Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
-1-
ION EXCHANGE COMPOSITE MATERIAL BASED ON PROTON CONDUCTIVE
SILICA PARTICLES DISPERSED IN A POLYMER .MATRIX
TECHNICAL FIELD
The present invention relates to a composite material based on proton
conductive silica particles dispersed in a polymer matrix. The present
invention also
relates to a method for producing the above composite material, and forming
membranes therewith, that can for example be used for electrochemical devices,
particularly for proton exchange membranes in fuel cells, as
drying/humidifying
membranes, for gas or solvent conditioning, or as acid catalysis membranes.
BACKGROUND ART
Ion exchange materials have numerous uses in several technological fields
such as in electrochemical devices, for environmental needs, and in chemical
reactions. Among ion exchange materials, proton conductive materials are under
considerable studies because of the growing interest in clean power generation
for
which polymer electrolyte membrane fuel cells (PEMFC) are one of its important
representatives.
The proton conductivity of a material can be obtained, for example, by
incorporating proton exchange groups in the chemical structure of the
material. The
sulfonic acid function is one of the most efficient proton exchange group,
however
carboxylic or phosphonic acid groups or the like can also be used for proton
mobility.
Many developments on perfluorinated or partially fluorinated polymers or
copolymers bearing sulfonic .acid groups have taken place. This fariiily of
materials
can be found in the market under the commercial names of, for example, Nafion~
(Du Pont de Nemours and Co.) [US 3,282,875 ; US 4,330,654], Aciplex~ (Asahi
Chemical Industry), FlemionT"" (Asahi Glass KK) or Gore-Select~ (W.L. Gore)
[US
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
C
5,635,041 ; US 5,547,551 ; US 5,599,614]. A phase separation between the
hydrophilic acid regions and the hydrophobic fluorocarbon regions occurs and
seems
to contribute to the good proton conductivity in the material [T.D. Gierke,
G.E. Munn,
F.C. Wilson, J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687 ; M. Fujimura, T.
Hashimoto, H. Kawai, Macromolecules, 1981, 14, 1309]. Unfortunately, at high
temperatures (close to 100°C), water management becomes problematic,
mainly
because of the hydrophobicity of the fluorinated backbone of the material that
causes
a rapid dehydration of the membrane.
By comparison, non fluorinated but sulfonated polymers can also present good
proton conductivity with less critical dehydration effects. A strong chemical
structure,
preferably an aromatic based structure, is essential to give the material a
good
stability at high temperatures. Interesting properties for fuel cell
applications have
already been demonstrated for polymers based on, for example, poly(aromatic
ether
ketone)s ([US 6,355,149]), poly(aromatic ether sulfone) or polyphenylene ([US
5,403,675]).
To reduce dimensional variations between the wet and dry states of the
material and to enhance its water retention, some inorganic fillers can be
added to
the sulfonated polymer. In that case, proton conductivity is ensured by the
organic
phase while the inorganic phase helps retaining water and reduces material
expansion ["Proceedings of 1998 Fuel Cell Seminar", November 16-19, Palm
Spring,
California].
The combination of the advantageous properties of the inorganic and organic
phases is encountered in numerous developments of composite material dealing
with
the formation of a stable continuous proton conductive phase. In these
developments, alkoxysilane derivatives are polymerized via sol-gel or co-
condensation processes to lead mainly to three-dimensionally cross-linked
silicon-
oxygen based structures ([EP 1223632A2], [EP 056089981], [US 6,277,304]). Such
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
C
-3-
kind of composite materials are promising but the control of their
preparations is not
easy and is often difficult to achieve. Moreover, such kind of structure does
not easily
offer some ion exchange capacity. Simpler composite preparations can present
interesting solutions for the challenges of electrochemical devices, such as
fuel cell
membranes.
Japanese Patent Application PH 11-336986 published on June 8, 2001 under
Publication Number P2001-155744 and filed in the name of Toyota Central R & D
Labs. Inc. describes a proton conductor based on a high molecular weight
electrolyte
comprising functionalized silica. Silica functionalized with sulfonic acid,
carboxylic
acid and phosphonic acid groups are mentioned. With respect to the
electrolyte, the
description is restricted to perfluoro sulfonic acid type polymers, styrene
divinyl
benzene sulfonic acid type polymers and styrene - ethylene - butadiene -
styrene
copolymers. In a specific example using sulfonated silica and a perfluoro
sulfonic
acid polymer, the membrane obtained has a current density of 0.5 volt at 1
A/cm2,
which is not satisfactory. No data is available on the current density of the
membrane
obtained in the only other example. It has to be presumed that it is
substantially the
same or inferior to that of the membrane of example 1. There is therefore a
need to
provide an improved membrane in which the current density will give
satisfaction.
Canadian Application No. 2,292,703 published on June 8, 2000 and filed in
the name of Universite Laval, discloses an electrolytic membrane made of a
polymer
matrix and a filler material that contributes to the enhancement of the proton
. conductivity of the membrane. In all the examples, the polymer matrix is
based on an
aromatic polyether ketone (PEEK) or a sulfonated derivative thereof (SPEEK)
while
the filler is BP04 or a heteropolyacid. This composite will not be time
resistant
because of the progressive solubilization of the filler into the polymer
matrix.
There therefore exists a need for a composite material based on an inorganic
phase dispersed in a polymer matrix that has a good proton exchange capacity,
that
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
C
-4-
can give membranes with excellent current density, that is time resistant, and
that
can be easily prepared.
SUMMARY OF THE INVENTION
It is an object of the present invention to overcome the problems mentioned
previously.
It is another object of the invention to provide an ion exchange composite
material that presents a relevant proton exchange capacity.
It is another object of the invention to provide a method for producing an ion
exchange composite material in a membrane form that can be easily prepared.
It is another object of the present invention to provide a composite material
adapted to form a membrane with good current density.
The above and other objects of the present invention may be achieved by
providing a
composite material comprising
acid functionali~ed silica particles,
the balance comprising a polymer based on poly(aromatic ether ketones), or
poly (benzoyl phenylene), or derivatives thereof,
the composite material being capable of providing a membrane with a current
density of at least about 1 A/cm2 under 0.6V.
The composite material may be used in membrane form.
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
C
-5-
DISCLOSURE OF INVENTION
In the composite material according to the invention, the silica particles are
preferably functionalized with sulfonic, carboxylic and/or phosphonic acid
groups,
sulfonic acid groups being preferred.
According to a preferred embodiment, the composite material of the invention
normally comprises at least about 10 weight percent, preferably 20 weight
percent of
functionalized silica particles.
The polymer used for constitute the polymer matrix may be acid
functionalized, for example with sulfonic, carboxylic and/or phosphonic acid
groups,
or derivatives thereof.
The acid groups may be covalently bonded to the silica particles and/or to the
polymer, for example through linear or ramified alkyl chains, linear or
ramified
aromatic chains, or a combination of alkyl and aromatic chains that are linear
or
ramified with a linear or ramified alkyl or aromatic chains, the chains
optionally
comprising heteroatoms and/or halogen atoms.
In the composite material according to the invention, the silica particles are
preferably characterized by:
i. a surface area of 10 m2 per gram to 1500 m2 per gram,
ii. silica particle dimension from 0,01 ~,m to 500 p,m,
iii. silica pore diameter from 0 angstrom to 500 angstroms.
Ion exchange groups are usually present in the silica particles in amounts
between 0.1 and 5.0 mmol/g.
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
C
-6-
The acid groups are normally present in the polymer in amounts varying
between 0 mmol/g and 5.0 mmol/g.
The membrane according to the invention are preferably intended for use in
fuel cells, for humidifying or drying, in conditioning gas or solvent, or as
an acid
catalytic membrane.
The composite material can be easily prepared in a membrane form usable for
electrochemical devices like proton exchange membranes for fuel cells,
humidifying
or drying membranes for gas or solvent conditioning, and acid catalytic
membrane.
The silica particles are functionalized with acid moieties and, when dispersed
inside the polymer matrix, they constitute an inorganic hydrophilic phase with
a
proton exchange capacity. The organic phase comprising the polymer matrix may
contain ion exchange groups that are initially present in the chemical
structure of the
polymer, or ion exchange groups bonded to the chemical structure of the
polymer to
enhance the proton conductivity of the composite material. The proton exchange
capacity is achieved by both the functionalized polymer matrix and the
dispersed
silica particles.
Several functional groups are appropriate to give the material a proton
exchange capacity. Preferred functionalities are acid groups, more preferably
sulfonic
groups (-S03H). Other acid groups can also be grafted to the structures to
give an
interesting proton conductivity such as carboxylic (-C02H) or phosphonic (-
P03H2)
acid groups.
The ion exchange groups are preferably covalently bonded to the chemical
structures of the organic and the inorganic phases. The chemical bonds are
preferably made of alkyl or aromatic chains or a combination of both, linear
or
ramified, and can contain eventually some heteroatoms or halogen atoms.
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
As mentioned above, various kinds of silica can be used for the formation of
the inorganic phase in the composite material. Preferred silica is porous
silica,
however other types may be used including but not limited to: amorphous
silica,
fumed silica, spherical silica, irregular silica, structured silica, molecular
sieve silica,
silesquioxane derivatives, and mixture thereof. The amount of silica particles
and
their average size play important roles in the formation of a continuous
hydrophilic
phase and in the mechanical properties of the material.
In the family of poly(aromatic ether ketones), the preferred polymer is the
poly(oxy-1,4-phenylene-oxy-1,4-phenylene-carbonyl-1,4-phenylene) (PEEK)
manufactured by Victrex (UK) and having the following formula:
0
o ~ o ~ c
~n
The glass transition temperature of PEEK is typically about 200 °C, and
it has
the required thermal and chemical resistance to lead to a strong composite.
Sulfonation is a common way to modify a polymer structure by grafting sulfonic
acid groups that give the sulfonated material proton exchange capacity. The
capacity
of proton mobility depends on the amount and on the dispersion of the acid
groups in
the material.
Several studies are currently available on the sulfonation of this kind of
structures. One of the most suitable sulfonation methods for use in the
present
invention is with a sulfonation in concentrated H2S04, as described in EP 8895
and
later in Br. Polym. J., vol. 17, 1985, p. 4. This sulfonation reaction is less
damageable
for the polymer than the chlorosulfonation route because no significant chain
scission
or degradation occurs. Once the polymer is sulfonated, the corresponding
formula for
the sulfonated PEEK is typically:
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
Q
_g_
O
O ~ 0 ~ C ~ O
S03H ~x ~ n-x
The degree of sulfonation corresponds to x/n, with x corresponding to the
number of repeat units carrying one sulfonic acid group. Then, PEEK with 100%
sulfonation has one acid group per repeat unit, or one acid group per three
aromatic
rings. The number of sulfonic acid groups per gram of sulfonated polymer
determines
the ion exchange capacity (IEC) of the polymer. For example, a 100% sulfonated
PEEK has an IEC of 2,9 mmol/g.
The amount of sulfonic acid groups bonded to the aromatic rings depends on
several parameters such as temperature, time, concentration of polymer in the
acid.
Many properties of the sulfonated PEEK (SPEEK) such as its proton capacity,
solubility, water retention, and expansion coefficients vary with its
sulfonation rate,
i.e. with its ion exchange capacity
For the inorganic phase, the use of silica functionalized with sulfonic acid
groups presents not only the advantage of the proton conductivity, but also a
better
efficiency in water retention than the non functionalized silica. Typically,
the water
retention of acid silica is twice higher than usual silica. For example, the
water
retention of acid silica is about 30% instead of 15% with usual silica in an
environment under 70% of relative humidity.
The structure of silica also plays an important role in water retention. For
example, a low bulk density structure increases the water retention in
comparison to
a high bulk density silica mainly because of its higher specific area.
Typically, a low
bulk density structure can take twice more water than a high bulk density
structure.
For example, the water retention of silica with a low bulk density structure
is about
15% comparatively to 7% for silica with a high bulk density structure under
70% of
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
C
-9-
relative humidity. Moreover, a large surface area, as encountered in a low
bulk
density structure, improves the loading of the acid functionality in the
inorganic
compound. For example, the loading of a functionalized low bulk density silica
is
typically 1,7 mmol/g while it is typically twice less with only 0.9 mmol/g for
a porous
high bulk density silica.
Low bulk density sulfonic acid silica can be typically prepared via a co-
condensation process as described, for example, in Chem. Mater. 2000, Vol. 12,
p.2448. Sulfonic acid groups can also be grafted on high bulk density silica
using, for
example, the method described in J. Chromato. 1976, Vo1.117, p.269. Several
types
of bonding are possible to link the sulfonic acid groups to the silica
particles. In the
present invention, preferred but not limited bonding deals with a propylphenyl
chain.
The link may also comprise any kinds of alkyl derivatives or aromatic
derivatives and
combination thereof, with or without heteroatoms and/or halogens in the
chemical
structure.
The composite material is prepared by adding the acid silica particles into
the
polymer matrix and mixing both homogenously. A preferred method proceeds via a
polymer solution in which the silica particles or a silica suspension in the
same
solvent or in a miscible solvent of the polymer solution are added. The
suspension is
then homogenized before being spread in a uniform thin layer and dried.
Satisfying
mixture may also be obtained without using a solvent such as a melting phase
based
process.
The mechanical properties of the composite material depend mainly on the
ones of the polymer matrix and on the silica content. Mechanical properties
determine the lower limit of a film thickness that can be manipulated without
breaking. A polymer that is too rigid does not allow enough deformation of a
thin film
without breaking while structures that are too flexible do not hold the
composite
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
-10-
material in a thin film form. In the same way, too many inorganic particles
prevent a
good tear resistance and make,the film particularly brittle.
The solubility properties of the composite material depend particularly on the
ones of the polymer matrix. As previously mentioned, the solubility of the
polymer
depends on the temperature and on its ion exchange capacity. The maximum
temperature at which the material may be used in a particular liquid such as
water for
the hydrated state is directly related to the solubility properties of the
polymer.
Sufficient silica in the composite material, that may vary between 10 to 30
weight
percent enhances proton conductivity to a degree that depends on the density
of the
corresponding silica used.
In the present invention, many parameters can easily be changed to adjust the
properties of the final composite. Typically, the following parameters have to
be
considered for the formulation of the composite material: solvent solubility,
utilization
temperature, thickness of the material in final form, and the expected ion
exchange
value. The corresponding sulfonation rate of the polymer matrix is then
determined.
The characteristics of the silica are subsequently evaluated considering
mainly the
porosity needs depending on the desired acid loading and water retention.
BRIEF DESCRIPTION OF DRAWINGS
The invention is illustrated by means of the annexed drawings in which
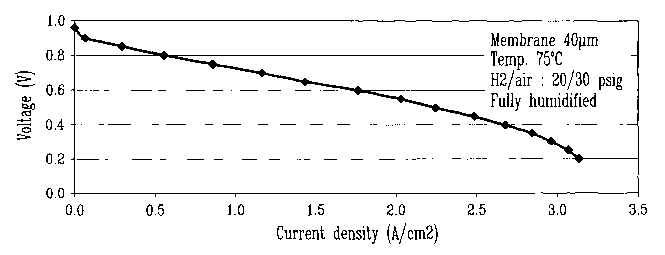
FIGURE 1 is a polarization curve of current density versus voltage of a
membrane according to the invention.
The invention is also illustrated by means of the following non limiting
examples.
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
-11-
EXAMPLE 1
Sulfonation of PEEK
SPEEK with 55% of sulfonation is obtained, for example, by stirring 50g of
PEEK in 2 I of H2S04 (95-98% in H20) for 48 hours at room temperature. The
solution is poured in H20 and the solid phase, corresponding to sulfonated
PEEK
(SPEEK), is washed vigorously 2 to 3 times in 5 I of pure water. The isolated
solid is
firstly dried in an oven at about 70 °C for one night and then, after
another washing, it
is dried at 100 °C under vacuum for several days. About 40 g of SPEEK
is obtained
(yield ~ 80%). Elementary analysis gives the sulfur content of the sulfonated
polymer
and the corresponding ion exchange capacity (IEC) is then calculated. An IEC
of
1,6~0,1 mmol/g is obtained, corresponding to a sulfonation rate of about 55%.
EXAMPLE 2
Composite film preparation
a) 1 g of 55% sulfonated PEEK (SPEEK55) is solubilized in 10 ml of
dimethylformamide (DMF) at room temperature and filtered on filter paper. A
suspension of 0.2707 g of sulfonic acid grafted silica in 2 ml of DMF is added
to the
clear polymer solution. After stirring, the homogenous mixture is spread out
over a
385 cm2 glass substrate before being dried at 70 °C for several days.
After the
complete evaporation of the solvent, the film is easily removed from the glass
substrate by immersion in water. Once dried, the thickness of the composite
film,
made of 80% in weight of a 55% sulfonated PEEK and of 20% in weight of acid
silica,
is 40~10 wm.
b) 0.1755 g of SPEEK55 is solubilized in 1.7 ml of DMF and filtered. 0.0195
g of sulfonic acid grafted silica is added to the polymer solution. After
homogeneization, the mixture is spread out over a 25 cm2 glass substrate. Once
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
-12-
dried, the composite film, comprising 90% in weight of a 55% sulfonated PEEK
and
10% in weight of acid silica, has a thickness of 50 pm.
~Yennpi ~ ~
Electrode deposition on composite films for fuel cell testing
Commercial Pt/C electrodes (Pt/Vulcan XC-72 from ElectroChem Inc.) are
stuck on composite films by spreading a small amount of SPEEK55 10% DMF
solution (w/v) on the side of the two electrodes that sandwich the membrane.
Assemblies are dried under vacuum at room temperature for one day, under
vacuum
at 60 °C for one night, and at 80 °C for several days.
EXAMPLE 4
Performance comparison
The performance obtained with a membrane according to the present
invention is compared to that obtained with a membrane according to JP 2001-
155744 (example 1 ).
The composite material of the Japanese reference contains an inorganic
phase mixed inside a polymer solution at 5% (w/v). The inorganic phase is
fumed
silica grafted with phenylsilane as coupling agent and is thereafter reacted
with
H2S04 cc. The organic phase is the binding agent of the inorganic phase. In
the
present case, Nafion~, a perFluorinated polymer bearing sulfonic acid groups,
is
used. For the experimentation, the fuel cell is operated at 80 °C under
an H2/air
atmosphere at 22 psig. Under voltage from 0.6 V to 0.7 V, the fuel cell
generates a
current density of 0.5 A/cm2 while under 0.5 V, it generates 1 A/cm2. It will
be noted
that the membrane according to the Japanese reference contains 1 weight
percent
CA 02480345 2004-09-27
WO 03/083985 PCT/CA03/00435
-13-
silica, while the membrane according to the present invention contains 20
weight
percent silica.
The composite material according to the present invention contains an
inorganic phase mixed inside a polymer solution at 10% (w/v). The inorganic
phase
contains silica obtained by co-condensation and fu.nctionalized by
chlorosulfonation.
The organic phase is SPEEK. For the experimentation, the fuel cell is operated
at 75
°C under an H2/air atmosphere at 20/30 psig. Under a voltage of 0.7 V,
the fuel cell
generates a current density of 1 A/cm2, under 0.6 V, it generates 1.7 A/cm2 to
1.8
A/cm2, and under 0.5 V, it generates 2.2 A/cm2 to 2.3 A/cm2.
Under similar operating conditions, the present invention generates a much
higher current density than that of the Japanese patent, as will be seen from
FIGURE
1 wherein the material used is made of 20 weight percent silica containing 1.4
mmol
of sulfonic acid groups per gram and 80 weight percent of SPEEK55 prepared as
in
example 1.
It is understood that the invention is not restricted to the above
embodiments and that many modifications are possible within the scope of the
appended claims.