Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
DESCRIPTION
STABILIZED PHARMACEUTICAL COMPOSITION
IN LYOPHILIZED FORM
TECHNICAL FIELD
The present invention relates to a stabilized
pharmaceutical composition in lyophilized form containing
a cyclic polypeptide compound. More particularly, the
present invention relates to a stabilized pharmaceutical
composition in lyophilized form containing a cyclic
polypeptida compound or a salt thereof and a stabilizer.
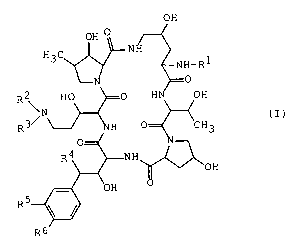
The cyclic polypeptide compound of the present
invention is represented by the following general formula
(I)
OH
OH O
N
H3C
NH-R1
R~ HO 0 H OH
\N (I)
R 3"~ H O=_~C H 3
R
R6
r
wherein
R1 is hydrogen or aryl group,
R~ is hydrogen or.acyl group,
R3 is lower alkyl which has one or more hydroxy or
protected hydroxy,
R4 is hydrogen or hydroxy,
R5 is hydrogen, hydroxy, lower alkoxy or hydroxysulfonyloxy,
1
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
and
R6 is hydroxy or acyloxy,
or a salt thereof.
The cyclic polypeptide compound (I) or a salt thereof
has an antimicrobial activity, particularly an antifungal
activity and a (3 -1, 3-glucan synthase inhibiting action, and
is useful for preventing and treating various kinds of
infectious diseases including Pneumocystis carinii
infection, e.g., carinii pneumonia.
BACKGROUND ART
The cyclic polypeptide compound represented by the
above formula (I) are disclosed by WO 01/60846.
The cyclic polypeptide compound ( I ) or a salt thereof
is generally unstable to light, humidity, acids, heat and
the like. Therefore, there is desired development of
pharmaceutical preparations in which the cyclic polypeptide
compound (I) or a salt thereof is stabilized.
DISChOSURE OF INVENTION
The present invention provides a stabilized
pharmaceutical composition in lyophilized form containing
a cyclic polypeptide compound (I) or a salt thereof and a
stabilizer.
Suitable examples and illustration of the various
definitions in the above and subsequent descriptions of the
present specification, which the present invention intends
to include within the scope thereof, are explained in detail
as follows:
The term "lower" is used to intend a group having 1 to
6 carbon atom(s), unless otherwise provided.
Suitable example of "one or more" may be the number of
2
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
1 to 6, in which the preferred one may be the number of 1
to 3, and .the most preferred one may be the number of 1 or
2.
Suitable example of "halogen" may be fluorine, chlorine,
bromine, iodine and the like.
Suitable example of "lower alkoxy" may include straight
or branched one such as methoxy, ethoxy, propoxy, isopropoxy,
butoxy, isobutoxy, tert-butoxy, pentyloxy, tent-pentyloxy,
neo-pentyloxy, hexyloxy, isohexyloxy and the like.
Suitable example of "higher alkoxy" may include straight
or branched one such as heptyloxy, octyloxy,
3,5-dimethyloctyloxy, 3,7-dimethyloctyloxy, nonyloxy,
decyloxy,.undecyloxy, dodecyloxy, tridecyloxy,
tetradecyloxy, hexadecyloxy, heptadecyloxy, octadecyloxy,
nonadecyloxy, icosyloxy, and the like.
Suitable example of "lower alkyl" may include straight
or branched one having 1 to 6 carbon atom ( s ) , such as methyl,
ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl,
tert-butyl, pentyl, tert-pentyl, neo-pentyl, hexyl,
isohexyl and the like.
Suitable example of "higher alkyl" may include straight
or branched one such as heptyl, octyl, 3,5-dimethyloctyl,
3,7-dimethyloctyl, nonyl, decyl, undecyl, dodecyl, tridecyl,
tetradecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl,
icosyl, and the like.
Suitable example of "aryl" and "ar" moiety may include
phenyl which may have lower alkyl (e. g., phenyl, mesityl,
xylyl, tolyl, etc. ) , naphthyl, anthryl, indanyl, fluorenyl,
and the like, and this "aryl" and "ar" moiety may have one
or more halogen.
Suitable example of "aroyl" may include benzoyl, toluoyl,
naphthoyl, anthrylcarbonyl, and the like.
Suitable example of "heterocyclic group" may include
unsaturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclic group containing 1 to 4 nitrogen
3
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
atom(s), for example, pyrrolyl, pyrrolinyl, imidazolyl,
pyrazolyl, pyridyl, dihydropyridyl, pyrimidyl, pyrazinyl,
pyridazinyl, triazolyl (e. g., 4H-1,2,4-triazolyl,
1H-1,2,3-triazolyl, 2H-1,2,3-triazolyl, etc.), tetrazolyl
(e. g. 1H-tetrazolyl, 2H-tetrazolyl, etc.), etc.;
saturated 3 to 8-membered (more preferably 5 or
6-membered) hetero~nonocyclic group containing 1 to 4 nitrogen
atom(s), for example, pyrrolidinyl, imidazolidinyl,
piperidyl, piperazinyl, azetidinyl, etc.;
unsaturated condensed heterocyclic group containing 1
to 4 nitrogen atom(s), for example, indolyl, isoindolyl,
indolinyl, indolizinyl, benzimidazolyl, quinolyl,
isoquinolyl,~ indazolyl, benzotriazolyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclic group containing 1 or 2 oxygen
atom ( s ) and 1 to 3 nitrogen atom ( s ) , for example, oxazolyl,
isoxazolyl, oxadiazolyl (e. g., 1,2,4-oxadiazolyl,
1,3,4-oxadiazolyl, 1,2,5-oxadiazolyl, etc.), etc.;
saturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclic group containing 1 or 2 oxygen
atom ( s ) and 1 to 3 nitrogen atom ( s ) , for example, morpholinyl,
sydnonyl, morpholino, etc.;
unsaturated condensed heterocyclic group containing 1
or 2 oxygen atoms) and 1 to 3 nitrogen atom(s), for example,
benzoxazolyl, benzoxadiazolyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 or 2 sulfur
atom ( s ) and 1 to 3 nitrogen atom ( s ) , for example, thiazolyl,
isothiazolyl, thiadiazolyl (e. g., 1,2,3-thiadiazolyl,
1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl,
1,2,5-thiadiazolyl, etc.), dihydrothiazinyl, etc.;
saturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclic group containing 1 or 2 sulfur
atom ( s ) and 1 to 3 nitrogen atom ( s ) , for example thiazolidinyl,
thiomorpholinyl, thiomorpholino, etc.;
4
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
unsaturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclic group containing 1 or 2 sulfur
atom(s), for example, thienyl, dihydrodithiinyl,
dihydrodithionyl, etc.;,
unsaturated condensed heterocyclic group containing 1
or 2 sulfur atoms) and l to 3 nitrogen atoms) , for example,
benzothiazolyl, benzothiadiazolyl, imidazothiadiazolyl,
etc.;
unsaturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclicgroupcontaininganoxygenatom,
for~example, furyl etc.;
saturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclic group containing 1 or 2 oxygen
atom(s), for example, tetrahydrofuran, tetrahydropyran,
dioxacyclopentane, dioxacyclohexane, etc.;
unsaturated 3 to 8-membered (more preferably 5 or
6-membered) heteromonocyclicgroupcontaininganoxygenatom
and 1 or 2 sulfur atoms) , for example, dihydrooxathiinyl,
etc. ;
unsaturated condensed heterocyclic group containing 1
or 2 sulfur atom ( s ) , for example benzothienyl, ben~zodithiinyl,
etc.;
unsaturated condensed heterocyclic group containing an
oxygen atom and 1 or 2 sulfur atom(s), for example,
benzoxathiinyl, etc.; and the like, and this "heterocyclic
group" may have one or more suitable substituent (s) selected
from the group consisting of lower alkyl, oxo,
cyclo(lower)alkyl, hydroxy(lower)alkyl,
carboxy(lower)alkanoyl which may have amino and
heterocycliccarbonyl.
Suitable example of "cyclo(lower)alkyl" may include
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and the
like, and this "cyclo (lower) alkyl" may have one or more lower
alkyl.
5
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Suitable example of "cyclo (lower) alkyloxy" may include
cyclopropyloxy, cyclobutyloxy, cyclopentyloxy,
cyclohexyloxy, and the like. '.
Suitable example of "acyl group" may include carboxy;
carbamoyl; mono or di(lower)alkylcarbamoyl (e. g.,
methylcarbamoyl, dimethylcarbamoyl,~, ethylcarbamoyl,
diethylcarbamoyl, etc.); aliphatic acyl, aromatic acyl,
arylaliphatic acyl and heterocyclic-aliphatic acyl derived
from carboxylic acid, carbonic acid, carbamic acid, sulfonic
acid; and the like.
Aliphatic acyl such as lower or higher alkanoyl (e. g. ,
formyl, acetyl, propanoyl, butanoyl, 2-methylpropanoyl,
pentanoyl, 2,2-dimethylpropanoyl, hexanoyl, heptanoyl,
octanoyl, nonanoyl, decanoyl, undecanoyl, dodecanoyl,
tridecanoyl, tetradecanoyl, pentadecanoyl, hexadecanoyl,
heptadecanoyl, octadecanoyl, nonadecanoyl, icosanoyl,
etc.);
lower or higher alkoxycarbonyl (e. g., methoxycarbonyl,
ethoxycarbonyl, t-butoxycarbonyl, t-pentyloxycarbonyl,
heptyloxycarbonyl, etc.); lower alkenyloxycarbonyl (e. g.,
vinyloxycarbonyl, propenyloxycarbonyl, allyloxycarbonyl,
butenyloxycarbonyl, butedienyloxycarbonyl,
pentenyloxycarbonyl, hexenyloxycarbonyl, etc.);
lower or higher alkylsulfonyl (e. g., methylsulfonyl,
ethylsulfonyl, etc.);
lower or higher alkoxysulfonyl (e. g., methoxysulfonyl,
ethoxysulfonyl, etc.); or the like;
Aromatic acyl such as
aroyl (e. g., benzoyl, toluoyl, naphthoyl, etc.);
ar(lower)alkanoyl [e. g., phenyl(C1-C6)alkanoyl (e. g.,
phenylacetyl, phenylpropanoyl, phenylbutanoyl,
phenylisobutanoyl,phenylpentanoyl,phenylhexanoyl,etc.),
naphthyl(C1-C6)alkanoyl (e. g., naphthylacetyl,
naphthylpropanoyl, naphthylbutanoyl, etc.), etc.];
G
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
ar(lower)alkenoyl [e. g., phenyl(C3-C6)alkenoyl (e. g.,
phenylpropenoyl, phenylbutenoyl, phenylmethacryloyl,
phenylpentanoyl, phenylhexenoyl, etc.),
naphthyl(C3-C6)alkenoyl (e. g., naphthylpropenoyl,
naphthylbutenoyl, etc.), etc.]:
ar(lower)alkoxycarbonyl [e.g., phenyl(C1-C6
)alkoxycarbonyl (e. g., benzyloxycarbonyl, etc.),
fluorenyl(C1-C6)alkoxy-
carbonyl (e.g., fluorenylmethyloxycarbonyl, etc.), etc.]o
aryloxycarbonyl (e. g., phenoxycarbonyl,
naphthyloxycarbonyl, etc.);
aryloxy(lower)alkanoyl (e. g., phenoxyacetyl,
phenoxypropionyl, etc.);
arylcarbamoyl (e. g., phenylcarbamoyl, etc.);
arylthiocarbamoyl (e. g., phenylthiocarbamoyl, etc.);
arylglyoxyloyl (e. g., phenylglyoxyloyl,
naphthylglyoxyloyl, etc.);
arylsulfonyl which may have 1 to 4 lower alkyl (e. g.,
phenylsulfonyl, p-tolylsulfonyl, etc.);
aroyl (e.g., beIlZOy1) substituted with one or more suitable
substituent(s); or the like; .
Heterocyclic acyl such as
heterocycliccarbonyl;
heterocyclic(lower)alkanoyl (e. g., heterocyclicacetyl,
heterocyclicpropanoyl, heterocyclicbutanoyl,
heterocyclicpentanoyl, heterocyclichexanoyl, etc.);
heterocyclic(lower)alkenoyl~(e.g., heterocyclicpropenoyl,
heterocyclicbutenoyl, heterocyclicpentenoyl,
heterocyclichexenoyl, etc.);
heterocyclicglyoxyloyl; or the like;
in which suitable "heterocyclic" moiety in the terms
"heterocycliccarbonyl", "heterocyclic(lower)alkanoyl",
"heterocyclic(lower)alkenoyl" and
"heterocyclicglyoxyloyl" can be referred to aforementioned
"heterocyclic" moiety.
7
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Suitable example of "acyl group" of R1 can be referred
to aforementioned "acyl group", in which the preferred one
may be lower alkoxycarbonyl, higher alkanoyl and benzoyl
substituted with one or more suitable substituent(s).
Suitable example of "suitable substituent (s) " in the
term of "benzoyl substituted with one or more suitable
substituent(s)" maybe thiadiazolyl substituted with phenyl
having phenylsubstituted with morphlinohaving loweralkyl,
thiadiazolyl substituted with phenyl having a suitable
substituent selected from the group consisting of lower
alkoxy(lower)alkoxy and lower alkoxy(higher)alkoxy,
piperazinyl substituted with phenyl having piperidyl
substituted with a suitable substituent selected from the
group consisting of phenyl having lower alkoxy (lower) alkoxy,
cyclo(lower)alkyloxy and lower alkoxy(lower)alkylthio,
piperazinyl substituted with phenyl having phenyl
substituted with morpholino having lower alkyl,
imidazothiadiazolyl substituted with phenyl having
piperidyl substituted with a suitable substituent selected
from the group consisting of lower alkoxy (lower) alkoxy and
lower alkoxy(lower)alkylthio,
imidazothiadiazolyl substituted with phenyl having
lower alkoxy(lower)alkoxy, '
phenyl subsutituted with piperazinyl having phenyl
substituted with morpholino having lower alkyl,
isoxazolyl substituted with phenyl having lower
alkoxy(lower)alkoxy,
isoxazolyl ,substituted with phenyl having higher
alkoxy substituted with morpholino having lower alkyl,
thiadiazolyl substituted with phenyl having
piperazinyl substituted with cyclo(lower)alkyl which has
. one or more suitable substituent (s) selected from the group
consisting of lower alkyl, lower alkenyl, lower
alkoxy(higher)alkoxy and phenyl,
8
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
thiadiazolyl substituted with phenyl having
piperazinyl substituted with lower alkyl having
cyclo(lower)alkyl,
thiadiazolyl substituted with phenyl having piperidyl
substituted with one ormore suitable substituent ( s) selected
from the group consisting of cyclo(lower)alkyloxy, lower
alkoxy(lower)alkoxy and lower
alkoxy(lower)alkoxy(lower)alkyl,
thiadiazolyl substituted with phenyl having piperidyl
substituted with cyclo(lower)alkyl and lower alkoxy,
thiadiazolyl substituted with pyridyl having
piperazinyl substituted withcyclo(lower)alkylhavinglower
alkyl,
imidazothiadiazolyl substituted with phenyl having
piperidyl substituted with cyclo(lower) alkyl,
imidazothiadiazolyl substituted with phenyl having
piperazinylsubstituted withcyclo(lower)alkyl havinglower
alkyl,
phenyl substituted with plperazinyl navly
cyclo(lower)alkyl substituted with one or more suitable
substituent(s) selected from the group consisting of
cyclo (lower) alkyl which may have lower alkoxy, lower alkyl,
lower alkoxy and phenyl which may have lower alkoxy,
in which the preferred one may be thiadiazolyl
substituted with phenyl having phenyl substituted with
morpholino having dimethyl,
thiadiazolyl substituted with phenyl having a
substituent selected from the group consisting of
methoxyhexyloxy and methoxyheptyloxy,
piperazinyl substituted with phenyl having piperidyl
substituted with a substituent selected from the group
consisting of phenyl having methoxybutoxy, cyclohexyloxy
and methoxyhexylthio,
piperazinyl substituted with phenyl having phenyl
9
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
substituted with morpholino having dimethyl,
imidazothiadiazolyl substituted with phenyl having
piperidyl substituted with a substituent selected from the
group consisting of methoxypropox~y, methoxybutoxy,
methoxypentyloxy and methoxyhexylthio,
imidazothiadiazolyl substituted with phenyl having
methoxybutoxy, .
phenyl subsutituted with piperazinyl having phenyl
substituted with morpholino having dimethyl,
isoxazolyl substituted with phenyl having
methoxyhexyloxy,
isoxazolyl substituted with phenyl having heptyloxy
substituted with morphlino having dimethyl,
thiadiazolyl substituted with phenyl having
piperazinyl substituted with cyclohexyl which has one or
two substituent(s) selected from the group consisting of
methyl, methylene, methoxyheptyloxy, methoxyoctyloxy and
phenyl,
thiadiazolyl substituted with phenyl having
piperazinyl substituted with methyl which has a substituent
selected from the group consisting of cyclopentyl and
cyclohexyl,
thiadiazolyl substituted with phenyl having piperidyl
substituted with one or two substituent(s) selected from
the group consisting of cyclohexyl, methoxy, cyclohexyloxy,
methoxypentyloxy,methoxyhexyloxy,methoxybutoxymethyland
methoxypentyloxymethyl,
thiadiazolyl substituted with pyridyl having
piperazinyl substituted with cyclohexyl which has a
substituent selected from the group consisting of methyl
and ethyl,
imidazothiadiazolyl substituted with phenyl having
piperidyl substituted with cyclohexyl,
imidazothiadiazolyl substituted with phenyl having
piperazinyl substituted with cyclohexyl having methyl,
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
phenyl substituted with piperazinyl having cyclohexyl
substituted with one or two substituent(s) selected from
thegroup consistingofethyl,t-butyl,methoxy,cyclopentyl,
cyclohexyl which may have methoxy or dimethyl, and phenyl
which may have methoxy.
The suitable example of ~~acyl group" for R1 may be
benzoyl which has thiadiazolyl substituted with phenyl
. having phenyl substituted with morpholino having dimethyl,
benzoyl which has thiadiazolyl substituted with phenyl
having a substituent selected from the group consisting of
methoxyhexyloxy and methoxyheptyloxy,
benzoyl which has piperazinyl substituted with phenyl
having piperidyl substituted with a substituent selected
from the group consisting of phenyl having methoxybutoxy,
cyclohexyloxy and methoxyhexylthio,
benzoyl which has piperazinyl substituted with phenyl
having phenyl substituted with morpholino having dimethyl,
benzoyl whichhas imidazothiadiazolyl substituted with
phenyl having piperidyl substituted with a substituent
selected from the group consisting of methoxypropoxy,
methoxybutoxy, methoxypentyloxy and methoxyhexylthio,
benzoyl which has imidazothiadiazolyl substituted with
phenyl having methoxybutoxy,
benzoyl which has phenyl subsutituted with piperazinyl
having phenyl substituted with morpholino having dimethyl,
benzoyl which has isoxazolyl substituted with phenyl
having methoxyhexyloxy,
benzoyl which has isoxazolyl substituted with phenyl
havingheptyloxysubstituted withmorphlinohavingdimethyl,
benzoyl which has thiadiazolyl substituted with phenyl
having piperazinyl substituted with cyclohexyl which has
one or two substituent (s) selected from the group consisting
of methyl, methylene, methoxyheptyloxy, methoxyoctyloxyand
phenyl,
11
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
benzoyl which has thiadiazolyl substituted with phenyl
having piperazinyl substituted with methyl which has a
substituent selected from the group consisting of cyclopentyl
and cyclohexyl,
benzoyl which has thiadiazolyl substituted with phenyl
having piperidyl substituted with one or two substituent ( s )
selected from the group consisting of cyclohexyl, methoxy,
cyclohexyloxy, methoxypentyloxy, methoxybutoxymethyl and
methoxypentyloxymethyl,
l0 benzoyl which has thiadiazolyl substituted with pyridyl
having piperazinyl substituted with cyclohexyl which has
a substituent selected from the group consisting of methyl
and ethyl,
benzoyl which has imidazothiadi~azolyl substituted with
phenyl having piperidyl substituted with a substituent
selected from the group consisting of methoxyhexyloxy,
cyclohexyl and methoxyhex~yl,
benzoyl which has imidazothiadiazolyl substituted with
phenylhaving piperazinylsubstituted withcyclohexylhaving
methyl,
benzoyl which has phenyl substituted with piperazinyl
having cyclohexyl substituted with one or two substituent ( s )
selected from the group consisting of ethyl, t-butyl, methoxy,
cyclopentyl, cyclohexyl which may have methoxy or dimethyl,
and phenyl which may have methoxy.
The suitable example of "lower alkyl" in the term of
"lower alkyl which has one or more hydroxy or protected
hydroxy" may be methyl, ethyl, propyl, isopropyl, butyl,
pentyl and hexyl.
The suitable example of ~~hydroxy protective group"
in the term of unprotected hydroxy" may be phenyl (lower) alkyl
which may have one or more suitable substituent(s) (e. g.,
benzyl, 4-methoxybenzyl, trityl, etc.), tri-substituted
silyl [e. g., tri(lower)alkylsilyl(e.g., trimethylsilyl,
12
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
t-butyldimethylsilyl, etc.), etc.], tetrahydropyranyl and
the like.
The suitable example of "lower alkyl which has one or
more hydroxy or protected hydroxy" may be dihydroxypropyl,
dihydroxyisopropyl, trihydroxybutyl, tetrahydroxypentyl,
pentahydroxyhexyl and diacetyloxyisopropyl.
The suitable example of "acyl group" of R2 may be acetyl,
2-acetyloxypropionyl, metylsulfonyl, 2,5-diaminopentanoyl,
benzyloxycarbonyl, fluorenylmethoxycarbonyl,
allyloxycarbonyl and tert-butoxycarbonyl.
The suitable example of "acyl" moiety of "acyloxy" may
be lower alkenyloxycarbonyl, and the most preferred one may
be allyloxycarbonyl.
The suitable example of "acyloxy" may be lower
alkenyloxycarbonyloxy, and the more preferred one may be
allyloxycarbonyloxy.
Particularly, the preferred examples of the cyclic
polypeptide compound (I) of the present invention are as
follows
the compound (I), wherein
R1 is hydrogen, lower alkoxycarbonyl, higher alkanoyl
or benzoyl substituted with one or more suitable
substituent(s),
R~ is hydrogen,
R3 is lower alkyl which has one or more hydroxy,
R4 is hydrogen or hydroxy;
R5 is hydroxy or hydroxysulfonyloxy; and
R6 is hydroxy.
And, more preferred one may be the compound (I)
wherein
13
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
R1 is hydrogen, lower alkoxycarbonyl, higher alkanoyl
or benzoyl substituted with one or more suitable
substituent(s),
R~ is hydrogen,
R3 is lower alkyl which has. two hydroxy,
R4 is hydrogen or hydroxy;
R5 is hydroxy or hydroxysulfonyloxy; and
R6 is hydroxy.
And, still more preferred one may be the compound ( I )
wherein
R1 is benzoyl substituted with a suitable substituent
selected from the group consisting of
thiadiazolyl substituted with phenyl having phenyl
substituted with morphlino having lower alkyl,
thiadiazolyl substituted with phenyl havingasuitable
substituent selected from the group consisting of lower
alkoxy(lower)alkoxy and lower alkoxy(higher)alkoxy,
piperazinyl substituted with phenyl having piperidyl
substituted with a suitable substituent selected from the
group consisting of phenyl having lower alkoxy (lower)'alkoxy,
cyclo(lower)alkyloxy and lower alkoxy(lower)alkylthio,
piperazinyl substituted with phenyl having phenyl
substituted with morpholino having lower alkyl,
imidazothiadiazolyl~substituted with phenyl having
piperidyl substituted with a suitable substituent selected
from the group consisting of lower alkoxy (lower) alkoxy and
lower alkoxy(lower)alkylthio,
imidazothiadiazolyl substituted with phenyl having
lower alkoxy(lower)alkoxy,
phenyl subsutituted with piperazinyl having phenyl
substituted with morpholino having lower alkyl,
isoxazolyl substituted with phenyl having lower
alkoxy(lower)alkoxy, ,
isoxazolyl substituted with phenyl having higher
14
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
alkoxy substituted with morpholino having lower alkyl,
thiadiazolyl substituted with phenyl having
piperazinyl substituted with cyclo(lower)alkyl which has
one or more suitable substituent ( s ) selected from the group
consisting of lower alkyl, lower alkenyl, lower
alkoxy(higher)alkoxy and phenyl,
thiadiazolyl substituted with phenyl having
piperazinyl substituted with lower alkyl having
cyclo (lower) alkyl,
thiadiazolyl substituted with phenyl having piperidyl
substituted with one or more suitable substituent ( s ) selected
from the group consisting of cyclo(lower)alkyloxy, lower
alkoxy(lower)alkoxy and lower
alkoxy(lower)alkoxy(lower)alkyl,
thiadiazolyl substituted with phenyl having piperidyl
substituted with cyelo(lower)alkyl and lower alkoxy,
thiadiazolyl substituted with pyridyl having
piperazinylsubstituted withcyclo(lower)alkylhavinglower
alkyl,
imidazothiadiazolyl substituted with phenyl having
piperidyl substituted with cyclo(lower) alkyl,
imidazothiadiazolyl substituted with phenyl having
piperazinylsubstituted withcyclo(lower)alkylhavinglower
alkyl, and
phenyl substituted with piperazinyl having
cyclo(lower)alkyl substituted with one or more suitable
substituent(s) selected from the group consisting of
cyclo (lower) alkyl which may have lower alkoxy, lower alkyl,
lower alkoxy and phenyl which may have lower alkoxy,
R' is hydrogen,
R3 is lower alkyl which has two hydroxy,
R4 is hydrogen or hydroxy;
R5 is hydroxy or hydroxysulfonyloxy; and
R6 is hydroxy.
15
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
And, the most preferred one may be the compound (I)
Wherein
R1 is benzoyl which has thiadiazolyl substituted with
phenyl having piperazinyl substituted with
cyclo(lower)alkyl which has lower alkyl,
benzoyl which has thiadiazolyl substituted with
phenyl having piperidyl substituted with
cyclo(lower)alkyloxy,
benzoyl which has phenyl substituted with
piperazinyl having cyclo(lower)alkyl substituted with
cyclo(lower)alkyl and lower alkoxy,
benzoyl which has thiadiazolyl substituted with
phenylhaving piperidylsubst,ituted with cyclo(lower)alkyl,
R~ is hydrogen,
R3 is lower alkyl which has two hydroxy,
R4 is hydrogen or hydroxy;
R5 is hydroxy or hydroxysulfonyloxy~ and
R6 is hydroxy.
The suitable salt of the cyclic polypeptide compound
(I) is soluble in water, and a pharmaceutically acceptable
salt including salt with base and acid addition salt. Such
a salt may be prepared by treating the cyclic polypeptide
compound ( I ) with an appropriate base or acid according to
the conventional method.
As a salt with bases, may be mentioned salt with
inorganic base such as alkali metal salt (e.g., sodium salt,
potassium salt, etc.), alkaline earth metal salt (e. g.,
calcium salt, magnesium salt, etc.), ammonium salt and the
like; salt with organic base such as organic amine salt (e. g. ,
triethylamine salt, diisopropyleth ylamine salt, pyridine
salt,picolinesalt, ethanolaminesalt, triethanolaminesalt,
dicyclohexylaminesalt,N,N'-dibenzylethylenediaminesalt,
etc.)~ and the like.
As acid addition salt, may be mentioned inorganic acid
16
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
addition salt (e. g., hydrochloride, hydrobromide, sulfate,
phosphate, etc.); and organic carboxylic or sulfonic acid
addition salt (e. g., formate, acetate, trifluoroacetate,
maleate, tartrate, fumarate, methnesulfonate,
benzenesulfonate, toluenesulfonate, etc.). Further, may
also be mentioned salt with basic or acidic amino acid (e.g.,
salt with arginine, aspartic acid, glutamic acid, etc.).
The cyclic polypeptide compound (I) of the present
invention may also include possible conformers and a pair
or more of stereoisomers such as geometric isomers and optical
isomers which may exist due to asymmetric carbon atoms.
The amount of the cyclic polypeptide compound (I) or
a salt thereof contained in the composition for a single
unit dosage of the present invention is 0.1 to 400 mg, more
preferably 1 to 200 mg, still more preferably 5 to 100 mg,
specifically 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60,
70, 75, 80, 85, 90, 95 and 100 mg.
Thepreferredlyophilizedcomposition may beconsisting
of the cyclic polypeptide compound (I) or a salt thereof
and a stabilizer, optionally adding a pH adjuster and/or
a pH buffer.
As the stabilizer, po.lysaecharides may be mentioned.
The suitable examples of the polysaccharide are dextran
(e. g. , dextran 40, dextran 70, etc. ) , starch, cellulose and
hyaluronic acid. The polysaccharide contained in the
pharmaceutical composition of the present invention may be
a -monohydrate, a -anhydride, (3 -anhydride or a combination
thereof.
The amount of the stabilizer used in the pharmaceutical
composition of the present invention should be at least
sufficient for stabilizing the cyclic polypeptide compound
(I) or a salt thereof in the composition. In order to
1~
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
stabilize the cyclic polypeptide compound ( I ) , one part by
weight of the stabilizer with respect to one part by weight
of the cyclic polypeptide compound (I) or a salt thereof
in the present composition is sufficient at least. The
stabilizer may also serve as a carrier or an excipient. Thus
the use amount of stabilizer does not have a particular upper
limit and may be determined in consideration of the weight
or volume of the composition with respect to a unit dose
of the compound and the like. However, such amount is
preferably 1 to 50 parts by weight, more preferably 5 to
parts by weight, with respect to one part by weight of
the cyclic polypeptide compound (I) or a salt thereof, though
it varies depending upon the kind and the used amount of
the cyclic polypeptide compound (I) or a salt thereof, its
15 preparation form and/or the like.
Thepharmaceuticalcompositionofthepresentinvention
may be produced according to methods known in the art with
using additives if necessary. Here, Basic Lecture on
Development of Pharmaceuticals XI 20 Production of
20 Pharmaceuticals (the second volume) (editedbyKyo7aunn Tsuda
and Hisashi Nogami and published by Chi~yo Shoten) is
mentioned for reference.
The lyophilized composition may be obtained by preparing
an aqueous solution of the cyclic polypeptide compound ( I )
or a salt thereof and a stabilizer, optionally adding a pH
buffer and/or a pH adjuster.
Suitable example of the pH buffer may be amino acid (e . g. ,
glycine,h-arginine,h-asparticacid,h-glutamieacid, etc.),
sodium L-glutamate monohydrate, ethanolamine, trometamol,
and the like, and the amount of the pH buffer is preferably
1 to 30 parts by weight, more preferably 10 to 20 parts by
weight, with respect to one part by weight of the cyclic
polypeptide compound ( I ) or a salt thereof, though i~t varies
depending upon the kind and the used amount of the cyclic
polypeptide compound (I) or a salt thereof, its preparation
18
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
form and/or the like.
Suitable example of the pH adjuster may be inorganic
base (e. g., sodium hydroxide, potassium hydroxide, calcium
hydroxide, magnesium hydroxide), organic base (e. g.,
triethylamine, diisopropylethylamine, pyridine, picoline,
ethanolamine, triethanolamine, dicyclohexylamine,
N,N'-dibenzylethylenediamine, etc.), inorganic acid (e. g.,
hydrochloric acid, hydrobromic acid, sulfuric acid,
phosphoric acid, etc. ) , organic carboxylic or sulfonic acid
(e . g. , formic acid, acetic acid, trifluoroacetic acid, malefic
acid, tartaric acid, fumaric acid, methanesulfonic acid,
benzenesulfonic acid, toluenesulfonic acid, etc), citric
acid anhydrous, and the like, as required to attain pH 9.0
to 10.0, preferably pH 9.5 to 10.0, and then lyophilizing
the resulting solution in vial according to a conventional
method. Thus, the stabilized pharmaceutical composition in
lyophilized form, when dissolved in purified water,
preferably gives a solution of pH 9. 0 to 10. 0, more preferably
pH 9.5 to 10Ø
It is preferable that the thus prepared composition in
lyophilized form is sealed and stored with shading. The
lyophilized composition can be loaded in each vial in the
solution form before lyophilizing or in lyophilized powder
form after lyophilizing.
Since the cyclic polypeptide compound (I) or a salt
thereof is not satisfactorily stable to humidity, it is
necessary that the lyophilized composition of the present
invention contains 3. 4 % by weight or less of water, preferably
3.0 0, more preferably 2.0 0.
Usually the stabilized pharmaceutical composition in
lyophilized form is dissolved in isotonic sodium chloride
solution as required and used as an injection solution. The
pharmaceutical composition of the present invention may be
used as an .injection preparation which requires some
compounding before use.
19
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
The present invention is now described in further detail
by way of example and test examples, which should not be
construed to limit the scope of the invention.
Compositional Example 1
The compound of Example 2 0.2g
Dextran 40 ~g
Glycine 0.3g
Sodium hydroxide in a suitable amount
Dextran 40 and glycine were dissolved in purified water
(30mL) at ambient temperature, and the compound of Example
_2 was dispersed into the solution. The compound of Example
_2 was dissolved at pH of approximately 9. 8 with adding 0. 4 0
aqueous sodium hydroxide solution. After adding purified
water, total 40mL of the solution was obtained. Each 2mL
of the resulting solution was filled into a lOmL size of
Type I tubing glass vial. The solution in the respective
vials was lyophilized by using the lyophilizer (Hull 2FS5C
manufactured by Hull Corp. ) by the conventional method to
obtain lyophilized compositions each containing lOmg of the
compound of Example 2.
Compositional Example 2
Lyophilized compositions each containing l0mg of the
compound of Example 1 are obtained in the similar manner
as Compositional Example 1.
Compositional Example 3
'Lyophilized compositions each containing l0mg of the
compound of Example 3 are obtained in the similar manner
as Compositional Example 1.
Compositional Example 4
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Lyophilized compositions each containing lOmg of the
compound of Example 4 are obtained in the similar manner
as Compositional Example 1 .
Compositional Example 5
The compound of Example 2 0.1258
Dextran 40 1.258
Glycine 0.18758
Sodium hydroxide in a suitable amount
Dextran 40 and glycine were dissolved in purified water
(20mL) at ambient temperature, and the compound of Example
2 was dispersed into that solution. The compound of Example
2 was dissolved at pH of approximately 9. 8 with adding 0 . 4 0
15 aqueous sodium hydroxide solution. After adding purified
water, total 25mL of the solution was obtained.
Each 1mL of the resulting solution was filled into a
lOmL size of Type I tubing glass vial. The solution in the
respective vials was lyophilized by using the lyophilizer
20 (Hull 2FS5C manufactured by Hull Corp. ) by the conventional
method to obtain lyophilized compositions each containing
5m8 of the compound of Example 2.
Compositional Example 6
25 The compound of Example 2 5g
Dextran 40 508
L-arginine 17.48
Anhydrous Citric Acid in a suitable amount
30 Dextran 40 and L-arginine were dissolved in purified
water (800mL) at ambient temperature, and the solution was
added with.the compound of Example 2 avoiding bubbling under
gently stirring. After adding 21o aqueous citric acid
solution (about 0.5mL) to adjust pH 9.8 and adding purified
35 water for injection, total 1000mL of the solution was
21
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
obtained.
Each 1mL of the resulting solution was filled into a
lOmL size of Type I tubing glass vial. The solution in the
respective vials was lyophilized by using the lyophilizer
(Hull 2FS5C manufactured by Hull Co.) by the conventional
method to obtain lyophilised compositions each containing
5mg of the compound of Example 2.
Compositional Example 7
Lyophilized compositions each containing 5mg of the
compound of Example 4 are obtained in the similar manner
as Compositional Example 6 . "
Compositional Example 8
Lyophilized compositions each containing 5mg of the
compound of Example 5 are obtained in the similar manner
as Compositional Example ~ except that trometamol is used
instead of L-arginine.
Compositional Example 9
Lyophilized compositions each containing 5mg of the
compound of Example 1 are obtained in the similar manner
as Compositional Example 5 except that trometamol is used
instead of L-arginine.
Compositional Example 10
The compound of Example 2 5g
Dextran 40 50g
L-arginine 70g
Anhydrous Citric Acid in a suitable amount
Dextran 40 and L-arginine were dissolved in water for
injection (800mL) at ambient temperature, and the solution
was added with the compound of Example 2 avoiding foaming
under gently stirring. After adding 20o aqueous citric acid
22
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
solution (about 25mL) to adjust pH 9.8 and adding water for
injection, tota1s1000mL of the solution was obtained.
Each 1mL of the resulting solution was filled into a
lOmL size of Type I tubing glass vial. The solution in the
respective vials was lyophilized by using the lyophilizer
(Hu11~2FS5C manufactured by Hull Co.) by the conventional
method to obtain lyophilized compositions each containing
5mg of the compound of Example 2.
Compositional Example 11
Lyophilized compositions each containing 5mg of the
compound of Example 7 are obtained in the similar manner
as Compositional Example 10 .
Test Example 1
Effect of stabilizer in stabilizing lyophilized
compositions of the compound of Example 2
The compound of Example 2 and dextran 40, lactose, as
a stabilizer, were dissolved completely in 1 or 2mL of
glycine-NaOH buffer solution (pH 9.8). The resulting
solutions were lyophilized and maintained at 70°C in glass
vials. Nine days after, the resulting compositions were
tested on their appearance, the residual amount of the
compound of Example 2, and pH. As a control, a solution of
the compound of Example 2 without any stabilizers was used.
The results are shown in Table 1.
23
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Table 1
Stabilizers Test Items 0 hours After 9 days
Control: nil Appearance Yellow mass Yellow mass
Residual 100.0 56.5
Amount (%)
pH* 9.78 9.75
Dextran 40 Appearance Yellow mass Yellow mass
(100mg) Residual 100.0 >90
Amount ( o )
pH* 9.80 9.71
Lactose Appearance Yellow mass Brown melt
(100mg) Residual 100.0 <80
Amount (%)
pH* 9.71 4.70
Each vial was contained l0mg of the compound of Example
_2.
*pH of reconstituted solutions of compositions in 2mZ
of purified water.
As is obvious from Table 1, the lyophilized composition
of the compound of Example 2 and dextran 40 was significantly
stable as compared with the one not containing any stabilizer
or containing other stabilizers, such as lactose.
Test Example 2
Stability test of lyophilized composition containing
5mg of the compound of Example 2
The pharmaceutical compositions obtained in
Compositional Example6were storedat varioustemperatures.
The results are shown in Table 2.
24
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
TahlP 7
Storage Test Items
Conditions Residual
Appearance pH*
Amount ( o )
0 hours Yellow
100.0 9.69
mass
After 3 months
Yellow
at 40C and a 75 >95 9. 72
0
mass
humidity
After 3 months
Yellow
at 25C and a 60 >95 9 . 71
0
mass
humidity
Each vial was contained 5mg of the compound of Example
_2.
*pH of reconstituted solutions of compositions in 1mZ of
purified water
As is obvious from Table 2, the residual amount of the
compound of Example 2 after stored at 40°C or 25°C for 3 months
was more than 950.
The following Examples are given for the purpose of
illustrating the present invention in more detail.
The Starting Compounds used and the Object
Compounds obtained in the following Examples 1 to 7
are given in the table as below, in which the formulas
of the starting compounds are in the upper column, and
the formulas of the object compounds are in the lower
column, respectively.
25
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Example Formula
No.
OH 0
HO HO 0 ' N.N . _
HO~H3C'~~.... N H \ / l~ ~ \ / ~O~CH2)50CH3
N S
0 o H~ OH
b '
HO' H O~H3
0 l~
1('u'0H
D QH
H03S0 .
l
0
1
OH O
HO 0 - N,N _
HO H3C'~~ N -I~,. H \ / / J~S~ \ / ~O I CH2 ) 50CH3
NH ~ 0
HO o H~ OH
HO'~ H o : H3
0 g l~
~~''OH
DH
H03S0 \
HO
OH O
HO 0 ~--~'
H3C~. ~. Nn ? H ~ N
0
o H~ OH
H2N HO" H o 'CH3
0 g ~~(~ N
~~''OH
~D H ~
H03S0 \. ! - 'CHg
2 H
OH O
HO O ~-i
N~ ~N
HO H3C,...~ O ~ ~I
HO~ 0 H~ OH
H HO' H o~Hg l \
O ~~''OH
_ ~DH ~
H03S0 \ / - 'CH3
HO
26
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Example Formula
No.
OH 0
HO 0 - ~'
HO H30'" N j q \ / \ / ~ \ /
~--N :..~ ~O
HOJ p H OH
O~ HO' ~H O~H3
O H ~(~
~~~'OH .
D 0UH
Na03S0 \
0
3
OH
HO ~ ' 0
N
H 0 H 3 C".. H '--'
~--~ H ~ O
HO-i o H~ OH
H0~ H O .CH3
0
~~~''OH
~DH
H03S0 \ / .
HO
OH O
HO 0
HO H3C'~N H \ / ~ .
HON ~NY O
0 H OH
O'~OHO
H o~H3
J o H l~
N~'OH
DH
H03S0
o~ . ~N~.
4 =Lo-~o
OH p
H3CH~:. 0 N~ H \ / ~ \
HO ~ 0
o H~ OH
HO~HH~,. H O~H3
0 l~
~''OH
_ DH
H03S0 \ /
. HO
27
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Example Formula
No.
OH
HO O ~
HgC~, '. N~ NHS
O
HO 0 H~ OH
HO-J I HO' H O~CH3
Fmoc O
N~"'OH
DH
H03S0
HO
OH
O
HO O ~ ~,~ ~
NH \ H!~~5~(CH2)qOCHg
H3C "...~~ ~ ~ NO
HO o HN OH
J H HO,; H O . H3
HO O H
N ~""OH
_ OH
HO3S0
HO
OH
HO O
......~N~ NH2
HO
O
HO~ p H~ OH
0 HO g 0
r ~0 0
' 1 / ~ ~~""OH
OH
H03S0
HOr
6
OH O ,
HO HO O N~ H ~~ NSN ~ ~ N~O
...... O
HO 0 HN OH
H H O" g
0 ~ 1'~
"'OH
_ OH
H03SO ~
HO
2~
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Example Formula
No.
OH
HO ~ ~'
NH ? NHS
H O ,....
O
H0~ o HN OH
HO' H O
O p H
= 1 / ~ N~~"OH
_ OH
H03S0
7 HO
OH 0 N-N OMe
/ \
HO 0 N--IO_ ~~ / S ~ / N
HO ......~ ~O O
HO~ ~O HN OH
H HO H o
0 H
N
"~OH
_ OH
H03S0 ~ !
HO
Example 1
To a solution of the starting compound ( 1 ) ( 610 . 6
mg ) in a mixture of methanol ( 10 ml ) a'nd tetrahydrofuran
(25 ml) were successively added triphenylphosphine (32
mg), tetrakis(triphenylphosphine)palladium(0) (35
mg) and morpholine (106 ~.1) with stirring, and the
mixture was stirred at ambient temperature for 3.5
hours. Ethyl acetate (100 ml) was added, and the
resulting precipitate collected, washed with isopropyl
ether, and dried to give a crude pale yellow powder
(535 mg). The crude powder was dissolved sodium
hydroxide aqueous solution and subjected to column
chromatography on ODS (YMC-gel ODS-AM-S-50 (Trademark:
prepared by YMC Co., Ztd.)) (37% acetonitrile aqueous
29
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
solution). The fractions containing the object
compound were combined, and evaporated under reduced
pressure to remove acetonitrile. The residue was
lyophi 1i zed to give the obj ect compound ( 1 ) ( 293 . 7 mg ) .
IR (KBr) : 3355.5, 1633.4, 1608.3, 1529.3, 1517.7,
1463.7, 1444.4, 1267.0, 1230.4 cm-1
NMR ( DMSO-d6, b) : 0 . 98 ( 3H, d, J=6 . 7Hz ) , 1 . 10 ( 3H,
d, J=5 . 6Hz ) , 1 . 2-5 . 6 ( 65H, m) , 6. 71 ( 1H, d, J=8 . 1Hz ) ,
6.78 (1H, d, J=9.7Hz), 7.00 (1H, s), 7.09 (2H, d,
J=9.lHz) , 7.75 (2H, d, J=8. 7Hz) , 7 . 95 (4H, s) , 7. 3-8. 7
(7H, m) , 8.79 (1H, s)
MASS (m/z) : 1465.5 (M-H)-
Elemental Analysis Calcd.for C66H90N1202252'7H20:
C 49.74, H 6.58, N 10.55
Found : C 49.72, H 6.43, N 10.40
Example 2
The suspension of a mixture of the starting compound
( 2 ) ( 10 0 mg ) , 1 , 3-dihydroxyacetate ( 13 . 5 mg ) and acetic acid
(0.13 ml) in a mixture of methanol (1.5 ml) and
dimethylformamide(0.7m1)wasaddedsodiumcyanoborohydride
( 9 . 4 mg ) with stirring at ambient temperature, and the mixture
was stirred at the same temperature overnight. To the
reaction mixture was added ethyl acetate (20 ml). The
resulting precipitate was collected by filtration and dried
in vacuo. The precipitate was dissolved in a mixture of water
and 1N sodium hydroxide and the solution was subjected to
column chromatographyon ODS (Daiso-gel, SP-120-40/60-ODS-B
(Trademark: prepared by Daiso Co., Ltd.)) (50 ml) eluting
with 40o acetonitrile in water. The fractions containing
the object compound were collected and evaporated under
reduced pressure to remove acetonitrile. ,The residue was
lyophilized to give the object compound (2) (55 mg).
NMR ( DMSO-d6, 8) : 0 . 90 ( 3H, d, J=6 . 8Hz ) , 0 . 98 ( 3H,
d, J=6. 7Hz) , 1.11 (3H, d, J=5. 5Hz) , 1. 43-5.24 (62H,
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
m), 6.69-8.85 (17H, m)
ESI MASS (m/z) (Negative) : 1408.3 (M+)
Example 3
To a solution of starting compound (3) (0.22 g) in a mixture
of methanol (4 ml) and THF (1 ml) were successively added
triphenylphosphine (14 mg),
tetrakis(triphenylphosphine)palladi'um(0) (8 mg) and morpholine
(40 ~.l) with stirring and the mixture was stirred at ambient
temperature for 3 hours. The reaction mixture was concentrated
in vacuo. The resulting residue was dissolved in a mixture of pH
6.86 standard buffer solution and 1N sodium hydroxide, insoluble
materials were filtered off and the solution was subj ected to column
chromatography on ODS (Daiso-gel, SP-120-40/60-ODS-B (Trademark:
prepared by Daiso Co. , htd. ) ) (100 ml) eluting with 30 o acetonitrile
in water. The fractions containing the object compound were
collected and evaporated under reduced pressure to remove
acetonitrile. The residue was lyophilized to give object compound
(3) (85 mg).
IR (KBr): 1633, 1537, 1516, 1450, 1234 cm-1
NMR (DMSO-d6 + D20, 8): 0.98 (3H, d, J=7.09Hz), 1.05
(3H, d, J=7.OOHz), 1.15 (6H, d, J=6.21Hz), 1.60-2.30 (8H,
m), 2.75-3.45 (14H, m), 3.80-4.50 (10H, m), 4.81 (1H, br
s), 6.65-7.20 (8H, m), 7.50-7.80 (5H, m), 7.94 (2H, d,
J=8.49Hz)
ESI MASS (m/z)(Negative): 1416.4 (M++1)
Elemental Analysis Calcd. for C67H91N110215'7H20:
C 52.10, H 6.85, N 9.97
Found: C 52.29, H 6. 60, N 9. 61
The following comopound was obtained according to
a similar manner to that of Example 3.
Example 4
IR (KBr): 2931, 2854, 1632, 1510, 1446, 1385, 1325 cm-1
31
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
NMR (DMSO-d6, b): 0.97 (3H, d, J=6.7Hz), 1.12 (3H, d,
J=5. 5Hz) , 1. 08-2. 62 (23H, m) , 2. 62-4.50 (37H, m) , 4 . 66-5. 45
( 10H, m) , 6 . 70 ( 1H, d, J=8 . 1Hz ) , 6. 78 ( 1H, d, J=8 . 1Hz ) ,
6. 83-7 . 09 (7H, m) , 7 .34-8 . 00 (3H, m) , 7 . 80 (2H, d, J=8. 7Hz) ,
8.00-8.49 (2H, m), 8.71 (1H, s)
MASS (m/z): 1408.4 (M++1)
Elemental Analysis Calcd. for C66H95N110215'7H20v
C 51.59, H 7.15, N 10.03
Found: C 51.77, H 7.05, N 9.82
Example 5
To a solution of starting compound (5) (12.50 g)
and diisopropyleth.ylamine (3.67 ml) in
N,N-dimethylformamide (250 ml) was added
4-[2-[4-(4-methoxybutoxy)phenyl]imidazo-
[2,1-b][1,3,4]thiadiazol-6-yl]benzoic acid
benzotriazol-1-yl ester at room temperature. The
solution was stirred for 4 hours at the same temperature,
during which period additional
4-[2-[4-(4-methoxybutoxy)phenyl]imidazo[2,1-b][1,3
,4]-thiadiazol-6-yl]benzoic acid benzotriazol-1-yl
ester was added to the mixture. The reaction mixture
was then filtered. To the filtrate was added
piperidine (9.33 ml) at room temperature. The
solution was stirred for 1 hour at the same temperature.
Ethyl acetate was added to the reaction mixture. The
powder was collected by filtration to give crude
material (16.12 g). The crude material was purified
by column chromatography on ODS to give obj ect compound
(5) (11.10 g). ~ '
IR (KBr)~: 1659, 1633, 1529, 1518, 1466, 1444, 1255 cm-1
NMR (DMSO-d6, 8): 0.98 (3H, d, J=6.7Hz), 1.00 (3H, d,
J=5 . 8Hz) , 1 . 5-2 . 6 ( 12H, m) , 2 . 8-3 . 6 ( 33H, m) , 4 . 7-5 . 4 (
10H,
m) , 6 . 65-6 . 85 ( 2H, m) , 7 . 00 ( 1H, s ) , 7 . 15 ( 2H, d, J=8 . 9Hz ) ,
7. 3-7. 7 (2H, m) , 7. 90 (2H, d, J=8. 8Hz) , 7. 96 (4H, s) , 8. 0-8.5
32
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
(2H, m) , 8.71 (1H, s) , 8.85 (1H, s)
MASS (m/z) : 1392 (M++23)
Elemental Analysis Calcd.forC60H79N11022S2~5H20:
C 49.34, H 6.14, N 10.55
Found: C 49.30, H 6.23, N 10.53
The following compounds [Example 6 and 7] were obtained
according to a similar manner to that of Example 5.
Example 6
IR (F~Br) : 1664, 1635, 1605, 1446, 1410, 1350, 1329,
1281 cm-1
NMR (DMS~-d6, D20, ~) : 0. 98 (3H, d, J=6.7Hz) , 1. 10 (3H,
d, J=5.9Hz), 1.1-2.6 (21H, m), 2.8-4.5 (31H, m), 4.7-4.9
(2H, m) , 6. 7-~. 9 (2H, m) , 7. 0-7. 2 (3H, m) , 7. 85 (2H, d,
J=8.9Hz), 8.0-8.2 (4H, m)
ESI MASS (m/z) (Negative) : 1409.4 (M--1)
Elemental Analysis Calcd. for C~4H87N11021S2~6H2Q:
C 50.62, H 6.57, N 10.15
Found: C 50.40, H 6.61, N 9.92
Example 7
IR (I~Br) : 1664, 1628, 1605, 1446, 1417, 1279, 1084,
1047 cm-1
NMR (DMSO-d6+D20, 8): 0.8-1.3 (12H, m), 1.5-2.6 (16H,
m) , 2. 8-4 . 5 (32H, m) , 4 .7-4 . 9 (2H, m) , 6. 7-6. 9 (2H, m) , 7 . 0-7 .2
(3H, m), 7.85 (2H, d, J=8.6Hz), 8.0-8.2 (4H, m)
ESI MASS (m/z)(Negative): 1423.5 (M--1)
The cyclic polypeptide compound ( I ) or a salt thereof
has an antifungal activity, particularly against the
following fungi.
Ac.remonium;
Absidia (e. g., Absidia corymbifera, etc);
Aspergillus (e. g. , I~spergillus clavatus, Aspergillus
33
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
flavus, Aspergillus fumigatus, Aspergillus nidulans,
Aspergillus niger, Aspergillus terreus, Aspergillus
versicolor, etc);
Blastomyces (e. g. , Blastomyces derma titidis, etc) ;
Candida (e. g. , Candida albicans, Candida glabrata,
Candida guilliermondii, Candida kefyr, Candida krusei,
Candida parapsilosis, Candida stellatoides, Candida
tropicalis, Candida utilis, etc.);
Cladosporium (e. g. , Cladosporium trichoides, etc) ;
Coccidioides (e. g., Coccidioides immitis, etc);
Cryptococcus (e. g., Cryptococcus neoformans, etc);
Cunninghamella (e. g. , ' Cunninghamella elegans, etc) ;
Dermatophyte;
Exophiala (e. g., Exophiala dermatitidis, Exophiala
spinifera, ete);
Epidermophyton (e. g. , Epidermophyton floccosum, etc) ;
Fonsecaea (e. g. , Fonsecaea pedrosoi, etc) ;
Fusarium (e. g. , Fusarium solani, etc) ;
Geotrichum (e. g., Geotrichum candiddum, etc);
Histoplasma (e. g., Histoplasma capsulatum var.
capsulatum, etc);
Malassezia (e. g. , Malassezia furfvr, etc) ;
Microsporum (e. g. , Microsporum cams, Microsporum
gypseum, etc);
Mucor;
Paracoccidioides (e. g. , Paracoccidioides brasiliensis,
etc) ;
Penicillium (e. g., Penicillium marneffei, etc);
Phialophora;
Pneumocystis (e. g. , Pneumocystis carinii, etc) ;
Pseudallescheria (e. g. , Pseudallescheria boydii, etc)
Rhizopus (e. g., Rhizopus microsporus var.
rhizopodiformis, Rhizopus oryzae, etc) ;
Saccharomyces (e. g., Saccharomyces cerevisiae, etc);
Scopulariopsis;
34
CA 02433263 2003-06-26
WO 02/053584 PCT/JPO1/11242
Sporothrix (e. g. , Sporothrix schenchii, etc) ;
Trichophyton (e. g. , Trichophyton mentagrophytes,
Trichophyton rubrum, etc);
Trichosporon (e. g., Trichosporon asahii, Trichosporon
cutaneum, etc).
The above fungi are well known to cause various infection
diseases in skin, hair, nail, oral mucosa, gastrointestinal
tract, bronchus,lung,endocardium,brain,meninges,urinary
organ, vaginal protion, oral cavity, ophthalmus, systemic,
kidney,bronchus,heart,externalauditorycanal,bone,nasal
cavity, paranasal cavity, spleen, liver, hypodermal tissue,
lymph duct, gastrointestine, articulation, muscle, tendon,
interstitial plasma cell in lung, and so on.
Therefore, the cyclic polypeptide compound (I) or a salt
thereof of the present composition is useful for preventing
and treating various infectious diseases, such as
dermatophytosis (e. g., trichophytosis, etc), pityriasis
versicolor, candidiasis, cryptococcosis, geotrichosis,
trichosporosis, aspergillosis, penicilliosis, fusariosis,
zygomycosis, sporotrichosis, chromomycosis,
coccidioidomycosis, histoplasmosis, blastomycosis,
paracoccidioidomycosis, pseudallescheriosis, mycetoma,
mycotic keratrtis, otomycosis, pneumocystosis, and so on.
A commercialpackage comprisingthe cyclic polypeptide
compound (I) or a salt thereof of the present composition
and a written matter associated therewith, wherein the
written matter states that the pharmaceutical composition
can or should be used for preventing or treating infections
disease.