Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
a ~ CA 02436273 2003-07-25
DESCRIPTION A X77
An active substance concentrate and a method of producing an active substance
concentrate
The invention refers to a water-soluble concentrate which comprises a
physiological active substance, which is insoluble or only soluble with
difficulty in
water. and a solubiliser, as well as a method for producing the concentrate.
Fat-soluble compounds. such as for example vitamin E. vitamin A and other
carotenoids or also coenzyme Qio, are absorbed in dependence of the presence
of
bile salts and pancreatic enzymes. A process of so-called micelle formation in
the
intestine precedes the absorption process: this is necessary so that the fat-
soluble
compounds can be "packed" and in this way the various barners of the
intestinal
mucosa overcome.
If the secretion of the bile fluid or pancreatic enzymes is disturbed. then
this results
in a so-called maldigestion or malabsorption of fat-soluble compounds. The
best
example of this is the illness cystic fibrosis where, due to deficient
generation of
pancreatic enzymes, the resorption of fat-soluble compounds is possible only
to a
very slight extent.
The special features of the absorption of fat-soluble micronutrients also
become
apparent in that the ingestion always increases when fat is offered
simultaneously.
Fat promotes on one hand the release of bile acid and pancreatic enzymes and,
on
the other hand, the formation of micelles, which then contain the said fat-
soluble
micronutrients.
Once the fat-soluble compounds have been ingested by the intestinal cells.
they are
then available there in a free form. i.e. they are no longer bound to micellar
constituents. In this free form they are then rendered "water-soluble" again
in that
they are integrated into the transporters formed within the intestinal cells
- , CA 02436273 2003-07-25
s
2
(lipoproteins - chylomicrons) and then released via the large lymphatic
channels
into the blood.
In order to be able to accept lipophilic compounds, the organism must render
them
water-soluble in two steps. The first step occurs in the intestine through the
formation of the micelles, out of which the substance is then released again
in the
intestinal cell, and the second step is the formation of lipoproteins for
transport in
the blood. Therefore lipophilic substances which have been rendered water-
soluble
(clear solutions), but not those which are just dispersed in the aqueous
medium
(turbid solutions). are more rapidly and more efficiently absorbed by the
organism
than the original lipophilic substance.
Only very little data is available on the bioavailability of lipophilic
micronutrients
that have been rendered water-soluble (clear solutions). A technique for
testing the
bioavailability of such compounds is provided by the so-called in-vitro
dissolution
methods. In this connection, it is found how far a compound dissolves in the
aqueous compartment or how far it is released from a certain galenic
formulation.
From US 6,048,566 it is known that Quo that has been rendered water-soluble,
in
contrast to Q,o from oily solutions or dispersions (turbid solutions), is
released by
up to 100 percent. This signifies though that Q,o applied in this way is
already
available in a higher concentration in free form in the inte~:inal lumen and
does not
need to be released first by micelle formation or by decomposition of the
lipids
surrounding the Q,a.
The object of the invention is therefore to improve the bioavailabilitv of
physiologically important substances. which are insoluble or only soluble with
difficulty in water, such as c.~-3-fatty acids, a-lipoic acid (thioctic acid),
ubichinons
(e.g. coenzyme Quo), phytosterins and others, and to simplify technologically
the
industrial processing of these substances.
The object of the invention is also to use the smallest possible amount of
solubilisers, in particular polysorbates. in the production of the
concentrates -
CA 02436273 2003-07-25
taking into account a tolerance range for ensuring complete, stable water
solubility
- so that the ADI values (ADI=Acceptable Daily Intake) for the polysorbates
according to the JECFA (=Joint FAO/WHO Expert Committee on Food Additives)
and SCG substances (SCG = Scientific Committee on Food (EU)) such as
tocopherols (e.g. a-tocopherols), w-3-fatty acids, a-lipoic acid (thioctic
acid) and
ubichinons (e.g. coenzyme Quo) are clearly undercut.
This object is solved according to the invention in that the substance which
is
insoluble or only soluble with difficulty in water is added to an excess of
heated
physiologically compatible solubiliser with an HLB value between 6 and 19,
especially Polysorbate 20 or Polysorbate 80, the mixture being stirred under
the
influence of heat until a clear, viscous intermediate product is produced and
the
intermediate product is then cooled to room temperature. If the active
substance is
to be provided in the aqueous phase, hot distilled water is added to the hot
intermediate product in such an amount that corresponds to the desired
concentration of active substance; it is then stirred to obtain homogeneity
and the
phase produced in this way is then cooled quickly to room temperature.
If the phase is to be free of water, a hot triglyceride, for example a light
vegetable
oil with a high linoleic acid content; and hot polysorbate are added to the
intermediate product in such amomis so as to give the desired concentration of
active substance and are stirred again under the influence of heat until the
concentrate becomes clear. After cooling the active substance is present in a
water-
free phase which can however be dissolved in water as required. This is
particularly suitable for administering the active substance in capsule form
which
in the long term tolerates only a very low water content.
Both the water-free and the aqueous phases are soluble in water or fat and/or
oil.
As measurements using single-phase chromatography show. the active substance
is
present both in the aqueous and in the water-free phase in molecular
aggregates
enclosed by polysorbate, whereby the polysorbate envelope in each case
exhibits a
CA 02436273 2003-07-25
diameter of about 30 nm; the polysorbate envelope with enclosed molecular
aggregate can be regarded as a micelle.
In the physiological field the micelle formation according to the invention
results in
a substantially improved bioavailability of the active substance. The active
substance which is'insoluble or only soluble with difficulty in water does not
first
need to be rendered ingestible for the intestinal lumen through interaction
with bile
secretions.
Embodiments of the invention are given in the subclaims and explained in the
following without restricting the generality of the claimed invention. The
enclosed
drawing shows
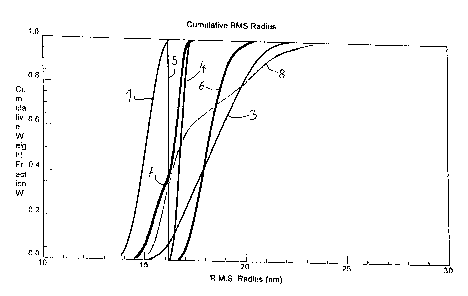
Figure l, a diagram of measured micelle radius distributions;
Figure 2. a schematic explanation of active substance micelles with the
coenzyme
Q,o as an example and
Figure 3, a schematic explanation of the arrangement of micelles which contain
an
active substance (coenzyme Q,o) and an auxiliary substance (linoleic acid):
Figure 4: a schematic representation of phytosterin micelles;
Figure 5: a schematic representation of the distribution of additional
linoleic acid
micelles around a phytosterin micelle. and
Figure 6: a schematic representation of a micelle of an w-3-fatty acid.
Example 1: Coenzyme Quo
In 77 parts by mass of Polysorbate 80 heated to 85°C, 23 parts by
mass of
coenzyme Qlo are introduced and the mixture stirred for about 5 min. at
85°C until
a clear viscous mass of a slightly yellow colour is produced. The mass
proportions
of coenzyme Quo to Polysorbate 80 in the intermediate product are 1:3.35 and
the
ratio of the molecular numbers is 1:2.56, because the molecular weight of
coenzyme Quo is 863.36 and that of Polysorbate 80 is 1130.00.
CA 02436273 2003-07-25
To obtain an aqueous phase of Quo from the intermediate product, which
contains a
Q,o concentration of about 3%, approximately 865 parts by weight of hot water
are
added to the hot intermediate product and stirred under the influence of heat
until a
clear liquid is produced. Then the liquid is cooled quickly (for example.
within
about 1 to 2 minutes) to room temperature (about 20°C) to give the
finished
aqueous phase of the coenzyme Quo. The mean particle radius present in the
phase
was measured by field flow fractionation (FFF) with a DAWN EOS detector from
Wyatt Technologie Deutschland GmbFI coupled to the chromatography column. As
Curve 1 in Figure 1 shows, the radius is, depending on the cumulative weight
fraction, between 14 nm and about 16 nm and therefore in the size range of the
micelles. From the mean micelle weight of about 1.586 x 106 determined in this
way, it can be calculated that each micelle features two molecular aggregates
in the
core. totalling about 400 molecules of coenzyme Quo which is enclosed by five
Polysorbate 80 molecular aggregates of the same type totalling about 1000
molecules of Polysorbate 80, as schematically illustrated in Figure 2.~
To obtain the water-free phase of 5% by wt. of coenzyme Q,o, as much
trigiyceride
and Polysorbate 80 are added while hot (85°C) to the hot intermediate
product such
that the mixture comprises about 5 parts of coenzyme Quo. about 16 parts of
triglyceride and about 79 parts of Polysorbate 80. For triglyceride, thistle
oil. is used:
here which, according to the guiding principles for edible fats and oils of
29/30.11.1983 in the version modified on 2/3.12.1986 (GM Leaflet No. 21, page
379 of 1.8.1987), exhibits a high linoleic acid content of about 67.8% to
about
83.2%. Linoleic acid is recommended due to its molecular size which is similar
to
coenzyme Quo (the molecular weight of linoleic acid is 725). The mixture is
stirred
hot until clarity is obtained and then slowly cooled. A water-free phase of
coenzyme Quo is obtained with a particle size, based on the quoted
measurements.
also in the micelle range. In contrast to the aqueous phase. with the said
method a
mean micelle diameter of 7.657 x 10' is measured from which it follows that
each
micelle in the core exhibits about 200 coenzyme Quo molecules and five
enclosing
molecular aggregates totalling 480 molecules of Polvsorbate 80. whereby this
CA 02436273 2003-07-25
6
micelle is surrounded by four other micelles of the same type, of which each
exhibits about 190 molecules of thistle oil or linoleic acid and a polysorbate
envelope of five molecular aggregates totalling about 480 molecules of
polysorbate. A schematic representation of this micelle formation is shown in
Figure 3.
The water-free phase has excellent storage properties and can be dissolved in
water
at body temperature as required. It is therefore suitable as an additive to
nutritional
supplements which are normally offered in gelatine capsules. An explanation of
the
special stability of the water-free phase can in some circumstances be seen in
that
the central micelle containing the coenzyme Quo is extensively protected by
the
four surrounding micelles containing the auxiliary substance. thistle oil,
i.e. mainly
linoleic acid, particularly from the penetration of polar molecules such as
HBO.
Example 2: Phytosterin
The starting point is the ADM phytosterol which can be obtained from ADM
Nutraceutical, Decatur, Illinois 62526. U.S.A. under the product code 040095.
This
product contains at least 90% phytosterins and in fact 40% - 58% beta-
sitosterin,
20% - 30% campesterin and 14% - 22% stigmasterin as well as up to about 5%
each of sitostanol and brassicasterol. In the Following this product is
briefly
designated as phytosterin. Of course, phytosterins from other manufacturers.
which
contain other compositions in other concentrations, can be treated as
described
below to give corresponding results. The invention is therefore not restricted
to
ADM phytosterol.
To produce an aqueous phase, about 320 g of polysorbate, preferably
Polysorbate
80 is heated to about 100°C. About 10 g of phytosterin is added to the
hot
polysorbate and the mixture is stirred. keeping it at a temperature of about
100°C,
for about 10 minutes. until a homogeneous and transparent phytosterin
concentration of about 3% is produced. The resulting water-free phase, heated
CA 02436273 2003-07-25
7
where necessary to about 40°C, can be dissolved as required in water at
about
20°C.
The said investigation shows that micelles with a molecular aggregate
arrangement
according to Figure 4 are present, whereby about 107 phytosterin "molecules"
are
present in the micelle core and about 207 polysorbate molecules in the
Polysorbate
80 envelope. The distribution of the micelle radii can be seen in Curve 3 in
Figure
l and lies between about 15 nm and about 22 nm.
To produce a water-free phase, first about 100 g of light vegetable oil, for
example
thistle oil, is heated to about 100°C. About 10 g of phytosterin is
added to the hot
thistle oil and the mixture stirred, keeping it at a temperature of about
100°C until,
for example after 10 minutes, the phvtosterin has completely dissolved. About
220 g of polysorbate, preferably Polysorbate 80, are added to this solution.
Stirnng
is continued at about 100°C until the mixture becomes transparent with
a slight
yellow colour. After cooling to room temperature. the water-free phase of a 3%
phytosterin concentration is obtained. It is soluble in water and oils and
remains
stable with respect to hydrochloric and gastric acids (pH < 1 ) and also with
respect
to heating effects. Measurements show that about 90% of the particles in the
water-
free phase have a radius of about 16 nm. The molecular aggregate arrangement
is
shown schematically in Figur° 5, according to which the phytosterin
micelle is
enclosed by four linoleic acid micelles.
The effect of cholesterol reduction of phytosterins has been known for decades
and
has also been proven through more recent clinical studies.
Example 3: w-3-fatty acid
As an example of an c~-3-fatty acid, the product Softgel, which can be
obtained
from Merck KGaA, Darmstadt, under the product number 1.00743.0200 HI-DHA
25 S. This product contains 25% - 28% decosahexaenoic acid (DH A), ~% - 8%
eicosapentaenoic acid (EPA) and in total about 34% - 40% of ca-3-fatty acid.
In the
CA 02436273 2003-07-25
8
following this product is briefly designated as omega-3-fatty acid. Of course,
w-3-
fatty acids from other manufacturers can be treated as described in the
following,
whereby analogous results are obtained.
As an example of the production of an c~-3-fatty acid concentrate, about 800 g
of
Polysorbate 80 are heated to about 160°C. Then, maintaining the
temperature.
about 200 g of omega-3-fatty acid is added and stirred for about S minutes
while
maintaining the temperature until a homogeneous mixture is obtained as an
intermediate product. The intermediate product produced in this way is
transparent
and retains its transparency also after slow cooling to room temperature. It
can be
dissolved as required in water at about 20°C after brief stirring
without turbidity or
sedimentation occurring. The intermediate product. which contains about 6% of
w-
3-fatty acids. exhibits micelles with a mean radius distribution as given in
Curve 4
of Figure l, as provided by measurements of the aqueous intermediate product
according to the said above method. The mean micelle diameter lies at about 33
nm. The molecular aggregate arrangement is shown Figure 6: reference is made
to
the figures entered there.
The clarity and water solubility of the aqueous intermediate product are
retained
even when gastric acid (hydrochloric acid) is added.
A kilogram of the intermediate product contains about 67 g of ca-3-fatty
acids. so
that about 3 to 4 g of this intermediate product covers the human daily
requirement
of ca-3-fatty acids.
Example 4: Isoflavones
The starting point is a Soya bean extract powder which can be obtained from
the
Archer Daniels Midland Company. U.S.A. under the trade name NOVASOY. This
product contains at least 40% by wt. of genistin, daidzin and glycitin and
their
aglycones in a quantity ratio of 1.3:1.0:0.3. Therefore l00 g of this said
extract
contain 20.0 g of genistin, 15.4 g daidzin and 4.6 g of glycitin, totalling 40
g of
CA 02436273 2003-07-25
9
isoflavones. In the following this product is briefly designated as genistin
isoflavone. Of course, an appropriate product from another manufacturer can be
used provided it contains isoflavones, in a different composition where
necessary.
For example, a Soya bean extract is obtainable from K.-W. Pfannenschmidt GmbH,
Hamburg, which contains about 7.58% genistin, 25.43% genistein, 5.48% daidzin
and 1.67% daidzein, i.e. about 40% isoflavones. Approximately the same results
can be achieved after the treatment described in the following.
About 166 g of the genistin isoflavone is trickled into about 834 g of
Polysorbate
80 heated to about 75°C and the mixture evenly stirred at this
temperature for about
half an hour. A clear, dark brown intermediate product is obtained without
sediment. If about I - 2 ml of this intermediate product is added to ten times
that
amount of distilled water at room temperature, a clear aqueous phase is
obtained.
As the measurements show, it contains micelles of which about 96.1 % have a
radius of about 16 nm to about 20 nm, as illustrated in Curve 5 in Figure 1.
According to an alternative process, about 100 g of the genistin isoflavone is
stirred
evenly in about 400 g of water which has previously been heated to about
60°C.
The mixture is stirred, maintaining the temperature, for about 10 minutes and
then
about 500 g of Polysorbate 80 is added with continuation of stirring and
during the
addition the temperature is increased to about 100°C. The stirring
process i~
continued at this temperature until a clear intermediate product is obtained.
Example 5: Quercetin
A quercetin dihydrate, which can be obtained from Sigma-Aldrich-Chemie GmbH,
Schnelldorf under the article number 83370-1006, can be used as a starting
source
of quercetin.
67 g of quercetin dihydrate are evenly stirred in about 280 g of water which
has
been previously heated to about 60°C. The mixture is stirred constantly
(using. say.
a magnetic stirrer), maintaining the temperature. for about 5 minutes and then
' CA 02436273 2003-07-25
during stirring about 653 g of Polysorbate 80 are added, whereby the
temperature is
increased to approximately 100°C. Stirring continues until a clear,
transparent
intermediate product containing about 6.7% of quercetin is obtained. An
aqueous
solution of this product shows micelles of which about 90% exhibit a radius
distribution between 17 nm and 19 nm (Curve 6 in Figure l ).
Example 6: Lycopene
The starting point is a product which can be obtained from BASE S.A.,
Switzerland, under the name Tomato Oleoresin. It contains about 40% lycopene
and is known in the following as Base lycopene. A product containing lycopene
can also be obtained from LycoRed Natural Products Industries Ltd, Beer-Sheba,
Israel.
About 100 g of water are heated to just 100°C and about SO g of Base
lycopene
added to the hot water. Maintaining the temperature, the mixture is vigorously
stirred for about 5 minutes until it becomes homogeneous and transparent. Then
about 850 g of Polysorbate 80 is heated to about 100°C and added to the
mixture.
The total mixtwe that is produced is stirred at about 100°C until a
homogeneous
and transparent intermediate product is obtained. After cooling to room or
body
temperature, the clarity and water solubility of the obtained aqueous phase
with a
2% lycopene content are retained. According to Curve 7 in Figwe 1. about 86%
of
the produced micelles have a radius of about 15 nm to about 16 nm.
Curve 8 in Figure 1 refers to pure Polysorbate 80 without active substance.
Example 7: Chondroitin sulphate
The polymeric galactosamine sulphate, chondroitin, supports the regeneration
of
overstressed cartilage tissue and reduces symptoms of osteoarthritis.
CA 02436273 2003-07-25
11
About 300 g of Polysorbate 80 is added to about 500 g of water heated to about
85°C and the mixture is stirred at about 85°C until (after about
5 minutes) the
mixture becomes homogeneous and transparent. Then about 200 g of pure
chondroitin sulphate are added to this mixture. This mixture is in turn
vigorously
stirred at the said temperature until a homogeneous and transparent
intermediate
product is obtained. After cooling to room or body temperature, the clarity
and
water solubility of the phase so obtained with approximately 20% chondroitin
sulphate content are retained.
Example 8: a-lipoic acid
About 870 g of polysorbate, preferably Polysorbate 80, are heated to about
120°C
and then about 130 g of a-lipoic acid - for example the product alpha lipoic
acid.
Art. No. 19991010 from K.-W. Pfannenschmidt GmbH, Hamburg - is added and
stirred for about 10 minutes maintaining the temperature until a homogeneous.
transparent mixture is obtained.
The intermediate product produced in this way can be dissolved as required
after
cooling with stirring in water at about 25°C. The clarity and water
solubility of the
water-free phase are retained even when gastric acid (hydrochloric acid) is
added to
its aqueous solution.
From the above it can be seen that from physiologically effective active
substances,
which are insoluble or difficult to dissolve in water, a phase of low active
substance
concentration. soluble in water and oils. can be obtained, which exhibits
micelles
with radii between about 10 nm and about 20 nm, through treatment with a
physiologically compatible solubiliser with an HLB value between 9 and 16.
preferably a polysorbate, in particular Polysorbate 80. under heat followed as
required by quick cooling to room temperature. This phase is very resistant.
primarily to acids such as gastric acid. Above all, it is simpler to deal with
than the
actual active substances. The resistance of the phase can still be increased
in that an
auxiliary substance such as linoleic acid is supplemented. As has been shown.
the
CA 02436273 2003-07-25
12
micelles containing the active substance are enclosed with a number of
micelles
containing the auxiliary substance which provide protection for the active
substance micelles.
The micelles formed according to the invention are very stable chemically.
microbiologically, 'mechanically and thermally. They contain proportionally a
much larger quantity of mainly lipophilic active substances than comparable
liposomes. The active substance concentrates or the formed active substance
phases
can be added with benefit to foodstuffs, nutritional supplements, skin. hair
and
dental care products as well as to cosmetic or pharmaceutical media. The
active
substance concentrates are absolutely stable in gastric acid. Due to the
micelle
formation the active substances are available to the organism substantially
quicker
then in active substances administered as emulsions. The resorption in the
intestinal
region renders participation of the bile acid superfluous.