Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Le A 34 838-Forei~n Countries Lin/walN'T
1 ~~a~~~'~.~~~ _
~, r' .~'~,~ ~.~.
'~..............._.,.........",._...-" ~ ~ ~ ~''~ :~ ,~ '~
Pronionic acid derivatives ~'""'-w-'.
The present invention relates to novel potent PPAR-alpha-activating compounds
for
treating, for example, coronary heart disease, and to their preparation.
In spite of many successful therapies, coronary heart disease (CHD) remains a
serious
public health problem. Treatment with statins, which inhibit HMG-CoA
reductase,
successfully lowers both LDL cholesterol plasma concentrations and the
mortality of
patients at risk; however, convincing treatment strategies for the therapy of
patients
having an unfavourable HDL/LDL cholesterol ratio or hypertriglyceridaemia are
still
not available to date.
Currently, fibrates are the only therapy option for patients of these risk
groups. They
act as weak agonists of the peroxisome-proliferator-activated receptor (PPAR)-
alpha
(Nature 1990, 347, 645-50). A disadvantage of the fibrates which have hitherto
been
approved is that their interaction with the receptor is only weak, requiring
high daily
doses and causing considerable side-effects.
WO 00/23407 describes PPAR modulators for treating obesity, atherosclerosis
and/or
diabetes.
It was an object of the present invention to provide novel compounds which can
be
used as PPAR-alpha modulators.
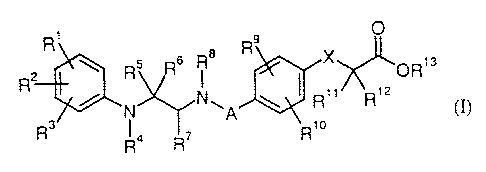
It has now been found that this object is achieved by compounds of the general
formula (I)
CA 02424540 2003-04-02
Ix A 34 838-Forei~-n Countries
-2-
R, O .
~ Rs R6 i 8 R x OR,s
I R" R, z
y
Rs R4 R~ R, o
in which
A represents a bond or represents a -CHZ- or -CH2CH2- group,
X represents O, S or CHZ,
R', Rz and R3 are identical or different and independently of one another each
represents hydrogen, (C,-C6)-alkyl, (C3-C~)-cycloalkyl, hydroxyl, (C,-C6)-
alkoxy, (C6-C,o)-aryloxy, halogen, trif7uoromethyl, tril7uoromethoxy, (Ci-C6)-
alkylaminosulphonyl, nitro or cyano,
or
R' and RZ are attached to two adjacent carbon atoms and together with these
form a
fused cyclohexane or benzene ring, the latter optionally being substituted by
a
(C,-C4)-alkylsulphonylmethyl group,
and
R3 is as defined above,
R4 represents hydrogen or (C,-Ca)-alkyl,
RS and R6 represent hydrogen or together with the carbon atom to which they
are
attached form a carbonyl group,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-3-
R7 represents hydrogen, (C~-C6)-alkyl, phenyl or benzyl, where the aromatic
radicals mentioned for their part may in each case be mono- to trisubstituted
by identical or different substituents from the group consisting of (C,-C6)-
alkyl, (CI-C6)-alkoxy, hydroxyl and halogen,
Rg represents hydrogen, (C6-Coo)-aryl or represents (C,-C4)-alkyl which for
its
part may be substituted by hydroxyl, trifluoromethoxy, (C,-C.~)-alkoxy or
phenoxy, which for their part are optionally mono- or disubstituted by
trifluoromethyl, or by (C6-C,o)-aryl or 5- or 6-membered heteroaryl having up
to three heteroatoms from the group consisting of N, O and S, where all aryl
and hetaroaryl rings mentioned may for their pan in each case be mono- to
trisubstituted by identical or different substituents from the group
consisting
of halogen, hydroxyl, (C,-C6)-alkyl, (C,-C6)-alkoxy, trifluoromethyl,
trifluoromethoxy, cyano, vitro and amino,
R~ and R'° are identical or different and independently of one another
each represents
hydrogen, (C,-C6)-alkyl, (C,-C6)-alkoxy, trifluoromethyl, trifluoromethoxy or
halogen,
R'1 and R~z are identical or different and independently of one another each
represents hydrogen or (C,-C6)-alkyl or together with the carbon atom to
which they are attached form a (C~-C~)-cycloalkyl ring,
and
R'3 represents hydrogen or represents a group which can hydrolysed and
degraded
to the corresponding carboxylic acid,
and their pharmaceutically acceptable salts, hydrates and solvates,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-4-
which have pharmacological action and can be used as medicaments or for
preparing
medicament formulations.
In the context of the invention, a hydrolysable group in the definition of R13
is a
group which, in particular in the body, leads to a conversion of the -C(O)OR13
grouping into the corresponding carboxylic acid (R13 = hydrogen). Such groups
are,
by way of example and by way of preference: benzyl, (C1-C6)-alkyl or (C3-C8)-
cycloalkyl which are in each case optionally mono- or polysubstituted by
identical or
different substituents from the group consisting of halogen, hydroxyl, amino,
(C,-C6)-alkoxy, carboxyl, (C,-C6)-alkoxycarbonyl, (C~-C6)-alkoxycarbonylamino
and
(C~-C6)-alkanoyloxy, and in particular (C,-C,~)-alkyl which is optionally mono-
or
polysubstituted by identical or different substituents from the Group
consisting of
halogen, hydroxyl, amino, (C1-C~)-alkoxy, carboxyl, (C~-C4)-alkoxycarbonyl,
(C,-C4)-alkoxycarbonylamino and (C1-Ca)-alkanoyloxy.
In the context of the invention, (C,-C6)-alkyl and (C,-C4)-alkyl represent a
straight
chain or branched alkyl radical having 1 to 6 or 1 to 4 carbon atoms,
respectively.
Preference is given to a straight-chain or branched alkyl radical having 1 to
4 carbon
atoms. The following radicals may be mentioned by way of example and by way of
preference: methyl, ethyl, n-propyl, isopropyl and tert-butyl.
In the context of the invention, (C6-C,o)-aryl represents an aromatic radical
having 6 to
10 carbon atoms. A preferred example is the aryl radical phenyl.
In the context of the invention, (C3-Ca)-cycloalkyl and (C4-C~)-cycloalkyl
represent a
cycloalkyl group having 3 to 8 and 4 to 7 carbon atoms, respectively. The
following
radicals may be mentioned by way of example and by way of preference:
cyclobutyl,
cyclopentyl and cyclohexyl.
In the context of the invention, (C1-C6)-alkoxy represents a straight-chain or
branched
alkoxy radical having 1 to 6 carbon atoms. Preference is given to a straight-
chain or
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-5-
branched alkoxy radical having 1 to 4 carbon atoms. The following radicals may
be
mentioned by way of example and by way of preference: methoxy, ethoxy, n-
propoxy,
isopropoxy, tent-butoxy, n-pentoxy and n-hexoxy.
In the context of the invention, (C6-Cloy-aryloxy represents an aromatic
radical
having 6 to 10 carbon atoms which is attached via an oxygen atom. A preferred
example is the aryloxy radical phenoxy.
In the context of the invention, (C~-C6)-alkoxycarbonyl represents a straight-
chain or
branched alkoxy radical having 1 to 6 carbon atoms which is attached via a
carbonyl
group. Preference is given to a straight-chain or branched alkoxycarbonyl
radical
having 1 to 4 carbon atoms. The following radicals may be mentioned by way of
example and by way of preference: methoxycarbonyl, ethoxycarbonyl,
n-propoxycarbonyl, isopropoxycarbonyl and tert-butoxycarbonyl.
In the context of the invention, (C,-C6)-alkoxycarbonylamino represents an
amino
group having a straight-chain or branched alkoxycarbonyl substituent which has
1 to
6 carbon atoms in the alkoxy radical and is attached via the carbonyl group.
Preference is given to an alkoxycarbonylamino radical having 1 to 4 carbon
atoms.
The following radicals may be mentioned by way of example and by way of
preference: methoxycarbonylamino, ethoxycarbonylamino, n-propoxycarbonylamino,
isopropoxycarbonylamino and ten-butoxycarbonylamino.
In the context of the invention, (C,-C6)-alkanoyloxy represents a straight-
chain or
branched alkyl radical having 1 to 6 carbon atoms which carries a doubly
attached
oxygen atom in the 1-position and is attached in the 1-position via a further
oxygen
atom. The following radicals may be mentioned by way of example and by way of
preference: acetoxy, propionoxy, n-butyroxy, i-butyroxy, pivaloyloxy,
n-hexanoyloxy.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-6-
In the context of the invention, (C1-C6)-alkylaminosulphonyl represents an
amino
group which is attached via a sulphonyl group and has a straight-chain or
branched
alkyl substituent having 1 to 6 carbon atoms. Preference is given to an
alkylaminosulphonyl radical having 1 to 4 carbon atoms. The following radicals
may
be mentioned by way of example and by way of preference: methylaminosulphonyl,
ethylaminosulphonyl, n-propylaminosulphonyl, isopropylaminosulphonyl and tert-
butylaminosulphonyl.
In the context of the invention, halogen represents fluorine, chlorine,
bromine and
iodine. Preference is given to chlorine or fluorine.
In the context of the invention, 5- or 6-membered heteroaryl having up to 3
heteroatoms selected from the group consisting of S, N and O generally
represents a
monocyclic heteroaromatic radical which is attached via a ring carbon atom of
the
heteroaromatic radical, or, if appropriate, via a ring nitrogen atom of the
heteroaromatic radical. The following radicals may be mentioned by way of
example
and by way of preference: furanyl, pyrrolyl, thienyl, thiazolyl, oxazolyl,
imidazolyl,
triazolyl, pyridyl, pyrimidyl, pyridazinyl. Preference is given to furanyl,
thienyl and
oxazolyl.
Depending on the substitution pattern, the compounds according to the
invention can
exist in stereoisomeric forms which are either like image and mirror image
(enantiomers) or which are not like image and mirror image (diastereomers).
The
invention relates both to the enantiomers or diastereomers and to their
respective
mixtures. The racemic forms, like the diastereomers, can be separated in a
known
manner into the stereoisomerically uniform components.
Furthermore, certain compounds may be present in tautomeric forms. This is
known
to the person skilled in the art, and such compounds are likewise included
within the
scope of the invention.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
The compounds according to the invention can also be present as salts. In the
context
of the invention, preference is given to physiologically acceptable salts.
Physiologically acceptable salts can be salts of the compounds according to
the
invention with inorganic or organic acids. Preference is given to salts with
organic
acids such as, for example, hydrochloric acid, hydrobromic acid, phosphoric
acid or
sulphuric acid or to salts with organic carboxylic or sulphonic acids such as,
for
example, acetic acid, propionic acid, malefic acid, fumaric acid, malic acid,
citric
acid, tartaric acid, lactic acid, benzoic acid, or methanesulphonic acid,
ethanesulphonic acid, benzenesulphonic acid, toluenesulphonic acid or
naphthalene-
disulphonic acid.
Physiologically acceptable salts can also be salts of the compounds according
to the
invention with bases, such as, for example, metal or ammonium salts. Preferred
examples are alkali metal salts (for example sodium salts or potassium salts),
alkaline
earth metal salts (for example magnesium salts or calcium salts), and also
ammonium
salts which are derived from ammonia or organic amines, such as, for example,
ethylamine, di- or triethylamine, ethyldiisopropylamine, monoethanolamine, di-
or
triethanolamine, dicyclohexylamine, dimethylaminoethanol, dibenzylamine,
N-methylmorpholine, dihydroabietylamine, 1-ephenamine, methylpiperidine,
arginine, lysine, ethylenediamine or 2-phenylethylamine.
The compounds according to the invention can also be present in the form of
their
solvates, in particular in the form of their hydrates.
Preference is given to compounds of the general formula (I),
in which
A represents a bond or represents a -CH2- or -CHZCHZ- group,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
_g_
X represents O, S or CHZ,
R', RZ and R3 are identical or different and independently of one another each
represents hydrogen, (C~-C6)-alkyl, (C,-C6)-alkoxy, hydroxyl, halogen, tri-
fluoromethyl, trifluoromethoxy, vitro or cyano,
R~ represents hydrogen or (C~-C.~)-alkyl,
RS and R6 each represents hydrogen or together with the carbon atom to which
they
are attached form a carbonyl group,
R' represents hydrogen, (C,-C6)-alkyl, phenyl or benzyl, in which the aromatic
radicals mentioned for their pan may in each case be mono- to trisubstituted
by identical or different substituents from the group consisting of (C~-C6)-
alkyl, (C,-C6)-alkoxy, hydroxyl or halogen,
R8 represents hydrogen, (C6-C,o)-aryl or (C,-C4)-alkyl, which for its part is
optionally substituted by (C6-C,o)-aryl or 5- or 6-membered heteroaryl having
up to three heteroatoms from the group consisting of N, O and S, where all of
the ring systems mentioned may for their part in each case be mono- to
trisubstituted by identical or different substituents from the group
consisting
of halogen, hydroxyl, (C,-C6)-alkyl, (C,-C6)-alkoxy, trifluoromethyl,
trifluoromethoxy, cyano, vitro and amino,
R9 and R'° are identical or different and independently of one another
each represents
hydrogen, (C,-C6)-alkyl, (C,-C6)-alkoxy, trifluoromethyl, trifluoromethoxy or
halogen,
R" and R'Z are identical or different and independently of one another each
represents hydrogen or (C,-C6)-alkyl, or together with the carbon atom to
which they are attached they form a (Ca-C~)-cycloalkyl ring,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-9-
and
R'3 represents hydrogen or a group that can be hydrolysed and degraded to the
corresponding carboxylic acid,
and their pharmaceutically acceptable salts, hydrates and solvates.
Particular preference is given to compounds of the general formula (I),
in which
A represents a -CHZ- or -CHZCH~- group,
1 ~ X represents O, S or CHZ,
R', RZ and R3 are identical or different and independently of one another each
represents hydrogen, (C,-C4)-alkyl, (C,-C4)-alkoxy, chlorine, fluorine, tri-
fluoromethyl, trif)uoromethoxy, vitro or cyano,
R'~ represents hydrogen or methyl,
RS and R6 each represent hydrogen or together with the carbon atom to which
they
are attached form a carbonyl group,
R' represents hydrogen, (C,-C4)-alkyl or benzyl,
R8 represents hydrogen, phenyl, benzyl or 5-membered heteroarylmethyl having
up to two heteroatoms from the group consisting of N, O and S, where the
aromatic ring systems mentioned for their part may in each case be mono- to
trisubstituted by identical or different substituents from the group
consisting
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 10-
of chlorine, fluorine, bromine, hydroxyl, (C1-C.~)-alkyl, (C,-C.r)-alkoxy,
trifluoromethyl and amino,
R9 and R'° are identical or different and independently of one another
each represents
hydrogen, (C~-C3)-alkyl, (C,-C3)-alkoxy, trifluoromethyl, fluorine or
chlorine,
R" and R'Z are identical or diffferent and independently of one another each
represents hydrogen, methyl or ethyl, or together with the carbon atom to
which they are attached they form a cyclopentyl or cyclohexyl ring,
and
R'3 represents hydrogen or represents a group that can be hydrolysed and
degraded to the corresponding carboxylic acid,
and their pharmaceutically acceptable salts, hydrates and solvates.
Very particular preference is given to compounds of the general formula (I),
in which
A represents a -CHZ- or -CH2CHZ- group,
X represents O, S or CH2,
R' represents hydrogen, methyl or methoxy,
R2 and R3 are identical or different and independently of one another each
represents
methyl, trifluoromethyl, methoxy, trifluoromethoxy, chlorine or fluorine,
R4 represents hydrogen,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-11-
RS and R6 together with the carbon atom to which they are attached form a
carbonyl
group,
R7 represents methyl, ethyl, n-propyl or, in particular, hydrogen,
Rg represents phenyl, furanylmethyl or thienylmethyl, where the ring systems
mentioned for their part may in each case be mono- or disubstituted by
identical or different substituents from the group consisting of methyl and
ethyl,
R9 and R'° are identical or different and each represents hydrogen or
methyl in
particular hydrogen,
R" and R'Z are identical or different and each represents hydrogen or methyl
in
particular methyl,
and
R'3 represents a group which can be hydrolysed and degraded to the
corresponding carboxylic acid, or, in particular, represents hydrogen,
and their pharmaceutically acceptable salts, hydrates and solvates.
The general or preferred radical definitions listed above apply both to the
end
products of the formula (I) and, correspondingly, to the starting materials
and
intermediates required in each case for the preparation.
The individual radical definitions given in the respective combinations or
preferred
combinations of radicals are, independently of the respective given
combination of
radicals, also replaced by any radical definitions of other combinations.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-12-
Of particular importance are compounds of the formula (I) in which R4 is
hydrogen.
Of particular importance are compounds of the formula (I) in which RS and R6
together with the carbon atom to which they are attached form a carbonyl
group.
Of particular importance are compounds of the formula (I) in which
R~ represents hydrogen, methyl or methoxy,
and
Rz and R3 are identical or different and independently of one another each
represents
methyl, isopropyl, ten-butyl, cyclohexyl, trif7uoromethyl, methoxy,
trifluoromethoxy, chlorine or fluorine.
Of particular importance are compounds of the formula (I) in which
R8 represents phenyl, furanylmethyl, thienylmethyl or oxazolylmethyl, where
the
ring systems mentioned for their part may in each case be mono- or
disubstituted by methyl, or represents 2-methoxyethyl.
Of very particular importance are compounds of the formula (IA)
R, O
O ~ a ~ I X OH
/ N~N~A ~ H3C CH3 (IA)~
R3 H
in which
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-13-
A represents a -CHZ- or -CH2CH2- group,
X represents O or S,
RI represents hydrogen, methyl or methoxy,
RZ and R3 are identical or different and independently of one another each
represents
methyl, isopropyl, tert-butyl, cyclohexyl, trifluoromethyl, methoxy, trifluoro-
methoxy, chlorine or fluorine,
and
Rg represents phenyl, furanylmethyl, thienylmethyl or oxazolylmethyl, where
the
ring systems mentioned for their pan may in each case be mono- or
disubstituted by methyl, or represents 2-methoxyethyl.
Moreover, we have found a process for preparing the compounds of the general
formula (I) according to the invention, characterized in that
[A] compounds of the general formula (II)
s O
Ra R X
O-T
HOOC N~ ~ R" R
(B),
Rio
R
in which
A, X, R', Rg, R9, R'°, R~~ and R'2 are each as defined above
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 14-
and
T represents benzyl, (C,-C6)-alkyl or a polymeric support suitable for
solid-phase synthesis,
are initially, with activation of the carboxylic acid group in (II), reacted
with
compounds of the general formula (III)
R'
z \
R
NH
R3 z
in which
R~, RZ and R3 are each as defined above,
to give compounds of the general formula (Ia)
R, s O
a R
Rz \ O I I X O_T
N Nw \ R" R~z (Ia)~
R~ A Rio
in which
A, X, T, R', R2, R3, R', Rg, R9, R'°, R' ~ and R~Z are each as defined
above,
or
[B) compounds of the general formula (IV)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-15-
O
Ra R X
O-T
HN~A ~ ~ R" R,2 (IV),
R, o
in which
A, X, T, Rg, R9, R'°, R~ ~ and R~z are each as defined above,
are, in the presence of a base, reacted with compounds of the general formula
(V)
R'
O
R
N O (V),
R3
in which
I5 R', R2, R3 and R' are each as defined above
and
Q is a suitable leaving group, for example halogen, mesylate or tosylate,
preferably bromine or iodine,
likewise to compounds of the general formula (Ia)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-16-
the compounds of the general formula (Ia) are, if appropriate according to
known
methods for amide alkylation or amide reduction, converted into compounds of
the
general formula (Ib)
O
R s R
R2 \ Rs s ~ ~ X O_T
/ N NBA \ R'i R~2
Rs R4 R~ Rio
in which
A, X, T, R', RZ, R3, R4, R5, R6, R', Rg, R9, R'°, R' ~ and R''' are
each as defined above
then converted with acids or bases into the corresponding carboxylic acids of
the
general formula (Ic)
9 O
R s R
RZ \ RS Rs i ~ X OH
/ N NwA \ R" R~2 ( )
Ic ,
Rs R4 R~ Rio
in which
A, X, R', R2, R3, R4, R5, R6, R', R8, R9, R'°, R" and R'Z are each as
defined above,
and these are, if appropriate according to known methods for esterification,
modified
further by reaction with compounds of the general formula (VI)
R'3-Z (VI),
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-17-
in which
R'3 is as defined above
and
Z represents a suitable leaving group for example halogen, mesylate or
tosylate
or represents a hydroxyl group.
The process according to the invention is generally carried out at atmospheric
pressure. However, it is also possible to carry out the process under elevated
pressure
or under reduced pressure (for example in a range of from 0.5 to 5 bar).
Solvents which are suitable for the process are customary organic solvents
which do
not change under the reaction conditions. These include ethers, such as
diethyl ether,
dioxane, tetrahydrofuran, glycol dimethyl ether, or hydrocarbons, such as
benzene,
toluene, xylene, hexane, cyclohexane or mineral oil fractions, or halogenated
hydrocarbons, such as dichloromethane, trichloromethane, carbon tetrachloride,
dichloroethylene, trichloroethylene or chlorobenzene, or ethyl acetate,
pyridine,
dimethyl sulphoxide, dimethylformamide, N,N'-dimethylpropyleneurea (DMPU),
N-methylpyrrolidone (NMP), acetonitrile, acetone or nitromethane. It is also
possible
to use mixtures of the solvents mentioned.
Solvents which are preferred for process step (II) + (III) --~ (Ia) are
dichloromethane
and dimethylformamide. For process step (IV) + (V) --~ (Ia), preference is
given to
dimethylformamide.
The process step (II) + (III) -~ (Ia) according to the invention is generally
carried out
in a temperature range of from 0°C to +100°C, preferably from
0°C to +40°C. The
process step (IV) + (V) --~ (Ia) is generally carried out in a temperature
range of from
0°C to +120°C, preferably from +50°C to +100°C.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-18-
The auxiliaries used for the amide formation in process step (II) + ()II] -~
(Ia) are
preferably customary condensing agents, such as carbodiimides, for example,
N,N'-
diethyl-, N,N'-dipropyl-, N,N'-diisopropyl-, N>N'-dicyclohexylcarbodiimide
(DCC),
N-(3-dimethylaminoisopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), or
carbonyl compounds, such as carbonyldiimidazole, or 1,2-oxazolium compounds,
such as 2-ethyl-5-phenyl-1,2-oxazolium 3-sulphate or 2-tert-butyl-5-methyl-
isoxa-
zolium perchlorate, or acylamino compounds, such as 2-ethoxy-1-ethoxycarbonyl-
1,2-dihydroquinoline, or propanephosphonic anhydride, or isobutyl
chloroformate, or
bis-(2-oxo-3-oxazolidinyl)-phosphoryl chloride or benzotriazolyloxy-
tris(dimethyl-
amino)phosphonium hexafluorophosphate, or O-(benzotriazol-1-yl)-N,N,N',N'-
tetramethyluronium hexafluorophosphate (HBTU), 2-(2-oxo-1-(2H)-pyridyl)-
1,1,3,3-
tetramethyluronium tetrafluoroborate (TPTU) or O-(7-azabenzotriazol-1-yl)-
N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU), if appropriate in
combination with further auxiliaries such as 1-hydroxybenzotriazole or N-
hydroxysuccinimide, and the bases used are preferably alkali metal carbonates,
for
example sodium carbonate or bicarbonate or potassium carbonate or bicarbonate,
or
organic bases, such as trialkylamines, for example triethylamine, N-methyl-
morpholine, N-methylpiperidine or diisopropylethylamine. Particular preference
is
given to the combination of EDC, N-methylmorpholine and 1-
hydroxybenzotriazole,
of EDC, triethylamine and 1-hydroxybenzotriazole and of HATU and
diisopropylethylamine.
Suitable bases for the reaction (IV) + (V) ~ (Ia) are the customary inorganic
bases,
such as alkali metal alkoxides, such as, for example, lithium hydroxide,
sodium
hydroxide or potassium hydroxide, alkali metal carbonates or alkaline earth
metal
carbonates, such as sodium carbonate, potassium carbonate, calcium carbonate
or
cesium carbonate, or sodium bicarbonate or potassium bicarbonate, or organic
bases,
such as trialkylamines, for example triethylamine, N-methylmorpholine, N-
methyl-
piperidine or diisopropylethylamine. Preference is given to sodium
bicarbonate.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 19-
The hydrolysis of the carboxylic acid esters in the process step (Ia) or (Ib) -
~ (Ic) is
carried out by customary methods by treating the esters in inert solvents with
bases,
the salts that are initially formed being converted by treatment with acid
into the free
carboxylic acids. In the case of the tert-butyl esters, the hydrolysis is
preferably
carried out using acids.
Suitable solvents for the hydrolysis of the carboxylic acid esters are water
or the
organic solvents which are customary for ester cleavage. These preferably
include
alcohols, such as methanol, ethanol, propanol, isopropanol or butanol, or
ethers, such
as tetrahydrofuran or dioxane, dimethylformamide, dichloromethane or dimethyl
sulphoxide. It is also possible to use mixtures of the solvents mentioned.
Preference
is given to water/tetrahydrofuran and, in the case of the reaction with
trifluoroacetic
acid, to dichloromethane and, in the case of hydrogen chloride, to
tetrahydrofuran,
diethyl ether, dioxane or water.
Bases suitable for the hydrolysis are the customary inorganic bases. These
preferably
include alkali metal hydroxide or alkaline earth metal hydroxide, such as, for
example, sodium hydroxide, lithium hydroxide, potassium hydroxide or barium
hydroxide, or alkali metal carbonates, such as sodium carbonate or potassium
carbonate, or sodium bicarbonate. Particular preference is given to using
sodium
hydroxide or lithium hydroxide.
Suitable acids are, in general, trifluoroacetic acid, sulphuric acid, hydrogen
chloride,
hydrogen bromide and acetic acid, or mixtures thereof, if appropriate with
addition of
water. Preference is given to hydrogen chloride or trifluoroacetic acid in the
case of
the tert-butyl esters and to hydrochloric acid in the case of the methyl
esters.
In the case of compounds of the general formula (Ia) or (Ib) prepared by solid-
phase
synthesis and attached to a polymeric support via the carboxylic acid group,
the
cleavage from the resin to give the compounds of the general formula (Ic) is
likewise
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-20-
carried out by the above-described customary methods for carboxylic acid ester
hydrolysis. Here, preference is given to using trifluoroacetic acid.
When carrying out the hydrolysis, the base or the acid is generally employed
in an
amount of from 1 to 100 mol, preferably from 1.5 to 40 mol, based on 1 mole of
the
ester.
The hydrolysis is generally carned out in a temperature range of from
0°C to +100°C,
preferably from 0°C to +50°C.
The compounds of the general formula (II) are novel, and they can be prepared
by
initially
[a] reacting compounds of the general formula (VII)
9
R X
O-T
H g ~ R" R~2 (VII)>
Rio
in which
X, T, R9, R'°, R~ ~ and R'2 are each as defined above
and
B represents a bond or a methylene group
in the presence of a suitable reducing agent with compounds of the general
formula
(VIII)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-21-
R' 4-~z
in which
R14 [a-1] has the meaning of R8 given above
or
O
[a-2] represents a group of the formula R'5 O
R'
in which
R' is as defined above
and
R'S represents (C,-C4)-alkyl or tr;methylsilyl,
to give compounds of the general formula (IX)
O
R X
O-T
RyN~B ~ ~ R» R~2 (IX),
Rio
in which
B, X, T, R9, R'°, R", R12 and R'4 are each as defined above,
then reacting these compounds in the presence of a base with compounds of the
general formula (X)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-22-
R 16-Y
in which
R'6 in the case of process variant [a-1] represents a group of the formula
O
R15 O
R'
in which R' and R'S are each as defined above
or,
in the case of process variant [a-2] has the meaning of R8 given above
and
Y represents a suitable leaving group, such as, for example halogen, mesylate
or
tosylate, preferably bromine or iodine,
to give compounds of the general formula (XI)
R9 X
R' I O-T
R» R~2 (~)
R' S O
O R8 H Rio
in which
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-23-
B, X, T, R', R8, R9, R'°, RI', R'Z and R'S are each as defined
above ,
and finally selectively hydrolysing the carboxylic acid ester grouping -COORS
in
these compounds to the carboxylic acid,
or
[b] reacting compounds of the general formula (X)I)
s O
R X
O-T
I R~~ R,2 (~)
R, o
in which
A, X, T, R9, R'°, R" and R'2 are each as defined above
in the presence of a suitable reducing agent with compounds of the general
formula
(
R"-CHO (XllI),
in which
R'~ represents hydrogen, (C6-C,°)-aryl, 5- or 6-membered heteroaryl
having up to
three heteroatoms selected from the group consisting of N, O and S, or
represents (C,-C3)-alkyl which for its part may be substituted by hydroxyl,
trifluoromethoxy, (C,-C4)-alkoxy or phenoxy, which for their part are
optionally mono- or disubstituted by trifluoromethyl, or by (C6-C,°)-
aryl or 5-
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-24-
to 6-membered heteroaryl having up to three heteroatoms from the group
consisting of N, O and S, where all aryl and heteroaryl rings mentioned may
for their part in each case be mono- to trisubstituted by identical or
different
substituents from the group consisting of halogen, hydroxyl, (C1-C6)-alkyl,
(C,-C6)-alkoxy, trifluoromethyl, trifluoromethoxy, cyano, vitro and amino,
to give compounds of the general formula (XIV)
O
R" R X
O-T
" , 2 (xlv),
\ R R
R, o
in which
A, X, T, R9, R'°, R", R'Z and R" are each as defined above,
then reacting these compounds in the presence of a base with compounds of the
general formula (XV)
O
RCS O Y
(XV),
R'
in which
R', R'S and Y are each as defined above
to give compounds of the general formula (XVI)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-25-
OR'\ R' X
O-T
o ~z
R~s ~ NwA \ R R (XVI).
Rio
in which
A, X, T, R', R9, R'°, R", R'Z, R'S and R" are each as defined
above,
and finally selectively hydrolysing the carboxylic acid ester grouping -COORS
in
these compounds to the carboxylic acid.
The entire process can also be carried out as solid-phase synthesis. In this
case, the
compounds of the general formula (VII) or (XII) are attached as carboxylic
acid
esters to a suitable support resin, the further reactions are carried out on
solid phase
and the target compound is finally cleaved off from the resin. Solid-phase
synthesis
and the attachment and the cleavage from the resin are customary standard
techniques. To mention but one example from the extensive literature,
reference is
made to the publication "Linkers for Solid Phase Organic Synthesis", Ian W.
James,
Tetrahedron 55, 4855-4946 ( 1999).
The reaction (VII) + (VIII) ~ (IX) or (XII) + (XIII) --~ (XIV) is carried out
in the
solvents which are customary for reductive amination and inert under the
reaction
conditions, if appropriate in the presence of an acid. The solvents include,
for
example, water, dimethylformamide, tetrahydrofuran, dichloromethane, dichloro-
ethane, or alcohols such as methanol, ethanol, propanol, isopropanol or
butanol; it is
also possible to use mixtures of the solvents mentioned. Preference is given
to
methanol and ethanol in each case with addition of acetic acid.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-26-
Suitable reducing agents for the reaction (VII) + (VIII) -~ (IX) or (XII) +
(X~ --~ (XN) are complex aluminium hydrides or boron hydrides, such as, for
example, diisobutylaluminium hydride, sodium borohydride, sodium
triacetoxyborohydride, sodium cyanoborohydride or tetrabutylammonium
borohydride, or else catalytic hydrogenation in the presence of transition
metal
catalysts such as, for example, palladium, platinum, rhodium or Raney nickel.
Preferred reducing agents are sodium cyanoborohydride, sodium
triacetoxyborohydride and tetrabutylammonium borohydride.
The reaction (VII) + (VIII) -~ (IX) or (XII) + (XIII) -+ (XIV) is generally
carried out
in a temperature range of from 0°C to +40°C.
The reaction (IX) + (X) -~ (XI) or (XN) + (XV) ~ (XVI) is carried out in the
customary solvents which are inert under the reaction conditions. Preference
is given
to dimethylformamide, tetrahydrofuran and dioxane.
Suitable bases for the reaction (IX) + (X) ~ (XI) or (XIV) + (XV) ~ (XVI) are
the
customary inorganic or organic bases. Preference is given to triethylamine.
The reaction (IX) + (X) ~ (XI) or (XIV) + (XV) -~ (XVI) is generally carried
out in
a temperature range of from 0°C to +100°C.
The reaction (XI) ~ (II) or (XVI) ~ (II) is carried out in the solvents which
are
customary for ester cleavage and inert under the reaction conditions. In the
case of
the ester hydrolysis, these are preferably tetrahydrofuran, dioxane and
alcohols, such
as methanol and ethanol, in each case in a mixture with water. In the case of
the
cleavage of silyl esters, preference is given to using dioxane or
tetrahydrofuran.
Suitable bases for the reaction (XI) ~ (II) or (XVI) --~ (II) are, in the case
of the
hydrolysis, the customary inorganic bases. Preference is given to lithium
hydroxide,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-27-
sodium hydroxide and potassium hydroxide. In the case of the cleavage of silyl
esters, preference is given to using tetrabutylammonium fluoride.
The reaction (XI) ~ (II) or (XVI) -~ (II) is generally carried out in a
temperature
range of from 0°C to +100°C.
The compounds of the general formula (N) correspond to the compounds of the
general formula (IX) or (XIV) and can be prepared as described above.
The compounds of the general formulae (III), (V), (VI), (VII), (VIII), (X),
(XII),
(XI)?) and (XV) are commercially available, known or can be prepared by
customary
methods (cf., for example, P.J. Brown et al., J. Med. Chem. 42, 3785-88
(1999)].
The compounds of the formula (I) according to the invention have a surprising
and
useful spectrum of pharmacological activity and can therefore be used as
versatile
medicaments. In particular, they are suitable for treating coronary heart
diseases, for
the prophylaxis of myocardial infarction and for the treatment of restenosis
after
coronary angioplasty or stenting. The compounds of the formula (I) according
to the
invention are preferably suitable for treating arteriosclerosis and
hypercholesterolaemia, for increasing pathogenically low HDL levels and for
lowering elevated triglyceride, fibrinogen and LDL levels. In addition, they
can be
used for treating obesity, diabetes, for treating the metabolic syndrome
(glucose
intolerance, hyperinsulinaemia, dyslipidaemia and high blood pressure owing to
insulin resistance), hepatic fibrosis and cancer.
The activity of the compounds according to the invention can be examined, for
example, in vitro by the transactivation assay described in the experimental
section.
The activity of the compounds according to the invention in vivo can be
examined,
for example, by the tests described in the experimental section.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-28-
Suitable administration forms for administering the compounds of the general
formula (17 are all customary administration forms, i.e. oral, parenteral,
inhalative,
nasal, sublingual, rectal or external, for example transdermal, preferably
oral or
parenteral, administration forms. In the case of parenteral administration,
particular
mention has to be made of intravenous, intramuscular and subcutaneous
administration, for example as a subcutaneous depot. Very particular
preference is
given to oral administration.
Here, the active compounds can be administered on their own or in the form of
preparations. Preparations suitable for oral administration are, inter alia,
tablets,
capsules, pellets, sugar-coated tablets, pills, granules, solid and liquid
aerosols,
syrups, emulsions, suspensions and solutions. Here, the active compound has to
be
present in such an amount that a therapeutic effect is obtained. In general,
the active
compound can be present in a concentration of from 0.1 to 100% by weight, in
particular from 0.5 to 90% by weight, preferably from 5 to 80% by weight. In
particular, the concentration of active compound should be 0.5 - 90% by
weight, i.e.
the active compound should be present in amounts sufficient to reach the
dosage
range stated.
To this end, the active compounds can be converted in a manner known per se
into
the customary preparations. This is carried out using inert non-toxic
pharmaceutically
suitable excipients, auxiliaries, solvents, vehicles, emulsifiers and/or
dispersants.
Auxiliaries which may be mentioned are, for example: water, non-toxic organic
solvents, such as, for example, paraffins, vegetable oils (for example sesame
oil),
alcohols (for example ethanol, glycerol), glycols (for example polyethylene
glycol),
solid carriers, such as natural or synthetic ground minerals (for example talc
or
silicates), sugar (for example lactose), emulsifiers, dispersants (for example
polyvinylpyrrolidone) and glidants (for example magnesium sulphate).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-29-
In the case of oral administration, the tablets may, of course, also contain
additives
such as sodium citrate, together with additives such as starch, gelatine and
the like.
Aqueous preparations for oral administration may furthermore comprise flavour
improvers or colorants.
In the case of oral administration, preference is given to administering
dosages of
from 0.001 to 5 mgJkg, preferably from 0.005 to 3 mg/kg, of body weight per
24 hours.
The embodiments below illustrate the invention. The invention is not limited
to the
examples.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-30-
The following abbreviations used represent:
Ac Acetyl
Bu Butyl
TLC Thin-layer chromatography
DCI Direct chemical ionization (in MS)
DCM Dichloromethane
DIC Diisopropylcarbodiimide
DMAP 4-N,N-Dimethylaminopyridine
DMF N,N-Dimethylformamide
DMSO Dimethyl sulphoxide
EDC N'-(3-Dimethylaminopropyl)-N-ethylcarbodiimide
x HC1
EI Electron impact ionization (in MS)
ESI Electron spray ionization (in MS)
Et Ethyl
sat. Saturated
HATU O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium
hexafluorophosphate
HOBt 1-Hydroxy-1H-benzotriazole x HZO
HPLC High-pressure, high-performance liquid chromatography
LC-MS Liquid chromatography-coupled mass spectroscopy
Me Methyl
MS Mass spectroscopy
NMR Nuclear magnetic resonance spectroscopy
RF Reflux
Rf . Retention index (in TLC)
RT Room temperature
R~ Retention time (in HPLC)
TBAF Tetrabutylammonium fluoride
TBAI Tetrabutylammonium iodide
TFA Trifluoroacetic acid
THF Tetrahydrofuran
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-31-
Starting materials I
Example I-1
tent-Butyl 2-methylpropionate
~ok
With ice-cooling, a solution of 73.0 g (0.985 mol) of ten-butanol, 190 g
(1.877 mol)
of triethylamine and 0.573 g (0.0047 mol) of DMAP in 750 ml of dichloromethane
is
treated with a solution of 100 g (0.939 mmol) of isobutyryl chloride in 150 ml
of di-
chloromethane, and the mixture is then stirred overnight. 500 ml of 2 M
hydrochloric
acid are then added, the aqueous phase is extracted with dichloromethane and
the
combined organic phases are washed with water, sat. NaHC03 solution and sat.
NaCI
solution, dried over sodium sulphate and concentrated. Distillative
purification of the
crude product gives 65.5 g (48%) of tent-butyl 2-methylpropionate.
'H-NMR (200 MHz, CDC1;): 8 = 1.11 (d, 6H); 1.44 (s, 9H); 2.42 (sept., 1H).
Example I-2
tent-Butyl 3-(4-bromophenyl)-2,2-dimethylpropionate
O
\ O
Br / .
At -78°C, 34.7 ml (69.4 mmol) of a 2 M lithium diisopropylamide
solution are
slowly added dropwise to a solution of 10.0 g (69.34 mmol) of ten-butyl
2-methylpropionate (Example I-1) in 100 ml of tetrahydrofuran. After the
addition
has ended, the mixture is stirred at -78°C for 1 h, and a solution of
15.76 g
CA 02424540 2003-04-02
Le A 34 838-Foreia-n Countries
-32-
(63.04 mmol) of 4-bromobenzyl bromide in 10 ml of tetrahydrofuran is then
added
and the mixture is stirred at -78°C for 1 h. The reaction is then
warmed to room
temperature and poured into 100 ml of 1 N hydrochloric acid, the phases are
separated and the aqueous phase is extracted 3x with diethyl ether. The
combined
organic phases are washed with NaHC03 solution, dried over sodium sulphate and
freed from the solvent under reduced pressure. Distillative purification of
the residue
under oil pump vacuum gives 16.75 g (85%) of ten-butyl 3-(4-bromophenyl)-2,2-
dimethylpropionate.
'H-NMR (200 MHz, DMSO): 8 = 1.06 (s, 6H); 1.38 (s, 9H); 2.74 (s, 2H); 7.10 (d,
2H); 7.47 (d, ZH).
Example I-3
tent-Butyl 3-(4-formylphenyl)-2,2-dimethylpropionate
O
~O
O~ /
At -75°C, 13.5 ml (22.98 mmol) of a 1.7 M ten-butyllithium solution in
pentane are
slowly added to a solution of 6.00 g (19.16 mmol) of tent-butyl 3-(4-
bromophenyl)-
2,2-dimethylpropionate (Example I-2) in 80 ml of tetrahydrofuran, the
temperature
being kept below -60°C. The mixture is stirred for 15 min, and 1.82 g
(24.90 mmol)
of N,N-dimethylformamide are then added and the mixture is stirred at -
75°C for a
further 4 h. The mixture is slowly warmed to -20°C and, with vigorous
stirring,
admixed with 20 ml of water, and then warmed to room temperature. The aqueous
phase is extracted 3x with diethyl ether and the combined organic phases are
dried
over sodium sulfphate/sodium carbonate and freed from the solvent under
reduced
pressure. Distillation of the residue under oilpump vacuum gives 2.54 g (51%)
of
ten-butyl 3-(4-formylphenyl)-2,2-dimethylpropionate.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-33-
'H-NMR (300 MHz, CDC13): 8 = 1.16 (s, 6H); 1.42 (s, 9H); 2.90 (s, ZH); 7.32
(d,
2H); 7.78 (d, 2H); 9.98 (s, 1H).
Example I-4
tent-Butyl2-(4-formylphenoxy)-2-methylpropionate
O
O
O
O~ ~ /
A solution of 24.4 g (200 mmol) of 4-hydroxybenzaldehyde in 100 ml of dimethyl-
formamide is treated with 97.75 g (300 mmol) of caesium carbonate and stirred
at
90°C for 1 h. A solution of 66.93 g (300 mmol) of ten-butyl 2-
bromisobutyrate in
100 ml of dimethylformamide is then added dropwise, and the mixture is stirred
at
90°C overnight. The dimethylformamide is distilled off under reduced
pressure and
the residue is then taken up in ethyl acetate, washed 2x with water, 2x with 1
N
aqueous sodium hydroxide solution and lx with sat. NaCI solution, dried over
sodium sulphate and freed from the solvent under reduced pressure. This gives
16.6 g
(31%) of tent-butyl 2-(4-formylphenoxy)-2-methylpropionate.
~H-NMR (200 MHz, CDC13): 8 = 1.40 (s, 9H); 1.63 (s, 6H); 6.90 (d, 2H); 7.78
(d,
2H); 9.88 (s, 1H).
Example I-5
ten-Butyl 3-(4-{ [(2-furylmethyl)amino]methyl }phenyl)-2,2-dimethylpropionate
O
O ~ \ ~y0
HN / '/
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-34-
At room temperature, a solution of 1.00 g (3.81 mmol) of tent-butyl 3-(4-
formylphenyl)-2,2-dimethylpropionate (Example I-3) and 0.37 g (3.81 mmol) of
furfurylamine in 10 ml of dichIoroethane is stirred for 30 min, 1.21 g (5.72
mmol) of
S sodium triacetoxyborohydride are added and the mixture is then stirred at
room
temperature for 22 h. 6 ml of sat. NaHC03 solution and 10 ml of ethyl acetate
are
then added, the phases are separated, the aqueous phase is extracted Zx with
ethyl
acetate and the combined organic phases are dried over sodium sulphate. The
solvent
is removed under reduced pressure, and chromatographic purification on silica
jel
(cyclohexane -> cyclohexane/ethyl acetate 10:1 -> 2:1) then gives 720 mg (55%)
of
tent-butyl 3-(4-{ [(2-furylmethyl)amino]methyl } phenyl)-2,2-
dimethylpropionate.
~H-NMR (300 MHz, CDC13): 8 = 1.11 (s, 6H); 1.42 (s, 9H); 1.62 (broad s, 1H);
2.70
(s, 2H); 3.76 (s, 2H); 3.80 (s, 2H); 6.18 (d, 1H); 6.32 (dd, 1H); 7.10 (d,
2H); 7.20 (d,
2H); 7.35 (d, 1H).
Example I-6
ten-Butyl 3-[4-(anilinomethyl)phenyl]-2,2-dimethylpropionate
O
\ O
HN ~ /
Similarly to the procedure of Example I-5, 200 mg (0.762 mmol) of ten-butyl 3-
(4
formylphenyl)-2,2-dimethylpropionate (Example I-3), 71 mg (0.762 mmol) of
aniline
and 210 mg (0.991 mmol) of sodium triacetoxyborohydride in 2 ml of
dichloroethane
are converted into 223 mg (86%) of ten-butyl 3-[4-(anilinomethyl)phenyl]-2,2
dimethylpropionate.
'H-NMR (400 MHz, CDC13): 8 = 1.I 1 (s, 6H); 1.42 (s, 9H); 2.81 (s, 2H); 3.98
(broad
s, 1H); 4.29 (s, 2H); 6.64 (d, 2H); 6.71 (t, 1H); 7.12 (d, 2H); 7.17 (t, 2H);
7.25 (d,
2H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-35-
Example I-7
ten-Butyl 2,2-dimethyl-3-(4-{ [(4-methylphenyl)amino]methyl }phenyl)propionate
O
/ ~ O
HN ~ /
Similarly to the procedure of Example I-5, 200 m~ (0.762 mmol) of tent-butyl 3-
(4-
formylphenyl)-2,2-dimethylpropionate (Example I-3), 82 mg (0.762 mmol) of
toluidine and 210 m~ (0.991 mmol) of sodium triacetoxyborohydride in 2 ml of
dichloroethane are convened into 206 mg (76%) of tent-butyl 2,2-dimethyl-3-(4-
{ [(4-
methylphenyl)amino]methyl }-phenyl)propionate.
'H-NMR (400 MHz, CDC13): 8 = 1.11 (s, 6H); 1.42 (s, 9H); 2.23 (s, 3H); 2.81
(s,
2H); 3.87 (broad s, 1H); 4.27 (s, 2H); 6.57 (d, 2H); 6.98 (d, 2H); 7.12 (d,
2H); 7.25
(d, 2H).
15-
Example I-8
Methyl 2-{ [4-(2-ten-butoxy-1,1-dimethyl-2-oxoethoxy)benzyl]amino } butyrate
~O
O
O ~ O O
N
Similarly to the procedure of Example I-5, 1.20 g (4.54 mmol) of ten-butyl 2-
(4-
formylphenoxy)-2-methylpropionate (Example I-4) and 0.70 ~ (4.54 mmol) of
methyl
DL-2-aminobutyrate are reacted at room temperature with 0.92 g (9.08 mmol) of
CA 02424540 2003-04-02
Le A 34 838-Forei ~n Countries
-36-
triethylamine and 1.44 g (6.81 mmol) of sodium triacetoxyborohydride in 10 ml
of
dichloroethane. A further 0.9 g (4.25 mmol) of sodium triacetoxyborohydride
and
0.35 g (2.27 mmol) of methyl DL-2-aminobutyrate are added and the mixture is
heated at 40°C for 3 h, giving 1.47 g (89%) of methyl 2-{ [4-(2-tent-
butoxy-1,1-
dimethyl-2-oxoethoxy)benzylJamino}butyrate.
'H-NMR (300 MHz, DMSO): 8 = 0.84 (t, 3H); 1.38 (s, 9H); 1.47 (s, 6H); 1.57
(dt,
2H); 2.29 (broad s, 1H); 3.08 (t, 1H); 3.47 (d, 1H); 3.62 (s, 3H); 3.65 (d,
1H); 6.73
(d, 2H); 7.18 (d, 2H).
Example I-9
ten-Butyl 3-(4-{ [(2-ethoxy-2-oxoethyl)(2-furylmethyl)amino]methyl }phenyl)-
2,2-
dimethylpropionate
O
O
O '~ ~ ~O
A solution of 600 mg (1.75 mmol) of ten-butyl 3-(4-{ [(2-furylmethyl)amino]-
methyl}phenyl)-2,2-dimethylpropionate (Example I-5), 323 mg (0.87 mmol) of
tetra-
n-butylammonium iodide and 265 mg (2.62 mmol) of triethylamine in 10 ml of THF
is treated with 438 mg (2.62 mmol) of ethyl bromoacetate and heated at reflux
overnight. After cooling, the mixture is concentrated under reduced pressure,
the
residue is taken up in water and ethyl acetate, the aqueous phase is extracted
2x with
ethyl acetate and the combined organic phases are washed with NaCI solution
and
dried over sodium sulphate. The solvent is removed under reduced pressure, and
chromatographic purification on silica gel (cyclohexane -> cyclohexane/ethyl
acetate
10:1) then gives 702 mg (94%) of ten-butyl 3-(4-{ [(2-ethoxy-2-oxoethyl)(2-
furylmethyl)amino]methyl }phenyl)-2,2-dimethyipropionate.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-37-
'H-NMR (400 MHz, CDC13): 8 = 1.11 (s, 6H); 1.27 (t, 3H); 1.42 (s, 9H); 2.80
(s,
2H); 3.31 (s, 2H); 3.76 (s, 2H); 3.84 (s, 2H); 4.17 (q, 2H); 6.20 (d, 1H);
6.32 (dd,
1H); 7.10 (d, 2H); 7.25 (d, 2H); 7.38 (d, 1H).
Example I-IO
ten-Butyl 3-(4-{ [N-(2-ethoxy-2-oxo)ethyl-N-phenylamino]methyl } phenyl)-2,2-
dimethylpropionate
O
O / ~ ~ O
/\ 'I N /
~O
Similarly to the procedure of Example I-9, 198 mg (0.583 mmol) of ten-butyl 3-
[4-
(anilinomethyl)phenyl]-2,2-dimethylpropionate (Example I-6), 108 m~ (0.292
mmol)
of tetra-n-butylammonium iodide, two portions of in each case 89 m~ (0.875
mmol)
of triethylamine and three portions of in each case 146 mb (0.875 mmol) of
ethyl
bromoacetate in 2 ml of tetrahydrofuran and 2 ml of dimethylformamide dive 191
mg
(77%) of tent-butyl 3-(4-{ [(2-ethoxy-2-oxoethyl)(2-phenyl)amino]methyl
}phenyl)-
2,2-dimethylpropionate.
'H-NMR (200 MHz, CDC13): b = 1.11 (s, 6H); 1.25 (t, 3H); 1.42 (s, 9H); 2.70
(s,
2H); 4.05 (s, 2H); 4.20 (q, 2H); 4.62 (s, 2H); 6.69 (d, 2H); 6.?3 (t, 1H);
7.07 - 7.25
(m, 6H).
Example I-11
ten-Butyl 3-(4-{ [N-(2-ethoxy-2-oxo)ethyl-N-(4-methylphenyl)amino]methyl }-
phenyl)-2,2-dimethylpropionate
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-38-
O
O / ~ O
~N I / /~
~o
Similarly to the procedure of Example I-9, 181 mg (0.512 mmol) of ten-butyl
2,2-dimethyl-3-(4-{ [(4-methylphenyl)amino]methyl }phenyl)propionate (Example
I-
7), 95 mg (0.256 mmol) of tetra-n-butyl ammonium iodide, two portions of in
each
case 78 mg (0.768 mmol) of triethylamine and three portions of in each case
128 mg
(0.768 mmol) of ethyl bromoacetate in 2 ml of tetrahydrofuran and 2 ml of
dimethylformamide give 176 mg (78%) of tent-butyl 3-(4-{ [N-(2-ethoxy-2-
oxo)ethyl-
N-(4-methylphenyl)-amino]methyl } phenyl)-2,2-dimethylpropionate.
'H-NMR (200 MHz, CDC13): 1.11 (s, 6H); 1.25 (t, 3H); 1.42 (s, 9H); 2.22 (s,
3H);
2.80 (s, 2H); 4.02 (s, 2H); 4.19 (q, 2H); 4.59 (s, 2H); 6.60 (d, 2H); 7.00 (d,
2H); 7.10
(d, 2H); 7.17 (d, 2H).
Example I-12
N-[4-(3-tent-Butoxy 2,2-dimethyl-3-oxopropyl)benzylJ-N-(2-furylmethyl)glycine
O
O
0 1 ~ ~ ~O
~ 'N
HO-
A solution of 785 mg (1.83 mmol) of tent-butyl 3-(4-{ [(2-ethoxy-2-oxoethyl)(2-
furylmethyl)amino]methyl}phenyl)-2,2-dimethylpropionate (Example I-9) in 15 ml
of ethanol is admixed with 5.5 ml (5.5 mmol) of 1 N aqueous sodium hydroxide
solution and heated at 80°C for 1 h. After cooling, the mixture is
concentrated under
reduced pressure' and the residue is taken up in a little water, acidified
with 1 N
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-39-
hydrochloric acid and extracted 3x with ethyl acetate. The combined organic
extracts
are washed 2x with sat. NaCI solution, dried over sodium sulphate and freed
from the
solvent under reduced pressure. This gives 728 mg (99°70) of N-[4-(3-
ten-butoxy-
2,2-dimethyl-3-oxopropyl)benzyl]-N-(2-furylmethyl)glycine.
IH-NMR (200 MHz, DMSO): 8 = 1.06 (s, 6H); 1.37 (s, 9H); 2.74 (s, 2H); 3.24 (s,
2H); 3.76 (s, 2H); 3.84 (s, 2H); 6.32 (m, 1H); 6.41 (m, 1H); 7.11 (d, 2H);
7.26 (d,
2H); 7.63 (d, 1H); 12.20 (broad s, 1H).
Example I-13
N-[4-(3-ten-Butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-phenylglycine
I~ O
O / ~ O
I ~ /~
HO
Similarly to the procedure of Example I-12, 175 mg (0.411 mmol) of ten-butyl 3-
(4-
{ [(2-ethoxy-2-oxoethyl)(2-phenyl)amino]methyl } phenyl)-2,2-
dimethylpropionate
(Example I-10) and 1.23 ml (1.23 mmol) of 1 N aqueous sodium hydroxide
solution
in 3 ml of ethanol give 162 mg (99%) of N-[4-(3-ten-butoxy-2,2-dimethyl-3-
oxopropyl)benzyl]-N-phenylglycine.
'H-NMR (300 MHz, DMSO): 8 = 1.04 (s, 6H); 1.36 (s, 9H); 2.73 (s, 2H); 4.12 (s,
2H); 4.56 (s, 2H); 6.56 (d, 2H); 6.61 (t, 1H); 7.07 (d, ZH); 7.11 (d, 2H);
7.19 (d, 1H);
12.53 (broad s, 1H).
Example I-14
N [4-(3-ten-Butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-(4-methylphenyl)glycine
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-40-
\ O
O / \ O
I / /\
HO
Similarly to the procedure of Example I-12, 153 mg (0.348 mmol) of ten-butyl
3-(4-( [N-(2-ethoxy-2-oxo)ethyl-N-(4-methylphenyl)amino]methyl } phenyl)-2,2-
dimethylpropionate (Example I-11) and 1.23 ml (1.23 mmol) of 1 N aqueous
sodium
hydroxide solution in 3 ml of ethanol give 141 mg (99°l0) of N-[4-(3-
tent-butoxy-2,2-
dimethyl-3-oxopropyl)benzyl]-N-(4-methylphenyl)glycine.
'H-NiVIR (300 MHz, DMSO): 8 = 1.04 (s, 6H); 1.36 (s, 9H); 2.14 (s, 3H); 2.72
(s,
2H); 4.08 (s, 2H); 4.52 (s, 2H); 6.48 (d, 2H); 6.90 (d, 2H); 7.08 (d, 2H);
7.18 (d, 2H);
12.48 (broad s, 1 H).
Example I-15
2-{[4-(2-ten-Butoxy-l,l-dimethyl-2-oxoethoxy)benzyl]amino}butyric acid
O
O \ O O
I/
HO
Similarly to the procedure of Example I-12, 750 mg (2.05 mmol) of methyl 2-{
[4-(2-
tent-butoxy-1,1-dimethyl-2-oxoethoxy)benzyl]amino}butyrate (Example I-8) and
6.20 ml (6.20 mmol) of 1 N aqueous sodium hydroxide solution in 6 ml of
ethanol
give 640 mg (89°l0) of 2-{ (4-(2-ten-butoxy-1,1-dimethyl-2-
oxoethoxy)benzyl]-
amino}butyric acid.
'H-NMR (300 MHz, DMSO): S = 0.91 (t, 3H); 1.40 (s, 9H); 1.51 (s, 6H); 1.84 (m,
2H); 3.25 (broad s, 1H); 3.57 (t, 1H); 3.99 (s, 2H); 6.81 (d, 2H); 7.38 (d,
2H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-41 -
Working Examples 1
Example 1-1
tent-Butyl 3-(4-{ [(2-(2,4-dimethylphenyl)amino-2-oxoethyl)(2-
furylmethyl)amino]-
methyl}phenyl)-2,2-dimethylpropionate
O
O
/ ~ O ~ \ ~ ~O
\ N~N /
H
At 0°C, 88 mg (0.648 mmol) of 1-hydroxy-1H-benzotriazole, 124 mg
(0.648 mmol)
of 1-ethyl-3-(3-dimethylamino)propylcarbodiimide hydrochloride, 151 mg
(1.494 mmol) of N-methylmorpholine and 3 mg (0.025 mmol) of 4-dimethyl-
aminopyridine are added to a solution of 200 mg (0.498 mmol) of N-[4-(3-tert-
butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-(2-furylmethyl)glycine (Example I-
12)
and 91 mg (0.747 mmol) of 2,4-dimethylaniline in 8 ml of dimethylformamide,
and
the solution is stirred at this temperature for 1 h. The mixture is then
stirred at room
temperature for 9 h and then admixed with 10 ml of water. The aqueous phase is
extracted 2x with ethyl acetate and the combined organic phases are washed
with 1 N
hydrochloric acid, sat. NaHC03 solution and sat. NaCI solution, dried over
sodium
sulphate and freed from the solvent under reduced pressure. Chromatographic
purification of the residue on silica gel (cyclohexane/ethyl acetate 10:1 ->
3:1) gives
228 mg (91%) of ten-butyl 3-(4-{[(2-(2,4-dimethylphenyl)amino-2-oxoethyl)(2-
furylmethyl)amino]methyl } phenyl)-2,2-dimethylpropionate.
'H-NMR (200 MHz, CDC13): 8 = 1.10 (s, 6H); 1.40 (s, 9H); 2.26 (s, 3H); 2.28
(s,
3H); 2.80 (s, 2H); 3.29 (s, 2H); 3.71 (s, 2H); 3.74 (s, 2H); 6.25 (d, 1H);
6.32 (dd,
1H); 6.99 (m, 2H); 7.11 (d, 2H); 7.23 (d, 2H); 7.37 (d, 1H); 7.84 (d, 1H);
9.12 (broad
s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-42-
Example 1-2
tent-Butyl 3-(4-{ [(2-(4-methoxy-2,5-dimethylphenyl)amino-2-oxoethyl)(2-furyl-
methyl)amino]methyl } phenyl)-2,2-dimethylpropionate
/O / O
Similarly to the procedure of Example 1-1, 200 m~ (0.498 mmol) of N-(4-(3-tert-
butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-(2-furylmethyl)glycine (Example I-
12),
113 mg (0.?47 mmol) of 4-methoxy-2,5-dimethylaniline, 88 mg (0.648 mmol) of
1-hydroxy-1H-benzotriazole, 124 mg (0.648 mmol) of 1-ethyl-3-(3-dimethylamino)-
propylcarbodiimide hydrochloride, 151 mg (1.494 mmol) of N-methylmorpholine
and 3 mg (0.025 mmol) of 4-dimethylaminopyridine in 8 ml of dimethylformamide
are convened into 241 mg (90%) of ten-butyl 3-(4-{ [(2-(4-methoxy-2,5-
dimethylphenyl)amino-2-oxoethyl)(2-furylmethyl)amino]methyl }phenyl)-2, 2-di-
methylpropionate.
'H-NMR (200 MHz, DMSO): b = 1.05 (s, 6H); 1.35 (s, 9H); 2.08 (s, 3H); 2.14 (s,
3H); 2.75 (s, 2H); 3.18 (s, 2H); 3.69 (s, 2H); 3.74 (s, 3H); 3.76 (s, 2H);
6.35 (d, 1H);
6.41 (dd, 1H); 6.75 (s, 1H); 7.11 (d, 2H); 7.28 (d, 2H); 7.31 (s, 1H); 7.61
(d, 1H);
9.02 (broad s, 1 H).
Example 1-3
ten-Butyl 3-(4-{ [N-(2-(2,4-dimethylphenyl)amino-2-oxo)ethyl-N-phenylamino]-
methyl } phenyl)-2,2-dimethylpropionate
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 43 -
O
O ~ ~ O
~ I ~N I ~ /~
N
H
Similarly to the procedure of Example 1-1, 65 mg (0.164 mmol) of N-[4-(3-tert-
butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-phenylglycine (Example I-13), 30 m~
(0.245 mmol) of 2,4-dimethylaniline, 29 m~ (0.213 mmol) of 1-hydroxy-1H-
benzotriazole, 41 mg (0.213 mmol) of 1-ethyl-3-(3-dimethylamino)propylcarbo-
diimide hydrochloride, 50 mg (0.491 mmol) of N-methylmorpholine and 0.2 mg
(0.002 mmol) of 4-dimethylaminopyridine in 2 ml of dimethylformamide are
converted into 65 m~ (79%) of ten-butyl 3-(4-{ [N-(2-(2,4-dimethylphenyl)amino-
2-
oxo)ethyl-N-phenylamino]methyl }phenyl)-2,2-dimethylpropionate.
~H-NMR (200 MHz, CDC13): b = 1.10 (s, 6H); 1.41 (s, 9H); 1.90 (s, 3H); 2.26
(s,
3H); 2.79 (s, 2H); 4.09 (s, 2H); 4.66 (s, 2H); 6.80 - 6.95 (m, 4H); 6.98 (d,
1H); 7.12
(s, 4H); 7.27 (m, 2H); 7.67 (d, 1 H); 8.11 broad s, 1 H).
Example 1-4
ten-Butyl 3-(4-{ [N-(2-(4-methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-
phenyl-
amino]methyl } phenyl)-2,2-dimethylpropionate
I ~ O
O ~ O / ~ O
~N
N
H
Similarly to the procedure of Example 1-1, 65 m~ (0.164 mmol) of N-[4-(3-tert-
butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-phenylglycine (Example I-13), 37 mg
(0.245 mmol) of 4-methoxy-2,5-dimethylaniline, 29 mg (0.213 mmol) of 1-hydroxy-
1H-benzotriazole, 41 m~ (0.213 mmol) of 1-ethyl-3-(3-dimethylamino)propylcarbo-
CA 02424540 2003-04-02
Le A 34 838-Forei7n Countries
diimide hydrochloride, 50 mg (0.491 mmol) of N-methylmorpholine and 0.2 mg
(0.002 mmol) of 4-dimethylaminopyridine in 2 ml of dimethylformamide are
convened into 78 mg (90%) of ten-butyl 3-(4-{ [N-(2-(4-methoxy-2>5-
dimethylphenyl)amino-2-oxo)ethyl-N-phenylamino]methyl } phenyl)-2,2-dimethyl-
propionate.
'H-NMR (200 MHz, CDCl3): $ = 1.11 (s, 6H); 1.42 (s, 9H); 1.96 (s, 3H); 2.16
(s,
3H); 2.80 (s, ZH); 3.77 (s, 3H); 4.09 (s, 2H); 4.67 (s, 2H); 6.57 (s, 1H);
6.83 (dd, 1H);
6.89 (d, 2H); 7.13 (s, 4H); 7.24 (d, 2H); 7.34 (m, 1H); 7.94 (broad s, 1H).
E~cample 1-5
ten-Butyl 3-(4-{ [N-(2-(2,4-dimethylphenyl)amino-2-oxo)ethyl-N-(4-
methylphenyl)-
amino]methyl }phenyl)-2,2-dimethylpropionate
O
/ ~ O \ ~ ~O
\ N~N /
H
Similarly to the procedure of Example 1-1, 50 mg (0.121 mmol) of N-[4-(3-tert-
butoxy-2,2-dimethyl-3-oxopropyl)benzyl]-N-(4-methylphenyl)glycine (Example
I-14), 22 mg (0.182 mmol) of 2,4-dimethylaniline, 21 mg (0.158 mmol) of
1-hydroxy-1H-benzotriazole, 30 mg (0.158 mmol) of 1-ethyl-3-(3-
dimethylamino)propylcarbodiimide hydrochloride, 37 mg (0.364 mmol) of
N-methylmorpholine and 0.1 ma (0.001 mmol) of 4-dimethylaminopyridine in 2 ml
of dimethylformamide are converted into 40 mg (64%) of tent-butyl -3-(4-{ [N-
(2-
(2,4-dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)amino]methyl }phenyl)-
2,2-dimethylpropionate.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 45 -
'H-NMR (300 MHz, CDC13): 8 = 1.10 (s, 6H); 1.40 (s, 9H); 1.92 (s, 3H); 2.27
(s,
6H); 2.79 (s, 2H); 4.02 (s, 2H); 4.58 (s, 2H); 6.80 (d, 2H); 6.91 (s, 1H);
6.98 (d, 1H);
7.06 (d, 2H); 7.11 (d, 2H); 7.13 (d, 2H); 7.67 (d, 1H); 8.18 (broad s, 1H).
Example 1-6
tent-Butyl 3-(4-{ [N-(2-(4-methoxy-2,~-dimethylphenyl)amino-2-oxo)ethyl-N-(4-
methylphenyl)amino)methyl } phenyl)-2,2-dimethylpropionate
I \ O
O ~ O / \ O
\ I ~N I ~ /\
N
H
Similarly to the procedure of Example 1-1, 50 m~ (0.121 mmol) of N-[4-(3-tert-
butoxy-2,2-dimethyl-3-oxopropyl)benzylJ-N-(4-methylphenyl)glycine (Example
I-14), 28 mg (0.182 mmol) of 4-methoxy-2,~-dimethylaniline, 21 mg (0.158 mmol)
of 1-hydroxy-1H-benzotriazole, 30 mg (0.158 mmol) of 1-ethyl-3-(3-dimethyl-
amino)propylcarbodiimide hydrochloride, 37 mg (0.364 mmol) of
N-methylmorpholine and 0.1 m~ (0.001 mmol) of 4-dimethylaminopyridine in 2 ml
of dimethylformamide are convened into 58 mg (88°l0) of ten-butyl-3-(4-
{ (N-(2-(4-
methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)amino]-
methyl } phenyl)-2,2-dimethylpropionate.
'H-IVMR (300 MHz, CDCI;): 8 = 1.10 (s, 6H); 1.41 (s, 9H); 1.96 (s, 3H); 2.15
(s,
3H); 2.26 (s, 3H); 2.79 (s, 2H); 3.77 (s, 3H); 4.02 (s, 2H); 4.60 (s, 2H);
6.57 (s, 1H);
6.80 (d, 2H); 7.07 (d, 2H); 7.10 (d, 2H); 7.13 (d, 2H); 7.37 (s, 1 H); 8.01
(broad s,
1 H).
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-46-
Example 1-7
ten-Butyl 2-(4-{ [(1-{ [(2,4-dimethylphenyl)amino]carbonyl } propyl)-
amino)methyl }-
phenoxy)-2-methylpropionate
O
O
N
H
Similarly to the procedure of Example 1-1, 320 m~ (0.90 mmol) of 2-{ [4-(2-
tert-
butoxy-1,1-dimethyl-2-oxoethoxy)benzyl]amino}butyric acid (Example I-15),
160 mg (1.36 mmol) of 2,4-dimethylaniline, 160 mg (1.18 mmol) of 1-hydroxy-1H-
benzotriazole, 230 mg (1.18 mmol) of 1-ethyl-3-(3-dimethylamino)propylcarbo-
diimide hydrochloride, 270 m? (2.71 mmol) of N-methylmorpholine and 1 mg
(0.01 mmol) of 4-dimethylaminopyridine in 5 ml of dimethylformamide are
converted into 190 mg (46%) of ten-butyl 2-(4-{ [( 1-{ [(2,4-dimethylphenyl)-
amino]carbonyl }propyl)amino]methyl } phenoxy)-2-methylpropionate.
'H-NMR (200 MHz, CDC13): 8 = 0.99 (t, 3H); 1.43 (s, 9H); 1.56 (s, 6H); 1.74
(m,
2H); 2.21 (s, 3H); 2.28 (s, 3H); 3.22 (dd, 1H); 3.69 (d, 1H); 3.82 (d, 1H);
6.83 (d,
2H); 6.98 (s, 1H); 7.02 (d, 1H); 7.18 (d, 2H); 7.93 (d, 1H); 9.32 (broad s,
1H).
Example 1-8
ten-Butyl 2-(4-{ [(1-{ [(4-methoxy-2,5-dimethylphenyl)amino]carbonyl }propyl)-
amino]methyl }phenoxy)-2-methylpropionate
O
O ~ O \ O O
~i
N
H
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 47 -
Similarly to the procedure of Example 1-1, 320 mg (0.90 mmol) of 2-{ [4-(2-
tert-
butoxy-1,1-dimethyl-2-oxoethoxy)benzyl]amino}butyric acid (Example I-15),
210 mg (1.36 mmol) of 4-methoxy-2,5-dimethylaniline, 160 mg (1.18 mmol) of 1-
hydroxy-1H-benzotriazole, 230 mg (1.18 mmol) of 1-ethyl-3-(3-
dimethylamino)propylcarbodiimide hydrochloride, 270 mg (2.71 mmol) of N-
methylmorpholine and 1 mg (0.01 mmol) of 4-dimethylaminopyridine in 5 ml of
dimethylformamide are converted into 130 mg (30%) of ten-butyl 2-(4-{ ((1-{
[(4-
methoxy-2,5-dimethylphenyl)amino]carbonyl } propyl)amino]methyl } phenoxy)-2-
methylpropionate
~H-NMR (200 MHz, CDC1;): ~ = 0.99 (t, 3H); 1.44 (s, 9H); 1.56 (s, 6H); 1.74
(m,
2H); 2.19 (s, 3H); 2.22 (s, 3H); 3.22 (dd, 1H); 3.71 (d, 1H); 3.80 (s, 3H);
3.82 (d,
1H); 6.64 (s, 1H); 6.83 (d, 2H); 7.19 (d, 2H); 7.6~ (s, 1H); 9.13 (broad s,
1H).
E~cample 1-9
3-(4-{ [(2-(2,4-Dimethylphenyl)amino-2-oxoethyl)(2-furylmethyl)amino]methyl }-
phenyl)-2,2-dimethylpropionic acid
O
O
O \ ~~ \0H
\ N~"~N /
H
A solution of 192 mg (0.380 mmol) of ten-butyl 3-(4-{ [(2-(2,4-dimethylphenyl)-
amino-2-oxoethyl)(2-furylmethyl)amino]methyl } phenyl)-2,2-dimethylpropionate
(Example 1-1) in 1 ml of dichloromethane is treated with 1 ml of
trifluoroacetic acid
and stirred at room temperature for 2 h. The mixture is then concentrated
under
reduced pressure, the residue is taken up in ethyl acetate and the organic
phase is
washed 2x with water, lx with 20% strength sodium acetate solution, lx with
water
and lx with sat. NaCI solution, dried over sodium sulphate and freed from the
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 48 -
solvent under reduced pressure. Chromatographic purification of the residue on
silica
gel (dichloromethane -> dichloromethane/methanol 20:1) gives 150 mg (88%) of 3-
(4-{ [(2-(2,4-dimethylphenyl)amino-2-oxoethyl)(2-furylmethyl)amino]methyl }-
phenyl)-2,2-dimethylpropionic acid.
'H-NMR (200 MHz, CDCl3): 8 = 1.16 (s, 6H); 2.26 (s, 3H); 2.28 (s, 3H); 2.87
(s,
2H); 3.30 (s, 2H); 3.71 (s, 2H); 3.74 (s> 2H); 6.26 (d, 1H); 6.32 (dd, 1H);
6.99 (m,
2H); 7.12 (d, 2H); 7.24 (d, 2H); 7.37 (d, 1H); 7.83 (d, 1H); 9.12 (broad s,
1H).
Example 1-10
3-(4-{ [(2-(4-Methoxy-2,5-dimethylphenyl)amino-2-oxoethyl)(2-furylmethyl)-
amino]methyl}phenyl)-2,2-dimethylpropionic acid
O
O O
O ~ \ /~ OOH
\ N~N /
Similarly to the procedure of Example 1-9, 170 mg (0.318 mmol) of ten-butyl 3-
(4-
{ [(2-(4-methoxy-2,5-dimethylphenyl)amino-2-oxoethyl)(2-furylmethyl)amino]-
methyl }phenyl)-2,2-dimethylpropionate (Example 1-2) are reacted with 1 ml of
trifluoroacetic acid in 1 ml of dichloromethane to give 133 mg (87%) of 3-(4-(
[(2-(4-
methoxy-2,5-dimethylphenyl)amino-2-oxoethyl)(2-furylmethyl)amino]methyl }-
phenyl)-2,2-dimethylpropionic acid.
'H-NMR (200 MHz, DMSO): 8 = 1.04 (s, 6H); 2.07 (s, 3H); 2.13 (s, 3H); 2.76 (s,
2H); 3.18 (s, 2H); 3.70 (s, 2H); 3.74 (s, 3H); 3.76 (s, 2H); 6.39 (d, 2H);
6.87 (s, 1H);
7.12 (d, 2H); 7.28 (d, 2H); 7.30 (s, 1H); 7.61 (s, 1H); 9.02 (broad s, 1H);
12.18
(broad s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-49-
Example 1-11
3-(4-{ [N-(2-(2,4-Dimethylphenyl)amino-2-oxo)ethyl-N-phenylamino]methyl }-
phenyl)-2,2-dimethylpropionic acid
O
O / ~ OH
I ~N I ~ /\
'N
H
Similarly to the procedure of Example 1-9, 48 m? (0.096 mmol) of tert-butyl 3-
(4-
{ (N-(2-(2>4-dimethylphenyl)amino-2-oxo)ethyl-N-phenylamino]methyl }phenyl)-
2,2-
dimethylpropionate (Example 1-3) are reacted with 1 ml of trifluoroacetic acid
in
2 ml of dichlormethane to give 36 mg (85%) of 3-(4-{ [N-(2-(2,4-dimethyl-
phenyl)amino-2-oxo)ethyl-N-phenylamino]methyl }phenyl)-2,2-dimethylpropionic
acid.
~H-NMR (200 MHz, CDC13): 8 = 1.19 (s, 6H); 1.90 (s, 3H); 2.26 (s, 3H); 2.87
(s,
2H); 4.08 (s, 2H); 4.66 (s, 2H); 6.80 - 6.9~ (m, 4H); 6.98 (d, 1H); 7.14 (s,
4H); 7.27
(m, 2H); 7.67 (d, 1 H); 8.08 (broad s, 1 H).
Example 1-12
3-(4-{ [N-(2-(4-Methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-phenylamino]-
methyl}phenyl)-2,2-dimethylpropionic acid
I ~ O
O / ~ OH
I ~N I ~ /\
N
H
Similarly to the procedure of Example I-9, 61 mg (0.115 mmol) of ten-butyl 3-
(4-
{ [N-(2-(4-methoxy-2,5-dimethylphenyI)amino-2-oxo)ethyl-N-phenylamino]methyl }-
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-50-
phenyl)-2,2-dimethylpropionate (Example 1-4) are reacted with 1 ml of
trifluoroacetic acid in 2 ml of dichloromethane to give 46 mg (85%) of 3-(4-{
[N-(2-
(4-methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-phenylamino]methyl } phenyl)-
2,2-dimethylpropionic acid.
'H-NMR (200 MHz, CDC13): 8 = 1.19 (s, 6H); 1.94 (s, 3H); 2.15 (s, 3H); 2.86
(s,
2H); 3.77 (s, 3H); 4.08 (s, ZH); 4.66 (s, 2H); 6.56 (s, 1H); 6.83 (dd, 1H);
6.88 (d,
2H); 7.13 (s, 4H); 7.24 (d, 2H); 7.34 (m, 1H); 7.93 (broad s, 1H).
Example 1-13
3-(4-{ [N-(2-(2,4-Dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)amino]-
methyl }phenyl)-2,2-dimethylpropionic acid
O
O / '~ O H
~N
N
H
Similarly to the procedure of Example 1-9, 23 mg (0.049 mmol) of ten-butyl 3-
(4-
{ [N-(2-(2,4-dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)amino]methyf }-
phenyl)-2,2-dimethylpropionate (Example 1-5) are reacted with 1 ml of
trifluoroacetic acid in 2 ml of dichloromethane to give 20 mg (91 %) of 3-(4-{
[N-(2-
(2,4-dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)amino]methyl }phenyl)-
2,2-dimethylpropionic acid.
'H-NMR (200 MHz, CDC13): 8 = 1.17 (s, 6H); 1.92 (s, 3H); 2.25 (s, 6H); 2.86
(s,
2H); 4.02 (s, 2H); 4.60 (s, 2H); 6.79 (d, 2H); 6.91 (s, 1H); 6.98 (d, 1H);
7.06 (d, 2H);
7.13 (s, 2H); 7.17 (d, 2H); 7.68 (d, 1H); 8.19 (broad s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-51 -
Example 1-14
3-(4-{ [N-(2-(4-Methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-(4-
methylphenyl)-
amino]methyl }phenyl)-2,2-dimethylpropionic acid
O
O / O / ~ \ OH
N
~N
H
Similarly to the procedure of Example 1-9, 40 m~ (0.073 mmol) of ten-butyl 3-
(4-
{ [N-(2-(4-methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)-
amino]methyl}phenyl)-2,2-dimethylpropionate (Example 1-6) are reacted with 1
ml
of trifluoroacetic acid in 2 ml of dichloromethane to dive 33 mg (93%) of 3-(4-
{ [N-
(2-(4-methoxy-2,5-dimethylphenyl)amino-2-oxo)ethyl-N-(4-methylphenyl)amino]-
methyl}phenyl)-2,2-dimethylpropionic acid.
'H-NMR (200 i'VE-lz, CDC13): S = 1.18 (s, 6H); 1.96 (s, 3H); 2.15 (s, 3H);
2.26 (s,
3H); 2.86 (s, 2H); 3.76 (s, 3H); 4.03 (s, 2H); 4.61 (s, 2H); 6.57 (s, 1H);
6.80 (dd, 2H);
7.07 (d, 2H); 7.14 (s, 4H); 7.36 (s, 1H); 8.02 (broad s, 1H).
Example 1-1~
2-(4-{ [(1-{ [(2,4-Dimethylphenyl)amino]carbonyl }propyl)amino)methyl
}phenoxy)-2-
methylpropionic acid
O
O \ O OH
\ N ~ /
N
H
CA 02424540 2003-04-02
Le A 34 838-Forei ~n Countries
-52-
Similarly to the procedure of Example 1-9, 170 mg (0.374 mmol) of ten-butyl 2-
(4-
{ [(I-{ [(2,4-dimethylphenyl)amino]carbonyl } propyl)amino]methyl }phenoxy)-2-
methylpropionate (Example 1-7) are reacted with 0.72 ml (9.35 mmol) of
trifluoroacetic acid in 3 ml of dichloromethane to give 113 mg (72%) of 2-(4-{
j(1-
S {[(2,4-dimethylphenyl)amino]carbonyl?propyl)amino]methyl}phenoxy)-2-methyl-
propionic acid.
'H-NMR (300 MHz, CDC13): 8 = 1.01 (t, 3H); 1.53 (d, 6H); 1.95 (m, 2H); 2.10
(s,
3H); 2.23 (s, 3H); 3.67 (broad s, 1H); 4.02 (m, 1H); 4.55 (m, 1H); 6.61 (d,
2H); 6.82
(d, 1H); 6.89 (s, 1H); 7.10 (d, 2H); 7.11 (s, 1H); 9.53 (broad s, 1H).
Example 1-16
2-(4-{ [(1-{ [(4-'Vlethoxy-2,5-dimethylphenyl)amino]carbonyl}propyl)amino]-
methyl }phenoxy)-2-methylpropionic acid
O
O / O ~ O OH
\ N ~ /
N
H
Similarly to the procedure of Example 1-9, 115 mg (0.237 mmol) of tent-butyl 2-
(4-
{ [(1-{ [(4-methoxy-2,5-dimethylphenyl)amino]carbonyl }propyl)amino]-methyl }-
phenoxy)-2-methylpropionate (Example 1-8) are reacted with 0.46 ml (5.93 mmol)
of
trifluoroacetic acid in 3 ml of dichloromethane to give 100 mg (93%) of 2-(4-{
[(1-
{ [(4-methoxy-2>5-dimethylphenyl)amino]carbonyl }propyl)amino]methyl }phenoxy)-
2-methylpropionic acid.
'H-NMR (300 MHz, CDC13): 8 = 1.05 (t, 3H); 1.55 (d, 6H); 1.97 (m, 2H); 2.10
(s,
6H); 3.75 (s, 3H); 3.78 (m, 1H); 4.08 (m, 2H); 4.50 (m, 2H); 6.50 (s, 1H);
6.64 (d,
2H); 6.94 (s, 1H); 7.14 (d, 2H); 7.65 (s, 1H); 9.38 (broad s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Forei~ Countries
-53-
Starting materials II
Example II-1
l,l-Dimethylethyl 2-[(4-bromophenyl)thio)-2-methyl-propanoate
4-Bromothiophenol (100 g) and tert-butyl 2-bromoisobutyrate (118 g) are
dissolved
in 1 1 of ethanol and treated with 29 g of KOH. The mixture is stirred under
reflux for
2 h and cooled, and the KBr is filtered off. The filtrate is concentrated and
the residue
is recrystallized from n-hexane. This gives 93.6 g of a colourless solid.
~H-NNLR (200 MHz, CDCl3): 1.48 (s> 15H); 7.38 (m, 4H).
Example II-2
1,1-Dimethylethyl 2-[(4-formylphenyl)thio]-2-methyl-propanoate
1.0 g of 1,1-dimethylethyl 2-[(4-bromophenyl)thio]-2-methyl-propanoate is
dissolved
in 20 ml of THF and treated with 189 ml (3.02 mmol, 1 eq) of n-butyllithium
solution in hexane. Directly afterwards, 0.46 ml of dimethylformamide are
added and
the mixture is warmed to room temperature and stirred for 1 hour. The reaction
is
quenched by addition of 1 ml 1 N HCI, the mixture is concentrated and the
residue is
taken up in ethyl acetate. The mixture is extracted with sat. NaHC03 solution
and
with NaCI solution and dried (MgSO,~). Chromatographic purification
(dichloromethane) gives 550 mg of a pale yellow oil.
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R~ = 4.86 min ([M+H]+ -
281 ).
Example II-3
1,1-Dimethylethyl 2-[[4-[[(2-furanylmethyl)amino)methyl]phenyl]thio]-2-methyl-
propanoate
550 mg of 1,1-dimethylethyl 2-[(4-formylphenyl)thio]-2-methyl-propanoate and
381 mg of furfurylamine are initially charged in 100 ml of methanol and
treated with
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-54-
1 ml of glacial acetic acid. The mixture is stirred at room temperature for 15
min,
briefly brought to the boil and then, at 0°C, admixed a little at a
time with 493 mg of
sodium cyanoborohydride. The mixture is stirred overnight at room temperature
and
then treated with 1 N HCl and stirred for 30 min. The mixture is then made
basic
using NaZC03 solution and extracted 2x with ethyl acetate. The organic phase
is
washed (sat. NaCI solution) and dried (MgS04). Concentration and
chromatographic
purification (dichloromethane/ethyl acetate 10+1) gives 430 mg of a colourless
oil.
~H-NMR (300 MHz, CDCI3): 1.42 (s, 15H); 3.79 (s, 2H); 3.80 (s, 2H); 6.15 (m,
1H);
6.28 (m, 1H); 7.25-7.45 (m, 5H).
Example II-4
1,1-Dimethylethyl 2-[[4-[2-[(2-ethoxy-2-oxoethyl)(2-
furanylmethyl)amino]methyl]-
phenyl]thio]-2-methyl-propanoate
5.4 g of 1,1-dimethylethyl 2-[[4-[[(2-furanylmethyl)amino]methyl]phenylJthio]-
2-
methyl-propanoate are dissolved in 270 ml of tetrahydrofuran and treated with
2.27 g
of triethylamine and 3.74 g of ethyl bromoacetate and 14.83 g of tetra-n-butyl-
ammonium iodide. The mixture is stirred at 90°C for 48 h, cooled and
mixed with
water and ethyl acetate. The organic phase is separated off and washed twice
with sat.
NaCI solution. The mixture is dried (MgSO~) and concentrated and the residue
is
purified chromatographically (cyclohexane/ethyl acetate 5+1), giving 6.4 g of
a
colourless oil.
~H-NMR (CDCI3, 200 MHz): 1.28 (t, 3 H, J=8.7 Hz); 1.40 (s, 9H); 1.42 (s, 6H);
3.32 (s, 2H); 3.78 (s, 2H); 3.84 (s, 2H); 4.15 (q, J=8.7 Hz); 6.17 (m, 1H);
6.30 (m,
1H); 7.23-7.45 (m, SH).
Example II-5
2-[[4-[2-[(Carboxymethyl)(2-furanylmethyl)amino]methyl]phenylJthioJ-2-methyl-
1,1-dimethylethyl propanoate
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-55-
192 mg of 1,1-dimethylethyl 2-([4-[2-[(2-ethoxy-2-oxoethyl){2-furanylmethyl)-
amino]methyl]phenyl]-thio]-2-methyl-propanoate are initially charged in 5 ml
of
ethanol and treated with 0.4 ml 1 N NaOH. The mixture is stirred at
80°C for 1 h.
The mixture is checked by TLC (CHZCI~/methanol=10+1) and then cooled and
concentrated, and the residue is dissolved in a little water. The mixture is
acidified
using 1 N HCl and extracted three times with ethyl acetate. The combined
organic
phases are washed 2x with water and 2x with sat. NaCI solution and dried over
MgSO~. The mixture is concentrated, applied to silica gel and purified by
flash
chromatography using CHZC1~ -> CH~CIz/methanol 50+1 -> 25+1. This gives 132 mg
of a colourless oil which solidifies under high vacuum.
'H-NMR (DMSO, 200 MHz): 1.32 (s, 9H); 1.39 (s, 6H); 3.18 (s, 2H); 3.22 (s,
2H);
3.23 (s, 2H); 6.27 (m, 1H); 6.40 (m, 1H); 7.34 (d, 2H, J= 9.0 Hz); 7.50 (d,
2H, J=9.0
Hz); 7.59 (m, 1H); 12.38 (broad s, 1H).
Example II-6
1,1-Dimethylethyl 2-[[4-[2-[(2-furanylmethyl)amino]ethyl]phenyl]thin]-2-methyl-
propanoate
4.0 g of 1,1-dimethylethyl 2-[[4-(2-aminoethyl)phenyl]thio]-2-methyl-
propanoate
[(P.]. Brown et al., J. Med. Chern. 42, 3785-88 (1999)] are dissolved in 100
ml of
methanol and treated with 2.6 g of furfural. 9.3 ml of glacial acetic acid are-
added
and the mixture is boiled briefly (10 min). The mixture is then cooled to
0°C, and
4.25 g of sodium cyanoborohydride are added a little at a time. The mixture is
then
stirred at room temperature overnight. 1 N HCl is added until the mixture is
acidic,
and the mixture is stirred for 30 min. The mixture is concentrated slightly
and made
basic using sat. NaHC03 solution. The mixture is then extracted twice with
ethyl
acetate and the extracts are washed (sat. NaCI solution) and dried and
concentrated.
Chromatographic purification (dichloromethane/methanol 15+1) gives 2.4 g of
the
title compound as a colourless oil.
Rf (Dichloromethane/methanol 10+1) = 0.57.
CA 02424540 2003-04-02
L,e A 34 838-Foreign Countries
-56-
Example II-7
1,1-Dimethylethyl 2-[[4-[2-[(2-ethoxy-2-oxoethyl)(2-furanylmethyl)amino]ethyl]-
phenyl]thio]-2-methyl-propanoate
2.4 g of l,l-dimethylethyl 2-[[4-[2-[(2-furanylmethyl)amino]ethyl]phenyl]thio]-
2-
methyl-propanoate, 1.5 g of ethyl bromoacetate, 0.97 g of triethylamine and
7.08 g of
tetra-n-butylammonium iodide are dissolved in 100 ml of tetrahydrofuran and
heated
at reflux overnight. Ethyl acetate and water are added, and the mixture is
extracted
with water and sat. NaCI solution. Concentration and chromatography (petroleum
ether/ethyl acetate 10+1) gives 1.38 g of the title compound.
'H-NMR (DMSO, 200 MHz): 1.18 (t, 3H, J=7.8 Hz); 1.37 (s, 15H); 2.77 (m 4H);
3.32 (s, 2H); 3.81 (s, 2H); 4.06 (q, 2H, J=7.8 Hz); 6.21 (m, 1H); 6.34 (m,
1H); 7.16
(d, 2H, J=9.6 Hz); 7.32 (d, 2H, J=9.6 Hz); 7.58 (m, 1H).
Example II-8
l, l-Dimethylethyl 2-[[4-[2-[(carboxymethyl)(2-
furanylmethyl)amino]ethyl]phenyl]-
thio]-2-methyl-propanoate
1.0 g of 1>1-dimethylethyl 2-[[4-[2-[(2-ethoxy-2-oxoethyl)(2-
furanylmethyl)amino]-
ethyl]phenyl]thio]-2-methyl-propanoate is treated with 6.5 ml of 1 N NaOH in
10 ml
of ethanol. The mixture is stirred at 80°C for 1 h, concentrated,
dissolved in water
and acidified with 1 N HCI. Three extractions with ethyl acetate and
chromatography
(dichloromethane/methanol 5+1) gives 744 mg as a colourless oil.
1H-NMR (DMSO, 200 MHz): 1.36 (s, 15H); 2.75 (m, 4H); 3.20 (s, 2H); 3.72 (s,
2H);
6.18 (m, 1H); 6.88 (m, 1H); 7.12 (d, 2H, J=9.5 Hz); 7.32 (d, 2H, J=9.5 Hz);
7.56 (m,
1 H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-57-
Example II-9
1,1-Dimethylethyl 2-[[4-[[(2-methoxyethyl)amino]methyl]phenyl]thio]-2-methyl-
propanoate
7.9 g of 1,1-dimethylethyl 2-[(4-formylphenyl)thioJ-2-methyl-propanoate and
4.23 g
of methoxyethylamine are initially charged in 100 ml of methanol and admixed
with
19 ml of acetic acid. The mixture is stirred at RT for 15 min, boiled briefly
and then,
at 0°C, admixed a little at a time with 8.9 g of sodium
cyanoborohydride. The
mixture is stirred at room temperature overnight and then admixed with 1 N HC1
and
stirred for 30 min. The mixture is then made basic using sodium carbonate
solution
and extracted 2 x with ethyl acetate. The organic phase is washed with sat.
sodium
chloride solution and dried over magnesium sulphate. Concentration and
chromatographic purification give 5.6 g (58%) of a colourless oil.
'H-NMR (200 MHz, CDCI;): 8 = 1.38 (s, 6H), 1.42 (s, 9H), 2.45 (m, 3H, CHI +
NH), 3.37 (s, 3H), 3.88 (s, 2H), 7.25-7.52 (m, 4H).
Example II-10
1,1-Dimethylethyl 2-[[4-[[(2-(5-methylfuranmethyl)amino]methylJphenyl]thioJ-2-
methyl-propanoate
8.0 g 1,1-dimethylethyl 2-[(4-formylphenyl)thio)-2-methyl-propanoate and 6.3 g
of
5-methyl-2-furanmethanamine are initially charged in 100 ml of methanol and
treated
with 16 ml of acetic acid. The mixture is stirred at RT for 15 min, boiled
briefly and
then, at 0°C, admixed a little at a time with 5.7 g of sodium
cyanoborohydride. The
mixture is stirred at room temperature overnight and then admixed with 1 N HC1
and
stirred for 30 min. The mixture is then made basic using sodium carbonate
solution
and extracted 2 x with ethyl acetate. The organic phase is washed with sat.
sodium
chloride solution . and dried over magnesium sulphate. Concentration and
chromatographic purification gives 4.8 g (45%) of a colourless oil which tends
to
decompose and is stored at -25°C.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-58-
1H-NMR (200 MHz, CDCI3): 8 = 1.42 (s, 15H), 1.72 (s, 1H, NH), 2.28 (s, 3H),
3.79
(s, 2H), 3.78 (s, 2H), 5.88 (m, 1H), 6.03 (m, 1H), 7.28 (dd, 2 H, J=llHz),
7.45 (m;
2H, J=llHz).
Example II-11
2-Bromo-N-(2,4-dimethylphenyl)-acetamide
117 g of triethylamine and 140 ~ of 2,4-dimethylaniline are dissolved in 21 of
methylene chloride, and a solution of 233 g of alpha-bromoacetyl bromide in
400 ml
of methylene chloride is added with ice-cooling, at at most 15°C>
within 30 min.
After a reaction time of 30 min, the precipitate is filtered off with suction,
the residue
is dissolved in 3 1 of methylene chlor;de and combined with the filtrate and
washed
twice with 2 1 of water and 2 I of sat. sodium chloride solution. The mixture
is dried
over sodium sulphate, filtered off with suction and concentrated, and the
residue is
recrystallized from ethanol. This dives 193 ~ of the title compound.
Example II-12
2-Bromo-N-(2,4-dichlorophenyl)-acetamide
This compound was prepared similarly to Example II-11 from 4.2 g of 2,4-
dichloro-
aniline and 5.76 g of bromoacetyl bromide and 2.89 g of triethylamine in
methylene
chloride. This have 5.9 g (80.4%) of the title compound.
Rf (Dichloromethane): 0.38
MS (EI pos.): M+ = 283.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-59-
Working Examples 2
Example 2-1
ten-Butyl 2-[[4-[[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-furanylmethy1)-
amino]-methyl]phenyl]thio]-2-methyl-propanoate
Method a):
250 mg of 2-[[4-[2-[(carboxymethyl)(2-furanylmethyl)amino]methyl]-phenyl]thio]-
2
methyl-1,1-dimethylethyl propanoate, 89 mg of hydroxybenzotriazole, 249 ml of
triethylamine, 82 mg of 2,4-dimethylaniline and 131 mg of N'-(3
dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride are dissolved in 5 ml
of
dichloromethane. The mixture is stirred at room temperature for 20 h and
extracted
with 1 N NaOH, 1 N HCI, water and sat. NaCI solution. The combined organic
phases are dried (MgSO;~) and purified chromatographically
(dichloromethane/ethyl
acetate 25+1). This gives 200 mg of a viscous oil.
LC-MS: Acetonitrilel30~o aqueous HCIlwater (gradient): R, = 4.87 min ([M+H]+ -
523).
Method b):
1.5 g of 1,1-dimethylethyl 2-[[4-[[(2-furanylmethyl)amino]methyl]phenyl]thio]-
2-
methyl-propanoate (Example II-3) and 1.1 g of 2-bromo-N-(2,4-dimethylphenyl)-
acetamide (Exari-lple II-9) are dissolved in 20 ml of DMF and treated with 0.4
g of
sodium bicarbonate. The mixture is heated at 90°C overnight,
concentrated and
purified chromatographically (dichloromethane/ethyl acetate 10:1 and 5:1).
This
gives 2.1 g of the title compound.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-60-
Example 2-2
tert-Butyl 2-[[4-[[[2-[(2,4,6-trimethylphenyl)aminoj-2-oxoethylj(2-
furanylmethyl)-
amino]-methyl]phenyljthioj-2-methyl-propanoate
250 mg of 2-[[4-[2-[(carboxymethyl)(2-furanylmethyl)aminojmethylj-phenyljthioj-
2-
methyl-1,1-dimethylethyl propanate, 90 mg of hydroxybenzotriazole, 250 ml of
triethylamine, 80 mg of 2,4,6-trimethylaniline and 130 mg of N'-(3-
dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride are dissolved in 5 ml
of
dichloromethane. The mixture is stirred at room temperature for 20 h and
extracted
with 1 N NaOH, 1 N HCI, water and sat. NaCI solution. The combined organic
phases are dried (MgSO,~) and purified chromatographically
(dichloromethane/ethyl
acetate 25+1). This gives 210 mg of a viscous oil.
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R, = 5.32 min ([M+Hj+ -
537).
Example 2-3
tert-Butyl 2-[[4-[[(2-[(2,5-dimethyl-4-methoxyphenyl)amino]-2-oxoethylj(2-
furanyl-
methyl)-aminojmethyljphenyl]thioj-2-methyl-propanoate
250 mg of 2-[[4-[2-[(carboxymethyl)(2-furanylmethyl)amino]-ethyl]-phenyljthioj-
2-
methyl-1>1-dimethylethyl propanoate, 90 mg of hydroxybenzotriazole, 2~0 ml of
triethylamine, 80 mg of 2,5-dimethyl-4-methoxyaniline and 130 mg of N'-(3-
dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride are dissolved in 5 ml
of
dichloromethane. The mixture is stirred at room temperature for 20 h and
extracted
with 1 N NaOH, 1 N HCI, water and sat. NaCI solution. The combined organic
phases are dried (MgSO~) and purified chromatographically
(dichloromethane/ethyl
acetate 25+1). This gives 190 mg of a viscous oil.
LC-MS: Acetonitrile/30% aqueous HCI/water (Gradient): R~ = 4.90 min ([M+H]+ _
552).
CA 02424540 2003-04-02
Ix A 34 838-Forei~ Countries
-61 -
Example 2-4
tent-Butyl 2-[[4-[[[2-[(2-methyl-4-methoxyphenyl)amino]-2-oxoethyl](2-furanyl-
methyl)amino]-methyl]phenyl]thio]-2-methyl-propanoate
250 mg of 2-[[4-[2-[(carboxymethyl)(2-furanylmethyl)amino]-ethyl]-phenyl]thio]-
2-
methyl-1,1-dimethylethyl propanoate, 90 m~ of hydroxybenzotriazole, 250 ml of
triethylamine, 80 mg of 2-methyl-4-methoxylaniline and 130 m~ of N'-(3-
dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride are dissolved in 5 ml
of
dichloromethane. The mixture is stirred at room temperature for 20 h and
extracted
with 1 N NaOH, 1 N HCI, water and sat. NaC1 solution. The combined organic
phases are dried (MgSO:~) and purified chromato~raphically
(dichloromethane/ethyl
acetate 25+1). This dives 190 mg of a viscous oil.
LC-MS: Acetonitrile/30% aqueous HC1/water Gradient): R~ = 4.69 min ([M+H]+ -
538).
Example 2-~
2-[[4-j[[2-[(2,4-Dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)amino]-
methyl]phenyl]thio]-2-methyl-propanoic acid
w o 0
OH
N \
~~O
90 mg of tert-butyl 2-[[4-[[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-
furanylmethyl)amino]-methyl]phenyl]thio]-2-methyl-propanoate are dissolved in
5 ml of dichloromethane and reacted with 0.1 ml of trifluoroacetic acid. The
mixture
is stirred at room temperature for 4 h and then concentrated and purified
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-62-
chromatographically (dichloromethane/methanol 100+1). This gives 80 mg of the
title compound as a solid foam.
Rf(Dichloromethane/methanol 10+1) = 0.3
'H-N1VIR (400 MHz, D6-DMSO): S = 1.34 (s, 6H, CH3), 2.16 (s, 3H, CH3), 2.23
(s,
3H, CH3), 3.24 (s, 2H, CHZ), 3.76 (s, 2H, CHZ), 3.78 (s, 2H, CHZ), 6.38-6.40
(m, 2H,
2x furanyl-H), 6.93-6.95 (d, 2H, Ar-H), 7.0 (s, 1H, Ar-H), 7.38-7.51 (m, 4H,
Ar-H),
7.60-7.61 (m, 1H, furanyl-H), 9.14 (s, 1H, NH).
MS (ESI pos.): m/z = 467 ([M+H]+), m/z = 489 ([M+Na]+)
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R~ = 3.76 min ([M+H]+ _
467).
E~cample 2-~a
2-[[4-[[[2-[(2,4-Dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)amino]-
methyl]phenyl]thio]-2-methyl-propanoic acid dicyclohexylammonium salt
O S O
~OH
E
HN
500 mg of 2-[[4-[[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)-
amino]methyl]phenyl]thio]-2-methyl-propanoic acid (Example 2-5) are dissolved
in
500 mg of acetonitrile, and 194 mg of dicyclohexylamine are added. Water is
added,
some of the acetonitrile is distilled off until the mixture becomes turbid and
the
mixture is lyophilized. This gives 445 mg of a powder.
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): Rt = 3.76 min ([M+H]+ -
467).
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-63-
Example 2-Sb
2-[ [4-[ [ [2-[(2,4-Dimethylphenyl)amino]-2-oxoethyl ] (2-furanylmethyl)amino]-
methyl]phenylJthio]-2-methyl-propanoic acid hydrochloride
O O
OH
N \
/~O x HC!
1.20 g of 2-[[4-[[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-furanylmethy1)-
amino]-methyl]phenyl]thio]-2-methyl-propanoic acid (Example 2-5) are dissolved
in
100 ml of ethyl acetate and admixed with 1N HCl/diethyl ether until the
mixture
becomes turbid. The resulting crystals are filtered off with suction and
washed with
dry ether. This gives 1 g of the title compound.
M.p.: 158°C (from ethanol/diethyl ether).
Example 2-6
2-[[4-[[[2-[(2,4,6-Trimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)amino]-
methyl]phenyl]thio]-2-methyl-propanoic acid
\ O O
OH
N \
/~O
210 mg of ten-butyl 2-([4-[[[2-[(2,4,6-trimethylphenyl)amino]-2-oxoethyl](2-
furanylmethyl)-amino]methyl]phenyl]thio]-2-methyl-propanoate are dissolved in
5 ml of dichloromethane and reacted with 1 ml of trifluoroacetic acid. The
mixture is
CA 02424540 2003-04-02
Le A 34 838-Forei~~n Countries
stirred at room temperature for 4 h and then concentrated and purified
chromatographically (dichloromethanelethyl acetate 50+1). This dives 187 m~ of
the
title compound as a solid foam.
'H-NMR (DMSO, 200 MHz): 1.42 (s, 6H); 2.04 (s, 6H); 2.23 (s, 3H); 3.58 (broad
s,
2H); 4.05 (s, 2H); 4.12 (s, 2H); 6.55 (m, 2H); 6.87 (s, 2H); 7.48 (d, 2H, J=
9.0 Hz);
7.51 (d, 2H, J= 9.0 Hz); 7.72 (m, 1H); 9.40 (broad s, 1H).
Example 2-7
2-[[4-[[[2-[(2,5-Dimethyl-4-methoxyphenyl)amino]-2-oxoethyl](2-furanylmethyl)-
amino]methylJphenyl]thio]-2-methyl-propanoic acid
O
O S O
~OH
E
190 m~ of tert-butyl 2-[[4-[[[2-[(2,5-dimethyl-4-methoxyphenyl)amino]-2-
oxoethyl]-
(2-furanyl-methyl)amino]methyl]phenylJthio]-2-methyl-propanoate are dissolved
in
5 ml of dichloromethane and reacted with 1 ml of trifluoroacetic acid. The
mixture is
stirred at room temperature for 20 h and then concentrated and purified
chromatojraphically (dichloromethanelmethanol 50+1). This gives 166 mg of the
title compound as a solid foam.
'H-NMR (DMSO, 200 MHz): 1.39 (s, 6H); 2.08 (s, 3H); 2.11 (s, 3H); 3.7 (s, 3H);
4.00 (broad s, 4H); 6.48 (m, 1H); 6.51 (m, 1H); 6.76 (s, 1H); 7.08 (s, 1H);
7.48 (m,
4H); 7.72 (m, 1H); 9.35, (broad s, 1H); 12.65 (broad s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
Example 2-8
2-[[4-[[[2-[(2-Methyl-4-methoxyphenyl)amino]-2-oxoethyl)(2-furanylmethyl)-
amino)methyl]phenyl]thio]-2-methyl-propanoic acid
O ~ O O
OH
N \
~~O
5
200 mg of tert-butyl 2-[[4-[[[2-[(2-methyl-4-methoxyphenyl)amino]-2-
oxoethyl](2-
furanyl-methyl)amino]methyl]phenyl]thio]-2-methyl-propanoate are dissolved in
5 ml of dichloromethane and reacted with 1 ml of trifluoroacetic acid. The
mixture is
10 stined at room .temperature for 20 h and then concentrated and purified
chromatographically (dichloromethane/methanol 50+1). This gives 174 mg of the
title compound as a solid foam.
~H-NMR (DMSO, 200 MHz): 1.38 (s, 6H); 2.12 (s, 3H); 3.7 (s, 3H); 3.80 (broad
s,
2H); 4.00 (broad s, 2H); 6.45 (m, 1H); 6.5~ (m, 1H); 6.65 (m, 1H); 6.78 (m,
1H);
15 7.25 (m, 1H); 7.48 (m, 4H); 7.71 (m, 1H); 9.37 (broad s, 1H); 12.65 (broad
s, 1H).
Example 2-9
25
tert-Butyl 2-([4-(2-[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-
furanylmethyl)-
amino]-ethyl]phenyl]thio]-2-methyl-propanoate
104 mg of 1,1-dimethylethyl 2-([4-[2-[(carboxymethyl)(2-furanylmethyl)amino]-
ethyl]phenyl]thio]-2-methyl-propanoate, 36 mg of hydroxybenzotriazole, 0.1 ml
of
triethylamine, 29 mg of 2,4-dimethylaniline and 53 mg of N'-(3-dimethyl-
aminopropyl)-N-ethylcarbodiimide hydrochloride are dissolved in 5 ml of
dichloromethane. The mixture is stirred at room temperature for 20 h and
extracted
with 1 N NaOH, 1 N HCI, water and sat. NaCI solution. The combined organic
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-66-
phases are dried (MaS04) and purified chromatographically
(dichloromethane/ethyl
acetate 5+1). This gives 190 mg of a viscous oil.
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R~ = 5.3 min ([M+H]+ -
537).
'H-NMR (CDC13, 200 MHz): 1.38 (s, 9H); 1.40 (s, 6H); 2.08 (s, 3H); 2.28 (s,
3H);
2.82 (m, 4H); 3.32 (s, 2H); 3.78 (s, 2H); 6.22 (m, 1H); 6.95 (m, 1H); 7.00 (m,
1H);
7.05 (d, 2H, J=10.0 Hz); 7.3~ (d, 2H, J=10.0 Hz), including: (m, 1H); 7.79 (m,
1H);
8.95 (broad s, 1H); 12.60 (broad s, 1H).
Example 2-10
tert-Butyl 2-[[4-[2-[[2-[(2,5-dimethyl-4-methoxyphenyl)amino]-2-oxoethyl](2-
furanylmethyl)-amino]ethyl]phenyl]thio]-2-methyl-propanoate
98 mg of 1,1-dimethylethyl 2-[[4-[2-[(carboxymethyl)(2-furanylmethyl)amino]-
ethyl]phenyl]thio]-2-methyl-propanoate, 33 mg of hydroxybenzotriazole, 0.09 ml
of
triethylamine, 34 mg of 2,5-dimethyl-4-methoxyaniline and 49 mg of N'-(3-
dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride are dissolved in 5 ml
of
dichloromethane. The mixture is stirred at room temperature for 20 h and
extracted
with 1 N NaOH, 1 N HCI, water and sat. NaCI solution. The combined organic
phases are dried (MgSO.~) and purified chromatographically
(dichloromethane/ethyl
acetate S+1). This gives 48 m~ of a viscous oil.
TLC: R f = 0.65 (dichloromethane/ethyl acetate =10+1).
Example 2-11
2-[[4-[2-[[2-[(2,4-Dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)amino]-
ethyl]phenyl]thio]-2-methyl-propanoic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-67-
O
HO
I / N N
I\
O
O
38 mg of tert-butyl 2-[[4-[2-[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-
furanylmethyl)-amino]ethyl]phenyl]thio]-2-methyl-propanoate are dissolved in ~
ml
of dichloromethane and treated with 0.27 ml of trifluoroacetic acid. The
mixture is
stirred at room temperature for 24 h and co-evaporated with toluene, and the
residue
is chromatographed (dichloromethane/methanol 10+1). This gives 33 mg of a
colourless oil.
LC-MS: Acetonitrile/30% aqueous HCI/water (gradient): R~ = 3.38 min ([M+H)+ -
481).
Example 2-12
2-[[4-[2-[[2-((2,5-Dimethyl-4-methoxyphenyl)amino]-2-oxoethyl](2-
furanylmethyl)-
amino]ethyl]phenyl)thio)-2-methyl-propanoic acid
O
HO
H
/ N~N \
!O~ I r O
O
30 mg of tert-butyl 2-[[4-[2-[[2-[(2,5-dimethyl-4-methoxyphenyl)amino)-2-
oxoethyl)(2-furanyl-methyl)amino]ethyl]phenyl]thio]-2-methyl-propanoate are
dissolved in 5 ml of dichloromethane and treated with 0.20 ml of
trifluoroacetic acid.
The mixture is stirred at room temperature for 24 h and co-evaporated with
toluene,
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-68-
and the residue is chromato~aphed (dichloromethane/methanol 10+1). This gives
27 mg of an oil which turns dark when exposed to the atmosphere.
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R~ = 3.78 min ([M+H]+ -
511).
'H-NMR (DMSO, 200 MHz): 1.35 (s, 9H); 2.05 (s, 3H); 2.10 (s, 3H); 2.82 (m,
4H);
3.25 (s, 2H); 3.72 (s, 3H); 3.82 (s, 2H); 6.33 (m, 2H); 6.72 (m, 1H); 7.1~ (d,
2H,
J=9.8 Hz); 7.24 (d, 2H, 1=9.8 Hz), including: (m, 1H); 7.62 (m, 1H); 8.88
(broad s,
1H); 12.55 (broad s, 1H).
The following exemplary compounds were prepared in a similar manner:
Example 2-13
2-Methyl-2-[[4-[[[(5-methyl-2-furanyl)methyl][2-oxo-2-[(2,4-dichlorophenyl)-
amino]ethyl]amino]methyl]phenyl]thioJ-propanoic acid
O
CI / CI00 \ S OH
\ I ~N ! /
N
H
Yield: 343 mg (68%).
'H-NMR: (200 MHz, CDC13): 8 = 1.50 (s, 6H, 2xCH3), 2.19 (s, 3H, CH3), 3.38 (s,
ZH, CHz), 3.78 (s, 2H, CHZ), 3.83 (s, 2H, CHZ), 4.30 (s, br, 1H, COOH), 5.85
(m,
1H, furanyl-H), 6.16 (m, 1H, furanyl-H), 7.18-7.49 (m, 6H, Ar-H), 8.30 (m, 1H,
Ar
H), 9.68 (s, 1 H, NH).
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R~ = 3.42 min ([M+H]+ _
521)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-69-
Example 2-14
2-Methyl-2-[(4-[[[(5-methyl-2-furanyl)methyl] (2-oxo-2-[(2,4,6-
trichlorophenyl)-
amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
ij o
CI / CI O \ S OH
\ I ~N I /
~N
H
CI
Yield: 90 mg (36°l0)
'H-NMR (200 MHz, CDCl3): 8 = 1.53 (s, 6H, 2xCH3), 2.29 (s, 3H, CH3), 3.75 (s,
2H, CHz), 4.2~ (s, 2H, CHI), 4.28 (s, 2H, CHZ), 5.95 (m, 1H, furanyi-H), 6.49
(m,
1H, furanyl-H), 7.35 (s, 2H, Ar-H), 7.38-7.51 (m, 4H, Ar-H), 9.51 (s, 1H, NH).
LC-MS: Acetonitrile/30% aqueous HC1/water Gradient): R~ = 3.05 min ([M+H]+=
555)
Example 2-15
2-Methyl-2-[[4-[[[(5-methyl-2-furanyl)methyl][2-oxo-2-[(2,4,6-
trimethylphenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
O
/ O ~ S OH
\ I ~N I /
~N
H
Yield: 46 m~ (26°l0)
LC-MS: Acetonitrile/30% aqueous HCl/water (jradient): R~ = 4.18 min ([M+H]+ -
494)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-70-
Example 2-16
2-Methyl-2-([4-[[[(5-methyl-2-furanyl)methyl] [2-oxo-2-[(2,4-dimethylphenyl)-
amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
/ ~ O
/ O \ S OH
\ I ~N I /
~N
H
Yield: 183 mg (41%)
LC-MS: Acetonitrile/30% aqueous HC1/water Gradient): R~ = 2.80 min ([M+H]+ -
481)
Example 2-17
2-Methyl-2-[(4-[[[(~-methyl-2-furanyl)methyl][2-oxo-2-[(2,5-dimethyl-4-methoxy-
phenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
/l o
O / O \ S OH
\ I ~N I /
N
Yield: 149 m~ (67%)
LC-MS: Acetonitrile/30% aqueous HCl/water (jradient): R, = 4.10 min ((M+H]+ -
511)
Example 2-18
2-Methyl-2-[[4-[[[(5-methyl-2-furanyl)methyl] [2-oxo-2-[(4-chloro-2-
trifluoromethyl-
phenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-71 -
O
CI / ~ \ S OH
\ I ~N I /
~N
H
C F3
Yield: 63 mg (22%)
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R~ = 3.48 min ([M+H]+ -
555)
Example 2-19
2-Methyl-2-[[4-[[[(5-methyl-2-furanyl)methyl] [2-oxo-2-[(4-methoxy-2-methyl-
phenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
O
/ O \ S OH
\ I ~N I /
~N
H
Yield: 24 mg (18%)
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R, = 2.59 min ([M+H]+ -
497)
Example 2-20
2-[[4-[[[2-[(2,5-Dimethyl-4-methoxy-phenyl)amino]-2-oxoethyl](2-methoxyethyl)-
amino]methyl]phenyl]thio]-2-methyl-propionic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-72-
O O
O / O \ S OH
\ I ~N I /
-N
H
Yield: 60 mg (60%)
LC-MS: Acetonitrile/30% aqueous HCI/water (gradient): R~ = 2.15 min ([M+H)+ -
475).
Example 2-21
2-Methyl-2-[[4-[[[(5-methyl-2-furanyl)methyl] [2-oxo-2-[(2,4-
bistrifluoromethyl-
phenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
0
CF3 / ~ \ S OH
\ I ~N I /
~N
H
CF3
Yield: 16 mg (20%)
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R~ = 3.59 min ([M+H)+ -
589)
Example 2-22
2-Methyl-2-[[4-[[((5-methyl-2-furanyl)methyl] [2-oxo-2-[(2-methyl-4-trifluoro-
methoxy-5-chlorophenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
73 -
F\ /F C~ / ~ O
F O / O \ S OH
\ I ~N I /
~N
H
Yield: 89 mg (81%)
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R, = 3.36 min ([M+H]+ -
585)
Example 2-23
2-Methyl-2-[[4-[[[(5-methyl-2-furanyl)methyl] [2-oxo-2-[(2-trifluoromethyl-4-
trifluoromethoxyphenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
F' I F ~ ~ O
F O / O \ S OH
\ I ~N I /
~N
H
F F
F
Yield: 22 mg (34%)
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R~ = 3.52 min ((M+H]+ -
605)
E~cample 2-24
2-[[4-[[[2-[[2,4-Bis(trifluoromethyl)phenyl]amino]-2-oxoethyl](2-methoxyethyl)-
amino]methyl]phenyl]thio]-2-methyl-propanoic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-74-
F ~ O
F ~ O \ S OH
\ I ~N I /
-N
H
F F
F
Yield: 26 mg (20%)
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R~ = 3.05 min ([M+H]+ -
553).
Example 2-2~
2-[(4-[[[2-[[2,4-Dichlorophenyl]amino]-2-oxoethyl](2-methoxyethyl)amino]-
methyl]phenyl]thio]-2-methyl-propanoic acid
O
CI / CI 00~ \ S OH
\ I ~N I /
N
H
Yield: 61 mg (27%).
~H-NMR (300 MHz, CDC13): S = 1.38 (s, 6H, 2xCH3), 2.82 (m, 2H, CHZ), 3.23 (s,
3H, OMe), 3.32 (s, 2H, CHz), 3.50 (m, 2H, CHZ), 3.73 (s, 2H, CHZ), 5.28 (s,
1H,
COOH), 7.15-7.48 (m, 6H, Ar-H), 8.35 (m, 1H, Ar-H), 9.90 (s, 1H, NH).
LC-MS: Acetonitrile/30% aqueous HCl/water (gradient): R, = 2.76 min ([M+H]+ -
485).
Example 2-26
2-[[4-[[[2-[[2,4-Dimethylphenyl]amino)-2-oxoethyl](2-methoxyethyl)amino]-
methyl]phenyl]thio]-2-methyl-propanoic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 75 -
O
/ O ~ \ S OH
\ I ~N I /
N
H
Yield: 50 mg (75%)
'H-NMR (200 MHz, CDC13): S = 1.50 (s, 6H, 2xCH3), 2.15 (s, 3H, Me), 2.28 (s,
3H,
Me), 3.34 (s, 3H, OMe), 3.40 (m, 2H, CHZ), 3.68 (m, 2H, CHZ), 3.83 (s, ZH,
CHZ),
4.32 (s, 2H, CHz), 5.40 (s, 1H, COOH), 7.00 (m, 2H, Ar-H), 7.32-7.52 (m, 7H,
Ar-
H), 9.00 (s, 1 H, NH).
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R~ = 2.22 min ([M+H]+ -
445).
Example 2-27
2-Methyl-2-[[4-[[[(2-thiophenyl)methyl][2-oxo-2-[(2-methyl-4-trif7uoromethoxy-
5-
chlorophenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
F\ /F C~ / ~ O
F O / O \ S OH
\ I ~N I /
~N
H
Yield: 200 mg (99%)
'H-NMR (300 MHz, CDC13): 8 = 1.50 (s, 6H, 2xCH3), 2.20 (s, 3H, Me), 3.61 (s,
2H,
CHZ), 4.20 (s, 2H, CHZ), 4.48 (s, 2H, CH2), 5.60 (s, 1H, COOH), 7.00 (m, 2H,
Ar-H),
7.02-7.17 (m, 3H, Ar-H and thienyl-H), 7.36 (m, 3H, Ar-H), 7.50 (m, 2H, Ar-H),
8.00 (s, 1H, Ar H), 8.88 (s, 1H, NH).
LC-MS: Acetonitrile/30% aqueous HCI/water (gradient): R~ = 3.40 min ([M+H/]+ -
587).
CA 02424540 2003-04-02
Le A 34 838-Forei~ Countries
-76-
Example 2-28
2-Methyl-2-[[4-[([(2-thiophenyl)methyl][2-oxo-2-[(2-trifluoromethyl-4-
trif7uoro-
methoxy-phenyl)amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
F~ F / I O
F~'O
/ p ~ S OH
\ I ~N I /
~N
H
FF F
Yield: 80 mg (98%)
LC-MS: Acetonitrile/30% aqueous HCl/water Gradient): R~ = 3.56 min ([M+H]+ _
606).
Example 2-29
2-Methyl-2-[[4-[[[(2-thiophenyl)methyl] [2-oxo-2-[(2-methyl-4-methoxy-phenyl)-
amino]ethyl]amino]methyl]phenyl]thio]-propanoic acid
I // o
O / p \ S OH
\ I ~N I /
~N
H
Yield: 83 mg (83%)
LC-MS: Acetonitrile/30% aqueous HC1/water (gradient): R~ = 2.74 min ([M+H]+ -
498).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
_ 77 _
Example 2-30
2-Methyl-2-[[4-[[[(2-furanyl)methyl][2-oxo-2-[(2,4-dimethoxyphenyl)amino]-
ethyl]amino]methyl]phenyl]thio]-propanoic acid
O
/O / O \ S OH
\ I ~N I /
~N
H
/O
Yield: 75 m~ (60%)
LC-MS: Acetonitrile/30% aqueous HCI/water (gradient): R, = 4.19 min ([M+H]+ -
499).
Example 2-31
2-[ (4-[ [ [2-((2-Methyl-4-methoxyphenyl)amino]-2-oxoethyl ] (2-
methoxyethyl)amino]-
methyl]phenyl]thio]-2-methyl-propanoic acid
O O~
HO
N /
O N
H
Yield: 65 % of theory
'H-NMR (300 MHz, CDCI3): 8 = 1.51 (s, 6H); 2.18 (s, 3H); 3.34 (s, 3H); 3.37-
3.45
(m, 2H); 3.65-3.75 (m, 2H); 3.77 (s, 3H); 3.89 (s, 2H); 4.34 (s, 2H); 6.67-
6.78 (m,
2H); 7.35-7.44 (m, 3H); 7.52 (d, 2H); 9.05 (s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
78 _
Example 2-32
2-[[4-[[[2-[(2,4,6-Trimethylphenyl)amino]-2-oxoethyl](2-methoxyethyl)amino]-
methyl]phenyl]thin]-2-methyl-propanoic acid
O O~
HO
/ N
/
O N
H
S
Yield: 89 % of theory
~H-NMR (300 MHz, CDC13): 8 = 1.51 (s, 6H); 2.12 (s, 6H); 2.25 (s, 3H); 3.35
(s,
3H); 3.38-3.54 (m, 2H); 3.65-3.77 (m, 2H); 3.85-3.94 (m, 2H); 4.30-4.45 (m,
2H);
6.87 (s, 2H); 7.39 (d, 2H); 7.53 (d, 2H); 8.82 (br s, 1H).
Starting materials III
Example III-1
ten-Butyl (4-formylphenoxy)acetate
O
/ O.
O
O~ \
At room temperature, 31.60 g (281.48 mmol) of potassium tert-butoxide and
52.70 g
(270.22 mmol) of tent-butyl bromoacetate are added to a solution of 27.50 g
(225.18 mmol) of 4-hydroxybenzaldehyde in 200 ml of dioxane, and the mixture
is
heated at the boil overnight. 1 1 of water is added, and the mixture is then
extracted
with diethyl ether, washed with 1 N sodium hydroxide solution, water and
saturated
sodium chloride solution and dried over magnesium sulphate, and the solvent is
CA 02424540 2003-04-02
Le A 34 838-Foreien Countries
-79-
distilled off. Flash chromatography on silica gel (cyclohexane ->
cyclohexane/ethyl
acetate 20:1 -> 10:1 -> 5:1) gives, after recrystallization from pentane, the
target
compound.
Yield: 31%
Melting point: 58 - b0°C
E~cample III-2
tent-Butyl 2-(4-formylphenoxy)-2-methylpropanoate
O
O
O~
24.42 g (200 mmol) of 4-hydroxybenzaldehyde are dissolved in 250 ml of
N,N-dimethylformamide and treated with 27.64 g (200 mmol) of potassium
carbonate. At 100°C, 53.5 g (240 mmol) of tert-butyl a-bromoisobutyrate
are added
dropwise. The mixture is stirred for another hour, a further 200 mmol of
potassium
carbonate and 240 mmol of tent-butyl a-bromoisobutyrate are added and, after
4 hours at 100°C, 1 1 of water is added. Following extraction with
diethyl ether,
washing with 1 N aqeuous sodium hydroxide solution and saturated sodium
chloride
solution and drying over magnesium sulphate, the solvent is distilled off and
the
residue is purified by flash chromatography on silica gel (cyclohexane ->
cyclohexane/ethyl acetate 20:1 -> 10:1 -> 5:1) and dried under reduced
pressure. The
target compound is obtained in the form of colourless crystals in a yield of
42%.
'H-NMR (200 MHz, CDC13): 8 = 1.40 (s, 9 H), 1.62 (s, 6 H), 6.91 (d, 2 H), 7.79
(d, 2
H), 9.88 (s, 1H).
MS (ESI): 265 [M+H]+.
The following compounds are obtained similarly to the procedure of Example llT-
2:
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-80-
Example III-3
Ethyl 2-(4-formylphenoxy)-2-methylbutanoate
O
O O
S
Yield: 11.71%
'H-NMR (200 MHz, CDC13): 8 = 1.00 (t, 3 H), 1.22 (t, 3 H), 1.61 (s, 3 H), 1.90
-
2.20 (m, 2 H), 4.24 (q, 2 H), 6.90 (d, 2 H), 7.80 (d, 2 H), 9.85 (s, 1 H).
MS (ESI): 251 [M+H]+, 273 [M+Na]+.
Example III-4
ten-Butyl 2-[(3-bromophenyl)sulphanyl]-2-methylpropanoat
O
O
Br
Yield: 87%
'H-NMR (200 MHz, CDC13): 8 = 1.43 (s, 9 H), 1.45 (s, 6 H), 7.14 - 7.28 (m, 1
H),
7.39 - 7.53 (m, 2 H), 7.67 (t, 1 H).
MS (DCI/NH3): 348 [M+NH.~+].
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-81 -
Example III-5
ten -Butyl 2-(3-formylphenoxy)-2-methylpropanoate
O
O
Or
Yield: 35%
'H-NMR (300 MHz, CDC13): cS = 1.44 (s, 9 H), 1.61 (s, 6 H), 7.14 (dd, 1 H),
7.31-
7.35 (m, 1 H), 7.41 (t, 1 H), 7.45- 7.52 (m, 1 H).
MS (DCI/NH3): 282 [M+NH.~+].
Example III-6
tent-Butyl 2-(3-bromophenoxy)-2-methylpropanoate
O
O
Br
Yield: 21%
' H-NMR (300 MHz, CDC13): 8 = 1.44 (s, 9H), 1.56 (s, 6 H), 6.74 - 6.83 (m, 1
H),
7.00 - 7.04 (m, 1 H), 7.06 - 7.11 (m, 2H).
MS (DCI/NH3): 332 [M+NH4+].
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-$2-
Example III-7
ten-Butyl 2-[4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)phenoxyJ-2-methyl-
propanoate
O O
N ~ ~ O O
O
Yield: 24%
Melting point: 142 - 143°C
Example III-8
tert-Butyl 2-[(3-for-mylphenyl)sulphanyl]-2-methylpropanoate
\ S O
O
i
O
At -78°C, 30.00 g (90.56 mmol) of the compound from Example III-4 are
dissolved
in tetrahydrofuran and treated with 36.2 ml of a 2.5 M n-butyllithium solution
in
hexane. 13.94 ml (181.12 mmol) of N,N-dimethylformamide are then added. After
30 min, the mixture is warmed to room temperature and stirred for 1 hour. 30
ml of
1 N hydrochloric acid are added, the solvent is distilled off, the residue is
extracted
with ethyl acetate and the extract is washed with saturated sodium bicarbonate
solution and sodium chloride solution and then dried over magnesium sulphate.
Following flash chromatography on silica gel (dichloromethane), the target
compound is purified by NP-HPLC (cyclohexane/ethyl acetate) and obtained in a
yield of 10%.
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-83-
1H-IVI~IR (300 MHz, CDC13): 8 = 1.43 (s, 9 H), 1.46 (s, 6 H), 7.50 (t, 1 H),
7.77 -
7.80 (m, 1 H), 7.87 (d, 1 H), 7.98 - 8.05 (m, 1 H), 10.00 (s, 1 H).
MS (DCI/NH3): 298 [M+NH~+].
Example III-9
tent-Butyl 2-{ 3-[2-( 1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethenyl]phenoxy)-
2-
methyl-propanoate
O
N
O
O
O
O
In an autoclave, 14.93 ~ (47.37 mmol) of the compound from Example III-6,
10.25 g
(59.21 mmol) of vinylphthalimide, 0.39 g (1.27 mmol) of tris-o-tolylphosphine,
0.07 g (0.32 mmol) [lacuna] and 21.78 g (21.23 mmol) of triethylamine are
heated
at 130°C. Water/methanol is added, and the precipitate is then filtered
off with
suction and recrystallized from cyclohexane/ethyl acetate.
Yield: 66%.
'H-NMR (200 MHz, CDC13): S = 1.40 (s, 9 H), 1.50 (s, 6 H), 6.73 (dd, 1 H),
6.86 -
6.93 (m, 1 H), 7.16 (t, 1 H), 7.21-7.34 (m, 2 H), 7.43 (d, 1 H), 7.80 - 8.00
(m, 4 H).
MS (DCI/NH3): 425 [M+NH.~+].
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-84-
Example III-10
ten-Butyl 2-{ 3-[2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethyl]phenoxy }-2-
methyl-propanoate
15.00 ~ (36.81 mmol) of the compound from Example III-9 are dissolved in 200
ml
of tetrahydrofuran and stirred overnight in a hydrogen atmosphere under
atmospheric
pressure in the presence of a suspension of 2.00 g (2.16 mmol) of Wilkinson's
catalyst in 40 m1 of ethanol. Two flash chromato~raphies on silica gel
(cyclohexane/dichloromethane 10:1 -> cyclohexane/ethyl acetate 10:1 -> 5:1 and
cyclohexane -> cyclohexane/dichloromethane -> dichloromethane) give the title
compound in a yield of 64%.
~H-NMR (200 MHz, CDC13): 8 = 1.45 (s, 9 H), 1.52 (s, 6 H), 2.85 -3.00 (m, 2
H),
3.82-3.95 (m, 2 H), 6.65 - 6.80 (m, 2 H), 6.88 (d, 1 H), 7.15 (t, 1 H), 7.62 -
7.76 (m,
2 H), 7.77 - 7.89 (m, 2 H).
MS (ESI): 432 [M+Na+], 841 [2M+Na+]
Example III-11
tent-Butyl2-(4-aminophenoxy)-2-methylpropanoate
O
HZN ~ ~ O O
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
_8~_
18.88 g (49.50 mmol) of the compound from Example III-7 are dissolved in 25 ml
of
ethanol and, with 12.04 ml (247.49 mmol) of hydrazine hydrate, heated at the
boil for
2 h and then stirred at room temperature for 12 hours. The precipitate is
separated off
and washed with ethanol and the filtrate is concentrated and then diluted with
1 1 of
diethyl ether. This solution is washed with 1 N sodium hydroxide solution and
saturated sodium chloride solution and dried over magnesium sulphate. The
solvent
is removed, giving the title compound in a yield of 87%.
Melting point: 87 - 88°C.
The following compound is obtained similarly to the procedure of Example III-
11:
E~cample III-12
tert-Butyl 2-[3-(2-aminoethyl)phenoxy]-2-methylpropanoate
O
O
NHZ
Yield: 70%
'H-NMR (200 MHz, CDC13): 8 = 1.31 (broad s, 2 H), 1.44 (s, 9 H), 1.56 (s, 6
H),
2.69 (t, 2 H), 2.94 (t, 2 H), 6.64 - 6.75 (m, 2 H), 6.81 (d, 1 H), 7.15 (t, 1
H).
MS (EI): 279 (M +].
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-86-
Example III-13
tart-Butyl 2-(4-{ [(2-furylmethyl)amino]methyl }phenoxy)-2-methylpropanoate
O
O
O ~ \ H
N ~~~~
O
20.00 g (75.67 mmol) of the compound from Example III-2 and 7.3~ g (75.67
mmol)
of 2-furfurylamine are stirred at room temperature with 24.06 g (113.50 mmol)
of
sodium triacetoxyborohydride in 350 ml of 1,2-dichloroethane for 5 hours.
Saturated
sodium bicarbonate solution and ethyl acetate are added to the reaction
mixture. The
organic phase is dried over magnesium sulphate and the solvent is distilled
off, and
the residue is then purified by flash chromatography on silica gel
(cyclohexane ->
cyclohexane/ethyl acetate 10:1 -> 2:1). The target compound is obtained in a
yield of
72°l0.
~H-NMR (200 MHz, CDC13): 8 = 1.61 (broad s, 1 H), 1.44 (s, 9 H), 1.55 (s, 6
H),
3.71 (s, 2 H), 3.77 (s, 2 H), 6.17 (d, 1 H), 6.26-6.36 (m, 1 H), 6.70 - 6.88
(m, 2 H),
7.18 (d, 2 H), 7.32-7.40 (m, 1 H).
MS (ESI): 346 [M+H]+.
Example III-14
ten-Butyl2-{4-[(2-furylmethyl)amino]phenoxy}-2-methylpropanoate
O
O N ~ ~ ~ O
H
4.79 g ( 19.06 mmol) of the compound from Example III-11 and 1.83 g ( 19.06
mmol)
of furfural are dissolved in 80 ml of 1,2-dichloroethane and, in the presence
of 6.06 g
(28.59 mmol) of sodium triacetoxyborohydride, stirred at room temperature for
5 hours. Saturated sodium bicarbonate solution and ethyl acetate are added to
the
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
_87_
reaction solution. The organic phase is dried over magnesium sulphate and the
solvent is distilled off, and the residue is then purified by flash
chromatography on
silica gel (cyclohexane -> cyclohexane/ethyl acetate 10:1 -> 2:1) and by NP-
HPLC
(cyclohexane/ethyl acetate 10:1). The target compound is obtained in a yield
of 79%.
1H-NIV1R (200 MHz, CDCI;): b = 1.46 (s, 9 H), 1.48 (s, 6 H), 3.80 (broad s, 1
H),
4.26 (s, 2 H), 6.21 (d, 1 H), 6.25-6.35 (m, 1 H), 6.50-6.61 (m, 2 H), 6.72-
6.85 (m, 2
H), 7.30 -7.39 (m, 1 H).
MS (DCI/NH;): 332 [M+H+], 349 [M+NH~+].
Erample III-1~
tent-Butyl 2-[4-[[(2-ethoxy-2-oxoethyl)(2-furanylmethyl)amino]methyl]phenoxy]-
2-
methyl-propanoate
O
O O I ~ O
N
O O
18.14 g (52.50 mmol) of the compound from Example III-13, 11 ml of
triethylamine
and 1.10 g (2.97 mmol) of tetra-n-butyl ammonium iodide are initially charged
in
200 ml of tetrahydrofuran and treated with 8.77 ml (78.75 mmol) of ethyl
bromoacetate, and the mixture is stirred at room tempeature for 1 hour and at
60°C
for 2 hours. Water and ethyl acetate are then added to the mixture and the
mixture is
washed with saturated sodium chloride solution and dried over magnesium
sulphate,
and, after removal of the solvent, the residue is purified by flash
chromatography on
silica gel (cyclohexane/ dichloromethane 4:1 -> cyclohexane/ethyl acetate
10:1 -> 5:1). The yield of target compound is quantitative.
1H-NMR (300 MHz, CDC13): 8 = 1.26 (t, 3 H), 1.43 (s, 9H), 1.55 (s, 6 H), 3.30
(s, 2
H), 3.71 (s, 2 H), 3.83 (s, 2 H), 4.15 (q, 2 H), 6.19 (d, 1H), 6.28-6.34 (m, 1
H), 6.77 -
6.85 (m, 2 H), 7.22 (d, 2 H), 7.35-7.41 (m, 1 H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
_88-
MS (ESI): 432 [M+HJ+.
Example III-16
tert-Butyl 2-[4-[[(carboxymethyl)(2-furanylmethyl)aminoJmethyl]phenoxyJ-2-
S methyl-propanoate
O
p O \ O
/ N
O OH
22.01 g (51.00 mmol) of the compound from Example III-15 are stirred at
80°C in
78~ ml of ethanol in the presence of 6.12 g (153.00 mmol) of sodium hydroxide
for
1 hour. The solvent is distilled off and water is added, and the mixture is
then
acidified using 1 N hydrochloric acid and extracted with ethyl acetate. The
extract is
then washed with water and saturated sodium chloride solution and dried over
magnesium sulphate. The amount of solvent is reduced and the product is then
filtered off with suction and dried, giving the target compound in a yield of
74%.
Melting point: 1~2 - 155°C
Example III-17
2-Bromo-N-[4-isopropyl-2-(trifluoromethyl)phenyl]acetamide
F
F F
H
Br~N
O
50 g (246.06 mmol) of 4-isopropyl-2-(trifluoromethyl)aniline and 27.39 g
(270.66 mmol) of triethylamine are initially charged in 1000 ml of
dichloromethane.
CA 02424540 2003-04-02
L,e A 34 838-Foreign Countries
-89-
At 0°-5°C, 54.63 g (270.66 mmol) of bromoacetyl bromide,
dissolved in 200 ml of
dichloromethane, are added dropwise. The mixture is stirred at room
temperature for
20 hours. The reaction mixture is then extracted successively with water, 1 N
hydrochloric acid, water, saturated sodium bicarbonate solution and water. The
organic phase is dried over sodium sulphate and the solvent is removed under
reduced pressure. The residue is purified chromatographically. The product is
recrystallized from cyclohexane/n-pentane, filtered off with suction and dried
under
reduced pressure at 40°C for 20 hours. This gives 32.45 g (41% of
theory) of the title
compound.
I0 'H-NMR (300 MHz, CDC13): ~ = 1.25 (d, 6H); 2.95 (sept., 1H); 4.05 (s, 2H);
7.45 (d,
1H); 7.49 (s, 1H); 8.02 (d, 1H); 8.50 (br s, 1H).
Example III-18
2-Bromo-N-(4-tent-butyl-2-methylphenyl)acetamide
p I \ ,\
Br~
N
H
5.5 g (33.69 mmol) of 4-tert-butyl-2-methylaniline and 3.75 g (37.06 mmol) of
triethylamine are initially charged in 150 ml of dichloromethane. At 0°-
5°C, 7.48 g
(37.06 mmol) of bromoacetyl bromide, dissolved in 90 ml of dichloromethane,
are
added dropwise, and a light-brown precipitate is formed. The mixture is
stirred at
room temperature overnight. 150 ml of ethyl acetate are then added to the
reaction
mixture, which is extracted successively with water, 1 N hydrochloric acid,
water,
saturated sodium bicarbonate soluton and water. The organic phase is dried
over
magnesium sulphate and freed from the solvent under reduced pressure. The
residue
is purified chromatographically. The product is recrystallized from ethyl
acetate and
n-pentane, filtered off with suction and dried under reduced pressure at
40°C. This
gives 6.53 g (68% of theory) of the title compound.
CA 02424540 2003-04-02
Le A 34 838-Forei~n Countries
-90-
'H-NMR (400 MHz, CDCI3): b = 1.3 (s, 9H); 2.3 (s, 3H); 4.06 (s, 2H); 7.20-7.23
(m,
1H); 7.25 (d, 1H); 7.7 (d, 1H); 8.05 (br s, 1H).
Example III-19
2-Bromo-N-(4-cyclohexyl-2-methylphenyl)acetamide
H
~ N~Br
I IO
Yield: 41.0% of theory
'H-NMR (200 MHz, CDC13): ~ = 1.20-1.50 (m, 5H); 1.65-1.95 (m, 5H); 2.28 (s,
3H);
2.35-2.55 (m, 1H); 4.07 (s, 2H); 7.00-7.13 (m, 2H); 7.69 (d, 1H); 8.05 (br s,
1H).
E~cample III-20
2-Bromo-N-(5,6,7,8-tetrahydro-1-naphthalenyl)acetamide
O
~Br
H
Yield: 95.6% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.70-1.90 (m, 4H); 2.55-2.70 (m, 2H); 2.75-2.85
(m, 2H); 4.08 (s, 2H); 6.95 (d, 1H); 7.14 (t, 1H); 7.69 (d, 1H); 8.09 (br s,
1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-91-
Example III-21
2-Bromo-N-[4-(1-naphthyloxy)-2-(trifluoromethyl)phenyl]acetamide
F
H
N
B r~
O
Yield: 80.5% of theory
'H-NMR (200 MHz, CDCl3): b = 4.08 (s, 2H); 7.01 (d, 1H); 7.18 (dd, 1H); 7.30-
7.62
(m, 4H); 7.70 (d, 1H); 7.85-8,17 (m, 3H); 8.47 (br s, 1H).
Example III-22
2-Bromo-N-[5-chloro-2-(2-naphthyloxy)phenyl] acetamide
CI
H
N
Br~
O O
Yield: 77.9% of theory
'H-NMR (200 MHz, CDC13): 8 = 3.99 (s, 2H); 6.88 (d, 1H); 7.06 (dd, 1H); 7.21-
7.36
(m, 2H); 7.38-7.57 (m, 2H); 7.68-7.79 (m, 1H); 7.80-7.95 (m, 2H); 8.51 (d,
1H); 8.85
(br s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Forei~-n Countries
-92-
Example III-23
N-[2,4-Bis(trifluoromethyl)phenyl]-2-bromoacetamide
F
F F
H F
N
Br~ F F
O
Yield: 28% of theory
'H-Ni~IR (200 MHz, CDC13): cS = 4.10 (s, 2H); 7.80-7.91 (m, 2H); 8.50 (d, 1H);
8.80
(br s, 1 H).
Example III-24
2-Bromo-N-(2-ethoxy-1-naphthyl)acetamide
O
Br~
H
O
Yield: 24% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.46 (t, 3H); 4.10-4.30 (m, 4H); 7.26-7.30 (d,
1H);
7.36 (t, 1H); 7.50 (t, 1H); 7.70-7.87 (m, 3H); 8.07 (br s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-93-
Example III-2~
2-Bromo-N-{ 5-((ethylsulphonyl)methyl]-1-naphthyl } acetamide
O,,S O
\ \
/ /
NH
O
Br
Yield: 16% of theory
~H-NMR (200 MHz, CDC13): 8 = 1.37 (t, 3H); 1.54 (s, 1H); 2.91 (q, 2H); 4.20
(s,
2H); 4.72 (s, 2H); 7.53-7.70 (m, 3H); 7.90-8.11 (m, 3H); 8.65 (br s, 1H).
Example III-26
2-Bromo-N-[5-chloro-2-methyl-4-(trifluoromethoxy)phenyl]acetamide
CI
OF F
O
Br~N \ F
H
Yield: 84.0% of theory
~H-NMR (200 MHz, CDCl3): 8 = 2.35 (s, 3H); 4.08 (s, 2H); 7.18 (s, 1H); 8.05-
8.20
(m, 2H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-94-
Example III-27
4-Methyl-1,3-oxazole-5-carbaldehyde oxime
CH3
~ N.
O OH
0.50 ~ (4.50 mmol) of 4-methyl-1,3-oxazole-5-carbaldehyde [prepared from the
corresponding alcohol CChem. Ber. 1961, 1248) by Swern oxidation (Tetrahedron
34,
1651 (1978))] is initially charged in 3 ml of water and treated with 0.66 g
(9.45 mmol) of hydroxylamine hydrochloride in 2 ml of water. 0.68 ~ (4.95
mmol) of
potassium carbonate is then added. After 2 h, the mixture is filtered off with
suction
and the product is washed with water and dried at room temperature. The yield
is
0.41 g (72.2% of theory).
'H-NMR (200 MHz, DMSO): 8 = 2.21 (s, 3H); 8.20 (s, 1H); 8.33 (s, 1H); 11.48
(s,
1 H).
Example III-28
(4-Methyl-1,3-oxazol-5-yl)methylamine
CH3
N
NHZ
O
4.00 g (31.72 mmol) of 4-methyl-1,3-oxazole-5-carbaldehyde oxime are initially
charged in 70 ml of acetic acid. At room temperature, 47.70 g (729.50 mmol) of
zinc
dust are added in small portions. The mixture is stirred at room temperature
for
2 hours and the zinc dust is then filtered off with suction and washed twice
with
50 ml of acetic acid. Under reduced pressure, the filtrate is freed from the
solvent.
The residue is treated with 20% strength aqueous sodium hydroxide solution
until a
pH of 11 is reached. During the addition, white crystals precipitate out.
These are
Le A 34 838-Foreign Countries
-9~-
triturated with ethyl acetate and filtered off with suction. Under reduced
pressure, the
combined filtrates are freed from the solvent, and the residue is then
purified
chromatographically. This gives 1.34 g (38% of theory) of the title compound.
'H-NMR (300 MHz, CDC13): cS = 1.5 (s, 2H); 2.1~ (s, 3H); 3.83 (s, 2H); 7.73
(s, 1H).
Example III-29
1,1-Dimethylethyl 2-[(4-bromophenyl)thio]-2-ethyl-butanoate
Br ~ ~ S O
O
The synthesis was carried out similarly to Example II-1 from 4-bromothiophenol
and
1,1-dimethylethyl 2-bromo-2-ethyl-butanoate [preparation, for example,
similarly to
Liebigs Ann. Chem. 725, 106-115 (1969); J. Am. Chem. Soc. 77, 946-947 (1955),
and bromination with N-bromosuccinimide or bromine, for example similarly to
Tetrahedron Lett. 1970, 3431; J. Org. Chem. 40, 3420 (1975)].
Yield: 15.9% of theory
~H-N1~IR (300 MHz, CDC13): b = 0.96 (t, 6H); 1.58-1.74 (m, 4H); 7.28-7.3~ (m,
2H);
7.39-7.46 (m, 2H).
Example III-30
1,1-Dimethylethyl 2-ethyl-2-[(4-formylphenyl)thio]-butanoate
O
\O S w
O
H
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-96-
The synthesis was carried out similarly to Example II-2 using the compound
from
Example III-29 as starting material.
Yield: 70.4% of theory
'H-NMR (300 MHz, CDCl3): 8 = 0.96 (t, 6H); 1.64-1.87 (m, 4H); 7.60 (d, 2H);
7.78
(d, 2H); 10.1 (s, 1H).
Example III-31
tent-Butyl 2-ethyl-2-[(4-{ [(2-furylmethyl)amino]methyl }phenyl)-
sulphanyl]butanoate
O
O S
H
N O
The synthesis was carried out similarly to Example III-13, using the compound
from
Example III-30 and furfurylamine as starting materials.
Yield: 83.1 % of theory
'H-NMR (300 MHz, CDC13): S = 0.93 (t, 6H); 1.43 (s, 9H); 1.60-1.7~ (m, 4H);
3.78
(s, 4H); 6.18 (d, 1H); 6.28-6.35 (m, 1H); 7.25 (d, 2H); 7.35-7.38 (m, 1H);
7.43 (d,
ZH).
Example III-32
ten-Butyl 2-methyl-2-[4-({[(4-methyl-1,3-oxazol-5-yl)methyl]amino}methyl)-
phenoxy]-propanoate
O
O O
O
NH
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-97-
1.25 g (4.73 mmol) of tent-butyl 2-(4-formylphenoxy)-2-methylpropanoate
(Example
I-4) and 0.64 g (5.67 mmol) of (4-methyl-1,3-oxazol-5-yl)methylamine (Example
III-
28) are together initially charged in 1,2-dichloroethane. At room temperature,
1.50 g
(7.09 mmol) of sodium triacetoxyborohydride are added. The reaction mixture is
stirred at room temperature for 4 hours and then admixed with saturated sodium
bicarbonate solution and extracted with ethyl acetate. The organic phase is
dried over
magnesium sulphate and freed from the solvent under reduced pressure. The
residue
is purified chromatographically on silica gel (dichloromethane/methanol 30:1)
and
then dried under reduced pressure. This gives 1.104 g (65% of theory) of the
title
compound.
'H-NMR (200 MHz, CDC1;): S = 1.45 (s, 9H); 1.55 (s, 6H); 2.11 (s, 3H); 3.70
(s,
2H); 3.77 (s, 2H); 6.70-6.90 (m, 2H); 7.10-7.20 (m, 2H); 7.29 (s, 1H); 7.75
(br s,
1 H).
The following compounds were obtained similarly to the procedure of Example
III-32:
Example III-33
tent-Butyl 2-(4-( [(2-methoxyethyl)amino]methyl }phenoxy)-2-methylpropanoate
O
O O ~ \ H
/ N~Oi
Yield: 92.8% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.44 (s, 9H); 1.55 (s, 6H); 2.48 (br s, 1H); 2.83
(t,
2H); 3.35 (s, 3H); 3.54 (t, 2H); 3.77 (s, 2H); 6.75-6.86 (m, 2H); 7.19 (d,
2H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-98-
Example III-34
tert-Butyl 2-methyl-2-[4-( { [(5-methyl-2-furyl)methyl] ami no } methyl)-
phenoxy]-
propanoate
O
O O \
H
/ N O
Yield: 55.1% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.44 (s, 9H); 1.55 (s, 6H); 2.27 (s, 3H); 3.71
(s,
4H); 5.83-5.92 (m, IH); 6.00-6.08 (m, 1H); 6.75-6.88 (m, 2H); 7.12-7.24 (m,
2H).
Example III-35
tert-Butyl 2-[(4-{ [(2-methoxyethyl)amino]methyl }phenyl)-sulphanylJ-2-methyl-
propanoate
O
O S ~ \
H
N~\Oi
4.00 g (14.27 mmol) of tert-butyl 2-[(4-formylphenyl)sulphanyl]-2-
methylpropanoate
(Example II-2) and 1.07 g (14.27 mmol) of 2-methoxyethylamine are dissolved in
80 ml of 1,2-dichloroethane and, after 30 min and after 10 hours, admixed with
4.54 g (21.40 mmol) of sodium triacetoxyborohydride. The reaction is checked
by
TLC, and ethyl acetate and saturated sodium bicarbonate solution are then
added and
the product is extracted with ethyl acetate. The organic phase is washed with
1 N HCl
and dried over magnesium sulphate, and the product is, after distillative
removal of
the solvent, purified by silica gel chromatography (ethyl acetate/cyclohexane
1:1).
Yield: 2.69 g (55.6% of theory)
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-99-
'H-NMR (300 MHz, CDC13): 8 = 1.45 (s, 15 H); 2.96 (t, 2H); 3.37 (s, 3H); 3.72
(t,
2H); 4.13 (s, 2H); 7.52 (s, 4H).
The following compound was obtained similarly to the procedure of Example III-
35:
Example III-36
tert-Butyl 2-methyl-2-{ [4-( { ((4-methyl-1,3-oxazol-5-yl)methyl]amino }
methyl)-
phenyl]-sulphanyl }propanoate
O
O S \ N
~ ~ N O
Yield: 68.8% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.43 (s, 15H); 2.12 (s, 3H); 3.77 (s, 2H); 3.78
(s,
2H); 7.22- 7.33 (m, 2H); 7.46 (d, 2H); 7.7~ (s, 1H).
Working Examples 3
Example 3-1
ten-Butyl2-[4-[[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)-
amino]methyl]phenoxy]-2-methyl-propanoate
O
O O \ O
N
O N \
H
Le A 34 838-Foreign Countries
- 100 -
0.50 g ( 1.25 mmol) of the compound from Example III-16, 0.23 g ( 1.88 mmol)
of
2,4-dimethylaniline, 0.22 g (1.63 mmol) of 1-hydroxy-1H-benzotriazole, 0.31 g
(1.63 mmol) of EDCxHCI, 0.38 g (3.7~ mmol) of 4-methylmorpholine and 0.01 g
(0.08 mmol) of 4-dimethylaminopyr7dine are stirred in 30 ml of
S N,N-dimethylformamide at 0°C for 2 hours and then at room temperature
overnight.
Water is added and the mixture is extracted with ethyl acetate, and the
organic phases
are then washed with 1 N hydrochloric acid, water, saturated sodium
bicarbonate
solution and saturated sodium chloride solution and then dried over magnesium
sulphate. The solvent is distilled off and the residue is purified by flash
chromatography on silica gel (cyclohexane/dichloroethane 2:1 ->
cyclohexane/ethyl
acetate 10:1 -> 4:1). Recrystallization from n-heptane gives the target
compound in a
yield of 78%.
Melting point: 90 - 91°C.
Example 3-2
ten-Butyl 2-[4-[[[2-[(2,4-dimethylphenyl)methylamino]-2-oxoethyl](2-furanyl-
methyl)amino]methyl]phenoxy]-2-methyl-propanoate
O
O O I \ O
N
\)
O N
At 0°C, 0.51 g (1.00 mmol) of the compound from Example 3-1 and
0.04 g
(1.10 mmol) of sodium hydride are stirred for 30 min and then admixed with
0.07 ml
(1.10 mmol) of iodomethane and then with water. The mixture is extracted with
ethyl
acetate, the extract is washed with water and saturated sodium chloride
solution and
dried over magnesium sulphate, the solvent is distilled off and the residue is
purified
by flash chromatography on silica gel (cyclohexane/dichloromethane 3:1 ->
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 101 -
dichloromethane -> dichloromethane/ethyl acetate 15:1). Recrystallization from
n-pentane gives the target compound in a yield of 51 %.
Melting point: 80 - 81°C.
S Example 3-3
ten-Butyl 2-[4-[[[2-[(2,4-dimethylphenyl)amino]ethyl](2-furanylmethyl)-amino]-
methyl]phenoxy]-2-methyl-propanoate
O
O O \ O
/ N
N \
H
In 5 ml toluene, 0.25 g (0.50 mmol) of the compound from Example 3-1 is
treated
with 0.30 ml of 2 M borane-dimethyl sulphide solution in tetrahydrofuran, and
the
mixture is heated at the boil for 2 hours. The mixture is then stirred in the
presence of
5 ml of 2 N sodium carbonate solution for 1 hour and the organic phase is
washed
with water and with saturated sodium chloride solution. The organic phase is
dried
over magnesium sulphate and the solvent is distilled off, and the residue is
then
purified using silica gel flash chromatography (cyclohexane/dichloromethane
3:1 ->
cyclohexane/ethyl acetate 10:1). This gives the target compound in a yield of
37%.
'H-NMR (200 MHz, CDC13): b = 1.43 (s, 9 H), 1.55 (s, 6 H), 2.15 (s, 3 H), 2.22
(s, 3
H), 2.73 - 2.87 (m, 2 H), 3.09 - 3.22 (m, 2 H); 3.57 (s, 2 H), 3.63 (s, 2 H),
6.12 -
6.19 (m, 1 H), 6.28 - 6.35 (m, 1 H), 6.47 (d, 1 H), 6.73 - 6.95 (m, 4 H), 7.20
(d, 1 H),
7.34 - 7.40 (m, 1 H).
MS (ESI): 493 [M+H]+, 985 [2M+H]+.
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 102 -
Example 3-4
2-[4-[[[2-[(2,4-Dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)amino]-
methyl]phenoxy]-2-methyl-propionic acid
° I \~
HO O ~ O
/ N
O N
H
7.09 g (14.00 mmol) of the compound from Example 3-1 and 3~ ml of
trifluoroacetic
acid are stirred at room temperature in 35 ml of dichloromethane for 2 hours.
The
solvent is distilled off and the residue is then dissolved in ethyl acetate,
washed with
water, 20 per cent strength sodium acetate solution and saturated sodium
chloride
solution and then dried over magnesium sulphate. The solvent is removed and
the
residue is then purified by flash chromatography on silica gel
(dichloromethane ->
dichloromethane/ethyl acetate 5:1 -> 2:1 -> 1:1). The gives the target
compound in a
yield of 82°l0.
'H-NMR (200 MHz, CDC13): b = 1.57 (s, 6 H), 2.24 (s, 3 H), 2.27 (s, 3 H), 3.31
(s, 2
H), 3.67 (s, 2 H), 3.75 (s, 2 H), 6.22 - 6.36 (m, 2 H), 6.88 (d, 2 H), 6.93 -
7.03 (m, 2
H), 7.23 (d, 2 H), 7.34 - 7.40 (m, 1 H), 7.78 (d, 1 H), 8.00 (broad s, 1 H),
9,09 (s, 1
H).
MS (ESI): 451 [M+H]+, 901 [2M+H]+.
The following compounds are obtained similarly to the procedure of Example 3-
4:
Le A 34 838-Foreign Countries
-103-
Example 3-~
2-[4-[[[2-[(2,4-Dimethylphenyl)methylamino]-2-oxoethyl](2-furanylmethyl)amino]-
methyl]phenoxy]-2-methyl-propionic acid
O
HO
V fV
Yield: 85%
'H-NMR (200 NIHz, CDCI;): 8 = 1.46 (s, 6 H), 1.92 (s, 3 H), 2.24 (s, 3 H),
2.73 (q, 2
H), 3.00 (s, 3 H), 3.30 (broad s, 1 H), 3.63 (d, 2 H), 3.78 (d, 2 H), 6.19 (d,
1 H), 6.30-
6.40 (m, 1 H), 6.74 (d, 2 H), 6.80 - 7.10 (m, 5 H), 7.52 - 7.57 (m, 1 H).
MS (ESI): 465 [M+H]+, 487 [M+Na]+.
Example 3-6
2-[4-[[[2-[(2,4-Dimethylphenyl)amino]ethyl](2-furanylmethyl)amino]-methyl]phen-
oxy]-2-methyl-propionic acid
O
O i
HO ~ O x 2 CF3COOH
N
N \
H
Yield: 60%
'H-NMR (200 MHz, DMSO-db): 8 = 1.49 (s, 6 H), 2.01 (s, 3 H), 2.12 (s, 3 H),
2.55 -
2.72 (broad m, 2 H), 2.97 - 3.20 (broad m, 2 H), 3.46 - 3.78 (m, 4 H), 4.40
(broad s,
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 104 -
1 H), 6.20 - 6.50 (m, 3 H), 6.68 - 6.88 (m, 4 H), 7.12 - 7.30 (m, 2 H), 7.56 -
7.68 (m,
1 H), 13.00 (broad s, 1 H).
MS (ESI): 437 [M+I-i]+, 873 [2M+H)+.
Example 3-7
1,1-Dimethylethyl 2-[4-[[(2-methoxyethyl)[2-[(4-(1-methylethyl)-2-(trifluoro-
methyl)phenyl)amino)-2-oxoethyl]amino]methyl]phenoxy]-2-methyl-propionate
O F F
O F
o ~ i
0.533 g (1.65 mmol) of ten-butyl 2-(4-{ [(2-methoxyethyl)amino]methyl?phenoxy)-
2-
methylpropanoate (Example III-33) is initially charged in 6 ml of
dimethylformamide. At room temperature, 0.588 g (1.81 mmol) of 2-bromo-N-[4-
isopropyl-2-(trifluoromethyl)phenyl)acetamide (Example III-17) and 0.152 g
(1.81 mmol) of sodium bicarbonate are added. The mixture is set at 90°C
for 2 hours.
The reaction mixture is then allowed to cool, and water is added. The mixture
is
extracted once with ethyl acetate and the organic phase is washed three times
with
water and once with saturated sodium chloride solution. The organic phase is
dried
over sodium sulphate and freed from the solvent under reduced pressure. The
residue
is purified chromatographically on silica gel (cyclohexane/ethyl acetate 4:1)
and the
product is then dried under reduced pressure. This gives 0.885 g (95% of
theory) of
the title compound.
'H-NNgt (400 MHz, CDC13): 8 = 1.25 (d, 6H); 1.42 (s, 9H); 1.55 (s, 6H); 2.80
(t,
2H); 2.93 (sept., 1H); 3.28 (s, 3H); 3.30 (s, 2H); 3.54 (t, 2H); 3.70 (s, 2H);
6.80 (d,
2H); 7.20 (d, 2H); 7.39 (dd, 1H); 7.45 (d, 1H); 8.17 (d, 1H); 9.65 (br s, 1H).
Le A 34 838-Foreign Countries
105 -
Example 3-8
2-[4-[[(2-Methoxyethyl)[2-[ [4-( 1-methylethyl)-2-
(trifluoromethyl)phenyl]amino]-2-
oxoethyl]amino]methyl]phenoxy]-2-methyl-propionic acid
O F F
O F
O H
HO / ( ~N /
N O
0.842 ~ (1.49 mmol) of the compound from Example 3-7 is initially charged in
10 ml
of dichloromethane. At room temperature, 10 ml of trifluoroacetic acid are
added.
The reaction mixture is stirred at room temperature for 2 hours. The mixture
is then
concentrated under reduced pressure using a rotary evaporator. The residue is
taken
up in ethyl acetate and washed with water, 20% strength sodium acetate
solution,
water and saturated sodium chloride solution. The organic phase is dried over
magnesium sulphate and freed from the solvent under reduced pressure. The
product
is purified chromatographically on silica gel (dichloromethane/methanol 30:1)
and
then dried under reduced pressure. This gives 0.648 g (85% of theory) of the
title
compound.
~ H-NMR (200 MHz, CDC13): 8 = 1.26 (d, 6H); 1.55 (s, 6H); 2.81 (t, 2H); 2.91
(sept.,
1H); 3.28 (s, 3H); 3.31 (s, 2H); 3.55 (t, 2H); 3.72 (s, 2H); 6.90 (d, 2H);
7.25 (d, 2H);
7.35-7.49 (m, 2H); 8.12 (d, 1H); 9.62 (br s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 106 -
Example 3-9
2-(4-[[(2-Methoxyethyl)[2-[[4-(1-methylethyl)-2-(trifluoromethyl)phenyl]amino]-
2-
oxoethyl]aminoJmethyl]phenoxyJ-2-methyl-propionic acid hydrochloride
O F F
O F
O H
HO / I N~N / I
11O
x HCI
0.4 ~ (0.78 mmol) of the compound from Example 3-7 are dissolved in 4 ml of
ethyl
acetate. At 40°C, first 8 ml of 1N hydrochloric acid (in diethyl ether)
and then 12 ml
of diethyl ether are added. The mixture is allowed to stand at 4°C for
one hour. The
precipitated crystals are filtered off with suction, washed with a mixture of
ethyl
acetate and diethyl ether (ratio 1:1) and then dried at 40°C under
reduced pressure for
hours. This gives 0.362 ~ (84.S~o of theory) of the title compound.
'H-NMR (200 MHz, DMSO): 8 = 1.22 (d, 6H); 1.55 (s, 6H); 2.94-3.08 (m, 1H);
3.28
(s, 3H); 3.30-3.40 (m, 2H); 3.60-3.80 (m, 2H); 4.00-4.20 (m, 2H); 4.30-4.50
(m, 2H);
15 6.86 (d, 2H); 7.20-7.70 (m, SH); 10.25 (br s, 1H); 13.18 (br s, 1H).
Example 3-10
1,1-Dimethylethyl 2-[4-[[[2-[(4-cyclohexyl-2-methylphenyl)amino]-2-oxoethyl](2-
methoxyethyl)-amino]methyl]phenoxy]-2-methyl-propionate
O
O
O H
O ~ I N 11N ~ I
O
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 107 -
0.303 g (0.94 mmol) of tert-butyl 2-(4-{ [(2-methoxyethyl)amino]methyl }
phenoxy)-2-
methylpropanoate (Example III-33) is initially charged in 5 ml of
dimethylformamide. At room temperature, 0.319 g ( 1.03 mmol) of 2-bromo-N-(4-
cyclohexyl-2-methylphenyl)acetamide (Example III-19) and 0.086 g (1.03 mmol)
of
sodium bicarbonate are added. The mixture is stirred at 90°C for 2
hours. The
reaction mixture is then allowed to cool, and water is added. The mixture is
extracted
with ethyl acetate and the organic phase is washed with water and saturated
sodium
chloride solution. The organic phase is dried over sodium sulphate and freed
from the
solvent under reduced pressure. The residue is purified chromatographically on
silica
gel (cyclohexane/ethyl acetate 3:1) and the product is dried under reduced
pressure.
This gives 0.464 g (90% of theory) of the title compound.
'H-NMR (300 MHz, CDC1;): 8 = 1.20-1.45 (m, 14H); 1.50 (s, 6H); 1.70-1.90 (m,
5H); 2.25 (s, 3H); 2.36-2.48 (m, 1H); 2.80 (t, 2H); 3.25 (s, 5H); 3.5 (t, 2H);
3.69 (s,
2H); 6.80 (d, 2H); 6.98- 7.06 (m, 2H); 7.15-7.25 (m, 2H); 7.85 (d, 1H); 9.25
(br s,
1H).
Example 3-11
2-[4-[[[2-[(4-Cyclohexyl-2-methylphenyl)amino]-2-oxoethyl](2-methoxyethyl)-
amino]methyl]phenoxy]-2-methyl-propionic acid hydrochloride
O
O
O H
HO
~~O
x HCI
0.398 g (0.72 mmol) of the compound from Example 3-10 is initially charged in
5 ml
of dichloromethane. At room temperature, 5 ml of trifluoroacetic acid are
added. The
reaction mixture is stirred at room temperature for 2 hours. The mixture is
then
concentrated under reduced pressure using a rotary evaporator. The residue is
taken
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 108 -
up in ethyl acetate and washed with water, 20% strength sodium acetate
solution,
water and saturated sodium chloride solution. The organic phase is dried over
magnesium sulphate and freed from the solvent under reduced pressure. The
product
is purified chromatographically on silica gel (dichloromethane/methanol 30:1).
With
heating, the residue is dissolved in dichloromethane, 1N hydrochloric acid in
diethyl
ether is added and n-heptane is added dropwise until the mixture becomes
slightly
turbid. The product is filtered off with suction, washed with diethyl ether
and dried at
40°C under reduced pressure. This gives 0.187 g (49% of theory) of the
title
compound.
~H-NMR (300 MHz, CDC13): 8 = 1.15-1.47 (m, SH); 1.55 (s, 6H); 1.68-1.90 (m,
5H);
2.25 (s, 3H); 2.36-2.49 (m, 1H); 2.85 (t, 2H); 3.28 (s, 3H); 3.30 (s, 2H);
3.52 (t, 2H);
3.72 (s, 2H); 6.87 (d, 2H); 6.99-7.10 (m, 2H); 7.25 (d, 2H); 7.80 (d, 1H);
9.25 (br s,
1 H).
The following compounds were obtained similarly to the procedure of Examples 3-
7
and 3-10:
Example 3-12
1,1-Dimethylethyl 2-[4-[[[2-[[2,4-bis(trifluoromethyl)phenyl]amino]-2-
oxoethyl][(5-
methyl-2-furanyl)-methyl]amino]methyl]phenoxy]-2-methyl-propionate
O O / I O~
'-O
F
F
F F
F F
Yield: 88% of theory
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 109 -
'H-IVNIR (300 MHz, CDCI3): b = 1.40 (s, 9H); 1.55 (s, 6H); 2.15 (s, 3H); 3.30
(s,
2H); 3.65 (s, 4H); 5.85 (m, 1H); 6.12 (d, 1H); 6.81 (m, 2H); 7.20 (m, 2H);
7.25 (m,
1H); 7.35 (s, 1H); 8.57 (d, 1H); 9.85 (br s, 1H).
Example 3-13
1,1-Dimethylethyl 2-[[4-[[[2-[[5-chloro-2-methyl-4-(trifluoromethoxy)phenyl]-
amino]-2-oxoethyl] [(4-methyl-5-oxazolyl)methyl]amino]methyl]phenyl]thio]-2-
methyl-propionoate
O N
O S ~ ~ O CI F
/ N / O\1 /F
O N ~ I F
H
Yield: 80.2% of theory
'H-NMR (300 MHz, CDCI3): 8 = 1.40 (s, 9H); 1.41 (s, 6H); 2.14 (s, 3H); 2.29
(s,
3H); 3.32 (s, 2H); 3.73 (s, 2H); 3.77 (s, 2H); 7.13 (s, 1H); 7.23-7.31 (m,
2H); 7.49 (d,
2H); 7.78 (s, 1H); 8.30 (s, 1H); 9.05 (s, 1H).
Le A 34 838-Foreign Countries
- 110 -
Example 3-14
1,1-Dimethyl-ethyl 2-[(4-[[[2-[[5-chloro-2-methyl-4-(trifluoromethoxy)phenyl]-
amino]-2-oxoethyl](2-furanylmethyl)amino]methyl]phenyl]thio]-2-methyl-
propionate
o 1y
o s I ~ o ci F
N / O~F
O N \ I F
H
Yield: 85.1% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.39 (s, 9H); 1.41 (s, 6H); 2.30 (s, 3H); 3.31
(s,
2H); 3.74 (s, 4H); 6.28 (d, 1H); 6.31-6.35 (m, 1H); 7.12 (s, 1H); 7.27 (d,
2H); 7.35-
7.38 (m, 1H); 7.48 (d, 2H); 8.31 (s, 1H); 9.19 (s, 1H).
Example 3-1~
1,1-Dimethylethyl 2-[[4-[[[2-[(2,4-dimethylphenyl)amino]-2-oxoethyl](2-furanyl-
methyl)amino]-methyl]phenyl]thio]-2-ethyl-butanoate
O I
o s I ~ o'
/ N /
~I
O N
H
Yield: 73.4% of theory
'H-NMR (200 MHz, CDC13): 8 = 0.95 (t, 6H); 1.41 (s, 9H); 1.55-1.78 (m, 4H);
2.26
(s, 3H); 2.28 (s, 3H); 3.30 (s, 2H); 3.73 (s, 2H); 3.74 (s, 2H); 6.20-6.38 (m,
2H);
6.90-7.08 (m, 2H); 7.28 (d, 2H); 7.35-7.50 (m, 3H); 7.75-7.88 (m, 1H); 9.05
(s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 111 -
Example 3-16
1,1-Dimethylethyl 2-j[4-[[[2-[(4-cyclohexyl-2-methylphenyl)amino]-2-
oxoethyl](2-
methoxyethyl)-amino]methyl]phenyl]thio]-2-methyl-propionoate
O O~
O S \
/ N
O N
H
Yield: 81.9°!0 of theory
~H-NMR (200 MHz, CDC13): 8 = 1.31-1.47 (m, 18H); 1.70-1.95 (m, 6H); 2.20-2.31
(m, 4H); 2.35-2.51 (m, 1H); 2.82 (t, 2H); 3.28 (s, 5H); 3.51 (t, 2H); 3.77 (s,
2H); 7.03
(d, 2H); 7.31 (d, 2H); ?.46 (d, 2H); 7.83 (d, 1H); 9.24 (s, 1H).
Example 3-17
1,1-Dimethylethyl 2-[j4-[[j2-[[4-(1,1-dimethylethyl)-2-methylphenyl]amino]-2-
oxoethyl](2-methoxy-ethyl)amino]methyl]phenyl]thio]-2-methyl-propionate
O O~
p s \
N
O N
H
Yield: 82.9% of theory
1H-NMR (300 MHz, CDCl3): 8 = 1.29 (s, 12 H); 1.40 (s, 9H); 1.42 (s, 6H); 2.82
(t,
2H); 3.29 (s> 5H); 3.51 (t, 2H); 3.77 (s, 2H); 7.13-7.40 (m, 4H); 7.40-7.53
(m, 2H);
7.86 (d, 1H); 9.26 (br s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
112
Example 3-18
l,l-Dimethylethyl 2-[[4-[[[2-[[5-chloro-2-methyl-4-(trifluoromethoxy)phenyl]-
amino]-2-oxoethyl](2-furanylmethyl)amino)methyl]phenyl]thio]-2-ethyl- butanoate
O
O S I \ O CI F
N / O~F
\ I ~'F
O N
H
Yield: 86.8% of theory
~H-NMR (200 MHz, CDC13): 8 = 0.94 (t, 6H); 1.41 (s, 9H); 1.55-1.75 (m, 4H);
2.30
(s, 3H); 3.31 (s, 2H); 3.73 (s, 2H); 3.75 (s, 2H); 6.24-6.38 (m, 2H); 7.12 (s,
1H); 7.26
(d, 2H); 7.36 (d, 1H); 7.44 (d, 2H}; 8.31 (s, 1H); 9.19 (s, 1H).
Example 3-19
1,1-Dimethylethyl 2-([4-[[(2-[[5-chloro-2-methyl-4-(trifluoromethoxy)phenyl]-
amino)-2-oxoethyl](2-methoxyethyl)amino]methyl]phenyl]thio]-2-methyl-
propionate
O O~
O S \ CI
N / OF F
O N \ F
H
Yield: 57.4% of theory
'H-NMR (300 MHz, CDCl3): 8 = 1.40 (s, 9H); 1.41 (s, 6H); 2.29 (s, 3H); 2.83
(t,
2H); 3.27 (s, 3H); 3.29 (s, 2H); 3.51 (t, 2H); 3.77 (s, 2H); 7.11 (s, 1 H);
7.30 (d, 2H);
7.46 (d, 2H); 8.29 (s, 1H); 9.44 (s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 113
Example 3-20
l,l-Dimethylethyl 2-methyl-2-[4-([[2-[(4-( 1-methylethyl)-2-(trifluoromethyl)-
phenyl]amino]-2-oxoethyl] [(5-methyl-2-furanyl)methyl]amino)methyl]phenoxy]-
propionate
F F
O ~ F
O O
O
N /
.~. N
Yield: 94% of theory
1H-NMR (400 MHz, CDC13): 8 = 1.25 (d, 6H); 1.40 (s, 9H); 1.55 (s, 6H); 2.17
(s,
3H); 2.88 (sept., IH); 3.25 (s, 2H); 3.15 (m, 4H); 5.85 (m, 1H); 6.10 (d, 1H);
6.81 (d,
2H); 7.21 (d, 2H); 7.35 (m, 1H); 7.43 (m, 1H); 8.15 (d, 1H); 9.67 (s, 1H).
Example 3-21
1,1-Dimethylethyl 2-[4-[[[2-((2-ethoxy-1-naphthalenyl)amino]-2-oxoethyl][(5-
methyl-2-furanyl)-methyl]amino]methyl]phenoxy]-2-methyl-propionate
O
O
O \
/ N ~O
O
HN
O / ( \
\ /
Yield: 95% of theory
'H-NMR (200 MHz, CDC13): S = 1.30 (t, 3H); 1.43 (s, 9H); 1.54 (s, 6H); 2.25
(s,
3H); 3.44 (s, 2H); 3.78-3.82 (m, 4H); 4.15 (q, 2H); 5.89-5.94 (m, 1H); 6.15-
6.18 (m,
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 114 -
1H); 6.84 (d, 2H); 7.20-7.38 (m, 4H); 7.45 (t, 1H); 7.65 (d, 1H); 7.75-7.85
(m, 2H);
9.05 (br s, 1H).
Example 3-22
l , l -Dimethylethyl 2-methyl-2-[4-[[[(5-methyl-2-furanyl)methyl] [2-oxo-2-
[(5,6,7,8-
tetrahydro-1-naphthalenyl)amino]ethyl]amino]methyl]phenoxy]-propionate
O
O
0 1~ N
0
0
HN
Yield: 91 % of theory
~H-NMR (300 MHz, CDC13): F = 1.40 (s, 9H); 1.55 (s, 6H); 1.70-1.95 (m, 4H);
2.20
(s, 3H); 2.65-2.82 (m, 4H); 3.24 (s, 2H); 3.67 (s, 4H); 5.86-5.90 (m, 1H);
6.10-6.14
(d, 1H); 6.78-6.93 (m, 3H); 7.08 (t, 1H); 7.22 (d, 2H); 7.89 (d, 1H); 9.20 (br
s, 1H).
Example 3-23
1,1-Dimethylethyl 2-[4-[[[2-[(2,4-dichloropheny()amino]-2-oxoethyl](2-methaxy-
ethyl)amino]methyl)-phenoxy]-2-methyl-propionate
O Ci
O
O O / N
N~ ~ CI
Yield: 87% of theory
Le A 34 838-Forei~ Countries
- 115
'H-NI~~ (300 MHz, CDC13): 8 = 1.39 (s, 9H); 1.53 (s, 6H); 2.81 (t, 2H); 3.24
(s,
3H); 3.29 (s, 2H); 3.51 (t, 2H); 3.70 (s, 2H); 6.80 (m, 2H); 7.10-7.30 (m,
3H); 7.38
(d, 1H); 8.42 (d, 1H); 9.93 (br s, 1H).
Example 3-24
1,1-Dimethylethyl 2-[4-( [(2-methoxyethyl)[2-[[4-( 1-naphthalenyloxy)-2-
(trifluoro-
methyl)phenyl)amino]-2-oxoethyl]amino]methyl]phenoxy]-2-methyl-propionate
O F F
O F
o ~ ~ ~ N
N~ ~ O
w
Yield: 95.5% of theory
'H-NMR (300 MHz, CDC1;): S = 1.41 (s, 9H); 1.55 (s, 6H); 2.80 (t, 2H); 3.28
(s,
3H); 3.30 (s, 2H); 3.54 (t, 2H); 3.70 (s, 2H); 6.80 (d, 2H); 6.95 (d, 1H);
7.13-7.25 (m,
3H); 7.34 (d, 1H); 7.40 (t, 1H); 7.47-7.58 (m, 2H); 7.66 (d, 1H); 7.89 (dd,
1H); 8.07-
8.21 (m, 2H); 9.68 (br s, 1H).
Example 3-2~
1,1-Dimethylethyl 2-(4-[([2-[[5-[(ethylsulphonyl)methyl]-1-naphthalenyl]amino]-
2-
oxoethyl](2-methoxyethyl)amino]methyl]phenoxy]-2-methyl-propionate
O O
O H
O / ~ ~,( N ~ ~ S=O
w. N 1O w O,
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
- 116
Yield: 91 % of theory
' H-NMR (200 MHz, CDCl3): 8 = 1.20-1.37 (m, 12H); 1.55 (s, 6H); 2.83-2.94 (m,
4H); 3.22 (s, 3H); 3.39 (s, 2H); 3.55 (t, 2H); 3.77 (s, 2H); 4.77 (s, 2H);
6.81 (d, 2H);
7.15-7.30 (in, 2H); 7.50-7.70 (m, 3H); 7.91 (d, 1H); 8.12 (d, 1 H); 8.22 (d, 1
H); 10.18
(br s, 1H).
Example 3-26
1,1-Dimethylethyl 2-methyl-2-[4-[[[2-[[4-(1-methylethyl)-2-(trif)uoromethyl)-
phenyl ) amino]-2-oxoethyl] [(4-methyl-5-oxazolyl)methyl]
amino]methyl]phenoxy]-
propionate
N F
F
O ~ ~ F
O O
~O / ~ N /
N
Yield: 83.5% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.25 (d, 6H); 1.40 (s, 9H); 1.55 (s, 6H); 2.10
(s,
3H); 2.85-3.00 (sept., 1H); 3.28 (s, 2H); 3.66 (s, 2H); 3.75 (s, 2H); 6.82 (d,
2H); 7.20
(d, 2H); 7.38 (dd, 1H); 7.40-7.45 (m, 1H); 7.75 (s, 1H); 8.14 (d, 1H); 9.45
(br s, 1H).
Example 3-27
1,1-Dimethylethyl 2-[4-[[(2-[[2,4-bis(trifluoromethyl)phenyl]amino]-2-
oxoethyl][(4-
methyl-5-oxazolyl)-methyl]amino]methyl]phenoxy]-2-methyl-propionate
O
O
O / I N F
CA 02424540 2003-04-02
Le A 34 838-Foreign Countr'ses
- 117 -
Yield: 79.5% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.40 (s, 9H); 1.55 (s, 6H); 2.11 (s, 3H); 3.30
(s,
2H); 3.68 (s, 2H); 3.76 (s, 2H); 6.81 (d, 2H); 7.18 (d, 2H); 7.70-7.80 (m,
2H); 7.86 (s,
1 H); 8.56 (d, 1 H); 9.71 (br s, 1 H).
Example 3-28
l , l -Dimethylethyl 2-[4-[[j2-[ [4-( 1,1-di methylethyl)-2-
methylphenyl]amino]-2-
oxoethyl](2-methoxy-ethyl)amino]methyl]phenoxy]-2-methyl-propionate
O
O
O H
O / ( ~N ~
N O
Yield: 81 % of theory
'H-NNIR (300 MHz, CDC13): 8 = 1.30 (s, 9H); 1.40 (s, 9H); 1.55 (s, 6H); 2.38
(s,
3H); 2.80 (t, 2H); 3.29 (s, 5H); 3.50 (t, 2H); 3.70 (s, 2H); 6.80 (d, 2H);
7.15-7.25 (m,
4H); 7.78 (d, 1 H); 9.30 (br s, 1 H).
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 118 -
Example 3-29
1,1-Dimethylethyl 2-[4-[[[2-[[2,4-bis(trifluoromethyl)phenyl]amino]-2-
oxoethyl](2-
methoxyethyl)-amino]methyl]phenoxy]-2-methyl-propionate
O
O o w
/ N~./~Oi
O
HN
F F ~ \
F
F F
F
Yield: 80% of theory
'H-NMR (300 MHz, CDCI;): s = 1.39 (s, 9H); 1.55 (s, 6H); 2.82 (t, 2H); 3.28
(s,
3H); 3.33 (s, 2H); 3.52 (t, 2H); 3.71 (s, 2H); 6.80 (d, 2H); 7.18 (d, 2H);
7.78 (d, 1H);
7.84 (s, 1H); 8.60 (d, 1H); 9.98 (br s, 1H).
Example 3-30
1,1-Dimethylethyl 2-[4-[[[2-[[2,4-bis(trif)uoromethyl)phenyl]amino]-2-
oxoethyl](2-
furanylmethyl)-amino]methyl]phenoxy]-2-methyl-propionate
I \~
O O \ O F
N
/ I FF
O
F F
Yield: 84°l0 of theory
Le A 34 838-Foreign Countries
- 119 -
'H-NMR (300 MHz, CDCl3): 8 = 1.40 (s, 9H); 1.55 (s, 6H); 3.30 (s, 2H); 3.65
(s,
2H); 3.75 (s, 2H); 6.20-6.30 (m, 1H); 6.30-6.38 (m, 1H); 6.82 (d, 2H); 7.18
(d, 2H);
7.36-7.39 (m, 1H); 7.75 (d, 1H); 7.90 (s, 1H); 8.60 (d, 1H); 9.82 (br s, 1H).
Example 3-31
1,1-Dimethylethyl 2-[[4-[[[2-[[2,4-bis(trifluoromethyl)phenyl]amino]-2-
oxoethyl](2-
furanylmethyl)-amino]methyl]phenyl]thio]-2-methyl-propionate
0
p S ~ O F
N ~ F
F
O N
H F
F F
to
Yield: 92% of theory
'H-NMR (300 MHz, CDCl3): 8 = 1.40 (s, 9H); 1.45 (s, 6H); 3.31 (s, 2H); 3.74-
3.80
(m, 4H); 6.25 (d, 1H); 6.30-6.38 (m, 1H); 7.22-7.40 (m, 3H); 7.50 (d, 2H);
7.78 (d,
1H); 7.90 (s, 1H); 8.61 (d, 1H); 9.78 (br s, 1H).
The following compounds were obtained similarly to the procedure of Example 3-
8:
Example 3-32
2-[4-[[[2-[[2,4-Bis(trifluoromethyl)phenyl]amino]-2-oxoethyl][(5-methyl-2-
furanyl)-
methyl]amino]methyl]phenoxy]-2-methyl-propionic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 120 -
O
HO O ~ I O
,O
~N - F
F
F F
F F
Yield: 83.4% of theory
'H-NMR (300 MHz, CDC13): b = 1.56 (s, 6H); 2.15 (s, 3H); 3.29 (s, 2H); 3.69
(s,
2H); 3.71 (s, 2H); 5.80-5.88 (m, 1H); 6.13 (d, 1H); 6.89-6.98 (m, 2H); 7.20-
7.35 (m,
2H); 7.74 (d, 1H); 7.86 (s, 1H); 8.56 (d, 1H); 9.79 (s, 1H).
Example 3-33
2-[[4-[[[2-[[5-Chloro-2-methyl-4-(trifluoromethoxy)phenyl]amino]-2-
oxoethyl][(4-
methyl-5-oxazalyl)methyl]amino]methyl]phenyl]thio]-2-methyl-propionic acid
O N
HO S I ~ O C1 F
N / O~F
O N \ I F
H
Yield: 62.8% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.47 (s, 6H); 2.11 (s, 3H); 2.28 (s, 3H); 3.35
(s,
2H); 3.74 (s, 2H); 3.77 (s, 2H); 7.11 (s, 1H); 7.20-7.30 (m, 2H); 7.49 (d,
2H); 7.80 (s,
1H); 8.28 (s, 1H); 9.04 (s, 1H).
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 121 -
Example 3-34
2-[[4-[[[2-[[5-Chloro-2-methyl-4-(trifluoromethoxy)phenyl)aminoJ-2-oxoethyl](2-
furanylmethyl}amino]methyl]phenylJthioJ-2-methyl-propionic acid
~ \~
HO S ( ~ ° Cl F
N / O~F
O N \ I F
H
Yield: 90.9% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.46 (s, 6H); 2.28 (s, 3H); 3.32 (s, 2H); 3.75
(s,
4H); 6.30 (dd, 2H); 7.10 (s, 1H); ?.29 (d, 2H); 7.36 (d, 1H); 7.48 (d, 2H);
8.27 (s,
1H); 9.16 (s, 1H).
Example 3-3~
2-[[4-[[[2-[(2,4-Dimethylphenyl)aminoJ-2-oxoethylJ(2-furanylmethyl)aminoJ-
methyl]phenylJthioJ-2-ethyl-butanoic acid
O
HO S \ O~
/ N /
I
O N
H
Yield: 96.6% of theory
'H-NMR (300 MHz, CDC13): 8 = 0.97 (t, 6H); 1.60-1.90 (m, 4H); 2.25 (s, 3H);
2.28
(s, 3H); 3.31 (s, 2H); 3.73 (s, 2H); 3.74 (s, 2H); 6.26 (d, 1H); 6.29-6.35 (m,
1H);
6.95-7.05 (m, 2H); 7.29 (d, 2H); 7.37 (d, 1H); 7.46 (d, 2H); 7.80 (d, 1H);
9.03 (s,
1 H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 122 -
Example 3-36
2-[[4-[[[2-[[5-Chloro-2-methyl-4-(trifluoromethoxy)phenyl]amino]-2-oxoethyl](2-
furanylmethyl)amino]methyl]phenyl]thio]-2-ethyl-butanoic acid
S
O
HO S I ~ O/ C!
/ N / OF F
F
O N
H
Yield: 90.9% of theory
'H-NMR (300 MHz, CDC13): 8 = 0.96 (t, 6 H); 1.58-1.8? (m, 4H); 2.28 (s, 3H);
3.31
(s, 2H); 3.73 (s, 2H); 3.76 (s, 2H); 6.26 (d, 1H); 6.30-b.36 (m, 1H); 7.10 (s,
1H); 7.27
(d, 2H); 7.34-7.40 (m, 1H); 7.4~ (d, 2H); 8.28 (s, 1H); 9.16 (s, 1H).
Example 3-37
2-[[4-[[[2-[[5-Chloro-2-methyl-4-(trifluoromethoxy)phenyl]amino]-2-oxoethyl](2-
methoxyethyl)amino]methyl]phenyl]thio]-2-methyl-propionic acid
O O~
HO
N F
O N
H
Yield: 83.9% of theory
'H-NMR (200 MHz, CDC13): S = 1.46 (s, 6H); 2.27 (s, 3H); 2.84 (t, 2H); 3.27
(s,
3H); 3.31 (s, 2H); 3.50 (t, 2H); 3.77 (s, 2H); 7.10 (br s, 1H); 7.31 (d, 2H);
7.48 (d,
2H); 8.24 (s, 1H); 9.43 (s, 1H).
Le A 34 838-Foreign Countries
-123-
Example 3-38
2-Methyl-2-[4-[[[2-[[4-( 1-methylethyl)-2-(trif3uoromethyl)phenyl]amino]-2-
oxoethyl][(5-methyl-2-furanyl)methyl]amino]methyl]phenoxy]-propionic acid
\, F F
O ~~ F
O O
HO
N ~ 1
N
Yield: 91 % of theory
'H-NMR (200 MHz, CDC13): 8 = 1.25 (d, 6H); 1.55 (s, 6H); 2.17 (s, 3H); 2.91
(sept.,
1H); 3.28 (s, 2H); 3.7 (s, 4H); 5.80-5.90 (m, 1H); 6.13 (d, 1H); 6.90 (m, 2H);
7.17-
7.30 (m, 2H); 7.32-7.47 (m, 2H); 8.12 (d, 1H); 9.55 (br s, 1H).
Example 3-39
2-[4-[[[2-[(2-Ethoxy-1-naphthalenyl)amino]-2-oxoethyl][(5-methyl-2-furanyl)-
methyl]amino]methyl]phenoxy]-2-methyl-propionic acid
o \~ ~ 1
Ho o ~ ~ N ~ 1
N
O
Yield: 64°10 of theory
'H-NMR (300 MHz, CDC13): S = 1.25-1.32 (t, 3H); 1.60 (s, 3H); 2.25 (s, 3H);
3.45
(s, 2H); 3.82 (s, 4H); 4.15 (quart., 2H); 5.94 (m, 1H); 6.17 (d, 1H); 6.90-
7.00 (m,
2H); 7.23-7.46 (m, 5H); 7.60-7.70 (m, 1H); 7.75-7.80 (m, 2H); 9.05 (br s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 124 -
Example 3-40
2-Methyl-2-[4-[[[(5-methyl-2-furanyl)-methyl][2-oxo-2-[(5,6,7,8-tetrahydro-1-
naphthalenyl)amino)ethyl)amino)methyl]phenoxy)-propionic acid
O
O / ~ N / I
HO
.~ N
Yield: 76% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.55 (s, 6H); 1.75-1.95 (m, 4H); 2.20 (s, 3H);
2.36
(t, 2H); 2.78 (t, 2H); 3.30 (s, 2H); 3.69 (s, 4H); 5.89 (m, 1H); 6.12 (d, 1H);
6.83-6.94
(m, 4H); 7.09 (t, 1H); 7.20-7.32 (m, 1H); 7.85 (d, 1H); 9.15 (s, 1H).
Example 3-41
2-[4-[[[2-[(2,4-Dichlorophenyl)amino)-2-oxoethyl](2-methoxyethyl)amino]methyl]-
phenoxy]-2-methyl-propionic acid
O C1
O
HO O / ~N /
' N 1O '~ Ci
Yield: 69% of theory
'H-NMR (300 MHz, CDCl3): 8 = 1.55 (s, 6H); 2.82 (t, 2H}; 3.28 (s, 3H); 3.00
(s,
2H); 3.54 (t, 2H); 3.75 (s, 2H); 6.90 (m, 2H); 7.18-7.36 (m, 3H); 7.39 (d,
1H); 8.40
(d, 1H); 9.90 (br s, 1H}.
Example 3-42
2-[4-[[(2-Methoxyethyl)[2-[[4-( 1-naphthalenyloxy)-2-(trifluoromethyl)phenyl)-
amino)-2-oxoethyl]amino]methyl]phenoxy]-2-methyl-propionic acid
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 125 -
O F F
O F
O H
HO
\ I N ~ N '~. ' O
O
w
Yield: 74% of theory
'H-NMR (300 MHz, DMSO): 8 = 1.45 (s, 6H); 2.72 (t, 2H); 3.18 (s, 3H); 3.25 (s,
2H); 3.47 (t, 2H); 3.68 (s, 2H); 6.78 (d, 2H); 7.10 (d, 1H); 7.21 (d, 2H);
7.28 (dd,
1 H); 7.40 (d, 1 H); 7.48-7.66 (m, 3H); 7.80 (d, 1 H); 7.90 (d, 1 H); 8.05 (t,
2H); 9.60
(br s, 1 H).
E~cample 3-43
2-[4-[[[2-[[5-[(Ethylsulphonyl)methyl]-1-naphthalenyl]amino]-2-oxoethyl](2-
methoxyethyl)amino]methyl]phenoxy]-2-methyl-propionic acid
1
O O H
HO O / I N N ~ ~ .S=O
O
Yield: 40.5% of theory
'H-NMR (400 MHz, CDZCIZ): S = 1.34 (t, 3H); 1.46 (s, 6H); 2.83-3.04 (m, 4H);
3.24
(s, 3H); 3.37 (s, 2H); 3.32-3.64 (m, 2H); 3.78 (s, 2H); 4.72 (s, 2H); 6.83 (d,
2H); 7.31
(d, 2H); 7.46-7.65 (m, 3H); 7.90 (d, 1H); 8.04-8.20 (m, 2H); 10.10 (br s, 1H).
Le A 34 838-Foreign Countries
- 126 -
Example 3-44
2-Methyl-2-[4-[[[2-[(4-( 1-methylethyl)-2-(trifluoromethyl)phenyl]amino]-2-
oxoethyl][(4-methyl-5-oxazolyl)methyl]amino]methyl]phenoxy]-propionic acid
N~ F
F
O F
H
O O
HO / ~ ~N / I
N 1'O w
Yield: 69% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.25 (d, 6H); 1.58 (s, 6H); 2.09 (s, 3H); 2.82-
3.04
(sept., 1H); 3.30 (s, 2H); 3.66 (s, 2H); 3.76 (s, 2H); 6.90 (d, 2H); 7.25 (d,
2H); 7.35-
7.48 (m, 2H); 7.80 (s, 1H); 8.11 (d, 1H); 9.40 (br s, 1H).
Example 3-45
2-[4-[[[2-([2,4-Bis(trifluoromethyl)phenyl]amino]-2-oxoethyl][(4-methyl-5-
oxazolyl)methyl]amino]methyl]phenoxy]-2-methyl-propionic acid
N\ _ F
O \ %O
O
HO / ~ N~ F
1'O
Yield: 76% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.60 (s, 6H); 2.10 (s, 3H); 3.32 (s, 2H); 3.70
(s,
2H); 3.77 (s, 2H); 6.90 (d, 2H); 7.21 (d, 2H); 7.73-7.90 (m, 3H); 8.55 (d,
1H); 9.68
(br s, 1 H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-127-
Example 3-46
2-[4-[[[2-([2,4-Bis(trifluoromethyl)phenyl]amino]-2-oxoethylJ(2-methoxyethyl)-
amino]methyl]phenoxy]-2-methyl-propionic acid
O F F
O F
O H
HO / ~ N~N / I F
'1O
Yield: 77°l0 of theory
'H-NMR (300 MHz, CDCI;): 8 = 1.55 (s, 6H); 2.84 (t, 2H); 3.25 (s, 3H); 3.35
(s,
2H); 3.55 (t, 2H); 3.75 (s, 2H); 6.90 (d, 2H); 7.15-7.30 (m, 2H); 7.75 (d,
1H); 7.88 (s,
1H); 8.59 (d, IH); 9.91 (br s, IH).
Example 3-47
2-[4-[[[2-([2,4-Bis(trif>uoromethyl)phenyl]amino)-2-oxoethyl](2-furanylmethyl)-
amino]methyl)phenoxy]-2-methyl-propionic acid
HO °
F
O
t- r
Yield: 9I°fo of theory
jH-NMR (300 MHz, CDCl3): 8 = 1.57 (s, 6H); 3.30 (s, 2H); 3.70 (s, 2H); 3.77
(s,
2H); 6.30 (dd, 2H); 6.88 (d, 2H); ?.20-7.35 (m, 2H); 7.37-7.42 (m, 1H); 7.75
(d, IH);
7.86 (s, 1 H); 8.56 (d, 1 H); 9.80 (br s, 1 H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
_ 12g _
Example 3-48
2-([4-[[[2-[[2,4-Bis(triouoromethyl)phenyl]amino]-2-oxoethyl](2-furanylmethyl)-
amino]methyl]phenyl]thio]-2-methyl-propionic acid
o I ~~
HO S \ O F
N
FF
F
O H
F F
Yield: 91% of theory
~H-NMR (300 MHz, CDC13): b = 1.46 (s, 6H}; 3.32 (s, 2H); 3.75 (s, 4H); 6.25
(dd,
2H); 7.20-7.40 (m, 3H); 7.50 (d, 2H); 7.78 (d, 1H}; 7.90 (s, 1H); 8.59 (d,
1H); 9.78
(br s, 1 H).
The followinj compounds were obtained similarly to the procedure of Examples 3-
9
and 3-11:
1 S Example 3-49
2-[[4-[[[2-[(4-Cyclohexyl-2-methylphenyl)amino]-2-oxoethyl](2-methoxyethyl)-
amino]methyl]phenyl]thio]-2-methyl-propionic acid hydrochloride
O O~
HO S \
N
x HCt O H
Yield: 53.3°!0 of theory
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 129 -
'H-NMR (300 MHz, DMSO): 8 = I.20-1.48 (m, 12H); 1.62-1.87 (m, 5H); 2.14 (s,
3H); 3.27 (s, 3H); 3.51 (br s, 2H); 3.74 (br s, 2H); 4.12 (br s, 2H); 4.51 (br
s, 2H);
7.02 (d, 2H); 7.16-7.30 (br s, 1H); 7.46-7.68 (m, 4H); 9.93 (br s, 1H); 10.36
(br s,
1H); 12.74 (br s, 1H).
Example 3-50
2-[[4-[[[2-[[4-(l,l-Dimethylethyl)-2-methylphenyl]amino)-2-oxoethyl](2-
methoxyethyl)amino]methyl]phenyl]thio]-2-methyl-propionic acid hydrochloride
O O~
HO
I / N
I y
x HCI O H
Yield: 85.3% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.29 (s, 9H); 1.56 (s, 6H); 2.26 (s, 3H); 2.86
(t,
2H); 3.29 (s, 3H); 3.35 (s, 2H); 3.53 (t, 2H); 3.74 (s, 2H); 6.88 (d, 2H);
7.1~-7.26 (m,
4H); 7.79 (d, 1H); 9.26 (s, 1H).
Example 3-51
2-Methyl-2-[4-[[[2-[[4-( 1-methylethyl)-2-(trifluoromethyl)phenyl)amino)-2-oxo-
ethyl][(5-methyl-2-furanyl)methyl]amino]methyl]phenoxy)-propionic acid hydro-
chloride
F F
O ~ F
O O
N',1~ ~ .
HO / N / I
x HCI
CA 02424540 2003-04-02
Le A 34 838-Forei~ Countries
- 130 -
Yield: 99% of theory
'H-NMR (300 MHz, DMSO): 8 = 1.20 (d, 6H); 1.50 (s, 6H); 2.27 (br s, 3I-~; 2.96-
3.05 (sept., 1H); 3.95 (br s, 2H); 4.31 (br s, 4H); 6.17 (br s, 1H); 6.63 (br
s, 1H); 6.85
(d, 2H); 7.46-7.57 (m, 5H); 10.23 (br s, 1H); 10.55 (br s, 1H); 13.15 (br s,
1H).
Example 3-52
2-[4-[([2-[(4-( l,l-Dimethylethyl)-2-methylphenyl]amino]-2-oxoethyl](2-methoxy-
ethyl)amino]methyl]phenoxy]-2-methyl-propionic acid hydrochloride
I
O
O
HO O ~ ' N /
N
x HCl
Yield: 54% of theory
LC-MS: 470 [M+]
Example 3-53
2-[4-[([2-[(2,4-Dimethylphenyl)amino]-2-oxoethyl](2-furanylmethyl)amino]-
methyl]phenoxy]-2-methyl-propionic acid sodium salt
of o
0
\ N \ O-
~N
0.015 g (0.03 mmol) of the compound from Example 3-4 is dissolved in 0.5 ml of
ethanol and treated with 0.3 ml of 1N aqueous sodium hydroxide solution. The
reaction mixture is stirred for another 5 min and then concentrated using a
rotary
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 131 -
evaporator. The residue is taken up in a little toluene and the solvent is
removed
under reduced pressure. The product is then dried under reduced pressure for
20 hours. This gives 0.015 g (95.5% of theory) of the title compound.
'H-NMR (200 MHz, CDC13): 8 = 1.21 (s, 6H); 2.10-2.20 (m, 6H); 3.16 (s, 2H);
3.58-
3.64 (m, 4H); 6.18-6.25 (m, 2H); 6.73-6.82 (m, 2H); 7.09-7.35 (m, 3H); 7.71
(d, 1H);
9.00 (br s> 1H).
The following compounds were obtained similarly to the procedure of Examples 3-
7
and 3-10:
Example 3-S4
1 > 1-Dimethylethyl 2-[[4-[[[2-[[4-( 1-methylethyl)-2-
(trifluoromethyl)phenyl]amino]-
2-oxoethyl](2-methoxyethyl)amino]methyl)phenyl]thio]-2-methyl-propionate
O F F
O F
O S / ~ N / I
.~ N~ .
Yield: 61% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.24 (d, 6H); 1.39 (s, 9H); 1.42 (s, 6H); 2.80
(t,
2H); 2.90-3.1 (m, 1H); 3.28 (s, 3H); 3.32 (s, 2H); 3.53 (t, 2H); 3.78 (s, 2H);
7.25-
7.50 (m, 6H); 8.14 (d, 1H); 9.62 (br s, 1H).
Example 3-5~
1,1-Dimethylethyl 2-methyl-2-[[4-[[[2-[[4-(1-methylethyl)-2-(trifluoromethyl)-
phenyl]amino]-2-oxo-ethyl] [(4-methyl-5-oxazolyl)methyl]amino]methyl]phenyl]-
thio]propionate
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
- 132 -
O N
p S \. O
/ N
O H
F F
Yield: 66% of theory
1H-NMR (300 MHz, CDCI;): & = 1.25 (d, 6H); 1.40 (s, 9H); 1.43 (s, 6H); 2.10
(s,
3H); 2.90-3.10 (m, 1 H); 3.29 (s, 2H); 3.70-3.80 (m, 4H); 7.30-7.55 (m, 6H);
7.77 (s,
1H); 8.13 (d, 1H); 9.40 (br s, 1H).
Example 3-~6
1,1-Dimethylethyl 2-[[4-[[[2-[(2-methyl-4-methoxyphenyl)amino]-2-oxoethyl][(4-
methyl-5-oxazolyl)-methyl]amino]methyl]phenyl]thin]-2-methyl-propionate
O N
\ O
O
N / O~
\
O N
H
Yield: 86% of theory
~H-NMR (200 MHz, CDC13): 8 = 1.41 (s, 9H); 1.43 (s, 9H); 2.13 (s, 3H); 2.24
(s,
3H); 3.31 (s, 2H); 3.70-3.81 (m, 7H); 6.68-6.80 (m, 2H); 7.30 (d, 2H); 7.50
(d, 2H);
7.67-7.75 (m, 1H); 7.78 (s, 1H); 8.80 (br s, 1H).
Example 3-57
1,1-Dimethyl-ethyl 2-[(4-[[[2-[[2,4-bis(trifluoromethyl)phenyl]amino]-2-
oxoethyl]-
[(4-methyl-5-oxazolyl)methyl]amino]methyl]phenyl]thio]-2-methyl-propionate
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Ix A 34 838-Foreign Countries
-133-
O N
O S \ O~ F
N ~ F
F
O N
H F
F F
Yield: 84% of theory
~H-NUIR (200 MHz, CDC13): ~ = 1.40 (s, 9H); 1.42 (s, 6H); 2.11 (s, 3H); 3.32
(s,
2H); 3.74-3.82 (m, 4H); 7.29 (d, 2H); 7.49 (d, 2H); 7.70-7.85 (m, 2H); 7.87
(s, 1H);
8.57 (d, 1 H); 9.67 (br s, 1 H).
Example 3-58
1,1-Dimethylethyl 2-[4-[([2-[(4-cyclohexyl-2-methylphenyl)amino]-2-
oxoethyl]((4-
methyl-S-oxazolyl)-methyl]amino]methyl]phenoxy]-2-methyl-propionate
O N
O O \
I / N
/ I ~./
O N
H
Yield: 54.8% of theory
~H-NMR (200 MHz, CDC13): & = 1.30-1.46 (m, 14 H); 1.55 (s, 6H); 1.62-1.94 (m,
6H); 2.12 (s, 3H); 2.26 (s, 3H); 3.29 (s, 2H); 3.65 (s, 2H); 3.74 (s, 2H);
6.82 (d, 2H);
6.98-7.08 (m, 2H); 7.18 (d, 2H); 7.77 (s, 1H); 7.83 (d, 1H); 8.96 (br s, 1H).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 134 -
Example 3-~9
1,1-Dimethyl-ethyl 2-[4-[[[2-[[4-(1,1-dimethylethyl)-2-methylphenyl]amino]-2-
oxo-
ethyl] [(4-methyl-5-oxazolyl)methyl]amino]methyl]phenoxy]-2-methyl-propionate
O N
O ~ O
O
N
O H
Yield: 64.7% of theory
'H-NiVIR (200 iVIHz, CDC13): 8 = 1.29 (s, 9H); 1.41 (s, 9H); 1.55 (s, 6H);
2.12 (s,
3H); 2.28 (s, 3H); 3.29 (s, 2H); 3.65 (s, 2H); 3.75 (s, 2H); 6.82 (d, 2H);
7.10-7.30 (m,
4H); 7.77 (s, 1H); 7.85 (d, 1H); 8.98 (br s, 1H).
Example 3-60
2-[[4-[[[2-[[4-(1-Methylethyl)-2-(trifluoromethyl)phenyl]amino]-2-oxoethyl](2-
methoxyethyl)amino]methylJphenyl]thio]-2-methyl-propionic acid
F
O
O
S H
/ ( N
HO N
0
0.248 g (0.43 mmol) of the compound from Example 3-54 is initially charged in
5 ml
of dichloromethane. At room temperature, 5 ml of trifluoroacetic acid are
added. The
reaction mixture is stirred at room temperature for 2 hours and then
concentrated
under reduced pressure using a rotary evaporator. The residue is taken up in
ethyl
acetate and extracted with water, 20% strength sodium acetate solution, water
and
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
135
saturated sodium chloride solution. The organic phase is dried over magnesium
sulphate and freed from the solvent under reduced pressure. The product is
purified
chromatographically on silica gel (dichloromethane, dichloromethanelmethanol
30:1)
and then dried under reduced pressure. This jives 197 mg (88% of theory) of
the title
compound.
'H-NMR (200 MHz, CDC13): 8 = 1.25 (d, 6H); 1.49 (s, 6H); 2.80 (t, 2H); 2.85-
3.00
(m, 1H); 3.30 (s, 3H); 3.32 (s, 2H); 3.49-3.59 (m, 2H); 3.80 (s, 2H); 7.24-
7.53 (m,
6H); 8.12 (d, 1H); 9.58 (br s, 1H).
The following compounds were obtained similarly to the procedure of Example 3-
60:
Example 3-61
2-Methyl-2-[[4-[[[2-[[4-( 1-methylethyl)-2-(trifluoromethyl)phenyl]amino]-2-
oxo-
ethyl][(4-methyl-5-oxazolyl)methyl]amino]methyl]phenyl]thio]-propionic acid
O N
'\ O
HO
N
O
F F
Yield: 81 °lo of theory
'H-NMR (300 MHz, CDCl3): 8 = 1.25 (d, 6H); 1.50 (s, 6H); 2.07 (s, 3H); 2.85-
3.00
(m, 1H); 3.39 (s, 2H); 3.74-3.78 (m, 4H); 7.30 (d, 2H); 7.36-7.53 (m, 4H);
7.79 (s,
1H); 8.I 1 (d, 1H); 9.39 (br s, 1H).
Le A 34 838-Foreign Countries
- 136 -
Example 3-62
2-( [4-[[[2-[(2-Methyl-4-methoxyphenyl)amino]-2-oxoethyl ] ( (4-methyl-5-
oxazolyl)-
methyl]amino]methyl]phenyl]thio]-2-methyl-propionic acid
O N
S
HO
/ N ~ O~.
~l
O N
H
Yield: 84% of theory
'H-NMR (200 MHz, CDC13): 8 = 1.51 (s, 6H); 2.08 (s, 3H); 2.23 (s, 3H); 3.35
(s,
2H); 3.70-3.82 (m, 7H); 6.70-6.80 (m, 2H); 7.28 (d, 2H); 7.48 (d, 2H); 7.63-
7.73 (m,
1H); 7.80 (s, 1H); 8.81 (br s, 1H).
Example 3-63
2-[(4-([[2-([2,4-Bis(trifluoromethyl)phenyl]amino]-2-oxoethyl][(4-methyl-5-
oxazolyl)methyl]amino]methyl]phenyl]thio]-2-methyl-propionic acid
O N
f \
HO S ' ~ O F
/ N / F
F
O N
H F
F F
Yield: 76% of theory
'H-NMR (300 MHz, CDC13): 8 = 1.49 (s, 6H); 2.09 (s, 3H); 3.35 (s, 2H); 3.74-
3.80
(m, 4H); 7.29 (d, 2H); 7.49 (d, 2H); 7.75-7.82 (m, 2H); 7.87 (s, 1H); 8.56 (d,
1H);
9.66 (br s, 1 H).
CA 02424540 2003-04-02
CA 02424540 2003-04-02
Le A 34 838-Forei?n Countries
137 -
Example 3-64
2-[4-([(2-((4-Cyclohexyl-2-methylphenyl)amino]-2-oxoethyl)[(4-methyl-5-
oxazolyl)-
methyl]amino]methyl]phenoxy]-2-methyl-propionic acid
O N
HO O ~ O
/ N
O N
H
Yield: 80% of theory
LC-l~IS: Acetonitrile / 30% aqueous HCllwater (gradient): R~ = 2.64 min
([l~I+H]+ _
534).
Example 3-6~
2-[4-[[[2-((4-(1,1-Dimethylethyl)-2-methylphenyl]amino]-2-oxoethyl][(4-methyl-
5-
oxazolyl)methyl]amino]methyl]phenoxy]-2-methyl-propionic acid
O N
HO O
/ N
/I
O N
H
Yield: 80% of theory
LC-MS: Acetonitrile / 30% aqueous HCl/water (gradient): R~ = 2.43 min ((M+H]+
_
508).
Le A 34 838-Foreign Countries
-138-
Working Examples 4
Example 4-1
S 2-[4-(([2-[(2,5-Dimethylphenyl)amino]-2-oxoethyl](2-
furanylmethyl)amino]methyl]-
phenoxyJ-2-methyl-propanoic acid
0
o ° i , ° off
H
Step a)
Wang resin (from Rapp Polymere, Order No. H 1011) (48.0 g, 14.06 mmol of
reactive groups) is suspended in dichloromethane. 2-(4-Formylphenoxy)-2-methyl-
propionic acid [G.J. Ellymes, C. Glynis, J. Chem. Soc. Perkin Trans. 2, 1993,
43-48]
(8.78 g, 42.18 mmol), diisopropylcarbodiimide (10.65 ~, 84.35 mmol) and DMAP
(3.44 ~, 28.12 mmol) are added, and the mixture is then shaken at room
temperature
for 18 h. The mixture is then filtered and the resin is washed with
dichloromethane,
DMF and methanol, giving resin A.
Step b)
Resin A (2.50 g, 0.72 mmol of reactive groups) and 2-furfurylamine (352 mg,
3.62 mmol) are suspended in 20 ml of trimethyl orthoformate. The mixture is
shaken
at room temperature for 20 h and then filtered, and the resin is washed with
DMF.
The resin is then suspended in 20 ml of DMF, tetrabutylammonium borohydride
(559 mg, 2.17 mmol) and acetic acid (0.42 ml, 7.25 mmol) are added and the
mixture
is shaken at room temperature for 7 h. The mixture is then filtered and the
resin is
washed with dichloromethane, DMF and methanol, giving resin B 1.
CA 02424540 2003-04-02
Le A 34 838-Forei ~n Countries
- 139 -
Step c)
Resin B 1 (2.5 g, 0.72 mmol of reactive ~ oups) is suspended in 40 ml of
dioxane and
treated with triethylamine (3.03 ml, 21.75 mmol) and trimethylsilyl
bromoacetate
(2.38 ml, 14.5 mmol). The mixture is shaken at 60°C overnight. The
mixture is then
filtered and the resin is washed with dichloromethane, DMF and methanol. The
protective silyl group is removed by suspending the resin in 25 ml of dioxane
and
treating it with tetrabutylammonium fluoride solution (1 M in THF, 1 ml). The
mixture is shaken at room temperature for 1 h and then filtered. The resin is
then
washed with dichloromethane, DMF and methanol, living resin C1.
Step d)
Resin C1 (2.5 g, 0.72 mmol of reactive groups) is suspended in 20 ml of DMF
and
treated with diisopropylethylamine (656 mg, 5.08 mmol), HATU (1.38 g, 3.63
mmol)
and 2,5-dimethylaniline (615 mg, 5.08 mmol). The mixture is shaken at room
temperature for 18 h and then filtered, and the resin is washed with
dichloromethane,
DMF and methanol. The resin is then suspended in a mixture of dichloromethane
and
trifluoroacetic acid. The mixture is shaken at room temperature for 30 min and
then
filtered and evaporated. The target compound is obtained as a colourless film.
LC-MS: R~ = 3.68 min; [M+H]+ = 451.3 ( 100%), [M-H]+ = 449.3 ( 100%)
[Method: Symmetry C18 column (Waters), flow rate: 0.5 ml/min, oven temp.
40°C,
pressure 400 bar, gradient: t=0 min: 10% A, 90% B; t=4.0 min: 90% A, 10% B;
t=6.0 min: 90% A, 10% B; t=6.1 min 10% A, 90% B; t=7.5 min 10% A, 90% B. A:
CH3CN + 0.1 % HCOOH; B: H20 +0.1 % HCOOH].
'H-NMR (d6-DMSO): S = 1.4 (s, 6H), 2.3 (s, 3H), 2.4 (s, 3H), 3.3 (s, 2H), 3.7
(s,
2H), 3.8 (s> 2H), 6.3 (d, 1H), 6.4 (d, 1H), 6.8 (d, 1H), 6.9 (d, 2H), 7.05 (d,
IH), 7.2
(m, 2H), 7.4 (s, 1H), 7.8 (s, 1H).
Example 4-2
2-[4-[[j2-[(4-Methoxy-2,5-dimethylphenyl)amino)-2-oxoethyl](2-furanylmethyl)-
amino]-methyl]phenoxy]-2-methyl-propanoic acid
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
140 -
I 1~~ °
° ~ ~ o °~ ~ ° off
~H
Resin C1 from Example 4-1 step c) (2.5 g, 0.72 mmol of reactive groups) is
suspended in 20 ml of DNLF and treated with diisopropylethylamine (656 mg,
S 5.08 mmol), HATU (1.38 g, 3.63 mmol) and 2,S-dimethyl-4-methoxyaniline
(756 m~, 5.08 mmol). The mixture is shaken at room temperature for 18 h and
then
filtered, and the resin is washed with dichloromethane, DNIF and methanol. The
resin
is then suspended in a mixture of dichloromethane and trifluoroacetic acid.
The
mixture is shaken at room temperature for 30 min and then filtered and
evaporated.
The target compound is obtained as a colourless film.
LC-MS: RI = 3.48 min; [M+H]+ = 481.226 (100%), [M-H]+ = 479.226 ( 100%)
[Method: Symmetry C 18 column ('Vaters), flow rate: 0.5 mllmin, oven temp.
40°C,
pressure 400 bar, gradient: t=0 min: 10% A, 90% B; t=4.0 min: 90% A, 10% B;
t=6.0 min: 90% A, 10% B; t=6.1 min 10% A, 90% B; t=7.5 min 10% A, 90% B. A:
CH3CN + 0.1 % HCOOH; B: H20 +0.1 % HCOOH].
E~tample 4-3
2-[4-[[[2-[(4-Methoxy-2,5-dimethylphenyl)amino]-2-oxoethyl](2-thienylmethyl)-
amino]-methyl]phenoxy]-2-methyl-propanoic acid
I 1~~ °
° ' ~ o S ~ ( ° off
H
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-141-
Step a)
Resin A from Example 4-1 step a) {2,50 g, 0.72 mmol of reactive groups) and
2-aminomethylthiophene (409 mg, 3.62 mmol) are suspended in 20 ml of trimethyl
orthoformate. The mixture is shaken at room temperature overnight and then
filtered,
and the resin is washed with DMF. The resin is then suspended in 20 ml of
DIvlF,
treated with tetrabutylammonium borohydride (559 mg, 2.17 mmol) and acetic
acid
(0.42 ml, 7.25 mmol) and shaken at room temperature for 7 h. The mixture is
then
filtered and the resin is washed with dichloromethane, DMF and methanol,
giving
resin B2.
Step b)
Resin B2 (2.5 g, 0.72 mmol of reactive jroups) is suspended in 40 ml of
dioxane and
treated with triethylamine (3.03 ml, 21.75 mmol) and trimethylsilyl
bromoacetate
(2.38 ml, 14.5 mmol). The mixture is shaken at 60°C overnight. The
mixture is then
filtered and the resin is washed with dichloromethane, DMF and methanol. The
protective silyl jroup is removed by suspending the resin in 25 ml of dioxane
and
treating it with tetrabutylammonium fluoride solution ( 1 M in THF, 1 ml). The
mixture is shaken at room temperature for 1 h and then filtered. The resin is
then
washed with dichloromethane, DMF and methanol, giving resin C2.
Step c)
Resin C2 (2.5 ~, 0.72 mmol of reactive groups) is suspended in 20 ml of DNIF
and
treated with diisopropylethylarnine (656 mg, 5.08 mmol), HATU (1.38 g, 3.63
mmol)
and 2,5-dimethyl-4-methoxyaniline (657 mg, 5.08 mmol). The mixture is shaken
at
room temperature for 18 h and then filtered, and the resin is washed with
dichloromethane, DMF and methanol. The resin is then suspended in a mixture of
dichloromethane and ttifluoroacetic acid. The mixture is shaken at room
temperature
for 30 min and then filtered and evaporated. The target compound is obtained
as a
colourless film.
LC-MS: R~ = 3.90 min; [M+H]+ = 497.4 ( 100%), [M-H]+ = 495.4 (100%)
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 142
[Method: Symmetry C18 column (Waters), flow rate: 0.5 mlimin, oven temp.
40°C,
pressure 400 bar, gradient: t=0 min: 10% A, 90% B; t=4.0 min: 90% A, 10% B;
t=6.0 min: 90% A, 10% B; t=6.1 min 10% A, 90% B; t=7.5 min 10% A, 90% B. A:
CH3CN + 0.1% HCOOH; B: H20 +0.1% HCOOH].
1H-NMR (d6-DMSO): b = 1.5 (s, 6H), 2.1 (s, 3H), 2.2 (s, 3H), 3.3 (s, 2H), 3.7
(s,
2H), 3.8 (s, 3H), 4.0 (s, 2H), 6.8-7.5 (m, 9H).
The following compound is obtained in a similar manner:
Example 4-4
2-[4-[[[2-[(4-Methoxy-2,5-dimethylphenyl)amino]-2-oxoethyl][(5-methyl-2-
furany1)-
methyl]-amino]methyl]phenoxy]-2-methyl-propanoic acid
1 1~~ °
\ o ° ~ ~ ° off
/ N~N \
H
LC-MS: R~ = 2.76 min; [M+H]+ = 495 ( 100%), [M-H]+ = 493 ( 100%)
[Method: Symmetry C 18 column (Waters), flow rate: 0.5 mllmin, oven temp.
40°C,
pressure 400 bar, gradient: t=0 min: 10% A, 90% B; t=4.0 min: 90% A, 10% B;
t=6.0 min: 90% A, 10% B; t=6.1 min 10% A, 90% B; t=7.5 min 10% A, 90% B. A:
CH3CN + 0.1 % HCOOH; B: H20 +0.1 % HCOOH).
Example A
Cellular transactivation assay:
Test principle:
A cellular assay is used to identify activators of the peroxisome proliferator-
activated
receptor alpha (PPAR-alpha).
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-143-
Since mammalian cells contain different endogenous nuclear receptors which may
complicate an unambiguous interpretation of the results, an established
chimera
system is used in which the ligand binding domain of the human PPARa receptor
is
S fused to the DNA binding domain of the yeast transcription factor GAL4. The
resulting GAL4-PPARa chimera is co-transfected and stably expressed in CHO
cells
having a reporter construct.
Cloning:
The GAL4-PPARa expression construct contains the ligand binding domain of
PPARa (amino acids 167-468), which is PCR-amplified and cloned into the vector
pcDNA3.1. This vector already contains the GAL4 DNA binding domain (amino
acids 1-147) of the vector pFC2-dbd (Stratagene). The reporter construct,
which
contains five copies of the GAL4 binding site upstream of a thymidine kinase
1~ promoter, expresses firefly luciferase (Photinus pyralis) following
activation and
binding of GAL4-PPARa.
Transactivation assay (luciferase reporter):
CHO (chinese hamster ovary) cells are sown in DNIEMlFI2 medium (BioWhittaker),
supplemented by 10% foetal calf serum, 1% penicillin/streptomycin (GIBCO), at
a
cell density of 2 x 103 cells per well in a 384 well plate (Greiner). The
cells are
cultivated at 37°C for 48 h and then stimulated. To this end, the
substances to be
tested are taken up in CHO-A-SFM medium (GIBCO), supplemented by 10% foetal
calf serum, 1% penicillin/streptomycin (GIBCO) and added to the cells. After a
stimulation period of 24 hours, the luciferase activity is measured using a
video
camera. The relative light units measured give, as a function of the substrate
concentration, a sigmoidal stimulation curve. The ECso values are calculated
using
the computer programme GraphPad PRISM (Version 3.02).
In this test, the compounds according to the invention of Examples 3-4, 3-6, 3-
60,
1-9, 2-7 and 2-12 show ECSO values of from 0.04 to 200 nM.
CA 02424540 2003-04-02
Le A 34 838-Foreiran Countries
- 144 -
Example B
Determination of fibrinogen:
To determine the effect on the plasma fibrinogen concentration, male Wistar
rats are
treated with the substance to be examined for a period of 4-9 days, by
administration
via a stomach tube or by mixing the substance with the feed. In terminal
anaesthesia,
citrated blood is then obtained by heart puncture. The plasma fibrinogen
levels are
determined using the Clauss method (Clauss A., Acta Haematol. 17, 237-46
(1957)]
by measuring the thrombin time using human fibrinogen as standard. In some
cases,
parallel determinations are carried out using a turbidometric method [Becker
U.,
Bartl K., Wahlefeld A. W., Thrombosis Res. 35, 475-84 (1984)] where batroxobin
is
used instead of thrombin.
Example C
Description of the test for finding pharmacologically active substances which
increase apoprotein A1 (ApoAl) and HDL cholesterol (HDL-C) concentrations
in the serum of transgenic mice transfected with the human ApoAl gene
(hApoAl): '
The substances to be examined in vivo for their HDL-C-increasing activity are
administered orally to male transgenic hApoAl mice. One day prior to the start
of the
experiment, the animals are randomized into groups with the same number of
animals, generally n = 7-10. During the entire experiment, the animals have
drinking
water and feed ad libitum. The substances are administered orally once a day
for
7 days. To this end, the test substances are dissolved in a solution of
Solutol HS 15 +
ethanol + saline (0.9%) in a ratio of 1+1+8 or in a solution of Solutol HS 15
+ saline
(0.9%) in a ratio of 2+8. The dissolved substances are administered in a
volume of
10 ml/kg of body weight using a stomach tube. Animals which have been treated
in
exactly the same manner but have only been given the solvent (10 ml/kg of body
weight), without test substance, serve as control group.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
-145-
Prior to the first administration of substance, a blood sample from each of
the mice is
taken by puncture of the retroorbital venous plexus, to determine ApoAl, serum
cholesterol, HDL-C and serum triglycerides (TG) (zero value). Subsequently,
using a
stomach tube, the test substance is administered for the first time to the
animals.
S 24 hours after the last administration of substance (on day 8 after the
start of the
treatment), another blood sample is taken from each animal by puncture of the
retroorbital venous plexus, to determine the same parameters. The blood
samples are
centrifuged and, after the serum has been obtained, cholesterol and TG are
determined photometrically using an EPOS Analyzer 5060 (Eppendorf-Geratebau,
Netheler & Hinz GmbH, Hamburg). The said determinations are carried out using
commercial enzyme tests (Boehringer Mannheim, Mannheim).
To determine the HDL-C, the non-HDL-C fraction is precipitated using 20% PEG
8000 in 0.2 M glycine buffer pH 10. From the supernatant, the cholesterol is
determined UV-photometrically (BIO-TEK Instruments Inc. USA) in a 96-~,vell
plate
using a commercial reagent (Ecoline 25, Merck, Darmstadt).
Human mouse ApoAl is determined with a Sandwich ELISA method using a
polyclonal antihuman ApoAl and a monoclonal antihuman ApoAl antibody
(Biodesign International, USA). Quantification is carried out UV-
photometrically
(BIO-TEK Instruments, USA) using peroxidase-coupled anti-mouse-IGG antibodies
(KPL, USA) and peroxidase substrate (KPL, USA).
The effect of the test substances on the HDL-C concentration is determined by
subtracting the value measured for the 1st blood sample (zero value) from the
value
measured for the 2°d blood sample (after the treatment). The mean of
the differences
of all HDL-C values of one group is determined and compared to the mean of the
differences of the control group.
Statistical evaluation is carried out using student's t-test, after the
variances have
been checked for homogeneity.
CA 02424540 2003-04-02
Le A 34 838-Foreign Countries
- 146 -
Substances which increase the HDL-C of the treated animals in a statistically
significant (p<0.05) manner by at least 20%, compared to the control group,
are
considered to be pharmacologically effective.
CA 02424540 2003-04-02