Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
1
CANCER
The present invention relates to methods for the diagnosis, imaging and
treatment of cancer cells. In particular ~t relates to the use of a tumour
related molecule
which is present at a higher density on cancer cells than normally dividing
cells in the
diagnosis, imaging, prophylaxis and treatment of cancer.
The chromosomal location of tumour suppressor genes (TSG) has often been fiist
indicated by tumour-associated deletion and loss of heterozygous alleles., The
successful identification of these genes has frequently relied on. the
isolation of
10. candidate TSG from within much srrialler homozygously deleted regions,
mapping
within the larger region of allele loss (1). Various deletion mapping studies
have-
indicated that several distinct regions within the chromosomal 3 loci are
involved in
the onset and/or progression of lung cancer. Recent studies include a recent
extensive
study of 1 S 1 lung tumour biopsies and cell lines with 28 markers of
polymorphic loci
(2) on chromosome 3. Using FISH, homozygous deletions at 3p12, 3p14 and 3p21
have been shown to also exist in biopsy material (4).
Despite thorough investigation of genes within these homozygous deletions none
has
emerged as a "classic" TSG with allele loss surrounding the gene on one
chromosome and point mutations in the gene in the remaining homologue. However
it is becoming increasingly recognised that tumour suppressor genes may be
inactivated by epigenetic mechanisms (6). Several genes on chromosome 3 fall
into
this category. An isoform of the 123F2/RASSFI gene and the FHIT gene are
particularly noteworthy as both have reduced or aberrant expression in lung
(and
other) tumours and have been shown to suppress tumorigenicity following
transfection into tumour cell lines (6,7) Inactivation of the Fhit gene in
mice results
in gastric and sebaceous gland tumour formation in mutant mice challenged
intragastrically with carcinogen (8) Therefore, there exists a need in the art
to
identify other tumour suppressor molecules. Such molecules may be useful in
the
prophylaxis and/or treatment of tumours.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
2
Cancers may be detected by treating samples of tissues with agents known to
bind to
defined types of cancer cell. Such detection generally requires removal of
tissue
samples from a vertebrate. Suitable agents include antibodies and the like.
Some
cancers can be detected by imaging: radiological methods(e.g. X-rays) are the
most
familiar. Although valuable, there are problems of selectivity-i.e. other non-
malignant
nodules are detected and sensitivity-i.e. very small clusters of tumour cells
e.g.
micrometasteses are undetectable.
In an attempt to overcome these problems other methods of obtaining an image
of a
tumour are being evaluated. For many years tumour -specif c antigens were
sought but
generally have not been found in solid tumours. It is becoming apparent that
tumour
targets need not be expressed exclusively by tumours to be of value. What is
required
is that the tumour over-expresses a cell surface antigen compared to
surrounding
tissue.
Recently, sigma receptor-binding benzamides have been used in the diagnosis
and as
therapeutic agents for human prostate tumours (27). In this study, it was
found that a
very high density of sigma receptors is expressed on the androgen-independent
human-
prostate tumour cell line (DU-145). Both radiolabelled and non-radiolabelled
benzamides were shown to bind selectively and with a high affinity to human
prostate
tumour cells xenografted to nude mice. The three compounds tested all showed a
fast
clearence from the blood pool and a high uptake and retention in the tumour..
They
also showed a dose-dependent inhibition of cell colony formation in two
different
human prostate cancer cell-lines. There remains a need in the art, thereofore
to identify
further cell surface molecules the cell surface density of which is different
when
normally dividing cells are compared with cancer cells. Identification of such
molecules will be of use in the imaging and/or diagnosis of cancer.
Previously, The present inventors have described a large homozygous
deletion at 3p12-13 in an SCLC line, U2020. Following construction of a
physical
map of this region, CpG island mapping was used to identify the gene location
(9) .
One such gene, named DUTTl (deleted in U2020) was found to map within smaller
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
3
homozygous deletions in two other tumour cell lines (9). The DUTTIlROBOI gene
is widely expressed in mammals and codes for a receptor with a domain
structure of
the NCAM family (13). Several lung tumour cell lines including NIH-H219X have
previously been shown to possess one or more deletions within the gene
encoding
DUTT1, therefore implicating DUTT1 in lung cancer.
The present inventors set out to overcome the problems of the prior art. In
particular,
they set out to identify one or more molecules which play a role in the onset
or
progression of lung tumour formation. In addition they set out to establish a
method
for the imaging, diagnosis, prophylaxis and/or treatment of cancer cells.
Summary of the invention
The present inventors have surprisingly found that the level of the tumour
suppressor
protein DUTT1 is high in cancer cells, including carcinoma insitu (pre-
invasive cancer
cells), and is low on non-cancerous epithelium, for instance bronchial
epithelium. In
addition, negligible protein is detected on epithelial stem cells. This is a
surprising
finding and contrary to expectations as the present inventors have shown, as
described
herein (Figures 1 to 4), that decreasing the functional activity of DUTT1 in
cells,
particularly cells comprising the lung causes the appearance of bronchial
epithelial
hyperplasia in lung cells.
In a first aspect, the present invention provides a method for the early
detection of
cancer in a population of cells comprising the steps of:
( 1 ) providing a population of cells
(2) assaying the cell population for an increased level of DUTT1 in any one or
more of
those cells as compared with normally dividing cells.
Thus in a second aspect, the present invention provides a method for the early
detection of cancer in a population of cells comprising the steps of
(1) providing a population of cells
(2) assaying those cells and establishing a reference level of DUTT1,
(3) obtaining a population of cells for diagnosis,
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
4
(4) assaying the cell population of step 3, for an increased level of DUTT1 in
any one
or more of those cells when compared with the reference level of DUTT1.
In a .further aspect, the present invention provides a method for the
diagnosis of cancer
in a population of cells comprising the steps o~
(1)providing a population of cells
(2)assaying the cell population for an increased level of DUTT1 in any one or
more of
those cells as compared with normally dividing cells.
Thus in a further aspect still, the present invention provides a method for
the diagnosis
of cancer in a population of cells comprising the steps of
(1)providing a population of cells
(2)assaying those cells and establishing a reference' level of DUTT1, .
(3)obtaining a population of cells for diagnosis,
(4) assaying the cell population of step 3, for an increased level of DUTT1 in
any one
or more of those cells when compared with the reference level of DUTTl.
Common characteristics of 'cancer' as herein defined include the ability of a
cell to
undergo endless replication, loss of contact inhibition, invasiveness and the
ability to
metastasize. That is, when the cell divides in an uncontrollable way and can
not
recognise its own natural boundary, the cancer cells obtain the ability to
spread to
other areas of the body. Mutations within the nucleic acid of one or more
cells are
involved in the onset of cancer. Often, more than one nucleic acid mutation or
other
aberrant cellular event is required for the development of tumours (bundles of
aberrantly. dividing cells), that is tumour formation is a mufti-signal event.
In the
context of the present invention cancer cells include any cells which exhibit
any one or
more of the following features aberrant cell division, aberrant contact
inhibition,
aberrant cell differentiation as compared with cells behaving normally within
their
native environment, the ability. of the cell to invade tissues, and the
ability to
metastasise. The definition of 'cancer cells' in the context of the present
invention,
therefore includes within its scope tumour cells and also cells prior to the
formation of
tumours in so far as they possess one or more of the requisite characteristics
listed
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
above. In addition the term cancer cells according to the present invention
includes
metastatic cells.
The method of the present invention is suitable for the diagnosis and/or
detection of
5 many forms of cancer. In particular, the cancer may be one or more selected
from the
group consisting of: epithelial cancer, sarcoma and lymphoma. In a preferred
embodiment of the above two aspects of the invention, the cancer is lung
cancer,
advantageously it is human lung cancer. Advantageously, the cells for
diagnosis
include bronchial epithelial cells and/or bronchial hyperplasia of epithelium,
and/or
cells derived from lymph nodes Preferably, the method is an in vitro method. .
In the context of the present invention, the term the 'early detection' of
cancer means
the detection of cancer prior to the onset of one or more clinical signs of
cancer in a
patient. Clinical signs of cancer will be known to those skilled in the art
and includes
the formation of tumours and metastases. The early detection of cancer
One skilled in the art will appreciate that the cell population of steps (1)
and (3) may
be the same population. In practice steps (1) to (4) may be performed
simultaneously,
or separately. Alternatively, several steps may be performed simultaneously,
and
others separately.
A reference level of IaUTT l may be established using one or more agents
selected
from the group consisting of: anti-DUTT1 antibodies, DUTT1 binding peptides
and
small molecules which bind to DUTT1.. Advantageously, DUTT1 antibodies are
used.
Suitable methods for measuring DUTT1 levels using these agents will be
familiar to
one skilled in the art and are described herein.
In addition ox alternatively DUTT1 binding agents may be bound to a population
of
cells, and a reference level of DUTT1 established by.directly comparing
cancerous and
non-cancerous cells within the same cell population. This has the advantage
that
protein levels in cancer cells and surrounding normally dividing cells can be
compared
easily and simultaneous, and that quantitative measurements of DUTT1 levels do
not
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
6
need to be made. Cells binding an increased level of DUTT1 binding agent,
compared
with the normal cells will be easily distinguishable.
Advantageously, the DUTT1 binding agents will comprise or have associated with
them means for their detection. Suitable means may be naturally occurring or
synthetic
molecules and will be familiar to those skilled in the art, and are described
herein.
In a further aspect, the present invention provides, the use of a DUTT1
binding agent
in the diagnosis of cancer.
The use according to the present invention is suitable for the diagnosis of
many forms
of cancer. In particular, the cancer may be one or more selected from the
group .
consisting of: epithelial cancer, sarcoma and lymphoma. In a preferred
embodiment of
the above two aspects of the invention, the cancer is lung cancer,
advantageously it is
human lung cancer. Advantageously, the cells for diagnosis include bronchial
epithelial cells and/or bronchial hyperplasia of epithelium, and/orcells
derived from
lymph nodes Preferably, the method is an in vitro method.
Suitable DUTT1 binding agents for diagnosis are as herein described,.
Preferably the
DUTT1 binding agent is an antibody raised against DUTT1.
Due to the surprising finding that that the level of the tumour suppressor
DUTT1 is
higher on cancer cells including carcinoma in situ cells, than on non-cancer
cells, then
DUTT1 binding agents can be used as a method of imaging or visualising cancer
cells.
Thus in a further aspect, the present invention provides a method for the
selective
labelling of cancer cells comprising the step of treating one or more cancer
cells with a
DUTTl binding agent.
In yet a further aspect, the present invention provides the use of a DUTT1
binding
agent in the selective labelling of cancer cells.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
7
Cells suitable for selective labelling include any cell which expresses DUTT1,
preferably at high levels. Suitable cells include epithelial cancer cells,
sarcoma cells
and lymphoma cells. In a preferred embodiment of the invention, the cells are
lung
cancer cells, preferably human lung cancer cells. Suitably, the cancer cells
are
metastatic cells and/or primary cancer cells.
Advantageously, the method is for the selective labelling of cancer cells
selected from
the group consisting of: human cancer cells, human epithelial cancer cells,
human
sarcoma cells, human haematopoietic cells.
Suitable DUTT1 binding agents are as described herein.
The term 'labelling' in the context of the present invention means the
selective binding
of an agent to cells, in this case to cancer cells and not to normally
dividing cells.
Advantageously the labelling agent comprises or has associated with it means
permitting the detection of the label. Suitable means include fluorescent,
phosphorescent, or radio-active agents as herein described. One skilled in the
art will
appreciate that this list is not intended to be exhaustive. Suitable means for
detection of
the labelled cells will vary according to whether the labelled cells are to be
detected in
vitro or in vivo. Suitable in vitro methods include but are not limited to
autoradiography and fluorescence or phosphorescence detection. Suitable, in
vivo
methods include but are . not limited to nuclear magnetic resonance,
autoradiography
and so on. Those skilled will be aware of other suitable methods. In this way
the
method of this aspect of the present invention may be used for the in vivo
imaging of
cancer cells, preferably metastatic cancer cells. Advantageously, the method
is used
for the imaging of human cancer cells. Most preferably, the method is used for
the
imaging of human cancer cells, which may be primary cancer cells or metastatic
cells
within the body of a patient.
Suitable DUTT1 binding agents include any one or more selected from the group
consisting of the following: anti-DUTT1 antibodies, DUTT1 binding peptides and
small molecules which bind to DUTT1. Advantageously, DUTT1 antibodies are
used.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
8
In a further aspect, the present invention provides a method for the imaging
of cancer
cells comprising the step of treating one or more cancer cells with one or
more DUTT1
binding agents wherein the DUTT1 binding agent further comprises detection
means.
In the context of the present invention the term 'imaging' refers to the
generation of a
2 dimensional or 3 dimensional picture/image of the cancer cells. Where the
imaging
is performed within an in vivo environment, it may allow the relative position
of the
cancer cells within the in vivo environment, preferably the human body to be
established. Due to the high density of DUTT1 found on lung cancer cells
including
pre-invasive cancer cells, a high signal to noise ratio can be achieved,
allowing high
sensitivity imaging. Such a high signal to noise ratio is particularly
important in the
detection of small bundles of cancer cells, that is metastatic cells. One
skilled in the art
will appreciate though that this method is also of use in the imaging of
primary cancer .
cells.
Preferably the method is for the in viyo imaging .of .cancer cells. More
preferably it is
for the imaging of human cancer cells. Advantageously, the method is for the
imaging
of cancer cells selected from the group consisting of~ human epithelial cancer
cells,
human sarcoma cells, human haerriatopoietic cancer cells.
In a further aspect still, the present invention provides a composition
comprising
DUTT1, or a binding agent thereof.
In a further aspect still, the present invention provides a method for the
internalisation
of one or more DUTTI binding agents comprising the step of treating a cell
with one
or more DUTT1 binding agents.
In the context of the present invention, the term 'treating' refers to the
process of
bringing one or more cells into contact with a DUTT1 binding agent. Suitable
methods
for 'treating' cells will depend upon whether the procedure is carried out in
vivo or in
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
9
vitro and are described herein. In a preferred embodiment of this aspect of
the
invention, the method occurs in vivo
As herein defined, the term 'internalisation' means bringing one or more
molecules
into the interior of the cell. Advantageously, it describes bringing one or
more
molecules into the cytoplasm of a cell. In the present case at least one
molecule is a
DUTT1 binding agent. Suitable DUTT1 binding agents are herein described.
Cells suitable for treatment with a DUTT1 binding agent according to the
method of
the present invention includes any cell which expresses DUTT1. Suitable cells
include
epithelial cells. Those skilled in the art will be aware of other suitable
cell types.
Suitable DUTT1 binding agents are herein described. Advantageously, the DUTT1
binding agent is a antibody raised against DUTT1. Preferably, it is a
monoclonal
antibody.
In a final aspect, the present invention provides the use of DUTT1, and/or a
binding
agent thereof in the preparation of a medicament for the prophylaxis or
treatment of
cancer.
In one embodiment of this aspect of the invention, the use is in the treatment
of lung
cancer. In a further embodiment of this aspect of the invention, the use is in
the
treatment of epithelial cancer. In a further embodiment still, the use is in
the treatment
of sarcoma. In a further preferred embodiment, the use is in the treatment if
lymphoma.
Brief description of the figures
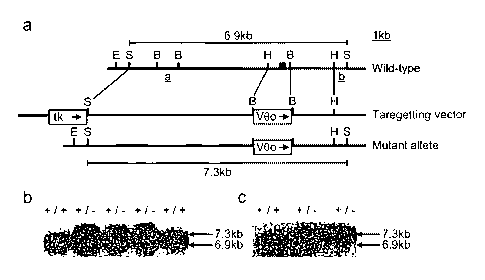
Fig. 1. Targeted mutation of the DuttllRobol gene .A, the genomic structure of
the
targeted locus and the targeting construct and mutant allele. The filled box
represents
exon 2. Fragments a and b are the hybridisation probes used in (B) below. The
outer
dashed lines indicate the fragment used for homologous recombination. E EcoRI,
S
SacI, B BamHI. B, Following transfection of the targeting construct into ES
cells,
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
Sacl digested DNA was prepared from 6418 resistant ES(+/-) and control ES
cells(+/+) and analysed by Southern blot analysis The filter was hybridised to
probe
[a] which flanks the site of homologous recombination at the 3'end, and with
probe
[b] external to the homologous integration, not shown. Two clones showing
correct
5 homologous recombination (i.e. both wild type, 6.9kb and mutant, 7.3 kb
alleles)
were injected into blastocysts and the resulting chimaeras bred with C57BL/6
mice
to obtain transmission of the mutant allele in the germ-line, results not
shown. C,
Finally mice with germ-line transmission, DuttllRobol +~ , were inter-crossed
to
obtain offspring which were homozygous (-/-) for the targeted mutation. Sacl
10 digested tail DNA from offspring from this inter-cross was screened by
Southern
blotting with probe [a].
Fig. 2. Duttl/Robol protein analysis (24). A, Western blotting analysis of
protein
isolated from the organs shown from wild type (+/+) and DuttllRobol ~ (-/-)
day 15
embryos using antiserum raised against a C-terminal DUTT1/ROBO1 peptide. B,
phase contrast image of a 4 micrometre transverse section of paraformaldehyde
fixed
normal new-born lung at x200 magnification C, detection by
immunohistochemistry
of Duttl/Robol protein using anti-peptide antiserum in (A) in bronchial
'epithelium
with Cy-3-TSA amplification system (NEN Life Sciences) applied to the same
section as in (B) x200 magnification.
Fig. 3. Gross morphological phenotypes of wild-type and DuttllRobol-~ mice. A
wild
type mouse and its lungs shown.below (+/+) compared to a DuttllRobol-~ mouse
and its lungs shown below (-/-)
Fig. 4. . Histological analysis of lungs of DuttllRobol-~ mice and wild-type
littermates. Paraformaldehyde fixed 4 micrometre sections of lung tissue were
H&E
stained and photographed. A, wild-type lung, embryo day E15.5 x 400
magnification, B, DuttllRobol-~- lung, embryo day 15.5, x 400 magnification,
C,
wild-type newborn lung, x 100 magnification, D, DuttllRobol'~' newborn lung x
100
magnification (arrow indicates bronchi), E, wild-type adult lung x 100
magnification, F, DuttllRobol-~- adult lung. x 100 magnification, G, wild-type
adult
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
11
lung at x 400 magnification, H, DuttllRobol-~' adult lung, at x 400
magnification, I,
DuttllRobol-~- adult lung, at x 400 magnification, focal dysplasia indicated
by arrow.
Populations of mice were established from two independent ES clones: both
gave indistinguishable abnormal lung pathology.
Fig 5. Detection by immunohistochemistry of DUTT1/ROBO1 protein using
anti-peptide antibody in a section of formalin fixed human squamous cell
carcinoma of the lung .
Fig 6. Detection by immunohistochemistry of DUTTI/ROBOl protein using
anti-peptide antibody in a section of formalin fixed human normal bronchial
epithelium
Definitions
Common characteristics of 'cancer' include the ability of the cancer cell to
undergo endless replication, loss of contact inhibition, invasiveness and the
ability to metastasise. That is, when the cell divides in an uncontrollable
way and can not recognise its own natural boundary, the cancer cells
obtain the ability to spread to other areas of the body. Mutations within the
nucleic acid of one or more cells are involved in the onset of cancer. Often,
more than one nucleic acid mutation or other aberrant cellular event is
required for the development of tumours (bundles of aberrantly dividing
cells), that is tumour formation is a multi-signal event. In. the context of
the
present invention cancer cells include any cells which exhibit any one or
more of the following features aberrant cell division, aberrant contact
inhibition, aberrant cell differentiation as compared with cells behaving
normally within their native environment, the ability of the cell to invade
tissues, and the ability to metastasise. The definition of 'cancer cells' in
the
context of the present invention, therefore includes within its scope tumour
cells and also cells prior to the formation of tumours in so far as they
possess one or more of the requisite characteristics listed above. In
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
12
addition the term cancer cells according to the present invention includes
metastatic cells.
A 'tumour suppressor molecule' is a molecule one function of which is to
suppress tumourigenesis. Certain cancers have been found to be associated with
mutant suppressor genes for example p53 and RB. However as indicated above ..
often more than one abherent cell component or signal is required to initiate
and/or to cause the progression of cancer.
'Bronchial epithelial hyperplasia'. The. airway of the lung in descending
order of size are bronchi, bronchioles and alveoli. Bronchial refers to the
first
two. . The lung consists of mesenchymal cells and epithelial cells which line
the airways. Hyperplasia means over growth. The epithelium is normally a
sheet of cells one layer thick but when it becomes hyperplastic it thickens
and
becomes disorderly. Bronchial epithelial hyperplasia. as herein defined is
thus
disordered cell morphology in the cell layer lining the main airways of the
lung
This form of hyperplasia is associated with the early stages of lung (and
other)
cancers and the term cancer as herein defined includes within its scope
bronchial epithelial hyperplasia.
'Specific labelling of a cell' in the context of the present invention means
the
selective/specific binding of a labelling agent to a cell. That is, .that when
a
labelling agent is exposed to a cell population, only those cells showing
certain
characteristics will bind to the labelling agent. Generally, the
characteristics
include the presence of certain cell surface features, for example the
presence of
a surface antigen which binds to the labelling agent.
An 'Antibody' (for example IgG, IgM, IgA, IgD or IgE) or fragment (such as a
FAb, F(Ab')2, Fv, disulphide linked Fv, scFv, diabody) whether derived from
any species naturally producing an antibody, or created by recombinant DNA
technology; whether isolated from serum, B-cells, hybridomas, transfectomas,
yeast or bacteria).
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
13
DETAILED DESCRIPTION OF THE INVENTION
General Technigues
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art (e.g., in
cell
culture, molecular genetics, nucleic acid chemistry, hybridisation techniques
and
biochemistry). Standard techniques are used for molecular, genetic and
biochemical
methods (see generally, Sambrook et al., Molecular Cloning: A Laboratory
Manual, 2d
ed. (1989) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. and
Ausubel et al.; Short Protocols in Molecular Biology (1999) 4't' Ed, John
Wiley &
Sons, Inc. which are incorporated herein by reference) and chemical methods.
In
addition Harlow & Lane., A Laboratory Manual Cold Spring Harbor, N.Y, is
referred
to for standard Immunological Techniques.
Method for the diagnosis of cancer in a vertebrate
In a first aspect, the present invention provides a method for the early
diagnosis of
cancer in a population of cells comprising the steps of:
( 1 )providing a population of cells
(2)assaying the cell population for an increased level of DUTT1 in any one or
more of
those cells as compared with normally dividing cells.
In a second aspect, the present invention provides a method for the early
diagnosis of
cancer in a population of cells comprising the steps of
(1)providing a population of cells
(2)assaying those cells and establishing a reference level of DUTT1,
(3)obtaining a population of cells for diagnosis,
(4) assaying the cell population of step 3, for an increased level of DUTTI in
any one
or more of those cells when compared with the reference level of DUTT1.
In a further aspect, the present invention provides a method for the diagnosis
of cancer
in a population of cells comprising the steps of:
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
14
(1)providing a population of cells
(2)assaying the cell population for an increased level of DUTT1 in any one or
more of those cells as compared with normally dividing cells.
In a further aspect still, the present invention provides a method for the
diagnosis of
cancer in a population of cells comprising the steps of
(1)providing a population of cells
(2)assaying those cells and establishing a reference level of DUTT1,
(3)obtaining a population of cells for diagnosis,
(4)assaying the cell population of step 3, for an increased level of DUTT1 in
any one or more of those cells when compared with the reference level of
DUTT 1
~A) Cells for dia ng osis.
Cell samples are obtained from a vertebrate for treatment using methods
familiar to
those skilled in the art. In the case of small vertebrates the animals may be
sacrificed,
and the tissue for diagnosis extracted. Tissue slices may be prepared using
methods .
familiar to those skilled in the art.
Alternatively, cells for diagnosis may be obtained by performing a biopsy
(removal of
a small amount of tissue from a vertebrate, which is preferably alive).
(B) Establishing a reference level of DUTT1
Techniques such as Northern blotting and Western blotting of cell extracts
followed by
hybridisation with agents which bind to DUTT1 may be used in the measurement
of
DUTT1 levels. Agents which bind to DUTTI include monoclonal and polyclonal
antibodies, DUTT1 binding peptides and small molecules which bind to DUTT1. In
addition or alternatively DUTT1 binding agents may be bound to a population of
cells,
and a reference level of DUTT1 established by directly comparing cancerous
andwon-
cancerous cells within the same cell population. This has the advantage that
protein
levels in cancer cells and surrounding normally dividing cells can be compared
easily
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
and simultaneous, and that quantitative measurements of DUTT1 levels do not
need to
be made..
(Bi)DUTT1 bindingagents
S (a) Antibodies and peptides
Antibodies raised against DUTT1, and DUTT1 peptides may be prepared using
standard laboratory techniques. Either recombinant proteins or those derived
from
natural . sources can be used to generate antibodies. For example; the protein
(or
"immunogen") is administered to challenge a mammal such as a monkey, goat,
rabbit
10 or mouse. The resulting antibodies can be collected as polyclonal sera, or
antibody-
producing cells from the challenged animal can be immortalized (e.g. by fusion
with
an immortalizing fusion partner to produce a hybridoma), which cells then
produce
monoclonal antibodies.
15 Polyclonal antibodies
The antigen protein is either used alone or conjugated to a conventional
carrier
in order to increases its immunogenicity, and an antiserum to. the peptide-
carrier
conjugate is raised in an animal, as described above. Coupling of a peptide to
a carrier
protein and immunizations may be performed as described (Dymecki et al. (
1992) J.
Biol. Chem., 267: 4815). The serum is titered against protein antigen by ELISA
or
alternatively by dot or spot blotting (Boersma and Van Leeuwen (1994) J.
Neurosci.
Methods, 51: 317). The serum is shown to react strongly with the appropriate
peptides
by ELISA, for example, following the procedures of Green et al. (1982) Cell,
28: 477.
Monoclonal antibodies
. Techniques for preparing monoclonal antibodies are well known, and
monoclonal antibodies may be prepared using any candidate antigen, preferably
bound
to a .carrier, as described by Arnheiter et al. (1981) Nature, 294, 278.
Monoclonal
antibodies are typically obtained from hybridoma tissue cultures or from
ascites fluid
obtained from animals into which the hybridoma tissue was introduced.
Nevertheless,
monoclonal antibodies may be described as being "raised against" or "induced
by" a
protein.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
16
After being raised, monoclonal antibodies are tested for function and
specificity by any
of a number of means. Similar procedures can also be used to test recombinant
antibodies produced by phage display or other in vitro selection technologies.
Monoclonal antibody-producing hybridomas (or polyclonal sera) Gan be screened
for
antibody binding to the immunogen, as well. Particularly preferred
immunological
tests include enzyme-linked immunoassays (ELISA), immunoblotting and
immunoprecipitation (see Volley, (1978) Diagnostic Horizons, 2: 1,
Microbiological
Associates Quarterly Publication, Walkersville, MD; Volley et al. (1978) J.
Clin.
Pathol., 31: 507; U.S. Reissue Pat. No. 31,006; UK Patent 2,019,408; Butler
(1981)
Methods Enzymol., 73: 482; Maggio, E. (ed.), (1980) Enzyme Immunoassay, CRC
Press, Boca Raton, FL) or radioimmunoassays (RIA) (Weintraub, B., Principles
of
radioimmunoassays, Seventh Training Course on Radioligand Assay Techniques,
The
Endocrine Society, March 1986, pp. I-5, 46-49 and 68-78), all to detect
binding of the
1 S antibody to the immunogen against which it was immunogen must .be labeled
to
facilitate such detection. Techniques for labelling antibody molecules are
well known
to those skilled in the art (see Harlow and Lane (1989) Antibodies, Cold
Spring Harbor
Laboratory, pp. 1-726.).
(Bii) RNA (Northern) blotting and Western (antibody) blotting
Northern and Western blotting may be performed using methods familiar to those
skilled in the art and detailed in Sambrook et al., Molecular Cloning: A
Laboratory
Manual, 2d ed. (1989) Cold Spring Harbor Laboratory Press, Cold Spring Harbor,
N.Y. and Ausubel et al., Short Protocols in Molecular Biology (1999).
(C) Assaying tissue samples for binding to DUTT1 bindine a ents
Tissue samples may be prepared using methods familiar to those skilled in the
art and
DUTT1 detected using any of the reagents referred to above. An example of a
typical
protocol is detailed below:
Tissues or whole embryos are fixed in 4% paraformaldehyde in PBS buffer for at
least
24h, paraffin embedded and processed to give 4 mm sections. Sections were
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
17
deparaffmised and endogenous peroxide quenched. After blocking, Duttl/Robol
was
detected using the polyclonal antisera raised against DUTT1 followed by donkey
biotin-conjugated anti-rabbit secondary antibody (SantaCruz USA) and
amplification
with the Vectastain ABC kit (Vector Laboratories USA) as per the manufacturers
protocol. Positive staining was detected using nickel enhanced
diaminobenzidine
tetrahydrochloride (DAB) and counterstained with Fast Red. Slides were mounted
with Vectashield-mounting medium: Staining was blocked by preincubation of the
immunising peptide with the antisera. Sections were stained with haematoxylin
and
eosin (H&E).
The functional portion of the DUTT1 binding agent, when the DUTT1 binding
agent,
is used for diagnosis, usually comprises and may consist of a radioactive atom
for
scintigraphic studies, for example technetium 99m (99mTC) or iodine-123
(~23I). '
Selective labelling of cancer cells
In a further aspect, the present invention provides a method for the selective
labelling
of cancer cells comprising the step of treating one or more cancer cells with
a DUTT1
binding agent.
The term 'labelling' according to. the present invention means the selective
binding of
an agent to cells, in this case to cancer cells and not to normally dividing
cells.
Advantageously the labelling agent comprises or has associated with it means
permitting the detection of the label. Suitable means include fluorescent,
phosphorescent, or radio-active agents, or radioopaque molecules or agents,
such as
metal particles, which are readily visualised within an embryo or a cell mass.
Suitable cancer cell, particularly lung cancer cell labelling agents include
antibodies
raised against DUTTI as herein defined, .DUTT1 binding peptides, and/or small
molecule agonists which mimic the binding of the natural ligand to the
receptor.
Particularly indicated are immunostaining and FACS techniques. Suitable
fluorophores
are known in the art, and include chemical fluorophores and fluorescent
polypeptides,
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
18
attached to immunoglobulin molecules by incorporating binding sites therefor
into the
immunoglobulin molecule during the synthesis thereof.
Preferably, the fluorophore is a fluorescent protein, which is advantageously
GFP or a
mutant thereof. GFP and its mutants may be synthesised together with the
immunoglobulin or target molecule by expression therewith as a fusion
polypeptide,
according to methods well known in the art. For example, a transcription unit
may be
constructed as an in-frame fusion of the desired GFP and the immunoglobulin or
target, and inserted into a vector as described above, using conventional PCR
cloning
and ligation techniques.
Antibodies may be labelled with any agent capable of generating a signal. The
signal
may be any detectable signal, such as the induction of the expression of a
detectable
gene product. Examples of detectable gene products include bioluminescent
polypeptides, such as luciferase and GFP, polypeptides detectable by specific
assays,
such as ~i-galactosidase and CAT, and polypeptides which modulate the growth
characteristics of the host cell, such as enzymes. required for metabolism
such as HIS3,
or antibiotic resistance genes such as 6418. In a preferred aspect of the
invention, the
signal is detectable at the cell surface. For example, the signal may be a
luminescent
or fluorescent signal, which is detectable from outside the cell and allows
cell sorting
by FACS or other optical sorting techniques.
Preferred is the use of optical immunosensor technology, based on optical
detection of
fluorescently-labelled antibodies. Immunosensors are biochemical detectors
comprising an antigen or antibody species coupled to a signal transducer which
detects
the binding of the complementary species (Rabbany et al., 1994 Crit Rev Biomed
Eng
22:307-346; Morgan et al., 1996 Clin Chem 42,:193-209). Examples of such
complementary species include the antigen Zif 268 and the anti-Zif 268
antibody.
Immunosensors produce a quantitative measure of the. amount of antibody,
antigen or
hapten present in a complex sample such as serum or whole blood (Robinson 1991
Biosens Bioelectron 6:183-191). The sensitivity of immunosensors makes them
ideal
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
19
for situations requiring speed and accuracy (Rabbany et al., 1994 Crit Rev
Biomed Eng
22:307-346).
Detection techniques employed by immunosensors include electrochemical,
piezoelectric or optical detection of the immunointeraction (Ghindilis et al.,
1998
Biosens Bioelectron 1:113-131). An indirect immunosensor uses a separate
labelled
species that is detected after binding by, for example, fluorescence or
luminescence
(Morgan et al., 1996 Clin Chem 42:193-209). Direct immunosensors detect the
binding by a change in potential difference, current, resistance, mass, heat
or optical
properties (Morgan et al., 1996 Clin Chem 42:193-209). Indirect immunosensors
may
encounter fewer problems due to non-specific binding (Attridge et al., 1991
Biosens
Bioelecton 6:201-214; Morgan et al., 1996 Clin Chem 42:193-209). such as GFP
and
mutants thereof (see WO 97/28261).
1 S Imaging of cancer cells
A method for the imaging of cancer cells comprising the step of treating one
or. more
cancer cells with one or more DUTTl binding agents wherein the DL1TT1 binding
agent further comprises detection means.
Suitable detection means as herein defined include molecules/agents which can
be
readily detected when associated with, or form a component of the specific
labelling
agent as herein defined, when present within an in vivo environment,
preferably the
human body.
(1) Emission of radioactive particles
Examples of suitable radioactive agents/molecules include technetium 99m
(99mTC) or
iodine-123 (~23I). Tumours can then readily be visualised by detecting the
emission of
radioactive particles using methods known to those skilled in the art.
(2) Nuclear Magnetic resonance (NMR)/ma~netic resonance imaging (MRI)
Detection molecules/agents such as iodine-123, iodine-313, indium-111,
fluorine-19,
carbon-13, nitrogen-15, oxygen-17, gadolinium, manganese or iron allow
visualisation
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
of cancer cells using NMR. This has the advantage that a whole body scanning
can be
performed.
(3)Positron Emission Tomography ET
5 This method differs from standard nuclear magnetic resonance imaging in that
short
lived isotopes are used which emit positrons. The positrons then interact with
normal
tissue electrons to produce pairs of 511 KeV photons which can then be
detected using
a very sensitive positron emission tomographic camera.
Suitable detection means for use iri PET include 11 C methionine and FDG.
10 Descriptions of procedures and protocols for using PET are familiar to
those skilled- in
the art..
Further uses of DUTT1, and/or DUTTl binding a~ent/s.
In a final aspect, the present invention provides the use of DUTT1, and/or a
binding
15 agent thereof in the preparation of a medicament for the prophylaxis or
treatment of
cancer.
Therapeutic and prophylactic uses of DUTT1 and/or binding agents thereof
involve the
administration of the above to a recipient mammal, such as a human.
Substantially pure DUTTl and/or binding agents thereof of at least 90 to 95%
homogeneity are preferred for administration to a mammal, and 98 to 99% or
more
homogeneity is most preferred for pharmaceutical uses, especially when the
mammal
is a human. Once purified, partially or to homogeneity as desired, the DUTT1
and/or
binding agents thereof may be used diagnostically or therapeutically
(including
extracorporeally) or in developing and performing assay procedures using
methods
knov~m to those skilled in the art.
DUTT1 and binding agents thereof, may be effective in treating cancer related
diseases. The present invention includes the method of treating cancer related
disease
with an effective amount of DUTT1 or DUTTl binding agents, according to the
present invention. The DUTT1 and DUTTl binding agents' of the present
invention
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
21
can be provided as isolated and substantially purified proteins and protein
fragments in
pharmaceutically acceptable compositions using formulation methods known to
those
of ordinary skill in the art. These compositions can be administered by
standard
routes. These include but are not limited to: oral, rectal, ophthalmic
(including
intravitreal or intracameral), nasal, topical (including buccal and
sublingual),
intrauterine, vaginal or . parenteral (including subcutaneous,
intraperitoneal,
intramuscular, intravenous, intradermal, intracranial, intratracheal, and
epidural)
transdermal, intraperitoneal, intracranial, intracerebroventricular,
intracerebral,
intravaginal, intrauterine, or parenteral (e.g., intravenous, intraspinal,
subcutaneous or
intramuscular) routes.
The DUTT1 and DUTT1 binding agents may conveniently be presented in unit
dosage
form and may be prepared by . conventional pharmaceutical techniques. Such
techniques include the step of bringing into association the active ingredient
and the
pharmaceutical carriers) or excipient(s). In general, the formulations are
prepared by
uniformly and intimately bringing into association the active ingredient with
liquid
carriers or finely divided solid carriers or both, and then, if necessary,
shaping the
product.
In addition, the DUTT1 and DUTT1 binding agents of the present invention may
be
incorporated into biodegradable polymers allowing for sustained release of the
compound, the polymers being implanted in the vicinity of where drug delivery
is
desired, for example, at the site of a tumor or implanted so that the DUTT1
binding
agent or fragment is slowly released systemically. The biodegradable polymers
and
their use are described, for example, in detail in Brem et al (J.- Neurosurg
1991
74:441-446). Osmotic minipumps may also be used to provide controlled delivery
of
high concentrations of DUTT1 or binding agents thereof, including fragments
thereof
through cannulae to the site of interest, such as directly into a metastatic
growth or into
the vascular supply to that tumor.
The DUTTl and DUTT1 binding agents of the present invention may be linked to
cytotoxic agents which are infused in a manner designed to maximize delivery
to the
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
22
desired location. For example, ricin-linked high affinity DUTT1, and/or
binding
agents thereof are delivered through a cannula into vessels supplying the
target site or
directly into the target. Such agents are also delivered in a controlled
manner through
osmotic pumps coupled to infusion cannulae.
S
Preferred unit 'dosage formulations are those containing a daily dose or unit,
daily
sub-dose, as herein above Tecited, or an appropriate fraction thereof, of the
administered ingredient. It should be understood that in addition to the
ingredients,
particularly mentioned above, the formulations of the present invention may
include
IO other agents conventional in the art having regard to the type of
formulation in
question.
DUTT1 or binding agents thereof may be administered in any suitable way,
usually
parenterally, for example intravenously or intraperitoneally, in standard
sterile, non-
15 pyrogenic formulations of diluents and carriers, for example isotonic
saline (when
administered intravenously). Once DUTT 1 or binding agents has bound to the
target
cells and been cleared from the bloodstream (if necessary), which typically
takes a day
or so, the pro-drug is administered, usually as a single infused dose, or the
tumour is
imaged. If needed, because DUTT1 or the DUTT1 binding agent may be
20 immunogenic, cyclosporin or some other immunosuppressant can be
administered to
provide a longer period for treatment but usually this will not be necessary.
The timing between administrations of DUTT 1 binding agents and/or fragments
thereof may be optimised in a non-inventive way since tumour/normal tissue
ratios of
25 conjugate (at least following intravenous delivery) are highest after about
4-6 days,
whereas at this time the absolute amount of DUTT1 binding agents, or fragments
of
bound to the tumour, in terms of percent of injected dose per gram, is lower
than at
earlier times.
30 Therefore, the optimum interval between administration of DUTT1 binding
agent
and/or fragments thereof will be a compromise between peak tumour
concentration of
and the best distribution ratio between tumour and normal tissues. The dosage
of the
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
23
DUTT1 binding agent or fragment thereof will chosen by the physician according
to
the usual criteria. At least in the case of methods employing a targeted
enzyme such as
(3-glucosidase and intravenous amygdalin as a toxic pro-drug, 1 to 50 daily
doses of
0.1 to 10.0 grams per square metre of body surface area, preferably 1.0-S.0
g/m2 are
likely to be appropriate. . For oral therapy, three doses per day of 0.05 to
lO.Og,
preferably 1.0-S.Og, for, one to fifty days may be appropriate. The dosage of
DUTT1
binding agent or fragment thereof will similarly be chosen according to normal
criteria, particularly with reference to the type, stage and location of the
tumour and
the weight of the patient. The duration of treatment will depend in part upon
the
rapidity and extent of any immune reaction .to the DUTTl binding agent, or
fragment
thereof.
When used in a compound for selective destruction of the tumour, the
functional
portion of DUTT1 binding agent, or fragment thereof may comprise a highly
radioactive atom, such as iodine-131, rhenium-186, rhenium-188, yttrium-90 or
lead-
212, which emits enough energy to destroy neighbouring cells, or a cytotoxic
chemical
compound such as methotrexate, adriamicin, vinca alkaliods (vincristine,
vinblastine,
etoposide), daunorubicin or other intercalating agents.
The radio- or other detection agents may be incorporated in the DUTT1 binding
agent
and/or fragments thereof in known ways. For.example, a DUTT1 binding peptide
may
be biosynthesised or may be synthesised by chemical amino acid synthesis using
suitable amino acid precursors involving, for example, fluorine-19 in place
'of
hydrogen. Labels such as 99mTc, iz3h lsb~~ ~8s~ ~d ulln can be attached via a
.
cysteine residue in the peptide. Yttrium-90 can be attached via. a lysine
residue. The
IODOGEN method (Fraker et al (1978) Biochem. Biophys. Res. Commun. 80: 49-57
can be used to incorporate iodine-123. "Monoclonal Antibodies in
Immunoscinigraphy" (Chatal, CRC Press 1989) describes other methods in detail.
Pharmaceutical compositions comprising an effective amount of DUTT1 binding
agent
and/or a fragment thereof can be used in the prophylaxis, suppression or
treatment of
cancer related disorders. Such disorders include but not limited to: solid
tumours;
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
24
blood born tumours such as leukemias; tumor metastasis; benign tumours, for
example
hemangiomas, acoustic neuromas, neurofibromas, trachomas, and ' pyogenic
granulomas; rheumatoid arthritis; psoriasis; ocular angiogenic diseases, for
example,
diabetic retinopathy, retinopathy of prematurity, macular degeneration,
corneal graft
rejection, neovascular glaucoma, retrolental fibroplasia, rubeosis; Osler-
Webber
Syndrome; myocardial angiogenesis; plaque neovascularization; telangiectasia;
hemophiliac joints; angiofibroma; wound granulation; corornay collaterals;
cerebral
collaterals; arteriovenous malformations; ischeniic limb angiogenesis;
neovascular
glaucoma; retrolental fibroplasia; diabetic neovascularization; heliobacter
related
diseases, fractures, vasculogenesis, hematopoiesis, ovulation, menstruation
and
placentation.
In ,the instant application, the term "prevention" involves . administration
of the
protective composition prior to the induction of the disease. "Suppression"
refers to
administration of the composition after an inductive event, but prior to the
clinical
appearance of the disease. "Treatment" involves administration of the
protective
composition after disease symptoms become manifest.
The invention is further described, for the purposes of illustration only, in
the
following examples which are in no way limiting of the invention.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
EXAMPLES
Example 1
DNA seauencin~ and conti~ assembly of mouse Duttl/Robol cDNA clones. A
mouse brain cDNA library, purchased from Stratagene (La Jolla, CA) and a mouse
5 13.5 day embryo cDNA library, purchased from Life Technology were screened
with
human DUTTIlROBOI cDNA clones. Positive cDNA clones were sequenced and
overlapped to generate a contig map that was entered into the Genbank data
base. All
sequencing reactions were performed using the Sanger dideoxy chain termination
method using the Sequenase version 2 kit (United States Biochemical, USB). The
10 sequence of the cDNA clones was independently confirmed (Oswell Research
Products Ltd., Lab 5005, Southampton).
Example 2
Generation of DuttllRobol mice (see Fig. 1). A genomic library of E14. TG2a ES
15 cell DNA cloned in lambda2001 and was screened with a mouse DuttllRobol
cDNA
clone corresponding to exon 2 and positive clones purified and subcloned. An
8.0 kb
SacI fragment containing exon 2 of the mouse DuttllRobol gene was used for
construction of the targeting vector. A neo expression cassette ( 16) (pMC 1-
neo
poly[A]), corrected to the wild-type neo sequence (17), was used to replace a
0.7 kb
20 genomic HindIII, BamHI fragment spanning sequences coding for exon 2 of the
Duttl gene. The replacement sequence was flanked 5' by 4.4 kb and 3' by 1.4 kb
of
genomic sequence. A thymidine kinase gene expression cassette (18) was ligated
to a
unique XbaI site at the 5' of the longer homologous arm. The final vector was
linearized with HindIII for electroporation. CCB ES cells were maintained on
mouse
25 embryonic feeders. Twenty-five micrograms of HindIII-digested targeting
vector
DNA were used to electroporate 1 x 10' CCB ES cells. SacI digested genomic DNA
from cell clones surviving 6418 (GIBCO) at 400 g/ml and Gancyclovir at 2.5 M
selection was subjected to Southern blot analysis. Positively targeted clones
were
confirmed using a 5' 0.7 kb BamHI fragment internal probe and a 0.4 kb
HindIII/SacI probe external to the homologous sequence on genomic DNA digested
with SacI. ES cells from two independent clones were used for injection into
blastocysts derived from C57BL/6 mice. Blastocysts were transferred to pseudo-
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
26
pregnant females, and chimaeric offspring were detected by the presence of
agouti
colour on a non-agouti background. Chimaeric males were mated to C57BL/6
females to produce ES cell-derived offspring. Their genotype was confirmed by
Southern blot analysis of tail DNA. Mice heterozygous for the gene targeting
event,
i.e. with a deletion of exon 2 of the DuttllRobol gene, were inter-crossed to
generate
homozygotes.
Example 3]
RNA analysis. Total RNA was prepared from various tissues with Trizol Reagent
(GIBCO BRL). Reverse transcription reactions were performed on total RNA (5
micrograms) in 20microlitres containing 0.5 microgms of random hexamer
(Pharmacia) 25 mM Tris-HCI, pH 8.3, 50 mM KCI, 2.0 mM dithiothreitol, 5.0 mM
MgCl2, 1 mM each of dATP, dCTP, dGTP & dTTP, 1 u/microlitre of RNasin
ribonuclease inhibitor (Promega, Madison, WI), and 10 u/microgm of SUPER RT
(HT Biotechnology Ltd, Cambridge, England). Each RT reaction was at
42°C for 40
minutes. The product of RT was diluted five-fold and amplified by PCR. (30
cycles
of lmin denaturation at 95°, lmin annealing at SS° and lmin
extension at 72°~. The
primers used were: exon 1 forward 5'-AGGGATTGACAAGCCTCCGG-3', exon 2
reverse 5'-AGCTACCTCCAGCGATGCGT-3', exon 3 reverse 5'-
CATCTTTATCATCCAGGGGT-3'. The products were visualised following
electrophoresis on agarose gels.
Example 4
Western blotting. Protein lysates were prepared with a lysis buffer composed
of 9
M urea, 75 mM Tris-HCl pH 7.5 and 0.15 M beta-mercaptoethanol as described
(19).
Protein concentration was determined using the Bradford assay, (Bio-Rad
Laboratories). For Western blotting, 50 microgm of protein lysate were
subjected to
SDS-PAGE on a 10% polyacrylamide gel and transferred onto a nitrocellulose
membrane (BioTrace; Gelman Sciences). Transfer was assessed by Ponceau S
staining (Bio-Rad). Filters were incubated with a polyclonal antisera raised
against
the C-terminal peptide of DUTT1/ROBOl (CYERGEDNNEELEETES) followed by
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
27
HRP-conjugated mouse anti-rabbit IgG and visualised by enhanced
chemiluminscence (Amersham, Pharmacia).
Example 5
Histoloeical analysis and immunohistochemistrY. Tissues or whole embryos were
fixed in 4% paraformaldehyde in PBS buffer for at least 24h, para~n embedded
and
processed to give 5 micrometre sections. Sections were stained with
haematoxylin
and eosin (H&E). For immunohistochemistry, Duttl/Robol was detected using the
polyclonal antisera described above followed by donkey biotin-conjugated anti-
rabbit secondary antibody and Cy-3 conjugated Streptavidin: Slides were
mounted
with Vectashield-mounting medium. Surfactant protein-C was detected using a.
commercially available antibody (SantaCruz) followed by donkey biotin-
conjugated
anti-goat secondary antibody and Cy-3 conjugated Streptavidin.
Example 6
Generation of Duttl/Robol mutant mice. The DuttllRoliol. gene was disrupted in
mice by targeted replacement of exon 2 with the neomycin phosphotransferase
gene,
producing a mutant form of the gene unable to code for the first
immunoglobulin
domain (F'ig.lA). This exactly reproduces a truncated DUTTIlROBOI transcript'
detected in the human lung cancer cell line NIH-H219X (9): Following
transfectiori,
DNA from 6418-resistant embryonic stem cell (ES) clones was analysed by
Southern blotting to identify correctly targeted clones by hybridisation with
probe
(a) within the targeting construct (Fig. 1 B) and with probe (b) external to
the
homologous integration. Two such clones were ' injected into blastocysts and'
resulting chimaeras bred with C57BL/6 mice. Tail DNA from offspring was
screened by Southern blotting to identify mice carrying the mutant form of the
gene
in their germ-line (Fig. l C). Heterozygotes were interbred to produce -
homozygous
offspring. RT-PCR was used to confirm that exon 1 and 3 sequences were
contiguous and that exon 2 was absent in the transcript from DuttllRobol-~'
mice
(result not shown).
Example 7
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
28
Duttl/Robol protein in mutant and normal mice. In all organs from homozygous
offspring examined by Western blotting (embryos and new-born mice), the
resulting
protein was reduced to about one half of the level detected in wild-type
organs (Fig.
2A). The Duttl/Robol protein is shorter by 20kDa due to loss of the first
immunoglobulin domain encoded by exon 2 (Fig. 2A). Immunohistochemistry
was performed using a polyclonal antibody raised against the C terminus of
human
DUTT1/ROBO1 (Clark, KJ, JX, EH and PHR unpublished observations), which
recognises the mouse protein. This detected the protein at high levels in
epithelial
cells lining bronchi but at low or undetectable levels in adjacent mesenchyme
and
cells lining alveoli (Fig. 2B).
Example 8
DuttllRobol-~- mice freguently die at birth due to respiratory failure. Mice
heterozygous for the DuttllRobol deletion were born at the expected frequency
and
had no obviously abnormal phenotype. At birth, DuttllRobol-~- mice failed to
feed
(Fig 3), were usually inactive with laboured breathing and nearly two-thirds
(22/36)
died within the first twenty-four hours. At autopsy, lungs were 'frequently
dark red
(Fig. 3) and sank in fixative suggesting inadequate inflation. Following
fixation and
H&E staining, lungs from DuttllRobol-~ mice and their normal littermates were
compared. The most striking feature of lungs from DuttllRobol-~ newborn mice W
as
increased cellularity of the mesenchyme resulting in reduction of the size of
terminal
air spaces (Fig. 4D); ~ alveolar septa were reduced in number and thicker than
in the
wild-type littermates(Fig 4C); the bronchioles were of an irregular cross
section (Fig.
4D). The overall appearance was consistent with a developmental delay of 0.5
to 1.0
day. To determine if the abnormal phenotypes were present before birth, lungs
from
day E15.5 and day 18.5 were similarly examined., This showed that the abnormal
lung morphology was already established at day 15.5 (Fig. 4A&B).
Example 9
Search for other phenotypic changes in DuttllRobol'~- mice. All newborn mice
which die at birth were examined for macroscopic abnormalities in addition to
their
abnormal lungs. None was found except for three mice with diaphragmatic
hernias.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
29
The heart, kidneys and muscle were examined microsopically but no abnormal
histology was detected. In view of the function of Drosophila Robo in the
control of
axonal migration and the high level of expression of the protein in the brain
of
newborn mice (Fig. 2A) and in foetal brain (Clark, KJ, JX, EH and PHR
unpublished
observations), , brains and spinal chords of mutant mice were examined.
Transverse
serial sections of spinal chord from E12.5 and E15.5 embryos were examined by
H&E staining for thickening of the ventral commissures underlying the floor
plate of
the spinal chord. However, no differences could be detected between
DuttllRobol-~~
and normal embryos (results not shown).
Example 10
Development of bronchial epithelial hyperplasia in DuttllRobol-~- mice
surviving
birth. Our results show that the DuttllRobol targeted germline mutation, which
emulated the naturally. occurring somatic mutation found in the NCIH219X lung
tumour cell line, has a profound effect on lung development. Some homozygous
mice
do survive to adulthood and are capable of breeding (39%). The DuttllRobol-~-
mice
surviving beyond the first 24h after birth appeared to develop normally but
commonly
showed signs of morbidity at ages ranging from three weeks onwards. Following
autopsy and tissue examination, bronchial epithelial hyperplasia was observed
as a
common feature. In some lungs, papillary hyperplasia was seen throughout the
entire
bronchial tree (Fig. 4F&H), contrasting with the uniform cuboidal appearance
of the
bronchial epithelium of normal adult mice (Fig. 4E&G). Very occasionally,
focal
dysplasia was observed .characterised by increased epithelial cell layers,
large
pleomorphic nuclei and reduced cytoplasm (Fig. 4I). Thus while differences in
gross
lung morphology are .less distinct in surviving post-natal homozygotes,
specific
bronchial epithelial alterations are apparent in these mice.
Example 11
Production and purification of soluble Fabs
Soluble Fab's were produced as described by Roovers et al (1998). The cultures
were
inoculated with E. coli TG1 (K12, D(lac pro), supE, thi, hsdDSlF' traD36,
proA+B+,
lacl9, IacZDMlS) harbouring the Fab in pCESI. Individual bacterial clones were
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
picked and production of soluble Fab was induced by activation of the upstream
Lac Z
promoter with isopropyl-(3-1-thiogalactopyranoside (IPTG) as described by
Marks et
al (1991).
5 ELISA
An ELISA using soluble Fabs was performed on purified, recombinant DUTT1 in
order to identify binding Fabs from the individual clones selected. ELISA
plates
(Costar, Cambridge, MA, USA) were coated overnight with 1 p.g m1-1 DUTTl in
phosphate-buffered saline (PBS), washed three times with PBS-T [PBS, 0.5%
(v/v)
10 Tween 20], three times with PBS and blocked for 1 h at room temperature
(RT) with
2% MPBS [2% (w/v) Marvel - skimmed milk powder - in PBS]. After blocking,
induced bacterial supernatants were added [50% (v/v) in 2% MBS] and incubated
for
1.5 h at RT.. Bound antibody fragments were detected with a further antibody
[50%
(v/v) hybridoma supernatant in 2% MPBS], peroxidase-conjugated rabbit anti-
mouse
15 immunoglobins [Dako, Glustrup, Denmark; 0.1% (v/v) in 2% MPBS] and stained
with
trimethylbenzidine (TMB) and hydrogen peroxide. Optical density was measured
at
450 nni. This assay is described in detail by Roovers et al (1998 ibicl).
Competition ELISA
20 For competition ELISA, DUTT1 binding of soluble Fab antibody fragments was
detected in the presence of excess whole murine monoclonal antibody.
Specificity ELISA
The assay was carried out as described above but the wells were coated with
antigens
25 such as 10 pg/ml Bovine Serum Albumin (Sigma) in PBS, 3 mg/ml Hen Egg White
Lysozyme (Boehringer Mannheim) in 0.1 M NaHC03 (pH 9.6), 10 p.g/ml Tetanus
Toxoid in 0.1 M NaHC03 (pH 9.6) and 0.5 pg/ml EGP-2 in PBS.
CA 02438330 2003-08-29
WO 03/029488 PCT/GB02/04445
31
Example 12
Polyclonal antibody and Immunohistochemistry
A polyclonal antisera was raised in rabbits against the. C-terminal peptide of
DUTT1/ROBO1 (CYERGEDNNEELEETES) by Zeneca (Cambridge Research
Biochemicals UK). The antisera specifically recognised a band at approximately
190kDa in cells transfected with either a DUTT1 or ROBO1 expression vector.
Staining was blocked by preincubation of the immunising peptide with the
antisera.
Tissues or whole embryos were fixed in 4% paraformaldehyde in, PBS buffer for
at
least 24h, paraffin embedded and processed to give 4 micorometre sections.
Sections
were deparaffinised and endogenous peroxide quenched. After blocking,
Duttl/Robol
was detected using the polyclonal antisera described above followed by donkey
biotin-
conjugated anti-rabbit secondary antibody (SantaCruz USA) and amplification
with
the Vectastain ABC kit (Vector Laboratories USA) as per the manufacturers
protocol.
Positive staining was detected using nickel enhanced diaminobenzidine
tetrahydrochloride (DAB) and counterstained with Fast Red. Slides were mounted
with Vectashield-mounting medium. Staining was blocked by .preincubation of
the
immunising peptide with the antisera. Sections were stained with haematoxylin
and
eosin (H&E).
All publications mentioned in the above specification are herein incorporated
by
reference. Various modifications and variations of the described methods and
system
of the invention will be apparent to those skilled in the art without
departing from the
scope and spirit of the invention. Although the invention has been described
in
connection with specific preferred embodiments, it should be understood that
the
invention as claimed should not be unduly limited to such specific
embodiments.
Indeed, various modifications of the described modes for carrying out the
invention
which are obvious to those skilled in molecular biology or related fields are
intended
to be within the scope of the following claims.