Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02481265 2006-07-28
ROCHE DIAGNOSTICS CORPORATION
CELL FOR ELECTROCHEMICAL ANALYSIS OF A SAMPLE AND METHOD
BACKGROUND OF THE INVENTION
This application is a Divisional of Application No. CA 2,329,563, filed
December 22, 2000.
T he present invention relates to test cells for electrochemical analysis.
Test cells for electrochemical analysis are well known. They have been used to
determine
the concentration of various analytes from biological samples, particularly
from blood.
Cells for electrochemical analysis are described in U.S. Patent Nos.
5,413,690; 5,762,770
and 5,798,031; as well as in International Publication No. W099/13101.
' o An electrochemical biosensor typically includes a sensor strip. The sensor
strip includes a
space that holds the sample to be analyzed, may include reagents to be
released into the
sample, and includes an electrode set. The electrode set normally includes an
insulating
substrate, and electrodes that contact the sample, which have contact pads for
electrically
connecting the electrodes to the electronics of an analysis apparatus.
15 SUMMARY OF THE INVENTION
According to an aspect of the invention, a cell for electrochemical analysis
is provided,
comprising a body having a chamber. and a hair of electrodes onnosinu each
other within
the chamber comprising a metal rod extending through the body transverse to
the
longitudinal direction and removed within the capillary channel. According to
a preferred
?o embodiment, at least one reagent is provided within the capillary channel.
The cell may
be part of a plurality of such cells connected in seriatim.
According to a further aspect of the invention, a method of making a cell for
electrochemical analysis is provided, comprising molding a body with a metal
rod,
forming a capillary channel transverse to the metal rod, and removing the
metal rod from
25 within the capillary channel thereby forming a pair of opposing electrodes.
According to
a preferred embodiment, the method further comprises depositing at least one
reagent
within the capillary channel. According to a further aspect of the invention,
the method
comprises molding a body as a parallel- row of cell bodies with a metal rod
transverse to
the row of cell bodies.
CA 02481265 2006-07-28
la
In accordance with one aspect of the present invention there is provided a
method of making
a cell for electrochemical analysis of a liquid sample comprising: forming a
body of dielectric
material with a rod of electrically conductive material embedded therein;
removing dielectric
material and electrically conductive material to form a chamber within the
body; wherein the
chamber has a size and location such that the rod of electrically conductive
material is
divided by a gap.
In accordance with another aspect of the present invention there is provided a
method of
making a cell for electrochemical analysis of a liquid sample comprising:
forming a cylinder
of a dielectric material with a rod of electrically conductive material
passing through the
cylinder in a direction perpendicular to the longitudinal axis of the
cylinder; removing
dielectric material and electrically conductive material to form a cylindrical
chamber
concentric with the longitudinal axis; wherein the size and location of the
chamber are such
that the rod of electrically conductive material is divided with a gap between
a first portion
that terminates at the inner wall of the chamber on one side of the chamber
and a second
portion that terminates at a inner wall of the chamber on an opposite side of
the chamber.
In accordance with yet another aspect of the present invention there is
provided a method of
making a cell for electrochemical analysis, comprising: molding a body with an
electrically
conductive rod; forming a capillary channel in the body transverse to the
electrically
conductive rod; and, removing the electrically conductive rod from within the
capillary
channel thereby forming a pair of opposing electrodes.
In accordance with a further aspect of the present invention there is provided
a method of
making a cell for electrochemical analysis, comprising: molding a body as a
parallel row of
cell bodies with an electrically conductive rod transverse to the row of cell
bodies; forming a
plurality of parallel capillary channels in the body transverse to the
electrically conductive
rod, one capillary channel for each cell body; and, removing the electrically
conductive rod
from within each capillary channel.
CA 02481265 2004-10-21
2
According to a further aspect of the invention, a method of electrochemically
analyzing a
sample is provided, comprising drawing the sample within a cell for
electrochemical analysis
of the type described above, and applying a difference in electrical potential
across the
electrodes.
Many fluid samples may be analyzed according to the numerous aspects of the
invention. For
example, human body Iluids such as whole blood, blood serum, urine, and
cerebrospinal fluid
may be measured. Also fermentation products and in environmental substances,
which
potentially contain environmental contaminants, may be measured.
BRIEF DESCRIPTION OF THE DRAWINGS
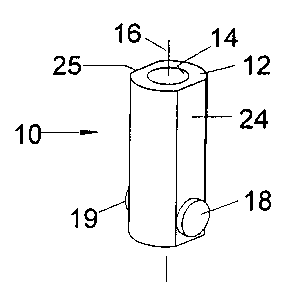
Figure 1 presents a perspective view of an cell for electrochemical analysis
according to an
aspect of the invention.
Figure 2 presents a top plan view of the Figure 1 cell.
Figure 3 presents a side cross-sectional view taken along line :~-3 of Figure
2.
Figure 4 presents a side cross-section view taken along line 4-4 of Figure 2.
Figure 5 presents a perspective view of a body comprising a plurality of cell
bodies, according
to a further aspect of the invention.
Figure 6 presents a perspective cross-sectional view taken along line 6-6 of
Figure 5.
Figure 7 presents the perspective cross-sectional view of Figure 6 with a
reagent deposited
within the cells.
Figure 8 presents a perspective of a cell for electrochemical analysis
according to a further
aspect of the invention.
Figure 9 presents a perspective view of a body comprising a plurality of cell
bodies, according
to a further aspect of the invention.
Figure 10 presents a schematic view of a sample analysis method and apparatus.
Figure 11 presents a top plan view of a rotary clip for use with the cell of
the invention.
Figure 12 presents a side plan view of a linear clip for use with the cell of
the invention.
CA 02481265 2004-10-21
3
Figure 13 presents an end view of a cell according to an aspect of the
invention.
Figure 14 presents an end view of a cell having a chamber with an oblong cross-
section,
according to a further aspect of the invention.
Figure 15 is a side cross-sectional view of a cell having a chamber that is
enlarged at one end,
according to a further aspect of the invention.
Figure 16 is a side cross-sectional view of a cell having a chamber that is
enlarged at one end,
according to a further aspect of the invention.
Figure 17 is an end view of a cell according to a further aspect of the
invention.
Figure 18 is a side cross-sectional view of the Figure 17 cell taken along
line 18-18 of Figure 17.
Figure 19 is an enlarged top pan view of a rod according to an aspect of the
invention.
Figure 20 is a side view of the Figure 19 rod.
Figure 21 is an end view of a cell according to a further aspect of the
invention.
Figure 22 is a side cross-sectional view of the Figure 21 cell taken along
line 22-22 of Figure 21.
DETAILED DESCRIPTION
Various aspects of the invention are presented in Figures 1-22, which are not
drawn to scale
and wherein like components are numbered alike. Referring now to Figures 1-4,
numerous
views of a cell 10 for electrochemical analysis are presented according to an
aspect of the
invention. Figure 1 presents a perspective view of the cell 10, Figure 2
presents a top plan
view, Figure 3 presents a side cross-sectional view taken along line 3-3 of
Figure 2, and Figure
4 presents a side cross-sectional view taken along line 4-4 of Figure 2.
The cell 10 comprises a cell body 12 of dielectric material having a chamber
14 extending in a
longitudinal direction 16. In the example presented in t~igures 1-4, the cell
body 12 is an
annular wall that defines the chamber 14. A pair of electrodes 18 and 19
opposing each other
within the chamber comprise a rod of electrically conductive material
extending through the
cell body 12 transverse to the longitudinal direction 16 and removed within
the chamber 14.
The chamber 14 divides the rod of electrically conductive material, thereby
forming the pair of
opposing electrodes 18 and 19. The rod of electrically conductive material
preferably extends
CA 02481265 2004-10-21
4
in a direction perpendicular to the longitudinal axis 16 of the cell body 12
(particularly if
cylindrical) or the chamber 14. As used herein, the term "perpendicular" is
intended to
indicate an angle on the order of 90°, and is intended to include
moderate deviations from
exactly 90° to the extent that functionality of the electrochemical
cell is not adversely effected.
Some variation is inevitable in a manufacturing process.
The pair of electrodes 18 and 19 penetrate the annular wall of the cell body
12 within the
chamber 14. The metal rod, and hence the electrodes 18 and 19, may be circular
in cross
section (as shown), or square, rectangular, triangular, polygonal, or any
other shape suitable
for an electrode. According to a preferred embodiment, the chamber 14 is a
capillary channel
and extends all the way through the cell body I2.
As best shown in Figure 4, the size and location of the chamber 14 are such
that the rod of
electrically conductive material is divided with a dielectric gap 40 between a
first portion 42
that terminates at the inner wall of the chamber 14 on one side of the chamber
14 and a
second portion 44 that terminates at the inner wall of the chamber 14 on an
opposite side of
the chamber 14. The rod of electrically conductive material~passes from one
side to the other,
but is divided. The gap 40 is presented from another view in Figure 13 as seen
looking into
one of the ends of cell 10. Another view of the gap 40 is presented in Figure
14 wherein the
chamber 14 is oblong transverse to the axis of the rod. According to a
preferred embodiment,
the gap 40 is within the range, inclusive, of 1 micrometer to 3000
micrometers. According to a
more preferred embodiment, the gap 40 is within the range, inclusive, of 5-
1000 micrometers.
According to a particularly preferred embodiment, the gap 40 is on the order
25 micrometers.
Referring again to Figures 1-4, the cell body 12 is preferably injection
molded around the rod,
thus embedding the rod in the cell body 12, and the rod is removed from within
the chamber
14 by, for example, mechanical or laser drilling using machining methods known
in the art
thereby reducing the rod to individual electrodes 18 and 19. The diameter of
the hole drilled
is preferably slightly larger than the diameter of the rod so that the two
electrodes are
separated and electrically insulated from each other, as shown in Figure 4.
The hole drilled
may be circular in cross-section. The chamber 14 may be partially molded, and
the chamber
14 may be fully or partially formed by removing the dielectric material
forming the body 12.
Examples of metals that may be implemented in forming the electrodes 18 and 19
include
aluminum, carbon (such as graphite), cobalt, copper, gallium, gold, indium,
iridium, iron,
lead, magnesium, mercury (as an amalgam), nickel, niobium, osmium, palladium,
platinum,
rhenium, rhodium, selenium, silicon (such as highly doped polycrystalline
silicon), silver,
CA 02481265 2004-10-21
tantalum, tin, titanium, tungsten, uranium, vanadium, zinc, zirconium,
mixtures thereof, and
alloys or metallic compounds of these elements. Preferably, the electrode set
is constructed of
gold, platinum, palladium, iridium, or alloys of these metals, since such
noble metals and their
alloys are unreactive in biological systems. The rod may be a material or a
metal other than a
5 noble metal, for example graphite or copper. In such case, the surface of
the electrodes 18 and
19 within the chamber may be plated with a noble metal after the metal rod is
removed from
within the chamber 14, for example by immersion or electroless plating.
The volume of chamber 14 within the electrochemical cell may be relatively
small, such as 5
microliters or less. Volumes as small as 1 microliter or less are envisioned
in the practice of
the invention. Volume of the chamber 14 may be reduced by reducing the height
of the cell
10 in the longitudinal direction 16, by reducing the diameter of the chamber
14, and/or by
making the chamber oblong, as presented in Figure 14.
Referring now to Figures 15 and 16, the chamber 14 need not have a constant
cross-section
section. For example, it may have a smaller diameter at one end of the body
than the other.
Referring now specifically to Figure 15, a cell 100 having a body 112 and a
chamber 114 is
presented wherein the chamber 114 is 'enlarged on one end by a plurality of
concentric circular
sections 102, each section 102 closer to the end of the cell 100 having a
larger diameter than
the previous one. Referring now specifically to Figure 16; a cell 200 having a
body 212 and a
chamber 214 is presented wherein the chamber 214 is enlarged on one end and
reduces in
diameter with curvilinear sloping sides to the reduced diameter on the other
end. Enlarging
the chamber on one end facilitates applying the sample to the cell
particularly if done
manually.
Referring now to Figure 5, a perspective view of an embodiment is presented
wherein the cell
10 is part of a plurality of cells 10 connected in seriatim. Figure 6 presents
a cross=sectional
view of the cells 10 of Figure 5 taken along line 6-6 of Figure 5. Each
chamber 14 divides the
rod of electrically conductive material. Figure 7 presents a view identical to
Figure 6, except
the cells 10 further comprise at least one reagent 20 within the chamber,l4.
In the example
presented, the at least one reagent 20 is deposited on the cell body 12 within
the chamber 14
and overlying the electrodes 18 and 19. The cells 10 connected in seriatim as
shown in Figures
5-7 may be used in that form, or may be separated into individual cells 10, as
shown in Figures
1-4.
The reagent may be deposited, for example, by dipping the cell into the
reagent in liquid form
to a depth that deposits the reagent at the desired level within the chamber
14. For a capillary
CA 02481265 2004-10-21
6
chamber 14, the reagent may be drawn into the cell body 12 via capillary
action. The reagent
may reach an equilibrium level that may correspond to the desired level within
the chamber
I4. If the desired level is less than the equilibrium level, then the cell 10
is dipped for a period
of time that is less than the time it takes for the reagent to reach the
equilibrium level with the
chamber I4. If the desired level is greater than the equilibrium level, then
the cell 10 is dipped
a greater distance into the reagent.
According to a further aspect of the invention, with reference to Figures 1-4,
a method of
making a cell 10 for electrochemical analysis is provided, comprising molding
a cell body 12
with a metal rod, forming a chamber 14 transverse to the metal rod, removing
the metal rod
from within the chamber 14 thereby forming a pair of opposing electrodes 18
and 19. The
method may further comprise depositing at least one reagent within the chamber
14, for
example, by drawing the reagent into the chamber 14 in liquid form via chamber
action. As
presented in Figures 5-7, the method may further comprise forming a plurality
of parallel
chambers 14 in the cell body 12 and removing the metal rod from within each
chamber 14.
The chamber I4 may be at least partially formed while molding the cell body
12.
According to a further aspect of the invention, with reference to Figures 5-7,
a method of
making a cell 10 for electrochemical analysis is provided, comprising molding
a body 22 as a
parallel row of cell bodies 12 with a metal rod transverse to the row of cell
bodies 12, forming
a plurality of parallel chambers 14 in the body 22 transverse to the metal
rod, one chamber 14
for each the cell body 12, and removing the metal rod from within each chamber
14. The
method may further comprise separating the cell bodies 12, thereby forming
individual cells
10.
The cell body 12 of Figures 1-4 is cylindrical with a pair of opposing planar
sides 24 and 25
aligned with the electrodes 18 and 19 and extending in the longitudinal
direction 16, and the
body 22 of Figures 5-7 is molded as a row of discrete cell bodies I2
interconnected by the
metal rod that forms the electrodes 18 and 19. Referring now to Figure 8
(first drawing sheet),
a cell 10 is presented having cylindrical cell body I2 without the planar
sides 24 and 25. The
cross-sectional shape of cell body 12 may also be square, rectangular,
polygonal, or any other
shape suitable for use in a cell 10. Referring now to Figure 9 (third drawing
sheet), the body
22 may comprise a parallel row of cell bodies having a cylindrical cross-
section. In the
example presented, the body 22 is monolithic. It may be implemented in the
monolithic
form, or planar sides 24 and 25 (Figures I-7) may be formed by machining the
body 22, or the
cell bodies 12 may be otherwise rendered discrete and interconnected by the
metal rod.
CA 02481265 2004-10-21
Referring now to Figure 10, a method of electrochemically analyzing a sample
26, comprising
drawing the sample into a cell 10 for electrochemical analysis and applying a
difference in
electrical potential, indicated as V, across the electrodes 18 and 19. An
electrochemical
reaction commences, particularly where the electrodes 18 and 19 are closest
together, that is
S indicative of a chemical property of the sample. The indication may be in
the form of a
current, an impedance, or other measurement, as is known in the art. The
method may further
comprise suspending at least one reagent in the sample, preferably by
depositing the at least
one reagent being deposited on the cell body 12 within the chamber 14 before
drawing the
sample 26 into the chamber.
Placing the electrodes 18 and 19 close together is advantageous as closer
proximity tends to
decrease the time it takes to make a measurement. The novel manufacturing
method of the
invention creates a pair of opposing fingers in the side wall of the cell I2
that are in very close
proximity, as best shown in Figures 4 and 6. The center of the chamber and the
center of the
metal rod are preferably aligned, and the diameter of the hole drilled through
the metal rod
while forming the electrodes 18 and 19 is preferably slightly larger than the
diameter of the rod
so that the two electrodes are separated and electrically insulated from each
other, but
preferably not larger than needed to reliably and repeatedly separate the
electrodes, taking
manufacturing tolerances and other manufacturing process variations into
account.
An analysis device 28 (shown in phantom) is typically provided to measure
current, impedance,
or other property. The analysis device may be provided with an electrical
connector, and the
electrochemical cell 10 is inserted into the electrical connector in contact
with the electrodes 18
and 19, manually or by an automatic feeding mechanism. The electrochemical
cell 10 may be
used in individual form, as presented in Figures 1-4 and 8; and/or as an
interconnected row as
presented in Figures 5-? and 9 with an appropriate electrical connector that
contacts each set of
electrodes 18 and 19. Examples of measuring apparatus that may be adapted for
use with the
cells of the present invention are disclosed in U.S. Patent Nos. 4,963,814;
4,999,632; 4,999,582;
and 5,243,516, and European Patent Application EP I 042 66? by Beaty et al.
Referring now to Figures 11 and 12, a rotary clip 30 and a linear clip 32 are
presented,
according to a further aspect of the invention, for product packaging of the
electrochemical cell
10. The cells 10 may be stacked horizontally, vertically,. andlor helically
within the clips 30
and 32, The bodies may also be oriented radially or circumferentially in the
rotary clip 30. The
rotary clip 30 may be configured as a carousel. The cells may or may not be
connected in
CA 02481265 2004-10-21
8
seriatim within the clips 30 and 32. The clips 30 and 32 are particularly
desirable for use with an
automatic analysis device 28.
Referring now to Figures 17 and 18, a cell 300 is presented according to a
further aspect of the
invention. Figure 18 is a cross-sectional view taken along line 18-18 of
Figure 17. Cell 300 has a
S body 312, a chamber 314, and electrodes 318 and 319. The chamber 314 is
oblong transverse to
the axis of the rod that forms the electrodes 318 and 319, arid the rod is
removed from within the
chamber 3I4, as previously described herein. Referring now to Figures 19 and
20, a top plan
view and a side elevational view of a rod 320 is presented of the type used to
form,the electrodes
318 and 319. The rod 320 comprises a disk 322 with fingers 324 extending
therefrom on opposite
sides of the disk 322. The rod 320 may be formed, by example, by periodically
stamping disks
322 in a rod of constant cross-section.
Referring now to Figures 21 and 22, a cell 400 is presented according to a
further aspect of the
invention. Cell 400 has a body 412, a chamber 414, and the electrodes 318 and
319. The chamber
414 is oblong transverse to the axis of the rod that forms the electrodes 318
and 319, and the rod
is removed from within the chamber 414, as previously described herein. The
chamber 414 is
enlarged on one end thereby forming a funnel shape. The various features of
the numerous
embodiments presented herein may be used alone, or in combination with one or
more other
features, thus creating innumerable variations all according to aspects of the
invention.
The reagent 20 provides electrochemical probes for specific analytes. The
choice of specific
reagent 20 depends on the specific analyte or analytes to be measured, and are
well known to
those of ordinary skill in the art, An example of a reagent that may be used
in cell 10 of the
present invention is a reagent for measuring glucose from a whole blood
sample. A non-limiting
example of a reagent for measurement of glucose in a human blood sar~nple
contains 62.2 mg
polyethylene oxide (mean molecular weight of 100-900 kilodaltons), 3.3 mg
NATROSOL~
250M, 41.5 mg AVICEL~ RC-591 F, 89.4 rng monobasic potassium phosphate, 157.9
mg
dibasic potassium phosphate, 437.3 mg potassium ferricyanide, 46.0 mg sodium
succinate, 148.0
mg trehalose, 2.6 mg TRITONTM X-100 surfactant, and 2,000 to 9,OOU.units of
enzyme activity
per gram of reagent. The enzyme is prepared as an enzyme solution from 12.5 mg
coenzyme
PQQ and 1.21 million units of the apoenzyme of quinoprotein glucose
dehydrogenase. This
reagent is further described in WO 99/30152.
CA 02481265 2004-10-21
9
When hematocrit is to be determined, the reagent includes oxidized and reduced
forms of a reversible
electroactive compound (potassium hexacyanoferrate (IIl) ("ferricyanide") and
potassium
hexacyanoferrate (II) ("ferrocyanide"), respectively), an electrolyte
(potassium phosphate butter), and
a microcrystalline material (Avicel~ RC-591F - a blend of 88% microcrystalline
cellulose and 12%
sodium carboxymethyl-cellulose, available from FMC Corp.). Concentrations of
the components
within the reagent before drying are as follows: 400 millimolar (mM)
ferrocyanide, 55 mM
ferrocyanide, 400 rnM potassium phosphate, and 2.0% (weight: volume) Avicel~.
A further
description of the-reagent for a hematocrit assay is found in U.S" Patent No.
5,385,846. A hernatocrit
reagent is preferably not deposited on the surface of the electrodes 18 and
19. It may be deposited
1 0 within the chamber 14 at an end opposite to the electrodes 18 and 19.
Other non-limiting examples of enzymes and mediators that may be used in
measuring particular
analytes in sensor 20 of the present invention are listed below in Table 1.
The electrochemical cell of
the invention may have a plurality of reagents deposited on the cell body
within the chamber.
CA 02481265 2004-10-21
TABLE 1
Analyte Enzymes Mediator . Additional Mediator
(Oxidized Forrn)
and Diaphorase
genase Ferri
Cholesterol Oxidase Benzoquinone
2,5-Dichloro-1,4-
Benzoquinone or
Phenazine Ethosulfate
FiDL Cholesterol EsteraseFerricyanide 2,6-Dimethyl-1,4-
Cholesterol and Cholesterol Benzoquinone
Oxidase
2,5-Dichloro-1,4-
Benzoquinone or
Phenazine Ethosulfate
TriglyceridesLipoprotein Lipase,Ferricyanide Phenazine Methosulfate
or
Glycerol Kinase, Phenazine
and
Glycerol-3-PhosphateEthosulfate
Oxidase
Lactate Lactate Oxidase Ferricyanide 2,6-Dichloro-1,4-
Lactate Lactate Dehydrogenase Ferricyanide
and Diaphorase Phenazine
Ethosulfate, or
Phenazine
Methosulfate
Lactate Diaphorase Ferricyanide Phenazine Ethosulfate, or
Dehydro- Phenazine Methosulfate
In some of the examples shown in Table l, at least one additional enzyme is
used as a reaction
catalyst. Also, some of the examples shown in Table 1 may utilize an
additional mediator,
5 which facilitates electron transfer to the oxidized form of the mediator. ~
The additional
mediator may be provided to the reagent in lesser amount than the oxidized
form of the
mediator. While the above assays are described, it is appreciated that a
variety of
electrochemical assays may be conducted with sensor 10 in accordance with this
disclosure.
Bilirubin Bilirubin Oxidase 1-Methoxy-
Phenazine
Methosulfate
Uric Acid Uricase Ferricyanide
CA 02481265 2004-10-21
11
According to a preferred embodiment, the reagents are applied in liquid form
and dried. As
used herein, the term "dry" or "dried" is intended to mean removing water from
the reagent to
the point where it is immobile, chemically stable, and reactive when it comes
in contact with
the sample. The cell of the~present invention may also include microspheres,
as described in
European application EP 1 113 263 entitled "MICROSPHERE CONTAINING SENSOR",
inventors Raghbir Singh Bhullar and Brian S. Hill, based on a priority
document filed December
23, 1999. The microspheres decrease sample size and improve flow of the sample
within the
cell. A reagent may be deposited on the microspheres.
Referring again to Figures 5-7, in one embodiment, the body 12 cells 10 are
formed by
injection molding polycarbonate around a solid gold rod on the order 500
micrometers in
diameter. A suitable rod is available from ENGELHARD - CLAL LP, ofNew Jersey,
U.S.A.
The chamber 14 is mechanically drilled having a diameter o:n the order of 500
micrometers.
The central axis of the drilling operation is aligned with the central axis of
the rod, and
perpendicular thereto. The actual diameter of the rod is typically slightly
less than the nominal
diameter of 500 micrometers. Conversely, the actual diameter of the chamber
drilled is
typically slightly larger than the nominal diameter of 500 micrometers. The
result is that the
rod is divided into to electrodes separated by dielectric gap 40 having a
desirable width. The
cell 10 according to this embodiment has a length in the longitudinal.
direction on the order of
36 millimeters and an outside diameter on the order of 16 millimeters. The
flat faces 24 and 25
are on the order of 14 millimeters apart.
Products made by the methods disclosed herein are also represent further
aspects of the
invention. Although the invention has been described and illustrated with
reference to specific
illustrative embodiments thereof, it is not intended that the invention be
limited to those
illustrative embodiments. Those skilled in the art will recognize that
variations and
modifications can be made without departing from the true scope and spirit of
the invention as
defined by the claims that follow. It is therefore intended to include within
the invention all
such variations arid modifications as fall within the scope of the appended
claims and
equivalents thereof.