Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
INFLATION DEVICE AND METHODS OF USE
10
FIELD OF THE INVENTION
The present invention relates to inflation devices, more particularly the
present
invention relates to inflation devices utilized to inflate an inflatable
medical device, for
example, the inflation device may be utilized to inflate a balloon disposed
upon a catheter,
guidewire, cannula, or similar medical devices.
DESCRIPTION OF THE PRIOR ART
Medical devices having inflatable balloons contained thereon are commonly
utilized
for many procedures. For example, a catheter having an inflatable balloon
disposed
2o thereupon may be utilized to occlude a vessel, expand a vessel, or deploy a
medical device
such as a stmt. During these procedures, typically a guidewire is advanced to
an area where
it is desired to deploy an inflatable balloon. A catheter or similar device
having a balloon
mounted thereupon is then advanced over the guidewire until the balloon is
positioned as
desired. The balloon is then inflated by filling the chamber of the balloon
with an inflation
fluid, typically saline or contrast solution. Typically, the balloon is
inflated to a known
1
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
pressure, wherein an undetermined volume of fluid is used to obtain the
desired
diameter and pressure.
These balloons are typically constructed of a compliant material such as C-
Flex,
urethane or polyvinyl chloride, or similar materials where the durromoeter and
expansion and
contraction forces may be controlled. The outer surfaces of the balloon must
be smooth and
contain no rough edges or areas, which may abrade the vessel wall or cause
trauma to the
vessel. Additionally, it is desirable to provide a balloon, which has a low
profile when no
inflation fluid has been introduced into the chamber of the balloon. The
benefit of having a
to low profile balloon is to provide better tractability or maneuverability of
the medical device
to which the balloon is affixed. A potential side effect of have a low profile
balloon is that
due to the materials durrometer, a greater amount of fluid pressure is
necessary to inflate the
balloon. Typically balloons are inflated using a syringe filled within
inflation fluid wherein
the syringe is in fluid communication with the chamber of the balloon.
While a syringe coupled to the chamber of the balloon is an effective method
of
inflating the balloon there are some dangers associated with this method.
Particularly, it is
difficult to control the diameter of the inflated balloon through the use of a
syringe because a
small change in volume and pressure within the syringe may translate to a
larger force within
2o the chamber of the balloon. For example, if it is desirable to inflate the
balloon to Smm, the
user must inject a given amount of fluid into the chamber of the balloon and
retain that
amount by closing a valve or holding the syringe plunger in a fixed position.
Though, in the
process of closing the valve, the user may apply a force to the syringe
therein causing the
balloon to inflate to a diameter greater than what is desired, or
alternatively to a diameter
smaller than desired wherein the vessel is not occluded properly. Still
further, even though
2
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
the manufacturer of the medical device having the balloon disposed thereupon
may supply a
syringe the supplied syringe may not be utilized to inflate the balloon.
Therefore, it is
possible that a syringe having a different diameter barrel or markings on the
barrel which are
slightly different than those on the supplied syringe which may lead to
over/under inflation
and/or damage to the vessel.
Further still, with recent advancements it has become possible to form a
guidewire
having an inflatable balloon and a valve that may be selectively opened and
closed for
inflation/deflation of the balloon. While this device eliminates the need for
a separate
to occlusion catheter it presents other problems in that a syringe can no
longer be utilized to
inflate/deflate the balloon without the use of an adapter. Though, adapters
are presently
available they have many shortcomings, for example they require multiple steps
in order to
prepare and use them which introduces many points for errors to be made in
preparing the
system. Additionally, these adapters further require the user to utilize a
separate syringe that
15 must be attached to the adapter to perform inflation/deflation of the
balloon.
Therefore, there is a need for an apparatus that will allow a user to
accurately
inflate/deflate a balloon. Furthermore, there is a need for an apparatus that
will allow the
balloon to be accurately inflated/deflated repeatedly.
There is also a need for an inflation device that allows the user to inflate
the balloon
in small increments accurately, such that the overall diameter of the inflated
balloon may be
carefully controlled.
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
There is also a need for an inflation device that is easy to operate and does
not require
multiple pieces to be assembled during a surgical procedure in order to
utilize the inflation
device.
Still further, there is a need for an inflation device which may be utilized
to open a
valve assembly of a medical device, accurately inflate a balloon to a desired
diameter, and
then be removed from the medical device so that the medical device may be
utilized for other
procedures, such as being utilized to occlude a vessel with the inflated
balloon as well as
serve the function of a guidewire.
t0
SUMMARY OF THE PRESENT INVENTION
In accordance with the present invention there is provided a device for
inflating
balloons disposed upon medical devices, wherein the balloons require accurate
volume
inflation. The device including a main body, a locking cover, an inflation
knob coupled to a
15 fluid chamber, a first seal knob disposed within the main body, wherein the
seal knob is
operatively coupled to the seal lever, and a second seal knob disposed within
a shuttle
assembly coupled to the main body, wherein the second seal knob is operatively
coupled to
the seal lever. The device further includes a shuttle assembly coupled to a
locking knob,
wherein the shuttle assembly opens and closes a valve assembly disposed upon a
medical
2o device inserted into a valve chamber of the main body.
In accordance with yet another embodiment in accordance with the present
invention,
there is provided an inflation device, wherein the inflation device is
configured to actuate a
valve assembly of an inflatable medical device. The inflation device includes
a main body
z5 having a fluid inlet, a vacuum outlet and a fluid reservoir. The inflation
device further
4
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
includes an inflation chamber, wherein the inflation chamber is configured to
receive an
inflatable medical device therein, wherein the inflation chamber includes a
device configured
for actuating a valve assembly disposed on the inflatable medical device.
In accordance with the present invention there is provided a method of
inflating an
inflatable medical device, the method includes the steps of (a) disposing a
medical device
having an inflation port within an inflation chamber of an inflation device,
(b) creating a
vacuum within the inflation chamber and within an inflation lumen of the
inflatable medical
device, wherein the inflation chamber and inflation lumen are in fluid
communication with an
l0 inflatable balloon disposed on the inflatable medical device; (c) providing
an inflation fluid,
wherein the inflation fluid is in fluid communication with the inflation
lumen; and (d)
displacing a predetermined volume of inflation fluid to inflate the inflatable
balloon to a
known diameter.
In accordance with the present invention there is provided yet another method
for
inflating a balloon disposed upon a medical device, the medical device
including a low
profile valve and a balloon disposed upon a distal end portion. The method
including the
steps of, providing an inflation device, the inflation device comprising a
main body, an
inflation chamber, a device for opening an closing the valve chamber, and a
device for
opening the valve assembly of the medical device. Opening the inflation
chamber for
2o insertion of a proximal end portion containing a low profile valve. Closing
the inflation
chamber therein creating a fluid tight chamber about the valve assembly of the
medical
device and opening the valve of the medical device. Creating a vacuum within
the inflation
device and medical device. Providing a source of inflation fluid, wherein the
vacuum draws
the inflation fluid into the inflation device, valve assembly, inflation
chamber and a fluid
reservoir. Providing an inflation knob coupled to the fluid reservoir, wherein
a rotational
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
force applied to the inflation knob causes fluid to enter the inflation
chamber and inflate the
balloon, closing the valve assembly of the medical device and releasing the
proximal end
portion of the medical device from the valve chamber.
DETAILED DESCRIPTION OF THE DRAWINGS
In the following detailed portion of the present description, the invention
will be
explained in greater detail with reference to the drawings, wherein:
Figure 1. is an isometric plan view of the inflation device according to the
present invention illustrating the seal lever in a closed position;
to Figure 2. is an isometric plan view of the inflation device according to
the
present invention illustrating the seal lever in an open position, wherein the
inflation device is
capable of receiving a valve assembly of an inflatable medical device;
Figure 3. is a side view of the inflation device according to the present
mvenhon;
t 5 Figure 4. is a top view of the inflation device according to the present
invention;
Figure 5. is side view of the main body of the inflation device according to
the
present invention prior to assembly;
Figure 6. is a top view of the main body illustrating the inflation knob,
selector
20 knob, and first seal knob as assembled in the main body;
Figure 7 Is a cross-sectional side view of the inflation device according to
the
present invention taken about line A-A of Figure l;
Figure 8 is a partial cross-sectional side view of the projection of the main
body illustrating the first seal knob and seal disposed therein, taken about
line B-B of Figure
25 1;
6
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Figure 9 is a side view of the shuttle assembly according the present
invention;
Figure 10. is a top view of the shuttle assembly according to the present
invention;
Figure 11. is a cross-sectional side view of the shuttle assembly according to
the
present invention, wherein there is shown the shuttle body, shuttle, second
seal knob, and
seal;
Figure 12. is a side view of the shuttle according to the present invention,
wherein a second seal knob is disposed within the proximal end portion of the
shuttle;
Figure 13. is a side view of the locking knob and locking knob rod in
accordance
to with the present invention;
Figure 14. is a side view of the inflation knob of the inflation device
according
to the present invention;
Figure 15. is a bottom view of the seal lever of the inflation device
according to
the present invention;
Figure 16. is a side view of the seal lever of the inflation device according
to the
present invention;
Figure 17A. is an isometric top view of the inflation device according to the
present invention, wherein a medical device having a valve assembly and an
inflatable
balloon is being inserted within the inflation chamber;
2o Figure 17B. is a top view of the inflation device according to the present
invention
illustrating a vacuum syringe and contrast syringe coupled to the inflation
device;
Figure 18. is an isometric top view of the inflation device according to the
present invention, wherein the seal lever and locking knob have been closed
therein opening
the valve of the medical device for inflation of the balloon;
7
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Figure 19. is an isometric view illustrating an exemplary alternative
embodiment
of an inflation device in accordance with the present invention;
Figure 20. is a plan side view of the alternative embodiment of the inflation
device in accordance with the present invention;
Figure 21. is a cross-sectional plan view of the main body if the inflation
device
in accordance with the alternative embodiment of the present invention;
Figure 22. is a cross-sectional side view of the shuttle assembly in
accordance
with an alternative embodiment of the present invention;
Figure 23. is an isometric view of the second seal knob in accordance with an
1 o alternative embodiment of the present invention;
Figure 24. is a cross-sectional view of the first seal knob, shuttle assembly
and
second seal knob as assembled in the alternative embodiment of the inflation
device;
Figure 25. is a top view illustrating the inflation device prepared for use
wherein a
proximal end of a medical device to be inflated is disposed within the
inflation chamber and
an vacuum syringe and contrast syringe are connected to the inflation device;
Figure 26. is a functional flow diagram illustrating a method of use of the
present
invention;
Figure 27. is a top view illustrating the inflation device prepared for use
wherein a
proximal end of a medical device to be inflated is disposed within the
inflation chamber;
2o Figure 28. is a functional flow chart illustrating the method of utilizing
the present
invention to inflate a balloon on a medical device;
Figure 29. is an exploded view of an alternative embodiment of the inflation
knob
incorporating a selector valve in accordance with the present invention;
8
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Figure 30. is a top view of the alternative embodiment of the inflation knob
in
accordance with the present invention, wherein the selector valve is opened to
the vacuum
port;
Figure 31. is a top view of the alternative embodiment of the inflation knob
in
accordance with the present invention, wherein the selector valve is opened to
the vacuum
port and the contrast port;
Figure 32. is a top view of the alternative embodiment of the inflation knob
in
accordance with the present invention, wherein the selector valve has been
moved to seal the
vacuum port and the contrast port;
1o Figure 33. is a cross-sectional view of yet another alternative embodiment
of the
inflation device in accordance with the present invention;
Figure 34. is an expanded perspective view of an alternative embodiment of the
inflation device in accordance with the present invention; and
Figure 35. is a cross-sectional side view of the alternative embodiment of the
inflation device illustrated in Figure 34.
DETAILED DESCRIPTION OF A PREFERRED EMBODIMENT
In accordance with the present invention, there is provided an inflation
device for use
with expandable medical devices such as those disclosed in co-pending U.S.
Patent
2o Application No. 09/822,823 filed on June 15, 2001 entitled "Balloon
Occlusion Device
Having a Proximal Valve", the entirety of which is herein incorporated by
reference. The
inflation device includes a main body, a locking knob, a locking knob rod, a
seal lever, first
and second seal knobs, a fluid chamber, an inflation control knob, a fluid
inlet, a vacuum
inlet, a selector knob, and a shuttle for opening and closing a valve assembly
of a medical
device.
9
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
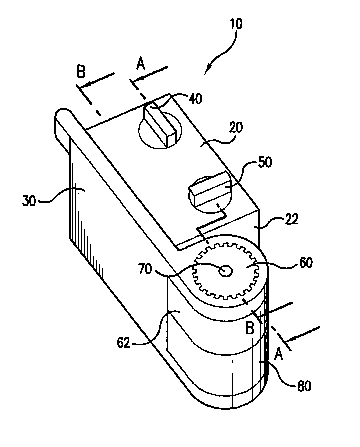
Referring now to Figure 1, there is shown a perspective view of the inflation
device
in accordance with the present invention. As shown in Figure 1, the seal lever
30 is shown
in a closed and locked position. Referring now to Figure 2, there is shown a
perspective view
5 of the inflation device 10 wherein the seal lever 30 is in an open unlocked
position, wherein
the inflation device 10 is capable of receiving a medical device (not shown)
within the
aperture 70 for inflation of an inflatable member disposed upon the medical
device. As
shown in Figures 1 and 2, the inflation device 10 further includes a main body
20 having a
fluid chamber 53, a vacuum port 47, a fluid inlet 45, a chamber 64 for
receiving a locking
to knob, and a projection 62 extending from the distal end portion 22. Each
element of the
inflation device 10 will be described in greater detail below with reference
to the
corresponding figures.
Referring now to Figure 3, there is shown a side view of the inflation device
10 in
accordance with the present invention, wherein Figure 3 illustrates the
inflation device 10 as
assembled for use. Referring now to Figure 4, there is shown a top view of the
inflation
device 10 in accordance with the present invention illustrating the various
parts as assembled
upon the main body 20 of the inflation device 10.
Refernng now to Figure 5, there is shown a side view of the main body 20 in
accordance with the present invention. The main body 20 includes a proximal
end portion
21, and a distal end portion 22. Wherein as shown in Figure 5, the selector
knob 40, inflation
knob 50, and the first locking knob 60 have been disposed within the main body
20. The
main body 20, further includes a plurality of conduits 57, as shown in Figures
7 and 8,
disposed therethrough, wherein the conduits 57 are in fluid communication with
the fluid
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
inlet 45, vacuum inlet 47, fluid chamber 53, and the distal end 65 of the
extension 67
protruding from the projection 62. The function of the conduits 57 will be
described in
greater detail below with reference to the use of the inflation device 10.
Refernng now to the projection 62 extending from the distal end portion 22 of
the
main body 20, as shown in Figures 5 and 6. The projection includes an aperture
61 (not
shown) for receiving a first seal knob 60, wherein the first seal knob 60 may
be threadably
disposed within the chamber 61. The first seal knob 60 engages a seal 75
disposed within a
chamber 61 of the projection 62, wherein, as the first seal knob 60 is
advanced into the
t0 chamber 61 the seal 75 is compressed. The projection 62 further includes an
extension
member 67, wherein the extension member 67 includes a seal 66 disposed
radially thereabout
as shown.
Referring now to Figure 7, there is shown a cross-sectional side view of the
main
15 body ZO in accordance with the present invention. As shown in Figures 7 and
8, the fluid
chamber 53 is in fluid communication with end of the extension member 67, and
the vacuum
port 47 (not shown) through the conduits 57 disposed within the main body 20.
Additionally,
the fluid inlet port 45 is in fluid communication with the end of the
extension member 67,
therein coupling the vacuum port 47 to the fluid inlet port 45 and to the
fluid chamber 53.
In an alternative embodiment, the fluid inlet port may not be necessary; the
inflation
device may be pre-filled with contrast solution where all air within the fluid
reservoir and
associated conduits has been removed. In this embodiment, the vacuum port may
also be
eliminated because it would not be necessary to draw a vacuum in order to
eliminate air
within the fluid chamber or conduits because this would have been accomplished
during the
11
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
manufacture of the device. In yet another embodiment, it is contemplated that
the fluid inlet
may be replaced by an assembly that is designed to accept a pre-filled fluid
cartridge, wherein
the assembly includes a means for piercing a seal on the pre-filled cartridge
therein emptying
the contents of the cartridge into the inflation device. Additionally, the use
of the terms
"fluid" or "contrast" shall be understood to define any fluids that my be
utilized to inflate the
balloon disposed on the medical device. For example, fluids utilized to
inflate balloons
during surgical procedures include carbon dioxide, saline and similar fluids.
The main body 20 may be constructed of biocompatible materials such as
titanium,
to stainless steel or plastics. In a preferred embodiment the main body is
constructed of a
biocompatible plastic such as polycarbonate. In a preferred embodiment the
main body is
manufactured as a unitary body as shown in Figures 1, 2, and 3. It is further
contemplated
that the main body may be constructed of a plurality of pieces that may be
assembled
utilizing a biocompatible adhesive, sonic welding, or similar procedures.
Still further, in a
15 preferred embodiment the main body 20 may be constructed wherein the main
body is clear
or opaque, therein allowing an operator to visually view the functionality of
the inflation
device 10 as well as visually determine if any air bubbles remain in the
conduits 57 or fluid
chamber 53. Additionally, the user may visually determine if the medical
device is leaking
after inflation because the fluid level in the fluid chamber 53 may decline
without movement
20 of the inflation knob 50.
Refernng now to Figure 8, there is shown a cross-sectional side view of the
projection
62 of the main body 20 in accordance with the present invention. As shown in
Figure 8, the
projection 62 includes an aperture 70 and a chamber 61 disposed therein. The
chamber 61 is
25 adapted to receive a first seal knob 60 as described above, wherein the
first seal knob 60
12
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
contains an aperture 70 extending from the proximal end portion to the distal
end portion.
The chamber 61 is further adapted to receive a seal 75 as shown. The seal 75
further includes
an aperture 70 disposed therethrough, wherein the apertures of the seal, seal
knob and
projection are in axial alignment. The chamber 61 is further adapted to engage
the first seal
knob 60. For example, the first seal knob 60 and chamber 61 may be threaded
respectively,
wherein the first seal knob 60 may be advanced within the chamber 61 by
applying a radial
force to the proximal end portion of the first seal knob 60. It is
contemplated that other
methods may be utilized to engage the first seal knob within the chamber,
therefore the
example above should not be considered limiting in any manner and should be
considered
t0 exemplary. In the event that a threaded connection is utilized to advance
or withdraw the
first seal knob 60 within the chamber 61 of the projection 62, an appropriate
thread must be
utilized. That is, the thread pitch chosen must be sufficient to advance or
retract the first seal
knob 60 a sufficient amount during use as will be described in greater detail
below.
The first seal knob 60 may be constructed of a biocompatible material such as
those
listed above. In a preferred embodiment, the first seal knob 60 is constructed
of
biocompatible plastic such as delrin, polycarbonate, nylon or similar
biocompatible plastics,
which may be sterilizable. Additionally, the first seal knob 60 may be
manufactured using
known techniques such as injection molding or machining.
As shown in Figure 8, the seal 75 is disposed within the chamber 61 of the
projection
62, wherein the seal 75 is sized accordingly to contact the walls of the
chamber 61 when
disposed therein. The seal 75 may be constructed of biocompatible materials
such as
silicone, urethane, delrin, rubber, latex, pebax, kraton, alcryn, and other
similar materials that
are conventionally utilized to construct seals in medical devices. The seal 75
is constructed
13
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
having a diamond shaped cross-sectional profile. The diamond cross-sectional
profile of the
seal 75 is important, in that when the first seal knob 60 compresses the seal,
the aperture 70
disposed through the seal compresses and becomes smaller thus gripping
anything passed
through the aperture formed in the seal.
As shown in Figure 8 and described above, the projection 62 further includes
an
extension 67, wherein the aperture 70 is axially disposed through the
extension 67 as shown.
Additionally, the extension 67 further includes a gasket seal 66 disposed
radially thereabout
and adjacent a distal end portion. The projection 62 further includes a first
fluid line 57 and a
t0 second fluid line 57' disposed adjacent the aperture 70. The first and
second fluid lines are
respectively coupled to the fluid inlet, fluid chamber and vacuum inlet as
will be described in
greater detail below with reference to the methods of use of the inflation
device 10.
Referring now to Figures 9-11, there is shown the shuttle assembly 80 in
accordance
~ 5 with the present invention. The shuttle assembly 80 includes a shuttle
body 82, shuttle 87
and second seal knob 85. The shuttle 87 further includes a first chamber 88
having an
aperture 89 disposed therein, and a second chamber 88' wherein the second
chamber 88' is
adapted to receive a seal 75 as described above. The shuttle 87 is slidably
disposed within
the shuttle body 82. As shown in Figures 9-11, the shuttle 87 may include at
least one groove
20 83 in order to align the shuttle properly within the shuttle body 82. In
addition to the groove,
the shuttle 87 may have a geometric shape such that the shuttle may not rotate
within the
shuttle body 82 when the shuttle is slidably disposed therein. The shuttle
body may further
contain chambers 81, wherein the chambers 81 may be threaded to receive at
least one screw
or bolt that may be utilized to retain the shuttle assembly when the shuttle
assembly is
14
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
assembled with the main body 20. Alternatively, it is contemplated that other
methods may
be utilized to secure the shuttle assembly 80 to the main body 20.
As shown in Figure 10, the first chamber 88 disposed within the shuttle is
adapted to
receive the extension 67 of the projection 62. The gasket seal 66 radially
disposed about the
extension 67 forms a fluid tight seal between the extension 67 and the chamber
88, therein
forming a valve chamber 90. It shall be understood that the projection,
shuttle assembly and
their respective related components maybe referred to additionally herewith as
the inflation
chamber.
to
Referring now to Figure 12, there is shown a side view of the shuttle 87 in
accordance
with the present invention. As shown in Figure 12, the shuttle 87 further
includes a plurality
of gear teeth 86 disposed within the sidewall of the shuttle 87. Additionally,
as shown, the
distal end portion of the shuttle is adapted to receive a second seal knob 85.
The second
is locking knob 85 may be threadably engaged within the distal end portion of
the shuttle 87. In
a preferred embodiment the first and second seal knobs are threadably engaged
within the
corresponding structures. In addition to being threadably engaged within their
corresponding
structures, each seal knob and corresponding structure is threaded in opposite
directions.
That is in a preferred embodiment, the first seal knob and corresponding
structure includes a
2o right hand thread, which, when turned in a clockwise direction, the seal
knob is advanced into
the projection. In a preferred embodiment the second seal knob and shuttle
have left hand
threads, which, when turned in a counter clockwise direction the second seal
knob is
advanced into the shuttle. Thus, in a preferred embodiment, the seal knobs
must have
opposite direction advancing mechanisms. The purpose of the oppositely
threaded seal knobs
25 will be described in greater detail with reference to the methods of use of
the inflation device.
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Refernng now to Figure 11, there is shown a cross-sectional view of the
shuttle
assembly 80 according to the present invention. As shown in Figure 11 and
described above,
the first chamber 88 of the shuttle 87 is adapted to receive the extension 67
and form a fluid
tight seal therein. Additionally, the shuttle includes a second chamber 88',
wherein a seal 75
such as the one described above is disposed therein. The seal is compressed by
the
advancement of the second seal knob within the second chamber 88' of the
shuttle 87. The
compression of the seal 75 by the second seal knob therein causes the aperture
disposed
through the seal to compress and grip anything disposed through the aperture.
The shuttle
body 82 may further include an aperture (not shown) disposed adjacent the gear
teeth 86
therein exposing the gear teeth to a (seal) locking knob projecting from an
aperture 64
disposed within the main body 20 of the inflation device as will be described
in greater detail
below.
The shuttle body 82 may be constructed of biocompatible materials such as
those
described above. In a preferred embodiment the shuttle body and second seal
knob are
constructed of biocompatible plastics such as polycarbonate or delrin, or
polyvinyl chloride.
The shuttle is preferably constructed of a biocompatible material such as
titanium, stainless
steel, injection-molded nylon, or similar plastics having good mechanical
properties, which
2o are, enable to withstand the torque of the locking knob.
Referring now to Figure 13 there is shown the locking knob SS in accordance
with the
present invention. As shown in Figure 13 the locking knob includes an
elongated shaft 56
extending from a knob portion 54. The distal end portion 57 of the elongated
shaft 56 is
adapted to engage the gear teeth 86 disposed upon the outer surface of the
shuttle 87. The
16
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
knob portion 54 of the locking knob includes means for locking the seal lever
30 of the
inflation device 10 when the seal lever 30 is in a closed position.
The locking knob is rotatably disposed within the aperture 64 of the main body
20 of
the inflation device 10. The distal end portion 57 extends beyond the distal
end portion 21 of
the main body 20, wherein the knob portion 54 of the locking knob is received
within a relief
formed within the proximal end portion of the main body 20. Additionally, the
locking knob
55 may further include a locking device (not shown). Wherein the locking
device engages a
locking surface of the seal lever 30, wherein when the locking device engages
the locking
to surface the seal lever cannot be accidentally opened. This safety feature
is important in that it
prevents the accidental opening of the seal lever when the valve assembly of
the medical
device is open. For example, if the locking device was not present and the
seal lever was
accidentally opened during use when the valve assembly of the medical device
is open, the
fluid within the inflation device would be expelled through the aperture 70
instead of being
utilized to inflate the balloon. Therefore, the balloon may not be inflated
properly, or may
become deflated during use.
Referring now to Figure 14, there is shown the inflation knob 50 in accordance
with
the present invention. The inflation knob 50 includes a proximal end portion,
a distal end
2o portion and a plurality of threads 51 disposed therebetween. As shown in
Figures l, 2, and 7
the inflation knob 50 is disposed within the fluid chamber 53 of the main body
20. As shown
in Figure 7, the inflation chamber 53 further includes a first seal 54 and a
second seal 55
disposed adjacent to a plurality of threads. The seals engage the distal end
portion of the
inflation knob SO and provide a fluid tight seal between the fluid chamber 53
and the
atmosphere. Additionally, the distal end portion of the inflation knob may be
modified to
17
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
increase or decrease the amount of fluid, which is held within, or displace
from the fluid
chamber. For example, if it is desired to increase the volume of fluid in the
fluid chamber the
distal end portion of the inflation knob would be made shorter, the converse
is true if it were
desirable to decrease the volume of fluid in the fluid chamber. Still further,
by being able to
adjust the volume of the chamber by varying the distal end portion of the
inflation knob 50,
the inflation device may be custom configured to various inflatable medical
devices. Still
further, it is contemplated that the inflation knob may be disposed within the
fluid reservoir
using other connection means. For example, the inflation knob may be slidaby
disposed
within the main body and in communication with the fluid reservoir.
Referring now to Figures 15 and 16 there is shown the seal lever 30 in
accordance
with the present invention. The seal lever includes a proximal end portion 31
a distal end
portion 37. The distal end portion 37 further includes at least one projection
35 extending
therefrom, wherein the projection 35 is adapted to be gripable for opening and
closing the
seal lever 30. The seal lever 30 further includes a groove (not shown)
disposed adjacent the
distal end portion, wherein the groove is adapted to receive a locking device
disposed upon
the locking knob 55, the function of which was described above.
Disposed at the proximal end portion 31 of the seal lever 30 are at least two
apertures
32 and 34. The apertures 32 and 34 are adapted to receive the first and second
seal knobs 60
and 85 respectively. As shown in Figure 15, the apertures may be formed having
a pattern
such as a gear, wherein the outer diameter of the seal knobs are formed having
a
corresponding gear pattern such that when the seal lever 30 is assembled with
the elements
described above the form the inflation device 10, the radial motion of opening
the seal lever
is translated into linear motion of the seal knobs. It shall be understood
that the gear pattern
18
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
shown and described should be considered merely exemplary and should not be
considered
limiting in any manner. For example, other geometric shapes such as square,
octagonal,
pentagonal, etc. may be utilized.
The seal lever may be manufactured of biocompatible materials such as those
listed
above. In a preferred embodiment the locking cover is manufactured of a
biocompatible
plastic such as polycarbonate, polyvinyl chloride, delrin, or similar
plastics, which are
capable of sterilization. The seal lever may be manufactured using
conventional
manufacturing methods such as machining or injection molding.
to
Referring now to Figures 17 -18, there is illustrated the inflation device 10
in use in
accordance with the present invention. As previously described, the inflation
device includes
a main body, a seal lever, a plurality of seal knobs operatively coupled to
the seal lever, an
inflation control knob, a fluid inlet, a vacuum inlet.
The inflation device 10 prepared for use by assembling the various parts
described
above to form a functional unit. The inflation device is assembled by first
attaching the
shuttle assembly 80 to the main body 20 through the use of a fastening device
(not shown).
Examples of an appropriate fastening device may include screws, press-fit
clips, one-way
clips, adhesives, sonic welding, or similar methods or devices, which may be
used to join two
or more pieces together. Prior to assembling the shuttle assembly 80 with the
main body 20,
the shuttle 87 is disposed within the shuttle chamber formed within the
shuttle~body 82. Still
further, prior to assembling the shuttle assembly 80 onto the main body 20, a
seal 66 is
disposed about the extension 67 of the projection 62. After assembling the
shuttle assembly
19
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
with the main body 20 of the inflation device, a valve chamber 90 is formed
between the
extension 67 and the first chamber 88 of the shuttle 87.
The locking knob 55 may then be inserted into a chamber 64 formed in the main
body
20 of the inflation device 10. The gear teeth 57 disposed upon the distal end
portion of the
locking knob 55 couple with the gear teeth 86 on the shuttle 87. Wherein
rotational motion
of the locking knob will cause the shuttle to be linearly translated within
the shuttle body 82.
The proximal end portion 31 of the seal lever 30 is then disposed about the
first and
l0 second seal knobs, wherein the gear teeth within the apertures of the seal
lever align with the
gear teeth of the seal knobs.
Refernng now to Figure 17A there is shown the inflation device 10 in
accordance
with the present invention being prepared for use. As shown in Figure 17A,
a.proximal end P
15 of an inflatable medical device 99 is disposed within the inflation chamber
of the inflatable
device 10. Prior to insertion of the proximal end, the seal lever 30 is
translated into an
unlocked open position as shown. By placing the seal lever into the open
position as shown,
both the first and second seal knobs are advanced outwardly therein releasing
pressure on the
seals within the inflation chamber, therein allowing a medical device to be
disposed within
2o the inflation chamber.
Referring now to Figure 17B, there is shown the inflation device 10 in
accordance
with the present invention, wherein a syringe filled with a contrast solution
is prepared and
coupled to the fluid inlet 45. The fluid inlet may comprise a luer fitting or
other standard
25 fitting that may be coupled with a conventionally available syringe. A
second syringe is
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
coupled to the vacuum inlet 47, wherein the vacuum inlet may comprise a luer
fitting or
similar fitting which may be coupled to a conventional syringe. Prior to
drawing a vacuum,
the seal lever is closed and locked as shown in Figure 18, closing the seal
lever advances the
first and second seal knobs, therein compressing the seals about the diameter
of the medical
device therein forming a fluid tight chamber. In addition to forming a fluid
tight chamber,
the rotation of the locking knob 55 advanced the shuttle assembly, therein
opening a low
profile valve disposed on the proximal end of the inflatable medical device.
After closing and locking the seal lever as described above a vacuum is drawn
by
to pulling back on the plunger of the vacuum syringe, the selector knob 40 is
then turned to
open the contrast valve wherein contrast flows from the contrast syringe
through the plurality
of conduits 57, into the inflation chamber and fluid reservoir and then into
the vacuum
syringe. At this time the inflation knob is then turned to start position and
stops the flow of
contrast into the inflation device. The contrast valve is then closed wherein
the vacuum
15 syringe and the contrast syringe may then be detached from the inflation
device.
The inflatable medical device 99 may then be removed from the inflation
chamber by
twisting the locking knob 55 counter-clockwise, wherein the low profile valve
assembly of
the inflatable medical device is closed. The seal lever 30 may then be rotated
to release the
20 proximal end of the inflatable medical device from the inflation chamber.
At this time, the
inflation device is considered to be primed and ready for use.
After the inflatable medical device 99 is placed within the patient at a
desired location
using known conventional techniques, the proximal end P of the inflatable
device may then
25 be re-inserted into the inflation chamber, the seal lever rotated to engage
the seals and the
21
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
locking knob turned clockwise to open the low profile valve assembly disposed
on the
proximal end of the inflatable medical device. The inflation knob may then be
rotated to
inflate the balloon disposed on the inflatable medical device to a known
diameter. The
inflatable balloon disposed on the inflatable medical device is inflated to a
known diameter
through the use of a known volume of fluid. Unlike conventional inflatable
medical devices
that are inflated to a desired diameter utilizing an unknown volume of fluid,
the inflation
device 10 in accordance with the present invention is configured to provide
consistent
inflation diameter of the balloon through the use of a known volume of fluid.
1 o The inflatable medical device 99 shown and described above having a low
profile
valve disposed upon the proximal end portion, may be the device shown and
described in
U.S. Patent Application No. 09/822,823 filed one June 15, 2001 entitled
"Balloon Occlusion
Device Having a Proximal Valve" the entirety of which is incorporated by
reference, is
inserted into the valve chamber 90 of the proximal end portion and advanced
until the
15 proximal end of the medical device 100 contacts the second seal knob 85.
Refernng now to Figure 19 there is shown an exemplary embodiment of an
alternative inflation device in accordance with the present invention. As
shown in Figure 19
the inflation device 100 comprises a main body, a seal lever, a seal knob, an
inflation port, a
20 contrast inlet, a vacuum port, and an inflation knob.
In accordance with the exemplary alternative embodiment of the inflation
device 100
in accordance with the present invention, similar reference numbers are
utilized to denote
similar elements as described above with regard to the inflation device 10.
22
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
As shown Figure 19, there is shown a perspective view of the inflation device
100 in
accordance within an alternative embodiment according to the present
invention. As shown
in Figure 19, the seal lever 130 is shown in a closed and locked position.
Referring now to
Figure 20, there is shown a side view of one side of the inflation device 100.
As shown in
Figure 20, the inflation device includes a contrast inlet and a vacuum port,
each of which will
be described in detail below.
Referring now to Figure 21, there is shown a cross-sectional view of the
inflation
device 100 in accordance with the present invention. As shown in Figure 21,
the inflation
device 100 further includes a projection 162 and a shuttle assembly 180, each
of which define
an inflation chamber, wherein the inflation chamber is configured to receive
the proximal end
of an inflatable medical device. The inflation device further includes a fluid
chamber 153,
wherein the fluid chamber 153 is in fluid communication with the inflation
chamber, the
contrast inlet and the vacuum port through a plurality of conduits 157.
Refernng now to the
projection 162 as shown in Figure 21, the projection 162 further includes an
extension
member 167. The extension member 167 includes a seal 166 disposed adjacent to
a distal
end thereof, wherein the extension member and the seal is configured to
receive and retain
the shuttle assembly as shown in Figures 19 and 20. The extension member 167
further
includes a plurality of conduits 157 and 157' formed therein, wherein the
conduits are in fluid
2o communication with the fluid chamber 153. The projection 162 is further
configured to
threadably receive a first seal knob 160. The proximal end of the first seal
knob 160 is
configured to be engaged by a portion of the seal lever 130 as shown in Figure
19. The first
seal knob 160 further includes an aperture 170 disposed therethrough. The
aperture 170
forms a portion of the inflation chamber and is configured to receive a
proximal end of an
inflatable medical device. The projection 162 further includes a seal 175
disposed at the
23
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
proximal end of the first seal knob 160. The seal 175 includes an aperture
formed
therethrough, wherein the aperture is sized to accept the proximal end of an
inflatable
medical device therethrough, wherein the seal when compressed by the first
seal knob both
retains and fluidly seals about the medical device passed therethrough.
Still further, as shown in Figure 21, the inflation device 100 includes a
locking knob
155, wherein the locking knob has a proximal end and a distal end. A plurality
of gear teeth
144 being formed at the proximal end and a knob 154 being formed at the distal
end. The
locking knob 155 further includes retaining means 146, wherein the retaining
means may
comprise a raised portion formed along a portion of the shaft 156 of the
locking knob 1 ~5.
As shown in Figure 21, the retaining means is received within a corresponding
groove 101
formed in the housing of the inflation device 100. The retaining means 146 is
configured to
detachably retain the locking knob 155 within the main body 120 of the
inflation device 100,
while further allowing the disassembly of the locking knob from the inflation
device 100.
The retaining means 146 ensures that during use, the user cannot accidentally
pull back on
the locking knob therein disassembling the inflation device 100. Referring now
to the distal
end of the locking knob wherein the knob 154 is disposed thereon. As shown in
Figure 21,
the knob 154 further includes means for limiting the rotation of the locking
knob 155. The
limiting means includes a groove 143 formed in the knob portion 154 and a
projection 123
2o formed within the main housing 120. In use, the groove 143 interfaces with
the projection
123, thus limiting the rotational travel of the locking knob 155.
Refernng now to Figure 22 there is shown a cross-sectional view of the shuttle
assembly 180 in accordance with the present invention. As shown in Figure 22,
the shuttle
assembly comprises a shuttle body 182 and a shuttle 187. The shuttle body 182
further
24
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
includes a first chamber 188 and a second chamber 188'. The first chamber 188
is
configured to receive the extension 167 of the projection 162. The seal 166
disposed on the
end of the extension 167 is slidably received within the chamber 188 therein
forming a fluid
tight seal within the chamber. As shown in Figure 22, the second chamber 188'
is configured
to receive a second seal knob 191, a seal 198, and a seal retaining member
197. The first and
second chambers are coupled by an aperture 189, wherein the aperture is sized
and
configured to receive the proximal end of a medical device therethrough. In
addition, the
seal 198 and the seal-retaining member 197 each include an aperture formed
therethrough.
t 0 As shown in Figure 22, a first side of the seal 198 is formed having a
concave surface,
wherein the second side of the seal is formed having a substantially flat
surface, wherein the
flat surface of the seal is disposed against the seal-retaining member. The
seal 198 may be
formed of materials such as silicone, kraton, pebax, and other materials that
are suitable for
seals. The seal-retaining member 197 is formed of a rigid or substantially
rigid material such
t 5 as plastic, aluminum, stainless steel or other suitable materials.
The second seal knob 191 is configured to be threadably received within the
second
chamber 188'. The threads disposed on the second seal knob 191 are opposite
those formed
on the first seal knob 160. For example the first seal knob 160 may have right
hand threads,
2o thus the second knob would be formed having left hand threads, thus when
the seal lever 130
is moved between an opened position and a closed position, each seal knob is
advanced or
retraced from the inflation device 100.
As shown in Figure 22, the proximal end of the second seal knob 191 is
configured to
25 receive the seal lever 130 as shown in Figure 19. The distal end of the
second seal knob 191
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
includes a collet 195 formed therein. The collet 195 and the second seal knob
191 may be
integrally formed or alternatively the collet 195 and the second seal knob 191
may be formed
as separate pieces which are then joined utilizing know methods such as
friction fit, bonding,
welding, melting, sonic welding, or other similar processes.
Referring now to Figures 23, there is shown a perspective view of the second
seal
knob 190 in accordance with the present invention. As shown, the collet 195
includes a
plurality of grooves 197 formed therein and an aperture 196. As the second
locking member
190 is threadably advanced into the second chamber 188' of the shuttle 187,
the plurality of
t0 grooves 197 formed in the collet are compressed against the seal retaining
member 197; thus
closing and or reducing the diameter of the aperture 196 and compressing the
seal 198,
therein closing and/or reducing the diameter of the aperture disposed through
the seal. It
shall be understood that the projection, shuttle assembly and their respective
related
components maybe referred to additionally hereafter as the inflation chamber.
Referring now to Figures 24, there is shown a cross-sectional view of the
inflation
chamber of the inflatable medical device 100 in accordance with the present
invention. As
shown in Figure 24, a proximal end of an inflatable medical device 99 has been
disposed
within the inflation chamber. As shown, the proximal end of the medical device
99 passes
2o through the aperture 170 formed in the first seal knob 160, through seal
175, through seal 198
and seal retaining member 197, and into the aperture 196 formed in the second
seal knob 190.
As shown in Figure 24 the first and second seal knobs have been advanced by
rotating the
seal lever to a closed position, therein compressing the seals. The seal 175
forms a fluid tight
seal about the medical device and further retains the shaft of the medical
device within the
inflation chamber. The seal 198 is compressed by the second seal knob 190,
wherein the
26
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
second seal forms a fluid tight seal about the valve portion of the medical
device. As shown
in Figure 25, the distal end of the medical device is retained within the
collet 195 of the
second seal knob, thus, when the locking knob 155 is activated the shuttle
assembly is
translated, therein opening the low profile valve of the medical device as
shown. As shown
in Figure 24, the shuttle assembly is configured to translate with respect to
the inflation
chamber. By configuring the shuttle device to translate, a low profile valve
assembly
disposed on the proximal end of the inflatable medical device may be moved
from a closed
position to an open position, therein exposing the inflation lumen of the
inflatable medical
device. As shown in Figure 24, the collet of the second seal knob is
configured to grip and
1 o retain a portion of the valve assembly.
Referring now to Figure 25, there is shown the inflation device 100 prior to
use. As
shown in Figure 26, a syringe filled with a contrast solution is coupled to
the fluid inlet 145,
wherein a valve assembly 300 is fitted between the syringe and the inflation
device. The
valve assembly 300 allows a user to open and close the fluid path between the
contrast
syringe and the fluid inlet. A vacuum syringe is shown coupled to the vacuum
port 147, and
a medical device 99 is shown disposed within the inflation chamber of the
inflation device
100 in accordance with the present invention.
In use, the user prepares the inflation device according to the following
procedure.
The inflation device 100 is removed from the packaging material; if the seal
lever is disposed
in the closed position, the knob portion of the locking knob is rotated
counter clockwise
unlocking the seal lever 130. The seal lever is then rotated away from the
main body 120 of
the inflation device 100, therein uncompressing the seals disposed within the
inflation
chamber. Alternatively, the inflation device may be shipped wherein the
seal,lever is already
27
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
in the opened position. The proximal end of a medical device 99 is then placed
within the
inflation chamber. The seal lever 130 is then rotated to the position shown in
Figure 26,
therein compressing the seals about the diameter of the medical device and
compressing the
collet about the proximal end of the medical device.
The user then rotates the knob portion of the locking knob 155 clockwise. The
rotation of the locking knob engages a locking mechanism disposed on the knob
portion and
the seal lever 130. The gear teeth on the locking knob 155 engage a plurality
of gear teeth on
the shuttle body 182, therein the rotation of the gear teeth causes the
shuttle body 182 to be
l0 displaced within the shuttle assembly, therein opening the sealing member
of the valve
assembly disposed on the proximal end of the medical device.
The inflation knob 150 is turned to the "open" position and the valve assembly
300 is
turned to a closed position. The user then pulls back on and locks the plunger
of the vacuum
15 syringe therein developing a vacuum within the plurality of channels 157,
157', the fluid
reservoir 153, the inflation chamber and within the medical device. The user
then opens the
valve assembly 300 therein allowing contrast to flow from the contrast syringe
into the
plurality of channels, fluid reservoir, inflation chamber, medical device, and
out into the
vacuum syringe. During this process, the user will see air bubbles entering
the vacuum
20 syringe, as soon as the amount of air bubbles significantly changes or
become no longer
visible in the fluid entering the vacuum syringe the user then rotates the
inflation knob to the
"start" position and closes the contrast valve disposed on the contrast valve
disposed on the
contrast syringe. The vacuum syringe and the contrast syringe can then be
removed from the
inflation device.
28
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
The user may at this time inflate the balloon disposed on the inflatable
medical device
by rotating the inflation knob and visually inspecting the inflation of the
balloon. After
testing the inflation of the balloon, the user may then rotate the inflation
knob to the "deflate"
position or back to the start position, wherein the balloon disposed on the
inflation device
may then be deflated. After checking for proper inflation and deflation of the
balloon, the
inflation device is prepared and ready to be used. The medical device may then
be removed
from the inflation device, wherein the locking knob is rotated
counterclockwise, therein
causing the shuttle body to be translated therein closing the valve assembly
disposed on the
medical device. The rotation of the locking knob additionally releases the
locking
t0 mechanism therein allowing the seal lever 130 to rotate. The seal lever 130
may then be
rotated therein releasing pressure on the seals and collet; the medical device
can then be
removed from the inflation chamber of the inflation device.
The function of the inflation device may be better understood with reference
to the
functional flow diagram illustrated in Figure 26, wherein the functional flow
diagram
illustrates the steps describe above for preparing the inflation device for
use. Referring now
to Box 400, the locking knob is rotated counter clockwise and the seal lever
is rotated therein
opening the inflation chamber. Referring now to Box 410 the proximal end of an
inflatable
medical device is inserted within the inflation chamber. Referring now to Box
420, the seal
lever is rotated clockwise to a closed position, and the locking knob is
rotated clockwise to
lock the seal lever in place and open the low profile valve disposed on the
proximal end of
the inflatable medical device. Refernng now to Box 430, a contrast syringe and
a vacuum
syringe are removably connected to the respective ports on the inflation
device. Referring
now to Box 440, the plunger of the vacuum syringe is pulled back, therein
forming a vacuum
within the lumen of the inflatable medical device, inflation chamber, fluid
chamber and
29
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
respective conduits interconnecting each of the above, the plunger is affixed
to retain the
vacuum within the syringe. Refernng now to Box 450 the stopcock or valve
disposed
between the contrast syringe and the contrast inlet is rotated to allow
contrast to flow from
the contrast syringe through the inflation device and inflatable medical
device. Refernng
now to Box 460, the inflation knob is rotated to a "start" position, wherein
the contrast inlet
and the vacuum outlet are fluidly sealed. Referring now to Box 470 the
contrast syringe and
stopcock (valve) and the vacuum syringe are removed from the respective ports
disposed on
the inflation device. Referring now to Box 480, the inflation knob is rotated
from the "start"
position to "3mm" to inflate the balloon disposed on the distal end of the
inflatable medical
t0 device to check for leaks and to prepare the balloon for use. Referring now
to Box 490 the
inflation knob if rotated from the "3mm" mark back to "start" or "deflate",
wherein the
balloon is deflated. Referring now to Box 500, the locking knob is rotated
counter-clockwise
to close the valve assembly on the inflatable medical device and unlock the
seal lever, the
seal lever is then rotated counter-clockwise to release the pressure on the
seals in the inflation
chamber, wherein the proximal end of the inflatable medical device may then be
removed
from the inflation chamber. Referring now to Circle 510, the inflation device
is primed and
ready for use.
The inflatable medical device 99 can then be placed into the patient's
arterial system
to a desired location utilizing known placement methods such as flouoscopy.
When it is
desirable to inflate the balloon disposed on the medical device, the proximal
end of the
medical device is inserted into the inflation chamber; the seal lever is
rotated against the body
of the inflation device and locked by rotating the locking knob. The inflation
knob 150 can
then be rotated to inflate the balloon to a desired diameter as indicated in
Figure 28 by the
arrow labeled "I". The balloon can be inflated and deflated by rotating the
inflation knob
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
clockwise or counterclockwise as desired by the user, as shown in Figures 26
and 28, the
inflation device 100 may include markings on the top surface adjacent to the
inflation knob,
wherein the markings indicate known balloon diameters or known functions such
as "start",
"deflate", or "open". The inflation device 100 will remain primed and ready
for use, that is
the seal lever and locking knob may be rotated between an opened and closed
position
multiple times while inserting and removing the proximal end of the medical
device from the
inflation chamber, so long as the user does not rotate the inflation knob to
the "open" position
after priming the device according to the procedure above.
t0 Referring now to Figure 28, there is shown a functional flow diagram
illustrating the
method of using the inflation device 100 for inflating a balloon disposed on
an inflatable
medical device. Refernng now to Box 520, the proximal end of the inflatable
medical device
is disposed within the inflation chamber. Referring now to Box 530, the seal
lever is rotated
counter clockwise to engage the seals in inflation chamber, and the locking
knob is rotated
15 clockwise to lock the seal lever and open the valve assembly of the
inflatable medical device.
Referring now to Box 540, the inflation knob is then rotated from the "start"
or "deflate"
position to a marked balloon diameter location. Referring now to Diamond 550
it is then
determined if the inflatable medical device is to be removed or retained
within the patient. If
the device is not to be retained, then proceed to Box 555 wherein the
inflation knob is rotated
2o to the "deflate" position, and the locking knob and seal lever are rotated
counter-clockwise to
release the proximal end of the inflatable medical device. After rotating the
inflation knob to
deflate, proceed to Box 580, wherein the locking knob and seal lever are
opened to allow
removal of the proximal end of the medical device from the inflation chamber,
then proceed
to Diamond 590 to determine if the device is to be reinflated/reinserted.
Referring now to
25 Diamond 560 it is determined whether or not the proximal end of the
inflatable medical
31
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
device is to be removed from the inflation chamber of the inflation device. If
the proximal
end is to be removed then proceed to Box 556, wherein the locking knob is
rotated counter-
clockwise to close the valve assembly of the inflatable medical device and
unlock the seal
lever, then proceed to Box 580. At Box 580, the seal lever may then be rotated
counter-
s clockwise to release the proximal end of the inflatable medical device from
the inflation
chamber while retaining the balloon in an inflated state, when it is desired
to deflate or
increase the diameter of the balloon then proceed to Diamond 590 or Box 520.
If it is
determined in Diamonds 550 and 560, that the device is not to be removed from
the patient or
the proximal end is not to be removed from the inflation device, then proceed
to Box 570. At
t 0 Box 570, the size of the balloon may be adjusted by rotating the inflation
knob clockwise or
counterclockwise to increase or decrease the diameter of the balloon, after
the balloon has
been adjusted return to Diamond 550.
Referring now to Figures 29-32, there is shown an alternative embodiment of
the
15 inflation knob in accordance with the present invention. As shown in
Figures 27-30, the
inflation knob assembly 205 includes an inflation knob 250, a seal 260 and a
seal-retaining
member 252. The seal 260 further includes a recessed portion 262 and an
aperture 263
formed therethrough. The seal 260 may be constructed of materials such as
silicone, kraton
or other similar materials suitable for forming seals. Alternatively, the seal
260 may be
20 formed of a rigid or semi-rigid member including a seal disposed thereabout
as will be
apparent to one skilled in the art. As shown in Figure 27, the inflation knob
assembly 205 is
to be disposed within a fluid chamber formed in the main body of the inflation
device, a
portion of which is shown in Figure 27. The fluid chamber further includes a
fluid inlet 245
and a vacuum outlet 247 as shown.
32
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Referring now to Figure 29 there is shown the inflation knob assembly 205
assembled
within the fluid chamber of an inflation device. As shown, the recessed
portion is aligned
with the vacuum port 247, wherein a user may then pull back on the plunger of
a syringe
connected to the vacuum port thus developing a vacuum within the fluid
chamber, inflation
chamber, and the medical device.
Refernng now to Figure 30, after drawing a vacuum, the inflation knob is then
rotated
therein aligning the vacuum port 247 and the fluid inlet port 245, thus
allowing fluid to flow
from the fluid inlet port into the fluid chamber, inflation chamber and
medical device and out
through the vacuum port. After fluid has entered each of the above areas and
flows out of the
vacuum port, the user then rotates the inflation knob as shown in Figure 30,
therein sealing
off the fluid inlet port and the vacuum port. The inflation device may then be
utilized in
accordance with that described above.
It has been determined that in use, the inflation knob assembly 205 may
further
require a fluid flow restrictor disposed within the fluid inlet. The fluid
flow restrictor may
comprise a hydrophilic material that swells when exposed to fluid.
Alternatively, the fluid
flow restrictor may comprise an insert having a smaller diameter disposed
within the fluid
2o flow path. The fluid flow restrictor being utilized to slow the flow of
fluid through the fluid
chamber, inflation chamber, medical device and out the vacuum port. The
restrictor may be
necessary because the user may not have enough time to rotate the inflation
knob to the
position shown in Figure 32 to seal the fluid inlet and the vacuum port prior
to draining the
fluid from the syringe coupled to the fluid inlet and therein loosing the
vacuum, and thus
requiring re-priming of the system.
33
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Referring now to Figure 33 there is shown a cross-sectional view of yet
another
alternative embodiment of the inflation device in accordance with the present
invention. As
shown in Figure 33 the inflation device 300 includes a main body 320, a
locking cover 330,
an inflation knob (not shown), and a fluid inlet (not shown). Additionally, as
shown in
Figure 33, the inflation device 300 further includes means for generating a
vacuum 390. As
shown, the means for generating vacuum may comprise a plunger assembly 395.
The
plunger assembly 395 being coupled to the locking cover 330, wherein the
motion of closing
the locking cover advances a plunger 396 disposed within a chamber 397 formed
within the
t 0 main body of the inflation device 300. Although the plunger is illustrated
as being directly
coupled to the locking cover, it is contemplated that the plunger may be
activated utilizing
any known mechanical combination. For example, the plunger may be activated
through the
use of a series of gears, cables and pulleys, etc. Alternatively, the vacuum
may be formed
utilizing electromechanical means such as an electric motor coupled to a
vacuum pump or
similar means.
Referring now to Figure 34, there is shown yet another alternative embodiment
of the
inflation device in accordance with the present invention. As shown in Figure
34, the
inflation device 600 includes a main body 620, a seal lever 630, an inflation
knob 650, and a
removable inflation chamber 610. The removable inflation chamber 610 defined
by a
projection 662 and shuttle assembly 680, wherein the projection and shuttle
assemblies
further include seal knobs 660 and 685 (not shown). The inflation device 600
functions in
the same or similar manner to that as described above with regard to the
inflation devices 100
and 10 and therefore will not be described in greater detail.
34
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
Refernng now to Figure 35 there is shown the inflation device 600, wherein the
removable inflation chamber 610 is shown being detached from the main body 620
of the
inflation device. As shown, the removable inflation chamber 610 includes
projection 605
extending from the projection 662, wherein the projection 605 is configured to
be received
within an aperture formed in the main body 620. The projection 605 further
includes a seal
606 disposed radially thereabout, wherein the seal 606 is configured to form a
fluid tight seal
between the inflation chamber and the fluid reservoir disposed within the main
body 620.
The removable inflation chamber 610 further includes seal lever sections 633
wherein the
seal lever sections 633 a configured to receive the seal knobs 660 and 685
(not shown) in a
t0 similar manner to that shown in Figures 1 and 19. The seal lever sections
633 include
projections 636, wherein the projections are configured to be received within
grooves/apertures formed at the distal end 637 of the seal lever 630.
As shown in Figure 35, the main body further includes seal knob 655, wherein a
geared portion 567 of the seal knob extends beyond the distal end of the main
body. The
removable inflation chamber 610 includes an aperture configured to receive the
geared
portion of the seal know, wherein the geared portion is received within the
shuttle assembly
as described above. Although not shown, it is contemplated that the removable
inflation
chamber or the main body may include receiving means, wherein the receiving
means are
configured to detachably retain the two assemblies. For example, the receiving
means may
include assemblies such as a hook and a receiving slot, a protrusion and an
aperture wherein
the protrusion is frictionally received within the aperture.
As shown in Figures 34 and 35, the removable inflation chamber 610 is
configured to
allow the inflation device 600 to be manufactured in multiple components, some
of which
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
may be designed to be disposable (inflation chamber 610) and some of which may
be
designed to be reusable (main body 620, and related components). By forming
the inflation
chamber to be a removable replaceable assembly allows the inflation device to
be configured
to be utilized with other inflatable medical devices. For example, the
aperture through which
the proximal end of an inflatable medical device to be inflated may be made
having differing
diameters, wherein the proper diameter may be selected for use with the proper
diameter
inflatable medical device. Additionally, the removable section may reduce the
cost of the
device because only a portion of the device is disposed of after use. Still
further, the shuttle
assembly of the removable inflation chamber maybe configured to receive and
actuate
to different valve assemblies disposed upon the inflatable medical device to
be disposed within
the inflation chamber.
Still further, it is contemplated that the removable inflation chamber 610
maybe
configured to be coupled to an automatic inflation means (not shown). For
example, the
inflation chamber 610 maybe connected to a computer controlled console,
wherein the
console may be programmed or operated to inflate the balloon with a high
degree of accuracy
or inflate/deflate the balloon in combination with other surgical procedures.
Although the method of use of the inflation device has been described and
shown in
the above-referenced functional flow charts with regards to the inflation
device 100. It shall
be understood that the same or similar method may be utilized to use the
inflation device 10
in accordance with the present invention. Wherein the functional steps of the
inflation device
10 are similar to or the same as those described with regard to the inflation
device 100
wherein one skilled in the art could easily understand the difference between
the two devices.
Further still, while the inflation device has been shown and described in use
in preferred
36
CA 02455386 2003-12-15
WO 03/008031 PCT/US02/22231
embodiments this shall not be considered limiting in any manner, it shall be
understood that
one skilled in the art may undertake modifications to the above reference
device and methods
of use without departing from the scope and nature of the present invention.
Although the present invention has been described according to preferred
embodiments, this should not be considered limiting in any manner. For example
it is
contemplated that one skilled in the art may undertake modifications to the
invention
described herein without departing from the overall scope of the invention.
37