Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02472495 2004-09-23
PROCESS TO RECOVER BASE METALS
FIELD OF THE INVENTION:
This invention relates to the hydrometallurgical processing of metals from
sulphide feed materials.
DESCRIPTION OF THE RELATED ART.
The hydrometallurgical treatment of base metal sulphide feeds such as
chalcopyrite (copper) and
pentlandite (nickel) concentrates has been the subject of numerous
investigations, since it would constitute
an alternative to conventional smelting.
Several new processes have been developed, particularly for the treatment of
copper sulphide concentrates;
to cite a few of them: the INTEC process (atmospheric chloride process), the
thermophile bioleach
processes (known by the names BIOCOP, MINTEK/BACTECH), low temperature
pressure oxidative
leaching processes (known by the names ACTIVOX, MIM), medium temperature
pressure oxidative
leaching process (known by the names CESL, ANGLO AMERICAN, PHELPS DODGE,
DYNATEC), and
high temperature pressure oxidative leaching process (known by the names
PLACER DOME,
PLATSOL~).
Low temperature leaching processes (typically operating between 90 and
110°C), require ultra fine grinding
(< 10 microns) and long leach residence times ( > 2 hours) to achieve
acceptable copper recovery. Medium
temperature processes (typically operating at 130 to 170°C) have to
take special precautions to overcome
the potential negative effect of elemental sulphur, due to armouring of the
sulphide surfaces by elemental
sulphur for example. One process adds chloride ions, another adds a carbon
compound, while others add a
dispersant, all with the objective of mitigating the negative effect of
elemental sulphur.
CA 02472495 2004-09-23
High temperature pressure oxidation (HTPOX) processes (such as the PLACER DOME
process and the
PLATSOL~ process, which operate at 190 to 230°C) do not require
ultrafine grinding, are extremely fast,
and do not form elemental sulphur (forming instead sulphate, mostly in the
form of sulphuric acid). The
negative aspect of the high temperature process is that the equipment to
perform the process is relatively
more expensive. In addition oxidation of sulphide ions all the way to
sulphuric acid consumes much more
oxygen in the autoclave, and in those cases where there is no application for
the acid that is produced, it has
to be neutralized at the expense of a neutralizing agent.
It is an object of the invention to benefit from at least some of the
advantages of the low, medium and/or
high temperature leaching processes, while reducing at least some of their
disadvantages.
SUMMARY OF THE INVENTION:
In one of its aspects the present invention provides a hydrometallurgical
process to recover at least one base
metal from one or more base metal sulphide feeds comprising the steps of
- providing a base metal sulphide feed;
- processing at least a portion the feed to reduce the content of sulphur
therein to a predetermined
sulphur content level;
- directing some or all of the processed feed with or without some or all of
the unprocessed feed to
an oxidative leach step to carry out an oxidative leach step under conditions
sufficient to recover at
least a portion of the base metal from the feed, the predetermined sulphur
content level being
selected to provide a source of heat for the oxidative leach step and to
provide sufficient sulphate
ions to complex the portion of the base metal being recovered.
2
CA 02472495 2004-09-23
Preferably, the predetermined sulphur content level does not exceed that which
is required to provide a
source of heat for the oxidative leach step and to provide sufficient sulphate
to complex the portion of the
base metal being recovered.
The oxidative leach step may be carried out either under ambient pressure
conditions or above-ambient
pressure conditions, depending on a number of different factors, such as the
presence of PGM's, (in which
case the oxidative leach step may likely be carried out at above-ambient
pressure conditions), as well as
other economic and environmental factors. The oxidative leach step may be
carried out in an autoclave,
although it may also be carried out in another reactor such as an open tank or
atmospheric vessel.
In one embodiment, the directing step is carried out to recover substantially
all of the recoverable base
metal from the feed. However, the process may also be conducted to recover a
predetermined portion of
the base metal contained in the feed.
The entire feed or a portion of the feed may be subjected to the processing
step which may involve roasting
under either auto thermal conditions or externally heated conditions. The
roasting step itself may be a
partial roast (meaning that sulphides remain in the roast product) or a
substantially complete or "dead" roast
(meaning that substantially all sulphides have been removed from the roast
product).
In one embodiment, the roasting step occurs under auto thermal conditions or
under conditions where an
external heat source delivers heat thereto. The roasting step may occur in a
roasting vessel or chamber.
In one embodiment, the processing step may include the step of dividing the
base metal sulphide feed into a
first portion and a second portion, the first portion being subjected to the
roasting step to form a roasted
first portion. In this latter case, the second portion may be directed to an
oxidative leach step together with
at least a portion of the roasted first portion.
3
CA 02472495 2004-09-23
This may be carried out in one, or more than one, reaction vessels either
under atmospheric or non-
atmospheric conditions. The reaction vessels may themselves include a number
of compartments in series
along one or more flow paths which themselves may be parallel. Alternatively
or in addition, the flow path
may include a number of vessels ganged in series. The reaction vessels may
include one or more
autoclaves or agitation tanks and the like.
Thus, in one embodiment, the step of directing the feed includes injecting the
roasted first portion at
different locations in the one or more reaction vessels. In one example, the
one or more reaction vessels
includes one or more autoclaves and/or one or more agitation tanks. If
desired, one or more of the reaction
vessels may form one or more flow paths, which themselves may be in series or
parallel relationship. If
desired, one or more of the reaction vessels may include a number of
compartments in series along one or
more of the flow paths
In one embodiment, the process further comprises the step of subjecting the
first portion to auto thermal
roasting conditions in the presence of air or oxygen to produce the calcine
and sulphur dioxide (SOZ). In
this case, the SOZ is removed from the roaster and delivered to an Acid Plant
to be converted to sulphuric
acid (HZS04 ) or liquid SO2,
In one embodiment, the process further comprises the step of recovering
sulphur dioxide from the roasting
step and converting the sulphur dioxide to sulphuric acid.
In one embodiment, the roasted first portion is substantially entirely
depleted of sulphide. In this case, the
roasted first portion may be totally or partially blended with the second
portion prior to delivery to the
autoclave.
In one embodiment, the step of directing the feed includes injecting the
roasted first portion at different
locations in the autoclave or other reaction vessel as the case may be.
Alternatively, the roasted first
4
CA 02472495 2004-09-23
portion may be delivered to just one location in the autoclave, such as an
upstream end thereof, or at a
location upstream of the autoclave itself.
In one embodiment, the process further comprises the steps of
S
- collecting a pressure leach slurry from the autoclave; and thereafter
- directing an additional portion of the roasted first portion to the pressure
leach slurry.
Preferably, the oxidative leach step occurs at temperatures ranging from about
40 to about 250 degrees
Celsius except in circumstances where there is platinum or other precious
metals in the base metal sulphide
feed in which case a pressure vessel is required, such as an autoclave, and
preferred temperatures may
range from about of 190 to about 230 degrees Celsius when using the PLATSOL
process to recover the
precious metal. The gold, or platinum group metals may be recovered using
either the PLATSOL step or a
1 S subsequent cyanidation step, or another appropriate step.
Other temperature ranges may also be employed, provided the base metals are
dissolved andlor the
formation of elemental sulphur is minimized. For example, in the case of
secondary copper minerals such
as chalcocite and covellite, the temperature of the oxidative leach step may
be lower, since such materials
are easier to oxidize.
Preferably, the roasted first portion ranges from about 5 percent to about 100
percent of the base metal
sulphide feed material. Either portion 100% roasted or partially, the portion
that is roasted will be partially
roasted under mild conditions to benefit from more favourable kinetics and
thermodynamics that prevail in
2S the initial stages of roasting. It is expected that 40 to 100 percent of
the sulphur will be oxidized to SOz in
this step. The specific conditions for a particular sulphide feed will depend
on the feed itself which may
include chalcopyrite, nickel copper sulphide, nickel copper sulphide
containing PGMs and /or gold.
CA 02472495 2004-09-23
Preferably, the metal sulphide feed material is a concentrate formed from a
flotation step, although other
processes may be employed, such as gravity separation and magnetic separation.
For example, the first
portion may be a relatively high grade cleaner concentrate (having a sulphur
content, for example, ranging
from 20 to 40 percent), while the second portion may be a relatively low grade
cleaner tailings scavenger
concentrate (having a sulphur concentrate, for example, of less than 20
percent).
In one embodiment, the oxidative leach step generates a slurry temperature
within the autoclave ranging
from about 190 to about 250 degrees Celsius.
In one embodiment, the pulp density in the feed ranges from about 5 to about
30 percent.
In one embodiment, the first portion ranges from about 5 percent to about 100
percent of the feed.
In one embodiment, the one or more base metal sulphide feeds includes a
concentrate formed from a
flotation step, a gravity concentration step or a gravity/flotation
concentration step. In one example, the first
and second portions may originate from different feeds.
Preferably, the oxidative leach step generates a terminal sulphuric acid
concentration not exceeding 80 glL,
and more preferably not exceeding SOgIL, in order to capture iron constituents
in the feed material as stable
iron solid residues.
In one embodiment, the process includes the step of collecting a pressure
leach slurry from the autoclave.
In this case, the pressure leach slurry may contain residual free acid, in
which case a sufficient portion of
the roast product can be added to the pressure leach slurry to neutralize the
residual free acid.
In another embodiment, the oxidative leach step is carned out in a heap. In
this case, the material in the
heap should be prepared or arranged to allow effective distribution of the
leach solution therethrough. For
instance, the heap may be prepared by the use of granular carrier elements on
which the processed feed (or
6
CA 02472495 2004-09-23
the mixture with unprocessed feed as the case may be) has been deposited.
Other methods may also be
used or become available for agglomerating or establishing a porous matrix of
the processed feed in order
to receive the leach solution.
S In one embodiment, the oxidative leach step occurs in the presence of
sulphuric acid at a concentration not
exceeding 80 g/L, in order to facilitate the capture of unwanted iron
impurities in a stable hematite residue.
In another of its aspects, the present invention provides a hydrometallurgical
process to recover at least one
base metal from a base metal bearing sulphide feed material comprising the
steps of
- providing a base metal bearing sulphide feed material;
- splitting the base metal bearing sulphide feed material into a first portion
and second portion;
- removing substantially all the sulphide from the first portion to form a
sulphide depleted first
portion;
- collecting the sulphide depleted first portion;
- directing at least a portion of the sulphide depleted portion to an
autoclave;
- directing the second portion to the same autoclave to carry out an oxidative
pressure leach
step to recover the base metal from the feed, the second portion having
sufficient sulphide
content to act as the source of heat for the oxidative pressure leach step .
For those base metal sulphides containing gold or other "platinum group
metals" (PGM's), the latter may
be solubilized by adding a halide, more preferably a reactive chloride as
described below.
7
CA 02472495 2004-09-23
In still another of its aspects, the present invention provides a process to
recover a base metal, comprising
the steps of
- providing a feed material containing a base metal sulphide constituent with
a predetermined
amount of a sulphur-bearing constituent therein;
- directing the feed material to a reactor to carry out an oxidative leach
step under conditions
sufficient to recover an economic portion of the base metal from the feed
material
- wherein the providing step includes adjusting the predetermined amount of
the sulphur-bearing
constituent, so as not substantially to exceed the amount required to provide
a source of heat for
the oxidative leach step and to provide sufficient sulphate to complex the
portion of the base
metal being recovered.
In an embodiment, substantially all of the predetermined amount of sulphur-
bearing constituent in the feed
material upstream of the leach reactor is in a form other than a sulphate.
Preferably, substantially all of
the predetermined amount of sulphur-bearing constituent in the feed material
in the reactor is converted to a
sulphate for complexing with the base metal.
In an embodiment, the providing step includes the step of roasting at least a
portion of the feed material. If
desired, the feed material may be divided into a first portion and a second
portion, followed by roasting the
first portion.
If desired, the step of providing may include the step of providing a first
portion of the feed material with
substantially no sulphur-bearing constituent and a second portion containing a
known amount of the
sulphur-bearing constituent, and mixing the first and second portions.
In an embodiment, the roast product is substantially void of ferrite-bearing
materials.
8
CA 02472495 2004-09-23
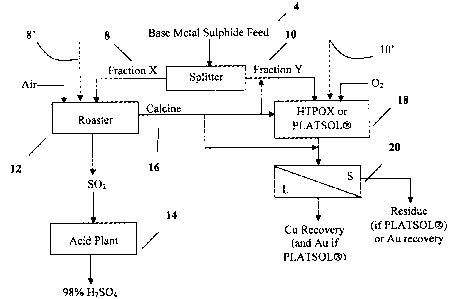
BRIEF DESCRIPTION OF THE DRAWINGS
Several preferred embodiments of the present invention will be provided, by
way of examples only, with
reference to the appended drawings, wherein:
Figure 1 is a flow chart illustrating a hydrometallurgical process to leach at
least one base metal; and
Figure 2 is a flow chat illustrating one example of the process of figure 1.
1 O DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring to figure 1, there is provided a hydrometallurgical process to leach
at least one base metal from a
base metal sulphide feed material 4 by utilizing the advantages of the high
temperature HTPOX processes.
The process involves dividing the feed material into a first portion X shown
at 8 and a second portion Y
shown at 10. It will be understood that, as an alternative, the first and
second portions may originate from
separate feeds, as represented by the dashed lines 8', 10'.
The first portion is then directed to a roasting station 12 where the first
portion 8 is roasted, in the presence
of air or oxygen-enriched air or oxygen, to form a roast product in the form
of a calcine 16, in which the
sulphur (that is elemental sulphur and other sulphur bearing constituents such
as sulphide materials) is
either partially or essentially completely depleted.
At least a fraction of the calcine 16 is then delivered to a feed to a closed
vessel in the form of an autoclave
18, together with the unroasted second portion 10, where the calcine 16 and
second portion 10 are subjected
to an oxidative pressure leach step in the autoclave 18 by oxygen or air to
recover at least some of the base
metal contained in the first or second portions.
9
CA 02472495 2004-09-23
The SOZ gas in the roasting step is converted to HZS04 in an acid plant 14,
for example at a concentration
of 93 percent or, optionally, about 98 percent for commercial grade HZS04. The
acid so produced can be
reused in the process, sold, or stored.
As will be described, a particular feature of the present method is that the
amount of the sulphur (in the
form of elemental sulphur and sulphide) present in the combined feed to the
leaching process is sufficient
to provide auto thermal conditions for the oxidative pressure leach step to
occur at the required
temperature, while the first portion 8 is oxidized using air or oxygen, or
oxygen-enriched air in an
inexpensive roasting step to reduce, if not substantially eliminate, oxygen-
consuming sulphur constituents
therein.
In this example, the base metal sulphide feed material 4 may be a concentrate
of chalcopyrite (CuFeSz) or
of pentlandite (Fe,Ni~Sg or other materials such as pyrite, arsenopyrite,
bornite, enargite, chalcocite,
sphalerite, pyrrhotite, covellite, cobaltite, millerite or combinations of one
or more thereof. The
concentrate may be produced by a number of well known processes such as
flotation or gravity processes.
When considering chalcopyrite concentrates (CuFeS2), it is apparent that there
are 2 moles of sulphur per
mole of copper, and that in the HTPOX process (PLACER DOME, PLATSOL~), all the
sulphur eventually
has to be neutralized to form gypsum, in addition to all the sulphur
originating from other sulphides in the
concentrate such as pyrite, pyrrhotite and others.
The proportions of the base metal sulphide feed material fractions X and Y can
depend on many factors,
such as total sulphide in the concentrate and the acid-consuming properties of
gangue minerals in the
concentrate. Preferably, the amount of un-roasted portion proceeding directly
to the autoclave 18 must be
suffcient to generate auto thermal conditions for the combined feed. In other
words, the unroasted portion
provides sufficient oxygen-consuming sulphur constituents so that their
oxidation generates sufficient heat
in the autoclave 18 to drive the reactions occurring within the autoclave 18
substantially without another
(such as external) source of heat. However, there may be some cases where
supplemental heat is necessary
CA 02472495 2004-09-23
or desirable. For example, there may be cases where the temperature of the
autoclave 18 may be monitored
and, should the sulphur content of the feed momentarily drop below a minimum
value, a supplemental heat
source may be introduced to make up the difference, that is to compensate for
the loss of heat.
It is desirable that there be sufficient sulphate formed in the autoclave 18
to react with the base metals to
form base metal sulphates which will thus become soluble and leave the
autoclave 18 in a leach solution.
In this case, a significant portion of the sulphates is likely to be formed in
the autoclave 18, though the roast
product may also be a source of sulphates in cases where the roasting step is
carried out in conditions
forming them. Preferably, the roasting step may be configured to effectively
eliminate sulphate since the
sulphates otherwise present in the roast product will increase the amount of
lime needed for acid
neutralization, adding cost to the process. Sulphate formation may be
minimized in the roasting step by
selecting appropriate temperature and partial oxygen pressure conditions. It
is, thus, desirable that there be
sufficient sulphides in the feed entering the autoclave 18 , not only to
provide the source of heat to drive the
reactions occurring therein, but also to provide sufficient sulphates for the
complexing of base metals of
interest (Cu, Ni, Co, Zn).
In one example, the size of the first portion 8 may vary between about 5% and
about 100% of the total base
metal sulphide concentrate feed 4.
The calcine 16 may be delivered to the autoclave 18 after being totally or
partially blended with the second
portion 10. Alternatively a portion or substantially all of the calcine 16 may
be injected or otherwise
deposited to the autoclave 18 at different locations therein.
In some cases, it may be desirable to direct still another portion of the
calcine 16 to the pressure leach
slurry produced after being recovered from the autoclave 18. This can be
useful in some cases to
neutralize excess acid in the leach slurry without having to use lime.
11
CA 02472495 2004-09-23
The autoclave 18 may be conditioned to carry out a high temperature POX
process which can be a simple
high temperature oxidation process. Alternatively, a reactive halide (such as
chloride) constituent may be
added if the sulphide concentrate and calcine contain precious metals such as
gold and the platinum group
metals (PGM's) by using the well known PLATSOL~ conditions, for example using
the conditions of U.S.
Patent 6,315,812 entitled OXIDATIVE PRESSURE LEACH RECOVERY USING HALIDE IONS,
which
issued November 13, 2001, the entire subject matter of which is incorporated
herein by reference. In this
case, the gold and PGM's may be solubilized in a halide complex in the leach
solution.
In some applications, it may be advantageous to produce two concentrates in
the initial flotation or
gravitylflotation process, a high grade cleaner concentrate and a lower grade
cleaner tailings scavenger
concentrate and the overall recovery of valuable metals in the flotation or
gravitylflotation process may be
improved by adopting this approach. The cleaner concentrate may contain a high
concentration of sulphides
and base metals, and thus may constitute the first portion because it can be
readily treated in a roaster,
producing a concentrated stream of sulphur dioxide gas for conversion to
sulphuric acid. The lower grade
cleaner tailings concentrate would contain some gangue minerals, which have
minimal negative impact on
the operation of the autoclave, but which are recovered to enhance the overall
recovery of valuable metals
(particularly gold and PGMs ) in the overall process.
The pulp density (that is the concentration of solids per unit volume) in the
feed to the autoclave 18 in
HTPOX processes (PLACER DOME and PLATSOL~) is dependent on the concentration
of the sulphide in
the concentrate and the operating temperature in the autoclave 18. For example
the pulp density is usually
quite low and in the 5 to 30% range for two reasons:
(i) Oxidation of sulphides is an exothermal process. The above pulp densities
( 5 to 30%)
produce auto thermal conditions with high sulphide grade concentrate, where
the heat
generated by the oxidative pressure leach step generates the required
operating
temperature in the autoclave 18 ( 190 to 230°C for HTPOX) . At higher
pulp densities,
heat may need to be extracted from the autoclave 18, which in some cases
increases
12
CA 02472495 2004-09-23
operating costs; at lower pulp densities, the feed to the autoclave 18 may
need to be
preheated to maintain the required temperatures in the autoclave 18, which
adds to the
capital cost of the plant. Operating at the optimum pulp density for a given
sulphur
content of the feed produces the most favourable economics for the process.
(ii) A typical chalcopyrite concentrate will have a sulphide concentration of
about 30%,
which will potentially generate up to 1 ton of sulphuric acid per ton of
concentrate, when
fully oxidized. To produce a stable iron residue of hematite during pressure
leaching
(which is desirable for environmental as well as processing reasons), the
sulphuric acid
concentration in the autoclave must be maintained at <80 gIL, and preferably
<50 g/L.
This means that the pulp density in the feed to the autoclave 18 should be
adjusted to take
into account the acid consuming gangue mineral constituents in the
concentrate. For
example, the required pulp density to produce hematite in the residue may be
less than
10% in some cases.
Notwithstanding the economic benefits of operating the oxidative leach step
under auto thermal conditions
in the autoclave and the benefits of producing a stable iron residue such as
hematite, it may, in some cases,
be desirable to feed the autoclave at a much higher pulp density, to reduce
the size of the reaction vessel
and lower the capital cost of the reaction vessel, and to minimize the amount
of water that wastefully
consumes heat in the process.
The calcine 16 that is added to the feed to the autoclave 18 in the present
process is an acid-consumer, so
by blending the first portion 8 into the autoclave feed with the unroasted
second portion 10, it will be
possible to feed the autoclave 18 at much higher pulp densities, while still
meeting the requirements for
auto thermal operation and residual sulphuric acid of <80 gIL. This will
positively impact the capital cost
of the overall process by significantly reducing the size of the capital-
intensive autoclave 18. For example,
doubling the overall pulp density to about 20% solids will more than halve the
size of the autoclave 18
compared to stand alone HTPOX or PLATSOL~ processes.
13
CA 02472495 2004-09-23
The calcine 16 can be totally or partially blended with the unroasted
concentrate of the second portion 10
prior to entering the autoclave 18, or it could be injected at different
locations inside the autoclave 18, or
part of the calcine 15 may be added to the pressure leached slurry after
discharging from the autoclave 18,
to neutralize the residual free acid under properly selected conditions. The
process will generate a hematite
S iron residue, which is easily discarded without substantial short or long
term impact to the environment.
If the concentrate contains gold or PGM minerals, the precious metals can be
recovered from the combined
calcinel6/HTPOX leach residue by conventional means such as the cyanidation
process, since the residue
does not contain elemental sulphur. Gold and the PGMs can also be recovered
directly into the acidic
autoclave liquor by using PLATSOL~ conditions.
The advantage of PLATSOL~over conventional cyanidation for the treatment of
the oxidized concentrate
are:
- the capital and operating expenses of the cyanidation plant are avoided
1S
-the high consumption of cyanide associated with treatment of base metal leach
residues can be
substantially avoided;
-the PLATSOL~ process efficiently recovers gold as well as PGMs, whereas
cyanidation only
recovers gold efficiently;
-the PLATSOL~ process is a viable alternative to cyanidation for gold recovery
in those regions of
the world where permitting of the cyanidation process is restricted and;
2S -gold can be recovered directly from the oxidized, acidic slurry by carbon
in pulp, prior to solid
liquid separation, reducing the risk of gold losses during solid liquid
separation;
Thus, examples of the present process may have one or more of the following
advantages:
14
CA 02472495 2004-09-23
-relatively fast kinetics for base metal (such as Cu, Ni) leaching and iron
precipitation as hematite;
-minimal environmental impact from the hematitic tailings;
- generally no requirement for ultrafine grinding;
-no requirement for the addition of chloride (unless PLATSOL~), nor carbon,
nor dispersant, to
avoid passivation of the sulphide mineral surfaces;
-excellent base metal (Cu, Ni, others) recoveries (>98%);
-low oxygen consumption;
-low neutralizing agent consumption;
-no new equipment required, but a combination of a well known roasting and
HTPOX autoclave
equipment, operating under proven, well-established conditions, similar to
refractory gold plants
for the autoclave and copper nickel roasting for the roaster.
-relatively easy gold recovery from the POX residue, with low consumption of
cyanide, since it
does not contain elemental sulphur, or during HTPOX if applying the PLATSOL~
process.
-direct recovery of PGMs via the PLATSOL~ process at minimal incremental cost
versus base
metal recovery costs;
-the potential for increasing overall recovery of pay metals to the
concentrate by producing a high
grade (cleaner) concentrate for processing in the roaster and a lower grade
(cleaner tailings)
scavenger concentrate for treatment in the autoclave;
CA 02472495 2004-09-23
-low operating costs because a significant amount of sulphide is oxidized in
the roasting step with
the inexpensive oxidant air, (versus oxidizing all the sulphide with expensive
oxygen in the
HTPOX process), and because concentrated sulphuric acid is produced from the
roaster, which can
either be used in the process or sold to generate additional revenue, versus
the requirement to
S neutralize autoclave acid with limestone or other agents; and
-low capital costs because the size of the autoclave can be reduced by:
a) diverting some of the concentrate to the roaster (Fraction X); and
b)feeding the remaining concentrate (Fraction Y) to a small autoclave at
higher pulp
density.
Embodiments of the present invention will be described with reference to the
following examples which are
presented for illustrative purposes only and are not intended to limit the
scope of the invention.
EXAMPLE:
One example of high temperature autoclaving of a chalcopyrite concentrate is
shown in Figure 2. When a
chalcopyrite concentrate is processed at high temperature (>200°C) in
an oxidizing environment in an
autoclave, the main reaction can be written as:
CuFeS2 + 1~ ~z + Hz0 --~ CuS04 +'/z Fe203 + HzS04
4
Under these conditions, copper is produced in the autoclave discharge as fully
soluble copper sulphate, iron
2S is precipitated in the residue as hematite Fe203, and two moles of SOQ are
produced per mole of
chalcopyrite treated; that S04 will have to be neutralized, consuming
limestone.
16
CA 02472495 2004-09-23
Therefore, the chemical operating costs of such a process (HTPOX) are oxygen
(for the oxidation inside the
autoclave) and limestone (to neutralize the acid generated in the autoclave).
In other words, these
consumptions can be calculated for a chalcopyrite concentrate as 2.14 kg
OZ/kgCu and 3.14 kg CaC03/kg
Cu or, assuming a typical 25% Cu concentrate, as 618 kg OZJT conc and 910 kg
CaC03/T conc. While the
costs of oxygen and limestone vary widely, in many cases their combined cost
in the HTPOX process will
be equivalent to 10% or more of the value of the copper recovered. There are
no heating costs, since the
oxidation reactions are generating sufficient heat that the process may be
operated in a diluted or relatively
low concentration manner to maintain the temperature. (i.e. for example 10%
solids for a 25% S
chalcopyrite concentrate).
If portions of the chalcopyrite concentrate are roasted prior to blending with
unroasted materials following
the present process, several events should happen at the same time:
1. The autoclave can now be operated at higher °fo solids, since the %S
of the blend is lower and
there is less need to dilute the autoclave feed to control the temperature.
For example, a blend at I S% sulphur may be operated at 20% solids, which
means that a plant
would need an autoclave volume less than half the size of the autoclave that
would have to
process 100% unroasted feed. The capital savings would compensate for the
addition of a
small roaster and small acid plant to roast a portion of the feed. The roaster
would use air
instead of oxygen to oxidize the sulphur; or it could use oxygen-enriched air
or oxygen, but it
would be low pressure oxygen instead of high pressure oxygen as used during
pressure
oxidation.
2. Oxygen and limestone consumption would be significantly reduced.
Because of the lower sulphur content of the blend feeding the autoclave,
oxygen consumption
in the autoclave, and limestone neutralization costs downstream of the
autoclave, may be
17
CA 02472495 2004-09-23
significantly reduced.
Table 1 below shows the oxygen and limestone consumptions calculated for
various blends,
assuming all the sulphur is originating from the chalcopyrite. Various tests
were conducted
on a Canadian chalcopyrite concentrate, whereby various blends of unroasted
concentrate and
dead roasted concentrate were treated in an autoclave at 225°C for 2
hours. The process
intended is illustrated in Figure 2.
TABLE 1 Application to a 22.5% Cu, 32.3% S Canadian concentrate
l A Consum %S in % Cu Acid generated
i dons
e in
C
a pprox. OZ CaC03 AutoclaveExtractedin roaster
c % Solids
n
Feed to autoclave
k conc conc feed conc
0 7.5 688 1009 32.3 98.0 0
8.5 595 809 25.9 98.8 192
10.0 484 709 22.7 97.8 288
12.0 415 609 19.5 99.9 384
15.0 347 509 16.3 95.3 480
Results indicate that after dead roasting a portion of the chalcopyrite
concentrate, up to 50%
15 of calcine could be blended with the unroasted concentrate. This blending
did not affect
copper extraction, but resulted in a 40-50% reduction of oxygen and limestone
consumptions.
The resulting blend still had sufficient sulphide sulphur for the autoclave
operation to be auto-
thermal (no heating costs), but also to operate at higher %solids. This will
significantly
reduce the capital expenditure (capex) of the autoclave circuit and subsequent
solid/liquid
20 separation circuit, to compensate for the additional cost of a small
roaster and acid plant.
Moreover, about 480 kg of acid is generated in the roaster.
3. Sales of pure acid
25 The portion of the blend going through the roaster will generate commercial
grade sulphuric
acid. This acid could be sold, or used up internally (i.e. heap leach
operation), generating
additional revenues.
18
CA 02472495 2004-09-23
4. Recovery of gold
If gold is present in the chalcopyrite concentrate, it can be easily recovered
from the HTPOX
residue (mainly hematite Fez03), using cyanidation for example. In that case,
cyanide
consumption as CNS will be low since the HTPOX conditions oxidize the sulphide
species to
sulphate and very little elemental sulphur remains in the final residue.
Alternatively, if PLATSOL~ conditions are used during the autoclaving, gold
will be
extracted together with copper and can be recovered from the leach solution by
various
methods.
While the present invention has been described for what are presently
considered the preferred
embodiments, the invention is not so limited. To the contrary, the invention
is intended to cover various
modifications and equivalent arrangements included within the spirit and scope
of the appended claims.
The scope of the following claims is to be accorded the broadest
interpretation so as to encompass all such
modifications and equivalent structures and functions.
19