Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02441099 2009-06-10
52171-14
Title: PROANTHOCYANIDINS FOR THE TREATMENT OF AMYLOID
AND ALPHA-SYNUCLEIN DISEASES
TECHNICAL FIELD
The invention relates to methods and compositions for treatment and prevention
of
amyloid, NAC (i.e. non-amyloid component) and a-synuclein diseases, such as
Alzheimer's
disease and Parkinson's disease, and to method of isolation of new compounds
for the same;
particularly it relates to polyphenolic compositions and methods of using same
to treat these
same diseases; more particularly it relates to proanthocyanidin and related
compounds for
treatment and prevention of amyloid, NAC and a-synuclein diseases.
BACKGROUND OF THE INVENTION
Alzheimer's disease is characterized by the accumulation of a 39-43 amino acid
peptide
termed the beta-amyloid protein or AB, in a fibrillar form, existing as
extracellular amyloid
plaques and as amyloid within the walls of cerebral blood vessels. Fibrillar
AB amyloid
deposition in Alzheimer's disease is believed to be detrimental to the patient
and eventually
leads to toxicity and neuronal cell death, characteristic hallmarks of
Alzheimer's disease.
Accumulating evidence implicates amyloid, and more specifically, the
formation, deposition,
accumulation and/or persistence of AB fibrils, as a major causative factor of
Alzheimer's
disease pathogenesis. In addition, besides Alzheimer's disease, a number of
other amyloid
diseases involve accumulation of AB fibrils, including Down's syndrome,
disorders involving
1
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
congophilic angiopathy, hereditary cerebral hemorrhage of the Dutch type, and
inclusion body
myositosis.
Parkinson's disease is another human disorder characterized by the formation,
deposition, accumulation and/or persistence of abnormal fibrillar protein
deposits that
demonstrate many of the characteristics of amyloid. In Parkinson's disease, an
accumulation of
cytoplasmic Lewy bodies consisting of filaments of a-synuclein/NAC are
believed important in
the pathogenesis and as therapeutic targets. New agents or compounds able to
inhibit a-
synuclein/NAC formation, deposition, accumulation and/or persistence, or
disrupt pre-formed
a-synuclein/NAC fibrils (or portions thereof) are regarded as potential
therapeutics for the
treatment of Parkinson's disease.
A variety of other human diseases also demonstrate amyloid deposition and
usually
involve systemic organs (i.e. organs or tissues lying outside the central
nervous system), with
the amyloid accumulation leading to organ dysfunction or failure. These
amyloid diseases
(discussed below) leading to marked amyloid accumulation in a number of
different organs and
tissues are known as systemic amyloidoses. In other amyloid diseases, single
organs may be
affected such as the pancreas in 90% of patients with type 2 diabetes. In this
type of
amyloidosis, the beta-cells in the islets of Langerhans in pancreas are
believed to be destroyed
by the accumulation of fibrillar amyloid deposits consisting primarily of a
protein known as
islet amyloid polypeptide (IAPP). Inhibiting or reducing such amyloid
accumulation is believed
to lead to new effective treatments for type 2 diabetes. In Alzheimer's
disease, Parkinson's and
"systemic" amyloid diseases, there is currently no cure or effective
treatment, and the patient
usually dies within 3 to 10 years from disease onset.
The amyloid diseases include, but are not limited to, the amyloid associated
with
Alzheimer's disease, Down's syndrome, hereditary cerebral hemorrhage with
amyloidosis of
the Dutch type, and inclusion body myositosis (Askanas et al, Ann. Neurol.
43:521-560, 1993)
(wherein the specific amyloid is referred to as beta-amyloid protein or A13),
the amyloid
associated with chronic inflammation, various forms of malignancy and Familial
Mediterranean
Fever (wherein the specific amyloid is referred to as AA amyloid or
inflammation-associated
amyloidosis), the amyloid associated with multiple myeloma and other B-cell
dyscrasias
(wherein the specific amyloid is referred to as AL amyloid), the amyloid
associated with type II
diabetes (wherein the specific amyloid protein is referred to as amylin or
islet amyloid
2
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
polypeptide), the amyloid associated with the prion diseases including
Creutzfeldt-Jakob
disease, Gerstmann-Straussler syndrome, kuru and animal scrapie (wherein the
specific amyloid
is referred to as PrP amyloid), the amyloid associated with long-term
hemodialysis and carpal
tunnel syndrome (wherein the specific amyloid is referred to as beta2-
microglobulin amyloid),
the amyloid associated with senile cardiac amyloid and Familial Amyloidotic
Polyneuropathy
(wherein the specific amyloid is referred to as transthyretin or prealbumin),
and the amyloid
associated with endocrine tumors such as medullary carcinoma of the thyroid
(wherein the
specific amyloid is referred to as variants of procalcitonin). In addition,
the a-synuclein protein
which forms fibrils, and is Congo red and Thioflavin S positive, is found as
part of Lewy bodies
in the brains of patients with Parkinson's disease, Lewy body disease (Lewy in
Handbuch der
Neurologie, M. Lewandowski, ed., Springer, Berline pp.920-933, 1912; Pollanen
et al, J.
Neuropath. Exp. Neurol. 52:183-191, 1993; Spillantini et al, Proc. Natl. Acad.
Sci. USA
95:6469-6473, 1998; Arai et al, Neurosc. Lett. 259:83-86, 1999), and multiple
system atrophy
(Wakabayashi et al, Acta Neuropath. 96:445-452, 1998). For purposes of this
disclosure,
Parkinson's disease, due to the fact that fibrils develop in the brains of
patients with this disease
(which are Congo red and Thioflavin S positive, and which contain predominant
beta-pleated
sheet secondary structure), should be regarded as a disease that also displays
the characteristics
of an amyloid-like disease.
Discovery and identification of new compounds or agents as potential
therapeutics to
arrest amyloid formation, deposition, accumulation and/or persistence that
occurs in
Alzheimer's disease, Parkinson's disease, type II diabetes, systemic AA
amyloidosis, and other
amyloidoses are desperately sought.
Polyphenols are an incredibly diverse group of compounds (Ferreira et al,
Tetrahedron
48:1743-1803,1992) that widely occur in a variety of plants, some of which
enter into our food
chain. Although some of the polyphenols are considered to be nonnutritive,
interest in these
compounds has arisen because of their possible beneficial effects for health.
For example,
quercetin (a flavanoid) has been shown to possess anticarcinogenic activity in
experimental
studies (Kato et al, Carcinogenesis 4:1301-1305, 1983; Deschner et al,
Carcinogenesis 7:1193-
1196, 1991). Catechin and epicatechin (flavan-3-ols) have been shown to
inhibit Leukemia
virus reverse transcriptase activity (Chu et al, J. Natural Prods. 55:179-183,
1992). Statistical
reports have shown that stomach cancer mortality is significantly lower in the
tea producing
3
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
districts of Japan. Epigallocatechin gallate has been reported to be the
pharmacologically active
material in green tea that inhibits mouse skin tumors (Mimoto et al, Carcino
eg nesis 21:915-919,
2000). Ellagic acid has also been shown to possess anticarcinogenic activity
in various animal
tumor models (Inoue et al, Biol. Pharm. Bull. 18:1526-1530, 1995). However,
none of the
literature teaches or suggests that proanthocyanidins, and procyanidins,
particularly epicatechin-
epicatechin dimers or trimers or other oligomers, epicatechin-catechin dimers
or the like, or
analogs or derivatives thereof, have any benefit for the inhibition of amyloid
or a-synuclein/
NAC fibril formation, and/or cause a disruption of pre-formed amyloid or a-
synuclein/NAC
fibrils.
DISCLOSURE OF THE INVENTION
Methods pertaining to the isolation, identification and use of anti-amyloid
compounds
derived from plant material, and the surprising discovery that
proanthocyanidins are potent
inhibitors of amyloid and a-synuclein/NAC fibrillogenesis, and cause a potent
disruption/
disassembly of pre-formed fibrils for a variety of amyloid and a-synuclein
diseases are
disclosed. Exemplary compounds are identified to serve as potent amyloid
fibril inhibiting
agents, including procyanidins, such as epicatechin-epicatechin, catechin-
epicatechin dimers,
epiafzelechin-epicatechin dimers, epicatechin-epicatechin-epicatechin trimers,
as well as other
epicatechin and/or catechin oligomers for the treatment of amyloid diseases
including, but not
limited to, Alzheimer's disease, type II diabetes, and systemic AA
amyloidosis, as well as
inhibiting a-synuclein or non-amyloid component (NAC) fibril formation for the
treatment of
Parkinson's and Lewy body disease.
Also disclosed are methods for preparing and isolating such compounds, as well
as new
uses for them, especially as amyloid and a-synuclein/NAC fibril disrupting
agents. This
invention is also directed to methods for inhibiting or eliminating amyloid
fibril formation,
deposition, accumulation and/or persistence in a number of different amyloid
diseases by
treatment of patients with proanthocyanidins, such as procyanidins of the A, B
and C types, or
other monomers, dimers, trimers and multimers of epicatechin and catechin.
Exemplary
compounds are substituted epicatechin-epicatechin or catechin-epicatechin
dimers, such as
epicatechin-413--8-epicatechin or catechin-4a-48-epicatechin, and
epiafzelechin-4B--8-
epicatechin, or other proanthocyanidin oligomers.
4
CA 02441099 2010-02-08
52171-14
Also disclosed are methods of isolation, identification and use of amyloid-
inhibiting
compounds derived from plant material for the therapeutic intervention of
Alzheimer's disease,
type II diabetes, Parkinson's disease, systemic AA amyloidosis and other
disorders involving
amyloid fibril accumulation; more particularly, it relates to methods of
isolating amyloid-
inhibiting compounds from Uncaria tomentosa and related plants, and other
known
proanthocyanidin producing plants, and to the use of those compounds.
A surprising discovery is noted that specific extraction methods (and
individual
compounds derived from such extraction methods) when applied to the inner bark
and root parts
of Uncaria tomentosa, otherwise known as Una de Gato (or Cat's claw), lead to
the isolation
and purification of single compounds (such as "compound H2" identified as an
epicatechin-
epicatechin dimer; "compound Hl" identified as a catechin-epicatechin dieter,
"compound K2"
identified as an epicatechin-epicatechin-epicatechin trimer), and "compound
KI" identified as a
epiafzelechin-epicatechin dimer, all of which act as impressive inhibitors of
Alzheimer's
disease beta-amyloid protein (AB) formation and growth, Parkinson's disease ac-
synuclein fibril
formation and growth, and causes disruption/ dissolution of pre-formed
Alzheimer's,
Parkinson's and type II diabetes fibrils.
Previously our studies have led to the identification of a natural substance
derived from
the Amazon rain forest woody vine, Uncaria tonzentosa, and referred to as PTI-
00703.
See for instance US Patent Nos. 6,939,570; 6,346,280 and 6,264,994, which
describe the initial discovery of derivatives of Uncaria tomentosa and related
plant matenat
extracts as inhibitors of amyloidosis of Alzheimer's disease, type II diabetes
and other arnyloid
disorders. This was followed up by the parent application to this case,
which used assay-guided affinity fractionation
and reverse phase high pressure liquid chromatography (HPLC) methodology to
isolate, test and
characterize the most active water-soluble ingredients within PTI-00703
(collectively referred to
as "PTI-777") that appear to account for the majority of the AB
fibrillogenesis inhibitory
activity.
In these latter disclosures, it is discussed how PTI-777 and its individual
fractions as
isolated by HPLC were tested in relevant in vitro and/or animal models, and
found to
consistently demonstrate inhibition of AB fibrillogenesis. Also described were
extraction
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
methods for the isolation of PTI-777 and its individual fractions and/or
components. Further
purification and in vitro testing of each of the PTI-777 compounds, as well as
initial structural
characterization studies suggested that the amyloid inhibitor compounds
derived from Uncaria
tomentosa are small molecules (-200-500 molecular weight) that belong to the
general class of
aromatic polyphenolic compounds. Two such compounds, chlorogenic acid
(C16H1809; FW
354.3 1)(earlier referred to as "Fraction F") and epicatechin (C15H1406; FW
290.27)(earlier
referred to as "Fraction J") were purified and identified by analytical
techniques. In addition,
data indicates that "fraction H" isolated from PTI-777 was a most potent
inhibitor of amyloid
fibrillogenesis. In addition, PTI-777 has the ability to enter the brain as
demonstrated by
radiolabelling experiments, indicating that it has the potential to be useful
as a therapeutic agent
for Alzheimer's disease, Parkinson's disease, and other central nervous system
disorders
involving deposition and accumulation of fibrillar proteins, such as type 2
diabetes and systemic
AA amyloidosis.
We have now further purified, isolated and identified additional major
components of
PTI-777, and demonstrated a further surprising discovery that such single
compounds (which
belong to the general class of proanthocyanidins) are potent amyloid
inhibiting agents.
Compound H2, by mass spectroscopy studies, was shown to be a major component
of PTI-777,
and purified, and finally identified (as described herein) as epicatechin-4B-8-
epicatechin, also
known as procyanidin B2. Compound HI, also a major component of PTI-777, was
purified and
identified (as described herein) as catechin-4a--->8-epicatechin, also known
as procyanidin B4.
Compound K2, a component of PTI-777, was purified and identified as
epicatechin-4B--8-
epicatechin-43--8-epicatechin, also known as procyanidin C1. Compound Ki, a
component of
PTI-777, was purified and identified as epiafzelechin-43-8-epicatechin.
Efficacy of each of these proanthocyanidins as a potent inhibitor of
Alzheimer's AB
amyloidosis, Parkinson's disease a-synuclein/NAC fibrillogenesis, and type II
diabetes IAPP
fibrillogenesis, is disclosed herein, and supports the conclusion that
procyanidins in particular,
and proanthocyanidins in general, are useful compounds for the treatment of
amyloidosis and
related fibrillogenesis associated with Alzheimer's disease, Parkinson's
disease, type 2 diabetes,
systemic AA amyloidosis and other amyloid diseases.
A method of treating a human or other a mammal suffering from, or subject to,
an
amyloid disease, or any disease characterized by a-synuclein or NAC
fibrillogenesis is
6
CA 02441099 2009-06-10
52171-14
disclosed. Any mammal may be the subject or the disease or condition, or
simply
be a mammal that is subject to the disease or condition. Amyloid disease as
used
herein includes but is not limited to the various known and disclosed
amyloidoses
discussed herein. A disclosed treatment for an amyloid disease is intended to
cover a like treatment for the corresponding amyloidosis, and vice-versa. The
same is true for a-synuclein diseases. The term "fibrillogenesis" refers to
the fibril,
plaque and tangle-like forming propensities of the various substituent
proteins
and/or precursor proteins disclosed herein, whether or not any particular
degree of
fibrillogenesis has progressed, or is expected to progress, to any particular
recognized amyloidosis or to an amyloid or a-synuclein disease. In general,
treatment of fibrillogenesis as disclosed is intended to include and cover
treatment
of any amyloidosis or any amyloid or a-synuclein disease corresponding to,
following from, otherwise related to, that fibrillogenesis.
According to another aspect of the present invention, there is
provided use of a therapeutically effective amount of epicatechin-4p -- >8-
epicatechin in the treatment of amyloid, a-synuclein or NAC fibrillogenesis in
a
subject.
According to still another aspect of the present invention, there is
provided use of a therapeutically effective amount of epicatechin-43->8-
epicatechin in the manufacture of a medicament for the treatment of amyloid,
a-synuclein or NAC fibrillogenesis in a subject.
According to yet another aspect of the present invention, there is
provided a pharmaceutical composition for treating amyloid, a-synuclein or
NAC fibrillogenesis in a subject, comprising a therapeutically effective
amount of
epicatechin-4(3-*8-epicatechin and a pharmaceutically acceptable carrier or
diluent.
According to a further aspect of the present invention, there is
provided a commercial package comprising epicatechin-4(3-->8-epicatechin,
together with instructions for the use thereof in the treatment of amyloid,
a-synuclein or NAC fibrillogenesis.
7
CA 02441099 2009-06-10
52171-14
"Treatment" is also intended in every possible instance to include
and cover "in vitro treatment", whether for experimental or screening purposes
and
the like, and whether or not the in vitro treatment leads to, or is ever
intended to
lead to, treatment of a like fibrillogenesis, or any amyloid or a-synuclein
disease
corresponding to that fibrillogenesis, in a mammalian subject.
The method includes administering to the mammal a therapeutically
effective amount of any proanthocyanidin, or proanthocyanidin compound, that
may be found in the group of proanthocyanidin and proanthocyanidin compounds
characterized by either Formula I or Formula II, or both (see Figures 54-56).
The
group also includes proanthocyanidins characterized by oligomeric combinations
of Formula I and Formula II (see Figure 56), and also includes any
pharmaceutically acceptable salt of any of the foregoing proanthocyanidins.
As discussed in more detail elsewhere herein, proanthocyanidins
(also referred to herein as PA) include a variety of structural shapes and
oligomeric forms. Formulae I and II are intended each to represent one general
form of an oligomeric unit effective to make up the various disclosed
oligomers.
For instance some proanthocyanidin oligomers are well characterized by
Formula I, which is to say that the general structure stated by Formula I is a
valid
generalization of each unit of the oligomer. An example of a proanthocyanidin
characterized by Formula I is epicatechin-40-8-epicatechin, a dimer where two
epicatechin units, each a particular instance of, and conforming to, Formula
I, are
joined from the 4 carbon
7a
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
atom of one unit, to the 8 carbon atom of the other unit, thus effecting a so-
called 4-8 linkage.
In like manner, proanthocyanidin oligomers entirely characterized by Formula
II will have all
their units joined from the 4 carbon atom of one unit to the 6 carbon atom of
the adjacent unit,
thus effecting a so-called 4-6 linkage, and so on.
Some proanthocyanidins can not be well characterized by only Formula I or
Formula II,
in fact presenting in one unit a Formula I configuration and in another unit a
Formula II
configuration. For instance, a proanthocyanidin having a unit in a 4-6 linkage
to a second unit
which itself has a 4-8 linkage to a third unit is not strictly either a
Formula I or a Formula II
compound, but is actually an oligomeric combination of Formula I and Formula
II, where
among other characteristics, each unit may be susceptible of more than one
characterization
(viz. Formula I or Formula II). In the example just given, the middle of the
three units has both
it's 4 carbon and 6 carbon link sites filled and is therefore a Formula II
unit (it may not be
possible to specify with particularity what the first unit is, since as
terminal .unit it has only one
carbon linked); the third unit however has it's 8 carbon link site filled and
whether or not it is a
terminal unit, it will most likely conform to Formula I (except in the
relatively rare occurrence
of the third unit itself linking at it's 6 carbon site to a fourth unit,
making it a unit with it's 6
and 8 carbon link sites filled - which fits neither formula, though it may be
nonetheless a useful
compound for treatment). Thus, an oligomer with some units 4-6 (or 6-4) linked
and some 4-8
(or 8-4 linked) will show units in both Formula I and Formula II
configurations, but be neither
a Formula I nor a Formula II compound, but will be instead an oligomeric
combination of
Formula I and Formula II.
Based upon observations discussed herein, it is believed that
proanthocyanidins
characterized as above, or elsewhere herein, particularly with oligomer units
numbering in the
range of 2 - 20 (that is, where n in either Formula is an integer value from 2
to 20) will all be
efficacious to some significant degree for treating the diseases and
conditions disclosed herein.
It is believed as well, though not discussed to any extent, and also based
upon observations
discussed herein, that proanthocyanidins not precisely adhering to the above
formulaic
prescriptions (such as the presence of one or more variant linkages, like the
aforementioned 6-8
or 8-6 unit linkage, or even a 6-6 unit linkage) will also be efficacious to
some significant
degree for treating the diseases and conditions disclosed herein.
8
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
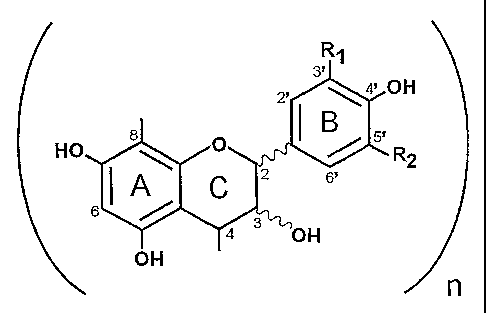
In the formulae shown, R, and R2 are independently selected from hydrogen and
hydroxy; R3 is selected from the group consisting of hydrogen, optionally
substituted 0-
glycosyl, -C(O)-(optionally substituted aryl), and -C(O)-(optionally
substituted heteroaryl);
and R4 is selected from the group consisting of hydrogen, catechin,
epicatechin, epiafzelechin,
and gallates of catechin and epicatechin. The lines at the 2-, 3- and 4-
position denote optional
R and S (sometimes and alternatively referred to as a and 13) stereochemical
configurations.
Generally the configuration at the 4-position is trans to the configuration at
the 3-position. The
lines at the 4- and 8-positions in Formula I and at the 4- and 6- positions in
Formula II denote
possible oligomer bonds between individual units as earlier discussed, and the
each of the
substitutions at R,, R2, R3, and R4, and each of the configurations at the 2-,
3-, and 4-positions,
and each of the oligomer bond configurations of 4-8 and 4-6 are independently
selected for
each individual unit and may be different for unit in the oligomer series of
units, though often
the units of shorter oligomers are homogenous with one another.
Preferred oligomers with have from 2 to 5 units, or even 2-3 units. The chiral
configuration at each 2- carbon position is preferably R as opposed to S. In
some embodiments
some or all of the units have an R3 that is either hydrogen, 2,3-
dihydroxybenzoyl, 3,4-
dihydroxybenzoyl; 2,3,4-trihydroxybenzoyl, or 3,4,5-trihydroxybenzoyl, and
preferably each
R3 is hydrogen and each R, is hydroxy and each R2 is hydrogen. R3 may also be
an optionally
substituted 0-glycosyl.
Another method of treatment of an amyloid disease, or a disease characterized
by a-
synuclein or NAC fibrillogenesis, in a mammalian subject, is also disclosed.
The method
includes the step of administering to the subject a therapeutically effective
amount of a
proanthocyanidin. The proanthocyanidin is preferably a procyanidin oligomer
having from 2
to 20, and more preferably 2-5, flavanoid units. Each flavanoid unit can
advantageously be
one of the catechins, including catechin, epicatechin, epiafzelechin,
gallocatechin,
galloepicatechin, epigallocatechin and the gallates of the catechins. The
flavanoid unit can also
be one of the flavanols, flavonols, flavandiols, leucocyanidins, or
anthocyanidins.
The particular amyloid disease to be treated can be Alzheimer's disease,
Down's
syndrome, hereditary cerebral hemorrhage with amyloidosis of the Dutch type,
inclusion body
myositosis, the amyloidosis of chronic inflammation, the amyloidosis of
malignancy and
Familial Mediterranean Fever, the amyloidosis of multiple myeloma and B-cell
dyscraisa, the
9
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
amyloidosis of type 2 diabetes, the amyloidosis of prion diseases, Creutzfeldt-
Jakob disease,
Gerstmann-Straussler syndrome, kuru, scrapie, mad cow disease, the amyloidosis
associated
with long-term hemodialysis, the amyloidosis with carpal tunnel syndrome,
senile cardiac
amyloidosis, Familial Amyloidotic Polyneuropathy, the amyloidosis associated
with endocrine
tumors, systemic AA amyloidosis, AL amyloidosis, AB amyloidosis or PrP
amyloidosis, but
particularly Alzheimer's disease.
The particular a-synuclein or NAC fibrillogenesis to be treated can be the
fibrillogenesis associated with Lewy body disease, Parkinson's disease or
multiple system
atrophy.
A method of treatment of amyloid, a-synuclein or NAC fibrillogenesis, in an in
vitro
environment, is also disclosed. The method includes the step of administering
into the in vitro
environment a therapeutically effective amount of a proanthocyanidin.
Preferably the
proanthocyanidin is a procyanidin which is an oligomer of any or all of
epicatechin, catechin,
epiafzelechin, epicatechin gallates or catechin gallates.
The procyanidin may advantageously be a procyanidin that is an A, B or C type
procyanidin. The procyanidin is preferably a dimer or trimer of epicatechin
and/or catechin
units, such as the dimers of the type B1, B2, B3, B4, B5, B6, B7, and B8 type
procyanidins. In
one embodiment, the procyanidin dimer is epicatechin-4B->8-epicatechin; in
another
embodiment, the procyanidin dimer is catechin-4cc->8-epicatechin; in still
another, the
procyanidin is the epicatechin trimer epicatechin-4B-->8-epicatechin-413-->8-
epicatechin; in yet
another embodiment the procyanidin is the dimer epiafzelechin-4B-~8-
epicatechin.
The method may also include an administration step to deliver the procyanidin
to the
subject by way of oral administration, parenteral injection, intraperitoneal
injection,
intravenous injection, subcutaneous injection, intramuscular injection,
topical administration,
or aerosol spray administration.
A pharmaceutical composition or agent is also disclosed. It is a
therapeutically
effective amount of a proanthocyanidin (PA) together with a pharmaceutically
acceptable
carrier, diluent, or excipient, or the like. The therapeutic amount of the PA
is selected for
efficacy in treating an amyloid, a-synuclein or NAC fibrillogenesis in a
mammalian subject.
Disclosed compositions are delivered in therapeutic dosages in the range of
about 10 mg/kg to
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
1,000 mg/kg of body weight of the subject, and preferably in the range of
about 10 mg/kg to
100 mg/kg of body weight of the subject.
The proanthocyanidin is preferably epicatechin or one or more of the dimers
and
trimers of epicatechin and catechin, or a mixture thereof, as well as the
pharmaceutically
acceptable analogs and derivatives thereof. Preferred proanthocyanidins are
the procyanidin
dimer epicatechin-4B->8-epicatechin, the procyanidin dimer catechin-4a->8-
epicatechin, the
procyanidin dimer epiafzelechin-4B-48,epicatechin, and the procyanidin trimer
epicatechin-
4B-48-epicatechin-4B- 48-epicatechin.
When the composition is a mixture of two or more proanthocyanidins, they may
also be
advantageously selected from epicatechin and the dimers and trimers of
epicatechin and
catechin and the pharmaceutically acceptable analogs and derivatives of these
compounds. It
is believed that a mixture of two or more procyanidins such as the dimers and
trimers of
epicatechin and catechin and/or their pharmaceutically acceptable analogs and
derivatives may
be employed to therapeutic advantage, and in particular, a mixture of two or
more
proanthocyanidins such as epicatechin-4B--.8-epicatechin, catechin-4a-8-
epicatechin,
epiafzelechin-4B->8-epicatechin, and epicatechin-41-48-epicatechin-4B-8-
epicatechin. It is
believed that a mixture of substantially pure proanthocyanidins as a
pharmaceutical
composition is especially advantageous and has not been earlier suggested in
the art.
Disclosed compositions contain one or more proanthocyanidins, each
proanthocyanidin
present in the composition in a proportion percentage or percentage purity
that "significantly
exceeds" a proportion percentage of the same proanthocyanidin's natural
presence in a plant,
or in an extract from the plant. For example, suppose that a particular
proanthocyanidin is
present in a plant in a percentage by weight of 0.01 percent, and is present
in an extract of the
plant in a percentage by weight of 1.0 percent. In a disclosed composition
then, the same
proanthocyanidin is present in the composition in a percentage by weight that
is significantly
greater than 0.01 percent or 1.0 percent, say 10 percent. Other
proportionalities along this line
may be applied as well, such as percentage composition or percentage presence
by volume, or
percent purity. By way of further example, without limiting the scope of
invention to this or
other examples, a PA is present in a tablet to be delivered orally in
accordance with the
disclosure herein. The PA is an isolated PA present in a percentage purity of
98.5% (that is, the
PA is 98.5% pure, as measured by conventional purity indicia, such as for
example the
11
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
characteristic single sharp peak band on an HPLC). The particular PA is
however only a 15%
ingredient by weight in the tablet. The PA is known to be present in a fruit
in a dry weight
percentage of 0.06, while certain fruit extracts are known to contain up to
0.75 percent dry
weight percent of the same PA. In this example, the PA is proportionally more
present in the
tablet than in the extract by a ratio of 20:1, and this is one measure of
significantly exceeding
the natural proportion percentage presence in a plant or extract of the plant.
In general, a PA present in a therapeutically administered dose form that has
a
percentage of the PA (by weight, dry weight, volume, or purity) that is 10
times (or more)
greater than the natural percentage presence of the same PA in a plant is a
percentage that
"significantly exceeds" the natural percentage presence of the PA in the
plant. When speaking
of extracts of a plant in this context, it should be noted that only
conventional or natural
extracts are to be considered (juices, concentrates and the like, or extracts
known and used for
other purposes), not new extracts prepared after the priority date of this
disclosure the effect of
which is to concentrate the particular PA so as to negative a finding of
"significantly
exceeding", as just defined. It should also be noted that in some cases, a
finding of
"significantly exceeding" may be justified with a ratio of as little as 2:1,
but more preferably as
great as 50:1 to 100:1.
In the example of a single PA compound with an excipient to make up the
composition,
it may be convenient merely to note and compare the percent purity of the
compound in the
composition, rather than its overall weight percentage, for purposes of the
"significantly
exceeding" standard, as claimed. In the case of mixtures of PA's it can be
appropriate to view
a combined percentage composition of the mixed PA's in the therapeutic dosage
and compare
that figure to a combined percentage presence of the same PA's in the natural
plant or extract.
In any event, the purpose of the disclosed standard of measurement is to set
forth a fair
margin by which a claimed composition exceeds reading on the active
ingredients' natural
occurrence in plants and conventional extracts of plants.
Preferred compositions will contain proanthocyanidin that is at least
substantially pure.
Proanthocyanidin that is in substantially pure isolated or synthetic form may
be advantageously
employed as well. In general "pure" means better than 95% pure, and
"substantially pure" PA
means a PA purified by extraction or other known means or means disclosed
herein such that
the PA is present in the therapeutic dosage with only those impurities that
can not readily nor
12
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
reasonably be removed by the extraction or purification processes. "Isolated"
means that the
PA in question is not accompanied in the therapeutic form by significant
quantities of other
PA's. An "isolated pure" compound is a compound in isolated purified form such
as is
conventional for active ingredients in the pharmaceutical industry.
Methods of isolation of a proanthocyanidin from a plant material containing
proanthocyanidins are disclosed. One method includes a) dissolving the plant
material with
methanol or the like non-polar solvent, b) loading the methanol-extracted
plant material onto a
silica gel column, c) eluting the column with a series of increasing
proportions of methanol in
chloroform to elute the proanthocyanidins, d) separating the proanthocyanidins
in the extract
by reverse phase HPLC, and e) collecting and freeze drying the separated and
isolated
proanthocyanidin, now deemed thereby to be "pure". The series of methanol in
chloroform
elutions will beneficially include at least elutions of 10% methanol in
chloroform, 20%
methanol in chloroform, 40% methanol in chloroform, 50% methanol, and 100%
methanol in
chloroform. A preferred plant material is derived from Uncaria tomentosa.
A proanthocyanidin composition made from the disclosed isolation process is
also
disclosed. The composition eluted from the silica gel column with the 20%
methanol in
chloroform step of the series will contain primarily procyanidin dimers and
trimers; the
proanthocyanidin composition eluted from the silica gel column with the 40%
methanol in
chloroform step will contain primarily procyanidin trimers and tetramers; the
composition
eluted from the silica gel column with the 50% methanol in chloroform step
will contain
primarily procyanidin trimers, tetramers, pentamers, and hexamers; and the
composition eluted
from the silica gel column with the 100% methanol in chloroform step will
contain primarily
procyanidins tetramers, pentamers, hexamers, and oligomers of greater than six
units.
A second isolation method includes a) dissolving the plant material with
ethanol or the
like non-polar solvent, b) loading the ethanol-extracted plant material onto a
LH20 column, c)
eluting the column with ethanol, followed by a series of increasing
proportions of acetone in
ethanol (and/or methanol) to elute the proanthocyanidins, d) separating the
proanthocyanidins
in the extract by reverse phase HPLC, and e) collecting and freeze drying the
separated and
isolated proanthocyanidin, now deemed thereby to be "pure". The series of
acetone in ethanol
(and/or methanol) elutions will beneficially include at least elutions of 5%
acetone in ethanol,
13
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
10% acetone in ethanol, 50% acetone in ethanol, 50% acetone in methanol and
100%
methanol. A preferred plant material is derived from Uncaria tomentosa.
A proanthocyanidin composition made from the second disclosed isolation
process is
also disclosed. The composition eluted from the LH 20 column with ethanol will
contain
primarily procyanidin dimers and trimers; the proanthocyanidin composition
eluted from the
LH2O column with the 5% acetone in ethanol step will contain primarily
procyanidin dimers
and trimers; the proanthocyanidin composition eluted from the LH2O column with
the 10%
acetone in ethanol step will contain primarily procyanidin dimers and trimers;
the
proanthocyanidin composition eluted from the LH2O column with the 50% acetone
in ethanol
step will contain primarily procyanidin dimers, trimers, and tetramers; the
proanthocyanidin
composition eluted from the LH2O column with the 50% acetone in methanol step
will contain
primarily procyanidin trimers, tetramers, pentamers, hexamers and oligomers of
greater than
six units; and the proanthocyanidin composition eluted from the LH2O column
with the 100%
methanol step will contain primarily procyanidin trimers, tetramers,
pentamers, hexamers and
oligomers of greater than six units.
A further method of treatment of an amyloid disease, or a disease
characterized by a-
synuclein or NAC fibrillogenesis, in a mammalian subject, is also disclosed.
The method
includes administering to the subject a therapeutically effective amount of
the
proanthocyanidin isolated by way of the disclosed isolation process.
In disclosed methods, the amyloid disease for treatment is selected from the
group
consisting of amyloid diseases associated with Alzheimer's disease, Down's
syndrome,
hereditary cerebral hemorrhage with amyloidosis of the Dutch type, inclusion
body myositosis,
the amyloidosis associated with type 2 diabetes, the amyloidosis associated
with chronic
inflammation, various forms of malignancy, and Familial Mediterranean Fever,
the
amyloidosis associated with multiple myeloma and other B-cell dyscrasias, the
amyloidosis
associated with the prion diseases including Creutzfeldt-Jakob disease,
Gerstmann-Strausller
syndrome, kuru, animal scrapie, and mad cow disease, the amyloidosis
associated with long-
term hemodialysis and carpal tunnel syndrome, the amyloidosis associated with
endocrine
tumors such as medullary carcinoma of the thyroid, and the a-synuclein disease
is selected
from the group consisting of Parkinson's disease, Lewy body disease and
multiple system
atrophy.
14
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Another composition is disclosed as well. It includes a pharmaceutically
acceptable
carrier, diluent, or excipient, or the like, and a proanthocyanidin (PA), or
proanthocyanidin
compound, that may be found in the group of proanthocyanidin and
proanthocyanidin
compounds characterized by either Formula I or Formula II, or both (see
Figures 54-56). The
group also includes proanthocyanidins characterized by oligomeric combinations
of Formula I
and Formula II (see Figure 56), and also includes any pharmaceutically
acceptable salt of any
of the foregoing proanthocyanidins. In the formula, n is an integer in the
range of 2 to 20 and
preferably 2-5 or even 2-3.
The PA is selectably present in the composition in an amount effective to
treat an
amyloid disease, or a disease characterized by a-synuclein or NAC
fibrillogenesis, in a
mammalian subject.
In the formulae shown, R, and R2 are independently selected from hydrogen and
hydroxy; R3 is selected from the group consisting of hydrogen, optionally
substituted 0-
glycosyl, -C(O)-(optionally substituted aryl), and -C(O)-(optionally
substituted heteroaryl);
and R4 is selected from the group consisting of hydrogen, catechin,
epicatechin, and gallates of
catechin and epicatechin. The lines at the 2-, 3- and 4-position denote
optional R and S
(sometimes and alternatively referred to as (x and 13) stereochemical
configurations. Generally
the configuration at the 4-position is trans to the configuration at the 3-
position. The lines at the
4- and 8-positions in Formula I and at the 4- and 6- positions in Formula II
denote possible
oligomer bonds between individual units as earlier discussed, and the each of
the substitutions
at R1, R2, R3, and R4, and each of the configurations at the 2-, 3-, and 4-
positions, and each of
the oligomer bond configurations of 4-8 and 4-6 are independently selected for
each individual
unit and may be different for unit in the oligomer series of units, though
often the units of
shorter oligomers are homogenous with one another.
The invention is described with reference to specific embodiments, plant
species and
parts, methods, procedures and the like. However, it will be recognized by
those skilled in the
art that various chemical substitutions can be made within the disclosed
compounds without
departing from the spirit and scope of the invention. In particular, it is
known that polyphenols
including flavanoids, procyanidins and proanthocyanidins can be isolated
and/or purified from
plant materials by a number of different methods. It will further be
recognized that these
alternate methods, and consequent changes in other steps of the method, such
as use of different
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
solvents or different columns for purification, and of procyanidins and
proanthocyanidins from
a composition of partially purified polyphenols, fall within the scope of the
presently disclosed
plant-derived extracts, and compounds derived thereof.
New methods for the treatment of the amyloid diseases are disclosed. The
amyloid
diseases include, but are not limited to, the amyloid associated with
Alzheimer's disease,
Down's syndrome, hereditary cerebral hemorrhage with amyloidosis of the Dutch
type,
inclusion body myositosis (wherein the specific amyloid is referred to as beta-
amyloid protein
or AB), the amyloid associated with chronic inflammation, various forms of
malignancy and
Familial Mediterranean Fever (wherein the specific amyloid is referred to as
AA amyloid or
inflammation-associated amyloidosis), the amyloid associated with multiple
myeloma and other
B-cell dyscrasias (wherein the specific amyloid is referred to as AL amyloid),
the amyloid
associated with type II diabetes (wherein the specific amyloid protein is
referred to as amylin or
islet amyloid polypeptide), the amyloid associated with the prion diseases
including Creutzfeldt-
Jakob disease, Gerstmann-Straussler syndrome, kuru and animal scrapie (wherein
the specific
amyloid is referred to as PrP amyloid), the amyloid associated with long-term
hemodialysis and
carpal tunnel syndrome (wherein the specific amyloid is referred to as beta2-
microglobulin
amyloid), the amyloid associated with senile cardiac amyloid and Familial
Amyloidotic
Polyneuropathy (wherein the specific amyloid is referred to as transthyretin
or prealbumin), and
the amyloid associated with endocrine tumors such as medullary carcinoma of
the thyroid
(wherein the specific amyloid is referred to as variants of procalcitonin). In
addition, the a-
synuclein protein which forms fibrils, and is Congo red and Thioflavin S
positive, is found as
part of Lewy bodies in the brains of patients with Parkinson's disease, Lewy
body disease
(Lewy in Handbuch der Neurologie, M. Lewandowski, ed., Springer, Berline
pp.920-933, 1912;
Pollanen et al, J. Neuropath. Exp. Neurol. 52:183-191, 1993; Spillantini et
al, Proc. Natl. Acad.
Sci. USA 95:6469-6473, 1998; Arai et al, Neurosc. Lett. 259:83-86, 1999), and
multiple system
atrophy. For purposes of this disclosure, Parkinson's disease, due to the fact
that fibrils develop
in the brains of patients with this disease (which are Congo red and
Thioflavin S positive, and
which contain predominant beta-pleated sheet secondary structure), should be
regarded as a
disease that also displays the characteristics of an amyloid-like disease.
Use of the inner bark and/or roots from Uncaria tomentosa (also referred to as
Una de
Gato or Cat's claw) to isolate and use the amyloid inhibiting compounds for
the treatment of
16
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
amyloid formation, deposition, accumulation and/or persistence in Alzheimer's
disease, type II
diabetes other amyloidoses, and Parkinson's disease are disclosed. Una de Gato
or Cat's claw is
also referred to as, but not limited to, Paraguayo, Garabato, Garbato casha,
Tambor huasca, Una
de gavilan, Hawk's claw, Nail of Cat, and Nail of Cat Schuler.
Use of extracts and/or compound derivatives thereof from plant matter related
to the
Rubiciaceae family, which includes but is not limited to the Uncaria genus,
for the treatment of
amyloid formation, deposition, accumulation and/or persistence in Alzheimer's
disease, type II
diabetes, other amyloidoses and Parkinson's disease are disclosed.
Use of extracts and/or compounds derived thereof from plant matter related to
the
various Uncaria species, which may include but not limited to, Uncaria
tomentosa, Uncaria
attenuata, Uncaria elliptica, Uncaria guianensis, Uncaria pteropoda, Uncaria
bernaysli,
Uncariaferra DC, Uncaria kawakamii, Uncaria rhyncophylla, Uncaria calophylla,
Uncaria
gambir, and Uncaria orientalis are also disclosed.
Use of commercially available pills, tablets, caplets, soft and hard gelatin
capsules,
lozenges, sachets, cachets, vegicaps, liquid drops, elixers, suspensions,
emulsions, solutions,
syrups, tea bags, aerosols (as a solid or in a liquid medium), suppositories,
sterile injectable
solutions, sterile packaged powders, bark bundles and/or bark powder which
contain Uncaria
tomentosa and related plant materials for use to obtain extractable plant
material, to treat
patients with Alzheimer's disease, type II diabetes other amyloidoses, and
Parkinson's disease
are disclosed.
Use of polyphenols contained within Uncaria tomentosa and related plant
materials for
the treatment of amyloid formation, deposition, accumulation and/or
persistence in Alzheimer's
disease, type 11 diabetes, other amyloidoses and Parkinson's disease are
disclosed.
It has been surprisingly discovered that proanthocyanidin type compounds
purified from
Uncaria tomentosa have anti-amyloid and anti-a-synuclein/NAC activity.
Accordingly the
present invention provides Uncaria tomentosa extracts and individual compounds
derived
thereof. The extract preferably comprises polyphenols(s), such as polyphenols
of a least one
proanthocyanidin selected from, but not limited to, epicatechin, catechin,
epiafzelechin,
procyanidin B2, procyanidin oligomers 2 though 10, preferably 2 through 5 or 4
through 10,
procyanidin B4, procyanidin Cl, and derivatives thereof.
17
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Use of the proanthocyanidins contained within Uncaria tomentosa and related
plant
materials for the treatment of amyloid formation, deposition, accumulation
and/or persistence in
Alzheimer's disease, type II diabetes, other amyloidoses and Parkinson's
disease are disclosed.
Use of the procyanidins contained within Uncaria tomentosa and related plant
materials
for the treatment of amyloid formation, deposition, accumulation and/or
persistence in
Alzheimer's disease, type II diabetes, other amyloidoses and Parkinson's
disease are disclosed.
Use of epicatechin-4B-)8-epicatechin, also known as procyanidin or
proanthocyanidin
B2, for the treatment of amyloid formation, deposition, accumulation and/or
persistence in
Alzheimer's disease, type II diabetes, other amyloidoses and Parkinson's
disease is disclosed.
Use of catechin-4a->8-epicatechin, also known as procyanidin or
proanthocyanidin B4,
for the treatment of amyloid formation, deposition, accumulation and/or
persistence in
Alzheimer's disease, type II diabetes, other amyloidoses and Parkinson's
disease is disclosed.
Use of epicatechin-4B--->8-epicatechin-4B->8-epicatechin, also known as
procyanidin or
proanthocyanidin C1, for the treatment of amyloid formation, deposition,
accumulation and/or
persistence in Alzheimer's disease, type II diabetes, other amyloidoses and
Parkinson's disease
is disclosed.
Use of epiafzelechin-4B-8-epicatechin, for the treatment of amyloid formation,
deposition, accumulation and/or persistence in Alzheimer's disease, type II
diabetes, other
amyloidoses and Parkinson's disease is also disclosed.
Methods to isolate the active amyloid inhibitory proanthocyanidins present
within
Uncaria tomentosa and related plant materials for use as potent agents which
inhibit amyloid
formation, amyloid deposition, amyloid accumulation, amyloid persistence,
amyloid protein-
amyloid protein interactions, and/or cause a dissolution/disruption of pre-
formed or pre-
deposited amyloid fibrils in Alzheimer's disease, type II diabetes, systemic
AA amyloidosis,
other amyloidoses and Parkinson's disease are also disclosed.
Compositions and methods involving administering to a subject a therapeutic
dose of
proanthocyanidins, epicatechin-4B-48-epicatechin, catechin-4a-+8-epicatechin,
epiafzelechin-
4B-8-epicatechin, epicatechin-4B->8-epicatechin-4B>8-epicatechin or analogs or
derivatives
thereof (as disclosed herein) that inhibits amyloid deposition are disclosed.
Accordingly, the
compositions and methods of the invention are useful for inhibiting
amyloidosis in disorders in
which amyloid deposition occurs. The compounds of the invention can be used
therapeutically
18
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
to treat amyloidosis or can be used prophylactically in a subject susceptible
to amyloidosis. The
methods of the invention are based, at least in part, in directly inhibiting
amyloid fibril
formation, inhibiting amyloid fibril growth, and/or causing
dissolution/disruption of preformed
amyloid fibrils.
Pharmaceutical compositions for treating amyloidosis are disclosed. The
pharmaceutical
compositions include a therapeutic compound of the invention in an amount
effective to inhibit
amyloid deposition and a pharmaceutically acceptable vehicle.
The proanthocyanidin composition of the invention which can be administered as
a
pharmaceutical composition is disclosed. The pharmaceutical composition, may
include, but is
not limited to, a proanthocyanidin extract or purified compound, and a
pharmaceutically
acceptable carrier, such as lactose, cellulose, or equivalent, or contained
within a
pharmaceutical dosage, such as a capsule or tablet.
Use of any and all synthetic compounds made similar to procyanidins,
proanthocyanidins, epicatechin-413-->8-epicatechin (i.e. procyanidin B2),
catechin-4a-->8-
epicatechin (i.e. procyanidin B4), epicatechin-413-a8-epicatechin-46-48-
epicatechin (i.e.
procyanidin Cl), epiafzelechin-46--8-epicatechin, or analogs or derivatives
thereof, including
proanthocyanidins B1, B2, B3, B4, B5, B6, B7, B8, Cl or C2, for use as potent
agents which
inhibit amyloid formation, amyloid deposition, amyloid accumulation, amyloid
persistence,
amyloid protein-amyloid protein interactions, and/or cause a dissolution/
disruption of pre-
formed or pre-deposited amyloid fibrils in Alzheimer's disease, type II
diabetes, systemic AA
amyloidosis, other amyloidoses and Parkinson's disease is disclosed.
Preventing or treating amyloidosis in a mammal by administering a
proanthocyanidin
composition, which may include but not limited to, a proanthocyanidin extract,
a
proanthocyanidin compound, a proanthocyanidin polymer or mixture thereof, to
the mammal in
an amount and for a time sufficient to prevent, reduce, or eliminate amyloid
formation,
deposition, accumulation and/or persistence, and thereby lead to effective
treatments for
Alzheimer's disease, Parkinson's disease, type 2 diabetes, systemic AA
amyloidosis, and other
amyloid disorders is disclosed.
A method of isolation to purify and identify the procyanidins,
proanthocyanidins,
epicatechin-4B-8-epicatechin (i.e. procyanidin B2), catechin-4a-->8-
epicatechin (i.e.
procyanidin B4), epicatechin-4B-8-epicatechin-4B-a8-epicatechin (i.e.
procyanidin Cl) and
19
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
epiafzelechin-4B-48-epicatechin, or analogs or derivatives thereof from
Uncaria tomentosa are
disclosed. In one such method, an extract prepared to produce commercially
obtained pills,
tablets, caplets, soft and hard gelatin capsules, lozenges, sachets, cachets,
vegicaps, liquid drops,
elixers, suspensions, emulsions, solutions, syrups, tea bags, aerosols (as a
solid or in a liquid
medium), suppositories, sterile injectable solutions, sterile packaged
powders, bark bundles
and/or bark powder, using the methods described in the present invention.
Methods of extraction as described herein to provide purified compounds from
Uncaria
tomentosa and related plant materials for promoting mental alertness and for
inhibiting the
formation of brain amyloid deposits in a subject are disclosed.
Purified compounds from Uncaria tomentosa and related plant materials for
mental
acuity; to promote mental alertness; to provide nutritional support for age or
related cognitive or
memory decline; to promote cognitive well being; to support brain function; to
improve
cognitive ability, mental performance or memory; to promote concentration and
mental
sharpness; to improve mental vitality; to promote greater mental clarity and
alertness; to
improve short term memory, for age associated cognitive or memory decline; to
support normal
brain function; to enhance learning or memory; to improve concentration; to
enhance mental
performance; to reduce mental decline; to reduce likelihood of age related
brain disorders; to
maintain good brain health; to reduce, eliminate, prevent, inhibit or
disrupt/dissolve amyloid
fibril or protein deposits, brain associated amyloid fibril deposits or brain
associated amyloid
protein deposits, amyloid fibril formation and growth or age associated
amyloid fibril formation
and growth, brain associated amyloid fibril formation and growth; to support
healthy pancreatic
function; to promote pancreatic function by helping to promote normal insulin
function; to
reduce, eliminate, prevent, inhibit or disrupt/dissolve amyloid fibril or
protein deposits, and
pancreas associated amyloid fibril formation and growth, are also disclosed.
Amyloid and Amyloidosis
Amyloid is a generic term referring to a group of diverse but specific
extracellular
protein deposits which all have common morphological properties, staining
characteristics, and
X-ray diffraction spectra. Regardless of the nature of the amyloid protein
deposited all
amyloids have the following characteristics: 1) showing an amorphous
appearance at the light
microscopic level, appearing eosinophilic using hematoxylin and eosin stains;
2) staining with
Congo red and demonstrating a red/green birefringence as viewed under
polarized light
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
(Puchtler et al., J. Histochem. Cytochem. 10:355-364, 1962), 3) containing a
predominant beta-
pleated sheet secondary structure, and 4) ultrastructurally consisting of non-
branching fibrils of
indefinite length and with a diameter of 7-10 nm. Amyloidoses and "amyloid
diseases" today
are classified according to the specific amyloid protein deposited. The
amyloids include, but
are not limited to, the amyloid associated with Alzheimer's disease, Down's
syndrome,
hereditary cerebral hemorrhage with amyloidosis of the Dutch type and
inclusion body
myositosis (where the specific amyloid is referred to as beta-amyloid protein
or AB), the
amyloid associated with chronic inflammation, various forms of malignancy and
familial
Mediterranean fever (where the specific amyloid is referred to as AA amyloid
or inflammation-
associated amyloid), the amyloid associated with multiple myeloma and other B-
cell dyscrasias
(where the specific amyloid is referred to as AL amyloid), the amyloid
associated with type II
diabetes (where the specific amyloid is referred to as amylin or islet
amyloid), the amyloid
associated with the prion diseases including Creutzfeldt-Jakob disease,
Gerstmann-Straussler
syndrome, kuru, and scrapie (where the specific amyloid is referred to as PrP
amyloid), the
amyloid associated with long-term hemodialysis and carpal tunnel syndrome
(where the
specific amyloid is referred to as beta2 microglobulin amyloid), the amyloid
associated with
senile cardiac amyloid and familial amyloidotic polyneuropathy (where the
specific amyloid is
referred to as prealbumin or transthyretin amyloid), and the amyloid
associated with endocrine
tumors such as medullary carcinoma of the thyroid (where the specific amyloid
is referred to as
variants of procalcitonin).
Although amyloid deposits in clinical conditions share common physical
properties
relating to the presence of a beta-pleated sheet conformation, it is now clear
that many different
chemical types exist and additional ones are likely to be described in the
future. It is currently
thought that there are several common pathogenetic mechanisms that may be
operating in
amyloidosis in general. In many cases, a circulating precursor protein may
result from
overproduction of either intact or aberrant molecules (for example, in plasma
cell dyscrasias),
reduced degradation or excretion (serum amyloid A in some secondary amyloid
syndromes and
beta2-microglobulin in long-term hemodialysis), or genetic abnormalities
associated with
variant proteins (for example, familial amyloidotic polyneuropathy).
Proteolysis of a larger
protein precursor molecule occurs in many types of amyloidosis, resulting in
the production of
lower molecular weight fragments that polymerize and assume a beta-pleated
sheet
21
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
conformation as tissue deposits, usually in an extracellular location. The
precise mechanisms
involved and the aberrant causes leading to changes in proteolytic processing
and/or
translational modification are not known in most amyloids.
Systemic amyloid diseases which include the amyloid associated with chronic
inflammation, various forms of malignancy and familial Mediterranean fever
(i.e. AA amyloid
or inflammation-associated amyloidosis) (Benson and Cohen, Arth. Rheum. 22:36-
42, 1979;
Kamei et al, Acta Path. Jpn. 32:123-133, 1982; McAdam et al., Lancet 2:572-
573, 1975;
Metaxas, Kidney Int. 20:676-685, 1981), and the amyloid associated with
multiple myeloma
and other B-cell dyscrasias (i.e. AL amyloid) (Harada et al., J. Histochem.
Cytochem. 19:1-15,
1971), as examples, are known to involve amyloid deposition in a variety of
different organs
and tissues generally lying outside the central nervous system. Amyloid
deposition in these
diseases may occur, for example, in liver, heart, spleen, gastrointestinal
tract, kidney, skin,
and/or lungs (Johnson et al, N. Engl. J. Med. 321:513-518, 1989). For most of
these
amyloidoses, there is no apparent cure or effective treatment and the
consequences of amyloid
deposition can be detrimental to the patient. For example, amyloid deposition
in the kidney
may lead to renal failure, whereas amyloid deposition in the heart may lead to
heart failure. For
these patients, amyloid accumulation in systemic organs leads to eventual
death generally
within 3-5 years. Other amyloidoses may affect a single organ or tissue such
as observed with
the AB amyloid deposits found in the brains of patients with Alzheimer's
disease and Down's
syndrome: the PrP amyloid deposits found in the brains of patients with
Creutzfeldt-Jakob
disease, Gerstmann-Straussler syndrome, and kuru; the islet amyloid (amylin)
deposits found
in the islets of Langerhans in the pancreas of 90% of patients with type II
diabetes (Johnson et
al, N. Engl. J. Med. 321:513-518, 1989; Lab. Invest. 66:522 535, 1992); the
beta2-microglobulin amyloid deposits in the medial nerve leading to carpal
tunnel syndrome as
observed in patients undergoing long-term hemodialysis (Geyjo et al, Biochem.
Biophys. Res.
Comm. 129:701-706, 1985; Kidney Int. 30:385-390, 1986); the
prealbumin/transthyretin
amyloid observed in the hearts of patients with senile cardiac amyloid; and
the
prealbumin/transthyretin amyloid observed in peripheral nerves of patients who
have familial
amyloidotic polyneuropathy (Skinner and Cohen, Biochem. Biophys. Res. Comm.
99:1326-
1332, 1981; Saraiva et al, J. Lab. Clin. Med. 102:590-603, 1983; J. Clin.
Invest. 74:104-119,
1984; Tawara et al, J. Lab. Clin. Med. 98:811-822, 1989).
22
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Alzheimer's Disease and the Aging Population
Alzheimer's disease is a leading cause of dementia in the elderly, affecting 5-
10% of
the population over the age of 65 years (A Guide to Understanding Alzheimer's
Disease and
Related Disorders, Jorm, ed., New York University Press, New York, 1987). In
Alzheimer's
disease, the parts of the brain essential for cognitive processes such as
memory, attention,
language, and reasoning degenerate, robbing victims of much that makes us
human, including
independence. In some inherited forms of Alzheimer's disease, onset is in
middle age, but
more commonly, symptoms appear from the mid-60's onward. Alzheimer's disease
today
affects 4-5 million Americans, with slightly more than half of these people
receiving care at
home, while the others are in many different health care institutions. The
prevalence of
Alzheimer's disease and other dementias doubles every 5 years beyond the age
of 65, and
recent studies indicate that nearly 50% of all people age 85 and older have
symptoms of
Alzheimer's disease (2000 Progress Report on Alzheimer's Disease, National
Institute on
Aging/National Institute of Health). 13% (33 million people) of the total
population of the
United States are age 65 and older, and this percentage will climb to 20% by
the year 2025
(2000 Progress Report on Alzheimer's Disease).
Alzheimer's disease also puts a heavy economic burden on society. A recent
study
estimated that the cost of caring for one Alzheimer's disease patient with
severe cognitive
impairments at home or in a nursing home, is more than $47,000 per year (A
Guide to
Understanding Alzheimer's Disease and Related Disorders). For a disease that
can span from 2
to 20 years, the overall cost of Alzheimer's disease to families and to
society is staggering. The
annual economic toll of Alzheimer's disease in the United States in terms of
health care
expenses and lost wages of both patients and their caregivers is estimated at
$80 to $100 billion
(2000 Progress Report on Alzheimer's Disease).
Tacrine hydrochloride ("Cognex"), the first FDA approved drug for Alzheimer's
disease, is a acetylcholinesterase inhibitor (Cutler and Sramek, N. Engl. J.
Med. 328:808 810,
1993). However, this drug has showed limited success in producing cognitive
improvement in
Alzheimer's disease patients and initially had major side effects such as
liver toxicity. The
second more recently FDA approved drug,-donepezil ("Aricept"), which is also
an
acetylcholinesterase inhibitor, is more effective than tacrine, by
demonstrating slight cognitive
improvement in Alzheimer's disease patients (Barner and Gray, Ann.
Pharmacotherapy 32:70-
23
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
77, 1998; Rogers and Friedhoff, Eur. Neuropsych. 8:67-75, 1998), but is not
believed to be a
cure. Therefore, it is clear that there is a need for more effective
treatments for Alzheimer's
disease patients.
Amyloid as a Therapeutic Target for Alzheimer's Disease
Alzheimer's disease is characterized by the deposition and accumulation of a
39-43
amino acid peptide termed the beta-amyloid protein, AB or B/A4 (Glenner and
Wong,
Biochem. Biophys. Res. Comm. 120:885-890, 1984; Masters et al., Proc. Natl.
Acad. Sci. USA
82:4245-4249, 1985; Husby et al., Bull. WHO 71:105-108, 1993). AB is derived
by protease
cleavage from larger precursor proteins termed beta-amyloid precursor proteins
(or BPPs) of
which there are several alternatively spliced variants. The most abundant
forms of the BPPs
include proteins consisting of 695, 751 and 770 amino acids (Tanzi et al.,
Nature 331:528-530,
1988; Kitaguchi et al., Nature 331:530-532, 1988; Ponte et al., Nature 331:525-
527, 1988).
The small AB peptide is a major component which makes up the amyloid deposits
of
"plaques" in the brains of patients with Alzheimer's disease. In addition,
Alzheimer's disease
is characterized by the presence of numerous neurofibrillary "tangles",
consisting of paired
helical filaments which abnormally accumulate in the neuronal cytoplasm
(Grundke-Iqbal et
al., Proc. Natl. Acad. Sci. USA 83:4913-4917, 1986; Kosik et al., Proc. Natl.
Acad. Sci. USA
83:4044-4048, 1986; Lee et al., Science 251:675-678, 1991). The pathological
hallmark of
Alzheimer's disease is therefore the presence of "plaques" and "tangles", with
amyloid being
deposited in the central core of the plaques. The other major type of lesion
found in the
Alzheimer's disease brain is the accumulation of amyloid in the walls of blood
vessels, both
within the brain parenchyma and in the walls of meningeal vessels that lie
outside the brain.
The amyloid deposits localized to the walls of blood vessels are referred to
as cerebrovascular
amyloid or congophilic angiopathy (Mandybur, J. Neuropath. Exp. Neurol. 45:79-
90, 1986;
Pardridge et al., J. Neurochem. 49:1394-1401, 1987).
For many years there has been an ongoing scientific debate as to the
importance of
"amyloid" in Alzheimer's disease, and whether the "plaques" and "tangles"
characteristic of
this disease were a cause or merely a consequence of the disease. Within the
last few years,
studies now indicate that amyloid is indeed a causative factor for Alzheimer's
disease and
should not be regarded as merely an innocent bystander. The Alzheimer's AB
protein in cell
culture has been shown to cause degeneration of nerve cells within short
periods of time (Pike
24
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
et al., Br. Res. 563:311-314, 1991; J. Neurochem. 64:253-265, 1995). Studies
suggest that it is
the fibrillar structure (consisting of a predominant beta-pleated sheet
secondary structure),
characteristic of all amyloids, that is responsible for the neurotoxic
effects. AB has also been
found to be neurotoxic in slice cultures of hippocampus (Harrigan et al.,
Neurobiol. Agin
16:779-789, 1995) and induces nerve cell death in transgenic mice (Games et
al., Nature
373:523-527, 1995; Hsiao et al., Science 274:99-102, 1996). Injection of the
Alzheimer's AB
into rat brain also causes memory impairment and neuronal dysfunction (Flood
et al., Proc.
Natl. Acad. Sci. USA 88:3363-3366, 1991; Br. Res. 663:271-276, 1994).
Probably, the most convincing evidence that AB amyloid is directly involved in
the
pathogenesis of Alzheimer's disease comes from genetic studies. It has been
discovered that
the production of AB can result from mutations in the gene encoding, its
precursor, beta
amyloid precursor protein (Van Broeckhoven et al., Science 248:1120-1122,
1990; Murrell et
al., Science 254:97-99, 1991; Haass et al., Nature Med. 1:1291-1296, 1995).
The identification
of mutations in the beta-amyloid precursor protein gene which causes early
onset familial
Alzheimer's disease is the strongest argument that amyloid is central to the
pathogenetic
process underlying this disease. Four reported disease-causing mutations have
now been
discovered which demonstrate the importance of AB in causing familial
Alzheimer's disease
(reviewed in Hardy, Nature Genet. 1:233-234, 1992). All of these studies
suggest that
providing a drug to reduce, eliminate or prevent fibrillar AB formation,
deposition,
accumulation and/or persistence in the brains of human patients will serve as
an effective
therapeutic.
Discovery and identification of new compounds or agents as potential
therapeutic
agents to arrest amyloid deposition, accumulation and/or persistence that
occur in Alzheimer's
disease and other amyloidoses are desperately sought.
Parkinson's Disease and a-Synuclein Fibril Formation
Parkinson's disease is a neurodegenerative disorder that is pathologically
characterized
by the presence of intracytoplasmic Lewy bodies (Lewy in Handbuch der
Neurologie, M.
Lewandowski, ed., Springer, Berlin, pp. 920-933, 1912; Pollanen et al., J.
Neuropath. Exp.
Neurol. 52:183-191, 1993), the major components of which are filaments
consisting of a-
synuclein (Spillantini et al., Proc. Natl. Acad. Sci. USA_95:6469-6473, 1998;
Arai et al.,
Neurosc. Lett. 259:83-86, 1999), a 140-amino acid protein (Ueda et al., Proc.
Natl. Acad. Sci.
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
U.S.A. 90:11282-11286, 1993). Two dominant mutations in a-synuclein causing
familial early
onset Parkinson's disease have been described suggesting that Lewy bodies
contribute
mechanistically to the degeneration of neurons in Parkinson's disease
(Polymeropoulos et al.,
Science 276:2045-2047, 1997; Kruger et al., Nature Genet. 18:106-108, 1998).
Recently, in
vitro studies have demonstrated that recombinant a-synuclein can indeed form
Lewy body-like
fibrils (Conway et al., Nature Med. 4:1318-1320, 1998; Hashimoto et al., Brain
Res. 799:301-
306, 1998; Nahri et al., J. Biol. Chem. 274:9843-9846, 1999). Most
importantly, both
Parkinson's disease-linked a-synuclein mutations accelerate this aggregation
process that
suggests that such in vitro studies may have relevance for Parkinson's disease
pathogenesis.
a-Synuclein aggregation and fibril formation fulfills of the criteria of a
nucleation-dependent
polymerization process (Wood et al., J. Biol. Chem. 274:19509-19512, 1999). In
this regard a-
synuclein fibril formation resembles that of Alzheimer's beta-amyloid protein
(AB) fibrils. a-
Synuclein recombinant protein, and non-amyloid component (known as NAC), which
is a 35-
amino acid peptide fragment of a-synuclein, both have the ability to form
fibrils when
incubated at 37 C, and are positive with amyloid stains such as Congo red
(demonstrating a
red/green birefringence when viewed under polarized light) and Thioflavin S
(demonstrating
positive fluorescence) (Hashimoto et al., Brain Res. 799:301-306, 1998; Ueda
et al., Proc. Natl.
Acad. Sci. U.S.A 90:11282-11286, 1993).
In addition, accumulation of a-synuclein/NAC is also a cytopathological
feature
common to Lewy body disease and multiple system atrophy (Wakabayashi et al,
Acta
Neuropath. 96:445-452, 1998; Piao et al, Acta Neuropath. 101:285-293, 2001).
Multiple
system atrophy is a sporadic neurodegenerative disease in adults characterized
by neuronal and
glial cytoplasmic inclusions, containing a-synuclein/NAC.
Parkinson's disease a-synuclein/NAC fibrils, like the A13 fibrils of
Alzheimer's disease,
also consist of a predominant beta-pleated sheet structure. It is therefore
believed that
compounds found to inhibit Alzheimer's disease AB amyloid fibril formation can
also be
anticipated to be effective in the inhibition of a-synuclein and/or NAC fibril
formation. These
compounds would therefore also serve as therapeutics for Parkinson's disease,
in addition to
having efficacy as a therapeutic for Alzheimer's disease and other amyloid
disorders.
26
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Islet Amyloid Polvpeptide (IAPP) and Type 2 Diabetes
Islet amyloid deposits are observed in -90% of patients with well-established
type 2
diabetes and would appear to be a characteristic feature of the disease
process (Westermark, J.
Med. Sci. 77:91-94,1972; Clark et al, Diabetes Res. 9:151-159,1988). In many
patients the
deposits are widespread and affect many islets. The degree of islet
(predominantly B-cell) mass
that has been replaced by amyloid may be a marker for the severity of the
diabetic disease
process, with those individuals requiring insulin treatment having the
greatest islet mass
reduction and amyloid formation (Westermark, Amyloid: Int. J. Exp. Clin.
Invest. 1:47-
60,1994). Since islet amyloid has been observed in autopsy samples obtained
from different
populations, it appears to be a phenomenon common to the disease rather than
to a
subpopulation of individuals with the syndrome (Westermark, J. Med. Sci. 77:91-
94,1972;
Clark et al, Diabetes Res. 9:151-159,1988). The prevalence of islet amyloid
deposits increases
with age (Bell, Am. J. Path. 35:801-805, 1959), which is not surprising
because normal aging
is associated with a deterioration in glucose tolerance and an increased
prevalence of type 2
diabetes (Davidson, Metabolism 28:687-705, 1979).
The major protein in islet amyloid is a 37-amino acid peptide known as islet
amyloid
polypeptide (IAPP) or amylin. IAPP is a known normal secretory product of the
pancreatic B-
cells (Kanh et al, Diabetes 39:634-638,1990) that is stored in insulin-bearing
cytoplasmic
granules (Clark et al, Cell Tissue Res. 257:179-185, 1989). It has long been
questioned
whether the deposition of islet amyloid is involved in or merely a consequence
of the
pathogenesis of type 2 diabetes. However, a number of studies now suggest that
in fact islet
amyloid formation, deposition and persistence may be an important primary
factor leading to
B-cell dysfunction and cell death, hyperglycemia, and in the development of
type 2 diabetes.
IAPP has been hypothesized to have an important role in the pathogenesis of
type 2
diabetes through its impairment of B-cell function and reduction of B-cell
mass (Johnson et al,
N. Engl. J. Med. 321:513-518,1989). Besides being able to form islet amyloid
deposits that
replace B-cell mass, amyloid fibrils appear to damage islets directly. Studies
in which islets
were incubated in the presence of human or rat IAPP demonstrated that human
IAPP formed
amyloid fibrils in a concentration-dependent manner and was associated with
the death of
pancreatic islet B-cells (Lorenzo et al, Nature 368:756-760,1994). Cell death
did not occur in
27
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
the presence of rat IAPP that does not form amyloid fibrils (Lorenzo et al,
Nature 368:756-
760,1994).
Studies involving transgenic mouse models have allowed further insight into
the role of
islet amyloid in the pathogenesis of type 2 diabetes. More recent studies do
suggest that
development of LAPP-derived islet amyloid does not depend on hyperglycemia and
is
progressive (Verchere et al, Proc. Natl. Acad. Sci. U.S.A. 93:3492-3496,
1996). In these latter
studies hyperglycemia developed in only 31% of male transgenic mice, and in
14% of male
nontransgenic animals. When pancreatic sections from these mice were examined,
islet
amyloid was found in every transgenic mouse with diabetes. However, two-thirds
of male
transgenic animals that were normoglycemic also developed islet amyloid
deposits indicating
that hyperglycemia was not a prerequisite for islet amyloid formation. The
data from these and
other studies further suggested that human IAPP fibrils may be cytotoxic to B-
cells and thus
could produce early alterations in islet function (Lorenzo et al, Nature
368:756-760, 1994;
Janson et al, Diabetes 47:A250, 1998). Islet amyloid deposition appears to be
an early feature
of the islet lesion of type 2 diabetes and progressive accumulation of islet
amyloid is associated
with further B-cell mass reduction (Clark et al, Diabetes Res. 9:151-159,1988;
Westermark and
Wilander, Diabetologia 15:417-421,1978). Thus, a progressive reduction in
islet mass caused
by increased amyloid deposition is associated with a progressive impairment in
insulin
secretion, reduction in glucose tolerance, and eventually the development of
fasting
hyperglycemia. The studies in transgenic animals suggest not just that
hyperglycemia is
associated with the development of islet amyloid, but that amyloid contributes
to the
development of hyperglycemia by replacing B-cells. These studies as a whole
suggest that islet
amyloid formation plays a central role in the development of B-cell failure of
type 2 diabetes.
Therefore, agents or compounds able to inhibit or disrupt islet amyloid (i.e.
IAPP or amylin)
formation, deposition, accumulation or persistence may lead to new potential
treatments for
type 2 diabetes.
Uncaria tomentosa
The herb Uncaria tomentosa, also known as "Una de Gato" (in Spanish) or "Cat's
claw" (in English) refers to a woody vine that grows within the Peruvian
Amazon rain forest.
This slow growing vine takes 20 years to reach maturity, and can grow over 100
feet in length
as it attaches and wraps itself around the native trees. It is found
abundantly in the foothills, at
28
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
elevations of two to eight thousand feet. The vine is referred to as "Cat's
claw" because of its
distinctive curved claw-like thorns that project from the base of its leaves.
The native Indian
tribes traditionally have boiled the inner bark and root of the herb to make a
tea decoction and
regard Uncaria tomentosa as a sacred medicinal plant. The highly effective
properties
contained within the inner bark of this plant are believed to have a profound
and positive
influence on the body, although scientific medical data is generally lacking
on its potential
benefits in humans. The alkaloids and phytochemicals in the inner bark of
Uncaria tomentosa
are almost identical to those found in the root, and harvesting this way
preserves the plant and
provides for the future of the rainforest.
Some of the active substances present in Uncaria tomentosa are alkaloids,
which occur
in the plant and its watery extract as a complex bound to tannins. In this
form, only little of
them can be activated. The complexes get split by the acid milieu of the
stomach; the alkaloids
get transformed into their hydrochloride form, and in this way, get well
absorbed. A darker
Uncaria tomentosa extract means more tannin is present and beneficial
alkaloids are locked up
with the tannins, which have formed a non-bioavailable and poorly absorbed
complex. A light
golden color of Uncaria tomentosa suggests that there are less tannins, and
more alkaloids
available in the extract.
Uncaria tomentosa is one of the most important plants in the South American
Peruvian
rainforest. A number of oxindole alkaloids have already been isolated from the
inner bark of
this plant. Two US patents (US patent #4,844,901 and US patent #4,940,725)
describe the
isolation and use of six oxindole alkaloids from Uncaria tomentosa, which are
believed to be
"suitable for the unspecified stimulation of the immunologic system". These
oxindole alkaloids
are believed to provide a general boost to the immune system as well as have a
profound effect
on the ability of white blood cells and macrophages to phagocytize harmful
microorganisms
and foreign matter. The most immunologically active alkaloid appears to be
alloisopteropodine, isomer A, a pentacyclic oxindole alkaloid (US patent
#4,940,725).
Although some health care providers have suggested that Uncaria tomentosa may
be
used to treat a variety of ailments, nowhere has there been any use or
suggestion of use, of this
plant or extracts thereof, or compounds derived thereof, for the treatment of
amyloid
formation, deposition, accumulation and/or persistence, such as that which
occurs in the
amyloidoses, including Alzheimer's disease and Parkinson's disease. The
present invention
29
CA 02441099 2009-06-10
52171-14'
clearly demonstrates the effectiveness of Uncaria tonzentosa derived
compounds, including
procyanidins and proanthocyanidins, for the treatment of amyloidosis
associated with
Alzheimer's disease, type 2 diabetes, systemic AA amyloidosis, and other
amyloid diseases, as
well as for the treatment of a-synuclein fibril formation and accumulation,
such as that
observed in patients with Parkinson's disease.
Proan(hocyanidins, Procyanidins, Flavanoids and Tannins
Proanthocyanidins are polyphenolic molecules occurring naturally in fruits,
berries and
other plant material. These molecules belong to the flavanoid family of
compounds. The
flavanoid polyphenolics include the catechins, anthocyanins, and
proanthocyanidins.
Proanthocyanidins are also known in the art as condensed tannins,
leucoanthocyanidins,
leucodelphinins, leucocyanins, anthocyanogens, epicatechin-catechin polymers
or
procyanidins. For a review of procyanidins and proanthocyanidins, see Santos-
Buelga and
Scalbert, J. Sc. Food Agri. 80:1094-1117,2000, which is discussed in detail
below.
Proanthocyanidin oligomers or polymers useful for the present anti-amyloid
activity
are comprised of monomeric units of leucoanthocyanidins. Leucoanthocyanidins
are generally
monomeric flavanoids which include catechins, epicatechins, gallocatechins,
galloepicatechins,
flavanols, flavonols, and flavan-3,4-diols, leucocyanidins and anthocyanidins.
The
therapeutically effective proanthocyanidin polymers have from 2 to 20
flavanoid units, and
more preferably from 2 to 11 flavanoid units.
Proanthocyanidins polymers or oligomers are known to have varying numbers of
flavanoid units, and have been reported for example in Mattice et al,
Phytochem. 23:1309-
1311, 1984; Czochanska et al, J.C. S. Chem. Comm. 375, 1979; Jones et al,
Photochemistry,
15:1407-1409,1976.
Procyanidins, also referred to as proanthocyanidins, are polymeric or
oligomeric
compounds composed of epicatechin and catechin residues. Disclosed compounds
include
dimers of epicatechin and catechin residues, and trimers of epicatechin.
Catechin and
epicatechin residues may be combined in all possible combinations in polymeric
procyanidins
up to molecular weights of up to about 10,000 daltons. Proanthocyanidin
polymers are known
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
to have a varying number of flavanoid units. The polymers preferably contain
two to fifteen
monomeric flavanoid subunits, most preferably two to ten subunits.
Tannins are classically divided into 2 groups. Hydrolysable tannins are esters
of
phenolic acids and a polyol, usually glucose. The phenolic acids are either
gallic acid in
gallotannins or other phenolic acids derived from the oxidation of galloyl
residues in
ellagitannins. Proanthocyanidins, forming the second group of tannins, are far
more common in
our diet. They are polymers made of elementary flavan-3-ol units. A key
feature of
proanthocyanidins is that they yield anthocyanidins upon heating in acidic
media, hence their
name (reviewed in Santos-Buelga and Scalbert, J. Sc. Food Agri. 80:1094-
1117,2000).
Structurally, tannins possess 12-16 phenolic groups and 5-7 aromatic rings per
1000
units of relative molecular mass (E. Haslam, Practical Polyphenoics-from
Structure to
Molecular Recognition and Physiological Action, Cambridge University Press,
Cambridge,
1998). This feature, together with their high molecular weight, clearly makes
the tannins and
similar phenolic polymers found in processed products such as red wine or
black tea different
both in structure and properties from the low-molecular-weight phenolic acids
and monomeric
flavanoids. The phenolic polymers, formed by enzymatic and/or chemical
transformation of
simple flavanols, proanthocyanidins and other phenolic compounds, are called
tannin-like
compounds.
Proanthocyanidins are polymeric flavan-3-ols whose elementary units are linked
by C-
C and occasionally C-O-C bonds. The flavan-3-ol units have the typical C6-C3-
C6 flavanoid
skeleton. The three rings are distinguished by the letters A, B and C (see
Figure 1). They differ
structurally according to the number of hydroxyl groups on both aromatic rings
and the
stereochemistry of the asymmetric carbons on the heterocycle. The most common
proanthocyanidins in food are procyanidins with a 3', 4'-dihydroxy
substitution on the B ring
and prodelphinidins with a 3', 4', 5'-trihydroxy substitution. Procyanidins or
mixed
procyanidins/prodelphinidins are most common in food. Propelargonidins with 4'-
hydroxy B-
rings are relatively rare in food sources, bit notably disclosed herein in the
form of
epiafzelechin. The three carbons C2, C3, C4 of the flavanol heterocycle are
asymmetric and
may occur in different configurations. With some very rare exceptions, the
configuration of C2
is R. Flavan-3-ol units with the 2S configuration are distinguished by the
prefix enantio(ent-).
The stereochemistry of the C2-C3 linkage may be either trans (2R, 3S) or cis
(2R, 3R) as in
31
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
(+)-(gallo)catechin and (-)-epi(gallo)catechin polymers respectively. The
interflavan bond at
C4 is always trans with respect to the hydroxy group at C3 (E. Haslam,
Practical Polyphenoics-
from Structure to Molecular Recognition and Physiological Action, Cambridge
University
Press, Cambridge, 1998).
The most usual interflavanol linkages are C-C bonds established between the C4
of
one flavanoid unit ("extension or upper unit"). Such proanthocyanidins belong
to the so-called
B-type (dimeric) and C type (trimeric) proanthocyanidins. Compounds with
doubly linked
units (one C-C and one C-O; "A type linkage") have also been reported in some
food sources
such as tea leaf, cocoa and cranberry fruits (LJ. Porter, Flavans and
proanthocyanidins, in The
Flavanoids-Advances in Research Since 1986, Ed. by JB Harborne, Chapman and
Hall,
London, pp.23-55, 1994). In these A-type proanthocyanidins an additional ether
linkage
between the C2 of the upper unit and the oxygen-bearing C7 or C5 of the lower
one is formed
in addition to the usual C4-C8 or C4-C6 bond.
Initially oligomeric proanthocyanidins were named by an alpha-numeric system,
with a
letter A, B or C to describe the type of interflavanol linkage; a number was
added to the letter
as they were detected (Thompson et al, J. Chem. Soc. Perkins Trans. 1: 1387-
1399, 1972). A
new nomenclature was later introduced to name an increasing number of new
structures. It is
based on that utilized for the polysaccharides (Hemingway et al, J. Chem. Soc.
Perkins Trans.
1:1387-1399, 1972). In this nomenclature, the elementary units of the
oligomers are designated
with the name of the corresponding flavan-3-ol monomers. The interflavanol
linkage and its
direction are indicated with an arrow (4-) and its configuration at C4 is
described as a or B.
In type-A doubly linked proanthocyanidins, both linkages are indicated. It is
unnecessary to
indicate to indicate oxygen in the additional ether bond since it is obvious
from the substitution
pattern of catechin lower units (LJ Porter, in The Flavanoids-Advances in
Research since 1980,
Ed. by JB Harborne, Chapman and Hall, London, pp. 21-62, 1988). For instance,
according to
this nomenclature, procyanidin dimer B 1 becomes epicatechin-4B-8-catechin and
dimer A2
becomes epicatechin-2B-->7, 4B-->8-epicatechin.
Flavanol units can bear various acyl or glycosyl substituents. The most common
acyl
substiuent is gallic acid which forms an ester with the hydroxyl n the C3
position, as in tea
(Nonaka et al, Chem. Pharmaceutic. Bull. 31:3906-3914, 1983) and wine (Prieur
et al,
Phyochem. 36:781-784, 1994). Several glycosylated proanthocyanidin oligomers
have also
32
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
been characterized. The sugar is generally linked to the hydroxyl group at the
C3 position
(Ishimaru et al, Phytochmistry 26:1167-1170, 1987; Zhang et al, Phytochemistry
27:3277-
3280, 1988), but also at the C5 position (Gujer et al, Phytochemistry 25:1431-
1436, 1986).
Although proanthocyanidins heterosides are less frequently reported than other
flavanoid
glycosides, their occurrence may be underestimated, as sugars are frequently
associated with
purified proanthocyanidin polymers (Porter et al, Phytochemistry 24:567-569,
1985; Mathews
et al, J. Agric. Food Chem. 45:1195-1201, 1997). Such variations, and other
variations
disclosed herein, are included with the scope of disclosure of the disclosed
proanthocyanidins.
More recently, the introduction of electrospray mass spectrometry techniques
coupled to
liquid chromatography led to a more detailed characterization of
proanthocyanidin polymers.
Such methods were employed in the present invention to identify procyanidins
and
proanthocyanidins derived from Uncaria tomentosa which demonstrate potent anti-
amyloid and
anti-a-synuclein/NAC activity.
BRIEF DESCRIPTION OF THE DRAWINGS
FIGURE 1 is an illustration demonstrating the basic structure of
proanthocyanidins, including
proelargonidins (where R,, R2 = H); procyanidins (where R1= H, R2 = OH); and
prodelphinins
(where R1, R2 = OH).
FIGURE 2 is a HPLC tracing using method 1 (see Example 2, Table 1 for details)
demonstrating the separation of PTI-777. Using this method, there is a good
separation of Hl
and H2 peaks.
FIGURE 3 is a HPLC tracing using method 2 (see Example 2, Table I for details)
demonstrating the separation of Hl and H2 from PTI-777 following silica gel
chromatography
and elution with 20% methanol in chloroform.
FIGURE 4 is a HPLC tracing using method 1 (see Example 2, Table 1 for details)
demonstrating the separation of mostly Hl (with less H2) after fractioning PTI-
777 using silica
gel chromatography, followed by HPLC.
FIGURE 5 is a HPLC tracing using method 1 (see Example 2, Table 1 for details)
demonstrating the isolation of pure H2 from PTI-777, after fractionating PTI-
777 using silica
gel chromatography, followed by HPLC.
FIGURE 6 is a 111 NMR spectrum of peak H2 derived from PTI-777.
33
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
FIGURE 7 is a 13C NMR spectrum of peak H2 derived from PTI-777.
FIGURE 8 is a 13C NMR spectrum of peak H2 in deteroacetone (instead of
deuteromethanol).
FIGURE 9 is a'H NMR spectrum of peak H2 in deteroacetone (instead of
deuteromethanol).
FIGURE 10 is the peracetate structure of a sample of pure H2 following
acetylation.
FIGURE 11 is the 'H NMR spectrum of the H2 peracetate in CDC13.
FIGURE 12 is the 13C NMR spectrum of the H2 peracetate in CDC13.
FIGURE 13 is the CIGAR'H-13C correlation spectrum (low resolution) of the H2
peracetate.
FIGURE 14 is the CIGAR 'H-13C correlation spectrum (high resolution) of the H2
peracetate.
FIGURE 15 is the NOESY correlation spectrum of the H2 peracetate.
FIGURE 16 is the NOESY correlation spectrum of the H2 peracetate.
FIGURE 17 is the NOESY correlation spectrum of the H2 peracetate.
FIGURE 18 is the structure of H2 identified to be epicatechin-413-48-
epicatechin.
FIGURE 19 is the 'H NMR spectrum of peak Hl.
FIGURE 20 is the 13C NMR spectrum of peak Hl.
FIGURE 21 is the peracetate structure of a sample of pure Hi following
acetylation.
FIGURE 22 is the 'H NMR spectrum of the H1 peracetate.
FIGURE 23 is the13C NMR spectrum of the Hl peracetate.
FIGURE 24 is the CIGAR 'H -13C correlation spectrum (low resolution) of the Hl
peracetate.
FIGURE 25 is the CIGAR 1H -13C correlation spectrum (high resolution) of the
H1
peracetate.
FIGURE 26 is the structure of Hl identified to be catechin-4a--->8-
epicatechin.
FIGURE 27 is a HPLC tracing demonstrating separation of peak K2.
FIGURE 28 is a HPLC tracing demonstrating separation of a pure peak K2.
FIGURE 29 is a -ve ion electrospray mass spectrum of K2.
FIGURE 30 is the 'H NMR spectrum of K2.
FIGURE 31 is the 111 NMR spectrum of the K2 peracetate.
FIGURE 32 is the "C NMR spectrum of the K2 peracetate.
FIGURE 33 is a CIGAR 'H-"C correlation spectrum (low resolution) of the K2
peracetate.
FIGURE 34 is a CIGAR 'H-13C correlation spectrum (high resolution) of the K2
peracetate.
FIGURE 35 is the peracetate structure of K2.
34
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
FIGURE 36 is the structure of K2 determined to be epicatechin-4B-48-
epicatechin-4B->8-
epicatechin.
FIGURE 37 is a graph of a Thioflavin T fluorometry assay demonstrating the
dose-dependent
disruption/disassembly of pre-formed AB 1-42 fibrils by proanthocyanidins
(compounds H2,
Hl and K2).
FIGURE 38 is a black and white figure of a SDS-PAGE/Western blot further
demonstrating
the disruption of AB 1-42 fibrils, even in monomeric form by proanthocyanidins
(compounds
H2, Hl and K2).
FIGURE 39 is a graph of a circular dichroism spectroscopy assay demonstrating
compound
H2 (referred to as PTC38 in this figure) causes a marked
disruption/disassembly of 13-sheet
structure in AB 1-42 fibrils at 7 days following incubation.
FIGURE 40 is a graph of a circular dichroism spectroscopy assay demonstrating
compound
H2 (referred to as PTC38 in this figure) causes a marked
disruption/disassembly of B-sheet
structure in AB 1-40 fibrils at 7 days following incubation.
FIGURE 41 is a graph of a Thioflavin T fluorometry assay demonstrating the
dose-dependent
disruption/disassembly of pre-formed NAC fibrils by proanthocyanidins
(compounds H2, H1
and K2).
FIGURE 42 is a graph of a Thioflavin T fluorometry assay demonstrating the
dose-dependent
disruption/disassembly of pre-formed IAPP fibrils by proanthocyanidins
(compounds H2, H1
and K2).
FIGURE 43 is the peracetate structure of Kl.
FIGURE 44 is the structure of KI determined to be epiafzelechin-4B->8-
epicatechin.
FIGURE 45 is the -ve ion electrospray mass spectrum of Kl.
FIGURE 46 is the 13C NMR spectrum of Kl.
FIGURE 47 is the 1H NMR spectrum of Kl.
FIGURE 48 is the 1H NMR spectrum of the Kl peracetate.
FIGURE 49 is the 13C NMR spectrum of the Kl peracetate.
FIGURE 50 is the CIGAR 1H -13C correlation spectrum (low resolution) of the KI
peracetate.
FIGURE 51 is the CIGAR 'H -13C correlation spectrum (medium resolution) of the
K1
peracetate.
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
FIGURE 52 is the CIGAR 'H - 13C correlation spectrum (high resolution) of the
Kl
peracetate.
FIGURE 53 is the CIGAR 111 -13C correlation spectrum (high resolution) of the
Ki
peracetate.
FIGURE 54 is an illustration of general Formula I for the structure of
proanthocyanidins.
FIGURE 55 is an illustration of general Formula II for the structure of
proanthocyanidins.
FIGURE 56 is an alternate example of proanthocyanidin structure.
FIGURE 57 is a flowchart of an isolation process for proanthocyanidins.
BEST MODE OF CARRYING OUT THE INVENTION
Further Definitions
In this disclosure, the following terms shall have the following meanings,
without
regard to whether the terms are used variantly elsewhere in the literature or
otherwise in the
known art.
"Proanthocyanidins" includes "procyanidins"; "procyanidins" are a specific
class of
"proanthocyanidins".
"Mammal" and "mammalian subject" includes, but is not limited to, humans and
non-
human mammals, such as companion animals (cats, dogs, and the like), lab
animals (such as
mice, rats, guinea pigs, and the like) and farm animals (cattle, horses,
sheep, goats, swine, and
the like).
"Pharmaceutically acceptable excipient " means an excipient that is useful in
preparing
a pharmaceutical composition that is generally safe, non-toxic, and desirable,
and includes
excipients that are acceptable for veterinary use as well as for human
pharmaceutical use. Such
excipients may be solid, liquid, semisolid, or, in the case of an aerosol
composition, gaseous.
"Pharmaceutically acceptable salts" means salts that are pharmaceutically
acceptable
and have the desired pharmacological properties. Such salts include salts that
may be formed
where acidic protons present in the compounds are capable of reacting with
inorganic or
organic bases. Suitable inorganic salts include those formed with the alkali
metals, e.g. sodium
and potassium, magnesium, calcium, and aluminum. Suitable organic salts
include those
formed with organic bases such as the amine bases, e.g. ethanolamine,
diethanolamine,
triethanolamine, tromethamine, N-methylglucamine, and the like. Such salts
also include acid
36
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
addition salts formed with inorganic acids (e.g. hydrochloric and hydrobromic
acids) and
organic acids (e.g. acetic acid, citric acid, maleic acid, and the alkane- and
arene-sulfonic acids
such as methanesulfonic acid and benzenesulfonic acid). When there are two
acidic groups
present, a pharmaceutically acceptable salt may be a mono-acid-mono-salt or a
di-salt; and
similarly where there are more than two acidic groups present, some or all of
such groups can
be salified.
A "therapeutically effective amount" in general means the amount that, when
administered to a subject or animal for treating a disease, is sufficient to
effect the desired
degree of treatment for the disease. A "therapeutically effective amount" or a
"therapeutically
effective dosage" preferably inhibits, reduces, disrupts, disassembles
amyloidosis, fibril
formation, deposition, accumulation and/or persistence, or a disease
associated with a-
synuclein/NAC fibril formation in a patient by at least 20, more preferably by
at least 40%,
even more preferably by at least 60%, and still more preferably by at least
80%, relative to
untreated subjects. Effective amounts of a proanthocyanidin or procyanidin, or
other disclosed
compositions for treatment of a mammalian subject are about 1 mg to about
10,000 mg/kg of
body weight of the subject, but more preferably from about 10 mg/kg/body
weight to 100
mg/kg body weight. A broad range of disclosed composition dosages are believed
to be both
safe and effective.
"Treating" or "treatment" of a disease includes preventing the disease from
occurring in
a mammal that may be predisposed to the disease but does not yet experience or
exhibit
symptoms of the disease (prophylactic treatment), inhibiting the disease
(slowing or arresting
its development), providing relief from the symptoms or side-effects of the
disease (including
palliative treatment), and relieving the disease (causing regression of the
disease). "Treating"
amyloidosis or "amyloid diseases" includes any one or more of the following:
preventing,
inhibiting, reducing, disassembling, disrupting, and disaggregating amyloid
fibrils and amyloid
protein deposits, such as AB and the other amyloids referred to herein.
"Treating" an a-synuclein disease or "treating a-synuclein or NAC
fibrillogenesis"
includes any one or more of the following: preventing, inhibiting, reducing,
disassembling,
disrupting, and disaggregating a-synuclein/NAC fibrils and a-synuclein/NAC-
associated
protein deposits, such as those in Lewy body disease, Parkinson's disease and
multiple system
atrophy.
37
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
"NAC" (non-amyloid component) is a 35-amino acid peptide fragment of a-
synuclein,
which also, like a-synuclein, has the ability to form amyloid-like fibrils
when incubated at
37 C, and are positive with amyloid stains such as Congo red (demonstrating a
red/green
birefringence when viewed under polarized light) and Thioflavin S
(demonstrating positive
fluorescence) (Hashimoto et al., Brain Res. 799:301-306, 1998; Ueda et al.,
Proc. Natl. Acad.
Sci. U.S.A 90:11282-11286, 1993). Inhibition of NAC fibril formation,
deposition,
accumulation, aggregation, and/or persistence is believed to be effective
treatment for a
number of diseases involving a-synuclein, such as Parkinson's disease, Lewy
body disease and
multiple system atrophy.
"Fibrillogenesis" refers to the presence of amyloid fibrils or fibrils formed
containing
a-synuclein and/or NAC. Inhibition of such fibrillogenesis with a therapeutic
compound may
include, but not limited to, treating, inhibiting, preventing, or managing
such amyloid, amyloid
fibril, a-synuclein and/or NAC fibril formation, deposition, accumulation,
aggregation and/or
persistence in a mammalian subject.
"A pharmaceutical agent" or "pharmacological agent" or "pharmaceutical
composition"
refers to a compound or combination of compounds used for treatment,
preferably in a pure or
near pure form. In the specification, pharmaceutical or pharmacological agents
include the
proanthocyanidins and procyanidins as examples. Disclosed pharmaceutical or
pharmacological compounds or compounds in compositions, are purified to 80%
homogeneity,
and preferably 90% homogeneity. Compounds and compositions purified to 99.9%
homogeneity are believed to be advantageous. As a test or confirmation, a pure
compound on
HPLC would yield a single sharp-peak band.
The disclosed compounds and compositions may possess one or more chiral
centers,
and can therefore be produced as individual stereoisomers or as mixtures of
stereoisomers,
depending on whether individual stereoisomers or mixtures of stereoisomers of
the starting
materials are used. Unless indicated otherwise, the description or naming of a
compound or
group of compounds is intended to include both the individual stereoisomers
and mixtures
(racemic or otherwise) of stereoisomers. Methods for the determination of
stereochemistry and
the separation of stereoisomers are well known to a person of ordinary skill
in the art [see the
discussion in Chapter 4 of March J: Advanced Organic Chemistry, 4th ed. John
Wiley and
Sons, New York, NY, 1992].
38
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
"Optionally substituted glycosyl" is glycosyl optionally substituted with up
to three
anionic substituents selected from sulfate, sulfonate, phosphate, phosphonate,
and carboxylate,
each optionally esterified with optionally substituted alkyl, optionally
substituted aryl, or
optionally substituted heteroaryl; examples include glucosyl, galactosyl,
rhamnogalactosyl, and
the like.
"Aryl" is a cyclic (monocyclic, condensed bicyclic, or linked bicyclic) group
having
from 5 to 12 ring carbon atoms, and sufficient ring unsaturation that the
group is "aromatic" as
that term is conventionally used, e.g. phenyl, naphthyl, biphenylyl, and the
like. A "heteroaryl"
group is an "aryl" group as just defined in which from 1 to 4 of the ring
carbon atoms have
been replaced by 0, S or NR (where R is hydrogen or C1_6 alkyl), e.g.
pyrrolyl, furanyl, thienyl,
benzofuranyl, and the like. A "substituted aryl or heteroaryl" is an aryl or
heteroaryl group as
just defined substituted by 1 to 3, preferably adjacent, hydroxyl groups, and
up to 5 non-
interfering substituents. A non-interfering substituent is a substituent that
does not adversely
affect the pharmacological activity of the compound and is not otherwise
pharmacologically
undesirable. Suitable non-interfering substituents include halogen, and C1_6
alkyl and C1-6
alkoxy, each optionally substituted with up to five halogen atoms.
Disclosed compounds for pharmacological or pharmaceutical treatment of an
amyloid
disease, or for treatment of a-synuclein/NAC fibrillogenesis, will include and
not be limited to
proanthocyanidins, procyanidins, anthocyanins, condensed tannins,
leucoanthocyanidins,
leucocyanins, anthocyanogens, epicatechin-catechin polymers or oligomers,
flavanoids, flavan-
3,4-diols, propelargonidins, and A-type, B-type and C-type procyanidins.
It has now been surprisingly found that Uncaria tomentosa compounds and
extracts
exhibit potent anti-amyloid activity. The individual compounds found to
exhibit such activity
belong to the general class of compounds known as polyphenols, and more
specifically
procyanidins and proanthocyanidins. Disclosed are methods involving the steps
of isolation to
acquire individual compounds of the invention including, but not limited to
procyanidins B2,
B4 and Cl. The extracts and compounds having anti-amyloid and anti-a-
synuclein/NAC
inhibitory activity can be purified by a variety of methods disclosed herein,
including solvent
extraction techniques, gel permeation chromatography, preparative high
performance liquid
chromatography or a combination of such techniques.
39
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Anti-amyloid and anti-a-synuclein/NAC compositions and compounds containing
the
proanthocyanidins can be prepared in accordance with standard techniques well
known to
those skilled in the pharmaceutical art. Such compositions can be administered
in dosages and
by techniques well known to those skilled in the art taking into consideration
such factors such
as age, sex, weight, and condition of the particular patient, and the route of
administration. The
compositions can be co-administered or sequentially administered with other
potential anti-
amyloid agents, or anti-a-synuclein/NAC agents; again taking into
consideration such factors
as the age, sex, weight and condition of the particular patient, and the route
of administration.
Examples of compositions useful to effect the disclosed aims include solid
compositions for oral administration such as capsules, tablets, pills, and the
like, as well as
chewable solid formulations, to which the present invention may be well
suited; liquid
preparations for orifice, e.g. oral, nasal, administration such as
suspensions, syrups or elixers;
and preparations for parental, subcutaneous, intradermal, intramuscular or
intravenous
administration (e.g. injectable administration) such as sterile suspensions or
emulsions. The
active proanthocyanidin compound may be in admixture with a suitable carrier,
diluent, or
excipient such as sterile water, physiological saline or the like. The active
anti-amyloid
compounds of the invention can be provided in lyophilized form for
reconstituting, for
instance, in isotonic, aqueous, saline buffer.
These compounds can be purified, e.g., compounds or combinations thereof can
be
substantially pure; for instance, purified to apparent homogeneity. Purity is
a relative concept,
and the numerous Examples demonstrate isolation of inventive compounds or
combinations
thereof, as well as purification thereof, such by the methods exemplified a
skilled artisian can
obtain a substantially pure compound or combination thereof, or purify them to
apparent
homogeneity (e.g., purity by HPLC; observation of a single chromatographic
peak). As
defined herein, a substantially pure compound or combination of compounds is
at least about
70% pure, more advantageously at least 80-% pure, at least 90% pure, more
preferably greater
than 90% pure, e.g., at least 90-95% pure, or even purer such as greater than
95% pure, e.g.,
99.99% pure.
Polyphenols, (+)-catechin and (-)-epicatechin, are used herein to exemplify
the types of
polyphenol oligomers that may be prepared by the method of the present
invention. The
linkages between adjacent, the polyphenol monomers, (+)-catechin and (-)-
epicatechin, are
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
from position 4 to position 6 or from position 4 to position 8; and this
linkage between position
4 of a monomer and position 6 and 8 of the adjacent monomeric units is
designated herein as
(4-->6) or (4->8).
Moreover, stereoisomers of the oligomers are encompassed within the scope of
the
invention. The stereochemistry of the substituents on a flavanoid monomer of
the oligomer
may be described in terms of their relative stereochemistry, "alpha/beta" or
"cis/trans", or in
the terms of the absolute stereochemistry, R/S. The term "alpha" indicates
that the substituent
is oriented below the plane of the flavan ring, whereas "beta" indicates that
the substituent is
oriented above the plane of the ring. The term "cis" indicates that the two
substituents are
oriented on the same face of the ring, whereas "trans" indicates that the two
substituents are
oriented on opposite faces of the ring.. The terms R and S are used to denote
the arrangement
of the substituents about a sterogenic or "chiral" center, based on the
ranking of the groups
according to the atomic number of the atoms directly attached to that
stereogenic center. For
example, the polyphenol, (+)-catechin, may be defined as (2R, trans)-2-(3',4'-
dihydroxyphenyl)-3,4-dihydo-2H-1-benzopyran-3,5,7-triol, or as (2R, 3S)-flavan-
3,3', 4', 5,7-
pentaol. Interflavan (polyphenol-polyphenol) bonding is often characterized
using the relative
terms a/B or cis/trans; a/B is used herein to designate the relative
stereochemistry of the
interflavan bonding.
There are multiple stereochemccal linkages between position 4 of a monomer and
position 6 and 8 of the adjacent monomer; and the stereochemcial linkages
between
monomeric units is designated as (4(x--*6) or (48-.6) or (4a--48) or (4B->8)
for linear
oligomers. When catechin is linked to another catechin or epicatechin, the
linkages are
advantageously (4(x->6) or (4(x-8). When epicatechin is linked to catechin or
another
epicatechin, the linkages are advantageously (46-46) or (4B-->8).
In addition to carbon position 4, a bond to carbon position 2 has alpha or
beta
stereochemistry, and a bond to carbon position 3 has alpha or beta
stereochemistry (e.g., (-)-
epicatechin or (+)-catechin).
Examples of preferred compounds include, but are not limited to, dimers,
epicatechin-
4B-8-epicatechin and epicatechin-4B>6-epicatechin, wherein epicatechin-4B--8-
epicatechin
is preferred; trimers, [epicatechin-(48--8)]27epicatechin, [epicatechin-(4B-
>8)]2 catechin and
[epicatechin-(4B->6)]2 epicatechin, wherein [epicatechin-(4B-->8)]2-
epicatechin is preferred;
41
CA 02441099 2009-06-10
52171-14
tetramers, [epicatechin-(4I3--8)]3-epicatechin; [epicatechin-(48-48)]3
catechin; and
[epicatechin-(4B->8)12epicatechin-(4B--6)-catechin, wherein [epicatechin-(48-
8)]3
epicatechin is preferred; and pentamers, [epicatechin-(48--8)]4 epicatechin;
[epicatechin-
(48--X8)]3-epicatechin-(48--6)-epicatechin; [epicatechin-)(4B-8)]3-epicatechin-
(46--j6)-
catechin; [epicatechin-(4B-- 8)]3epicatechin0(48--8)-catechin; and
[epicatechin-(48-8)]3-
epicatechin-(4B--6)-catechin, wherein [epicatechin-(4B--*8)]4-epicatechin is
preferred.
It will be understood from the detailed description that the aforementioned
list is
exemplary and is provided to illustrate the types of compounds that may be
prepared by the
methods of the present invention and it is not intended as an exhaustive list
of the inventive
compounds encompassed by the present invention.
One skilled in the art will appreciate that rotation of a number of bonds
within the
oligomer of the invention may be restricted due to steric hindrance,
particularly if the oligomer
is substituted, such as with benzyl groups. Accordingly, all possible
regioisomers and
stereoisomers of the compounds of the invention are encompassed within the
scope of the
invention.
Proanthocyanidins can not only be extracted and purified from Uncaria
tonientosa as
described in the present invention, but also from other various plants such as
grape, kaki
(Japanese persimmon), betel palm, apple, barley, cocoa leaf, cocoa liqueur,
dark chocolate,
Nest-leaf, rhubarb, cinnamon, adzuki bean, raspberry, etc. They can also be
obtained by
conventional chemical synthesis.
Regarding chemical synthesis of procyanidins and proanthocyanidin, a method of
producing dimers of epicatechin or catechin is disclosed in Journal of
Chemical Society, Parkin
Transaction I, pp. 1535-1543, 1983. To chemically produce proanthocyanidins
for use as
disclosed, is referred to in US Patent #6,165,912 (Tuckmantel et al; Dec
5/2000) and US Patent
#6,207,842 B 1 (Romanczyk Jr. et al; Mar 27/2001).
Furthermore, while procyanidins or proanthocyanidins derived from Uncaria
tornentosa
are disclosed, persons skilled in the art will appreciate by means of this
disclosure and envision
synthetic and alternate extraction routes to obtain the active compounds.
Accordingly,
synthetic polyphenols or procyanidins or proanthocyanidins or their
derivatives which include,
but are not limited to glycosides, gallates, esters, and the like are included
within the scope of
the invention.
42
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Disclosed are methods pertaining to the isolation, identification and use of
anti-amyloid
compounds derived from plant material, and the surprising discovery that
proanthocyanidins are
potent inhibitors of amyloid and a-synuclein/NAC fibrillogenesis, and cause a
potent
disruption/disassembly of pre-formed fibrils for a variety of amyloid and a-
synuclein diseases.
Exemplary compounds identified to serve as potent amyloid fibril inhibiting
agents include
procyanidins, such as epicatechin-epicatechin, catechin-epicatechin,
epiafzelechin-epicatechin
dimers, epicatechin-epicatechin-epicatechin trimers, as well as other
epicatechin and/or catechin
oligomers for the treatment of amyloid diseases including, but not limited to,
Alzheimer's
disease, type II diabetes, and systemic AA amyloidosis, as well as inhibiting
a-synuclein or
non-amyloid component (NAC) fibril formation for the treatment of Parkinson's
and Lewy
body disease.
Also disclosed are methods for preparing and isolating such compounds, as well
as new
uses for them, especially as amyloid and a-synuclein/NAC fibril disrupting
agents. This
invention is also directed to methods for inhibiting or eliminating amyloid
fibril formation,
deposition, accumulation and/or persistence in a number of different amyloid
diseases by
treatment of patients with proanthocyanidins of the A, B and C types,
including monomers,
dimers, trimers and multimers of epicatechin and catechin. An exemplary
procyanidin
compound is a substituted epicatechin-epicatechin or catechin-epicatechin
dimer, such as
epicatechin-4B->8-epicatechin, catechin-4a->8-epicatechin, or epiafzelechin-4B-
*8-
epicatechin, or other oligomers.
Methods of isolation, identification and use of amyloid-inhibiting compounds
derived
from plant material are disclosed for the therapeutic intervention of
Alzheimer's disease, type 2
diabetes, Parkinson's disease, systemic AA amyloidosis and other diseases
involving amyloid
fibril formation and accumulation, especially methods of isolating amyloid
inhibiting
compounds from Uncaria tomentosa and related plants, and to the use of those
compounds.
Pharmacology and Utility,
The disclosed compounds act to inhibit or prevent amyloid fibril formation,
inhibit or
prevent amyloid fibril growth, and/or cause disassembly, disruption, and/or
disaggregation of
preformed amyloid fibrils and amyloid protein deposits. Their activity can be
measured in vitro
by methods such as those discussed in Examples 4 through 7 while their
activity in vivo against
43
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
amyloidoses can be measured in animal models, such as those of Alzheimer's
disease and in
humans by a method such as that discussed in Example 11.
The disclosed compounds also act to inhibit or prevent a-synuclein/NAC fibril
formation, inhibit or prevent a-synuclein/NAC fibril growth, and/or cause
disassembly,
disruption, and/or disaggregation of preformed a-synuclein/NAC fibrils and a-
synuclein/NAC-associated protein deposits. Their activity can be measured in
vitro by methods
similar to those discussed in Examples 4 through 7 below.
The therapeutic ratio of a compound can be determined, for example, by
comparing the
dose that gives effective anti-fibril (anti-amyloid or anti-a-synuclein/NAC
activity in a suitable
in vivo model in a suitable animal species such as the .mouse, with the dose
that gives
significant weight loss (or other observable side-effects) in the test animal
species.
Pharmaceutical compositions and administration
In general, compounds will be administered in pure isolated form in
therapeutically
effective amounts by any of the usual modes known in the art, either singly or
in combination
with at least one other compound of this invention and/or at least one other
conventional
therapeutic agent for the disease being treated. A therapeutically effective
amount may vary
widely depending on the disease, its severity, the age and relative health of
the animal being
treated, the potency of the compound(s), and other factors. As anti-fibril
agents, therapeutically
effective amounts of compounds of this invention may range from 1-1000 mg/Kg
body weight;
for example, 10-100 mg/Kg. A person of ordinary skill in the art will be
conventionally able,
and without undue experimentation, having regard to that skill and to this
disclosure, to
determine a therapeutically effective amount of a compound for the treatment
of amyloidosis
or a-synuclein/NAC fibril formation.
In general, compounds will be administered as pharmaceutical compositions by
one of
the following routes: oral, topical, systemic (e.g. transdermal, intranasal,
or by suppository), or
parenteral (e.g. intramuscular, subcutaneous, or intravenous injection).
Compositions may take
the form of tablets, pills, capsules, semisolids, powders, sustained release
formulations,
solutions, suspensions, elixirs, aerosols, or any other appropriate
compositions; and comprise at
least one compound of this invention in combination with at least one
pharmaceutically
acceptable excipient. Suitable excipients are well known to persons of
ordinary skill in the art,
and they, and the methods of formulating the compositions, may be found in
such standard
44
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
references as Alfonso AR: Remington's Pharmaceutical Sciences, 17th ed., Mack
Publishing
Company, Easton PA, 1985. Suitable liquid carriers, especially for injectable
solutions, include
water, aqueous saline solution, aqueous dextrose solution, and glycols.
In particular, the compound(s) - optimally only one such compound is
administered in
any particular dosage form - can be administered, orally, for example, as
tablets, troches,
lozenges, aqueous or oily suspension, dispersible powders or granules,
emulsions, hard or soft
capsules, or syrups or elixirs. Compositions intended for oral use may be
prepared according to
any method known in the art for the manufacture of pharmaceutical compositions
and such
compositions may contain one or more agents selected from the group consisting
of sweetening
agents, flavoring agents, coloring agents and preserving agents in order to
provide
pharmaceutically elegant and palatable preparations.
Tablets contain the compound in admixture with non-toxic pharmaceutically
acceptable
excipients that are suitable for the manufacture of tablets. These excipients
may be for
example, inert diluents, such as calcium carbonate, sodium carbonate, lactose,
calcium
phosphate or sodium phosphate; granulating and disintegrating agents, for
example, maize
starch or alginic acid; binding agents, for example, maize starch, gelatin or
acacia, and
lubricating agents, for example, magnesium'stearate or stearic acid or tale.
The tablets may be
uncoated or they may be coated by known techniques to delay disintegration and
absorption in
the gastrointestinal tract and thereby provide a sustained action over a
longer period. For
example, a time delay material such as glycerol monostearate or glycerol
distearate may be
employed. Formulations for oral use may also be presented as hard gelatin
capsules wherein
the compound is mixed with an inert solid diluent, for example, calcium
carbonate, calcium
phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient
is mixed with
water or an oil medium, for example, peanut oil, liquid paraffin or olive oil.
Aqueous suspensions contain the compound in admixture with excipients suitable
for
the manufacture of aqueous suspensions. Such excipients are suspending agents,
for example,
sodium carboxymethylcellulose, methylcellulose, hydroxypropylmethyl cellulose,
sodium
alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia; dispersing or
wetting agents
may be naturally occurring phosphatides, for example lecithin, or condensation
products of an
alkylene oxide with fatty acids, for example polyoxyethylene stearate, or
condensation
products of ethylene oxide with long chain aliphatic alcohols, for example,
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with
partial esters
derived from fatty acids such as hexitol such as polyoxyethylene sorbitol
monooleate, or
condensation products of ethylene oxide with partial esters from fatty acids
and a hexitol
annhydrides, for example, polyethylene sorbitan monooleate. The aqueous
suspensions may
also contain one or more preservatives, for example, ethyl or n-propyl p-
hydroxybenzoate, one
or more coloring agents, one or more flavoring agents, or one or more
sweetening agents, such
as sucrose or saccharin.
Oily suspensions may be formulated by suspending the compound in a vegetable
oil,
for example arachis oil, olive oil, sesame oil, or coconut oil or in a mineral
oil such as liquid
paraffin. The oily suspensions may contain a thickening agent, for example
beeswax, hard
paraffin or cetyl alcohol. Sweetening agents, such as those set forth below,
and flavoring
agents may be added to provide a palatable oral preparation. These
compositions may be
preserved by the addition of an antioxidant such as ascorbic acid. Dispersible
powders and
granules suitable for preparation of an aqueous suspension by the addition of
water provide the
active ingredient in admixture with a dispersing or wetting agent, a
suspending agent and one
or more preservatives. Suitable dispersing or wetting agents and suspending
agents are
exemplified by those already described above. Additional excipients, for
example sweetening,
flavoring and agents, may also be present.
The compounds may also be in the form of oil-in-water emulsions. The oily
phase may
be a vegetable oil, for example olive oil or arachis oils, or a mineral oil,
for example liquid
paraffin or mixtures of these. Suitable emulsifying agents may be naturally-
occurring gums, for
example gum acacia or gum tragacanth, naturally occurring phosphatides, for
example soy
bean, lecithin, and occurring phosphatides, for example soy bean, lecithin,
and esters or partial
esters derived from fatty acids and hexitol anhydrides, for example sorbitan
monooleate, and
condensation products of the said partial esters with ethylene oxide, for
example
polyoxyethylene sorbitan monooleate. The emulsion may also contain sweetening
and
flavoring agents. Syrups and elixirs may be formulated with sweetening agents,
for example,
glycerol, sorbitol or sucrose. Such formulations may also contain a demulcent,
a preservative
and flavoring and coloring agents.
The compound can also be administered by injection or infusion, either
subcutaneously
or intravenously, or intramuscularly, or intrasternally, or intranasally, or
by infusion techniques
46
CA 02441099 2009-06-10
52171-14
in the form of sterile injectable or oleaginous suspension. The compound may
be in the form of
a sterile injectable aqueous or oleaginous suspensions. These suspensions may
be formulated
according to the known art using suitable dispersing of wetting agents and
suspending agents
that have been described above. The sterile injectable preparation may also be
a sterile
injectable solution or suspension in a non-toxic parenterally-acceptable
diluent or solvent for
example, as a solution in 1,3-butanediol. Among the acceptable vehicles and
solvents that may
be employed are water, Ringer's solution and isotonic sodium chloride
solution. In addition,
sterile, fixed oils are conventionally employed as a solvent or suspending
medium. For this
purpose any bland fixed oils may be conventionally employed including
synthetic mono- or
diglycerides. In addition fatty acids such as oleic acid find use in the
preparation of injectables.
Dosage regimens can be adjusted to provide the optimum therapeutic response.
For example,
several divided dosages may be administered daily or the dosage may be
proportionally
reduced as indicated by the exigencies of the therapeutic situation.
It is especially advantageous to formulate the compounds in dosage unit form
for ease
of administration and uniformity of dosage. Dosage unit form as used herein
refers to
physically discrete units suited as unitary dosages for the subjects to be
treated; each
containing a therapeutically effective quantity of the compound and at least
one pharmaceutical
excipient. A drug product will comprise a dosage unit form within a container
that is labeled or
accompanied by a label indicating the intended method of treatment, such as
the treatment of
an amyloid disease, such as Alzheimer's disease, or of a disease associated
with a-
synuclein/NAC fibril formation, such as Parkinson's disease.
The following non-limiting Examples are given by way of illustration only and
are not
considered a limitation of this invention, many apparent variations of which
are possible
without departing from the spirit or scope thereof.
EXAMPLES
Example 1:
Isolation of Amyloid Inhibitory Components from Uncaria tomentosa and PTI-777
We have previously reported in US Publication No. US 2002 - 0086067A1,
US Patent Nos. 6,607,758; 6,929,808 and US Publication No. US 2003 - 0017998A1
pertaining to the
47
CA 02441099 2009-06-10
52171-14
discovery of amyloid inhibitory components in an extract of the rain forest
woody vine,
Uncaria tomentosa, with regards to the treatment of neurological disorders
involving beta-
amyloid protein (AB) or a-synuclein/NAC fibrillogenesis and other amyloid
disorders. We have
previously reported the discovery that a methonolic extract of the powdered
bark of Uncaria
tomentosa contains potent amyloid inhibitory activity that is relatively
concentrated in a mixture
of compounds consisting of mainly polyphenols. Tests of samples of pure
oxindole alkaloids
that were known to be major components of Uncaria tomentosa also demonstrated
in these
previous studies that oxindole alkaloids were not responsible for the amyloid
inhibitory activity.
Previously we isolated and identified two polyphenolic compounds from the
methanolic
extract of Uncaria tomentosa known as PTI-777; and these were demonstrated to
be
chlorogenic acid and epicatechin. The methanolic extract of Uncaria tonzentosa
found to exhibit
potent amyloid inhibitory activity was previously referred to as "PTI-777". As
described in
previously reported US Patent No. 6,929,808; PTI-777 represents a group of
approximately 11 major fractions referred to as fraction F, fraction G,
fraction H, fraction I, fraction J, fraction K1, fraction K2, fraction L,
fraction M, fraction N and
fraction 0, which were isolated from the powdered bark of Uncaria tomentosa.
Some of these
fractions, as demonstrated in the present invention, were further purified to
one or two major
components as was done with fraction H (now found to contain 2 major
components referred to
as H1 and H2 as described below).
Using PTI-777 as a starting point, as described in US Patent
No. 6,929,080; we disclose details of methods of isolation and identification
of
further major components contained within PTI-777, referred to as compounds
H2, Hl, K2 and
K1, which all belong to the general class of proanthocyanidins, and which were
all found to
possess potent amyloid and a-synuclein/NAC inhibitory activity. In addition,
we disclose and
teach new methods for the isolation of such anti-amyloid/ anti-a-synuclein/NAC
compounds,
and proanthocyanidins from Uncaria tomentosa, and other plants.
Varying the methods of high pressure liquid chromatography (HPLC) earlier
reported by
us in our applications cited above, and by using a lower % of acetonitrile
(described in detail
below), we obtained a trace where the main peak H, came off at the same
relative retention time
as earlier reported, and which also resulted in a further separation of some
of the peaks (i.e. H1
and H2), previously seen to be overlapping (i.e. as fraction H). In a further
variation, and noting
48
CA 02441099 2009-06-10
52171-14
that one of our HPLC traces was run at 1.5 min/ml, we found that running the
elution at 2
mUmin obtained a trace almost identical to the 1.5 ml/min trace.
Epicatechin, together with catechin, epigallocatechin and various catechin
gallates are
known to be present in green tea (see our US Patent Publication No. US 2002 -
0086067A1;
and also Baumann et al, J. Natural Prod. 64:353-355,2001, and Zeeb et al,
Anal.
Chem.. 72:5020-5026, 2000). In some of the reports of the isolation of some of
these catechins,
'sephadex LH2O gel in particular, along with other gels, and also solvent
partitions, and other
HPLC methods have been discussed. In addition, silica gel chromatography,
eluting with a
gradient of methanol in chloroform has also been used on compounds of a
polarity similar to the
catechins, for example, flavanoid and iridoid glycosides (Kim et al, J. Nat.
Prods. 64:75-78,
2001; Sang et al, J. Nat. Prods. 64:799-800, 2001; Calls et al, J. Nat. Prods.
64:961-964, 2001).
We had already used a Sephadex LH2O gel in an earlier step in the purification
of the
extract PTI-777. Further work was therefore concentrated on the use of reverse
phase (RP) C18
silica or silica gel chromatography. Using a variety of solvent systems, we
analyzed the use of
RP C18 and silica gel thin layer chromatography (TLC) behavior of the methanol
extract of
PTI-777. RP C18 silica showed all the material to be in one main spot on the
TLC, but with
silica we could see several separate spots.
We therefore tried separation of a small sample of extract PTI-777 by silica
gel
chromatography. The extract appeared to be both light and air sensitive, so
the column was used
in limited light, with a rapid solvent gradient, to minimize decomposition on
the silica. HPLC
analysis of the column fractions showed that we had discovered new methods to
perform good
separation.
In particular fraction 9 (see methods 1 and 2) was almost pure epicatechin,
and fractions
11 to 13 contained mostly peak H2, with its underlying H1 peak. Other
fractions showing
interesting concentrations of other peaks, in particular K1 was concentrated
in fraction 10,
whilst K2 was concentrated in fraction 14. The total recovery of useful
material was 50%, but
given the separation of some of the peaks, this appeared to be a good way to
separate a large
amount of material to give fractions rich in various peaks.
49
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Example 2:
Isolation and Identification of Peak H2 from PTI-777 as an Epicatechin-
Epicatechin Dimer
General Experimental Procedures
All solvents were distilled before use and were removed by rotary evaporation
under
vacuum at temperatures up to 20-60 . Octadecyl functionalised silica gel (C18)
was used for
reversed-phase (RP) flash chromatography, and Merck silica gel 60, 200-400
mesh, 40-63 pm,
was used for silica gel flash chromatography. TLC was carried out using Merck
DC-
plastikfolien Kieselgel 60 F2541 first visualised with a UV lamp, and then by
dipping in 5%
aqueous ferric chloride solution. Optical rotations were measured on a Perkin-
Elmer 241
polarimeter. Mass, ultraviolet (UV), and infra-red (IR) spectra were recorded
on Kratos MS-
80, Shimadzu UV 240, and Perkin-Elmer 1600 FTIR instruments, respectively. NMR
spectra,
at 25-, were recorded at 500 or 300 MHz for 111 and 125 or 75 MHz for 13C on
Varian INOVA-
500 or VXR-300 spectrometers. Chemical shifts are given in ppm on the S scale
referenced to
the solvent peak CH3OH at 3.30, CD3OD at 49.3 ppm, CHC13 at 7.25, CDC13 at
77.0; (CH3)2CO
at 2.15 and (CD3)2CO at 30.5.
HPLC Conditions to Isolate Peaks H2 and H1
The analytical HPLC equipment consisted of a Waters 717 autosampler, 600 pump
and
controller, and a 2487 UV detector controlled by Omega software. Samples were
analyzed by
using an RP-18 semi-preparative column (Phenomenex Jupiter 5 pm C18 300A, 250
x 10 mm)
with a guard column (Phenomenex SecurityGuard cartridge containing a C18 ODS 4
x 3 mm,
5 m column) fitted at 30 C.Samples (5 pl) were analyzed using a mobile phase
flow rate of
5.0 mL/min, with UV detection at 280 nm.
Solvent A - CH3CN containing 0.1% TFA
Solvent B - H2O containing 0.1% TFA
HPLC Method I
Time (minutes) Solvent A Solvent B
0 11 89
20 11 89
100 0
31 11 89
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
HPLC Method 2
Time (minutes) Solvent A Solvent B
0 8 92
20 8 92
30 100 0
31 8 92
Samples were generally run under HPLC method 1, unless otherwise stated.
An Example of the Silica Gel Fractionation of PTI-777
A sample of the extract PTI-777 (1 g) was dissolved in methanol (2 ml) then
loaded
onto a silica gel (10g) column, prepared in chloroform. Elution of this column
with increasing
proportions of methanol in chloroform gave 45 fractions.
Table 1 Example of a Silica Gel Column Fractionation of PTI-777
Solvent Fractions Peaks present Weight
10% MeOH in CHC13 50 ml 1-11 nothing 0 mg
12 - 15 J and pre-F 45 mg
16-19 J,KI 16 mg
20% MeOH in CHC13 50 ml 20 - 24 Hi, H2 134 mg Figure 3
25-30 H1,H2,K2 101 mg
40% MeOH in CHC13 50 ml 31 - 34 H1,H2 mostly K2 39 mg
50% MeOH in CHC13 50 ml 35 - 40 Hl, H2, K2, L post L 226 mg
100% MeOH 100 nil 41 - 45 Hl, H2, K2, others 228 mg
The main component of peak H, called H2, of the PTI-777 was isolated by a
series of
chromatographic techniques, monitored by HPLC (Figures 2-5). We initially
separated the
PTI-777 extract by column chromatography over silica gel (Table l)(and
tracings were
monitored by HPLC using method 1), whereby elution with 20% methanol in
chloroform gave
a fraction rich in the two components of peak H (134 mg)(with the HPLC tracing
using method
2, shown in Figure 3). An HPLC method was developed to separate the two main
components
of peak H on a preparative scale (i.e. HPLC method 2), to give us a mostly
pure Hl (16
mg)(HPLC method 1, Figure 4) and pure H2 (23 mg) (HPLC method 1, Figure 5).
A -ve ion electrospray mass spectrum of H2 gave a clean 100% ion at 577
daltons. This
is appropriate for the molecular ion (M+-H) of a molecular formula of
C30H260121 such as a
51
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
dimer of two epicatechin, or isomeric units. Epicatechin has previously been
isolated from the
PTI-777 extract and is described in the parent case to this application.
A 'H NMR spectrum (Figure 6) of peak H2 showed unusual broadening of the
signals,
whilst the 13C NMR (Figure 7) showed sharp and broad signals, consistent with
some kind of
flavonol dimer. We were surprised to see no signals in the 5.8 - 6.3 ppm
region of the 'H
spectrum, or in the 90 - 99 ppm region of the 13C NMR spectrum, where the
characteristic H-
6/H-8 and C-6/C-8 signals would appear. Running the NMR spectra in
deuteroacetone (Figures
8, 9) instead of deuteromethanol, showed the expected signals to be present,
indicating that in
deuterated protic solvents, an exchange of these H-6 and H-8 protons for
deuterons took place.
Broadening of signals is often due to restricted rotation within a molecule.
This rotation
can be sped up by running the spectrum at higher temperatures which gives
sharper signals.
We therefore ran the 'H and 13C NMR spectra first at 40 C, then at 50 C.
Unfortunately,
although there were definite signs of sharpening of the signals, there were
also new signals,
showing either rearrangement or degradation of H2.
Peak H2 Data Summary
Aliquots (14 x 70 1) of fractions 20 - 24 (134 mg in 1 ml)(Table 1) from the
above
silica gel column were separated by HPLC using method 2. The peaks between
14.5 and 16.2
and between 16.2 and 19.0 minutes were collected, then freeze dried to give
two products,
about 80% pure H1, retention time 15.1 minutes (HPLC method 2)( 16 mg) as a
white solid;
and pure H2 (23 mg) as a white solid, retention time 16.9 minutes (HPLC method
2).
-ve electrospray mass spectroscopy 577 (M+-H, 100%)
molecular weight of H2 = 578
1H NMR (CD3OD): 2.81 (1H, br d), 2.95 (1H, br d), 3.92 (1H, s), 4.30 (1H, br
s), 4.65 (IH, br
s), 4.98 (br s), 5.07 (1H, br s), 6.70 - 7.20 (6H, m).
13C NUR (CD3OD): 29.97 (C-41), 37.50 (C-4u), 67.33, 73.83, 77.45, 80.23,
101.00, 115.71,
116.27, 116.31, 119.77, 132.41, 145.96 and 146.22.
1H NMR ((CD3)2CO): 2.83 (114, m, H-41), 2.96 (114, m, H-41), 4.10 (1H, s),
4.41 (1H, br s),
4.83 (1H, s, H-4u), 5.07 (111, br s), 5.20 (1H, br s), 6.07 (1H, s), 6.09 (1H,
s), 6.12 (1H, s), 6.80
- 7.20 (6H, m) and 7.50 - 8.30 (6H, br s, OHs)
52
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
13C NMR ((CD3)2CO): 30.3, 37.6, 67.1, 73.6, 77.6, 79.9, 96.6, 97.1, 97.7,
101.3, 115.5 -
116.2,119.9,120.2,132.5,133.1,145.6-146.1,156.4-160Ø
UV (MeOH) X max (log a) 210 (4.79), 227sh (4.48) and 280 (3.81) nm;
[0124 589nm +29.00, [0]24 577nm +19.80, [0]24 546nm +5.70,[-124
43Snm-87.40, [0]24 405nm-106.10
(c 0.2, MeOH)
Acetylation of H2
Since the compound H2 was unstable under the conditions necessary to
ultimately
prove its structure by NMR spectroscopy, we had to make a stable derivative.
Acetylation of a
sample of pure H2 gave a peracetate (Figure 10), which was purified by column
chromatography over silica gel. A larger sample of this peracetate, identical
by NMR and TLC,
was also obtained by silica gel separation of the two main products from
acetylation of a
fraction rich in HI and H2.
For these studies, a sample of H2 (7 mg) was dissolved in a mixture of acetic
anhydride
(0.5 ml) and pyridine (0.5 ml). The mixture stood at room temperature for 18
hours, then the
solvents were removed in vacuo. Purification by column chromatography over
silica gel,
eluting with 20% ethyl acetate in dichloromethane gave an H2 peracetate (6 mg)
as a colorless
gum. The NMR data is collated in Table 2.
One and 2D NMR experiments (see Figures 11, 12)(Table 2) of the H2 peracetate
showed it to be a decaacetate. Two sets of signals were seen in both the 'H
and 13C NMR
spectra, in a ratio of three to one. These were due to rotational isomers
(atropisomers), shown
by opposite phase cross peaks in the nuclear Overhauser enhancement
spectroscopy (NOESY)
spectrum to interconvert in the time frame of the NMR experiment. It would not
be possible to
separate these atropisomers by chromatography. If they could be separated by
crystallization,
they would revert to a mixture upon taking into solution for biological assay.
We solved the
structure using the signals of the major atropisomer.
The presence of two flavan-3-ol units could be seen from the four 13C signals
for the C-
2 and C-3 positions in the 60 - 80 region, as well as a signal at 26.65 for
the free C-4 position
of the lower unit and. a signal at 33.99 for the linked C-4 of the upper unit.
A CIGAR 1H -13C
correlation experiment (Figures 13, 14) showed that the two units were
connected from the
4(u) position to the 8(1) position, by the correlations from H-4(u) to C-8(l)
and C-8a(l). The
53
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
stereochemistries at C-2 and C-3 of both upper and lower units was shown to be
the same as in
epicatechin by the similar chemical shifts of the 'H and 13C signals for the
lower unit, as well as
the similar low coupling constants between H-2 and H-3 in both units. The
stereochemistry of
the linkage was shown to be 4B->8 from the NOESY interactions (Figures 15-17),
in particular
the lack of an interaction between H-2(u) and H-4(u), and the presence of an
interaction
between H-2(u) and H-6'(1), and between H-3(u) and H-6'(1). The structure of
the natural
product H2 was therefore assigned to be epicatechin-48->8-epicatechin (Figure
18).
Epicatechin-4B->8-epicatechin is also known as procyanidin B2 or
proanthocyanidin
B2. Our NMR data of H2 match partial NMR data published on procyanidin B2
(Kashiwada et
al, Chem. Pharm. Bull. 38:888-893, 1990; Porter et al, J. Chem. Soc. Perkin
1:1217-1221,
1982), and our data of the H2 peracetate (Figure 10; Table 2) exactly matched
the published
data on peracetylated procyanidin B2 (Franck et al, ACH Models in Chemistry
136:511-517,
1999). The optical rotation of +29.0 compared to a literatures value of +25
showed the
absolute stereochemistry to be the same as found previously.
Table 2: 500 MHz NMR data of the H2 Peracetate in Deuterochloroform, Major
Atropisomer.
lower Ca Hb CIGARC H-C selected NOE interactions
2 77.00 4.53, br s 31, (4)1, (8a)l, 11, 2'l, 6'I 31, 41, 2'1, 6'1
3 66.81 5.09, br d, 5 (4)1, 4a1, 3-Oacl 21, 41, 2'1, 6'1
4 26.65 2.85 br d 18 21, 31, 4al, 51, 8a1 2, 3
2.92 dd 18, 5
4a 111.68
5 149.07
6 110.33 6.64, s 4a1, 51, 71, 81
7 147.82
8 116.75
8a 154.21
1' 134.46
2' 122.46 7.00, d 2 61
3' 141.64
4' 141.97
5' 122.78 7.02, d, 7 1'1, 3'I, 4'1, 6'I
6' 125.06 6.87, dd, 2, 7
3 OAc 170.38 1.97s
5 OAc 167.97 51, 2.27 s
7 OAc 169.98 71, 2.35 s
3'/4' OAc 168.31 12.27s
4'/3' OAc 167.86 12.02s
54
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
upper
2 73.61 5.56, br s 3u, (4u), Vu, 2'u, 6'u 3u, 2'u, 6'u, 2'I, 6'1
3 71.06 5.15, dd, 1,2 3-OAcu, 4u, 4au, (81) 2u, 4u, 2'u, 6'u
4 33.99 4.45, d 2 2u, 3u, 4au, 5u, 71, 81, 8au,8a1 3u, 2'u, 6'u, 2'l, 6'l
4a 111.57
147.91
6 108.63 6.22, d J 1 Hz 4au, 5u, 7u, 8u
7 149.15
8 107.23 5.98, d, J 1 Hz 4au, 6u, 7u, 8au
8a 155.35
1' 136.55
2' 122.21 7.35, d, 2 6'u
3' 141.75
4' 141.99
5' 123.11 7.16, d, 7 1'u, 3'u, 4'u
6' 124.43 7.26, dm, J 7Hz
3 OAc 169.74 1.86s
5 OAc 169.06 5u, 1.86 s
7 OAc 168.87 7u, 2.17 s
3'/4'OAc 168.31 2.27 s
4'/3'OAc 167.87 12.02s
ashift in ppm. bShift in ppm, multiplicity, couplings in Hz. cBrackets
indicate weak correlations,
I = lower unit, u = upper unit. dRecorded for H-2, H-3 and H-4 only. OAc
methyl groups not shown
Example
5 Isolation and Identification of Peak H1 from PTI-777
General Experimental Procedures
The minor component of peak H, referred to as Hl, of the PTI-777 extract was
also
isolated by a series of chromatographic techniques, monitored by HPLC (see
Example 1,
Experimental Procedures for details). We initially separated the original PTI-
777 extract (see
HPLC tracing, Figure 2) by column chromatography over silica gel, where 20%
methanol in
chloroform gave a fraction rich in the two components of peak H (134 mg). An
HPLC method
was developed to separate the two main components of peak H on a preparative
scale)(see
HPLC tracing, Figure 3), to give us a mostly pure H1 (16 mg)(see HPLC tracing,
Figure 4) and
pure H2 (23 mg).
A -ve ion electrospray mass spectrm of H1 gave a clean 100% ion at 577
daltons. This
is appropriate for the molecular ion (M-H) of a molecular formula of C30H26012
(molecular
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
weight 578), such as a dimer of two epicatechin, or isomeric units. We had
previously isolated
and identified epicatechin from the PTI-777 extract.
A 'H NMR spectrum (Figure 19) and 13C NMR spectrum (Figure 20) of peak Hl
showed two sets of signals, consistent with some kind of flavonol dimer, with
major and minor
atropisomers present.
Acetylation of a sample of Hl gave a peracetate (Figure 21), which was
purified by
column chromatography over silica gel. A larger sample of this peracetate,
identical by NMR
and TLC, was also obtained by silica gel separation of the two main products
from acetylation
of a fraction rich in Hl and H2.
One and 2D NMR experiments (Figures 22, 23) (Table 3) on the Hi peracetate
showed
it to be a decaacetate. The structure was solved using the signals of the
dominant atropisomer.
The presence of two flavan-3-ol units could be seen from the four 13C signals
in the 60 - 80
region (Figure 23), as well as a signal at 26.56 for the free C-4 position of
the lower unit and a
signal at 36.72 for the coupled C-4 of the upper unit (Figure 23). The
positions of the 13C
signals and the small couplings of the 1H signals of the lower unit were
typical of an
epicatechin unit, whilst the positions of the 13C signals and the much larger
couplings of the IH
signals of the upper unit were typical of a coupled catechin (Fletcher et al,
JCS Perkin 1:1628-
1637, 1977).
A CIGAR'H -13C correlation experiment (Figures 24, 25) showed that the two
units
were connected from the 4(u) position to the 8(I) position, by the
correlations from H-4(u) to
C-8(I) and C-8a(I).
The structure of the natural product Hl was therefore determined to be
catechin-4a-8-
epicatechin, also known as procyanidin B4 or proanthocyanidin B4 (Figure 26).
Our NMR data
on compound HI matched partial NMR data published on procyanidin B4 (Thompson
et al,
JCS Perkin 1:1387-1399, 1972; Fletcher et al, JCS Perkin 1:1628-1637, 1977)
and our data of
acetylated compound Hl (Figure 21; Table 3) matched partial NMR data published
on
peracetylated procyanidin B4 (Thompson et al, JCS Perkin 1:1387-1399, 1972;
Fletcher et al,
JCS Perkin 1:1628-1637, 1977). The optical rotation of -102 (MeOH) for
compound H1
compared to a literature value of -193 (EtOH)(Thompson et al, JCS Perkin
1:1387-1399,
1972) showed the absolute stereochemistry to be the same as found previously.
56
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Peak Hl Data Summary
Aliquots (14 x 70pl) of fractions 20 - 24 (134 mg in 1 ml)(Figure 3) from the
silica gel
column were separated by HPLC using method 2 (as described in Example 1). The
peaks
between 14.5 and 16.2 and between 16.2 and 19.0 minutes were collected, then
freeze dried to
give two products, about 80% pure HI, retention time 15.1 Method 2 (16
mg)(Figure 4) as a
white solid; and pure H2 (23 mg) as a white solid.
-ve electrospray m.s. 577 (M+-H, 100%)
molecular weight 578
1H NMR ((CD3)2CO)(major isomer, partial data) :2.92 (1H, dd, 2, 16, H-41),
3.02 (1H, dd, 5,
16, H-41), 4.34 (1H, bs, H-31),4.54 (1H, d, 10, H-4u), 4.64 (1H, dd, 8, 10, H-
3u), 4.79 (1H, d,
8, H-2u), 5.09 (1H, s, H-21), 5.94 (1H, d, 2, H-6u), 5.96 (1H, d, 2, H-8u) and
6.15 (1H, s, H-61).
13C NMR ((CD3)2CO)(major isomer, partial data ):30.30 (determined from HSQC
correlation), 38.85, 67.49, 73.89, 80.52, 83.99, 96.76, 97.81, 98.02, 106.66,
108.29 and 116.11.
IJV (MeOH) ? max (log c) 209 (4.71), 225sh (4.44) and 281 (3.65) nm;
[ ]24 589nm-103.0 , [ c]24 577nm-118.3 , [-]24 546nm-153.1 , [ 124 435nm-
379.9o, [-124 405nm-469.1
(c 0.2, MeOH)
Acetvlation of Hi Protocol
A sample of a fraction rich in peaks 111 and H2, from a second silica gel
column of
PTI-777 (50 mg) was dissolved in a mixture of acetic anhydride (0.5 ml) and
pyridine (0.5 ml).
The mixture stood at room temperature for 18 hours, then the solvents were
removed in vacuo.
Purification by column chromatography over silica gel, eluting with 20% ethyl
acetate in
dichloromethane gave the H2 peracetate (38 mg) followed by the H1 peracetate 5
(15 mg) as a
colorless gum. The NMR data is shown in Table 3 below.
30
57
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Table 3: 500 MHz NMR data of the H1 eracetate in deuterochloroform, major
atropisomer.
Ce Hb CIGAR
lower H-C
7.10 5.00, br s I
6.62 5.21, br d, J 5Hz 41,21
6.56 .75 br d J 18Hz I, 31, 4a1, 51, 8a1
3.00 dd J 18, 5Hz as above
a 110.36
148.29
109.49 6.62,s al, 51, 71, 81
147.57
116.86
a 153.46
1' 135.16A
' 121.81 5.90, d J 2Hz not defined
31 142.10*
141.96"
' 123.00^ .14, d, J 7Hz 1'I, 3'I, 4'I, 6'I
124.82* 5.86, dd, 2, 7Hz not defined
8.99 14.81 d 8 u
upper
0 5.70 t 8 u, 4u
6.72 .52 d 8 3au, 8a1, 71, 81, 4au
a 115.01
149.48*
108.13 5.57 d 1 not defined
149.20*
110.06 5.52 d 1 not defined
a 155.69
1' 135.21
21 122.7 6.961 not defined
31 141.71*
' 141.41 *
123.62* .09 d 7
' 124.96 16.89 m au, 2'u
ashift in ppm. bShift in ppm, multiplicity, couplings in Hz.
I = lower unit, u = upper unit. OAc groups not shown.
Exam In a 4;
Isolation and Identification of Peak K2 from PTI-777
General Experimental Procedures
The major component of peak K, called K2, of the PTI-777 extract was also
isolated by
a series of chromatographic techniques, monitored by HPLC (see Example 2,
Experimental
Procedures for details). We initially separated the original PTI-777 extract
by column
58
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
chromatography over silica gel, when 40% methanol in chloroform gave a
fraction rich in the
major component of peak K (Table 1). Preparative HPLC on a fraction rich in K2
(Figure 27),
using method 1 (see example 1) gave a pure sample of peak K2 (Figure 28). A -
ve ion
electrospray mass spectroscopy of this showed it to have a molecular ion M+ of
866 (Figure
29). This is appropriate for a molecular formula of C45H38018 (molecular
weight = 866), such as
a trimer of three epicatechin or catechin units. The initial 1H NMR (Figure
30) showed there to
be similar broad peaks to that seen in H2, so it was decided to acetylate the
compound to
definitely identify the structure of K2.
A further fraction from the silica gel column which was rich in peak K2 was
acetylated
as before (in Examples 1 and 2) to enable us to obtain more material for
structure elucidation.
The peracetate of K2 was purified by column chromatography over silica gel.
One (Figures 31, 32) and 2D NMR experiments (Figures 33, 34) were carried out
on
the K2 peracetate. Two sets of signals were seen in both the 'H and 13C NMR
spectra, in a ratio
of three to one. These were due to optional isomers (atropisomers) as
discussed in Example 2.
We solved the structure using the signals of the major isomer (see Table 4
below).
The positions of the 13C signals and the small couplings of the 'H signals of
the lower
unit was typical of epicatechin, and the positions of the 13C signals and the
small couplings of
the 'H signals of the other two units were typical of coupled epicatechin
(Fletcher et al, J.C.S.
Perkin 1:1628-1637, 1977). The presence of three flavan-3-ol units could be
seen from the six
13C signals in the 60-80 region, as well as a signal at 26.39 for the free C-4
position of the
lower unit and signals at 34.36 and 35.04 for the coupled C-4's of the other
units.
A CIGAR 1H -13C correlation experiment (Figures 33, 34) showed that the two
units
were connected from the 4 (upper) positions to the 8 (lower) positions, by the
correlations from
H-4(u) to C-8(m) and C-8a(m), and from H-4(m) to C-8(l) and C-8a(l).
K2 peracetate was therefore determined to be the structure shown in Figure 35.
Our
NMR data on the K2 peracetate shown in Figure 35 matched partial NMR data
published on
procyanidin Cl (Porter et al, J.C.S. Perkin 1:1217-1221, 1982; Hemingway et
al, J.C.S. Perkin
1:1209-1216, 1982), but we could not find any 13C NMR data published on
structure K2
(Figure 36). The optical rotation of +60.9 (MeOH) for structure K2 compared
to the literature
value of +92 (H20) showed it to have the same absolute stereochemistry as
that published. K2
59
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
was therefore identified as epicatechin-413-~8-epicatechin-4B-8-epicatechin or
procyanidin
CI (Figure 36).
Peak K2 Data Summary,
Aliquots (8 X 70 l) of fractions 35-40 (226 mg in 1.Oml) from the above silica
gel
column were separated by HPLC using method 1 (as described in Example 2). The
peak
between 12.90 and 15.70 minutes was collected. P88-21-2, retention time 15.1
minutes (5
mg)(Figures 27, 28) which is peak K2.
13C NMR ((CD3)2CO)(partial data on major isomer): 29.3(determined from HSQC
correlation), 37.6, 37.6, 67.10, 72.68, 73.69, 77.48, 77.61, 79.85.
UV (MeOH) ? max (log c) 211 (4.95), 226sh (4.66) and 281 (3.94) nm;
[a]24sa9nm +60.9 , [a]24577nm +53.2 , [a]24546nm +40.00 (c 0.2, MeOH)
Acetylation of K2 Protocol
A sample of K2 (5 mg) was dissolved in a mixture of acetic anhydride (0.5 ml)
and
pyridine (0.5 ml). The mixture was kept at room temperature for 18 hours, then
the solvents
were removed in vacuo. Purification by column chromatography over silica gel,
eluting with
20% ethyl acetate in dichloromethane gave the K2 peracetate (2 mg) as a
colorless gum.
A fraction rich in K2 (34 mg)(Figure 28) was dissolved in a mixture of acetic
anhydride
(0.5 ml) and pyridine (0.5 ml). The mixture was kept at room temperature for
18 hours, then
the solvents were removed in vacuo. Purification by column chromatography over
silica gel,
eluting with 20% ethyl acetate in dichloromethane gave the K2 peracetate (15
mg) as a
colorless gum. NMR data is shown in Table 4 below.
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Table 4: 500 MHz NMR data of the K2 peracetate in deuterochioroform, major
atropisomer..
upper C H CIGAR
4.70 5.37 bs 1'u, 3u
3 0.47 5.35 bs 3-OAcu, 4au, 8m
4.36 4.76s u, 3u, 4au, 5u, 7m, 8m, 8au, 8am,
a 111.61
149.88
108.12 .75 d 2
109.30 .64 d 2
a 154.88
1' 135.44
middle
4.94 5.40 bs I'm
1.29 5.38 bs 3-OAcm, 4am, 81
35.04 4.69s m, 3m, 4am, 5m, 71, 81, 8a1
a 112.17
148.58
110.95 6.70s ram, 5m, 7m, 8m
147.59
117.72
a 151.87
1' 135.15
lower
7.00 5.19s 1'I, 31
6.57 5.69 m aI
6.39 .98 m al, 51, 8a1
a 109.96
148.49
110.63 .63 s al, 51, 71, 81
147.18
117.60
a 151.73
1' 135.72
ashift in ppm. bShift in ppm, multiplicity, couplings in Hz.
I = lower unit, m = middle unit, u = upper unit. Ring C and OAc groups not
shown.
Example
Efficacy of Proanthocyanidins H2, Hl and K2 as Disruptors of AB Fibrils
In the next set of studies, we demonstrate that pure compounds H2 (epicatechin-
4B--8-
epicatechin), Hl (catechin-4a--8-epicatechin) and K2 (epicatechin-4B-8-
epicatechin-4B->8-
epicatechin) isolated as described in Examples 1-3, were tested for efficacy
in different
amyloid and a-synuclein/NAC diseases. In a first set of studies, the efficacy
of these
61
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
proanthocyanidin pure compounds were tested for their ability to cause a
potent disassembly/
disruption of pre-formed amyloid fibrils of Alzheimer's disease (i.e.
consisting of AB 1-42
fibrils).
In one study, Thioflavin T fluorometry was used to determine the effects of
H2, H1, K2
and EDTA (as a negative control) on disassembly/dissolution of pre-formed AB 1-
42 fibrils
(Figure 37). In this assay Thioflavin T binds specifically to fibrillar
amyloid, and this binding
produces a fluorescence enhancement at 485 nm that is directly proportional to
the amount of
amyloid fibrils formed. The higher the fluorescence the greater the amount of
amyloid fibrils
formed (Naki et al, Lab. Invest. 65:104-110, 1991; Levine III, Protein Sc.
2:404-410, 1993;
Amyloid Int. J. Exp. Clin. Invest. 2:1-6, 1995).
In this study, 25 pM of pre-fibrillized AB 1-42 (Bachem Inc) was incubated at
37 C for
1 week either alone, or in the presence of EDTA, H2, Hl, or K2 at an AB:test
compound
weight ratios of 1:0.1, 1:0.01, 1:0.001 or 1:0.0001. Following 3-days or 7 -
days of co-
incubation, 500 of each incubation mixture was transferred into a 96-well
microtiter plate
containing 150 l of distilled water and 50 1 of a Thioflavin T solution (i.e.
500mM Thioflavin
T in 250 mM phosphate buffer)(pH 6.8). The fluorescence was read at 485 nm
(444 nm
excitation wavelength) using an ELISA plate fluorometer after subtraction with
buffer alone or
compound alone, as blank.
The results of day 7 incubations are presented here, but similar results were
obtained as
early as 3 days. As shown in Figure 37, whereas EDTA caused no significant
inhibition of AB
1-42 fibrils at all concentrations tested, compound H2 caused a dose-dependent
disruption/
disassembly of preformed AB 1-42 fibrils, with a significant (p<0.01) 29 +/-
4% disruption
when used at an AB:H2 wt/wt ratio of 1:0.01, and a significant (p<0.01) 73 +/-
2% disruption
when used at an AB:H2 wt/wt ratio of 1:0.1 (i.e. 1:1 molar ratio). Similarly,
compound Hl
caused a dose-dependent disruption/ disassembly of preformed AB 1-42 fibrils,
with a
significant 16 +/- 3% disruption when used at an A8:H1 wt/wt ratio of 1:0.001;
a significant 33
+/- 6% disruption when used at an AB:H1 wt/wt ratio of 1:0.01; and a
significant 54 +/- 8%
disruption when used at an AB:H1 wt/wt ratio of 1:0.1 (i.e. 1:1 molar ratio).
Compound K2
also caused a dose-dependent disruption/disassembly of preformed AB 1-42
fibrils, with a
significant 27 +/- 4% disruption when used at an AB:K2 wt/wt ratio of 1:0.01;
and a significant
60 +/- 19% disruption when used at an AB:K2 wt/wt ratio of 1:0.1 (i.e. 1:1
molar ratio). This
62
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
study indicated that the proanthocyanidins H2, H1 and K2 were potent
disruptors of
Alzheimer's disease type AB fibrils, and exerted their effect in a dose-
dependent manner.
The disruption of AB 1-42, even in its monomeric form, was confirmed by a
study
involving the use of SDS-PAGE and Western blotting methods. In this latter
study, triplicate
samples of pre-fibrillized AB 1-42 (25 M) was incubated at 37 C for 3 and 7
days either alone
or in the presence of compound H2, Hl, K2 or EDTA (as a negative control). 5 g
of each
sample was then filtered through a 0.2 m filter. Protein recovered from the
filtrate was then
loaded, and ran on a 10-20% Tris-Tricine SDS-PAGE, blotted to nitrocellulose
and detected by
ECL using an AB-antibody (clone 6E10; Senetek). As shown in Figure 38, AB 1-42
was
detected as a -4 kilodalton band (i.e. monomeric AB) following incubation
alone, or in the
presence of EDTA, at both 3 and 7 days. AB 1-42 monomers were not detected
following
incubation of AB 1-42 with either H2, H1 or K2 by 7 days of co-incubation (Fig
38) suggesting
that these compounds were capable of causing a disappearance of monomeric AB 1-
42.
Disappearance of AB 1-42 was already apparent even following 3 days of
incubation with H2
and H1, whereas K2 took longer (i.e. 7 days) to have a full effect. This study
confirmed that
the proanthocyanidins H2, H1 and K2 were also capable of causing a
disruption/removal of
monomeric AB 1-42.
Example 6
Disruption of Pre-Formed AB 1-42 and 1-40 Fibrils by Proanthocyanidin Compound
H2
as Revealed by Circular Dichroism Spectroscopy
Circular dichroism (CD) spectroscopy is a method used to determine the effects
of test
compounds to disrupt pre-formed amyloid fibrils. In one study, as shown here
circular
dichroism spectroscopy was used to determine the effects of pure compound H2
(i.e.
(epicatechin-43-)8-epicatechin) on disruption of B-pleated sheet structure of
pre-formed AB 1-
42 and 1-40 fibrils of the types found in the brains of patients with
Alzheimer's and related
disorders. For this study, AB 1-42 or AB 1-40 peptides (Bachem Inc., Torrance,
CA) were first
dissolved in 2mM NaOH solution, maintaining the pH of these solutions above
10. The peptides
were then dissolved in PBS containing 10% TFE, and the pH was adjusted to 7.2.
AB 1-40 or
AB 1-42 was incubated in the absence or presence of compound H2 at an AB:H2
weight/weight
ratio of 1:0.1 (i.e. molar ratio of 1:1). At 3 and 7 days following
incubation, CD spectra were
recorded on a AVIV 202 spectropolarimeter with 50 M of AB and H2 compound
mixtures. All
63
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
spectra were collected with 0.1 cm quartz cell using a thermstated cuvette
holder. Wavelength
traces were scanned from 260-195 nm at 0.5 nm increments with a bandwidth of 1
nm and
averaged over a time of 5 seconds; the temperature was held constant at 25 C.
All spectra
reported are an average of 4 scans.
As shown in Figure 39, AB 1-42 alone in 10% TFE PBS buffer showed the typical
CD
spectra of an amyloid protein with significant 13-sheet structure, as
demonstrated by the sharp
minima observed at 218 nm. However, in the presence of H2 compound (at a 1:1
molar ratio)
(noted as PTC38 in Figure 39), a marked disruption of 13-sheet structure in AB
1-42 fibrils was
evident (with a significant increase in random coil or (X-helix) as shown by
the flattening out of
the minima observed at 218 nm (compare to AB 1-42 alone)(Figure 39). This was
observed at
both 3 (not shown) and 7 days (Figure 39) following co-incubation of AB 1-42
fibrils with H2.
This study clearly demonstrated that H2 compound had the ability to
disrupt/disassemble the
beta-pleated sheet structure characteristic of AB 1-42 fibrils.
As shown in Figure 40, AB 1-40 alone in 10% TFE PBS buffer also showed the
typical
CD spectra of an amyloid protein with significant B-sheet structure, as
demonstrated by the
sharp minima observed at 218 nm. However, in the presence of H2 compound (at a
1:1 molar
ratio)(noted as PTC38 in Figure 40), a nearly complete disruption/disassembly
of B-sheet
structure in AB 1-40 fibrils was evident (with a significant increase in
random coil or (x-helix) as
shown by the complete flattening out of the minima observed at 218 nm (compare
to AB 1-40
alone)(Figure 40). This was observed at both 3 (not shown) and 7 days (Figure
40) following
co-incubation of AB 1-40 fibrils with H2. This study clearly demonstrated that
H2 compound
had the ability to disrupt/disassemble the beta-pleated sheet structure
characteristic of AB 1-40
fibrils. Both AB 1-42 and AB 1-40 are known to be present in the amyloid
deposits in the brains
of patients with Alzheimer's disease and related disorders. This study
confirms the efficacy of
proanthocyanidins (in this particular case the epicatechin-epicatechin dimer)
as potent
inhibiting/disrupting agents of amyloid fibrils, and confirms the previous
examples using
Thioflavin T fluorometry and SDS-PAGE/ECL type assays that compound H2 and the
other
proanthocyanidins are potent anti-amyloid agents.
64
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Example 7:
Efficacy of Proanthocyanidins H2, H1 and K2 as Disruptors of a-Synuclein/NAC
Fibrils
Parkinson's disease is a neurodegenerative disorder that is pathologically
characterized
by the presence of intracytoplasmic Lewy bodies (Lewy in Handbuch der
Neuroloaie, M.
Lewandowski, ed., Springer, Berlin, pp. 920-933, 1912; Pollanen et al., J.
Neuropath. Exp.
Neurol. 52:183-191, 1993), the major components of which are filaments
consisting of a-
synuclein (Spillantini et al., Proc. Natl. Acad. Sci. USA 95:6469-6473, 1998;
Arai et al.,
Neurosc. Lett. 259:83-86, 1999), a 140-amino acid protein (Ueda et al., Proc.
Natl. Acad. Sci.
U.S.A. 90:11282-11286, 1993). a-Synuclein recombinant protein, and non-amyloid
component
(known as NAC), which is a 35-amino acid peptide fragment of a-synuclein, both
have the
ability to form fibrils when incubated at 37 C, and are positive with amyloid
stains such as
Congo red (demonstrating a red/green birefringence when viewed under polarized
light) and
Thioflavin S (demonstrating positive fluorescence) (Hashimoto et al., Brain
Res. 799:301-306,
1998; Ueda et al., Proc. Natl. Acad. Sci. U.S.A 90:11282-11286, 1993).
Inhibition, disruption/
disassembly of pre-formed a-synuclein and/or NAC fibrils, are believed to
serve as future
therapeutics for the treatment of Parkinson's and Lewy body disease.
In the next study, we therefore determined the efficacy of proanthocyanidins,
specifically H2, H1 and K2 as disruptor of pre-formed NAC fibrils. Thioflavin
T fluorometry
was used to determine the effects of H2, Hl, K2 and EDTA (as a negative
control) on
disassembly/dissolution of pre-formed NAC fibrils (Figure 41). In this study,
25 M of pre-
fibrillized NAC (Bachem Inc) was incubated at 37 C for 1 week either alone, or
in the presence
of EDTA, H2, H1, or K2 at an NAC:test compound weight ratios of 1:0.1, 1:0.01,
1:0.001 or
1:0.0001. Following 3 or 7 days of co-incubation, 5O 1 of each incubation
mixture was
transferred into a 96-well microtiter plate containing 150 l of distilled
water and 500 of a
Thioflavin T solution (i.e. 500mM Thioflavin T in 250 mM phosphate buffer (pH
6.8). The
fluorescence was read at 485 nm (444 nm excitation wavelength) using an ELISA
plate
fluorometer after subtraction of buffer alone as blank.
The results of day 7 incubations are presented here (Figure 41), but similar
results were
obtained as early as 3 days. As shown in Figure 41 whereas EDTA caused no
significant
inhibition of NAC fibrils at all concentrations tested, compound H2 caused a
dose-dependent
disruption/disassembly of preformed NAC fibrils, with a significant (p<0.01)
27 +/- 27%
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
disruption when used at a NAC:H2 wt/wt ratio of 1:0.01; and a significant
(p<0.01) 77 +/- 2%
disruption when used at a NAC:H2 wt/wt ratio of 1:0.1 (i.e. 1:1 molar ratio).
Similarly,
compound H1 caused a dose-dependent disruption/ disassembly of preformed NAC
fibrils,
with a significant 31 +/- 16% disruption when used at a NAC:H1 wt/wt ratio of
1:0.01; and a
significant 64 +/- 3% disruption when used at a NAC:H1 wt/wt ratio of 0.1
(i.e. 1:1 molar
ratio). Compound K2 also caused a dose-dependent disruption/disassembly of
preformed NAC
fibrils, with a significant 20 +/- 27% disruption when used at an NAC:K2 wt/wt
ratio of 1:0.01;
and a significant 39 +/- 12% disruption when used at an NAC:K2 wt/wt ratio of
1:0.1 (i.e. 1:1
molar ratio). This study indicated that the proanthocyanidins H2, Hi and K2
were also potent
disruptors of NAC fibrils, and exerted their effect in a dose-dependent
manner. It is expected
that similar efficacy of these proanthocyanidins will be also observed for
disruption/disassembly of a-synuclein fibrils.
Example 8:
Efficacy of Proanthocyanidins H2, Hl and K2 as Disruptors of Type 2 Diabetes
Amyloid
Fibrils
Islet amyloid deposits are observed in -90% of patients with well-established
type 2
diabetes and would appear to be a characteristic feature of the disease
process (Westermark, J.
Med. Sci. 77:91-94,1972; Clark et al, Diabetes Res. 9:151-159,1988). In many
patients, the
deposits are widespread and affect many islets. The degree of islet
(predominantly B-cell) mass
that has been replaced by amyloid may be a marker for the severity of the
diabetic disease
process, with those individuals requiring insulin treatment having the
greatest islet mass
reduction and amyloid formation (Westermark, Amyloid: Int. J. Exp. Clin.
Invest. 1:47-
60,1994).
The major protein in type 2 diabetes islet amyloid is a 37-amino acid peptide
known as
islet amyloid polypeptide (IAPP) or amylin. IAPP is a known normal secretory
product of the
pancreatic B-cells (Kanh et al, Diabetes 39:634-638,1990) that is stored in
insulin-bearing
cytoplasmic granules (Clark et al, Cell Tissue Res. 257:179-185, 1989). IAPP
has been
hypothesized to have an important role in the pathogenesis of type 2 diabetes
through its
impairment of B-cell function and reduction of B-cell mass (Johnson et al, N.
Engl. J. Med.
321:513-518,1989). Besides being able to form islet amyloid deposits that
replace B-cell mass,
amyloid fibrils appear to damage islets directly. Studies as a whole suggest
that islet amyloid
66
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
formation plays a central role in the development of B-cell failure of type 2
diabetes. Therefore,
agents or compounds able to inhibit or disrupt islet amyloid (i.e. LAPP)
formation, deposition,
accumulation or persistence, and/or cause a disruption/dissolution or
disassembly of pre-
formed IAPP fibrils are believed to lead to the discovery of new therapeutic
compounds for the
treatment of type 2 diabetes.
Therefore in the next study, we determined the efficacy of the
proanthocyanidins, H2,
Hl and K2 as disruptors/causing disassembly of pre-formed IAPP fibrils.
Thioflavin T
fluorometry was used to determine the effects of H2, H1, K2 and EDTA (as a
negative control)
on disassembly/dissolution of pre-formed IAPP fibrils (Figure 42). In this
study, 25 M of pre-
fibrillized IAPP (Bachem Inc) was incubated at 37 C for 1 week either alone,
or in the
presence of EDTA, H2, Hl, or K2 at an IAPP:test compound weight ratios of
1:0.1, 1:0.01,
1:0.001 or 1:0.0001. Following 3 or 7 days of co-incubation, 50 1 of each
incubation mixture
was transferred into a 96-well microtiter plate containing 150 1 of distilled
water and 50 1 of a
Thioflavin T solution (i.e. 500 mM Thioflavin T in 250 mM phosphate buffer (pH
6.8). The
fluorescence was read at 485 nm (444 nm excitation wavelength) using an ELISA
plate
fluorometer after subtraction of buffer alone as blank. The results of day 7
incubations are
presented here (Figure 42), but similar results were obtained as early as 3
days. As shown in
Figure 42 whereas EDTA caused no significant inhibition of IAPP fibrils at all
concentrations
tested, compound H2 caused a dose-dependent disruption/disassembly of
preformed IAPP
fibrils, with a significant (p<0.01) 36 +/- 5% disruption when used at a
IAPP:H2 wt/wt ratio of
1:0.01; and a significant (p<0.01) 83 +/- 1% disruption when used at a IAPP:H2
wt/wt ratio of
1:0.1 (i.e. 1:1 molar ratio). Similarly, compound H1 caused a dose-dependent
disruption/
disassembly of preformed IAPP fibrils, with a significant 35 +/- 4% disruption
when used at a
IAPP:H1 wt/wt ratio of 1:0.01; and a significant 79 +/- 1% disruption when
used at a IAPP:H1
wt/wt ratio of 0.1 (i.e. 1:1 molar ratio). Compound K2 also caused a dose-
dependent
disruption/disassembly of preformed IAPP fibrils, with a significant 26 +/- 4%
disruption when
used at an IAPP:K2 wt/wt ratio of 1:0.01; and a significant 62 +/- 1%
disruption when used at
an IAPP:K2 wt/wt ratio of 1:0.1 (i.e. 1:1 molar ratio). This study indicated
that the
proanthocyanidins H2, H1 and K2 were also potent disruptors of IAPP fibrils,
and exerted their
effect in a dose-dependent manner. This study also indicates that
proanthocyanidins are
expected to be useful for the treatment of IAPP amyloidosis in type 2
diabetes.
67
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Example 9:
Isolation and Identification of Peak K1 from PTI-777
General Experimental Procedures:
A sample of the PTI-777 extract (1 gram) was dissoved in ethanol (2 ml) and
then loaded
onto a sephadex LH2O (10g) column, prepared in ethanol. Elution of this column
with ethanol
(1000ml), followed by 5% acetone in ethanol (400m1), 190% acetone in ethanol
(200m1), then
50% acetone in methanol (200m1) gave 120 (12 ml fractions).
Table 5: Sephadex LH2O Column Fractionation of PTI-777
Solvent Fractions Peaks present Weight
EtoH 25-29 other, J 4 mg
30-35 J, other 36 mg
36-37 J, and Kl 5 mg
38-42 Kl 22 mg
43-48 Kl, H2, other 13 mg
49-56 H2 89 mg
57-71 H2, Hl 89 mg
72-83 Hl 38 mg
5% acetone in ethanol 84-90 Hl, other 23 mg
91-92 other, Hi, K2 7 mg
10% acetone in ethanol 93-100 K2 40 mg
50% acetone in ethanol 101-114 mix 92 mg
50% acetone in methanol 115-119 mix + post-L 400 mg
100% methanol 120-end post-L only 187 mg
The analytical HPLC conditions for monitoring of K1 and the K1 acetate
(described
below) are under the same conditions as in Example 2, where method 1 was used.
Isolation of Peak Kl:
Fractions 38 to 42 (see Table 5 above) contained compound K1 (22 mg) as a pale
brown gum. The retention time of this K1 peak was 15.0 minutes as monitored by
HPLC
Method 1.
68
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
For acetylation of Kl to help determine the structure, a sample of KI (15 mg)
was
dissoved in a mixture of acetic anhydride (0.5 ml) and pyridine (0.5 ml). The
mixture stood at
room temperature for 18 hours, then the solvents were removed in vacuo to give
the Kl
peracetate (16 mg) as a colourless gum. The NMR data is shown in Table 6
below.
Identification of K1 and the KI peracetate:
The minor component of peak K, called Kl, of the PTI-777 extract was isolated
by
column chromatography over sephadex LH2O, monitored by HPLC. Elution with 95%
ethanol
followed by increasing amounts of acetone and water, followed by methanol,
gave pure peak
K1 in fractions 38 to 42 (see Table 5). The structure of the Kl peracetate is
shown in Figure
43, whereas the structure of Kl is shown in Figure 44. To arrive at these
structures, the
following analysis and results were obtained.
A -ve ion electrospray mass spectrum of Kl gave a clean 100% ion at 561
daltons
(Figure 45). This is appropriate for the molecular ion (M+-H) of a molecular
formula of
C301126011(molecular weight = 562), such as a mixed dimer of one epicatechin,
or isomeric unit
and one epiafzelechin, or isomeric unit. The 13C NMR of Kl showed signals
consistent with
some kind of flavanol dimer (Figure 46). The 'H NMR of Kl showed there to be
similar broad
peaks to that seen in compound H2 (Figure 47), so it was decided to acetylate
the compound to
determine the final structure. Acetylation of a sample of pure Kl gave a
peracetate (structure
shown in Fig. 43). The 'H and 13C NMR spectrum of the Kl peracetate are shown
in Figures 48
and 49, respectively. Two sets of signals were seen in both the 'H and 13C NMR
spectra, in a
ratio of three to one. These were due to rotational isomers (atropisomers). We
solved the
structure using the signals of the major atropisomer (see Table 6 below).
The presence of two flavan-3-ol units could be seen from the four 13C signals
for the C-
2 and C-3 positions in the 60-80 region, as well as a signal at 26.61 for the
free C-4 position of
the lower unit and a signal at 34.14 for the linked C-4 of the upper unit. A
CIGAR 'H-13C
correlation experiment (Figures 50-53) showed that the two units were
connected from the 4(u)
position to the 8(1) position, by the correlation between H-4(u) and C-8(l).
The
stereochemistries at C-2 and C-3 of both upper and lower units was shown to be
the same as in
epicatechin by the similar chemical shifts of the 'H and 13C signals for the
lower unit, as well as
the similar low coupling constants between H-2 and H-3 in both units. The
lower flavan-3-ol
unit was shown to be epicatechin by CIGAR correlations from H-2(1) to C-2' and
C-6' signals
69
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
of a 3',4'-dioxygenated aromatic ring. The upper flavan-3-ol unit was
identified by CIGAR
correlations from H-2(u) to equivalent, C-2'/C-6' signals of a 4'-oxygenated
ring. This
constitutes an epiafzelechin unit. The structure of the natural product K1 was
therefore
assigned to be epiafzelechin-4B-->8-epicatechin. This compound is a known
compound
(Kashiwada et al, Chem. Pharma. Bull. 36:39-47, 1988; Morimoto et al, Chem.
Pharm. Bull.
34:888-893, 1990). Our NMR data on the structure for Kl matched partial NMR
data
published on epiafzelechin-4B- 8-epicatechin. The optical rotation of -1.4
compared to a
literature value of +29 showed uncertain absolute stereochemistry.
Peak Kl Data Summary
-ve electrospray mass spectroscopy 561 (M+-H, 100%)
molecular weight 562
'H NMR ((CD3)2CO):2.88 (2H, m, H-41), 3.59 (1H, br s, OH), 3.73 (1H, br s,
OH), 4.11 (1H,
s), 4.39 (1H, br s), 4.84 (1H, s H-4u), 5.07 (1H, br s), 5.26 (1H, s), 6.08
(1H, s), 6.09 (1H, s),
6.12 (1H, s), 6.80 (1H, m), 6.85 (2H, d, J 8Hz), 6.97 (1H, m), 7.19 (1H, br
s), 7.37 (2H, d, J
8Hz) and 7.40-8.20 (7H, br s, OHs)
13C NMR ((CD3)2CO): 37.69, 67.11, 73.47, 77.70, 79.95, 96.63, 97.10, 97.74,
101.30, 115.62,
116.14, 119.84, 129.89, 132.48, 145.79, 145.98, 156.78 and 158.28.
UV (MeOH) X max (log s) 216 (4.89), 227 sh (4.74) and 280 (4.04) nm;
[aj4 589.-1.4 [a] 577,-23.1 , [x124546.-62.3 , (c 0.1, MeOH).
Table 6: 500 MHz NMR data of the K1 Peracetate in Deuterochloroform, Major
Atropisomer.
upper Ca Hb CIGARc H-C
2 73.96 5.58 s 3u, 1'u, 2'u/6'u
3 71.18 5.18 dd 1,2 none observed
4 34.14 4.44 d2 2u, 3u, 4au, 5, 8au, 81, (8a1)
4a 111.66
5 147.81
6 108.52 6.22 d 2 none observed
7 149.04
8 107.23 5.98 d 2 none observed
8a 154.15
1' 135.23
2' 127.72 7.44 d 8 2u, (4'u)
3' 121.357.07d8 4'u
4' 150.50
5' 121.357/07d8 4'u
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
6' 127.72 7.44 d 8 2u, (4'u)
lower
2 77.16 4.55s 31, 11, 2'l, 6'1
3 66.77 5.10 m
4 26.61 2.85 d 18 31, 5 I, 4a I, 8a I
2.91 dd 18, 5 31, 51, 4a 1, 8a I
4a 111.59
147.91
6 110.27 6.64 s (21), 4a 1, 51, 71, 81
7 149.12
8 116.79 4au, 6u, 7u, 8au
8a 155.55
1' 134-48
2' 122.44 7.03 m 21, 6' l
3' 141.59
4' 141.90
5' 122.71 7.04 m 6' I
6' 124.98 6.87 dd 8, 2 none observed
ashift in ppm. bShift in ppm, multiplicity, couplings in Hz. CBrackets
indicate weak correlations,
I = lower unit, u = upper unit. OAc groups not shown.
Example 10=
5 Therapeutic Applications
Proanthocyanidins act as potent inhibitors/disruptors and/or causing
disassembly of
amyloid fibrils (regardless of the type of amyloid protein present. Examples
are shown for AB,
NAC and IAPP fibrils), as well as a potent inhibitor/disruptor of a-
synuclein/NAC fibrils. Both
procyanidin dimers and trimers are shown specifically to inhibit such
fibrillogenesis, and our
ongoing studies suggest that procyanidin tetramers and oligomers (greater than
tetramers) are
also able to exert such amyloid fibril inhibiting effects. Thus, preferred
therapeutic applications
include the use of proanthocyanidins and procyanidins for the treatment of
amyloid diseases,
and diseases which include a-synuclein/NAC fibrillogenesis.
The proanthocyanidins of the present invention were discovered, isolated and
identified
from the plant Uncaria tolnentosa. However, it is probable that similar
amyloid/a-synuclein/
NAC inhibitory activity is observed with any proanthocyanidin regardless of
the source (i.e.
plant or food), and will include proanthocyanidins that can be synthesized by
methods known
to those knowledgeable and skilled in the art.
71
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Preparations of proanthocyanidins compounds for parenteral administration
include
sterile aqueous or non-aqueous solutions, suspensions, emulsions, which may
contain axillary
agents or excipients which are known in the art. Pharmaceutical or
pharmacological
compositions such as tablets, pills, caplets, soft and hard gelatin capsules,
lozenges, sachets,
cachets, vegicaps, liquid drops, elixers, suspensions, emulsions, solutions,
syrups, tea bags,
aerosols (as a solid or in a liquid medium), suppositories, sterile injectible
solutions, sterile
packaged powders, can be prepared according to routine method and are known in
the art.
Proanthocyanidins of the present invention may be administered by any means
that
achieve their intended purpose, for example to treat amyloid diseases, such as
Alzheimer's
disease or type 2 diabetes, or other pathologies involving a-synuclein/NAC
fibrillogenesis,
using a proanthocyanidin described herein, in the form of a pharmaceutical or
pharmacological
composition.
For example, administration of such a composition may be by various parenteral
routes
such as subcutaneous, intravenous, intradermal, intramuscular,
intraperitoneal, intranasal,
transdermal or buccal routes. Alternatively, or concurrently, administration
may be by the oral
route. Parenteral administration can be by bolus injection or by gradual
perfusion over time. A
preferred mode of using a proanthocyanidin pharmaceutical composition of the
present
invention is by oral administration or intravenous application.
A typical regimen for preventing, suppressing or treating amyloid pathologies,
such as
Alzheimer's disease amyloidosis, comprises administration of an effective
amount of a
proanthocyanidin over a period of one or several days, up to and including
between one week
to about 10 years.
It is understood that the dosage of the proanthocyanidin of the present
invention
administered in vivo or in vitro will be dependent upon the age, sex, health,
and weight of the
recipient, kind of concurrent treatment, if any, frequency of treatment, and
the nature of the
effect desired. The most preferred dosage will be tailored to the individual
subject, as is
understood and determinable by one of skill in the art, without undue
experimentation.
The total dose required for each treatment may be administered by multiple
doses or in
a single dose. A proanthocyanidin or procyanidin compound may be administered
alone or in
conjunction with other therapeutics directed to amyloid disease or a-
synuclein/NAC
fibrillogenesis, such as Alzheimer's disease or Parkinson's disease, as
described herein.
72
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
Effective amounts of a proanthocyanidin compound for treatment, are about 10
mg to about
1,000 mg/kg body weight, and preferably from about 10 mg to 100 mg/kg body
weight, such as
10, 20, 30, 40, 50, 60, 70, 80, 90 or 100 mg/kg body weight.
Preparations for parenteral administration include sterile aqueous or non-
aqueous
solutions, suspensions and emulsions, which may contain axillary agents or
excipients that are
known in the art. Pharmaceutical compositions containing a proanthocyanidin of
the present
invention may include all compositions where the proanthocyanidin is contained
in an amount
effective to achieve its intended purpose. In addition, a pharmaceutical
composition may
contain suitable pharmaceutically acceptable carriers, such as excipients,
carriers and/or
axillaries that facilitate processing of the active compounds into
preparations which can be
used pharmaceutically.
Pharmaceutical compositions comprise at least one proanthocyanidin compound
may
also include solutions for administration intravenously, subcutaneously,
dermally, orally,
mucosally, rectally, or may be by injection or orally, and contain from about
0.01 to 100 %,
preferably about 95-100% of active compound together with the excipient.
Pharmaceutical
compositions for oral administration include pills, tablets, caplets, soft and
hard gelatin
capsules, lozenges, sachets, cachets, vegicaps, liquid drops. elixers,
suspensions, emulsions,
solutions and syrups.
The proanthocyanidin compounds for Alzheimer's and Parkinson's disease, and
other
central nervous system disorders may be optimized to cross the blood-brain
barrier. Methods of
introductions include but are not limited to systemic administration,
parenteral administration,
i.e. via an intraperitoneal, intravenous, perioral, subcutaneous,
intramuscular, intraarterial,
intradermal, intramuscular, intranasal, epidural or oral routes. In a
preferred embodiment for
the treatment of central nervous system disorders, a proanthocyanidin compound
may be
directly administered to the cerebrospinal fluid by intraventricular
injection. In a specific
embodiment, it may be desirable to administer a proanthocyanidin compound
locally to the
areas of tissue in need of treatment; this may be achieved by, for example,
and not by way of
limitation, local infusion during surgery, topical application, by injection,
by infusing a
cannulae with osmotic pump, by means of a catheter, by means of a suppository,
or by means
of an implant.
73
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
In yet another embodiment, a proanthocyanidin compound may be delivered in a
controlled release system, such as an osmotic pump. In yet another embodiment,
a controlled
release system can be placed in proximity to the therapeutic target, i.e. the
brain, thus requiring
only a fraction of the systemic dose.
Example 11:
Clinical Testing in Alzheimer's Patients for Example
Five to fifty women are selected for a clinical study. The women are post-
menopausal,
i.e. have ceased menstruating for between 6 and 12 months prior to the study's
initiation, have
been diagnosed with early stage Alzheimer's disease, and expected to have
worsening
symptoms of Alzheimer's disease within the study period, but are in good
general health
otherwise. The study has a placebo group, i.e. the women are divided into two
groups, one of
which receives the compound of this invention and the other receives a
placebo. The patients
are benchmarked as to memory, cognition, reasoning, and other symptoms
associated with
Alzheimer's disease. Women in the test group receive a therapeutic dose of the
compound by
the oral route. They continue this therapy for 6-36 months. Accurate records
are kept as to the
benchmarked symptoms in both groups and at the end of the study these results
are compared.
The results are compared both between members of each group and also the
results for each
patient are compared to the symptoms reported by each patient before the study
began. Activity
of the compound is illustrated by an attenuation of the typical cognitive
decline and/or
behavioral disruptions associated with Alzheimer's disease.
Utility of the compounds is evidenced by activity in at least one of the above
assays.
INDUSTRIAL APPLICABILITY
To date there is no suggested usage of proanthocyanidin compounds for
treatment of
amyloid diseases, amyloidoses, amyloid fibrillogenesis or diseases
characterized by NAC or a-
synuclein fibrillogenesis. It is believed that cost effective treatment for
these conditions
throughout the world is now at hand, and that this disclosure readily puts
into the hands of
health providers and health officials worldwide a means to alleviate much
suffering and
economic loss.
74
CA 02441099 2003-09-10
WO 02/076381 PCT/US02/04764
While this invention has been described in conjunction with specific
embodiments and
examples, it will be apparent to a person of ordinary skill in the art, having
regard to this
disclosure, that equivalents of the specifically disclosed materials and
techniques will also be
applicable to this invention; and such equivalents are intended to be included
within the
following claims.