Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
STIMULATION FOR DELIVERY OF MOLECULAR THERAPY
Field of the Invention
The present invention provides a novel stimulatory device for the controlled
production
of angiogenic growth factors. More specifically, the present invention
provides a subthreshold
pulse generator for the local production of vascular endothelial growth
factor.
Background of the Invention
Coronary artery disease (CAD) results from arteriosclerosis of blood vessels
serving the
heart. Arteriosclerosis is a hardening and narrowing of the arteries. Often
the arteries of the
heart can suddenly become so severely blocked that there is inadequate blood
supply to areas of
the heart, leading to the occurrence of a myocardial infarction. The area of
damage where the
reduced blood flow has occurred is called the ischemic area. The ischemic area
of the heart,
because it does not get adequate blood flow, is starved of oxygen and
nutrients. Tlus blockage, if
not treated quickly, can lead to severe tissue damage. Often surgical
procedures are used to graft
new blood vessels to the ischemic area to improve circulation. Alternatively,
angioplasty or
stenting of the blocked blood vessel is done to reopen or maintain blood flow.
However, by-
passing or reopening of the arteries is often not possible because of
limitations of present
methodologies and the risk to the patient from surgical intervention.
Damage from ischemia from insufficient blood circulation can also occur in
blood vessels
peripheral to the heart. Peripheral arterial occlusive disease (PAOD), caused
by arteriosclerosis
or by formation of vascular blood clots from diseases such as diabetes, often
leads to loss of
external limbs.
One way to address the need for improved blood flow to ischemic tissue is to
generate
new blood vessels. Angiogenic factors are known to directly participate in the
formation of new
blood vessels. Local administration of recombinant angiogenic growth factors,
such as basic
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
2
fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF),
can salvage
ischemic areas of myocardial and skeletal muscle tissue in animal models. A
number of
approaches have been developed to deliver these factors to ischemic areas in
hope of developing
new blood vessels, including direct injection, electroporation, and delivery
using retroviral
vectors.
The direct inj ection of angiogenic growth factors has many problems
associated with it,
most notably problems with effective delivery of the factors into the cells.
Electroporation is a
possible method of delivery of genetic materials encoding angiogenic factors;
however, the
transfection efficiency is still very low and the high-energy pulses directed
to the tissue often kill
many healthy cells. Alternatively, others have sought to develop viral based
gene delivery
systems to directly produce angiogenic factors in vivo; however, this approach
requires
considerably more development before it is considered to be a safe and
effective therapy.
Although extensive research continues in the areas of gene delivery, very
little has been reported
on methods to control and regulate gene expression in vivo. The inability to
effectively deliver
the agent to the target tissue, therefore, is one of the major limitations of
the use of such agents.
During delivery of the angiogenic factors the effectiveness is often destroyed
or lost.
Recent work has been published related to using electrical fields to stimulate
natural
production of angiogenic growth factors. WO 00/27466 describes use of constant
voltage
sources to generate electrical fields for stimulating angiogenesis. The
described voltages are on
the order of 50-300 volts/cm, which would also stimulate contractile responses
during
stimulation. Stimulation of angiogenesis without causing a contractile muscle
response would be
advantageous. In a recent publication (Circulation, 1999;99:2682-2687) it was
reported that low-
voltage electrical stimulation of skeletal muscle induced de novo synthesis of
VEGF protein and
promoted angiogenesis. Further work is needed in this research area. Even with
the known
methods in the art, there still exists a need for additional and more
effective subthreshold devices
and more efficient methods for the controlled delivery of angiogenic growth
factors to promote
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
3
angiogenesis in muscle tissue, and methodologies that can be used to stimulate
angiogenesis in
cardiac and vascular tissue.
Summary of the Invention
The present invention addresses a number of problems existing in the prior art
with
respect to controlled Iocal delivery of angiogenic factors. Various
embodiments of the present
invention provide solutions and to one or more of the problems existing in the
prior art with
respect to delivery of angiogenic factors. The present invention provides a
novel electrical pulse
generator for angiogenesis and production angiogenic growth factors.
The present invention provides an electrical pulse generator for providing
subthreshold
pulses. The present device can be adapted to a range of subthreshold pulses by
modulating the
time, frequency, and delivery of a given stimulus. The present generator
allows the use of a
constant voltage, regardless of the distance between electrodes by allowing a
variable field
density. The present generator allows for control over the amplitude of the
voltage and for
charge balancing of the delivered and recovered charged pulse.
In another embodiment, the subthreshold pulse generator can be used
externally, but
preferably is designed and configured to be irnplantable. The subthreshold
pulse generator
includes a power supply and a control mechanism interconnected with the power
supply.
Optionally, the pulse generator can be used with a plurality of electrodes in
electrical
communication with the power supply. The present generator is also capable of
checking the
lead continuity at a predesignated time.
The invention also provides a subthreshold pulse generator for a patient in
need thereof.
In one aspect, the invention includes a method for reducing or repairing
tissue injury or disease
by providing a means for regulating angiogenic growth factor production. In
another aspect the
subthreshold stimulation provided is sufficient to stimulate angiogenesis in
the targeted body
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
4
tissue. In yet another aspect, the present invention provides a novel method
of pacing that is
capable of stimulating cells or tissues for the controlled expression of
angiogenic factors.
These and other obj ects and features of the invention will become more fully
apparent
when the following detailed description is read in conjunction with the
accompanying drawings.
Brief Description of the Drawings
The following drawings depict certain embodiments of the invention. They are
illustrative only and do not limit the invention as otherwise disclosed
herein.
Figure I : Subthreshold Stimulation of Heart Tissues For Production of VEGF.
Figure 1 is an overview of one mode of operation for subthreshold stimulation
of cardiac
tissue.
Figure 2: Simplified Schematic of The Output Circuit for Subthreshold
Stimulation
Figure 2 illustrates the schematic of the output circuitry of a subthreshold
stimulation
device for a pulse generator.
Figure 3: Equivalent Circuit of the Subthreshold Stimulation During the Output
Stage
Figure 3 illustrates the schematic of the output circuitry of a subthreshold
device for a
pulse generator during the output stage.
Figure 4: Subthreshold Stimulation Sequence
Figure 4 illustrate a pacing scheme for providing a series of subthreshold
stimulations.
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
lure 5: Pulse Generator for Subthreshold Stimulation
Figure 5 shows a block diagram of a circuit for pulse generator capable of
delivering
electrical stimulation to the target tissue cells.
F~ure 6: Schematic of The Output Circuit for Subthreshold Stimulation
Figure 6 illustrates the schematic of the output circuitry of a subthreshold
stimulation
device for a subthreshold pulse generator.
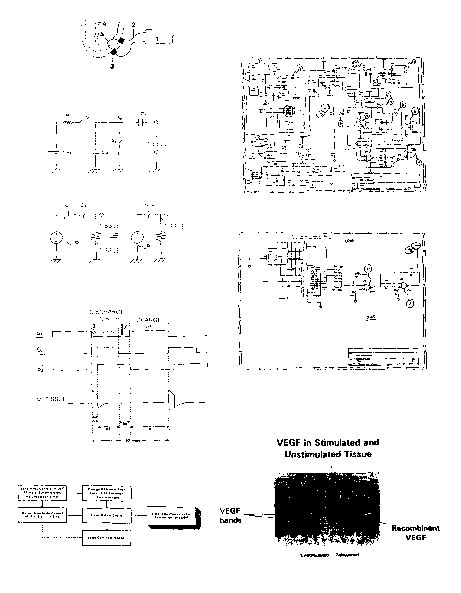
lure 7: VEGF Production in Stimulated and Unstimulated Tissue.
Figure 7 shows a western blot of VEGF protein in stimulated and unstimulated
vascular
tissue.
Detailed Description of the Invention
Definitions
"Angiogenic factors" are a group of substances that promote angiogenesis in a
tissue.
These factors include, but are not limited to, vascular endothelial growth
factor (VEGF) and
fibroblast growth factor (FGF) and all natural analogs found encoded in the
genome of the
patient that are structurally and/or functionally related members of these
factors.
The term "mature protein" or "mature polypeptide" as used herein refers to the
forms) of
the protein produced by expression in a mammalian cell. It is generally
hypothesized that, once
export of a growing protein chain across the rough endoplasmic reticulum has
been initiated,
proteins secreted by mammalian cells have a signal sequence which is cleaved
from the complete
polypeptide to produce a "mature" form of the protein. Often, cleavage of a
secreted protein is
not uniform and may result in more than one species of mature protein. The
cleavage site of a
secreted protein is determined by the primary amino acid sequence of the
complete protein and
generally cannot be predicted with complete accuracy. However, cleavage sites
for a secreted
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
6
protein may be determined experimentally by amino-terminal sequencing of the
one or more
species of mature proteins found within a purified preparation of the protein.
"Operably coupled" refers to the transference of an electrical stimulus by a
subthreshold
pulse generator to a tissue. A subthreshold pulse generator operably coupled
with tissue or cells
refers to a configuration where an electrical stimulus is delivered to the
tissue or cells to cause an
increase in available angiogenic factors. Usually the stimulus is delivered
from the subthreshold
pulse generator through leads to electrodes attached to the tissue
The terms "treating", "treatment", and "therapy" as used herein refer to
curative therapy,
prophylactic therapy, and preventive therapy. An example of "preventive
therapy" is the
prevention or lessening of a targeted disease or related condition thereto.
For example,
subthreshold stimulation can be used prophylactically to promote angiogenesis
as a preventive
effort to avoid the occurrence of a myocardial infarction. Those in need of
treatment include
those already with the disease or condition as well as those prone to having
the disease or
condition to be prevented. The terms "treating", "treatment", and "therapy" as
used herein also
describe the management and care of a patient for the purpose of combating a
disease or related
condition, and includes the administration of at least one subthreshold
electrical pulse to an
ischemic area to improve blood flow to the tissue.
"Chronic" administration refers to administration of an electrical stimulus in
a continuous
mode as opposed to an acute mode, so as to maintain the initial therapeutic
effect (activity) for an
extended period of time.
"Intermittent" administration is treatment that is not consecutively done
without
interruption and is repeated in the course of time.
"Ischemia" is defined as an insufficient supply of blood to a specific organ
or tissue. A
consequence of decreased blood supply is an inadequate supply of oxygen and/or
nutrients to the
organ or tissue. Prolonged ischemia may result in injury to the affected organ
or tissue. "Anoxia"
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
7
refers to a virtually complete absence of oxygen in the organ or tissue,
which, if prolonged, may
result in death of the organ or tissue.
"Ischemic injury" refers to cellular and/or molecular damage to an organ or
tissue as a
result of a period of ischemia and/or ischemia followed by reperfusion.
"Hypoxic condition" is defined as a condition under which a particular organ
or tissue
receives an inadequate supply of oxygen.
"Anoxic condition" refers to a condition under which the supply of oxygen to a
particular
organ or tissue is cut off.
"Reperfusion" refers to the resumption of blood flow in a tissue following a
period of
ischemia.
The term "patient" as used herein refers to any mammal, including humans,
domestic and
farm animals, and zoo, sports, or pet animals, such as cattle (e.g., cows),
horses, dogs, sheep,
pigs, rabbits, goats, cats, and non-domesticated animals such as mice and
rats. In a preferred
embodiment of the invention, the mammal is a human, dog, rabbit, or mouse.
A "therapeutically effective amount" as referred to herein is the minimal
amount of
subthreshold stimulation that is necessary to impart a therapeutic benefit or
a desired biological
effect to a patient. For example, a "therapeutically effective amount" for a
patient suffering from
ischemia is such an amount which induces, ameliorates, or otherwise causes an
improvement in
the amount of angiogenic factors available or otherwise improve circulation in
the tissue. For
example, a "therapeutically effective stimulus" is the amount of electrical
stimulation necessary
to express a therapeutically effective amount of an angiogenic protein in an
amount to provide a
therapeutic benefit or provides at least one measurable improvement in
circulation.
The term "pace" as used herein is the act of issuing an electrical
subthreshold stimulus
delivered to the cellular tissue delivered a subthreshold pulse generator.
"Pacing" generally
refers to the act of repeatedly issuing an electrical stimulation to the
tissue, as in the present case,
delivering a series of subthreshold stimulations to the tissue.
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
"Pharmacologically effective stimulus" is the amount of stimulus needed to
provide a
desired level of an angiogenic protein in the patient. The precise amount of
stimulation or pacing
needed will depend upon numerous factors, e.g., such as the specific
angiogenic factor involved,
the delivery stimulus employed, characteristics of stimulus provided, its
intended use, and patient
considerations. These determinations can readily be determined by one skilled
in the art in view
of the information provided herein.
The teen "administer an electrical stimulus" means to deliver electrical
stimulation to a
tissue. As applied in the present invention, the electrical stimulus is
delivered to the tissue by a
subthreshold pulse generator.
"Threshold" versus "subthreshold" stimulation refers to a relative level of
applied
stimulation. "Threshold" stimulation as used herein, refers to a level of
stimulation to evoke a
gross tissue electrical or mechanical response in the excited tissue, e.g. the
minimum electrical
stimulus needed to consistently elicit a cardiac depolarization for a heart
contraction or to elicit a
skeletal muscle movement. Generally, threshold stimulation is greater than 1.0
volt.
Subthreshold stimulation refers to the application of electrical stimulation
to tissue at levels low
enough not to elicit a gross electrical or mechanical response from the
tissue, such as to not cause
cardiac depolarization or muscle contraction. A subthreshold stimulus can be
achieved by
keeping either the voltage amplitude and/or the duration of the electrical
pulses below the
threshold response levels for gross motor or nerve responses. Generally,
subthreshold stimulus is
less than or equal to 1.0 volt. Subthreshold stimulation allows one to deliver
electrical
stimulation to the tissue to increase the levels of angiogenic protein
available without having the
unwanted side effects due to the stimulation of nerve or muscle cells, such as
unwanted
contraction and or uncomfortable tactile sensations, and the like.
As used herein, a number of terms for measured physical parameters have been
abbreviated: amplitude may be expressed in volts (V) or millivolts (mV);
current may be
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
9
expressed in amperes (amps) or milliamperes (mamps); and pulse width,
frequency, or timing in
milliseconds (msec); and energy in joules (J) or millijoules (mJ).
Description
In general the present invention relates to a subthreshold pulse generator for
producing an
electrical field near to or within a targeted body tissue and methods of
treating damaged or
ischemic tissue by stimulating angiogenesis. In one embodiment the electrical
field is delivered
directly to the target area located between a plurality of electrodes. As an
example, Figure 1
(Fig. 1) illustrates a subthreshold pulse generator (1) creating a
subthreshold electrical field (5) to
the lower ventricle of the heart (4) through a pair of leads (2) and
electrodes (3).
Subthreshold stimulation has been demonstrated to promote production of
angiogenic
growth factors. The promotion of angiogenic response by the present device
thereby serves as a
significant adjunct over current surgical methods of intervention to re-
establish circulation to
ischemic tissue areas.
Purpose of the Subthreshold Pulse
The present invention provides a novel subthreshold electrical pulse generator
(also
referred to herein as subthreshold pulse generator, pulse generator, or
generator). The pulse
generator has the essential feature of being capable of providing an
electrical stimulus or series of
electrical subthreshold stimulations or pulses (pacing). The subthreshold
electrical stimulus or
pulses are used to induce angiogenesis in targeted cells or tissues. In one
embodiment, the
electrical stimulator provides a subthreshold stimulation to activate
transcription of at least one
angiogenic factor. The objective of the subthreshold stimulation is not to
excite the tissue for
mechanical contraction but to selectively activate angiogenesis.
It is envisioned that different stimulation therapies may be given in
conjunction with a
course of subthreshold stimulation therapy. At times, particularly when
considered with benefits
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
of threshold electrical stimulation of traditional pacemakers, it may be
advantageous to combine
the features of a traditional pacemaker with components for subthreshold
stimulation.
pulse generator operation parameters
5 The controlled output voltage from the subthreshold electrical pulse
generator can be
adjusted for a wide range of tissue impedances, such as from 35 S2 to
infinity. Through a variety
of unique combinations of voltage and timing settings the present device
provides a unique
mechanism to increase production of angiogenic factors while not evoking
contractile responses
The present generator allows the use of a constant voltage, regardless of the
distance
10 between electrodes, by allowing a variable field density. The present
generator allows for
control over the amplitude of the voltage and for charge balancing of the
delivered and recovered
charged pulse. As a specific embodiment, the subthreshold pulse generator
allows the use o~ a
constant voltage by allowing a variable field density of about 30% of the
targeted voltage, more
preferably of about 20% of the targeted voltage, and even more preferably of
about 10%, and
most preferably of about 5% of the targeted voltage. As will be illustrated
later, the subthreshold
voltage is at a constant level, allowing the field intensity to vary across
the tissue. Effective
results were obtained in the in vitro experiments having a variable field
intensity in vitro (see
Experiment l, Table 1) as well as with stimulating VEGF production in vivo
(see Experiment 2,
Figure 7).
In one embodiment, the subthreshold pulse generator is capable of delivering
an electric
field to the targeted body tissue of 0 to 1.5 V output in steps of 0.1 V,
wherein the electric field is
generally less than or equal to about 1 V/cm, and more preferably less than
about .5 V/cm, and
even more preferably about 0.1 V/cm. I
In yet another embodiment, the subthreshold field can be produced by a number
of pulses
with a frequency about 10 Hz to about 100 Hz, and preferably with a frequency
of about 25 Hz to
about 85 Hz, and more preferably with a frequency of about 40 Hz to about 70
Hz, and even
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
11
more preferably with a frequency of about 50 Hz to about 60 Hz, and most
preferably with a
frequency of about 50 Hz.
In a further embodiment, the stimulation period is less than the pulse cycle
(1/frequency).
The stimulation period can be chosen to be between about 100 cosec and .O1
cosec, between
about 50 cosec and .OS cosec, and between 3 cosec and .1 cosec, wherein the
actual value can be
less than about 20 cosec, preferably less than about 10 cosec, more preferably
less than about 3
cosec or any value between O.lms and 3.0 ms.
Subthreshold Stimulation
The schematic of the output circuitry in Figure 2 is a simplified illustration
of the
schematic of the operating circuitry for a subthreshold pulse generator. Fig.
2 is useful for
setting basic design requirements of a subthreshold pulse generator: S" SZ,
and S3 are switches
that are opened and closed during the operating cycle, R is a circuit
resistor, Vs is the battery,
and CH and C~ are capacitors. For example, if the component values can be
chosen as follows:
VS=2.SVolts, R=25SZ, C~ CH 10~,F, one can calculate that CH will have 0.110
volts at the end of
10 cosec charging phase as shown in Figure 4. By this illustration, one
skilled in the art could
choose a number of settings that would provide CH at any given set of
subthreshold output
voltages. Figure 3 shows the equivalent circuit of the output stage during the
stimulation phase.
V~ represents the initial condition on the CH. In this case, because CH, C~
and Rhssue are
connected in series, one can combine CH and C~ into Ceq = 5 qF. Voltages seen
at the electrodes
are given by: VT;ssue(t) = VcH(0) f 1- exp [-t / (CEqRTissue)~~' If, for
example, the output voltage is
allowed to change by only 10 %, then the VTissue(t) will vary between 0.110
volts and 0.090 volts.
That would indicate that V~H(0) = 0.110, and VT;SSUe(t) (0.3msec) = 0.090.
Rewriting the equation
for the tissue voltage, 0.090 = 0.110 { 1- exp [-t / ( CEQRT;SS"e)~}, t =
0.3msec or 0.090 = 0.110 f
1 - exp [-0.3x10-3 / (5 x10-6x RT;ssue)]~ and solving for RT;ssue one can find
that RT;ssue = 35 S2. In
other words, the minimum tissue impedance that one can drive will be 35 S~,
with output voltage
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
12
staying in the 90-110 mV range. Use of the above settings in the pulse
generator provides one
example for (1) a pulse generator for subthreshold stimulation; (2) controlled
output voltage for a
wide range of tissue impedances (35 S2 to infinity); (3) a pacing output for
subthreshold
stimulation where the objective is not to excite the tissue for mechanical
contraction but to
increase the amounts of angiogenic factors available by providing a set of
variables equal to or
less than a 1.0 volt subthreshold stimulus.
Figure 4 (Fig. 4) exemplifies a set of subthreshold stimulation parameters.
Fig 4.
illustrates the timing diagram of the electrical pulses and charging of
capacitors, to provide the
illustrated pulse train: pulse frequency of SOHz (20 msec); stimulatory pulse
of 0.3 msec to the
tissue (Vtissue) and a 6.7 msec discharge (recharge with opposite polarity for
charge balance).
The remaining 13 msec of the pulse cycle electrodes are floating, and
capacitors are recharged.
Figure 5. (Fig. 5) is a block diagram of a subthreshold stimulator employed in
chronic
animal studies. The basic stimulus repetition frequency of 50 Hz is generated
by the clock/timer
at upper left. This triggers timers that control the 1 msec discharge and 5
msec recharge phases
of each output pulse (top center). Pulse amplitude is controlled by 4-bit DAC
(left center). The
pulse output circuit (center) utilizes the timing and amplitude information to
generate the actual
output pulse which is, in turn, delivered to electrodes and the tissue (right
center). The pulse
output circuit (bottom center) optionally incorporates a lead continuity
monitor to check for lead
or electrode malfunction. Output amplitude may be adjusted (dashed line) based
on conditions
of increased electrode resistance, or turned off if a lead breaks or shorts.
An alternative
stimulation setting of 2 Hz (upper left) is employed for evaluation of the
stimulation safety
margin (pacing threshold) in conjunction with the output amplitude control.
Figure 6 (Fig. 6) shows the stimulator circuit used during in vivo
experiments..
Explanations of general symbols used in the circuit diagram are as follows:
U: Integrated Circuit
R: Resistor
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
13
C: Capacitor
SW: Switch
D: Diode
Vcc: Positive terminal of the power
supply (battery)
Vee: Negative terminal of the power
supply (battery)
JP: Jumper terminal for off board
connections
Below
is a
list
of components
labeled
specifically:
(1) U2: Main oscillator keeping the stimulator timing
at 50 Hz
(20 milk-seconds)
(2) UlB: Timer to control the stun pulse width, wluch
closes S2
shown as (13).
(3) UlA: Timer to control the discharge duration,
which closes S3,
shown as (7).
(4) Cl is the holding capacitor, CH, with the value
1 of 10 micro-
Farads.
(5) C10 is the coupling capacitor, C~, with the
value of 6.8 micro-
Farads.
(6) JP2 is the header where the stimulation electrodes
are attached.
(7) U5: Switch S3, when closed discharges the coupling
capacitor.
(8) R9: Resistor in series with the tissue being
stimulated that is
used to measure the stimulation current
intensity.
(9) SW2: Switch to test lead integrity using the
lead integrity indicator
shown as (10)
(10) LED: Light emitting diode used as lead integrity
indicator
(11) U7C: Digital to analog converter which is used
to set the stimulation
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
14
amplitude, Vadj, shown as (14).
(12) U7D: Inverter/driver for the adjustable stimulation amplitude
determined by ( 11 ).
(13) U3: Switch S3, when closed, delivers charge from the holding
capacitor to the tissue connected to (6).
(14) Vadj: adjustable stimulation amplitude determined by (11)
The subthreshold stimulator circuit of Figure 6A (Fig 6A) and continued on
Figure 6B
(Fig. 6B) operates essentially to produce the stimulation waveform shown in
Fig. 2. The
generated stimulus is a periodic signal which main oscillator (1) is set to
produce 50 Hz digital
pulses, and provides the main clock. Timers (2) and (3) produce the
stimulation and discharge
pulses, respectively, from the clock, again as shown on Figure 2. These pulses
are used to close
the switches (13) and (7), which correspond to SZ and S3 on Figure 2.
Terminals 1 and 4 of the
on board comzector (6) is where the leads going to the tissue are attached.
During the
stimulation, energy stored on holding capacitor (6) passes through switch
(13), the coupling
capacitor (5), and series resistor (8) to reach terminal (6) before arriving
at the tissue. Voltage
drop on series resistor (8) can be monitored from terminal (6) to get an
indication of the current
being passed through the tissue. Switch (9) can be used to monitor the lead
integrity using the
lead integrity indicator (10). Stimulation amplitude adjuster (14) is set by
the digital to analog
converter (11) followed by the inverter/driver (12).
additional features of the subthreshold pulse generator
In another embodiment, the subthreshold pulse generator can.be used
externally, but
preferably is designed and configured to be implantable. The present device
can be implanted
into the body and the electrical components sealed from the body tissues and
fluids. Ideally, the
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
implantable device has a volume of about 50 cm3, preferably about 40 cm3, more
preferably
about 30 cm3, even more preferably about 20 cm3, and most preferably about 10
cm3.
It is envisioned that the electrical pulse generator can be implanted or can
be external to
the body. Ideally, the subthreshold pulse generator is implanted.
5
electrodes and leads
The subthreshold pulse generator includes a power supply and a control
mechanism
interconnected with the power supply. Optionally, the pulse generator can be
used with
electrodes in electrical communication with the power supply. In another
embodiment he
10 subthreshold stimulation provided is sufficient to stimulate angiogenesis
in the targeted body
tissue. The present generator is also capable of checking the lead continuity
at a predesignated
time. W other preferred embodiments, electrodes and leads can be used with the
subthreshold
pulse generator. In a preferred embodiment, the electrodes axe configured in a
manner consisting
of bipolar or multiple electrode configurations.
15 The electrodes are made of conductive metals or organic polymers, or
combinations of
the two. For example, they can be made of platinium, gold, zirconium, iridium,
titanium, certain
carbons, stainless steel, silver, copper, tin, nickel, iron, or lithium, or
various mixtures, alloys, or
amalgams thereof. Design of the electrodes can take on a number of different
shapes and sizes,
depending on the nature of the target tissue. In the case of heart muscle or
other muscle tissues,
the electrodes can consist of a straight pin, screw, patch, or the like, which
can further comprise
various barbs, hooks, or alternate structures for affixing the electrode.
As yet another embodiment, various types of electrical leads similar to those
exemplified
herein or commonly used with other implantable pulse generators can be used to
connect to the
power source.
A number of suitable electrodes can function to provide the electrical
stimulation. In one
feature, the electrode is a surface coil electrode, or heart wire. The surface
electrode may be
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
16
constructed of a platinum alloy or other biocompatible metals. The electrode
can be a coil, a
cylinder, a wire, or any other shape.
Electrode placement can be done in one of two ways: In the preferred
embodiment,
electrodes are advanced to the vicinity of the tissue of the heart where the
angiogenesis is
desired, using the venous system, and left in place. Alternatively, it is
possible to place the
electrodes in place using minimally invasive surgical procedures, which would
allow access to
locations that are beyond the reach of the catheters in the vasculature. In
either case, bipolar or
unipolar stimulation can be applied to generate the electrical fields in the
tissue to trigger the
electrically responsive promoter. Bipolar stimulation is the preferred method.
The placement of the electrodes would be determined primarily by the method
used to
implant the electrodes. If the electrodes are placed via a transvenous route
then the electrodes
should be placed as close as possible to the implanted cells, ischemic tissue,
or target area for
angiogenesis, understanding that patient anatomy may not allow close proximity
of the
electrodes. If a non-transvenous implant technique is used, then the
stimulating electrodes can
usually be placed very close to the ischemic area.
subthreshold stimulation of patients and cells
The subthreshold stimulation provided is sufficient to stimulate angiogenesis
in the
targeted body tissue of a patient. As one embodiment, the pulse generator is
used to modulate
transcription of angiogenic growth factors by the delivery of subthreshold
electrical fields.
The invention also provides a subthreshold pulse generator for a patient in
need of
subthreshold stimulation therapy. In one embodiment, the invention includes a
method for
reducing or repairing tissue injury by providing a means for regulating
angiogenic growth factor
production. In one aspect the pulse generator is effective in delivering
subthreshold pulses that
mediate the repair of injured muscle tissue, such as where ischemic injury has
occurred. The
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
1~
method may be applied to damaged cardiac or peripheral muscle tissue by
providing a
therapeutic stimulus to the surrounding cardiac or muscle tissue or cells. In
an alternative
embodiment, vascular muscle tissue is stimulated using the subthreshold pulse
generator.
Subthreshold stimulation of vascular tissue includes stimulation of arteries
and veins in a patient.
In one feature of the invention, the present system can be used to treat
peripheral arterial
occlusive disease (PAOD) or coronary arterial disease (CAD) or stroke, by
delivery of a
therapeutically effective amount of subthreshold stimulation. It is envisioned
that treatment of
peripheral arterial occlusive disease (PAOD) or coronary arterial disease
(CAD) is achieved by
stimulation of angiogeuc proteins, such as VEGF and FGF, to enhance blood
vessel formation
(angiogenesis).
The present invention also provides a novel method of stimulating cells for
controlled
expression of angiogenic factors. In one preferred embodiment the stimulated
cells are muscle
cells. In another preferred embodiment the cells are muscle cells, and more
preferably, heart,
smooth, or skeletal muscle cells. As a preferred embodiment, subthreshold
pulses are proved to
enhance the cellular production of endogenous angiogenic growth factors of the
transplanted
cells. In an alternative embodiment, the present device can be used in vitro
to pre-stimulate cells
which may then be transplanted to the heart. In this process, cells in culture
are stimulated in a
subthreshold field and used for transplantation. In this process cells may be
taken from the
patient (autologous cell transplantation) or used from a different patient of
the same species
(allogenic cell transplantation) or from a different species (xenogenic cell
transplantation).
Transplanted cells or grafts may be derived from auto-, allo- or xeno-graphic
sources.
Transplanted or grafted cells for heart tissue used with pre- or post
subthreshold stimulation can
be chosen from the group consisting of adult cardiomyocytes, pediatric
cardiomyocytes, fetal
cardiomyocytes, adult fibroblasts, fetal fibroblasts, adult smooth muscle
cells, fetal smooth
muscle cells, endothelial cells, and skeletal myoblasts (see US patentNo.
6,099,832 and
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
18
procedures described herein for isolation of various cell types). A number of
additional
procedures are known and described in the art for isolating various primary
cell types.
Examples
The present invention is further described by the following examples. The
examples are
provided solely to illustrate the invention by reference to specific
embodiments. These
exemplifications, while illustrating certain specific aspects of the
invention, do not portray the
limitations or circumscribe the scope of the invention.
Materials and Assays
Human VEGF in samples was quantified using the Human VEGF Immunoassay by
Quantikine~. The protocol followed was essentially as described in the
Quantikine~
Catalog (Number DVE00). The Human VEGF Immunoassay employs a sandwich enzyme
immunoassay technique. A monoclonal antibody specific for VEGF is pre-coated
on micro-
titer plates. Standards and samples are pipetted into wells and any VEGF
present is bound
by the immobilized antibody. After washing away any unbound substances, an
enzyme-
linked polyclonal antibody specific for VEGF is added to the wells. Following
a wash to
remove any unbound antibody-enzyme reagent, a substrate solution is added to
the wells
and color develops in proportion to the amount of VEGF bound in the initial
step. The color
development is stopped and the intensity of the color is measured.
Example 1:
Sterile 6 well culture plates (Corning) were seeded with cells on six well
culture inserts
using SmGM growth media; CZCl2 cells (mouse myoblasts) were seeded at 7.S x
103/cm2; Human
Coronary Smooth Muscle Cells (HCASMC) were seeded at 2.5 x 103/cm2. After two
days'
growth (confluent), the wells were washed twice with electrical stimulation
medium (DMEM
with 1% bovine serum albumin). 2.0 ml of serum free medium was added but
without any fetal
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
19
bovine serum in the medium. Wells contained approximately 4 ml of total growth
medium,
approximately 2.0 ml inside well insert and 2.0 ml outside well insert. The
cells were electrically
stimulated using a circular graphite electrode for 8 hours per stimulation
condition. Cells were
stimulated at one volt in the stimulation chamber for 1 msec stimulation pulse
width with a 4
msec discharge pulse width. The escape period was adjusted to achieve the
desired frequency.
Samples were harvested after 22 hours post-stimulation. Cell culture
supernatants were removed
from the well. Any debris or floating cells were removed by centrifuging the
supernatants at 300
RPM ~or 5 minutes prior to freezing the samples at -85° C. A cell count
was done on all wells of
the culture plate.
Frozen supernatants were thawed and quantified for the amount of VEGF in the
samples
using the Quantikine Human VEGF Immunoassay. The results (Table 1) indicated
that
subthreshold stimulation increased the amount of VEGF found in the samples.
Table 1
Cells Control 24 Hz 50Hz
(seeding density)(pg/103 cells)(pg/103 cells) (pg/103 cells)
CZC12 0 .104 .0S56
HCASMC
(2 x 104) 0.410 0.360 0.670
(4 x 104) 1.920 1.920 3.140
Example 2: In Vivo Subthreshold Stimulation In Canine Model of Regional
Ischemic
Cardiomyopathy.
Dogs were initially anesthetized with intramuscular morphine sulfate (4
mg/kg)/ A bolus
injection of pentothal (20 mg/kg) was given followed by continuous inhaling of
isoflurane
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
(0.5%-2% in oxygen) after endotracheal intubation. A left lateral thoracotomy
was performed,
and the pericardium opened. A micromanometer pressure transducer (MPC-500,
Millar
Tnstruments, Houston, Texas) was inserted into the left ventricle through an
apical incision. Pairs
of 5 MHz ultrasonic crystals also was implanted in the area supplied by the
distal left anterior
5 descending (LAD) and left circumflex (LCX) arteries just distal to the first
diagonal branch of
measurement of coronary flow. Two heart wire electrodes were inserted in the
LAD perfusion
area. A catheter was inserted into the left atrium for injection of colored 15
um microspheres to
measure regional myocardial blood flow. Ameriod constrictors were placed on
the LAD artery
proximal to the flow probe. All wires and tubing were tunneled subcutaneously
and brought out
10 through the skin of the dorsal neck. The thoracotomy incision was closed in
layers, and a
regimen of broad spectrum antibiotics and analgesia was initiated throughout
the reperfusion
period.
The basic chronology of the experimental protocol after surgery was divided
into three
periods. The first period (recovery period) occurred in the first week after
surgery. After surgery
15 to instnunent the dogs, the dogs were allowed to recover. During week one,
microspheres were
injected for evaluating resting coronary blood flow (CBF) at one week. The
second period
occurred during weeks 2 through 5 after surgery, and included weekly
monitoring of regional
stroke work, CBF (LAD and LCX), and left ventricular pressure (LVP) in
addition to
microsphere injections for evaluating resting CBF. The third period, week 5
through 6 the
20 stimulation occurred for 5 days.
During the six week period, all hemodynamic signals were recorded using an
analog-
digital converter with sampling at 250 Hz. Regional blood flow was assessed
using colored
microspheres (I S uM) to quantify the blood flow in the epi- and
endomyocardium at the end of
the recovery phase (first period), following development of ischemia (second
period), and
following field stimulation (tlurd period). Tissue and reference blood samples
were analyzed in
a Spectra Max 250 Microplate Reader Spectrophotometer. Myocardial blood flow
was
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
21
calculated in the subepicardial and subendocardial region of nonischemic and
ischemic zones.
The potential loss of microspheres from chronic postischemic tissue is
corrected by using a rate
factor of baseline flow in nonischemic tissue applied to ischemic tissue blood
flow data (e,g,
ischemic/nonischemic flow).
At the start of week 6 subthreshold pulsing was delivered to the heart using a
subthreshold stimulator operated at SOHz, O.1V, with 0.3 ms pulses. The
subthreshold stimulator
had an operational range of 0 to 1.5 V output in steps of 0.1 V for all 16
allowed settings, pulse
widths of 0.1, 0.3, 1.0, and 3.0 m by a series of 4 slide switches. As a
result of this pulse output
flexibility, the stimulator also had the ability to determine pacing threshold
and thus the margin
or extent to which the O.1V, SOHz pulses are subthreshold. This is done by
setting the device to
an assessment mode of 1-3 Hz while stepping up the amplitude to look for VOO
pacing capture.
Stimulation was delivered via a set of myocardial pacing wires or "Heart-
Wires" that were
connected to the stimulator through a set of unipolar IS-1 leads to a biopolax
IS-1 comlector. In
addition, the pulse generator contains a combination battery level OIL and
pacing wire continuity
OIL indicator with a visible LED light.
The LAD heart area was stimulated for 5 days during week six before
terminating the
experiment. Sample heart tissue was collected from both stimulated and
unstimulated dogs.
Heart tissue samples were taken and prepared for Western blot analysis using a
VEGF antibody
(Fig. 7). Total protein was extracted from postexperimental cardiac tissue.
100 ug of protein was
loaded onto each lane of a SDS-Page gel, and run for 15 minutes at 200 Volts.
The lanes were
exposed to a polyclonal antibody for human VEGF (Santa Cruz). This antibody
was blocked by a
molecule that blocks the
antibody's binding epitope (data not shown), and hence is specific for the
VEGF site. Lane 8 - 11 = control group. Lane 8 (nonischemic transmural); Lane
9 (ischemic
subepicardium); Lane 10 (ischemic subendocardium); Lane (right ventricle);
Lanes 12 - 1 S are
treatment (field stimulation) group: Lane 12 (transmural nonischemic); Lane 13
(ischemic
CA 02440514 2003-09-04
WO 02/070065 PCT/US02/06800
22
subepicardium); Lane 14 (ischemic subendocardium); Lane 15 (right ventricle).
Bands for VEGF
appear at approximately 26 kD molecular weight. Two bands appear, typical of
the two
fragments seen for VEGF. Notice that bands consistent with VEGF appear only in
Lanes 12 - 15,
i.e. in the treated group. Gels were processed on the same day to avoid
variability.