Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02425164 2007-10-22
25771-796
1
(4-ACYLAMINOPIPERIDIN-1-YL) ACETAMIDES
AS NEUROKININ ANTAGONISTS
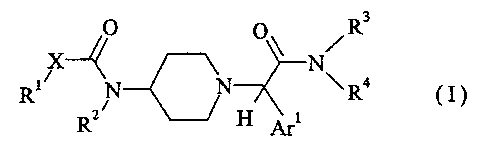
The invention relates to new compounds of formula I,
R3
X O 0 N
~
R. N N R.4 (I)
RZ H Ar
wherein the groups Ar, R', R2, R3 , R4 and X have the meanings given in the
claims and
1o specification, processes for preparing them as well as their use as
pharmaceutical
compositions, and the pharmaceutically acceptable salts thereof, processes for
preparing them
and pharmaceutical compositions containing thege compounds. The compounds are
valuable
neurokinin (tachykinin) antagonists.
15 Background to the invention
The compounds of formula I are partly covered by the broad general formula of
International
Patent Application W096/32386 . Hovi~ever, this does not disclose any
compounds in which
the amide group is substituted with a 2-phenyl-ethyl group and the piperidyl
group in the 4
position is substituted with a substituted urethane or urea group. The
compounds described in
20 this international patent application are neurokinin antagonists with a
broad spectrum of
activity.
The problem of the present invention is to provide new neurokinin antagonists
with an
enhanced activity. This problem is now solved according to the invention by
the preparation
25 of the new compounds of formula I. .
CA 02425164 2003-04-07
1l1155-ff Boehringer Ingelheim Pharma KG
2
Detailed description of the invention
Surprisingly it has been found that the activity of the new NK, receptor
antagonists of formula I is
dramatically increased compared with the known compounds.
{ The invention therefore relates to new compounds of formula I
O O~ R3
N
Ri N -C - N \R4 (I)
R2 H Arl
or the pharmaceutically acceptable salts thereof,
wherein
R' denotes Ci-C6-alkyl or Ar2,
R2 denotes hydrogen, Ci-C6-alkyl or Cs-C6-cycloalkylmethyl, or
R' and RZ taken together denote a C2-C3-alkylenediyl group optionally
substituted by one or two
oxo groups (=O),
X denotes 0 or NRS,
Ar' and A? independently of one another denote unsubstituted phenyl or phenyl
which is I-
to 5-substituted by halogen, hydroxy, Ci-C4-alkyl, Ci-Ca-alkoxy,
Ci-C4-fluoroalkyl, Ci-Ca-fluoroalkoxy or -OCH2O-;
R3 denotes 2-phenyl-ethyl, wherein the phenyl group may be substituted by 1 to
3
substituents, while the substituents, independently of one another, are
selected
from among halogen, hydroxy, Ci-Ca-alkyl, Ci-Ca-alkoxy, Ci-C4-fluoroalkyl,
C i-Ca-fluoroalkoxy;
R4 denotes hydrogen, CI-Ca-alkyl, C3-Cs-cycloalkyl, CH2COOH, -CH2C(O)NH2,
-OH or phenyl-Ci-Ca-alkyl ; and
R5 denotes hydrogen or Ci-Cs-aHyl.
In the foregoing and in what is to follow, the terms "alkyl" and " alkoxy" as
used with reference to
the groups R', R2, R3, R4 or the substituents of Arl or A? denote straight-
chain or branched,
saturated hydrocarbon groups with up to 6 carbon atoms, preferably 1 to 4
carbon atoms,
particularly methyl, ethyl, n-propyl, i-propyl, n-butyl, tert-butyl, methoxy,
ethoxy, n-propoxy or
i-propoxy.
CA 02425164 2003-04-07
1/1155-ff Boebringer Ingelheim Pharma KG
3
In the foregoing and in what is to follow, the terms " fluoroalkyl" and
"fluoroalkoxy" as used with
reference to the group R3 or the substituents of Ar denote straight-chain or
branched, fluorine-
substituted hydrocarbon groups with up to 4 carbon atoms and up to 9 fluorine
atoms, preferably 1
or 2 carbon atoms and up to 5 fluorine atoms, particularly trifluoroethyl,
pentafluoroethyl, 2,2,2-
trifluoroethyl, 2-fluoroethyl, difluoromethoxy, trifluoromethoxy,
pentafluoroethoxy, 2,2,2-
trifluoroethoxy or 2-fluoroethoxy.
The compounds according to the invention are valuable neurokinin (tachykinin)
antagonists which
io have substance P-antagonistic properties. They are useful for treating and
preventing neurokinin-
mediated illnesses and additionally have a dramatically increased effect.
Compounds of general formula I may have acid groups, mainly carboxyl groups,
and/or basic
groups such as, for example, amino functions. Compounds of general formula I
may therefore
be in the form of internal salts, salts with pharmaceutically useable
inorganic acids such as
hydrochloric acid, sulphuric acid, phosphoric acid, sulphonic acid or organic
acids (such as
for example maleic acid, fumaric acid, citric acid, tartaric acid or acetic
acid) or salts with
pharmaceutically useable bases such as alkali or alkaline earth metal
hydroxides or
carbonates, zinc or ammonium hydroxides or organic amines such as, for
example,
diethylamine, triethylamine, triethanolamine etc.
The compounds according to the invention may occur as racemates, or they may
be obtained
as pure enantiomers, i.e. in the (R)- or (S)-form. Compounds which occur as
racemates or as
the (S)-forrn are preferred.
The compounds according to the invention are valuable neurokinin (tachykinin)
antagonists
which have substance P-antagonistic properties. They are useful for treating
and preventing
neurokinin-mediated illnesses:
Treatment or prevention of inflammatory and allergic complaints of the
airways, such
as asthma, chronic bronchitis, hyperreactive airways, emphysema, rhinitis,
COPD, pulmonary
hypertension, cystic fibrosis, coughs;
of the eyes, such as conjunctivitis and iritis,
CA 02425164 2003-04-07
111155-ff Boehringer Ingelheim Pharma KG
..~ .
4
of the skin, such as dermatitis in contact eczema, neurodermatitis, pruritus,
urticaria,.psoriasis,
sunburn, burns, insect bites, rosacea, itching, sensitive or hypersensitive
skin,
of the gastro-intestinal tract, such as gastric and duodenal ulcers,
ulcerative colitis, Crohn's
disease, inflammatory bowel disease, initable colon, Hirschsprung's disease,
motility
problems;
of the joints or bones, such as rheumatoid arthritis, reactive arthritis,
arthrosis, osteoporosis
and Reiter's syndrome;
of the bladder, such as irritable bladder, incontinence, urinary urgency,
urethritis, colic and
cystitis.
Also for the treatment of diseases of the central nervous system such as
dementia,
Alzheimer's disease, schizophrenia, psychoses, anxiety states, alcohol or drug
dependency,
sexual dysfunctions, eating disorders, depression, headaches (e.g. migraine or
tension
headaches), epilepsy; Parkinson's disease, stroke,
treatment of Herpes zoster as well as postherpetic pain, tumours,
collagenoses, a dysfunction
of the deferent urinary tracts, haemorrhoid, nausea and vomiting, triggered
for example by
radiation or cytostatic therapy or motion, and painful conditions of all
kinds.
2o The invention therefore also relates to the use of the compounds of formula
I as curative
agents and pharniaceutical preparations which contain these compounds. They
are preferably
used on humans. The compounds according to the invention may be given
intravenously,
subcutaneously, intramuscularly, intraperitoneally, intranasally, by
inhalation, transdermally,
optionally assisted by iontophoresis or enhancers known from the literature,
and by oral
route.
For parenteral administration the compounds of formula I or their
physiologically acceptable
salts, may be put into solution, suspension or emulsion, possibly with
substances
conventionally used for this purpose such as solubilisers, emulsifiers or
other adjuvants.
Suitable solvents include, for example: water, physiological saline solutions
or alcohols, e.g.
ethanol, propanediol or glycerol, sugar solutions such as glucose or mannitol
solutions or a
mixture of various solvents.
CA 02425164 2003-04-07
1/1155-ff Boehringer Ingelheim Pharma KG
In addition, the compounds may be administered by the use of implants, e.g. of
polylactide,
polyglycolide or polyhydroxybutyric acid or intranasal preparations.
Compounds of formula I, wherein R4 denotes Cl-C4-alkyl, particularly methyl,
are preferred.
5
Also preferred are compounds of formula I wherein Ar is unsubstituted phenyl
or 2,3-
methylenedioxyphenyl, particularly unsubstituted phenyl.
Preferred compounds of formula I are those wherein R3 denotes 2-phenylethyl,
wherein the phenyl
1o group may be substituted by 1 to 3 substituents, wherein the substituents
are selected independently
of one another from among halogen, hydroxy, methyl, methoxy, trifluoromethyl,
trifluoromethoxy,
particularly wherein R3 is 2-(3,5-bis-trifluoromethylphenyl)-ethyl.
Particularly preferred compounds of formula I are those wherein the group -
NR3R4 is
CF3
N CF3
CH3
In a preferred aspect the invention relates to compounds of formula I, wherein
R' denotes a CI-C3-alkyl, particularly methyl, phenyl or Cl-C3-alkoxyphenyl
group, particularly
4-methoxyphenyl,
X denotes NH, and
R2 denotes a hydrogen atom.
In another preferred aspect the invention relates to compounds of formula I,
wherein
R' and R2 taken together denote an ethylene-l,2-diyl, 1-oxoethylene-l,2-diyl,
propylene-1,3-diyl, 1-
oxopropylene-1,3-diyl or 1-oxobutylene-1,3-diyl group, and
X denotes 0, NH or NCH3.
Particularly preferred are NK1 receptor antagonists of formula I, wherein the
group
CA 02425164 2003-04-07
I 1/1155-ff Boebringer Ingeiheim Phazma KG
6
RI-X-CO-NRz-
is a group selected from the formulae A-1 to A-8:
~
H H
,,N
I~C y OyN
0 A-1 0
A-2
p CH
O 3
{ O i
N O
N\ ~
0 A-3 A-4
C"3
O NyO
H
N.I-I N O
~
CH 'N
3 A-5 A-6
N Y N~ yN,,
I I N
O H3C~' O
A-7 A-8
The following compounds are particularly preferred:
CF3
N
N
H3C Y
~ N O
N CF
3
\ CH3
= CA 02425164 2003-04-07
111155-ff BOehringer Ingellieim Phazma KG
7
CF3
OyN
O
O N-'YAN
CF3
CH3
O
O 3
O \
N N I
~ /
i I CF 3
lVTT
/ 1.t13
CH3
O N
CF 3
N
o
N
O
,, -lyAN
CF
3
, KT
~1~
CH3
O N
~3
N
OII
CH3 N\ ~
Y N CF3
~3
CA 02425164 2003-04-07
1/1155-ff Boelninger Ingelheim Pharma KG
8
H
N O
CF3
N
0
N
CF3
CH3
H H CFs
NyN 0
N~
3
~ lj CF
CH3
H H CF3
\ NyN
~ O
O / O --"A
I N CF3
CH3 L+H3
0~0
3
CN,,,o O
CF
3
O CH3
<
0
CA 02425164 2003-04-07
1/1155-ff Boebringer Ingelheim Phanna KG
9
H
H3CCF3
N O
H f ~.
NN CF
3
O CH3
O
The compounds may be prepared in a manner known per se.
Advantageous methods are illustrated and described in the following diagram
The compounds of general formula I may be prepared by reacting an
amide of formula II
O R 3
" NJ
x- R4 (II)
H Ar
wherein X denotes a suitable leaving group, preferably halogen,
alkylsulphonyloxy,
particularly methylsulphonyloxy, or arylsulphonyloxy, particularly p-
tolylsulphonyloxy,
with a piperidine of general formula III
O
X1NN-H Rl ( ~ )
RZ
in an inert solvent in the presence of abase.
This process is illustrated by means of the following Diagram 1 for compounds
wherein Ar is
phenyl, R3 is bis-(trifluoromethyl)-phenylethyl and R4 is methyl. However, the
process can be used
analogously for all compounds of formula I. The compounds of fonnula III are
known or may be
prepared analogously to methods known per se.
CA 02425164 2003-04-07
1/1155-ff Boehringer Ingelheim Phazma KG
Diagram I
O 0
HO QH CH3SOZC1 ~CSO2OOH
CF3
~ \
HN ~ CF3
CI-i3
CF'3
H3CSOZO I /
N CF3
CH3
R1~X~0
RziN TEA
H
t~
R'~X~/ O CF3
R2/N ` O
N V `N \' CF
3
CH 3
The reactant for this piperazine derivative is obtained as shown in Diagraazn
1, on the right.
(R)-Mandelic acid is reacted with methanesulphonic acid halide to obtain (R)-2-
(methanesulphonyloxy)-acetic acid. This is then reacted with a coupling
reagent and the
5 correspondingly substituted phenethylamine to obtain the corresponding
amide, or it is
converted into the corresponding acid halide (e.g. with SOC12/S02C12) and then
converted
with the suitably substituted phenethylamine into the corresponding amide. In
the last step the
amide thus obtained is reacted with the piperidine derivative described above,
while during
the substitution of inethanesulphonate C-N-linking takes place with
simultaneous reversal of
CA 02425164 2003-04-07
1/1155-ff Baehringer Ingelheim Pharma KG
11
the chiral centre. The reaction is carried out in an inert solvent, preferably
a polar aprotic
solvent such as, for example, DMF, dimethyl acetamide, ethylmethylketone or
acetonitrile in
the presence of a base, preferably an inorganic base such as, for example,
K2C03, NaHCO3
or CaCO3, or organic bases such as, for example, tertiary amines, preferably
triethylamine,
Hiinig base, pyridine or N-methylmorpholine, at between 0 C and 120 C,
typically between
C and 80 C. The reaction time is generally between 0.5 h and 48 h.
The compounds and compositions according to the invention will now be
illustrated by the
Examples which follow. The skilled person is aware that the Examples serve
only as an
1o illustration and are not to be regarded as limiting.
A Example of the synthesis of compounds according to the invention
Example 1
N-[2-(3,5-bis-trifluoromethyl-phenyl)-ethyl]-N-methyl-2-[4-(3-methyl-ureido)-
piperidin-l-
y,l]-2-phenyl-acetamide
6.8 g of 4-(3-methylureido)-piperidine are refluxed together with 19.2 g of N-
[2-(3,5-bis-
trifluoromethyl-phenyl)-ethyl]-2-methanesulphonyloxy-N-methyl-2-phenyl-
acetamide
(prepared analogously to the method described in WO 99/62893 ) and 6.8 ml of
triethylamine
in 400 ml of acetone for 8 hours. Then the solution is evaporated down,
combined with
saturated sodium hydrogen carbonate solution and extracted with ethyl acetate.
The extract is
dried, the solvent is eliminated in vacuo and the residue is chromatographed
with methylene
chloride / methano19:1 over silica gel. The fractions found to be uniform by
TLC are
combined and the solvent is eliminated in vacuo. N-[2-(3,5-bis-tifluormethyl-
phenyi)-ethyl]-
N-methyl-2-[4-(3-methyl-ureido)-piperidin-l-yl]-2-phenyl-acetamide is
crystallised from the
residue with ethanolic hydrochloric acid and ether in the form of the
hydrochloride, yielding
7.1 g of colourless crystals.
'H-NMR (250 MHz, CD3OD) S ppm = 7.93 - 7.33 (81-L m); 5.58; 5.38 (1H, 2s);
4.03 - 2.59
(5H, m); 3.05 (4H, m); 2.98; 2.90 (3H, 2s); 2.74; 2.70 (3H, 2s); NH in the
solvent blind peak
4.89; 2.27 - 1.52 (4H, m). Most signals are split by amide rotation.
CA 02425164 2003-04-07
111155-ff Boehringer Ingelheim Phaima KG
12
Example 2
(S)-N-[2-(3,5-bis trifluoromethyl-phenyl)-ethyl] N-methyl-2-[4-(2-oxo-
[1.3]oxazinan-3-yl)-
piperidin-1-yl]-2-phenyl-acetamide
5.4 g of 4-(2-oxo -[ 1. 3 ]oxazinan-3 -yl)-piperidine are refluxed together
with 12.5 g of (R)-N-
[2-(3,5-bis-trifluoromethyl-phenyl)-ethyl]-2-methanesulphonyloxy N-methyl-2-
phenyl-
acetamide (prepared from D-(-)-mandelic acid) and 4.5 ml of triethylamine in
250 ml of
acetone for 6 hours. Then the solution is concentrated by evaporation,
combined with
saturated sodium hydrogen carbonate solution and extracted with ethyl acetate.
The extract is
io dried, the solvent is eliminated in vacuo and the residue is
chromatographed with methylene
chloride / methanol 9:1 over silica gel. The fractions found to be uniform by
TLC are
combined and the solvent is eliminated in vacuo. (S)-N-[2-(3,5-bis-
trifluoromethyl-phenyl)-
ethyl]-N-methyl-2-[4-(2-oxo-[1,3]oxazinau-3-yl)-piperidin 1 yl]-2-phenyl-
acetamide is
crystallised from the residue with ethanolic hydrochloric acid and ether in
the form of the
hydrochloride. 7.5 g of light beige crystals are obtaine(i
'H-NMR (250 MHz, CD3OD) S ppm = 7.88 - 7.55 (8H, m); 5.47; 5.28 (iH, 2s); 4.24
(2H, t,
J = 5.8 Hz); 4.13 (IH, m); 4.06 - 2.69 (6H, m); 3.04 (4H, m); 3.00; 2.89 (3H,
2s); 2.34 - 1.71
(6H, m). Most signals are split by amide rotation.
Rotational value [a]D20 = +36,7 (c=1; methanol)
Examples 3 to 10 may be prepared analogously.
R1~X~0 CF
3
RZ~N O
"'A
N CF3
RS ' CH3
R6
CA 02425164 2003-04-07
1/1155-ff Boebringer Ingellheim Phatioaa KG
13
Example R X- R- R R
3 -O-CH2-C=O- H H
4 -N(CH3)-C=O-CH2CH2- H H
N(CH3)-C=O-CH2CH(CHs)- H H
6 -NH-CH2CH2- H H
7 phenyl-NH- H- H H
8 4-methoxy-phenyl-NH- H- H H
9 -O-CH2-CH2CH2- -O-CH2-O-
methyl-NH- H -O-CH2-O-
5 B Results of investigations into the compound according to the invention:
The receptor affinity to the NKl-receptor (substance P-receptor) is determined
on human
lymphoblastoma cells (IM-9) with cloned NKl-receptors, by measuring the
displacement of
12sI-labelled substance P. The Kl-values thus obtained show the efficacy of
the compounds.
lo The compounds according to the invention were compared with the compounds
of the following
fonnulae known from International Patent Application W096/32386 :
CA 02425164 2003-04-07
1/1155-ff Boehringer Ingeiheim Pharma KG
14
B-6 O 0 0 H CF 3
N N
H3C N~ ~ \
1 \ CF3
B-7
O O N CF3
N~
H N N
H
CF3
These compounds correspond to the compounds of Examples 6 and 7, wherein the 2-
bis-
trifluoromethylphenyl-ethyl group has been replaced by a bis-
trifluoromethylbenzyl.
The results are listed in Table I:
Example No. K; [nM]
1 0.7
2 1.7
3 0.7
4 0.6
5 0.6
6 3.5
B-6 165.0
7 0.8
B-7 432.0
CA 02425164 2003-04-07
1/1155ff Boeluinger Ingelheim Pharma KG
C Formulations of compounds according to the Invention
Injectable solution
200 mg active substance *
5 1.2 mg monopotassium dihydrogen phosphate = KH2PO4 )
0.2 mg disodium hydrogen phosphate = ) (buffer)
NaH2PO4.2H20 )
94 mg sodium chloride ) (isotonic agent)
or )
lo 520 mg glucose )
4 mg albumin (protease protection)
q.s. sodium hydroxide solution )
q.s. hydrochloric acid ) ad pH 6
ad 10 ml water for injections
Znjectable solution
200 mg active substance*
94 mg sodium chloride
or
2o 520 mg glucose
4 mg albumin
q.s. sodium hydroxide solution )
q.s. hydrochloric acid ) ad pH 9
ad 10 ml water for injections
CA 02425164 2003-04-07
1/1155-ff Boehringer Ingeiheim Pharnia KG
16
Lyophilisate
200 mg active substance*
520 mg mannitol (isotonic agent/bulking agent)
4 mg albumin
solvent 1 for lyophilisate
ml water for injections
solvent 2 for lyophilisate
mg Polysorbat 80 = Tween 80
10 (surfactant)
.-,
10 ml water for injections
* active substance: compound according to the invention, e.g. one of Examples
1 to 8
dose for humans weighing 67 kg: 1 to 500 mg