Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02471620 2004-06-23
t , WO 03/059905 PCT/CH02/00725
- 1 -
Pyrrolidonecarboxamides
The present invention relates to pyrrolidonecarboxamide
derivatives.
In particular, the invention relates to pyrrolidone-
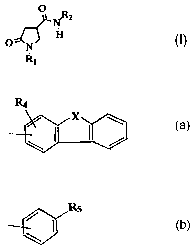
carboxamides of the formula
o NR
z
Fi
O N
R1 I
in which
RI is phenyl which is optionally monosubstituted or
disubstituted in the phenyl radical by alkyl,
alkoxy, dialkylamino, halogen or trifluoromethyl,
benzyl, phenylethyl or a-hydroxyphenylethyl;
naphthyl or naphthylmethyl; thienyl-, furyl-,
pyridyl-, 1-alkylpyrrolidin-2-yl-, pyrrolidino- or
morpholino-alkyl; or cycloalkyl which can
optionally possess a fused-on benzene ring;
R2 is a radical of the formula
Itg
or
~E ~ i w
ta) Sb)
X is -CH2-, -CO-, -0- or -NR3-;
R3 is hydrogen or alkyl;
R4 is hydrogen or alkoxy;
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 2 -
R5 is phenyl, heteroalkyl, aryloxy, alkoxy, alkanoyl
or -NR6R';
R6 is hydrogen, alkyl, aralkyl, cycloalkylalkyl or
alkoxycarbonylalkyl; and
R' is aryl, heteroaryl, alkyl, hydroxyalkyl or acyl;
to pharmaceutically utilizable acid addition salts of
basic compounds of the formula I, to pharmaceutically
utilizable salts of acid compounds of the formula I
with bases, to pharmaceutically utilizable esters of
compounds of the formula I which contain hydroxyl or
carboxyl groups, and to hydrates or solvates thereof.
Since the pyrrolidonecarboxamides of the formula I
contain at least one asymmetric C atom, they can be
present as optically pure enantiomers, as mixtures of
enantiomers, such as racemates, or, where appropriate,
as optically pure diastereomers, as mixtures of
diastereomers, as diastereomeric racemates or as
mixtures of diastereomeric racemates.
WO 01/07409 Al relates to carbazole derivatives whose
general formula partially overlaps with the above
formula I but does not specifically describe a single
compound covered by the above formula I and, further-
more, does not contain any sufficiently concrete
general pointers in the direction of compounds of the
above formula I.
The compounds defined at the outset are novel and are
distinguished by possessing valuable pharmacodynamic
properties. They inhibit the interaction of the neuro-
peptide Y (NPY) with one of the neuropeptide receptor
subtypes (NPY-Y5) and are suitable, in particular, for
preventing and treating arthritis, diabetes and,
especially, eating disturbances and obesity.
The present invention relates to the above compounds as
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
' ~ WO 03/059905 PCT/CH02/00725
- 3 -
such and as therapeutic active compounds; to processes
and intermediates for preparing them; to pharma-
ceuticals which comprise one of the above compounds;
and to the use of the above compounds for preventing
and treating arthritis, diabetes and, especially,
eating disturbances and obesity or for producing
corresponding pharmaceuticals.
In the present description, the term "alkyl" denotes,
on its own or in combination, a branched or unbranched
saturated hydrocarbon radical having from 1 to 8 carbon
atoms, preferably having from 1 to 6 carbon atoms and,
particularly preferably, having from 1 to 4 carbon
atoms. Examples of these radicals are methyl, ethyl,
n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl,
tert-butyl, the isomeric pentyls, the isomeric hexyls
and the isomeric octyls; methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl and the like are
preferred.
The term "cycloalkyl" denotes, on its own or in
combination, a saturated cyclic hydrocarbon radical
having 3-8 carbon atoms, preferably having from 3 to
6 carbon atoms, which can be substituted, for example
by alkyl groups, such as methyl, and which can possess
a fused-on benzene ring. Examples of cycloalkyl groups
which are optionally substituted by alkyl are
cyclopropyl, methylcyclopropyl, dimethylcyclopropyl,
cyclobutyl, methylcyclobutyl, cyclopentyl, methylcyclo-
pentyl, cyclohexyl, methylcyclohexyl, dimethylcyclo-
hexyl, cycloheptyl and cyclooctyl; examples of
cycloalkyl radicals having a fused-on benzene ring are
1-indanyl, 2-indanyl and the like.
The term "hydroxyalkyl" denotes, on its own or in
combination, an alkyl group, as described above, with
one or two H atoms, preferably one H atom, being
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 4 -
replaced with a hydroxyl group. Examples are
hydroxymethyl, hydroxyethyl, hydroxypropyl and the
like.
The term "alkoxy" denotes, on its own or in
combination, an alkyl radical, as described above,
which is linked by way of an oxygen bridge. Examples
are methoxy, ethoxy and the like.
The term "alkanoyl" denotes, on its own or in
combination, an alkyl group, as described above, which
is linked by way of a CO bridge. Examples are acetyl,
3-methylbutyryl, 2,2-dimethylpropionyl and the like.
The term "aryl" denotes, on its own or in combination,
a phenyl or naphthyl group, preferably a phenyl group,
which can carry up to four, preferably from one to
three and particularly preferably one or two,
substituents. Examples of such substituents are alkyl,
hydroxyalkyl, alkoxy, alkoxyalkyl, nitro, fluoro,
bromo, chloro, hydroxy, dialkylamino and the like.
Particularly preferred substituents are alkyl and
alkoxy. Examples of these aryl groups are phenyl,
methylphenyl, dimethylphenyl, ethylphenyl, isopropyl-
phenyl, methoxyphenyl, methoxymethylphenyl, dimethyl-
aminophenyl, phenylaminophenyl and the like.
The term "aralkyl" denotes, on its own or in
combination, an alkyl group, as described above, in
which at least one H atom is replaced with an aryl
group, as described above, in particular with a phenyl
or naphthyl group, which can carry one or more
substituents, such as alkyl or alkoxy groups. Examples
of these aralkyl radicals are benzyl, phenethyl,
2-(3,4-dimethoxyphenyl)ethyl and the like.
The term "heteroaryl" denotes, on its own or in
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
. ~ WO 03/059905 PCT/CH02/00725
- 5 -
combination, an aromatic monocyclic, bicyclic or
tricyclic heterocyclic ring system having from 5 to 10,
preferably from 5 to 6, ring members which contains
from one to four, preferably from one to two,
heteroatoms which are selected, independently of one
another, from nitrogen, oxygen and sulphur. Examples of
these heteroaryl groups are pyridyl, pyrimidinyl,
thiazolyl, thiophenyl, furanyl, tetrazolyl, carbazolyl
and the like. These heteroaryl groups can be
substituted, expediently monosubstituted, disubstituted
or trisubstituted, with suitable substituents primarily
being alkyl, alkoxy, amino or aryl groups. Examples are
2-pyridyl, 2-thienyl, 4,6-dimethyl-2-pyrimidinyl and
the like.
The term "acyl" denotes, alone or in combination, an
alkanoyl group, as described above, or a cycloalkyl,
aryl, aralkyl or heteroaryl group, as described above,
which is linked by way of a CO bridge. Examples are, as
mentioned above, acetyl, 3-methylbutyryl and
2,2-dimethylpropionyl as well as cyclopropanecarbonyl,
benzoyl, phenylacetyl, 2-methoxybenzoyl, 4-methoxy-
benzoyl, 3-fluorobenzoyl, benzo[1,3]dioxole-5-carbonyl,
furan-2-carbonyl and the like.
The term "pharmaceutically utilizable salts" relates to
those salts which do not impair the biological effect
and properties of the free bases or free acids and
which are not undesirable biologically or in some other
way. The salts are formed from the free bases using
inorganic acids, such as hydrochloric acid, hydrobromic
acid, sulfuric acid, nitric acid, phosphoric acid and
the like, preferably hydrochloric acid, or using
organic acids, such as acetic acid, propionic acid,
glycolic acid, pyruvic acid, oxalic acid, malefic acid,
malonic acid, succinic acid, tartaric acid, salicylic
acid, citric acid, benzoic acid, mandelic acid,
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 6
methanesulfonic acid, p-toluenesulfonic acid and the
like. The free acids can form salts with inorganic
bases or with organic bases. Preferred salts with
inorganic bases are, but not exclusively, sodium salts,
potassium salts, lithium salts, ammonium salts, calcium
salts, magnesium salts and the like. Preferred salts
with organic bases are, but not exclusively, salts with
primary, secondary and tertiary amines, substituted
amines, including all naturally occurring substituted
amines, cyclic amines and basic ion exchange resins,
such as isopropylamine, trimethylamine, diethylamine,
triethylamine, tripropylamine, ethanolamine, lysine,
arginine, N-ethylpiperidine, piperidine, polyamine
resins and the like. Compounds of formula I can also be
present as zwitterions.
The invention also includes pharmaceutically suitable
esters of compounds of the formula I which contain
hydroxyl or carboxyl groups. ~~Pharmaceutically suitable
esters" means that, in compounds of the formula I,
corresponding functional groups are derivatized to form
ester groups such that they are once again retrans-
formed, in vivo, into their active form. On the one
hand, COOH groups can be esterified. Examples of
suitable esters of this nature are the alkyl esters and
aralkyl esters. Preferred esters of this nature are the
methyl, ethyl, propyl, butyl and benzyl esters as well
as the (R/S)-1-[(isopropoxycarbonyl)oxy]ethyl esters.
The ethyl esters and the isomeric butyl esters are
particularly preferred. On the other hand, OH groups
can be esterified. Examples of these compounds contain
physiologically acceptable and metabolically labile
ester groups, such as methoxymethyl ester,
methylthiomethyl ester and pivaloyloxymethyl ester, and
similar ester groups.
Preferred possible meanings for R1 are phenyl, 4-tolyl,
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
2,5-dimethylphenyl, 2-isopropylphenyl, 3-methoxyphenyl,
2-methyl-5-methoxyphenyl, benzyl, 2-phenylethyl,
2-(2-pyridyl)ethyl, 2-(2-thienyl)ethyl, 2-indanyl and
2-morpholinoethyl. Other preferred possible meanings
for RI are cycloheptyl, 2-hydroxy-2-phenylethyl,
2-thienylmethyl, 2-furanylmethyl, 4-chlorobenzyl,
3-fluorophenyl, 2-chlorobenzyl and 2,4-dimethoxybenzyl
as well as 2-naphthyl, naphthalen-1-ylmethyl, 4-di-
methylaminophenyl, 2-pyrrolidin-1-ylethyl, 1-methyl-
pyrrolidin-2-ylethyl, 4-isopropylphenyl and 3,5-bis-
trifluoromethylphenyl.
Particularly preferred possible meanings for RI are
2,5-dimethylphenyl, 2-isopropylphenyl and 2-methyl
5-methoxyphenyl.
Preferred possible meanings for R2 are biphenyl-4-yl,
4-methoxyphenyl, 4-phenoxyphenyl, 4-dimethylamino-
phenyl, 4-diethylaminophenyl, 4-phenylaminophenyl,
4-[N-ethyl-N-(2-hydroxyethyl)amino]phenyl, 4-(N-ethyl-
N-isopropylamino)phenyl, 4-N-(4,6-dimethyl-2-pyrimi-
dinyl)aminophenyl, 4-[N-ethyl-N-(4,6-dimethyl-2-pyrimi-
dinyl)amino]phenyl, 4-[N-methyl-N-(4,6-dimethyl-
2-pyrimidinyl)amino]phenyl, 4-acetylphenyl, 9H-fluoren-
2-yl, 9-oxo-9H-fluoren-2-yl and 9-ethylcarbazol-3-yl.
Other preferred possible meanings for R2 are
4-(N-ethoxycarbonylmethyl-N-phenylamino)phenyl, 4-(N-
ethyl-N-phenylamino)phenyl, 4-(N-methyl-N-phenylamino-
phenyl, 4-(N-cyclopropylmethyl-N-phenylamino)phenyl,
4-(N-isobutyl-N-phenylamino)phenyl, 4-(2-methoxy-
benzoylamino)phenyl, 4-(2,2-dimethylpropionylamino)-
phenyl, 4-(3-methylbutyrylamino)phenyl, 4-(cyclo-
propanecarbonylamino)phenyl, 4-(3-fluorobenzoylamino)-
phenyl and 4-[(furan-2-carbonyl)amino]phenyl as well as
biphenyl-3-yl, 9H-fluoren-1-yl, 2-methoxydibenzofuran-
3-yl, 4-(N-isopropyl-N-phenylamino)phenyl, 4-(N-benzyl-
N-phenylamino)phenyl, 4-acetylaminophenyl, 4-benzoyl-
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
~ ~ WO 03/059905 PCT/CH02/00725
_ g _
aminophenyl, 4-phenylacetylaminophenyl, 4-
[(benzo[1,3]dioxole-5-carbonyl)amino]phenyl and 4-(4-
methoxybenzoylamino)phenyl.
Particularly preferred possible meanings of RZ are
9-ethyl-9H-carbazol-3-yl, 4-[N-ethyl-N-(4,6-dimethyl-
2-pyrimidinyl)amino]phenyl, 4-[N-methyl-N-(4,6-di-
methyl-2-pyrimidinyl)amino]phenyl, 4-(4,6-dimethyl-
2-pyrimidinyl)amino]phenyl, 4-phenylaminophenyl,
4-diethylaminophenyl, 4-(N-ethyl-N-isopropylamino)-
phenyl, 4-(N-ethoxycarbonylmethyl-N-phenylamino)phenyl,
4-(N-ethyl-N-phenylamino)phenyl, 4-(N-methyl-N-phenyl-
amino)phenyl and 2,4-dimethoxybenzyl.
Representative examples of preferred compounds of
formula I are:
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-(2-pyridin-
2-ylethyl)pyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-hydroxy-
2-phenylethyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Diethylaminophenyl)-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-(2-thiophen-
2-ylethyl)pyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(4-chlorobenzyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-thiophen-
2-ylmethylpyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-furan-2-ylmethyl-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(5-methoxy-
2-methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylisopropylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- g -
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-phenethylpyr-
rolidine-3-carboxamide;
rac. Ethyl [(4-{[I-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carbonyl]amino}phenyl)phenylamino]acetate;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-cycloheptyl-5-oxo-
pyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-{4-[Ethyl-(2-hydroxyethyl)amino]phenyl}-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(3-methoxyphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-chlorobenzyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-1-(2,5-dimethylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-phenyl-
pyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-5-oxo-1-p-tolylpyrrolidine-3-carboxamide;
rac. N-[4-(Isopropylphenylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 10 -
3-carboxamide;
rac. N-[4-(Methylphenylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Methylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(4-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(3-fluorophenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Phenylaminophenyl)-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-(5-methoxy-
2-methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-benzyl-5-oxopyr-
rolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
5-oxo-1-p-tolylpyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-indan-2-yl-5-oxo-
pyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(2-hydroxy-2-phenylethyl)-5-oxo-pyrrolidine-
3-carboxamide;
rac. N-(Biphenyl-4-yl)-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide;
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-carbox-
amide;
rac. N-[4-(Isopropylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Phenylaminophenyl)-1-(2-isopropylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
P898II-FINAL VERSION.DOC
' ' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 11 -
phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(3-methoxyphenyl)-5-oxopyrrolidine-3-carbox-
amide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-5-oxo-1-m-tolylpyrrolidine-3-carboxamide;
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-1-(2,5-
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. Ethyl [(4-{[1-(5-methoxy-2-methylphenyl)-5-oxopyr-
rolidine-3-carbonyl]amino}phenyl)phenylamino]acetate;
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-indan-2-yl-5-oxo-
pyrrolidine-3-carboxamide; and
rac. N-[4-(Isobutylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide.
Other representative examples of preferred compounds of
formula I are:
rac. N-[4-(2-Methoxybenzoylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(2,2-Dimethylpropionylamino)phenyl]-1-
(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-morpholin-4-yl-
ethyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-p-
tolylpyrrolidine-3-carboxamide; and
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2,4-dimethoxy-
benzyl)-5-oxopyrrolidine-3-carboxamide; as well as
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-1-
(4-dimethylaminophenyl)-5-oxopyrrolidine-3-carboxamide;
and
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 12
rac. N-[4-(4,6-Dimethylpyrimidin-2-yl)methylamino-
phenyl]-1-(4-dimethylaminophenyl)-5-oxopyrrolidine-
3-carboxamide.
Compounds of the formula I can be prepared, according
to the invention, by reacting a pyrrolidonecarboxylic
acid of the formula III (see scheme below), in which R1
has the meaning mentioned at the outset, or a reactive
derivative thereof, with an amine of the formula IV, in
which R2 has the meaning mentioned at the outset, or a
reactive derivative thereof. Any stereoisomeric
mixtures, such as racemates, which are obtained can, if
desired, be resolved using generally customary methods.
In order to prepare the corresponding pyrrolidone
carboxylic acid of the formula III, it is possible, for
example, to take the following route, with the
substituents and indices given in the following scheme
having the meanings mentioned at the outset unless
otherwise indicated; this route consists in reacting an
amine of the formula II, such as aniline or the like,
in a solvent, such as water, dioxane, ethanol or the
like, at elevated temperature, with itaconic acid
(Buzas et al., Chim Ther 7, 398-403, 1972).
Scheme
O OH
U OH O OH H Rz O ~ z
zN ~ H
R Z '"' O N ~ O N
Ri Ri
The compounds of the formula I can be prepared by
reacting a pyrrolidonecarboxylic acid of the
formula III with an amine of the formula IV. For this,
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
~ . WO 03/059905 PCT/CH02/00725
- 13 -
the pyrrolidonecarboxylic acid of the formula III is
expediently converted, where appropriate in a suitable
solvent, such as toluene, into the corresponding acid
chloride using a halogenating agent such as SOC12 or
POC13. This reactive derivative of the pyrrolidone-
carboxylic acid of the formula III is then reacted with
an amine of the formula IV in a suitable solvent, such
as methylene chloride, in the presence of a base, such
as triethylamine.
In a process variant, the pyrrolidonecarboxylic acid of
the formula III is reacted with an amine of the
formula IV in the added presence of a coupling reagent,
such as EDC, DCC or BOP, in a solvent, such as DMF,
and, where appropriate, in the presence of a base, such
as triethylamine.
Compounds of the formula I in which R2 is a radical of
the formula (b) , R5 is -NR6R~ and R~ is acyl can also be
prepared by acylating a corresponding compound in which
R' is hydrogen, such as rac. N-(4-aminophenyl)-1-(2,5-
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide, for
example using acetyl chloride, isovaleryl chloride,
cyclopropylcarbonyl chloride, benzoyl chloride,
phenylacetyl chloride, 2-methoxybenzoyl chloride,
piperonyloyl chloride, pivaloyl chloride, 4-
methoxybenzoyl chloride, 3-fluorobenzoyl chloride and
the like.
While only some of the pyrrolidonecarboxylic acids of
the formula III are known, they can be prepared by
methods which are known per se and with which every
skilled person is familiar, for example using the
abovementioned method (Buzas et al., Chim Ther 7, 398-
403, 1972); furthermore, some of the examples which
follow contain information regarding the preparation of
particular pyrrolidonecarboxylic acids of the
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 14
formula III.
Some of the amines of the formula IV are also known or
can be prepared by methods which are known per se; some
of the examples which follow also contain information
regarding the preparation of particular amines of the
formula IV.
Insofar as the starting compounds of the formulae III
and IV, and also the nitro precursors of the compounds
of the formula IV, are novel, they also form part of
the subject matter of the present invention. Thus, the
following compounds of the formula IV and their
nitroprecursors, in particular:
ethyl [(4-nitrophenyl)phenylamino]acetate;
ethyl [(4-aminophenyl)phenylamino]acetate;
cyclopropylmethyl(4-nitrophenyl)phenylamine;
N-cyclopropylmethyl-N-phenylphenylene-1,4-diamine;
isobutyl(4-nitrophenyl)phenylamine;
N-isobutyl-N-phenylphenylene-1,4-diamine;
ethyl [(4-nitrophenyl)phenylamino]pentanoate;
ethyl [(4-aminophenyl)phenylamino]pentanoate;
benzyl(4-nitrophenyl)phenylamine; and
N-benzyl-N-phenylphenylene-1,4-diamine;
as well as the following compounds of the formula III:
rac. 1-indan-2-yl-5-oxopyrrolidine-3-carboxylic acid;
rac. 1-naphthalen-2-yl-5-oxopyrrolidine-3-carboxylic
acid;
rac. 1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine
3-carboxylic acid;
rac. 5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxylic acid;
rac. 1-cycloheptyl-5-oxopyrrolidine-3-carboxylic acid;
rac. 1-(2-hydroxy-2-phenylethyl)-5-oxopyrrolidine-
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
. WO 03/059905 PCT/CH02/00725
- 15 -
3-carboxylic acid;
rac. 5-oxo-1-(2-pyrrolidin-1-ylethyl)pyrrolidine-
3-carboxylic acid;
rac. 1-[2-(1-methylpyrrolidin-2-yl)ethyl]-5-oxopyr-
rolidine-3-carboxylic acid; and
rac. 1-(3-fluorophenyl)-5-oxopyrrolidine-3-carboxylic
acid;
are part of the subject matter of the present
invention.
As mentioned at the outset, the compounds of the
formula I and their pharmaceutically utilizable salts
and esters are novel and possess valuable
pharmacological properties. In particular, they inhibit
the interaction of the neuropeptide Y (NPY) with one of
the neuropeptide receptor subtypes (NPY-Y5). NPY is a
regulatory, 36-amino acid peptide belonging to the
pancreatic polypeptide family. NPY is the most
widespread neuropeptide in the central and peripheral
nervous systems and has prominent and complex effects
on nutrient uptake, anxiety, depression, circadian
rhythm, sexual function, reproduction, memory function,
migraine, pain, epileptic seizures, blood pressure,
cerebral hemorrhages, shock, sleep disturbance,
diarrhea, etc.
NPY interacts with a heterogeneous population of at
least five NPY receptor subtypes, i.e. Y1-Y5, which
activate adenylate cyclase using a G protein. One of
the most prominent effects is the induction of nutrient
uptake in vertebrates. Recent investigations involving
the selective activation and blocking of the individual
NPY receptors have shown that it is principally the
NPY-Y5 receptor which is responsible for appetite-
inducing signals.
P898II-FINAL VERSION.DOC
' ~ CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 16
Obesity is an important and increasing problem in the
industrialized world. Obesity is associated with a
variety of diseases such as non-insulin-dependent
diabetes (type II diabetes), high blood pressure,
coronary diseases of the heart, dyslipidemia etc., and
has an influence on the life expectancy and quality of
life of the population affected. For this reason, there
is a need for pharmaceutical substances which exert an
influence on eating habits. The NPY-Y5 receptor is a
possible target for a corresponding pharmacological
intervention. Using a low molecular weight compound to
inhibit this receptor represents an attractive
possibility for preventing or treating the above
diseases.
Because of their property of inhibiting the interaction
of neuropeptide Y with the neuropeptide Y5 receptor
subtype, the compounds of the formula I, and their
pharmaceutically utilizable salts and esters, are
suitable for preventing and treating arthritis,
diabetes and, in particular, eating disturbances and
obesity.
The valuable pharmacodynamic properties of the novel
compounds according to the invention can be
demonstrated using the methods which are described
below.
Cloning the mouse NPY-Y5 rece for cDNAs
The full-length cDNA which contains the mouse NPY-Y5
(mNPY-Y5) receptor coding was amplified from mouse
brain cDNA using specific primers, which were
custom-made on the basis of published sequences, and
employing Pfu DNA polymerase (Stratagene). The
amplification product was subcloned into a mammalian
expression vector pcDNA3 using EcoRI and XhoI
restriction sites. Positive clones were sequenced; one
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 17 -
clone, which contained the published sequence, was
selected for preparing stable cell clones.
Stable transfection
Human embryonic kidney 293 (HEK293) cells were
transfected with 10 ~g of mNPYS DNA using Lipofectamine
reagent (Gibco BRL) in accordance with the
manufacturer's instructions. Two days after the
transfection, the geneticin selection (1 mg/ml) was
initiated and several stable clones were isolated. One
of the clones was used for further pharmacological
characterization.
Radioligand competition binding
Human embryonic kidney cells (HEK293) which express
recombinant mouse NPY-Y5 receptors (mNPY-Y5) were
disrupted by being frozen/thawed three times in
hypotonic Tris buffer (5 mM, pH 7.4, 1 mM MgCl2), after
which they were homogenized and centrifuged at 72 000 G
for 15 minutes. The precipitate was washed twice with
Tris buffer (pH 7.4) containing 25 mM MgCl2, 250 mM
sucrose, 0.1 mM phenylmethylsulfonyl fluoride and
0.1 mM 1,10-phenanthroline, then resuspended in the
same buffer and stored in aliquots at -80°C. The
protein was determined by the Lowry method using bovine
serum albumin (BSA) as the standard.
The competition binding analysis was carried out in
250 ~1 of 25 mM Hepes buffer (pH 7.4, 2.5 mM CaCl2,
1 mM MgCl2, to bovine serum albumin and 0.010 sodium
azide) which 5 ~g of protein, 100 pM l2sl-labeled
peptide YY (PYY) and 10 ~1 of a DMSO solution
containing increasing quantities of DMSO solution
containing unlabeled test compound. After a one-hour
incubation at 22°C, the bound ligand was separated from
the unbound ligand by means of filtration through a
glass fiber filter. Nonspecific binding was determined
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 18
in the presence of 1 ~M unlabeled PYY. Specific binding
is defined as the difference between total binding and
nonspecific binding. An ICso value is defined as the
concentration of the antagonist which displaces 500 of
the lasI-labeled neuropeptide Y. This concentration is
determined by linear regression analysis following
logit/log transformation of the binding values.
In the above-described test, preferred compounds
according to the invention exhibit ICSO values of less
than 1000 nM, while particularly preferred compounds
exhibit ICSO values of less than 100 nM and very
particularly preferred compounds exhibit ICSO values of
less than 50 nM.
The results which were obtained in the above-described
test using representative compounds of the formula I as
test compounds are compiled in the following table.
Substance NPY5
ICso
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1- 0.003
(2-pyridin-2-ylethyl)pyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-hydroxy- 0.008
2-phenylethyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(4-Diethylaminophenyl)-1-(2,5-dimethyl- 0.009
phenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-(2-thio-0.010
phen-2-ylethyl)pyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(4-chloro- 0.010
benzyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-thiophen-0.010
2-ylmethylpyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-furan-2-yl- 0.010
methyl-5-oxopyrrolidine-3-carboxamide
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 19
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-isopropyl- 0.010
phenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(5-methoxy- 0.010
2-methylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-[4-(Ethylisopropylamino)phenyl]-1-(2,5-di- 0.010
methylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1- 0.012
phenethylpyrrolidine-3-carboxamide
rac. Ethyl [(4-{[1-(2,5-dimethylphenyl)-5-oxopyr- 0.013
rolidine-3-carbonyl]amino}phenyl)phenylamino]-
acetate
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-cycloheptyl-5-0.015
oxopyrrolidine-3-carboxamide
rac. N-{9-[(4,6-Dimethylpyrimidin-2-y1)ethylamino]-0.015
phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrol-
idine-3-carboxamide
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 20
rac. N-{4-[Ethyl-(2-hydroxyethyl)amino]phenyl}- 0.016
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carbox-
amide
rac. N-[4-(Ethylphenylamino)phenyl]-1-(2,5- 0.017
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(3-methoxy- 0.020
phenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-chloro- 0.020
benzyl)-5-oxopyrrolidine-3-carboxamide
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-0.020
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carbox-
amide
rac. N-{4-[(4,6-Dimethylpyrimidin-2- 0.020
yl)methylamino]phenyl}-1-(2,5-dimethylphenyl)-5-
oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-phenyl-0.021
pyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2- 0.022
yl)methylamino]phenyl}-5-oxo-1-p-tolylpyrrolidine-
3-carboxamide
rac. N-[4-(Isopropylphenylamino)phenyl]-1-(2,5-di-0.022
methylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-[4-(Ethylphenylamino)phenyl]-1-(2-isopropyl-0.023
phenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-0.024
phenyl}-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxamide
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-0.025
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-
carboxamide
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-0.026
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide
rac. N-[4-(Methylphenylamino)phenyl]-1-(2,5-di- 0.026
methylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-[4-(Methylphenylamino)phenyl]-1-(2- 0.020
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 21
isopropylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(4-isopropyl-0.030
phenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(3-fluoro- 0.030
phenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(4-Phenylaminophenyl)-1-(2,5- 0.030
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-0.030
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide
rac. N-[4-(Ethylphenylamino)phenyl]-1-(5-methoxy-0.030
2-methylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2- 0.031
yl)methylamino]phenyl}-1-(2-isopropylphenyl)-5-
oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-benzyl-5- 0.032
oxopyrrolidine-3-carboxamide
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-0.032
5-oxo-1-p-tolylpyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-indan-2-yl- 0.032
5-oxopyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-0.033
phenyl}-1-(2-hydroxy-2-phenylethyl)-5-oxo-pyrrol-
idine-3-carboxamide
rac. N-(Biphenyl-4-yl)-1-(2,5-dimethylphenyl)-5- 0.034
oxopyrrolidine-3-carboxamide
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]- 0.034
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-
carboxamide
rac. N-[4-(Isopropylphenylamino)phenyl]-1-(2- 0.034
isopropylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(4-Phenylaminophenyl)-1-(2-isopropylphenyl)-0.041
5-oxopyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2- 0.041
yl)methylamino]phenyl}-1-(5-methoxy-2-
methylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-0.044
phenyl}-1-(3-methoxyphenyl)-5-oxopyrrolidine-3-car-
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 22 -
boxamide
rac. N-(4-Isopropylphenyl)-1-(2,5-dimethylphenyl)-0.045
5-oxopyrrolidine-3-carboxamide
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-1-0.045
(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-0.046
phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide
rac. Ethyl [(4-{[1-(5-methoxy-2-methylphenyl)-5- 0.046
oxopyrrolidine-3-
carbonyl]amino}phenyl)phenylamino]acetate
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]- 0.047
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carbox-
amide
rac. N-[4-(Ethylphenylamino)phenyl]-1-indan-2-yl-0.049
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-morpholin-4-0.01
ylethyl)-5-oxopyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-p-tolyl-0.03
pyrrolidine-3-carboxamide
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2,4-dimethoxy-0.02
benzyl)-5-oxopyrrolidine-3-carboxamide
Methods which are well known and familiar to every
skilled person can be used to bring the compounds
according to the invention into suitable pharmaceutical
forms for administration. Examples of these
administration forms are tablets, lacquered tablets,
sugar-coated tablets, capsules, solutions for
injection, etc. Excipients and auxiliary substances
which are suitable for producing these pharmaceutical
administration forms are likewise well known and
familiar to every skilled person. In addition to one or
more compounds according to the invention, these
administration forms can also comprise additional
pharmacological active compounds.
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 23 -
The attending physician has to adjust the dosage of the
compounds according to the invention, or of the
administration forms which comprise them, in dependence
on the particular requirements of the patient. In
general, a daily dose of 0.1-20 mg, preferably 0.5-
5 mg, of a compound according to the invention per kg
of the patient's body weight ought to be appropriate.
The following examples are intended to clarify the
invention without, however, restricting its scope in
any way.
Example 1 (R1 is phenyl)
20.5 mg (0.1 mmol) of rac. 5-oxo-1-phenylpyrrolidine-3-
carboxylic acid (Buzas et al., Chim Ther 7, 398-403,
1972), dissolved in 0.5 ml of methylene chloride/DMF
(9:1), were added to solid phase coupling reagents
(DCC, loading 1.7 mmol/g). The mixture was shaken for
5 minutes after which 13.6 mg (0.1 mmol) of N,N-
dimethyl-p-phenylenediamine, dissolved in 0.5 ml of
methylene chloride/DMF (9:1), were added and the
mixture was shaken at room temperature overnight. The
solid was then filtered off and the filtrate was
evaporated; the residue was dissolved in 1 ml of
methylene chloride and methylisocyanate polystyrene
(1.8 mmol/g) (solid phase scavenger) was then added to
the solution, which was shaken at room temperature for
12 hours and then filtered; Tris(2-aminoethyl)amine
polystyrene (3.4 mmol/g) was then added to the
filtrate, which was shaken at room temperature for
12 hours and then filtered; the filtrate was
evaporated. This resulted in 18 mg of colorless rac. N-
(4-dimethylaminophenyl)-5-oxo-1-phenylpyrrolidine-3-
carboxamide, MS (M+H) 324.3, MS (M-H) 322.5.
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 24 -
Example 2 (Rl is phenyl)
The following products were prepared in analogy with
example 1 and using the amines which are listed below:
a) from 4-phenoxyaniline, rac. N-(4-phenoxyphenyl)-5-
oxo-1-phenylpyrrolidine-3-carboxamide, MS(M+H) 373.3,
MS(M-H) 371.4.
b) from 2-(3,4-dimethoxyphenyl)ethylamine, rac. N-[2-
(3,4-dimethoxyphenyl)ethyl]-5-oxo-1-phenylpyrrolidine-
3-carboxamide, MS(M+H) 369.3.
c) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyrim-
idine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-5-oxo-1-phenylpyrrolidine-3-carboxamide,
MS(M+H) 430.3, MS(M-H) 428.5.
d) from 2-aminofluorene, rac. N-(9H-fluoren-2-yl)-5
oxo-1-phenylpyrrolidine-3-carboxamide, MS(M+H9) 369.3,
MS(M-H) 367.4.
e) from 3-aminobiphenyl, rac. N-(biphenyl-3-yl)-5-oxo
1-phenylpyrrolidine-3-carboxamide, MS(M+H) 357.2,
MS(M-H) 355.4.
f) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, N-[4-(4,6-dimethylpyrimidin-2-ylamino)phenyl]-
5-oxo-1-phenylpyrrolidine-3-carboxamide, MS(M+H) 402.3,
MS(M-H) 400.5.
g) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-5-oxo-1-phenylpyrrolidine-3-carboxamide,
MS(M+H) 416.3, MS(M-H) 414.5.
h) from N-phenyl-1,4-phenylenediamine, rac. N-(4-
P898II-FINAL VERSION.DOC
~
~ CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 25
phenylaminophenyl)-5-oxo-1-phenylpyrrolidine-3-carbox-
amide, MS(M+H) 372.2, MS(M-H) 370.5.
i) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-
9H-carbazol-3-yl)-5-oxo-1-phenylpyrrolidine-3-carbox-
amide, MS(M+H) 398.2, MS(M-H) 396.3.
P898II-FINAL VERSION.DOC
. ~ CA 02471620 2004-06-23
. WO 03/059905 PCT/CH02/00725
- 26 -
Example 3 (Rl is benzyl)
a) The following products were prepared from rac.
1-benzyl-5-oxo-1-pyrrolidine-3-carboxylic acid, in
analogy with example l, using the amines which are
listed below:
al) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-benzyl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 444.3, MS(M-H) 442.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-benzyl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 430.4, MS(M-H) 428.5.
a3) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-benzyl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 412.1, MS(M-H) 410.3.
b) The rac. 1-benzyl-5-oxo-1-pyrrolidine-3-carboxylic
acid which was required for example 3a was prepared
from benzylamine and itaconic acid in analogy with a
protocol published by Buzas et al. (Chim Ther 7, 398-
403 (1972)).
Example 4 (R1 is 2,5-dimethylphenyl)
a) The following products were prepared from rac.
1-(2,5-dimethylphenyl)-5-oxo-1-pyrrolidine-3-carboxylic
acid, in analogy with example 1, using the amines which
are listed below:
al) from 4'-aminoacetophenone, rac. N-(4-acetylphenyl)-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 351.3, MS(M-H) 349.5.
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
~ ~ WO 03/059905 PCT/CH02/00725
- 27 -
a2) from 3-aminobiphenyl, rac. N-(biphenyl-3-yl)-1-
(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 385.3, MS(M-H) 383.4.
a3) from 3-amino-2-methoxydibenzofuran, rac. N-(2-meth-
oxydibenzofuran-3-yl)-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 429.2, MS(M-H) 427.4.
a4) from 2-amino-9-fluorenone, rac. N-(9-oxo-9H-
fluoren-2-yl)-1-(2,5-dimethylphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 411.2, MS(M-H) 409.4.
a5) from 2-aminofluorene, rac. N-(9H-fluoren-2-yl)-1-
(2,5-dimethylphenyl)-5-oxopyrrlidine-3-carboxamide,
MS(M+H) 397.3, MS(M-H) 395.5.
a6) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-(2,5--dimethylphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 458.4, MS(M-H) 456.5.
a7) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-car-
boxamide, MS(M+H) 430.4, MS(M-H) 428.5.
a8) from N-(4-aminophenyl-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-(2,5-dimethylphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 444.4, MS(M-H) 442.5.
a9) from N-phenyl-1,4-phenylenediamine, rac. N
(4-phenylaminophenyl)-1-(2,5-dimethylphenyl)-5-oxopyr
rolidine-3-carboxamide, MS(M+H) 400.3, MS(M-H) 398.5.
a10) from N,N-dimethyl-p-phenylenediamine, rac. N-(4-
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 28 -
dimethylaminophenyl)-1-(2,5-dimethylphenyl)-5-
oxopyrrolidine-3-carboxamide, MS(M+H) 352.3,
MS(M-H) 350.5.
a11) from p-methoxyaniline, rac. N-(4-methoxyphenyl)-1-
(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 339.2, MS(M-H) 337.4.
alt) from N-ethyl-N-(2-hydroxyethyl)-p-phenylene-
diamine, rac. N-{4-[ethyl-(2-
hydroxyethyl)amino]phenyl}-1-(2,5-dimethylphenyl)-5-
oxopyrrolidine-3-carboxamide, MS(M+H) 396.4, MS(M-
H) 394.5.
a13) from 4-amino-N-ethyl-N-isopropylaniline, rac. N-
[4-(ethylisopropylamino)phenyl]-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide, MS(M+H) 394.4,
MS(M-H) 392.5.
a14) from 4-amino-N,N-diethylaniline, rac. N-(4-di-
ethylaminophenyl)-1-(2,5-dimethylphenyl)-5-oxopyrrolid-
ine-3-carboxamide, MS(M+H) 380.4, MS(M-H) 378.5.
al5) from 1-amino-9-fluorene, rac. N-(9H-fluoren-1-yl)-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 397.3, MS(M-H) 395.5.
alb) from 4-aminobiphenyl, rac. N-(biphenyl-4-yl)-1-
(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 385.3, MS(M-H) 383.4.
all) from ethyl [(4-aminophenyl)phenylamino]acetate
(example 4b2), rac. ethyl [(4-{[1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carbonyl]amino}phenyl)phenylamino]-
acetate, MS(M+H) 486.4, MS(M-H) 484.5.
alb) from N-cyclopropylmethyl-N-phenylphenylene-1,4-
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 29
diamine (example 4c2), rac. N-[4-(cyclopropylmethyl-
phenylamino)phenyl]-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 454.15, MS(M-H) 454.5.
a19) from N-isobutyl-N-phenylphenylene-1,4-diamine
(example 4d2), rac. N-[4-(isobutylphenylamino)phenyl]-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 456.4, MS(M-H) 454.5.
a20) from N-methyl-N-phenylphenylene-1,4-diamine
(example 4e2), rac. N-[4-(methylphenylamino)phenyl]-1-
(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 414.3, MS(M-H) 412.5.
a21) from ethyl [(4-aminophenyl)phenylamino]pentanoate
(example 4f2), rac. ethyl 5-[(4-{[1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carbonyl]amino}phenyl)-
phenylamino]pentanoate, MS(M+H) 528.5.
a22) from N-benzyl-N-phenyl-1,4-diamine (example 4g2),
rac. N-[4-(benzylphenylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide, MS(M+H) 490.3,
MS(M-H) 488.5.
a23) from N-isopropyl-N-phenyl-1,4-diamine
(example
4h2), rac. N-[4-(isopropylphenylamino)phenyl]-1-(2,5-
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 442.4, MS(M-H) 440.5.
a24) from N-ethyl-N-phenyl-1,4-diamine (example 4i2),
rac. N-[4-(ethylphenylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide, MS(M+H) 428.4,
MS(M-H) 426.5.
b) The rac. 2,5-dimethylphenyl-5-oxo-1-pyrrolidine-3-
carboxylic acid which was required for example 4a was
prepared in analogy with example 3b) but using 2,5-
dimethylaniline in place of benzylamine.
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 30 -
bl) The ethyl [(4-aminophenyl)phenylamino]acetate
required in example 4a17) was prepared as follows:
62 mg of sodium hydride dispersion (600), and then
178 ~1 of ethyl bromoacetate, were added to a solution
of 300 mg of 4-nitrodiphenylamine in 3 ml of DMF. The
reaction mixture was stirred at room temperature for
16 hours and then at 50°C for 4 hours, after which it
was cooled down and diluted with 3 ml of toluene; the
solution was then filtered. The filtrate was evaporated
and the residue was purified by chromatography on
silica gel (pentane/toluene). This resulted in 197 mg
of pure ethyl [(4-nitrophenyl)phenylamino]acetate.
b2) The 197 mg of ethyl [(4-nitrophenyl)phenyl-
amino]acetate which were obtained as described in
example 4bl were dissolved in 2 ml of methanol after
which 20 mg of palladium/charcoal catalyst were added
and the mixture was hydrogenated at room temperature
for 3 hours. After the reaction mixture had been
filtered and the filtrate evaporated, 173 mg of ethyl
[(4-aminophenyl)phenylamino]acetate, MS(M+H) 271.1,
were obtained.
cl) The cyclopropylmethyl(4-nitrophenyl)phenylamine was
prepared in analogy with example 4b1 but using
(bromomethyl)cyclopropane in place of ethyl
bromoacetate.
c2) The N-cyclopropylmethyl-N-phenylphenylene-1,4-
diamine, MS(M+H) 239.3, was prepared in analogy with
example 4b2 but using the product from example 4c1.
dl) The isobutyl(4-nitrophenyl)phenylamine was prepared
in analogy with example 4b1 but using 3-bromo-2-
methylpropane in place of ethyl bromoacetate.
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
. . WO 03/059905 PCT/CH02/00725
- 31 -
d2) The N-isobutyl-N-phenylphenylene-1,4-diamine,
MS(M+H) 241.3, was prepared in analogy with example 4b2
but using the product from example 4d1.
el) The methyl(4-nitrophenyl)phenylamine was prepared
in analogy with example 4bl but using methyl iodide in
place of ethyl bromoacetate.
e2) The N-methyl-N-phenylphenylene-1,4-diamine, MS(M+H)
199.3, was prepared in analogy with example 4b2 but
using the product from example 4e1.
f1) The ethyl [(4-nitrophenyl)phenylamino]pentanoate
was prepared in analogy with example 4b1 but using
ethyl bromopentanoate in place of ethyl bromoacetate.
f2) The ethyl [(4-aminophenyl)phenylamino]pentanoate,
MS(M+H) 313.2, was prepared in analogy with example 4b2
but using the product from example 4f1.
gl) The benzyl (4-nitrophenyl)phenylamine was prepared
in analogy with example 4b1 but using benzyl bromide in
place of ethyl bromoacetate.
g2) The N-benzyl-N-phenylphenylene-1,4-diamine, MS(M+H)
275.3, was prepared in analogy with example 4b2 but
using the product from example 4g1.
hl) The isopropyl(4-nitrophenyl)phenylamine was
prepared in analogy with example 4b1 but using 2-bromo-
propane in place of ethyl bromoacetate.
h2) The N-isopropyl-N-phenylphenylene-1,4-diamine,
MS(M+H) 227.3, was prepared in analogy with example 4b2
but using the product from example 4h1.
il) The ethyl(4-nitrophenyl)phenylamine was prepared in
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 32 -
analogy with example 4bl but using bromoethane in place
of ethyl bromoacetate.
i2) The N-ethyl-N-phenylphenylene-1,4-diamine, MS(M+H)
213.3, was prepared in analogy with example 4b2 but
using the product from example 4i1.
Example 5 (R1 is indan-2-yl)
a) The following products were prepared from rac.
1-indan-2-yl-5-oxopyrrolidine-3-carboxylic cid, in
analogy with example l, using the amines which are
listed below:
al) from 3-aminobiphenyl, rac. N-(biphenyl-3-yl)-1-
indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS (M+H) 397. 3, MS (M-H) 395. 5.
a2) from 3-amino-2-methoxydibenzofuran, rac. N-(2-
methoxydibenzofuran-3-yl)-1-indan-2-yl-5-oxopyrrolid-
ine-3-carboxamide, MS(M+H) 441.2, MS(M-H) 439.5.
a3) from 2-amino-9-fluorenone, rac. N-(9-oxo-9H-
fluoren-2-yl)-1-indan-2-yl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 423.2, MS(M-H) 421.4.
a4) from 2-aminofluorene, rac. N-(9H-fluoren-2-yl)-1-
indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 409.3, MS(M-H) 407.5.
a5) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-indan-2-yl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 470.4, MS(M-H) 468.5.
a6) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
. . WO 03/059905 PCT/CH02/00725
- 33 -
phenyl]-1-indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 442.4, MS(M-H) 440.5.
a7) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-indan-2-yl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 456.3, MS(M-H) 454.5.
a8) from N-phenyl-1,4-phenylenediamine, rac. N-(4-
phenylaminophenyl)-1-indan-2-yl-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 412.3, MS(M-H) 410.5.
a9) from 1-amino-9-fluorene, rac. N-(9H-fluoren-1-yl)-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 409.3, MS(M-H) 407.5.
a10) from 4-aminobiphenyl, rac. N-(biphenyl-4-yl)-1-
indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 397.3, MS(M-H) 395.5.
all) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-indan-2-yl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 438.4, MS(M-H) 436.3.
alt) from N,N-dimethyl-p-phenylenediamine, rac. N-(4-
dimethylaminophenyl)-1-indan-2-yl-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 364.3, MS(M-H) 362.1.
a13) from ethyl [(4-aminophenyl)phenylamino]acetate
(see example 3a22), rac. ethyl ({4-[(1-indan-2-yl-5-
oxopyrrolidine-3-carbonyl)amino]phenyl}phenylamino)-
acetate, MS(M+H) 498.3, MS(M-H) 496.5.
al4) from N-cyclopropylmethyl-N-phenylphenylene-1,4-di-
amine (see example 3a23), rac. N-[4-(cyclopropylmethyl-
phenylamino)phenyl]-1-indan-2-yl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 466.4, MS(M-H) 464.5.
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 34
a15) from N-isobutyl-N-phenylphenylene-1,4-diamine
(example 4d2), rac. N-[4-(isobutylphenylamino)phenyl]-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 468.3, MS(M-H) 466.5.
a16) from N-methyl-N-phenylphenylene-1,4-diamine
(example 4e2), rac. N-[4-(methylphenylamino)phenyl]-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 426.3, MS(M-H) 424.5.
a17) from ethyl [(4-aminophenyl)phenylamino]pentanoate
(example 4f2), rac. ethyl 5-({4-[(1-indan-2-yl-5-
oxopyrrolidine-3-carbonyl)amino]phenyl}phenylamino)-
pentanoate, MS(M+H) 540.4, MS(M-H) 538.5.
alb) from N-benzyl-N-phenylphenylene-1,4-diamine
(example 4g2), rac. N-[4-(benzylphenylamino)phenyl]-1-
indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 502.3, MS(M-H) 500.5.
al9) from N-isopropyl-N-phenylphenylene-1,4-diamine
(example 4h2), rac. N-[4-(isopropylphenylamino)phenyl]-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 454.4, MS(M-H) 452.5.
a20) from N-ethyl-N-phenylphenylene-1,4-diamine
(example 4i2), rac. N-[4-(ethylphenylamino)phenyl]-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 440.3, MS(M-H) 438.5.
b) The rac. 1-indan-2-yl-5-oxopyrrolidine-3-carboxylic
acid required for example 5a was prepared in analogy
with example 3b) but using indan-2-amine in place of
benzylamine.
Example 6 (Rl is 2-naphthyl)
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 35 -
a) The following products were prepared from rac. 1
naphthalen-2-yl-5-oxopyrrolidine-3-carboxylic acid, in
analogy with example 1, using the amines which are
listed below:
al) from 4-phenoxyaniline, rac. N-(4-phenoxyphenyl)-1-
naphthalen-2-yl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 423.4, MS(M-H) 421.3.
a2) from N,N-dimethyl-p-phenylenediamine, rac. N-(4-di-
methylaminophenyl)-1-naphthalen-2-yl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 374.3, MS(M-H) 372.5.
b) The rac. 1-naphthalen-2-yl-5-oxopyrrolidine-
3-carboxylic acid required for example 6a was prepared
in analogy with example 3b) but using 2-naphthylamine
in place of benzylamine.
Example 7 (R1 is 2-isopropylphenyl)
a) The following products were prepared from rac. 1-(2
isopropylphenyl)-5-oxopyrrolidine-3-carboxylic acid, in
analogy with example 1, using the amines which are
listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-1-(2-isopropylphenyl)-5-oxopyrrolidine-3-car-
boxamide, MS(M+H) 444.4, MS(M-H) 442.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 458.4, MS(M-H) 456.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 36
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 472.2, MS(M-H) 470.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-
phenylaminophenyl)-1-(2-isopropylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 414.3, MS(M-H) 412.5.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(2-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 440'.4, MS(M-H) 438.3.
a6) from ethyl [(4-aminophenyl)phenylamino]acetate (see
example 3a22), rac. ethyl [(4-{[1-(2-isopropylphenyl)-
5-oxopyrrolidine-3-carbonyl]amino}phenyl)phenylamino]-
acetate, MS(M+H) 500.3, MS(M-H) 498.5.
a7) from N-cyclopropylmethyl-N-phenylphenylene-1,4-di-
amine (see example 3a23), rac. N-[4-(cyclopropylmethyl-
phenylamino)phenyl]-1-(2-isopropylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 468.4, MS(M-H) 466.5.
a8) from N-isobutyl-N-phenylphenylene-1,4-diamine
(example 4d2), rac. N-[4-(isobutylphenylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 470.4, MS(M-H) 468.5.
a9) from N-methyl-N-phenylphenylene-1,4-diamine
(example 4e2), rac. N-[4-(methylphenylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 428.4, MS(M-H) 426.5.
a10) from ethyl [(4-aminophenyl)phenylamino]pentanoate
(example 4f2), rac. ethyl 5-[(4-{[1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carbonyl]amino}phenyl)-
phenylamino]pentanoate, MS(M+H) 542.4, MS(M-H) 540.6.
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 37
all) from N-benzyl-N-phenylphenylene-1,4-diamine
(example 4g2), rac. N-[4-(benzylphenylamino)phenyl]-1-
(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 504.3, MS(M-H) 502.5.
alt) from N-isopropyl-N-phenylphenylene-1,4-diamine
(example 4h2), rac. N-[4-(isopropylphenylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 456.4, MS(M-H) 454.5.
a13) from N-ethyl-N-phenylphenylene-1,4-diamine
(example 4i2), rac. N-[4-(ethylphenylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 442.4, MS(M-H) 440.5.
b) The rac. 1-(2-isopropylphenyl)-5-oxopyrrolidine-3-
carboxylic acid required for example 7a was prepared in
analogy with example 3b) but using isopropylamine in
place of benzylamine.
Example 8 (R1 is 2-phenylethyl)
a) The following products were prepared from rac. 5-
oxo-1-phenethylpyrrolidine-3-carboxylic acid, in
analogy with example l, using the amines which are
listed below:
al) from 4-phenoxyaniline, rac. N-(4-phenoxyphenyl)-5-
oxo-1-phenethylpyrrolidine-3-carboxamide,
MS(M+H) 401.3, MS(M-H) 399.5.
a2) from N,N-dimethyl-p-phenylenediamine, rac. N-(4-
dimethylaminophenyl)-5-oxo-1-phenethylpyrrolidine-3-
carboxamide, MS(M+H) 352.3, MS(M-H) 350.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 38
amino]phenyl}-5-oxo-1-phenethylpyrrolidine-3-carbox-
amide, MS(M+H) 458.4, MS(M-H) 456.5.
a4) from 3-aminobiphenyl, rac. N-(biphenyl-3-yl)-5-oxo
1-phenethylpyrrolidine-3-carboxamide, MS(M+H) 385.2,
MS(M-H) 383.4.
a5) from 2-amino-9-fluorenone, rac. N-(9-oxo-9H-
fluoren-2-yl)-5-oxo-1-phenethylpyrrolidine-3-carbox-
amide, MS(M+H) 411.3, MS(M-H) 409.4.
a6) from 2-aminofluorene, rac. N-(9H-fluoren-2-yl)-5-
oxo-1-phenethylpyrrolidine-3-carboxamide,
MS(M+H) 397.3, MS(M-H) 395.5.
a7) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-5-oxo-1-phenethylpyrrolidine-3-carboxamide,
MS(M+H) 430.3, MS(M-H) 428.5.
a8) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-5-oxo-1-phenethylpyrrolidine-3-carbox-
amide, MS(M+H) 444.3, MS(M-H) 442.5.
a9) from N-phenyl-1,4-phenylenediamine, rac. N-(4-phen-
ylaminophenyl)-5-oxo-1-phenethylpyrrolidine-3-carbox-
amide, MS(M+H) 400.3, MS(M-H) 398.5.
a10) from 4-aminobiphenyl, rac. N-(biphenyl-4-yl)-
5-oxo-1-phenethylpyrrolidine-3-carboxamide,
MS(M+) 385.4, MS(M-H) 383.4.
all) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-5-oxo-1-phenethylpyrrolidine-3-
carboxamide, MS(M+H) 426.3, MS(M-H) 424.4.
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 39 -
b) The rac. 5-oxo-1-phenethylpyrrolidine-3-carboxylic
acid required for example 8a was prepared in analogy
with example 3b) but using phenethylamine in place of
benzylamine.
Example 9 (R1 is 5-methoxy-2-methylphenyl)
a) The following compounds were prepared from rac.
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-carbox-
ylic acid, in analogy with example l, using the amines
which are listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 446.4, MS(M-H) 444.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrol
idine-3-carboxamide, MS(M+H) 460.4, MS(M-H) 458.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrol
idine-3-carboxamide, MS(M+H) 474.0, MS(M-H) 472.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-phen-
ylaminophenyl)-1-(5-methoxy-2-methylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 416.3, MS(M-H) 414.5.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(5-methoxy-2-methylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 442.6, MS(M-H) 440.3.
a6) from ethyl [(4-aminophenyl)phenylamino]acetate (see
example 3a22), rac. ethyl [(4-{[1-(5-methoxy-2-meth-
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
~ WO 03/059905 PCT/CH02/00725
- 40 -
ylphenyl)-5-oxopyrrolidine-3-carbonyl]amino}phenyl)-
phenylamino]acetate, MS(M+H) 502.3, MS(M-H) 500.5.
a7) from N-cyclopropylmethyl-N-phenylphenylene-1,4-di-
amine (see example 3a23), rac. N-[4-(cyclopropylmethyl-
phenylamino)phenyl]-1-(5-methoxy-2-methylphenyl)-5-oxo-
pyrrolidine-3-carboxamide, MS(M+H) 470.4, MS(M-H) 468.5.
a8) from N-isobutyl-N-phenylphenylene-1,4-diamine
(example 4d2), rac. N-[4-(isobutylphenylamino)phenyl]-
1-(5-methoxy-2-methylphenyl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 472.3, MS(M-H) 470.5.
a9) from N-methyl-N-phenylphenylene-1,4-diamine
(example 4e2), rac. N-[4-(methylphenylamino)phenyl]-1-
(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 430.3, MS(M-H) 428.5.
a10) from ethyl [(4-aminophenyl)phenylamino]pentanoate
(example 4f2), rac. ethyl 5-[(4-{[1-(5-methoxy-2-meth-
ylphenyl)-5-oxopyrrolidine-3-carbonyl]amino}phenyl)-
phenylamino]pentanoate, MS(M+H) 544.5, MS(M-H) 542.6.
all) from N-benzyl-N-phenylphenylene-1,4-diamine
(example 4g2), rac. N-[4-(benzylphenylamino)phenyl]-1-
(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 506.3, MS(M-H) 504.5.
a12) from N-isopropyl-N-phenylphenylene-1,4-diamine
(example 4h2), rac. N-[4-(isopropylphenylamino)phenyl]-
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 458.4, MS(M-H) 456.5.
a13) from N-ethyl-N-phenylphenylene-1,4-diamine
(example 4i2); rac. N-[4-(ethylphenylamino)phenyl]-1-
(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 444.4, MS(M-H) 442.5.
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
. , WO 03/059905 PCT/CH02/00725
- 41 -
b) The rac. 1-(5-methoxy-2-methylphenyl)-5-oxopyrrol-
idine-3-carboxylic acid required for example 9a was
prepared in analogy with example 3b) but using
5-methoxy-2-methylaniline in place of benzylamine.
Example 10 (R1 is morpholinoethyl)
a) The following products were prepared from rac. 1-
(2-morpholin-4-ylethyl)-5-oxopyrrolidine-3-carboxylic
acid, in analogy with example 1, using the amines which
are listed below:
al) from 2-aminofluorene, rac. N-(9H-fluoren-2-yl)-1-
(2-morpholin-4-ylethyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 406.4, MS(M-H) 404.5.
a2) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-(2-morpholin-4-ylethyl)-5-
oxopyrrolidine-3-carboxamide, MS(M+H) 467.3, MS(M-
H) 4465.5.
a3) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(2-morpholin-4-ylethyl)-5-oxopyrrol-
idine-3-carboxamide, MS(M+H) 435.5, MS(M-H) 433.3.
b) The rac. 1-(5-methoxy-2-methylphenyl)-5-
oxopyrrolidine-3-carboxylic acid required for example
l0a was prepared in analogy with example 3b) but using
4-(2-aminoethyl)morpholine in place of benzylamine.
Example 11 (R1 is thien-2-ylethyl)
a) The following products were prepared from rac.
5-oxo-1-(2-thiophen-2-ylethyl)pyrrolidine-3-carboxylic
acid, in analogy with example l, using the amines which
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 42 -
are listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-5-oxo-1-(2-thiophen-2-ylethyl)pyrrolidine-3-
carboxamide, MS(M+H) 436.3, MS(M-H) 434.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-5-oxo-1-(2-thiophen-2-ylethyl)pyrrolid
ine-3-carboxamide, MS(M+H) 450.3, MS(M-H) 448.4.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-5-oxo-1-(2-thiophen-2-ylethyl)pyrrolid
ine-3-carboxamide, MS(M+H) 464.5, MS(M-H) 462.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-
(4-phenylaminophenyl)-5-oxo-1-(2-thiophen-2-ylethyl)-
pyrrolidine-3-carboxamide, MS(M+H) 406.2, MS(M-H) 404.4.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-5-oxo-1-(2-thiophen-2-ylethyl)pyrrolid-
ine-3-carboxamide, MS(M+H) 432.2, MS(M-H) 430.2.
b) The rac. 5-oxo-1-(2-thiophen-2-ylethyl)pyrrolidine-
3-carboxylic acid required for example 11a was prepared
in analogy with example 3b) but using 2-thiophene-
ethylamine in place of benzylamine.
Example 12 (R1 is 2-pyridin-2-ylethyl)
a) The following products were prepared from rac. 5-
oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-3-carboxylic
acid, in analogy with example 1, using the amines which
are listed below:
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 43 -
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxamide, MS(M+H) 431.3, MS(M-H) 429.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxamide, MS(M+H) 445.3, MS(M-H) 443.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxamide, MS(M+H) 459.3, MS(M-H) 457.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-phen-
ylaminophenyl)-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolid-
ine-3-carboxamide, MS(M+H) 401.3, MS(M-H) 399.5.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolid-
ine-3-carboxamide, MS(M+H) 427.5, MS(M-H) 425.4.
b) The rac. 5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-3-
carboxylic acid required for example 12a was prepared
in analogy with example 3b) but using 2-(2-
aminoethyl)pyridine in place of benzylamine.
Example 13 (R1 is p-tolyl)
a) The following products were prepared from rac.
5-oxo-1-p-tolylpyrrolidine-3-carboxylic acid, in
analogy with example 1, using the amines which are
listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-5-oxo-1-p-tolylpyrrolidine-3-carboxamide,
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
. . WO 03/059905 PCT/CH02/00725
- 44 -
MS(M+H) 416.4, MS(M-H) 414.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-5-oxo-1-p-tolylpyrrolidine-3-carboxamide,
MS(M+H) 430.4, MS(M-H) 428.4.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-5-oxo-1-p-tolylpyrrolidine-3-carboxamide,
MS(M+H) 444.3, MS(M-H) 442.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-
phenylaminophenyl)-5-oxo-1-p-tolylpyrrolidine-3-
carboxamide, MS(M+H) 386.3, MS(M-H) 384.4.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-5-oxo-1-p-tolylpyrrolidine-3-carbox-
amide, MS(M+H) 412.1, MS(M-H) 410.3.
b) The rac. 5-oxo-1-p-tolylpyrrolidine-3-carboxylic
acid required for example 13a was prepared in analogy
with example 3b) but using p-toluidine in place of
benzylamine.
Example 14 (R1 is m-methoxyphenyl)
a) The following products were prepared from rac. 1-(3
methoxyphenyl)-5-oxopyrrolidine-3-carboxylic acid, in
analogy with example 1, using the amines which are
listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-1-(3-methoxyphenyl)-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 432.2, MS(M-H) 430.5.
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 45 -
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-(3-methoxyphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 446.4, MS(M-H) 444.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-(3-methoxyphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 460.3, MS(M-H) 458.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-phen-
ylaminophenyl)-1-(3-methoxyphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 402.2, MS(M-H) 400.4.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(3-methoxyphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 428.2, MS(M-H) 426.3.
b) The rac. 1-(3-methoxyphenyl)-5-oxopyrrolidine-3-car-
boxylic acid required for example 14a was prepared in
analogy with example 3b) but using m-anisidine in place
of benzylamine.
Example 15 (Rl is cycloheptyl)
a) The following products were prepared from rac.
1-cycloheptyl-5-oxopyrrolidine-3-carboxylic acid, in
analogy with example 1, using the amines which are
listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-
ylamino)phenyl]-1-cycloheptyl-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 422.5, MS(M-H) 420.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 46 -
amino]phenyl}-1-cycloheptyl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 436.6, MS(M-H) 434Ø
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-cycloheptyl-5-oxopyrrolidine-3-carbox-
amide, MS(M+H) 450.6, MS(M-H) 448.6.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-
phenylaminophenyl)-1-cycloheptyl-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 392.4, MS(M-H) 390.5.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-cycloheptyl-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 418.5, MS(M-H) 426.3.
b) The rac. 1-cycloheptyl-5-oxopyrrolidine-3-carboxylic
acid required for example 15a was prepared in analogy
with example 3b) but using cycloheptylamine in place of
benzylamine; MS(M+H) 226.1, MS(M-H) 224.1.
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
, , WO 03/059905 PCT/CH02/00725
- 47 -
Example 16 (Rl is naphthalen-1-ylmethyl)
a) The following products were prepared from rac.
1-naphthalen-1-ylmethyl-5-oxopyrrolidine-3-carboxylic
acid, in analogy with example l, using the amines which
are listed below:
a1) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-1-naphthalen-1-ylmethyl-5-oxopyrrolidine-3-car-
boxamide, MS(M+H) 466.3, MS(M-H) 464.3.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-naphthalen-1-ylmethyl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 480.4, MS(M-H) 478.5
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-naphthalen-1-ylmethyl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 494.4, MS(M-H) 492.5.
b) The rac. 1-naphthalen-1-ylmethyl-5-oxopyrrolidine-
3-carboxylic acid required for example 16a was prepared
in analogy with example 3b) but using 1-naphthyl-
methylamine in place of benzylamine; MS(M+H) 270.1,
MS(M-H) 268.1.
Example 18 (R1 is 2-hydroxy-2-phenylethyl)
a) The following products were prepared from rac. 1-
(2-hydroxy-2-phenylethyl)-5-oxopyrrolidine-3-carboxylic
acid, in analogy with example l, using the amines which
are listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)phen-
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 48 -
yl]-1-(2-hydroxy-2-phenylethyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 446.4, MS(M-H) 444.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-(2-hydroxy-2-phenylethyl)-5-oxopyrrol-
idine-3-carboxamide, MS(M+H) 460.6, MS(M-H) 458.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-(2-hydroxy-2-phenylethyl)-5-oxopyrrol-
idine-3-carboxamide, MS(M+H) 475.6, MS(M-H) 472.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-phen-
ylaminophenyl)-1-(2-hydroxy-2-phenylethyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 416.3, MS(M-H) 414.5.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(2-hydroxy-2-phenylethyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 442.6, MS(M-H) 440.3.
b) The rac. 1-(2-hydroxy-2-phenylethyl)-5-oxopyrrolid-
ine-3-carboxylic acid required for example 18a was
prepared in analogy with example 3b) but using 2-
hydroxy-2-phenylethylamine in place of benzylamine;
MS(M+H) 250.1, MS(M-H) 248.1.
Example 18 (R1 is m-tolyl)
a) The following products were prepared from rac.
5-oxo-1-m-tolylpyrrolidine-3-carboxylic acid, in
analogy with example l, using the amines which are
listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-5-oxo-1-m-tolylpyrrolidine-3-carboxamide,
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 49 -
MS(M+H) 416.3, MS(M-H) 414.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-5-oxo-1-m-tolylpyrrolidine-3-carboxamide,
MS(M+H) 430, MS(M-H) 428.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-5-oxo-1-m-tolylpyrrolidine-3-carboxamide,
MS(M+H) 444.6, MS(M-H) 442.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-phen-
ylaminophenyl)-5-oxo-1-N-tolylpyrrolidine-3-carbox-
amide, MS(M+H) 386.3, MS(M-H) 384.5.
b) The rac. 5-oxo-1-m-tolylpyrrolidine-3-carboxylic
acid required for example 18a was prepared in analogy
with example 3b) but using m-toluidine in place of
benzylamine; MS(M+H) 220.1, MS(M-H) 218.1.
Example 19 (R1 is 2-thienylmethyl)
a) The following product was prepared from rac. 5-oxo-
1-(2-thienylmethyl)pyrrolidine-3-carboxylic acid
(Maybridge), in analogy with example l, using the amine
which is listed below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-5-oxo-1-thiophen-2-ylmethylpyrrolidine-
3-carboxamide, MS(M+H) 418.1, MS(M-H) 416.2.
Example 20 (R1 is 2-furylmethyl)
a) The following products were prepared from rac. 1-(2-
furylmethyl)-5-oxopyrrolidine-3-carboxylic acid
(Maybridge), in analogy with example 1 using the amines
P898II-FINAL VERSION.DOC
- ' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 50 -
which are listed below:
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)phen-
yl]-1-furan-2-ylmethyl-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 406.3, MS(M-H) 404.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)methyl-
amino]phenyl}-1-furan-2-ylmethyl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 420.5, MS(M-H) 418.5.
a3) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-1-furan-2-ylmethyl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 434.6, MS(M-H) 432.5.
a4) from N-phenyl-1,4-phenylenediamine, rac. N-(4-
phenylaminophenyl)-1-furan-2-ylmethyl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 376.3, MS(M-H) 474.5.
a5) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-furan-2-ylmethyl-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 401.9, MS(M-H) 400.1.
Example 21 (Rl is p-chlorobenzyl)
a) The following product was prepared from rac. 1-(4-
chlorobenzyl)-5-oxopyrrolidine-3-carboxylic acid
(Maybridge), in analogy with example l, using the amine
which is listed below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(4-chlorobenzyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 446.1, MS(M-H) 444.1.
Example 22 (R1 is p-dimethylaminophenyl)
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
. . WO 03/059905 PCT/CH02/00725
- 51 -
a) In analogy with example l, and using suitable
amines, rac. 1-(4-dimethylaminophenyl)-5-oxopyrrolid
ine-3-carboxylic acid can be converted into products of
the formula I.
al) from N-(4-aminophenyl)-4,6-dimethyl-2-pyrimidine-
amine, rac. N-[4-(4,6-dimethylpyrimidin-2-ylamino)-
phenyl]-1-(4-dimethylaminophenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 445.2, MS(M-H) 443.5.
a2) from N-(4-aminophenyl)-N-methyl-4,6-dimethyl-2-pyr-
imidine-amine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)-
methylamino]phenyl}-1-(4-dimethylaminophenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 459.4, MS(M-H) 457.5.
b) The rac. 1-(4-dimethylaminophenyl)-5-oxopyrrolidine-
3-carboxylic acid required for example 22a was prepared
in analogy with example 3b but using N,N-dimethyl-
p-phenylenediamine in place of benzylamine;
MS(M+H) 249.1, MS(M-H) 247.1.
Example 23 (Rl is 2-pyrrolidin-1-ylethyl)
a) The following product was prepared from rac. 5-oxo-
1-(2-pyrrolidin-1-ylethyl)pyrrolidine-3-carboxylic
acid, in analogy with example l, using the amine which
is listed below:
al) from N-(4-aminophenyl)-N-ethyl-4,6-dimethyl-2-pyr-
imidine, rac. N-{4-[(4,6-dimethylpyrimidin-2-yl)ethyl-
amino]phenyl}-5-oxo-1-(2-pyrrolidin-1-ylethyl)pyrrolid-
ine-3-carboxamide, MS(M+H) 451.2, MS(M-H) 449.3.
b) The rac. 5-oxo-1-(2-pyrrolidin-1-ylethyl)pyrrolid-
ine-3-carboxylic acid required for example 23a was
prepared in analogy with example 3b) but using 1-
P898II-FINAL VERSION.DOC
- ' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 52 -
(2-aminoethyl)pyrrolidine in place of benzylamine;
MS(M+H) 227.1, MS(M-H) 225.1.
Example 24 (R1 is 1-methylpyrrolidin-2-ylethyl)
a) The following product was prepared from rac. 1-[2-
(1-methylpyrrolidin-2-yl)ethyl]-5-oxopyrrolidine-3-car-
boxylic acid, in analogy with example l, using the
amine which is listed below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-[2-(1-methylpyrrolidin-2-yl)ethyl]-5-
oxopyrrolidine-3-carboxamide, MS(M+H) 433.4,
MS(M-H) 431.3.
b) The rac. 1-[2-(1-methylpyrrolidin-2-yl)ethyl]-5-oxo-
pyrrolidine-3-carboxylic acid required for example 24a
was prepared in analogy with example 3b) but using 2-
(2-aminoethyl)-1-methylpyrrolidine in place of
benzylamine; MS(M+H) 241.2, MS(M-H) 239.1.
Example 25 (R1 is 4-isopropylphenyl)
a) The following product was prepared from rac. 1-
(4-isopropylphenyl)-5-oxopyrrolidine-3-carboxylic acid,
in analogy with example 1, using the amine which is
listed below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(4-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 440.4, MS(M-H) 438.3.
b) The rac. 1-(4-isopropylphenyl)-5-oxopyrrolidine-
3-carboxylic acid required for example 25a was prepared
in analogy with example 3b) but using 4-isopropyl-
aniline in place of benzylamine; MS(M+H) 248.1,
MS(M-H) 246.1.
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 53 -
Example 26 (Rl is 3,5-bis-(trifluoromethyl)-phenyl)
a) The following product was prepared from rac. 1-
(3,5-bis-(trifluoromethyl)-phenyl)-5-oxopyrrolidine-3-
carboxylic acid, in analogy with example 1, using the
amine which is listed below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(3,5-bistrifluoromethylphenyl)-5-
oxopyrrolidine-3-carboxamide, MS(M+H) 534.4.
b) The rac. 1-(3,5-bistrifluoromethylphenyl)-5-oxopyr
rolidine-3-carboxylic acid required for example 26a was
prepared in analogy with example 3b) but using 3,5
bis(trifluoromethyl)aniline in place of benzylamine.
Example 27 (R1 is 3-fluorophenyl)
a) The following product was prepared from rac. 1-(3-
fluorophenyl)-5-oxopyrrolidine-3-carboxylic acid, in
analogy with example 1, using the amine which is listed
below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(3-fluorophenyl)-5-oxopyrrolidine-3-
carboxamide, MS(M+H) 416.1, MS(M-H) 414.2.
b) The rac. 1-(3-fluorophenyl)-5-oxopyrrolidine-3-
carboxylic acid required for example 27a was prepared
in analogy with example 3b) but using 3-fluoroaniline
in place of benzylamine; MS(M+H) 224.2, MS(M-H) 222.1.
Example 28 (R1 is 2-chlorobenzyl)
a) The following product was prepared from rac. 1-
(2-chlorobenzyl)-5-oxopyrrolidine-3-carboxylic acid, in
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
, . WO 03/059905 PCT/CH02/00725
- 54 -
analogy with example l, using the amine which is listed
below:
al) from 3-amino-9-ethylcarbazole, rac. N-(9-ethyl-9H-
carbazol-3-yl)-1-(2-chlorobenzyl)-5-oxopyrrolidine-
3-carboxamide, MS(M+H) 446.2, MS(M-H) 444.2.
b) The rac. 1-(2-chlorobenzyl)-5-oxopyrrolidine-3-car-
boxylic acid required for example 28a was prepared in
analogy with example 3b) but using 2-chlorobenzylamine
in place of benzylamine; MS(M+H) 254.1, MS(M-H) 252.1.
Example 29 (Enantiomerically pure compounds)
The rac. N-(9-ethyl-9H-carbazol-3-yl)-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide prepared as
described in example 7a5) can be resolved into the two
enantiomers
(a) (R)-N-(9-ethyl-9H-carbazol-3-yl)-1-(2-iso-
propylphenyl)-5-oxopyrrolidine-3-carboxamide;
and
(b) (S)-N-(9-ethyl-9H-carbazol-3-yl)-1-(2-
isopropylphenyl)-5-oxopyrrolidine-3-
carboxamide
by means of HPLC on a LichroCART (R, R) Whelk-O1 column
using a solvent gradient (n-hexane + 0.5% acetic
acid/isopropanol + 0.5% acetic acid).
Example 30 (Enantiomerically pure compounds)
The following racemic compounds can be resolved into
the corresponding enantiomers in analogy with example
29:
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-(2-pyridin-
2-ylethyl)pyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-hydroxy-
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
. . WO 03/059905 PCT/CH02/00725
- 55 -
2-phenylethyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Diethylaminophenyl)-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-(2-thiophen-
2-ylethyl)pyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(4-chlorobenzyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-thiophen-
2-ylmethylpyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-furan-2-ylmethyl-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(5-methoxy-
2-methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylisopropylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-phenethylpyr-
rolidine-3-carboxamide;
rac. Ethyl [(4-{[1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carbonyl]amino}phenyl)phenylamino]acetate;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-cycloheptyl-5-oxo-
pyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-{4-[Ethyl-(2-hydroxyethyl)amino]phenyl}-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(3-methoxyphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-chlorobenzyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-1-(2,5-dimethylphenyl)-5-oxopyrrolidine-
3-carboxamide;
P898II-FINAL VERSION.DOC
' CA 02471620 2004-06-23
. WO 03/059905 PCT/CH02/00725
- 56 -
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-phenyl-
pyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-5-oxo-1-p-tolylpyrrolidine-3-carboxamide;
rac. N-[4-(Isopropylphenylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-5-oxo-1-(2-pyridin-2-ylethyl)pyrrolidine-
3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-[4-(Methylphenylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Methylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(4-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(3-fluorophenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Phenylaminophenyl)-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
1-indan-2-yl-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Ethylphenylamino)phenyl]-1-(5-methoxy-
2-methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-benzyl-5-oxopyr-
rolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-
5-oxo-1-p-tolylpyrrolidine-3-carboxamide;
P898II-FINA1, VERSION.DOC
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 57 -
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-indan-2-yl-5-oxo-
pyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(2-hydroxy-2-phenylethyl)-5-oxo-pyrrolidine-
3-carboxamide;
rac. N-(Biphenyl-4-yl)-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide;
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-
1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-3-carbox-
amide;
rac. N-[4-(Isopropylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Phenylaminophenyl)-1-(2-isopropylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-1-(5-methoxy-2-methylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(3-methoxyphenyl)-5-oxopyrrolidine-3-carbox-
amide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)methylamino]-
phenyl}-5-oxo-1-m-tolylpyrrolidine-3-carboxamide;
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-1-(2,5-
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(4,6-Dimethylpyrimidin-2-yl)ethylamino]-
phenyl}-1-(2-isopropylphenyl)-5-oxopyrrolidine-
3-carboxamide;
rac. Ethyl [(4-{[1-(5-methoxy-2-methylphenyl)-5-oxopyr-
rolidine-3-carbonyl]amino}phenyl)phenylamino]acetate;
rac. N-[4-(Cyclopropylmethylphenylamino)phenyl]-
1-(2-isopropylphenyl)-5-oxopyrrolidine-3-carboxamide;
and
rac. N-[4-(Ethylphenylamino)phenyl]-1-indan-2-yl-5-oxo-
pyrrolidine-3-carboxamide.
Example 31 (R1 is 2,5-dimethylphenyl)
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 58 -
a) 29 ~1 of Hunig's base and 1 equivalent of the acid
chloride listed below were added to a solution of 50 mg
of rac. N-(4-aminophenyl)-1-(2,5-dimethylphenyl)-5-
oxopyrrolidine-3-carboxamide in 0.5 ml of methylene
chloride. The reaction mixture was stirred overnight at
room temperature and then evaporated and the residue
was chromatographed on silica gel using ethyl acetate/
ethanol (8:2). The evaporated product fractions in each
case yielded approx. 30 mg of product. This method was
used to prepare the following compounds:
al) with acetyl chloride, rac. N-(4-acetylaminophenyl)-
1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-carboxamide,
MS(M+H) 366.3, MS(M-H) 364.4.
a2) with isovaleryl chloride, rac. N-[4-(3-methyl-
butyrylamino)phenyl]-1-(2,5-dimethylphenyl)-5-
oxopyrrolidine-3-carboxamide, MS(M+H) 408.3, MS(M-
H) 406.4.
a3) with cyclopropylcarbonyl chloride, rac. N-
[4-(cyclopropanecarbonylamino)phenyl]-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide, MS(M+H) 392.3,
MS(M-H) 390.4.
a4) with benzoyl chloride, rac. N-(4-benzoylamino-
phenyl)-1-(2,5-dimethylphenyl)-5-oxopyrrolidine-3-car-
boxamide, MS(M+H) 428.3, MS(M-H) 426.4.
a5) with phenylacetyl chloride, rac. N-(4-phenyl-
acetylaminophenyl)-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 442.4, MS(M-H) 440.5.
a6) with 2-methoxybenzoyl chloride, rac. N-[4-(2-meth-
oxybenzoylamino)phenyl]-1-(2,5-dimethylphenyl)-5-oxo-
pyrrolidine-3-carboxamide, MS(M+H) 458.2, MS(M-H) -
456.5.
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
. . WO 03/059905 PCT/CH02/00725
- 59 -
a7) with piperonyloyl chloride, rac. N-{4-[(benzo-
[1,3]dioxole-5-carbonyl)amino]phenyl}-1-(2,5-dimethyl-
phenyl)-5-oxopyrrolidine-3-carboxamide, MS(M+H) 472.3,
MS(M-H) 470.5.
a8) with pivaloyl chloride, rac. N-[4-(2,2-dimethyl-
propionylamino)phenyl]-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 408.3, MS(M-H) 406.4.
a9) with 4-methoxybenzoyl chloride, rac. N-[4-(4-meth-
oxybenzoylamino)phenyl]-1-(2,5-dimethylphenyl)-5-oxo-
pyrrolidine-3-carboxamide, MS(M+H) 458.2,
MS(M-H) 456.4.
a10) with 3-fluorobenzoyl chloride, rac. N-[4-
(3-fluorobenzoylamino)phenyl]-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide, MS(M+H) 446.3,
MS(M-H) 444.4.
all) with 2-furoyl chloride, rac. N-{4-[(furan-2-car-
bonyl)amino]phenyl}-1-(2,5-dimethylphenyl)-5-oxopyr-
rolidine-3-carboxamide, MS(M+H) 418.4, MS(M-H) 416.4.
b) The rac. N-(4-aminophenyl)-1-(2,5-dimethylphenyl)-5-
oxopyrrolidine-3-carboxamide required in example 31a)
was prepared as follows:
b1) 1.18 g of p-nitroaniline, 1.95 g of N-(3-di-
methylaminopropyl)-N'-ethylcarbodiimide hydrochloride
(EDC HCl), 2.22 ml of Hunig's base and 1.04 g of 4-
(N,N-dimethylamino)pyridine were added consecutively to
a solution of 2 g of 1-(2,5-dimethylphenyl)-5-oxo-1-
pyrrolidine-3-carboxylic acid (example 4b) in 28 ml of
methylene chloride. The reaction mixture was stirred at
40°C for 3 hours and then taken up in ethyl acetate and
washed with water until neutral. The organic phase was
P898II-FINAL VERSION.DOC
~
- CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 60
evaporated and this resulted in 2.5 g of rac. N-
(4-nitrophenyl)-1-(2,5-dimethylphenyl)-5-oxopyrrolid-
ine-3-carboxamide, MS(M+H) 354.1, MS(M-H) 352.3.
b2) The 2.5 g of rac. N-(4-nitrophenyl)-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide which were
obtained as described in example 3lbl) were dissolved
in 70 ml of methanol and 70 ml of methylene chloride
after which 0.5 g of palladium/charcoal catalyst was
added and the mixture was hydrogenated overnight at
room temperature. After the reaction mixture had been
filtered and the filtrate evaporated, 2.3 g of rac. N-
(4-aminophenyl)-1-(2,5-dimethylphenyl)-5-oxopyrrolid-
ine-3-carboxamide, MS(M+H) 324.3, MS(M-H) 322.4, were
obtained.
Example 32 (Enantiomerically pure compounds)
The following racemic compounds can be resolved into
the corresponding enantiomers in analogy with example
29:
rac. N-(4-Acetylaminophenyl)-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(3-Methylbutyrylamino)phenyl]-1-(2,5-dimeth-
ylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(Cyclopropanecarbonylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Benzoylaminophenyl)-1-(2,5-dimethylphenyl)-
5-oxopyrrolidine-3-carboxamide;
rac. N-(4-Phenylacetylaminophenyl)-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
P898II-FINAL VERSION.DOC
- ' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 61 -
rac. N-[4-(2-Methoxybenzoylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(Benzo[1,3]dioxole-5-carbonyl)amino]-1-(2,5-
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(2,2-Dimethylpropionylamino)phenyl]-1-(2,5-
dimethylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4-Methoxybenzoylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(3-Fluorobenzoylamino)phenyl]-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-{4-[(Furan-2-carbonyl)amino]phenyl}-1-(2,5-di-
methylphenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2-morpholin-4-yl-
ethyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-(9-Ethyl-9H-carbazol-3-yl)-5-oxo-1-p-tolylpyr-
rolidine-3-carboxamide; and
rac. N-(9-Ethyl-9H-carbazol-3-yl)-1-(2,4-
dimethoxybenzyl)-5-oxopyrrolidine-3-carboxamide.
Example 33 (Enantiomerically pure compounds)
The following racemic compounds can be resolved into
the corresponding enantiomers in analogy with example
29:
rac. N-[4-(Isobutylphenylamino)phenyl]-1-(2-isopropyl-
phenyl)-5-oxopyrrolidine-3-carboxamide;
rac. N-[4-(4,6-Dimethylpyrimidin-2-ylamino)phenyl]-1-
(4-dimethylaminophenyl)-5-oxopyrrolidine-3-carboxamide;
P898II-FINAL VERSION.DOC
~
' CA 02471620 2004-06-23
WO 03/059905 PCT/CH02/00725
- 62 -
and
rac. N-[4-(4,6-Dimethylpyrimidin-2-yl)methylamino-
phenyl]-1-(4-dimethylaminophenyl)-5-oxopyrrolidine-
3-carboxamide.
Example 34 (R1 is 2,4-dimethoxybenzyl)
a) rac. N-(9-Ethyl-9H-carbazole-3-yl)-1-(2,4-dimethoxy-
benzyl)-5-oxopyrrolidine-3-carboxamide was pre ared
from rac. 1-(2,4-dimethoxybenzyl)-5-oxopyrrolidine-3-
carboxylic acid, in analogy with example 1, using 3-
amino-9-ethylcarbazole; MS(M+H) 472.4, MS(M-H) 470.2.
b) The 1-(2,4-dimethoxybenzyl)-5-oxo yrrolidine-3-car-
boxylic acid required for example 34a was prepared in
analogy with example 3b) but using 2,4-
dimethoxybenzylamine; MS(M+H) 280.1, MS(M-H) 278.1.
Example A
A compound of the formula I can be used, in a manner
known per se, as the active compound for producing
tablets of the following composition:
Per tablet
Active compound 200 mg
Microcrystalline cellulose 155 mg
Corn starch 25 mg
Talc 25 mg
Hydroxypropylmethyl cellulose 20 m
425 ma
Example B
A compound of the formula I can be used, in a manner
known per se, as the active compound for producina
P898II-FINAL VERSION.DOC
CA 02471620 2004-06-23
. WO 03/059905 PCT/CH02/00725
- 63 -
capsules of the following composition:
Per capsule
Active compound 100 mg
Corn starch 20 mg
Lactose 95 mg
Talc 4.5 mg
Magnesium stearate 0.5 mg
220.0 ma
P898II-FINAL VERSION.DOC