Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
01-1188 ff-Ko, l t 111 CA 02435372 2003-07-18
BOEHRINGER INGELHEIM PHARMA KG
75058fft.205
Process for purring 20 S~-Camptothecine
s The invention relates to a process for purifying 20(5)-camptothecine
contaminated by a vinyl-
camptothecine derivative. _.
Background to the invention
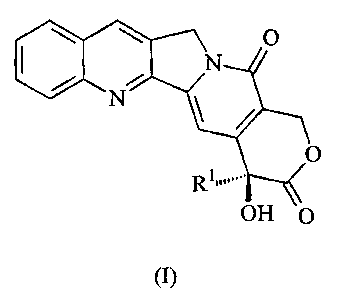
20(5)-camptothecine (20(5)-CPT) is a natural alkaloid of formula (I)
O
io (~
wherein Rl denotes ethyl.
OH O
20{S)-CPT and its derivatives, being topoisomerase I inhibitors, have tumour-
inhibiting
properties (e.g. Giovanelle, B.C. et al., Cancer Research, 51: 302-3055, 1991,
European
15 Patent applications EP 0 074 256, EP 0 088 642, US Patents US 4,473,692, US
4,545,880, US
4,604,463 and International Patent Application WO 92/05785).
;,
20(5)-CPT can be obtained as a crude product from the Chinese tree Camptotheca
acuminata
{Nyssaceae) (Wall M. et al., J. Am. Chem. Soc. 88: 3888-3890, 1966) or from
the Indian tree
20 Nothapodytes foetida (nimmoniana) (formerly known as: Mappie foetida Miers)
(Govindachari, T.R. et al., Phytochemistry 11: 3529-3531, 1972), inter alia.
These crude products, particularly the one obtained from Nothapodytes foetida,
contain 20(S)-
CPT contaminated by a CPT derivative of formula (I) wherein Rl denotes vinyl
(20-vinyl-
25 CPT).
Traditionally, the crude products are purified by complex chromatographic
methods or by
converting the camptothecine into the aqueous phase and eliminating impurities
by extraction
Ol-1188 prio-Ko 2/1l CA 02435372 2003-07-18
_2-
with water-insoluble solvents (e.g. WO 94/19353). However, contamination by 20-
vinyl-CPT
cannot be efficiently dealt with by these methods.
The problem of the present invention is therefore to provide a process which
allows the 20(S)-
CPT starting product to be purified without using complex chromatographic
methods.
Description of the invention
Surprisingly, it has been found that 20(S)-CPT can be virtually completely
freed from
contamination with 20-vinyl-CPT by first treating the starting material with
an aqueous base,
lo hydrogenating and subsequently acidifying it and then isolating the
product.
The invention thus relates to a process for purifying 20(S)-camptothecine
which comprises
the following steps:
(a) combining an aqueous base and a starting material containing 20(S)-
camptothecine,
thereby converting the lactone ring of the 20(S)-camptothecine into a
carboxylate salt;
(b) hydrogenating the resulting mixture in the presence of a transition metal
catalyst;
(c) acidifying the aqueous phase, thereby forming camptothecine crystals;
(d) adding a polar aprotic solvent; and
(e) separating off the camptothecine crystals.
The invention further relates to a process for preparing 20(S)-camptothecine
of formula (I)
wherein Rl denotes ethyl, from 20-vinyl-camptothecine of formula (I) wherein
Rl denotes
vinyl, which comprises the following steps:
(a) combining an aqueous base and the starting material containing 20(S)-
camptothecine, forming a compound of formula (II),
O
l N OH
N
OMet
R;,""~
OH O
wherein
Ri denotes vinyl; and
01-11 ~8 prio -~Ko 3 / 11 CA 02435372 2003-07-18
-3-
Met denotes a metal;
(b) hydrogenating the resulting mixture in the presence of a transition metal
catalyst;
(c) acidifying the aqueous phase to form camptathecine crystals;
(d) adding at least one polax aprotic solvent; and
(e) separating off the camptothecine crystals.
Detailed descn~tion of the invention
to The term "starting material containing camptothecine " as used above and
hereinafter refers to
a contaminated material containing 20(S)-CPT, crude camptothecine,
camptothecine-
containing plant extracts, synthetic camptothecine, derivatives of
camptothecine as described
for example in International Patent Application WO 92/05785, or reaction
products
containing camptothecine.
Preferably, the starting material is a natural crude product which is obtained
in particular from
Nothapadytes foetida. As a rule, it is a mixture of the compound of formula
{I) wherein Rl
denotes ethyl, and the compound of formula (I) wherein Rl denotes vinyl. It
generally
contains 0.9 to 1.5 wt.-%, preferably 1.0 to 1.4 wt.-% of the vinyl compound.
In addition, the
2o starting material may contain other camptothecine derivatives such as, for
example, 9-
methoxy-CPT, 10-methoxy-CPT, 11-methoxy-CPT, 10-hydroxy-CPT and 11-hydroxy-
CPT.
As a rule, the starting material contains up to 1 wt.-% of one or more of
these additional CPT
derivatives, particularly 0.2 to 0.8 wt.% of 9-methoxy-CPT.
The term "aqueous base" as used above and hereinafter in connection with step
(a) of the
purification process according to the invention relates to a base which
generates enough
hydroxide ions in the aqueous medium, preferably in pure water, to convert the
lactone group
of the camptothecine derivatives contained in the starting material completely
into the
corresponding hydroxycarboxylates. Metal hydroxides are preferred,
particularly alkali metal
or alkaline earth metal hydroxides such as lithium hydroxide, sodium
hydroxide, potassium
hydroxide or calcium hydroxide. Sodium hydroxide is most preferred
01-1188prio-Ko 4/11 CA 02435372 2003-07-18
-4-
The metal hydroxide is preferably used in the form of a dilute aqueous
solution, preferably in
the form of a 1 to 25 %, particularly a 3 to 10 % aqueous solution. As a rule,
sufficient metal
hydroxide is used to make the camptothecine derivatives go completely into
solution;
preferably, 1 to 20 mol, more preferably 5 to 15 mol, particularly 7.5 to 12.5
mol of metal
hydroxide are used per 1 mol of starting material.
In step (b) a transition metal catalyst, preferably a heterogeneous transition
metal catalyst,
particularly platinum, platinum oxide, nickel, palladium or rhodium on a
carrier material such
as activated charcoal or aluminium oxide is added to the resulting mixture.
Palladium on
to activated charcoal containing 1 to 15 wt.%, preferably 2 to 10 wt.%,
particularly about 5
wt.-% of palladium is particularly preferred.
The quantity of transition metal catalyst is selected so as to ensure total
hydrogenation of the
vinylic CPT derivative. Preferably, 0.01 to 0.50 parts by weight, particularly
0.02 to 0.10
1s parts by weight of transition metal catalyst (including carrier materials)
are used, based on 1
part by weight of the starting material.
The resulting mixture is subjected to the action of hydrogen gas, preferably
at a temperature
of - 20 °C to 100 °C, particularly 10 °C to 40 °C,
most preferably at about room temperature.
The hydrogen pressure is not critical per se; the hydrogenation is preferably
carried out at
normal pressure or at slightly raised pressure, particularly at 0.9 to 5.0
bar, most preferably at
about 1 bar.
2s Under these conditions, hydrogenation is generally complete within 1 to 20
hours, preferably
4 to 15 hours, particularly 6 to 10 hours.
After the hydrogenatian has ended, the transition metal catalyst is preferably
eliminated by
filtration, and the resulting reaction mixture is acidified in step (c). The
acidification can be
3o done with an inorganic or organic acid. Preferred acids are inorganic acids
such as HCI, HBr,
HI, HN03, H~POa, Ha50a, or aliphatic carboxylic acids such as acetic acid and
trifluoroacetic
acid or mixtures of these acids, particularly concentrated hydrochloric acid.
Using the chosen
acid, the pH is adjusted to 3.0 to 6.0, preferably 3.5 to 5.0, particularly
about 4.0 to 4.5. The
01-11 &8 prio -KO .S l1 ~ CA 02435372 2003-07-18
-$-
reaction with the acid is generally carried out at a temperature of 0
°C to 100 °C, preferably
30 °C to 80 °C, particularly 50 °C to 60 °C.
In a particularly preferred embodiment, acidification is carried out with 2 to
20 parts by
weight, preferably 4 to 9 parts by weight, particularly 6 to 8 parts by weight
of concentrated
hydrochloric acid, based on 1 part by weight of starting material.
Under the conditions described, lactonisation to form the CPT is generally
complete within 10
to 180 minutes, preferably 1 S to 60 hours, particularly within about 30
minutes.
to
The reaction mixture obtained by acidification is generally in the form of a
pure suspension.
To improve the crystallisation in step (d) one or more polar aprotic solvents
are added thereto.
Suitable solvents of this kind are preferably sulphoxides such as
dimethylsulphoxide (DMSO)
or amides and urea derivatives of formula
R3
R~C~N~Ra
I!
is O
wherein
R2 denotes hydrogen or a Cl-4 alkyl group, particularly hydrogen or methyl;
R3 and R4 independently of each other denote a Ci-a alkyl group, particularly
methyl; or
R2 and R3 together denote a -(CHZ)m- or a NRS-(CH2)n- group, while
! . 2o RS denotes a Cl-4 alkyl group;
m is 3 or 4, particularly 3; and
nis2or3,
particularly selected from among N,N-dimethylformamide (DMF), N,N-
dimethylacetamide
(DMA), N-methylpyrrolidone (NMP), N,N-dimethylethylene urea (DMEI~ and N,N-
25 dimethylpropylene urea (DMPL~ or mixtures of these solvents, most
preferably DMF.
As a rule, 10 to 100 parts by weight, preferably 20 to 80 parts by weight,
particularly 30 to 50
parts by weight of the polar aprotic solvent are used, based on 1 part by
weight of the starting
material used.
DI-1188 prio-.Ko 6111 CA 02435372 2003-07-18
-6-
The treatment with the polar aprotic solvent may be carned out at any desired
temperature.
The reaction mixture is preferably stirred at a temperature of 30 °C to
120 °C, particularly 80
to 100 °C and then slowly cooled to ambient temperature.
The CPT crystals thus obtained are easily separated from the liquid phase in
step (e),
preferably by decanting, centrifuging, spinning, squeezing out or filtration,
particularly by
filtration.
As a rule, the CPT crystals thus obtained are washed with an alcohol,
preferably methanol,
1o ethanol or isopropanol, particularly methanol, and dried.
The advantage of the procedure according to the invention is the high
space/time yield and the
high yield and purity of the 20(S)-camptothecine produced, which is obtained
without any
chromatographic purification substantially free from contaminants containing
vinyl groups.
The Examples that follow serve to illustrate some processes for purifying
camptothecine
carned out by way of example. They are intended only as possible methods given
as
examples, without restricting the invention to their content.
2o Example 1
10.45 g of a crude product containing camptothecine, obtained from
Nothapodytes foetida,
containing 1.33 % of 20-vinyl-CPT and 0.47 % of 9-methoxy-CPT, is taken up in
260 ml of a
a
2 N sodium hydroxide solution and 0.6 g of palladiumlactivated charcoal (5 %)
are added.
The mixture is treated with hydrogen for 8 hours at ambient temperature under
a pressure of 1
bar.
Then the reaction mixture is filtered and combined at 50-60 °C with 80
ml of concentrated
hydrochloric acid and adjusted to a pH of 4.0 to 4.5.
so The suspension formed is combined with 400 ml of DMF and stirred far 2.5
hours at 90-
100°C. The resulting suspension is slowly cooled to ambient temperature
and filtered. The
CPT crystals obtained are washed with 100 ml of methanol and dried at
55°C in vacuo.
01-1188 prio-.KO 7/1l CA 02435372 2003-07-18
- _.
9.85 g (94.2 % of material put in) of 20(S)-camptothecine are obtained,
containing less than
0.05 % of 20-vinyl-CPT and 0.11 % of 9-methoxy CPT.
Example 2
10.45 g of a crude product containing camptothecine, obtained from
Nothapodytes foetida,
containing 1.33 % of 20-vinyl-CPT and 0.47 % of 9-methoxy-CPT is taken up in
260 ml of a
2 N sodium hydroxide solution and 0.6 g of palladiumJactivated charcoal (5 %)
are added.
The mixture is treated with hydrogen for 8 hours at ambient temperature under
a pressure of 1
1 o bar.
Then the reaction mixture is filtered and combined at 50-60 °C with 300
ml of a 10%
sulphuric acid and adjusted to a pH of 4.0 to 4.5.
The suspension formed is combined with 500 ml of DMF and stirred for 2.5 hours
at 90-
100°C. The resulting suspension is slowly cooled to ambient temperature
and filtered. The
CPT crystals obtained are washed with 100 ml of methanol and dried at
55°C in vacuo.
9.67 g (92.6 % of material put in) of 20(S)-camptothecine are obtained,
containing 0.09 % of
9-methoxy CPT, the content of 20-vinyl-CPT being below the detection
threshold.