Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
NON-ASPIRATING TRANSITIONAL
VISCOELASTICS FOR USE IN SURGERY
FIELD OF THE INVENTION
The present invention relates to the field of viscous and viscoelastic
materials
suitable for use in surgical procedures. In particular, non-aspirating
viscoelastics,
including transitional viscoelastics (having non-shear related variable
viscosities), which
,o may be left in situ at the close of surgery are disclosed. Methods of using
transitional
viscoelastics in surgery, especially ophthalmic surgery are also disclosed.
BACKGROUND OF THE INVENTION
Viscous or viscoelastic agents used in surgery may perform a number of
different
functions, including without limitation maintenance and support of soft
tissue, tissue
manipulation, lubrication, tissue protection, and adhesion prevention. It is
recognized
that the differing rheological properties of these agents will necessarily
impact their
zo ability to perform these functions, and, as a result, their suitability for
certain surgical
procedures. See, for example, U.S. Patent No. 5,273,056.
Cataracts are opacities of the ocular lens which generally arise in the
elderly. In
order to improve eyesight, the cataractous lens is surgically removed and an
artificial
intraocular lens is inserted in its place. During these surgical procedures,
viscoelastic
a5 materials are typically injected in the anterior chamber and capsular bag
to prevent
collapse of the anterior chamber and to protect tissue from damage resulting
from
physical manipulation.
A number of viscous or viscoelastic agents (hereinafter "agents") are known
for
ophthalmic surgical use. For example, Viscoat~ (Alcon Laboratories, Inc.)
which
3o contains sodium hyaluronate and chondroitin sulfate; Healori and Healon~ GV
(Pharmacia Corp.), Amvisc Regular and Amvisc~ Plus (IOLAB), and Vitrax°
(Allergan) all of which contain sodium hyaluronate; and Cellugel~ (Alcon)
which
contains hydroxypropylmethylcellulose (HPMC) are alI useful in cataract
surgery. They
are used by the skilled ophthalmic surgeon for several purposes, including
maintenance
1
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
of the anterior chamber of the eye and protection of ophthalmic tissues during
surgery,
particularly corneal endothelial cells, and as an aid in manipulating
ophthalmic tissues.
While alI of the agents described above may be used during cataract surgery,
each
has certain recognized advantages and disadvantages. See, U.S. Patent No.
5,273,056.
Generally, however, all such agents having sufficient viscosity and
pseudoplasticity to be
useful in ophthalmic surgery will, if left in the eye at the close of surgery,
result in a
transient increase in intraocular pressure ("IOP") known as an "IOP spike."
(See,
Obstbaum, Postoperative pressure elevation. A rational approach to its
prevention and
management, J. Cataract Refractive Surgery 18:1 (1992).) The pressure increase
has been
,o attributed to the agent's interference with the normal outflow of aqueous
humor through
the trabecular meshwork and Schlemm's canal. (See, Berson et al., Obstruction
of
Aqueous Ou~ow by Sodium Hyaluronate in Enucleated Human Eyes, Am. J.
Ophthalmology, 95:668 (1983); Olivius et al., Intraocular pressure after
cataract
surgery with Healon~, Am. Intraocular Implant Soc. J. 11:480 (1985); Fry,
,s Postoperative intraocular pressure rises: A comparison of Healon, Amvis,
and Yiscoat,
J. Cataract Refractive Surgery 15:415 (1989).) IOP spikes, depending on their
magnitude and duration, can cause significant and/or irreversible damage to
susceptible
ocular tissues, including, without limitation, the optic nerve.
Thus, the ease with which an agent can be removed from the surgical site;
ao typically by aspiration, has traditionally been considered an important
characteristic in
the overall assessment of the agent's usefulness in cataract surgery. By
removing the
agent before the close of surgery, the surgeon hopes to minimize or avoid any
significant
IOP spike. Unfortunately, however, removal of agents which are relatively
dispersive
(as opposed to cohesive) or which adhere to the oculax tissue is often
difficult and may
z5 cause additional trauma to the eye.
Exogenous dilution of the viscoelastic has been suggested to alleviate IOP
spikes.
See U.S. Patent No. 4,328,803. Depending, however, on the particular
viscoelastic and
the surgical technique employed, IOP spike may still be a problem. More
recently, it has
been suggested that the administration of degradative agents ~to break down
conventional
3o viscous or viscoelastic agents in the eye can reduce or avoid the
occurrence of IOP
spikes. See, e.g., U.S. Patent No. 5,792,103., Such an approach requires not
only the
administration of a second, enzymatic agent into the eye, the biocompatability
of which
2
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
must be assured; but also means for adequately mixing the two agents in a
special
apparatus.
Viscoelastics have also been promoted as drug delivery devices for
pharmaceutical agents which are administered when the viscoelastics are
applied during
s surgery. For example, U.S. Patent No. 5,811,453 (Yanni et al.) discloses
viscoelastics
containing anti-inflammatory compounds and methods of using these enhanced
viscoelastics in cataract surgery. While this approach may ameliorate ocular
inflammation resulting from surgical trauma, such an approach still possesses
the
significant limitation of presenting IOP spike problems, as described above.
,o Consequently, these enhanced viscoelastics still need to be aspirated out
at the close of
surgery.
There is, therefore, a need for an improved means for reducing or avoiding IOP
spikes associated with the use of conventional viscous or viscoelastic agents
in
ophthalmic surgery, especially cataract surgery. More specifically, we
conceived the
15 need for an improved viscous or viscoelastic agent having a variable or
transitional
viscosity such that it will, without the addition of degradation agents,
become
substantially less viscous after its purpose has been served in surgery, such
agents being
hereinafter referred to as transitional viscoelastics. A significant amount of
such a
transitional viscoelastic may then be left in the eye by the surgeon to be
eliminated by
zo the body's natural processes without creating a dangerous IOP spike.
Transitional viscosities are known to occur in certain systems. In the
ophthalmic
field, systems are known in which a liquid forms a gel after application to
the eye. For
example, such gelations may be triggered by a change in pH. See, Gurney et
al., "The
Development and Use of In Situ Formed Gels, Triggered by pH" Biopharm. Ocul.
Drug
Zs Delivery, (1993) pp. 81-90. Temperature sensitive gelation systems have
also been
observed for certain ethyl (hydroxyethyl) cellulose ethers (EHECs) when mixed
with
particular ionic surfactants at appropriate concentrations (see, Carlsson et
al., "Thermal
Gelation of Nonionic Cellulose Ethers and Ionic Surfactants in Water" Colloids
Surf.,
volume 47, pages 147-65 (1990)) and for systems of pure methylethyl cellulose
(U.S.
3o Patent No. 5,618,800 (Kabra et al.)) More recently, in U.S. Patent No.
6,177,544, a
modified collagen for ophthalmic use was disclosed, which was reported to lose
viscosity
upon denaturation to facilitate removal. However, no commercial embodiments of
such
3
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
material are believed to be available. It is also known that carrageenans can
be tailored
to adjust their viscosity transitions to different temperature ranges. (See,
Verschueren et
al. "Evaluation of various carrageenans as ophthalmic viscolysers" STP Plaarma
Sci;
volume 6, pages 203-210 (1996), and Picullel et al., "Gelling Carrageenans,"
Food
Polysaccharides arid Their Applications, Ed: Stephen, A.M., Marcel Dekker:New
York,
volume 67, pages 204-44 (1995).) Finally, gellan gum (Gelrite~) is known to
form a
gel on contact with specifications. Greaves et al., "Scintigraphic Assessment
of an
Ophthalmic Gelling Vehicle in Man and Rabbit," Curr. Eye Res., volume 9, page
415
(1990). Gellan systems have been suggested for use as a vehicle for ophthalmic
,o medications (Rozier et al., "Gelrite: A Novel, Ion-Activated, IrZ Situ
Gelling Polymer for
Ophthalmic Vehicles. Effect on Bioavailability of Timolol," Iht. J. Plaa~m.,
volume 57,
page 163 (199)), and one gellan system is currently being marketed with
timolol, a beta
blocker, as a glaucoma medication.
The use of a non-collagen based transitional viscosity viscoelastic agent as
an
,5 effective surgical tool, however, especially in ophthalmic surgery, has
neither been
disclosed or suggested in the art. To be most effective for use as an
ophthalmic surgical
tool, the agent, in addition to having the desired initial and transitional
viscosities over
the prescribed temperature range, would preferably meet the following
requirements:
physiologically acceptable osmolality and pH; relatively short viscosity
transition time;
zo clear (without turbidity); biocompatible; and sterilizable. The
transitional viscoelastics
of the present invention are believed to satisfy these requirements.
SUMMARY OF THE INVENTION
25 The present invention is directed to improved viscous or viscoelastic
agents for
use in surgical procedures, especially ophthalmic surgical procedures. More
specifically,
the present invention is directed to any such agent with the desired initial
viscosity that
yields an acceptable IOP spike profile in the IOP Spike Model described below.
The
improved agents of the present invention include transitional viscous or
viscoelastic
so polymeric agents suitable for use in ophthalmic surgery. As used herein,
the term
"transitional viscoelastic" means such an agent which maintains high viscosity
during the
surgical procedure, but rapidly loses viscosity after the close of surgery so
as to reduce or
4
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
avoid the occurrence of dangerous IOP spikes, and to reduce or obviate the
need for
active removal of the viscoelastic at the end of the surgical procedure.
Appreciating that the surface temperature of the eye tissues during surgery
will
approximate room temperature, or about 25°C or less, we have discovered
agents that
will maintain suitable viscosity at that temperature, but will rapidly lose
viscosity at a
slightly higher temperature (i.e., body temperature, approximately 37
°C). The loss of
viscosity, which occurs without the exogenous addition of a degradation agent,
results
primarily from the warming of the eye back to body temperature after the
surgery is
complete.
,o The stability of the transitional viscoelastics of the present invention -
is a
particularly important feature of the present invention. If the agent
undergoes substantial
hydrolysis, oxidation or other degradation prior to use, the agent may lose
its viscous
properties and yield a non-useful or less useful viscoelastic. The preferred
transitional
viscoelastic compositions of the present invention are substantially stable,
exhibiting less
,s than 1 % degradation for up to six months at storage temperatures. These
compositions
will yield little or no IOP spike (as defined below) when used and allowed to
remain in
the eye after routine cataract surgery.
Substances suitable for use as transitional viscoelastics include, without
limitation, hydrophobically modified polysaccharides or mucopolysaccharides
such as
2o hyaluronic acid and its salts (HA) (with or without surfactants); dialyzed
polyampholytes
or dialyzed mixtures of oppositely charged polyelectrolytes; polysaccharides
or
mucopolysaccharides such as HA with cationic hydrophilic polymers;
polysaccharides
and hydrophilic synthetic polymers with temperature dependent conformational
transitions; and combinations thereof. Preferred are hydrophobically modified
~s polysaccharides or mucopolysaccharides. Most preferred are hydrophobically
modified
HAs, especially HA-amides.
DETAILED DESCRIPTION OF THE DRAWINGS
so FIG. 1 is a graph depicting viscosity as a function of concentration for
dodecylamide HA of the present invention and control HA.
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
FIG. 2 is a graph depicting viscosity as a function of shear rate for
octylamide
HA of the present invention.
FIG. 3 is a graph depicting the transitional viscosity of octylamide HA of the
present invention.
FIG. 4 is a graph depicting the viscosity stability of autoclaved dodecylamide
HA
of the present invention.
FIG. 5 is a graph depicting the stability of the transitional behavior of
hexadecylamide HA of the present invention.
FIG. 6 is a graph depicting the rate of hydrolysis of esterified HAs of the
present
,o invention.
FIG. 7 is a diagrammatic representation of the IOP Spike Model of the present
invention.
FIG. 8 is an exploded elevation of a perfusion apparatus of the present
invention.
FIG. 9a is a top plan view of an eye for use in a perfusion apparatus.
15 FIG. 9b is a side view of the eye of FIG. 9a.
FIG. 10 is a cross sectional view of the perfusion apparatus of the present
invention FIG. 8 with the inclusion of an anterior segment.
FIG. 11 is a graph depicting the effects of traditional viscoelastics and a
transitional viscoelastic of the present invention on IOP using the IOP Spike
Model of
zo the present invention.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
The present invention is directed to viscoelastic materials, and especially to
transitional viscoelastic materials, compositions and methods of use. The
primary use of
25 the transitional viscoelastics is in surgical applications where the
transitional viscoelastic
is applied during surgery in its more viscous state and, following surgery,
loses
substantial viscosity ira situ. A preferred use of the transitional
viscoelastics is in cataract
surgery, where the viscoelastic is instilled i) in the anterior chamber of the
eye to
maintain the dome and protect the exposed tissues; and/or ii) in the posterior
chamber to
3o inflate the capsular bag. Following surgery, the viscoelastic remaining in
the eye is
heated by the body to ambient body temperature, loses its viscosity, and is
more readily
removed (than non-transitional viscoelastics) by the eye's processes. The
major
6
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
advantage of this preferred use is the avoidance of the IOP spike that may
occur with
other systems. Thus, another advantage of this use is that it allows the
surgeon the
traditional advantages of a viscoelastic without the disadvantage of having to
thoroughly
aspirate the viscoelastic out of the surgical site following completion of the
surgery. As
stated above, such aspiration is time consuming and presents additional risk
to the
patient. .
The transitional viscoelastics of the present invention typically exhibit a
viscosity
loss of 70% or more, without substantial hydrolysis, when such materials
undergo a
temperature change of from about room temperature or surgical temperature
,o (approximately 17-26°C) to about body temperature (approximately 35-
38°C).
As stated above, the preferred transitional viscoelastics of the present
invention
will be substantially stable. As used herein, "substantially stable" refers to
viscoelastics
that only lose 1% or less of their hydrophobic side chains by hydrolysis,
oxidation or
other degradation, when such viscoelastics are stored refrigeration
temperatures of
,s approximately 4°C for up to 6 months.
The transitional property of the present invention viscoelastics is preferably
reversible. The reversible viscosity property of the preferred embodiments
allows the
transitional viscoelastics to be heated prior to use, e.g., heat
sterilization, and then
recooled for surgical application.
2o Additional preferred properties of the transitional viscoelastics of the
present
invention include: (1) a transition time of less than about two hours post
surgery; (2)
optically~clear gels with little or no turbidity; (3) safe adherence to ocular
tissue (i.e.,
capability of providing a protective coating to delicate tissues); and (4)
biocompatibility.
As stated above, when used in cataract surgical procedures, the most important
~s feature of the transitional viscoelastics of the present invention is that
they will cause
little or no IOP spike following such surgery. For purposes of the present
invention, a
transitional viscoelastic material will be deemed to exhibit "little or no IOP
spike" if 0.5
ml of a 10% solution (i.e., the actual product composition diluted to 10% of
its original
concentration with a buffered isotonic salt solution) yields an IOP spike
which is not
3o more than an average of about 10 mm Hg above the baseline IOP in a
validated IOP
spike model (the "IOP Spike Model") as described below.
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
While bound by no theories, we postulate that the transitional viscoelastic
character of the compositions of the present invention may be attributable to
physical
associations between relatively low molecular weight molecules resulting in a
viscosity
beyond what would be expected from such low molecular weight molecules at a
given
concentration. Typically, the transitional viscoelastics of the present
invention are
modified viscoelastics wherein hydrophobic side chains have been covalently
linked to
the viscoelastic compounds. The unmodified viscoelastics may be substituted in
varying
degrees with various moieties to yield transitional viscoelastics of the
present invention.
For example, all of the appropriate side chains (e.g., for esters and amide
transitional
,° viscoelastics described further below, the carboxylate side chains)
of the viscoelastics
could be substituted (i.e., 100% substitution) or only a fraction of the side
chains, e.g.,
15% substitution. In general, transitional viscoe~lastics will be derived from
known
viscoelastics and modified to exhibit the properties discussed above. Examples
of
commercially available viscoelastics useful in the preparation of transitional
viscoelastics
15 include salts of hyaluronic acid, (e.g., sodium hyaluronate (HA)),
chondroitin sulfate
(CS) and hydroxypropylmethylcellulose (HPMC) and a combination of HA and CS.
Other viscoelastics useful in preparing transitional viscoelastics include
dialyzed
polyampholytes, such as, carboxymethylcellulose.
The transitional viscoelastics may be composed of viscoelastic polymers of
2o varying molecular weight. Generally, the average molecular weight of the
non-modified
polymer will range from 50,000 to 1,000,000 daltons. For HA-based transitional
viscoelastics, the average molecular weight of the non-modified HA polymer
backbone
will preferably range from about 120,000 to about 400,000 daltons (weight
average
molecular weight) and from about 50,000 to about 350,000 daltons (number
average
~5 molecular weight). These preferred non-modified HAs at conventional
concentrations
will exhibit insufficient viscosity for the preferred surgical purposes. The
molecular
weight of viscoelastics may be estimated by the method of gel permeation
chromatography (GPC), also referred to as size exclusion chromatography (SEC),
with
detection by light scattering or against standards of known molecular weight
using
so refractive index detection. Molecular weight typically affects the degree
of viscosity of
these known viscoelastics. All of the molecular weights pertaining to the
transitional
viscoelastics described herein, unless otherwise noted, are the number average
molecular
s
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
weights of the unmodified viscoelastic polymer prior to modification to yield
a
transitional viscoelastic.
The HAs (free acid and salt form) may be modified to exhibit the above
properties and hence be useful as transitional viscoelastics of the present
invention. For
example, dodecyl moieties may be covalently linked to the backbone carboxylic
acid
groups of the HAs to form dodecyl esters thereof. As used herein, HAs modified
by
esterification of their side chains with various moieties are referred to as
"HA-esters."
Examples of esters that may be substituted on the carboxylate groups of HA
include, but
are not limited to, alkyl groups such as methyl, ethyl, propyl, isopropyl,
butyl, sec-butyl,
,o tert-butyl, pentyl, neopentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl,
dodecyl,
tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyh octadecyl, nonadecyl,
or any
other alkyl groups containing up to 30 carbons; cycloalkyl groups, e.g.
cyclohexyl; and
aryl groups, e.g. phenyl; and any isomers of the foregoing groups. Such
substituents
may optionally be further substituted and may optionally contain hetero atoms
selected
from the group consisting of O, N and S. Such HA-esters are available from
Fidia
Advanced Biopolymers (Abano Terme, Italy), or may also be synthesized by
methods
known in the art, e.g., U.S. Patent Nos. 5,466,461; 5,616,568; and 5,652,347;
the
contents of which are by this reference incorporated herein. The degree and
type of such
substitution will affect the low-shear or apparent viscosity and low-shear
cohesion, as
zo well as the elevated temperature viscosity and cohesion. Preferred are
hydrophobic
substituents.
Since there are several factors influencing the Theological properties of
compositions of the present invention (e.g., type and degree of substitution
and average
molecular weight and concentration of the viscoelastic polymer (unmodified),
various
zs compositions of varying parameters may yield similar Theological
properties. For
example, a 0.88% w/v solution of a 200 kDal HA that is 14% substituted with
dodecylated carboxyl groups, a 1.2% w/v solution of a 200 kDal HA that is 11
substituted.with dodecylated carboxyl groups, and a 2.55% w/v solution of a
200 kDal
HA that is 4% substituted with hexadecylated carboxyl groups all display
similar
3o viscosity and transitional behaviors. The transitional viscoelastics of the
present
invention may thus be characterized by a "Viscosity Factor," which is
determined by the
9
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
following formula:
concentration x molecular weight x percent - Viscosity
(w/v%) (unmodified substitution Factor
polymer in
kilodaltons)
Transitional viscoelastics of the present invention will have Viscosity
Factors ranging
from about 200 to about 50,000. Preferred transitional viscoelastics of the
present
invention will have Viscosity Factors ranging from about 1000 to about 20,000.
Most
preferred are those transitional viscoelastics having Viscosity Factors from
about 2000 to
about 10,000.
,o Just as one skilled in the art will appreciate that compositions of varying
parameters may yield similar rheological properties (see preceding paragraph),
it will
also be appreciated that because of the interplay of the parameters,
compositions with the
same or similar Viscosity Factor may have significantly different rheological
properties.
The Viscosity Factor is only a general indicator of the suitability of a
viscoelastic
composition for the presently contemplated purposes. Those skilled in the art
will
further appreciate that by modifying one or more of the parameters, optimal
rheological
properties for a given purpose may be achieved.
Preferred modified hyaluronates include the partial amide modification of the
carboxylate groups of HA with alkyl or aryl groups to form alkyl or aryl
amides of HA.
zo As used herein, such molecules are referred to as "HA-amides." Examples of
amides
that may be substituted on the carboxylate groups of HA include, but are not
limited to,
alkyl groups such as methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-
butyl, pentyl,
neopentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, tridecyl,
tetradecyl,
pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, or any other alkyl
groups
zs containing up to 30 carbons; cycloalkyl groups, e.g. cyclohexyl; and aryl
groups, e.g.
phenyl; and any isomers of the foregoing groups. Such substituents may
optionally be
further substituted and may optionally contain hetero selected from the group
consisting
of O, N and S. The most preferred amide substituting group is dodecyl.
to
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
The degree of substitution may also vary. In general, the substitution will be
from about 2 to 60%. The preferred substitution level will be from about 5 to
40%.
Preferred amide substituting groups are octyl, dodecyl, and hexadecyl; dodecyl
being the
most preferred. For the octylamide HAs, the preferred variables are:
substitution level
of 30 to 40%; polymeric concentration of 1 to 3% by weight; and molecular
weight of
the unmodified HA of 200 to 350 kilodaltons ("kDal") (weight average) or 120
to 230
kDal (number average). For the dodecylamide HAs, the preferred variables will
be:
substitution level of 5 to 32%, more preferably 15 to 25%, and most preferably
10-20%;
polymeric concentration of 0.35 to 1.2% by weight; and molecular weight of the
,o unmodified HA of 200 to 350 kDal (weight average) or 120 to 230 kDal
(number
average). Alternatively, a lower molecular weight unmodified HA may be used.
Preferred parameters for such lower molecular weight transitional viscoelastic
material
would be: unmodified HA with a molecular weight of 50 to 150 kDal (weight
average),
amide (preferably dodecyl) substitution level of 25 to 40%, and a polymeric
concentration of 0.5 to 2% (wt./v.). For the hexadecylamide HAs, the preferred
variable
will be: substitution level of 5 to 15%; polymeric concentration of 0.3 to
0:~% by weight;
and molecular weight of the unmodified HA of 200 to 350 kDal (weight average)
or 120
to 230 kDal (number average). Substitution levels may be determined by NMR as
described in Example 11. In most instances, the substitution levels specified
in the
zo examples herein were provided by the supplier of the HA-amides, Fidia
Advanced
Biopolyrners.
The transitional viscoelastic compositions of the present invention will
generally
have sufficient zero shear viscosity to be useful in viscosurgical procedures.
Typically
such zero shear viscosities will be at least 1 Pa-s at 25°C. Preferred
are compositions
as exhibiting zero shear viscosities from about 5 to 10,000 Pa-s at
25°C. Most preferred are
those exhibiting zero shear viscosities from about 40 to 1000 Pa-s at
25°C.
The HA-amides may be obtained commercially from Fidia Advanced
Biopolymers (Abano Terme, Italy), may be synthesized by methods described by
Danishefsky and Siskovic in "Conversion of Carboxyl Groups of
Mucopolysaccharides
so in Amides of Amino Acid Esters," Carbohydrate Res. Volume 16, pages 199-205
(1971), Bulpitt and Aeschlimann "New strategy for chemical modification of
hyaluronic
acid: Preparation of functionalized derivatives and their use in the formation
of novel
11
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
biocompatible hydrogels," Biomed. Mater. Res. Volume 47, pages 152-169 (1999),
or
may be synthesized by other methods. WO 00/01733 (Bellini et al.), which
discloses
amides of HA and a process for their preparation, is by this reference
incorporated
herein. This reference generally discloses the use of such amides as vehicles
for drug
delivery for use in viscoelastic surgery or in ophthalinic surgery, but does
not disclose or
suggest the novel compositions and methods of the present invention.
Other transitional viscoelastics of the present invention include modified HAs
wherein the hydrophobic group is linked to the HA structure through the
hydroxyl
moieties, the N-acetamide moieties, or the carboxyl groups, and have been
converted to
,o form hydrophobic amine ("HA-amines"), ether ("HA-ethers"), thioether ("HA-
thioethers") and alkyl ("HA-alkyls") side chains. Examples of such
transitional
viscoelastics include HA alkyl ethers, HA alkylamines, HA alkyl thioethers, HA
alkylcarbamates HA alkylthiocarbamates, HA alkylthioureas, and HA alkylureas,
in
which.the alkyl group can be methyl, ethyl, propyl, isopropyl, butyl, sec-
butyl, tert-butyl,
,5 pentyl, neopentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl,
tridecyl,
tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, or any ~
other alkyl
group containing up to 30 carbons; and any isomer of the alkyl group,
including
cycloallcyl and aryl isomers. Such transitional viscoelastics may, for
example, ~be
prepared by methods disclosed in March, Advanced Organic Chemistry -
Reactions,
zo Mechanisms, and Structure, John Wiley & Sons: New York, 4th Edition, 1992.
Chondroitin sulfate (CS) of varying molecular weights may be modified
similarly
to the HAs, described above, in order to yield transitional viscoelastics of
the present
invention. For example, the carboxylate groups may be amidated in analgous
fashion as
described above with HA. Additionally, the hydroxyl or N-acetamide moieties of
25 chondroitin sulfates may be converted into hydrophobic amines, ethers,
thioethers,
carbamates, thiocarbamates, ureas, and thioureas in the same manner as
described above
fox HA using the same alkyl, cycloalkyl and aryl substituents in order to
yield
transitional viscoelastics of the present invention. Examples of such
transitional
viscoelastics include CS alkyl ethers, CS alkylamines, CS alkyl thioethers, CS
3o alkylcarbamates, CS alkylthiocarbamates, CS alkylthioureas, and CS
alkylureas, in
which the alkyl group can be methyl, ethyl, propyl, isopropyl, butyl, sec-
butyl, tert-butyl,
pentyl, neopentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl,
tridecyl,
12
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, or any
other alkyl
group containing up to 30 carbons; and any structural isomers of such group,
including
cycloalkyl and aryl isomers. Such transitional viscoelastics may be prepared
by methods
disclosed in March, Advanced Organic Chemistry - Reactions, Mechanisms, and
s Structure, John Wiley & Sons: New York, 4th Edition, 1992.
The following Examples 1-6 are examples of preferred compositions of the
present invention:
Example 1
Ingredient Amount (% w/w)
10% dodecylamide substituted HA (200 kDal)0.45
sodium salt
Dibasic Sodium Phosphate (Anhydrous) 0.056
Monobasic Sodium Phosphate (Monohydrate) 0.004 a
Sodium Chloride 0.84
Hydrochloric Acid / Sodium Hydroxide QS pH to 7.4
Water QS
Example 2
Ingredient Amount (% w/w)
10% substituted dodecylamide-HA 0.8
(200 kDal)
or 8% substituted hexadecylamide-HA
(200
kDal)
Dibasic Sodium Phosphate (Anhydrous)0.056
Monobasic Sodium Phosphate (Monohydrate)0.004
Sodium Chloride 0.84
Hydrochloric Acid / Sodium HydroxideQS to pH to 7.4
Water QS
13
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Examine 3
Ingredient Amount (% w/w)
10% substituted dodecylamide-HA, 0.2, 0.4, 0.6,
sodium 0.8 or
salt (200 kDal) 1.0%
Dibasic Sodium Phosphate (Anhydrous)0.056
Monobasic Sodium Phosphate (Monohydrate)0.004
Sodium Chloride 0.84
Hydrochloric Acid / Sodium Hydroxideto adjust pH to
7.4
Water ~ QS
Example 4
Ingredient Amount (% w/w)
32% substituted Octylamide-HA, 1.0
sodium salt
(200 kDal)
Dibasic Sodium Phosphate (Anhydrous)0.056
Monobasic Sodium Phosphate (Monohydrate)0.004
Sodium Chloride 0.84
Hydrochloric Acid / Sodium HydroxideQS to pH to 7.4
Water QS
14
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Example 5
In redient Amount (% wlw)
10% substituted dodecylamide-HA, 1.0
sodium
salt (200 kDal)
Dibasic Sodium Phos hate (Anhydrous)0.056
Monobasic Sodium Phosphate (Monohydrate)0.004
Sodium Chloride 0.84
Hydrochloric Acid / Sodium HydroxideQS to pH 7.4
Water QS
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Example 6
Ingredient Amount (% w/w)
10% dodecylamide substituted HA sodium 0.33, 0.37, 0.40,
salt 0.44, 0.5,
0.6 0.7 or 1
Dibasic Sodium Phosphate (Anhydrous) 0.2
Monobasic Sodium Phosphate (Monohydrate) 0.045
Sodium Chloride 0.7
Hydrochloric Acid / Sodium Hydroxide QS pH to 7.4
Water QS
The following Examples 7-18 illustrate the rheological properties of
compositions of the present invention.
Example 7
The rheological properties of HA-amide compositions of the present invention
were compared with an analogous non-transitional HA composition through
several
concentrations. The 10% substituted dodecylamide-HA formulations of Example 3
(0.2%, 0.4%, 0.6%, 0.8% and 1.0% w/v) and control compositions containing the
,s precursor non-modified HA at 0.2, 0.4, 0.6, 0.8 and 1.0%, were prepared to
the according
to the following procedure. Containers containing the various formulations
were capped
and heated for 4 days at 50°C, swirling occasionally to ensure that all
the dodecylamide-
HA or HA powder was immersed in the buffered liquid and that complete
dissolution
occurred. Each solution was then transferred to separate Scc syringes, capped,
and
zo centrifuged at 2500 RPM to remove bubbles. The loaded syringes were then
each
configured with an empty Scc syringe via a Dual-hub assembly. The syringes
were
cooled for one hour and mixed using 50 passes on their respective assemblies.
16
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
In order to perform rheological analyses, samples were aspirated onto a Bohlin
CS-10 (Constant Stress Rheometer) sample plate through 27 gauge needles, with
the
instrument set to viscometry mode. Sample viscosities were measured versus
shear rate,
using a shear stress of 1.09 to 52.89 Pa at 16°C. As close to a full
shear-rate sweep as
was feasible was used, taking into consideration possible sample loss due to
centripetal
forces at the high shear rate end. For each sample, the transitional behavior
(viscosity
versus temperature) was then characterized, using a temperature range of
10°C to 50°C
and a heating rate of 2.5°C/min. Each sample was processed using
identical parameters.
The summary results are contained in Table 1.
Table l:
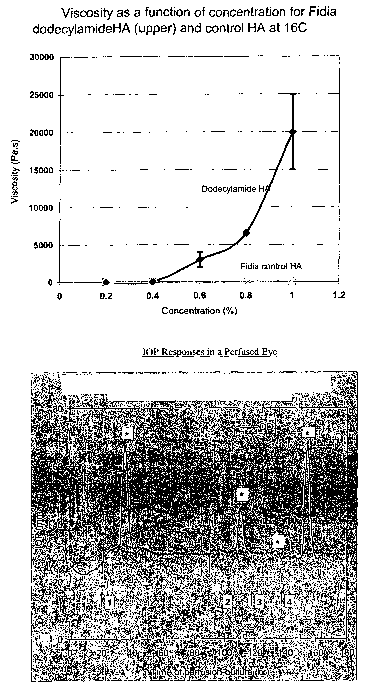
Viscosity of 10% Substituted Dodecylamide-HA versus control HA at
16°C
Concentration (% wlw)Viscosity at 1.36
Pa Shear, Pa-s
Control HA 10% Dodecylamide of
HA
0.2 0.0209 0.0300
0.4 0.0229 49.2
0.6 0.0262 2960
0.8 0.0300 6580
1.0 0.0330 19,900
As shown in Table 1, the dodecylamide-HA compositions were much more
viscous than the unmodified HA compositions. Also, an increase in the low
shear
viscosity through about 5 orders of magnitude was correlated with increasing
dodecylamide-HA concentration (i.e., from 0.2% to 1.0% w/v).
Example 8
zo
The composition of Example 4 was rheologically evaluated. The composition
was prepared in a method similar to that disclosed in Example 6.
This sample was processed Theologically using the Bohlin CS-10 controlled
stress rheometer for viscosity versus shear-rate using the same procedures as
described in
1~
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Example 6, with a shear stress range of 0.41 to 32.58 Pa. The results are
contained in
Table 2.
Table 2:
s Viscosity versus Shear Stress at 16°C for 32% substituted Octylamide-
HA
Shear Stress (Pa) Viscosi (Pa-s)
0.416 995
0.676 961
1.09 670
1.78 342
2.89 166
4.69 79.0
7.61 27.0
12.36 7.88
20.07 2.12
32.58 0.65
As shown in Table 2, the 1% solution demonstrated a low shear viscosity of
about 1000 Pa-s.
,° In an analogous experiment, the viscosity of the composition was
tested through a
temperature range of 17°C to 37°C, with a shear stress of 1.781
Pa. The results are
reported in Table 3.
is
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Table 3:
Viscosity versus Temperature for 1
solution of 32% substituted Octylamide-HA
Tem erature (C) Viscosi (Pa-s)
17 261
22 155
27 97.0
32 47.3
37 29.5
As shown in Table 3, and illustrated in Figure 3, the transitional viscosity
loss
behavior of this material correlates linearly with increasing temperature. The
total
viscosity loss over the temperature range was about 88%.
,o Example 9
The storage stability of preferred compositions of the present invention, as a
measure of retained viscosity, was observed in the following experiment. The
dodecylamide-HA of Example 2 was incubated at 4°C and room temperature
("RT," i.e.,
,5 21-23°C) through 5.5 months. At a given time point, an aliquot of
each composition was
taken and rheological analysis (the shear stress range for this example was
from 0.16 to
52.89 Pa.) of the sample was performed. The results are listed in Table 4 and
illustrated
in Figure 4.
19
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Table 4:
Viscosity Stability of 0.8% solution of 10% substituted Dodecylamide-HA
Time (months) at RT Viscosity at 16C and with a 7.61
Pa
Shear Stress (Pa-s)
0 30.0
1 14.2
2 18.1
5.5 18.1
s
As shown in Table 4, dodecylamide-HA compositions experienced an initial shift
of 16 Pa-s at low shear conditions in viscosity between the zero time control
and 1 month
incubation time point. However, the viscosity was stable from the 1 month
incubation
time point to the 5.5 month incubation time point.
,o The transitional stability of hexadecylamide-HA compositions stored at
4°C
through 5.5 months was also tested. The results are disclosed in Table 5.
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Table 5:
Viscosity versus Temperature Stability Analysis of 8% substituted
Hexadecylamide-HA Stored in Phosphate Suffered Saline at 4°C
Temperature (C) Viscosity at
Shear Stress
of 2.89 Pa (Pa-s)
Storage Time, Storage Time, Storage Time,
0 months 2 months 5.5 months
25 36.2 55.5 59.8
28 25.3 39.8 42.6
31 17.3 28.4 29.7
34 12.6 20.3 21.3
37 8.8 14.0 14.8
As shown in Table 5 and Figure 5, the viscosity of all compositions
transitioned
through the temperature change from 25°C to 37°C, losing between
70% and 80% of
their viscosity at a shear stress of 2.89 Pa. This compares to a viscosity
loss of only
about 35% for hyaluronic acid solutions of similar starting viscosities.
Example 10
The stability of a dodecylamide-HA composition of the present invention was
observed with the following experiment. The 1% w/v dodecylamide-HA composition
of
,5 Example 7 was incubated at 4°C, room temperature (21-23°C)
and 37°C through 6
months. At the appropriate time, an aliquot of the composition was taken and
the
chemical stability of the dodecylamide-HA was analyzed using capillary gas
chromatography (GC). GC was performed on the Hewlett Packard
21
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
5890A GC system equipped with a flame ionization detector (FID), using the
following
parameters:
GC Parameter Value
Injection port temperature 250C
Initial oven temperature 60C
Oven temperature program 60C to 300C
at 10/min.
Hold at 300C
for 5 min.
Helium, flow rates Split 100 mL min
Septum purge 0.3 mL/min
Column 1.2 mL/min
Make up (FID) 30 mL/min
~
Compressed air (FID) 395 mL/min
Hydrogen (FID) 23.5 mL/min
s The column used was a DBS fused silica capillary column (30 meters in length
with an inner diameter of 0.32 mm and a film thickness of 1.0 mm) from J&W
Scientific;
(Folsom, CA).
After a given incubation time, samples were mixed with one weight equivalent
of
a mixture of two parts ethyl acetate to one part ethanol (also by weight) and
incubated at
,0 50°C for one hour. To this mixture was added three more parts by
weight of the ethyl
acetate-ethanol mixture. This second addition caused precipitation of the
polysaccharide,
which is centrifuged down, and the supernatant was analyzed by GC for the
presence of
the breakdown hydrophobic group, dodecylamine. If 100% hydrolysis of the side
chains
of the dodecylamine-HA occurred, full precipitation of the 10% substituted
,s dodecylamine-HA, would result in 42 ppm dodecylamine in the supernatant.
The test
results showed that less than 1 ppm of dodecylamine was present in the
supernatants of
the processed composition samples, through the various temperature and time
incubations. These results indicated that the amide linkage of the
transitional
viscoelastic was very stable in the buffered composition.
22
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Examule 11
NMR Analysis for Determination of Hydrophobic Group Substitution Level of
Hydrophobically-modified Hyaluronic Acid (HM-HA) Compounds
Into 4 mL glass vials, was added 3-5 mg of a HM-HA material. Into the same
vial was
added 0.8 mL of water and the vial then agitated on a vortex mixer for 5 to 10
seconds.
The sample vial was placed into an oven set at 50°C and heated
overnight (15-20 hours)
to dissolve. The next day a hyaluronate lyase (600-900 units/sealed ampule,
Cat.
No. H-1136, Sigma Chemical Co.) enzyme solution was prepared by snapping open
the
sealed ampule and adding 0.8 mL of water to the ampule containing
approximately 900
,o units of enzyme (1 unit/uL). To the vial was then added 100 uL (0.1 mL) of
the enzyme
solution. The vial was capped and placed into a 37 C oven overnight (1S-20
hrs).
The next day, the vial was removed from the oven and 100 uL (0.1 mL) of
deuterium
oxide (99.6% atom-% D, Cat. No. 42,345-9 Aldrich Chemical Co.) was added to
the vial.
r
After mixing, the solution was transferred into a NMR tube using a glass
disposable
transfer pipette. The sample was then analyzed to obtain a proton NMR spectrum
on a
600 MHz Bruker NMR instrument capable of operation in a moisture suppression
mode
with accurate integration of peak signals.
Using the NMR spectrum of the enzyme-treated HM-HA sample, the hydrophobic
substitution level was calculated from integration values by adding together
the integral
values for the 3 signals indicative of the hydrophobic residue from 0.8 to 1.3
ppm versus
the 2 to 4 signals at 2.0-2.1 ppm for the N-acetylmethyl group.
The three signals between 0.8-1.3 ppm originate from the hydrogen atoms bonded
to CZ
to Cn carbons in the hydrophobic group, which contains n carbon atoms. The
hyaluronate lyase enzyme has no interfering signals in the hydrophobic group
region or
the N-acetylmethyl signal region. Since the N-acetylmethyl group is on every
repeat unit
23
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
in the HA structure, the hydrophobic substitution level may be calculated from
the ratio
of integral values for the hydrophobic group to that of the N-acetylmethyl
group.
Therefore, the calculation of the degree of substitution of a HM-HA substance
with a
straight chain normal alkyl group [-(CHZ)p_1CH3] as its hydrophobic
substitutent with n
carbons can be given by the equation:
[ Integrals 0.8-l.Sppm/(2(n-1)+1) ]
hydrophobic substitution = X 100
,o . [ Integrals 2.0-2.1 ppm / 3]
24
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Example 12
The following example demonstrates the lesser stability of less preferred
s viscoelastic agents of the present invention. Compositions analogous to
those of
Examples 1-6, wherein the viscoelastic agent is replaced with either a 15%
dodecyl ester-
HA, sodium salt (approx. 200 kDa) or 43% or 52% benzyl ester-HA, sodium salt
(approx. 200 kDa final, modified viscoelastic) were prepared in similar manner
to the
method disclosed in Example 7. The compositions were incubated at 4°C,
RT and 37°C
,o through 9.5 weeks. The dodecyl or benzyl alcohol (the breakdown products of
the
respective hydrophobic ester side chains) was quantified using the GC method
of
Example 10.
After a given incubation time, samples were mixed with four volumes of
acetone,
which caused precipitation of the polysaccharide. The precipitated
polysaccharide was
,5 then centrifuged down and the supernatant was analyzed by GC for the
presence of the
appropriate alcohol.
Full hydrolysis of the dodecyl or benzyl ester-HA side chains would result in
70
ppm dodecyl alcohol or 400 ppm benzyl alcohol in the supernatant,
respectively. The
results are disclosed in Table 6.
zo
Table 6:
Percent Hydrolysis of HA-Esters Stored in Phosphate Buffered Saline at
4°C
Composition Storage Time % Hydrolysis
at
4C
52% benzyl ester-HA3.5 weeks 1.8
43% benzyl ester-HA9 weeks 5.0
43% benzyl ester-HA9.5 weeks 12.8
15% dodecyl ester-HA6 weeks 6.8
15% dodecyl ester-HA9.5 weeks 12.1
As illustrated above, hydrolysis of the comparative modified viscoelastics was
zs greater than 1 % through various time points. Because it is desirable for
viscoelastic
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
compositions to have storage stability (i.e., viscoelastic products typically
require a two
year shelf life expiration date), viscoelastics exhibiting the above described
rates of
hydrolysis are considered to be less useful in compositions of the present
invention.
s Example 13
A 3% solution of benzyl ester of HA at 50% carboxylic acid substitution
(approximately 200 kDa) was prepared in phosphate buffer with sodium chloride
and in
citrate/acetate buffer with balanced salts. These solutions formed optically
clear,
viscoelastic gels which were easily aspirated through a 27 gauge needle. These
solutions
,o had low shear viscosities comparable to Viscoat~ or Provisc at 25°C
(k.e.,
approximately 200 Pa-s) and were shear thinning like Provisc~ or Viscoat~ at
25°C.
These solutions showed a significant drop in viscosity from approximately 200
Pa-s at
surgical temperature (25°C) to 20 Pa-s at body temperature
(37°C).
Example 14
,s A 1% solution of the dodecyl ester of HA at 14.3% carboxylic acid
substitution
(approximately 200 kDa) was prepared in citrate/acetate buffer with balanced
salts to
form a clear, viscoelastic solution. This solution showed a comparable
rheological
profile to Provisc° or Viscoat~. Viscosity at 25°C and at shear
rates below 0.085/s was
approximately 90 Pa-s. Shear thinning began at 0.24/s with a viscosity of 75
Pa-s. At
zo 5.4/s viscosity had dropped to approximatelyl6 Pa-s. At 31 °C, low
shear viscosity was
only about 45 Pa-s. At a constant shear stress of 2.89 Pa, the viscosity of
this
formulation dropped from approximately 100 Pa-s at 25°C to about 25 Pa-
s at 37°C.
Example 15
zs A 0.75% solution of the dodecyl ester of HA at 14.3% carboxylic acid
substitution (approximately 200 kDa) was prepared in citrate/acetate buffer
with
balanced salts to form a clear, viscoelastic solution. This solution showed a
low shear
viscosity of about 25 Pa-s at 25°C and was shear thinning to below 0.1
Pa-s at 534/s.
This formulation also showed a decrease in viscosity at constant shear stress
(1.1 Pa)
3o from about 25 Pa-s at 25°C to about 5 Pa-s at 37°C.
26
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Example 16
A 2% solution of the dodecyl ester of HA at 14.3% carboxylic acid substitution
(approximately 200 kDa) was prepared in citrate/acetate buffer with balanced
salts to
form a clear, thick solution. This solution (approximately 2cc) was autoclaved
at 125°C
for about 20 minutes exposure time and was cooled with slow exhaust. After
cooling to
room temperature, the formulation retained enough viscosity to yield a useful,
viscoelastic gel.
Example 17
,o A 5% solution of the hexadecyl ether of carboxymethylcellulose (containing
hexadecyl moieties ether linked to 5% of the repeating monosaccharide units
and at
approximately 100 kDa) was prepared in phosphate buffers with sodium chloride.
The
solution was clear and qualitatively formed a viscous gel.
,s Example 18
A solution of a 1.0 wt% of 20% dodecylamide of HA, sodium salt with 3%
chondroitin sulfate and was prepared in PBS by slowly dissolving at
50°C for two days.
Another sample containing only 1.0 wt% of 20% dodecylamide of HA, sodium salt
was
likewise prepared in PBS by slowly dissolving at 50°C for two days.
After dissolving
zo the samples were each transferred into separate 10-mL syringes and
subjected to 100
passes through a dual hub connector to another empty syringe to provide
adequate
mixing. The samples were centrifuged to remove air bubbles and aspirated
through a 27-
gauge needle onto the sample plate of a Bohlin CS-10 Constant Stress
Rheometer.
Rheological analysis was performed to obtain plots of viscosity versus shear
rate at 25°C
zs for both samples. Both samples gave apparent viscosity values of
approximately 90 Pa-s
in the low shear plateau region of the viscosity versus shear rate plot.
2~
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Example 19
The following is a description of the IOP Spike Model.
IOP Spike Model:
The IOP Spike Model employs (1) a perfusion apparatus, pump and pressure
transducer/recorder as depicted diagrammatically in Figure 7; (2) perfusion
medium; (3)
dissected human eyes; and (4) the viscoelastic materials) to be tested.
,0 1. Perfusion Medium
The perfusion medium used in the IOP Spike Model is prepared by adding 5 mL
of a penicillin-streptomycin solution (10,000 units/mL penicillin (base) from
penicillin G
and 10,000 ~g/mL streptomycin (base) from streptomycin sulfate) and 0.85 mL of
a
gentamicin solution (10 mg/mL) to 500 mL of a cell culture medium (Dulbecco's
modified Eagle's medium, low glucose, with L-alanyl-L-glutamine and pyruvate
(Life
Technologies, Grand Island, NY)). The perfusion medium is then filtered using
a
500 mL sterile filter unit (0.2 ~m pore size) and stored at 4°C
(brought .to 37°C before
use).
zo 2. Eye Preparation
Cadaver eyes useful in the IOP Spike Model must: i) not be older than 24-36
hours post-mortem when prepared for use in the model; ii) be stored as whole
eyes in
moist chambers; iii) be devoid of HIV, hepatitis or other infectious agents;
and iv) not
have undergone ocular surgeries such as glaucoma filtration, scleral buckle
implantation
zs or IOL implanation. Anterior segment 7 (see Fig. 10) of a human eye is
prepared by the
following dissection method:
The eye is carefully trimmed of excess muscle or connective tissue using
straight,
fine scissors (Katena No. K4-7440), and placed in a container containing a
povidone
iodine solution (1% free iodine) at 25°C for approximately 2 minutes.
The eye is then
3o removed from the iodine solution, rinsed thoroughly with saline solution,
and positioned
such that the cornea 3 is centered on top (see Figure 9a). Refernng to Figures
9a and 9b,
the sclera 5 is then scored (using a sterile ophthalmic crescent knife (Alcon
No. 8065-
940001) with 24 evenly spaced linear cuts 9 extending radially from the limbus
towards
2s
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
the ora serrata (each cut not to exceed 50% depth of the sclera and about 5 mm
length) to
open the episcleral veins and provide an exit route for the perfusion medium.
Refernng
to Figure 9b, the globe is then cut into two halves along the horizontal plane
11
approximately midway between the equatorial plane 13 and the scleral plane 15.
The
anterior (top) half of the eye is separated from the posterior (bottom) half
which is
discarded. The anterior half is turned over so that the cornea is facing down,
and residual
vitreous is then carefully removed from the anterior half using Graefe forceps
(Katena
No. KS-421). The zonules are then cut with Wescott scissors (Katena No. K4-
4100) and
the lens removed from the anterior segment using the Graefe forceps. Dressing
forceps
,o (Katena No.. KS-4010) are then used to remove the iris, and the choroid is
circumferentially cut at the ora seratta with the Wescott scissors. Any
residual pigment
from inside the sclera is then removed with the dressing forceps. Remaining
anterior
segment 7 is then rinsed two times with perfusion medium to wash out pigment,
tissue
remnants, or other debris.
29
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
3. Perfusion Apparatus
The perfusion apparatus used in the IOP Spike Model is a modified version of
that described in the perfusion systems of Johnson and Tschumper and of Clark
et al.
(Johnson and Tschumper, "Human trabecular meshwork organ culture: a new
method,"
s Invest. Ophthalmol. Vis. Sci., 28:945-953 (1987); and Clarke, et al.,
"Dexamethasone-
Induced Ocular Hypertension in Perfusion-Cultured Human Eyes," Invest.
Ophthalmol.
Iris. Sci., 36(2):478-489 (1995)). The critical modifications to the prior
systems are the
reduction of the chamber volume from about 0.8-1.0 mL to about 0.5-0.6 mL to
more
nearly approximate the volume of the pseudophakic human anterior segment
volume,
,o and the inversion of the chamber during perfusion. The volume reduction is
achieved by
a protruding island on the platform of the chamber which reduces the space
within the
dome of the anterior segment and should prevent or reduce stagnation of the
viscoelastic
solution in the posterior dead space, i.e. posterior to the trabecular
meshwork. Turning
the chamber upside down (relative to the prior systems) during perfusion is
believed to
15 prevent stagnation of the viscoelastic from occurnng at the corneal
concavity, as such
viscoelastic should be effectively mixed by the perfusand which comes out of
the
elevated island in the direction of that concavity.
Perfusion apparatus 1 is illustrated in Figs. 7, 8 and 10. Apparatus 1 is
comprised
of base 2, cylinder 4, island 6, o-ring 8, a plurality of screws 10 and cap
12. In use,
2o apparatus 1 also comprises anterior segment 7.
Base 2 is disk-shaped having top 22, bottom 24 and side 26, and containing
channels 14 and 16, platform 18 and threads I9, shaped and sized, to receive
screws 10.
Channel 14 communicates with opening 28 of side 26 and opening 20 of island 6.
Channel 16 communicates with opening 30 of side 26 and opening 32 of platform
18.
~5 Channels 14 and 16 are shaped and sized in order to provide for the precise
flow
(channel 14) and accurate pressure measurement (channel 16) of perfusion
medium, to
and from apparatus 1. Opening 28 is sized and shaped to receive a fitting (to
be
connected to infusion line 29) and opening 30 also is sized and shaped to
receive a fitting
(to be connected to transducer line 31). The fittings are standard connectors
known in
3o the art to be useful for receiving tubing or other cylindrical lines.
Platform 18 protrudes
from base 2, has side 34 and is conically shaped and sized to receive anterior
segment 7.
Island 6 protrudes from the center of platform 18.
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Referring to Figs. 8 and 10, cylinder 4 is coaxially and permanently situated
on
top 22 of base 2, and extends flush therefrom. Island 6 has opening 20,
contains a
portion of channel 14 and extends from platform 18. O-ring 8 has annular,
concave
interior edge 36 and a plurality of holes 38 sized and shaped for receiving
through o-ring
8, screws 10. Edge 36 is sized and shaped such that, when apparatus 1 is put
in use, the
compression of o-ring 8 against base 2 sandwiches the periphery of anterior
segment 7
between edge 36 and side 34 of platform 18, forming anterior chamber 42. As
described
above, the volume of anterior chamber 42 has been designed by the inventors to
approximate the combined volumes of anterior chamber and crystallin lens of a
human
,o eye (generally about 0.5-0.6 mL).
As shown in Figs. 8 and 10, cap 12 is shaped and sized to receive a portion of
cylinder 4 and forming closed space 40. The preferred perfusion apparatus for
the IOP
Spike Model will employ a polysulfone base 2, a polysulfone island 6, a
polysulfone o
ring 8, nylon screws 10, a transparent polysulfone cylinder 4, medical steel
channels 14
and 16, and a transparent polystyrene cap 12.
4. Assembly and Preparation of Perfusion Apparatus:
Prior to use, apparatus 1 is disassembled and the individual parts are
autoclaved
or cold sterilized and then soaked in a laminar flow chamber with a sporicidin
zo disinfecting solution (SOmI/L water) followed by an overnight rinse in
sterile deionized
water.
Refernng to Figs. 7 and 10, perfusion medium is fed to pump, which in turn is
connected to infusion line 29, which will infuse perfusion medium through
channel 14
and into chamber 42; and transducer line 31 is connected to calibrated
pressure
Zs transducer and recorder capable of recording the pressure of chamber 42.
Apparatus 1 is
then reassembled by first connecting fittings disposed at openings 28 and 30
to the
infusion and transducer lines, respectively. Apparatus 1 is then arranged with
top 22
facing up. Anterior segment 7 is placed on platform 18, cornea side up. Slight
perfusion
medium flow is then applied via syringe through channel 16 in order to
properly seat
so segment 7 on platform 18. O-ring 8 (which is approximately 1.5 inches in
diameter,
outer circumference and 0.710-0.736 inches in inner diameter) is then placed
over
segment 7. It is important that o-ring 8 seats well with segment 7 in order to
avoid leaks
31
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
(a slightly different diameter o-ring 8 may be used in order to improve the
seat). Screws
are inserted through holes 38 and into threads 19 and tightened evenly to
ensure that
o-ring 8 is evenly seated. As o-ring 8 is tightened onto the periphery of
segment 7, the
flow applied through channel 16 should be relieved, such that excessive
pressure is not
5 applied to segment 7 upon seating. Screws 10 are torqued against base 2 such
that the
periphery of segment 7 is tightly sandwiched between platform 18 and o-ring 8,
but not
so tightly that segment 7 is ruptured. Using syringes, perfusion medium is
then pushed
through channel 14 while simultaneously pulling perfusion medium out channel
16
through opening 30, slanting base 2 such that any bubbles present will flow
out of
,° chamber 42 via channel 16. After the bubbles have been purged,
channels 14 and 16 are
closed to perfusion medium flow. Apparatus 1 is then returned to level with
top 22
facing up.
Cap 12 is then placed over cylinder 4, thereby forming space 40. Apparatus 1
is
then inverted so that bottom 24 is facing up, and placed in a tissue culture
incubator
,5 (Nuaire) maintaining humidified atmosphere (5%C02 / 95% air) at
37°C. Transducer
line 31 and infusion line 29 should be positioned against the seal of the
incubator door,
so as not to be damaged or crimped when the door is closed. Pressure
transducer should
be kept level with apparatus 1. In this configuration apparatus 1 is now ready
to be used
in the IOP Spike Model.
zo
32
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
5. Initial perfusion:
The perfusion line is opened and the pump operated (setting "6" for Harvard
Model No. 944) so that perfusion medium flows freely through opening 28 and
channel
14. The pump is allowed to run until the pressure rises to about 5-10 mm Hg,
the pump
s speed is then decreased to about 2 ~,L/min (setting "12") thereafter.
Segment 7 is
perfused for up to about 24 hours prior to injection of the viscoelastic
candidate. If a
stable baseline at a pressure of between 10-40 mm Hg is not established within
24 hours,
the flow rate can be adjusted repeatedly for a perfusion volume of 2-3 ml each
time until
a stable baseline IOP is achieved. If the problem is not resolved for another
24 hours,
,o and subsequent flow rate adjustment and flushing steps have not remedied
the problem
within 48 hours, anterior segment 7 should be considered unreliable and the
perfusion
terminated.
6. Viscoelastic injection and perfusion:
15 Once perfused anterior segment 7 has generated a stable baseline IOP
between
10-40 mm Hg for a period of no less than 4 hours (perfusion rate at between
about 1.75-
2.05 ~,L/min) it is ready for IOP spike studies. Typically, such a point is
reached 24
hours after initial perfusion. The Model is first validated with injection of
a "positive".
control. The positive control is 0.5 mL of diluted sodium hyaluronate
(approximatley
zo 750kDa1 available from Lifecore Biomedical, Inc., Chaska, MN), which is
prepared by
diluting one part of 3.5% HA in buffering solution to nine parts of the same
buffering
solution, wherein each 1 mL of the buffering solution contains approximately
0.45 mg
sodium dihydrogen phosphate hydrate, 2.00 mg disodium hydrogen phosphate, 4.3
mg
sodium chloride (with Water For Injection, USP grade, q.s.) and has a neutral
pH. The
25 positive control of 0.5 mL diluted HA (0.35%) is injected into a tubing
loop of similar
volume attached to mufti-valve assembly 33 on perfusion line 29, and mufti-
valve
assembly 33 is switched to permit the complete sample volume to be flushed
into
perfusion apparatus 1. Thus, the rate of injection is determined by the rate
of perfusion.
IOP is continuously monitored and recorded. Any IOP spike above the baseline
IOP is
30 observed and recorded. If the positive control results in an IOP spike of
between 20-80
. mm Hg above baseline within 24 hours of injection, the Model is considered
validated,
and may be used to test candidate transitional viscoelastics.
33
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
Generally, transitional viscoelastic samples may be inj ected in a single
anterior
segment 2 times, at one day intervals (the first injection being that of the
positive
control). Sample viscoelastics should be diluted to one tenth the original
concentration
using the same buffering solution used to prepare the control sample. A stable
and
acceptable baseline IOP should be reached before each new injection, as
indicated by the
decline of any IOP spike generated by a preceding viscoelastic sample. If the
baseline
IOP is not regained within the range of 10-40 mm Hg within one day, any
further
perfusion in a given anterior segment should be discontinued.
As stated above, a transitional viscoelastic of the present invention (i.e.
causing
,o little or no IOP spike) will exhibit an average spike of 10 mm Hg or less
above baseline.
Examule 20
A transitional viscoelastic of the present invention, AL-12488, 43%
substituted
benzyl ester modified HA (approximately 200 kDal) was tested in the IOP Spike
Method
described above and compared to the positive control (Benchmark) and 200 kDal
HA
from Fidia. The results of the study are represented graphically in Figure 11.
The arrows
indicate the various injections. Injections 1 and 4 were 0.35% positive
control HA,
injection 2 was 0.35% Fidia unmodified HA, and injection 3 was 0.35% AL-12488.
All
inj ection samples were 0.5 ml in volume. The asterisks indicate the
individual IOP spikes
zo resulting from the injections. Only the transitional viscoelastic (AL-
12488) can be
characterized as exhibiting little or no IOP spike, as the spike observed
therefor in the
IOP Spike Model is not more than, and in fact is considerably less than, about
10 mm Hg
above the baseline IOP.
Those skilled in the art will appreciate that the suitability of a given
transitional
viscoelastic for a particular step in a surgical procedure will depend upon
such things as
the viscoelastic's concentration, average molecular weight, viscosity,
pseudoplasticity,
elasticity, rigidity, adherence (coatability), cohesiveness, molecular charge,
and
osmolality in solution. The viscoelastic's suitability will depend further on
the
functions) which the viscoelastic is expected to perform and the surgical
technique
so being employed by the surgeon.
34
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
An appropriate buffer system (e.g., sodium phosphate, sodium acetate or sodium
borate) may be added to the compositions to prevent pH drift under storage
conditions.
Because all or a significant portion of the transitional viscoelastics of the
present
invention may be left irz the eye at the close of surgery, these viscoelastics
are uniquely
s adapted to serve the dual roles ofviscosurgical tool and dnzg delivery
device.
Ophthalmic drugs suitable for use in the compositions of the present invention
include, but are not limited to: anti-glaucoma agents, such as beta-blockers
including
timolol, betaxolol, levobetaxolol, carteolol, miotics including pilocarpine,
carbonic
anhydrase inhibitors, prostaglandins, seratonergics, muscarinics, dopaminergic
agonists,
,o adremexgic agonists including apraclonidine and brimonidine; anti-infective
agents
including quinolones such as ciprofloxacin, and aminoglycosides such as
tobramycin and
gentamicin; non-steroidai and steroidal anti-inflammatory agents, such as
suprofen,
diclofenac, ketorolac, rimexolone and tetrahydrocortisol; growth factors, such
as EGF;
immunosuppressant agents; and anti-allergic agents including olopatadine. The
ophthalmic
15 drug may be present in the form of a pharmaceutically acceptable salt, such
as timolol
maleate, brimonidine tartrate or sodium diclofenac. Compositions of the
present invention
may also include combinations of ophthalmic drugs, such as combinations of (l)
a beta-
blocker selected from the group consisting of betaxolol and timolol, and (ii)
a prostaglandin
selected from the group consisting of latanoprost; 15-keto latanoprost;
fluprostenol
2° isopropyl ester (especially IR-[1a(2),2[3(IE,3.R*),3a,5a]-7-[3,5-
dihydroxy 2-[3-hydroxy-
4-[3-(trifluoromethyl)-phenoxy]-1-butenyl]cyclopentyl]-5-heptenoic acid, 1-
methylethyl
ester); and isopropyl [2R(lE,3R),3S(4Z),4R]-7-[tetrahydro-2-[4-(3-
chlorophenoxy)-3-
hydroxy-I -butenyl]-4-hydroxy-3-furanyl]-4-heptenoate.
In the event a pharmaceutical agent is added to the transitional
viscoelastics, such
25 agents rnay have limited solubility in water and therefore may require a
surfactant or
other appropriate co-solvent in the composition. Such co-solvents typically
include:
polyethoxylated castor oils, Polysorbate 20, 60 and 80; PluronicC~? F-68, F-84
and P-I03
(BASF Corp., Parsippany NJ, USA); cyclodextrin; or other agents known to those
skilled
in the art. Such co-solvents are typically employed at a level of from about
0.01 to 2
3° wt.°I°. It may also be desirable to add a
pharmaceutically acceptable dye to the
viscoelastic to improve visualization of the viscoelastic during surgery
and/or to stain
ocular tissue (especially the capsular bag during capsulorhexis in cataract
surgery) for
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
improved visualization of such tissue. The use of such dyes in conventional
viscoelastics
is described in WO 99/58160. Preferred dyes include trypan blue, trypan red,
brilliant
crysyl blue, and indo cyanine green. The concentration of the dye in the
viscoelastic
solution will preferrably be between about 0.001 and 2 wt.%, and most
preferably
between about 0.01 and 0.1 wt%. However, it will be appreciated by those
skilled in the
art that any such additive (pharmaceutical agents, co-solvents, or dyes) may
only be
employed to the extent that they do not detrimentally affect the viscoelastic
properties of
the compositions of the present invention.
The methods of the present invention may also involve the use of various
,o viscoelastic agents having different adherent or cohesive properties. Those
skilled in the
art will recognize that the compositions of the present invention may be
employed by the
skilled surgeon in a variety of surgical procedures.
Given the advantages of each type of viscoelastic, the surgeon may employ
various viscoelastic compositions of the present invention in a single
surgical procedure.
While the use of the transitional viscoelastic of the present invention have
not been
previously disclosed for use in surgeries, U.S. Patent No. 5,273,056
(McLaughlin et al.)
discloses methods which exploit the use of compositions employing
viscoelastics of
varying viscoelastic properties during a given ocular surgery, the entire
contents ~of
which are incorporated herein by reference.
2o For example, for portions of surgical procedures involving
phacoemulsification.
and/or iz-rigation/aspiration, e.g., cataract surgery, it is generally
preferable to use a
viscoelastic agent that possesses relatively greater adherent properties and
relatively
lesser cohesive properties. Such viscoelastic agents are referred to herein as
"adherent"
agents. The cohesiveness of a viscoelastic agent in solution is thought to be
dependent,
zs at least in part, on the average molecular weight of that agent. At a given
concentration,
the greater the molecular weight, the greater the cohesiveness. Those portions
of surgical
procedures involving manipulation of delicate tissue are generally better
served by
viscoelastic agents that possess relatively greater cohesive properties and
relatively lesser
adherent properties. Such agents are referred to herein as "cohesive" agents.
For
3o cohesive agents such as these, which are being employed primarily for
tissue
manipulation or maintenance purposes as opposed to protective purposes, a
functionally
desirable viscosity will be a viscosity sufficient to permit the skilled
surgeon to use such
36
CA 02428066 2003-05-05
WO 02/36096 PCT/USO1/44063
agent as a soft tool to manipulate or support the tissue of concern during the
surgical
steps) being performed.
For other viscoelastic agents, which are being employed primarily for
protective
purposes ("adherent" agents) as opposed to tissue manipulation purposes, a
functionally
s desirable viscosity will be a viscosity sufficient to permit a protective
layer of such agent
to remain on the tissue or cells of concern during the surgical steps) being
performed.
Such viscosity will typically be from about 3,000 cps to about 60,000 cps (at
shear rate
of 2 sec'1 and 25° C), and preferably will be about 40,000 cps. Such
adherent agents are
capable of providing the protective function previously discussed, yet are not
prone to
,o inadvertent removal, which could jeopardize the delicate tissue being
protected.
Unfortunately, this same characteristic makes aspiration of such adherent
viscoelastics at
the end of surgery (as recommended for alb such commercially available
products in
cataract surgery) problematic for surgeons, and may result in the coated
tissues.being
subjected to trauma during the removal procedure. A significant advantage of
the
15 transitional viscoelastics of the present invention is that they may be
left in the surgical
site at the close of surgery thereby avoiding unnecessary trauma to the
affected soft
tissues.
Preferred methods of the present invention will employ the use of multiple
viscoelastics in a given surgical procedure, wherein at least one of such
viscoelastics is a
~o ~ transitional viscoelastic. In a most preferred embodiment of the
invention, a transitional
viscoelastic possessing superior adherent properties is used in cataract
surgery, at the
close of which some or all of the transitional viscoelastic is left in situ
and causes little or
no IOP spike.
The invention has been described by reference to certain preferred
embodiments;
25 however, it should be understood that it may be embodied in other specific
forms or
variations thereof without departing from its spirit or essential
characteristics. The
embodiments described above are therefore considered to be illustrative in all
respects
and not restrictive, the scope of the invention being indicated by the
appended claims
rather than by the foregoing description.
37