Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
1
IMPROVEMENTS IN OR RELATING TO COMPOSITIONS
This invention is concerned with containers and
packaging systems, and relates in particular to a
packaging system incorporating a water-soluble vessel.
The containers of the invention are particularly
useful for pharmaceuticals, pesticides, biocides,
compositions which are potentially toxic or damaging
or detrimental to health or the environment, and
washing or cleaning materials. As used herein, the
term "water-soluble" also encompasses "water-
dispersible"
Thermoformed water-soluble packages are known, but
some have limitations, one of which is caused by the
propensity of some thermoformed water-soluble films,
particularly those comprising a polyvinyl alcohol)
(PVOH) to undergo ~~shrink-back', which prevents the
volume of a formed cavity being fully utilized at the
filling stage. The present invention seeks to provide
improved containers, and packaging systems, especially
suited for containing pharmaceutical, pesticidal,
biocidal, compositions which are potentially toxic or
damaging or detrimental to health or the environment,
or washing or cleaning compositions.
The present invention provides a web comprising a
plurality of containers, each container comprising a
composition in an internal thermoformed or vacuum'
formed water-soluble vessel with a water-soluble lid
sealed thereto, each sealed vessel being enclosed
CONFIRMATION COPY
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
2
within an external thermoformed or vacuum formed
water-resistant or water-insoluble holder.
The present invention additionally provides a container
comprising a composition in an internal thermoformed or
vacuum formed water-soluble vessel with a water-soluble
lid sealed thereto, the sealed vessel being enclosed
within an external thermoformed or vacuum formed water-
resistant or water-insoluble holder.
The present invention also provides a method for making
a web or container as defined above which comprises:
a. positioning a first water-soluble film face
to face with a water-resistant or water-insoluble film,
the films being in intimate contact so that there is
no, or substantially no air trapped between them;
b. feeding the films on to the mould of a
forming machine with the water-resistant or water-
insoluble film being closest to the mould;
c. forming the films in the mould to make an
internal vessel cavity and an external holder;
d. filling the cavity with a composition;
e. positioning a water-soluble lid over the
filled vessel; and
f. sealing the lid to the water-soluble film.
The method may also optionally comprise:
g. cutting the water-soluble film and lid to
separate at least one of the vessels;
h. removing an area of waste film produced by
the cutting; and/or
i. sealing a water-insoluble or water-resistant
lid to the top of the holder.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
3
The present invention furthermore.provides the use of a
thermoformed or vacuum formed water-resistant or water-
insoluble holder to enclose a thermoformed or vacuum
formed water-soluble vessel enclosing a composition.
More specifically, the present invention provides one
or more of an easy-to-make, easy-to-fill and easy-to-
use package. For this purpose it proposes a packaging
system wherein each individual container of a
multiplicity of such containers all joined together in
a group side by side in a web comprises an inner
water-soluble or -dispersible encapsulate enclosed
wholly within a protective external water-insoluble
casing, and each of the encapsulate 'and the casing is,
separately, in the form of a thermoformed hollow body
and a lid and is made from two films bound together by
one common seal, so that each encapsulate is in
contact only with the inner wall of its casing. It is
possible for the vessel and holder to be in intimate
contact over substantially all of the inner surface of
the holder. When taking the vessel out of the holder,
the user may have a sensation of peeling away the
holder from the vessel.
In one aspect, therefore, this invention provides a
packaging system, useful for making a multiplicity of
individual containers all joined together in a group
side by side in a web, or an individual container,
wherein the container, or each individual container,
comprises an inner water-soluble or water-dispersible
encapsulate, in the form of a thermoformed vessel and
a lid, for example made from two films, especially two
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
4
films bound together by one common seal, enclosed
within a protective external water resistant/
/water-insoluble casing in the form of a thermoformed
holder, optionally with a lid, especially a removable
lid such as a peelable lid, and also made from two
films bound together by one common seal, so that each
encapsulate is in contact only with the inner wall of
the casing.
In such a packaging system each encapsulate - the
inner water-soluble or water-dispersible container,
e.g. an envelope - may be filled with any one or more
of a wide variety of materials, typical ones being
ingredients or compositions useful for
pharmaceuticals, pesticides, biocides, compositions.
which are potentially toxic or damaging or detrimental
to health or the environment,detergents, materials for
fabric washing or fabric care, for surface washing or
surface care, and for dishwashing, anal deodorants,
dyes, pigments, or water-treatment chemicals. The
fillings may take any appropriate form - they may for
instance be liquids, gels, pastes, solids, granules or
powders. The invention's packaging system is,
however, most useful for containing liquid
compositions. A typical such product is a thickened
liquid detergent formulation.
The material for the walls of the water-
soluble/-dispersible compartment - the encapsulate (or
envelope) - can be hot- or cold-water soluble or
dispersible, and can be flexible or rigid. Preferably,
it is a film, or a combination of two different films,
each of which may be a mono-Layer or a laminated film,
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
which is both water-soluble and flexible. By
cold-water soluble is meant, for example, a material
which is soluble in water at 20°C or less, while by
hot-water soluble is meant a material which is soluble
5 in water at 60°C or more. Material which. is soluble
between these temperatures can also be used. The
encapsulate can be made from films of different
grades, from films of different thicknesses, or from
films which have been perfumed or coloured to obtain
the desired characteristics, or from any combination
of these.
Preferred materials for the encapsulate are PVOH
(polyvinyl alcohol) and cellulose derivatives such as
cellulose ethers, for example hydroxypropyl methyl
cellulose (HPMC). An example of a preferred PVOH is
ethoxylated PVOH. The PVOH may be partially or fully
alcoholised or hydrolysed. For example it may be from
40 to 100%, preferably from 70 to 920, more preferably
about 88% or about 92%, alcoholised or hydrolysed. The
degree of hydrolysis is known to influence the
temperature at which the PVOH starts to dissolve in
water. 88% hydrolysis corresponds to a film soluble in.
cold (ie room temperature) water, whereas 92%
hydrolysis corresponds to a film soluble in warm water.
They are also generally injection-mouldable, and do not
hold a static charge. However, other water-soluble
compounds that can be used include polyglycolides and
polylactides, and polylactide-polyglycolide copolymers.
These materials may of course also, if necessary,
contain components such as plasticizers and mould
release agents which beneficially modify their
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
6
properties - and all of them can naturally include
other components such as colouring agents.
It has surprisingly been found to be possible by means
of this invention to thermoform films which were
previously thought to be difficult or impossible to
thermoform due to their brittle nature. An example of
such a film is an HPMC film.
The encapsulate film may be a single film, or a
laminated film as disclosed in GB-A-2,244,258. while a
single film may have pinholes, the two or more layers
20
of a laminated film are unlikely to have pinholes which
coincide.
The film may be produced by any process, for example by
extrusion and blowing or by casting. The film may be
unoriented, monoaxially oriented or biaxially oriented.
If the layers in the film are oriented, they usually
have the same orientation, although their planes of
orientation may be different if desired.
The layers in a laminate may be the same or different.
Thus they may each comprise the same polymer or a
different polymer. If it is desired to have a water-
soluble laminated film, each of the layers should be
water-soluble.
The thickness of the walls of the encapsulate is
conveniently in the range 20 to 500 ~.m, preferably 30
to 300 Vim, more preferably 35 to 200 ~,m, especially 40
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
7
to 160 Vim, more especially 40 to 150 ~,m and most
especially 40 to 120 ~,m.
A typical water-soluble film for the encapsulates
vessel is made of that variety of 75 ~,m PVOH available
as MONOSOL M8534. A typical film for the vessel's lid
is made of that variety of 60 ~.m PVOH available as
SOLUBLO.N PT60.
The thermoformed water-insoluble/resistant material
for the external protective casing is preferably semi-
rigid. Polyester and nylon/polyethylene laminates are
convenient for this purpose, preferably in their
amorphous form.
The thickness of the walls of the water-resistant or
insoluble compartment is conveniently in the range of
60 to 1000 ~.m, preferably 170 to 750 ~,m.
A typical water-insoluble/resistant film is 170 ~,m
thick and made from amorphous polyester APET; this is
suitable for both the holder and its lid.
The encapsulates vessel and the casing's holder are
essentially cavities or compartments the one for
containing the substance being packaged and the other
for containing, and protecting, the first one. These
can be of any suitable shape, but preferably they are
each in a slightly tapered cylindrical shape (perhaps
with a squarish cross-section), so that a good release
from the mould is achieved. In addition, with such a
shape one then fits neatly inside the other, so that
(as described hereinafter) a set of lined casings
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
8
without lid portions can be nested to form a
space-saving stack.
In a second aspect the invention provides a method for
making containers of the invention, and in particular
for making a web or group of such containers joined
side by side, in which method:
a. a thermoformable water-soluble or
-dispersible lining film, for example in sheet
form, is positioned face to face with a
thermoformable water-insoluble or -dispersible
carrier film in matching sheet form to make a
base web combination, the films being in intimate
contact such that there is no, or substantially
no ai-r trapped between them, and.this base web is
fed into a thermoformer and there moulded into a
sequence of cavity or bowl container shapes with
the water-soluble or -dispersible film as a
lining on the inside, for each shape the
combination being the encapsulates vessel within
the casing's holder;
b. the encapsulates vessel is filled with the
relevant material to.be packaged;
c. a water-soluble or -dispersible lid film,
for example in sheet form, is placed over the
thus-formed lined and filled container shapes,
and sealed to the lining film around the mouth of
each container shape, forming a lid for the
vessel therein, this lidded vessel being the
encapsulate, and the lid and lining films are
then cut through to separate each encapsulate
from its neighbours;
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
9
d. the areas of waste lid and lining film
between the encapsulates are removed, exposing
the underlying carrier film; and, optionally,
e. a water-insoluble lid film in matching sheet
form is placed over the thus-separated and
-spaced casing-borne encapsulates so as to cover
them, and is sealed, preferably removably sealed,
to the previously-exposed carrier film so as to
form for each casing's holder a lid which may
preferably be peeled back to expose the
encapsulate therein for subsequent removal and
use.
The web may be formed on a continuous basis, with a
line of, or many side-by-side rows/sets of,
containers. How many depends on the width of the
sheet; if narrow, like a tape, there might only be one
container (so that the method produces a strip of
containers), while if wide there might be two, three
or more containers in each row. Clearly, the length
of the web is indefinite. Once formed, of course, the
web can be cut into the more conveniently-sized packs
- of individual containers, pairs of containers or
two-by-two containers, say - required for sale. If
two or more containers are left conjoined, the
individual containers may have the same or different
sizes, and the compositions held in each container may
be the same or different. At that time the holder
lidding film, which is preferably peelable, is cut or
perforated to allow the lid of each pack to be removed
without damaging the hermetic seal of neighbouring
packs.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
We have surprisingly found that when thermoforming
both films together the inner water-soluble film does
not undergo as much thinning at corners of the mould
as when a film is thermoformed without an outer water-
5 soluble film. This is advantageous since it allows
thinner films to be used for the inner vessel,
allowing for quicker dissolution in water.
Although in this method the shaping, filling and
lidding is most likely to be done all at the same time,
10 it is of course possible to break it into quite widely
time-separated stages. Thus, the shaping could be
followed by the storing of the formed shapes as
"pre-forms", optionally having first cut the sequence
of containers into manageable and convenient lengths,
and at some later time these pre-forms could be filled,
and lidded. Moreover, it is a possibility, though not
a preferred one, not to apply the final, water-
insoluble or -dispersible film that forms the casing's
holder's lid but instead to leave the container as just
the encapsulate sitting inside its open-topped holder.
In a thermoforming process the film may be drawn down
or blown down into a mould. Thus, for example, the
film is heated to the thermoforming temperature using a
thermoforming heater plate assembly, and then drawn
down under vacuum or blown down under pressure into the
mould. Plug-assisted thermoforming and pre-
stretching the film, for example by blowing the film
away from the mould before thermoforming, may, if
desired, be used. One skilled in the art can choose an
appropriate temperature, pressure or vacuum and dwell
time to achieve an appropriate pocket. The amount of
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
11
vacuum or pressure and the thermoforming temperature
used depend on the thickness and type of the film and
on the polymer or mixture of polymers being used.
Thermoforming of PVOH films is known and described in,
for example, WO 00/55045.
A suitable forming temperature for PVOH or ethoxylated
PVOH is, for example, from 90 to 130°C, especially 90
to 120°C. A suitable forming pressure is, for example,
69 to 138kPa (10 to 20 p.s.i.), especially 83 to 117
kPa (12 to 17 p.s.i.). A suitable forming vacuum is 0
to 4 kPa (0 to 40 mbar), especially 0 to 2 kPa (0 to 20
mbar). A suitable dwell time is, for example, 0.4 to
2.5 seconds, especially 2 to 2.5 seconds.
While desirably conditions are chosen within the above
ranges, it is possible to use one or more of these
parameters outside the above ranges, although it may be
necessary to compensate by changing the values of the
other two parameters.
The inner vessel is then filled with the desired
composition. The pocket may be completely filled or.
only. partially filled. The composition may be a solid.
For example, it may be a particulate or granulated
solid, or a tablet. It may also be a liquid, which may
be thickened or gelled if desired. The liquid
composition may be non-aqueous or aqueous, for example
comprising less than or more than 5% total or free
water: The composition may have more than one phase.
For example it may comprise an aqueous composition and
a liquid composition which is immiscible with the
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
12
aqueous composition. It may also comprise a liquid
composition and a separate solid composition, for
example in the form of a ball, pill or speckles.
After the pocket has been filled, a vessel lid such as
a film is placed on top of the filled pocket and across
the sealing portion, and the films are sealed together
at the sealing portion. This film will usually be a
single-layered film but may be a laminated film to
reduce the possibility of pinholes allowing leakage
through the film. The film may be the same or
different as the film forming the cavity. Examples of
suitable films are those given for the film forming the
cavity.
Desirably the vessel film has a thickness which is less
than that of the film used for forming a cavity because
it would not generally be stretched so localised
thinning of the sheet will not occur. It is also
desirable to have a thickness which is less than that
of the film used to form a vessel cavity to ensure a
sufficient heat transfer through the film to soften the
base web if heat sealing is used.
The thickness of the covering film is generally from 20
to 160 ~.m, preferably from 40 to 100 ~.m, such as 40 to
80 ~,m or 40 to 60 ~,m.
The sealing effected in the method of the invention
can be done by any appropriate means - for example, by
an adhesive, heat welding or by ultrasonic, laser,
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
13
vibrations, spin, radio frequency or solvent welding,
or a combination thereof. Heat sealing, laser sealing
and solvent welding or combinations thereof are
preferred. A suitable adhesive is water or an aqueous
solution of PVOH. The inner seal is desirably water-
soluble.
If heat sealing is used, a suitable sealing temperature
is, for example, 120 to 195°C, for example 140 to
250°C. A suitable sealing pressure is, for example,
from 250 to 600 kPa. Examples of sealing pressures are
276 to 552 kPa (40 to 80 p.s.i.), especially 345 to 483
kPa (50 to 70 p.s.i.) or 400 to 800 kPa (4 to 8 bar),
especially 500 to 700 kPa (5 to 7 bar) depending on the
heat sealing machine used. Suitable sealing dwell
Z5 times are 0.4 to 2.5 seconds.
One skilled in the art can use an appropriate
temperature, pressure and dwell time to achieve a seal
of the desired integrity. While desirably conditions
are chosen within the above ranges, it is possible to
use one or more of these parameters outside the above
ranges,. although it might be necessary to compensate
by changing.the values of the other two parameters.
The composition may be any composition which is
intended to be released in an aqueous environment.
Thus, for example, it may be a pesticidal composition
such as a plant protection agent, for instance an
insecticide, fungicide, herbicide, acaricide, or
nematocide, or a plant growth regulator, a plant
nutrient, or a composition which is potentially toxic
or damaging or detrimental to health or the
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
14
environment. Such compositions are generally packaged
in total amounts of from 0.1 g to 7 kg, preferably 1g
to 5 kg, when in solid form. When in liquid or gelled
form, such compositions are generally packaged in total
amounts of from 1 ml to 10 litres, preferably 5m1 to 6
litres, especially from 10m1 to 1.5 litres.
The composition may also be a fabric care, surface care
or dishwashing composition. Thus, for example, it may
be a dishwashing, water-softening, laundry or detergent
composition, or a rinse aid. Such compositions may be
suitable for use in a domestic washing machine. The
composition may also be a disinfectant, antibacterial
or antiseptic composition, or a refill composition for
a trigger-type spray. Such compositions are generally
packaged in total amounts of from 5 to 100 g,
especially from 15 to 40 g. For example, a dishwashing
composition may weigh from 15 to 30 g, a water-
softening composition may weigh from 15 to 40 g.
The inner vessel may comprise two or more compartments,
particularly if the inner lidding film itself comprises
a compartment as disclosed in, for example,
GB-A-2,361,686.
If the article is for use in laundry washing, the
primary composition may comprise, for example, a
detergent, and the secondary composition may comprise a
bleach, stain remover, water-softener, enzyme or fabric
conditioner. The article may be adapted to release the
compositions at different times during the laundry
wash. For example, a bleach or fabric conditioner is
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
generally released at the end of a wash, and a water-
softener is generally released at the start of a wash.
An enzyme may be released at the start or the end of a
wash.
5
If the article is for use as a fabric conditioner, the
primary composition may comprise a fabric conditioner
and the secondary component may comprise an enzyme
which is released before or after the fabric
10 conditioner in a rinse cycle.
If the article is for use in dish washing the primary
composition may comprise a detergent and the secondary
composition may comprise a water-softener, salt,
15 enzyme, rinse aid, bleach or bleach activator. The
article may be adapted to release the compositions at
different times during the laundry wash. For example,
a rinse aid, bleach or bleach activator is generally
released at the end of a wash, and a water-softener,
salt or enzyme is generally released at the start of a
wash.
The primary and secondary compositions, if in liquid
form, may independently be anhydrous or comprise water,
for example at least 5 wt %, preferably at least 10 wt%
free or total water based on the weight of the aqueous
compositions. Desirably the compositions contain less
than 80 wto water.
The composition may contain surface active agents such
as an anionic, nonionic, cationic, amphoteric or
zwitterionic surface active agents or mixtures thereof.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
16
Examples of anionic surfactants are straight-chained or
branched alkyl sulfates and alkyl polyalkoxylated
sulfates, also known as alkyl ether sulfates. Such
surfactants may be produced by the sulfation of higher
Ca-CZO fatty alcohols .
Examples of primary alkyl sulfate surfactants are those
of formula:
ROS03-M+
wherein R is a linear CB-Cao hydrocarbyl group and M is
a water-solubilising ration. Preferably R is Clo-Cls
alkyl, for example C12-C14, and M is alkali metal such as
lithium, sodium or potassium.
Examples of secondary alkyl sulfate surfactants are
those which have the sulfate moiety on a "backbone" of
the molecule, for example those of formula:
CHI ( CHI ) n ( CHOS03-M'~ ) ( CH2 ) n,CH3
wherein m and n are independently 2 or more, the sum of
m+n typically being 6 to 20, for example 9 to 15, and M
is a water-solubilising ration such as lithium, sodium
or potassium.
Especially preferred secondary alkyl sulfates are the
(2,3) alkyl sulfate surfactants of formulae:
CHz (CH2) X (CHOS03-M+) CH3 and
3 0 CH3 ( CHz ) X ( CHOS03-Mt ) CH~CH3
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
17
for the 2-sulfate and 3-sulfate, respectively. In
these formulae x is at least 4, for example 6 to 20,
preferably l0 to 16. M is ration, such as an alkali
metal, for example lithium, sodium or potassium.
Examples of alkoxylated alkyl sulfates are ethoxylated
alkyl sulfates of the formula:
RO (C~H40) nS03-M~
wherein R is a C$-CZO alkyl group, preferably Clo-C18 such
as a C12-C16, n is at least 1, for example from 1 to 20,
preferably 1 to 15, especially 1 to 6, and M is a salt-
forming ration .such. as lithium; sodium, potassium,
ammonium, alkylammonium or alkanolammonium. These
compounds can provide especially desirable fabric
cleaning performance benefits when used in combination
with alkyl sulfates.
The alkyl sulfates and alkyl ether sulfates will
generally be used in the form of mixtures comprising
varying alkyl chain lengths and, if present, varying
degrees of alkoxylation.
Other anionic surfactants which may be employed are
salts of fatty acids, for example C$-C18 fatty acids,
especially the sodium or potassium salts, and alkyl,
for example C$-Clg, benzene sulfonates.
Examples of nonionic surfactants are fatty acid
alkoxylates, such as fatty acid ethoxylates, especially
those of formula:
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
18
R (C2H4p) npH
wherein R is a straight or branched C8-C16 alkyl group,
preferably a C9-C15, for example Clo-C14, alkyl group and
n is at least 1, for example from 1 to 16, preferably 2
to 12, more preferably 3 to 10.
The alkoxylated fatty alcohol nonionic surfactant will
frequently have a hydrophilic-lipophilic balance (HLB)
which ranges from 3 to 17, more preferably from 6 to
15, most preferably from 10 to 15.
Examples of fatty alcohol ethoxylates are those made
from alcohols of 12 to 15 carbon atoms and which
contain about 7 moles of ethylene oxide. Such
materials are commercially marketed under the
trademarks Neodol 25-7 and Neodol 23-6.5 by Shell
Chemical Company. Other useful Neodols include Neodol
1-5, an ethoxylated fatty alcohol averaging 11 carbon
atoms in its alkyl chain with about 5 moles of ethylene
oxide; Neodol 23-9, an ethoxylated primary C12-C13
alcohol having about 9 moles of ethylene oxide; and
Neodol 91-10, an ethoxylated C9-Clz primary alcohol
having about 10 moles of ethylene oxide.
Alcohol ethoxylates of this type have also been
marketed by Shell Chemical Company under the Dobanol
trademark. Dobanol 91-5 is an ethoxylated C9-C11 fatty
alcohol with an average of 5 moles ethylene oxide and
Dobanol 25-7 is an ethoxylated C12-Cis fatty alcohol with
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
19
an average of 7 moles of ethylene oxide..per mole of
fatty alcohol.
Other examples of suitable ethoxylated alcohol nonionic
surfactants include Tergitol 15-S-7 and Tergitol 15-S-
9, both of which are linear secondary alcohol
ethoxylates available from Union Carbide Corporation.
Tergitol 15-S-7 is a mixed ethoxylated product of a C11-
C15 linear secondary alkanol with 7 moles of ethylene
oxide and Tergitol 15-S-9 is the same but with 9 moles
of ethylene oxide.
Other suitable alcohol ethoxylated nonionic surfactants
are Neodol 45-11, which is a similar ethylene oxide
condensation proc3.uct of a fatty alcohol having 14-15
carbon atoms and the number of ethylene oxide groups
per mole being about 11. Such products are also
available from Shell Chemical Company.
Further nonionic surfactants are, for example, Clo-Cla
alkyl polyglycosides, such as Cla-C16 alkyl
polyglycosides, especially the polyglucosides. These
are especially useful when high foaming compositions
are desired. Further surfactants are polyhydroxy fatty
acid amides, such as C1o-C1$ N- (3-methoxypropyl)
glycamides and ethylene oxide-propylene oxide block
polymers of the Pluronic type.
Examples of cationic surfactants are those of the
quaternary ammonium type.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
The total content of surfactants in the..composition is
desirab1y,60 to 95 wt%, especially 75 to 90 wt%.
Desirably an anionic surfactant is present in an amount
of 50 to 75 wto, the nonionic surfactant is present in
5 an amount of 5 to 20 wt%, and/or the cationic
surfactant is present in an amount of from 0 to 20 wt%.
The amounts are based on the total solids content of
the composition, i.e. excluding any solvent which may
be present.
The composition, particularly when used as a laundry
washing or dishwashing composition, may also
independently comprise enzymes, such as protease,
lipase, amylase, cellulase and peroxidase enzymes.
Such enzymes are commercially available and sold, for
example, under the registered trade marks Esperesc,
Alcalasc and Savinasc by Nova Industries A/S and
Maxatasc by International Biosynthetics, Inc.
Desirably the enzymes are independently present in the
primary or secondary compositions in an amount of from
0.5 to 3 wt%, especially 1 to 2 wt%.
The composition may, if desired, comprise a thickening
agent or gelling agent. Suitable thickeners are
polyacrylate polymers such as those sold under the
trade mark CARBOPOL, or the trade mark ACUSOL by Rohm
and Haas Company. Other suitable thickeners are
xanthan gums. The thickener, if present, is generally
present in an amount of from 0.2 to 4 wto, especially
0.5 to 2 wto.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
21
Compositions used in dishwashing independently usually
comprise a detergency builder: Suitable builders are
alkali metal or ammonium phbsphates, polyphosphates,
phosphonates, polyphosphonates, carbonates,
bicarbonates, borates, polyhydroxysulfonates,
polyacetates, carboxylates such as citrates, and
polycarboxylates. The builder is desirably present in
an amount of up to 90 wt%, preferably 15 to 90 wt%,
more preferable 15 to 75 wt%, relative to the total
weight of the composition. Further details of suitable
components are given in, for example, EP-A-694,059, EP-
A-518,720 and WO 99/06522.
The composition can also optionally comprise one or
more additional ingredients. These include
conventional detergent composition components such as
further surfactants, bleaches, bleach enhancing agents,
builders, suds boosters or suds suppressors, anti-
tarnish and anti-corrosion agents, organic solvents,
co-solvents, phase stabilisers, emulsifying agents,
preservatives, soil suspending agents, soil release
agents, germicides, pH adjusting agents or buffers,
non-builder alkalinity sources, chelating agents, clays
such as smectite clays, enzyme stabilizers, anti-
limescale agents, colourants, dyes, hydrotropes, dye
transfer inhibiting agents, brighteners, and perfumes.
If used, such optional ingredients will generally
constitute no more than 10 wt%, for example from 1 to 6
wt%, the total weight of the compositions.
The builders counteract the effects of calcium, or
other ion, water hardness encountered during laundering
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
22
or bleaching use of the compositions herein. Examples
of such materials are citrate, succinate, malonate,
carboxymethyl succinate, carboxylate, polycarboxylate
and polyacetyl carboxylate salts, for example with
alkali metal or alkaline earth metal cations, or the
corresponding free acids. Specific examples are
sodium, potassium and lithium salts of oxydisuccinic
acid, mellitic acid, benzene polycarboxylic acids, C10-
C22 fatty acids and citric acid. Other examples are
organic phosphonate type sequestering agents such as
those sold by Monsanto under the trade mark bequest and
alkylhydroxy phosphonates. Citrate salts and C12-Cls
fatty acid soaps are preferred.
Other suitable builders are polymers and copolymers
known to have builder properties. For example, such
materials include appropriate polyacrylic acid,
polymaleic acid, and polyacrylic/polymaleic and
copolymers and their salts, such as those sold by BASF
under the trade mark Sokalan.
The builders generally constitute from 0 to 3 wto, more
preferably from 0.1 to 1 wt%, by weight of the
compositions.
Compositions which comprise an enzyme may optionally
contain materials which maintain the stability of the
enzyme. Such enzyme stabilizers include, for example,
polyols such as propylene glycol, boric acid and borax.
Combinations of these enzyme stabilizers may also be
employed. If utilized, the enzyme stabilizers
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
23
generally constitute from 0.1 to 1 wt% of the
compositions.
The composition may optionally comprise materials which
serve as phase stabilizers and/or co-solvents. Example
are Cz-C3 alcohols such as methanol, ethanol and
propanol. C1-C3 alkanolamines such as mono-, di- and
triethanolamines can also be used, by themselves or in
combination with the alcohols. The phase stabilizers
and/or co-solvents can, for example, constitute 0 to 1
wt%, preferably 0.1 to 0.5 wt%, of the composition.
The composition may optionally comprise components
which adjust or maintain the pH of the compositions at
optimum levels. The pH maybe from, for example, 1 to
13, such as 8 to 11 depending on the nature of the
composition. For example a dishwashing composition
desirably has a pH of 8 to 11, a laundry composition
desirable has a pH of 7 to 9, and a water-softening
composition desirably has a pH of 7 to 9. Examples of
pH adjusting agents are NaOH and citric acid.
In the present invention, if more than one container,
is formed at the same time from the same sheet, the
containers may then be separated from each other by,
cutting the portions between them, for example between
the sealed portions or flanges. Alternatively, they
may be left conjoined and, for example, perforations
providedibetween the individual articles so that they
can be easily separated at a later stage, for example
by a consumer. If the articles are separated, the
flanges may be left in place. However, desirably the
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
24
flanges are partially removed in order to provide an
even more attractive appearance. Generally the flanges
remaining should be as small as possible for aesthetic
purposes while bearing in mind that some flange is
required if the articles are in the form of
thermoformed containers to ensure the casing film and
the lidding film remain adhered to each other. A
flange having a width of 1 mm to 8 mm is desirable,
preferably 2 mm to 7 mm, most preferably about 5 mm.
The containers produced by the process of the present
invention, especially when used for a fabric care,
surface care or dishwashing composition, may have a
maximum dimension of 50 cm, excluding any flanges. For
example, a container may have a length of 1 to 50 cm,
especially 3.5 to 4.5 cm, a width of 1.5 to 50 cm,
especially 2 to 3 cm, and a height of 1 to 10 cm,
especially 1.25 to 1.75 cm.
Embodiments of the invention are now described, though
by way of illustration only, with reference to the
accompanying diagrammatic drawings in which:
Figure l shows the stages of the method of the
invention;
Figure 2 shows that moment in the method during which
the waste sealed lid/lining films are removed;
Figure 3 shows a section through a container of the
invention; and
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
Figures 4A&B show a pre-form made during the method of
the invention, and a stack of such pre-forms nested
together.
For convenience only a few embodiments are described.
5 However, any one or more of the individual features of
these embodiments may have general applicability in
all embodiments of the present invention.
Figure 1 shows a water-soluble PVOH film 12 being fed
from a roll into the thermoformer 13 (the actual
10 forming apparatus is not itself shown) in contact with
and on top of the carrier film web 11 to form a
combined base web 123. The films 12 and 11 pass
between rollers (not.shown) which place them in
intimate contact with substantially no air trapped
15 between them, before they pass to the thermoformer 13..
This combined base web is drawn through the machine
without the need for any special unwind or tension
control.
In the thermoforming process both the PVOH film 12 and
20 the carrier film 11 are formed simultaneously. The
PVOH film clings to the carrier film, and the carrier
film produces a cavity form which holds the formed
PVOH film within itself. If the combined base web 123
is examined closely after forming, it will be seen
25 that the PVOH film is held so well.within the carrier
film that it is not obvious without close physical
examination that there are two different films within
the formed base web.
It is important to understand that at this stage of
the process the two films 12,11 can be easily
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
26
separated to reveal a well-defined PVOH..cavity. If
left for a period of time, the formed PVOH film will .
start to shrink back, but the time required for this
to take place is considerably extended, compared to
the rate of shrink-back of a PVOH film which has not
been "carried" in this way. Full advantage of this is
taken when, after forming, the combined base web 123
cavity is then filled (by filling machine 14) with
whatever filling (33) is required; the shrink-back of
the PVOH film cavity is conditioned by that of the
carrier film, which is almost insignificant.
Once filled, there is constructed the desired water-
soluble encapsulate by sealing thereover (at the
thermoformer~s sealing station 16) a second PVOH,
film (15) as the top web (as noted earlier, the PVOH
top web film 15 may be of a different thickness or a
different type from the PVOH base web film 12 in order
to bestow different properties on the encapsulate).
In a typical application, the PVOH top web 15 is
thinner than the PVOH bottom web 12 as it has not had
to undergo forming (during which some thinning of the
film inevitably takes place, particularly at any
corners present in the cavity).
The filled and sealed water-soluble encapsulate is
still being carried by the carrier film. It is
important to understand that, although carried by the
carrier film throughout the sealing process, the
encapsulate has not become welded to the carrier film
during that sealing process. This is because PVOH -
or whatever the water-soluble or water-dispersible
film material is - is a hydrophilic material, while
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
27
the carrier film is (most preferably) a..hydrophobic
material (most water-insoluble films currently
preferred for thermoforming are hydrophobic). As a
result, it is not possible to form a heat weld between
the carrier film 11 and the PVOH film 12.
Figure 1 shows how the carrier film 11, rather than
being put to waste (as in the case of many other
carrying devices when their function has been
fulfilled), is used as an integral part of the
secondary (protective) packaging for the water soluble
encapsulate. First, though, it is necessary to
separate the individual water-soluble encapsulates
making up the sequence of these encapsulates. This is
effected by cutting - die-cutting (at cutting
station 17) - through the two water-soluble lid and
lining films (top and base webs 15,12) around the
seals but not through the underlying carrier film 11,
and then removing the waste "in-between" material at a
rewind station (18) (this is also shown in Figure 2).
This die-cutting process is conventional; it is
similar to that used in the flat bed die-cutting of
self-adhesive labels (in which only the self-adhesive
face material is cut, leaving the self-adhesive label
adhering to the uncut "siliconed" release material).
Figure 2 shows a plan view of this operation. After
cutting the combined water-soluble films (top and
bottom webs 15,12) at the die-cutting station 17,
leaving the die-cut-around portions (23) in place, the
waste (24) is removed upwards to a rewind station 18
leaving behind the now completely-separate water-
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
28
soluble encapsulates 25 held in the cavities of the
carrier film 11.
Once the water-soluble encapsulates have been die-cut,
and the PVOH lid/lining waste removed, a top web (19)
of water-resistant film is sealed over the sequence
(at heat-sealing station 100). This top web 19 of
water-resistant film, which need not necessarily be of
the same material or thickness as the carrier film 11,
needs only to be capable of being reliably (and
preferably peelably) sealed to the carrier film for
example, by means of an adhesive or welding by heat,
ultrasonic, laser, vibration, spin, radio frequency or
solvent welding or a combination thereof but during
this process care must be taken not to trap the edges
Z5 of the flanges of the water-soluble encapsulates in
the formed secondary seal. The finished sequence of
webbed containers can then be cut into groups, or into
individual containers, at a final cutting
station (111) .
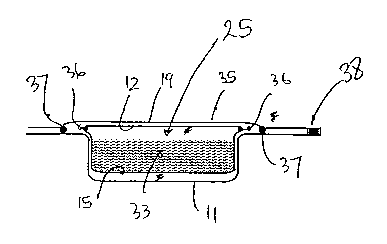
In Figure 3 there is shown a cross-sectional view of
one example of a single container (or pack) according
to the invention: The product (33) is enclosed
between the two layers of water-soluble lid and lining
film 15,12 sealed at the periphery (36), and the
resulting water-soluble encapsulate 25 is entirely
enclosed within the two water resistant films 11,19,
which are sealed around the periphery of the
encapsulate with peelable seals (37). This enables
the outer lid (35) to be readily peeled away from the
carrier film 11, allowing the soluble encapsulate 25
with its product 33 to be elegantly dispensed. If,
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
29
for any reason, the lid 35 is required to remain
attached to the carrier film 11 after peeling open the
pack, the lid may be additionally sealed on one side
only with a further seal (38) which is not peelable.
Typically, the top web of water resistant film 19 is
sealed to the carrier film 11 with a peelable seal
which, whilst hermetically sealing the pack from
ingress of contaminants, particularly water, also
allows a pull tab or other opening device (not shown)
located at the corner of each individual compartment
of the pack to be used to gain easy access to the
water-soluble encapsulate 25 contained within. The
product can then be unit dosed elegantly, leaving the
remainder of the encapsulates protected as in a
pharmaceutical blister pack.
A further embodiment of the invention is one where the
carrier film 11 is used as the only component of the
protective packaging of the water-soluble
encapsulate 25. It is still necessary to separate the
water-soluble encapsulates by die-cutting or stamping
through the two PVOH films (top and base webs 15,12)
around the seals.but not through the carrier film and,
in the case of die-cutting, the waste PVOH lid/lining
films are again removed at a rewind station 18.
However, here the process is interrupted after die-
cutting such that the second web of water-resistant
film 19 is not applied. The carrier film 11 within
which the water-soluble encapsulates 25 reside after
die-cutting may be retained and cut into trays
containing a plurality of packs for stacking or
display purposes.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
5
A yet further embodiment of the invention is described
with reference to Figure 4. The process illustrated
in Figure.l is interrupted before the. filling
stage 14, and the web is moved directly on to the
cutting and separation stages 17,18 in order to
produce empty so-called "pre-forms" (41) for storage
and later use. Figure 4 shows an example of such a
pre-form, and how several of these can be nested for
efficient storage provided that the cavity has a
10 sufficient taper, narrowing towards the base. It is
evident that the pre-forms, complete with their PVOH
liner, can be stored, and then at a later date filled
with product and fed through a simple lidding and
cutting machine (such as those produced by Tiromat and
15 Multivac) to produce the water-soluble encapsulates 25
which are each individually supported by the carrier
film but not completely protected by it.
The packaging system and containers of the invention
have many advantages, some of them being as follows:-
20 a) Shrinkage of the water-
soluble/dispersible film between forming
and sealing is virtually eliminated.
b) The water-soluble/dispersible film can
be thermoformed without losing its shape
25 and volume due to shrink-back.
c) The process can be run at increased
speeds with reduced risk of contamination
of the seals by spillage.
CA 02447202 2003-11-14
WO 02/092439 PCT/GB02/02113
31
d) The finished pack greatly improves
storage, handling and dispensing of the
product.
e) PVOH films with otherwise desirable
properties but especially susceptible to
shrink-back can be used efficiently.
f) As the PVOH base web film is supported
by the carrier film, the suction holes in
the cavity forming mould will not be in
contact with the PVOH film, thus
eliminating any risk of damage to the
PVOH film during forming.
g) The formed cavities of carrier & PVOH
films combined may be separated, singly
or in a plurality of units, before
filling with product. These unfilled
cavities may then be stored and used at a
later date. These "pre-forms" will nest
and store economically, and require less
sophisticated equipment to fill and seal.
h) A package can be produced.where the carrier
film is the required packaging and the PVOH
provides a "barrier" lining. The package will
then be able to contain many products that the
carrier film alone may not be able to contain,
such as perfumes, odours, oils, fats and greases.
The package will also act as a barrier to oxygen.