Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02478278 2004-08-20
1
TITLE OF THE INVENTION
MICRONUTRIENT SUPPLEMENT
FIELD OF THE INVENTION
[0001] The present invention relates to a micronutrient supplement
containing ingredients such as multivitamins, minerals, fatty acids, amino
acids,
plant extracts, and the like.
BACKGROUND OF THE INVENTION
[0002] Micronutrient compositions are commonly taken as dietary
aids; either as therapeutic preparations directed to a specific medical
problem
or as general nutritional supplements. Micronutrients may be broadly defined
as substances that are essential or helpful for the maintenance of normal or
enhanced metabolic function, but are not normally or sufficiently synthesized
in
the body and must thus be supplied from an exogenous source.
[0003] Given poor dietary habits of individuals and other factors, it
has become clear that the role of micronutrient compositions is substantial
when it comes to preventing fatigue, disease and optimizing cell maintenance
and development. This is particularly the case for individuals who lead a
stressful lifestyle, for pregnant women or those who engage in a large amount
.of physical exercise. Additionally, many drugs, some chronic diseases (e.g.
rheumatoid arthritis), certain cancer treatments, and alcoholism can all lead
to
a deficiency in one or more micronutrients.
[0004] It is has also been suggested that a significant portion of
preventable illnesses (which it is estimated absorbs as much as 70 per cent of
E:\Temp\-2451315.doc 08120/20
04
CA 02478278 2004-08-20
2
total health care costs in the United States) could be readily prevented
through
supplementing the diet with micronutrients. In addition to major health care
cost
savings other benefits of supplementation include better quality of life,
longer
life, and increased productivity. The level of supplements required for
effective
disease protection cannot be obtained through even the most healthful diet
(Bendich, Adrianne, et al. Potential health economic benefits of vitamin
supplementation. Western Journal of Medicine, Vol. 166, May 1997, pp. 306-
12).
[0005] Micronutrients are especially important to pregnant or
lactating women, ensuring an adequate provision of nutrients for the
developing
fetus and for the mother. It has become clear that the role of micronutrients
is
substantial when it comes to preventing fatigue, disease and optimizing cell
maintenance and development.
[0006] Many micronutrient supplements however, pose a potential
problem of nutrient-nutrient interactions. The presence or excess of one
nutrient in such a supplement may interact with another nutrient, thereby
adversely affecting its absorption. Iron for example is reported to inhibit
the co-
absorption of zinc and vice-versa (Hambridge et al., Obstet. Gynecol. 4:593-
596, 1987); zinc is reported to inhibit the co-absorption of copper (Festa et
al.,
Am.J. Clin. Nutr. 41:285-292, 1985); calcium is reported to interfere with the
co-
absorption of both iron and zinc (Seligman et al., Obstet. G ny ecol. 61:356-
362,
1983); and protein supplements are reported to increase urinary calcium losses
(Allen et al., Am.J. Clin. Nutr. 32: 741-749. 1979) and to increase vitamin B6
requirements (National Research Council Recommended Dietary Allowances,
10th ed., NatI. Acad. Press, Washington, D.C. 1989). Iron and copper are also
known to degrade folic acid and vitamin B12 when commingled.
E:1Temp1-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
3
[0007] Prior art efforts at multivitamins and nutritional aids have
often circumvented this problem by using greater doses of ill-absorbed or
degraded nutrients. Indeed, there has been a trend towards using mega doses,
of many nutrients. Such practice is a risky one since an overdose of many
nutrients, in particular metallic compounds, can be toxic, thereby achieving
the
opposite result of creating sickness rather than preventing it. One notable
example is iron.
[0008] Iron-deficiency anemia is a primary risk during pregnancy
because of the increasing red blood cell mass of the mother, the demands of
the fetus and placenta (more so in the second and third trimesters of
pregnancy), and blood losses during childbirth. Thus, prevention of iron
deficiency is of prime importance. A common problem with prior art
supplements is that little of the iron ingredients is actually absorbed in the
blood
stream. The known way to deal with this is to use larger doses of iron
ingredients which in turn triggers constipation, nausea when taken on an empty
stomach and a metallic taste (Solvell L.; Oral iron therapy: Side effects. In
Iron
Deficiency: Pathogenesis, Clinical Aspects, Therapy Edited by L Hallberg, HG
Harwerth and A Vannotti: London, Academic Press, 1970, pp. 573-583).
[0009] Another important micronutrient is folic acid. Studies have
revealed that folic acid may play an important role in preventing some types
of
cancers (e.g. Stolzenberg-Solomon, Rachael Z., et al. Dietary and other
methyl-group availability factors and pancreatic cancer risk in a cohort of
male
smokers. American Journal of Epidemiology, Vol. 153, April 1, 2001, pp. 680-
87), heart disease (Loria, Catherine M., et al. Serum folate and
cardiovascular
disease mortality among US men and women. Archives of Internal Medicine,
Vol. 160, November 27, 2000, pp. 3258-62.), and depression (Alpert, Jonathan
E. and Fava, Maurizio. Nutrition and depression: the role of folate. Nutrition
E:1Temp1-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
4
Reviews, Vol. 55, May 1997, pp. 145-49). It is also well established that
taking
folic acid before and during pregnancy as a nutritional supplement greatly
reduces risks of fetal diseases such as spina-bifida or cleft lip and palate.
If
use of the supplement containing folic acid is discontinued because of iron
intolerance, the benefits of the folic acid will be lost.
[0010] Thus, the importance of many of the ingredients present in
micronutrient supplements may not be overstated.
[0011] In the prior art, U.S. Patent No. 5,932,624, discloses vitamin
supplements comprising folic acid and vitamin B12, and which are essentially
free of antioxidants such as phytochemicals, certain vitamins, and minerals
such as iron and copper, which are known to destroy some of the vitamin B12
and folic acid. However, such vitamin supplement, in an effort to avoid co-
absorption problems provides an incomplete product, which fails to include
important components such as iron and copper.
[0012] U.S. Patent No. 5,976,568 provides examples of various
multivitamins some of them to be taken twice a day. However, the ingredients
of the morning and evening tablets are identical. The apparent purpose of the
twice-a-day formulation is to provide a second dose of ingredients, which may
have been used up during the day. Another drawback of the multivitamin
compositions proposed in this prior art is the presence of many competing
nutrients in a single dosage unit, e.g. iron, calcium and zinc.
[0013] Other problems often associated with patient non-compliance
with recommended use of multivitamins and nutritional supplements are related
to the large size of the tablets. For example, in the case of pregnant women,
because pregnant women are sometimes nauseated and because of their
E:1Temp1-2451315.doc 08120/20
04
CA 02478278 2004-08-20
normal instinct to avoid pharmaceutical products, the size and appearance of
current products is often enough to cause a pregnant woman to discontinue
taking the multivitamin or nutritional aid.
[0014] Published Canadian patent application No. 2,258,868
discloses an attempt as creating a slightly smaller tablet. The tablet
composition is said to provide high levels of calcium (calcium citrate) and
iron.
(carbonyl iron) while maintaining a smaller than usual size. Calcium citrate
is
described as enhancing the absorption of iron, zinc and magnesium, and as
being more soluble, better absorbed and better tolerated than traditional
calcium supplements. Carbonyl iron on the other hand, is described as having
a higher iron content as compared to the ferrous salts. The use of
UltradenseTM
calcium citrate and carbonyl iron allows the formulation to be compressed into
acceptably sized tablets.
[0015] Canadian patent application No. 2,144,751 and U.S. Patent
No. 5,494,678, discloses a multivitamin and mineral supplement for pregnant
women. The iron component is said to be ferrous sulfate, coated with a
pharmaceutically acceptable film forming material. The coating is said to
provide for the release of the ferrous sulfate in the intestine, thus
apparently
minimizing interactions between iron and divalent cations such as calcium
(also
in the supplement), in turn improving the iron bioavailability. Thus, the co-
absorption problem is dealt with by suggesting that absorption of various
components should be engineered to occur at different body sites.
[0016] U.S. Patent No. 4,431,634 discloses multi-mineral prenatal
dietary supplements, said to maximize the bioavailability of iron. This is
apparently accomplished by maintaining the amount of calcium compounds in
E:1Temp1-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
6
the supplement at 300 mg or less, and the amount of magnesium compounds
at 75 mg or less, per dosage unit.
[0017] Despite the foregoing efforts to improve micronutrient
supplements, there remains a need to develop micronutrient compositions
overcoming the drawbacks of prior art compositions.
[0018] The micronutrient supplement of the present invention seeks
to avoid deleterious co-absorption problems associated with co-mingled
ingredients.
[0019] The micronutrient supplement of the present invention also
seeks to provide rather small and palatable dosage units when compared to
those of the prior art.
[0020] In a preferred embodiment, the micronutrient supplement of
the present invention provides optimal nutritional components and amounts that
have been found to benefit both fetal growth and the mother's health
throughout the pregnancy.
[0021] The micronutrient supplement of the present invention also
allows the presentation of the tablet ingredients in the form of a plurality
of
different dosage units so that a patient can voluntarily take some ingredients
and not others in case they suffer from intolerance or side effects caused by
specific ingredients such as iron.
[0022] The present invention seeks to meet these and other needs.
E:1Temp1-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
7
[0023] The words "a" and "an," as used in this specification,
including the claims, denote "one or more." Specifically, the use of
"comprising," "having," or other open language in claims that claim a
combination or method employing "an object," denotes that "one or more of the
object" may be employed in the claimed method or combination.
SUMMARY OF THE INVENTION
[0024] In general terms, the present invention provides a
micronutrient supplement, the supplement being characterized by having at
least two types of dosage units designed to be taken at a predetermined time
interval.
[0025] In a preferred embodiment, micronutrients such as vitamin
and mineral supplements which are known or proven to be absorption-
competing when co-administered are thus be prepared as separate and distinct
dosage units and are administered at spaced time intervals so as to minimize
drop-offs in absorption and co-absorption problems.
[0026] Still in a preferred embodiment, micronutrients such as
vitamin and mineral supplements, which are known to potentially cause
deleterious side effects, for example constipation in the case of iron
supplements, can be grouped in a separate and distinct dosage unit. Therefore,
by virtue of the present invention a patient may temporarily stop taking a
type of
dosage unit of the invention and continue to take the other dosage unit(s) of
the
invention. The overall effect is to avoid discontinuing the use of supplements
entirely and thereby avoiding discontinuance of important ingredients
unrelated
to the side effects of some ingredients.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2007-07-25
8
[0027] Still in a preferred embodiment, the micronutrient supplement
is destined for pregnant women and provides optional nutritional components
and amounts that have been found to benefit both fetal growth and the
mother's health before, throughout and after pregnancy (post-partum).
[0028] The present invention also provides a micronutrient
supplement in the form of a kit comprising a plurality of types of dosage
units
along with instructions for taking the dosage units at spaced time intervals.
[0028.1] The present invention also relates to a micronutrient
supplement, comprising two distinct types of dosage units, wherein a first
type
of dosage units comprises iron, active metabolite, derivative or precursor
thereof and is essentially free of folic acid and wherein a second type of
dosage
units comprises folic acid, active metabolite, derivative or precursor thereof
and
is essentially free of iron.
[0028.2] The present invention also relates to a micronutrient
supplement kit for use in the treatment or prevention of micronutrient
deficiencies, in humans afflicted with or at risk of micronutrient
deficiencies,
according to a regimen comprising at least a daily intake of folic acid,
active
metabolite, derivative or precursor thereof, the kit comprising the following
components: (1) from about 7 days to about a month-supply of dosage units of
a first type, each dosage unit of the first type comprising iron, active
metabolite,
derivative or precursor thereof and being essentially free of folic acid, and
(2)
from about 7 days to about a month-supply of dosage units of a second type,
each dosage unit of the second type comprising folic acid, active metabolite,
derivative or precursor thereof and being essentially free of iron; and a
means
for having the components arranged in a way as to facilitate compliance with
the regimen, the means including instructions for daily intake of a dosage
unit
CA 02478278 2009-02-27
8a
of the second type and indications for a dosage unit of the first type to be
either
(a) taken daily at a predetermined time interval from the second type; or (b)
cycled by periodical discontinuation when and if side-effects are encountered.
[0028.3] The present invention also relates to the use of a micronutrient
supplement or kit as defined above for treating or preventing a micronutrient
deficiency, more specifically in women of child bearing age, still more
specifically when the women are in gestation, recently gave birth or are
contemplating becoming pregnant.
[0028.4] The present invention also relates to a kit for use in the treatment
or prevention of micronutrient deficiencies, in women of child bearing age
afflicted with or at risk to micronutrient deficiencies, the kit containing
the
following components: (1) from about 7 days to about a month-supply of
tablets of a first type, each tablet of the first type comprising a
pharmaceutically
acceptable form of iron while being free of folic acid, and (2) from about 7
days
to about a month-supply of tablets of a second type, each tablet of the second
type comprising a pharmaceutically acceptable form of folic acid and calcium
while being free of iron, the tablets of the first and second types being of a
similar small size for ease of swallowing; and a means for having the
components arranged in a way as to facilitate compliance with the regimen the
kit being for daily use during a period from about 7 days to about a month, a
delay intake of the tablets of the first type being discontinuable.
[0028.5] The present invention also relates to a use of folic acid and iron
in a micronutrient supplement for the treatment or prevention of micronutrient
deficiencies in women of child bearing age afflicted with or at risk to
micronutrient deficiencies,
CA 02478278 2009-02-27
8b
wherein the micronutrient supplement consists of two distinct types of
micronutrient tablets, a first type of tablets comprising a pharmaceutically
acceptable form of iron while being free of folic acid and a second type of
tablets comprising a pharmaceutically acceptable form of folic acid and
calcium
while being free of iron,
wherein the tablets of the first and second types are of a size for ease of
swallowing, and
wherein the micronutrient supplement is provided daily for about 7 days
to about a month of a tablet of the first type and of the second type at a
predetermined time interval of at least 4 hours, with a rest period from 0 day
to
about a month with a daily use of a tablet of the second type only.
BRIEF DESCRIPTION OF THE DRAWINGS
[0029] Having thus generally described the invention, reference will now
be made to the accompanying drawings, showing by way of illustration a
preferred embodiment thereof, and in which:
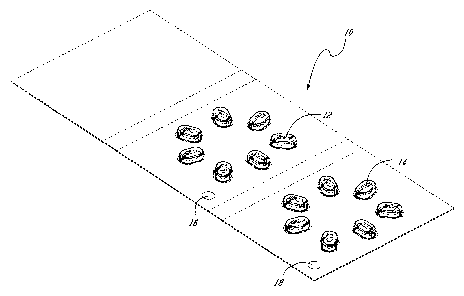
[0030] Figure 1 shows a perspective view of an example of a kit of the
present invention and more specifically an individual blister pack of a week's
worth of the supplement of the present invention having an array of a first
type
of dosage unit to be taken at a given time of day and an array of a second
type
of dosage unit to be taken at another time of day.
DESCRIPTION OF THE PREFERRED EMBODIMENT
[0032] The present invention will now be described by means of an
illustrative and preferred embodiment.
[0033] In a most preferred embodiment, the invention discloses a
micronutrient supplement in the form of two distinct dosage units to be taken
at
CA 02478278 2008-05-20
8c
[0031] DESCRIPTION OF THE PREFERRED EMBODIMENT
[0032] The present invention will now be described by means of an
illustrative and preferred embodiment.
[0033] In a most preferred embodiment, the invention discloses a
micronutrient supplement in the form of two distinct dosage units to be taken
at
CA 02478278 2004-08-20
9
spaced time intervals. Ideally, the time interval will be 12 hours, however,
the
time interval may be as short as 4 hours.
[0033] The two distinct dosage units and the time interval
recommended between ingestion of the distinct dosage units will of course be
the domain of those of skill in the art and may afford variations. The two
distinct
dosage units and recommended time intervals between ingestion is primarily
aimed at minimizing known or eventual vitamin-vitamin, vitamin-mineral and
mineral-mineral deleterious interactions.
[0034] An added benefit of the two distinct dosage units is the
possibility for a patient to discontinue taking the type of dosage unit
causing
unwanted side effects while continuing with the other type of dosage unit.
[0035] An added benefit of the two distinct dosage units is the
possibility for the manufacturer to produce a smaller and more palatable
dosage unit, which improves patient compliance when compared to unpalatable
large dosage units. The possibility for the manufacturer to produce smaller
units is not only related to the fractionating of a daily dose into two dosage
units
but also because of the reduction of ingredient interactions which caused
manufacturers to use greater quantities of ill-absorbed ingredients.
[0036] More specifically, in the most preferred embodiment of the
invention, the calcium and iron ingredients are placed in distinct and
different
dosage units so as to avoid their known propensity to mutually interfere with
their absorption.
[0037] Also in a most preferred embodiment, the folic acid and iron
ingredients are placed in distinct and different dosage units so as to allow
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
discontinuance of the iron-containing dosage unit while maintaining ingestion
of
the folic acid-containing dosage unit.
[0038] Also in a most preferred embodiment, the amount of zinc
present in the micronutrient supplement of the present invention, has been
reduced in order to further improve the iron bioavailability.
[0039] Furthermore, the presently disclosed micronutrient
supplement comprises a greater iron/vitamin C ratio (1:3.4), further improving
iron bioavailability.
[0040] In a most preferred and convenient embodiment, the
micronutrient supplement of the present invention will be provided with
instructions to take a first type of dosage unit in the morning and a second
type
of dosage unit in the evening. In a most preferred embodiment, the folic acid
ingredient will be present in the evening dosage unit while the iron
ingredient
will be present in the morning dosage unit. In cases where a patient suffers
from constipation as a result of the iron supplement, the patient would be
able
to halt the morning unit while continuing to take the important evening unit
comprising folic acid. It is indeed of interest that patients have enough
folic
acid in their bodies given its potential prophylactic affect against certain
types
of cancers, heart disease, depression and for preventing certain types of
birth
defects.
[0041] Thus the present invention provides a micronutrient
supplement formulation having distinct dosage units to be taken at spaced
apart time intervals. Most conveniently, one type of dosage unit can be taken
in
the morning and the second type in the evening. Hence, the AM and PM
formulations contain different ingredients. Each set of ingredients is aimed
at
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
11
providing optimal nutritional components and amounts, while concurrently
minimizing the undesired problems of the conventional unitary formulations.
[0042] In a preferred embodiment, the micronutrient supplement of
the present invention will feature an AM dosage unit composition comprising:
provitamin A (beta-carotene), vitamin E (di-u.-tocopheryl acetate), vitamin C
(ascorbic acid), vitamin B1 (thiamine mononitrate), vitamin B2 (riboflavin),
vitamin B3 (niacinamide), vitamin B6 (pyridoxine HCI), vitamin B5 pantothenic
acid (calcium pantothenate), magnesium (magnesium oxide), iodine (potassium
iodide), iron (ferrous fumarate), copper (cupric oxide), zinc (zinc oxide),
and
pharmaceutically acceptable excipients;
[0043] and the PM dosage unit composition will comprise: vitamin D3
(cholecalciferol), vitamin B12 (cyanocobalamin), folic acid, calcium (calcium
carbonate) and pharmaceutically acceptable excipients.
[0044] Description of various preferred ingredients in best mode
[0045] When referring to quantities of preferred ingredients
reference is made to quantities of pure substance regardless of form. For
example, when referring to quantities of calcium or iron, reference is made to
elemental calcium and elemental iron as opposed to quantities of calcium
carbonate and ferrous fumarate. It is to be understood that adequate
quantities
of calcium carbonate and ferrous fumarate would be used to contain the
chosen amount of elemental calcium or iron.
[0046] Throughout this disclosure, when referring to the term about,
was is to be understood is a variation of plus or minus 20%wt.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
12
[0047] Beta-carotene or provitamin A is a precursor to vitamin A.
Beta carotene is a potent antioxidant that appears to work synergistically
with
several vitamins, minerals and antioxidants. Beta-carotene is provided in the
present micronutrient formulation in amounts of about 250 to 5000 I.U.; and
most preferably about 2700 W.
[0048] Vitamin B1 (thiamine mononitrate) is an essential water-
soluble B-vitamin playing an important role in the metabolism of
carbohydrates.
It is critical for the transmission of high-frequency impulses in the central
nervous system. Vitamin B1 is provided in the present micronutrient
formulation in amounts of about 0.5 to 10 mg; most preferably about 3.0 mg.
[0049] Vitamin B2 (riboflavin) is an essential water-soluble B-vitamin
that is required for the repair and growth of tissues as well as for DNA
synthesis. It also assists in the metabolism of nutrients. Vitamin B2 is
provided
in the present micronutrient formulation in amounts of about 0.5 to 10 mg and
most preferably about 3.4 mg.
[0050] Vitamin B3 (niacinamide) is the amide form of the vitamin
Niacine, and is an essential constituent of coenzymes I and II, occurring in a
wide variety of enzyme systems, and which are involved in the anaerobic
oxidation of carbohydrates. Vitamin B3 is provided in the present
micronutrient
formulation in amounts of about 2 to 50 mg and most preferably about 20.0 mg.
[0051] Vitamin B6 (Pyridoxine HCI) is a term commonly used for a
group of vitamins consisting of pyridoxine, pyridoxal, pyridoxal-5-phosphate,
pyridoxamine, and pyridoxamine-5-phosphate . These vitamins are important in
protein and amino acid metabolism and are required to synthesize hemoglobin.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
13
Vitamin B6 is provided in the present micronutrient formulation in amounts of
about 2 to 100 mg and most preferably about 10.0 mg.
[0052] Vitamin B12 (cyanocobalamine) is an essential water-soluble
B vitamin that is provided in the present micronutrient formulation in amounts
of about 2 to 50 mcg and most preferably about 12.0 mcg.
[0053] Folic acid is a water soluble B-vitamin that helps build healthy
cells. Folic acid is necessary for the synthesis of RNA and DNA. Folic acid is
provided in the present micronutrient formulation in amounts of about 0.1 to
mg and most preferably about 1.1 mg. Since folic acid is water soluble, it is
readily eliminated from the body, and therefore has to be taken daily to help
prevent, for example, neural tube defects in the fetus. During periods of
rapid
growth, such as during pregnancy and fetal development, the body's
requirement for this vitamin increases. Patients having enough folic acid in
their
bodies can decrease the risk of some types of cancers, heart disease and even
depression. The U.S. Public Health Service currently recommends 400
micrograms of folic acid every day.
[0054] Vitamin B5 Pantothenic acid (calcium pantothenate) is a
water-soluble vitamin that plays an active role in the metabolism of proteins,
fats and carbohydrates. It is also involved in the synthesis of sterols,
hormones,
porphyrins and acetylcholine. Pantothenic acid is provided in the present
micronutrient formulation in amounts of about 0.5 to 20 mg and most
preferably about 5.0 mg.
[0055] Pharmaceutically acceptable forms of certain of the B
vitamins include, but are not limited to, thiamine mononitrate or thiamine
hydrochloride, niacin or niacinamide; and pyridoxine hydrochloride.
E:1Temp1-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
14
[0056] Vitamin C (ascorbic acid) is an essential water-soluble
vitamin that functions as an antioxidant. It is critical in producing and
maintaining collagen and promotes. wound healing. It is also important in
producing hormones that regulate basal metabolic rate and body temperature.
Vitamin C (ascorbic acid) is provided in the present micronutrient formulation
in
amounts of about 10 to 1000 mg and most preferably about 120.0 mg.
Pharmaceutically acceptable salts of ascorbic acid include, but are not
limited
to sodium or calcium ascorbate.
[0057] Vitamin D3 (cholecalciferol) is an essential fat-soluble vitamin
whose major biological function is to maintain normal blood levels of calcium
and phosphorus. Vitamin D3 is provided in the present micronutrient
formulation in amounts of about 10 to 1000 I.U. and most preferably about
250.0 I.U. vitamin D3 (cholecalciferol). The vitamin D3 used in the present
formulation can include any of the forms of vitamin D that is a precursor to
cholecalciferol.
[0058] Vitamin E (dl-a-tocopheryl acetate) is a fat-soluble vitamin
functioning as an antioxidant protecting lipid membranes from oxidation.
Vitamin E (dl-a-tocopheryl acetate) is provided in the present micronutrient
formulation in amounts of about 1 to 500 I.U. and most preferably about 30
I.U.
Vitamin E can also be present as a, a -,y-, or.8-tocopheryl, or as a mixture
or as
an isomer thereof, such as dl-a-tocopheryl acetate or a-tocopheryl acetate.
Salts of vitamin E include, but are not limited to, an acetate, or acid
succinate
salt.
[0059] Calcium (calcium carbonate) is required for adequate bone
formation and maintenance, as well as for diverse metabolic functions. Calcium
is involved in the transmission of nerve impulses, muscle contraction and
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
relaxation, blood clotting, structure and function of cell membranes and
vitamin
B12 absorption. Women are advised to increase their calcium intake
substantially during pregnancy. Calcium is provided in the present
micronutrient
formulation in amounts of about 10 to 1500 mg and most preferably about
300.0 mg, in the form of suitable amounts of calcium carbonate to equate to
the
required amount of calcium. Calcium carbonate relies on stomach acid to
dissolve. Supplemental calcium is beneficial for the skeletal system.
[0060] Iron (ferrous fumarate) is an essential mineral playing an
important role in the transport of oxygen to tissues throughout the body via
hemoglobin and myoglobin. Iron is provided in the present micronutrient
formulation in the form of ferrous fumarate, corresponding to amounts of
elemental iron of about 2 to 300 mg and most preferably about 35 mg.
[0061] Magnesium (magnesium oxide) is an essential mineral for
many biological processes. Magnesium is provided in the present micronutrient
formulation in the form of magnesium oxide, in amounts of about 5 to 200 mg
and most preferably about 50 mg. Magnesium can be incorporated in the
present micronutrient formulation in various forms such as an oxide, a
sulfate,
or the like.
[0062] Zinc (zinc oxide) is a trace mineral essential to cell
multiplication, tissue regeneration and wound healing. It is required in many
enzymatic functions throughout the body, and also helps regulate the immune
system and insulin metabolism. Zinc is provided in the present micronutrient
formulation in the form of zinc oxide, in amounts of about 1 to 50 mg and most
preferably about 15 mg. Zinc can be incorporated in the present formulation in
various forms such as an oxide, a phosphate, a chloride, a sulfate, a nitrate,
a
gluconate, or the like, as well as metallic zinc.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
16
[0063] Copper (cuprous oxide) is a trace mineral essential for red
blood cell formation. Copper is provided in the present micronutrient
formulation in the form of cupric oxide, in amounts of about 0.5 to 10 mg and
most preferably about 2.0 mg. Copper can be incorporated in the present
micronutrient formulation in various forms such as a sulfate, a nitrate, a
chloride, a carbonate, an oxide, a hydroxide, an iodide, a glutamate, an
aspartate, a citrate, or the like.
[0064] Iodine (potassium iodide) is essential for proper thyroid
functioning. Iodine is provided in the present micronutrient formulation in
the
form of a potassium salt, wherein the iodine is present in amounts of about
0.05
to 1 mg and most preferably about 0.15 mg.
[0065] The vitamins and minerals and other nutritional aids
incorporated in the micronutrient of the present invention are of food-grade,
approved for use in humans (U.S. Pharmacopoeia); they may be obtained from
various distributors known to one of skill in the art.
[0066] Further micronutrients, including but not limited to vitamin A,
vitamin K, fatty acids (including linoleic acid, linolenic acid, and omega-3
fatty
acids), phosphorous, selenium, boron, biotin, choline, inositol, chromium,
molybdenum, cobalt, fluorine, manganese, nickel, potassium, or the like, may
be added to the micronutrient formulation of the present invention, provided
they do not interfere with the components already described.
[0067] The micronutrient formulation of the present invention
preferably contains the active ingredients described above, and may contain
non-active excipients such as for example fillers or binders, disintegrating
agents, lubricating agents, silica flow conditioners and stabilizing agents.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
17
[0068] Disintegrating agents are included in the present formulation
to assist in the dissolution of the tablet. Disintegrating agents are well
known in
the art and include, but are not limited to alginic acid,
carboxymethylcellulose,
carboxymethylcellulose sodium, hydroxypropylcellulose (low substituted),
microcrystalline cellulose, powdered cellulose, colloidal silicon dioxide,
sodium
croscarmellose, crospovidone, methylcellulose, polacrilin potassium, povidone,
sodium alginate, sodium starch glycolate, starch, disodium disulfite, disodium
edathamil, disodium edetate, disodiumethylenediaminetetraacetate (EDTA),
crosslinked polyvinylpyrollidines, pregelatanized starch, carboxymethyl
starch,
sodium carboxymethylstarch, microcrystalline cellulose. A preferred
disintegrating agent consists of sodium crosscarmellose, and is provided in
the
present dosage unit formulation in amounts of about 2 to 100 rng preferably
about 30 to 40 mg.
[0069] Lubricating agents are included in the present formulation to
assist in the compression of the formulation. Lubricating agents are well
known
in the art and include, but are not limited to calcium stearate, canola oil,
glyceryl
palmitosstearate, hydrogenated vegetable oil (type I), magnesium oxide,
magnesium stearate, mineral oil, poloxamer, polyethylene glycols, sodium
lauryl sulfate, sodium stearate fumarate, stearic acid, talc, zinc stearate,
glyceryl behapate, magnesium lauryl sulfate, boric acid, sodium benzoate,
sodium acetate, sodium benzoatelsodium acetate (in combination) and D,L-
leucine. Preferred lubricants consists of magnesium stearate and sodium lauryl
sulfate and are provided in the present AM multi-vitamin formulation in
amounts
of about 1 to 20 mg and most preferably equal amounts of about 3 to 4 mg.
[0070] Fillers or binders well known in the art, are included in the
present formulation and include, but are not limited to acacia, alginic acid,
calcium phosphate (dibasic), carboxymethylcellulose, carboxymethylcellulose
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2005-07-11
18
sodium, hydroxyethylcelIulose, hydroxypropylcellulose,
hydroxypropylmethylcellulose, dextrin, dextrates, sucrose, tylose,
pregelatinized starch, calcium sulfate, amylose, glycine, bentonite, maltose,
sorbitol, ethylcellulose, disodium hydrogen phosphate, disodium phosphate,
disodium pyrosulfite, polyvinyl alcohol, gelatin, glucose, guar gum, liquid
glucose, compressible sugar, magnesium aluminum silicate, maltodextrin,
polyethylene oxide, polymethacrylates, povidone, sodium alginate,
microcrystalline cellulose, starch and zein. Preferred fillers or binders
consists
of microcrystalline cellulose and starch, and are provided in the present
morning dosage unit in amounts of 10 to 500mg and most preferably about 180
mg and 55 mg respectively.
[0071] Many other pharmaceutically acceptable tableting agents
such as fillers or binders, lubricating agents, disintegrating agents, silica
flow
conditioners and stabilizing agents known in the pharmaceutical arts can be
used in the formulation and tableting of the micronutrient formulation of the
present invention (see, e.q. Remington: The Science and Practice of
Pharmacy, 20th Edition, 2000, Lippincott Williams & Wilkins; Kibbe: Handbook
of Pharmaceutical Excipients, 3rd Edition, 2000, American Pharmaceutical
Association). As used herein, pharmaceutically acceptable is any agent
suitable for use in humans without undue side effects, such as irritation,
toxicity, or allergic response.
CA 02478278 2004-08-20
19
Example I
[0072] The following is an example of a morning dosage unit core
formulation:
Table 1: Core ingredients:
Item Ingredient Label mg/Tab.
# Claim
1. Beta Carotene 2700 IU
2. Vitamin E 30 IU
3. Vitamin C 120mg
4. Vitamin B1 3mg
5. Vitamin B2 3.4mg
6. Vitamin B3 20mg
7. Vitamin Bs 10mg
8. Pantothenic Acid 5mg
9. Magnesium 50mg
10. Iodine 0.15mg
11. Iron 35mg
12. Copper 2mg
13. Zinc 15mg
14. Cross carmellose 35
Sodium
15. Sodium Lauryl 3.5
Sulphate
16. Microcrystalline 180
Cellulose PH102
17. Starch 1500 55
18. Magnesium 3.5
Stearate
[0073] The following is an example of an evening dosage unit core
formulation:
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
Table 2: Core ingredients:
Item # Ingredient Label Claim Mg/Tab.
1. Vitamin D3 2501U
2. Calcium 300mg
3. Vitamin B12 12mcg
4. Folic Acid 1.1 mg
5. Cross carmellose 30
Sodium
6. Sodium Lauryl 3
Sulfate
7. Magnesium 3
Stearate
Dispensing Kit
[0074] Referring now to Figure 1, the product of the present invention may be
conveniently marketed as a dispensing kit containing distinct dosage units
grouped by type. Blister packs [10] of a week's worth of the supplement of the
present invention having an array [12] of a first type of dosage unit to be
taken
at a given time of day and an array [14] of a second type of dosage unit to be
taken at another time of day. Conveniently, 5 blister packs can be grouped in
a
box (not shown) for sale as monthly dosage packs. Advantageously, the
package of dosage units will contain a 30 day supply, as four 7-day blister
packs and one 2-day blister pack.
[0075] In the case of pregnant women or those wishing to become
pregnant, Ideally, a woman expecting to become pregnant may start taking the
multivitamin and mineral supplement of the present invention at least three
months prior to pregnancy, thereafter during the entire pregnancy and during a
postpartum period of at least three months.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
21
[0076] Still referring to Figure 1, the blister pack includes graphical
means [16] and [18] permitting a patient to differentiate between the morning
and evening dosage types. These means may be, for example, a color code or
diagrams surrounding a particular array of dosage units of the same type be it
morning or evening.
[0077] Another benefit of the blister pack is that micronutrient
supplements often have an unpleasant odor. By providing a "blister pack", each
tablet is confined to its individual blister, significantly reducing odor
emanations.
[0078] The cores of the formulations of the present invention are
preferably coated to achieve a chosen wear resistance, aesthetic appearance,
external finish or dissolution profile. Enteric, seal or color coats can be
used.
This may be accomplished by tablet coating procedures well known to those
skilled in the pharmaceutical arts, such as for example pan coating or spray
coating.
[0079] In a most preferred embodiment, the morning dosage unit of
the present invention is provided with a sprayed-on Opadry PinkTM coating and
polished with carnauba wax to avoid sticking. Still in a most preferred
embodiment the evening dosage unit is provided with a sprayed-on Opadry
Blue TM coating and also polished with carnauba wax.
[0080] It is to be understood that although a preferred embodiment
of the invention is in the form of oral tablets, other dosage units and routes
of
administration could be used such as sublingual, rectal, intravenous, topical,
etc.
E:\Temp\-2451315.doc 08/20/20
04
CA 02478278 2004-08-20
22
[0081] Although the present invention has been described
hereinabove by way of preferred embodiments thereof, it can be modified
without departing from the spirit and nature of the subject invention as
defined
in the appended claims.
E:\Temp\-2451315.doc 08/20/20
04