Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
CHEMOPROTECTANT FOR GASTRIC TOXICITY
The present invention was partially supported under NIH grant #NS33618. The
United States Government may have certain rights in this invention.
Technical Field of the Invention
The invention provides chemoprotectant agents that may be administered in
conjunction with cytotoxic agents that target carcinoma type cancers. More
particularly,
the cytotoxic agent targets Lewis Y glycoproteins on gastric epithelium, with
or without
addition of conventional chemotherapeutic agents. Infra-arterial
administration of a thiol-
based chemoprotective agent will target the cytotoxic agent to reduce
immunoconjugate
and chemotherapy side effects without decreasing anti-tumor efficacy.
Background of the Invention
N-acetylcysteine (NAC) is an analog of cysteine with strong anti-oxidant and
free
radical scavenging activity. In addition to direct chemoprotective activity,
when
administered to a mammal NAC is deacylated and enters a cellular synthetic
pathway for
production of glutathione. Glutathione is involved in many cellular processes
that may
have importance for the resistance of tumors to cytotoxic drugs, including
anti-oxidant,
drug conjugation, and drug extrusion. Thus, NAC can mimic short term effects
of
glutathione as well as increasing glutathione for later protective activity.
This is especially
important when intracellular glutathione is artificially reduced in an effort
to enhance the
cytotoxic properties of chemotherapeutic drugs, by pretreatment with
buthionine
sulfoximine (BSO).
A potential problem with any chemoprotectant is the possibility of
deactivating the
anti-tumor effect of the chemotherapy or radiation therapy. The goal of
chemoprotection
is to reduce unwanted toxicities of chemotherapy or radiotherapy without
affecting
efficacy. Therefore, there is a need in the art to improve pharmacokinetics
and
biodistribution of chemoprotectant agents so that they will be more effective
if they can be
delivered in a tissue-specific manner. In other words, to maximize their
delivery to the
breast, gastrointestinal tract, lung, cervix, and ovary while minimizing
systemic delivery.
There axe several thiol-based chemoprotectant agents that contain a thio,
thiol,
aminothiol or thioester moiety. These include N-acetyl cysteine (NAC), sodium
thiosulfate (STS), GSH ethyl ester, D-methionine, and Ethyol (WR2721). Ethyol
is also
marketed in the United~States under the generic name of Amifostine. GSH ethyl
ester is
an experimental thiol not yet marketed for clinical use, but is representative
of the class of
thiols that is converted directly to glutathione.
NAC is currently marketed in the United States under an orphan indication for
oral
and infra venous (iv) administration for overdosing with acetaminophen. NAC
has also
been shown to be a chemoprotectant when administered in combination with a
vanadate
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
compound (U.S. Patent 5,843,481; and Yarbo (ed) Serrain. Oracol. 10 [Suppl
1]56-61,
1983). In addition, NAC has been shown to be a mucoregulatory drug used for
the
treatment of chronic bronchitis (Grassi and Morandini, Eur. J. Clin.
Pharr3aacol. 9:393-
396, 1976; Multicenter Study Group, Eur. J. Respir. Dis. 61: [Suppl.]93-108,
1980; and
Borman et al., Eur. .I. Respir. Dis. 64:405-415, 1983).
In plasma, NAC can be present in its intact, reduced forms as well as in
various
oxidized forms. It can be oxidized to a disulfide by reacting with other low
molecular
weight thiols, such as cysteine and glutathione. NAC can be oxidized by
reaction the thiol
groups of plasma proteins. There are bioanalytical methods for the
determination of
NAC in plasma, including Cotgreave and Moldeus, Bioplrarm. Drug Disp. 8:365-
375,
1987; and Johansson and Westerlund, J .. Chromatogr. 385:343-356, 1986 that
also permit
a determination of other forms of NAC. Moreover, cysteine and cystine have
been
identified as major metabolites of NAC. The excreted urinary product was
inorganic
sulfate together with small amounts of taurine and unchanged NAC. According to
the
label indications for NAC manufactured by American Regent Laboratories
Shirley, NY),
vials of NAC are produced as a sterile solution for oral administration
diluted with water
or soft drinks. NAC is initially diluted in the venous pool when administered
iv and then
rapidly eliminated from the systemic circulation by the liver. Thus, very
little of the initial
dose of NAC is available to systemic tissues for entry into the glutathione
pathway and
potential chemoprotection.
Another thiol-containing chemoprotectant is sodium thiosulfate or STS. Its
chemical formula is Na2SaO3 and it has been used clinically for cyanide
poisoning and for
nephrotoxicity caused by cisplatin. STS is cleared rapidly from circulation
primarily by
the kidney. The plasma half life after a bolus injection is about 17 minutes.
STS can also
inactivate platinum agents due to a covalent binding to platinum agents at
molar excess
>40:1 (STS:platinum). STS is currently used as a chemoprotectant against
carboplatin
chemotherapy-induced hearing loss (Neuwelt, JPET 1998).
Tumor selective monoclonal antibodies (mA.bs) can be used as delivery systems
for chemotherapeutic agents, toxins, and enzyme prodrug therapies based on
their
potential to discriminate neoplastic cell populations relative to normal
tissues. A marine
mAb, BR96 (IgG1) has been developed which binds to a Lewis Y (Ley)-related
antigen
abundantly expressed at the surface of cells from carcinomas of the lung,
breast, ovary and
colon while having low reactivity with most normal human tissues (Trail et
al., Cancer
Res. 52:5693-5700, 1992; Trail et al., Science 261:212-215, 1993. Remsen et
al.,
Neurosurgery, 46:704-709, 2000). The BR96 antibody was conjugated to
doxorubicin
(DOX) to produce a targeted immunoconjugate. DOX is a broad spectrum antitumor
agent frequently used in the treatment of leukemia, breast carcinoma and other
cancers,
but its efficacy is limited by dose dependent toxicities including bone marrow
suppression
and cardiotoxicity. The conjugation of the drug to the antibody produced an
immunoconjugate, BR96-DOX, with reduced systemic toxicity, and with high
specificity
2
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
against carcinomas that express the Lewis Y antigen. BR96-DOX has been shown
to be
an effective and safe agent against several tumor types growing as
subcutaneous
transplants in animal models including human lung adenocarcinoma, colon
carcinoma, and
breast carcinoma. BR96-DOX, in combination with conventional chemotherapeutic
agents such as carboplatin or Taxol (pacletaxel), has synergistic antitumor
effect. Next
generation antibodies targeting the Lewis Y antigen should also be effective
immunoconj ugates.
Unfortunately, normal human gastric cells can express the Lewis Y antigen.
Therefore, the dose-limiting toxicity of BR96-DOX is gastro-intestinal
toxicity or gastritis
(Seleh et al., J. Clip. Oncol. 2000). Similar gastritis can be expected from
any
immunoconjugate that targets the Lewis Y antigen. This GI toxicity must be
reduced for
this effective experimental approach to be successful in clinical trials.
Immunoconjugate toxicity may be increased by combination with conventional
chemotherapy. However, conventional chemotherapy does not induce gastritis on
its own.
NAC protects against chemotherapy induced systemic toxicity, not inclusive of
gastric
toxicity.
Therefore, there is a need in the art to find better ways to use thiol-based
chemoprotectants, such as NAC and STS, and to take advantage of their
pharmacokinetic
properties. There is a need in the art to reduce BR96-DOX toxicity in
patients' gastric
cells. There is a need to reduce the GI toxicity of all Lewis Y targeting
immunoconjugates, with or without addition of other commonly used
chemotherapeutic
agents. There is also a need to reduce end-organ toxicity so that higher dose
chemotherapeutic treatment regimens can be used against head and neck as well
as brain
tumors with limited drug access, that avoid dose-limiting side effects.
Summary of the Invention
A method for treating or mitigating the side effects of a cytotoxic cancer
therapy
for carcinoma type cancers is described. One or a plurality of cytotoxic
agents and a thiol-
based chemoprotectant agent are administered infra-arterially, wherein the
infra-axterial
administration is through a catheter placed into an artery that provides blood
flow to an
organ most susceptible to toxic side effects of the cytotoxic agent. In one
embodiment, the
thiol-based chemoprotectant agent is a compound selected from the group
consisting of N-
acetyl cysteine (NAC), sodium thiosulfate (STS), GSH ethyl ester, D-
methionine, Ethyol,
and combinations thereof. In another embodiment, the cytotoxic agent is
selected from the
group consisting of chimeric anti-Lewis Y monoclonal antibodies conjugated to
a
cytotoxic agent, used either alone or in combination with unconjugated,
platinum
compounds, taxanes (e.g., paclitaxel), steroid derivatives, anti-metabolites,
vinca
alkaloids, adriamycin and doxarubicin, etoposide, arsenic derivatives,
intercalating agents,
alkylating agents (e.g., melphalan) and combinations thereof. Preferably, the
cytotoxic
agent is a monoclonal antibody to the Lewis Y glycoprotein. In a preferred
embodiment,
3
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
the monoclonal antibody is BR96-~oxorubicin. Most preferably, the dose of
the'thiol-
based chemoprotectant agent per procedure is from about 200 mg/m2 to about 40
g/ma.
Most preferably, the daily dose of NAC agent during chemotherapy is from about
400
mg/m2 to about 1200 mg/m2.
A pharmaceutical composition for treatment of carcinoma type cancers for
administration via arterial catheter including a first agent that is a cancer
cytotoxic agent
and a second agent administered infra-arterially is disclosed, wherein the
first agent is a
cytotoxic compound that is used for cancer chemotherapy but is dose-limited
due to side
effects, and the second agent is a thiol-based chemoprotectant agent. In one
embodiment,
the first agent is selected from the group consisting of chimeric anti-Lewis Y
monoclonal
antibodies conjugated to a cytotoxic agent used either alone or in combination
with
unconjugated, platinum compounds, taxanes (e.g., paclitaxel), steroid
derivatives, anti-
metabolites, vinca alkaloids, adriarnycin and doxarubicin, etoposide, arsenic
derivatives,
intercalating agents, alkylating agents (such as melphalan), and combinations
thereof. In a
preferred embodiment, the chimeric monoclonal antibody is BR96-Doxorubicin.
Preferably, the second agent is administered in a pyrogen-free sterile
solution. Preferably,
the second agent is administered in a pyrogen-free, non-oxidized sterile
solution having a
reducing agent, and optionally a buffer to maintain pH at or near physiologic
pH and
optionally a metal chelating agent to bind up metal ions that can catalyze
oxidation of the
thiol-based chemoprotectant agent. Preferably, the thiol-based chemoprotectant
agent is
stored in a vial having a blanket of an inert gas. Most preferably, the inert
gas is selected
from the group consisting of argon, helium, nitrogen and mixtures thereof.
Preferably, the
reducing agent is selected from the group consisting of vitamin E, tocoperol,
dithiothreatal, mercaptoethanol, glutathione, and combinations thereof.
Preferably, the
buffer is one that is relatively non-toxic and can maintain a pH of between 6
and 8 (e.g.,
phosphate buffer, Tris buffer, Ringers solution, and the like). Preferably,
the thiol-based
chemoprotectant agent is a compound selected from the group consisting of N-
acetyl
cysteine (NAC), sodium thiosulfate (STS), GSH ethyl ester, D-methionine,
Ethyol, and
combinations thereof. Preferably, the daily dose of the thiol-based
chemoprotectant agent
during chemotherapy is from about 200 mg/m2 to about 2000 mg/mz. Most
preferably, the
dose of NAC per procedure is from about 400 mg/m2 to about 1200 mg/rn2.
A pharmaceutical composition for mitigating the gastrointestinal side effects
from
treatment of carcinoma type cancers with agents that bind to the Lewis Y
antigen
(administered alone, in combination with other cytotoxic agents, or conjugated
to other
cytotoxic agents) for administration via arterial catheter is disclosed
including an agent
administered infra-arterially, wherein the agent is a thiol-based
chemoprotectant agent.
Preferably, the Lewis Y antigen binding agent is a chimeric monoclonal
antibody,
optionally conjugated to a cytotoxic agent, and used either alone or in
combination with
unconjugated, platinum compounds, taxanes (e.g., paclitaxel), steroid
derivatives, anti-
metabolites, vinca alkaloids, adriamycin and doxorubicin, etoposide, arsenic
derivatives,
4
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
intercalating agents, alkylating agents (such as melphalan), and combinations
thereof. In a
preferred embodiment, the chimeric monoclonal antibody is BR96-Doxorubicin.
Preferably, the agent is administered in a pyrogen-free sterile solution
Preferably, the
agent is administered in a pyrogen-free, non-oxidized sterile solution having
a reducing
agent, and optionally a buffer to maintain pH at or near physiologic pH and
optionally a
metal chelating agent to bind up metal ions that can catalyze oxidation of the
thiol-based
chemoprotectant agent. Preferably, the thiol-based chemoprotectant agent is
stored in a
vial having a blanket of an inert gas. Most preferably, the inert gas is
selected from the
group consisting of argon, helicon, nitrogen and mixtures thereof. Preferably,
the reducing
agent is selected from the group consisting of vitamin E, tocoperol,
dithiothreatal,
rnercaptoethanol, glutathione, and combinations thereof. Preferably, the
buffer is one that
is relatively non-toxic and can maintain a pH of between 6 and 8 (e.g.,
phosphate buffer,
Tris buffer, Ringers solution, and the like). Preferably, the thiol-based
chemoprotectant
agent is a compound selected from the group consisting of N-acetyl cysteine
(NAC),
sodium thiosulfate (STS), GSH ethyl ester, D-methionine, Ethyol, and
combinations
thereof. Preferably, the daily dose of the thiol-based chemoprotectant agent
during
chemotherapy is from about 200 mg/m2 to about 2000 mg/m2. Most preferably, the
dose
of NAC per procedure is from about 400 mg/m2 to about 1200 mg/m2.
The invention will best be understood by reference to the following detailed
description of the preferred embodiment, taken in conjunction with the
accompanying
drawings. The discussion below is descriptive, illustrative and exemplary and
is not to be
taken as limiting the scope defined by any appended claims.
Brief Description of the Drawings
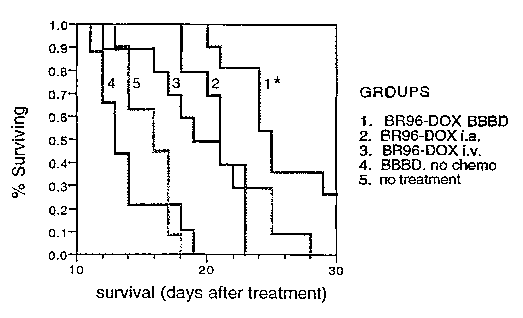
Figure 1 shows a graph representing the efficacy of BR96-DOX against human
small cell lung carcinoma cells implanted in the brain of the nude rat.
Figure 2a shows a graph representing the NAC dose response for chemoprotection
against the cytotoxicity of alkylating chemotherapeutics.
Figure 2b shows the dose/response for NAC chemoprotection of BR96-DOX
cytotoxicity in normal human gastric cells.
Figure 3 shows a graph representing protection against BR96-DOX gastric cell
toxicity.
Figure 4 shows a graph representing protection against BR96-DOX gastric cell
toxicity when cells are pretreated with BSO.
Figure 5 shows a graph representing the effect of BSO and NAC on BR96-DOX
cytotoxicity.
Figure 6 shows another graph representing the effect of BSO and NAC on BR-96-
DOX cytotoxicity.
Figure 7 shows a graph representing the effects of BSO on BR96-DOX
cyctotoxicity.
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
Figure 8 shows another graph representing the effects of BSO on BR96-DOX
cyctotoxicity.
Figure 9 shows an anatomical diagram of major arteries and the top level for
placing the catheter for administration of the thiol-based chemoprotectant
agent.
Detailed Description of the Invention
Chemoprotection with NAC and/or STS can reduce BR96-DOX toxicity in
cultured gastric cells. With its delivery optimized, NAC and/or STS reduce
BR96-DOX
toxicity in normal GI tract cells in patients, even when the immunoconjugate
is given in
combination with conventional chemotherapeutic agents.
Figure 1 shows a graph representing the efficacy of BR96-DOX against human
small cell lung carcinoma (SCLC) cells implanted in the brain of the nude rat.
A Kaplan-
Meier survival graph is shown for rats with intracerebral xenografts of B.5 LX-
1 cells with
low Lewis Y antigen expression. BR96-DOX immunoconjugate was administered with
or
without optimizing brain delivery using propofol anesthesia. There was a
significant
increase in survival in animals which received BR96-DOX following osmotic
blood brain
barrier diffusion (BBBD) (p<.0001). There was no difference in survival when
BR96-
DOX was administered either i.a, or i.v. without BBBD, and both groups were
significantly better than the controls (BBBD + saline, no treatment, p<.0001).
There was
no difference in survival between either control group.
Figure 2a shows the protection of alkylating chemotherapy cytotoxicity by
increasing doses of NAC. Cytotoxicity was assessed in cultured B.5 LX-1 SCLC
cells,
using the WST colorometric assay for live cells. Cells were treated with
approximately
LD90 dose of chemotherapy (melphalan = 20 ~M, carboplatin = 200 ~,M, cisplatin
= 20
~.M). Immediately following chemotherapy, NAC chemoprotectant was added at the
indicated concentration. NAC protected against cell death by all three
chemotherapeutic
drugs, with half maximal protection found between 0.1 mg/ml NAC and 0.3 mg/ml
NAC.
Figure 2b shows the dose/response for NAC protection against BR96-DOX
toxicity in normal human gastric cells. Cytotoxicity was assessed in cultured
normal
human gastric cells (NHGC), using the WST colorometric assay for live cells.
Cells were
treated with approximately LD90 dose of BR96-DOX, immediately followed by NAC
at
the indicated concentrations. NAC was protective against BR96-DOX toxicity in
the
range of 1 mg/ml to 3 mglml, 10-fold higher than the concentration required
for
chemoprotection against the alkylating chemotherapeutics.
Figures 3 and 4 represent graphically the percent viable gastric cells when
treated
with BR96-DOX alone and in combination with the various alternative
chemoprotectants.
Figure 3 records the results without the administration of BSO while Figure 4
records the
results after pretreatment with BSO to reduce intracellular glutathione
levels. A greater
percentage of viable gastric cells were measured with the administration of
NAC and
BR96-DOX without the administration of BSO than with the administration of BSO
6
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
(compare Figure 3 with Figure 4, particularly bar number 3 from left). The
administration
of NAC with BR96-DOX increased the percentage of viable gastric cells
regardless of
BSO administration. Addition of GSH ethyl ester provided the second highest
amount of
viable gastric cells.
S Figures 5 and 6 represent the effect of BSO and NAC on BR96-DOX cytotoxicity
in gastric carcinoma cells. As the dosage of BR96-DOX is increased, fewer
cells survive.
The greatest amount of cells survived when the combination of NAC, BSO, and
BR96-
DOX was administered, compared with the least survival with the administration
of BR96-
DOX and BSO without NAC.
Figures 7 and 8 represent the administration of BR96-DOX either alone of in
combination with BSO. As expected, the combination of BR96-DOX and BSO reduces
the percentage of viable cells to zero.
Modulation of glutathione (GSH) levels may alter the toxicity of
chemotherapeutic
agents. In vivo cytoenhancement with Buthionine Sulfoximine (BSO) was
investigated
and found to reduces cellular GSH levels and chemoprotection with N-
acetylcysteine
(NAC) and sodium thiosulfate (STS); the two later agents can mimic GSH.
Modulation of
GSH levels with BSO treatment enhances the chemotherapeutic cytotoxicity of
intra-
carotid carboplatin and melphalan. Aortic infusion increases chemoprotectant
delivery to
systemic tissue with resultant bone marrow protection, but CNS delivery is
negligible. In
one embodiment, chemoprotection is valuable in the clinical setting if
chemotherapy (~
Chemo) and chernoprotectant can be physically and/or temporally separated by
infra-carotid infusion of alkylators and aortic infusion of chemoprotectant.
Pharmaceutical Formulations
Techniques for the formulation and administration of the compounds of the
instant
application may be found in "Remington's Pharmaceutical Sciences" Mack
Publishing
Co., Easton, PA, latest addition. Suitable routes of administration are infra-
arterial.
The compositions and compounds of the present invention may be manufact~zred
in
a manner that is itself known, e.g., by means of conventional mixing,
dissolving,
emulsifying, encapsulating, entrapping, or lyophilizing processes.
Pharmaceutical
compositions for use in accordance with the present invention thus may be
formulated in
conventional manner using one or more physiologically acceptable carriers
comprising
excipients and auxiliaries that facilitate processing of the active compounds
into
preparations, which can be used pharmaceutically. Proper formulation is
dependent upon
the route of administration chosen.
For injection, the compounds of the invention may be formulated in aqueous
solutions, preferably in physiologically compatible buffers, such as Hank's
solution,
Ringer's solution, or physiological saline buffer. The compounds may be
formulated for
parenteral administration by injection, e.g., by bolus injection or continuous
infusion.
Formulations for injection may be presented in unit dosage form, e.g., in
ampoules or in
mufti-dose containers, with an added preservative. The compositions may take
such forms
7
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
as suspensions, solutions or emulsions in oily or aqueous vehicles, and may
contain
formulary agents such as suspending, stabilizing and/or dispersing agents.
Pharmaceutical formulations for parenteral administration include aqueous
solutions of the active compounds in water-soluble form. Additionally,
suspensions of the
active compounds may be prepared as appropriate oily injection suspensions.
Suitable
lipophilic solvents or vehicles include fatty oils such as sesame oil, or
synthetic fatty acid
esters, such as ethyl oleate or triglycerides, or liposomes. Aqueous injection
suspensions
may contain substances that increase the viscosity of the suspension, such as
sodium
carboxyrnethyl cellulose, sorbitol, or dextran. Optionally, the suspension may
also contain
suitable stabilizers or agents that increase the solubility of the compounds
to allow for the
preparation of highly concentrated solutions.
Alternatively, the active ingredient may be in powder form for constitution
with a
suitable vehicle, e.g., sterile pyrogen-free water, before use.
A therapeutically effective dose refers to that amount of the compound that
results
in a reduction in the development or severity of reduction in renal function.
Toxicity and
therapeutic efficacy of such compounds can be determined by standard
pharmaceutical,
pharmacological, and toxicological procedures in cell cultures or experimental
animals,
e.g., for determining the LD50 (the dose lethal to 50% of the population) and
the ED50
(the dose therapeutically effective in 50% of the population). The dose ratio
between toxic
and therapeutic effects is the therapeutic index and it can be expressed as
the ratio between
LD50 and ED50. Compounds that exhibit high therapeutic indices are preferred.
The data
obtained from cell culture assays or animal studies can be used in formulating
a range of
dosage for use in humans. The dosage of such compounds Iies preferably within
a range
of circulating concentrations that include the ED50 with little or no
toxicity. The dosage
may vary within this range depending upon the dosage form employed and the
route of
administration utilized. The individual physician in view of the patient's
condition can
choose route of administration and dosage the exact formulation. (Fingl et
al., 1975, in
"The Pharmacological Basis of Therapeutics", Ch. 1).
The amount of composition administered will, of course, be dependent on the
subject being treated, on the subject's weight, the severity of the
affliction, the manner of
administration and the judgment of the prescribing physician.
The thiol-based chemoprotectant agent is administered infra-arterially
according to
the present invention and in order for systemic tissues to be exposed to an
initial dose of
the thiol-based chemoprotectant agent in high enough concentration by
chemoprotective
effective effect and before getting to the venous circulation and being
eliminated by the
liver.
Synthesis
Each thiol-based chemoprotectant agent, such as NAC or STS, can be synthesized
by conventional methods and are commercially available as a sterile solution.
Pyrogen-
8
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
free solutions for intra-arterial administration and those with buffers for
physiologic pH
administrations can be made by conventional techniques.
The results of the following examples suggest that cyto-enhancement and
chemoprotection may be effective in combination with BR96-DOX treatment. A
NHGC
cell line and a human gastric carcinoma cell line (AGS) were obtained. Both
cell lines
were homogeneously highly positive for immunocytochemical staining with the
BR96
antibody directed against the Lewis Y antigen. BSO enhanced cytotoxicity in
the
carcinoma cells, but did not increase toxicity in the normal gastric cells,
the site of dose-
limiting toxicity of BR96-DOX. Conversely, NAC protected the normal gastric
cells from
BR96-DOX toxicity, but did not protect the carcinoma cells.
Example 1
The dose response curves for BR96-DOX and doxorubicin with or without the
addition of buthionine sulfoximine (BSO) at a concentration of 100 ~,M were
assessed.
The half maximal cytotoxic dose of BR96-DOX administered to AGS cells was
approximately 1 ~,g/ml in the absence of BSO. Pretreatment with BSO reduced
the EC50
to approximately 0.6 ~.g/ml. BSO treatment also increased the maximum
cytotoxicity of
BR96-DOX from 70% to 100 % cell kill. Pretreatment with BSO also shifted the
half
maximal cytotoxic dose of doxorubicin from 0.1 ~g/ml to approximately 0.05
~,g/ml, but
did not enhance the maximum cytotoxicity of doxorubicin, as doxorubicin alone
killed
nearly 100% of cells at maximal doses. In a second experiment, BSO did not
significantly
shift the EC50 of BR96-DOX in AGS carcinoma cells grown in an unsupplemented
medium, but did increase the maximal cell kill from 75% to 100%.
In NHGC cells, as well as in the low-expressor and high-expressor subclones of
the LX-1 SCLC cell line, BSO did not enhance the cytotoxicity of either BR96-
DOX or
doxorubicin.
Example 2
Chemoprotection in the gastric cells was examined by using NHGC normal gastric
cells wherein the chemoprotective agent N-acetylcysteine (NAC) was at least
partially
protective against BR96-DOX cytotoxicity. The level of protection was
variable, reducing
cell kill by 25% in experiment 1, 95% in experiment 2, and 55% in experiment
3. NAC
was protective independent of the presence of BSO. Other chemoprotective
agents tested
were not as effective as NAC, with only GSH ethyl ester yielding significant
protection
(cell kill reduced by 15-20%) and no significant effect of sodium thiosulfate
or d-
Methionine.
In contrast to the NHGC cells, NAC was not significantly effective at reducing
BR96-DOX cytotoxicity in the AGS gastric carcinoma cells. NAC did reverse the
enhanced cytotoxicity induced by BSO treatment, but did not alter the response
to BR96-
DOX in cells not treated with BSO.
9
CA 02420897 2003-02-27
WO 02/17962 PCT/USO1/27296
Example 3
NAC biodistribution was determined with radiolabelled tracer (n=12). Blood and
tissue GSH levels were measured with a colorometric kit 9 (n=19). For bone
marrow
toxicity studies, rats were treated with or without BSO (10 g/m2 i.p. b.i.d. x
3 days),
followed by chemotherapy consisting of infra-carotid carboplatin (200 mg/m2),
melphalan
(10 mg/m2) and etoposide phosphate (100 mg/m2) (n=61). The dose of NAC was
1200
mg/m2 and STS was 8 gm/m2. White blood cell and platelet counts were obtained
prior
to, at 6 days and 9-10 days after chemotherapy. BSO treatment for 3 days
reduced blood
and tissue GSH levels by 50-65% even in brain and intracerebral tumor in nude
rats. BSO
pretreatment enhanced the bone marrow toxicity of combination chemotherapy.
Intraarterial administration of radiolabelled NAC in the right carotid artery
resulted in high
delivery to the right cerebral hemisphere, however, infusion of NAC via a new
"aortic
infusion" technique, retrograde in the left external carotid artery with the
left internal
carotid artery occluded to prevent infusion of the brain, reduced brain
delivery to
negligible levels while increasing systemic delivery. When NAC was
administered via
"aortic infusion" before infra-carotid chemotherapy (no BSO), the magnitude of
the bone
marrow toxicity nadir at day 6 was markedly reduced (no NA : platelets 215 ~
126,
granulocytes 146 ~ 160; with NAC: platelets 470 ~ 234, granulocytes 785 ~ 494,
which by
non-parametric analysis gave a p value of < 0.02). Virtually no
myelosuppression
occurred if both NAC and STS were given via "aortic infusion" even in BSO-
treated
animals.
The discussion above is descriptive, illustrative and exemplary and is not to
be
taken as limiting the scope defined by any appended claims.