Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
01/12311FF
CA 02455324 2004-O1-27
s,
1
Method for sealing medicinal capsules for inhalation and apparatus for
sealing them
The present invention relates to a new method of sealing plastic capsules or
inhalers, inhalation capsules thus produced and an apparatus which is
specially adapted to carry out the process according to the invention. The
capsules produced by the process according to the invention are disposable
and preferably contain a single dose of a pharmaceutical formulation in the
form of a powder or liquid intended to be administered by inhalation and are
suitable by their form and function for use in powder inhalers or liquid
nebulisers for producing aerosols. Aerosols thus produced can be inhaled,
for example, in order to administer a pharmaceutical formulation to the lungs.
Prior Art
Capsules containing pharmaceutical preparations are widely used in the
treatment and diagnosis of diseases. The capsules may be administered
orally or are used in specific medical devices such as powder inhalers. As a
rule, the capsules consist of two parts, a capsule body (body) and a capsule
cap (cap) which are pushed telescopically into one another. However, multi-
sectional capsules are also known. The capsules generally consist of
gelatine, particularly hard gelatine. For some special purposes, the capsules
are occasionally made of water-soluble plastics which are easily digested by
the patient so that when administered orally the active substance is released
in certain sections of the gastro-intestinal tract. Some examples of various
capsule materials are give hereinafter.
EP 0460921 describes capsules consisting of chitosan and starch, powdered
cereal, oligosaccharides, methacrylic acid-methylacrylate, methacrylic acid-
ethylacrylate, hydroxypropylmethyl-cellulose acetate, succinate or phthalate.
01112311F F
CA 02455324 2004-O1-27
2
The capsule material is characterised in that the contents are only released
in the large bowel.
GB 938828 discloses capsules for radioactive substances for therapeutic or
diagnostic use. The capsules consist of water-soluble gelatine,
methylcellulose, polyvinylaicohol or water-soluble non-toxic thermoplasts.
EP 0312760 describes a method of sealing hard gelatine or starch capsules
with a specific sealing agent. The seam in the capsules may be displaced
from the central plane of the longitudinal axis of the capsule.
DE 3430764 discloses another method of sealing hard gelatine capsules. In
this method the capsules are first filled and the two capsule halves are
fitted
together telescopically. Then, by lifting the cap relative to the body of the
capsule, a contact zone is exposed on the capsule body, but the capsule
must not be opened. In a subsequent step the contact zone is then made
"tacky" so that the cap can then be pushed back onto its original position and
brought into contact with the contact zone. This process requires high
precision when carrying it out particularly as it is important to avoid
deforming
the capsule when the cap is pushed back onto the capsule body which has
been made tacky by heating and thereby been made more prone to
deformation. Page 32 of the application states that tools with no tolerances
or play of any kind are needed in order to hold and guide the capsule
sections.
US 4991377 discloses another process for sealing keratin or gelatine
capsules. In this process the sealed capsule which consists of two
telescopically connected sections is treated with hot air at its weld seam.
During the process the lower part of the capsule rests on a holder. The
patent specification does not give any hint as to how to avoid deformation of
the capsules softened by the blast of heat nor how to avoid heating or
carbonisation of other parts of the capsule outside the seam zone. Nor is
01112311F F
CA 02455324 2004-O1-27
3
there any mention of how the quality of the contents of the capsule is
affected
by the heat produced by the welding.
WO 00107572 discloses capsules for inhalers of the kind according to the
invention which consist of indigestible plastic. We hereby refer expressly to
this patent document and the object disclosed therein. The capsules
described therein are sealed analogously to standard commercial hard
gelatine capsules, i.e. the capsule cap is placed telescopically on the
capsule
body. The seam which is necessarily produced between the cap and the
body may optionally be welded, glued or banded to reduce the steam
permeability. Alternatively, the entire cap may be covered with a continuous
protective film or the gap in the seam may be filled with a filler. There are
no
details of the methods of sealing the cap, particularly no mention of any
welding processes.
Various thermal welding methods are known from the prior art for welding
plastic materials. These include ultrasonic welding, hot plate and hot tool
welding, hot gas welding, rotary welding, high frequency voltage welding or
induction welding.
By contrast, processes of this kind for sealing plastic capsules for medicinal
inhalers, which are subject to certain limiting conditions with regard to
their
use, are not known.
It has now been found that these welding processes cannot readily be
transferred to the welding of the capsule halves described in WO 00107572.
The limiting conditions mentioned above, which prevent the simple transfer of
the methods known from the prior art to capsules for inhalation, include the
fact, for example, that the capsules are filled with a pharmaceutical
formulation the pharmaceutical quality of which must not be impaired during
the welding process.
Another condition is laid down by the dimensions and thickness of the
capsules to be welded, particularly the thin walls of such capsules. This is
0111231/FF
CA 02455324 2004-O1-27
4
necessary to allow the capsule to be used in a standard commercial inhaler
analogously to the hard gelatine capsules currently in common use. In fact, it
has to be capable of being opened easily. If the conventional methods are
applied to such a capsule, holes will rapidly be burnt into the capsule during
the welding process, particularly in those parts of the capsule which are
outside the area being welded. This area where the seam is to be formed is
naturally in a region where the walls of the two parts to be joined together
overlap. Next to this area there are parts where there is no such overlap. In
these parts the capsule can more easily be damaged by the welding process.
There is also the danger that if the capsule walls are too thick any weld seam
at the junction will not be properly sealed.
Description of the Invention
The present invention is intended to provide sealed plastic capsules for
medicinal inhalation devices which contain a pharmaceutical formulation.
Preferably, these capsules contain a single dose of the formulation. The
capsules are also referred to as single dose capsules within the context of
the present invention.
An inhaler which is preferred for the present purpose is described for
example in WO 94/28958 (HandiHaler~) to which reference is hereby made.
The present invention solves the problem described above by providing a
new welding method in which only the part of an inhalation capsule to be
welded is welded by means of an energy beam in the form of a jet of hot gas
or a laser beam. At the same time, the capsule material is preferably
warmed only at the seam.
Thus, one aim of the invention is to provide a welding process in which the
parts of a plastic capsule which are not to be welded are not heated to the
melting point of the plastic.
01112311FF
CA 02455324 2004-O1-27
A further aim is to provide a welding process for plastic capsules in which
the
individual parts of a plastic capsule of this kind for inhalers are firmly
held
together.
A further objective is to connect the individual parts of a plastic capsule to
one another so that they cannot be separated again without damaging the
capsule. This makes it easier for the user to tell that the capsules have not
already been opened.
A further aim is to provide a welding process for plastic capsules in which
there is no damage to the capsule caused by overpressure produced in the
capsule by the welding process, or any such overpressure is minimised.
A further aim is to provide a welding process for plastic capsules in which
the
pharmaceutical quality of the pharmaceutical formulation in the capsule,
which is a formulation for inhalation according to the invention, is not
jeopardised during welding.
A further aim of the invention is to weld the seam of inhalation capsules of
the kind described above on an industrial scale under GMP conditions (good
manufacturing practice).
The capsules to be welded consist of non-water-soluble, preferably water-
repellent plastics which themselves do not substantially affect the
pharmaceutical quality of the contents but which improve the usability of the
filled capsules in terms of their function, their period of use andlor the
climatic
region and which are advantageous at various stages from manufacture to
use.
The capsule according to the invention consists of at least two parts, a
capsule body (body) and at least one capsule cap (cap), which are joined
together in such a way as to form a stable sealed cavity of a defined volume
which contains the pharmaceutical formulation. The dimensions of the
capsule are such that it can be used in common powder inhalers for capsules
0111231/FF
CA 02455324 2004-O1-27
6
as described for example in the patents DE 3345722 (Ingelheim M Inhaler ),
EP 0591136 (Ingelheim Inhaler) or in published German application DE
4318455 ("HandiHalerC~"). Size 3 capsules are particularly preferred.
In one embodiment the plastic of the capsule is indigestible by humans so
that if it is taken orally the active substance is not released. The advantage
of this is that accidental swallowing of the capsule cannot lead to any
lasting
effects on health. This applies particularly to small children or older
people.
Preferably, plastics are used which may be processed by injection or blow
moulding andlor plastics which can be processed to form the capsule cap or
body without the use of any mould release agents which may cause the
contents to adhere to the walls of the capsule. The advantage of this is that
the inside of the cap or body does not have to be cleaned to remove mould
release agent in order to conform to the official requirements (e.g. according
to DAB (Deutsches Arzneibuch)) which restrict the use of mould release
agents for primary packaging. Thermoplasts which allow the halves of the
capsule to be welded together are preferred.
Preferred materials have the property of having as little powder as possible,
preferably no powder, adhering to them.
Suitable plastics for the process according to the invention include
polyethylene LD (low density), polyethylene HD (high density), polystyrene,
acrylonitrile-butadiene-styrene, polypropylene, polymethylmethacrylate,
polyvinyl chloride, polyoxymethylene, polycarbonate, polyester or
polyethylene terephthalate.
In preferred embodiments the plastic is polyethylene, particularly
polyethylene with a density of between 900 and 1000 kg/m3, preferably 960
kglm3 (high density polyethylene).
In a preferred embodiment the plastic has no marked adhesion for
pharmaceuticallchemical substances, particularly for particles of a size
01/1231/FF
CA 02455324 2004-O1-27
7
capable of entering the lungs, so that when the capsule is used in one of the
inhalers described above the entire contents of the capsule can be released.
This has the advantage of enabling a more accurate dosing, particularly of
the fine fraction destined for the lungs.
In another embodiment the capsule consists of a plastic with a Shore
hardness D of 65 to 73. A plastic with this hardness does not shatter when
pierced or cut open but at the same time is rigid enough so that the hole
formed does not close up on its own. The advantage of such a material is
that no bits of the capsule can be broken off during the opening, piercing or
cutting open of the capsule in the powder inhaler, which might then be
breathed in during inhalation.
In one embodiment the plastic capsule is so stable that it will withstand a
force of up to 15 N along the longitudinal or transverse axis. The advantage
of this is that the capsule is better adapted to the stresses which act on the
capsule during manufacture, filling, packaging, transporting and the like.
In another embodiment the material of the capsule has a permeation
coefficient for steam of less than 10-'3 kg/(m s Pa), preferably less than 1.3
x
1O-'4 kg/(m s Pa). Preferably, the coefficient is between 10-'5 and 5 x 10-'6
kg/(m s Pa), most preferably between 5 x 10-'6 and 2 x 10-'6 kg/(m s Pa).
The advantage of this property is that the contents of the capsule are
protected from moisture even in geographical zones with a high relative
humidity.
The capsule according to the invention can be used in all kinds of powder
inhalers in which the preparation which is to be inhaled is introduced by
means of a capsule.
In a preferred embodiment the cap and body of the capsule are similarly
cylindrical in shape, consisting of an inherently closed casing with one
closed
and one open end. The shape and size of the cap and capsule are such that
0111231IFF
CA 02455324 2004-O1-27
the open end of the body can be pushed telescopically into the open end of
the cap so that the cap is firmly attached to the body.
In a preferred embodiment the cap and the body are in the form of a cylinder
of circular cross-section with a convex, virtually hemispherical, closed
underside and both consist of high density polyethylene with a density of
between 950 and ~ 000 kglm3.
In a special embodiment the cap and body are provided with closure means
which are advantageous for temporarily andJor finally closing the capsule.
In one such embodiment there may be dot-like elevations on the inner
surface of the cap and somewhat larger dot-shaped depressions on the outer
surface of the body, which are so arranged that when the capsule is closed
the elevations engage in the depressions. Alternatively, the elevations may
be provided on the outer surtace of the body and the depressions on the
inner surface of the cap. Arrangements in which the elevations or
depressions are arranged in rings or spirals around the casing are preferred.
Instead of the dot-like shape of the elevations and depressions these may
also extend in a continuous ring around the surface of the cap or body. The
latter engagement means are preferred. One or more encircling annular
elevations are formed on the inner surface of the cap and the outer surface of
the body so that in the closed position of the capsule an elevation on the cap
is adjacent to an elevation on the body in each case.
In the embodiments with these annular depressions andlor elevations the
latter may be continuous or interrupted. In another embodiment, elevations
are formed on the outside of the body close to its open end and holes are
formed in the cap close to the open end so that the elevations on the body
engage in the holes in the cap in the closed position of the capsule. The
elevations may be such that the cap can be opened at any time without
damage to the capsule or so that once it has been sealed the capsule cannot
be opened again without destroying it.
0111231 IF F
CA 02455324 2004-O1-27
9
Capsules with one or more such engagement mechanisms (detents) (e.g.
two encircling grooves) are preferred.
Capsules with at least two such engagement means which secure the two
capsule parts with different degrees of strength are particularly preferred.
In
one such part a first latching means may be provided close to the openings
of the cap and body and a second may be provided somewhat closer to the
closed end of the capsule parts. The first engagement means secure the two
capsule parts less strongly than the second.
The advantage of this embodiment is that the cap and body of the capsule
can be temporarily joined together using the first engagement means before
the capsule is filled. Then, in order to fill the capsule, the two sections
are
separated again. After filling, the two capsule parts are pushed together
until
the second set of latching means firmly secure the capsule sections.
In another embodiment a bead is formed on the outside of the body, running
around the body perpendicular to the connecting axis between the cap and
body. The bead acts as a stop for the capsule when the latter is pushed over
the body to prevent impact between the cap and the body. The region
between the open end of the body and the bead corresponds to the part of
the body over which the cap can be pushed. The bead is located on the
body so that the cap can be pushed far enough over the body to achieve a
firm seal between the cap and body. In other words the bead is not located
right at the open end of the body, for example. The side of the bead which
points towards the open end of the body stands up as a perpendicular edge
an the outer wall of the body so that when the capsule is closed the cap
cannot be pushed beyond the bead. The side of the bead facing the closed
end of the body may take the form of a virtually right-angled edge or may
flatten out towards the closed end of the body. The provision of a virtually
right-angled edge may be advantageous when the capsule is fitted loosely in
a capsule holder, while the variant with a bead that flattens out is suitable
for
a firm fit of the capsule. The bead may be continuous or interrupted.
01 /1231/F F
CA 02455324 2004-O1-27
In a preferred embodiment the bead flattens out continuously towards the
closed end of the body and stands on the capsule body with its side oriented
towards the open end of the body in a perpendicular position. The height of
the edge thus formed is such that in the closed state of the capsule the edge
does not project beyond the cap, with the result that the transition from the
cap to the body is smooth.
Alternatively, instead of the bead, the diameter of the capsule body may be
abruptly reduced at one point so that the diameter oriented towards the
opening is smaller (or larger) than the diameter oriented towards the closed
end of the body. The cap and body may be constructed so that in the closed
state the capsule is smooth at the seam.
In another alternative, the shape of the wall of the cap in the opening region
is precisely the reverse of the shape of the wall of the opening of the
capsule.
In other words the wall of the capsule widens out in the region of the opening
to form an internal edge. in this embodiment, too, the outer casing is
preferably smooth. In this embodiment the cap is pushed onto the capsule
until the edge of the cap and the edge of the capsule make contact with each
other.
In another preferred embodiment the bead is located on the inside of the
capsule body. The edge of the bead on which the cap rests in the closed
position of the cap then faces the outside of the capsule body. In such a
case the cap is not fitted onto the capsule body but inserted in the capsule
body. The cap and body may be constructed so that in the closed state the
capsule is smooth at its seam. Within the scope of the present invention the
two possible methods of fitting the capsule cap over or into the capsule body
are described as being of equal value, i.e. wherever one of the two
possibilities is described, the other is also applicable.
01112311FF
CA 02455324 2004-O1-27
11
In another embodiment the edge of the capsule body is in the form of a U-
shaped return in which the edge of the opening of the capsule cap is
inserted.
In another embodiment both openings have a U-shaped return of this kind.
In yet another embodiment the edge of the openings of the cap and body
widen out and are smooth in the direction pointing towards the opening.
The thickness of the walls of the cap and body may vary over the entire
region. Thus, the wall thickness is generally greater in the rounded parts of
the cap or body or at the point on the body where the bead is formed, than in
the areas where the walls extend in a straight line. In one embodiment, the
walls of the cap and body have a thickness of 0.1 mm to 0.5 mm, the capsule
preferably having a mean thickness of 0.1 mm to 0.4 mm, more preferably
0.2 mmto0.4mm.
The capsule body has a thickness of 0.15 mm to 0.35 mm, preferably 0.225
mm to 0.275 mm, most preferably 0.25 mm in the region of its opening,
particularly at its edge.
The capsule cap has a thickness of 0.25 mm to 0.45 mm, preferably 0.325
mm to 0.375 mm, most preferably 0.35 mm, in the region of its opening,
particularly at its edge.
The length of the capsule is 8 mm to 30 mm, preferably 13 to 17 mm, most
preferably 15.5 mm to 16 mm. The diameter of the capsule is 4 mm to 7 mm,
preferably 5.3 mm to 6.3 mm. A diameter of 5.75 to 5.95 mm is particularly
preferred.
A preferred capsule is 15.9 mm long, with a body 5.57 mm in diameter and a
cap 5.83 mm in diameter. The preferred wall thickness of the capsule body
is 0.25 mm and that of the cap is 0.35 mm.
0111231/FF
CA 02455324 2004-O1-27
12
In one possible embodiment, bumps are formed on the outside of the capsule
while in another embodiment three or more ribs are provided extending
parallel to the longitudinal axis of the capsule. The advantage of these
arrangements is that the capsule can be removed from a capsule holder, as
used for example in the powder inhalers mentioned above, in such a way that
it is not damaged or pulled open. The ribs or bumps may extend over the
entire outer surface of the capsule or may cover only a part of it.
Alternatively, they may be formed only on the cap or only in that part of the
body which is outwardly visible in the closed state. The ribs run parallel to
the longitudinal axis of the capsule and ensure that the capsule is fixed
vertically in said capsule holder. In the case of a capsule of circular cross-
section the ribs are preferably arranged so that the cross-section of the
capsule is not rotationally symmetrical about the central axis. In such an
embodiment the ribs may be farmed only in that part of the body which is
visible in the closed state of the capsule. This embodiment prevents the
capsule from becoming jammed in a capsule holder.
In an embodiment without a bead but with ribs on the part of the body which
is visible in the closed position of the cap, the ribs are formed so that the
ends of the ribs oriented towards the open end of the body perform the same
function as the bead, namely to act as a stop for the cap when the cap is
combined with the body.
In another embodiment the outer surfaces of the cap and body describe a
hollow cylinder of round, oval, triangular, rectangular, hexagonal, octagonal
or polygonal cross-section, in which the top is open and the bottom is closed
in each case. The closed underside may be flat or convex. The angular
embodiments have the advantage that they can be stored more compactly
than the round ones.
In one embodiment the elongation of the capsule (distance from the closed
end of the body to the closed end of the cap in relation to the diameter when
the capsule is closed) is greater than 1, in another embodiment the
01f1231/FF
CA 02455324 2004-O1-27
13
elongation is equal to 1 and in yet another embodiment the elongation is less
than 1. The advantage of the latter is that the body has a larger opening for
filling it up. In one of the embodiments with an elongation equal to 1 the cap
and body are such that the closed capsule is spherical in shape, which may
be advantageous for automatically loading a powder inhaler with the capsule
from a reservoir.
The description shows that the capsule according to the invention is
particularly suitable for holding powdered pharmaceutical formulation of any
kind suitable for inhalation. In one particular embodiment the capsule
contains as active substance cromoglycic acid, reproterol, beclomethasone,
terbutalin, salbutamol, salmeterol, ketotifen, orciprenaline, fluticasone,
insulin, ipratropium, tiotropium, dexamethasone, bambuterol, tiotropium,
budesonide, fenoterol, clenbuterol, prednisolone, prednisone, prednylidene,
methylprednisolone, formoterol or nedocromil, the pharmacologically
acceptable salts or mixtures thereof or another cortisone preparation or
atropine derivative suitable for inhalation.
In a preferred embodiment the capsule contains ipratropium bromide or
tiotropium bromide.
Apart from powder fillings the welded capsules may also hold liquids and are
then suitable for the latest liquid inhalers.
The sealing of the minimum of two telescopically fitting sections of the
capsule is preferably done using a process in which a locally tightly
restricted
stream of energy consisting of hot gas or laser light is guided in a relative
movement at least once around the perpendicular axis of the overlapping
area of the capsule elements so that in this area the capsule material is
melted but not destroyed and a weld seam is produced in this area.
0111231 /F F
CA 02455324 2004-O1-27
14
The rotary movement can either be produced by rotating the capsule about
its axis, perpendicular to the plane of the weld seam, in the beam of energy
or by guiding the beam of energy around the capsule.
The capsule seam is then welded within one revolution or several
revolutions.
Welding processes in which the welded seam of capsule material is
produced within several revolutions are preferred as this allows better
control
of the welding process. For example this will prevent holes from being burnt
into the capsule wall.
Preferably, in the process according to the invention, hot gas and laser light
are used as the energy sources. If laser light is used the thermal energy
required for welding is induced in the capsule material by absorption.
The welding process itself may take place either directly at the seam
between the two capsule sections or in the overlap between the two capsule
halves.
The weld seam thus formed is produced as one or more continuous lines
along the circumference of the capsule parallel to and between the two
planes which span the openings of the cap and the body. Preferably, the
seam is tightly sealed all round by means of the process according to the
invention.
In addition to closed lines forming the weld seam it is also possible to use
spiral lines. However, the configuration of the weld seams is not restricted
to
straight shapes but may also include zigzags or meandering lines or any
other shape running around the exterior of the capsule. The weld seam may
also be in the form of spot welds.
01112311FF
CA 02455324 2004-O1-27
Preferably, the weld seam is formed at a point where the capsule body and
cap are just beginning to overlap. Depending on the method used, a number
of weld seams may be provided. These may be formed parallel to one
another, for example.
In mufti-sectional capsules, weld seams are needed at all the connecting
points to achieve the required stability and seal. In two-part and multi-part
capsules, a plurality of adjacent weld seams increases the reliability of the
seal.
Depending on the size of the capsule body and cap the position of the weld
seams may be located centrally with respect to the longitudinal axis.
However, an asymmetric position is preferred in order to obtain the maximum
possible spacing from the capsule contents.
This may also be achieved for example if either the capsule body is longer
than the capsule cap or vice versa. In this context the term length denotes
the distance between the opening of each of the two capsule halves and the
opposite closed side. Preferably, the seam is located in the top third of the
closed capsule. This means that the proportion of the capsule from the
closed base to the point where the above-mentioned bead is formed, which
marks the point up to which the open end of the capsule cap is pushed over
the opening of the capsule body onto said body, is about two thirds of the
total length of the sealed capsule.
A capsule of this kind has the advantage that the fill height of the
formulation
within the capsule can be below the seam, so that the danger of impairing the
quality of the formulation by the welding process is significantly reduced.
The fill level of the capsule may be adapted to suit the welding temperature
and the temperature sensitivity of the pharmaceutical formulation.
01 /12311FF
CA 02455324 2004-O1-27
16
The present invention therefore also relates to a plastic capsule of this kind
with its weld seam positioned asymmetrically. The other characteristics of
the capsule according to the invention were described in detail at the
beginning.
The use of a measuring device makes it possible to judge the welding
temperature and the flow characteristics of the capsule material during the
welding process and hence to control the process. The welding process
preferably takes place with feedback of the data from the measuring device
in order to achieve a constantly high quality for the weld seam. In this way
it
is possible to ensure a uniform influx of energy at the weld seam.
The feedback balances out any fluctuations in the thickness of the material,
for example, and any environmental influences by adjusting the speed of
rotation of the relative rotation or the energy supplied. In this context, it
is
preferable to melt as small an area as possible and to measure the
temperature or radiation precisely at this point. The weld seam is then
produced by rotation.
In the process according to the invention the energy supplied and the relative
speed of rotation are preferably adapted to one another so that the capsule
material is brought to the point where it is just beginning to melt. By
repeating this process once or a number of times a weld seam formed from
the material is produced.
The following are the temperatures required to bring the preferred capsule
materials to the point where they are beginning to melt:
The examples must not be interpreted as restricting the materials.
Material Processing
Temperature
Polyethylene LD 160C - 260C
Polyethylene HD 260C - 300C
0111231 /F F
CA 02455324 2004-O1-27
17
Polystyrene 170C - 280C
Acrylonitrile-Butadiene- 210C - 275C
Styrene
Polypropylene 250C - 270C
Polymethylmethacrylate 210C - 240C
Polyvinylchloride 170C - 210C
Polyoxymethylene 200C - 210C
In accordance with the melting temperature, the beam of energy, its distance
from the capsule surface and its mean delay time on the surface of the
capsule are adjusted so that the capsule material is melted without any holes
being burnt in the capsule wall.
The speed of rotation of the energy beam around the capsule, or of the
capsule in the energy beam, may be between 0.01 revolutions per second
and a maximum of 40 revolutions per second. In the case of laser welding it
is preferably 0.1 to 20 revolutions per second while in the case of hot gas
welding it is preferably 0.2 to 2 revolutions per second.
This results in a circumferential speed of the preferred capsule, with a
length
of 15.9 mm and a cap diameter of 5.83 mm and a body diameter of 5.57 mm,
of about 0.18 mm per second up to 732 mm per second. In the laser welding
process the circumferential speed is preferably 1.8 mm per second to 366
mm per second and in the case of hot gas welding it is 3.7 mm to 37 mm per
second.
The number of revolutions of the capsule/energy beam may be up to 40 for
one welding process, preferably up to 20. In laser welding it is preferably 2
to
3 and in hot gas welding it is 5 to 8.
In the welding process according to the invention the capsules and the
energy source are preferably brought together at a specific speed of
advance. Speeds of advance of 0.1 cm per second to 10 cm per second are
01112311F F
CA 02455324 2004-O1-27
18
preferred, whilst a speed of advance of 1 cm per second to 5 cm per second
is particularly preferred.
Using the process according to the invention the capsules can be welded in a
cycle time of preferably less than 10 seconds. Processes in which the
capsules are welded within 5 seconds are more preferred while processes in
which welding takes place within 1 second are even more preferred.
Thus, a further advantage of the process according to the invention is
achieved: the short welding duration avoids the formation of bubbles caused
by the heating of the gas enclosed in the capsule.
The capsules produced by the process according to the invention preferably
have an axis of symmetry Cn (n=the symmetry number) and a plane of
symmetry which is ideally perpendicular thereto. This latter criterion is
restricted to cylindrical capsules whose weld seam is central in relation to
the
longitudinal axis. Rotationally symmetrical capsules are particularly
preferred.
It is not essential for the circumference of the capsules to be circular. The
circumference may also be polygonal or elliptical.
The preferred external shape of the capsule is smooth. Plastic capsules
according to published German application DE 198 35 346 A1 are particularly
preferred.
In the case of laser welding and embodiments of the capsules with a
polygonal or elliptical cross-section the capsule seams are either irradiated
with an average dose at an average working distance with an unfocused
beam or preferably with an active beam adjustment, the focus following the
rotation synchronously in accordance with the geometry of the capsule, or
most preferably with intensity regulation which equalises any loss of focus by
increasing the power.
01112311F F
CA 02455324 2004-O1-27
19
Before the actual welding process, preparatory measures have to be taken.
These are necessary in order to take account of welding requirements
regarding the energy source used, the construction of the capsules from a
geometric point of view and the choice of material as well as the choice of
additives, e.g. dyes, which may be added to one or more parts of the
capsule. Dyes are preferably used in laser welding.
The advantage of having the dyes in the plastic is that the dyes absorb the
laser light in the plastic and thereby heat up the plastic locally to cause
the
material to fuse.
The dyes used are adjusted to the frequency of the laser light and the
quantity of heat required.
The dyes used are those which will not affect the pharmaceutical quality of
the formulation in the capsule. Preferably, food colourings are used.
Examples of particularly preferred dyes are:
Dye Colour
Iron Oxide Red = E 172 =Fe203 red
FD&C Red 3 = E 127 = C2oH61405Na2''H20 red
=
Erythrosine
beta-carotene = E 160a = C4oH5s yellow
Iron Oxide Yellow = E 172 = Fe0(OH) yellow
FD&C Blue 2 = E 132 = C,6HsN208S2Na2 blue
=
01112311FF
CA 02455324 2004-O1-27
Indigotine
Chlorophylline = E 141 = C34H31N4~6CUNa3 green
Or
C34H31 N4~6CUK3
Caramel = E 150a or E 150d = "burnt" sugarbeige
Titanium dioxide = E171 white
Of these, the inorganic pigments are particularly preferred.
The additives may either be applied to the finished capsule sections (e.g. by
spraying, printing, painting or dipping) and then be fused on during the
welding process or they may be incorporated in the capsule material during
the manufacture of the capsules, by a masterbatch method.
In laser welding the circumference has to be totally irradiated to ensure that
the entire circumference of the capsule is fully welded. It is possible to use
a
linear optical device which welds the entire circumference simultaneously.
Preferably, the capsule and light beam are moved relative to each other and
as a result the focal spot is guided along the circumference of the capsule.
This may be done, for example, by rotating the capsule in the light beam of
the laser.
Alternatively, the laser beam may be guided around the capsule by means of
rotating mirrors. Relative rotation of the capsule and laser beam by means of
a holder which allows the angle of rotation and laser activity to be
synchronised is particularly preferred.
Preferably, a radiation measuring device is used in laser welding as a
measuring device for feedback control of the welding apparatus.
0111231 /FF
CA 02455324 2004-O1-27
21
The feedback equalises any fluctuations e.g. in the thickness of the material,
its reflectivity, the focusing and other environmental influences by adjusting
either the speed of rotation or preferably the laser output. In this context,
it is
preferable to melt the smallest possible spot of seam material while
measuring the temperature or radiation. The weld seam is then produced by
relative rotation of the capsule around the laser beam as described above.
The laser power required for welding depends on the optical device used (the
size of the focal spot), the speed of advance, the surface quality of the
material (e.g. its roughness), the optical qualities of the material (e.g.
transparency) and the necessary melting temperature of the materials. For
example, green plastic capsules were welded with a 5 watt argon ion laser
beam (wavelength all line, 514 nm and 488 nm), focusing was done with a
microscope lens (magnification 10X). The same capsules could also be
welded using an infra-red laser (wavelength around 900 nm) with a radiation
output of about 100 watts.
Using the laser welding process according to the invention the weld seam
can be produced either immediately or during the second or even
subsequent repeat irradiations. When the process is repeated the capsule
and light source are preferably rotated relative to another so that the point
which has been irradiated once has not yet cooled down again when it meets
the laser beam again. The focus of the laser beam is preferably selected to
be precisely the right size to generate only a little heat on the inside of
the
capsule wall and localised on the seam of the capsule.
The width of the weld seam can be adjusted by a rapid oscillating movement
of the focal spot in directions other than the direction of advance of the
welding. Preferably, the width is adjusted by optically imaging the laser
radiation on a focal spot preferably less than 1 mm in size. A focal spot
diameter of less than 0.5 mm is particularly preferred.
0111231/FF
CA 02455324 2004-O1-27
22
In hot gas welding the total welding of the entire circumference of the
capsule
requires that the capsule be heated uniformly around its circumference. This
can be achieved by the use of one or more nozzles which may be crescent-
shaped or annular, for example, which simultaneously weld the entire
circumference. However, relative movement of the capsule and a flat beam
generated by a corresponding nozzle with a flat rectangular opening is
preferred. The melting zone thus produced can then be guided around the
circumference of the capsule until the weld is complete. It is particularly
preferred to rotate the capsule by means of a holder which allows
synchronisation between the angle of rotation and the influx of hot gas.
When producing individual capsules it is advantageous to use one nozzle or
a plurality of nozzles with a small cross-section.
For industrial mass production, one (or two) long slotted nozzles should be
used and a plurality of capsules are guided past said nozzle or nozzles all at
once with continuous rotation.
In the case of hot gas welding, crescent-shaped slotted nozzles may
advantageously be used if the process involves rotating the capsules in the
hot stream of gas.
The dimensions of the slots of the nozzle are designed so that a flow of heat
is obtained which is very restricted in its height. Preferably, the height of
the
slot opening is up to 3 mm, preferably up to 2 mm, most preferably up to 1
mm. The length of the opening is variable. In the case of non-radial weld
seams, the length like the height should be restricted to 3 mm, preferably 2
mm, most preferably 1 mm. In the case of weld seams running radially or
spirally around the capsule the length of the nozzle slot is of less
importance.
The height of the weld seam is determined by the height of the nozzle jet. In
the case of a weld seam running radially around the capsule the region of the
capsule on which the weld seam is to be formed runs parallel to the
longitudinal side of the nozzle arrangement in the energy flow. The length of
the nozzle arrangement and the temperature of the energy flow together with
01/1231/FF
CA 02455324 2004-O1-27
23
the melting point of the capsule material determine the retention time of the
capsule in the energy beam. In other words, the longer the nozzle opening
and the hotter the energy beam the faster the capsule has to be rotated
parallel to this direction. In other words the weld seam around the capsule
extends parallel to the length of the nozzle arrangement.
In such cases, the length of the slotted nozzle may be several centimetres or
may even run into metres. An elongated slotted nozzle of this kind is
particularly advantageous if a plurality of capsules are to be welded one
after
another on a conveyor belt.
The process according to the invention is perfectly controlled so that the
weld
seam is not produced immediately but only on the second or subsequent
repeat exposure to the hot gas. This repetition is preferably achieved by
rotating the capsule and hot gas nozzle relative to one another before the
heated point has cooled down again.
The temperature of the gas required for welding depends on the melting
ranges of the plastics used and their distance from the hot gas nozzle.
However, in order to apply the narrowest possible jet of heating gas to the
seam the capsule is moved past the nozzle at a very short distance from it,
preferably at a distance of 5 mm.
The width of the hot gas jet on the weld point is 1 mm to 2 mm.
In another aspect the present invention relates, in addition to the welding
process and the capsule thus obtained, to a capsule holder adapted to the
welding process.
The capsule holder according to the invention consists of two separate
moulds the inner configuration of which is dish-shaped. The dish-shaped
mould is constructed so that one mould holds the capsule body tightly while
01112311F F
CA 02455324 2004-O1-27
24
the other holds the cap, and in the closed state only the seam area which is
to be welded is not covered by the holder.
The advantage of this arrangement is that only the area of the capsule to be
welded is able to come into contact with the energy beam and the other
areas of the capsule are protected from the beam.
Preferably up to 3 mm and most preferably 0.5 to 1 mm of the edge of the
capsule half which is to provide the seam is left exposed.
The outer shape of the holder is unimportant but it must allow the hot gas to
flow away. For example, the two parts of the holder may be cylindrical in
shape.
The top part and bottom part of the capsule holder are joined together so that
the position of the tools is maintained even when the connection between the
individual parts is just beginning to melt, without exposing the capsule to
any
torsion or tension. This is particularly important for the welding process if
the
dimensional stability of the capsule is reduced by heating up a partial
region.
The purpose of the capsule holders is to hold the capsules and guide them
past the energy source for the welding process. At the same time they serve
to cool the areas of the capsule walls which are not to be welded and thus
also protect the formulation in the capsule.
So as to protect the formulation from the conditions of the welding process
the fill level of the capsule is preferably below the weld seam, in the
protected
area.
The capsule holders may be constructed so as to cool the capsules.
Systems with water cooling or Pettier elements may be suitable for this, for
example.
0111231IFF
CA 02455324 2004-O1-27
The capsule holder may be made of metal, e.g. aluminium, copper, stainless
steel or heat resistance plastics, e.g. polytetrafluoroethylene (=Teflon~).
Preferably at least part of the two halves of the capsule holder is connected
to a rotating device so that the capsule can be rotated about the energy
source. Preferably, the capsule holder rotates about its own axis which is
spanned by the top and bottom parts of the holder.
A plurality of capsule holders may be arranged on one conveyor, preferably
in a row. In this case the holder for the capsule body is connected to a
conveyor. Over it and parallel thereto runs a second conveyor to which the
holder for the capsule cap is attached so that each capsule with its body is
located in the holder provided and the capsule cap is located in the holder
provided for it. The conveyors are constructed so that the two halves of the
capsule holders can be moved apart after the welding so that the capsules
are left behind in one of the two halves, preferably the one for the original
capsule body. This may be done, for example, by having the conveyors
consist of conveyor belts which diverge after the welding station, initially
in
the same plane. After that the capsules can be removed from the holder.
In another embodiment the holders may be attached to the conveyor by a
telescopically extendable arm. These arms are extended towards the other
arm in each case when the capsule is welded so that the capsule is protected
by the two extremities of the holder in a manner described above. After the
welding process the arms are retracted again so that the two halves of the
holder are moved apart.
In this way the capsules can be brought up to the energy source.
Preferably, the holders are mounted so as to be rotatable about themselves
on the conveyor. As soon as the capsule has been conveyed into the energy
beam the capsule holder as a whole rotates about itself and thereby rotates
the capsule in the energy beam so that the weld seam is formed all around
the capsule.
0111231/FF
CA 02455324 2004-O1-27
26
In the case of capsule holders which are not rotatably mounted the energy
beam may be moved around the capsule accordingly.
During the welding an overpressure is produced by the heating in the
capsule. This pressure constitutes a considerable risk, as it may lead to the
formation of bubbles in the heated wall of the capsule if the capsule is
heated
excessively. The capsule holder according to the invention also
advantageously provides a remedy to this source of danger.
Additionally, the capsule holder also protects the parts of the capsule which
are not to be welded and the formulation in the capsule.
The process according to the invention produces capsules containing
pharmaceutical compositions for inhalers, which are totally sealed so that the
pharmaceutical substance cannot escape from the capsule unless the
capsule is destroyed.
The advantage of the capsules according to the invention is that they have a
very low steam permeability, particularly at the seam, and are therefore
suitable for use in various climatic regions, e.g. in climatic region 3 with a
high relative humidity, without any impairment of the pharmaceutical quality
of the formulation. These capsules also have various advantages at other
stages of the life of the capsule from its manufacture to its use, in terms of
its
usability as a carrier of pharmaceutical preparations, the method of
administering the contents, the durability of the contents and/or the
suitability
of the capsules for use in various countries. One other advantage of the
capsule materials according to the invention is that they do not have a
tendency to bind powdered materials to themselves, so that precise metering
of the fine fraction destined for the lungs is made easier.
These capsules may also be used in non-medicinal aerosols.
01 /12311FF
Description of the Figures
CA 02455324 2004-O1-27
27
The Figures show various embodiments of the capsules according to the
invention by way of example but are provided solely as an illustration without
restricting the scope of the invention.
Fig. 1 shows the simplest and most preferred embodiment of the capsule
according to the invention in lateral cross-section.
Figs. 2a and 2b each show a different embodiment of the capsule with a
tapering bead on the body in lateral cross-section.
Fig. 3 shows an embodiment of the capsule with an edge-shaped bead on
the body in lateral cross-section.
Fig. 4 shows an embodiment of the capsule with a tapering bead on the body
and an annular recess on the body and cap in lateral cross-section.
Fig. 5 shows an embodiment of the capsule with a tapering bead on the body
and an annular recess on the body and cap in front view.
Fig. 6 shows an embodiment of the capsule with a tapering bead on the body
and dot-shaped recesses or elevations on the body and cap in front view.
Fig. 7 shows an embodiment of the capsule with a tapering bead on the body
and dot-shaped elevations on the body and dot-shaped holes in the cap in
front view.
Fig. 8 shows an embodiment of the capsule with ribs on the body in front
view.
Fig. 9 shows the capsule of Fig. 7 in horizontal cross-section.
01112311F F
CA 02455324 2004-O1-27
28
Figs. 10a, 10b and 10c show embodiments of the capsule with different
cross-sections.
Figs. 11 a - g show different embodiment of capsules 1 with non-centrally
arranged closure points between the cap and the body to form the weld seam
13.
Figs. 12 a - h show capsules with various forms of weld seam (straight,
spiral, meandering, zigzag, spot welds, parallel diagonally extending non-
continuous weld seams).
Fig. 13 shows a capsule holder.
Fig. 14 shows a capsule holder in the energy beam of a laser.
Fig. 15 shows a capsule holder with a capsule around which the laser beam
is guided.
Figure 16 shows a capsule holder in the energy beam of a hot gas nozzle.
Fig. 17 shows a capsule in which the seam between the cap 2 and body 3 is
formed perpendicular to the longitudinal axis of the capsule.
Fig. 18 shows a capsule consisting of two caps 2 and a body 3.
An embodiment with a spherical capsule is not shown. Figure 1 shows the
simplest embodiment of the capsule 1 according to the invention, in cross-
section. The capsule 1 consists of the cap 2 and the body 3 which are fitted
telescopically one inside the other. The cap 2 and body 3 are of the same
configuration and each have a convex underside 4. Figure 2a shows a
cross-section through an embodiment in which a bead 5 is formed on the
body 3 of the capsule 1; this bead tapers towards the closed end of the body.
With its side oriented towards the open end of the body, the bead 5 stands
01/1231/FF
CA 02455324 2004-O1-27
29
virtually perpendicularly on the body. The edge thus formed limits the part of
the body over which the cap 2 can be telescopically pushed. Another
embodiment is shown in Figure 2b. The cross-section shows that this
embodiment differs from the one shown in Figure 2a in that the wall thickness
of the cap 2 or body 3 is not the same over the entire area but varies over
individual sections. In addition, the convex undersides 4 of the cap or body
each have a concave indentation at the apex.
Figure 3 shows an embodiment in which the bead 5 rests more or less at
right angles to the body, both towards the top of the body and towards its
underside.
The embodiment in Figure 4 is a further development of the embodiment in
Figure 2a in which an annular recess 6 or 7 is formed in the cap 2 or body 3
to improve the closure of the capsule 1.
Figure 5 shows a front view of the embodiment shown in cross-section in
Figure 4.
Figure 6 shows another variant of the invention with dot-shaped depressions
8 and 9, in front view.
Figure 7 shows an alternative form of the capsule 1 in which elevations 10
are formed on the body 3 near the open end and holes 11 are formed in the
cap 2 near the open end so that the elevations 10 is engaged in the holes 11
when the capsule is closed.
Figure 8 shows an embodiment of the capsule 1 viewed from outside,
wherein the ribs 12 are formed on the body 3.
Figure 9 shows the body 3 of the embodiment shown in cross-section in
Figure 7. The cross-section shows that the three ribs 12 are not rotationally
symmetrical about the central axis of the body. Figure 10a shows a capsule
01/12311FF
CA 02455324 2004-O1-27
1 of rectangular cross-section while Figure 10b shows one with a triangular
cross-section and Figure 10c shows one with an octagonal cross-section.
Figs. 11 a - g show various embodiments of capsules 1 with non-centrally
arranged closures between the cap and the body to form the weld seam 13.
Fig. 11 a shows a closure in which an inwardly offset edge 5 is formed on the
outside of the body and an outwardly directed edge 5 is formed on the inside
of the cap. The connection is reinforced by a detent, preferably two detents.
Fig. 11 b shows an embodiment in which only the body has an inwardly offset
edge 5. The cap and body adjoin one another smoothly. Fig. 11 c shows an
embodiment in which an inwardly offset edge 5 is formed only on the cap.
The body does not have this feature. The cap and body adjoin one another
smoothly. Fig. 11d shows an analogous embodiment in which, in the
overlapping area of the cap and body, one or more bumps and one or more
depressions corresponding to them is or are formed on the counterpart in
order to achieve a better temporary closure before welding. Figure 11e
shows an embodiment in which the closure is such that the body does not
have any closure features, the cap has a U-shaped return 21 at its open end
which can be fitted over the open edge of the wall of the body. Figs. 11 f and
g show embodiments in which the thickness of the walls thicken the open
ends 22 of the body and cap in order to produce a wider contact zone in the
closure region between the two capsule halves.
Fig. 12 a - i show capsules 1 with various forms of weld seam 13: a), c) and
d) being straight, e) spot welds, f) zigzag shaped, g) and h) parallel,
diagonally extending, non-continuous weld seams, b), i) spiral shaped.
Fig. 13 shows a capsule holder 14 consisting of the holder 15 for the capsule
body 3 and a holder 16 for the capsule cap 2.
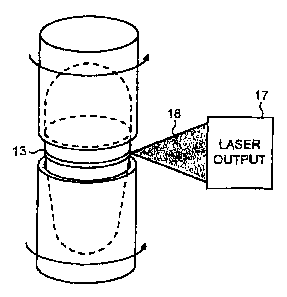
Fig. 14 shows a capsule holder 14 in the energy beam 18 of a laser 17.
01112311FF
CA 02455324 2004-O1-27
31
Fig. 15 shows a capsule holder 14 with a capsule 1 around which the laser
beam 18 is guided by means of two mirrors 19.
Figure 16 shows a capsule holder 14 in the energy beam of a hot gas nozzle
20. The nozzle may also be wider so that a plurality of capsules in a row can
be moved past the slot-shaped nozzle at the same time.
Fig. 17 shows a capsule in which the seam between the cap 2 and body 3 is
formed perpendicularly to the longitudinal axis of the capsule.
Fig. 18 shows a capsule consisting of two caps 2 and a body 3. In this case
a body is a tube open on two sides, each opening being closed off by a cap
2.
In all the embodiments, the features described for closing the capsule with
regard to the cap and body may also be arranged reciprocally, i.e. the
closure features located on the cap may be provided on the body and vice
versa.
Examples
Typical operating data for hot air blowers:
Steinel hot air blower 1800 W, electronically regulated. Special nozzle with
nozzle opening 1 mm x 7 mm, hot air temperature about 250° C at the
nozzle
outlet. Rotation of the capsule by stepping motor slower than 3 revolutions
per second at a distance of 10 mm.
Operating data for lasers:
The operating data are determined by the particular laser used. A rate of 0.75
seconds per capsule is achieved with a light output of about 30 Watts.
The average retention time at a welding station is approximately 22
milliseconds in the case of laser welding with a focal spot 0.1 mm in
01112311FF
CA 02455324 2004-O1-27
32
diameter, if the capsule performs 2 revolutions within 8 seconds,
corresponding to a circumferential speed of 4.6 mm per second. A capsule
15.9 mm long with a body 5.57 mm in diameter and a cap 5.83 mm in
diameter was used, the wall thickness of the capsule body being 0.25 mm
and that of the cap being 0.35 mm.
In the case of a 1.5 Watt argon ion laser a green capsule is used.
In hot gas welding, an average retention time of about 0.26 seconds is
obtained for the same capsule if a jet of hot air about 1 mm high and 7 mm
wide is used and the capsule is rotated 12 times in the jet within 8 seconds.
The speed at the capsule circumference is then about 27 mm per second.
Examples of capsules:
Length of capsule bodies: 22.210.46 mm; 20.22 ~0.46 mm; 20.9810.46 mm;
18.4 ~0.46 mm; 16.61 10.46 mm; 15.2710.46 mm; 13.59 ~0.46 mm; 12.19
10.46 mm; 9.3 10.46 mm.
Length of capsule cap: 12.95 10.46 mm; 11.7410.46 mm; 11.99 ~0.46 mm;
10.72 10.46 mm; 9.78 10.46 mm; 8.94 ~0.46 mm; 8.0810.46 mm; 7.21 ~0.46
mm; 6.2 ~0.46 mm.
External diameter of capsule bodies: 9.55 mm; 8.18 mm; 7.36 mm; 7.34 mm;
6.63 mm; 6.07 mm; 5.57 mm; 5.05 mm; 4.68 mm.
External diameter of capsule caps: 9.91 mm; 8.53 mm; 7.66 mm; 7.64 mm;
6.91 mm; 6.35 mm; 5.83 mm; 5.32 mm; 4.91 mm.
Total length of sealed capsule: 26.1 t0.3 mm; 23.3 t0.3 mm; 24.2 t0.3 mm;
21.7 t0.3 mm; 19.4 ~0.3 mm; 18.0 t0.3 mm; 15.9 t0.3 mm; 14.3 t0.3 mm;
11.1 t0.3 mm.
01112311FF
CA 02455324 2004-O1-27
33
Capsule volumes: 1.37 ml; 0.95 ml; 0.78 ml; 0.50 ml; 0.37 ml; 0.30 ml; 0.21
ml; 0.13 ml.
Weight of capsules: 163 mg; 118 mg; 110 mg; 96 mg; 76 mg; 61 mg; 48 mg;
38 mg; 28 mg.