Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02460875 2004-03-23
1
LITBIL14-BBASZD ZLTCTROC~'i.MICALLY ACTIVS MATERIALS AND
PREPARATION THEREOF
THIS IS A DIVISIONAL OF CANADIAN PATZNT APPLICATION SZRIAL
NO.: 2,394,318 FILZD ON DTCZb=R 22, 2000
Field of the invwntion
This invention relates to improved materials usable as
electrode active materials and to their preparation.
Background of the Invention
Lithium batteries are prepared from one or more lithium
electrochemical cells containing electrochemically active
(electroactive) materials. Such cells typically include an
anode (negative electrode), a cathode (positive electrode),
and an electrolyte interposed between spaced apart positive
and negative electrodes. Batteries with anodes of metallic
lithium and containing metal chalcogenide cathode active
material are known. The electrolyte typically comprises a
salt of lithium dissolved in one or more solvents, typically
nonaqueous (aprotic) organic solvents. Other electrolytes
are solid electrolytes typically called polymeric matrixes
that contain an ionic conductive medium, typically a
metallic powder or salt, in combination with a polymer that
itself may be ionically conductive which is electrically
insulating. By convention, during discharge of the cell,
the negative electrode of the cell is defined as the anode.
Cells having a metallic lithium anode and metal chalcogenide
cathode are charged in an initial condition. During
discharge, lithium ions from the metallic anode pass through
the liquid electrolyte to the electrochemical
CA 02460875 2004-03-23
2
active (electroactive) material of the cathode whereupon
they release electrical energy to an external circuit.
It has recently been suggested to replace the
lithium metal anode with an insertion anode, such as a
lithium metal chalcogenide or lithium metal oxide.
Carbon anodes, such as coke and graphite, are also
insertion materials. Such negative electrodes are used
with lithium- containing insertion cathodes, in order to
form an electroactive couple in a cell. Such cells, in
an initial condition, are not charged. In order to be
used to deliver electrochemical energy, such cells must
be charged in order to transfer lithium to the anode from
the lithium- containing cathode. During discharge the
lithium is transferred from the anode back to the
cathode. During a subsequent recharge, the lithium is
transferred back to the anode where it re-inserts. Upon
subsequent charge and discharge, the lithium ions (Li')
are transported between the electrodes. Such
rechargeable batteries, having no free metallic species
are called rechargeable ion batteries or rocking chair
batteries. See U.S. Patent Nos. 5,418,090; 4,464,447;
4,194,062; and 5,130,211.
Preferred positive electrode active materials
include LiCoO2, LiMn,Oa, and LiNiO-,. The cobalt compounds
are relatively expensive and the nickel compounds are
difficult to synthesize. A relatively economical
positive electrode is LiMn,Oq, for which methods of
synthesis are known. The lithium cobalt oxide (LiCoO2),
the lithium manganese oxide (LiMn:,O1,), and the lithium
nickel oxide (LiNiO-,) all have a common disadvantage in
that the charge capacity of a cell comprising such
cathodes suffers a significant loss in capacity. That
is, the initial capacity available (amp hours/gram) from
LiMn2O4, LiNi02, and LiCoO_ is less than the theoretical
CA 02460875 2004-03-23
3
capacity because significantly less than 1 atomic unit of
lithium engages in the electrochemical reaction. Such an
initial capacity value is significantly diminished during
the first cycle operation and such capacity further
diminishes on every successive cycle of operation. For
LiNiO2 and LiCoO, only about 0.5 atomic units of lithium
is reversibly cycled during cell operation. Many
attempts have been made to reduce capacity fading, for
example, as described in U.S. Patent No. 4,828,834 by
Nagaura et al. However, the presently known and commonly
used, alkali transition metal oxide compounds suffer from
relatively low capacity. Therefore, there remains the
difficulty of obtaining a lithium-containing electrode
material having acceptable capacity without disadvantage
of significant capacity loss when used in a cell.
CA 02460875 2004-03-23
4
Summary of the Invention
The invention provides novel lithium-mixed
metal materials which, upon electrochemical interaction,
release lithium ions, and are capable of reversibly
cycling lithium ions. The invention provides a
rechargeable lithium battery which comprises an electrode
formed from the novel lithium-mixed metal materials.
Methods for making the novel lithium-mixed metal
materials and methods for using such lithium-mixed metal
materials in electrochemical cells are also provided.
The lithium-mixed metal materials comprise lithium and at
least one other metal besides lithium. Preferred
materials are lithium-mixed metal phosphates which
contain lithium and two other metals besides lithium.
Accordingly, the invention provides a rechargeable
lithium battery which comprises an electrolyte; a first
electrode having a compatible active material; and a
second electrode comprising the novel materials. In one
aspect, the novel materials are lithium-mixed metal
phosphates which preferably used as a positive electrode
active material, reversibly cycle lithium ions with the
compatible negative electrode active material. Desirably,
the lithium-mixed metal phosphate is represented by the
nominal general formula Li~MIt,MI I,( PO4 ) a. Such compounds
include LilMIrMII,,POq and Li.,MIr,MIIr, (POq) ,; therefore, in an
initial condition 0:5 a<_ 1 or 0 5 a S 3, respectively.
During cycling, x quantity of lithium is released where
0<_ x<_ a. In the general formula, the sum of b plus c
is up to about 2. Specific examples are
Li1MI1_yMIIyPOq and Li,,MI~_yMIIy (PO4) õ wherein "y" is defined
hereinafter.
In one aspect, MI and MII are the same. In a
preferred aspect, MI and MII are different from one
CA 02460875 2004-03-23
another. At least one of MI and MII is an element
capable of an oxidation state higher than that initially
present in the lithium-mixed metal phosphate compound.
5 Correspondingly, at least one of MI and MII has more than
one oxidation state in the phosphate compound, and more
than one oxidation state above the ground state M . The
term oxidation state and valence state are used in the
art interchangeably.
In another aspect, both MI and MII may have
more than one oxidation state and both may be oxidizable
from the state initially present in the phosphate
compound. Desirably, MII is a metal or semi-metal having
a +2 oxidation state, and is selected from Groups 2, 12
and 14 of the Periodic Table. Desirably, MII is selected
from non-transition metals and semi-metals. In one
embodiment, MII has only one oxidation state and is
nonoxidizable from its oxidation state in the lithium-
mixed metal compound. In another embodiment, MII has
more than one oxidation state. Examples of semi-metals
having more than one oxidation state are selenium and
tellurium; other non-transition metals with more than one
oxidation state are tin and lead. Preferably, MII is
selected from Mg (magnesium), Ca (calcium), Zn (zinc), Sr
(strontium), Pb (lead), Cd (cadmium), Sn (tin), Ba
(barium), and Be (beryllium), and mixtures thereof. In
another preferred aspect, MII is a metal having a +2
oxidation state and having more than one oxidation state,
and is oxidizable from its oxidation state in lithium-
mixed metal compound.
Desirably, MI is selected from Fe (iron), Co
(cobalt), Ni (nickel), Mn (manganese), Cu (copper), V
(vanadium), Sn (tin), Ti (titanium), Cr (chromium), and
CA 02460875 2004-03-23
6
mixtures thereof. As can be seen, MI is preferably
selected from the first row of transition metals and
further includes tin, and MI preferably initially has a
+2 oxidation state.
In one aspect, the product LiMI,_yMIIyPO4 may
have an olivine structure and the product Li_,MIl_y(PO4)3 is
a rhombohedral or monoclinic Nasicon structure. In
another aspect, the term "nominal formula" refers to the
fact that the relative proportion of atomic species may
vary slightly on the order of 2 percent to 5 percent, or
more typically, 1 percent to 3 percent. In still another
aspect, any portion of P (phosphorous) may be substituted
by Si (silicon), S (sulfur), and/or As (arsenic); and any
portion of 0 (oxygen) may be substituted by halogen,
preferably F (fluorine). These aspects are also disclosed
in U.S. Patent Application Serial Numbers 09/105,748
issued as U.S. 6,136,472 on October 24, 2000, 09/274,371
issued as U.S. 6,153,333 on November 28, 2000 and in U.S.
Patent No. 5,871,866 issued February 16, 1999; each of
the listed applications and patents are co-owned by the
assignee of the present invention.
The metal phosphates are alternatively
represented by the nominal general formulas such as
Lil_.MI1_yMI IyP04 (0 <_ x<_ 1), and Li,_.{MI:_yMI Iy ( PO4 ) 3
signifying capability to release and reinsert lithium.
The term "general" refers to a family of compounds, with
M, x and y representing variations therein. The
expressions 2-y and 1-y each signify that the relative
amount of MI and MII may vary. In addition, as stated
above, MI may be a mixture of metals meeting the earlier
stated criteria for MI. In addition, MII may be a
mixture of metallic elements meeting the stated criteria
CA 02460875 2004-03-23
7
for MII. Preferably, where MII is a mixture, it is a
mixture of 2 metallic elements; and where MI is a
mixture, it is a mixture of 2 metals. Preferably, each
such metal and metallic element has a +2 oxidation state
in the initial phosphate compound.
According to a preferred embodiment, the invention
provides a compound represented by the nominal general
formula
L].aFel_yMyPOa
wherein 0 < y < 1, a is about 1, and M is at least one
selected from the group consisting of Mg, Ca, Zn, Sr, Pb,
Cd, Sn, Ba, Be, and mixtures thereof.
Another preferred embodiment of the invention is a
compound having the nominal general formula LiMII_yMIIyPO4
wherein MI is selected from the group consisting of Fe,
V, Sn, Ti, Cr, and mixtures thereof, and MII is selected
from the group consisting of Mg, Ca, Zn, Sr, Pb, Cd, Sn,
Ba, Be, and mixtures thereof, a is about 1, and wherein y
is greater than zero and less than one.
Still another preferred embodiment of the invention is a
compound having the nominal general formula L].aMIl_yMIIyP04
wherein MI is at least one transition metal selected from
Groups 4 to 11 inclusive of the Periodic Table and has a
+2 valence state, MII is at least one metallic element
which is selected from Groups 2, 12, and 14 of the
Periodic Table and has a +2 valence state, a is about 1,
and wherein 0 < y < 1, with the proviso that when MI is
Co, Ni, or Mn, MII is not Ge, Mg or Zn, and with the
proviso that when MI is Cu, MII is not Zn.
Another preferred embodiment of the invention is a
compound having the nominal general formula Li,MI1:yMIIyPO4
CA 02460875 2004-03-23
8
wherein MI is selected from the group consisting of Fe,
Co, Ni, Mn, Cu, V, Sn, Ti, Cr, and mixtures thereof, and
MII is selected from the group consisting of Ca, Sr, Pb,
Cd, Sn, Ba, Be, and mixtures thereof, a is about 1, and
wherein y is greater than zero and less than one.
Still another preferred embodiment of the invention is a
compound having the nominal general formula Li,MII_yMIIyPO4
wherein MI is selected from the group consisting of Fe,
Ni, Mn, V, Sn, Ti, Cr, and mixtures thereof, and MII is
selected from the group consisting of Ca, Sr, Pb, Cd,
Sn, Ba, Be, and mixtures thereof, a is about 1, and
wherein y is greater than zero and less than one.
Still another preferred embodiment of the invention is a
compound having the nominal general formula Li,MIl_YMIIYPO4
wherein MI is selected from the group consisting of Fe,
Cu, V, Sn, Ti, Cr, and mixtures thereof, and MII is
selected from the group consisting of Mg, Ca, Sr, Pb, Cd,
Sn, Ba, Be, and mixtures thereof, a is about 1, and
wherein y is greater than zero and less than one.
In another aspect of the present invention, there is
provided, in a preferred embodiment, an electrode
comprising an active material represented by the nominal
general formula LiaMI,_õMIIy,POa wherein MI is selected from
the group consisting of Fe, V, Sn, Ti, Cr, and mixtures
thereof, and MII is selected from the group consisting of
Mg, Ca, Zn, Sr, Pb, Cd, Sn, Ba, Be, and mixtures thereof,
a is about 1, and wherein y is greater than zero and less
than one.
Another preferred embodiment of the invention is an
electrode comprising an active material which is a
compound having the nominal general formula LiMIl_YMIIYPO4
wherein MI is selected from the group consisting of Fe,
CA 02460875 2004-03-23
9
Co, Ni, Mn, Cu, V, Sn, Ti, Cr, and mixtures thereof, and
MII is selected from the group consisting of Ca, Sr, Pb,
Cd, Sn, Ba, Be, and mixtures thereof, a is about 1, and
wherein y is greater than zero and less than one.
Still another preferred embodiment of the invention is an
electrode comprising an active material which is a
compound having the nominal general formula LiMII_yMIIyP04
wherein MI is selected from the group consisting of Fe,
Ni, Mn, V, Sn, Ti, Cr, and mixtures thereof, and MII is
selected from the group consisting of Ca, Sr, Pb, Cd,
Sn, Ba, Be, and mixtures thereof, a is about 1, and
wherein y is greater than zero and less than one.
Another preferred embodiment of the invention is an
electrode comprising an active material which is a
compound having the nominal general formula Li,MIl_yMIIYPO4
wherein MI is selected from the group consisting of Fe,
Cu, V, Sn, Ti, Cr, and mixtures thereof, and MII is
selected from the group consisting of Mg, Ca, Sr, Pb, Cd,
Sn, Ba, Be, and mixtures thereof, a is about 1, and
wherein y is greater than zero and less than one.
In another aspect of this invention there is provided a
lithium ion battery; a preferred embodiment of this
aspect is a lithium ion battery comprising:
a first electrode having an active material
represented by the nominal general formula
Li,Fe,_YMYPO4
wherein 0 < y < 1, a is about 1, and M is at least
one selected from the group consisting of Mg, Ca,
Zn, Sr, Pb, Cd, Sn, Ba, Be, and mixtures thereof;
a second electrode which is a counterelectrode to
said first electrode; and
an electrolyte between said electrodes.
CA 02460875 2004-03-23
Another preferred embodiment of this invention is a
lithium ion battery comprising:
a first electrode having an active material
represented by the nominal general formula Li,MIl_
5 yMIIYPOQ wherein MI is selected from the group
consisting of Fe, V, Sn, Ti, Cr, and mixtures
thereof, and MII is selected from the group
consisting of Mg, Ca, Zn, Sr, Pb, Cd, Sn, Ba, Be,
and mixtures thereof, a is about 1, and wherein y is
10 greater than zero and less than one;
a second electrode which is a counterelectrode to
said first electrode; and
an electrolyte between said electrodes.
A further aspect of the preferred embodiment of this
invention is a lithium ion battery comprising:
a first electrode having an active material which is
a compound represented by the nominal general
formula Li,MI,_YMII,,PO4 wherein MI is at least one
transition metal selected from Groups 4 to 11
inclusive of the Periodic Table and has a +2 valence
state, MII is at least one metallic element which is
selected from Groups 2, 12, and 14 of the Periodic
Table and has a +2 valence state, a is about 1, and
wherein 0 < y < 1, with the proviso that when MI is
Co, Ni, or Mn, MII is not Ge, Mg or Zn;
a second electrode which is a counterelectrode to
said first electrode; and
an electrolyte between said electrodes.
A further preferred embodiment of this invention is a
lithium ion battery comprising:
a first electrode having an active material which is
a compound having the nominal general formula Li,MII_
YMIIyP04 wherein MI is selected from the group
consisting of Fe, Co, Ni, Mn, Cu, V, Sn, Ti, Cr, and
CA 02460875 2004-03-23
11
mixtures thereof, and MII is selected from the group
consisting of Ca, Sr, Pb, Cd, Sn, Ba, Be, and
mixtures thereof, a is about 1, and wherein y is
greater than zero and less than one, a second
electrode which is a counterelectrode to said first
electrode; and
an electrolyte between said electrodes.
A further embodiment of this invention is a lithium ion
battery comprising:
a first electrode having an active material which is
a compound having the nominal general formula
LiaMII_yMIIyPO4 wherein MI is selected from the group
consisting of Fe, Ni, Mn, V, Sn, Ti, Cr, and
mixtures thereof, and MII is selected from the group
consisting of Ca, Sr, Pb, Cd, Sn, Ba, Be, and
mixtures thereof, a is about 1, and wherein y is
greater than zero and less than one;
a second electrode which is a counterelectrode to
said first electrode; and
an electrolyte between said electrodes.
Another aspect of the preferred embodiment of this
invention is a lithium ion battery comprising:
a first electrode having an active material which is
a compound having the nominal general formula LiaMIl_
yMIIYP04 wherein MI is selected from the group
consisting of Fe, Cu, V, Sn, Ti, Cr, and mixtures
thereof, and MII is selected from the group
consisting of Mg, Ca, Sr, Pb, Cd, Sn, Ba, Be, and
mixtures thereof, a is about 1, and wherein y is
greater than zero and less than one;
a second electrode which is a counterelectrode to
said first electrode; and
an electrolyte between said electrodes.
CA 02460875 2004-03-23
12
The active material of the counter electrode is
any material compatible with the lithium-mixed metal
phosphate of the invention. Where the lithium-mixed
metal phosphate is used as a positive electrode active
material, metallic lithium, lithium-containing material,
or non-lithium-containing material may be used as the
negative electrode active material. The negative
electrode is desirably a nonmetallic insertion material.
Desirably, the negative electrode comprises an active
material from the group consisting of metal oxide,
particularly transition metal oxide, metal chalcogenide,
carbon, graphite, and mixtures thereof. It is preferred
that the anode active material comprises a carbonaceous
material such as graphite. The lithium-mixed metal
phosphate of the invention may also be used as a negative
electrode material.
In another embodiment, the present invention
provides a method of preparing a compound of the nominal
general formula Li,MI,,MI I, ( PO4 ),i where 0 < a<_ 3; the sum
of b plus c is greater than zero and up to about 2; and
0< d S 3. Preferred compounds include Li;MI,,MII, (P04) 3
where b plus c is about 2; and LiMI1,MIIcPOq where b plus c
is about 1. The method comprises providing starting
materials in particle form. The starting (precursor)
materials include a lithium-containing compound, one or
more metal containing compounds, a compound capable of
providing the phosphate (PO4)-' anion, and carbon.
Preferably, the lithium-containing compound is in
particle form, and an example is lithium salt.
Preferably, the phosphate-containing anion compound is in
particle form, and examples include metal phosphate salt
and diammonium hydrogen phosphate (DAHP) and ammonium
dihydrogen phosphate (ADHP). The lithium compound, one
or more metal compounds, and phosphate compound are
included in a proportion which provides the stated
CA 02460875 2004-03-23
13
nominal general formula. The starting materials are
mixed together with carbon, which is included in an
amount sufficient to reduce the metal ion of one or more
of the metal-containing starting materials without full
reduction to an elemental metal state. Excess quantities
of carbon and one or more other starting materials (i.e.,
5 to 10% excess) may be used to enhance product quality.
A small amount of carbon, remaining after the reaction,
functions as a conductive constituent in the ultimate
electrode formulation. This is an advantage since such
remaining carbon is very intimately mixed with the
product active material. Accordingly, large quantities of
excess carbon, on the order of 100% excess carbon are
useable in the process. The carbon present during
compound formation is thought to be intimately dispersed
throughout the precursor and product. This provides many
advantages, including the enhanced conductivity of the
product. The presence of carbon particles in the
starting materials is also thought to provide nucleation
sites for the production of the product crystals.
The starting materials are intimately mixed and
then reacted together where the reaction is initiated by
heat and is preferably conducted in a nonoxidizing, inert
atmosphere, whereby the lithium, metal from the metal
compound(s), and phosphate combine to form the
LiaMI,,MII, (P04)d product. Before reacting the compounds,
the particles are intermingled to form an essentially
homogeneous powder mixture of the precursors. In one
aspect, the precursor powders are dry-mixed using a ball
mill, such as zirconia media. Then the mixed powders are
pressed into pellets. In another aspect, the precursor
powders are mixed with a binder. The binder is selected
so as to not inhibit reaction between particles of the
powders. Therefore, preferred binders decompose or
evaporate at a temperature less than the reaction
CA 02460875 2004-03-23
14
temperature. Examples include mineral oils (i.e.,
glycerol, or C-18 hydrocarbon mineral oil) and polymers
which decompose (carbonize) to form a carbon residue
before the reaction starts, or which evaporate before the
reaction starts. In still another aspect, intermingling
is conducted by forming a wet mixture using a volatile
solvent and then the intermingled particles are pressed
together in pellet form to provide good grain-to-grain
contact.
Although it is desired that the precursor
compounds be present in a proportion which provides the
stated general formula of the product, the lithium
compound may be present in an excess amount on the order
of 5 percent excess lithium compared to a stoichiometric
mixture of the precursors. And the carbon may be present
at up to 100% excess compared to the stoichiometric
amount. The method of the invention may also be used to
prepare other novel products, and to prepare known
products. A number of lithium compounds are available as
precursors, such as lithium acetate (LiOOCCH3)1 lithium
hydroxide, lithium nitrate (LiN03), lithium oxalate
(Li2C20q) , lithium oxide (Li2O), lithium phosphate
(Li3PO4) , lithium dihydrogen phosphate (LiH,P04) 1 lithium
vanadate (LiVO3) , and lithium carbonate (Li2CO3) . The
lithium carbonate is preferred for the solid state
reaction since it has a very high melting point and
commonly reacts with the other precursors before melting.
Lithium carbonate has a melting point over 600 C and it
decomposes in the presence of the other precursors and/or
effectively reacts with the other precursors before
melting. In contrast, lithium hydroxide melts at about
400 C. At some reaction temperatures preferred herein of
over 450 C the lithium hydroxide will melt before any
significant reaction with the other precursors occurs to
an effective extent. This melting renders the reaction
CA 02460875 2004-03-23
very difficult to control. In addition, anhydrous LiOH
is highly hygroscopic and a significant quantity of water
is released during the reaction. Such water needs to be
removed from the oven and the resultant product may need
5 to be dried. In one preferred aspect, the solid state
reaction made possible by the present invention is much
preferred since it is conducted at temperatures at which
the lithium-containing compound reacts with the other
reactants before melting. Therefore, lithium hydroxide
10 is useable as a precursor in the method of the invention
in combination with some precursors, particularly the
phosphates. The method of the invention is able to be
conducted as an economical carbothermal-based process
with a wide variety of precursors and over a relatively
15 broad temperature range.
The aforesaid precursor compounds (starting
materials) are generally crystals, granules, and powders
and are generally referred to as being in particle form.
Although many types of phosphate salts are known, it is
preferred to use diammonium hydrogen phosphate (NH4) 2HP04
(DAHP) or ammonium dihydrogen phosphate (NH4)HZPO4 (ADHP) .
Both ADHP and DAHP meet the preferred criteria that the
precursors decompose in the presence of one another or
react with one another before melting of such precursor.
Exemplary metal compounds are Fe-,03, Fe309, V 05, V021
LiVO3, NH4VO;1 Mg (OH) :, Cao, MgO, Ca (OH) ~,, MnO2, Mn2031
Mn3 (P04) 21 CuO, SnO, Sn0=., TiO~, Ti-.Oi, Cr,Oõ PbO2, PbO,
Ba(OH)2, BaO, Cd(OH),. In addition, some starting
materials serve as both the source of inetal ion and
phosphate, such as FePOa, Fe, ( POq ), Zn, ( POQ ),,., and
Mg3(P04)2. Still others contain both lithium ion and
phosphate such as Li,PO; and LiH.POa. Other exemplary
precursors are H,,PO., (phosphoric acid) ; and P2O5 (P4010)
phosphoric oxide; and HPO.t meta phosphoric acid, which is
a decomposition product of P.05. If it is desired to
CA 02460875 2004-03-23
16
replace any of the oxygen with a halogen, such as
fluorine, the starting materials further include a
fluorine compound such as LiF. If it is desired to
replace any of the phosphorous with silicon, then the
starting materials further include silicon oxide (Si02).
Similarly, ammonium sulfate in the starting materials is
useable to replace phosphorus with sulfur.
The starting materials are available from a
number of sources. The following are typical. Vanadium
pentoxide of the formula V-,0, is obtainable from any
number of suppliers including Kerr McGee, Johnson
Matthey, or Alpha Products of Davers, Massachusetts.
Vanadium pentoxide has a CAS number of 1314-62-1. Iron
oxide Fe303 is a common and very inexpensive material
available in powder form from the same suppliers. The
other precursor materials mentioned above are also
available from well known suppliers, such as those listed
above.
The method of the invention may also be used to
react starting materials in the presence of carbon to
form a variety of other novel products, such as gamma-
LiV205 and also to produce known products. Here, the
carbon functions to reduce metal ion of a starting metal
compound to provide a product containing such reduced
metal ion. The method is particularly useful to also add
lithium to the resultant product, which thus contains the
metallic element ions, namely, the lithium ion and the
other metal ion, thereby forming a mixed metal product.
An example is the reaction of vanadium pentoxide (V205)
with lithium carbonate in the presence of carbon to form
gamma-LiV205. Here the starting metal ion V+sV+s is
reduced to V+9V+" in the final product. A single phase
gamma-LiV205 product is not known to have been directly
and independently formed before.
CA 02460875 2004-03-23
17
As described earlier, it is desirable to
conduct the reaction at a temperature where the lithium
compound reacts before melting. The temperature should
be about 400 C or greater, and desirably 450 C or
greater, and preferably 500 C or greater, and generally
will proceed at a faster rate at higher temperatures.
The various reactions involve production of CO or CO2 as
an effluent gas. The equilibrium at higher temperature
favors CO formation. Some of the reactions are more
desirably conducted at temperatures greater than 600 C;
most desirably greater than 650 C; preferably 700 C or
greater; more preferably 750 C or greater. Suitable
ranges for many reactions are about 700 to 950 C, or
about 700 to 800 C.
Generally, the higher temperature reactions
produce CO effluent and the stoichiometry requires more
carbon be used than the case where CO2 effluent is
produced at lower temperature. This is because the
reducing effect of the C to M reaction is greater than
the C to CO reaction. The C to CO, reaction involves an
increase in carbon oxidation state of +4 (from 0 to 4)
and the C to CO reaction involves an increase in carbon
oxidation state of +2 (from ground state zero to 2).
Here, higher temperature generally refers to a range of
about 650 C to about 1000 C and lower temperature refers
to up to about 650 C. Temperatures higher than 1200 C
are not thought to be needed.
In one aspect, the method of the invention
utilizes the reducing capabilities of carbon in a unique
and controlled manner to produce desired products having
structure and lithium content suitable for electrode
active materials. The method of the invention makes it
possible to produce products containing lithium, metal
and oxygen in an economical and convenient process. The
CA 02460875 2004-03-23
18
ability to lithiate precursors, and change the oxidation
state of a metal without causing abstraction of oxygen
from a precursor is heretofore unexpected. These
advantages are at least in part achieved by the
reductant, carbon, having an oxide whose free energy of
formation becomes more negative as temperature increases.
Such oxide of carbon is more stable at high temperature
than at low temperature. This feature is used to produce
products having one or more metal ions in a reduced
oxidation state relative to the precursor metal ion
oxidation state. The method utilizes an effective
combination of quantity of carbon, time and temperature
to produce new products and to produce known products in
a new way.
Referring back to the discussion of
temperature, at about 700 C both the carbon to carbon
monoxide and the carbon to carbon dioxide reactions are
occurring. At closer to 600 C the C to C0, reaction is
the dominant reaction. At closer to 800 C the C to CO
reaction is dominant. Since the reducing effect of the C
to CO2 reaction is greater, the result is that less
carbon is needed per atomic unit of metal to be reduced.
In the case of carbon to carbon monoxide, each atomic
unit of carbon is oxidized from ground state zero to plus
2. Thus, for each atomic unit of metal ion (M) which is
being reduced by one oxidation state, one half atomic
unit of carbon is required. In the case of the carbon to
carbon dioxide reaction, one quarter atomic unit of
carbon is stoichiometrically required for each atomic
unit of metal ion (M) which is reduced by one oxidation
state, because carbon goes from ground state zero to a
plus 4 oxidation state. These same relationships apply
for each such metal ion being reduced and for each unit
reduction in oxidation state desired.
CA 02460875 2004-03-23
19
It is preferred to heat the starting materials
at a ramp rate of a fraction of a degree to 10 C per
minute and preferably about 2 C per minute. Once the
desired reaction temperature is attained, the reactants
(starting materials) are held at the reaction temperature
for several hours. The heating is preferably conducted
under non-oxidizing or inert gas such as argon or vacuum.
Advantageously, a reducing atmosphere is not required,
although it may be used if desired. After reaction, the
products are preferably cooled from the elevated
temperature to ambient (room) temperature (i.e., 10 C to
40 C). Desirably, the cooling occurs at a rate similar
to the earlier ramp rate, and preferably 2 C/minute
cooling. Such cooling rate has been found to be adequate
to achieve the desired structure of the final product.
It is also possible to quench the products at a cooling
rate on the order of about 100 C/minute. In some
instances, such rapid cooling (quench) may be preferred.
The present invention resolves the capacity
problem posed by widely used cathode active material. It
has been found that the capacity and capacity retention
of cells having the preferred active material of the
invention are improved over conventional materials.
Optimized cells containing lithium-mixed metal phosphates
of the invention potentially have performance improved
over commonly used lithium metal oxide compounds.
Advantageously, the new method of making the novel
lithium-mixed metal phosphate compounds of the invention
is relatively economical and readily adaptable to
commercial production.
Another feature of one embodiment of the
invention includes an electrochemical cell or battery
based on lithium-mixed metal phosphates. Still another
feature is to provide an electrode active material which
CA 02460875 2004-03-23
combines the advantages of good discharge capacity and
capacity retention. It is also a desirable feature of
the present invention to provide electrodes which can be
manufactured economically. Yet another feature of one
5 embodiment is to provide a method for forming electrode
active material which lends itself to commercial scale
production for preparation of large quantities.
These and other objects, features, and
10 advantages will become apparent from the following
description of the preferred embodiments, claims, and
accompanying drawings.
CA 02460875 2004-03-23
21
Brief Description of the Drawings
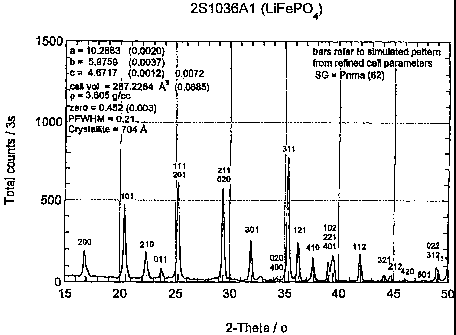
Figure 1 shows the results of an x-ray
diffraction analysis, of the LiFePOa prepared according
to the invention using CuKa radiation, A= 1.5405A.
Bars refer to simulated pattern from refined cell
parameters, Space Group, SG = Pnma (62). The values are
a = 10.2883A (0.0020), b = 5.9759A (0.0037), c = 4.6717A
(0.0012) 0.0072, cell volume = 287.2264A; (0.0685).
Density, p = 3.605 g/cc, zero = 0.452 (0.003). Peak at
full width half maximum, PFWHM = 0.21. Crystallite size
from XRD data = 704A.
Figure 2 is a voltage/capacity plot of LiFePO4-
containing cathode cycled with a lithium metal anode
using constant current cycling at 0.2 milliamps per
square centimeter in a range of 2.5 to 4.0 volts at a
temperature of about 23 C. The cathode contained 19.0mg
of the LiFePO4 active material, prepared by the method of
the invention. The electrolyte comprised ethylene
carbonate (EC) and dimethyl carbonate (DMC) in a weight
ratio of 2:1 and included a 1 molar concentration of
LiPF6 salt. The lithium-metal-phosphate containing
electrode and the lithium metal counter electrode are
maintained spaced apart by a glass fiber separator which
is interpenetrated by the solvent and the salt.
Figure 3 shows multiple constant current
cycling of LiFePOa active material cycled with a lithium
metal anode using the electrolyte as described in
connection with Figure 2 and cycled, charge and discharge
at 0.2 milliamps per square centimeter, 2.5 to 4.0
volts at two different temperature conditions, 23 C and
60 C. Figure 3 shows the excellent rechargeability of
the lithium iron phosphate/lithium metal cell, and also
CA 02460875 2004-03-23
22
shows the excellent cycling and specific capacity (mAh/g)
of the active material.
Figure 4 shows the results of an x-ray
diffraction analysis, of the LiFe,,,,,Mgo.1PO4 prepared
according to the invention, using CuKa radiation, X
1.5405A. Bars refer to simulated pattern from refined
cell parameters SG = Pnma (62). The values are a
10.2688A (0.0069), b 5.9709A (0.0072), c = 4.6762A
(0.0054), cell volume = 286.7208A (0.04294), p = 3.617
g/cc, zero = 0.702 (0.003), PFWHM = 0.01, and crystallite
= 950A.
Figure 5 is a voltage/capacity plot of
LiFe0,9Mg0,1PO4-containing cathode cycled with a lithium
metal anode using constant current cycling at 0.2
milliamps per square centimeter in a range of 2.5 to 4.0
volts. Other conditions are as described earlier with
respect to Figure 2. The cathode contained 18.9mg of the
LiFeo,gMgo,1PO4 active material prepared by the method of
the invention.
Figure 6 shows multiple constant current
cycling of LiFeo..,Mg0.,PO4 cycled with a lithium metal
anode using the electrolyte as described in connection
with Figure 2 and cycled, charge and discharge at 0.2
milliamps per square centimeter, 2.5 to 4.0 volts at two
different temperature conditions, 23 C and 60 C. Figure
6 shows the excellent rechargeability of the lithium-
metal-phosphate/lithium metal cell, and also shows the
excellent cycling and capacity of the cell.
Figure 7 is a voltage/capacity plot of
LiFeo,8Mgo,2P04-containing cathode cycled with a lithium
metal anode using constant current cycling at 0.2
milliamps per square centimeter in a range of 2.5 to 4.0
CA 02460875 2004-03-23
23
volts at 23 C. Other conditions are as described earlier
with respect to Figure 2. The cathode contained 16mg of
the LiFeo,eMgo_,PO4 active material prepared by the method
of the invention.
Figure 8 shows the results of an x-ray
diffraction analysis, of the LiFe(0,<,Ca(0.1PO4 prepared
according to the invention, using CuKcx radiation, A=
1.5405A. Bars refer to simulated pattern from refined
cell parameters SG = Pnma (62). The values are a =
10.3240A (0.0045), b 6.0042A (0.0031), c = 4.6887A
(0.0020), cell volume = 290.6370A (0.1807), zero = 0.702
(0.003), p = 3.62 g/cc, PFWHM = 0.18, and crystallite =
680A.
Figure 9 is a voltage/capacity plot of
LiFeo,8Cao,2P04-containing cathode cycled with a lithium
metal anode using constant current cycling at 0.2
milliamps per square centimeter in a range of 2.5 to 4.0
volts at 23 . Other conditions are as described earlier
with respect to Figure 2. The cathode contained 18.5mg
of the LiFeo,RCao, PO4 active material prepared by the
method of the invention.
Figure 10 is a voltage/capacity plot of
LiFeo,eZno,2P04-containing cathode cycled with a lithium
metal anode using constant current cycling at 0.2
milliamps per square centimeter in a range of 2.5 to 4.0
volts at 23 C. Other conditions are as described earlier
with respect to Figure 2. The cathode contained 18.9mg
of the LiFe0,6Zn0, ,POa active material prepared by the
method of the invention.
Figure 11 shows the results of an x-ray
diffraction analysis of the gamma-Li.,.V:.O,, (x = 1, gamma
LiV2O5) prepared according to the invention using CuKoc
CA 02460875 2004-03-23
24
radiation A= 1.5405A. The values are a = 9.687A (1), b
= 3.603A (2), and c = 10.677A (3); phase type is gamma-
LiXV205 (x = 1) ; symmetry is orthorhombic; and space group
is Pnma.
Figure 12 is a voltage/capacity plot of gamma-
LiV205-containing cathode cycled with a lithium metal
anode using constant current cycling at 0.2 milliamps
per square centimeter in a range of 2.5 to 3.8 volts at
23 C. Other conditions are as described earlier with
respect to Figure 2. The cathode contained 21mg of the
gamma-LiV205 active material prepared by the method of
the invention.
Figure 13 is a two-part graph based on multiple
constant current cycling of gamma-LiV,O,, cycled with a
lithium metal anode using the electrolyte as described in
connection with Figure 2 and cycled, charge and discharge
at 0.2 milliamps per square centimeter, 2.5 to 3.8
volts. In the two-part graph, Figure 13 shows the
excellent rechargeability of the lithium-metal-
oxide/lithium metal cell. Figure 13 shows the excellent
cycling and capacity of the cell.
Figure 14 shows the results of an x-ray
diffraction analysis of the Li3V,(P04)3 prepared according
to the invention. The analysis is based on CuKa
radiation, A= 1.5405A. The values are a = 12.184A (2),
b = 8.679A (2), c = 8.627A (3), and (3 = 90.457 (4).
Figure 15 shows the results of an x-ray
diffraction analysis of Li.,V.(PO4); prepared according to
a method described in U.S. Patent No. 5,871,866. The
analysis is based on CuKa radiation, A= 1.5405A. The
values are a = 12.155A (2), b = 8.711A (2), c 8.645A
CA 02460875 2004-03-23
(3); the angle beta is 90.175 (6); symmetry is
Monoclinic; and space group is P21/n.
Figure 16 is an EVS (Electrochemical Voltage
5 Spectroscopy) voltage/capacity profile for a cell with
cathode material formed by the carbothermal reduction
method of the invention. The cathode material is 13.8mg
of Li3V2(P09)3 . The cell includes a lithium metal counter
electrode in an electrolyte comprising ethylene carbonate
10 (EC) and dimethyl carbonate (DMC) in a weight ratio of
2:1 and including a 1 molar concentration of LiPF6 salt.
The lithium-metal-phosphate containing electrode and the
lithium metal counter electrode are maintained spaced
apart by a fiberglass separator which is interpenetrated
15 by the solvent and the salt. The conditions are 10 mV
steps, between about 3.0 and 4.2 volts, and the critical
limiting current density is less than or equal to 0.1
mA/ cm2 .
20 Figure 17 is an EVS differential capacity
versus voltage plot for the cell as described in
connection with Figure 16.
Figure 18 shows multiple constant current
25 cycling of LiFeo8BMgp22PO4 cycled with a lithium metal
anode using the electrolyte as described in connection
with Figure 2 and cycled, charge and discharge at 0.2
milliamps per square centimeter, 2.5 to 4.0 volts at two
different temperature conditions, 23 C and 60 C. Figure
18 shows the excellent rechargeability of the lithium-
metal-phosphate/lithium metal cell, and also shows the
excellent cycling and capacity of the cell.
Figure 19 is a graph of potential over time for
the first four complete cycles of the LiMgo1,Feo.9PO4/MCMB
graphite cell of the invention.
CA 02460875 2004-03-23
26
Figure 20 is a two-part graph based on multiple
constant current cycling of LiFeo9,,Mg0,=1PO4 cycled with an
MCMB graphite anode using the electrolyte as described in
connection with Figure 2 and cycled, charge and discharge
at 0.2 milliamps per square centimeter, 2.5 to 3.6
volts, 23 C and based on a C/10 (10 hour) rate. In the
two-part graph, Figure 20 shows the excellent
rechargeability of the lithium-metal-phosphate/graphite
cell. Figure 20 shows the excellent cycling and capacity
of the cell.
Figure 21 is a graph of potential over time for
the first three complete cycles of the gamma-LiV205/MCMB
graphite cell of the invention.
Figure 22 is a diagrammatic representation of a
typical laminated lithium-ion battery cell structure.
Figure 23 is a diagrammatic representation of a
typical multi-cell battery cell structure.
CA 02460875 2004-03-23
27
Detailed Description of the Preferred Embodiments
The present invention provides lithium-mixed
metal-phosphates, which are usable as electrode active
materials, for lithium (Li') ion removal and insertion.
Upon extraction of the lithium ions from the lithium-
mixed-metal-phosphates, significant capacity is achieved.
In one aspect of the invention, electrochemical energy is
provided when combined with a suitable counter electrode
by extraction of a quantity x of lithium from lithium-
mixed-metal-phosphates Liõ_xMIMII,, (PO4),,. When a quantity
x of lithium is removed per formula unit of the lithium-
mixed-metal phosphate, metal MI is oxidized. In another
aspect, metal MII is also oxidized. Therefore, at least
one of MI and MII is oxidizable from its initial
condition in the phosphate compound as Li is removed.
Consider the following which illustrate the mixed metal
compounds of the invention: LiFe,_ySnYP04, has two
oxidizable elements, Fe and Sn; in contrast,
LiFel_,,MgP04 has one oxidizable metal, the metal Fe.
In another aspect, the invention provides a
lithium ion battery which comprises an electrolyte; a
negative electrode having an insertion active material;
and a positive electrode comprising a lithium-mixed-
metal-phosphate active material characterized by an
ability to release lithium ions for insertion into the
negative electrode active material. The lithium-mixed-
metal-phosphate is desirably represented by the nominal
general formula Li,MI4MII,(PO4),,. Although the metals MI
and MII may be the same, it is preferred that the metals
MI and MII are different. Desirably, in the phosphate
compound MI is a metal selected from the group: Fe, Co,
Ni, Mn, Cu, V, Sn, Ti, Cr and mixtures thereof, and MI is
most desirably a transition metal or mixture thereof
CA 02460875 2004-03-23
28
selected from said group. Most preferably, MI has a +2 valence
or oxidation state.
In another aspect, MII is selected from Mg, Ca, Zn, Sr,
Pb, Cd, Sn, Ba, Be, and mixtures thereof. Most preferably, MII
has a +2 valence or oxidation state. The lithium-mixed-metal-
phosphate is preferably a compound represented by the nominal
general formula Lia_xMIbMII, (P04) d, signifying the preferred
composition and its capability to release x lithium.
Accordingly, during cycling, charge and discharge, the value of x
varies as x greater than or equal to 0 and less than or equal to
a. The present invention resolves a capacity problem posed by
conventional cathode active materials. Such problems with
conventional active materials are described by Tarascon in U.S.
Patent No. 5,425,932, using LiMn2O4 as an example. Similar
problems are observed with LiCoO2, LiNiO2, and many, if not all,
lithium metal chalcogenide materials. Other patents e.g. US
5,869,208, 5,882,821, 5,670,273, etc. reference or disclose
electrodes, both cathode and anode, which contain the LiCoO2,
LiMn2O4 and LiNi02materials, or are used in combination with
other electrode compositions. Such prior art is representative
of coiled electrodes, in which the cathode or anode (as the case
may be) is constructed according to conventional techniques with
cathodes having a film of a foil or metal wire net with a
thickness of e.g. 5 pm - 100 pm. Other prior art also teaches
10-200 pm of film thickness for various types of batteries, e.g.
US 5,741,472. The present invention demonstrates that
significant capacity of the cathode active material is utilizable
and maintained.
A preferred novel procedure for forming the lithium-
mixed-metal-phosphate LiaMIbMII, (PO4)d compound active material
will now be described. In addition, the preferred novel
CA 02460875 2004-03-23
28A
procedure is also applicable to formation of other lithium metal
compounds, and will be described as such. The basic procedure
will be described with reference to exemplary starting materials
but is not limited thereby. The basic process comprises
conducting a reaction between a lithium compound, preferably
lithium carbonate (Li2CO3)1 metal compound(s), for example,
vanadium pentoxide (V205) 1 iron oxide (Fe203), and/or manganese
hydroxide, and a phosphoric acid derivative, preferably the
phosphoric acid ammonium salt, diammonium hydrogen phosphate,
(NHq ) 2H (P04) . Each of the precursor
CA 02460875 2004-03-23
29
starting materials are available from a number of
chemical outfits including Aldrich Chemical Company and
Fluka. Using the method described herein, LiFePO4 and
LiFeo99Mgo11PO4, Li3V'(POa) 3 were prepared with approximately
a stoichiometric amount of Li,COI, the respective metal
compound, and (NH4)_HPO4. Carbon powder was included with
these precursor materials. The precursor materials were
initially intimately mixed and dry ground for about 30
minutes. The intimately mixed compounds were then
pressed into pellets. Reaction was conducted by heating
in an oven at a preferred ramped heating rate to an
elevated temperature, and held at such elevated
temperature for several hours to complete formation of
the reaction product. The entire reaction was conducted
in a non-oxidizing atmosphere, under flowing pure argon
gas. The flow rate will depend upon the size of the oven
and the quantity needed to maintain the atmosphere. The
oven was permitted to cool down at the end of the
reaction period, where cooling occurred at a desired rate
under argon. Exemplary and preferred ramp rates,
elevated reaction temperatures and reaction times are
described herein. In one aspect, a ramp rate of
2 /minute to an elevated temperature in a range of 750 C
to 800 C was suitable along with a dwell (reaction time)
of 8 hours. Refer to Reactions 1, 2, 3 and 4 herein. In
another variation per Reaction 5, a reaction temperature
of 600 C was used along with a dwell time of about one
hour. In still another variation, as per Reaction 6, a
two-stage heating was conducted, first to a temperature
of 300 C and then to a temperature of 850 .
The general aspects of the above synthesis
route are applicable to a variety of starting materials.
Lithium-containing compounds include Li-,0 (lithium
oxide), LiH2PO4 (lithium hydrogen phosphate),
Li2C209(lithium oxalate), LiOH (lithium hydroxide),
CA 02460875 2004-03-23
LiOH.H20 (lithium hydroxide monohydride), and LiHCO3
(lithium hydrogen carbonate). The metal compounds(s) are
reduced in the presence of the reducing agent, carbon.
The same considerations apply to other lithium-metal- and
5 phosphate-containing precursors. The thermodynamic
considerations such as ease of reduction, of the selected
precursors, the reaction kinetics, and the melting point
of the salts will cause adjustment in the general
procedure, such as, amount of carbon reducing agent, and
10 the temperature of reaction.
Figures 1 through 21 which will be described
more particularly below show characterization data and
capacity in actual use for the cathode materials
15 (positive electrodes) of the invention. Some tests were
conducted in a cell comprising a lithium metal counter
electrode (negative electrode) and other tests were
conducted in cells having a carbonaceous counter
electrode. All of the cells had an EC:DMC-LiPF6
20 electrolyte.
Typical cell configurations will now be
described with reference to Figures 22 and 23; and such
battery or cell utilizes the novel active material of the
25 invention. Note that the preferred cell arrangement
described here is illustrative and the invention is not
limited thereby. Experiments are often performed, based
on full and half cell arrangements, as per the following
description. For test purposes, test cells are often
30 fabricated using lithium metal electrodes. When forming
cells for use as batteries, it is preferred to use an
insertion positive electrode as per the invention and a
graphitic carbon negative electrode.
A typical laminated battery cell structure 10
is depicted in Figure 22. It comprises a negative
CA 02460875 2004-03-23
31
electrode side 12, a positive electrode side 14, and an
electrolyte/separator 16 there between. Negative
electrode side 12 includes current collector 18, and
positive electrode side 14 includes current collector 22.
A copper collector foil 18, preferably in the form of an
open mesh grid, upon which is laid a negative electrode
membrane 20 comprising an insertion material such as
carbon or graphite or low-voltage lithium insertion
compound, dispersed in a polymeric binder matrix. An
electrolyte/separator film 16 membrane is preferably a
plasticized copolymer. This electrolyte/separator
preferably comprises a polymeric separator and a suitable
electrolyte for ion transport. The electrolyte/separator
is positioned upon the electrode element and is covered
with a positive electrode membrane 24 comprising a
composition of a finely divided lithium insertion
compound in a polymeric binder matrix. An aluminum
collector foil or grid 22 completes the assembly.
Protective bagging material 40 covers the cell and
prevents infiltration of air and moisture.
In another embodiment, a multi-cell battery
configuration as per Figure 23 is prepared with copper
current collector 51, negative electrode 53,
electrolyte/separator 55, positive electrode 57, and
aluminum current collector 59. Tabs 52 and 58 of the
current collector elements form respective terminals for
the battery structure. As used herein, the terms "cell"
and "battery" refer to an individual cell comprising
anode/electrolyte/cathode and also refer to a multi-cell
arrangement in a stack.
The relative weight proportions of the
components of the positive electrode are generally: 50-
90% by weight active material; 5-30% carbon black as the
electric conductive diluent; and 3-20% binder chosen to
CA 02460875 2004-03-23
32
hold all particulate materials in contact with one
another without degrading ionic conductivity. Stated
ranges are not critical, and the amount of active
material in an electrode may range from 25-95 weight
percent. The negative electrode comprises about 50-95%
by weight of a preferred graphite, with the balance
constituted by the binder. A typical electrolyte
separator film comprises approximately two parts polymer
for every one part of a preferred fumed silica. The
conductive solvent comprises any number of suitable
solvents and salts. Desirable solvents and salts are
described in U.S. Patent Nos. 5,643,695 and 5,418,091.
One example is a mixture of EC:DMC:LiPFG in a weight
ratio of about 60:30:10.
Solvents are selected to be used individually
or in mixtures, and include dimethyl carbonate (DMC),
diethylcarbonate (DEC), dipropylcarbonate (DPC),
ethylmethylcarbonate (EMC), ethylene carbonate (EC),
propylene carbonate (PC), butylene carbonate, lactones,
esters, glymes, sulfoxides, sulfolanes, etc. The
preferred solvents are EC/DMC, EC/DEC, EC/DPC and EC/EMC.
The salt content ranges from 5% to 65% by weight,
preferably from 8% to 35% by weight.
Those skilled in the art will understand that
any number of methods are used to form films from the
casting solution using conventional meter bar or doctor
blade apparatus. It is usually sufficient to air-dry the
films at moderate temperature to yield self-supporting
films of copolymer composition. Lamination of assembled
cell structures is accomplished by conventional means by
pressing between metal plates at a temperature of about
120-160 C. Subsequent to lamination, the battery cell
material may be stored either with the retained
plasticizer or as a dry sheet after extraction of the
CA 02460875 2004-03-23
33
plasticizer with a selective low-boiling point solvent.
The plasticizer extraction solvent is not critical, and
methanol or ether are often used.
Separator membrane element 16 is generally
polymeric and prepared from a composition comprising a
copolymer. A preferred composition is the 75 to 92%
vinylidene fluoride with 8 to 25% hexafluoropropylene
copolymer (available commercially from Atochem North
America as KYNAR FLEX7") and an organic solvent
plasticizer. Such a copolymer composition is also
preferred for the preparation of the electrode membrane
elements, since subsequent laminate interface
compatibility is ensured. The plasticizing solvent may
be one of the various organic compounds commonly used as
solvents for electrolyte salts, e.g., propylene carbonate
or ethylene carbonate, as well as mixtures of these
compounds. Higher-boiling plasticizer compounds such as
dibutyl phthalate, dimethyl phthalate, diethyl phthalate,
and tris butoxyethyl phosphate are particularly suitable.
Inorganic filler adjuncts, such as fumed alumina or
silanized fumed silica, may be used to enhance the
physical strength and melt viscosity of a separator
membrane and, in some compositions, to increase the
subsequent level of electrolyte solution absorption.
In the construction of a lithium-ion battery, a
current collector layer of aluminum foil or grid is
overlaid with a positive electrode film, or membrane,
separately prepared as a coated layer of a dispersion of
insertion electrode composition. This is typically an
insertion compound such as LiMn,04 (LMO) , LiCoO2, or
LiNiO21 powder in a copolymer matrix solution, which is
dried to form the positive electrode. An
electrolyte/separator membrane is formed as a dried
coating of a composition comprising a solution containing
CA 02460875 2004-03-23
34
VdF:HFP copolymer and a plasticizer solvent is then
overlaid on the positive electrode film. A negative
electrode membrane formed as a dried coating of a
powdered carbon or other negative electrode material
dispersion in a VdF:HFP copolymer matrix solution is
similarly overlaid on the separator membrane layer. A
copper current collector foil or grid is laid upon the
negative electrode layer to complete the cell assembly.
Therefore, the VdF:HFP copolymer composition is used as a
binder in all of the major cell components, positive
electrode film, negative electrode film, and
electrolyte/separator membrane. The assembled components
are then heated under pressure to achieve heat-fusion
bonding between the plasticized copolymer matrix
electrode and electrolyte components, and to the
.collector grids, to thereby form an effective laminate of
cell elements. This produces an essentially unitary and
flexible battery cell structure.
Examples of forming cells containing metallic
lithium anode, insertion electrodes, solid electrolytes
and liquid electrolytes can be found in U.S. Patent Nos.
4, 668, 595; 4, 830, 939; 4, 935, 317; 4, 990, 413; 4, 792, 504;
5, 037, 712; 5, 262, 253; 5, 300, 373; 5, 435, 054; 5, 463, 179;
5,399,447; 5,482,795 and 5,411,820. Note that the older
generation of cells contained organic polymeric and
inorganic electrolyte matrix materials, with the
polymeric being most preferred. The polyethylene oxide
of 5,411,820 is an example. More modern examples are the
VdF:HFP polymeric matrix. Examples of casting,
lamination and formation of cells using VdF:HFP are as
described in U.S. Patent Nos. 5,418,091; 5,460,904;
5, 456, 000; and 5, 540, 741; assigned to Bell Communications
Research.
CA 02460875 2004-03-23
As described earlier, the electrochemical cell
operated as per the invention, may be prepared in a
variety of ways. In one embodiment, the negative
electrode may be metallic lithium. In more desirable
5 embodiments, the negative electrode is an insertion
active material, such as, metal oxides and graphite.
When a metal oxide active material is used, the
components of the electrode are the metal oxide,
electrically conductive carbon, and binder, in
10 proportions similar to that described above for the
positive electrode. In a preferred embodiment, the
negative electrode active material is graphite particles.
For test purposes, test cells are often fabricated using
lithium metal electrodes. When forming cells for use as
15 batteries, it is preferred to use an insertion metal
oxide positive electrode and a graphitic carbon negative
electrode. Various methods for fabricating
electrochemical cells and batteries and for forming
electrode components are described herein. The invention
20 is not, however, limited by any particular fabrication
method.
CA 02460875 2004-03-23
36
Formation of Active Materials
EXAMPLE I
Reaction 1 (a) . LiFePOa formed from FePO4
FeP04 + 0.5 Li,C03 + 0.5 C -4 LiFePO4 + 0.5 CO2 + 0.5 CO
(a) Pre-mix reactants in the following proportions
using ball mill. Thus,
1 mol FePOq 150.82g
0.5 mol Li~CO; 36.95g
0.5 mol carbon 6.Og
(but use 100% excess carbon -> 12.OOg)
(b) Pelletize powder mixture
(c) Heat pellet to 750 C at a rate of 2 /minute in
flowing inert atmosphere (e.g. argon). Dwell
for 8 hours at 750 C under argon.
(d) Cool to room temperature at 2 /minute under
argon.
(e) Powderize pellet.
Note that at 750 C this is predominantly a CO
reaction. This reaction is able to be
conducted at a temperature in a range of about
700 C to about 950 C in argon as shown, and
also under other inert atmospheres such as
nitrogen or vacuum.
EXAMPLE II
CA 02460875 2004-03-23
37
Reaction 1 (b) . LiFePOa formed from Fe2O3
0.5 Fe203 + 0.5 LiCO; +(NH4),HPO4 + 0.5 C-+ LiFePO4 +
0.5 C02 + 2 NH,, + 3/2 H20 + 0.5 CO
(a) Premix powders in the following proportions
0.5 mol Fe_=03 79.85g
0.5 mol Li_CO, 36.95g
1 mol (NH4) 2HP0q 132 . 06g
0.5 mol carbon 6.OOg
(use 100% excess carbon -4 12.OOg)
(b) Pelletize powder mixture
(c) Heat pellet to 750 C at a rate of 2 /minute in
flowing inert atmosphere (e.g. argon). Dwell
for 8 hours at 750 C under argon.
(d) Cool to room temperature at 2 /minute under
argon.
(e) Powderize
EXAMPLE III
Reaction 1(c) . LiFePO4 - from Fe, (P04) 2
Two steps:
CA 02460875 2006-10-20
38
Part I. Carbothermal preparation of Fel,(PO,)2
3/2 Fe1O 3 + 2 (NH.,) _HPO,, + 3/2 C -> Fe, (PO.,) , +
3/2 Co + 4NH, + 5/2 H,0
(a) Premix reactants in the following proportions
3/2 mol Fe,O2, 239.54g
2 mol (NHa)_ HPO;1 264.12g
3/2 mol carbon 18.OOg
(use 100 '0 excess carbon -> 36.00g)
(b) Pelletize powder mixture
(c) Heat pellet to 800 C at a rate of 2 /minute in
flowing inert atmosphere (e.g. argon). Dwell
for 8 hours at 750 C under argon.
(d) Cool to room temperature at 2 C/minute under
argon.
(e) Powderize pellet.
Part II. Preparation of LiFePOa from the Fe,(P04)2 of
Part I.
Li3PO4 + Fe3 (P0g) z -> LiFePOg (a) Premix reactants in the following
proportions
1 mol Li,P0a 115.79g
1 mol Fe,(PO,;), 357.48g
(b) Pelletize powder mixture
(c) Heat pellet to 750 C at a rate of 2 /minute in
flowing inert atmosphere (e.g. argon). Dwell
for 8 hours at 750 C under argon.
CA 02460875 2004-03-23
39
(d) Cool to room temperature at 2 C/minute under
argon.
(e) Powderize pellet.
EXAMPLE IV
Reaction 2 (a). LiFeo,<,Mgo,IPO-0 (LiFe,_YMgYP04) formed from
FePO4
0. 5 Li2CO3 + 0. 9 FePO4 + 0.1 Mg ( OH ) 2+ 0.1 ( NHq ) ZHP04 +
0.45C -4 LiFeO99Mg1,.1POq + 0.5C02 + 0.45C0 + 0.2NH3 +
0.25 H20
(a) Pre-mix reactants in the following proportions
0.50 mol Li_'CO3 = 36.95g
0.90 mol FePOa = 135.74g
0.10 mol Mg(OH)2 = 5.83g
0.10 mol (NH4 )2HP0q = 1.32g
0.45 mol carbon - 5.40g
(use 100% excess carbon -+ 10.80g)
(b) Pelletize powder mixture
(c) Heat to 750 C at a rate of 2 /minute in argon.
Hold for 8 hours dwell at 750 C in argon
(d) Cool at a rate of 2 /minute
(e) Powderize pellet.
EXAMPLE V
CA 02460875 2004-03-23
Reaction 2(b) . LiFeo,.,Mg0,1PO4 (LiFe,_,,MgõPO4) formed from
Fe203
5 0. 50 Li2CO3 + 0.45 Fe_O,j + 0. 10 Mg (OH) .+(NH4) 2HP04 +
0.45C -). LiFeo,,MgO.1PO4 + 0.5 CO2 + 0.45 CO + 2 NH3 +
1.6 H20
10 (a) Pre-mix reactants in following ratio
0.50 mol Li_C0; = 36.95g
0.45 mol Fe~O; = 71.86g
0.10 mol Mg(OH)-2 = 5.83g
15 1.00 mol (NH4) 2HP04 - 132.06g
0.45 mol carbon - 5.40g
(use 100% excess carbon -*10.80g)
20 (b) Pelletize powder mixture
(c) Heat to 750 C at a rate of 2 /minute in argon.
Hold for 8 hours dwell at 750 C in argon
25 (d) Cool at a rate of 2 /minute
(e) Powderize pellet.
CA 02460875 2004-03-23
41
EXAMPLE VI
Reaction 2(c) . LiFe,,,gNigo_1P04 (LiFe1_õMgYP04) formed from
LiH2PO4
1.0 LiH2POq + 0.45 Fe2O_l + 0.10 Mg (OH), + 0.45C -+
LiFeo_9Mgo,,P04 + 0.45 CO + 1.1 H,O
(a) Pre-mix reactants in the following proportions
1.00 mol LiH-,P0a = 103.93g
0.45 mol Fe2O3 = 71.86g
0.10 mol Mg(OH) - 5.83g
0.45 mol carbon = 5.40g
(use 100% excess carbon -> 10.80g)
(b) Pelletize powder mixture
(c) Heat to 750 C at a rate of 2 /minute in argon.
Hold for 8 hours dwell at 750 C in argon
(d) Cool at a rate of 2 /minute
(e) Powderize pellet.
EXAMPLE VII
Reaction 3. Formation of LiFe0 ,9yCa0 ,11P04
(LiFel_YCayPO4) from Fe,O3
0.50 LiZC03 ; + 0.45 Fe.O3 + 0.1 Ca (OH), + (NH4) 2HP04 +
0.45C -> LiFe(0..,Cao11POq + 0.5 CO2 + 0.45 Co + 2 NH3 +
1.6 H20
(a) Pre-mix reactants in the following proportions
CA 02460875 2004-03-23
42
0.50 mol Li2CO, = 36.95g
0.45 mol Fe203 = 71.86g
0.10 mol Ca(OH) = 7.41g
1.00 mol (NHq) 2HPO9 = 132.06g
0.45 mol carbon = 5.40g
(100% excess carbon -* 10.80g)
(b) Pelletize powder mixture
(c) Heat to 750 C at a rate of 2 /minute in argon.
Hold for 8 hours dwell at 750 C in argon
(d) Cool at a rate of 2 /minute
(e) Powderize pellet.
EXAMPLE VIII
Reaction 4. Formation of LiFe.,,,Zn(,.,PO4
(LiFe,_YZnYP04) from Fe_0,.
0.50 Li2CO3 + 0.45 Fe,03 + 0.033 Zn, (PO4)2 +
0.933 (NH4) 2HP04 + 0.45 C--* LiFeo99Zno,IP09 + 0.50 CO2 +
0.45 CO + 1.866 NH,+ 1.2 H.0
Pre-mix reactants in the following proportions
0.50 mol Li,CO,, = 36.95g
0.45 mol Fe_203 = 71.86g
0.033 mol Zn, (POa) 2 = 12.74g
0.933 mol (NH4) 2HPO4 = 123.21g
0.45 mol carbon = 5.40g
(100% excess carbon -4 10.80g)
CA 02460875 2004-03-23
43
(b) Pelletize powder mixture
(c) Heat to 750 C at a rate of 2 /minute in argon.
Hold for 8 hours dwell at 750 C in argon
(d) Cool at a rate of 2 /minute
(e) Powderize pellet.
EXAMPLE IX
Reaction 5. Formation of gamma-LiV,OS (y)
V205 + 0.5 Li2C03 + 0.25 C -~ LiV205 + 3/4 C02
(a) Pre-mix alpha V,,Os, Li2CO, and Shiwinigan Black
(carbon) using ball mix with suitable media.
Use a 25% weight excess of carbon over the
reaction amounts above. For example, according
to reaction above:
Need: 1 mol V2OS 181.88g
0.5 mol Li~CO336.95g
0.25 mol carbon 3.OOg
(but use 25% excess carbon -> 3.75g)
(b) Pelletize powder mixture
(c) Heat pellet to 600 C in flowing argon (or other
inert atmosphere) at a heat rate of
approximately 2 /minute. Hold at 600 C for
about 60 minutes.
CA 02460875 2004-03-23
44
(d) Allow to cool to room temperature in argon at
cooling rate of about 2 /minute.
(e) Powderize.pellet using mortar and pestle
This reaction is able to be conducted at a
temperature in a range of about 400 C to about 650 C in
argon as shown, and also under other inert atmospheres
such as nitrogen or vacuum. This reaction at this
temperature range is primarily C-+ CO. Note that the
reaction C-+ CO primarily occurs at a temperature over
about 650 C (HT, high temperature); and the reaction C
CO2 primarily occurs at a temperature of under about
650 C (LT, low temperature) . The reference to about
650 C is approximate and the designation "primarily"
refers to the predominant reaction thermodynamically
favored although the alternate reaction may occur to some
extent.
EXAMPLE X
Reaction 6. Formation of Li,,V, (P04) ~
V205 + 3/2 Li'C03 + 3 (NH4) 2HP04 + C Li,IV2 (P04) 3+ 2 CO
+ 3/2 CO, + 6 NH3 + 9/2 H20
(a) Pre-mix reactants above using ball mill with
suitable media. Use a 259,; weight excess of
carbon. Thus,
1 mol V2O1181.88g
3/2 mol Li-.CO3 110.84g
3 mol (NH4 ) 2HP04 396.18g
1 mol carbon 12.O1g
.9 CA 02460875 2004-03-23
(but use 25% excess carbon -> 15.O1g)
(b) Pelletize powder mixture
5 (c) Heat pellet at 2 /minute to 300 C to remove CO2
(from Li,CO;) and to remove NHõ H,O. Heat in
an inert atmosphere (e.g. argon). Cool to room
temperature.
10 (d) Powderize and repelletize
(e) Heat pellet in inert atmosphere at a rate of
2 C/minute to 850 C. Dwell for 8 hours at 850 C
15 (f) Cool to room temperature at a rate of 2 /minute
in argon.
(e) Powderize
20 This reaction is able to be conducted at a
temperature in a range of about 700 C to about 950 C in
argon as shown, and also under other inert atmospheres
such as nitrogen or vacuum. A reaction temperature
greater than about 670 C ensures C -> CO reaction is
25 primarily carried out.
Characterization of Active Materials
and Formation and Testing of Cells
30 Referring to Figure 1, the final product
LiFePO41 prepared from Fe'O3 metal compound per Reaction
1(b), appeared brown/black in color. This olivine
material product included carbon that remained after
reaction. Its CuKoc x-ray diffraction pattern contained
35 all of the peaks expected for this material as shown in
Figure 1. The pattern evident in Figure 1 is consistent
with the single phase olivine phosphate, LiFePO4. This
CA 02460875 2004-03-23
46
is evidenced by the position of the peaks in terms of the
scattering angle 2 6(theta), x axis. The x-ray pattern
showed no peaks due to the presence of precursor oxides
indicating that.the solid state reaction is essentially
entirely completed. Here the space group SG = pnma (62)
and the lattice parameters from XRD refinement are
consistent with the olivine structure. The values are a
= 10.2883A (0.0020), b = 5.9759 (0.0037), c = 4.6717A
(0.0012) 0.0072, cell volume = 287.2264A' (0.0685).
Density, p = 3.605 g/cc, zero = 0.452 (0.003). Peak at
full width half maximum, PFWHM = 0.21. Crystallite size
from XRD data = 704A.
The x-ray pattern demonstrates that the product
of the invention was indeed the nominal formula LiFePO4.
The term "nominal formula" refers to the fact that the
relative proportion of atomic species may vary slightly
on the order of 2 percent to 5 percent, or more
typically, 1 percent to 3 percent, and that some portion
of P may be substituted by Si, S or As; and some portion
of 0 may be substituted by halogen, preferably F.
The LiFePOq1 prepared as described immediately
above, was tested in an electrochemical cell. The
positive electrode was prepared as described above, using
19.0mg of active material. The positive electrode
contained, on a weight % basis, 85% active material, 10%
carbon black, and 5;s EPDM. The negative electrode was
metallic lithium. The electrolyte was a 2:1 weight ratio
mixture of ethylene carbonate and dimethyl carbonate
within which was dissolved 1 molar LiPF,;. The cells were
cycled between about 2.5 and about 4.0 volts with
performance as shown in Figures 2 and 3.
Figure 2 shows the results of the first
constant current cycling at 0.2 milliamps per square
CA 02460875 2004-03-23
47
centimeter between about 2.5 and 4.0 volts based upon
about 19 milligrams of the LiFePOq active material in the
cathode (positive electrode). In an as prepared, as
assembled, initial condition, the positive electrode
active material is LiFePO4. The lithium is extracted
from the LiFePOq during charging of the cell. When fully
charged, about 0.72 unit of lithium had been removed per
formula unit. Consequently, the positive electrode
active material corresponds to Li,_;:FeP04 where x appears
to be equal to about 0.72, when the cathode material is
at 4.0 volts versus Li/Li'. The extraction represents
approximately 123 milliamp hours per gram corresponding
to about 2.3 milliamp hours based on 19 milligrams active
material. Next, the cell is discharged whereupon a
quantity of lithium is re-inserted into the LiFePO4. The
re-insertion corresponds to approximately 121 milliamp
hours per gram proportional to the insertion of
essentially all of the lithium. The bottom of the curve
corresponds to approximately 2.5 volts. The total
cumulative capacity demonstrated during the entire
extraction-insertion cycle is 244mAh/g.
Figure 3 presents data obtained by multiple
constant current cycling at 0.2 milliamp hours per square
centimeter of the LiFePOq versus lithium metal counter
electrode between 2.5 and 4.0 volts. Data is shown for
two temperatures, 23 C and 60 C. Figure 3 shows the
excellent rechargeability of the LiFePO4 cell, and also
shows good cycling and capacity of the cell. The
performance shown after about 190 to 200 cycles is good
and shows that electrode formulation is very desirable.
Referring to Figure 4, there is shown data for
the final product LiFeO,9Mgp,1PO91 prepared from the metal
compounds Fe20, and Mg (OH) ,-4 Mg (OH) 2, per Reaction 2(b) .
Its CuKa x-ray diffraction pattern contained all of the
CA 02460875 2004-03-23
48
peaks expected for this material as shown in Figure 4.
The pattern evident in Figure 4 is consistent with the
single phase olivine phosphate compound, LiFeo.9Mgo11PO4_
This is evidenced by the position of the peaks in terms
of the scattering angle 2 A(theta), x axis. The x-ray
pattern showed no peaks due to the presence of precursor
oxides indicating that the solid state reaction is
essentially entirely completed. Here the space group SG
= Pnma (62) and the lattice parameters from XRD
refinement are consistent with the olivine structure.
The values are a = 10.2688A (0.0069), b = 5.9709A
(0.0072), c 4.6762A (0.0054), cell volume = 286.7208A
(0.04294), p 3.617 g/cc, zero = 0.702 (0.003), PFWHM =
0.01, and crystallite = 950A.
The x-ray pattern demonstrates that the product
of the invention was indeed the nominal formula
LiFeo,9Mgo11PO4, The term "nominal formula" refers to the
fact that the relative proportion of atomic species may
vary slightly on the order of 2 percent to 5 percent, or
more typically, 1 percent to 3 percent, and that some
substitution of P and 0 may be made while maintaining the
basic olivine structure.
The LiFeO,.,Mg0,.1POa, prepared as described
immediately above, was tested in an electrochemical cell.
The positive electrode was prepared as described above,
using 18.9mg of active materials. The positive
electrode, negative electrode and electrolyte were
prepared as described earlier and in connection with
Figure 1. The cell was between about 2.5 and about 4.0
volts with performance as shown in Figures 4, 5 and 6.
Figure 5 shows the results of the first
constant current cycling at 0.2 milliamps per square
centimeter between about 2.5 and 4.0 volts based upon
CA 02460875 2004-03-23
49
about 18. 9. milligrams of the LiFeo.,,Mgo_1PO4 active
material in the cathode (positive electrode). In an as
prepared, as assembled, initial condition, the positive
electrode active material is LiFeo,.,Mgo_,PO4. The lithium
is extracted from the LiFeo,4Mgo_1PO4 during charging of
the cell. When fully charged, about 0.87 units of
lithium have been removed per formula unit.
Consequently, the positive electrode active material
corresponds to Li1_,.Fe(0..,Mgo11POq where x appears to be equal
to about 0.87, when the cathode material is at 4.0 volts
versus Li/Li+. The extraction represents approximately
150 milliamp hours per gram corresponding to about 2.8
milliamp hours based on 18.9 milligrams active material.
Next, the cell is discharged whereupon a quantity of
lithium is re-inserted into the LiFeo.QMgo11P04 . The re-
insertion corresponds to approximately 146 milliamp hours
per gram proportional to the insertion of essentially all
of the lithium. The bottom of the curve corresponds to
approximately 2.5 volts. The total cumulative specific
capacity over the entire cycle is 296 mAhr/g. This
material has a much better cycle profile than the
LiFeP04. Figure 5(LiFeo,.aMgo,,P04) shows a very well
defined and sharp peak at about 150 mAh/g. In contrast,
Figure 2(LiFePOa) shows a very shallow slope leading to
the peak at about 123 mAh/g. The Fe-phosphate (Figure 2)
provides 123 mAh/g compared to its theoretical capacity
of 170 mAh/g. This ratio of 123/170, 72'25 is relatively
poor compared to the Fe/Mg-phosphate. The Fe/Mg-
phosphate (Figure 5) provides 150 mAh/g compared to a
theoretical capacity of 160, a ratio of 150/160 or 94%.
Figure 6 presents data obtained by multiple
constant current cycling at 0.2 milliamp hours per square
centimeter of the LiFe;;,9Mgo.1PO4 versus lithium metal
counter electrode between 2.5 and 4.0 volts. Figure 6
shows the excellent rechargeability of the
CA 02460875 2004-03-23
Li/LiFeo.9Mgo,1POq cell, and also shows good cycling and
capacity of the cell. The performance shown after about
150 to 160 cycles is very good and shows that electrode
formulation LiFeo,,Mgo,,P09 performed significantly better
5 than the LiFeP04. Comparing Figure 3(LiFePO4) to Figure
6(LiFeo,9Mgo,1P04) it can be seen that the Fe/Mg-phosphate
maintains its capacity over prolonged cycling, whereas
the Fe-phosphate capacity fades significantly.
10 Figure 7 shows the results of the first
constant current cycling at 0.2 milliamps per square
centimeter between about 2.5 and 4.0 volts based upon
about 16 milligrams of the LiFeo_AMgo,2PO4 active material
in the cathode (positive electrode). In an as prepared,
15 as assembled, initial condition, the positive electrode
active material is LiFeo.HMgo,2POa. The lithium is
extracted from the LiFeQ_IMgo,-'POa during charging of the
cell. When fully charged, about 0.79 units of lithium
have been removed per formula unit. Consequently, the
20 positive electrode active material corresponds to
LiFeo8BMgo.2PO4 where x appears to be equal to about 0.79,
when the cathode material is at 4.0 volts versus Li/Li'.
The extraction approximately 140 milliamp hours per gram
corresponding to about 2.2 milliamp hours based on 16
25 milligrams active material. Next, the cell is discharged
whereupon a quantity of lithium is re-inserted into the
LiFeo8BMgo22PO9. The re-insertion corresponds to
approximately 122 milliamp hours per gram proportional to
the insertion of essentially all of the lithium. The
30 bottom of the curve corresponds to approximately 2.5
volts. The total cumulative specific capacity over the
entire cycle is 262 mAhr/g.
Referring to Figure 8, there is shown data for
35 the final product LiFen,4Cao,1P04, prepared from Fe203 and
Ca(OH)2 by Reaction 3. Its CuKa x-ray diffraction
CA 02460875 2004-03-23
51
pattern contained all of the peaks expected for this
material as shown in Figure S. The pattern evident in
Figure 8 is consistent with the single phase olivine
phosphate compound, LiFeo.,,CaO11PO4. This is evidenced by
the position of the peaks in terms of the scattering
angle 2 8(theta), x axis. The x-ray pattern showed no
peaks due to the presence of precursor oxides indicating
that the solid state reaction is essentially entirely
completed. Here the space group SG = Pnma (62) and the
lattice parameters from XRD refinement are consistent
with olivine. The values are a = 10.3240A (0.0045), b
6.0042A (0.0031), c = 4.6887A (0.0020), cell volume =
290.6370A (0.1807), zero = 0.702 (0.003), p = 3.62 g/cc,
PFWHM = 0.18, and crystallite = 680A. The x-ray pattern
demonstrates that the product of the invention was indeed
the nominal formula LiFea,9Cao.1P04.
Figure 9 shows the results of the first
constant current cycling at 0.2 milliamps per square
centimeter between about 2.5 and 4.0 volts based upon
about 18.5 milligrams of the LiFeo.kCao,2P04 active
material in the cathode (positive electrode). In an as
prepared, as assembled, initial condition, the positive
electrode active material is LiFeo8RCao.2PO4. The lithium
is extracted from the LiFeo.rCao,,P0a during charging of
the cell. When fully charged, about 0.71 units of
lithium have been removed per formula unit.
Consequently, the positive electrode active material
corresponds to LiFeo8aCaO2~PO4 where x appears to be equal
to about 0.71, when the cathode material is at 4.0 volts
versus Li/Li*. The extraction represents approximately
123 milliamp hours per gram corresponding to about 2.3
milliamp hours based on 18.5 milligrams active material.
Next, the cell is discharged whereupon a quantity of
lithium is re-inserted into the LiFeO8RCa0 ,2PO4. The re-
insertion corresponds to approximately 110 milliamp hours
CA 02460875 2004-03-23
52
per gram proportional to the insertion of nearly all of
the lithium. The bottom of the curve corresponds to
approximately 2.5 volts. The total specific cumulative
capacity over the entire cycle is 233 mAhr/g.
Figure 10 shows the results of the first
constant current cycling at 0.2 milliamps per square
centimeter between about 2.5 and 4.0 volts based upon
about 18.9 milligrams of the LiFeO,yZno22'PO9 olivine active
material in the cathode (positive electrode). In an as
prepared, as assembled, initial condition, the positive
electrode active material is LiFeo,,Znp,2PO41 prepared from
Fe203 and Zn,, (P04) 2 by Reaction 4. The lithium is
extracted from the LiFeo.AZno. POQ during charging of the
cell. When fully charged, about 0.74 units of lithium
have been removed per formula unit. Consequently, the
positive electrode active material corresponds to Lil_
,Fe0.8Zn0.2P04 where x appears to be equal to about 0.74,
when the cathode material is at 4.0 volts versus Li/Li'.
The extraction represents approximately 124 milliamp
hours per gram corresponding to about 2.3 milliamp hours
based on 18.9 milligrams active material. Next, the cell
is discharged whereupon a quantity of lithium is re-
inserted into the LiFeo,eZno.,P04. The re-insertion
corresponds to approximately 108 milliamp hours per gram
proportional to the insertion of nearly all of the
lithium. The bottom of the curve corresponds to
approximately 2.5 volts.
Referring to Figure 11, the final product
LiV2O51 prepared by Reaction 5, appeared black in color.
Its CuKcx x-ray diffraction pattern contained all of the
peaks expected for this material as shown in Figure 11.
The pattern evident in Figure 11 is consistent with a
single oxide compound gamma-LiV,O-,. This is evidenced by
the position of the peaks in terms of the scattering
CA 02460875 2004-03-23
53
angle 2 0(theta), x axis. The x-ray pattern showed no
peaks due to the presence of precursor oxides indicating
that the solid state reaction is essentially entirely
completed.
The x-ray pattern demonstrates that the product
of the invention was indeed the nominal formula gamma-
LiV205. The term "nominal formula" refers to the fact
that the relative proportion of atomic species may vary
slightly on the order of 2 percent to 5 percent, or more
typically, 1 percent to 3 percent.
The LiV,O5 prepared as described immediately
above, was tested in an electrochemical cell. The cell
was prepared as described above and cycled with
performance as shown in Figures 12 and 13.
Figure 12 shows the results of the first
constant current cycling at 0.2 milliamps per square
centimeter between about 2.8 and 3.8 volts based upon
about 15.0 milligrams of the LiV2O5 active material in
the cathode (positive electrode). In an as prepared, as
assembled, initial condition, the positive electrode
active material is LiV-.Os. The lithium is extracted from
the LiV2O5 during charging of the cell. When fully
charged, about 0.93 unit of lithium had been removed per
formula unit. Consequently, the positive electrode
active material corresponds to Li,_,;V,O~ where x appears to
be equal to about 0.93, when the cathode material is at
3.8 volts versus Li/Li'. The extraction represents
approximately 132 milliamp hours per gram corresponding
to about 2.0 milliamp hours based on 15.0 milligrams
active material. Next, the cell is discharged whereupon
a quantity of lithium is re-inserted into the LiV205. The
re-insertion corresponds to approximately 130 milliamp
hours per gram proportional to the insertion of
CA 02460875 2004-03-23
54
essentially all of the lithium. The bottom of the curve
corresponds to approximately 2.8 volts.
Figure 13 presents data obtained by multiple
constant current cycling at 0.4 milliamp hours per square
centimeter (C/2 rate)of the LiV,O-, versus lithium metal
counter electrode between 3.0 and 3.75 volts. Data for
two temperature conditions are shown, 23 C and 60 C.
Figure 13 is a two part graph with Figure 13A showing the
excellent rechargeability of the LiV,O,. Figure 13B
shows good cycling and capacity of the cell. The
performance shown up to about 300 cycles is good.
Referring to Figure 14, the final product
Li3V2 (P04) 3, prepared by Reaction 6, appeared green/black
in color. Its CuKcx x-ray diffraction pattern contained
all of the peaks expected for this material as shown in
Figure 14. The pattern evident in Figure 14 is
consistent with a single phosphate compound Li3V2(P04)3 of
the monoclinic, Nasicon phase. This is evidenced by the
position of the peaks in terms of the scattering angle 2
0(theta), x axis. The x-ray pattern showed no peaks due
to the presence of precursor oxides indicating that the
solid state reaction is essentially entirely completed.
The x-ray pattern demonstrates that the product
of the invention was indeed the nominal formula
Li3V2(PO4)3, The term "nominal formula" refers to the fact
that the relative proportion of atomic species may vary
slightly on the order of 2 percent to 5 percent, or more
typically, 1 percent to 3 percent; and that substitution
of P and 0 may occur.
The Li3V2(P04) , prepared as described immediately
above, was tested in an electrochemical cell. The cell
was prepared as described above, using 13.8mg of active
CA 02460875 2004-03-23
material. The cell was prepared as described above and
cycled between about 3.0 and about 4.2 volts using the
EVS technique with performance as shown in Figures 16 and
17. Figure 16 shows specific capacity versus electrode
5 potential against Li. Figure 17 shows differential
capacity versus electrode potential against Li.
A comparative method was used to form
Li3V2(P04)3. Such method was reaction without carbon and
10 under H2-reducing gas as described in U.S. Patent No.
5,871,866. The final product, prepared as per U.S.
Patent No. 5,871,866, appeared green in color. Its CuKa
x-ray diffraction pattern contained all of the peaks
expected for this material as shown in Figure 15. The
15 pattern evident in Figure 15 is consistent with a
monoclinic Nasicon single phase phosphate compound
Li3V2(P04)3. This is evidenced by the position of the
peaks in terms of the scattering angle 2 8(theta), x
axis. The x-ray pattern showed no peaks due to the
20 presence of precursor oxides indicating that the solid
state reaction is essentially entirely completed.
Chemical analysis for lithium and vanadium by atomic
absorption spectroscopy showed, on a percent by weight
basis, 5.17 percent lithium and 26 percent vanadium. This
25 is close to the expected result of 5.11 percent lithium
and 25 percent vanadium.
The chemical analysis and x-ray patterns of
Figures 14 and 15 demonstrate that the product of Figure
30 14 was the same as that of Figure 15. The product of
Figure 14 was prepared without the undesirable H2
atmosphere and was prepared by the novel carbothermal
solid state synthesis of the invention.
35 Figure 16 shows a voltage profile of the test
cell, based on the Li,V_(P04){ positive electrode active
CA 02460875 2004-03-23
56
material made by the process of the invention and as
characterized in Figure 14. It was cycled against a
lithium metal counter electrode. The data shown in
Figure 16 is based on the Electrochemical Voltage
Spectroscopy (EVS) technique. Electrochemical and
kinetic data were recorded using the Electrochemical
Voltage Spectroscopy (EVS) technique. Such technique is
known in the art as described by J. Barker in Synth, Met
28, D217 (1989); Synth. Met. 32, 43 (1989); J. Power
Sources, 52, 185 (1994); and Electrochemica Acta, Vol.
40, No. 11, at 1603 (1995). Figure 16 clearly shows and
highlights the reversibility of the product. The positive
electrode contained about 13.8 milligrams of the
Li3V2(PO4)3 active material. The positive electrode
showed a performance of about 133 milliamp hours per gram
on the first discharge. In Figure 16, the capacity in,
and the capacity out are essentially the same, resulting
in essentially no capacity loss. Figure 17 is an EVS
differential capacity plot based on Figure 16. As can be
seen from Figure 17, the relatively symmetrical nature of
peaks indicates good electrical reversibility, there are
small peak separations (charge/discharge), and good
correspondence between peaks above and below the zero
axis. There are essentially no peaks that can be related
to irreversible reactions, since all peaks above the axis
(cell charge) have corresponding peaks below the axis
(cell discharge), and there is essentially no separation
between the peaks above and below the axis. This shows
that the carbothermal method of the invention produces
high quality electrode material.
Figure 18 presents data obtained by multiple
constant current cycling at 0.2 milliamp hours per square
centimeter of the LiFe(,88Mgo22PO4 versus lithium metal
counter electrode between 2.5 and 4.0 volts. Figure 18
shows the excellent rechargeability of the
CA 02460875 2004-03-23
57
Li/LiFeo,BMgo,2POq cell, and also shows good cycling and
capacity of the cell. The performance shown after about
110 to 120 cycles at 23 C is very good and shows that
electrode formulation LiFeo8SMgo22PO4 performed
significantly better than the LiFePO4. The cell cycling
test at 60 C was started after the 23 C test and was
ongoing. Comparing Figure 3(LiFePO4) to Figure 18
(LiFeo,BMgo,2PO4) 1 it can be seen that the Fe/Mg-phosphate
maintains its capacity over prolonged cycling, whereas
the Fe-phosphate capacity fades significantly.
In addition to the above cell tests, the active
materials of the invention were also cycled against
insertion anodes in non-metallic, lithium ion, rocking
chair cells.
The lithium mixed metal phosphate and the
lithium metal oxide were used to formulate a cathode
electrode. The electrode was fabricated by solvent
casting a slurry of the treated, enriched lithium
manganese oxide, conductive carbon, binder, plasticizer
and solvent. The conductive carbon used was Super P (MMM
Carbon). Kynar Flex 28010 was used as the binder and
electronic grade acetone was used as a solvent. The
preferred plasticizer was dibutyl phthalate (DPB). The
slurry was cast onto glass and a free-standing electrode
was formed as the solvent was evaporated. In this
example, the cathode had 23.1mg LiFeo,gMgo,,P04 active
material. Thus, the proportions are as follows on a
percent weight basis: 804, active material; 8% Super P
carbon; and 12% Kynar binder.
A graphite counter electrode was prepared for
use with the aforesaid cathode. The graphite counter
electrode served as the anode in the electrochemical
cell. The anode had 10.8 mg of the MCMB graphite active
CA 02460875 2004-03-23
58
material. The graphite electrode was fabricated by
solvent casting a slurry of MCMB2528 graphite, binder,
and casting solvent. MCMB2528 is a mesocarbon microbead
material supplied by Alumina Trading, which is the U.S.
distributor for the supplier, Osaka Gas Company of Japan.
This material has a density of about 2.24 grams per cubic
centimeter; a particle size maximum for at least 95% by
weight of the particles of 37 microns; median size of
about 22.5 microns and an interlayer distance of about
0.336. As in the case of the cathode, the binder was a
copolymer of polyvinylidene difluoride (PVdF) and
hexafluoropropylene (HFP) in a wt. ratio of PVdF to HFP
of 88:12. This binder is sold under the designation of
Kynar Flex 28010, showing it's a registered trademark.
Kynar Flex is available from Atochem Corporation. An
electronic grade solvent was used. The slurry was cast
onto glass and a free standing electrode was formed as
the casting solvent evaporated. The electrode
composition was approximately as follows on a dry weight
basis: 85% graphite; 12% binder; and 3% conductive
carbon.
A rocking chair battery was prepared comprising
the anode, the cathode, and an electrolyte. The ratio of
the active cathode mass to the active anode mass was
about 2.14:1. The two electrode layers were arranged
with an electrolyte layer in between, and the layers were
laminated together using heat and pressure as per the
Bell Comm. Res. patents. In a preferred method, the cell
is activated with EC/DMC solvent in a weight ratio of 2:1
in a solution containing 1 M LiPF, salt.
Figures 19 and 20 show data for the first four
complete cycles of the lithium ion cell having the
LiFeo,9Mgo,1P04 cathode and the MCMB2528 anode. The cell
comprised 23.1mg active LiFeo,s,Mgo.1P04 and 10.8mg active
CA 02460875 2004-03-23
59
MCMB2528 for a cathode to anode mass ratio of 2.14. The
cell was charged and discharged at 23 C at an approximate
C/10 (10 hour) rate between voltage limits of 2.50 V and
3.60 V. The voltage profile plot (Figure 19) shows the
variation in cell voltage versus time for the
LiFeQ99Mgo11PO4/MCMB2528 lithium ion cell. The symmetrical
nature of the charge-discharge is clearly evident. The
small degree of voltage hysteresis between the charge and
discharge processes is evidence for the low overvoltage
in the system, which is very good. Figure 20 shows the
variation of LiFeo.9Mgo,1P04 specific capacity with cycle
number. Clearly, over the cycles shown, the material
demonstrates good cycling stability.
Figure 21 shows data for the first three
complete cycles of the lithium ion cell having the gamma-
LiV205 cathode and the MCMB2528 anode. The cell prepared
was a rocking chair, lithium ion cell as described above.
The cell comprised 29.1mg gamma-LiV-,05 cathode active
material and 12.2mg MCMB2528 anode active material, for a
cathode to anode mass ratio of 2.39. As stated earlier,
the liquid electrolyte used was EC/DMC (2:1) and 1M
LiPF6. The cell was charged and discharged at 23 C at an
approximate C/10 (10 hour) rate between voltage limits of
2.50 V and 3.65 V. The voltage profile plot (Figure 21)
shows the variation in cell voltage versus time for the
LiV2O5/MCMB2528 lithium ion cell. The symmetrical nature
of the charge-discharge is clearly evident. The small
degree of voltage hysteresis between the charge and
discharge processes is evidence for the low overvoltage
in the system, which is very good.
In summary, the invention provides new
compounds LiaMIbMI I,_ ( POa ),i and gamma-LiV,O5, by new methods
which are adaptable to commercial scale production. The
Li1MI1_yMIIyPO4 compounds are isostructural olivine
CA 02460875 2004-03-23
compounds as demonstrated by XRD analysis. Substituted
compounds, such as LiFe,_,,Mg,,P04 show better performance
than LiFePOq unsubstituted compounds when used as
electrode active materials. The method of the invention
5 utilizes the reducing capabilities of carbon along with
selected precursors and reaction conditions to produce
high quality products suitable as electrode active
materials or as ion conductors. The reduction capability
of carbon over a broad temperature range is selectively
10 applied along with thermodynamic and kinetic
considerations to provide an energy-efficient, economical
and convenient process to produce compounds of a desired
composition and structure. This is in contrast to known
methods.
Principles of carbothermal reduction have been
applied to produce pure metal from metal oxides by
removal of oxygen. See, for example, U.S. Patent Nos.
2,580,878, 2,570,232, 4,177,060, and 5,803,974.
Principles of carbothermal and thermal reduction have
also been used to form carbides. See, for example, U.S.
Patent Nos. 3,865,745 and 5,384,291; and non-oxide
ceramics (see U.S. Patent No. 5,607,297). Such methods
are not known to have been applied to form lithiated
products or to form products without oxygen abstraction
from the precursor. The methods described with respect to
the present invention provide high quality products which
are prepared from precursors which are lithiated during
the reaction without oxygen abstraction. This is a
surprising result. The new methods of the invention also
provide new compounds not known to have been made before.
For example, alpha-V_0F, is conventionally
lithiated electrochemically against metallic lithium.
Thus, alpha-V20!, is not suitable as a source of lithium
for a cell. As a result, alpha-V OS is not used in an
CA 02460875 2004-03-23
61
ion cell. In the present invention, alpha-V205 is
lithiated by carbothermal reduction using a simple
lithium-containing compound and the reducing capability
of carbon to form a gamma-LiV2O!,. The single phase
compound, gamma-LiV_O.~ is not known to have been directly
and independently prepared before. There is not known to
be a direct synthesis route. Attempts to form it as a
single phase resulted in a mixed phase product containing
one or more beta phases and having the formula Li,V2O5
with O< x<_ 0.49. This is far different from the
present single phase gamma-Li,V.O, with x equal to one, or
very close to one. The flexibility of the process of the
present invention is such that it can be conducted over a
wide temperature range. The higher the temperature, the
more quickly the reaction proceeds. For example, at
650 C, conversion of alpha-V,05 to gamma-LiV205 occurs in
about one hour, and at 5000 it takes about 8 hours.
Here, about one quarter (1/4) atomic unit of carbon is
used to reduce one atomic unit of vanadium, that is,
V+5V'5 to V+5V'4. The predominate reaction is C to CO2
where for each atomic unit of carbon at ground state
zero, a plus 4 oxidation state results. Correspondingly,
for each 1/4 atomic unit of carbon, one atomic unit of
vanadium is reduced from V+5 to V+4. (See Reaction 5).
The new product, gamma-LiV,O~ is air-stable and suitable
as an electrode material for an ion cell or rocking chair
battery.
The convenience and energy efficiency of the
present process can also be contrasted to known methods
for forming products under reducing atmosphere such as H2
which is difficult to control, and from complex and
expensive precursors. In the present invention, carbon
is the reducing agent, and simple, inexpensive and even
naturally occurring precursors are useable. For example,
it is possible to produce LiFePO4 from Fe2Oj, a simple
CA 02460875 2004-03-23
62
common oxide. (See Reaction ib). The production of
LiFePO4 provides a good example of the thermodynamic and
kinetic features of the method. Iron phosphate is
reduced by carbon and lithiated over a broad temperature
range. At about 600 C, the C to CO: reaction
predominates and takes about a week to complete. At
about 750 C, the C to CO reaction predominates and takes
about 8 hours to complete. The C to CO 2 reaction
requires less carbon reductant but takes longer due to
the low temperature kinetics. The C to CO reaction
requires about twice as much carbon, but due to the high
temperature reaction kinetics, it proceeds relatively
fast. In both cases, the Fe in the precursor Fe203 has
oxidation state +3 and is reduced to oxidation (valence)
state +2 in the product LiFePO4. The C to CO reaction
requires that 1-i atomic unit of carbon be used for each
atomic unit of Fe reduced by one valence state. The CO
to CO2 reaction requires that 1/4 atomic unit of carbon
be used for each atomic unit of Fe reduced by one valence
state.
The active materials of the invention are also
characterized by being stable in an as-prepared
condition, in the presence of air and particularly humid
air. This is a striking advantage, because it
facilitates preparation of and assembly of battery
cathodes and cells, without the requirement for
controlled atmosphere. This feature is particularly
important, as those skilled in the art will recognize
that air stability, that is, lack of degradation on
exposure to air, is very important for commercial
processing. Air-stability is known in the art to more
specifically indicate that a material does not hydrolyze
in presence of moist air. Generally, air-stable
materials are also characterized by Li being extracted
therefrom above about 3.0 volts versus lithium. The
CA 02460875 2004-03-23
63
higher the extraction potential, the more tightly bound
the lithium ions are to the host lattice. This tightly
bound property generally confers air stability on the
material. The air-stability of the materials of the
invention is consistent with the stability demonstrated
by cycling at the conditions stated herein. This is in
contrast to materials which insert Li at lower voltages,
below about 3.0 volts versus lithium, and which are not
air-stable, and which hydrolyze in moist air.
While this invention has been described in
terms of certain embodiments thereof, it is not intended
that it be limited to the above description, but rather
only to the extent set forth in the following claims.
The embodiments of the invention in which an
exclusive property or privilege is claimed are defined in
the following claims.