Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02477005 2004-08-20
WO 03/072098 PCT/EP03/01560
Description:
Use of substituted 3-phenyl-5-alkoxy-1,3,4-oxdiazol-2-ones for prodncinq
medicaments with an inhibitory effect on pancreatic lipase
The invention relates to the use of substituted 3-phenyl-5-alkoxy-1,3,4-
oxdiazol-2-
ones for producing medicaments which have an inhibitory effect on pancreatic
lipase, PL.
Substituted 3-phenyl-5-alkoxy-1,3,4-oxdiazol-2-ones with an inhibitory effect
on
hormone-sensitive lipase are disclosed in WO 01/17981 (HMR 1999/L 052) and WO
01166531 (AVE-D 2000IA 015K).
It has now been found, surprisingly, that substituted 3-phenyl-5-alkoxy-1,3,4-
oxdiazol-2-ones show an inhibitory effect on pancreatic lipase, PL.
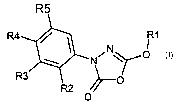
The invention therefore relates to the use of substituted 3-phenyl-5-alkoxy-
1,3,4-
oxdiazol-2-ones of the formula 1
R1
Ni ~ O
h--O
~~O
1
in which the meanings are:
R' C~-C6-alkyl, C3-C9-cycloalkyl, it being possible for both groups to be
substituted one or more times by phenyl, C~-C4-alkyloxy, S-C~-C4-alkyl,
N(C,-C4-alkyl)2, and far phenyl in turn to be substituted one or more times by
halogen, C~-C4-alkyl, C~-C4-alkyloxy, nitro, CF3; and
CA 02477005 2004-08-20
2
R2, R3, R4 and R5 independently of one another are hydrogen, halogen, vitro,
C~-C4-alkyl; C~-C9-alkyloxy which is substituted by fluorine, C6-Coo-aryl,
amino
or C~-C4-alkyl-amino;
Cs-C,o-aryl-C,-C4-alkyfoxy, C6-~,o-aryloxy, C6-C,o-aryl, Cs-C,o-aryloxy-C,-C4-
alkyl, Cs-C8-cycloalkyl or O-C3-C8-cycloalkyl, each of which may be
substituted once, twice or three times by halogen, CF3, C~-C4-alkyloxy or C~-
C4-alkyl;
SOZ-NH-C,-C6-alkyl, optionally substituted by N(C~-C6-alkyl)2, or SO~-NH-
(2,2,6,6-tetramethylpiperidin-4-yl), S02-NH-C3-Cs-cycfoalkyl, optionally
substituted one or more times by C~-C4-alkyl, or S02-N(C~-Cs-alkyl)2 or COX,
2-oxo-pyrrolidin-1-yl, 2,5-dimethylpyrrol-1-yl or NR6-A-R',
with the proviso that R2, R3, R4 and R5 are not simultaneously hydrogen, with
X ~ O-C~-Cs-alkyl, NH-C,-C6-alkyl, NH-C3-C8-cycloalkyl or N(C~-Cs-alkyl)2 and
N(C,-C6-alkyl)2 may also be pyrrolidino, piperidino, morpholino,
thiomorpholino
or piperazino, each of which may optionally be substituted by C~-C4-alkyl,
benzyf, Cs-Coo-aryl, CO-C~-C4-alkyl, CO-C6-C,o-aryl, CO-O-C~-C4-alkyl, S02-
C~-C4-alkyl or S02-C6-Coo-aryl;
R6 hydrogen, C,-C~-alkyl or C6-Coo-aryl-C~-C4-alkyl, where aryl may be
substituted by halogen, CF3, C~-C8-alkyloxy or C~-C4-alkyl;
A a single bond, CO~, SO~ or CONH;
n 1 or 2;
R' hydrogen;
C~-C~a-alkyl or C2-C~a-alkenyl, each of which may be substituted once to three
times by C~-C4-alkyl, halogen, CF3, C~-C4-aikyloxy, N(C~-C4-alkyl)Z, -COOH,
C~-C4-alkyloxycarbonyl, C6-C~2-aryl, C6-C~2-aryloxy, C6-C~2-arylcarbonyl,
C6-C,o-aryl-C~-C4-alkyloxy or oxo, where aryl in turn may be substituted by
halogen, C~-C4-alkyl, aminosuifonyl or methylmercapto;
CA 02477005 2004-08-20
3
C6-Coo-aryl-C~-C4-alkyl, C5-C8-cycloalkyl-C~-C4-alkyl, CS-C$-cycloalkyl, C6-
C~o-
aryl-CZ-Cs-alkenyl, Cs-Coo-aryl, biphenylyl, Biphenyl-C,-C4-alkyl, indanyl,
each
of which may be substituted once or twice by C,-C~$-alkyl, C,-C,8-alkyloxy,
C3-C8-cycloalkyl, COOH, hydroxyl, C~-C4-alkylcarbonyl, C6-C,o-aryl-C~-C4-
alkyl, C6-Coo-aryl-C~-C4-alkyloxy, C6-Coo-aryloxy, vitro, cyano, C~-Coo-aryl,
fluorosulfonyl, C,-C6-alkyloxycarbonyl, Cs-Coo-arylsulfonyloxy, pyridyl, NHS02-
C6-C,o-aryl, halogen, CF3 or OCF3, where alkyl may be substituted again by
C~-C4-alkyloxycarbonyl, CF3 or carboxyl, and aryl by halogen, CF3 or
C~-C4-alkyloxy;
or the group Het-(CH2)r,
with r = 0, 1, 2 or 3 and Het = saturated or unsaturated 5-7-membered
heterocycle which may be benzo-fused and substituted by C~-C4-alkyl,
C6-C,o-aryl, halogen, C~-C4-alkyloxy, C~-C4-alkyloxycarbonyl, C~-Coo-aryl-
C,-C4-alkyl; C6-Coo-aryl-C,-C4-alkylmercapto or vitro, where benzo-fused aryl
may in turn be substituted by halogen, C,-C4-alkyloxy or CF3 and alkyl in
arylalkyl by methoxy and CF3,
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
Said aryl radicals may optionally be substituted one. or more times by C~-C9-
alkyl,
C~-Cg-alkyloxy, halogen, trifluoromethyl. Said cycloalkyl radicals may
optionally be
substituted one or more times by C~-C4-alkyl, C6-Coo-aryl, and said alkyl
radicals may
be substituted by hydroxyl, di-C~-C4-alkylamino and fluorine. Halogen is
fluorine,
chlorine, bromine, preferably fluorine and chlorine. Alkyl, alkenyl, alkyloxy
etc. may
be branched or unbranched.
The preferred use is of compounds of the formula 1 in which the meanings are:
R' C,-C6-alkyl which may optionally be substituted by phenyl; andlor
R5 hydrogen; and/or
R2 hydrogen, halogen, C~-C4-alkyl, C~-C9-alkyfoxy or amino,
CA 02477005 2004-08-20
4
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
A further preferred use is of compounds of the formula 1 in which
R~ is hydrogen, C~-C4-alkyl, C6-Coo-aryl-C~-C4-alkyloxy which may optionally
be
substituted in the aryl moiety by halogen, or is NR6-A-R7 with
R6 = hydrogen or benzyl,
A - single bond and
R' = Cs-Coo-aryl-C~-C4-alkyl which may be substituted by halogen, CF3,
cyano, phenyl-C1-C4-alkyloxy, CF3-phenoxy, C5-Cg-cycloalkyl or
fluorosulfonyloxy;
C~-C~2-alkyl which may be substituted by C~-C4-alkyloxy, phenyl, CF3 or
phenyl-C~-C4-alkyloxy;
C2-C12-alkenyl or the group Het-(CHZ)r, with r = 0 or 1, and Het = saturated
or
unsaturated 5-7-membered heterocycle which may be benzo-fused and
substituted by C~-C4-alkyl or halogen, or
of the compounds of the formula 1 in which the meanings are:
R2' and R3 independently of one another hydrogen, C6-Coo-aryl, C3-C8-
cycloalkyl,
optionally C~-C4-alkyl-substituted C6-C,o-aryloxymethyl, optionally mono- or
poly-C~-C4-alkyl- or halogen-substituted O-benzyl, O-C6-Coo-aryl or O-C3-Cs-
cycloalkyl, mono- or poly-fluorine-, C6-Coo-aryl- or amino-substituted O-C~-C6-
alkyl, where amino in turn may be substituted one or more times by C,-C4-
alkyi, or S02-NH-C~-C6-alkyl, optionaNy substituted by N(C~-C6-alkyl)2, or SOZ-
NH-(2,2,6,6-tetramethylpiperidin-4-yl), S02-NH-C3-C$-cycloalkyl, substituted
by C~-C4-alkyl, S02-N(C,-C6-alkyl)2 or CO-N(C~-Cs-alkyl)2, and
N(C,-Cs-alkyl)Z may also be piperidino, morphofino or piperazino, each of
which may optionally be substituted by C~-C4-alkyl,
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
CA 02477005 2004-08-20
An additionally preferred use is of compounds of the formula 1 in which the
meanings are:
R4 hydrogen, 2-oxo-pyrrolidin-1-yl, 2,5-dimethylpyrrol-1-yl or C6-C,o-aryl-C,-
C4-alkyloxy, which may be substituted by halogen, and/or
5
of the compounds of the formula 1 in which the meanings are:
R4 = NR6-A-R', with
R6 = hydrogen or methyl,
A = single bond and
R' = hydrogen;
C~-C~2-alkyl which may be substituted once or twice by halogen;
C2-C,8-alkenyl which may be substituted once or twice by C~-C4-alkyl or
C~-Ca-alkyloxycarbonyl;
C6-Coo-aryl-C~-C4-alkyl which may be substituted by halogen, C~-C6-alkyloxy,
CF3, cyano, C5-Cs-cycloalkyl, C~-C4-alkyloxycarbonyl, C6-C,o-aryl-C,-C4-alkyl,
C6-Coo-aryl-C~-C~-alkyloxy, where aryl may be substituted again by halogen or
CF3;
C5-C8-cycloalkyl-C~-C4-alkyl;
or the group Het-(CH2)r;
with r = 1, 2 or 3 and Het = saturated or unsaturated 5-7-membered
heterocycle which may be substituted by halogen, C~-C4-alkyloxy or C~-C4-
alkyfoxycarbonyl, andJor
of compounds of the formula 1 in which the meanings are:
R4 = NR6-A-R' with
R6 = hydrogen,
A = -CO- and
R' = C~-C,8-alkyl which may be substituted by halogen, phenyl, phenoxy,
phenylcarbonyl or C,-Ca-alkyloxycarbonyl, where phenoxy in turn may be
substituted by methyl, halogen or methylmercapto;
C2-C~$-alkenyf which may be substituted by C6-Coo-aryl;
CA 02477005 2004-08-20
6
Cs-Coo-aryl which may be substituted by halogen, C~-C$-alkyl, phenyl-C~-C4-
alkyl, CF3, OCF3, fluorosulfonyl, C~-Ca-alkyloxycarbonyl, phenoxy, where aryl
in turn may be substituted by C~-C4-alkyloxy;
Cs-Coo-aryl-C~-Ca-alkyl, where alkyl may be substituted by methoxy or CF3,
and aryl by halogen;
or the group Het-(CH2)r,
with r = 0 and Het = saturated or unsaturated 5-7-membered heterocycle
which may be benzo-fused and substituted by C~-C4-alkyl, halogen, C~-C4-
alkyloxy, halophenyl or halobenzylmercapto, where benzo-fused aryl may in
turn be substituted by halogen or methoxy, and/or
of compounds of the formula 1 in which the meanings are:
R4 = N Rs-A-R', with
Rs = hydrogen,
A = -C02- and
R' = C,-C,s-alkyl which is substituted by CF3 or phenyl; -
Cs-C~o-arYl;
Cs-C,o-aryl-C~-C4-alkyl which is substituted by C~-C4-alkyl, halogen, CF3 or
OCF3, benzyloxy or phenyl;
or the group Het-(CH2)r,
with r = 0 or 1 and Het = saturated or unsaturated 5-7-membered heterocycle
which may be benzo-fused and substituted by C,-C4-alkyl or benzyl, and/or
R4 = N Rs-A-R', with
Rs = hydrogen,
A = -SOZ- and -
R' = C~-Cs-alkyl which may be substituted by CF3;
C2-C4-alkenyl which may be substituted by phenyl;
Cs-Coo-aryl which may be substituted by C,-Cs-alkyl, halogen, C~-C4-alkyloxy
or benzyl;
biphenylyl-C~-C~-alkyl, substituted by halogen;
or the group Het-(CH2)r,
CA 02477005 2004-08-20
7
with r = 0 and Het = saturated or unsaturated 5-7-membered heterocycle,
and/or
of compounds of the formula 1 in which the meanings are:
R4 = N R6-A-R', with
Rs = hydrogen,
A = -CO-NH- and
R' = C~-Coo-alkyl which may be substituted by C~-C4-alkyloxycarbonyl, N(C,-
C4-alkyl)2 or phenyl which may in tum be substituted by halogen or
aminosulfonyl;
C~-C1o-aryl which may be substituted by C~-Cs-alkyl, C~-C6-alkyfoxy, C,-Cs-
alkyloxycarbonyl, phenoxy, OCF3, benzyl or pyridyl, where alkyl may again be
substituted by C~-C4-alkyloxycarbonyl or carboxyl;
C5-Cs-cycloalkyl which may be substituted by hydroxyl, or indanyl;
or the group Het-(CH2)r,
with r = 0 or 1 and Het = saturated or unsaturated 5-7-membered heterocycle
which may be substituted by benzyl,
and of their pharmacologically acceptable salts and acid addition salts
fog producing a medicament with an inhibitory effect on pancreatic lipase.
A further preferred use is of compounds of the formula 1 in which the meanings
are:
R2 and R5 hydrogen,
R3 hydrogen, C6-Coo-aryl, O-C6-Coo-aryl, optionally C~-C4-alkyl-substituted
C6-Coo-aryloxymethyl, O-benzyl, mono- or poly-fluorine- or amino-substituted
O-C~-C6-alkyl, where amino in turn may be substituted one or more times by
C,-C4-alkyl, or optionally mono- or poly-C~-C4-alkyl-substituted O-C3-C8-
cyclaalkyl and
R4 hydrogen, C6-Coo-aryl, C3-C8-cycloalkyl, optionally mono- or poly-C~-C4-
alkyl-
or halogen-substituted O-C6-C,o-aryl or O-C3-C8-cycloalkyl, mono- or poly-
fluorine-substituted O-C~-C6-alkyl, SOZ-NH-C~-Cs-alkyl, optionally substituted
by N(C~-C6-alkyl)2, or S02-NH-(2,2,6,fi-tetramethylpiperidin-4-yl), S02-NH-C3-
CA 02477005 2004-08-20
Ca-cycloalkyl, substituted one or more times by C~-C4-alkyl, S02-N(C,-C6-
alkyl)2 or CO-N(C,-C6-alkyl)2, and
N(C~-C6-alkyl)2 is also piperidino, morpholino or piperazino, each ofi which
may optionally be substituted by C~-C4-alkyl,
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
The particularly preferred use is of compounds of the formula 1 in which
R' is methyl, ethyl, butyl, isopropyl or benzyl, and
RZ and R5 are hydrogen, and
R3 is hydrogen, trifluoromethoxy, trifluorobutoxy, 3,3,5,5-
tetramethylcyclohexyloxy, benzyloxy, phenoxy, phenyl, 2-diethylamino-
ethyloxy or 3-methylphenoxymethyl, and
R4 is hydrogen, trifluoromethoxy, 3,3,5,5-tetramethylcyclohexyloxy, phenoxy,
4-chlorophenoxy, cyclohexyl, phenyl, morpholinosulfonyl, 3,3,5-
trimethylcyclohexylaminosulfonyl, 2,2,6,6-tetramethylpiperidin-4-
ylaminosulfonyl, 2-(diisopropylaminoethyl)aminosulfonyl, 4-methylpiperazin-1-
ylsulfonyl, 3,3-dimethylpiperidinocarbonyl or 3,5-dichlorophenoxy, or
of compounds of the formula 1 in which
R' is methyl, ethyl, butyl, isopropyl or benzyl, and
RZ and R5 are hydrogen, and
R3 is hydrogen, trifluoromethoxy, 3,3,5,5-tetramethylcyclohexyloxy, benzyloxy
or
phenoxy and
R4 is hydrogen, trifluoromethoxy, 3,3,5,5-tetramethylcyclohexyloxy, phenoxy,
cyclohexyl, phenyl, morpholinosulfonyl or 3,3,5-trimethylcyclohexyl-
aminosulfonyl,
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
The very particularly preferred use is of compounds of the formula 1 in which
R' is C~-C4-alkyl,
R2 is hydrogen,
CA 02477005 2004-08-20
9
R3 is hydrogen, trifluoromethoxy, benzyloxy,
R4 is hydrogen, trifluoromethoxy, 4-chlorophenoxy, 4-trifluoromethylbenzoyl-
amino, and
R5 is hydrogen
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
A further very particularly preferred use is of compounds of the formula 1 in
which R'
is methyl,
and of their pharmacologically acceptable salts and acid addition salts
for producing a medicament with an inhibitory effect on pancreatic lipase.
An additional very particularly preferred use is of the compounds of the
formula 1
which are mentioned in Examples 86, 210, 212, 213, 216, 218, 220 and 229.
The invention relates to the use of compounds of the formula 1 in the form of
their
racemates, racemic mixtures and pure enantiomers, and to their diastereomers
and
mixtures thereof.
Pharmaceutically acceptable salts are particularly suitable for medical
applications
because of their greater solubility in water compared with the initial
compounds on
which they are based. These salts must have a pharmaceutically acceptable
anion
or cation. Suitable pharmaceutically acceptable acid addition salts of the
compounds
of the formula 1 are salts of inorganic acids such as hydrochloric acid,
hydrobromic
acid, phosphoric, metaphosphoric, nitric, sulfonic and sulfuric acids, and of
organic
acids such as, for example, acetic acid, benzenesulfonic, benzoic, citric,
ethanesulfonic, fumaric, gluconic, giycoiic, isethionic, lactic, lactobionic,
malefic,
malic, methanesuifonic, succinic, p-toluenesulfonic, tartaric and
trifluoroacetic acids.
It is particularly preferred to use the chloride salt and the tartaric acid
salt for medical
purposes. Suitable pharmaceutically acceptable basic salts are ammonium salts,
alkali metal salts (such as sodium and potassium salts) and alkaline earth
metal salts
(such as magnesium and calcium salts).
CA 02477005 2004-08-20
Salts with a pharmaceutically unacceptable anion likewise fall within the
scope of the
invention as useful intermediates for preparing or purifying pharmaceutically
acceptable salts and/or for use in non-therapeutic, for example in vitro,
applications.
5 The term "physiologically functional derivative" used herein refers to any
physiologically tolerated derivative of a compound according to the invention,
for
example an ester, which is able on administration to a mammal, such as, for
example, to humans, to form (directly or indirectly) such a compound or an
active
metabolite thereof.
A further aspect of this invention is the use of prodrugs of compounds of the
formula
1. Such prodrugs can be metabolized in vivo to a compound of the formula 1.
These
prodrugs may themselves be active or not.
The compounds of the formula 1 may also exist in various polymorphous forms,
for
example as amorphous and crystalline polymorphous forms. All polymorphous
forms
of the compounds of the formula 1 fall within the scope of the invention and
are a
further aspect of the invention.
A11 references hereinafter to "compound(s) of the formula 1" refer to
compounds) of
the formula 1 as described above and to the salts, solvates and
physiologically
functional derivatives thereof as described herein.
The amount of a compound of the formula 1 necessary to achieve the desired
biological effect depends on a number of factors, for example the specific
compound
chosen, the intended use, the mode of administration and the clinical
condition of the
patient. The daily dose is generally in the range from 0.3 mg to 100 mg
(typically
from 3 mg to 50 mg) per day and per kilogram of body weight, for example 3-10
mg/kg/day. An intravenous dose may be, for example, in the range from 0.3 mg
to
1.0 mg/kg, which can suitably be administered as infusion of 10 ng to 100 ng
per
kilogram and per minute. Infusion solutions suitable for these purposes may
contain,
for example, from 0.1 ng to 10 mg, typically from 1 ng to 10 mg, per
milliliter. Single
CA 02477005 2004-08-20
11
doses may contain, for example, from 1 mg to 10 g of the active ingredient.
Thus,
ampoules for injections may contain, for example, from 1 mg to 100 mg, and
single
dose formulations which can be administered orally, such as, for example,
tablets or
capsules, may contain, for example, from 1.0 to 1000 mg, typically from 10 to
600
mg. In the case of pharmaceutically acceptable salts, the above weight data
are
based on the weight of the salt of the compound of the formula 1. The
compounds of
the formula 1 can be used for prophylaxis or therapy of the abovementioned
states
themselves as compound, but they are preferably in the form of a
pharmaceutical
composition with a compatible carrier. The carrier must, of course, be
compatible in
the sense of compatibility with other ingredients of the composition and not
be
harmful to the patient's health. The carrier may be a solid or a liquid or
both and is
preferably formulated with the compound as single dose, for example as tablet,
which may contain from 0.05% to 95% by weight of the active ingredient.
Further
pharmaceutically active substances may likewise be present, including further
compounds of the formula 1. The pharmaceutical compositions according to the
invention may be produced by one of the known pharmaceutical methods which
essentially consist of mixing the ingredients with pharmacologically
acceptable
carriers and/or excipients.
Pharmaceutical compositions according to the invention are those suitable for
oral,
rectal, topical, peroral (for example sublingual) and parenteral (for example
subcutaneous,.intramuscular, intradermal or intravenous) administration,
although
the most suitable mode of administration depends in each individual case on
the
nature and severity of the condition to be treated and on the nature of the
compound
of the formula 1 used in each case. Coated formulations and coated slow-
release
formulations also fall within the scope of the invention. Acid- and gastric
fluid-
resistant formulations are preferred. Suitable gastric fluid-resistant
coatings comprise
cellulose acetate phthalate, polyvinyl acetate phthalate, hydroxypropylmethyl-
cellulose phthalate and anionic polymers of methacrylic acid and methyl
methacrylate.
CA 02477005 2004-08-20
12
Suitable pharmaceutical compounds for oral administration may be in the form
of
separate units such as, for example, capsules, cachets, pastilles or tablets,
each of
which contains a defined amount of the compound of the formula 1; as powder or
granules; as solution or suspension in an aqueous or nonaqueous liquid; or as
an oil-
in-water or water-in-oil emulsion. T hese compositions may, as already
mentioned, be
prepared by any suitable pharmaceutical method which includes a step in which
the
active ingredient and the carrier (which may consist of one or more additional
ingredients) are brought into contact. In general, the compositions are
produced by
uniform and homogeneous mixing of the active ingredient with a liquid and/or
finely
dispersed solid carrier, after which the product is shaped if necessary. Thus,
for
example, a tablet can be produced by compressing or shaping a powder or
granules
of the compound, where appropriate with one or more additional ingredients.
Compressed tablets may be produced by tabletting the compound in free-flowing
form, such as, for example, a powder or granules, where appropriate mixed with
a
binder, lubricant, inert diluent and/or one (or more) surface-
active/dispersing agents
in a suitable machine. Shaped tablets can be produced by shaping, in a
suitable
machine, the compound which is in powder form and has been moistened with an
inert liquid diluent.
Pharmaceutical compositions suitable for peroral (sublingual) administration
comprise suckable tablets which contain a compound of the formula 1 with a
flavoring, normally sucrose, and gum arabic or tragacanth, and pastilles which
contain the compound in an inert base such as gelatin and glycerol or sucrose
and
gum arabic.
Suitable pharmaceutical compositions for parenteral administration comprise
preferably sterile aqueous preparations of a compound of the formula 1, which
are
preferably isotonic with the blood of the intended recipient. These
preparations are
preferably administered intravenously, although administration can also take
place
by subcutaneous, intramuscular or intradermal injection. These preparations
can
preferably be produced by mixing the compound with water and making the
resulting
CA 02477005 2004-08-20
13
solution sterile and isotonic with blood. Injectable compositions according to
the
invention generally contain from 0.1 to 5% by weight of the active compound.
Suitable pharmaceutical compositions for rectal administration are preferably
in the
form of single-dose suppositories. These can be produced by mixing a compound
of
the formula 1 with one or more conventional solid carriers, for example cocoa
butter,
and shaping the resulting mixture.
Suitable pharmaceutical compositions for topical use on the skin are
preferably in the
form of an ointment, cream, lotion, paste, spray, aerosol or oil. Carriers
which can be
used are petrolatum, lanolin, polyethylene glycols, alcohofs and combinations
of two
or more of these substances. The active ingredient is generally present in a
concentration of from 0.1 to 15°l° by weight of the composition,
for example from 0.5
to 2%.
Transdermal administration is also possible. Suitable pharmaceutical
compositions
for transdermal applications may be in the form of single plasters which are
suitable
for long-term close contact with the patient's epidermis. Plasters of this
type suitably
contain the active ingredient in an aqueous solution which is buffered where
appropriate, dissolved and/or dispersed in an adhesive or dispersed in a
polymer: A
suitable active ingredient concentration is about 1 % to 35%, preferably about
3% to
15%. As a particular option, the active ingredient can be released by
electrotransport
or iontophoresis as described, for example, in Pharmaceutical Research, 2 (6):
318
(1986).
The following preparations serve to illustrate the invention without, however,
restricting it.
CA 02477005 2004-08-20
14
Example A
Soft gelatin capsules containing 100 mg of active ingredient per capsule:
per capsule
active ingredient 100 mg
triglyceride mixture
fractionated from coconut fat 400 mg
capsule content 500 mg
Example B
Emulsion containing 60 mg of active
ingredient per 5 ml:
per 100 ml of emulsion
active ingredient 1.2 g
neutral oii q.s.
sodium carboxymethylcellulose 0.6 g
polyoxyethylene stearate q.s.
glycerol, pure 0.2 to 2.0 g
flavoring q.s.
water (deionized or distilled) ad 100 ml
Example C
Rectal drug form containing 40 mg of active ingredient per suppository:
per suppository
active ingredient 40 mg
suppository base ad 2 g
Example D
Tablets containing 40 mg of active ingredient per tablet:
per tablet
active ingredient 40 mg
lactose 600 mg
corn starch 300 mg
CA 02477005 2004-08-20
soluble starch 20 mg
magnesium stearate 40 mg
1000 mg
5
Example E
Coated tablets containing 50
mg of active ingredient per
tablets:
per tablet
active ingredient 50 mg
10 corn starch 100 mg
lactose 60 mg
sec. calcium phosphate 30 mg
soluble starch 5 mg
magnesium stearate 10 mg
15 colloidal silica 5 mg
260 mg
EXample F
The following formulas are for producing the contents
suitable of hard gelatin
capsules:
a) active ingredient 100 mg
corn starch 300 mg
400 mg
b) active ingredient 140 mg
lactose 180 mg
com starch 180 mg
500 mg
Example G
Drops can be produced in accordance with the following formula (100 mg of
active
ingredient in 1 ml = 20 drops):
CA 02477005 2004-08-20
16
active ingredient 10 g
methyl benzoate 0.07 g
ethyl benzoate 0.03 g
ethanol, 96°f° 5 ml
demineralized water ad 100 ml
The compounds of the formula 1 can be prepared in various ways by methods
known per se.
R5 R5
H O_R1 H
R4 f \ N + O~ ~ R4 ~ ~ N O-R1
NH2 CI N--
R3 R2 R3 R2 H O
3
= a
R5 O R5
~CO_R1 --~ R4 ~ ~ N O
R4 N
N--~ N O~R1
R3 R2 H O R3 R2
5 1
For example, substituted 3-phenyl-5-alkoxy-1,3,4-oxdiazol-2-ones of the
formula 1
can be prepared by reacting hydrazines of the formula 2 with chloroformic
esters of
the formula 3 or other reactive carbonic ester derivatives, in which R', R2,
R3, R4 and
R5 are as defined above, to give the compounds of the formula 4, which are
acylated
with phosgene, carbonyldiimidazole, diphosgene or triphosgene, cyclized and
converted where appropriate by further chemical modification of the radicals
R2-R5,
such as, for example, by reduction of vitro to amino radicals by known
processes,
and subsequent acylation or alkylation, into compounds of the formula 1. Since
acids
are usually liberated in these reactions, promotion is advisable by adding
bases such
as pyridine, triethylamine, sodium hydroxide solution or alkali metal
carbonates. The
reactions can be carried out in wide temperature ranges. It has proved
advantageous as a rule to operate at 0°C to the boiling point of the
solvent used.
CA 02477005 2004-08-20
17
Examples of solvents employed are methylene chloride, THF, DMF, toluene, ethyl
acetate, n-heptane, dioxane, diethyl ether.
The hydrazines of the formula 2 can be prepared by known methods, for example
by
diazotization of the corresponding anilines and
R5 R5
hydrazine hydrate R4
R4 x v
NHz
R3 6 R2 R3 R2
_ 2
subsequent reduction by known methods or by nucleophilic substitution of
suitably
substituted phenyl derivatives 6 (X = F, CI, Br, I, OS02CF3) with hydrazine
hydrate.
Such suitable phenyl derivatives may be nitro-substituted halobenzenes,
preferably
fluoro- and chloronitrobenzenes, from which the compounds of the formula 1 can
be
prepared by known methods at a suitable point in the synthetic route by
reduction
and reaction with acylating or alkylating agents such as, for example, acid
chlorides,
anhydrides, isocyanates, chloroformic esters, sulfonyl chlorides or alkyl and
arylalkyf
halides, or by reductive alkylation with aldehydes.
The following examples illustrate the preparation methods in detail without
restricting
them.
Examples:
Example 1:
3-Methyl-4-nitrophenylhydrazine
5 g of hydrazine hydrate are slowly added dropwise to a solution of 15.9 g of
2-methyl-4-fluoronitrobenzene in 10 ml of N-methylpyrrolidone at room
temperature,
and the mixture is heated with stirring at 65°C for 4 hours. The
product is precipitated
CA 02477005 2004-08-20
18
by adding 70 ml of water and is filtered off with suction and recrystallized
from
isopropanol.
Yield:13.3 g rn.p.: 138°C
The following examples were prepared in an analogous way:
Example 2:
3-Fluoro-4-nitrophenylhydrazine
M.p.: 130°C
Example 3:
2-Chloro-4-nitrophenylhydrazine
M.p.:144°C
Example 4:
2-Methyl-4-nitrophenylhydrazine
M.p.:135°C
Example 5:
3-(4-Fluorobenzyloxy)-2-nitrophenylhydrazine
M.p.:164°C
The starting compound 2-fluoro-4-(4-fluorobenzyloxy)nitrobenzene (m.p.:
99°C) was
prepared by alkylation of 3-fluoro-4-nitrophenol with 4-fluorobenzyl chloride
in DMF
in the presence of potassium carbonate.
Example 6:
3-(4-Fluorobenzyloxy)-4-nitrophenylhydrazine (intermediate)
M.p.: 145°C
Example 7:
4-(4-Chlorophenoxy)-3-nitroaniline
CA 02477005 2004-08-20
19
1.4 g of potassium carbonate are added to a solution of 1.29 g of 4-
chlorophenol in
8 ml of DMF and, after stirring for 30 minutes, 1.6 g of 4-fluoro-3-
nitroaniline are
added, and the mixture is stirred at 100°C for 3 hours. After cooling,
80 ml of water
are added and, after briefly stirring, the precipitate is filtered off with
suction and
dried in vacuo at 40°C.
Yield: 2.0 g; m.p.: 101 °C
Example 8:
4-(4-Chlorophenoxy)-3-nitrophenylhydrazine
A solution of 0.52 g of sodium nitrite in 5 ml of water is added dropwise to a
stirred
mixture consisting of 1.9 g of 4-(4-chlorophenoxy)-3-nitroaniline, 25 ml of
concentrated hydrochloric acid and 25 ml of ethanol cooled to 0°C, and
the mixture
is then stirred at 0°C for 60 min and subsequently added dropwise to a
suspension
of 8.5 g of tin dichloride dihydrate in 8 ml of concentrated HCI. The
precipitate is
filtered off with suction, washed with water, suspended in 200 ml of water
under
nitrogen and decomposed with 100 ml of 30°I° strength sodium
hydroxide solution at
10-15°C. The oil which forms is extracted by shaking with ethyl acetate
and washed
with water, and the organic phase is dried with sodium sulfate. The product is
then
precipitated with isopropanolic HCI, filtered off with suction and dried in
vacuo.
Yield: 1.1 g; m.p.: 221 °C
Example 9:
Methyl N'-(4-nitro-2-methylphenyl)hydrazinoformate
0.43 ml of methyl chloroformate was cautiously added dropwise to a mixture
consisting of 0.84 g of 2-methyl-4-nitrophenylhydrazine, 15 ml of NMP and 2 ml
of
pyridine while cooling in ice, and the mixture was then stirred for 2 hours
while slowly
warming to RT. After dilution with 50 ml of water, the mixture was stirred
overnight
and the solid was dried in vacuo at 40°C.
Yield: 0.81 g; m.p.:153°C
CA 02477005 2004-08-20
The following examples were prepared in an analogous way:
Example 10:
Methyl N'-(4-nitrophenyl)hydrazinoformate (intermediate)
5 M.p.:179°C
Example 11:
Methyl N'-(3-fluoro-4-nitrophenyl)hydrazinoformate
M.p.: 127.4°C
Example 12:
Methyl N'-(3-methyl-4-nitrpphenyl)hydrazinoformate
M.p.: 159°C
Example 13:
Methyl N'-(2-chloro-4-nitrophenyl)hydrazinoformate
M.p.: 156°G
Example 14:
Methyl N'-(3-(4-fluorobenzyloxy)-4-nitrophenyl)hydrazinoformate (intermediate)
M.p.: 166°C
Example 15:
Methyl N'-(3-(4-fluorobenzyloxy)-2-nitrophenyl)hydrazinoformate
M.p.:193°C
Example 1 fi:
Methyl N'-(4-(4-chlorophenoxy)-3-nitrophenyl)hydrazinoformate
M.p.: 147°C
Example 17:
Methyl N'-(3-piperidino-4-nitrophenyl)hydrazinoformate (-)
CA 02477005 2004-08-20
21
M.p.: 131 °C
The latter compound and the compound of Example 18 were prepared by reacting
methyl N'-(3-fluoro-4-nitrophenyl)hydrazinoformate with piperidine and N-
benzyl-
piperazine, respectively, in NMP at 80°C.
Example 18:
Methyl N'-(3-(N-benzylpiperazino)-4-nitrophenyl)hydrazinoformate
M.p.: 15fi°C
Example 19:
5-Methoxy-3-(4-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
2.5 g of methyl N'-(4-nitrophenyl)hydrazinoformate and 5 ml of pyridine were
taken
up in 15 ml of methylene chloride and, while stirring and cooling in ice, 3 ml
of a 20%
strength solution of phosgene in toluene were added dropwise. This mixture was
left
to stand at room temperature overnight and was diluted with a further 10 ml of
methylene chloride and then washed 3 times with water. After drying over
sodium
sulfate, the mixture was concentrated in vacuo, and the product was purified
by
column chromatography (silica gel, solvents: methanol:methylene chloride =
2:98)
and recrystallized from isopropanol.
Yield:1.5 g m.p.: 151 °C
The following examples were prepared in analogy to Example 4:
Example 20:
5-Methoxy-3-(3-methyl-4-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 112°C
Example 21:
5-Methoxy-3-(4-(4-chlorophenoxy-3-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: oil
CA 02477005 2004-08-20
22
Example 22:
5-Methoxy-3-(3-(4-fluorobenzyloxy)-2-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 99°C
Example 23:
5-Methoxy-3-(2-methyl-4-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 111°C
Example 24:
5-Methoxy-3-(3-(4-fluorobenzyloxy)-4-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 137°C
Example 25:
5-Methoxy-3-(4-aminophenyl)-3-H-(1,3,4)oxdiazol-2-one
A mixture consisting of 1.4 g of 5-methoxy-3-(4-nitrophenyl)-3-H-
(1,3,4)oxdiazol-
2-one, 0.5 g of Pd/C and 20 ml of methanol is hydrogenated under atmospheric
pressure at room temperature until the calculated amount of hydrogen has been
taken up. The catalyst is then filtered off, and the solution is concentrated
in vacuo. -
The remaining semisolid residue is stirred with isopropanal and filtered off
with
suction.
Yield: 0.75 g; m.p.: 85°C
Example 26:
5-Methoxy-3-(2-amino-4-(4-fluorobenzyloxy)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: oil
Example 27:
5-Methoxy-3-(3-amino-4-(4-chlorophenoxy)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.:133°C
CA 02477005 2004-08-20
23
Example 28:
5-Methoxy-3-(4-amino-3-methylphenyl)-3-H-( 1,3,4)oxdiazof-2-one
M.p.: 114°C
Example 29:
5-Methoxy-3-(4-amino-3-(4-fluorobenzyfoxy)phenyl~3-H-(1,3,4)oxdiazol-2-one
M.p.: 195°C
Example 30:
5-Methoxy-3-(4-(4-chlorophenylacetylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
201 mg of 4-chlorophenylacetyl chloride are added dropwise to a mixture
consisting
of 200 mg of 5-methoxy-3-(4-aminophenyl)-3-H-(1,3,4)oxdiazol-2-one, 20 ml of
methylene chloride and 0.1 ml of pyridine cooled in ice, and the mixture is
stirred at
room temperature for 5 hours. Volatiles are removed in vacuo, and the residue
is
stirred with water and the solid is filtered off with suction and dried at
40°C in vacuo.
Yield: 318 mg; m.p.:161 °C
The following examples were prepared in an analogous way:
Example 31:
5-Methoxy-3-(4-(4-chlorophenylacetylamino)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-
2-one
M.p.: 190pC
Example 32:
5-Methoxy-3-(4-octanoylamino-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 110°C
Example 33:
5-Methoxy-3-(4-(4-heptylbenzoylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 155°C
CA 02477005 2004-08-20
24
Example 34:
5-Methoxy-3-(4-(4-butylphenylsulfonylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 135°C
Example 35:
5-Methoxy-3-(4-(4-chlorobutanoylaminor3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 137°C
Example 36:
5-Methoxy-3-(4-pivaloylamino-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 157°C
Example 37:
5-Methoxy-3-(4-(4-chlorophenylsulfonylamino)-3-methylphenyl)-3-H-
(1,3,4)oxdiazol-
2-one
M.p.: 147°C
Example 38:
5-Methoxy-3-(4-(1-naphthylsulfonylamino)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 123°C
Example 39:
5-Methoxy-3-(4-(2-phenylethenylsulfonylamino)-3-methylphenyl)-3-H-
(1,3,4)oxdiazol-
2-one
M.p.: 129°C
Example 40:
5-Methoxy-3-(4-(2,2,2-trifluoroethylsulfonylamino)-3-methylphenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 151°C
CA 02477005 2004-08-20
Example 41:
5-Methoxy-3-(4-(benzyloxycarbonylamino)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 115°C
5
Example 42:
5-Methoxy-3-(4-(3,4-dichlorophenylaminocarbonylamino)-3-methylphenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 210°C
The latter compound was obtained by reacting 5-methoxy-3-(4-amino-3-methyl-
phenyl)-3-H-(1,3,4)oxdiazol-2-one with equimolar amounts of 3,4-dichlorophenyl
isocyanate in toluene at 50°C.
Example 43:
5-Methoxy-3-(4-(4-chforophenylsulfonylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 169°C
EXample 44:
5-Methoxy-3-(4-(2-chlorophenylsulfonylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 171°C
Example 45:
5-Methoxy-3-(4-(3-chlorophenylsulfonylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 141 °C
Example 46:
5-Methoxy-3-(4-(4-chlorophenylacetylamino)-3-(4-fluorobenzyloxy)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.:167°C
CA 02477005 2004-08-20
26
Example 47:
5-Methoxy-3-(4-benzylsulfonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 153°C
Example 48:
5-Methoxy-3-(4-(-2-(4'-chlorobiphenyl)ethyl)sulfonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 165°C
Example 4J:
5-Methoxy-3-(4-isopropylsulfonylaminophenyl)-3-H-(1,3,4~xdiazol-2-one
M.p.: 190°C
Example 50:
5-Methoxy-3-(4-dimethylamino-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 71 °C
The latter compound was obtained by reacting 5-methoxy-3-(4-amino-3-methyl-
phenyl)-3-H-(1,3,4)oxdiazol-2-one with paraformaldehydeJformic acid in DMF at
room temperature and was purified by column chromatography (silica gel, ethyl
acetate:n-heptane = 1:1 ).
Example 51:
5-Methoxy-3-(4-(4-chlorobenzylamino)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: oil
The latter compound was obtained by reacting 5-methoxy-3-(4-amino-3-methyl-
phenyf)-3-H-(1,3,4)oxdiazol-2-one with 4-chlorobenzaldehyde/sodium borohydride
in
methanol/methylene chloride at room temperature and was purified by column
chromatography (silica gel, ethyl acetate:n-heptane = 1:1 ).
CA 02477005 2004-08-20
27
Example 52:
5- .Methoxy-3-(4-(2-oxopyrrolidin-1-yl)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: oil
The latter compound was prepared by reacting 5-methoxy-3-{4-(4-
chlorobutanoylamino)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-one with sodium
hydride in dioxane at room temperature and purifying the crude product by
column
chromatography (siilca gel, methylene chloride:methanol = 98:2).
Example 53:
5-Methoxy-3-(4-(4-oxopent-2-en-2-ylamino)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-
2-one
M.p.: 143°C
The latter compound was obtained by reacting 5-methoxy-3-{4-amino-3-methyl-
phenyl)-3-H-(1,3,4)oxdiazol-2-one with equimolar amounts of acetylacetone in
glacial
acetic acid at 80°C and was isolated by precipitation by adding water
and filtration.
Example 54:
5-Methoxy-3-(4-(2,5-dimethylpyrrol-1-yl)-3-methylphenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: oif
The latter compound was obtained by reacting 5-methoxy-3-{4-amino-3-methyl-
phenyl)-3-H-(1,3,4)oxdiazol-2-one with equimolar amounts of acetonylacetone in
glacial acetic acid at 80°C. Working up took place by dilution with
water, extraction
by shaking with ethyl acetate and column chromatography (silica gel, methylene
chloride) of the crude product obtained after concentration of the dried
organic
phase.
Example 55:
5-Methoxy-3-(3-(4-fluorobenzyloxy)-4-methylaminophenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 98°C
CA 02477005 2004-08-20
28
The latter compound was obtained as by-product of the hydrogenation of 5-
methoxy-
3-(3-(4-fluorobenzyloxy)-4-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one with
platinum
dioxide as catalyst in methanol at room temperature under atmospheric pressure
and after filtering off the catalyst, concentrating the reaction mixture and
column
chromatography (silica gel, methylene chloride).
The compounds of Examples 56-199 were prepared analogously to the above
examples.
Example 56:
5-Methoxy-3-(3-aminophenyl)-3-H-(1,3,4~xdiazol-2-one
M.p.: 95°C
Example 57:
5-Methoxy-3-(3-dibenzylaminophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 71 °C
Example 58:
5-Methoxy-3-(3-benzylaminophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: oil
Example 59:
5-Methoxy-3-(3-(pyrid-2-yl)aminocarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 81 °C
Example 60:
5-Methoxy-3-(3-(4-fluorobenzyloxy)-4-benzyloxycarbonylaminophenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: oil
Example 61:
CA 02477005 2004-08-20
29
5-Methoxy-3-(4-amino-2-methylphenyl)-3-H-( 1,3,4)oxdiazol-2-one
M.p.: oil
Example 62:
5-Methoxy-3-(3-methyl-4-(2-chlorobenzyloxycarbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 161°C
Example 63:
5-Methoxy-3-(4-amino-2-chlorophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 126°C
Example 64:
5-Methoxy-3-(2-chloro-4-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.:92°C
Example 65:
5-Methoxy-3-(2-methyl-4-benzyloxycarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-
one
M:p.: 112°C
Example 66:
5-Methoxy-3-(2-methyl-4-(4-trifluoromethoxybenzoylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 150°C
Example 67:
5-Methoxy-3-(2-chloro-4-benzyloxycarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 150°C
Example 68:
5-Methoxy-3-(3-fluoro-4.-nitrophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 127°C
CA 02477005 2004-08-20
Example 69:
5-Methoxy-3-(4-(4-t-butylbenzoylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p:: 173°C
5
Example 70:
5-Methoxy-3-(4-(4-chlorobenzyloxycarbonylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 177°C
10 Example 71:
5-Methoxy-3-{2-chloro-4-{4-heptylbenzoylamino)phenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 135°C
Example 72:
15 5-Methoxy-3-(4-(3,4-dichlorobenzoyfamino)phenyl)-3-H-(1,3,4)oxdiazol-2-orie
M.p.: 200°C
Example 73:
5-Methoxy-3-(4-(2-(4-chlorophenoxy)-2-methylpropionylamino)phenyl)-3-H-
20 (1,3,4)oxdiazol-2-one
M.p.: 153°C
Example 74:
5-Ethoxy-3-(3-methyl-4-benzyloxycarbonylaminophenyl}-3-H-(1;3,4)oxdiazol-2-one
25 M.p.:94°C
Example 75:
5-Isopropoxy-3-(3-methyl-4-benzyfoxycarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-
2-one
M.p.: 119°C
Example 76:
5-Isopropoxy-3-(3-methyl-4-butyloxycarbonylaminophenyl)-3-H-.(1,3,4)oxdiazol-2-
one
CA 02477005 2004-08-20
31
M.p.: 114°C
Example 77:
5-Isopropoxy-3-(3-methyl-4-(3-chlorophenylaminocarbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 201 °C
Example 78:
5-tert-Butoxy-3-(3-methyl-4-benzyloxycarbonylaminophenyi)-3-H-(1,3,4)oxdiazol-
2-one
M.p.: 113°C
Example 79:
5-Methoxy-3-(3-methyl-4-phenoxycarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-one
M.p.: 145°C
Example 80:
5-Methoxy-3-(3-methyl-4.-(pyrid-3-ylcarbonylamino)phenyl)-3-H-(1,3,4)oxdiazol-
2-one
M.p.: oil
Example 81:
5-Methoxy-3-(3-methyl-4-(indan-2-ylaminocarbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 206°C
Example 82:
5-Methoxy-3-(3-methyl-4-(pyrid-3-ylmethylaminocarbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 229°C
Example 83:
CA 02477005 2004-08-20
32
5-Methoxy-3-(3-methyl-4-(pyrid-3-ylmethoxycarbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 232°C
Example 84:
5-Methoxy-3-(3-fluoro-4-benzyloxycarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: oil
Example 85:
5-Methoxy-3-(3-fluoro-4-(4-trifluoromethyfbenzoylamino)phenyl)-3-H-
(1,3,4)oxdiazol-
2-one
M.p.: oil
Example 86:
5-Methoxy-3-(3-benzyloxy-4-(4-trifluoromethylbenzoylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 159°C
Example 87:
5-Methoxy-3-(3-fluoro-4.-(4-tert-butylbenzoylamino)phenyl)-3-H-(1,3,4)oxdiazol-
2-one
M.p.: 144°C
Example 88:
5-Methoxy-3-(3-methyl-4-(2,2,2-trifluoroethoxycarbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 141 °C
Example 89:
5-Methoxy-3-(3-methyl-4-piperidinocarbonylaminophenyl)-3-H-(1,3,4)oxdiazol-2-
one
M.p.: 154°C
CA 02477005 2004-08-20
33
Example 90:
5-Methoxy-3-(4-(6-methoxybenzofuran-2-yl-carbonylamino)phenyl)-3-H-
(1,3,4)oxdiazol-2-one
M.p.: 191°C
Further examples which were prepared by the processes described above and were
characterized by mass spectroscopy (M+1 ):
Example No. Chemical name: M+1 Mol.
wt.
91 N-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-362 361.4
3-methyl-benzenesulfonamide
92 3,4-Dimethoxy-N-[4-(5-methoxy-2-oxo- 408 407.4
[1,3,4]oxdiazol-3-yl)phenyl]benzenesulfonamide
93 Quinoline-8-sulfonic acid [4-(5-methoxy-2-oxo-399 398.4
[1,3,4]oxdiazol-3-yl)phenyl]amide
94 N-[4-(5-Methoxy-2-oxo-j1,3,4]oxdiazol-3-yl)phenyl]-415 414.3
5-nitro-isophthalic acid monomethyl ester
95 3-(2-Chlorophenyl)-5-methylisoxazole-4-carboxylic427 426.8
acid
[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-
yl)phenyl]amide
96 3,3,3-Trifluoro-2-methoxy-N-[4-(5-methoxy-2-oxo-424 423.3
[1,3,4]oxdiazol-3-yl)phenyl]-2-phenylpropionamide
97 2-Fluoro-N-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-330 329.3
yl)phenyl]-benzamide
98 Tetradecanoic acid [4-(5-methoxy-2-oxo- 418 417.5
[1,3,4]oxdiazol-3-yl)phenyl]amide
99 N-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-416 415.4
2-phenethyl-benzamide
100 N-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-479 478.4
2-(4-methoxyphenoxy)-5-nitrobenzamide
101 2-(4-Benzyfoxyphenyl)-N-[4-(5-methoxy-2-oxo-432 431.4
[1,3,4]oxdiazol-3-yl)phenyl]acetamide
102 N-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-492 491.5
3,3,3-triphenylpropionamide
103 N-(4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-448 447.3
3,5-bistrifluoromethylbenzamide
104 4-Cyano-N-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-337 336.3
yl)phenyl]-benzamide
105 Nonanoic acid [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-348 347.4
3-yl)phenyl]amide
CA 02477005 2004-08-20
34
106 Methyl 9-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-406 405.4
yl)phenylcarbamoyl]nonanoate
107 Undecanoic acid [4-(5-methoxy-2-oxo- 376 375.5
[1,3,4Joxdiazol-3-yl)phenyl]amide
108 4-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3- 394 393.3
yl)phenylcarbamoyl]-benzenesulfonyl fluoride
109 ~ 11-Phenoxyundecanoic acid [4-(5-methoxy-2-oxo-468 467.6
[1,3,4]oxdiazol-3-yl)phenyl]amide
110 N-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-416. 415.4
2,3-diphenylpropionamide
111 4-Chloro-N-[4-(5-methoxy-2-oxo-[1,3,4Joxdiazol-3-360 359.8
yl)phenyl]-2-methylbenzamide
112 6-Chloro-N-[4-(5-methoxy-2-oxo-[1,3,4Joxdiazol-3-347 346.7
yl)phenyl]nicotinamide
113 5-Fluoro-N-[4-(5-methoxy-2-oxo-[1,3,4Joxdiazol-3-344 343.3
yl)phenyl]-2-methylbenzamide
114 N-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-354 353.4
2,4,6-trimethylbenzamide
115 N-[4-(5-Methoxy-2-oxo-[1,3,4Joxdiazol-3-yl)phenyl]-388 387.4
3-naphthalen-2-ylacrylamide
116 5-Oxo-5-phenylpentanoic acid [4-(5-methoxy-2-382 381.4
oxo-[1,3,4]oxdiazol-3-yl)phenyl]amide
117 3-(2,4-Dichlorobenzylsulfanyl)thiophene-2-509 508.4
carboxylic acid [4-(5-methoxy-2-oxo-
[1,3,4]oxdiazol-3-yl)phenyl]amide
118 2-Fluoro-N-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-398 397.3
yl)phenyl]-4-trifluoromethyfbenzamide
119 1-Hexyl-3-[3-(5-methoxy-2-oxo-[1,3,4]oxdiazoi-3-335 334.4
yl)phenyl]urea
120 1-(4-Bromophenyl)-3-[3-(5-methoxy-2-oxo- 406 405.2
[1,3,4]oxdiazol-3-yl)phenyl]urea
121 1-[3-(5-Methoxy-2-oxo-[1,3,4Joxdiazol-3-yl)phenyl]-357 356.3
3-(2-methoxyphenyl)urea
122 Ethyl2-[3-[3-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-427 426.4
yl)phenyl]ureido]-3-phenylpropionate
123 1-(2,6-Diisopropylphenyl)-3-[3-(5-methoxy-2-oxo-411 410.5
[1,3,4]oxdiazol-3-yi)phenyl]urea
124 1-[3-(5-Methoxy-2-oxo-[1,3,4Joxdiazol-3-yl)phenyl]-363 362.4
3-octylurea
125 1-(4-Fluorobenzyl)-3-[3-(5-methoxy-2-oxo-359 358.3
[1,3,4Joxdiazol-3-yl)phenyl]urea
126 1-(2-Ethylphenyl)-3-[3-(5-methoxy-2-oxo- 355 354.4
[1,3,4]oxdiazol-3-yl)phenyl]urea
127 Ethyl 6-[3-[3-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-393 392.4
yl)phenyl]ureido]hexanoate
128 1-(2,6-Dimethoxyphenyl)-3-[3-(5-methoxy-2-oxo-387 386.4
[1,3,4Joxdiazol-3-yl)phenyl]urea
CA 02477005 2004-08-20
129 5-Methoxy-3-[4-[(thiophen-3- 304 303.3
ylmethyl)amino]phenyl]-3H-[1,3,4]oxdiazol-2-one
130 4-[[4-(5-Methoxy-2-oxo-[1,3,4)oxdiazol-3-437 436.3
yl)phenylamino]methyl]-benzonitrile trifluoroacetate
131 3-[4-(2-Bromo-4,5-dimethoxybenzylamino)phenyl]-437 436.3
5-methoxy-3H-[1,3,4]oxdiazol-2-one
132 3-[4-(3-Ethaxy-4-methoxybenzylamino)phenyl]-5-486 485.4
methoxy-3H-[1,3,4]oxdiazol-2-one trifluoroacetate
133 Methyl4-[[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-470 469.4
yl)phenylamino]methyl]benzoate trifluoroacetate
134 4-[[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-356 355.3
yl)phenylamino]methyl]phenyf acetate
135 5-Methoxy-3-[4- 388 387.3
(pentafluorophenylmethylamino)phenyl]-
3H-[1,3,4]oxdiazol-2-one
136 3-[4-(4-Benzyloxybenzylamino)phenyl]-5-methoxy-518 517.5
3H-[1,3,4]oxdiazol-2-one trifluoroacetate
137 3-[4-(3,3-Dichlorononylamino)phenyl]-5-methoxy-517 516.3
3H-[1,3,4]oxdiazol-2-one trifluoroacetate
138 2-[[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-323 322.3
yl)phenylamino]methyl]benzonitrile
139 3-[4-(Cyclohexylmethylamino)phenyl]-5-methoxy-304 303.4
3H-[1,3,4]oxdiazol-2-one
140 5-Methoxy-3-[4-(2,3,5- 515 514.7
trichlorobenzylamino)phenyl]-3H-[1,3,4]oxdiazol-2-
one trifluoroacetate
141 3-[4-(5-Bromo-2-fluorobenzylamino)phenyl]-5-509 508.2
methoxy-3H-[1,3,4]oxdiazol-2-one trifluoroacetate
142 3-[4-(4-Hexyloxybenzylamino)phenyl]-5-methoxy-512 511.5
3H-[1,3,4]oxdiazol-2-one trifluoroacetate
143 5-Methoxy-3-[4-[3-(3- 572 571.4
trifluoromethylphenoxy)benzyfamino]phenyl]-3H-
[1,3,4]oxdiazol-2-one trifluoroacetate
144 3-[4-[(2-Chloroquinolin-3-ylmethyl)amino]phenyl]-5-497 496.8
methoxy-3H-[1,3,4]oxdiazol-2-one trifluoroacetate
145 Methyl3-methoxy-5-[[4-(5-methoxy-2-oxo- 501 500.4
[1,3,4]oxdiazol-3-yl)phenylamino]methyl]pyridine-2-
carboxylate trifluoroacetate
146 4-[[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-454 453.5
yl)phenylamino]methyl]phenyl benzenesulfonate
147 2-(2,6-Dirnethyl-4-methylsulfanylphenoxy)-N-[3-(5-416 415.5
methoxy-2-oxo-[1,3,4]oxdiazol-3-
yl)phenyl]acetamide
148 1-(2,4-Difluorophenyl)-3-[4-(5-methoxy-2-oxo-363 362.3
[1,3,4]oxdiazol-3-yl)phenyl]urea
149 1-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenylJ-419 418.4
3-(4-phenoxyphenyl)urea
CA 02477005 2004-08-20
36
150 1-{2,6-Difluorophenyl)-3-[4-(5-methoxy-2-oxo-363 362.3
[1,3,4]oxdiazol-3-yl)phenyl]urea
151 1-Butyl-3-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-307 306.3
yl)phenyl]urea
152 1-(2-Ethoxyphenyl)-3-[4-(5-methoxy-2-oxo-371 370.4
[1,3,4]oxdiazol-3-yl)phenyl]urea
153 1-(2,6-Dibromo-4-fluorophenyl)-3-[4-(5-methoxy-2-503 502.1
oxo-[1,3,4]oxdiazoi-3-yf)phenyl]urea
154 1-(4-Butoxyphenyl)-3-[4-(5-methoxy-2-oxo-399 398.4
[1,3,4]oxdiazol-3-yl)phenyl]urea
155 1-[4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)phenyl]-411 410.3
3-(4-trifluoromethoxyphenyl)urea
156 1-Benzyl-3-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-341 340.3
yl)phenyl]urea
157 1-(3-Fluorophenyl~3-[4-(5-methoxy-2-oxo- 345 344.3
[1,3,4]oxdiazol-3-yl)phenyl]urea
158 Ethyl 6-[3-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-393 392.4
yl)phenyl]ureido]hexanoate
159 1-Biphenyl-4-yl-3-[4-(5-methoxy-2-oxo- 403 402.4
[1,3,4]oxdiazol-3-yl)phenylJurea
160 Butyl 2-[3-(4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-427 426.4
yl)phenyl]-ureido]benzoate
161 5-Methoxy-3-[3-(7-methoxy-3,7- 492 491.5
dimethyloctylamino)phenyl]-3H-[1,3,4]oxdiazol-2-
one trifluoroacetate
162 5-Methoxy-3-[3-[(thiophen-2- 418 417.4
ylmethyl)amino]phenyl]-3H-[1,3,4]oxdiazol-2-one
trifluoroacetate
163 3-(3-Hexylaminophenyl)-5-methoxy-3H- 406 405.4
[1,3,4]oxdiazol-2-one trifluoroacetate
164 5-Methoxy-3-[3-(3-phenylpropylamino)phenyl]-440 439.4
3H-[1,3,4]oxdiazoi-2-one trifluoroacetate
165 5-Methoxy-3-(3-undecylaminophenyl)-3H- 476 475.5
[1,3,4]oxdiazoi-2-one trifluoroacetate
166 5-Methoxy-3-[3-[3-(3- 572 571.4
trifluoromethylphenoxy)benzylamino]phenyl]-3H-
[1,3,4]oxdiazol-2-one trifluoroacetate
167 3-[3-[(2-Chloroquinolin-3-ylmethyl)amino]phenyl]-5-497 496.8
methoxy-3H-[1,3,4]oxdiazol-2-one trifluoroacetate
168 4-[[3-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-586 585.5
yl)phenylamino]methyl]phenyl 4-
fluorobenzenesulfonate trifluoroacetate
169 5-Methoxy-3-[3-(3,4,5- 466 465.3
trifluorobenzylamino)phenyl]-3H-[1,3,4]oxdiazol-2-
one trifluoroacetate
170 3-[3-(3,5-Bistrifluoromethylbenzylamino)phenyl]-5-548 547.3
methoxy-3H-[1,3,4]oxdiazol-2-one trifluoroacetate
CA 02477005 2004-08-20
37
171 3-(3-Dec-4-enylaminophenyi)-5-methoxy-3H- 460 459.5
[1,3,4]oxdiazol-2-one trifluoroacetate
172 3-[3-(3-Cyclopentyl-2- 600 599.6
phenethyloxybenzylamino)phenyl]-5-methoxy-3H-
[1,3,4]oxdiazol-2-one trifluoroacetate
173 4-[[3-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3- 437 436.3
yl)phenylamino]methyl]benzonitrile trifluoroacetate
174 5-Methoxy-3-[3-[(6-methylpyridin-2- 427 426.3
ylmethyl)amino]phenyl]-3H-[1,3,4]oxdiazol-2-one
trifluoroacetate
175 3-[3-(2-Benzyloxyethylamino)phenyl]-5-methoxy-455.4
456
3H-[1,3,4]oxdiazol-2-one trifluoroacetate
176 3-[3-(2,6-Difluorobenzylamino)phenyl]-5-methoxy-447.3
448
3H-[1,3,4]oxdiazol-2-one trifluoroacetate
M.p. C
177 Dodecanoic acid [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-93
yl)phenyl]amide
178 Octadec-9-enoic acid [4-(5-methoxy-2-oxo- 67
[1,3,4]oxdiazol-3-y1)phenyl]amide
179 2-Methoxyethyl [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-117
yl)-2-methylphenyl]carbamate
180 1-(4-Hydroxycyclohexyl)-3-(4-(5-methoxy-2-oxo-220
[1,3,4]oxdiazol-3-yl)-2-methyfphenyl]urea
181 1,1-Dibutyl-3-[4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)-Oil
2-methylphenyl]urea
182 5-Methoxybenzofuran-2-carboxylic acid [4-(5-methoxy-2-199
oxo-(1,3,4]oxdiazol-3-yl)-2-methylphenyl]amide
183 4-Methylpiperazine-1-carboxylic acid [4-(5-methoxy-2-Oi1
oxo-[1,3,4]oxdiazol-3-yl)-2-methylphenyl]amide
184 1-Methyfpiperidin-4-yl [4-(5-methoxy-2-oxo- 235
[1,3,4]oxdiazol-3-yl)-2-methylphenyl]carbamate
185 Cyclohexyl [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)-2-163
methylphenyl]carbamate
186 4-Benzylpiperidine-1-carboxylic acid (4-(5-methoxy-2-146
oxo-(1,3,4]oxdiazol-3-yl)-2-methylphenyl]amide
187 1-(2-Diisopropylaminoethyl)-3-[4-(5-methoxy-2-oxo-136
[1,3,4]oxdiazol-3-yl)-2-methylphenyl]urea
188 4-(2-{3-(4-(5-Methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)-2-200
methylphenyl]-ureido}ethyl)benzenesulfonamide
189 1-(1-Benzylpiperidin-4-yl )-3-[4-(5-methoxy-2-oxo-198
(1,3,4]oxdiazol-3-y1)-2-methylphenyl]urea
190 1-(4-Isopropylphenyl)-3-[4-(5-methoxy-2-oxo- 200
[1,3,4]oxdiazol-3-yl)-2-methylphenyl]urea
191 2-{3-[4-(5-Methoxy-2-oxo-[1,3,4]oxd iazol-3-yl246
)-2-
methylphenyl]ureido}-3-methylbutyric acid
192 1,2,3,4-Tetrahydronaphth-1-yl [4-(5-methoxy-2-oxo-159
(1,3,4]oxdiazol-3-yl)-2-methylphenyl]carbamate
CA 02477005 2004-08-20
38
193 1-Phenylethyl [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-yl)-Oi1
2-methylphenyl]carbamate
194 4-Isopropylbenzyl [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-3-88
yl)-2-methylphenyl]carbamate
195 4-Trifluoromethoxybenzyl [4-(5-methoxy-2-oxo- 82
[1,3,4]oxdiazol-3-yl)-2-methylphenyl]carbamate
196 3,5-Dichlorobenzyl [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-169
3-yl)-2-methylphenyl]carbamate
197 Biphenyl-2-ylmethyl [4-(5-methoxy-2-oxo-[1,3,4]oxdiazol-138
3-yl)-2-methylpheny!]carbamate
198 5-Chlorobenzofuran-2-carboxylic acid [4-(5-methoxy-2-210
oxo-[1,3,4]oxdiazol-3-yl)-2-methylphenyl]amide
199 5-Chlorobenzofuran-2-carboxylic acid [4-(5-methoxy-2-209
oxo-[1,3,4]oxdiazol-3-yl)phenyl]amide
Example 200:
4-Fluorobenzenesulfonic acid mvrpholide (intermediate)
20 g of morpholine were added dropwise to a solution of 19.5 g
4-fluorobenzenesulfonyi chloride in 100 ml of toluene cooled in ice and the
mixture
was heated to reflux for 1 hour. After cooling, it was concentrated in vacuo
and
stirred with water, and the precipitate was filtered off with suction, washed
with water
and recrystallized from isopropanol.
Yield:16.9 g, melting point: 140°C
Example 201:
4-Hydrazinobenzenesulfonic acid morpholide (intermediate)
5 g of 4-fluorobenzenesulfonic acid morpholide were dissolved in 15 ml of
N-methylpyrrolidone and, after addition of 2.5 g of hydrazine hydrate, heated
at
100°C for 1 hour. After cooling to room temperature, 75 ml of water
were added and
the mixture was stirred at room temperature. After 2 hours, the solid was
filtered off
with suction and recrystallized from isopropanol.
Yield: 3.2 g, melting point: 164°C
The following example was prepared analogously:
Example 202:
4-Hydrazinobenzenesulfonic acid (3,3,5-trimethylcyclohexyl)amide
(intermediate)
CA 02477005 2004-08-20
39
melting point: 129°C
Example 203:
4-(3,3,5,5-Tetramethylcyclohexyloxy)nitrobenzene (intermediate)
1.3 g of sodium hydride are added to a solution of 7.8 g of 3,3,5,5-
tetramethylcyclohexanol in 50 ml of dimethylformamide, and the mixture is
stirred at
40-50°C for 30 min. Then a total of 7.0 g of 4-fluoronitrobenzene is
added in
portions, and the mixture is then heated at 100°C for 3 hours and
cooled to room
temperature. Addition of 250 ml of ice-water is followed by stirring, and the
solid
which has formed is filtered off with suction and dried in vacuo.
Yield: 8.6 g, melting point: 70°C
Example 204:
4-(3,3,5,5-Tetramethylcyclohexyloxy)aniline (intermediate)
8.3 g of 4-(3,3,5,5-tetramethylcyclohexyloxy)nitrobenzene are hydrogenated in
500 ml of methanol in the presence of 400 mg of platinum dioxide under
atmospheric
pressure until hydrogen uptake ceases. After removal of the catalyst by
filtration, the
solution is evaporated in a rotary evaporator, and the residue, a gradually
solidifying
brownish oil, is used without further purification for further reactions.
Yield: 7.3 g
Example 205:
4-(3,3,5,5-Tetramethylcyclohexyloxy)phenylhydrazine hydrochloride
(intermediate)
A solution of 1.13 g of sodium nitrite in 7.5 ml of water is added dropwise to
a stirred
mixture, cooled to -10°C, consisting of 3.7 g of 4-(3,3,5,5-
tetramethylcyclo-
hexyloxy)aniiine, 7.5 m1 of water and 15.5 ml of concentrated HCI, and the
mixture is
then stirred at -10°C for 45 min and subsequently added dropwise to a
suspension
of 9.3 g of tin dichloride dihydrate in 7 ml of concentrated HCI. The
precipitate is
filtered off with suction, washed with water, suspended in 200 ml of water
under
nitrogen and decomposed with 100 ml of 30% strength sodium hydroxide solution
at
10-15°C. The new precipitate which forms is filtered off with suction,
washed with
CA 02477005 2004-08-20
water, taken up in 200 ml of ether and dried with sodium sulfate. The product
is then
precipitated with ethereal HC1, filtered off with suction and dried in vacuo.
Yield: 2.1 g, melting point: 171 °C
5 Example 206:
Ethyl N'-(4-morpholinosulfonylphenyl)hydrazinoformate (intermediate)
114 mg of ethyl chloroformate were cautiously added dropwise to a mixture
consisting of 0.275 g of 4-hydrazinobenzenesulfonic acid morpholide, 5 ml of
methylene chloride and 1 ml of pyridine while cooling in ice, and the mixture
was
10 then stirred while slowly warming to RT. After dilution with 10 ml of
water, the
product was extracted with ethyl acetate, and the ethyl acetate phase was
washed
several times with water, dried over sodium sulfate and concentrated. The oily
crude
product obtained in this way was reacted further without further purification.
Yield: 0.25 g
Example 207:
3-(4-Morpholinosulfonylphenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
The oil from Example 206 was taken up in 5 ml of methylene chloride and, while
stirring and cooling in ice, 1 ml of a 20% strength solution of phosgene in
toluene
was added. After standing at room temperature overnight, this mixture was
diluted
with a further 10 mi of methylene chloride and then washed 3 times with water.
After
drying over sodium sulfate, the mixture was concentrated in vacuo, and the
product
was purified by column chromatography (silica gel, solvents:
methanol:methylene
chloride = 2 : 98).
YieId:130 mg, melting point: 195°C
The following examples were prepared in analogy to Example 207:
Example 208:
3-(4-Morpholinosulfonylphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 164°C
CA 02477005 2004-08-20
41
Example 209:
3-(4-Trifluoromethoxyphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 52°C
Example 210:
3-(4-Trifluoromethoxyphenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: 63°C
Example 211:
3-(4-Trifluoromethoxyphenyl)-5-isopropoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 212:
3-(4-Trifluoromethoxyphenyl~5-butoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 213:
3-(4-Trifluoromethoxyphenyl)-5-benzyloxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 214:
3-(4-(3,3,5-Trimethylcyclohexylaminosulfonyl)phenyl)-5-methoxy-1,3,4-oxdiazol-
2-
one
melting point: 164°C
Example 215:
3-(4-(3,3,5,5-Tetramethylcyclohexyloxy)phenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: 111 °C
Example 216:
3-(3-Benzyloxyphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: oil
CA 02477005 2004-08-20
42
Example 217:
3-(3-Benzyioxyphenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: 85°C
Example 218:
3-(3-Trifluoromethoxyphenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 219:
3-(3-Trifluoromethoxyphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 220:
3-(3-Trifluoromethoxyphenyl)-5-isopropoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 221:
3-(4-(2,2,6,6-Tetramethylpiperidin-4-yl-aminosulfonyl)phenyl)-5-methoxy-1,3,4-
oxdiazol-2-one
melting point: resin
Example 222:
3-(4-(2,2,6,6-Tetramethylpiperidin-4-ylaminosulfonyl)phenyl)-5-isopropoxy-
1,3,4-
oxdiazol-2-one
melting point: resin
Example 223:
3-(4-(2-(Diisopropylaminoethyl)aminosulfonyl)phenyl)-5-methoxy-1,3,4-oxdiazol-
2-
one
melting point: oil
Example 224:
CA 02477005 2004-08-20
43
3-(4-(2-(Diisopropylaminoethyl)aminosulfonyl)phenyl)-5-isopropoxy-1,3,4-
oxdiazol-2-
one
melting point: oil
Example 225:
3-(4-(4-Methylpiperazin-1-yl-sulfonyl)phenyl)-5-isopropoxy-1,3,4-oxdiazol-2-
one
melting point: resin
Example 226:
3-(4-(4-Methylpiperazin-1-yl-sul#onyi)phenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: resin
Example 227:
3-(3-(4,4,4-Trifluorobutyloxy)phenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 228:
3-(3-(2-Diethylaminoethyloxy)phenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: resin
Example 229:
3-(4-(4-Chlorophenoxy)phenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 68°C
Example 230:
3-(4-(4-Chlorophenoxy)phenyl)-5-isopropoxy-1,3,4-oxdiazol-2-one
melting point: oil
Example 231:
3-(4-(3,3,5-Trimethylcyclohexylaminosulfonyl)phenyl)-5-isopropoxy-1,3,4-
oxdiazol-2-
one
melting point: oil
CA 02477005 2004-08-20
44
Example 232:
3-(3-Phenoxyphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 89°C
Example 233:
3-(3-Phenoxyphenyl)-5-ethoxy-1,3,4-oxdiazol-2-one
melting point: 50°C
Example 234:
3-(3-Phenoxyphenyl)-5-isoproxy-1,3,4-oxdiazol-2-one
melting point: 58°C
Example 235:
3-(4-Phenoxyphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 83°C
Example 236:
3-(4-Cyclohexylphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: resin
Example 237:
3-(3-(3,3,5,5-Tetramethylcyclohexyloxy)phenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 68°C
Example 238:
3-(4-Phenylphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: >260°C (decomp.)
Example 239:
3-(3-(3-Methylphenoxymethyl)phenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 47°C
CA 02477005 2004-08-20
Example 240:
3-(3-Phenylphenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: 80°C
5 Example 241:
3-(4-(3,3-Dimethylpiperidinocarbonyl)phenyl)-5-methoxy-1,3,4-oxdiazol-2-one
melting point: resin
Example 242:
10 3-(4-(3,3,5,5-Tetramethylcyclohexyloxy)phenyl)-5-isopropoxy-1,3,4-oxdiazol-
2-one
melting point: resin
The compounds of the formula 1 show an inhibitory effect on pancreatic lipase
(PL).
As PL inhibitors, they are able to prevent absorption of fat consumed with the
diet
15 and thus lead to a reduction in the fat uptake and the body weight or
prevent an
increase in body weight. The compounds of the formula 1 are particularly
suitable for
producing medicaments for the treatment of obesity and of diabetes mellitus of
type
1 and 2.
20 The activity of the compounds was assayed as follows:
1. Preparation of the substrate:
w 80 NI of tripalmitin (85 mM in chloroform) are mixed with 5,u1 of glycerol
tri[9,10(nr
3H]oleate (5 mCilml in toluene) in a 12 ml polypropylene vessel. Evaporation
in a
25 rotary evaporator (50°C) and addition of 4 ml of 200 mM TrislHCl (pH
7.6), 0.8% TX-
100 are followed by ultrasound treatment of the mixture (Branson B-12
sonifier,
output level 4, 3 X 2 min with 1 min intervals on ice) until a homogeneous
milky
suspension is produced.
30 2. Assay:
Lipase buffer: 80 mM TrislHCl (pH 7.6), 600 mM NaCI, 8 mM CaCl2, 8 mM
benzamidine, 2 mM Pefabloc (Roche Biochemicals) (add the inhibitors only on
the
CA 02477005 2004-08-20
46
day of the assay)
Pancreatic lipase: Enriched preparation from porcine pancreas (Sigma order No.
L-0382) dissolved in lipase buffer (100 000 unitsl500,u1)
Procedure:
5,u1 of test substance (in 100% DMSO) or DMSO (control) are mixed with 10 ~ul
of
substrate and 5 NI of lipase (in this sequence) and incubated at 30°C
(Eppendorf
Thermomixer, 350 min-') for 30 min. After addition of 325,u1 of
methanol/chloroform/n-heptane (10/9/7) and 105,u1 of 0.1 M K2C03, 0.1 M H3B03
(pH 10.5 adjusted with 1 M KOH) and vigorous mixing, the phases are separated
by
centrifugation (8000 rpm, Eppendorf centrifuge, 4°C). 140 NI portions
of the aqueous
supernatant {contains the liberated radiolabeled oleate; 70% recovery) are
transferred into 20 ml scintillation vials and mixed with 6 ml of
scintillation cocktail
(Beckman ReadySafe). After vigorously mixing and incubating at room
temperature
for 2 h, the radioactivity is measured in a liquid scintillation counter
(Beckman,
L8008, tritium channel with quench curve, measurement time 20 min).
Evaluation:
Substances are routinely tested in each concentration in three independent
incubation mixtures each with duplicate determination after phase separation
(SD <
0.02). Background values (reaction under the same conditions but without
lipase) are
subtracted from all values (corresponds predominantly to the content of
glycerol
trioleate or free oleate in the substrate preparation in the aqueous phase, <
5% of
the radioactivity employed). The inhibition of the pancreatic lipase enzymatic
activity
by a test substance is determined by comparison with an uninhibited control
reaction
(presence of lipase = 0% inhibition; absence of lipase 100% inhibition in each
case
after background correction). The lCSO is calculated from an inhibition plot
with up to
8 concentrations of the test substance. The software package GRAPH IT
(Eisevier-
BIOSOFT) is used for curve fitting and iCSO determination.
CA 02477005 2004-08-20
47
The compounds of the formula 1 showed the following effect in this assay
system:
Compound from IC-50
Example: wM
_- _ _. _ 86 1.5
210 0.7
212 0.5
213 0.5
216 0.8
218 0.7
220 --_1.8
229 ~ 0.6