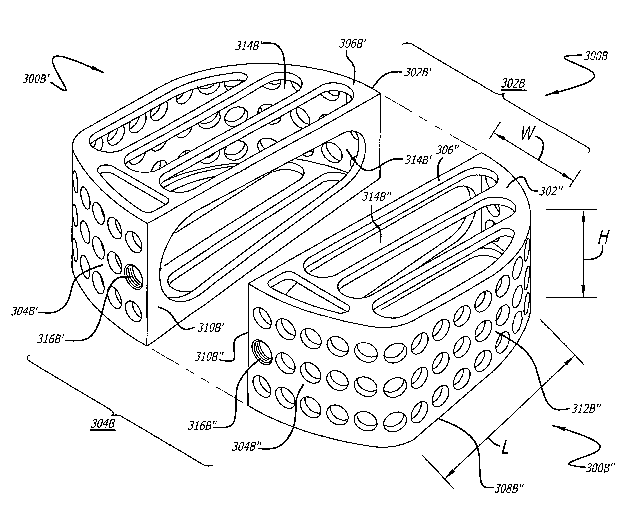
Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
What is claimed is:
1. An interbody spinal implant made of cortical bone for insertion at least in
part into an implantation space formed across the height of a disc space
between adjacent vertebral bodies of a human spine, the vertebral bodies
having an anterior aspect and a posterior aspect and a depth
therebetween, said implant comprising:
a leading end for insertion first into the disc space and a trailing end
opposite said leading end, said implant having a length from said leading
end to said trailing end;
opposed upper and lower portions between said leading and trailing
ends adapted to be placed at least in part within and across the height of
the disc space to contact and support the adjacent vertebral bodies, said
upper and lower portions being non-arcuate along at least a portion of the
length of said implant; and
opposite sides between said upper portion and said lower portion,
and between said leading and trailing ends, said opposite sides defining a
width of said implant, at least one of said opposite sides being at least in
part straight along at least a portion of the length of said implant, said
leading end configured in the shape of approximately one half of a circle
from one of said opposite sides to another of said opposite sides, the circle
having a diameter generally equal to the width of said implant;
said implant being manufactured from a bone ring obtained from a
major long bone of a human having a medullary canal, said implant
including at least a portion of the medullary canal passing through said
upper and lower portions to form at least a portion of a passage adapted to
hold bone growth promoting material for permitting for the growth of bone
from vertebral body to vertebral body through said passage.
2. The implant of claim 1, wherein said implant has a maximum width
between said opposite sides that is greater than one-half of the width of
the adjacent vertebral bodies into which said implant is adapted to be
inserted.
23
3. The implant of claim 1, wherein said implant has a maximum width
between said opposite sides that is less than one-half of the width of the
adjacent vertebral bodies into which said implant is adapted to be inserted.
4. The implant of claim 3, wherein said implant is adapted to be inserted side
by side a second of said implant into the disc space between the adjacent
vertebral bodies.
5. The implant of claim 1, wherein at least a portion of said leading end has
a
reduced height to facilitate insertion of said implant between the two
adjacent vertebral bodies.
6. The implant of claim 1, wherein said trailing end is adapted to conform
from side to side to at least a portion of the peripheral contour of at least
one of the anterior and posterior aspects of the vertebral bodies adjacent a
disc space into which said implant is inserted.
7. The implant of claim 1, wherein said passage is between said opposite
sides of said implant.
8. The implant of claim 1, wherein said passage intersects at least one of
said opposite sides.
9. The implant of claim 1, wherein said passage is between said leading and
trailing ends of said implant.
10. The implant of claim 1, wherein said implant has a mid-longitudinal axis
along the length, at least one of said opposite sides being at least in part
oriented generally parallel to the mid-longitudinal axis of said implant.
11. The implant of claim 1, wherein said opposite sides are at least in part
generally parallel one another.
12. The implant of claim 1, wherein at least a portion of said upper and lower
surfaces are in an angular relationship to each other from trailing end to
leading end for allowing angulation of the adjacent vertebral bodies
relative to each other.
13. The implant of claim 1, wherein said implant has a maximum length less
than and approximating the posterior to anterior depth of the vertebral
bodies.
14. The implant of claim 1, further comprising a bone engaging surface formed
on the exterior of at least said upper and lower portions for engaging the
adjacent vertebral bodies, said bone engaging surface including at least
24
one of a protrusion, a ratchet, a spike, a spline, surface roughenings, and
knurling.
15. The implant of claim 1, wherein said implant includes at least two
members, each member having a leading portion, a trailing portion, a top,
a bottom, and at least one side, each member being adapted to be placed
side by side with another of said members, said leading portion of said
members forming said leading end of said implant when placed side by
side.
16. The implant of claim 15, wherein said implant includes two of said
members, each member being a mirror image of the other.
17. The implant of claim 15, wherein each member includes at least a portion
of said passage.
18. The implant of claim 1, wherein said implant comprises at least in part of
a
bone growth promoting material.
19. The implant of claim 18, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
20. The implant of claim 1, in combination with a bone growth promoting
material.
21. The implant of claim 20, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
22. The implant of claim 1, wherein said implant is treated with a bone growth
promoting substance.
23. The implant of claim 1, wherein said implant is at least in part
resorbable.
24. The implant of claim 1, in combination with a chemical substance adapted
to inhibit scar formation.
25. The implant of claim 1, in combination with an antimicrobial material.
26. The implant of claim 1, wherein at least a portion of said implant is
treated
to promote bone ingrowth between said implant and said adjacent
vertebral bodies.
25
27. The implant of claim 1, further in combination with at least one spinal
fixation implant.
28. The implant of claim 1, wherein said trailing end is adapted to receive at
least one bone screw adapted to engage at least one vertebral body when
inserted through said implant.
29. The implant of claim 28, further comprising a lock for locking at least
one
bone screw to said implant.
30. The implant of claim 29, wherein said lock is made of one of cortical bone
and a bioresorbable material.
31. The implant of claim 28, wherein said screw is made of one of cortical
bone and a bioresorbable material.
32. An interbody spinal implant made of cortical bone for insertion at least
in
part into an implantation space formed across the height of a disc space
between adjacent vertebral bodies of a human spine, the vertebral bodies
having an anterior aspect and a posterior aspect and a depth
therebetween, said implant comprising:
a leading end for insertion first into the disc space and a trailing end
opposite said leading end, said implant having a length from said leading
end to said trailing end;
opposed upper and lower portions between said leading and trailing
ends adapted to be placed at least in part within and across the height of
the disc space to contact and support the adjacent vertebral bodies;
said implant having a maximum width that is greater than one-half
of the width of the adjacent vertebral bodies into which said implant is
adapted to be inserted, said leading end configured in the shape of
approximately one half of a first circle along the width of said implant, said
trailing end having a radius of curvature of a second circle along the width
of said implant, the second circle having a radius greater than the radius of
the first circle; and
said implant being manufactured from a bone ring obtained from a
major long bone of a human having a medullary canal, said implant
including at least a portion of the medullary canal passing through said
upper and lower portions to form a passage adapted to hold bone growth
26
promoting material for permitting for the growth of bone from vertebral
body to vertebral body through said passage.
33. The implant of claim 32, wherein said leading end and said trailing end of
said implant intersect at diametrically opposite points of the implant.
34. The implant of claim 32, wherein said width of said implant is
approximately equal to the diameter of the first circle.
35. The implant of claim 32, wherein said implant has a height from said upper
portion to said lower portion, the height of said implant being less than the
maximum width of said implant.
36. The implant of claim 32, wherein at least a portion of said leading end
has
a reduced height to facilitate insertion of said implant between the two
adjacent vertebral bodies.
37. The implant of claim 32, wherein said trailing end is adapted to conform
from side to side to at least a portion of the peripheral contour of at least
one of the anterior and posterior aspects of the vertebral bodies adjacent a
disc space into which said implant is inserted.
38. The implant of claim 32, wherein said implant has a perimeter, said
passage being within said perimeter of said implant.
39. The implant of claim 32, wherein said implant has a perimeter, said
passage intersecting at least a portion of said perimeter.
40. The implant of claim 32, wherein said passage is between said leading
and trailing ends of said implant.
41. The implant of claim 32, further comprising opposite sides between said
leading end and said trailing end.
42. The implant of claim 41, wherein at least one of said opposite sides is at
least in part straight along at least a portion of the length of said implant.
43. The implant of claim 41, wherein said implant has a mid-longitudinal axis
along the length, at least one of said opposite sides being at least in part
oriented generally parallel to the mid-longitudinal axis of said implant.
44. The implant of claim 41, wherein said opposite sides are at least in part
generally parallel one another.
45. The implant of claim 32, wherein at least a portion of said upper and
lower
surfaces are in an angular relationship to each other from trailing end to
27
leading end for allowing angulation of the adjacent vertebral bodies
relative to each other.
46. The implant of claim 32, wherein said implant has a maximum length less
than and approximating the posterior to anterior depth of the vertebral
bodies.
47. The implant of claim 32, further comprising a bone engaging surface
formed on the exterior of at least said upper and lower portions for
engaging the adjacent vertebral bodies, said bone engaging surface
including at least one of a protrusion, a ratchet, a spike, a spline, surface
roughenings, and knurling.
48. The implant of claim 32, wherein said implant includes at least two
members, each member having a leading portion, a trailing portion, a top,
a bottom, and at least one side, each member being adapted to be placed
side by side with another of said members, said leading portion of said
members forming said leading end of said implant when placed side by
side.
49. The implant of claim 47, wherein said implant includes two of said
members, each member being a mirror image of the other.
50. The implant of claim 48, wherein each member includes at least a portion
of said passage.
51. The implant of claim 32, wherein said implant comprises at least in part
of
a bone growth promoting material.
52. The implant of claim 51, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
53. The implant of claim 32, in combination with a bone growth promoting
material.
54. The implant of claim 53, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
55. The implant of claim 32, wherein said implant is treated with a bone
growth promoting substance.
28
56. The implant of claim 32, wherein said implant is at least in part
resorbable.
57. The implant of claim 32, in combination with a chemical substance
adapted to inhibit scar formation.
58. The implant of claim 32, in combination with an antimicrobial material.
59. The implant of claim 32, wherein at least a portion of said implant is
treated to promote bone ingrowth between said implant and said adjacent
vertebral bodies.
60. The implant of claim 32, further in combination with at least one spinal
fixation implant.
61. The implant of claim 40, further comprising a curved transition between at
least one of said opposite sides and said trailing end, said curved
transition forming at least part of an arc of a circle.
62. The implant of claim 32, wherein said trailing end is adapted to receive
at
least one bone screw adapted to engage at least one vertebral body when
inserted through said implant.
63. The implant of claim 62, further comprising a lock for locking at least
one
bone screw to said implant.
64. The implant of claim 63, wherein said lock is made of one of cortical bone
and a bioresorbable material.
65. The implant of claim 62, wherein said screw is made of one of cortical
bone and a bioresorbable material.
66. An interbody spinal implant made of a bone composite material for
insertion at least in part into an implantation space formed across the
height of a disc space between adjacent vertebral bodies of a human
spine, the vertebral bodies having an anterior aspect and a posterior
aspect and a depth therebetween, said implant comprising:
a leading end for insertion first into the disc space and a trailing end
opposite said leading end, said implant having a length from said leading
end to said trailing end;
opposed upper and lower portions between said leading and trailing
ends adapted to be placed at least in part within and across the height of
the disc space to contact and support the adjacent vertebral bodies, said
upper and lower portions being non-arcuate along at least a portion of the
length of said implant;
29
opposite sides between said upper portion and said lower portion,
and between said leading and trailing ends, said opposite sides defining a
width of said implant, at least one of said opposite sides being at least in
part straight along at least a portion of the length of said implant, said
leading end configured in the shape of approximately one half a circle from
one of said opposite sides to another of said opposite sides, the circle
having a diameter generally equal to the width of said implant; and
said implant being manufactured from a bone composite material,
said upper and lower portions of said implant including at least one
opening in communication with one another to form at least a portion of a
passage adapted to hold bone growth promoting material for permitting for
the growth of bone from vertebral body to vertebral body through said
passage.
67. The implant of claim 66, wherein said bone composite material includes at
least one of cortical bone fibers, bone filaments, bone particles and bone
dust.
68. The implant of claim 66, further comprising a binding material.
69. The implant of claim 59, wherein said binding material is at least one of
bioactive and bioresorbable.
70. The implant of claim 66, wherein said implant has a maximum width
between said opposite sides that is greater than one-half of the width of
the adjacent vertebral bodies into which said implant is adapted to be
inserted,
71. The implant of claim 66, wherein said implant has a maximum width
between said opposite sides that is less than one-half of the width of the
adjacent vertebral bodies into which said implant is adapted to be inserted.
72. The implant of claim 71, wherein said implant is adapted to be inserted
side by side a second of said implant into the disc space between the
adjacent vertebral bodies.
73. The implant of claim 66, wherein at least a portion of said leading end is
has a reduced height to facilitate insertion of said implant between the two
adjacent vertebral bodies.
74. The implant of claim 66, wherein said trailing end is adapted to conform
from side to side to at least a portion of the peripheral contour of at least
30
one of the anterior and posterior aspects of the vertebral bodies adjacent a
disc space into which said implant is inserted.
75. The implant of claim 66, wherein said passage is between said opposite
sides of said implant.
76. The implant of claim 66, wherein said passage intersects at least one of
said opposite sides of said implant.
77. The implant of claim 66, wherein said passage is between said leading
and trailing ends of said implant.
78. The implant of claim 66, wherein said implant has a mid-longitudinal axis,
at least one of said opposite sides being at least in part oriented generally
parallel to the mid-longitudinal axis of said implant.
79. The implant of claim 66, wherein said opposite sides are at least in part
generally parallel one another.
80. The implant of claim 66, wherein at least a portion of said upper and
lower
surfaces are in an angular relationship to each other from trailing end to
leading end for allowing angulation of the adjacent vertebral bodies
relative to each other.
81. The implant of claim 66, wherein said implant has a maximum length less
than and approximating the posterior to anterior depth of the vertebral
bodies.
82. The implant of claim 66, further comprising a bone engaging surface
formed on the exterior of at least said upper and lower portions for
engaging the adjacent vertebral bodies, said bone engaging surface
including at least one of a protrusion, a ratchet, a spike, a spline, surface
roughenings, and knurling.
83. The implant of claim 66, wherein said implant includes at least two
members, each member having a leading portion, a trailing portion, a top,
a bottom, and at least one side, each member being adapted to be placed
side by side with another of said members, said leading portion of said
members forming said leading end of said implant when placed side by
side.
84. The implant of claim 83, wherein said implant includes two of said
members, each member being a mirror image of the other.
31
85. The implant of claim 83, wherein each member includes at least a portion
of said passage.
86. The implant of claim 66, wherein said implant comprises at least in part
of
a bone growth promoting material.
87. The implant of claim 86, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
88. The implant of claim 66, in combination with a bone growth promoting
material.
89. The implant of claim 88, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
90. The implant of claim 66, wherein said implant is treated with a bone
growth promoting substance.
91. The implant of claim 66, wherein said implant is at least in part
resorbable.
92. The implant of claim 66, in combination with a chemical substance
adapted to inhibit scar formation.
93. The implant of claim 66, in combination with an antimicrobial material.
94. The implant of claim 66, wherein at least a portion of said implant is
treated to promote bone ingrowth between said implant and said adjacent
vertebral bodies.
95. The implant of claim 66, further in combination with at least one spinal
fixationimplant.
96. The implant of claim 66, wherein said trailing end is adapted to receive
at
least one bone screw adapted to engage at least one vertebral body when
inserted through said implant.
97. The implant of claim 96, further comprising a lock for locking at least
one
bone screw to said implant.
98. The implant of claim 97, wherein said lock is made of one of cortical bone
and a bioresorbable material.
99. The implant of claim 96, wherein said screw is made of one of cortical
bone and a bioresorbable material.
32
100. An interbody spinal implant made of a bone composite material for
insertion at least in part into an implantation space formed across the
height of a disc space between adjacent vertebral bodies of a human
spine, the vertebral bodies having an anterior aspect and a posterior
aspect and a depth therebetween, said implant comprising:
a leading end for insertion first into the disc space and a trailing end
opposite said leading end, said implant having a length from said leading
end to said trailing end;
opposed upper and lower portions between said leading and trailing
ends adapted to be placed at least in part within and across the height of
the disc space to contact and support the adjacent vertebral bodies;
said implant having a maximum width that is greater than one-half
of the width of the adjacent vertebral bodies into which said implant is
adapted to be inserted, said leading end configured in the shape of
approximately one half of a first circle along the width of said implant, said
trailing end having a radius of curvature of a second circle along the width
of said implant, the second circle having a radius greater than the radius of
the first circle; and
said implant being manufactured from a bone composite material,
said upper and lower portions of said implant including at least one
opening in communication with one another to form a passage adapted to
hold bone growth promoting material for permitting for the growth of bone
from vertebral body to vertebral body through said passage.
101. The implant of claim 100, wherein said bone composite material includes
at least one of cortical bone fibers, bone filaments, bone particles and
bone dust.
102. The implant of claim 100, further comprising a binding material.
103. The implant of claim 102, wherein said binding material is at least one
of
bioactive and bioresorbable.
104. The implant of claim 100, wherein said leading end and said trailing end
of
said implant intersect at diametrically opposite points of the implant.
105. The implant of claim 100, wherein said width of said implant is
approximately equal to the diameter of the first circle.
33
106. The implant of claim 100, wherein said implant has a height from said
upper portion to said lower portion the height of said implant is less than
the maximum width of said implant.
107. The implant of claim 100, wherein at least a portion of said leading end
has a reduced height to facilitate insertion of said implant between the two
adjacent vertebral bodies.
108. The implant of claim 100, wherein said trailing end is adapted to conform
from side to side to at least a portion of the peripheral contour of at least
one of the anterior and posterior aspects of the vertebral bodies adjacent a
disc space into which said implant is inserted.
109. The implant of claim 100, wherein implant has a perimeter and said
passage is at least in part with said perimeter of said implant.
110. The implant of claim 100, wherein said implant has a perimeter, said
passage intersecting at least a portion of said perimeter of said implant.
111. The implant of claim 100, wherein said passage is between said leading
and trailing ends of said implant.
112. The implant of claim 100, further comprising opposite sides between said
leading end and said trailing end.
113. The implant of claim 112, wherein at least one of said opposite sides is
at
least in part straight along at least a portion of the length of said implant.
114. The implant of claim 112, wherein said implant has a mid-longitudinal
axis
along the length, at least one of said opposite sides being at least in part
oriented generally parallel to the mid-longitudinal axis of said implant.
115. The implant of claim 112, wherein said opposite sides are at least in
part
generally parallel one another.
116. The implant of claim 100, wherein at least a portion of said upper and
lower surfaces are in an angular relationship to each other from trailing
end to leading end for allowing angulation of the adjacent vertebral bodies
relative to each other.
117. The implant of claim 100, wherein said implant has a maximum length less
than and approximating the posterior to anterior depth of the vertebral
bodies.
118. The implant of claim 100, further comprising a bone engaging surface
formed on the exterior of at least said upper and lower portions for
34
engaging the adjacent vertebral bodies, said bone engaging surface
including at least one of a protrusion, a ratchet, a spike, a spline, surface
roughenings, and knurling.
119. The implant of claim 100, wherein said implant includes at least two
members, each member having a leading portion, a trailing portion, a top,
a bottom, and at least one side, each member being adapted to be placed
side by side with another of said members, said leading portion of said
members forming said leading end of said implant when placed side by
side.
120. The implant of claim 119, wherein said implant includes two of said
members, each member being a mirror image of the other.
121. The implant of claim 119, wherein each member includes at least a portion
of said passage.
122. The implant of claim 100, wherein said implant comprises at least in part
of a bone growth promoting material.
123. The implant of claim 122, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
124. The implant of claim 100, in combination with a bone growth promoting
material.
125. The implant of claim 124, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
126. The implant of claim 100, wherein said implant is treated with a bone
growth promoting substance.
127. The implant of claim 100, wherein said implant is at least in part
resorbable.
128. The implant of claim 100, in combination with a chemical substance
adapted to inhibit scar formation.
129. The implant of claim 100, in combination with an antimicrobial material.
35
130. The implant of claim 100, wherein at least a portion of said implant is
treated to promote bone ingrowth between said implant and said adjacent
vertebral bodies.
131. The implant of claim 100, further in combination with at least one spinal
fixation implant.
132. The implant of claim 111, further comprising a curved transition between
at
least one of said opposite sides and said trailing end, said curved
transition forming at least part of an arc of a circle.
133. The implant of claim 100, wherein said trailing end is adapted to receive
at
least one bone screw adapted to engage at least one vertebral body when
inserted through said implant.
134. The implant of claim 133, further comprising a lock for locking at least
one
bone screw to said implant.
135. The implant of claim 134, wherein said lock is made of one of cortical
bone
and a bioresorbable material.
136. The implant of claim 133, wherein said screw is made of one of cortical
bone and a bioresorbable material.
137. An artificial interbody spinal implant for insertion at least in part
into an
implantation space formed across the height of a disc space between
adjacent vertebral bodies of a human spine, the vertebral bodies having an
anterior aspect and a posterior aspect and a depth therebetween, said
implant comprising:
a leading end for insertion first into the disc space and a trailing end
opposite said leading end, said implant having a length from said leading
end to said trailing end;
opposed upper and lower portions between said leading and trailing
ends adapted to be placed at least in part within and across the height of
the disc space to contact and support the adjacent vertebral bodies, said
upper and lower portions defining a height of said implant, said upper and
lower portions being non-arcuate along at least a portion of the length of
said implant; and
opposite sides between said upper portion and said lower portion,
and between said leading and trailing ends, said opposite sides defining a
width of said implant, at least one of said opposite sides being at least in
36
part straight along at least a portion of the length of said implant, said
leading end configured in the shape of approximately one half of a circle
from one of said opposite sides to another one of said opposite sides, the
circle having a diameter generally equal to the width of said implant, the
width of said implant being greater than the height of said implant;
said implant being manufactured from a material other than bone,
said upper and lower portions of said implant including at least one
opening in communication with one another and adapted to hold bone
growth promoting material for permitting for the growth of bone from
vertebral body to vertebral body through said implant.
138. The implant of claim 137, wherein said implant has a maximum width
between said opposite sides that is greater than one-half of the width of
the adjacent vertebral bodies into which said implant is adapted to be
inserted.
139. The implant of claim 137, wherein said implant has a maximum width
between said opposite sides that is less than one-half of the width of the
adjacent vertebral bodies into which said implant is adapted to be inserted.
140. The implant of claim 139, wherein said implant is adapted to be inserted
side by side a second of said implant into the disc space between the
adjacent vertebral bodies.
141. The implant of claim 137, wherein at least a portion of said leading end
has a reduced height to facilitate insertion of said implant between the two
adjacent vertebral bodies.
142. The implant of claim 137, wherein said trailing end is adapted to conform
from side to side to at least a portion of the peripheral contour of at least
one of the anterior and posterior aspects of the vertebral bodies adjacent a
disc space into which said implant is inserted.
143. The implant of claim 137, wherein said trailing end has a radius of
curvature of a second circle from side to side.
144. The implant of claim 143, wherein the radius of curvature said trailing
end
is greater than the radius of curvature of the leading end of said implant.
145. The implant of claim 137, wherein said at least one opening is between
said opposite sides of said implant.
37
146. The implant of claim 137, wherein said at least one opening intersects at
least one of said opposite sides.
147. The implant of claim 137, wherein said at least one opening is between
said leading and trailing ends of said implant.
148. The implant of claim 137, wherein said implant has a mid-longitudinal
axis
along the length, at least one of said opposite sides being at least in part
oriented generally parallel to the mid-longitudinal axis of said implant.
149. The implant of claim 137, wherein said opposite sides are at least in
part
generally parallel one another.
150. The implant of claim 137, wherein at least a portion of said upper and
lower surfaces are in an angular relationship to each other from trailing
end to leading end for allowing angulation of the adjacent vertebral bodies
relative to each other.
151. The implant of claim 137, wherein said implant has a maximum length less
than and approximating the posterior to anterior depth of the vertebral
bodies.
152. The implant of claim 137, further comprising a bone engaging surface
formed on the exterior of at least said upper and lower portions for
engaging the adjacent vertebral bodies, said bone engaging surface
including at least one of a protrusion, a ratchet, a spike, a spline, surface
roughenings, and knurling.
153. The implant of claim 137, wherein said implant includes at least two
members, each member having a leading portion, a trailing portion, a top,
a bottom, and at least one side, each member being adapted to be placed
side by side with another of said members, said leading portion of said
members forming said leading end of said implant when placed side by
side.
154. The implant of claim 153, wherein said implant includes two of said
members, each member being a mirror image of the other.
155. The implant of claim 153, wherein each member includes at least a portion
of said at least one opening.
156. The implant of claim 137, in combination with a bone growth promoting
material.
38
157. The implant of claim 156, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
158. The implant of claim 137, wherein said implant is treated with a bone
growth promoting substance.
159. The implant of claim 137, wherein said implant is at least in part
resorbable.
160. The implant of claim 137, in combination with a chemical substance
adapted to inhibit scar formation.
161. The implant of claim 137, in combination with an antimicrobial material.
162. The implant of claim 137, wherein at least a portion of said implant is
treated to promote bone ingrowth between said implant and said adjacent
vertebral bodies.
163. The implant of claim 137, in combination with at least one spinal
fixation
implant.
164. The implant of claim 137, wherein said trailing end is adapted to receive
at
least one bone screw adapted to engage at least one vertebral body when
inserted through said implant.
165. The implant of claim 164, further comprising a lock for locking at least
one
bone screw to said implant.
166. An artificial interbody spinal implant for insertion at least in part
into an
implantation space formed across the height of a disc space between
adjacent vertebral bodies of a human spine, the vertebral bodies having an
anterior aspect and a posterior aspect and a depth therebetween, said
implant comprising:
a leading end for insertion first into the disc space and a trailing end
opposite said leading end, said implant having a length from said leading
end to said trailing end;
opposed upper and lower portions between said leading and trailing
ends adapted to be placed at least in part within and across the height of
the disc space to contact and support the adjacent vertebral bodies;
said implant having a maximum width that is greater than one-half
of the width of the adjacent vertebral bodies into which said implant is
39
adapted to be inserted, said leading end configured in the shape of
approximately one half of a first circle along the width of said implant, said
trailing end having a radius of curvature of a second circle along the width
of said implant, the second circle having a radius greater than the radius of
the first circle; and
said implant being manufactured from a material other than bone,
said upper and lower portions of said implant including at least one
opening in communication with one another and adapted to hold bone
growth promoting material for permitting for the growth of bone from
vertebral body to vertebral body through said implant.
167. The implant of claim 166, wherein said leading end and said trailing end
of
said implant intersect at diametrically opposite points of said implant.
168. The implant of claim 166, wherein said width of said implant is
approximately equal to the diameter of the first circle.
169. The implant of claim 166, wherein said implant has a height from said
upper portion to said lower portion, the height of said implant being less
than the maximum width of said implant.
170. The implant of claim 166, wherein at least a portion of said leading end
has a reduced height to facilitate insertion of said implant between the two
adjacent vertebral bodies.
171. The implant of claim 166, wherein said trailing end is adapted to conform
from side to side to at least a portion of the peripheral contour of the
anterior aspect of the vertebral bodies adjacent a disc space into which
said implant is inserted.
172. The implant of claim 166, wherein said implant has a perimeter, said at
least one opening being within said perimeter of said implant.
173. The implant of claim 166, wherein said implant has a perimeter, said at
least one opening intersecting at least a portion of said perimeter of said
implant.
174. The implant of claim 166, wherein said at least one opening is between
said leading and trailing ends of said implant.
175. The implant of claim 166, further comprising opposite sides between said
leading end and said trailing end.
40
176. The implant of claim 175, wherein at least one of said opposite sides is
at
least in part straight along at least a portion of the length of said implant.
177. The implant of claim 175, wherein said implant has a mid-longitudinal
axis
along the length, at least one of said opposite sides being at least in part
oriented generally parallel to the mid-longitudinal axis of said implant.
178. The implant of claim 175, wherein said opposite sides are at least in
part
generally parallel one another.
179. The implant of claim 166, wherein at least a portion of said upper and
lower surfaces are in an angular relationship to each other from trailing
end to leading end for allowing angulation of the adjacent vertebral bodies
relative to each other.
180. The implant of claim 166, wherein said implant has a maximum length less
than and approximating the posterior to anterior depth of the vertebral
bodies.
181. The implant of claim 166, further comprising a bone engaging surface
formed on the exterior of at least said upper and lower portions for
engaging the adjacent vertebral bodies, said bone engaging surface
including at least one of a protrusion, a ratchet, a spike, a spline, surface
roughenings, and knurling.
182. The implant of claim 166, wherein said implant includes at least two
members, each member having a leading portion, a trailing portion, a top,
a bottom, and at least one side, each member being adapted to be placed
side by side with another of said members, said leading portion of said
members forming said leading end of said implant when placed side by
side.
183. The implant of claim 182, wherein said implant includes two of said
members, each member being a mirror image of the other.
184. The implant of claim 182, wherein each member includes at least a portion
of said at least one opening.
185. The implant of claim 166, in combination with a bone growth promoting
material.
186. The implant of claim 185, wherein said bone growth promoting material is
selected from one of bone, bone derived products, demineralized bone
41
matrix, mineralizing proteins, ossifying proteins, bone morphogenetic
protein, hydroxyapatite, and genes coding for the production of bone.
187. The implant of claim 166, wherein said implant is treated with a bone
growth promoting substance.
188. The implant of claim 166, wherein said implant is at least in part
resorbable.
189. The implant of claim 166, in combination with a chemical substance
adapted to inhibit scar formation.
190. The implant of claim 166, in combination with an antimicrobial material.
191. The implant of claim 166, wherein at least a portion of said implant is
treated to promote bone ingrowth between said implant and said adjacent
vertebral bodies.
192. The implant of claim 166, in combination with at least one spinal
fixation
implant.
193. The implant of claim 175, further comprising a curved transition between
at
least one of said opposite sides and said trailing end, said curved
transition forming at least part of an arc of a circle.
194. The implant of claim 16630, wherein said trailing end is adapted to
receive
at least one bone screw adapted to engage at least one vertebral body
when inserted through said implant.
195. The implant of claim 194, further comprising a lock for locking at least
one
bone screw to said implant.
42