Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02436423 2003-07-25
WO 02/070769 _ PCT/SE02/00372
STEEL ARTICLE
TECHNICAL FIELD
The invention concerns an article made of steel having features which are
desirable for
plastic mould steels intended to be used for any of the following fields of
application:
~ elements, e.g. screws and barrels for feeding and conducting plastic masses
in
machines for the manufacturing of plastic components, e.g. elements in
injection
moulding and extrusion assemblies, and
~ mould tools and tool parts for injection moulding of plastic materials.
Particularly the invention concerns objects of steel having excellent wear
resistance,
good corrosion resistance, hardenability, and tempering resistance as well as
adequate
toughness; features which make the steel suitable to be employed within said
fields of
application. The use of steel articles according to the invention, however, is
not limited
to said fields of application but can be employed also for a variety of other
applications,
!where said features are necessary or desirable, e.g. details in pumps for
feeding wearing
media and for wear parts in machines and other equipments, just to mention
some.
BACKGROUND OF THE INVENTION
For parts of the above mentioned fields of application there is today used a
steel which
is known under the trade name ELMAXTM, which is a high alloyed, powder
metallurgy
manufactured chromium-vanadium-molybdenum steel having good wear resistance
and
corrosion resistance. The steel has the following nominal chemical composition
in
weight %: 1.7 C, 0.8 Si, 0.3 Mn, 18.0 Cr, 1.0 Mo, 3.0 V, balance iron and
impurities.
The steel has a high wear resistance and corrosion resistance, which makes a
manufacturing of moulds for plastic moulding having a long working life
possible. The
steel is used e.g. in the electronic industry for the manufacturing of
couplings, contacts,
resistances, and integrated circuits, but can also be used in the food
industry, where
corrosion resistance is required from sanitary reasons, at the same time as
the wear
resistance is an essential factor.
However, there is a demand for a steel having a still better combination of
excellent
wear resistance, hardenability, tempering resistance, and corrosion
resistance,
particularly for elements such as screws and barrels for feeding and
conducting plastic
masses in equipments for injection moulding of plastic materials.
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
2
DISCLOSURE OF THE INVENTION
It is the purpose of the invention to provide steel articles which satisfy the
above
mentioned demands. This can be achieved therein that the article is
manufactured of a
spray formed steel material having a chemical composition in weight % and with
a
micro-structure which are stated in the appending patent claims.
Further, as far as the alloy elements which are included in the steel are
concerned, the
following applies.
Carbon shall exist in a sufficient amount in the steel in order, in the
hardened and
tempered condition of the steel, to form, in combination with vanadium, 3-8
vol-% MC-
carbides, in which M substantially is vanadium and, in combination with
chromium, 10-
vol-% M7C3-carbides, in which M substantially is chromium, the total amount of
MC-carbides and M7C3-carbides amounting to 14-25 vol-%, and also exist in
solid
15 solution in the martensitic matrix of the steel in the hardened condition
in an amount of
0.2-0.7 weight %, preferably 0.3-0.6 weight %. Suitably the amount of
dissolved
carbon in the matrix of the steel is about 0.5%. The total amount of carbon in
the steel,
i. e. carbon which is dissolved in the matrix of the steel plus the carbon
that is bound in
carbides shall be at least 1.7%, preferably at least 1.8%, while the maximum
content of
20 carbon may amount to 2.5%, preferably not more than 2.3%.
The article of the invention is manufactured by a technique which includes
spray
forming, in which drops of molten metal are sprayed against a rotating
substrate on
which the drops rapidly solidify to form a successively growing ingot. The
ingot then
can be hot worked by forging and/or rolling to desired shape. At the
solidification of the
drops said carbides are formed, which are evenly distributed in the ingot and
thence in
the final product. Due to the controlled rate of solidification of the drops,
which is
slower than during manufacturing of metal powder by atomising a stream of
molten
metal and rapid cooling of the formed droplets, but essentially more rapid
than during
conventional ingot manufacturing, continuous casting and/orESR-remelting, the
carbides have sufficient time to grow to a size which has turned out to be
very
favourable in the article according to the invention. Thus the MC-carbides can
be
caused to achieve an essentially rounded shape, such that at least 80 vol-% of
the MC-
carbides obtain a size in the longest extension of the carbides amounting to 1-
10 p,m,
preferably at least 5 Vim, while the M7C3-carbides typically achieve a more
elongated
shape than the MC-carbides, such that at least 80 vol-% of the MC-carbides get
a
maximum extension which amounts to 3-50 wm, preferably at least 10 ~.m.
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
Nitrogen optionally may be added to the steel in connection with the spray
forming to a
maximal amount of 0.20%. According to the preferred embodiment of the
invention,
however, nitrogen is not intentionally added to the steel but will
nevertheless exist as an
unavoidable element in an amount of max 0.15%, normally max 0.12%, and is then
not
a harmful ingredient. To the contrary, the nitrogen may have a favourable
effect by
forming vanadium and chromium carbonitrides in combination with carbon. Thus a
minor fraction of carbonitrides may be included in the above mentioned volume
contents of MC- and M7Cs-carbides.
Silicon is present as a residue from the manufacturing of the steel and exists
normally in
an amount of at least 0.1%, preferably at least 0.2%. The silicon increases
the carbon
activity in the steel and therefore contributes to affording the steel an
adequate hardness
without causing embrittlement problems. Silicon, however, is a strong ferrite
former
and must therefore not exist in amounts above 2.0%. Preferably, the steel does
not
contain more than max 1.0% silicon.
Manganese also exists as a residue from the manufacturing of the steel and
binds the
Ylow amounts of sulphur which may exist in the steel by forming manganese
sulphide.
Manganese therefore should exist in an amount of at least 0.1%, preferably in
an
amount of at least 0.2%. Manganese also promotes the hardenability, which is
favourable, but must not exist in amounts above 2.0% in order to avoid
embrittlement
problems. Preferably, the steel does not contain more than max 1.0% Mn. A
nominal
content of manganese is 0.5%.
Chromium shall exist in an amount of at least 12%, preferably in an amount of
at least
13% in order to afford the steel a desirable corrosion resistance. Furhter
chromium is an
important carbide former and forms M7C3-carbides together with carbon, which
carbides in combination with the MC-carbides contribute to a desired wear
resistance.
Chromium also strongly promotes the hardenability. The term hardenability
means the
capacity of achieving a high hardness more or less deep in the article that
shall be
hardened. The hardenability shall be sufficient for the article to be through
hardened
even if the article has comparatively large dimensions, without employing very
rapid
cooling in oil or water at the hardening operation, which might cause
dimension
changes. The hardness in the steel shall be at least 55 HRC, suitably 58-64
HRC, after
tempering. Chromium, however, is a strong ferrite former. In order to avoid
ferrite after
hardening from 1020-1150°C, the chromium content must not exceed 16%,
preferably
max 15.5%. A suitable chromium content is 13.2-14.5%, nominally 14.0%.
CA 02436423 2003-07-25
WO 02/070769 4 PCT/SE02/00372
Vanadium shall exist in the steel in an amount of 5.0-8.0% in order, together
with
carbon and possibly nitrogen, to form said MC-carbides or carbonitrides in the
martensitic matrix of the steel in the hardened and. tempered condition.
Preferably, the
steel contains at least 6.1% and max 7.5% V. A suitable vanadium content is
6.3-7.3%,
nominally 6.8% V.
In principle, vanadium may be replaced by niobium for the formation of MC-
carbides,
but for this twice as much niobium is required as compared with vanadium,
which is a
drawback. Further, niobium has the effect that the carbides will get a more
edgy shape
and be larger than pure vanadium carbides, which may initiate ruptures or
chippings and
therefore reduce the toughness of the material. This may be particularly
serious in the
steel of the invention, the composition of which has been optimised for the
purpose of
achieving an excellent wear resistance in combination with a high hardness and
tempering resistance, as far as the mechanical features of the material are
concerned.
The steel therefore must not contain more than max 0.1% niobium, preferably
max
0.04% niobium. According to the most preferred embodiment, niobium is
tolerated only
as an unavoidable impurity in the form of a residual element from the raw
materials
which are used in connection with the manufacturing of the steel.
Molybdenum shall exist in an amount of at least 2.1%, preferably at least
2.3%, in order
to afford the steel a desired hardenability in combination with chromium and
the limited
amount of manganese. Molybdenum also contributes to the corrosion resistance
of the
steel but is a strong ferrite former. The steel therefore must not contain
more than 3.5%
Mo, preferably max 3.0, suitably max 2.5%.
Iri principle, molybdenum may completely or partly be replaced by tungsten,
but for this
twice as much tungsten is required as compared with molybdenum, which is a
drawback. Also the use of any scrap will become more difficult. Therefore
tungsten
should not exist in an amount of more than max 1.0%, preferably max 0.5%. Most
conveniently, the steel should not contain any intentionally added tungsten,
which
according to the most preferred embodiment of the invention is tolerated only
as an
unavoidable impurity in the form of a residual element from the raw materials
which are
used in connection with the manufacturing of the steel.
Besides the mentioned alloy elements the steel does not need, and should not,
contain
any more alloy elements in significant amounts. Some elements are definitely
undesired, because they rnay have an undesired influence on the features of
the steel.
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
This is true, e.g. as far as phosphorus is concerned, which should be kept at
as low level
as possible, preferably at max 0.03%, in order not to have an unfavourable
effect on the
toughness the steel. Also sulphur in most respects is an undesired element,
but its
negative effect on, in the first place, the toughness, essentially can be
neutralised by
5 means of manganese, which forms essentially harmless manganese sulphides,
wherefore
sulphur may be tolerated in a maximal amount of 0.2% in order to improve the
machineability of the steel. Preferably, the steel, however, normally does not
contain
more than max 0.1%, preferably max 0.05% sulphur.
Further features and aspects of the invention will be apparent from the
following
description of performed experiments and from the appending patent claims.
BRIEF DESCRIPTION OF DRAWINGS
In the following description of performed experiments, reference will be made
to the
accompanying drawings, in which
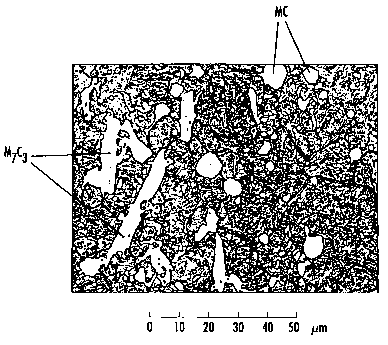
Fig. 1 is a photography which shows the micro-structure of a portion of an
article
according to the invention,
Fig. 2 shows tempering curves for a number of examined steel alloys,
Fig. 3 shows a section of the curves of Fig. 2 at a larger scale,
Fig. 4 in the form of a chart illustrates the hardenability of a steel
according to the
invention and of two reference materials with data from CCT- diagrams,
Fig. 5 shows the abrasive wear resistance of a steel according to the
invention and of
two reference materials, and
Fig. 6 illustrates the corrosion resistance of the examined materials in the
form of the
corrosion current, I~., from the polarisation curves of the materials.
DESCRTPTION OF PERFORMED TESTS
Materials
The chemical compositions of the materials which are included in the test
series are
stated in Table 1. Steels No. 1 and 2 are reference materials. Both are powder
metallurgy manufactured. Steel No. 1 is a commercial steel of type ELMA~~TM,
which
has been mentioned in the description of the background of the invention, and
steel No.
2 is another commercially available steel. Steels No. 3A and No. 4A refer to
aimed
compositions, while steel No. 3 and No. 4 are analysed compositions of two
steels, the
contents of vanadium of which lie in the lower and the upper section,
respectively, of
the widest aspects of the chemical composition of the steel of which the
axticle
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
6
according to the invention is made. The steels 3 and 4 have been manufactured
by the so
called spray forming technique, which also is referred to as the OSPRAY-
method,
according to which an ingot, which rotates about its longitudinal axis,
successively is
established from a molten material which in the form of drops which are
sprayed
against the growing end of the ingot that is produced continuously, the drops
being
caused to solidify comparatively rapidly once they have hit the substrate,
however not
as fast as when powder is produced and not as slow as in connection with
conventional
manufacturing of ingots or in connection with continuous casting. More
specifically, the
drops are caused to solidify so rapidly that formed MC- and M7C3-carbides will
grow
to the desired size according to the invention. The spray-formed ingots of
steel No. 3
and of steel No. 4 bade a mass of about 2.9 and about 2.2 tons, respectively.
The
diameter of the ingots was about 500 mm.
The spray-formed ingots of steel No. 3 and steel No. 4 were heated to a
forging
temperature of 1100°C and were forged to the shape of blanks for
further examinations.
Table 1 Chemical composition, weight-
Steel,C Si Mn S Cr Mo V Nb N Balance
No.
1 1.71 0.84 0.300.019 17.9 1.08 3.01 0.0150.104 Fe and
unavoidable
im urities
2 2:41 0.29 0.430.019 13.1 1.12 7.91 0.0030.083 -"-
3A* 1.85 0.50 0.40< 0.02014.0 2.30 6.00 - < 0.10='-
3 1.93 0.61 0.390.019 13.7 2.32 5.64 0.02 0.10 -"-
~
4A* 2.35 0.50 0.40< 0.02014.4 2.30 8.20 - < 0.10-"-
4 ='-
* Aimed compositions
In the studies which shall be explained in the following, steels No. 1, 2 and
3 were
tested with reference to
~ micro-structure
~ hardness versus austenitising and tempering temperature
~ hardenability
~ ductility
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
7
~ abrasive wear resistance
~ corrosion resistance
Micro-structure
The micro-structure of steels No. 1 and 2 is typical for powder metallurgy
manufactured
steels, which implies that all carbides are very small, max about 3 p.m, and
evenly
distributed in the matrix of the steel independent of its heat treatment. The
micro-
structure in the hardened, TA = 1120°C/30 min. and tempered,
525°C/2 x 2 h, condition,
of steel No. 3 is apparent from Fig. I, which shows a portion in the centre of
an
examined bar having the cross section 350 x 63.5 mm. In the matrix of the
steel, which
consists of tempered martensite, there are primary carbides of MC-type having
a
typically rounded shape and a size offrom about lpm up to max about 10~m, and
chromium carbides, M7C3, having a substantially more extended shape. The size
of the
chromium carbides was max about IS x 50 pm in the centre ofthe bar. In the
surface of
the bar, which also was examined but which is not shown in any picture, the MC-
carbides as well as the chromium carbides were somewhat smaller; up to about 6
~m
and up to about 8 x 30 Vim, respectively. A macro-etched cross section of the
rod also
Yevidenced that the structure is very even over the whole cross section.
The carbide content was examined by the point calculation method in a scanning
electron microscope. The measured total content of carbides in steel No. 3 was
20.4%,
of which 15.4% were rich in chromium (M7C3) and 5% were rich in vanadium (MC).
As far as steel No. 2 is concerned, the measured total content of carbides was
23.9 vol-
%, of Which 13.1% were rich in chromium (M7Cs) and 10.8% were rich in vanadium
(MC). The measured total content of carbides in steel No. I was I4%, of which
13%
were rich in chromium (M7C3) and 1% was rich in vanadium (MC). All carbide
contents
refer to vol-%. The heat treatment condition was TA =1120°C/30 min. +
250°C/2 x 2 h
for steel No. 2 and steel No. 3 and TA = 105C/30 min. + 250°C/2 x 2 h
for steel No. 1.
Hardness after heat treatment
In the soft annealed condition, the steel according to the invention has a
hardness
(Brinell-hardness) of 200-300 HB, typically about 250 HB. The influence of the
tempering temperature on the hardness after austenitising between 1080 and
1150°C is
shown in Fig. 2. Steel No. 3 exhibits a stronger secondary hardening than the
two
reference steels 1 and 2 after austenitising at 1120 and 1150°C and
reaches a hardness
of 63 HRC after tempering at 5252 x 2 h. A section of the region which
comprises the
hump on the tempering curves is shown at a larger scale in Fig. 3. Steel No. 2
had the
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
8
same hardness as steel No. 1 after austenitising at 1120°C, but a
substantially lower
tempering resistance than both steel No. 1 and No. 3.
Hardenability
The hardness versus the required time for cooling from 800 to 500°C
is shown
graphically in Fig. 4. It is apparent from this chart that the hardenability
of steel No. 3 is
significantly better than that of steel No. 1 and much better than that of
steel No. 2.
Toughness
The impact energy was examined, un-notched test specimens being used after
hardening
from TA = 1120°C/30 min for steels Nos. 2 and 3, and from TA =
1100°C/30 min,
respectively, for steel No. 1 after varying tempering temperatures between 200
and
550°C. The dimension of the bar of the examined steel, however, varied,
wherefore the
results are not fully comparable. However, it could be settled that the impact
energy of
all examined steels exceeded 10 J for all longitudinal samples, which
satisfies the
criteria as far as approvable impact toughness is concerned fox the intended
field of
application of the article according to the invention.
Abrasive wear
The wear resistance was examined in the form of a pin-to-pin test using Si02
as an
abrasive agent. As far as the dimensions and hardening temperatures of the
examined
samples are concerned the following applies. Steel No. 1: ~ 38 mm/TA
=1100°/30 min;
steel No. 2: Q~ 37 mm/TA =1120°C/30 min; steel No. 3: 350 x 63.5 mm/TA
=
1120°C/30 min. The results are apparent from the bar chart in Fig. 5.
This chart-
illustrates that steel No. 3 for all tempering temperatures exhibited the by
far best wear
resistance.
Corrosion resistance
The corrosion resistance was measured via potential curves in 0.05 M HaS04, pH
= 1.2.
I~. at the active peak defines the relative corrosion resistance, which means
that the
corrosion current should be as low as possible. In the bar chart in Fig. 6,
the different
materials are compared as a function of the heat treatment condition. Steel
No. 3 had the
best corrosion resistance after tempering up to at least 400°C. After
tempering at 525°C,
the corrosion resistance was reduced for all examined materials; steel No. 3
slightly
3 S more than steel No. 2 and considerably more than steel No. 1. It should,
however, be
observed, as far as this comparison is concerned, that steel No. 3 after
tempering had an
essentially higher hardness than the comparative materials.
CA 02436423 2003-07-25
WO 02/070769 PCT/SE02/00372
9
DISCUSSIGN
The described tests show that of steels according to the invention there can
be
manufactured articles having a very high wear resistance, which can be
attributed to a
combination of the hardness of the steel and its content of carbides in a
sufficient
S amount and of sufficient size. Another important factor is the hardenability
of the steel,
which is very good and better than comparable steels. Hardnesses of between S9
to 62
HRC in combination with an excellent corrosion resistance were measured after
tempering at 200 and 400°C and hardness of between 61 to 63 HRC after
tempering at
S00°C. By tempering at about SZS°C there can be achieved a
hardness peak of between
61 to 64 HRC. In the latter case some corrosion resistance is lost, but the
high hardness
can be utilised for certain applications where high requirements on the
corrosion
resistance do not exist. The invention thus provides a pronounced flexibility
with
reference to the adaptility of the usefulness of the steel for various
applications by
choice of a suitable heat treatment. Another important factor for the
usability of the steel
1S is its manufacturing, which is based on the spray forming technique, which
is essentially
more economical than powder metallurgy manufacturing.
!It should also be realised that the article according to the invention may
have any
conceivable shape, including spray formed ingots, blanks in the form of, e.g.,
plates,
bars, blocks, or the like, which normally are delivered by the steel
manufacturer in the
soft annealed condition with a hardness of 200-300 HB, typically about 2S0 HB
to the
customers for machining to final product shape, as well as the final product
which has
been hardened and tempered to intended hardness for the application in
question.