Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02444313 2003-10-03
CHEMIGAL HYDRIDE HYDROGEN GENERATION SYSTEM AND AN
ENERGY SYSTEM INCORPORATING THE SAME
FIELD OF INVENTION
(0001] This invention relates to a hydrogen generation system and
more particularly relates to a chemical hydride hydrogen generation system in
combination with a fuel cell system.
BACKGROUND TECHNOLOGY
(0002] Hydrogen has been recognized as an environmentally friendly
clean fuel of the future since it has various applications in power generation
systems. For example, hydrogen can be used as a fuel for fuel cells,
especially
proton exchange membrane fuel cells, which use hydrogen and air to produce
electricity, generating only water as a by-product. Fuel cells are being
developed
to replace traditional electricity generators because they produce clean,
environmentally friendly energy. However, these fuel cells require external
supply and storage devices for hydrogen. Extensive efforts have been made to
develop a safe and efficient way to store hydrogen, especially in mobile
applications. Conventional hydrogen storage technologies include liquid
hydrogen, compressed gas cylinders, dehydrogenation of compounds, chemical
adsorption into metal alloys and chemical storage as hydrides. However, each
of
these systems is either hazardous or bulky.
(0003) Another method of storing hydrogen has been proposed
recently. This method uses a classical chemical hydride, such as NaBH4, as a
hydrogen storage medium. The principle of this method is the reaction of the
chemical hydride with water in the presence of a catalyst to generate
hydrogen,
as shown in the equation below:
NaBH4 + 2H20 -~ 4H2 + NaB02 + HEAT
1
CA 02444313 2003-10-03
[0004) The borohydride, NaBH4, acts as both the hydrogen carrier and
the storage medium. Ruthenium, Cobalt, Platinum or alloys thereof can be used
as a catalyst in this reaction. It is to be noted that this reaction occurs
without a
catalyst in an acidic environment and only slightly under alkali conditions.
This
means the chemical hydride solution can be stored and has a shelf life under
alkali conditions. This reaction is efficient on a weight basis since half of
the
hydrogen produced comes from NaBH4 and the other half comes from H20.
Borohydride is a relatively cheap material, usually used in wastewater
processing, pharmaceutical synthesis, etc. Borohydride is also easier and
safer
to handle and transport than highly pressurized hydrogen or cryogenic
hydrogen.
As a result, there are some advantages to use borohydride as a method of
storing hydrogen as a fuel for fuel cells.
[0005) There are several known examples of hydrogen generation
systems that utilize chemical hydrides. One type of hydrogen generation system
comprises a closed vessel for containing chemical hydride and a mechanical
stirring mechanism disposed within the vessel for stirring the chemical
hydride
within the vessel. Water is injected into the vessel to react with chemical
hydride
and generated hydrogen is removed from the vessel through an outlet. The
stirring mechanism means is used to ensure sufficient contact between the
hydride and water while preventing the clumping of the hydride. Since the
hydride is in solid phase in this system, the stirring mechanism is
indispensable.
However, in such systems the stirring mechanism consumes energy, increases
the overall system weight and reduces system efficiency. Further, the noise
generated in the stirring operation is undesirable. In addition, the reaction
rate is
low, making the fuel unresponsive, useless or very hard to control. The system
also tends to be large and cumbersome.
[0006) Another type of hydrogen generation system employs a
chemical hydride solution. In this system an aqueous chemical hydride solution
is introduced to a catalyst bed to generate hydrogen. However, there are a
number of problems associated with this liquid phased system. First, the by-
product borate, in the above equation, NaB02 is less soluble then the reactant
borohydride, namely NaBH4. Specifically, NaB02 is only approximately 20%
2
CA 02444313 2003-10-03
soluble. This means that in order to generate hydrogen in a liquid phased
system, and thereby reduce the problems associated with the aforementioned
solid phased systems, the concentration of borohydride in the solution can
only
be about 20°lo which is much lower than borohydride's solubility in
water.
Therefore the achievable hydrogen density of the system is considerably
limited.
[0007] A further deficiency of the aforementioned examples is that
neither system is capable of responding in real time to the fuel (hydrogen)
needs
of the fuel cell. This ability is referred to as load following ability.
SUMMARY OF THE INVENTION
[0008] In order to overcome the aforementioned deficiencies
associated with the prior art, one aspect of the present invention provides an
energy system comprising:
a fuel cell stack for generating electricity from hydrogen and an oxidant to
form
water; a chemical hydride hydrogen generation system, comprising:
a storage means for storing a chemical hydride solution comprising a solution
of a chemical hydride solute in a solvent; a reactor containing a catalyst,
for
catalyzing reaction of the chemical hydride to generate hydrogen; a first pump
means connected between the storage means and the reactor in a first circuit,
for circulating the chemical hydride solution through the storage means and
the
reactor, so that the chemical hydride reacts to generate hydrogen in the
presence of the catalyst;
a first connection between the chemical hydride generation system and the
fuel cell stack for supplying hydrogen to the fuel cell stack; and
a heat transfer circuit including second connections between the chemical
hydride generation system and the fuel cell stack, for circulation of the
chemical
hydride solution through the fuel cell stack to effect heat transfer between
the
fuel cell stack and the chemical hydride solution
[0009] The chemical hydride solution can be a borohydride hydride
water solution. The solute of the solution can be in the form of MBxHy, in
which M
is a metal. Specifically, the solute can be NaBHa, LiBH4, KBHa, RbH4.
3
CA 02444313 2003-10-03
Alternatively, the solute can be NH3BH3. Preferably, the chemical hydride
solution is a water solution in which the solute is NaBH4 and less than 5%
LiBHa.
Preferably, to ensure the system works properly under low temperature, the
chemical hydride solution further includes a freezing point depressing agent.
The
freezing point depressing agent is preferably glycerol and concentration of
glycerol is no higher than 5%. More preferably, the concentration of glycerol
is
1 °I°. The solution preferably further includes alkaline
additives. More preferably,
the alkaline additive is selected from LiOH, KOH, and NaOH. More preferably,
the alkaline additive is 0.1% NaOH.
[0010] More preferably, the system further includes a flow control
means that operatively stops the first pump means when the hydrogen pressure
in the reactor reaches a first value and activates the first pump means when
the
hydrogen pressure in the reactor reaches a second value lower than the first
value. More preferably, the system further includes a heat exchanging means
for
the reactor that selectively removes heat from the reactor during normal
operation and heats up the reactor when the system works under low
temperature.
According to another aspect of the present invention, there is provided a
method
of operating a fuel cell stack and chemical hydride hydrogen generation system
with a reactor including a catalyst for generating hydrogen for the fuel cell
and
controlling the temperature of the fuel cell stack and the chemical hydride
generation system, the method comprising the steps of:
1) supplying a chemical hydride solution to the reactor, and permitting
the catalyst to catalyze reaction of the chemical hydride solution to generate
hydrogen;
2) supplying the hydrogen to the fuel cell stack and supplying an
oxidant to the fuel cell stack, for generation of electricity;
3) circulating the chemical hydride solution through the fuel cell stack
and the chemical hydride generation system, to effectively transfer heat
therebetween.
4
CA 02444313 2003-10-03
[0011] Preferably, the means for recovering the water generated in
the fuel cell includes a gas-liquid separator. More preferably, the system
further
includes a switch means that selectively allows the excess hydrogen leaving
the
fuel cell after reaction to be circulated back to the fuel cell, in the first
mode and
allows the hydrogen to be supplied to the catalytic burner from the fuel cell
in the
second mode. More preferably, the system further includes a first control
means
that operatively switches the switch means between the first and second modes.
[0012] Preferably, the means for supplying hydrogen generated in
the reactor to the fuel cell further includes a filtering means between the
reactor
and the fuel cell for purifying the hydrogen generated in the reactor before
the
hydrogen is supplied to the fuel cell.
[0013) In order to provide the energy system with load following
capability, the system further includes a second control means that
operatively
stops the first pump means when the hydrogen pressure in the reactor reaches a
first value and activates the first pump means when the hydrogen pressure in
the
reactor reaches a second value lower than the first value.
[0014] In order to ensure that the energy system works properly under
low temperature, the system further includes a heat exchanging means for the
reactor that selectively removes heat from the reactor during normal operation
and heats up the reactor when the system works under low temperature.
The fuel cell stack can comprise a single fuel cell or can include a plurality
of fuel
cells, and coolant ducts can be provided for the or each fuel cell.
[0015] The chemical hydride hydrogen generation system according to
the present invention provides a safe, clean, efficient and reliable hydrogen
generation system and an energy system in which the hydrogen generation
system and the fuel cell system operate synergistically. The hydrogen
generation system is safe in that low pressure hydrogen is generated and used
in the fuel cell instead of highly pressurized hydrogen. The system is also
environmentally safe in that the reaction products are harmless detergent base
5
CA 02444313 2003-10-03
chemicals. When novel borohydride solution is used, the system can operate at
as low as -22°C temperature. The pressure control means employed in the
system enables the system to follow the load of fuel cell stack as well as
meet
peak performance requirements. By capturing and recycling the water in the
fuel
cell exhaust and introducing it into the hydride solution, the system of the
present invention further enhances the energy density. Experiments show that
the chemical hydride hydrogen generation system according to the present
invention has achieved energy densities of 1.2 KWh/L and 0.8 KWh/kg, which is
comparable, if not advantageous to fuel cell systems currently available.
Hydrogen recycled through a filtration system could also allow for higher
system
efficiency as well as an increased chemical hydride energy density. The
circulation of the chemical hydride solution as a heat transfer fluid between
the
chemical hydride reactor and the fuel cell stack avoids the need for a
separate
heat transfer fluid and provides the advantage of mutually warming up the
chemical hydride reactor and the fuel cell stack, during initial start up,
thereby
shortening the time needed for the system to achieve optimum operation
condition, and further improving the system efficiency.
BRIEF DESCRIPTION OF DRAWINGS
(0016] For a better understanding of the present invention, and to show
more clearly how it may be carried into effect, reference will now be made to
the
accompanying drawings, which show, by way of example, preferred
embodiments of the present invention, and in which:.
(0017] Figure 1 is a schematic view of the first embodiment of the
energy system according to the present invention;
(0018] Figure 2 is a schematic view of the second embodiment of the
energy system according to the present invention;
(0019] Figure 3 is a diagram showing the hydrogen flow of the
energy system according to the present invention during operation;
6
CA 02444313 2003-10-03
(0020] Figure 4 is a diagram showing the relation of freezing point of
the chemical hydride solution according to the present invention with the
concentration of freezing point depressing agent in the solution;
(0021] Figure 5 is a diagram showing the relation of chemical hydride
solution temperature according to the present invention with the concentration
of
freezing point depressing agent in the solution;
[0022] Figure 6 is a diagram showing the comparison of two different
chemical hydride solutions;
[0023] Figure 7 is a schematic view of the third embodiment of the energy
system according to the present invention;
(0024] Figure 8 is a schematic view of the fourth embodiment of the
energy system according to the present invention; and
(0025] Figure 9 is a graph showing variation of coolant outlet
temperatures with time after start up for the chemical hydride reactor alone,
the
fuel cell stack alone and the combination of both.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
(0026] The features and advantage of the present invention will
become more apparent in the light of the following detailed description of
preferred embodiments thereof.
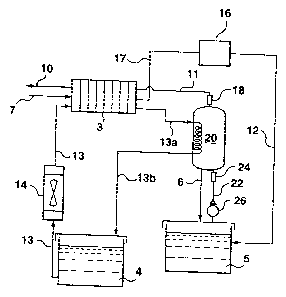
(0027] Referring to figure 1, the chemical hydride power generation
system according to the present invention combines a hydrogen generation
system and a fuel cell system. The hydrogen generation system includes a
chemical hydride storage tank 5 and a reactor 20. The fuel cell includes a
fuel
cell stack 3 and some peripherals, namely a coolant storage tank 4, a heat
exchanger 14 and a gas-liquid separator 16. In this embodiment, the present
7
CA 02444313 2003-10-03
invention is described by using NaBH4 as an example of the chemical hydride
used in the hydrogen generation system.
(0028] Chemical hydride is stored in the storage tank 5 in the form of
solution. When hydrogen is demanded by the fuel cell stack 3, the hydride is
supplied to the reactor 20 through a line 22 by means of a pump 26. The
reactor
20 contains a catalyst for the reaction. Therefore, the hydride reacts within
the
reactor 20 and hydrogen is generated. The generated hydrogen flows out
through a hydrogen outlet of the top of the reactor 20 and is supplied to an
anode inlet of the fuel cell stack 3 via a hydrogen line 11. As is known in
the art,
the hydrogen reacts on the anode of the fuel cell stack 3 and the unreacted
hydrogen leaves the fuel cell stack 3 through the anode outlet 10 thereof. The
unreacted hydride in the form of solution, together with the by-product NaB02
returns to the storage tank 5 through line 6.
(0029] In the fuel cell stack 3, oxidant, typically air is introduced through
an air inlet 7 into the cathode of the fuel cell stack 3. As is known to those
skilled
in the art, the oxygen in the air reacts at the cathode of the fuel cell stack
3 and
generates water as a product. The unreacted air leaves the fuel cell stack 3
through the cathode outlet thereof and flows out through a discharged airline
17
as shown in figure 1.
(0030] As previously mentioned , the by-product of the hydrogen
generation reaction, in this embodiment NaB02, is less soluble then the
reactant
NaBH4. Specifically, NaB02 is only approximately 20% soluble. Therefore, as
the
hydrogen generation reaction continues, the concentration of NaB02 in the
hydride solution stored in the storage tanks increases until it reaches the
solubility of the NaB02. If the reaction continues, NaB02 in solid phase will
occur
in the storage tank and may be supplied to the reactor 20, resulting in
clogging
of the hydrogen generation system. Thus, the reaction degrades and not enough
hydrogen will be supplied to the fuel cell stack 3. In conventional systems,
in
order to prevent this from happening, the initial concentration of the hydride
in
the solution has to be reduced to as low as 20% which is much lower than the
borohydride solubility in water, 40%. Consequently, the achievable hydrogen
8
CA 02444313 2003-10-03
storage density of the system is considerably limited. In this present
invention,
this problem is overcome by continuously introducing water into the hydride
tank
5. As the hydrogen generation continues, the increase of NaB02 concentration
in the solution is counteracted by the increase of solvent, i.e. water.
Therefore,
the initial concentration of the hydride can be higher than that is allowed in
conventional systems, which results in higher hydrogen density of the system.
[0031] Preferably, the operation of continuously introducing water into
the hydride tank 5 is achieved by recycling of the water entrapped in the fuel
cell
exhaust and supplying the water to the hydride solution. As shown in figure 1,
water is generated on the cathode of the fuel cell stack 3 and is exhausted
from
the fuel cell stack 3 together with the unreacted air. The exhaust mixture of
air
and water then flows to a gas-liquid separator 16 in which air and water are
separated. Therefore, the water is recovered. The recovered water is then
introduced through a recovered water line 12 into the chemical hydride
solution
in the storage tank 5. As water is a by-product of the fuel cell reaction, the
hydrogen generation system utilizes the readily available water in its
vicinity,
resulting in increased system efficiency. Generally, recovery of the exhaust
water can enable the initial concentration of the hydride to be increased by
at
least 50%, as is demonstrated in the following tables.
TABLE 1 Conventional NaBH4 water solution
NaBH4 H20 NaB02
Iteration WT% (g) (g) WT%
#
(g)
0.00 400.00 40.00% 1000.000.00 0.00%
1.00 350.00 36.75% 952.37 86.97 9.13%
2.00 300.00 33.1610904.73 173.9419.23%
3.00 250.00 29.17% 857.10 260,9030.44%
4.00 200.00 24.71 809.46 347.8742.98%
%
5.00 150.00 19.69% 761.83 434.8457.08%
6.00 100.00 14.00% 714.20 521.8173.06%
7.00 50.00 7.50% 666.56 608.7891.33!0
8.00 0.00 0.00% 618.93 695.74112.41
9
CA 02444313 2003-10-03
TABLE 2 NaBH4 water solution of the present invention
NaBH4 H20 NaB02 Recovered
H20
Iteration(g) UVT1o (g) (g) Wf% (g)
#
0.00 400.0040.00%1000.000.00 0.00% 0.00
1.00 350.0033.41 1047.6386.97 8.30% 95.27
%
2.00 300.0027.39%1095.27173.94 15.88% 95.27
3.00 250.0021.87%1142.90260.90 22.83% 95.27
4.00 200.0016.80%1190.54347.87 29.22% 95.27
5.00 150.0012.11 1238.17434.84 35.12% 95.27
%
6.00 100.007.78% 1285.80521.81 40.58% 95.27
7.00 50.00 3.75% 1333.44608.78 45.65% 95.27
8.00 0.00 0.00% 1381.07695.74 50.38% 95.27
(0032] Table 1 and table 2 respectively show the composition of
NaBH4, water and NaB02 in the solution during hydrogen generation reaction,
for a conventional solution and for a solution in accordance with the present
invention, in which the water is added as the reaction progresses. The initial
concentration of NaBH4 in both tables is 40%, that is to say, 400g NaBH4 in 1
L
water. As the experimental data show, the conventional solution begins to clog
when there is 300g of NaBH4 left in the solution. This is because the
concentration of NaBH4 is close to the initial 20% level, and exceeds this
when
the level of NaBHa has fallen to 250g. The solution of the present invention
(Table 2) only begins to clog when 250g of NaBH~ is left in the solution.
Again,
the table indicates that the critical 20% level for NaBH2 is exceeded just
before
the amount of NaBH4 falls to 250g.1t is clear that the present invention
considerably increases the hydrogen generation capacity of NaBH4 water
solution. The data shows that, by adding exhausted water, one can reduce the
concentration of NaB02 effectively enabling the NaBH4 level to be reduced
further before clogging occurs. Table 1 shows, as expected due to utilization
of
H20 to generated hydrogen, that the amount of H20 reduces by over one third at
the end of the experiment. Table 2 shows, due to the addition of the exhaust
water, that the total volume of water increases to close to 1400 g. and is at
1142.90 grams of at iteration 3. This would require the tank 5 to have a
larger
volume. However, the initial amount of NaBH4 is increased by 50g, that is 1/6
more than that in conventional systems. The increase of water happens only as
the reaction continues. In this example, the amount of water is only increased
by
CA 02444313 2003-10-03
approximately 1110, which means the recovery of water still has the effect of
increasing the energy density of the overall system.
[0033] Since the reaction in the reactor 20 is exothermic, necessary
cooling means has to be provided. Generally, cooling tubes can be provided
within the reactor 20 in which a cooling fluid flows through. As shown in
figure 1,
in the present invention, the coolant for the hydrogen generation reaction can
be
water or other commonly used coolants for the fuel cell stack 3. Coolant
enters
the reactor 20 via a coolant inlet thereof from the coolant line 13a, flows
through
the coolant tubes in the reactor 20 and leaves the reactor 20 via a coolant
outlet,
taking away the heat generated in the reactor 20. Then the coolant returns to
a
coolant storage tank 4 at the coolant line 13b and is circulated to the fuel
cell
stack 3. Generally, a heat exchanger 14 is provided in the coolant line 13
before
or after the coolant reaches the fuel cell stack 3 to maintain the coolant at
a low
temperature. Then the coolant flows through the fuel cell stack 3 and then
into
the reactor 20 and continues to circulate in the coolant loop. The heat
exchanger 14 can in principle be located at any location in the coolant lamp.
[0034] In operation, the fuel cell stack 3 generates electricity while
consuming the hydrogen supplied from the reactor 20. In order to provide the
hydrogen generation system with the load following ability, a pressure sensor
18
is provided at the hydrogen outlet of the reactor 20. The pressure sensor 18
is in
connection with a switch 24 in hydride supply line 22 and controls the
operation
of the same, and the switch 24 controls the pump 26 pumping the solution from
the tank 5 to the reactor 20. As the reaction in the reactor 20 continues, the
hydrogen is generated and supplied to the fuel cell stack 3. However, when the
fuel cell stack 3 operates in a condition that the hydrogen generation rate in
the
reactor 20 is more than the hydrogen consumption rate of the fuel cell stack
3,
the pressure of hydrogen in the reactor 20 increases until it reaches a
certain
value when the pressure sensor 18 activates the switch 24 to shut down the
pump, and hence cut the hydride supply to the reactor 20. Therefore the
reaction
in the reactor 20 stops. Then the fuel cell stack 3 continue to consume the
hydrogen, resulting in the pressure drop of hydrogen in the reactor 20 until
it
reaches a certain value when the pressure sensor 18 controls the switch 24 to
11
CA 02444313 2003-10-03
start the pump 26 and hence the hydride solution is supplied to the reactor 20
and in turn hydrogen is generated to meet the demand of the fuel cell stack 3.
Thus the system of the present invention has the capability to follow the load
as
well as meet peak performance requirements. Additionally, when the fuel cell
stack 3 shuts down, the pressure sensor 18 will immediately activate the
switch
24 to shut down the pump 26. The reactor 20 preferably has a vent (not shown)
so that the hydrogen present in the reactor at the time of shutdown and that
generated thereafter can be released, either into the environment or a storage
device. Hence the system can shut down completely in a relatively short time.
Figure 3 illustrates the hydrogen flow of the present system during operation
at a
constant rate, employing the pressure sensor 18 and the switch 24. As
illustrated, the hydrogen flow is stable throughout the operation. It is to be
understood that the drop of the hydrogen flow in the curve indicates the
process
of system shutdown.
[0035] In practice, the fuel cell and the hydrogen generation system
may work under low temperature. However, borohydride water solution freezes
at about 0°C. In the present invention, the solution can be a
borohydride water
solution with glycerol and sodium hydroxide. As can be seen in Figure 4, which
shows the relation of freezing point of the solution with the concentration of
the
glycerol, the addition of glycerol considerably lowers the freezing point of
the
solution. For example, the solution is stable and can still operate at as low
as -
22°C with 1 °!o of glycerol. Figure 5 shows the effects of
various concentrations of
glycerol on the freezing point of the solution. In Figure 5, the sudden
increase
in the solution temperature indicates that the solution starts to freeze since
the
crystallization process is exothermic. As the concentration of glycerol
increases,
even lower freezing points can be obtained and an approximately -35°C
freezing point is achieved with 16% glycerol. However, the solubility of the
borohydride, hence the hydrogen density of the overall hydrogen generation
system decreases with the increase in the concentration of glycerol.
Experiments show that the concentration of glycerol is preferably lower than
5%
and the best compromise between the freezing point and hydrogen density of
the solution is 1 % glycerol. 1 % of glycerol does not noticeably compromise
the
borohydride solubility but achieves a freezing point of -22°C.
12
CA 02444313 2003-10-03
[0036] In order to further ensure that the system works properly under
low temperature, the coolant in the present invention can also be used to heat
the system. In this situation, another heat exchanger may be added in the
coolant line 13 between the fuel cell stack 3 and the reactor 20 so that the
coolant can be further heated after it leaves the fuel cell stack 3. The
heated
coolant in turn heats the reactor 20 to facilitate the hydrogen generation
reaction.
[0037] Preferably, the chemical hydride solution further includes
alkaline additives, such as LiOH, KOH, NaOH to provide an alkaline condition,
which significantly slows the chemical hydride reaction, thereby lengthening
the
shelf life of the solution. NaOH is mostly used due to its relatively low mass
and
cost. A concentration of 0.1 % NaOH is adequate in raising the PH enough to
stabilize the solution.
(0038] For NaBH4, another additive, namely L;BH4 may be added into
NaBHd solution. This lighter material has a much higher hydrogen density than
NaBH4. However, the by-product of LiBH4 and water reaction, LiB02, only has a
solubility of 5% and the reaction of LiBH4 with water is much slower than that
of
NaBH4 and water. This means an addition of less than 5% LiBH4 to the NaBH4
solution will increase the hydrogen density of the solution without causing
precipitation of the by-product and without significantly affecting its load
following
ability. Figure 6 shows the comparison of NaBH4 solution and LiBHa-NaBH4
solution. The generally lower reaction rate and slow rise in temperature
indicate
that the mixed solution is more stable than pure NaBH4.
[0039] Now referring to figure 2, a second embodiment of the present
invention is shown. In this embodiment, similar components are indicated with
same reference numbers. As can be seen in figure 2, the chemical hydride
hydrogen generation system according to the present invention combines a
hydrogen generation system and a fuel cell system. The hydrogen generation
system generally includes a chemical hydride storage tank 5 and a reactor 20.
The fuel cell includes a fuel cell stack 3 and some peripherals, namely a
coolant
storage tank 4, a heat exchanger 14, a catalytic burner 2 and a water recovery
13
CA 02444313 2003-10-03
unit 1. In this embodiment, the present invention is also described using
NaBH4
as an example of the chemical hydride used in the hydrogen generation system.
[0040] The hydrogen is generated in the reactor 20 in the same manner
as that in the first embodiment. Likewise, the coolant loop is also identical
to that
in the first embodiment. Therefore, for simplicity and brevity, the
description of
the components will not be repeated.
[0041] In this embodiment, hydrogen enters the fuel cell stack 3 from
the hydrogen outlet of the reactor 20. Preferably, a filter 28 is provided in
the
hydrogen line 11 before the hydrogen enters the fuel cell stack 3 to remove
fine
aerosol particles in solution, catalyst and other particles (and this filter
can be
included in the first embodiment of Figure 1 ). As is known to those skilled
in the
art, a considerable portion of both air and hydrogen supplied to the fuel cell
stack
3 does not react. Rather, the excess hydrogen and air leave the fuel cell
stack 3
through the anode and cathode outlets thereof, respectively. Therefore, it is
preferable to recirculate the excessive hydrogen back to the fuel cell stack 3
for
reaction. For this purpose, a hydrogen recycle loop 15 and a catalytic burner
2
are provided in this embodiment. As shown in figure 2, a valve 9 and a
centrifugal pump 19 are provided respectively at the two ends of the hydrogen
recycle loop 15. Specifically, a centrifugal pump 19 is provided at the
junction of
the hydrogen recycle loop 15 and the hydrogen line 11 between the reactor 20
and fuel cell stack 3, and a valve 9 at the junction of the hydrogen recycle
loop
15 and the hydrogen line 11 between the fuel cell stack 3 and the catalytic
burner 2. When the fuel cell stack 3 is in operation, the pump 19 operates
continuously, creating a negative pressure to ensure the hydrogen generated in
the reactor 20 continuously flows from the reactor 20 to the fuel cell stack 3
via
the hydrogen line 11. Excessive hydrogen flows through the anode outlet 10 of
the fuel cell stack 3 to the valve 9. The valve 9 is in a position that closes
the
hydrogen line 11 from the anode outlet 10 to the catalytic burner 2, thereby
forcing the hydrogen to flow along the hydrogen recycle loop 15 and back to
the
fuel cell stack 3 for reaction by means of the pump 19. On a periodic basis,
the
valve 9 is turned to an open position so that the excess hydrogen flows to the
catalytic burner 2. As can be seen in figure 2, the exhaust of the fuel cell
from
14
CA 02444313 2003-10-03
the cathode thereof also flows into a catalytic burner 2 along the respective
line
17 thereof after leaving the fuel cell stack 3. In the catalytic burner, the
hydrogen
and the oxygen in the exhaust of the fuel cell react in the presence of an
appropriate catalyst to form water in the known manner, i.e. 2H2 + 02 --~
2H20.
Then the mixture of water and unreacted exhaust of the fuel cell flows from
the
catalytic burner 2 into a water recovery unit 1 which may be a gas-liquid
separator. The water is separated from the mixture and circulates to the
hydride
storage tank 5. Recognizing that there will usually be an excess of air or
hydrogen, an exhaust 8 is provided for venting residual gas into the
environment
from the water recovery unit 1. In practice, the opening of the valve 9 to let
hydrogen flow to the catalytic burner 2 may be controlled by a controlling
means,
for example a timer (not shown). The opening of the valve 9 also prevents the
fuel cell stack 3 from flooding due to the accumulation of water generated in
the
fuel cell reaction. The interval of opening valve 9 may be varied in various
operation conditions and optimized by experiments.
[0042] Reference will now be made to Figure 7, which shows a third
embodiment of the apparatus. Some components of this third embodiment are
similar to the earlier embodiments, and where applicable and for brevity, the
same reference numeral is used to described these components and the
description of these components is not repeated.
[0043] Fundamentally, this third embodiment of the apparatus is based on
two important realizations. The first is that, as mentioned above, the coolant
is
commonly water and the chemical hydride solution itself comprises mainly
water;
thus, it has been realized that it is possible to combine these two streams
and in
effect use the chemical hydride solution as the coolant. The second
realization,
which makes this possible, is that the operating temperature of the reactor 20
does not significantly deviate from that of the fuel cell 3. Essentially, they
can
operate at, generally, much the same temperature. An advantage that flows out
of this approach, which is detailed below, is that heat from the hydrogen
generation reaction can be used to heat up the fuel cell stack 3 in an initial
startup mode, and following startup, a single heat exchanger can be provided,
to
CA 02444313 2003-10-03
extract heat from the system and to maintain both the chemical reactor 20 and
the fuel cell stack 3 at a desired operating temperature.
[0044] In Figure 7, considering first the hydrogen generation scheme, the
hydride storage tank 5, as before, is connected through a line 22 containing a
pump 26 to the reactor 20. A bypass fine 30 is provided around the pump 26
including a pressure limiting check valve 32, set to maintain a desired
pressure.
The upper end of the reactor 20 is here connected by a line 34 to a liquid-gas
separator 36.
[0045] The separator 36 has a lower liquid outlet connected by the return
line 6
to the storage tank 5. In known manner, a solenoid valve 38 is connected to
and
controlled by a level switch 40, to maintain liquid level in the separator 36
within
desired limits. As indicated at 42, also in known manner, one or more pressure
transducers and a pressure controller can be provided, for monitoring the
pressure in the separator vessel 36, and connected to the pump 26 and a
further
level switch 44 on the tank 5. Lines 22, 34 and 6 form a circuit for
circulating the
chemical hydride solution through the reactor 20.
[0046] An upper, gas outlet of the liquid gas separator vessel 36 is
connected the hydrogen supply line 11, including the filter 28 for filtering
out any
aerosols present in the gas flow. For safety purposes, a pressure relief valve
46
is also connected to the outlet of the separator vessel 36, and arranged to
vent
to the exterior. A pressure reducing valve 48 reduces the gas pressure to, for
example 3 PSIG, or other desired value and a pressure indicator is provided at
50, also connected to the line 11. A solenoid valve 52 is provided in the line
11
immediately upstream of the fuel cell stack 3.
[0047] The hydrogen or anode outlet line 10 of the fuel cell stack 3 is
connected through a solenoid valve 54 to a hydrogen exhaust or vent 56. A
temperature transmitter 58 is provided for monitoring the temperature of
exhausted hydrogen. A catalytic burner (not shown), with its own oxidant input
16
CA 02444313 2003-10-03
can be connected to the hydrogen exhaust or vent 56, for consuming vented
hyd rogen .
A liquid-gas separator 86 is provided in the anode outlet line 10 for
separating
water from exhaust hydrogen. A hydrogen return line 60 is connected to the
outlet line 10 downstream of the separator 86 and includes a diaphragm pump
62, for recirculating the separated hydrogen through the fuel cell stack 3.
The
water recovered from the separator 86 is circulated back to hydride solution
storage tank 5 through a return line 87 to increase the volume of water as the
chemical hydride is consumed, and this compensates for the increasing
concentration of the less soluble byproduct NaBOa. Note that not all the
generated water need be supplied to the storage tank 5, and some can be
discharged, as required.
[0048] Turning to the air or oxidant circuit, ambient air is supplied through
the inlet line 7 to a main oxidant inlet, indicated at 70. The line 7 then
includes
an aerosol filter 72 and a pump 74, that pumps the air through an enthalpy
exchange device 76. The exchange device 76 exchanges heat and moisture
between the incoming and exhaust air streams, so that the incoming air stream
has desired temperature and humidity characteristics. The heated and
humidified air is then supplied to the fuel cell stack 3.
[0049) The exhaust or discharged airline 17 then has a temperature
transmitter 78, at the outlet of the fuel cell stack 3. The line 17 then
passes
through the enthalpy exchanger device 76 for exchanging heat and moisture as
mentioned above. The air is then passed to an air exhaust 80.
[0050] Now in accordance with this embodiment of the invention, the
chemical hydride solution is also used as a coolant. For this purpose, the
liquid-
gas separator 36has an additional outlet connected to a supply line 82,
connected to a coolant inlet port of the fuel cell stack 3. The line 82
includes a
respective temperature transmitter 84.
[0051) A coolant outlet port of the fuel cell stack 3 is connected to a return
17
CA 02444313 2003-10-03
line 88, also including a respective temperature transmitter 90. The return
line
then passes through an air-cooled heat exchanger 92 and a pump 94 before
returning to the reactor vessel 20. The pump 94 circulates the hydride
solution
from the reactor 20 and the liquid-gas separator 36 through the fuel cell
stack 3.
A further temperature transmitter 96 is provided immediately upstream of the
reactor vessel 20.
[0052] The temperature transmitter 90 is connected to a temperature
controller 98, which in turn is connected to the pump 94 and the motor of a
fan
100, for controlling the pump 94 and the fan 100. The fan 100 serves to blow
cooling air over the heat exchanger 92.
[0053) It will be understood that other pressure and temperature
transducers are provided as required, for example, a temperature transmitter
102, for monitoring the temperature in the reactor vessel 20.
[0054] Since the hydrogen generation reaction and the fuel cell reaction
are both exothermic, the heat generated in both reactions can be utilized in
order
to achieve optimum operating conditions rapidly. Accordingly, in a startup
mode,
a heater is not provided. Instead, during
startup, the cooling fan 100 is turned off, and the pump 94 operated to
circulate
the hydride solution through the cooling channels of the fuel cell stack 3.
Consequently, heat generated by both the fuel cell stack and generation of
hydrogen in the reactor vessel 20 are retained in the system, and both the
reactor vessel 20 and the fuel cell stack 3 heat up to desired operating
temperatures.
[0055] When the system is initially started-up, there is a lag time for
electricity generated by the fuel cell stack. This is because there is
insufficient
hydrogen available and the fuel cell stack is too cold therefore, initially
the
reactor 20 generates more heat and this heat is transferred by the flow of the
hydride solution to the fuel cell stack. This warms up the fuel cell stack and
enhances the fuel cell reaction. Therefore, the fuel cell reaction proceeds
faster
than when the stack starts from cold condition and the stack generates more
18
CA 02444313 2003-10-03
heat. When the fuel cell stack begins to produce electricity, the heat from
the fuel
cell stack 3 is transferred back to the reactor 20. The mutual heating allows
the
system to reach a steady state at a faster rate than conventional systems that
rely on some auxiliary heating device. However, the circuit through the
reactor
and the fuel cell stack in lines 82 and 88 can include a heater (not shown) to
further enhance system performance. Figure 9 shows graphs of the change in
coolant outlet temperature for the chemical hydride reactor and the fuel cell
stack. The outlet refers respectively to the chemical hydride outlet of the
reactor
when the reactor is running alone, the fuel cell stack anode outlet when the
fuel
cell stack is running alone, and also the fuel cell stack anode outlet when
the two
are running together. For all these results, the coolant used was deionised
water, but it is expected that the chemical hydride solution would give
similar
results. The solution is mainly water and has similar thermal properties to
water.
As shown for each of the Chemical hydride generator and the fuel cell stack
alone, heating occurs relatively slowly as indicated by the graphs of the
coolant
outlet temperature. On the other hand, for the combination of the chemical
hydride generator and the fuel cell stack together there is a much more rapid
warm up, again as demonstrated by the graph. This is believed to be due to the
fact that initial heat from the chemical hydride reaction accelerates heating
of the
fuel cell stack, which in turn starts to react at a higher rate and produce
significant heat more quickly, hence increasing the reaction rate of the
chemical
hydride reactor; together this leads to more rapid heat generation and heating
up
of the system.
[0056] Once the desired operating temperature is reached, then, as
sensed by the temperature transmitter 90, the fan 100 can be actuated, and the
system kept within a desired range of temperatures.
Another benefit associated with this embodiment is that the initial
concentration
of the chemical hydride solution can be further increased. The reason is that
the
unreacted chemical hydride solution picks up heat from the reactor when
flowing
through it and in turn warms up the storage tank. As is known, the solubility
of
the solutes in the solution increases with the temperature. Therefore, the
energy
density of the whole energy system is further increased.
19
CA 02444313 2003-10-03
(0057] A fourth embodiment of the present invention is shown in Figure 8.
Similar components are indicated with same reference number. In this
embodiment, chemical hydride solution is still used as a heat transfer fluid
as in
the third embodiment. However, a supply line, here indicated at 82a, branches
off from the chemical hydride supply line 22 at a location upstream of the
reactor
vessel 20. A portion of the chemical hydride solution from the storage tank 5
is
circulated through the supply line 82 to the fuel cell stack 3 as a coolant
while
the remaining of the chemical hydride is supplied to the reactor 20 for
generating
hydrogen. In the same manner as that in the third embodiment, the chemical
hydride solution in the line 82a flows through the coolant channels in the
fuel cell
stack 3 and take heat away from the stack. Then the solution is circulated
back
to the reactor 20 to cool the reactor 20 through a line 88. Similar heat
exchangers and temperature sensing and controlling devices as in the third
embodiment are provided in line 82a and line 88. For brevity, these and other
elements common to the third embodiment are not described. As a variant, there
is shown in Figure 8 two storage tanks for the chemical hydride, the two tanks
being provided for easy hook up and to enable switching from one to the other;
it
is possible that only one is working at any time.
(0058) In this fourth embodiment, although the chemical hydride solution
that is used as coolant still serves to transfer heat between the fuel cell
and the
chemical hydride generation system, it is taken from the chemical hydride
circuit
upstream from the reactor 20. Consequently, it has not been heated by the
reaction in the reactor 20, and is not used to warm up the reactor 20 and the
fuel
cell stack 3 in startup mode. Rather, this flow is used solely as coolant.
(0059] It is to be noted that in both the third and fourth embodiments, in
which some of the chemical hydride solution is used as coolant and flows
through the fuel cell stack, the coolant distribution manifold and channel of
the
fuel cell stack need to be insulated with non-conductive materials to prevent
the
possible shorting problems associated with the solution. This is because the
ions
in the solution may bypass the current between adjacent cells in the fuel cell
stack and hence reduce the output of the fuel cell.
CA 02444313 2003-10-03
(0060] The present invention has been described in detail in a number of
embodiments. It should be appreciated that the chemical hydride that can be
utilized in this invention includes but not limited to borohydride such as
NaBH4
and LiBHd, other types of chemical hydrides may also be used, such as B2H6,
LiAIH4, NH3BH3, etc. Likewise, the fuel cell stack 3 in the present invention
can
be any type of fuel cell using pure hydrogen as a fuel.
[0061] It is anticipated that those having ordinary skills in the art can
make various modifications to the embodiments disclosed herein after learning
the teaching of the present invention. For example, the number and arrangement
of components in the system might be different, different elements might be
used to achieve the same specific function. However, these modifications
should
be considered to fall under the protection scope of the invention as defined
in the
following claims.
21