight
heating
(Voigt and Biltz, Z. anorg. Chem., 133, 277, 1924). Even though bromine is the
more reactive reagent with gold, chlorine is more electronegative (VILM.
Latimer,
the Oxidation State of the Elements, pp. 56 and 62, Prentice Hall, 1952):
Ci- ~ Ci2 (-1.359 V);
Br -~ Br2 (-1.07 V].
[0046] It is possible to take advantage of this reactivity difference to
accelerate gold leaching from the oxidized ore, if a catalytic amount of a
bromide
CA 02418689 2003-02-11
16
is introduced into the leaching solution. The leaching solution is a brine
solution
having a high concentration of sodium chloride, i.e. from 275 to 300 g/I of
NaCI.
Lower salt concentrations yielded lower percentages of silver recovery, when
silver was associated with gold in the oxidized ore. A portion of the
concentrated
brine solution, also containing a trace (~-3 gll) of NaBr, is circulated in
the anodic
compartment of an electrolytic cell, at an appropriate rate, so as to dissolve
the
halogen liberated at the anode. As mentioned above, the bromide ion will be
reduced first, followed by some chloride ions so as to give a mixture of
halogens
dissolved in the brine solution. The brine solution containing dissolved CI2
and Br2
is mixed with fresh brine from a brine tank to provide a volume of liquid
necessary
to form a 20% slurry with the oxidized ore in a reactor kept at 35-
45°C. The slurry
is slowly stirred in order to prevent settling of the ore. The reacting mass
was not
aerated since aeration was neither improving the reaction rate nor the
reaction
yield, instead it resulted in the loss of dissolved halogens. Due to the trace
amounts of bromine in the system, the gold leaching process is believed to
involve the initial formation of gold tribromide (Eq. V):
2 Au + 3 Br2 --~ 2 Au,Br~
~0047~ The gold tribromide is then believed to be transformed,
because of the stronger oxidizing capacity of C12, into gold trichloride with
the
concomitant regeneration of elemental bromine (Eq. VI):
2AuBr3+3012--~2AuCl3+3Br2
CA 02418689 2003-02-11
17
[0048] A similar type of reaction is obtained for silver, the high
concentration of sodium chloride allowing the solubilization of the silver
halides by
complexation.
[0049] In the course of the leaching reaction, the other ions are
similarly solubilized, and exist at their maximum valency; copper as cupric
0
chloride, iron as ferric chloride, tin as stannic chloride, and arsenic as
arsenate
(As+5). Particularly with arsenic, the strong oxidizing environment leads to
the
precipitation of all the arsenic as an insoluble and inert ferric arsenate
(Eq. VII):
Fe3+ + AsO4 3 -~ FeAs04
[0050] The pH of the reaction mixture drops below 0.1 as the leaching
reaction proceeds. This strong acidification is an indication of the reaction
of
chlorine with water (Eq. VIII):
Ha0 + C12 ~ HCI + HOCI
[0051] The presence of hypochlorous acid could account for the
observed chloridation of gold by chlorine in the presence of water. A similar
equation can be written to describe the behavior of bromine, which is in
equilibrium with hydrobromic acid and hypobromous acid. The solubilized
species
can therefore be seen as a mixture of chlorides and hypochlorides, which
eventually end up as chlorides when the hypochlorous ion decomposes with the
concomitant evolution of nascent oxygen (Eq. IX):
t-IOCI ~ HCI + 11202
CA 02418689 2003-02-11
[0052] The production of nascent oxygen accounts in part for the very
strong oxidizing capability of the system without aeration of any sort.
[0053] The duration of the leaching, at 35-~.5°C in the reactor, ranges
from 2 to 3 hours. With exceedingly refractory ores it is necessary to extend
the
contact time to about 5 hours. Following the leaching, the slurry is filtered
or
centrifuged, producing a pregnant solution and a waste or sterile solid.
[0054] The sterile solid was first rinsed with brine in order to recover
any held-up values in the cake, followed by washing . with water to recover
any
salt. The so-obtained tailings contain arsenic as an. iron arsenate, and are
free of
sulfur and of soluble base metals. The pregnant solution is circulated over
carbon
to collect the gold and silver. Following the recovery of gold and silver from
the
carbon by known methods, these precious metals are obtained by electrowinning
or other standard techniques. The goldlsilver-free solution is then recovered
to be
further treated so as to collect the base metals.
Recovery of base metals (Figure 3)
[0055] The base metals to be obtained from the leaching of gold-
bearing polymetallic sulfiides are of two categories. The first category
contains
metals of relatively high commercial value, often obtained by
pyrometallurgical
operations. This category contains metals such as nickel, cobalt, copper,
zinc,
lead, tin and mercury. The second category contains metals of low economic
value, and comprises predominantly iron with considerably smaller amounts of
CA 02418689 2003-02-11
19
aluminum, titanium, chromium and traces of the p-bloc elements.
[0056] In order to isolate these two types of base metals, sodium
hydroxide is generated in the cathodic compartment of the electrolytic cell.
The
sodium hydroxide solution is accumulated in a caustic tank and is then used to
raise the pN of the previously produced barren solution, devoid of gold and
silver,
leaving the carbon columns, from below 1 to about 3-4. At a pH ranging from
about 3-4, any iron existing as Fe~3 is instantaneously precipitated by
hydrolysis
as a hydrated iron oxide. Titanium, aluminum and chromium react similarly
within
this pH range. The hydrated oxides are removed by filtration. The solids are
rinsed with brine in order to recuperate any base metals of values held up in
the
solid cake, followed by washing with water to remove any traces of salt. The
salt-
free mixture of oxides is then discarded as an insoluble and inert material of
little
or no commercial value.
[0057] The solution obtained from the filtration and the brine rinsings
contains the base metals of value. IVlercury if present, is separated by
amalgamation on pure copper. The ply of the mercury-free solution, pH between
3-4., is further raised using an additional portion of the sodium hydroxide
solution
to values above 8, causing all of the base metals {Ni, Co, Cu, Zn, Pb, Sn) to
precipitate as oxides or hydrated oxides. The oxides are removed from the
mixture by filtration and are rinsed with water to remove any traces of salt,
to
provide a concentrate of metals having significant commercial value. The
brine,
CA 02418689 2003-02-11
being free of metals, is recycled back to the fresh brine reservoir. The
rinsings are
concentrated by evaporation so as to give a brine solution of appropriate
concentration, and which is also recycled back to the fresh brine reservoir.
[0058) The implementation of the process of the present invention,
using a large variety of gold-bearing polymetallic sulfdes, provides for the
recovery of gold and silver in high yields, essentially always above 90
°/~ and
frequently above 95 %. The process of the present invention also provides for
the
recovery in high yields of the base metals of commercial value, frequently
above
95 %.
(0059) ~f all the base metals of little commercial value, iron is
generally the predominant one. Following the oxidation of the sulfides at 400-
600°C, the resulting iron oxide is quite inert and no more than about
20-25 °/~ of
this iron is leached, thus significantly decreasing the power consumption of
the
process. In fact, for a KVllh costing US$ 0.09, and with an efficiency at the
electrolytic cell of 80 %, each percent of iron in the ore would cost US$ 1.00
for
power to take care of, and each percent of base metals such as copper or zinc
in -
the ore would cost US$ 2.36 for power to extract. Thus, for an ore having 1
°/~
copper and 8 % iron, the value of recovered copper (US$ 16.50 at US$ 0.75/Ib
for
copper) covers all the electrolytical power costs (US$ 10.36) plus a fair
reserve
and no power imputations have to be made against the gold and silver values
recovered.
CA 02418689 2003-02-11
21
[0060) Using the process of the present invention, polymetallic ores
containing gold and/or silver which do not qualify for base metals extraction
either
because of a low base metal content, problems of enrichments by flotation or
other restrictions, can be treated economically from the return generated by
the
base metals in order to collect the precious metals. Consequently, the process
of
the present invention provides for an attractive alternative to the currently
available technologies, allowing the treatment of ores or tailings previously
not
attractive, at a profit.
[OOf1) The recycling of the brine solution, and the disposal of sulfurs
arsenic and metal oxides as stabte and inert solids, reduces the environmental
impacts of the operation to a minimum. Furthermore, the implementation of the
process of the present invention at near-ambient temperatures and atmospheric
pressure, reduces the investment per unit weight of ore to very competitive
values. Finally, the low temperature oxidation of sulfur being an exothermic
process, the energy consumption at that level is minimal and much lower than
the
corresponding electrochemical oxidation of sulfide to elemental sulfur.
[0062) The process of the present invention was implemented using a
variety of polymetallic ores and tailings containing gold and silver.
Example 1
[0063) A Canadian ore sample (90 g) from the Sudbury (Ontario) area
containing 4.5 g!T Au, 8 gIT Ag, 0.1 % As, 7.5 % S, 5.5 % Fe, 0.1 % Ni, 0.008
Co
CA 02418689 2003-02-11
22
and 0.5 % Cu was reduced to a particle size of about 140 mesh and heated at
585-600°C in an atmosphere composed of N2 (50%) and 50 % air, over a
period
of two hours in a VycorT"" tube heated externally in a LindbergT"" furnace.
The
temperature was measured inside the mass being oxidized. The external heating
was reduced when the oxidation began at around 400°C.
[0064, A small amount of a white deposit, arsenic oxide, could be
observed at the discharge side of the VycorT"" tube. The color of the oxidized
material changed from black to brown and the weight loss during the process
was
12 %.
[0065 A sample of the oxidized material (25.0 g) was placed in a
three-necked one liter flask, along with 500 g of water, 1508 of sodium
chloride
and 1.2 g of sodium bromide. The suspension was stirred magnetically and the
flask was closed so as to exclude air from entering the system.
[0066 The slurry was extracted from the flask through one of the
necks using a peristaltic pump, and was subsequently circulated through the
anodic compartment of an electrolytic cell, operating with a brine solution
having
the same concentration as the brine solution in the flask (anode of graphite,
operation at 2.5 V). The anodic fluid was returned to the flask after
dissolving
chlorine. The cell was operated on and off in such a manner as to maintain a
slight reddish coloration in the flask indicative of the presence of free
bromine.
CA 02418689 2003-02-11
23
(0067] The reaction flask was maintained at 40°C for a period of 2.5
hours after which it was filtered on a Buchner funnel. The solid was rinsed
three
times with a brine solution containing 300 g/l NaCI. The mixed filtrate and
rinsings
were very acid, having a pH below 1Ø The acidic filtrate and rinsings were
then
treated with 30 g of carbon (NoritT"" R~3515 so as to collect gold and silver.
The
sterile solid was then rinsed with water to completely remove any traces of
brine
{negative test to AgN03), dried at 110°C {16.8 g) and submitted to
elemental
analysis. The elemental analysis indicated that 96% of the gold and 94% of the
silver initially present in the oxidized material, were leached out and then
adsorbed on the carbon.
[0068] The barren solution following contacting with carbon was
combined with the aqueous rinsings and was submitted to elemental analysis.
The solution was found to be essentially free of gold and silver, and
contained
99% of the extracted iron, 98 % of the nickel and copper and 91 % of the
cobalt
present in the starting oxidized ore sample. Adjusting the pH at 3.5 with
sodium
hydroxide, resulted in the precipitation of the iron. Further raising the pH
to 8.5
precipitated the nickel, cobalt and copper. The brine, being essentially free
of
metals, is available for further use It was noted by elemental analysis that
the
bromine content in the brine did not change during the process, taking into
account the dilution induced by the rincings. Further is was f~und that the
gold
and silver content, following treatment, was below 0.05 gIT and 0.16 g/T
respectively, while 23% of the iron was extracted.
CA 02418689 2003-02-11
24
[0069) The process was repeated using several types of polymetallic
sulfides containing gold, silver or both, along with base metals of value. All
the
operational parameters were the same as in Example 1, except for the duration
of
the digestion. Those results are reported in Table !.
CA 02418689 2003-02-11
i ~Y ~ O ~ N
N ChO aO Oa O O
a
a cn
C~ O7 ~ O?
L
d
~ O
O O O ~
O O
o N tn N c0 a0 1~.. cp
Q o m o 0 0
0 ~ o
O ~Yc0 cDtC~~ t0 I~. M
O~ O ~ CO
~ 05 O
C
~ O
L
O O O O tn tty O tC'y tf>
D ~ N M M N M tp C~ M
L
~
~
v~ O
,~1 - t() M M tf~ O
O ~
r. M
~ Cn h-r COI~ d' CD r
U
L M ci~W M
_ M
O r- '-
~_ 1 t(7 O
T'
a
~
Z
O
C O ~'-O M ~ r ~
tn ~ M
~ ~-
r m ~ 00 0
Q7
_
O ch ,ON ~ M_ N
' 0 '
U 0 Q o o co ~ 'n
N
O
N o
N tt~ M a0 tc~ C~~6
o
O O
v O h.
'
O y 1 M O ~, M ~ M
U Q > N N
M
M M Lt> t1? N
O
a9
C
.U .U
O U 0
X ~ ? c E E > ~~ f.
~ O
C9 ~ t~ m U o . , n '
~ ~ ~
~
c c
a
L
c
* ~ m
cLo . cB
(JD ~ ~ r..~ O * U O
* ~ U
O td .~J .C N C (8
U cn ~ 'O tOp O fB N
Z
.
N U ~ ~ ~
n
~ ~ ~ o
c
7C p
N M V'tn CO t~ 00 ~ p7 w-
CA 02418689 2003-02-11
26
[0071, Although the present invention has been described
hereinabove by way of preferred embodiments thereof, it can be modified,
without departing from the spirit and nature of the subject irwention as
defined
in the appended claims.