Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02459962 2004-03-08
1
METHOD FOR THE PRODUCTION OF BLISTER COPPER
This invention relates to a pyrometallurgical method of producing blister
copper
in a smelting reactor, such as a suspension smelting furnace, directly from
its
sulfidic concentrate and/or finely ground copper matte.
A well=known method of the prior art is to produce raw copper or blister
copper
from a sulfidic concentrate in several stages, whereby the concentrate is
smelted in a suspension reactor, such as a suspension smelting furnace, with
air or oxygen-enriched air, which results in copper-rich matte containing 50 -
75
weight-% copper and slag. This kind of method is described in e.g. US patent
2,506,557. Copper matte formed in a suspension smelting furnace is converted
in for example a Pierce-Smith type converter or a flash converter into blister
copper and refined further in an anode furnace.
The production of blister copper from sulfidic concentrate directly in one
process step in a suspension reactor is economically viable within certain
boundary conditions. The greatest problems involved in the direct production
of
blister include copper deportment to slag and the large ambunt of slag formed.
The large amount of slag requires further treatment process step for copper
recovery, which affects the economic feasibility of the process.
If the copper content of the concentrate is high enough, typically at least 37
weight-% copper, as for example at the Olympic Dam smelter in Australia,
where the copper content of the concentrate normally exceeds 40 weight-%, it
is possible economically to produce blister directly in one stage. When using
the previously described concentrate the slag amount is moderate, but in order
to produce blister, which has low sulphur content, less than 1 weight-%
sulphur,
the oxidation conditions must be selected so that the produced slag contains
15
-25 weight-% copper.
Concentrate with a lower copper content can also be suitable for direct
blister
CA 02459962 2004-03-08
2
production, if it has an advantageous composition. For example, at the Glogow
smelter in Poland, blister copper is produced from concentrate in one stage,
since the iron content is low and the resulting amount of slag is not
significantly
high. The production of copper in one stage with the normal concentrates
causes slagging of all the iron and other gangues. This type of method is
described in the US patent 4,030,915.
The FI patent 104838 describes a method to produce blister copper in a
suspension reactor directly from a sulfidic copper concentrate, whereby the
concentrate, flux and oxygen-enriched air are fed into the reactor. The cooled
and finely-ground copper matte is fed into the suspension reactor along with
the
concentrate in order to bind the heat released from the concentrate and to
decrease the amount of slag relatively, whereby the degree of oxygen
enrichment of the air fed to the reactor is at least 50 % oxygen.
This FI patent 104838 is however, limiting the process to areas, where the
oxygen enrichment is higher than 50 % oxygen and on the other hand the
concentrate quality is limited to above 31 % copper in a concentrate. The
patent is limited to use both iron silicate slag (essentially free from
calcia) and
calcium ferrite slag (essentially free from silicate) depending on the
concentrate
quality.
The PCT patent application WO 00/09772 describes a method of smelting
copper sulphide concentrate by oxygen-smelting the copper sulphide
concentrate, and removing most of the iron in the copper sulphide concentrate
into slag as well as removing part or most of the sulphur therein as sulphur
dioxide S02, thereby obtaining copper from sulphide concentrate as white
metal, nearly white metal matte or blister copper. According to the method the
oxygen-smelting is carried out to produce; slag in which a weight ratio of
Ca0/(Si02+Ca0) is 0.3 to 0.6 (Ca0/Si02 = 0.43 to 1.5) and a weight ratio of
Fe/(FeOx+Si02+Ca0) is 0.2 to 0.5, and a white metal, nearly white metal matte,
or blister copper, by adding Si02 material and Ca0 material to the copper
CA 02459962 2004-03-08
3
sulphide concentrate as flux. The object of that PCT patent application WO
00/09772 is to provide a copper sulphide concentrate smelting process for
producing white metal or blister copper with continuous oxidation of copper
sulphide concentrate or matte at the temperature of 1300 °C or less,
without
magnetite complications, which is applicable for the treatment of copper
sulphide concentrate or matte containing Si02, with less loss of copper to
slag,
capable of recovering copper content of slag by flotation, with high
removability
of arsenic, antimony and lead into slag, and with less erosion of
refractories.
The PCT patent application WO 00109772, however, limits the suitable slag
composition area to a window, where the Ca0/Si02 ratio in the slag is lower
than 1.5 and where the silica content in the slag is relatively high, minimum
being about 12.4 % Si02 in the pure Ca0-Si02-FeOX system (Ca0 = 18.6 %).
As the lime content in the slag is increasing the silica content of the slag
has to
be increased, too, and the total slag amount increases accordingly. For
example when the CaO/(Ca0+Si02) ratio is 0.6 and the ratio
Fe/(Ca0+Si02+FeOX) decreases from 0.5 to 0.2 the slag amount is more than
doubled. The highest Ca0/Si02 ratio is 1.5.
The object of the invention is to eliminate drawbacks of the prior art and to
achieve an improved method to produce blister copper or high grade matte in a
suspension reactor directly from a sulfidic concentrate and/or finely ground
copper matte wherein both silica (Si02) and lime (Ca0) bearing materials are
also fed in order to form a slag, which is fluid at the temperature range of
1250
- 1350 °C. The essential novel features of the invention are apparent
from the
appended claims.
According to the method a copper sulphide concentrate and/or copper matte
with oxygen-containing gas is fed into a smelting reactor, such as a
suspension
smelting furnace, into which both silica (Si02) and lime (Ca0) bearing
materials
are also fed in order to form a slag so that the CaO/Si02 ratio in the slag is
higher than 1.5, and which slag is fluid at the temperature range of 1250 -
1350
CA 02459962 2004-03-08
4
°C. Essential to the slag fluidity is that the slag also contains
copper in oxidized
form at least 6 weight percent.
The method of the invention is based on the fact that oxidized copper in slag
fluxes effectively both magnetite and dicalcium silicate, which limits the
applicability of the Ca0-Si02-FeOX slag in the copper smelting. In the
oxidation
conditions, where the sulphur content in copper is below 0.8 weight %, part of
the copper in the concentrate and/or in the finely ground matte is oxidized
causing the fluxing effect, which allows the widening of the operation window,
i.e. eliminates the limitations Ca0/(Ca0+Si02) - 0.3 to 0.6 and
Fe/(Ca0+Si02+FeOX) = 0.2 to 0.5 as set in the method. of the PCT patent
application WO 00/09772.
The method of the invention produces blister copper or high grade matte in a
smelting reactor from a mixture of copper concentrate and/or matte as well as
silicate containing material and lime containing material. The cooled and
finely
ground copper matte is fed into the smelting reactor in order to produce
blister
copper with lower than 1.0 weight-% sulphur and a relatively low amount of
slag, in which the activity of lime is high in order to increase the slagging
of
arsenic and antimony, but in which the activity of silica is high in order to
eliminate lead from the blister copper.
The finely ground matte fed into the blister furnace may be matte produced in
any kind of known smelting furnace having a copper content of 60 - 78 weight-
%. A single suspension smelting unit may be designed directly as a blister
smelter depending on the copper content and composition of the available
concentrates and on the amount of the finely ground matte.
The slag is treated further in a single-stage or preferably two-stage slag
cleaning. The two-stage cleaning method includes either two electric furnaces
or an electric furnace and a slag concentrating plant. If the slag is treated
in a
' ' CA 02459962 2004-03-08
slag concentrating plant, the slag concentrate can be fed back into the
smelting
reactor. Blister copper goes for normal refining in an anode furnace.
If the production of the high-grade matte is carried out in a flash smelting
5 furnace, the slag produced in the blister smelting stage can be preferably
granulated and fed into the primary smelting furnace for copper recovery. The
economy of this depends on the amount of the concentrate in the feed mixture
and on the slag amount produced. The slag from the primary smelting furnace
goes then to a normal single-stage slag cleaning or directly disposed (an
electric furnace, a slag cleaning furnace or slag flotation) depending on the
copper content of the slag.
The invention is further described in more detail with reference to following
examples and to the appended drawings, where
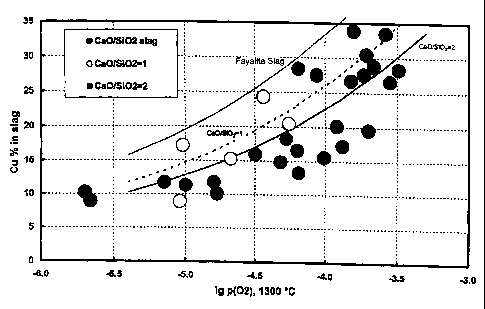
Fig. 1 shows copper content of different slag types as a function of
normalized
oxygen partial pressure (T=1300 °C) in blister copper according to the
example
1,
Fig. 2 shows the distribution coefficient of arsenic between slag and blister
copper in different slag types as a function of the normalized oxygen partial
pressure in blister copper according to the example 1,
Fig. 3 shows the distribution coefficient of lead between slag and blister
copper
in different slag types as a function of the normalized oxygen partial
pressure in
blister copper according to the example 1,
Fig. 4 shows the copper content of slag given in FeOx + Ca0 + Si02 = 100
diagram according to the example 1,
Fig. 5 shows the distribution coefficient of arsenic between slag and blister
shown in FeOx + Ca0 + Si02 = 100 diagram normalized to (% Cu) in slag = 20
according to the example 1,
Fig. 6 shows the distribution coefficient of lead between slag and blister
shown
in FeOx + Ca0 + Si02 = 100 diagram normalized to (% Cu) in slag = 20
according to the example 1, and
CA 02459962 2004-03-08
6
Fig. 7 shows the 200 cP viscosity temperature of the slag given in FeOX + Ca0
+ Si02 = 100 diagram normalized to (% Cu) in slag = 15 % according to the
example 1.
Example 1
Blister copper was produced in a suspension mini pilot smelting furnace in a
series of tests, where the copper containing raw materials were finely grained
copper matte (72.3 weight % Cu, 3.4 weight-% Fe, 20.3 weight-% S) and
copper concentrate (29.2 weight-% Cu, 33.7 weight% S, 21.0 weight % Fe):
The mixture of copper matte and concentrate (kg matte)/(kg matte + kg
concentrate)*100 was ranging between 50 - 100 %. The feed rate was 100 -
200 kg/h. The oxidation degree of blister copper produced was controlled by
the
oxygen coefficient (Nm3 O2/ton of feed), and the slag composition (Ca0/Si02,
Fe/Si02 in slag) was controlled by adding silica sand and lime to the feed.
After
each period, during which the process parameters were kept constant, the slag
and blister were tapped out of the settler of the mini pilot furnace and the
produced blister copper and slag was analysed. The average sulphur content of
the blister was 0.2 weight-% sulphur (0.01-0.89 % sulphur):
As an example results of one of the test periods is given as follows:
Matte feed rate 89.7 kg/h
Matte quality (3.4 % Fe, 18.2 % S, 0.26 % As, 0.2 % Pb) 72.3 % Cu
Concentrate feed rate 59.9 kg/h
Conc. quality (20.9 % Fe, 30.7 % S, 5.1 % Si02,
1.3 % As, 0.11 % Pb) 30.2 % Cu
Silica sand feed rate 0.5 kg/h
Lime feed rate 10.3 kg/h
Technical oxygen feed rate to concentrate burner 29.0 Nm3/h
Air feed rate to concentrate burner 31.0 Nm3/h
Oxygen enrichment 59.2
CA 02459962 2004-03-08
7
Oxygen coefficient 245.4 Nm302/t
Butane feed to reaction shaft and settler
in order to balance the heat losses 3.03 kg/h
Duration of test (feed on) 3h 10 min
Tapping temperature 1300 °C
Quality of blister produced:
Sulphur content 0.08 % S
Arsenic content 0.077 % As
Lead content 0.035 % Pb
Quality of slag produced:
Copper content 18.3 % Cu
Lime content 19.3 % Ca0
Silica content 7.6'
%
Si02
Iron content 28.2 % Fe
Arsenic content 0.68 % As
Lead content 0.28 % Pb
CaO/Si02 (w-%/w-%) ~
2.54
Fe/Si02 (w-%/w-%) 3.71
CaO/(Ca0+ Si02) (w-%/w-%) 0.72
Distribution coefficient of Arsenic between slag8.8
and blister
Distribution coefficient of Lead between slag 8.0
and blister
The applicability of the method is further described based on the results of
the
test runs and Figures 1 - 7.
Figure 1 shows copper content of different slag types as a function of
normalized oxygen partial pressure (T=1300 °C) in blister copper. It
can be
seen that when the Ca0/Si02 ratio (at a given Fe/Si02 ratio) of the slag
increases the copper content of the slag decreases. For comparison the copper
CA 02459962 2004-03-08
content of fayalite (iron silicate) slag is given in Figure 1, too. Compared
with
fayalite slag the copper content at the same oxygen potential is much lower.
Figure 2 shows the distribution coefficient of arsenic between slag and
blister
copper LpS~SIagICu) _ (% As in slag)/(% As in blister) in different slag types
as a
function of the normalized oxygen partial pressure in blister copper. It can
be
seen that when the CaOISi02 ratio (at a given FeISi02 ratio) of the slag
increases the distribution coefficient of arsenic, LAS~S~a~cu), increases. For
comparison the distribution coefficient of arsenic between iron silicate slag
and
blister copper is given in Figure 2, too. Compared with fayalite slag
distribution
coefficient of arsenic LAS~S~a~cu), the one of the CaOISi02 slag is higher at
the
same oxygen potential showing the much higher ability to remove arsenic from
blister.
Figure 3 shows the distribution coefficient of lead between slag and blister
copper Lpb~S~ag/Cu) _ (% Pb in slag)/(% Pb in blister) in different slag types
as a
function of normalized oxygen partial pressure in blister copper. It can be
seen
that when the CaO/Si02 ratio (at a given Fe/Si02 ratio) of the slag increases
the
distribution coefficient of lead, LPbtslag/Cu)~ slightly decreases. For
comparison the
distribution coefficient of lead between calcium ferrite slag and blister
copper is
given in Figure 3, too. Compared with calcium ferrite slag distribution
coefficient
of lead LPb~sia~c"», the one of the CaOISi02 slag is higher at the same oxygen
potential showing the higher ability to remove arsenic from blister.
Figure 4 shows the copper content of slag given in FeOx + Ca0 + Si02 = 100
diagram. The results are normalized to the temperature of 1300 °C and
to the
oxygen partial pressure of log pot = -4.5. It can be seen, that when operating
with FeOX + Ca0 + Si02 + copper oxide slag at a constant oxygen partial
pressure the copper content of slag is between 10-20 %, when the CaO/Si02
ratio is higher than 1.5 and the Ca0 content in Ca0+Si02+FeOX system is
higher than 20 %.
CA 02459962 2004-03-08
9
Figure 5 shows the distribution coefficient of arsenic between slag and
blister
shown in FeOX + Ca0 + Si02 = 100 diagram normalized to (% Cu) in slag = 20
%. The isodistribution lines based on the test results are also indicated.
When
the CaO/Si02 ratio is higher than 1.5, the distribution coefficient increases
when
the Ca0 content in the system increases.
Figure 6 shows the distribution coefficient of lead between slag and blister
shown in FeOX + Ca0 + Si02 = 100 diagram normalized to (% Cu) in slag = 20
%. When the CaO/Si02 ratio is higher than 1.5, the distribution coefficient of
lead increases, when the Ca0 content in the system is decreasing.
The viscosity of the stags in the pilot tests was low enough that they could
be
tapped out of the furnace through a normal tapping hole. In order to study the
viscosity behavior of the stags more detailed viscosity measurements were
carried out for some of the stags produced in the pilot tests. Figure 7 shows
the
200 cP viscosity temperature of the slag given in FeOx + Ca0 + Si02 = 100
diagram normalized to (% Cu) in slag = 15 %. The 200 cP viscosity temperature
increases when the Ca0 content of the slag is decreasing. Based on theoretical
calculations the solid magnetite formation is limiting the usability of this
kind of
slag as shown with the dashed line in Figure 7.
Now, the results in the Figures 1-7 indicate that the slag is fluid enough to
be
tapped out of the furnace, when the CaO/Si02 ratio of the slag is higher than
1.5 and that the Ca0 content of the slag calculated in FeOX + Ca0 + Si02 =
100 is higher than 20 % and when the copper content of the slag is higher than
8 % Cu in the slag.