Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
1
APPARATUS AND METHOD FOR PROTECTING THE
NEUROVASCULAR BUNDLE DURING CRYOSURGICAL
TREATMENT OF THE PROSTATE
FIELD AND BACKGROUND OF THE INVENTION
The present invention relates to an apparatus and method for protecting
the neurovascular bundle during cryoablation of tissues of the prostate. More
particularly, the present invention relates to heating the vicinity of the
neurovascular bundle while cooling pathological tissues in or near the
prostate
to cryoablation temperatures, thereby cryoablating pathological tissues while
protecting the neurovascular bundle from damage.
In recent years, cryoablation of pathological tissues has become an
increasingly popular method of treatment of prostate cancer and of benign
prostate hyperplasia ("BPH"). Cryoablation of pathological tissues is
typically
accomplished by utilizing imaging modalities such as x-ray, ultrasound, CT,
and MRI to identify a locus for ablative treatment, then inserting one or more
cryoprobes into that selected treatment locus, and cooling the treatment heads
of those cryoprobes sufficiently to cause the tissues surrounding the
treatment
heads to reach cryoablation temperatures, typically below about - 40 °
C. The
tissues thus cooled are thereby caused to loose their functional and
structural
integrity. Cancerous cells cease growing and multiplying, and cryoablated
tumor tissue material, whether from malignant tumors or from benign growths,
is subsequently absorbed by the body. Cryoablation may thus be used to treat
malignant tumors of the prostate, and to reduce prostate volume in cases of
BPH.
The principle danger and disadvantage of cryosurgical ablative
treatment of the prostate, however, is the danger of partially or completely
destroying the functional and structural integrity of non-pathological tissues
proximate to the treatment locus, thereby having a deleterious effect on the
health and quality of life of the treated patient.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
2
Various devices and methods have been proposed to enable cryoablation
of pathological prostate tissue while limiting damage to non-pathological
tissue. These fall roughly into two categories: devices and methods which
protect tissues by preventing excessive cooling of those tissues during a
cryoablation procedure in their vicinity, and methods devices and methods
which enable accurate placement of cryoprobes used in cryoablation, so as to
successfully concentrate the cooling effect of such cryoprobes at or near
pathological tissue, thereby minimizing unwanted cooling of non-pathological
tissue.
An example of the former category is the well-known technique of
introducing a heating device or a heated fluid into the urethra of a patient,
to
heat the urethra and tissues adjacent to it during cryoablation of portions of
the
prostate, thereby helping to protect the urethra from damage while prostate
tissues nearby are being cooled to cryoablation temperatures.
An example of the latter category is provided by U. S. Patent No.
6,142,991 to Schatzberger. Schatzberger describes a high resolution
cryosurgical method and device for treating a patient's prostate, including
the
steps of (a) introducing a plurality of cryosurgical probes to the prostate,
the
probes having a substantially small diameter, the probes being distributed
across the prostate, so as to form an outer arrangement of probes adjacent the
periphery of the prostate and an inner arrangement of probes adjacent the
prostatic urethra; (b) producing an ice-ball at the end of each of said
cryosurgical probes, so as to locally freeze a tissue segment of the prostate.
Schatzberger's apparatus includes (a) a plurality of cryosurgical probes
of small diameter, the probes being for insertion into the patient's organ,
the
probes being for producing ice-balls for locally freezing selected portions of
the organ; (b) a guiding element including a net of apertures for inserting
the
cryosurgical probes therethrough; and (c) an imaging device for providing a
set
of images, the images being for providing information on specific planes
located at specific depths within the organ, each of said images including a
net
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
3
of marks being correlated to the net of apertures of the guiding element,
wherein the marks represent the locations of ice-balls which may be formed by
the cryosurgical probes when introduced through said apertures of the guiding
element to said distinct depths within the organ.
Thus, Schatzberger's method and apparatus enable a surgeon to place a
set of cryoablation probes within a prostate with relatively high accuracy,
and
to operate those probes to ablate selected tissues while avoiding, to a large
extent, inadvertent and undesirable ablation of healthy tissues near the
ablation
site.
However, neither Schatzberger's technique nor any other known
technique has proven sufficiently accurate to prevent damage to peripheral
tissues in all cases. In particular, the neurovascular bundle, a prostatic
area rich
in blood vessels and in nerve tissues having cardinal importance in the
process
of erection of penis, is particularly vulnerable to damage by conventional
prostatic cryoablation procedures. The neurovascular bundle lies dorsolateral
to the prostate from the level of the seminal vesicles to the urethra, and is
embedded in the lateral pelvic fascia along the pelvic side wall.
Damage to the neurovascular bundle may cause loss of sexual potency.
Potent patients having an active sexual life are understandably reluctant to
risk
loss of potency as a result of cryosurgical treatment of the prostate, and
such
loss of potency unfortunately occurs in a non-negligible percentage of
patients
treated with conventional cryosurgery, as it does also in cases of treatment
of
prostate tumors by non-cryosurgical means.
Thus, there is a widely felt need for, and it would be highly
advantageous to have, a therapeutic approach to malignant prostate tumors and
to benign prostate hyperplasia, which approach enables cryoablation of
prostate
tissues while protecting the neurovascular bundle, thereby substantially
reducing or eliminating the danger that cryosurgical treatment of the prostate
will cause loss of erectile potency of the patient.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
4
Similarly, there is a widely felt need for, and it would be highly
advantageous to have, apparatus and method enabling selective protection of
sensitive and functionally important healthy tissues in close proximity to
pathological tissues whose cryoablation is desired, in numerous similar
contexts.
SUMMARY OF THE INVENTION
According to one aspect of the present invention there is provided a
method for protecting at least a portion of a neurovascular bundle while
cryoablating tissues of a prostate, comprising (a) positioning an operating
tip of
a cryoprobe in a vicinity of pathological tissue within a prostate; (b)
positioning
a heating probe in a vicinity of a neurovascular bundle; and (c) heating the
heating probe while cooling the operating tip of the cryoprobe to cryoablation
temperatures, thereby cryoablating pathological tissue near the operating tip
1 S while preventing freezing of tissue of the neurovascular bundle near the
heating
probe; thereby preventing damage to at least a portion of the neurovascular
bundle.
According to further features in preferred embodiments of the invention
described below, the method further comprises utilizing imaging modalities to
map the prostatic region of a patient, to locate pathological tissue to be
cryoablated, and to locate a neurovascular bundle to be protected from
cryoablation and from damage by freezing. Preferably, a guiding element is
used to guide placement of a cryoprobe in a vicinity of the located
pathological
tissue, and to guide placement of the heating probe in a vicinity of the
located
neurovascular bundle.
According to still further features in the described preferred
embodiments, the imaging modalities are selected from a group consisting of
CT imaging, x-ray imaging, and ultrasound imaging. According to a preferred
embodiment, ultrasound imaging of the prostate is obtained by insertion of an
ultrasound probe into the rectum of a patient.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
According to another aspect of the present invention there is provided a
cryoprobe having a shaft and a distal operating tip, the tip being operable to
cool to cryoablation temperatures tissues surrounding the tip, thereby
cryoablating the tissues, the shaft being designed and constructed to protect
S tissues adjacent to the shaft from cryoablation and from damage by freezing.
According to further features in preferred embodiments of the invention
described below, the shaft comprises an insulating element serving to
thermally
isolate cold regions within the shaft from external portions of the shaft
having
direct contact with tissues adjacent to the shaft. The insulating element may
be
formed as an insulating sheath comprising at least a partial vacuum.
According to further features in preferred embodiments of the invention
described below, the shaft comprises a heating element, which may be an
electrical resistance heating element, a microwave heating element, a radio
frequency heating element, or a fluid heating module.
According to further features in preferred embodiments of the invention
described below, the fluid heating module comprises a first passage for
delivery of a fluid to a volume within the shaft, and further comprises a
second
passage for exhausting the fluid from said volume of said shaft. The first
passage may be operable to deliver a pre-heated fluid to a portion of said
shaft,
or to deliver a compressed heating gas to a first Joule-Thomson orifice.
According to further features in preferred embodiments of the invention
described below, the shaft comprises a first Joule-Thomson orifice and the
operating tip comprises a second Joule-Thomson orifice. The cryoprobe
comprises a first gas input passage and a second gas input passage, the first
gas
input passage being operable to deliver compressed heating gas to the first
Joule-Thomson orifice while the second gas input passage delivers compressed
cooling gas to the second Joule-Thomson orifice.
According to still another aspect of the present invention there is
provided a method for protecting tissues adjacent to a shaft of a cryoprobe
while cryoablating tissues adjacent to an operating tip of said cryoprobe,
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
6
comprising (a) cooling the operating tip of the cryoprobe to cryoablation
temperatures, thereby cryoablating tissues adjacent to the operating tip; and
(b)
simultaneously heating a portion of the shaft, thereby preventing freezing of
tissues adjacent to the shaft, thereby protecting tissues adjacent to the
shaft
while cryoablating tissues adjacent to the operating tip.
A portion of the shaft, preferably the external portion adjacent to the
external wall of the shaft, may be heated by electrical resistance heating, by
microwave heating, by radio frequency heating, by circulating therein a
pre-heated fluid, and by Joule-Thomson heating.
The present invention successfully addresses the shortcomings of the
presently known configurations by providing an apparatus and method for
protecting healthy and functionally important tissue areas during cryoablation
of pathological tissues in their vicinity.
The present invention further successfully addresses the shortcomings of
1 S the presently known configurations by providing an apparatus and method
for
protecting the neurovascular bundle during cryoablation of nearby prostate
tissues, thereby substantially reducing or eliminating the probability that
loss of
erectile potency of the patient will result from cryoablative prostate
treatment.
Unless otherwise defined, all technical and scientific terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which this invention belongs. Although methods and materials similar or
equivalent to those described herein can be used in the practice or testing of
the
present invention, suitable methods and materials are described below. In case
of conflict, the patent specification, including definitions, will control. In
addition, the materials, methods, and examples are illustrative only and not
intended to be limiting.
Implementation of the method and system of the present invention
involves performing or completing selected tasks or steps manually,
automatically, or a combination thereof. Moreover, according to actual
instrumentation and equipment of preferred embodiments of the method and
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
7
system of the present invention, several selected steps could be implemented
by
hardware or by software on any operating system of any firmware or a
combination thereof. For example, as hardware, selected steps of the invention
could be implemented as a chip or a circuit. As software, selected steps of
the
invention could be implemented as a plurality of software instructions being
executed by a computer using any suitable operating system. In any case,
selected steps of the method and system of the invention could be described as
being performed by a data processor, such as a computing platform for
executing a plurality of instructions.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention is herein described, by way of example only, with
reference to the accompanying drawings. With specific reference now to the
drawings in detail, it is stressed that the particulars shown are by way of
example and for purposes of illustrative discussion of the preferred
embodiments of the present invention only, and are presented in the cause of
providing what is believed to be the most useful and readily understood
description of the principles and conceptual aspects of the invention. In this
regard, no attempt is made to show structural details of the invention in more
detail than is necessary for a fundamental understanding of the invention, the
description taken with the drawings making apparent to those skilled in the
art
how the several forms of the invention may be embodied in practice.
In the drawings:
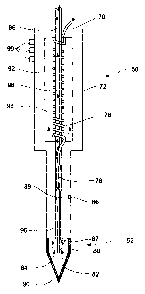
FIG. 1 is a simplified schematic of an exemplary cryoprobe, according
to the methods of prior art;
FIG. 2 is a simplified schematic of a manifold structure connecting a
plurality of cryosurgical probes to a common gas source, according to the
methods of prior art;
FIG. 3 is a simplified schematic of an alternative configuration of a
pre-cooling element, according to the methods of prior art;
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
8
FIG. 4 is a simplified schematic of an apparatus comprising an
ultrasound probe and a guiding element for guiding insertion of a plurality of
cryoprobes into a patient's body, according to the methods of prior art;
FIG. 5 is a simplified schematic showing a method of use of the
apparatus presented in Figure 4, according to the methods of prior art;
FIG. 6 is a simplified schematic showing a further step in the use of the
apparatus presented in Figure 4, according to the methods of prior art;
FIG. 7 is a simplified schematic representation of a prostrate treatment
apparatus according to an embodiment of the present invention;
FIG. 8 is an additional view of the treatment apparatus of Figure 7;
FIG. 9 is a simplified flow-chart of a recommended procedure for
cryoablation of a portion of an organ, such as a tumor of a prostate,
according
to an embodiment of the present invention;
FIG. 10 is a simplified schematic representation of a prostrate treatment
apparatus according to an additional preferred embodiment of the present
invention;
FIG. 11 is a simplified schematic of a cryoprobe comprising a cryogenic
treatment head and a heatable shaft, according to an embodiment of the present
invention;
FIG. 12 is a simplified schematic of an alternative construction of a
cryoprobe having a cryogenic treatment head and a heatable shaft, according to
an embodiment of the present invention; and
FIG. 13 is a simplified schematic of a cryoprobe having an insulated
shaft, according to an embodiment of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is of apparatus and method for protecting healthy
tissues from damage, while cryoablating pathological tissues nearby. More
particularly, the present invention relates to heating a first selected tissue
area
in or near a prostate, such as the neurovascular bundle area, while cooling to
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
9
cryoablation temperatures a second selected tissue area in or near a prostate,
such as benign or malignant tumor tissue, thereby cryoablating selected
pathological tissues while protecting the neurovascular bundle or other
selected
healthy tissues from damage. The invention can be used to protect the
neurovascular bundle during cryosurgery of prostate tissues, thereby reducing
the risk of adverse effects of prostate cryosurgery to penile erectile
functioning
of a patent so treated.
Before explaining at least one embodiment of the invention in detail, it
is to be understood that the invention is not limited in its application to
the
details of construction and the arrangement of the components set forth in the
following description or illustrated in the drawings. The invention is capable
of other embodiments or of being practiced or carried out in various ways.
Also, it is to be understood that the phraseology and terminology employed
herein is for the purpose of description and should not be regarded as
limiting.
1 S To enhance clarity of the following descriptions, the following terms
and phrases will first be defined:
The phrase "heat-exchanging configuration" is used herein to refer to
component configurations traditionally known as "heat exchangers", namely
configurations of components situated in such a manner as to facilitate the
passage of heat from one component to another. Examples of
"heat-exchanging configurations" of components include a porous matrix used
to facilitate heat exchange between components, a structure integrating a
tunnel
within a porous matrix, a structure including a coiled conduit within a porous
matrix, a structure including a first conduit coiled around a second conduit,
a
structure including one conduit within another conduit, or any similar
structure.
The phrase "Joule-Thomson heat exchanger" as used herein refers, in
general, to any device used for cryogenic cooling or for heating, in which a
gas
is passed from a first region of the device, wherein it is held under higher
pressure, to a second region of the device, wherein it is enabled to expand to
lower pressure. A Joule-Thomson heat exchanger may be a simple conduit, or
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
it may include an orifice through which gas passes from the first, higher
pressure, region of the device to the second, lower pressure, region of the
device. A Joule-Thomson heat exchanger may further include a
heat-exchanging configuration, for example a heat-exchanging configuration
5 used to cool gasses within a first region of the device, prior to their
expansion
into a second region of the device.
The phrase "cooling gasses" is used herein to refer to gasses which have
the property of becoming colder when passed through a Joule-Thomson heat
exchanger. As is well known in the art, when gasses such as argon, nitrogen,
10 air, krypton, C02, CF4, xenon, and N20, and various other gasses pass from
a
region of higher pressure to a region of lower pressure in a Joule-Thomson
heat
exchanger, these gasses cool and may to some extent liquefy, creating a
cryogenic pool of liquefied gas. This process cools the Joule-Thomson heat
exchanger itself, and also cools any thermally conductive materials in contact
therewith. A gas having the property of becoming colder when passing
through a Joule-Thomson heat exchanger is referred to as a "cooling gas" in
the
following.
The phrase "heating gasses" is used herein to refer to gasses which have
the property of becoming hotter when passed through a Joule-Thomson heat
exchanger. Helium is an example of a gas having this property. When helium
passes from a region of higher pressure to a region of lower pressure, it is
heated as a result. Thus, passing helium through a Joule-Thomson heat
exchanger has the effect of causing the helium to heat, thereby heating the
Joule-Thomson heat exchanger itself and also heating any thermally conductive
materials in contact therewith. Helium and other gasses having this property
are referred to as "heating gasses" in the following.
As used herein, a "Joule Thomson cooler" is a Joule Thomson heat
exchanger used for cooling. As used herein, a "Joule Thomson heater" is a
Joule Thomson heat exchanger used for heating.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
11
In discussion of the various figures described hereinbelow, like numbers
refer to like parts.
For purposes of better understanding the present invention, as illustrated
in Figures 6 - 13 of the drawings, reference is first made to the construction
and
operation of conventional (i.e., prior art) cryosurgery apparatus and
treatment
method as illustrated in Figures 1 - 6.
Referring to Figures 1-3, a cryosurgical apparatus according to methods
of prior art includes a plurality of cryosurgical probes.
Figure 1 presents a simplified schematic of an exemplary cryoprobe,
according to the methods of prior art.
Figure 1 presents a cryoprobe SO having an operating tip 52 including a
Joule-Thomson cooler for freezing a patient's tissue and a holding member 72
for holding by a surgeon. As shown in Figure 1, operating tip 52 includes at
least one passageway 78 extending therethrough for providing gas of high
pressure to orifice 80 located at the end of operating tip 52, orifice 80
being for
passage of high pressure cooling gas therethrough, so as to cool operating tip
52 and produce an ice-ball at its end 90.
When a high pressure cooling gas such as argon expands through orifice
80 it may liquefy, so as to form a cryogenic pool within chamber 82 of
operating tip 52, which cryogenic pool effectively cools surface 84 of
operating
tip 52. Surface 84 of operating tip 52 is preferably made of a heat conducting
material such as metal so as to enable the formation of an ice-ball at end 90
thereof.
Alternatively, a high pressure heating gas such as helium may be used
for heating operating tip 52 via a reverse Joule-Thomson process, so as to
enable treatment by cycles of cooling-heating, and further for preventing
sticking of the probe to the tissue when extracted from the patient's body,
and
to enable fast extraction when so desired.
When a high pressure heating gas such as helium expands through
orifice 80 it heats chamber 82, thereby heating surface 84 of operating tip
52.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
12
Operating tip 52 includes at least one evacuating passageway 96
extending therethrough for evacuating gas from operating tip 52 to the
atmosphere.
As shown in Figure l, holding member 72 may include a heat exchanger
for pre-cooling the gas flowing through passageway 78. Specifically, the upper
portion of passageway 78 may be in the form of a spiral tube 76 wrapped
around evacuating passageway 96, the spiral tube being accommodated within
a chamber 98. Thus, gas evacuated through passageway 96 may pre-cool the
incoming gas flowing through spiral tube 76.
As further shown in Figure 1, holding member 72 may include an
insulating body 92 for thermally insulating the heat exchanger from the
external environment.
Furthermore, operating tip 52 may include at least one thermal sensor 87
for sensing the temperature within chamber 82, the wire 89 of which extending
through evacuating passageway 96 or a dedicated passageway (not shown).
Probe 50 may further comprise one or more external thermal sensors 86,
preferably placed at some distance from operating tip 52, operable to report
on
temperatures induced in surrounding tissues by cooling of operating tip 52.
In addition, holding member 72 may include a plurality of switches 99
for manually controlling the operation of probe 50 by a surgeon. Such switches
may provide functions such as on/off, heating, cooling, and predetermined
cycles of heating and cooling by selectively and controllably communicating
incoming passageway 70 with an appropriate external gas container including a
cooling or a heating gas.
Attention is now drawn to Figure 2, which presents a simplified
schematic of a gas distribution module connecting a plurality of cryosurgical
probes 50 to a common gas source, according to the methods of prior art.
Figure 2 presents a gas distribution module 40, wherein each of
cryosurgical probes 50 is connected via a flexible connecting line 54 to a
connecting site 56 on a housing element 58, preferably by means of a linking
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
13
element 51. Cryosurgical probes 50 may be detachably connected to
connecting sites 56.
Preferably, evacuating passageway 96 extends through connecting line
54, such that the outgoing gas is evacuated through an opening located at
linking element 51 or at any other suitable location, e.g., manifold 55, see
below. Preferably, line 54 further includes electrical wires for providing
electrical signals to the thermal sensor and switches (not shown).
Each of cryosurgical probes SO is in fluid communication with a
manifold 55 received within a housing 58, manifold 55 being for distributing
the incoming high pressure gas via lines 57 to cryosurgical probes 50.
As shown, housing 58 is connected to a connector 62 via a flexible cable
60 including a gas tube (not shown), connector 62 being for connecting the
apparatus to a high pressure gas source and an electrical source.
The apparatus further includes electrical wires (not shown) extending
through cable 60 and housing 58 for providing electrical communication
between the electrical source and cryosurgical probes 50.
Preferably, housing 58 includes a pre-cooling element, generally
designated as 61, for pre-cooing the high pressure gas flowing to cryosurgical
probes 50. Preferably, pre-cooling element 61 is a Joule-Thomson cooler,
including a tubular member 48 received within a chamber 49, tubular member
48 including an orifice 59 for passage of high pressure gas therethrough, so
as
to cool chamber 49, thereby cooling the gas flowing through tubular member
48 into manifold 55.
Attention is now drawn to Figure 3, which presents an alternative
configuration of a pre-cooling element 61 according to the methods of prior
art,
wherein tubular member 48 is in the form of a spiral tube wrapped around a
cylindrical element 47, so as to increase the area of contact between tubular
member 48 and the cooling gas in chamber 49.
According to yet another configuration (not shown), housing 58 includes
a first tubular member for supplying a first high pressure gas to manifold 55,
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
14
and a second tubular member for supplying a second high pressure gas to
pre-cooling element 61. Any combination of gases may be used for cooling
and/or heating the gases flowing through such tubular members.
Alternatively, a cryogenic fluid such as liquid nitrogen may be used for
S pre-cooling the gas flowing through housing 58. Alternatively, an electrical
pre-cooling element may used for pre-cooling the gas.
Preferably, thermal sensors (not shown) may be located within cable 60
and manifold 55 for measuring the temperature of gas flowing therethrough.
Attention is now drawn to Figures 4-6, which present a prior art method
and apparatus utilizing an imaging device to form a three-dimensional grid of
the patient's treated organ, e.g., prostate, the three dimensional grid serves
for
providing information on the three dimensional shape of the organ. Each of a
set of cryosurgical probes is then inserted to a specific depth within the
organ
according to the information provided by the grid.
Figure 4 is a simplified schematic of an apparatus comprising an
ultrasound probe and a guiding element for guiding insertion of a plurality of
cryoprobes into a patient's body, according to the methods of prior art..
As shown in Figure 4, an ultrasound probe 130 is provided for insertion
into the patient's rectum, ultrasound probe 130 being received within a
housing
element 128. A guiding element 115 is connected to housing element 128 by
means of a connecting arm 126. As shown, guiding element 115 is in the form
of a plate 110 having a net of apertures 120, each aperture serves for
insertion
of a cryosurgical probe therethrough. Preferably, the distance between each
pair of adjacent apertures 120 is between about 2 millimeters and about 5
millimeters.
Attention is now drawn to Figure 5, which is a simplified schematic
showing a method of use of the apparatus presented in Figure 4.
As shown in Figure 5, ultrasound probe 130 is introduced to a specific
depth 113 within the patient's rectum 3. A net of marks 112 is provided on the
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
obtained ultrasound image 114, the net of marks 112 on image 114 being
accurately correlated to the net of apertures 120 on guiding element 115.
Thus, marks 112 on image 114 sign the exact locations of the centers of
ice-balls which may be formed at the end of the cryosurgical probes inserted
S through apertures 120 to the patient's prostate 2, wherein image 114 relates
to a
specific depth of penetration 113 of the cryosurgical probes into the prostate
2.
As shown in Figure 5, ultrasound probe 130 is gradually introduced to
various depths 113 of rectum 3, thereby producing a set of images 114, wherein
each image relates to a respective depth of penetration into the prostate 2.
10 Thus, each of images 114 relates to a specific plane perpendicular to the
axis of
penetration of the cryosurgical probes.
The set of images 114 provides a three dimensional grid of the prostate.
Such three-dimensional grid is then used for planning the cryosurgical
procedure.
15 For example, the introduction of a cryosurgical probe along a given axis
of penetration to a first depth may effectively destroy a prostatic tissue
segment, while introduction of the probe to a second depth may severely
damage the prostatic urethra.
Since the ice-ball is locally formed at the end of the cryosurgical probe,
each probe may be introduced to a specific depth so as to locally provide an
effective treatment to a limited portion of the prostate while avoiding the
damaging of non-prostatic or prostatic tissues located at other depths of
penetration.
Attention is now drawn to Figure 6, which is a simplified schematic
presenting a further step in the use of the apparatus presented in Figure 4,
according to the methods of prior art.
Figure 6 shows the insertion of an operating tip 52 of a cryosurgical
probe 50 through an aperture of guiding element 11 S into the prostate 2 of a
patient.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
16
Preferably, a plurality of cryosurgical probes are sequentially inserted
through apertures 120 of guiding element 115 into the patient's prostate,
wherein each probe is introduced to a specific depth, thereby providing
substantially local effective treatment to distinct segments of the prostatic
tissue while avoiding the damaging of other prostatic or non-prostatic tissue
segments.
Preferably, each of the cryosurgical probes includes a scale for
indicating the depth of penetration into the prostate.
Thus, it may be seen that the prior art apparatus and methods presented
by Figures 1-6 enable diagnostic mapping of areas to be treated within a
prostate, and further enable guiding a plurality of cryogenic probes into a
prostate in such a manner that the cryogenic probes are placed according to
the
planned treatment areas so mapped.
Preferred embodiments of the present invention may now be described,
utilizing the exemplary context of the prior art apparatus and methods
described hereinabove and presented in Figures 1-6. It is noted, however, that
the aforementioned prior art context is here described for exemplary purposes
only. The invention disclosed herein is not limited to the exemplary context.
In particular, alternative methods of diagnostic mapping may be utilized, such
as x-ray mapping, CT mapping with or without use of a contrast medium, MRI
mapping, ultrasound mapping not utilizing the anal probe described above, and
others. Cryoprobes dissimilar to cryoprobe 50 presented in Figure 1 may be
utilized in embodiments of the present invention, on condition that they are
capable of cooling tissues to cryoablation temperatures. Apparatus and
methods other than those depicted in figures 3-6 may be utilized to accurately
deliver one or more cryoprobes to a selected locus for cryoablation of tissues
thereat, and to accurately deliver one or more heating probes to selected
locations for protecting healthy tissue from freezing, as will be explained
hereinbelow.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
17
Attention is now drawn to Figure 7, which is a schematic representation
of a prostrate treatment apparatus according to a preferred embodiment of the
present invention.
Figure 7 is similar to Figure 6 in all respects, which the exception of the
presence of a heating probe 220 in addition to cooling probes 50. Heating
probe 220 may be passed through guiding element 115 into a planned location
in or near the prostate or other organ, where it may be used to heat tissues
during cryoablation procedures. Thus, while cryoprobes 50 are cooled to
cryoablated pathological tissues, heating probes 220 may be heated to protect
nearby tissues from the destructive effects of cooling induced by cryoprobes
S0.
Structurally, heating probes 220 may be similar or identical to
cryoprobes SO of Figure 1 and to probes 50 of Figure 2, and yet be
differentiated by the fact that whereas cooling probes 50 are connected to a
supply of cooling gas and use Joule-Thomson cooling to cool their operating
heads to cryoablation temperatures, heating probes 220 are connected to a
supply of heating gas and utilize Joule-Thomson heating to heat probes 220
during the surgical procedure, thereby protecting tissues in their vicinity
from
damage from cooling induced by cooling probes 50.
In alternate configurations, heating probes 220 may be heated by flow of
pre-heated fluids, by electrical resistance heating, by microwave heating, by
radio frequency heating, or by any other convenient form of heating. Yet, in a
particularly preferred embodiment, identical probes connectable both to a
cooling gas supply and to a heating gas supply may be utilized as cooling
probes 50 in a first context, and as heating probes 220 in a second context.
For
example, a same probe may be utilized as cooling cryoprobes 50 at a first
depth
of penetration, and as heating probe 220 at a second depth of penetration.
Attention is now drawn to Figure 8, which is another simplified
schematic representation of a prostrate treatment apparatus according to a
preferred embodiment of the present invention. Figure 8 is similar to Figure
7,
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
18
but additionally presents a thermal sensor unit 222, which may comprise a
thermocouple 224. Thermal sensor unit 222 is shown having been placed
between cryoprobes 50 and heating probes 220 in the prostate 2 of a patient,
where it may be used to monitor temperature changes resulting from cooling of
probes 50 and heating of probes 220. Data from thermal sensor unit 222, and
from thermal sensors within probes 50 and 220, may be monitored by a control
module 223, which may be operable to control heating of probes 220 and
cooling of probes 50, based on data received from these thermal sensors, under
algorithmic control.
Attention is now drawn to Figure 9, which is a simplified flow-chart of a
recommended procedure for cryoablation of a portion of an organ, such as a
tumor of a prostate. At step 300, an operator utilizes an imaging modality to
image a prostate to be treated. Step 300 may be accomplished utilizing the
anal
ultrasound probe and methodology described by Schatzberger. Alternatively,
other forms of ultrasound imaging, x-ray imaging, CT imaging (with or without
use of contrast medium), MRI imaging, or yet other medical imaging
modalities may be used to provide information concerning the prostate to be
treated.
According to an embodiment of the present invention, utilization of the
images gleaned from step 300 is twofold. At step 310, the images created at
step 300 are inspected to determine the locus of the pathological tissues to
be
cryoablated is obtained, as described by Schatzberger. (Preferably, a
three-dimensional mapping of the treatment locus is created.) At step 320, the
images created at step 300 are further inspected and the position of the
neurovascular bundles, or the position of other tissue which the surgeon
desires
to protect, is similarly determined and mapped.
At step 330, a guiding element such as guiding element 115 of Figure 6
is used to guide cooling probes such as cooling probe 50 of Figure 6 to the
treatment locus found at step 310.
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
19
At step 340, a guiding element is similarly utilized to guide one or more
heating probes 220 to the vicinity of the neurovascular bundles, or to the
vicinity of other tissues which the surgeon desires to protect.
At optional step 350, a guiding element such as guiding element 115
may be used to place thermal sensor units 222 in intermediate positions
between the desired locus of cryoablation and the position of the
neurovascular
bundle, or the position of other tissue which an operator desires to protect.
Output from thermal sensors may then be monitored, at step 370 below.
At step 360, the surgeon operates cooling probes 50 to cool the
pathological tissues to cryoablation temperatures, thereby cryoablating those
pathological tissues, and also heats the heating probes placed in step 340,
thereby protecting the neurovascular bundles or other selected tissues which
the
surgeon desires to protect.
At step 370, thermal sensors in cooling probes 50 and in heating probes
220 may be used to monitor the cooling of probes SO and the heating of probes
220, which cooling and heating may be adjusted according to the data returned
by these thermal sensors, thereby facilitating the adjustment of the
cooling/heating process so as to optimize cryoablation while ensuring that the
neurovascular bundle, or other protected tissues, are neither cooled to
destructive temperatures, nor exposed to excessive heating. If external
thermal
sensors were placed in optional step 350, these too may be monitored to
determine the temperature at a point or points between the cryoablation locus
and the position of the protected tissue, and cooling of cooling probes 50 and
heating of heating probe 220 may be adjusted accordingly, to optimize the
desired effect.
Additionally, at step 370, imaging modalities such as ultrasound may be
used to monitor the progression of freezing in the cryoablation process, and
in
particular to monitor the size and position of ice-balls formed around the
operating heads of cooling probes 50, and in particular to monitor the
effectiveness of the heating provided by heating probes 220 in limiting the
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
progression of formation of ice balls (frozen tissue areas) in the vicinity of
the
neurovascular bundle or other protected areas.
Control of cooling of cooling probes 50 and heating of heating probes
220 may be managed manually by a surgeon based on monitoring images and
5 data from thermal sensors in or near the probes. Alternatively, control of
the
heating and cooling of the probes may be managed by control unit 223, based
on data received electronically from the thermal sensors, under algorithmic
control.
Attention is now drawn to Figure 10, which presents a simplified
10 schematic representation of a prostrate treatment apparatus according to an
additional preferred embodiment of the present invention.
Figure 10 presents a probe 300 whose position with respect to the
patient's anatomy creates particular requirements, which probe 300 is designed
to satisfy. It is to be noted that a distal portion 320 of probe 300 is placed
15 within prostate 2, and may be thought of as being located in a region whose
cryoablation is desired. Shaft portion 312 of probe 300, however, is adjacent
to
the patient's neurovascular bundle, which it is desired to be protected from
freezing during cryoablation of the tissues surrounding distal portion 320. An
optimal solution to the situation depicted in Figure 10 requires that probe
300
20 be operable to cool distal portion 320, yet that tissues near shaft 312 be
protected from freezing. Figures 11-13 present designs for a probe 300 that
satisfies this requirement.
Attention is now drawn to Figure 11, which is a simplified schematic of
a cryoprobe having a cryogenic treatment head and a heatable shaft, according
to an embodiment of the present invention.
Figure 11 presents a cryoprobe 350 having a distal portion 320
implemented as a cryogenic operating tip 52, similar to that presented in
Figure
1 and discussed hereinabove. Probe 350 comprises a gas input passageway 78
operable to transport cooling gas, through a heat exchanging configuration
301,
to a Joule-Thomson orifice 80 in operating tip 52, for cooling operating tip
52
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
21
as described with respect to Figure 1. Expanded gas from operating tip 52 is
exhausted through exhaust passageway 96. Probe 350 of Figure 11 is
distinguished from probe SO of Figure 1, however, by the presence of an
electrical or electronic heating element 352 located in the proximal shaft
portion 312 of probe 350. Heating element 352 may be implemented as an
electrical heater resistance element 354, as a radio frequency heating element
356, as a microwave heating element 358, or as any other form of electrically
powered heater. Probe 350 is thus operable to satisfy the requirements
discussed above with respect to Figure 10, in that operating tip 52 may be
cooled to cryoablation temperatures, while shaft 312, corresponding to
proximal portion 310 of probe 300 Figure 10, is heated by heating element 352.
Heating of element 352 protects tissues outside shaft 312 from being frozen by
cold exhaust gasses in exhaust passageway 96. Heating element 352 also
protects tissues outside shaft 312 from freezing when tissues surrounding
operating tip 52 are cooled to cryoablation temperatures.
Attention is now drawn to Figure 12, which is a simplified schematic of
an alternative construction of cryoprobe 300, having a cryogenic treatment
head and a heatable shaft, according to an embodiment of the present
invention.
Figure 12 presents a cryoprobe 370 having a distal portion 320
implemented as a cryogenic operating tip 52, similar to that presented in
Figure
1 and in Figure 11, and discussed hereinabove. Probe 370 comprises a gas
input passageway 78 operable to transport cooling gas, through a heat
exchanging configuration 301, to a Joule-Thomson orifice 80 in operating tip
52, for cooling operating tip 52 as described with respect to Figure 1.
Expanded gas from operating tip 52 is exhausted through exhaust passageway
96. Probe 370 of Figure 12 is distinguished from probe 350 of Figure 11,
however, in that shaft 312 of probe 370 is operable to be heated by a fluid
heating process.
In one embodiment, pre-heated fluid may be supplied to probe 370
through input passageway 372, where it heats a portion of shaft 312 and is
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
22
subsequently exhausted through exhaust passageway 376. Exhaust passageway
376 is preferably formed as a volume surrounding internal portions of shaft
312
that contain, in passageways 78 and 96, cold gasses transported to and from
operating tip 52. Exhaust passageway 376 is also preferably formed so as to be
contiguous to outer wall 313 of shaft 312. Preferably, an insulating material
(not shown) is interposed between exhaust passageway 376 and internal
portions of shaft 312 such as cooling gas input passageway 78 and cooling gas
evacuation passageway 96.
In a preferred embodiment utilizing fluid heating, a portion of shaft 312
of probe 370 is heatable by Joule-Thomson heating. Input passageway 372 is
connectable to a source of compressed heating gas. Input passageway 372
transports compressed heating gas to a Joule-Thomson orifice 374.
Compressed heating gas passing through orifice 374 expands into exhaust
passageway 376, heats, and thereby heats outer wall 313 of shaft 312. Heated
expanded heating gas is then exhausted from shaft 312 through exhaust
passageway 376.
Probe 370 is thus operable to satisfy the requirements discussed above
with respect to Figure 10, in that operating tip 52 may be cooled to
cryoablation temperatures, while shaft 312 is heated by expanding heating gas
from Joule-Thomson orifice 374. Heating of shaft 312 protects tissues around
shaft 312 both from cold exhaust gasses in exhaust passageway 96, and from
cold induced by the cryoablation temperatures induced in tissues surrounding
operating tip 52.
Attention is now drawn to Figure 13, which is a simplified schematic of
a cryoprobe 300 having an insulated shaft, according to an embodiment of the
present invention.
Figure 13 presents a cryoprobe 380 having a distal portion 320
implemented as a cryogenic operating tip 52, similar to that presented in
Figure
1 and in Figures 11 and 12, and discussed hereinabove. Probe 380 comprises a
gas input passageway 78 operable to transport cooling gas, through a heat
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
23
exchanging configuration 301, to a Joule-Thomson orifice 80 in operating tip
52, where it may be used to cool operating tip 52 as described hereinabove
with
respect to Figure 1. Expanded gas from operating tip 52 is exhausted through
exhaust passageway 96. Probe 370 of Figure 13 is distinguished from probe 50
of Figure 1, however, in that shaft 312 of probe 380 comprises an insulating
element 382 which serves to insulate the outer surface 384 of shaft 312 from
cold temperatures induced in the walls of gas exhaust passageway 96 by cold
expanded cooling gasses passing therein. Insulating element 382 is preferably
implemented as a vacuum-containing sheath 386 extending along at least a
portion of shaft 312.
Probe 380 is thus operable to satisfy the requirements discussed above
with respect to Figure 10, in that operating tip 52 may be cooled to
cryoablation temperatures, while external surfaces of shaft 312 are not
substantially cooled by the cold cooling gasses, cooled by expansion in
operating tip 52, which pass therein after cooling operating tip 52.
It is appreciated that certain features of the invention, which are, for
clarity, described in the context of separate embodiments, may also be
provided
in combination in a single embodiment. Conversely, various features of the
invention, which are, for brevity, described in the context of a single
embodiment, may also be provided separately or in any suitable
subcombination.
Although the invention has been described in conjunction with specific
embodiments thereof, it is evident that many alternatives, modifications and
variations will be apparent to those skilled in the art. Accordingly, it is
intended to embrace all such alternatives, modifications and variations that
fall
within the spirit and broad scope of the appended claims. All publications,
patents and patent applications mentioned in this specification are herein
incorporated in their entirety by reference into the specification, to the
same
CA 02471904 2004-06-30
WO 03/059247 PCT/IL02/01062
24
extent as if each individual publication, patent or patent application was
specifically and individually indicated to be incorporated herein by
reference.
In addition, citation or identification of any reference in this application
shall
not be construed as an admission that such reference is available as prior art
to
the present invention.