Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
METAL ALLOY COMPOSITIONS AND PROCESS
BACKGROUND OF THE INVENTION
The Government has rights in this invention pursuant to Contract No. DE-
FC-07-98ID1 3618 awarded by the Department of Energy.
This invention relates to a method and apparatus for making metal
compositions containing degenerate dendrites.
Prior to the present invention, metal compositions have been made
containing up to about 65 weight percent degenerate dendrites. Such
compositions and their method of preparation are described in U.S. Patent Nos.
3,948,650, issued April 6, 1976 to Flernings et al and U.S. Patent 3,954,455,
issued May 4, 1976 to Flemings et al. As described by these patents, a metal
alloy
is heated to form a liquid-solid mixture which is vigorously agitated to
convert the
dendrites derived from the alloy to degenerate dendrites. The metal alloy is
cooled while being vigorously agitated to the point where the viscosity of the
high
fraction solid material formed by cooling cannot be overcome by increasing
shear
forces on the high fraction solid material. These compositions can be cast
directly or can be further solidified and subsequently reheated to form a
thixotropic composition which can be cast directly (thixocasting). Substantial
advantages are attained when casting the composition since the mold is not
exposed to the heat of fusion of the material solidified prior to casting.
Furthermore, the cast material experiences far less shrinkage upon
solidification
as compared to shrinkage as compared to an article cast from a totally liquid
metal
composition. However, the process for forming the high fraction solid while
continuously agitating it over a significant portion of the cooling cycle
causes
problems. The degree of agitation required by this process causes undesirable
entrapment of gas into the high fraction solid. In addition, the solid
degenerate
dendrites formed by the present commercial practice of the process contain a
large
proportion of entrapped liquid formed from eutectic metal compositions.
Furthermore, during the cooling cycle, some macrosegregation of solid and
liquid
1
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
occurs. The entrapped gas, entrapped eutectic metal compositions and solid
liquid
macrosegregation causes the overall metal alloy composition thus formed to be
non-uniform with resultant non-uniform physical characteristics such as
strength
characteristics.
The thixocasting process wherein the high fraction solid material is
completely solidified by cooling and then reheated to form a liquid-solid
composition is effected in part in order to form more of a microstructure
wherein
the solid degenerate dendrites are spherodized, resulting in more uniform
alloys.
However, the process is more costly since the solidified metal in the gates
and
runners of the forming apparatus must be reheated from the solid state to the
liquid state resulting in the loss of the desired degenerate dendrite
microstructure.
European Patent Application 96108499.3 (Publication No. EP0745
694A1) discloses a process for forming a liquid-solid metal alloy composition
which can be formed by casting. In this process, a melt of the alloy is formed
in
one or more first vessels. The melt then is transferred to an insulating
vessel
under cooling conditions wherein crystal nuclei form in the melt. The melt
then is
further cooled in the insulating vessel under conditions to effect formation
of
spheroidal solids which form on the nuclei to produce the liquid-solid
composition which is then cast. The melt is transferred into the insulating
vessel
either by moving it over a cooled inclined jig which diverts a melt stream
into the
insulating vessel or by pouring a plurality of metal alloy melts into the
insulating
vessel. When utilizing the latter mode of transfer, one of the melts is at a
temperature lower than the liquidus temperature of a second melt so that the
crystal nuclei form in the second melt. A major problem is that the poured
melts
entrap gas therein during the transfer and are retained therein due to the
presence
of solids. This results in a nonuniform final metal alloy composition having
defects caused by the entrapped gas. In addition, the cooling rate and degree
of
agitation are poorly controlled such that the crystal nuclei are limited in
number
and are not homogeneously dispersed in the liquid melt. This results in
degenerate
2
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
dendrites containing entrapped liquid and and in a formed metal alloy product
having nonuniform physical characteristics throughout its volume. Furthermore,
a
skin is formed on the bottom surface of the solidified product which then must
be
removed in order to obtain a desired homogeneous final metal alloy product.
European Patent Application No. 95 309498.4 (Publication NO. EPO
719606 Al) also discloses formation of a liquid-solid metal alloy composition
by
forming an alloy melt in a first vessel and transferring it to an insulating
vessel
over a cooled inclined surface to form crystal nuclei in the melt. The melt is
then
cooled to form a liquid-solid alloy composition containing spheroidal solids
which can be cast. Since this process relies on a pouring step in the presence
of
solids, it also has the disadvantage of entrapping gas while forming
spheroidal
solids nonhomogeneously distributed in the final metal alloy product.
U.S. Patents 5,144,998; 5,555,926; 5,901,778 and 5,865,240 also disclose
processes for forming a liquid-solid metal alloy composition which effects
formation of a metal alloy melt in a first vessel which then is transferred to
a
second vessel under poorly controlled cooling and agitation conditions to form
solid nuclei in the melt. Since the melt is transferred from one vessel to
another
while partially solidifying the melt, the problems associated with gas
entrapment
and nonhomogeneous sized spheroidal solids are encountered as set forth above.
Accordingly, it would be desirable to provide a skinless homogeneous
liquid-solid metal alloy compositions which are free from entrapped gas and
wherein the solid component is free of an eutectic composition. In addition,
it
would be desirable to form such compositions wherein the primary solids have
maximum sphericity which are homogeneously distributed throughout the volume
of the metal alloy composition. Such a metal alloy composition would be more
easily shaped to provide a product having homogeneous physical characteristics
throughout its volume. Furthermore, it would be desirable to provide a process
for forming such liquid-solid compositions wherein crystal nuclei are
homogeneously dispersed within a liquid melt so that homogeneously sized
3
CA 02422696 2003-03-17
71915-11
primary solids can be formed throughout the volume of the
liquid-solid metal alloy.
SUMMARY OF THE INVENTION
This invention is based on the discovery that a
skinless homogeneous liquid-solid metal alloy composition
can be formed from a molten metal alloy composition, free of
entrapped gas, substantially free of entrapped eutectic in
primary solids, and having primary solids which are
substantially spherical by controlling conditions of cooling
and vigorous agitation of a liquid precursor to the liquid-
solid metal alloy. It has been found that rapid cooling and
vigorous agitation can be effected for a short time over a
narrow temperature range near the liquidus temperature of
the molten metal alloy at a controlled cooling rate to form
solid particle nuclei. Agitation is then ceased in a batch
process or the liquid-solid alloy is removed from the source
of agitation in a continuous process while cooling is
continued so that the primary solids are formed on the solid
particle nuclei while avoiding the formation of a solid
dendritic network. The resultant skinless composition
comprises homogeneously sized primary spheroidal solid
particles substantially free of eutectic metal alloy
composition and which is free of entrapped gas. The
resultant liquid-solid metal alloy composition that can be
formed such as by casting.
According to one aspect of the present invention,
there is provided a process for forming a skinless metal
composition free of entrapped gas and having solid discrete
degenerate dendrites homogeneously distributed within a
liquid phase of said metal composition, said solid discrete
degenerate dendrites being substantially free of eutectics
4
CA 02422696 2003-03-17
71915-11
which comprises: (a) heating a metal alloy composition in a
vessel to form a liquid free of solids and comprising said
metal composition, (b) cooling said liquid while agitating
said liquid under conditions to form solid nuclei
homogeneously dispersed within said liquid while avoiding
entrapment of gas in said liquid at a cooling rate of
between about 2 degrees C per second and about 10 degrees C
per second to form a liquid-solid composition containing
between about 1 and about 10 weight fraction solid,
(c) ceasing agitation of said liquid or removing the liquid-
solid alloy from the source of agitation and (d) continuing
cooling said liquid while said liquid-solid composition is
quiescent until said metal composition is solid.
According to another aspect of the present
invention, there is provided a process for forming a
skinless metal composition free of entrapped gas and having
solid discrete degenerate dendrites homogeneously
distributed within a liquid phase of said metal composition
said solid discrete degenerate dendrites being substantially
free of eutectics which comprises: (a) heating a metal alloy
composition in a vessel to form a liquid free of solids and
comprising said metal composition, (b) cooling said liquid
while agitating said liquid under conditions to form solid
nuclei homogeneously dispersed within said liquid while
avoiding entrapment of gas in said liquid at a cooling rate
of between about 2 degrees C per second and about 10 degrees
C per second to form a liquid-solid composition containing
between about 1 and about 10 weight fraction solid,
(c) ceasing agitation of said liquid or removing the liquid-
solid alloy from the source of agitation, (d) continuing
cooling said liquid while said liquid-solid composition is
quiescent until said metal composition contains up to about
65 weight percent primary solids homogeneously dispersed in
4a
CA 02422696 2003-03-17
71915-11
a liquid secondary phase, and (e) shaping said liquid-solid
mixture formed in step (d).
BRIEF DESCRIPTION OF THE DRAWINGS
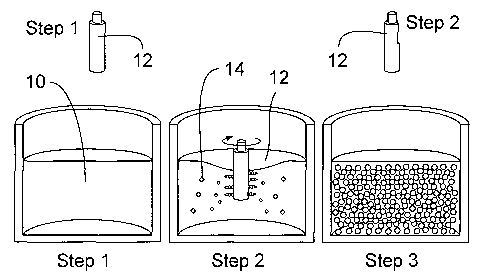
Fig. 1(a) is a schematic diagram illustrating the
process of this invention.
Fig. i(b) is a graph of temperature as a function
of time for the process of Fig. 1(a).
Fig. 2(a) is a photomicrograph of a metal
composition of Example 1(a) comprising primary solids,
secondary solid phase without reheating.
Fig. 2(b) is a graph of temperature as a function
of time for the process for producing the product of
Fig. 2 (a) .
Fig. 3(a) is a photomicrograph of a metal
composition of Example 1(a) comprising primary solids,
secondary solid phase with reheating.
4b
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
Fig. 3 (b) is a graph of temperature as a function of time for the process
for producing the product of Fig. 3 (a).
Fig. 4 (a) is a photomicrograph of a metal composition of the prior art
comprising primary solids, secondary solid phase and an eutectic metal alloy
composition entrapped within the primary solids.
Fig. 4 (b) is a graph of temperature as a function of time for the process
for producing the product of Fig. 4(a).
Fig. 5 (a) is a photomicrograph of a metal composition of the prior art
comprising primary solids, secondary solid phase and an eutectic metal alloy
composition entrapped within the primary solids.
Fig. 5(b) is a graph of temperature as a function of time for the process for
producing the product of Fig. 5 (a).
Fig. 6(a) is a photomicrograph of a metal composition of Example 1(b)
comprising primary solids, secondary solid phase without reheating.
Fig. 6(b) is a graph of temperature as a function of time for the process for
producing the product of Fig. 6 (a).
Fig. 7(a) is a photomicrograph of a metal composition of Example 1(b)
comprising primary solids, secondary solid phase with reheating.
Fig. 7 (b) is a graph of temperature as a function of time for the process
for producing the product of Fig. 7 (a).
Fig. 8(a) is a photomicrograph of a metal composition of Example 2
comprising primary solids, secondary solid phase without reheating.
Fig. 8(b) is a graph of temperature as a function of time for the process for
producing the product of Fig. 8 (a).
Fig. 9(a) is a photomicrograph of a metal composition of Example 2
comprising primary solids, secondary solid phase with reheating.
Fig. 9 (b) is a graph of tenlperature as a function of time for the process
for producing the product of Fig. (a).
1
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
Fig. 10 is a schematic diagram illustrating the continuous process of this
invention.
DESCRIPTION OF SPECIFIC EMBODIMENTS
The present invention provides a process for forming a skinless metal
alloy comprising primary solids substantially free of eutectic metal
compositions,
a secondary phase which is liquid or solid, primary solids having improved
sphericity homogeneously distributed throughout the volume of the metal alloy
which metal alloy is free of entrapped gas. By the phrase "substantially free
of
entrapped eutectic metal compositions" as used herein is meant less than about
2
% by volume, preferably less than 1 % by volume eutectic metal compositions
entrapped in the primary solids based of the volume of primary solids. The
primary solids of the metal alloy compositions of this invention are
characterized
by an overall improved sphericity as compared to metal alloy composition of
the
prior art. This results in providing metal alloys which are more easily formed
since the sphericity of the primary solids reduces friction within the metal
alloy
when it is moved during a shaping step as compared to metal alloys having
primary solids with irregular shapes or having dendritic elongations at their
surfaces.
The process of this invention comprises a first step of forming a metal
alloy liquid composition within a vessel. The liquid metal alloy composition
positioned within the vessel then can be cooled while vigorously agitating the
cooled alloy such as by stirring under conditions to form solid nuclei
particles
while avoiding entrapment of gas within the agitated alloy compositions.
Alternatively, the liquid metal alloy composition can be transferred to a
second
vessel under conditions to prevent formation of solids prior to cooling. Thus,
the
temperature of the walls of the second vessel can be above, at or below the
liquidus temperature of the metal alloy composition. When the wall temperature
is below the liquidus temperature, the wall temperature is raised to convert
any
formed solids to a liquid. In addition, the liquid metal alloy composition is
6
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
rendered quiescent so that entrapped gas, if any, floats to the liquid surface
without interference by the presence of solids and is removed from the liquid.
The alloy is vigorously agitated while being cooled in a manner such that the
solid
nuclei are distributed throughout the metal liquid alloy composition
substantially
homogeneously. The degree of agitation is such as to effect substantially
homogeneous distribution of the formed crystal nuclei while avoiding
entrapment
of gas. Agitation is effected while utilizing a rapid cooling rate range for a
short
time such as between about 1 second and about 1 minute, preferably between
about 1 and about 30 seconds over a temperature range corresponding to a
percent
solidification of the alloy of between about 1 and about 20 % weight fraction
solids, preferably between about 3 and about 7 % weight fraction solids while
the
liquid composition is cooled without agitation to effect forming the solid
nuclei.
Agitation can be effected utilizing a cool probe in any manner which avoids
excessive cavitation at the liquid surface thereby to avoid entrapment of gas
in the
liquid. The probe can be rendered cool by passing a heat exchange fluid, such
as
water therethrough. Representative suitable agitation means include one or a
plurality of cylindrical rods provided with an internal cooling means, a
helical
probe, or the like that preferably extends the depth of the liquid. The probe
extends into a portion of the depth of the liquid up to substantially 100 % of
the
depth of the liquid to promote homogeneous dispersion of the crystal nuclei.
Agitation then is ceased in a batch process or the liquid-solid alloy is
removed
from the source of agitation in a continuous process. The resultant liquid-
solid
metal alloy composition then is cooled within the vessel to effect formation
of
spheroidal solid particles about the solid nuclei particles up to a
concentration
wherein the spheroidal solid particles increase the viscosity of the overall
liquid-
solid composition where it can be moved into a formation step such as a
casting
step. Generally, the upper weight percent primary solids, is between about 40
and
about 65 percent and preferably contains 10 to 50 percent based on the total
weight of the liquid solid composition. Surprisingly, the formation of
spheroidal
7
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
solid particles without agitation is effected by coarsening without the
formation an
interconnected dendrite network. In addition, since agitation is effected only
for a
short period of time, the inclusion of entrapped gas within the alloy
composition
is avoided. In addition, it has been found that by operating in this manner,
macrosegregation of elements is minimized or eliminated throughout the volume
of the metal alloy product produced. The resultant liquid-solid composition
then
is formed such as by casting.
The metal alloy composition comprising the primary solids and the
secondary phase of the composition of this invention can be formed from a wide
variety of metals or alloys which, when frozen from a liquid state without
agitation form a dendritic network structure. When the composition of this
invention includes primary solid discrete particles, the composition contains
a
secondary phase which can be either solid or liquid. The secondary phase is
solid
when the metal composition is solid and liquid when the metal composition is
partially liquid. The secondary solid can be formed of one or more solid
compositions. The primary particles comprise small degenerate dendrites or
nodules which are generally spheroidal in shape and are formed as a result of
first
agitating the melt while cooling when the secondary phase is liquid followed
by
cooling the secondary phase of the partially molten alloy under a quiescent
condition without agitation. The primary solid particles are made up of a
single
phase having an average composition different from the average composition of
the surrounding secondary phase, which secondary phase can itself comprise
primary and secondary phases upon further solidification.
By the term "primary solid" as used herein is meant the phase or phases
solidified to form discrete degenerate dendrite particles as the temperature
of the
melt is reduced below the liquidus temperature of the metal into the liquid-
solid
temperature range after the solid nuclei are formed and prior to casting the
liquid-
solid slurry form. The primary solids are degenerate dendrites in that they
are
characterized by having smoother surfaces and less branched structures which
8
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
approach a more spherical configuration than normal dendrites and do not have
a
dendrite structure when interconnection of the primary particles is effected
to
form a network dendritic structure. In addition, the primary solids are
substantially free on eutectics. By the term "secondary solid" as used herein
is
meant the phase or phases that solidify from the liquid existing in the slurry
at a
lower temperature than at which the primary solid particles are formed after
formation of primary solids ceases. Normally solidified alloys have branched
dendrites separated from each other in the early stages of solidification,
i.e., up to
15 to 20 wt. percent solid, and develop into an interconnected network as the
temperature is reduced and the weight fraction solid increase. The composition
containing primary solids of this invention, on the other hand, prevents
formation
of the interconnected network by maintaining the discrete primary particles
separated from each other by the liquid phase even up to solid fractions of
about
65 weight percent.
The secondary solid which is formed during solidification from the liquid
phase subsequent to forming the primary solid contains one or more phases of
the
type which would be obtained during solidification by presently employed
casting
processes. That is, the secondary phase comprises solid solutions, or mixtures
of
dendrites, compounds and/or solid solutions.
The size of the primary particles depends upon the alloy or metal
composition employed, the temperature of the solid-liquid mixture and the time
the alloy spends in the solid-liquid temperature range. Thus, in general, the
size
of the primary particles depends on composition, thermo-mechanical history of
the slurry, number of crystal nuclei formed, cooling rate and can range from
about
1 to about 1,000 microns and are homogeneously sized throughout the metal
alloy
composition. It is preferred that the composition contain between 10 and 50
weight percent primary solids since these compositions have a viscosity which
promotes ease of casting or forming.
The compositions of this invention can be formed from any metal alloy
9
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
system or pure metal regardless of its chemical composition which, when frozen
from the liquid state without forming solid nuclei forms a dendritic
structure.
Even though pure metals and eutectics melt at a single temperature, they can
be
employed to form the composition of this invention since they can exist in
liquid-
solid equilibrium at the melting point by controlling the net heat input or
output to
the melt so that, at the melting point, the pure metal or eutectic contains
sufficient
heat to fuse only a portion of the metal or eutectic liquid. This occurs since
complete removal of heat of fusion in a slurry employed in the casting process
of
this invention cannot be obtained by equating the thermal energy supplied and
that removed by a cooler surrounding environment. Representative suitable
alloys
include lead alloys, magnesium alloys, zinc alloys, aluminum alloys, copper
alloys, iron alloys, nickel alloys, cobalt alloys. Examples of these alloys
are lead-
tin alloys, zinc-aluminum alloys, zinc-copper alloys, magnesium-aluminum
alloys, magnesium-aluminum-zinc alloys, magnesium-zinc alloys, aluminum-
silicon alloys, aluminum-copper-zinc-magnesium alloys, copper-tin bronzes,
brass, aluminum bronzes, steels, cast irons, tool steels, stainless tells,
super-alloys,
and cobalt-chromium alloys, or pure metals such as iron, copper or aluminum.
The following examples illustrate the present invention and are not
intended to limit the same.
Example Ia
A356 alloy processed in a high-density graphite crucible
The following is a detailed description of a method for producing A356
aluminum alloys with non-dendritic structures, with reference to Figures 1-5.
About 405 g of A356 aluminum alloy stock were melted in a high-density
graphite crucible 3 inches tall, with a 2.5 inch inner diameter, and a 0.25
inch wall
thickness. The crucible was placed inside an air-circulating resistance
furnace,
which was programmed to slowly cool the melt to a temperature 7 C above its
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
liquidus temperature. After holding at that temperature for several minutes, a
solid copper rod with a 0.5 inch diameter, rotating at 1236 rpm, and initially
at
room temperature, was introduced in the furnace through an opening in its top
and
immersed into the melt 1.8 inches. The immersed, rotating rod provided a
combination of rapid cooling and vigorous agitation of the melt. This led to a
rapid decrease of the melt temperature, which dropped below the liquidus
temperature, causing copious nucleation of primary aluminum particles. The
rotating rod remained in the melt for 15 seconds, dropping the melt
temperature to
615 C, about 2 C below the liquidus temperature, which corresponds to about 3
% fraction solid. After the combined cooling and agitation period, the rod was
removed from the melt, and the melt was cooled and solidified completely.
Figure 1(a) shows the three general processing steps of this invention. In
step 1,
the completely liquid melt 10 is formed prior to introducing rotating cold rod
12
into the melt 10. In step 2, crystal nuclei 14 are formed in the liquid melt
10 as a
result of contact with the rotating cold rod 12. In step 3, the rod 12 is
removed
from the liquid-solid metal composition 16 in which spheroidal primary solids
are
formed while cooling is continued at a cooling rate shown in Fig. 1(b) until
the
composition is solid.
Figure 2(a) shows the homogeneous structure found throughout the
volume of the solidified alloy after processing according to the above example
and the cooling curve shown in Figure 2(b). Figure 3(a) shows the same
material
after rapidly reheating to 590 C, isothermally holding for approximately 10
minutes, and quenching according to the temperature profile of Figure 3(b).
Figure 4(a) and (b) shows the microstructure and temperature profile of
reheated 3
inch diameter MHD billet (electromagnetically stirred) used commercially for
thixocasting. Figure 4(a) and (b) shows the microstructure and temperature
profile for reheated 1 inch diameter of a commercially available a Stress
Induced
Melt Activation (SIMA) processed billet.
The striking differences when comparing the microstructures of the
11
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
reheated material processed by the method described by this invention with
those
of the reheated 1VII-ID and SIMA billets, are the negligible amount of
entrapped
eutectic compositions, and the improved sphericity found in the reheated
material
processed by the method described by this invention. This difference is of
critical
importance during semisolid metal forming operations for which the material is
to
be used. At forming temperatures, the entrapped eutectic compositions are
liquid,
but do not contribute to the flow behavior of the semi-solid material, which
behaves as if it contained a larger amount of primary solid particles. When
significant amounts of entrapped liquid are present in unpredictable
quantities, as
shown in the reheated 1VBID material, flow behavior becomes difficult to
predict
and forming operations may yield large amounts of defective products.
Example lb
A356 alloy processed in a clay-graphite crucible
The following is a detailed description of a method for producing A356
aluminum alloys with non-dendritic structures, with reference to Figs. 6(a),
6(b),
7(a) and 7(b)..
About 540 g of A356 aluminum alloy stock were melted in a clay-graphite
crucible 5 inches tall, with a 3 inch inner diameter, and a 0.6 inch wall
thickness.
The crucible was placed inside an air-circulating resistance furnace, which
was
programmed to slowly cool the melt to a temperature slightly above its
liquidus
temperature. When the melt had been cooled to 3 C above the liquidus
temperature, a helix made with copper rod, rotating at 780 rpm, and initially
at
room temperature, was introduced in the furnace through an opening in its top
and
immersed into the melt 2 inches. The helix had a diameter of 1 inch and was
made from rod with a 0.25 inch diameter. The immersed, rotating helix provided
a combination of rapid cooling and vigorous agitation of the melt. This led to
a
rapid decrease of the melt temperature, which dropped below the liquidus
temperature, causing copious nucleation of primary aluminum particles. The
rotating helix remained in the melt for 30 seconds, dropping the melt
temperature
12
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
to 616 C, about 1 C below the liquidus temperature, which corresponds to
aboutl % fraction solid. After the combined cooling and agitation period, the
rod
was removed from the melt, and the melt was cooled and solidified completely.
Figure 6(a) shows the homogeneous structure found throughout the volume of the
solidified alloy after processing according to the above example and the
cooling
curve shown in Figure 6(b). Figure 7(a) shows the same material after rapidly
reheated to 590 C, isothermally holding for approximately 10 minutes, and
quenching according to the temperature profile in Figure 7(b).
Example 2
A356 alloy processed using an induction furnace
The following is a detailed description of a method for producing A356
aluminum alloys with non-dendritic structures, with reference to Figs 8(a),
8(b),
9(a) and 9(b).
About 590 g of A356 aluminum alloy stock were melted in a high-density
graphite crucible 4 inches tall, with a 3 inch inner diameter, and a 0.5 inch
wall
thickness. The crucible was placed inside an induction furnace, which was
programmed to superheat the melt roughly 50 C above its liquidus temperature.
The furnace power was then shut off and the melt was allowed to slowly cool in
the crucible. When the melt had been cooled to within 6 C above the liquidus
temperature, a helix made with copper rod, rotating at 1000 rpm, and initially
at
room temperature, was introduced in the furnace through an opening in its top
and
immersed into the melt 2 inches. The helix has a diameter of 1 inch and was
made from rod with a 0.25 inch diameter. The immersed, rotating helix provided
a combination of rapid cooling and vigorous agitation of the melt. This led to
a
rapid decrease of the melt temperature, which dropped below the liquidus
temperature, causing copious nucleation of primary aluminum particles. The
rotating helix remained in the melt for 32 seconds, dropping the melt
temperature
to 608 C, about 9 C below the liquidus temperature, which corresponds to
about
18 % fraction solid. After the combined cooling and agitation period, the rod
was
13
CA 02422696 2003-03-17
WO 02/24381 PCT/US01/29223
removed from the melt, and the melt was cooled and solidified completely.
Figure
8(a) shows the homogeneous structure found throughout the volume of the
solidified alloy after processing according to the above example and the
cooling
curve shown in Figure 8(b). Figure 9(a) shows the same material after rapidly
reheated to 590 C, isothermally holding for approximately 20 minutes, and
quenching according to the temperature profile in Figure 9(b).
As shown in Fig. 10 a continuous process for forming the metal alloy
compositions of this invention is illustrated. A vessel 20 comprises a first
subvesse122 for holding a completely liquid metal composition 24 which is
introduced from vessel 26. Subvesse128 is adapted to receive water cooled
rotating rod 30 and to receive the liquid metal composition through passageway
32 which can be opened or closed with conventional valve means (not shown).
Crystal nuclei 34 are formed in subvessel 28 in the manner described above.
The
liquid-solid composition 36 is removed from the agitating rod 30 in
subvesse128
either by gravity, under pressure or by a suitable pulling force on the
composition
36 so that it can be cooled in the absence of agitation to form a composition
that
can be shaped either directly or by being solidified and then reheated to form
a
liquid-solid composition.
14