Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
TITLE OF THE INVENTION
METHOD FOR PATTERNING METAL USING
NANOPARTICLE CONTAINING PRECURSORS
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to the patterning of a metal feature using a
metal
nanoparticles containing material and exposing it to radiation.
Discussion of the Background
Currently available technology for the micro fabrication of metal patterns
includes:
1) use of masks to define patterns of metal by deposition or etching (Shacham-
Diamand, Y., Inberg, A., Sverdlov, Y. & Croitoru, N., Electroless silver and
silver with
tungsten thin films for microelectronics and microelectromechanical system
applications.
Journal of the Electrochemical Society, 147, 3345-3349 (2000));
2) laser ablation of metal films to create patterns;
3) laser direct writing based on pyrollitic deposition of metal from vapor,
solution or
solid precursors; (Auerbach, A., On Depositing Conductors From Solution With a
Laser,
Journal of the Electrochemical Society, 132, 130-132 (1985); Auerbach, A.,
Optical-
Recording By Reducing a Metal Salt Complexed to a Polymer Host, Applied
Physics Letters,
45, 939, 941 (1984); Auerbach, A., Copper Conductors By Reduction of Copper
(I) Complex
in a Host Polymer, Applied Physics Letters, 47, 669-671 (1985); Auerbach, A.,
Method For
Reducing Metal-Salts Complexed in a Polymer Host With a Laser, Journal of the
Electrochemical Society, 132, 1437-1440 (1985)); and
4) light exposure and development of silver-halide based photographic film
followed
by electroless and electrochemical plating (Madou, M. & Florkey, J., From
batch to
continuous manufacturing of microbiomedical devices. Chemical Reviews, 100,
2679-2691
(2000); M. Madou., Fundaments of Microfabrication (CRC Press, Boca Raton,
1997);
Madou, M., Otagawa, T., Tierney, M. J., Joseph, J. & Oh, S. J., Multilayer
Ionic Devices
Fabricated By Thin-Film and Thick-Film Technologies. Solid State Ionics, 53-6,
47-57
(1992)).
-1-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Currently available methods are described, for example, in the following
publications:
Southward, R. E. et al., Synthesis of surface-metallized polymeric films by in
situ
reduction of (4,4,4-trifluoro-l-(2-thienyl)-1,3-butanedionato) silver(I) in a
polyimide matrix.
Journal of Materials Research, 14, 2897-2904 (1999);
Southward, R. E. & Thompson, D. W. Inverse CVD, A novel synthetic approach to
metallized polymeric films. Advanced Materials, 11, 1043-1047 (1999);
Gu, S., Atanasova, P., Hampden-Smith, M. J. & Kodas, T. T., Chemical vapor
deposition of copper-cobalt binary films. Thin Solid Films, 340, 45-52 (1999);
Jain, S., Gu, S., Hampden-Smith, M. & Kodas, T. T., Synthesis of composite
films.
Chemical Vapor Deposition, 4, 253-257 (1998);
Gu, S., Yao, X. B., Hampden-Smith, M. J. & Kodas, T. T., Reactions of
Cu(hfac)(2)
and Co-2(CO)(8) during chemical vapor deposition of copper-cobalt films.
Chemistry of
Materials, 10, 2145-2151 (1998);
Calvert, P. & Rieke, P., Biomimetic mineralization in and on polymers.
Chemistry of
Materials, 8, 1715-1727 (1996);
Hampden-Smith, M. J. & Kodas, T. T., Chemical-Vapor-Deposition of Metals. 2.
Overview of Selective CVD of Metals. Chemical Vapor Deposition, 1, 39-48
(1995);
Hampden-Smith, M. J. & Kodas, T. T., Chemical-Vapor-Deposition of Metals. 1.
an
Overview of CVD Processes. Chemical Vapor Deposition, 1, 8-23 (1995);
Xu, C. Y., Hampden-Smith, M. J. & Kodas, T. T., Aerosol-Assisted Chemical-
Vapor-
Deposition (AACVD) of Binary Alloy (Ag(x)Pd(l)-X, Cu(x)Pd(l)-X, Ag(x)Cu(1)-X)
Films
and Studies of Their Compositional Variation. Chemistry of Materials, 7, 1539-
1546 (1995);
and
Naik, M. B., Gill, W. N., Wentorf, R. H. & Reeves, R. R., CVD of Copper Using
Copper(I) and Copper(II) Beta-Diketonates. Thin Solid Films, 262, 60-66
(1995).
The above described methods are limited to direct production of two-
dimensional
patterns, and three-dimensional patterns must be built up by use of multilayer
or multistep
processes. Laser direct writing of metal lines allows for single step
microfabrication of one-
dimensional or two-dimensional patterns, but has mainly involved thermal
decomposition of
a metal precursor at a high temperature created by absorption of laser energy.
There is great
interest in an ambient temperature process for forming metal lines by laser
writing and for
directly writing three-dimensional metal patterns.
-2-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Swainson et al., in a series of patents, (U.S. 4,466,080; U.S. 4,333,165; U.S.
4,238,840; and U.S. 4,288,861) described the photoreduction of silver by using
conventional
dyes such as methylene-blue and others as silver photoreducing agents in
solution. Silver
coatings of surfaces following optical excitation of such silver ion and dye
solutions were
described. The presence of "certain reducing/chelating agents, such as o-
phenanthroline"
were described as being a fundamental component of the system. Swainson also
described
that by following similar methods one would not be able to write continuous
metal phases
within a solid matrix. In fact, the introduction to the sections that included
the metal
photoreduction stated that the previously generally preferred stabilized or
solid media are not
suitable for the production of products with a material complexity above a
certain level.
Accordingly, their examples used gaseous and liquid physical states which
according to
Swainson permit increased complexity of products by virtue of their
transportive capability.
In the solid state, the present inventors have indeed found that Swainson's
method does not
result in the formation of continuous metal.
Whitesides et al. described a multistep method for the generation of
conductive metal
features both in an article: Deng T., Arias, F., Ismagilov, R. F., Kenis, P.
J. A. & Whitesides,
G. M., Fabrication of metallic microstructures using exposed, developed silver
halide-based
photographic film. Analytical Chemistry, 72, 645-651 (2000); and in U.S.
5,951,881. The
key difference between the system described by Whitesides et al. and the
system of the
present invention is that they photochemically generate metal nanoparticles in
a gelatin and
in a subsequent step they use an electroless deposition of silver on the
silver crystals, so as to
develop it (Braun, E., Eichen, Y., Sivan, U. & Ben-Yoseph, G., DNA-templated
assembly
and electrode attachment of a conducting silver wire. Nature, 391, 775-778
(1998)), thus
forming a continuous metal structure. Moreover, in order to obtain real 3D
patterns they
have to perform multi-step construction of the device. The smallest dimension
of the lines
(30,um) described by Whitesides et al. is much larger than the one achievable
with the
method according to the present invention.
Reetz et al. described in an article and a patent titled : "Lithographic
process using
soluble or stabilized metal or bimetal clusters for production of
nanostructures on surfaces"
the fabrication via electron beam irradiation of continuous metal features
starting from
surfactant stabilized metal nanoparticles (Reetz, M. T., Winter, M., Dumpich,
G., Lohau, J. &
Friedrichowski, S. Fabrication of metallic and bimetallic nanostructures by
electron beam
-3-
CA 02430253 2009-12-17
induced metallization of surfactant stabilized Pd and Pd/Pt clusters. Journal
of the American
Chemical Society 119, 4539-4540 (1997); Dumpich, G., Lohau, J., Wassermann, E.
F.,
Winter, M. & Reetz, M. T. in Trends and New Applications of Thin Films 413-415
(Transtec
Publications Ltd, Zurich-Uetikon, 1998). Bedson et al., describe the electron
beam writing of
metal nanostructures starting from passivated gold clusters, that were
alkylthiol capped gold
nanoparticles. Bedson T. R, Nellist P. D., Palmer R. E., Wilcoxon J. P. Direct
Electron Beam
Writing of Nanostructures Using Passivated Gold Clusters. Microelectronic
Engineering 53,
187-190 (2000)).
The differences between what is described there and the present invention are:
1) Reetz et al's and Bedson et al's processes involve fusion of nanoparticles
rather
than the growth of nanoparticles based on the generation of metal atoms upon
excitation;
2) their starting materials are made solely of stabilized nanoparticles,
whereas we
teach the use of composite materials in which stabilized nanoparticles are
just one of the
components;
3) their irradiation method is solely electron-beam irradiation, while we
teach that
using suitable reducing agents our composite materials can be good precursors
for a wide
variety of stimulating radiation, electron-beams being just one of them; and
4) their nanoparticles are coated with ligands that provide only stabilization
solubilization properties, while our compositions for electron beam patterning
of metal are
composites based on nanoparticles, metal salt, and an excited dye reducing
agent, that can be
included by covalent attachment to a ligand on the nanoparticle.
The compositions and methods of excitation of dyes with strong multiphoton
absorption properties have been disclosed by Marder and Perry, U.S. Pat. No.
6,267,913
"Two-Photon or Higher-Order Absorbing Optical Materials and Methods of Use".
Some compositions and methods have been disclosed for the multiphoton
generation
of reactive species including the photogeneration of silver particles in a
patent application by
B. H. Cumpston, M. Lipson, S. R. Marder, J. W. Perry "Two-Photon or higher
order
absorbing optical materials for generation of reactive species" International
application No.
PCT/US99/08383. The method taught in International application No.
PCT/US99/08383
differs from those of this invention because in the prior application there is
no mention of the
use of metallic nanoparticles as precursors.
-4-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a process for 1) direct
fabrication of
one, two or three-dimensional microstructures of metal in a single processing
step, and 2) the
fabrication of nanometer scale metal patterns in one or two dimensional
patterns also in a
single processing step. Specifically, it is an object of the present invention
to provide a low
temperature process for forming metal lines by laser writing and for directly
writing three-
dimensional patterns.
These and other objects have been achieved by the present invention the first
embodiment which includes a method for growth of a pre-nucleated metal
nanoparticle,
comprising:
providing said pre-nucleated metal nanoparticle in a composite;
generating a metal atom by reducing a metal ion by exposure to radiation;
reacting said metal atom with said pre-nucleated metal nanoparticle, thereby
growing
a metal nanoparticle.
Another embodiment of the invention includes a method for growth of a pre-
nucleated metal nanoparticle, comprising:
forming a film from said pre-nucleated metal nanoparticle, a metal salt, a dye
and a
polymer matrix;
generating a metal atom by reducing a metal ion of said metal salt by exposure
to
radiation;
reacting said metal atom with said pre-nucleated metal nanoparticle, thereby
growing
a metal nanoparticle.
Yet another embodiment of the present invention includes a metal nanoparticle
containing composition, comprising:
a ligand coated metal nanoparticle;
a dye;
a metal salt; and
optionally a sacrificial donor.
Another embodiment of the present invention includes a metal nanoparticle
containing composition, comprising:
a ligand coated metal nanoparticle;
a dye;
-5-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
a metal salt; and
a matrix.
A further embodiment of the present invention includes a method, comprising:
subjecting one of the above metal nanoparticles containing compositions to
radiation,
thereby effecting a growth of said nanoparticles; and
forming a continuous or semi-continuous metal phase.
The present invention further includes a method, comprising:
forming a film from a metal nanoparticle, a metal salt, a dye and a polymer
matrix;
and
exposing said film to radiation, thereby producing a pattern of a conductive
metal.
BRIEF DESCRIPTION OF DRAWINGS
Figure 1. Schematic illustration of case 1 growth process.
Figure 2. Energy level scheme for sensitized metal ion reduction.
Figure 3. Illustration of writings of metal features in a nanoparticle
composite.
Figure 4. Optical transmission image, (top view) of a 3D structure (200x200x65
m)
written in a polymer matrix.
Figure 5. Optical image of the same structure shown in Fig. 4 on a larger
scale.
Figure 6. SEM image of a 3D metallic silver microstructure formed by two-
photon
writing in a composite film.
Figure 7. XPS spectrum and image of a set of silver lines.
Figure 8. Schematic drawing of the attachment of a ligand capped metal
nanoparticle
to a thiol functionalized glass substrate.
Figure 9. TEM images illustrating growth of metal nanoparticle in a composite
film
upon exposure to either one or three laser pulses from a ns pulsed laser.
Figure 10. Silver ribbon written with a two-photon irradiation.
Figure 11. Silver lines written using a one-photon excitation.
Figure 12. Optical micrograph of a copper square written by two-photon
excitation.
Figure 13. Spectrum of sample from control experiment.
Figure 14. SEM picture of the comer of a 3D metallic silver structure written
using
two-photon excitation.
-6-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Figure 15. TEM image of chemically synthesized nanoparticles used as a
precursor
in the composites.
Figure 16. Examples of a square and a line written and imaged using an SEM.
Figure 17. Laser and electron-beam induced growth of silver nanoparticles in a
nanoparticle/salt composite.
Figure 18. Transmission optical microscopy of a line written in a PVK film
doped
with AgBF4 and nAgl2.
Figure 19. Reflection image of a silver square embedded in a polymer
nanocomposite.
Figure 20. Schematic drawing of the slide/polymer/microfabricated line
configuration used to measure the conductivity of the grown wires.
Figure 21. Plot of an I(V) curve.
Figure 22. Metallic structures fabricated in nanocomposites by two-photon
scanning
laser exposure.
Figure 23. Optical set ups for the writing and reading of holograms.
Figure 24. Reconstructed holographic image.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to the use of metal nanoparticle containing
films in
conjunction with exposure to radiation to activate the growth and fusion of
such particles to
form continuous conducting metal patterns. In the process, one, two, or three
dimensional,
continuous conducting metal wires or other patterns may be formed.
The novel metal nanoparticle systems and methods of exposure for the direct
patterning of metal in three dimensions and with high resolution in the order
of microns to
nanometers, are unprecedented. Subjecting certain nanoparticle containing
compositions to
forms of excitation can result in growth of these particles, leading
ultimately to a continuous
(or semi-continuous) metal phase. Patterned excitation leads to formation of
corresponding
metallic patterns. Methods based on two types of compositions and involving
free space
optical exposure, near-field optical exposure or exposure with ionizing
radiation, such as
electrons from the conductive tip of an scanning probe microscope are
described herein.
In the process of the present invention, radiation from different sources
results in
different resolution. For example, a feature sizes of down to 300nm can be
achieved using a
-7-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
blue laser and one photon excitation. A feature size of down to 100nm, and
preferably down
to 50nm may be achieved using a near field light source. If two photon
excitation is used, the
feature size may be down to 100nm, preferably 50nm. Electron-beams allow for a
resolution
of l0nm to 300mn. Focused ion beam allow for a feature size of down to 5 to
10nm. An
extremely small feature size of down to 5nm may be achieved using a Scanning
Probe
Microscope tip.
The nanoparticles used in the method according to the present invention are
mainly
those coated with organic ligands. By ligand, we mean any molecule or ion that
has at least
one atom having a lone pair of electrons that can bond to a metal atom or ion.
By ligand, we
also mean unsaturated molecules or ions that can bond to a metal atom or ion.
Unsaturated
molecules or ions possess at least one t-bond, which is a bond formed by the
side-by-side
overlap of p-atomic orbitals on adjacent atoms. One example of an organic
ligand for silver,
gold, or copper nanoparticles is an n-alkylthiol ligand, which preferably has
an alkyl chain
length of 4 to 30 carbons. The coatings of the nanoparticles render them
soluble in common
organic solvents and processable by solution processing techniques. These
coatings can also
stabilize the nanoparticle with respect to aggregation and/or coalescence of
the metal core of
the particle. Throughout this disclosure, the term ligand coated nanoparticle
is used to
describe such stabilized particles. Furthermore, according to the present
invention,
nanoparticles with two or more different types of ligands, such as two
alkylthiol ligands of
different lengths exhibit increased solubility in organic solvents and polymer
matrices, such
as poly(vinyl carbazole) and a reduced tendency towards the formation of
aggregates
resulting from inter-digitation of ligands, compared to nanoparticles coated
with one type of
alkylthiol ligand, which are known to form aggregates with interdigitated
ligands. Voicu, R.,
Badia, A., Morin, F., Lennox, R.B. & Ellis, T. H., Thermal behavior of a self-
assembled
silver n-dodecanethiolate layered material monitored by DSC, PTIR, and C-13
NMR
spectroscopy. Chemistry of Materials, 12, 2646-2652 (2000); Sandhyarani, N.,
Pradeep, T.,
Chakrabarti, J., Yousuf, M. & Sahu, H. K. Distinct liquid phase in metal-
cluster superlattice
solids. Physical Review B, 62, 8739-8742 (2000); Sandhyarani, N. & Pradeep,
T., Crystalline
solids of alloy clusters. Chemistry of Materials, 12, 1755-1761 (2000); Badia,
A. et al., Self-
assembled monolayers on gold nanoparticles. Chemistry-a European Journal, 2,
359-363
(1996)). The use of nanoparticles with two or more types of ligands for the
formation of a
metal nanoparticle/polymer composite is advantageous because a higher
concentration of
-8-
9& 01. 474
2 2"JAN
{{' =1 it !{ I 1' .~= {l 1~ {I =='1 '~` 1=' {l' ..N . If 11A 1{'I 11,1; it J-
1. -R
particles may be achieved and the optical quality of the composite may be
higher, since the
reduction of aggregation leads to lower optical scattering compared to
composites including
nanoparticles with a single type of alkyithiol ligand. The ligand coated
nanoparticles can be
easily spin coated, casted or inserted as dopants into organic films or
diffused into inorganic
glasses prepared via sol-gel chemistry. Two classes of compositions are
described here.
They differ in the nature of the matrix:
Class I is a composition in which the ligand coated nanoparticles themselves
are the
matrix.
Class II is a composition in which a polymer, a glass or a highly viscous
liquid is the
matrix, and the nanoparticles are dopants.
It is known that photochemical reduction of metal ions, such as silver ions
(Ag+), by
suitable dye molecules leads to the formation of Ag atoms and the nucleation
of small,
nanometer-sized particles of Ag . However, a key problem with past attempts to
photochemically write continuous, conducting metal patterns is that the
limited supply of
metal ions in the precursor material, as well as the high degree of excitation
required to
provide nucleation centers makes the growth of continuous metal quite
difficult. Particles
formed are typically not interconnected and do not form a conductive path.
With this type of
product one would have to perform an additional wet chemical processing step
to "develop"
the particles to form conductive lines. However, according to the present
invention,
incorporation of ligand-coated silver nanoparticles into the precursor
material overcomes this
problem by providing both initial nucleation sites for growth of the metal,
and some starting
volume fraction of metal. Upon sufficient growth, the surface coverage of the
ligand on the
nanoparticle becomes insufficient to prevent the particle from undergoing
fusion with other
neighboring growing particles to form a larger metal phase. The surface
coverage of the
ligand on the nanoparticle becomes insufficient to prevent the particle from
undergoing
fusion with other neighboring growing particles to form a larger metal phase.
Thus, upon
sufficient growth the nanoparticles become highly interconnected and form well
conducting
pathways. The approach disclosed here allows for direct and simple,
photochemical
fabrication of microstructures of conductive metal, at low temperatures, such
as ambient
room temperature (21 'C).
One type of composition that is effective in the method according to the
present
invention involves a composite containing a) metal nanoparticles, b) a metal
salt and c) a dye,
-9-
AMENDED SHEET
CA 02430253 2009-12-17
capable of excited state reduction of the metal ions and possessing
appropriate light
absorption properties, and d) a polymer host material. Many variations on the
composition of
this composite are possible, including: 1) the type of metal nanoparticle, 2)
the type of metal
ion, 3) the counterion of the metal salt, 4) the structure of the dye, 5)
whether or not a
polymer host is used, and 6) the type of polymer host if one is used. By the
term dye, we
mean a molecule or ion that absorbs photons with wavelengths ranging from 300
nm to 1.5
m. Depending on the composition, metal nanoparticle/polymer nano-composites
with good
light transmission properties and large thickness, up to hundreds of
micrometers, preferably
up to 500 micrometers, more preferably up to 700 micrometers and most
preferably up to 900
micrometers, can be prepared. In the method according to the present
invention, such
composite can be exposed in a patterned manner with optical radiation or with
ionizing
radiation beams, either by use of a mask or by suitable scanning of a highly
confined beam of
radiation, to produce a pattern of conductive metal.
According to the present invention, compositions incorporating dye molecules
possessing two-photon absorption cross sections greater than or equal to 1 x
1050 cm4 photon
I sec-I and capable of excited-state reduction of the metal ions can be used
to create three
dimensional patterns of conductive metal. Examples of dye molecules with large
two-photon
absorption cross sections are described in U.S. Pat. No. 6,267,913. In the
present
embodiment, a tightly focused, high intensity laser beam tuned to the two-
photon absorption
band of the dye is used to localize the photoactivated growth of metal to a
small volume. The
ability to achieve high 3D spatial resolution arises from the fact that the
probability of
simultaneous absorption of two photons depends quadratically on the intensity
of the incident
laser light. If a tightly focused beam is used, the intensity is highest at
the focus and decreases
quadratically with the distance (z) from the focal plane, for distances larger
than the Rayleigh
length. Thus, the rate at which molecules are excited decreases very rapidly
(as z"4) with the
distance from the focus and the excitation is confined in a small volume
around the focus (of
the order of a,3, where 2 is the wavelength of the incident beam). The sample
or the focused
beam can be scanned and the intensity controlled to map out a three
dimensional pattern of
exposure and to produce 3D structures comprised of continuous metal.
In a preferred embodiment, Ag nanoparticles (with a ligand coating) are
combined
with AgBF4 salt, and an electron deficient two-photon absorbing dye in
polyvinylcarbazole
-10-
Al 01147791
IPA 2 2 J AN 2001
ii. " dits ii_al "= li it U ._i,'_ ,fi
to form a composite. In an example of exposure with radiation for writing of a
metal pattern,
100 fs laser pulses at a wavelength of 730 nm are focused onto the film
resulting in the.
formation of reflective and conductive Ag metal at the points of exposure.
Preferably, the
laser wavelength ranges from 157nm to 1.5 m for one-photon excitation and from
300nm to
3:0 m for two-photon excitation. The pulse width of the laser is preferably in
the order of
s 1 s to I Ofs for two-photon excitation. Arbitrary patterns of Ag metal can
be written by
moving the point of focus in the film. Writing of microscale lines,
rectangular shapes and
various 3D patterns of metal lines has been accomplished with this method.
Many variations
in the step of exposure with radiation are possible, as would be known to
those skilled in the
art of radiation induced change of materials properties. For example, electron
beams,
electrical current via a scanning probe tip, focused ion beams, y-radiation, x-
rays, UV-rays,
VUV-rays, neutron beams, and neutral atom beams may be used in the method of
the present
invention.
Formation and growth of metal nanoparticles
It is known that the formation of alkylthiolate coated metal nanoparticles
proceeds via
a nucleation-growth mechanism (Hostetler, M. J. et al., Alkanethiolate gold
cluster molecules
with core diameters from 1.5 to 5.2 nm: Core and monolayer properties as a
function of core
size. Langmuir, 14, 17-30 (1998)) that involves the formation of a layered
stoichiometric
compound as a first step as illustrated below for Ag:
nAg+ + nRSH - (AgSR)n + H+
followed by a second step involving growth. The second step can be due to the
presence of
silver zero atoms:
(AgSR)n + mA9 - Agn,(SR)n
with n'=n+m
or to the presence of an agent that reduces the metal ions of the layered
compound
themselves:
(AgSR)n -- Agõ(SR)n,+m'RSSR
with 2m'=n-m
Once generated, these nanoparticles are soluble materials that are processable
with
standard methods. In particular, their solubility in organic solvents allows
for a multiplicity
of processing techniques based on which films of nanoparticles or solid
matrices with
-11-
AMENDED SHEET
CA 02430253 2009-12-17
incorporated nanoparticles can be created. We teach that, with such a
nanoparticle film or
composite, the growth of nanoparticles may be driven such that they increase
in size, and
contact and fuse with other nanoparticles. When this process occurs to a
sufficient extent,
then a continuous metallic feature (single or polycrystalline) will be formed.
One important contribution of the present invention is that it teaches the
materials and
exposure conditions that allow the growth of pre-nucleated metal nanoparticles
in solid state.
By the term pre-nucleated metal nanoparticle we mean a metal nanoparticle
which has been
nucleated and grown in a preceding synthetic process. These conditions allow
nanoparticles
to grow to the point wherein they collapse in a continuous metallic feature.
Several growth processes are disclosed that vary in the method by which the
metal
atom (zero oxidation number) is generated from its ion.
The first case (case 1) makes use of the generation of the metal atom from the
metal
ion using an electron beam. The electron beam can directly reduce the silver
ion or can
generate a radical anion that then reduces the metal ion or can ionize a
molecule and the
electron then reduces the metal ion.
An advantage of case 1 is the versatility in the metal line resolution that
can be
achieved, which can range from several microns (with the use of a mask and a
large electron
beam) to few nanometers (with the use of conductive scanning probe microscopy
tips, for
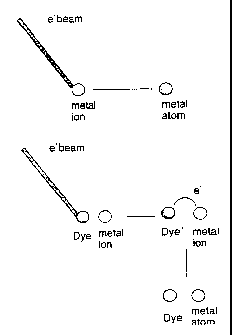
example). FIG. 1 is a schematic illustration of a case 1 growth process. In
the upper drawing
an injected electron reduces a metal ion; in the lower part it generates a
radical anion that
subsequently reduces the metal ion. In particular, conductive tip Atomic Force
Microscopy,
in which the tip of the microscope approaches a film in tapping mode, can be
used as source
of electrons. Once it is positioned within few nanometers away from the
surface of the
nanoparticles it injects electrons into the nanoparticle film to generate
metal lines whose
thickness may be just a few nanometers.
The second case (case 2) is that wherein metal ions are reduced to their zero
oxidation
number through a local increase in temperature which is caused by absorption
of light energy
(preferably a laser beam) by a dye molecule and the transfer of the absorbed
energy to heat.
Materials and methods for the non-linear local heating of materials are
described in U.S. Pat.
No. 6,322,931.
The third case (case 3) is to photoexcite a molecule so as to create an
excited state,
thus increasing the reducing potential of the molecule by a sufficient amount
that it can
-12-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
reduce the metal ion, whereas the ground state could not. In this process the
dye is oxidized
so, in many cases, a sacrificial donor may be required in order to regenerate
it. Figure 2
illustrates an energy level scheme for sensitized metal ion reduction. In the
first step (black)
an electron is promoted from the highest occupied molecular orbital (HOMO)
level of the
dye to one of its excited states. From this level the electron either goes
directly to the metal
ion (red) or first to the lowest unoccupied molecular orbital (LUMO) of an
electron
transporting material (blue) and then to the metal ion. Subsequently, an
electron may transfer
(green) from the HOMO of the sacrificial donor to the HOMO of the dye, thereby
regenerating the neutral dye. The sacrificial donor and the electron
transporting material are
not necessary.
The feature size resolution achievable in this case depends on the type of
photo
excitation. The lower limit on the feature size can be several microns is
diffraction limited
for one photon (case 3a) irradiation, is potentially smaller for multi-photon
irradiation, due to
thresholding effects (case 3b), and is in the order of tens of nanometers for
near field
irradiation (case 3c). In this case the limit is determined by the near-field
source dimension
and its position relative to the film.
Figure 3 illustrates the writing of metal features in a nanoparticle
composite. The red
cone represents the laser beam and the darker spot at its end represents the
focus of the beam.
The gray rectangle is the composite film containing the dye and the salt in
it, while the blue
circles represent the nanoparticles. Upon exposure, growth and coalescence, a
metal pattern
is formed. In the upper part (a), the metal atoms are formed in the beam focus
and they start
to migrate toward nanoparticles, then (b) the nanoparticle starts to grow, and
finally (c) a
continuous metal feature is formed. This scheme is appropriate to case 2 and 3
methods.
Preferred Embodiments
Preferred examples will be given in the following section to illustrate
methods of
fabrication of metal. These examples are by no means exhaustive and it should
be clear to
one skilled in the art that numerous other procedures can be employed based
upon the basic
principles of the invention disclosed herein. Two different classes of
compositions can be
used to generate metal features. In the first class the metal nanoparticles
act as their own
matrix and in the second class the metal nanoparticles are dopants in a host
matrix.
-13-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Class I
In the first embodiment the material is composed of
i) ligand coated metal nanoparticles coated by one or more types of organic
ligands.
In some cases it is advantageous to use a mixture of organic ligands, as
described above. In
addition, molecules as described in (ii) (below) could be attached to one or
more types of
ligands coating the particle.
ii) a molecule (dye) whose molecular orbital energy levels are suitable for
photoreduction of the corresponding metal salt or whose linear or nonlinear
optical
absorption is able to generate the sufficient heat to cause reduction the
metal salt. This
component can be dissolved in the nanoparticle matrix or covalently bonded to
the
nanoparticle as one of the ligands, or as the only ligand;
iii) a metal salt; and
iv) a sacrificial donor, that is a molecule whose molecular orbital levels are
of
appropriate energy to reduce the cation of the dye described in (ii) above,
which is formed
upon photoreduction of the metal ion or upon electron-beam exposure. In this
manner, the
original dye can be regenerated and can once again act as a reducing agent of
metal. The
component may be part of the host matrix structure. In some cases this
component may not
be necessary.
Preferred concentrations (in weight percent, based on the total weight of the
composition) for each component of the class I system are as follows and are
chosen so as to
add to a total of 100%:
Component (i): 55 to 100%, including all values and subvalues therebetween,
especially including 60, 65, 70, 75, 80, 85, 90 and 95%;
Component (ii): 0 to 15%, including all values and subvalues therebetween,
especially including 2, 4, 6, 8, 10, 12 and 14% (0 applies for the case where
the nanoparticles
have dye terminated ligands in their outside shell);
Component (iii): 0 to 15%, including all values and subvalues therebetween,
especially including 2, 4, 6, 8, 10, 12 and 14%;
Component (iv): 0 to 10%, including all values and subvalues therebetween,
especially including 2, 4, 6, and 8%.
Class II
-14-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
In the second embodiment a material acts as a host matrix in which the other
components i) - iv) are dispersed or dissolved:
v) a matrix that dissolves all the other components. This matrix can be:
a) a polymer;
b) a glass;
c) a highly viscous liquid;
d) a liquid crystalline material or polymer, or mesoscopic phase; and
e) a porous crystalline or amorphous solid.
In the case (a) of component (v) there could be circumstances in which it is
particularly advantageous to add an additional component (vi):
vi) a plasticizer, that is a molecule capable of lowering the glass transition
temperature of the polymer, thereby rendering its mechanical properties more
suitable for the
application.
In both cases the component (ii) is not necessary when the source of
irradiation is an
electron beam.
Preferred concentrations (in weight percent, based on the total weight
composition) of
the for each component of the class II system are as follows and are chosen so
as to add to a
total of 100%:
Component (i): 0.05% to 25%, including all values and subvalues therebetween,
especially including 0.1, 0.2, 0.5, 1, 2, 3, 4, 5, 10, 15 and 20%;
Component (ii): 0 to 15%, including all values and subvalues therebetween,
especially including 2, 4, 6, 8, 10, 12 and 14% (0 applies where the
nanoparticles have dye
terminated ligands in their outside shell, or either the host or the
plasticizer have a suitable
dye as subunit);
Component (iii): 0 to 25%, including all values and subvalues therebetween,
especially including 5, 10, IS and 20%;
Component (iv): 0 to 60%, preferably from 20% to 60%, including all values and
subvalues therebetween, especially including 10, 20, 30, 40 and 50%;
Component (v): 0.5-99.5%, including all values and subvalues therebetween,
especially including 10,, 20, 30, 40, 50, 60, 70 and 80%; and
Component (vi): 0 to 70%, including all values and subvalues therebetween,
especially including 10, 20, 30, 40, 50 and 60%.
-15-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Description of the components
Component (i): Metal Nanoparticles
Preferred examples of component (i) are:
il) metal (e.g. silver, gold, copper, and iridium) nanoparticles with
dimensions from 1
to 200 nm (diameter) coated with organic ligands (Kang, S. Y. & Kim, K.,
Comparative study
of dodecanethiol-derivatized silver nanoparticles prepared in one-phase and
two-phase
systems. Langmuir, 14, 226-230 (1998); Brust, M., Fink, J., Bethell, D.,
Schiffrin, D. J. &
Kiely, C., Synthesis and Reactions of Functionalized Gold Nanoparticles.
Journal of the
Chemical Society-Chemical Communications, 1655-1656 (1995); Brust, M., Walker,
M.,
Bethell, D., Schiffrin, D. J. & Whyman, R., Synthesis of Thiol-Derivatized
Gold
Nanoparticles in a 2-Phase Liquid-Liquid System. Journal of the Chemical
Society-
Chemical Communications, 801-802 (1994));
i2) nanoparticles composed of alloys of metals coated with organic ligands
(Link, S.,
Burda, C., Wang, Z. L. & El-Sayed, M. A., Electron dynamics in gold and gold-
silver alloy
nanoparticles: The influence of a nonequilibrium electron distribution and the
size
dependence of the electron-phonon relaxation. Journal of Chemical Physics,
111, 1255-1264
(1999); Link, S., Wang, Z. L. & El-Sayed, M. A., Alloy formation of gold-
silver
nanoparticles and the dependence of the plasmon absorption on their
composition. Journal of
Physical Chemistry B, 103, 3529-3533 (1999));
i3) uncoated metal nanoparticles (for the second embodiment) (Heilmann, A. &
Kreibig, U., Optical properties of embedded metal nanoparticles at low
temperatures.
European Physical Journal-Applied Physics, 10, 193-202 (2000)); and
i4) metallic nanoshells whose cores are semiconductor, metal oxide, silicate,
polymer,
or biopolymer nanoparticles and whose outer shells are metallic, the metallic
part being with
or without (for the second embodiment) an organic coating (Wiggins, J.,
Carpenter, E. E. &
O'Connor, C. J., Phenomenological magnetic modeling of An : Fe : Au nano-
onions. Journal
of Applied Physics, 87, 5651-5653 (2000); Carpenter, E. E. et al., Synthesis
and magnetic
properties of gold-iron-gold nanocomposites. Materials Science and Engineering
a-
Structural Materials Properties Microstructure and Processing, 286, 81-86
(2000)).
Components il, i2, i3 are made of nanoparticles that can be coated by organic
ligands.
These ligands are molecules that are essentially composed of three parts in
the following
-16-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
scheme: A-B-C.
Part A is a molecular or ionic fragment that has at least one atom having a
lone pair of
electrons that can bond to a metal nanoparticle surface, or is an unsaturated
molecular or
ionic fragment that can bond to the metal nanoparticle surface, and includes a
point of
attachment to connect the fragment to B. Some examples include: 'S-, e0-1 e02C-
, 'S-S-R,
e03S-, S2C-NR-, .02C-NR-l P(R1R2)-, N(R1R2)-, O(R,)-, P(OR1)(OR2)0-, and
S2(R)-, where
R, R1, and R2 may be independently selected from the group consisting of -H, a
linear or
branched alkyl chain containing 1 to 50 carbon atoms, phenyl or other aryl
groups, and hetero
aromatic groups.
Part B is an organic fragment that has two points of attachment, one for
connecting to
part A and one for connecting to part C. This fragment serves to provide bulk
around the
nanoparticle to help stabilize it against fusing with other nanoparticles.
Part B can be nothing
(a single bond) or can be independently selected from the group consisting of
a methylene
chain with 1 to 50 carbon atoms, a phenylene chain with 1 to 20 phenyls, a
thiophenylene
chain with 1 to 20 thiophenylenes, phenylene vinylene chains with 1 to 20
phenyl vinylenes,
branched hydrocarbon chains with 2 points of attachment, ethylene oxide chains
with 1 to 20
ethylene oxides, oligo(vinyl carbazole) chains with 1 to 20 vinyl carbazole
units and points
of attachment at each end of the chain.
Part C is a molecular fragment with one point of attachment that connects to
fragment
B. This group maybe used to impart specific functions to the exterior of the
ligand coated
nanoparticle such as, coinpatability with a matrix, photoreducing properties,
two-photon
absorption properties, self-assembly properties, chemical attachment
properties. Part C can
be independently selected from the group consisting of -H, phenyl, naphthyl,
anthryl, other
aryl groups, N-carbazoyl, a-fluorenyl, -SiOR3, -SiC13, any group described as
a possible Part
A fragment, photoreducing dyes, two-photon absorbing chromophores, a multi-
photon
absorbing chromophore methylene blue, oligonucleotide strand, peptide chain,
or any group
described as a possible part B fragment where one of the points of attachment
is substituted
with a hydrogen.
The preferred nanoparticles of the present invention may have a mixture of two
or
more types of ligands, each one with its own characteristic groups and
functionality.
For the sake of clarity some specific examples of ligands that have been used
for class
(il) are mentioned hereafter:
-17-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Ligand structure Ligand
Ligand chemical name label
SH Octanethiol 11
SH Dodecanethiol 12
SH Heptanethiol 13
-18-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
SH 8-(9H-carbazol-9-yl)octane-1- 14
%-N thiol
SH 8-(9H-carbazol-9-yl)dodecane-l- 15
N thiol
\ j
/~/ SH 3-mercaptopropanoic acid 16
HOOC
O 0
N N
0 0Bis[2-(dimethylamino)ethyl]2- 17
SH mercaptopentadioate
0
3-{2,5-bis[(E)-2-(4-
0/ 0 formyl-( phenyl)ethenyl]phenoxy} 18
propyl-4-(1,2-dithiolan-3 -
yl)butanoate
0
O
S
S
Examples of preferred metal and ligand combinations are the following:
Metal Ligand Given name
silver Il nAgl
silver I2 nAg2
silver 13 nAg3
silver 14 nAg4
-19-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
silver 17 nAg5
silver I1+I4 nAg6
silver I1+I2 nAg7
silver II+I7+I4 nAg8
silver I1+I4+I8 nAg9
gold Il nAul
gold 12 nAu2
copper I1 nCul
copper I1 nCu2
Component (ii) Photoreducing Dyes
Preferred examples of component (ii) are:
Class 1: Centrosymmetric bis(aldehyde)-bis(styryl)benzenes
R2
R1 O
RZ
- R2
O R1
R2
R1=H R2 =H la
R1=OCH3 R2=H lb
R1=OCH3 R2=OCH3 lc
R1=OC12H25 R2 =H ld
R1=OC12H25 R2 OCH3 le
Class 2: Non-centrosymmetric bis(aldehyde)-bis(styryl)benzenes
-20-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
R2
R1 O
R2
R2
O R3
R2
RI=H R2=H R3=H 2a
R1=OCH3 RZH R3=H 2b
R1=OCH3 RZOCH3 R3=H 2c
R1=OC12H25 R2=H R3H 2d
R1=OC12H25 R2=OCH3 R3H 2e
Class 3: Centrosymmetric acceptor terminated bis(styryl)benzenes
-21-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
R1
R3 -
R1 R2
R2 R1
R3
R1
CN la
R1=H R3=H R2= HC
CN
CN
R1=OCH3 R3=H R2= HC=~ lb
CN
<'S
N4 lc
R1=OCH3 R3=OCH3 R 2 = O N
HC O
S
N4 Id
R1=OC12H25 R3=H R2= O N
HC O
-22-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Class 4: Non-centrosymmetric acceptor terminated bis(styryl)benzenes
R1
R2
R, R4
R4 R1
R3
R1
CN
R1=H R2=OCH3 R3=H R4= HC la
CN
CN
R1=OCH3 R2=OC12H25 R3=H R2= HC==~ lb
CN
S
N g 4 -/ is
R1=OCH3 R2=H R3=OCH3 R2= O N
HC O
S
R1=0C12H25 R2=OCH3 R3=OCH3 R2= O le
HC O
Class 5: Other dyes
-23-
CA 02430253 2010-05-06
NC CN
The dye can be a donor-acceptor dye such as those described in U.S. 5,804,101;
U.S.
6,090,332; U.S 5,670,090; U.S. 5,670,091 and U.S. 5,500,156.
Component (iii:) Metal Salts
Preferred examples of component (iii) are any metal(I) soluble salt, including
silver
tetrafluoroborate (AgBF4); silver hexafluoroantimonate (AgSbF6); silver
diethyldithiocarbamate (C3H1ONSZAg);silver nitrate (AgNO);
trimethyl phosphite cuprous iodide (ICuP(OCH3)3); and chlorotrimethyl
phosphite gold
(C1AuP(OCH3)3).
Component iv): Matrices
The main requirement of this component is to have the ability to dissolve all,
the other
components and to form a homogeneous composite.
Preferred examples of component (v) are:
a) Polymer a,: poly(9-vinylcarbazole)
-24-
OCT
/US 01/47724
14 ! j1=., .': U =':: 4 1I u ~N a - 11, it i'.I 1y~rv e
l.. u v . IIit ., i. s='.:= ii' . J = fi_ 11'.- :I }::1 =~ +:
n
N
. o 0
In most of the examples given below the polymer actually used was the
secondary standard
(Aldrich chemicals) whose Mõ= 69,000;
Polymer a2: poly(2-{[11-(9H-carbazol-9-yl)undecanoyl]oxy}ethyl-2-
methylacrylate)
PCUEMA
Lyn
O
O
O
O
(C9H18)
Polymer a3: poly (4-chloro styrene); and
Polymer a4: poly (methylmethacrylate) PMMA;
b) SiOx, organically modified SiOx materials, TiOx, (SiOX)n(TiOx)m
c) viscous liquid host:
-25-
AMENDED SHEET
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
N
R
R=CH3 cl
R=OCH3 c2.
Other components
Preferred examples of component (iv) and of component (vi) are ethylcarbazole
which is a good plasticizers for polyvinyl carbazole, and a sacrificial donor;
N
C L 0
terminal di(9-carbazoyl) alkanes which are good plasticizer and sacrificial
donors;
N
N
n
0_<n<_10
-26-
f i
: flS 0l f4 'T2
M
IPA 2'JAk
and the following molecule which is a sacrificial donor:
N N
1 ~
NC CN
Applications of the present invention include: writing of metal line
diffraction
gratings for light waves in integrated optics, patterning of microelectrode
arrays for
applications in electrochemistry or biology, patterning of metal wires for
integrated circuit
interconnection, for example in hard wiring of security codes on chips, and in
chip repair.
Additional applications include: fabrication of nanometer size metal wires,
single electron
transistors, and other components for nanoelectronics applications; 3D
interconnection of
electronic components in multilevel integrated circuits; fabrication of
metallic devices for
microsurgical applications; antennae'and arrays thereof for terahertz
radiation, formation of
mirrors of different angles of inclination within a thin film metallic
photonic crystal and
photonic crystal waveguides, and metallic microsensors, micro-resonators and
micro-
electromechanical structures. This invention can be used for the wiring of
nanoscale and
single molecule based electronic devices.
Further, the present invention offers the following advantages over the
currently
available technology:
i) Continuous metal lines can be formed in three dimensional patterns with a
resolution in the micro- or nanoscale with few limitations on the shape of the
pattern.
ii) The process does not require the generation of high temperatures as needed
in
pyrollytic processes, and thus can be utilized in the integration of nanoscale
devices or in
conjunction with thermally sensitive substrates.
iii) Metal patterns or structures can be produced on a wide variety of
substrates.
Preferred substrates are silicon, glass or plastic substrates, all of which
may be covered with,
for example, indium-tin-oxide (ITO). Further preferred substrates are Au, Ag,
Cu, Al, SiO,s,
-27-
AMENDED SHEET
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
ITO, a hydrogel or a biocompatible polymer.
iv) The material systems are easy to process and simple to handle, as opposed
to
highly toxic gas phase organometallic precursors as typically used in chemical
vapor
deposition.
v) Inert atmospheres are not required.
vi) High vacuum equipment is not needed.
vii) The fact that the process is thresholded allows the sample to be handled
under
ambient lighting and thermal conditions, thus giving the samples exceptional
long-term
stability, for example, a shelf life of 8 months in the dark.
However, the process of the present invention is not limited to ambient
temperatures.
If Class I compositions are used, a temperature range of from -270 to 200 C is
preferred. If
Class II compositions are used, a temperature range of from -250 to 150 C is
preferred.
Thus, the present invention relates to a novel process for directly writing
three-
dimensional metal patterns in a material requiring low energy as described in
Examples 20
and 21, below and low temperature as stated above. The versatility of the
compositions with
regard to the type of metal nanoparticles used and the type of dye offers many
possibilities
for engineering of materials for specific applications.
There are many possible applications that can be embodied based on the present
invention.
One set of applications involves the ability to induce large changes in the
physical
properties in a matrix generated by the presence of dispersed nanoparticles (1-
100 nm),
dispersed small metal island (100 nm-100 ,um), quasi-continuous (percolated)
metal or
continuous metal lines. These modifications alter the optical properties, such
as refractive
index of a solid state matrix, and such changes in properties are useful in
optical data storage,
in creating diffractive optical devices, or in defining waveguiding regions
for integrated
optics, or writing of metal line diffraction gratings for light waves in
integrated optics.
This invention can be used for optical data storage in many formats.
Information can
be stored in 3D using two-photon excitation to write bits comprised of regions
containing
metal nanoparticles or metal islands. A focused beam is useful in this regard,
but crossing
beams or interfering beams, such as in holography, can be employed.
Another example of optical data storage is where the photosensitive metal
nanoparticle composite is used as an optical recording layer for recordable
compact disk-like
-28-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
applications.
A very attractive application is for ultra-high density 2D optical data
storage using
near field light source to write very small bits (' 100 nm or smaller).
Other optical applications include: fabrication of reflective polarizers,
switchable
gratings, and micromirrors.
A second set of applications of the present invention uses direct patterning
of
conductive metal features. They include: patterning of microelectrode arrays
for applications
in electrochemistry or biology, patterning of metal wires for integrated
circuit
interconnection, for example in hard wiring of security codes on chips, and in
chip repair.
Additional applications include: fabrication of nanometer size metal wires,
single electron
transistors, and other components for nanoelectronics applications; 3D
interconnection of
electronic components in multilevel integrated circuits; writing of contacts
on soft materials
such as organic light emitting diodes or organic field effect transistors;
fabrication of metallic
devices for microsurgical applications, such as needles and stents
(McAllister, D. V., Allen,
M. G. & Prausnitz, M. R., Micrafabricated microneedles for gene and drug
delivery. Annual
Review of Biomedical Engineering, 2, 289-313 (2000); Polla, D. L. et al.,
Microdevices in
medicine. Annual Review of Biomedical Engineering, 2, 551-576 (2000); Santini,
J. T.,
Richards, A. C., Scheidt, R., Cima, M. J. & Langer, R., Microchips as
controlled drug-
delivery devices. Angewandte Chemie-International Edition, 39, 2397-2407
(2000);.
Rymuza, Z., Control tribological and mechanical properties of MEMS surfaces.
Part 1:
critical review. Microsystem Technologies, 5, 173-180 (1999)); and micro-
electromechanical
structures (Walker, J. A., The future of MEMS in telecommunications networks.
Journal of
Micromechanics and Microengineering, 10, R1-R7 (2000); Spearing, S. M.,
Materials issues
in microelectromechanical systems (MEMS). Acta Materialia, 48, 179-196 (2000);
Lofdahl,
L. & Gad-el-Hak, M., MEMS applications in turbulence and flow control.
Progress in
Aerospace Sciences, 35, 101-203 (1999)). The materials and methods of this
invention can
be used for the wiring of nanoscale and single molecule based electronic
devices (Quake, S.
R. & Scherer, A., From micro- to nanofabrication with soft materials. Science,
290, 1536-
1540 (2000)).
Yet other applications can include uses of written metal features in hybrid
electrooptical applications, where both the electrical and optical properties
are exploited. An
example could be an electrode array shaped so to act as a diffractive grating
that may be
-29-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
backfilled by liquid crystalline material whose alignment is controlled by the
applied field.
The liquid crystal alignment would control the optical properties of the
grating.
Writing of conductive metal features is also of advantage to applications in
microfluidics. For example, in the fabrication of electroded channels to
control fluid flow, to
drive electrophoretic separations, to drive electrochemical reactions, or to
monitor the
dielectric properties of the channel contents.
Another application of the invention is the patterning or fabricating of
templates
(Ostuni, E., Yan, L. & Whitesides, G. M., The interaction of proteins and
cells with self-
assembled monolayers of alkanethiolates on gold and silver. Colloids and
Surfaces B-
Biointerfaces,15, 3-30 (1999)) which can be used for deposition, self-
assembly, or teraplated
growth of other materials or compounds. For example, patterned metals surfaces
can be used
for the generation of patterned arrays of self-assembled molecules such as
thiols, carboxylic
acid or other functionalized compounds. One can drive reactions at the metal
surfaces by
using gas phase, solution phase or solid phase reactants. The patterned
surfaces can also be
exploited for the patterned catalysis of chemical reactions.
Yet another application of the ability to write metal features in a free form
fashion is
to create electrode patterns which can be used to direct the growth and
interconnection of
neurons or other types of cells.
Having generally described this invention, a further understanding can be
obtained by
reference to certain specific examples which are provided herein for purposes
of illustration
only, and are not intended to be limiting unless otherwise specified.
Examples
Discussion of the Examples
The following discussion is meant to encompass a set of examples of some
important
experiments and to assist in the understanding of the examples section.
This discussion mainly focuses on the possibility to write 3D metallic
patterns with
multiphoton irradiation (class II, case 3). This, is a good test for all other
classes and cases
described above. In fact, most of the steps involved in the present invention
are common to
all the different cases described and namely they are
1. the synthesis of highly soluble nanoparticles,
2. the preparation of a homogeneous and optical quality matrix, and
-30-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
3. the post-writing processing and characterization.
These steps are common to all different kinds of writing processes. The
solution of
the problems involved in these parts constitutes a large part of the present
invention.
To generate a homogeneous matrix with good optical quality it is necessaries
to find
(i) a solvent or a solvent mixture capable of dissolving all the components
and (ii) a matrix (a
polymer) that is capable of dissolving all the components in solid state.
Preferred solvents
are chloroform, dichloromethane, acetonitrile, acetone, water, hexane,
heptane, pentane,
toluene, dichlorobenzene, dichloroethane and mixtures thereof. A solution of
chloroform/acetonitrile 20/1 in volume was found to be the best one for this
purpose and
polymers a, and a4 have the desired properties to efficiently dissolve a wide
variety of silver
salts, while PMMA shows the ability of dissolving copper salts. A more complex
problem is
the solubility of nanoparticles both is organic solvents and in solid
matrices.
It has been found that despite the wide variety of ligands that can be
attached on
nanoparticles their solubility remains limited. In order to overcome this
problem it is
important to use nanoparticles with a mixture of ligands. Examples of suitable
ligands have
been provided above. The use of a mixture of ligands adds entropy to the
system and mainly
limits the interdigitation between ligands that is the main cause for poor
solubility (Voicu, R.,
Badia, A., Morin, F., Lennox, R. B. & Ellis, T. H., Thermal behavior of a self-
assembled
silver n-dodecanethiolate layered material monitored by DSC, FTIR, and C- 13
NMR
spectroscopy. Chemistry of Materials, 12, 2646-2652 (2000); Sandhyarani, N.,
Pradeep, T.,
Chakrabarti, J., Yousuf, M. & Sahu, H. K., Distinct liquid phase in metal-
cluster superlattice
solids. Physical Review B, 62, 8739-8742 (2000); Sandhyarani, N. & Pradeep,
T., Crystalline
solids of alloy clusters. Chemistry of Materials, 12, 1755-1761 (2000); Badia,
A. et al., Self-
assembled monolayers on gold nanoparticles. Chemistry-a European Journal, 2,
359-363
(1996)). In addition, particular groups were used to make the particle more
soluble in their
host, e.g. carbazole terminated alkanethiol as one of the ligands to make the
particle soluble
in polyvinylcarbazole.
The best strategy to synthesize these nanoparticles was the use of a monophase
reaction in ethanol (Example 1), the simple addition of different ligands in
different ratios
was effective in obtaining particles with different ligands on their outer
shell and with a
drastically reduced enthalpy of melting (Example 2).
If the particles are soluble enough, the casting of the films becomes
relatively easy
-31-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
and can be done either via solvent evaporation (Example 8) or by spin coating
(Example 17).
In the first case the achievable range of thickness spans from few microns
(Example 13) to
200 gm. The maximum silver salt loading ratio achievable in polymer ai is 15%
(by weight)
for silver tetrafluoroborate; in the same polymer 5% is the maximum for dye
ld, and 3% is
the maximum for nAg6 (Example 9). The loading ratio maxima are slightly higher
in the
case of spin coating techniques.
The solid state growth of metal nanoparticle has been explored and proven
through a
series of experiments involving the photochemical reduction of silver ions in
a matrix. The
carbazole moiety plays the role of sacrificial anode too, thus allowing the
possibility of
doping the silver with a smaller amount of photoreducing dye.
Polyvinylcarbazole has a Tg of around 200'C so a plasticizer was used in order
to
lower the glass transition temperature to close to room temperature. A well
known
plasticizer for this polymer has been used: ethylcarbazole. The range of
compositions of the
film that was mostly used was (all percentages in weight) 30-50% of
plasticizer, 3-5% dye
1d, 10-15% AgBF41 0.2-3% nAg6. The amount of plasticizer includes all values
and
subvalues therebetween, especially including 33, 36, 39, 42, 45 and 48% by
weight. The
amount of dye includes all values and subvalues therebetween, especially
including 3.2; 3.4;
3.6; 3.8; 4.0; 4.2; 4.4; 4.6 and 4.8% by weight. The amount of AgBF4 includes
all values and
subvalues therebetween, especially including 10.5; 11; 11.5; 12, 12.5; 13;
13.5; 14 and 14.5%
by weight. The amount of nAg6 includes all values and subvalues therebetween,
especially
including 0.4; 0.6; 0.8; 1.0; 1.2; 1.4; 1.6; 1.8; 2.0; 2.2; 2.4; 2.6 and 2.8%
by weight.
Reference films with the same composition but without nanoparticle were also
made.
All the films made in this way had good optical quality and were perfectly
homogeneous by
naked eye inspection, though some defect could be observed, none was perfect
under the
microscope (60X magnification).
All films were irradiated with a tightly focused infrared light source (from
700 to 800
nm 100fs pulse-length) generated silver lines (Example 22) and or islands
(squares in
particular) while the corresponding reference film did not generate any
visible feature.
Further inspections of the reference film either using optical spectroscopy or
TEM
microscopy lead to the conclusion that small nanoparticles which have a large
size dispersion
are generated. The features generated in polymer films that contained metal
nanoparticles
could be separated from their matrix via dissolution of the matrix in an
appropriate solvent
-32-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
mixture and then studied with XPS (Example 10) and SEM techniques (Example 9).
XPS Results show that the generated features are made mainly of silver in its
zerovalent (metallic). The latter technique shows that three-dimensional
features can be
formed and that the generated lines are continuous up to a micron level.
A more complex experiment was done in order to study our process with a TEM
microscope: three identical films were castes of copper grids (Example 11) and
two of them
were exposed to irradiation with a nanosecond laser (532 nm), the first one
was irradiated by
a single laser shot (125 mJ) and the second one by three shots.
The result was that the average radius of the particle doubled after one laser
shot but
their number per unit area stayed the same, all in agreement with the proposed
mechanism
(Figure 9).
Many variations are possible, including the use of near field excitation. In
order to
check the feasibility of this variation the threshold power required for the
writing was check
and it was discovered to be approx. 108 W/m2 for single photon excitation
(Example 20) and
109 W/m2 for two photon excitation (Example 21). The power thresholds are
consistent with
those available for near field writing.
The experimental section contains many different kinds of films that have been
prepared using different kind of polymers (Example 15) or matrices, dyes
(Example 14), salts
or nanoparticles (Example 12).
Important issues in the developing step have been solved. In order to provide
a
chemical bond between the structures and the substrate a two step method for
functionalizing
the substrate was developed. In the first step a monolayer of thiol terminated
molecules is
created in the glass substrate. This monolayer is bound to the substrate via
trimethoxy silane
functionalities. In the second step a nanoparticle monolayer is introduced on
the first
monolayer and the particles are chemically bound to the thiols. This kind of
functionalized
substrate drastically improved the adhesion and success in the developing
step. Figure 8 is a
schematic drawing of the attachment of a ligand capped metal nanoparticle to a
thiol
functionalized glass substrate.
In order to test for the importance of the presence of silver nanoparticles in
the
precursor, we irradiated film F13 (Example 25) for more than one hour in a W
chamber to
see if any characteristic nanoparticle absorption band would arise in the
optical absorption
spectrum or if any metal feature could appear. Only a bleaching of the dye
band was
-33-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
observed and absolutely no evidence of continuos silver metal or nanoparticle
formation, (as
evidenced by optical absorption) was observed, even under such extreme
irradiation
conditions.
Functionalized Metal Nanoparticles for the fabrication of continuous metal
features
Silver nanoparticles were synthesized with coatings of different organic
ligands.
Some of the ligands possessed groups capable of reducing, from their excited
state, silver
ions to the neutral atom. The structures of these ligands are shown in the
schematic drawing
below. Three dye-ligands are used to synthesize electron and photo-active
nanoparticles. The
names and the ligand shell composition of the particles used in some
experiments are listed in
the legend below.
O
R \ / \
\ / \ R
O
HS
R = N02 19
N(CH2CH3)2 I10
HC=O III
Metal ligands Given Name
Silver 11 +12 +19 nAglO
Silver 11 +12 +110 nAgl 1
Silver 11 +12+111 nAgl2
Silver 19 nAgl3
The synthesis of the ligand coated particles was a place exchange reaction
(Hostetler,
M. J., Templeton, A. C. & Murray, R. W. Dynamics of place-exchange reactions
on mono
layer-protected gold cluster molecules. Langmuir 15, 3782-3789 (1999)).
Starting from a
solution of nAg7 and the desired ligand we were able to synthesize particles
with dye
molecules in their outside shell. A different synthesis was used to obtain
nanoparticles
-34-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
completely coated by dye attached ligands (Example 29). In this case silver
ions were
reduced with NaBH4 in the presence of ligand 19.
Mixtures of these particles and a silver salt gave rise to larger particles
(up to the
continuous limit) upon excitation (light or electron beams) both in solution
and the solid
state. A few examples, that are not representative of the full potentiality of
these particles,
will be discussed hereafter. The main advantage of these materials systems
that is that films
of these particles are precursors for both e-beam and light-induced growth of
continuous
metal features.
In order to test the reactivity of composite materials containing dye-coated
particles, a
series of experiments were performed on a set of four films:
Films containing nanoparticles with reducing dyes on their ligand shell (nAgl
1) and
2% wt. of silver salt (AgBF4). (F14, F18)
Films of nanoparticles with reducing dyes on their ligand shell (nAgl 1) and
no silver
salt. (F15, F19)
Films of nanoparticles with no reducing dyes on their ligand shell (nAg7) and
2% wt.
of silver salt (AgBF4). (F16, F20)
Films of nanoparticles with no reducing dyes on their ligand shell (nAg7) and
no
silver salt. (1717, F21)
The reactivity of films with a thickness of -20 nm was tested in a scanning
electron
microscope (SEM). Film (i) was shown to be an efficient precursor for
continuous metal
features. In fact, combinations of squares and lines could be written using
the electron beam
of the microscope. The unreacted film could be washed away following
patterning with
dichlororethane to reveal the remaining metal pattern. The structures before
and after the
washing are shown in Figure 16. The left image of Figure 16 shows an example
of a square
and a line written and imaged using an SEM on F 14. The right image shows an
example of a
part of a square and a line imaged with an SEM after removal of the unexposed
film. All the
other films showed were inactive with respect to the electron beam patterning.
The same films, cast on a glass substrate, were excited with laser beams in
order to
test their photochemical activity for metal patterning. On film (i) a series
of lines was written
using both visible (488 nm, 50 mW, one photon excitation) and infrared (730
nm, 250 mW,
two-photon excitation) light. The written pattern was imaged before and after
removal of the
unreacted nanoparticles by washing. Again Film (i) was shown to be
photochemically active
-35-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
in forming metal patterns. Film (ii) was shown to be active as well as, but at
higher incident
laser power (80 and 400 mW for one- and two-photon excitation, respectively).
All the lines
were written at a speed of 2 m/s. Films iii and iv were not photochemically
active in
patterning metal.
Similar experiments were conducted on films using a the electron beam of a
transmission electron microscope (TEM), with films cast on supporting Si3N4
grids. The
solution for film casting was the same as that used for the SEM tests, but
were diluted 10 fold
in order prepare sub-monolayer films. In some areas of these films, dense
regions of
particles could be found and in others well separated nanoparticle were
observed. In all four
films isolated nanoparticles with no neighboring particles showed no
significant
morphological change during electron beam exposure. Films (iii) and (iv)
showed no
morphological change even in the regions that were more dense in particles.
Films (i) and
(ii), in their more dense regions, showed growth of the silver particles and
their coalescence
to form semi-continuous regions. Film (i) was shown to react quickly under
electron
irradiation. The reaction was slower in the film (ii).
The photochemical reactivity was tested on the same set of films cast onto
four
separate grids. All the films were initially imaged quickly in the TEM, in
order to obtain
initial reference images, and then they were irradiated with 488 nm light for
240 min. with an
intensity of 1.5 W/cmz. The films were then imaged again in the TEM. Only film
(i) showed
morphological changes. Figure 17 illustrates the average changes for film (i)
after electron-
beam and light-induced growth. Figure 17 illustrates the laser and electron-
beam induced
growth of silver nanoparticles in a nanoparticle/salt composite. a, TEM image
of a composite
prior to laser exposure, showing a domain of ordered nanoparticles with a mean
radius of 6
nm. b, Image of composite following one-photon excitation at 488 rem for 240
min. with an
intensity of 1.5 W/cmz (to ensure depletion of the silver salt), showing
growth of particles. c,
Image of composite prior to electron-beam irradiation, showing a domain of
ordered
nanoparticles with the same mean radius as in a. d, Image of composite
following electron-
beam irradiation in the TEM instrument for 15 min, showing growth of particles
and
formation of a nearly consolidated metal domain. Scale bars: 50 rem.
The same set of tests were repeated with films based on nAgl0, nAgl2 and nAgl3
metal particles, and the results were identical to those described above.
A thick polyvinylcarbazole (PVK) film (F22) containing nAg12 and a silver salt
was
-36-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
cast in order to test whether such a composite material based on the
functionalized
nanoparticles would function as a precursor for the growth of continuous metal
features. The
film was mounted on a microfabrication stage and irradiated with 730 nm light
(80 mW). It
was found that a line of silver could be written and this line was imaged with
optical
microscopy (Figure 18). Figure 18 shows the transmission optical microscopy of
a line
written in a PVK film (F22) doped with AgBF4 and nAgl2. Scale bar 30 gm.
Growth of reflective and conductive metal islands and wires in a polymeric
matrix
Films with compositions described in Examples 39 and 40 were cast on glass
slides
for two-photon microfabrication. Squares patterns of silver were written using
rastered laser
scanning. The reflectivity of these squares was probed with a He-Ne laser
(632.8 nm). The
incident power was 2 mW and the incidence angle was -30 . The written squares
of silver
showed a reflectance of 25% whereas the unexposed polymeric composite showed a
reflectance of 3%. A reflection image of the square taken using a confocal
microscope
(514.5 nm) and an interference filter for 514.5 nm which was placed between
the scan head
and the microscope and which blocks any fluorescence and allows the passage of
the
reflected light to the detector, is shown in Figure 19 which illustrates the
reflection image of
a silver square (right) embedded in a polymer nanocomposite.
The conductance of silver lines written on a glass substrate with an array of
conductive pads the surface was measured. A series of parallel silver pads
were deposited on
a glass substrate using standard lithographic techniques. Half the slide was
masked to allow
subsequent contacting to the pads. A series of 5 parallel lines, 200 gm long
with a section of
1 gmz, were fabricated at the substrate surface and perpendicular to the pads
to make
electrical contact between the written lines and the pads. The resistance of
lines were
measured between pads separated by varying distances. Measurements were also
made
between control pads that were not connected by written lines. The bias
voltage was ramped
from -2 V to +2 V, the measured current between the pads not connected by
microfabricated
lines was in the range of the noise level of the instrument (0.1 pA)
indicating a huge
resistance. The average resistance measured between two neighboring pads
connected by the
microfabricated lines (32 m spacing) was 370 0. The resistivity (p)
microfabricated lines
was determined to be about 10-3 O cm, without correction for contact
resistance (Figure 20).
Figure 20 is a schematic drawing of the slide/polymer/microfabricated line
configuration
used to measure the conductivity of the grown wires.
-37-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Microfabrication of Copper and Gold microstructures via two-photon excitation.
Films loaded with copper nanoparticles, copper salts and a dye ld, as
described in
Example 41, were cast and a pattern of copper wires was microfabricated using
two photon
excitation. The same pattern was microfabricated in a gold nanoparticle
composite,
described in example 41. A 3D "stack of logs" structure was successfully
microfabricated in
both composites and demonstrates that the methods described herein are general
and can be
applied to a variety of metals. Both of the structures are shown in Figure 21
which is a plot
of the measured I(V) curve, showing a resistance of 373 0.
Holographic data storage via photoinduced growth of silver nanoparticles.
The use of metal nanoparticle containing composite materials for holographic
data
storage was demonstrated using films described in Example 43. Two laser beams
crossing at
90 (see Figure 22 for the optical set up) were used for holographic exposure.
One of the
beams (the image) was expanded and passed through a resolution test mask and
the other
beam served a plane wave reference. The holographic exposure was performed
with an Af'
ion laser (514.5 nm) with a total power of 200 mW. After exposure, the image
was
reconstructed with a diffraction efficiency of 8% and the reconstructed image
was captured
using a digital camera (see Figure 23).
Figure 22 shows metallic structures fabricated in nanocomposites by two-photon
scanning laser exposure a, TOM image of copper microstructure in a different
polymer
nanocomposite fabricated by two-photon laser exposure. b, TOM image of a gold
microstructure fabricated by two-photon laser exposure. Scale bars: 25 m,
scale bars.
Figure 23 shows on the left an optical set up for the writing of holograms. On
the
right an optical set up for the readings of holograms is shown. The blue
ellipsoids represent
focusing lenses, while the gray rectangles are mirrors. The faint gray
rectangle is a 50/50
beam-splitter. The black rectangle on the right is a beam stop.
Syntheses
All reagents were purchased from Aldrich and used as received. All solvents
used are
reagent grade unless specified.
-38-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Silver nanoparticles
Silver nanoparticles capped with a single type of li ag nd (nAg1=4)
All the syntheses were done using the following procedure:
340 mg of AgNO3 (2 mmol) were dissolved in 100 ml of absolute ethanol at 0 C
under vigorous stirring. An amount that varied from 2/9 to 2/3 of a millimole
of the chosen
ligand was dissolved in a small amount of ethanol and added. A saturated
ethanol (200 ml)
solution of NaBH4 was prepared and, 30 min after the addition of the ligand,
was added very
slowly (over 2 hours). The solution immediately turned yellow and then slowly
became very
dark. The solution was left stirring for additional 2 hours and then it was
put in a refrigerator
to flocculate.
On the next day the solution was vacuum filtered using a quantitative paper
filter
(VWR) with pore diameter of 1 gm and the filtered powder was washed twice with
ethanol
and various times with acetone.
The yields varied from 49 to 81 %.
Example 1:
Synthesis of dodecanethiol-coated silver nanoparticle (nAg1)
330 mg of AgNO3 ('2 mmol) were dissolved in 200 ml of absolute ethanol at 0 C
under vigorous stirring. 58 mg of dodecanethiol (2/6 mmol) were dissolved in
10 ml of
ethanol and added to the starting solution. A saturated ethanol (200 ml)
solution of NaBH4
was prepared and, 30 min after the addition of the ligand, was added very
slowly (2 hours).
The solution immediately turned yellow and then slowly became very dark. The
solution
was left stirring for other 2 hours and then it was put in a refrigerator to
flocculate.
On the next day the solution was filtered under vacuum using a quantitative
paper
filter (VWR) with a pore diameter of 1 gm and the filter powder was washed
twice with
ethanol and various times with acetone.
190.65 mg of a black powder were collected, giving a yield of 71 %.
Silver nanoparticle coated with two or more types of ligands
The syntheses were done using one of two different strategies. The first
strategy a)
involved a one step reaction in which nanoparticles are synthesized in the
presence of
-39-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
multiple ligands, the second strategy b) involved a two step reaction in which
nanoparticle
undergo a ligand exchange reaction to introduce a second type of ligand.
a) For the first strategy the following approach was used:
340 mg of AgNO3 (2 mmol) were dissolved in 100 ml of absolute ethanol at 0 C
under vigorous stirring. A 10 ml solution of the desired mixture of ligands
was prepared.
The molar ratio of the ligands (llligandA ) was between 1 and 0.25
llligandB
and the total amount was chosen so that the ratio between the moles of ligands
and the moles
of silver was between 2/9 to 2/3. 30 min after the addition of the ligand, a
saturated ethanol
(200 ml) solution of NaBH4 was prepared and, was added very slowly (over 2
hours). The
solution immediately turned yellow and then slowly became very dark. The
solution was left
stirring for other 2 hours and then it was put in a refrigerator to
flocculate.
On the next day the solution was filtered under vacuum using a quantitative
paper
filter (VWR) with pore diameter of 1 m and the filter powder was washed twice
with
ethanol and various time with acetone.
Some types of nanoparticles did not flocculate upon cooling and in those cases
the
solvent was evaporated under vacuum and then the residue was suspended in
water under
sonication for 15 min. The water was then left in the hood for 2h to
flocculate and then
filtered according to the standard procedure.
The yields varied from 30 to 75%.
Example 2:
Synthesis of octanethiol-thiol coated silver nanoparticle (nAg6).
340 mg of AgNO3 (2 mmol) were dissolved in 200 ml of absolute ethanol at 0 C
under vigorous stirring. 24 mg of octanethiol (1/6 mmol) were dissolved in 10
ml of ethanol
together with 156 mg of I4 (V/2 mmol) and added to the starting solution. A
saturated ethanol
(200 ml) solution of NaBH4 was prepared and, 30 min after the addition of the
ligands, was
added very slowly (2 hours). The solution immediately turned yellow and then
slowly
became very dark. The solution was left stirring for other 2 hours and then it
was put in a
refrigerator to flocculate.
On the next day the solution was filtered under vacuum using a quantitative
paper
filter (VWR) with pore diameter of 1 m and the filter powder was washed twice
with
-40-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
ethanol and various time with acetone.
Yield: 273 mg of a black-greenish powder.
b) the second strategy was an exchange reaction
This kind of reaction was done following a known method (Hostetler, M. J.,
Templeton, A. C. & Murray, R. W. Dynamics of place-exchange reactions on mono
layer-
protected gold cluster molecules. Langmuir 15, 3782-3789 (1999)).
Example 3:
Synthesis of octanethiol-is coated silver nanoparticle (nAg9).
Ligand exchange reaction on silver nanoparticles nAgl: The silver
nanoparticles nAgl
(85.4 mg) coated with octanethiol were dissolved by stirring overnight in
CH2C12. Then the
ligand I8 (14.2 mg, 0.024 mmol) is added and the dark brown solution is
stirred for 5 days in
the absence of light. The CH2C12 was removed in vacuum and the brown residue
is dispensed
in EtOH. The particles set down overnight and can be filtered off with
quantitative
filterpaper and washed several times with acetone.
Yield: 17 mg. Elemental analysis: nAg9: C: 26.30, H: 4.31, S: 7.38, Ag: 53.99
nAgl: C: 22.85, H: 4.62, S: 7.23, Ag: 62.50
Octanethiol: C: 66.19, H: 11.79, S: 22.07
I8: C: 70.66, H: 6.48, S: 9.81.
Calculation based on the elemental analysis give a weight ratio for the
ligands of ca. 85
Octanethiol and 15 % TMF-I-48. The calculated molar ratios are:
n18/nOctanethiol = 0.043
nAg/nOctanethiol + n18 - 1.78 in nAg9
nAJnOctanethiol = 2.25 in nAgl
'H NMR (CDC13): The 'H NMR reveals the spectrum of the ligand I8.
Preparation of gold nanoparticles
Gold nanoparticles were prepared according to the procedure of Brust (Brust,
M.,
Walker, M., Bethell, D., Schiffrin, D. J. & Whyman, R. Synthesis of Thiol-
Derivatized Gold
Nanoparticles in a 2-Phase Liquid-Liquid System. Journal of the Chemical
Society-
Chemical Communications, 801-802 (1994)).
-41-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Example 4:
Synthesis dodecanethiol-coated gold nanoparticle (nAul).
352.8 mg of HAuC14*3H2O (0.9 mmol) were dissolved in 30 ml of deionized water,
2.188 g of tetraoctylammoniumbromide (4 mmol) were dissolved in 80 ml of
toluene. The
two phases were mixed and stirred for 1 h. 170 mg of dodecanethiol (0.84 mmol)
were
dissolved in 10 ml of toluene and added. After 10 min 380 mg of NaBH4 were
dissolved in
25 ml of water and added all at once. Soon the organic layer became black.
After 2 h the
organic layer was separated and washed 3 times. The toluene was reduced to 10
ml under
vacuum and immediately diluted to 500 ml with ethanol and the solution put in
a refrigerator
overnight. On the next day the solution was filtered on a qualitative filter
paper and washed
with toluene multiple times.
mg of a black powder were collected.
Copper nanoparticles
Copper nanoparticle capped with a single type of ligand
237 mg of CuBF4*H20 (1 mmol) were dissolved in 100 ml of absolute ethanol
(degassed by argon bubbling for at least an hour) at 0 C under vigorous
stirring in Argon
atmosphere. An amount that varied from 2/9 to 2/3 of a millimole of the chosen
ligand was
dissolved in a small amount of ethanol and added. Solution immediately turned
bright
yellow. After 2h a saturated (100 ml) NaBH4 solution of degassed ethanol was
prepared and
was added very slowly (over 3 hours). The solution immediately turned dark
yellow and
then slowly became very dark. The solution was left stirring for other 2 hours
and then it was
put in a refrigerator to flocculate.
On the following day the solution was filtered under vacuum using a
quantitative
paper filter (VWR) with pore diameter of 1 m and the filter powder was washed
twice with
ethanol and various times with acetone. The whole reaction was made in
controlled
atmosphere.
Example 5:
Synthesis of dodecanethiol-coated copper nanoparticle ,nCul).
228 mg of CuBF4*H20 (0.96 mmol) were dissolved in 100 ml of absolute ethanol
-42-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
(degassed by argon bubbling for at least 2 h) at O 'C under vigorous stirring
in Argon
atmosphere. 51 mg of octanethiol (-1/3 mmol) were dissolved in a small amount
of ethanol
and added. Solution immediately turned bright yellow. After 2 h a saturated
(100 ml)
NaBH4 solution of degassed ethanol was prepared and was added very slowly (3
hours). The
solution immediately turned yellow and then slowly became very dark. The
solution was left
stirring for other 2 hours and then it was put in a refrigerator to
flocculate.
On the following day the solution was filtered under vacuum using a
quantitative
paper filter (VWR) with pore diameter of 1 gm and the filter powder was washed
twice with
ethanol and various times with acetone. The whole reaction was made in
controlled
atmosphere.
The yield was 40 mg of a black powder.
Copper nanoparticle coated with two or more Wes of ligands.
237 mg of CuBF4*H20 (1 mmol) were dissolved in 100 ml of absolute ethanol
(degassed by argon bubbling for at least an hour) at 0 C under vigorous
stirring in Argon
atmosphere. A 10 ml solution of the desired mixture of ligands was prepared
and added.
The molar ratio of the ligands ( r1ligandA ) was between 1 and 0.25 and the
total
'lligandB
amount was chosen so that the ratio between the moles of ligand and the moles
of silver was
between 2/9 to 2/3. After 2 h a saturated (100 ml) NaBH4 solution of degassed
ethanol was
prepared and was added very slowly (over 3 hours). The solution immediately
turned yellow
and then slowly became very dark. The solution was left to for other 2 hours
and then it was
put in a refrigerator to flocculate.
On the following day the solution was filtered under vacuum using a
quantitative
paper filter (VWR) with pore diameter of 1 gm and the filter powder was washed
twice with
ethanol and various time with acetone. The whole reaction was made in
controlled
atmosphere.
Example 6:
Synthesis of octanethiol-carbazolethiol coated copper nanoparticle (nCu3).
240 mg of CuBF4*H2O (0.974 mmol) were dissolved in 100 ml of absolute ethanol
(degassed by argon bubbling for at least 2 h) at 0 C under vigorous stirring
in Argon
-43-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
atmosphere. 76 mg of octanethiol (''/2 nimol) and 35 mg of dodecanethiol (-1/6
mmol) were
dissolved in a small amount of ethanol and added. Solution immediately turned
bright
yellow. After 2h a saturated (100 ml) NaBH4 solution of degassed ethanol was
prepared and
was added very slowly (3 hours). The solution immediately turned yellow and
then slowly
became very dark. The solution was left to for other 2 hours and then it was
put in a
refrigerator to flocculate.
On the following day the solution was filtered under vacuum using a
quantitative
paper filter (VWR) with pore diameter of 1 gm and the filter powder was washed
twice with
ethanol and various time with acetone. The whole reaction was made in
controlled
atmosphere.
The yield was 45 mg of a black powder.
Dye and li ag nd syntheses
Most of the molecules and polymers used in the Examples were prepared
according to
literature methods, the synthesis of the new molecules is described here:
19
OH
O O
\O
C27H24O4
Exact Mass: 412.17
Mol. Wt.: 412.48
C, 78.62; H, 5.86; 0, 15.52
4- {2-[4-[2-(4-formylphenyl)vinyl]-2-(3-
hydroxypropoxy)phenyl]vinyl}benzaldeh~de
(TMF-I-39): A solution of mono(diethyl)acetal terephthalaldehyde (1.75 ml, 8.8
mmol) and
diethyl 2-(3- { [tert-butyl(dimethyl)silyl]oxy}-propoxy)-4-
[(diethoxyphosphoryl)methyl]benzyl phosphonate (2.49 g, 4.4 mmol) in tetra
hydrofuran
-44-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
(THF) (100 ml) was cooled to 0 C with an ice bath. K2C03 (10 ml 1 M solution
in THF, 10
mmol)) was added slowly via a syringe and reaction is allowed to warm up to
room
temperature. After stirring overnight water was added followed by 1 M HCl (50
ml) and the
reaction mixture was stirred for another hour. The product was extracted with
CH2C12 and
chromatographed on silica. The first fraction eluted with CH2C12 was rejected
and the
product was then collected using ethylacetate as solvent. Crystallization from
CH2C 12 gave
the pure product as yellow solid (663 g).
'H NMR (CDC13): 10.01 (1H, s), 10.00 (1H, s), 7.88 (4H, t, J = 7.5 Hz), 7.67
(4H, t, J = 7.5
Hz), 7.60 - 7.64 (3H, m), 7.12 - 7.24 (4H, m), 4.30 (2H, t, J = 6.0 Hz), 3.97
(2H, t, J = 6.0
Hz), 2.20 (2H, tt, J = 6.0, 6.0 Hz), 1.61 (1H, br s) ppm; element. anal.:
calcd. C:
78.62 H: 5.86, found C: 78.36 H: 5.67.
S
S
O
0
0~ O
C35H3605S2
Exact Mass: 600.20
Mol. Wt.: 600.79
C, 69.97; H, 6.04; 0, 13.32; S, 10.67
3-{2,5-bis[(E)-2(4-formyl
~phenyl ethenyl]phenoxy}-propyl5-(1,2-dithiolan-3-vl)
pentanoate:
-45-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
A solution of 4-{2-[4-[2-(4-formylphenyl)vinyl]-2-(3-
hydroxypropoxy)phenyl]vinyl}-benzaldehyde (above) (200 mg, 0.49 mmol), lipoic
acid (100
mg, 0.49 mmol) and p-toluenesulfonic acid (20 mg, 0.10 mmol) was refluxed
overnight in the
minimum amount of CH2C12 (z20 ml) necessary to dissolve the chromophore. The
reaction
mixture was poured onto a column (A1203/CH2C12) and flash chromatographed with
CH2C12:Ethylacetate/10:1. The starting material was recovered using ethyl
alcohol (EtOH).
Yield: 90 mg (31 %) yellow solid.
1H NMR (CDC13): 9.974 (1H, s, CHO), 9.969 (1H, s, CHO), 7.86 (2H, d, J = 8.5
Hz), 7.85
(2H, d, J = 8.5 Hz), 7.65 (4H, d, J = 8.0 Hz), 7.57 - 7.62 (2H, m), 7.04 -
7.24 (5H, m), 4.36
(2H, t, J = 6,5 Hz), 4.19 (2H, t, J = 6.0 Hz), 3.49 (1H, m), 3.12 (1H, m),
3.05 (1H, m), 2.39
(1H, m), 2.31 (2H, t, J = 7.0 Hz), 2.25 (2H, m), 1.84 (1H, m), 1.57 - 1.69
(4H, m), 1.35 - 1.48
(2H, m) ppm. 13C NMR (CDC31): 191.86 (CHO), 191.77 (CHO), 173.65, 156.78,
144.08,
143.28, 138.09, 135.60, 135.38, 131.86, 130.47, 128.27, 128.00, 127.36,
127.16, 126.52,
126.30, 120.16, 110.30, 65.19, 61.33, 56.52, 40.42, 38.66, 34.76, 34.21,
29.89, 28.94, 24.85
ppm.
Polymer synthesis (a2)
-46-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
O Benzene/BTEAC/
+ Br-(CH2)10-COOH NaOH (50%), 60 C OO
N N
H (CH2)10-COOH
O
O
2-Hydroxyethylmethacrylate/
THF/DCC, r.t. 0
1
(CH2)10
N Cm
n
O O
Benzene/AIBN, 60 C
0
1
( IH2)1o
N
BTEAC = Benzyltriethylammonium chloride
DCC = 1,3-Dicyclohexylcarbodiimide
AIBN = 2,2'-Azobisisobutyronitrile
Synthesis of carbazole monomer cm_
To a solution of carbazole acid (5.0 g, 14.23 mmol) and 2
hydroxyethylmethacrylate
(2.0 g, 15.37 mmol) and 4-diinethylamino-pyridine (0.2 g) in THE (30 ml) was
added DCC
(3.7 g, 17.96 mmol) at room temperature. The reaction was carried out at this
temperature
-47-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
for 10 h. Solid was removed by filtration. After removal of THF, the crude
product was
purified by silica gel column using hexane/ethyl acetate (9:1) as eluent. The
pure product as
colorless oil was obtained in 4.2 g (63.6%).
'H-NMR (CDC13, TMS, 500 MHZ): 8 = 8.12 (d, 2 Harom. J = 7.5 Hz), 7.47 9m, 2
Harom), 7.42
(d, 2 Harom, J = 7.5 Hz), 7.24 (m, 2 Harom), 6.13 - (s, 1 H, C=C-H), 5.60 (s,
1 H, C=C-H), 4.3 5
(m, 4 H, 2 x OCH2), 4.30 (t, 2 H, NCH2, J = 7.5 Hz), 2.33 (t, 2 H, COCH2, J =
7.0 Hz), 1.95
(s, 3H, CH3), 1.88 (m, 2 H, CH2), 1.61 (m, 2 H, CH2), 1.24 (m, 12 H, 6 x CH2)
ppm.
13C-NMR (CDC13, 126 MHZ): S = 173.58, 167.07, 140.34, 135.87, 126.02, 125.51,
122.73,
120.29, 118.63, 108.59, 62.42, 61.82, 43.01, 34.09, 29.38, 29.35, 29.30,
29.14, 29.00, 28.92,
27.26, 24.84, 18.25 ppm.
Elemental Analysis for C29H37NO4 (463.61): Cald: C, 75.13; H, 8.04; N, 3.02.
Found:
C, 75.08; H, 7.83; N, 3.28.
Synthesis of carbazole polymer PCUEMA:
Carbazole monomer (2.7 g, 5.82 mmol) and AIBN (14.3 mg, 0.087 mmol) were
dissolved in dry benzene (5.0 ml) under nitrogen. The reaction mixture was
cooled with
liquid nitrogen. After one freeze-thaw-pump cycle the reaction was heated at
60 C for 60 h.
The polymer was precipitated in methanol and collected by filtration. The
polymer was
dissolved in THE solution and precipitated in methanol. The
dissolution/precipitation/
filtration sequence was repeated twice. After drying, the white polymer was
obtained in 2.65
g (98.1%) yield.
'H-NMR (CDCL3, TMS. 500 MHZ): 8 = 7.97 (d, 2 Harom, J = 8.0 Hz), 7.31 (m, 2
Harom), 7.24
(d, 2 Harom, J = 8.0 Hz), 7.09 (m, 2 Harom), 4.07 (m, 4 H, 2 x OCH2), 4.01 (s,
br, 2 H, NCH2),
2.17 (s, br, 2 H, COCH2), 1.68 (m, 2H, CH2), 1.45 (s, br, 2 H, CH2), 1.07 (m,
12 H, 6 x CH2),
0.93 (s, br, 2 H, CH2), 0.80 (s, br, 3 H, CH3) ppm. 13C-NMR (CDC13, 126 MHZ):
8 = 173.25,
140.30, 128.30, 125.48, 122.70, 120.26, 118.63, 108.56, 62.65, 61.16, 44.82,
42.89, 33.78,
29.44, 29.35, 29.28, 29.08, 28.90, 27.22, 24.74 ppm.
I and I5
-48-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Benzene/BTEAC/
+ Br-(CH2)n-Br NaOH (50%), 60 C
ONO N
I I
H (CH2)n- Br
DMSO/Thiourea/
NaOH, HC1
(CH2)n- SH
Example 7:
Synthesis of li ag nd I4
3.57g 9-carbazole-yl-octane-l-thiol ('10 mmol) were dissolved in 20 ml of
dimethylsulfoxide (DMSO), 1.52 mg of thiourea (-20 mmol) were added, the
solution was
vigorously stirred. After 2 days a concentrated aqueous solution of NaOH was
added
dropwise. Soon a red precipitated formed, during the addition the precipitated
redissolved
again and solution turned red. Addition was stopped upon reaching of pH 11
(checked using
pH paper). The solution was then neutralized adding dropwise HCl (aq. cone)
and it slowly
turned yellow. The organic was then extracted with diethyl ether (Et2O) and
washed with
water three times. The organic solvent was dried under vacuum, and the residue
was
collected.
Sample Preparation
In this section several examples of nanocomposite sample processing and
preparation
are given. All films hereafter reported have been tested and metallic silver
lines have been
successfully written using radiation in all cases on.
Samples were prepared by solvent casting or by spin coating, Most of the
samples
were cast in air atmosphere, in some cases the processing was done under argon
atmosphere.
All glass microscope slides were cleaned with the following procedure:
a) Sonication for 1 h in water and soap and extensive rinse with DI water
-49-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
b) Sonication for lh in spectroscopic grade methanol and rinse with absolute
ethanol
or isopropanol.
Glass slides with monolayer coatings of nanoparticles were processed after
cleaning
as follows (hereafter referred as monolayered slides):
a) Dipped for 10 min in a saturated isopropanol (reagent) solution of KOH, and
then
rinsed with DI water and dried using a nitrogen flow.
b) A solution of 75 ml of toluene, 0.5 ml of isopropylamide and 2 ml of 2-
mercaptopropyltrimethylsiloxane was prepared and kept at 60 C for an hour.
c) the slides were dipped in the solution for lh at 60'C, and then rinsed with
hexane
(spectrophotometric grade).
d) The samples were immersed overnight in hexane.
e) A CH2CI2 solution (2 mg/ml of nanoparticle nAg6) was solvent casted by
solvent
evaporation on the slides
f) The samples were immersed overnight in hexane.
ITO (Indium Tin Oxide) slides were cleaned simply by rinsing them in ethanol
on
them.
Nanocomposite film casting by solvent evaporation
A fixture for hold samples for casting by solvent evaporation was fabricated
and used
for all such castings this plate held substrates fixed in a horizontal
position and allowed for
control of the atmosphere. Under each slide 3 ml of reagent grade chloroform
were placed
prior to casting so to initially maintain the saturation of the atmosphere
with solvent vapor
upon introduction of the casting syrup. Each slide compartment was closed
using a watch
glass, so that the volume of air in which each slide was casted was approx. 15
cm3.
Solvents were degassed by freeze-pump-thaw cycle.
Example 8:
Fl Standard 100 m film
188.86 mg of polymer a, (poly 9-vinylcarbazole), 89.6 mg of ethylcarbazole,
2.59 mg
of nAg6, and 8.77 mg of dye Id were dissolved in 6 ml of degassed chloroform
under argon
atmosphere and left stirring overnight.
On the following day 22 mg of AgBF4 were dissolved in 0.02 ml of degassed
-50-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
acetonitrile and mixed to the chloroform solution. After 30 min the solution
was filtered with
a membrane filter (1 m pore size). 0.6 ml of the filtered solution were
casted on a 25 X 25
mm ITO slide.
Example 9:
F2 Standard 100 m film
660 mg of polymer a, (poly 9-vinylcarbazole), 329 mg of ethylcarbazole, 2.8 mg
of
nAg6, and 28 mg of dye Id were dissolved in 6 ml of degassed chloroform under
argon
atmosphere and left stirring overnight.
On the following day 110 mg of AgBF4 were dissolved in 0.33 ml of degassed
acetonitrile and 0.3 ml of those were added to the chloroform solution. After
10 min the
solution was filtered with a membrane filter (1 gm pore size). 2 ml of the
filtered solution
were casted on a 75 X 25 min glass slide.
Example 10:
F3 20.11m film with high loading of nanoparticles
92 mg of polymer a, (poly 9-vinylcarbazole) were dissolved in 5 ml of
dichloromethane (DCM), 12 mg of AgBF4 were dissolved in 5 ml of DCM and 1 ml
of
acetonitrile, 5 mg of dye Id were dissolved in 5 ml of DCM, 5.5 mg of nAgl
were dissolved
in 2 ml of chloroform. All solution were stirred for 2 h and then mixed
together. 2 ml of the
solution were carted on a 75 X 25 mm glass slide.
Example 11:
F4 Films for TEM
11.5 mg of polymer a, (poly 9-vinylcarbazole), 2.4 mg of AgBF4, 2.8 mg of dye
Id, 1
mg of nAgl were dissolved in 10 ml of DCM; solution was diluted 10 times and 2
1 of this
solution were carted on a carbon coated copper grid. Three identical films
were made in this
way.
Figure 9 shows TEM images illustrating growth of metal nanoparticle in a
composite
film upon exposure to either one or three laser pulses from a ns pulsed laser.
The upper TEM
image shows the system after one laser shot, the lower after three. In the
upper limit the
average radius has become 4.9 nm, in the non-irradiate sample it was 2.9. In
the lower image
-51-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
the average diameter is even bigger and larger metal islands can be seen.
Example 12:
FS Standard 100 m film with a different kind of nanoparticle
648 mg of polymer a, (poly 9-vinylcarbazole), 316 mg of ethylcarbazole, 2.75
mg of
nAg7, and 20.7 mg of dye Id were dissolved in 6 ml of degassed chloroform
under argon
atmosphere and left stirring overnight.
On the following day 219 mg of AgBF4 were dissolved in 0.6 ml of degassed
acetonitrile and 0.2 ml were mixed to the chloroform solution. After 30 min
the solution was
filtered with a membrane filter (1 m pore size). 0.4 ml of the filtered
solution were carted
on a 25 X 25 mm ITO slide.
Example 13:
F6 Standard 10 m film
64.5 mg of polymer a, (poly 9-vinylcarbazole), 38.1 mg of ethylcarbazole, 1.21
mg of
nAg6, and 3.31 mg of dye Id were dissolved in 6 ml of degassed chloroform
under argon
atmosphere and left stirring overnight.
On the following day 20.4 mg of AgBF4 were dissolved in 0.3 ml of degassed
acetonitrile and 0.1 ml were mixed to the chloroform solution. After 30 min
the solution was
filtered with a membrane filter (1 m pores). 0.4 ml of the filtered solution
were casted on a
75 X 25 mm glass slide.
Example 14:
F7 Standard 100 m film with a different dye
411.16 mg of polymer a, (poly 9-vinylcarbazole), 206.48 mg of ethylcarbazole,
2.28
mg of nAg6, and 19.72 mg of dye 2b were dissolved in 6 ml of degassed
chloroform under
argon atmosphere and left stirring overnight.
On the following day 50.8 mg of AgBF4 were dissolved in 0.3 ml of degassed
acetonitrile and mixed to the chloroform solution. After 30 min the solution
was filtered with
a membrane filter (1 m pores). 0.6 ml of the filtered solution were casted on
a 75 X 25 mm
monolayered glass slide.
-52-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Example 15:
F8 Standard 100 m film with a different polymer
95.1 mg of polymer a2 (PCUEMA), 0.71 mg of nAg6, and 1.8 mg of dye 1d were
dissolved in 1 ml of degassed chloroform under argon atmosphere and left
stirring overnight.
On the following day 9.5 mg of AgBF4 were dissolved in 0.05 ml of degassed
acetonitrile and mixed to the chloroform solution. After 30 min the solution
was filtered with
a membrane filter (1 m pores). 0.6 ml of the filtered solution were casted on
a 25 X 25 mm
monolayered glass slide.
Example 16:
F9 Standard 100 um film for copper generation
66.6 mg of polymer a4 (poly methylmethacrylate), 1.1 mg of nCul, and 3.08 mg
of
dye Id were dissolved in 0.6 ml of degassed chloroform under argon atmosphere
and left
stirring overnight.
On the following day 5 mg of ICuP(CH3)3 were dissolved in 0.05 ml of degassed
acetonitrile and mixed to the chloroform solution. After 30 min the solution
casted on a 25 X
25 mm glass slide
Spin Coated films
Example 17:
F10 Standard spin coated film
100 mg of polymer a, (poly 9-vinylcarbazole), 6 mg of ethylcarbazole, 3 mg of
nAg4,
and 4.5 mg of dye Id were dissolved in 1 ml of chloroform and left stirring
overnight.
On the following day 100 mg of AgBF4 were dissolved in 1 ml of acetonitrile
and 0.1
ml were added to the chloroform solution. After 30 min the solution was spin
coated on a
25X25 mm glass slide at 2000RPM for 20s. The obtained thickness was -'8 m, as
proved by
prism coupler measurements.
Nanoparticle containing viscous liquid
Example 18:
F11 Standard viscous liquid matrix film
-53-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
A quantity of host c, was heated using an heatgun and as soon as it flowed
freely it
was pipetted in a vial into order to weigh a fixed amount.
226 mg of host c1, 2 mg of nAg6, and 5.77 mg of dye Id were dissolved in 2 ml
of
chloroform and left stirring overnight.
On the following day 11 mg of AgBF4 were dissolved in 0.1 ml of acetonitrile
and
mixed to the chloroform solution. After 30 min the solution was casted on a 75
X 25 mm
glass slide.
Class I films
Example 19:
F12 Standard Class I film
5.23 mg of nAgl, and 0.5 mg of dye Id, and 0.5 mg of AgBF4 were dissolved in 2
ml
of chloroform left stirring overnight.
On the following day the solution was casted on a 75 X 25 mm glass slide.
Figure 10 shows a silver ribbon written with a two-photon irradiation (800 nm,
120fs)
(Example 19).
Metal writing in nanoparticle composites using one and two photon excitation
All writing experiments were performed using a femtosecond mode-locked
Ti:sapphire laser. Specifically a Spectra Physics system consisting of a
Tsunami (Ti:sapphire
laser) pumped by a Millenia (diodes pumped YAG laser) was used. The average
pulse length
was 120fs with a bandwidth of -20nm. Unless specified otherwise the wavelength
used was
760nm.
The sample was mounted on a micropositioner (Sutter MP-285). The laser beam
was
focused on the sample using an inverted microscope (Nikon). A computer
controlled both
the micropositioner and a shutter (Newport 846HP). The combination of the
micropositioner
movements and the opening/closing cycles of the shutter allowed patterned
exposures and
metallic structures to be written in the sample.
In order to locate the focus of the beam in the sample a two-photon microscopy
setup
was used, the beam was going through a Biorad MRC-1024 scanhead.
-54-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Writing using single photon excitation
In the case of writing using single photon excitation, the laser output light
was
frequency doubled double using a LBO doubling crystal and the remaining
fundamental light
left was filtered away using a combination of a crystal polarizer and an
infrared short pass
dielectric filter.
The rest of the set-up was identical to the twp-photon writing process, above
described. The focusing process was done using the scanhead in a confocal
fashion.
Example 20:
Threshold measurements for one-photon writing of silver
Film F1 (Example 8) was mounted on the micropositioner, in the standard way.
The
laser output wavelength was set at 860 nm (420 mW) and was not changed during
the
experiment. A filter wheel was placed in the set up so to be able to make
continuous and
controllable variations in the average power of the laser. The stage
translation speed was set
at 10 gm/s. The beam was focused in the sample using a 60X objective (NA 1.4).
It was
possible to write lines in the film everywhere and the behavior was mostly
uniform: so for a
comparison with the two-photon experiment the threshold was calculated at the
glass/film
interface. The power was gradually decreased and the success of the writing
process was
determined by optical microscopy. The writing threshold was found to be 0.09
mW. If we
make the hypothesis of a circular beamspot with a diameter of 1 m, we find a
threshold
intensity of approx. 108 W/m2 for these 120fs pulses.
For a pattern of lines written spaced of 5 m we were able to put an upper
limit to
their width using the optical images, this limit being 500 nm.
Figure 11 shows silver lines written using a one-photon excitation (430 nm),
lines are
clearly visible. The dark spots are defects in the film which was not of
optimal quality.
Writing using two-photon excitation.
Dye utilized herein as two-photon excitable photoreducing agents were known to
have a reasonably large two-photon cross-section thus allowing efficient two-
photon
excitation. This in combination with a high NA focusing system, allows writing
high
resolution lines in three-dimensional patterns in the matrix.
-55-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Example 21:
Threshold measurements for two-photon writing of silver
Film F1 (Example 8) was mounted on the micropositioner, in the standard way.
The
laser output wavelength was set at 760 nm (620 mW) and was not changed during
the
experiment. A filter wheel was placed in the set up so to be able to make
continuous and
controllable variations in the average power of the laser. The stage
translation speed was set
at 10 gm/s. The beam was focused in the sample using a 60X objective (NA 1.4).
Lines
were written everywhere in the film the behavior was mostly uniform, so to
have the
possibility to further develop the structures the threshold was calculated at
the glass/film
interface. The power was gradually decreased and the success of the writing
process was
determined by optical microscopy. The writing threshold was found to be 1.55
mW. If we
make the hypothesis of a circular beamspot with a diameter of 1 gm, we find an
intensity
threshold of approx. 1.5 109 W/m2.
For a pattern of lines written spaced of 5 gm we were able to put an upper
limit to
their width using the optical images, this limit being 1 gm.
Example 22:
Multiphoton writing and developing
Film F3 (Example 10) was mounted on the micropositioner, in the standard way.
The
laser output wavelength was set at 800 nm (400 mW) and was not changed during
the
experiment. The writing speed was set at 100 gm/s. The microscope objective
used was a
10X. Lines were written at the glass/film interface. A regular pattern of 6
sets of 5 lines
each was written. Each set was places 30 gm away from the previous and the
lines were
spaced 10 mm away to each other; all the lines were 500 gm long. After writing
the film was
placed in a DCM containing beaker and left there for 3 days. In doing these
the polymer was
washed away and the lines stayed on the substrate.
Example 23:
Multiphoton writing and developing
Film F2 (Example 9) was mounted on the micropositioner, in the standard way.
The
laser output wavelength was set at 760 nm (620 mW) and was not changed during
the
experiment. The stage translation speed was set at 10 gm/s. The beam was
focused in the
-56-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
sample using a 60X objective (NA 1.4) and an immersion oil was used. Lines
were written
everywhere in the film the behavior was mostly uniform. Many different
patterns were
written, the most significant one being a cage "like" structure with a 13
layer of sets of lines
each layer being 5 gm higher than the previous, each layer consisting of 20
lines 100 gm
long and spaced of 5 gm, each layer consisting of lines perpendicular to the
ones of the
previous layer.
After the writing process was done the film was removed from the
micropositioner,
cleaned from the immersion oil using a paper tissue and then put in a solution
of DCM and
acetonitrile (10:1). The polymer dissolved away leaving the structure on the
substrate.
Figure 4 illustrates an optical transmission image, (top view) of a 3D
structure
(200x200x65gm) written in a polymer a, matrix. The writing process was done
using a two-
photon excitation (760 nm, 120fs).
Figure 5 illustrates an optical image of the same structure shown in Fig. 4 on
a larger
scale, the optical quality of the matrix is clearly quite good.
Figure 6 is a SEM image of a 3D metallic silver microstructure formed by two-
photon
writing in a composite film, they revealed by washing to produce a free
standing structure on
the surface.
Example 24:
Multiphoton writing of copper structures
Film F9 (Example 16) was mounted on the micropositioner, in the standard way.
The
laser output wavelength was set at 760 nm (620 mW) and was not changed during
the
experiment. A square of copper was written using the a 1Ox lens. The result
was checked
via optical imaging.
Figure 12 shows an optical micrograph of a copper square (200 X 200 gm)
written by
two-photon excitation.
Example 25:
Control experiment. F13
mg of PVK where dissolved in 10 ml of dichloromethane, l mg of AgBF4 was
dissolved in the same solution, 1 mg of dye 1 was then added. The solution was
casted on a
75x25 mm glass slide.
-57-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
The film was put in a W chamber and irradiated at 419 nm with 15 lamps (5W
each).
The optical absorption was followed and the results are summarized in Figure
13, just the
bleaching of the nanoparticle band was observed.
Figure 13 shows a spectrum of the sample from control experiment showing the
absence of formation of metal nanoparticle in a film containing dyes and metal
salt but no
initial concentration of metal nanoparticle. Note the absence of the
absorption band of metal
nanoparticles following exposure. The solid line represent the spectrum of the
film 13, the
bands around 300 nm are due to the polymer itself while the band at 426 is
characteristic of
dye 1. The dashed line represents the same film irradiated for 30 min while
the dotted one
for 60 min.
Characterization
XPS
Metallic Silver lines written according to the procedures described above
(Example
22) were analyzed using XPS spectroscopy and their Auger Parameter was
measured to be
726.2 meV, perfectly matching the tabulated one for Ag . Images of the written
lines could
be recorded using the spectrometer in an imaging mode.
Figure 7 shows a XPS spectrum (above) and image (below) a set of silver lines.
The
Auger parameter obtained from the spectrum is 726.2, which is the same as that
tabulated one
for zerovalent silver.
SEM
The structures were coated with a very thin metallic layer (Au/Ir alloy)for
SEM
imaging. Figure 14 shows an SEM picture of the comer of a 3D metallic silver
structure
written using two-photon excitation. The multiple layers written are evident.
TEM characterization of metal nanoparticle growth.
A nanosecond YAG laser (20 Hz) doubled light (532 nm) was used in single shot
fashion for the following experiment. One of the amorphous carbon coated
copper grid with
the composite film on it (estimated thickness 50 nm) was not used and left as
a reference, the
second one received a single laser pulse, the third one received three laser
pulses. The
-58-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
average energy of each pulse was 120 J. The average radius of the particle in
the reference
grid was found to be 2.95 nm; in the second grid it was 4.95 nm, in the third
grid it was found
to be approx. 9.5 nm. Moreover the number of nanoparticle per unit area was
found not to
increase.
Figure 15 shows a TEM image of chemically synthesized nanoparticles (nAgl)
used
as a precursor in the composites. A solution of nanoparticles sample was
sonicated for a
minute in acetone and then one drop of the solution was dried on a copper grid
coated with
amorphous carbon. The microscope used was an Hitachi 8100.
Conductivity of silver lines
Tests were performed that demonstrated that the silver lines were electrically
conductive. In one test, probes from a volt-ohm meter were contacted to a
written pad of
silver and impedance of -80MO was measured over a distance of 200 mm. Since
good
electrical contact was not assured, this gives an upper limit on the impedance
of this length of
the line.
Exchange reaction on nAg7 with thiol functionalized dyes, general procedure:
The silver nanoparticles were dissolved in CH2C12 and stirred for two hour to
ensure
complete dissolution. The dye was added and the flask covered with aluminum
foil. After
stirring for 4 days the solvent was removed in vacuum with the moderate
heating to a
maximum of 35 C. Acetone was added and the precipitated nanoparticles
collected on
quantitative filter paper and washed with acetone until the solvent showed no
sign of
fluorescence in ultraviolet light. The nanoparticles were dried in air and
analyzed by
elemental analysis.
Example 26:
111 functionalized nanoparticles (nAg12):
Octylthiol/dodecylthiol protected silver nanoparticles (nAg7) (250 mg), 4-((E)-
2-{4-
[(E)-2-(4-formylphenyl)ethenyl]-2-[(11-mercaptoundecyl)oxy]-5-
methoxyphenyl} ethenyl)benzaldehyde (111) (14.1 mg, 0.025 mmol), CH2C12 (500
ml). Anal.
(%) for nAgl2 (duplicated analysis): C: 11.78 (11.70), H: 2.07 (2.01), S: 2.76
(2.86), Ag:
76.62 (76.13).
-59-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Example 27:
110 functionalized nanoparticles (nAgl1)
Octylthiol/dodecylthiol protected silver nanoparticles (nAg7) (150 mg), 11-
(2,5-
bis {(E)-2-[4-(diethylamino)phenyl]ethenyl}-4-methoxyphenoxy)undecan-1-thiol
(110) (50
mg, 0.076 mmol), CH2Clz (300 ml). Isolated yield: 80.5 mg. Anal. (%) for nAgl
1
(duplicated analysis): C: 19.41 (19.45), H: 2.85 (2.76), N: 0.62 (0.62), S:
3.06 (3.24), Ag:
71.10 (71.06).
Example 28:
19 functionalized nanoparticles nAg10 :
Octylthiol/dodecylthiol protected silver nanoparticles (nAg7) (150 mg), 11-{4-
methoxy-2,5-bis[(E)-2-(4-nitrophenyl)ethenyl]phenoxy}-1-undecanethiol (19) (46
mg, 0.076
mmol), CH2Clz (300 ml). Anal. (%) for nAg10 (duplicated analysis): C: 20.38
(20.40), H:
2.41 (2.26), N: 0.99 (0.96), S: 2.98 (2.86), Ag: 63.92 (63.97).
Example 29:
19 only functionalized nanoparticles (nA lg3):
116.5mg of silver nitrate were dissolved in '75m1 ethanol at 0 C. 138mg of 19
were
dissolved in a mixture of -100m1 acetone and ' 5m1 of dichloromethane. The dye
solution
was added to the silver nitrate solution and allowed to stir for 45 minutes.
75m1 of a saturated
sodium borohydride solution in ethanol were added dropwise over a four hours
period. The
solution was allowed to stir for an additional three hours. The solution was
stored in a
refrigerator overnight and allowed to decant. The precipitate was filtered and
washed with
water, acetone, and dichloromethane. 146.3mg of a black powder were collected.
Anal. (%)
for nAgl3: C :41.00, HA 10%, N: 2.88%, S: 3.57%, Ag: 37.51%.
Composition and preparation of films of nanoparticles
Example 30:
F14 Film of dye attached nanoparticles class (i), 20 nm thick
1 mg of nAgl2 was dissolved in 20 cc of chloroform and left to stir for 2
days. 2 mg of
AgBF4 were dissolved in 10 cc of acetonitrile; 0.1 cc of this solution were
added to the
-60-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
nanoparticle solution. 0.5 cc of the combined solution was cast on a 25X25 mm
ITO coated
glass.slides.
Example 31:
F 15 Film of dye attached nanoparticles class (ii). 20 nm thick
1 mg of nAgl2 was dissolved in 20 cc of chloroform and left to stir for 2
days. The film was
prepared casting 0.5 cc of the solution on a 25X25 mm ITO coated glass slides.
Example 32:
F 16 Film of dye attached nanoparticles class (iii). 20 nm thick
1 mg of nAg7 was dissolved in 20 cc of chloroform and left to stir for 2 days.
2 mg of
AgBF4 were dissolved in 10 cc of acetonitrile; 0.1 cc of this solution were
added to the
nanoparticle solution. 0.5 cc of the combined solution was cast on a 25X25 mm
ITO coated
glass slides.
Example 33:
F17 Film of dye attached nanoparticles class (iv). 20 nm thick
1 mg of nAg7 was dissolved in 20 cc of chloroform and left to stir for 2 days.
The film was
prepared casting 0.5 cc of the solution on a 25X25 mm ITO coated glass slides.
Example 34:
F 18 Film of dye attached nanoparticles class (i), submonolayer
1 mg of nAgl2 was dissolved in 20 cc of chloroform and left to stir for 2
days. 2 mg of
AgBF4 were dissolved in 10 cc of acetonitrile; 0.1 cc of this solution were
added to the
nanoparticle solution. 2 cc of the combined solution were diluted 10 times
with chloroform,
and 2 gl were deposited on a Si3N4 coated Si substrate (1 mm2).
Example 35:
F19 Film of dye attached nanoparticles class (ii), subinonolayer
1 mg of nAgl2 was dissolved in 20 cc of chloroform and left to stir for 2
days. 2 cc of the
solution were diluted 10 times with chloroform, and 2 l were deposited on a
Si3N4 coated Si
substrate (1 mmz).
-61-
CA 02430253 2003-05-27
WO 02/48432 PCT/US01/47724
Example 36:
F20 Film of dye attached nanoparticles class (iii), submonolayer
1 mg of nAg7 was dissolved in 20 cc of chloroform and left to stir for 2 days.
2 mg of AgBF4
were dissolved in 10 cc of acetonitrile; 0.1 cc of this solution were added to
the nanoparticle
solution. 2 cc of the combined solution were diluted 10 times with chloroform,
and 2 gl were
deposited on a Si3N4 coated Si substrate (1 mm2).
Example 37:
F21 Film of dye attached nanoparticles class (iv), submonolayer
1 mg of nAg7 was dissolved in 20 cc of chloroform and left to stir for 2 days.
2 cc of the
solution were diluted 10 times with chloroform, and 2 gl were deposited on a
Si3N4 coated Si
substrate (1 mm2).
Example 38:
F22 Polymer based film for nanoparticle growth
1 mg of nAgl2 was dissolved in 20 cc of chloroform and left to stir for 2
days. 200.6 mg of
PVK and 89 mg of ethylcarbazole were dissolved in 2 cc of the nanoparticle
solution and left
to stir for 1 day. 210 mg of AgBF4 were dissolved in 1 cc of acetonitrile; 0.1
cc of this
solution were added to the nanoparticle/polymer solution. The whole solution
was cast on a
25X75 mm glass slide.
Example 39:
F23 Film for reflectivity
271 mg of PCUEMA, 20.5 mg of ethylcarbazole, 1.67 mg of nAg6, and 7.16 mg of 1
d were
dissolved in 6 cc of chloroform and left to stir overnight. 27 mg of AgBF4
were dissolved in
0.2 cc of acetonitrile and added to the solution. The combined solution was
filtered using a 1
gm pores filter and 2 cc of the filtered solution were cast on a 25X75 mm
glass slide.
Example 40:
F24 Film for conductivity
2 mg of nAg6 were dissolved in 5 cc of chloroform and left to stir for 1 day.
The solution
was filtered with a 1 mm pores filter. 21 ing of PVK, 9 mg of ethylcarbazole,
and 1.3 mg of
-62-
CA 02430253 2009-12-17
1 d were dissolved in 0.3 cc of the nanoparticles solution and left to stir
for 2 hours. 20 mg of
AgBF4 were dissolved in 0.5 cc of acetonitrile, 0.05 cc of this solution were
added to the
polymer nanoparticle solution. The solution was cast of half of a tailor made
glass slide
(25X25 mm), while the other half was covered with Teflon tape.
The slide had on it a pattern of 40 parallel silver lines 150 m wide, 15 mm
long and
50 nm height. The lines were spaced of 32 m, and were fabricated using
standard e beam
lithography techniques
Example 41:
F25 Copper Microfabrication Film
The film was formed by dissolving 66 mg of poly(methylmethacrylate) PMMA, 1.1
mg of ligand coated copper nanoparticles nCul, 5 mg of CuP(CH3)31, and 3 mg of
dye Id in
0.6 ml of degassed CHC13 and cast on a 25X25 mm glass slide under an argon
atmosphere.
Example 42:
F26 Gold Microfabrication Film
The film was formed by dissolving 66 mg of PMMA, 1.1 mg of ligand coated gold
nanoparticles nAul, 5 mg of AuP(CH3)3Br, and 3 mg of dye Id in 0.6 ml of CHC13
and cast
on a 25X25 mm glass slide under an argon atmosphere.
Example 43:
F27 Film for Holography
271 mg of PVK, 20.5 mg of ethylcarbazole, 1.67 mg of nAg6, and 0.8 mg of Id
were
dissolved in 6 cc of chloroform and left to stir overnight. 27 mg of AgBF4
were dissolved in
0.2 cc of acetonitrile and added to the solution. The combined solution was
filtered using a 1
m pores filter and 2 cc of the filtered solution were cast on a 25X75 mm glass
slide.
Obviously, numerous modifications and variations on the present invention are
possible in light of the above teachings. It is therefore to be understood
that within the scope
of the appended claims, the invention may be practiced otherwise than as
specifically
described herein.
-63-