Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02480787 2004-09-29
DESCRIPTION
WORKED MOLYBDENUM-ALLOY MATERIAL, WHICH IS SUBJECTED TO
NITRIDING, HAVING HIGH CORROSION RESISTANCE, HIGH STRENGTH, AND
HIGH TOUGHNESS AND METHOD FOR MANUFACTURING THE WORKED
MOLYBDENUM-ALLOY MATERIAL
Technical Field
The present invention relates to a worked molybdenum-alloy
material, which is subjected to nitriding, having improved
strength, toughness, and corrosion resistance as a result of a
combination treatment of internal nitriding and external
nitriding, and a method for manufacturing the worked
molybdenum-alloy material subjected to nitriding.
Background Art
Molybdenum (Mo) that has, for example, a high melting
point (about 2600°C), relatively high mechanical strength
superior to other metals having high melting points, a low
thermal expansion coefficient, excellent electrical conduction
and thermal conduction properties, and a high corrosion
resistance to a melted alkali metal and hydrochloric acid, can
- 1 -
CA 02480787 2004-09-29
be applied to, for example, electrodes, components for vessels,
components for semiconductors, components for heat-resistant
structures, and materials for nuclear reactors.
A worked material having a worked structure exhibits high
toughness due to suppressed crack growth. However, in a
material recrystallized by heating (about 1050°C or more),
strength at high temperatures is not satisfactory because a
crack readily grows to cause embrittlement. Therefore, Mo-
Ti(0.5)-Zr(0.08)-C(0.03) (TZM) alloy and Mo-Nb(1.5)-Ti(0.5)-
Zr(0.03)-C(0.03) (TZC) alloy have been developed as molybdenum
alloys having improved strength at high temperatures.
The inventors found that, in a worked refractory-metal-
alloy such as an ultrafine-nitride-containing molybdenum alloy
formed by mufti-step internal nitriding treatment, high
toughness and high strength are achieved by maintaining a
worked structure in at least the surface region of the worked
material (patent document l, non-patent documents 1 to 3).
Molybdenum has excellent properties as described above.
However, molybdenum has no corrosion resistance against
oxidizing acids such as nitric acid and hot concentrated
sulfuric acid. Regarding the improvement of the corrosion
resistance, the inventors developed a highly corrosion-
- 2 -
CA 02480787 2004-09-29
resistant molybdenum-based composite material having a
molybdenum nitride (Mo2N) with a thickness of 0.5 to 10 ~m
produced by nitriding molybdenum and a molybdenum alloy (patent
document 2).
Patent document 1: Japanese Unexamined Patent Application
Publication No. 2001-73060.
Patent document 2: Japanese Unexamined Patent Application
Publication No. 11-286770.
Non-patent document 1: Masahiro Nagae, Jun Takada, Yoshito
Takemoto, Yutaka Hiraoka, and Tetsuo Yoshio. J. Japan Inst.
Metals, 64(2000)747-750.
Non-patent document 2: Masahiro Nagae, Jun Takada, Yoshito
Takemoto, Yutaka Hiraoka, and Tetsuo Yoshio. J. Japan Inst.
Metals, 64(2000)751-754.
Non-patent document 3: Masahiro Nagae, Jun Takada, Yoshito
Takemoto, and Yutaka Hiraoka. Materia Japan, 40(2001)666-667.
Disclosure of Invention
Only the metal tantalum (Ta) is useful as a material for
use in very severe corrosive conditions (for example, a boiling
concentrated sulfuric acid solution). However, tantalum has
low strength, in particular, its strength is low at high
- 3 -
CA 02480787 2004-09-29
temperatures; hence, it is inappropriate for an apparatus and a
structural material which require high strength. The above-
described highly corrosion-resistant molybdenum-based composite
material which is developed as an alternative to tantalum by
the inventors has a disadvantage in that a base material is
recrystallized during the manufacturing process to cause the
embrittlement of the entire material.
Accordingly, it is an object of the present invention to
provide an innovative material, which has properties which
cannot be achieved with conventional materials, i.e., having
satisfactory high corrosion resistance and high strength in
very severe corrosive conditions, for example, a 75o sulfuric
acid (H2S04) aqueous solution (180°C), in addition to high
strength at high temperatures and high toughness at low
temperatures, and to provide a method for effectively
manufacturing the innovative material.
The inventors found that a worked molybdenum-alloy
material having excellent corrosion resistance against
oxidizing acids in addition to high strength and high toughness
was effectively and inexpensively produced by subjecting a
worked molybdenum material to a combination treatment of
internal nitriding and external nitriding.
- 4 -
CA 02480787 2004-09-29
That is, a worked molybdenum-alloy material, subjected to
nitriding, which has high corrosion resistance, high strength,
and high toughness, includes fine nitride particles formed by
subjecting a nitride-forming-metal element dissolved to form a
solid solution in an untreated worked molybdenum-alloy material
to internal nitriding, the fine nitride particles being
dispersed inside the worked molybdenum-alloy material subjected
to nitriding; and a molybdenum nitride layer formed by
subjecting a worked structure or a recovered structure at the
surface of the untreated worked molybdenum-alloy material to
external nitriding, the molybdenum nitride layer being provided
at the surface of the worked molybdenum-alloy material
subjected to nitriding.
In the above-described worked molybdenum-alloy material
subjected to nitriding, the molybdenum nitride layer at the
surface of the worked molybdenum-alloy material subjected to
nitriding is composed of at least any one of ~MoN, ~Mo2N, and
,a-Mo2N .
In the above-described worked molybdenum-alloy material
subjected to nitriding, a layer between the molybdenum nitride
layer and the matrix in the inside of the worked molybdenum-
alloy material subjected to nitriding has a worked structure or
- 5 -
CA 02480787 2004-09-29
recOVered Structure.
In the above-described worked molybdenum-alloy material
subjected to nitriding, the inside of the worked molybdenum-
alloy material subjected to nitriding has a recrystallized
structure.
A method for manufacturing a worked molybdenum-alloy
material subjected to nitriding includes the steps of
subjecting an untreated worked alloy in which at least any one
of titanium, zirconium, hafnium, vanadium, niobium, and
tantalum is dissolved to form a solid solution in a molybdenum
matrix to mufti-step internal nitriding treatment including a
stepwise increase of the treatment temperature, and then
subjecting the worked alloy to external nitriding treatment.
In the method for manufacturing a worked molybdenum-alloy
material subjected to nitriding, the internal nitriding
treatment is performed with a nitrogen gas, and then the
external nitriding treatment is performed with an ammonia gas.
Brief Description of the Drawings
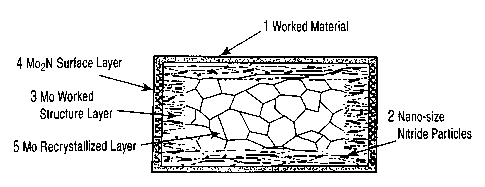
Fig. 1 is a schematic cross-sectional view of a worked
molybdenum-alloy material subjected to nitriding of the present
invention. Fig. 2 is a schematic view showing the structures
- 6 -
CA 02480787 2004-09-29
of a worked material at each step (1) to (3) of the internal
nitriding treatment in a manufacturing process of a worked
molybdenum-alloy material subjected to nitriding. Fig. 3 is a
graph showing the results of a corrosion test of a worked
molybdenum-alloy material, which is subjected to nitriding,
produced in EXAMPLE 1 and EXAMPLE 2 and also showing the result
of a pure molybdenum material in COMPARATIVE EXAMPLE. Fig. 4
shows a photograph (a), which is an alternative to a drawing,
of the cross-sectional structure of a worked molybdenum-alloy
material subjected to nitriding, and also shows a macro
photograph (b), which is an alternative to a drawing, after a
specimen of a worked molybdenum-alloy material subjected to
nitriding was tested by bending. The worked alloy shown in the
photographs (a) and (b) are produced in EXAMPLE 2.
Best Mode for Carrying Out the Invention
Fig. 1 is a schematic view showing an example of the
cross-sectional structure of a worked molybdenum-alloy material
subjected to nitriding of the present invention. The worked
molybdenum-alloy material subjected to nitriding shown in Fig.
1 has a triple-layer structure including a layer having nano-
size nitride particles 2 dispersed in the surface region of a
CA 02480787 2004-09-29
worked alloy material l; a molybdenum nitride (Mo2N) surface
layer 4 produced by subjecting a worked structure or a
recovered structure 3 to external nitriding; and a molybdenum
recrystallized layer 5. When a worked material composed of an
alloy is relatively thin, a worked structure can be completely
maintained through the entire worked material. In this case, a
double layer structure is produced without the molybdenum
recrystallized layer 5.
A worked material is produced by processing, for example,
rolling a dilute alloy which has a matrix composed of
molybdenum and in which at least any one of titanium (Ti),
zirconium (Zr), hafnium (Hf), vanadium (V), niobium (Nb), or
tantalum (Ta) is dissolved to form a solid solution. The term
"dilute alloy" means an alloy in which the content of the
solute elements) in a solid solution alloy is about 5 percent
by weight or less.
A worked molybdenum-alloy material, which is subjected to
nitriding, having high corrosion resistance, high strength, and
high toughness according to the present invention is
manufactured by an internal nitriding treatment including steps
(1) to (3) and an external nitriding treatment (4) described
below. Fig. 2 shows schematic views (1) to (3) illustrating
_ g _
CA 02480787 2004-09-29
the structures of a worked material at each step (1) to (3),
respectively, of the internal nitriding treatment including a
stepwise increase of the heating temperature.
(1) First nitriding step: A worked material is heated in a
nitriding atmosphere between a temperature 200°C lower than the
lower limit temperature of recrystallization and the upper
limit temperature of recrystallization to nitride a nitride-
forming-metal element. As a result, a worked material in which
ultrafine nitride particles are dispersed is formed. In this
first nitriding step, nitrogen is diffused into a worked
dilute-alloy material while maintaining a worked structure Xl
in the worked material. As a result, the nitride-forming-metal
element that is dissolved to form a solid solution in a matrix
is subjected to preferential nitriding to form subnano nitride
particles, which have diameters of about 1 nm to about 2 nm, in
the form of plates, the subnano nitride particles being
dispersed in the matrix. The term "preferential nitriding"
means a phenomenon in which a nitride-forming-metal element
alone is preferentially nitrided but a metal constituting a
matrix is not nitrided. A recrystallization temperature is
increased due to the pinning effect of the particles
precipitated during this nitriding step.
- 9 -
CA 02480787 2004-09-29
(2) Second nitriding step: The worked alloy produced by
the first nitriding step is heated at equal to or more than the
lower limit temperature of recrystallization of the worked
material in a nitriding atmosphere, thus leading to the grain
growth and the stabilization of the ultrafine nitride particles.
The grain growth and the stabilization of the precipitated
particles induced by this second nitriding step further
increase the recrystallization temperature. In nitriding,
recrystallization occurs inside a worked material but a worked
structure X2 still remains. When a worked material is
relatively thin (3 mm or less), a worked structure can be
completely maintained through the entire worked material.
(3) Third nitriding step and steps following the third
step: The worked material produced by the previous steps is
heated in a nitriding atmosphere at equal to or more than the
lower limit temperature of recrystallization of the worked
material, thus leading to the grain growth and the
stabilization of the nitride particles. An object of the third
step and steps following nitriding in the third step is to
further grow and to further stabilize the nitride particles
while retaining a worked structure X3. Bar-shaped nitride
particles having a thickness of about 10 nm and having a length
- 10 -
CA 02480787 2004-09-29
of about 50 nm are uniformly dispersed in the molybdenum matrix.
For example, fourth and fifth nitriding steps after the third
nitriding step can be performed, if necessary.
(4) External nitriding treatment: A molybdenum nitride
layer is formed by a strong nitriding treatment. An ammonia
gas atmosphere, a nitrogen gas atmosphere, a forming gas
atmosphere (the ratio of hydrogen gas to nitrogen gas is 1:9 to
5:5), and an atmosphere produced by subjecting each gas to
plasma discharge, may be used as a nitriding atmosphere.
Molybdenum nitride formed is at least any one of ~MoN, y-Mo2N,
or ~3-Mo2N. The external nitriding treatment is performed such
that a worked structure or a recovered structure remains
between the molybdenum nitride surface layer and the matrix of
the inside of the worked material.
Table 1 shows the relationship between the temperature of
heating treatment and the thickness of the surface layer of a
Mo-Ti-alloy (Ti content: 0.5 percent by weight). The layer
thickness increases with the increase in heating temperature.
It is better to increase the layer thickness in view of
corrosion resistance. However, it was found that toughness
(bending properties) was reduced with the increase in layer
thickness. Therefore, striking a balance between toughness and
- 11 -
CA 02480787 2004-09-29
corrosion resistance requires that the external nitriding
treatment (about 3 mm or less of layer thickness) be performed
at 900°C or less.
(Table 1)
Pure Mo Material subjected (Internal nitriding
to internal up to third step) +
nitriding up to (external nitriding)
third step (2.8 ~,m)
Yield
550 MPa 1190 MPa 1280 MPa
strength
Maximum
750 MPa 1020 MPa 1870 MPa
strength
A worked molybdenum-alloy material subjected to nitriding
of the present invention is useful for, for example, supporting
plates for semiconductors, ceramics, and metals; heaters for
high-temperature furnaces; components for high-temperature
furnaces; structural materials for chemical equipment and
apparatuses used in corrosive atmospheres (including high-
temperature incinerators); and materials for reactors with
supercritical solutions and/or subcritical solutions. In
- 12 -
CA 02480787 2004-09-29
addition, the worked molybdenum-alloy material subjected to
nitriding is also useful for, for example, acid-resistant
vessels and tubes for oxidizing acids such as sulfuric acid and
nitric acid; materials for apparatuses used in very severe
corrosive conditions (for example, a boiling concentrated
sulfuric acid solution); ultra-high-temperature heaters;
injection molds for metals; and injection nozzles for diesel
engines.
EXAMPLES
EXAMPLE 1
A worked Mo-Ti-alloy (Ti content: 1.0 percent by weight)
in the form of a plate having a side of 10 mm and a thickness
of 1 mm was subjected to internal nitriding up to the fourth
step at predetermined heating temperatures in a nitrogen gas
flow (1 atm). The profile of the heating temperature was set
as follows: 900°C ~ 950°C ~ 1200°C ~ 1500°C.
By this mufti-step nitriding treatment, the surface region
of the worked material (up to about 200 ~m in depth from the
surface) maintained a worked structure or a recovered structure
(the inside of the worked material consisted of a
recrystallized structure). In addition, fine titanium nitride
particles were precipitated and dispersed in the surface region.
- 13 -
CA 02480787 2004-09-29
Subsequently, external nitriding treatment was performed at
1000°C for 4 hours in an ammonia (NH3) gas flow (1 atm) to form
a molybdenum nitride (for example, y~Mo2N) layer having a
thickness of 14.0 ~m at the surface of the worked material.
This worked material had a triple layer structure as
follows: The surface of the worked material was composed of a
molybdenum nitride layer. The inside of the molybdenum nitride
layer was composed of a nitride layer of an element which is
dissolved to form a solid solution in a molybdenum matrix of a
worked structure or a recovered structure in which fine
titanium nitride (TiN) particles are precipitated and dispersed.
The inside of the nitride layer is composed of a molybdenum-
alloy layer having a structure with isometric and coarse
recrystallized grain.
Fig. 3 shows the results of a corrosion test in a boiling
75o concentrated sulfuric acid solution at 185°C in order to
evaluate corrosion resistance in severe corrosive conditions.
Fig. 3 also shows the results of pure molybdenum as a reference.
As shown in Fig. 3, the pure molybdenum was heavily corroded
and exhibited a high corrosion rate of 8 mm/year, while the
worked material (EXAMPLE 1) of the present invention was hardly
corroded and exhibited a corrosion rate of 0.076 mm/year. That
- 14 -
CA 02480787 2004-09-29
is, it was found that the worked material of the present
invention exhibited substantially complete corrosion resistance
((corrosion rate)<0.05 mm/year).
EXAMPLE 2
A worked Mo-Ti-alloy material (Ti content: 0.5 percent by
weight) was subjected to internal nitriding up to the third
step at predetermined heating temperatures in a nitrogen gas
flow (1 atm). The profile of the heating temperature was set
as follows: 900°C ~ 1200°C -~ 1500°C. The resulting Mo
alloy
subjected to the internal nitriding up to the third step was
further heated (external nitriding treatment) at 900°C for 4
hours in an ammonia gas flow (1 atm) to uniformly form a
molybdenum nitride (&-MoN, ~Mo2N) layer at the surface of the
worked material. The internal nitrided layer composed of a
worked structure or a recovered structure, in which fine
titanium nitride particles were precipitated and dispersed by
the multi-step nitriding treatment, had a thickness of 310 Vim.
The external nitrided layer composed of molybdenum nitride had
a thickness of 2.8 Vim. An X-ray diffraction pattern showed
that &-MoN and ~Mo2N were formed at the external nitrided
layer.
Fig. 3 shows the results of a corrosion test in a boiling
- 15 -
CA 02480787 2004-09-29
75o concentrated sulfuric acid solution at 185°C. The worked
material of EXAMPLE 2 was hardly corroded and exhibited a
corrosion rate of 0.046 mm/year. That is, the worked material
exhibited complete corrosion resistance ((corrosion rate)<0.05
mm/year) .
Table 2 shows the bending strength at room temperature
(yield strength and maximum strength) of a worked material
subjected to internal nitriding up to the third step (900°C
1200°C -~ 1500°C) and a worked material subjected to external
nitriding treatment (at 900°C for 4 hours) after internal
nitriding up to the third step. Fig. 4 shows a photograph (a)
of the cross-sectional structure and a macro photograph (b) of
a specimen subjected to the bending test.
(Table 2)
1100C 1000C 940C 900C 850C 800C
4 h 4 h 4 h 4 h 4 h 4 h
30 ~m 14.0 ~m 4.7 ~m 2.8 ~m 1.7 ~m 1.1 Nxn
As shown in Table 2, it was found that both yield strength
and maximum strength of the worked material (having a
molybdenum nitride layer thickness of about 2.8 Vim) subjected
- 16 -
CA 02480787 2004-09-29
to external nitriding treatment at 900°C for 4 hours in EXAMPLE
2 represented high stress values at the same level as those of
the material (highly strengthened and highly toughened)
subjected to only internal nitriding up to the third step.
That is, it was proved that a worked molybdenum-alloy
material subjected to nitriding of the present invention had
very high strength in addition to high corrosion resistance.
Industrial Applicability
The present invention provides a worked molybdenum-alloy
material, which is subjected to nitriding, having high strength
and high toughness in addition to high corrosion resistance
against oxidizing acids and thus can be used in the most
extreme corrosive conditions. The worked molybdenum-alloy
material is effectively and inexpensively produced by only
nitriding. The worked molybdenum-alloy material subjected to
nitriding contributes to enabling the practical use of
molybdenum materials in various applications such as materials
for apparatuses used in very severe corrosive conditions (for
example, a boiling concentrated sulfuric acid solution), ultra-
high-temperature heaters, injection molds for metals, and
injection nozzles for diesel engines, as well as various
- 1'7 -
CA 02480787 2004-09-29
applications of conventional molybdenum or molybdenum alloys.
- 18 -