Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
a
77486-17
CA 02463268 2004-04-05
SPECIFICATION
PROCESS FOR PURIFICATION OF AQUEOUS SOLUTION OF NICKEL
SULFATE CONTAINING COBALT AND CALCIUM
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
The present invention relates td a process for
purification of an aqueous solution of nickel sulfate
containing cobalt and calcium, more particularly an
industrially high efficiency process for :purification of the
aqueous solution of nickel sulfate containing cobalt and
calcium, the former being present at a high concentration,
which can increase cobalt treatment capacity and produce a
purified aqueous solution of nickel sulfate having reduced
cobalt and calcium concentrations by improving cobalt
extraction efficiency in the exchange reaction.
DESCRIPTION OF THE PRIOR ART
A process for purification of a crude aqueous
solution of nickel sulfate containing a variety of
impurities produces a high-purity nickel sulfate solution at
industrially high efficiency. The thus-produced high purity
aqueous solution of nickel sulfate can be treated by various
processes, adopted as required, to obtain high-purity nickel
compounds, e.g., nickel sulfate by concentration (e. g.,
crystallization), nickel oxide by roasting, and nickel
carbonate by neutralization with sodium carbonate.
Those nickel compounds; e.g., nickel sulfate,
oxide and carbonate, have various industrial applications.
These include materials for general electrolytic plating,
for electroless plating for computer hard disks, and for
1
CA 02463268 2004-04-05
77486-17
catalysts and batteries. Nickel sulfate, in particular, has
been extensively used as a material for plating and as a
material for secondary batteries. One of the raw materials
for nickel salts, e.g., nickel sulfate, is crude nickel
sulfate containing cobalt at a relatively low concentration.
A crude aqueous solution of nickel sulfate, produced by
treating a raw material containing cobalt at several
percent, is also used fairly extensively. These raw
materials include nickel matte, and compound hydroxide or
sulfate of nickel and cobalt. Therefore, the process for
producing a purified aqueous solution of nickel sulfate from
the crude solution or the like generally includes a step for
separating nickel and cobalt from each other. Cobalt is
rarer and more expensive than nickel, and is separated and
purified into various products, e.g., electrolytically
purified cobalt, and cobalt chloride and carbonate, to
improve economic efficiency of the above process. The
industrial applications of nickel salts often require
impurities present in the aqueous solution of nickel sulfate
to be minimized. These impurities include ammonia, sodium,
iron, zinc, copper, calcium and magnesium, in addition to
cobalt.
Solvent extraction is a normal choice for
purifying a crude aqueous solution of nickel sulfate
containing impurities. The solvent extraction processes
fall into two general concepts for purifying the solution;
(1) extraction of impurities in an organic phosphorous-
containing extractant, e.g:, acidic phosphonic or phosphinic
acid ester, and (2) extraction of nickel in an organic
extractant, followed by stripping of the nickel-loaded
organic phase with sulfuric acid.
However, each concept involves problems resulting
2
CA 02463268 2004-04-05
77486-17
from release of a hydrogen ion while impurities or nickel
present in a crude aqueous solution is being extracted with
an acid extractant, which invariably needs use of sodium
hydroxide or ammonia as a neutralizer.
When impurities are to be extracted from a crude
aqueous solution of nickel sulfate with an acid extractant,
for example, impurities (e. g., cobalt, calcium, iron, zinc,
copper or the like), which are generally extracted at a
lower pH level than nickel; can be separated in the
extractant by adjusting pH of the system to purify the
solution. However, this involves problems resulting from
contamination of the purified aqueous solution with the
sodium or ammonium ion, incorporated as a neutralizer
necessary for the extraction process.
On the other hand, when nickel is to be extracted
with an acid extractant from a crude aqueous solution of
nickel sulfate in the extractant, it is loaded together with
impurities, which are extracted at a lower pH level than
nickel. Stripping with sulfuric acid to recover nickel from
the above loaded extractant is difficult to remove all of
these impurity elements.
Some processes have been proposed to solve these
problems, where a crude aqueous solution of nickel sulfate
containing cobalt and other impurities is brought into
contact with an acid extractant treated beforehand to
extract nickel therein (such an extractant is hereinafter
sometimes referred to ws nickel-loaded, acid extractant) to
exchange (substitute) nickel in the nickel-loaded, acid
extractant with impurities, beginning with cobalt, which are
extracted in an acid extractant in preference to nickel, in
order to produce a purified aqueous solution of nickel
3
CA 02463268 2004-04-05
77486-17
sulfate and, at the same time, the organic extractant with
concentrated cobalt. These processes may be represented by
the ones described below:
(1) A solvent extraction process for separating impurities,
e.g., cobalt; calcium, magnesium and iron, from a crude
aqueous solution of nickel sulfate with a nickel-containing
alkyl phosphonic acid ester or alkyl phosphinic acid as an
extractant (disclosed in, e.g., Japanese Patent Laid-open
Publication No. 10-30135 (pages l and 2)).
(2) A nickel sulfate purification process comprising a
series of steps: an extraction step with an acid extractant
for separating nickel from a crude aqueous solution of
nickel sulfate containing sodium or ammonia at a high
concentration to prepare a nickel-loaded organic phase; a
step for washing the nickel-loaded organic phase prepared in
the preceding extraction step with a nickel-containing
washing solution; and an exchanging step fox exchanging
(substituting) nickel in the nickel-loaded organic phase
washed in the preceding step with impurities, e.g., cobalt,
present in the crude aqueous solution of nickel sulfate, by
reacting the organic phase with the crude solution
containing cobalt at a high concentration, to produce a
purified aqueous solution of nickel sulfate and, at the same
time, an organic phase in which cobalt is concentrated.
This process further comprises steps; a stripping step with
diluted sulfuric acid for selectively stripping nickel from
the impurity-loaded organic extractant prepared in the
preceding exchanging step; a stripping step with
hydrochloric acid for recovering cobalt from the organic
phase prepared in the preceding stripping step for selective
stripping of nickel; a step for washing the organic phase
prepared in the preceding cobalt recovery step; and a
4
CA 02463268 2004-04-05
r
77486-17
stripping step with sulfuric acid for separating other
impurities from the organic phase washed in the preceding
step; where part of the impurity-free organic phase prepared
in the preceding impurity stripping step is recycled back to
the extraction step as an acid extractant, and the remainder
is used for diluting the nickel-loaded organic phase
(disclosed in, e.g., Japanese Patent Laid-open Publication
No. 10-310437 (pages 1 to 5)).
These proposals have been contributing to
producing a high-purity, refined aqueous solution of nickel
sulfate from a crude aqueous solution of nickel sulfate
containing impurities and recovering cobalt. More recently,
however, demands have been increasing for processes which
can treat a crude aqueous solution of nickel sulfate
containing cobalt and other impurities at a higher
concentration to produce a highly pure aqueous solution of
nickel sulfate and, at the same time, improve efficiency of
recovering expensive cobalt.
To satisfy these demands, an application of the
above processes (disclosed in, e.g., Japanese Patent Laid-
open Publication No. 10-310437 (pages 1 to 5)) is expected,
because of their potential for producing a highly pure
aqueous solution of nickel sulfate from a crude aqueous
solution of nickel sulfate and, at the same time, an organic
solvent in which cobalt is concentrated. These processes
can be of industrially high efficiency, when they can have
increased capacity for treating a starting material of
higher cobalt concentration, because an existing production
system for purifying an aqueous solution of nickel sulfate
can be positively utilized for nickel/cobalt separation to
increase recovered cobalt production.
5
CA 02463268 2004-04-05
77486-17
Treatment of a starting material of higher cobalt
concentration will give a crude aqueous solution of nickel
sulfate of higher cobalt concentration. There are two
concepts to increase cobalt treatment capacity for the step
of exchanging nickel in the nickel-loaded organic phase with
cobalt and other impurities present in a crude aqueous
solution of nickel sulfate in these processes;
(1) increasing the flow rate of the nickel-loaded organic
phase to increase the amount of cobalt to be extracted per
unit time, and (2) increasing the cobalt concentration in
the organic phase from the exchanging reaction step. The
former concept of increasing the treatment flow rate is a
simple approach, but needs large investments to increase
capacity of the solvent extraction reaction facilities,
e.g., mixer settler, in proportion to the increased flow
rate to keep a residence time required for separation of the
nickel-loaded organic phase from a crude aqueous solution of
nickel sulfate, which may harm economic efficiency.
For the latter concept, on the other hand, it is
affective to increase the concentration of cobalt in the
exchanged organic phase by increasing the concentration of
an acid extractant in the nickel-loaded organic phase and
cobalt extraction efficiency. When phosphoric or phosphonic
acid is used as an acid extractant for the exchanging
reaction, 1 mol of cobalt or calcium can be extracted per
2 moll of the extractant. It is the maximum attainable
extraction rate of cobalt or calcium (hereinafter referred
to as the stoichiometric extraction rate). With
2-ethylhexylphosphonic acid mono-2-ethylhexyl ester as an
extractant, the stoichiometric extraction rate of cobalt is
I8.3g/L in the organic solvent containing the extractant at
20~ by volume and a diluent. On a commercial scale,
6
CA 02463268 2004-04-05
77486-17
however, cobalt extraction efficiency is generally low at 40
to 60~ of the stoichiometric extraction rate.
The low extraction rate results from intentional
control of the cobalt concentration to stabilize its
extraction, because (1) driving force for the extraction
reaction is decreased with an extractant having a larger
number of functional groups bound to the nickel or cobalt
ions than an extractant having a larger number of functional
groups bound to the hydrogen ions, (2) utilization of nickel
in the exchanging reaction is decreased, when pH level of
the reaction system is increased to increase extraction
capacity, which is accompanied by accelerated extraction of
nickel to increase its concentration in the exchanged
organic phase, and (3) an increased cobalt concentration in
the organic phase generally increases its viscosity, which
decreases efficiency for separating the organic phase from
the aqueous solution phase. Therefore, there are problems
to be solved also for increasing the cobalt concentration in
the exchanged organic phase to increase cobalt extraction
capacity.
Therefore, there are demands for methods which can
increase the cobalt concentration in the exchanged organic
phase, i.e., increase the cobalt extraction efficiency,
while controlling a viscosity increase of the phase, without
increasing plant capacity, in order to increase cobalt
treatment capacity.
SUMMARY OF THE INVENTION
It is an object of the present invention to
provide an industrially highly efficient process for
purification of an aqueous solution of nickel sulfate
containing cobalt and calcium, the former being present at a
7
CA 02463268 2004-04-05
77486-17
high concentration, which can increase cobalt treatment
capacity and produce a purified aqueous solution of nickel
sulfate with reduced cobalt and calcium concentrations by
improving cobalt extraction efficiency in the exchange
reaction, in consideration of the above-described problems
involved in the conventional techniques.
The inventors of the present invention extensively
studied an aqueous nickel sulfate solution purification
process based on solvent extraction involving an exchanging
reaction in a multi-stage, countercurrent reaction tank
system, attempting to attain the above object. As a result,
the inventors found that cobalt extraction efficiency can be
improved by bringing an aqueous solution phase of nickel
sulfate containing cobalt and calcium into contact with a
mixed organic solvent phase containing an extractant at a
specific concentration, and keeping the aqueous phase at a
specific pH level, and a calcium concentration and a nickel,
cobalt and calcium total concentration in the organic phase
at a specific level. Hence, the present invention was
achieved.
The present invention provides a process for
purification of an aqueous solution of nickel sulfate,
comprising:
a solvent extraction involving an exchanging
reaction in a multi-stage, countercurrent reaction tank
system, wherein:
an aqueous solution of nickel sulfate containing
cobalt and calcium as impurities is supplied as an aqueous
phase to a last stage of the reaction tank system and a
mixed organic solvent containing as an extractant
2-ethylhexylphosphonic acid mono-2-ethylhexyl ester loaded
8
CA 02463268 2004-04-05
77486-17
with nickel and diluted with a hydrocarbon to an extractant
concentration of 20 to 30% by volume, is supplied as an
organic phase to a first stage of the reaction tank system,
to bring these phases into contact with each other for the
exchanging reaction, and
the aqueous phase is kept at a pH level of 4.5 to
5.5 in the last stage, and a calcium concentration and a
nickel, cobalt and calcium total concentration in the
organic phase are kept at 0.4g/L or less and 25g/L or less,
respectively, in the last stage.
According to a first major embodiment of the
present invention, the mixed organic solvent contains the
extractant at a concentration of 25 to 30~ by volume.
According to a second major embodiment of the
present invention, the nickel, cobalt and calcium total
concentration in the organic phase is 20g/L or less in the
last stage.
According to a third major embodiment of the
present invention, the aqueous phase is kept at a pH level
of 4.8 to 5.2 in the last stage.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a graph showing viscosities of the
organic phase plotted against nickel, cobalt and calcium
total concentrations in the organic phase.
Figure 2 is a graph showing nickel, cobalt and
calcium total concentrations in the organic phase plotted
against pH levels for the exchanging reaction.
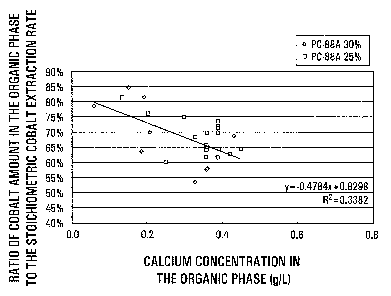
Figure 3 is a graph showing cobalt extraction
efficiencies (ratios of cobalt amounts in the organic phase
9
CA 02463268 2004-04-05
77486-17
to the stoichiometric cobalt extraction rate) plotted
against calcium concentrations in the organic phase.
DETAILED DESCRIPTION OF THE INVENTION
The process of the present invention for
purification of an aqueous solution of nickel sulfate
containing cobalt and calcium is described in detail.
The process of the present invention for
purification of an aqueous solution of nickel sulfate
containing cobalt and calcium, the former being present at a
high concentration, is of industrially high efficiency,
because it can increase cobalt treatment capacity without
increasing plant capacity by improving cabalt extraction
efficiency in the exchange reaction between the aqueous
solution and nickel-loadedacid extractant: As such, it is
suitable for an exchanging reaction step in a nickel sulfate
purification process comprising several steps; e.g.,
extraction step for producing the nickel-loaded organic
phase, step for exchanging with cobalt present in a crude
aqueous solution of nickel sulfate, step for stripping of
nickel from the exchanged organic phase in which cobalt is
concentrated, cobalt recovering step, and impurity stripping
step.
The process of the present invention for
purification of an aqueous solution of nickel sulfate
containing cobalt and calcium uses a multi-stage,
countercurrent reaction tank system, wherein the aqueous
solution of nickel sulfate is supplied as the aqueous phase
to the last stage and a mixed organic solvent containing an
extractant of 2-ethylhexylphosphonic acid mono-2-ethylhexyl
ester loaded with nickel and diluted with a hydrocarbon, is
supplied as the organic phase to the first stage, to bring
CA 02463268 2004-04-05
77486-17
these phases into contact with each other for an exchanging
reaction, the aqueous phase being kept at a given pH level
in the last stage, and the calcium concentration and the
nickel, cobalt and calcium total concentration in the
organic phase being kept at a given concentration in the
last stage.
(1) Aqueous solution of nickel sulfate
The aqueous solution of nickel sulfate to be
treated by the present invention contains Cobalt and
calcium. The particularly preferable solution is a crude
aqueous solution of nickel sulfate containing cobalt at a
high concentration, produced by treating a raw material
containing cobalt at several percent. The crude aqueous
solution, for example, contains nickel sulfate at 40-70 g/L,
cobalt sulfate at 20-50 g/L and calcium sulfate at
0.1-1 g/L, but always nickel sulfate is contained at a
higher concentration than cobalt sulfate. It may be
adjusted beforehand at a given pH level, to help adjust pH
level for the exchanging reaction.
(2) Mixed organic solvent containing a nickel-loaded acid
extractant
In the present invention, a nickel-loaded acid
extractant is prepared beforehand for the exchanging
reaction. In the present invention, 2-ethylhexylphosphonic
acid mono-2-ethylhexyl ester is used as the acid extractant,
which is diluted with a hydrocarbon to a given extractant
concentration to prepare the mixed organic solvent. The
extractant 2-ethylhexylphosphonic acid mono-2-ethylhexyl
ester can efficiently extract cobalt, calcium and magnesium.
It has a separation coefficient in a sulfuric acid solution
of cobalt/nickel: 650, calcium/nickel: 110 and
11
CA 02463268 2004-04-05
77486-17
magnesium/nickel: 50. It has a still higher separation
coefficient for zinc, iron and copper, by which is meant
that these elements are preferentially extracted.
Acid extractants are generally viscous, and are
mixed with a diluent before use. In the present invention,
it is diluted with a hydrocarbon. The hydrocarbon is not
limited for the present invention, and may be aliphatic or
aromatic, of which an alkylbenzene as an aromatic
hydrocarbon is particularly preferable. The mixed organic
solvent for the present invention contains the extractant
2-ethylhexylphosphonic acid mono-2-ethylhexyl ester at 20 to
30o by volume, preferably 25 to 30~. At below 20% by
volume, cobalt extraction rate per unit amount of the mixed
organic solvent is insufficient. At above 30o by volume, on
the other hand, the mixed organic solvent may be excessively
viscous to deteriorate organic solvent/aqueous phase
separation efficiency and cause unstable operation and
decreased productivity. The mixed organic solvent of the
above composition for the present invention is suitable for
exchanging of impurities present in the aqueous solution of
nickel sulfate, e.g., cobalt and calcium which are extracted
in preference to nickel, with nickel loaded beforehand by
the extractant.
It is an economically recommended practice to
recycle the exchanged organic phase containing impurities
back to a series of nickel stripping, cobalt recovery and
impurity stripping step in the nickel sulfate purification
process (disclosed in, e.g., Japanese Patent Laid-open
Publication No. 10-310437 (pages 1 to 5)), in order to
recover nickel remaining in the organic phase, recover
cobalt and remove impurities other than cobalt in the
respective step, and to clean and recover the organic phase
12
77486-17
for reuse.
CA 02463268 2004-04-05
The mixed organic solvent can load nickel by
common solvent extraction. For example, nickel can be
extracted from an aqueous solution of nickel sulfate as the
starting material with the acid extractant described above
in a multi-stage, countercurrent reaction tank system,
wherein the extractant is supplied to the first stage while
the aqueous solution to the last stage for the extraction
reaction at a pH of 5.0 to 7Ø It is preferable to keep a
concentration of nickel loaded in the mixed organic solvent
in excess of the stoichiometric level, so that the impurity
elements may be efficiently extracted by the exchanging
reaction. An insufficient amount of nickel may keep these
impurity elements in the purified aqueous solution of nickel
sulfate, even when the exchanging reaction proceeds
completely. At the stoichiometric level, on the other hand,
the exchanging reaction cannot proceed completely, because
nickel in the organic phase is depleted as the reaction
proceeds.
(3) Purification system and method
In the present invention, the reactor system for
the exchanging reaction may be selected from various multi-
stage, countercurrent reaction tank systems capable of
efficiently separating the organic and aqueous phase from
each other after contacting them. The particularly
preferable one is a continuous, multi-stage, countercurrent
mixer settler system, where the nickel-loaded mixed solvent
and the crude aqueous solution of nickel sulfate come into
contact with each other, the former supplied to the first
stage and the latter to the last stage. Therefore, the
purified aqueous solution of nickel sulfate is discharged
13
CA 02463268 2004-04-05
77486-17
from the first stage mixer settler, and the exchanged
organic phase containing cobalt from the last stage mixer
settler.
The exchanged organic phase preferably contains
nickel at 1 to 4.5g/L. Nickel in the organic phase is
depleted as the exchanging reaction proceeds. When it is
depleted to a concentration below lg/L, the nickel/cobalt
exchanging reaction proceeds insufficiently, to increase
concentration of cobalt in the purified aqueous solution of
nickel sulfate. At above 4.5g/L, on the other hand,
recovery of nickel in the purified aqueous solution of
nickel sulfate may be retarded. The nickel/cobalt
exchanging reaction will proceed smoothly at a nickel
concentration of 1 to 4.5g/L in the organic phase.
In the present invention, the aqueous phase in the
last stage of the mufti-stage, countercurrent reaction tank
system is adjusted at a pH level of 4.5 to 5.5, preferably
4.8 to 5.2. Increasing the pH level accelerates extraction
of nickel and cobalt in the organic phase. At a pH level
below 4.5, cobalt concentration in the exchanged organic
phase decreases to deteriorate cobalt extraction efficiency.
At a pH level above 5.5, on the other hand; nickel
concentration in the organic phase increases to deteriorate
the nickel recovery rate in the purified aqueous solution of
nickel sulfate, but also cobalt extraction efficiency
resulting from increased number of nickel atoms bound to
functional groups in the extractant to substantially reduces
number of functional groups to be bound to cobalt. A
conventional exchanging step with an acid extractant loaded
with nickel at an adequate concentration, releasing no
hydrogen ion, needs no neutralizer to have an adequate pH
level, because the exchanging stage can be kept at a pH
14
CA 02463268 2004-04-05
77486-17
level of 4 to 6, when the aqueous solution of nickel sulfate
to be supplied to the stage is adjusted beforehand at a pH
level in the above range. However, the present invention is
characterized by finer adjustment of pH level, which may be
realized with sulfuric acid.
Table 1 gives the relationship between the pH
level of the aqueous phase and the nickel concentration in
the organic phase in the last stage settler in the multi-
stage countercurrent reaction tank system, where a crude
aqueous solution of nickel sulfate is treated with a mixed
solvent containing nickel-loaded 2-ethylhexylphosphonic acid
mono-2-ethylhexyl ester as an extractant, diluted with an
alkyl benzene to a given extractant concentration.
Table 1
pH level of the Nickel concentration
No
aqueous phase in the in the organic phase
settler (g/L)
1 4.3 0.99
2 4.8 1.20
3 5.0 2.19
4 5.3 3.90
5 5.4
4.50
As shown in Table 1, the nickel concentration in
the organic phase decreases as the pH level decreases, and
the nickel/cobalt exchanging reaction proceeds smoothly at a
nickel concentration in the organic phase in the above-
CA 02463268 2004-04-05
77486-17
described range from 1 to 4.5g/L, which is achieved at a pH
level of 4.5 to 5.5:
It is essential according to the present invention
to keep the calcium concentration and the nickel, cobalt and
calcium total concentration in the organic phase each at a
given level in the last stage of the multi-stage
countercurrent reaction tank system. This prevents
viscosity increase of the organic phase and makes cobalt
extraction efficiency more stable than the conventional
operation without harming the nickel/cobalt exchanging
reaction.
The nickel, cobalt and calcium total concentration
in the organic phase is adjusted at 25g/L or less,
preferably 20g/L or less, often at least lOg/L, preferably
at least 15g/L, in the last stage of the multi-stage,
countercurrent reaction tank system for the present
invention. At a nickel; cobalt and calcium total
concentration in the organic phase above 25g/L, the mixed
organic solvent may be excessively viscous and this may
decrease efficiency for separating the organic phase from
the aqueous phase and cause an unstable operation and
notably deteriorated productivity.
Fig.l indicates viscosities of the organic phase
plotted against nickel, cobalt and calcium concentrations in
the organic phase, the relationship being similar to that
given in Table 1. As shown, the viscosity of the organic
phase starts to notably increase as the nickel, cobalt and
calcium total concentration in the organic phase increases
beyond 25g/L. The nickel, cobalt and calcium total
concentration also depends on the pH level for the
exchanging reaction.
16
b
CA 02463268 2004-04-05
77486-17
Fig.2 indicates nickel, cobalt and calcium total
concentrations in the organic phase plotted against pH
levels for the exchanging reaction in a range of high
extractant concentration in the organic phase, the
relationship being similar to that given :in Table 1. As
shown, the nickel, cobalt and calcium total concentration in
the organic phase increases as the pH level increases to
accelerate extraction. It is also noted that the total
concentration of nickel, cobalt and calcium extracted in the
organic phase increases as the extractant concentration
increases at the same pH level.
The calcium concentration in the organic phase is
adjusted at 0.4g/L or less in the last stage of the multi-
stage countercurrent reaction tank system for the present
invention. At a calcium concentration above 0.4g/L, cobalt
extraction efficiency may not be improved, but decreased to
40 to 600 of the stoichiometric extraction rate, which is on
a level with that associated with the conventional
operation.
Fig.3 indicates cobalt extraction efficiencies
(ratios of cobalt amounts in the organic phase to the
stoichiometric cobalt extraction rate) platted against
calcium concentrations in the organic phase, the
relationship being similar to that given in Table 1. The
solid line in Fig.3, obtained by the regression analysis,
indicates that a cobalt extraction efficiency of 60% or more
can be secured at a calcium concentration of 0.4g/L or less.
The calcium concentration is usually at least about 0.05g/L.
The relationship between the calcium concentration and the
viscosity of the organic phase in the last stage is not
clear, conceivably resulting from a locally increased
calcium concentration in the aqueous phase as the calcium
17
CA 02463268 2004-04-05
77486-17
concentration in the organic phase increases during the
exchanging reaction, to cause separation of insoluble
calcium sulfate and increased viscosity of the organic phase
when contaminated with calcium sulfate.
The nickel, cobalt and calcium total concentration
in the organic phase depends on the pH level of the
exchanging reaction at a given extractant concentration.
The concentration of calcium, which is distributed almost
completely in the organic phase, can be adjusted by
controlling the flow rate of the crude aqueous solution of
nickel sulfate or the amount of the organic phase for
dilution.
EXAMPLES
The present invention is described in more detail
by EXAMPLES, which by no means should be construed to limit
the present invention. The following analytical procedures
were used in EXAMPLES.
(1) Analysis of metals: Determined by atomic absorption
spec rometry
(2) Viscosity of the organic phase: Determined by a B-type
rotational viscometer
EXAMPLE 1
The exchanging step Was carried out by a 4-stage,
countercurrent, mixer settler system (effective mixer
volume: 300mL, effective settler volume: 3,OOOmL), where the
organic phase was supplied to the first stage of the system
and the aqueous phase to the fourth (last) stage.
A mixed organic solvent was prepared as the
organic phase by diluting 2-ethylhexylphosphonic acid mono-
18
CA 02463268 2004-04-05
77486-17
2-ethylhexyl ester (PC-88A~, Daihachi Chemical Industry)
with an alkylbenzene (Clean Sol G~, Nippon Qil Corporation)
to 25~ by volume and then treated to load nickel at 25g/L.
The organic phase was incorporated, as required, with the
organic phase for dilution, prepared by stripping step for
cobalt/calcium separation. A crude aqueous solution of
nickel sulfate containing nickel, cobalt and calcium at 50
to 60, 30 to 40 and 0.6g/L, respectively, was used as the
aqueous phase, which was adjusted at a pH level of 4.5 to
5Ø
These organic phase and aqueous phase were
supplied at 90 and 30 to 40mL/minute to adjust calcium load.
The aqueous phase was finely adjusted at a pH level of 4.8
to 5.2 with sulfuric acid in the settler in the fourth stage
of the mixer settler system. The mixer settler system was
continuously operated at 40 to 45°C for 8 hours or more
while keeping the calcium concentration and the nickel,
cobalt and calcium total concentration in the organic phase
at 0.40g/L or less and 20g/L or less, respectively, in the
fourth mixer settler. Samples were collected from the
exchanged aqueous phase and organic phase, as required, to
analyze the nickel, cobalt and calcium concentrations.
Table 2 gives nickel, cobalt anal calcium
concentrateons in the aqueous phase, i.e., purified aqueous
solution of nickel sulfate; discharged from the first mixer
settler and exchanged organic phase from the fourth mixer
settler; totaled nickel, cobalt and calcium concentration in
that organic phase; and efficiency of cobalt extracted in
that organic phase.
19
CA 02463268 2004-04-05
77486-17
Table 2
Composition
of
the
Composition Co
of
the
purified
aqueous
exchanged extraction
organic
phase
solution
of
nickel
No (g/L) efficiency
.
sulfate
(g/L)
(%)
Ni Co Ca Total Ni Co Ca
1 3.43 14.40 0.40 18.23 63 100 0.006 0.018
2 4.00 14.50 0.37 18.87 63 100 0.007 0.006
3 2.06 16.20 0.39 18.65 71 98.7 0.007 0.006
4 1.12 16.10 0.25 17.47 70 99.4 0.007 <0.005
4.12 15.36 0.37 19.87 67 102 0.006 <0.005
As shown in Table 2, efficiency of cobalt
extracted in the organic phase can be secured at 60% or
5 more, cobalt is mostly extracted in the aqueous phase, and
the purified crude solution of nickel sulfate containing
impurities at a very low concentration (cobalt: lOmg/L or
less, and calcium: 20mg/L or less) can be stably produced,
when the aqueous phase in the settler of the fourth mixer
settler is kept at a pH of 4.8 to 5.2, and the calcium
concentration and the nickel, cobalt and calcium total
concentration in the organic phase discharged from the
fourth stage of the mixer settler are kept at 0.40g/L or
less and 20g/L or less, respectively.
As described above, the process of the present
invention for purification of an aqueous solution of nickel
sulfate containing cobalt and calcium, the former being
CA 02463268 2004-04-05
77486-17
present at a high concentration, is of industrially high
efficiency, because it can increase cobalt treatment
capacity and produce a purified aqueous solution of nickel
sulfate with reduced cobalt and calcium concentrations by
improving cobalt extraction efficiency in the exchange
reaction. As such, it is of very high industrial value.
21