Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
Medical Gas Recirculation System
The present invention relates to an apparatus and method for recirculating
at least a binary gas mixture to a medical device such as a cardiac pulmonary
bypass oxygenator or an artificial ventilator.
More particularly the invention relates to an apparatus and method for
controlling the composition, pressure and flow rate of a recirculating gaseous
composition to a medical device, particularly to a cardiopulmonary bypass
oxygenator, and recycling the gaseous composition.
Medical devices- such as cardiopulmonary bypass oxygenators and artificial
ventilators or respirators require a reliable and constant source of gas for
safe and
reliable operation for use during the relevant medical procedures.
Usually, the gaseous compositions used for procedures with such devices
are various air/oxygen or nitrogen/oxygen mixtures, although in some
situations,
these devices may be used for administering other active agents to a patient.
For example, it is common to use a respirator for administering an
anaesthetic agent to anaesthetise a patient prior to undergoing certain
surgical
procedures. Xenon is known for use as an anaesthetic agent.
US-A-4989597 (Werner) discloses an apparatus for administration of at
least two gases, particularly oxygen and xenon, to a patient via a respiration
apparatus comprising a patient circuit and a drive circuit. The patient
circuit,
which enables rebreathing of the gas to make maximal use of valuable gases, is
provided with fresh gas input to replace exhaled carbon dioxide with oxygen
and
to supplement the xenon concentration. The drive circuit and the patient
circuit
are in open communication and the concentration of each of the components in
the patient circuit is independently monitored and controlled by addition of
small
quantities of one or other of the gases. The open communication between the
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-2-
patient circuit and the drive circuit results in an inherent equilibrium which
it is
stated allows the relative concentration of gases in the patient circuit to be
more
controllable. Xenon eventually accumulates in the drive circuit and can be
recovered therefrom by supply to a recovery bottle once a certain
concentration
has been reached.
More recently, xenon has been identified as being useful in the treatment of
neurointoxications, for example in WO-A-0053192. In particular, it is stated
that
xenon can reduce the release of neurotransmitters, particularly dopamine,
which
1o is caused by, for example, hypoxic situations such as an apoplexy or a
craniocerebral trauma. It is stated (on page 5, lines 15-18 of WO-A-0053192)
that
use of the cardiopulmonary bypass machine can cause an unidentified
neurointoxication, which significantly delays a patient's reconvalescence.
According to WO-A-0053192, xenon may be administered by an inhalation
method, or alternatively, may. be added directly to a cardiopulmonary bypass
machine. Further, WO-A-0108692 discloses the use of xenon as an NMDA
antagonist to, for example, provide neuroprotection, relieve neuropathic pain
or
inhibit synaptic plasticity.
Under normal circumstances, cardiopulmonary bypass oxygenators are
supplied with an oxygen/air or oxygen/nitrogen mixture on a once-through basis
after which the spent gas (comprising the remaining oxygen, nitrogen and
carbon
dioxide flushed from the patient's blood) is vented to atmosphere. However,
the
use of xenon, or any other high value gas, in a cardiopulmonary bypass
oxygenator would make this a very expensive procedure.
An apparatus and method for providing and recirculating gas to a medical
device, such as a cardiopulmonary bypass oxygenator or an artificial
ventilator,
which also enables recovery of the high value gas is highly desirable,
particularly
3 o when applied to a medical device used in an environment where space is at
a
premium.
CA 02484066 2011-02-04
-3-
Accordingly, in a first aspect of the invention there is provided an apparatus
providing and circulating to a medical device a medical gas mixture comprising
at
least two components, said apparatus comprising:-
a main gas circuit for recirculating the medical gas and comprising:-
a constant speed cirgulation pump for pumping gas through the main
circuit and increasing the gas pressure from a lower pressure to a highgr
pressure,
a pressure maintaining valve downstream of the pump and dividing the main
circuit into a higher pressure section and a lower pressure section in order
to
maintain a constant pressure in the higher pressure section,
a medical gas outlet in the higher pressure section,
a spent gas inlet in the lower pressure section,
a first feed gas supply inlet, preferably located in the higher pressure
section,
a second feed gas supply inlet, preferably located in the higher
pressure section,
a concentration determining means for measuring the concentration
of at least one component of the recirculating medical gas mixture and
generating a signal indicative of said concentration,
circuit volume regulating means for varying the volume of the main
circuit at a location in the lower pressure section for maintaining a
predetermined gas flow to the pump and generating a signal indicative of
said volume, and
means for venting gas from the main circuit;
a first feed gas supply conduit for supply to the first feed gas inlet of a
first
feed gas of predetermined composition;
first feed gas supply flow control means for controlling the flow of first
feed
gas through the first gas supply conduit in response to the signal from the
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-4-
concentration determining means to maintain constant the medical gas
composition at the pump inlet;
a second feed gas supply conduit for supply to the second feed gas inlet of
a second feed gas of predetermined composition different from the first feed
gas;
second feed gas supply flow control means for controlling the flow of
second feed gas through the second gas supply conduit in response to the
signal
from the circuit volume regulating means to maintain constant the
recirculating
1 o medical gas composition; and
a medical device supply circuit for connecting the medical device to the
main circuit to receive a portion of the medical gas from the medical gas
outlet
thereof and to return spent gas to the spent gas inlet thereof and comprising:
flow control means for controlling flow of the medical gas to the
medical device and
purification means for removing contaminant(s) from the spent gas.
In another aspect, the invention provides a medical device system
comprising a medical device connected to the medical device supply circuit of
an
apparatus of the first aspect supra.
Preferably, the pressure maintaining valve is a spill valve; i.e. a valve
which
opens wider in response to increased pressure to pass more gas into the lower
pressure section and.thereby maintain the pressure in the higher pressure
section. However, the valve could be a conventional pressure reduction valve.
Preferably, the circuit volume regulating means comprises expansion
bellows and the means for generating a signal indicative of the volume thereof
suitable is an infra-red level or, preferably, ultrasonic sensor for detecting
the level
of the expansion bellows in an expandable direction thereof.
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-5-
The apparatus preferably operates at a pressure of up to about 250 mbarg
(125 kPa) through the main circuit, more preferably up to about 150 mbarg (115
kPa) and may provide gas to the medical device at a pressure of up to about
100
mbarg (110 kPa), but preferably about 30 mbarg (103 kPa). The circulation pump
may circulate gas through the circuit at a rate of up to about 80 litres per
minute
(1/min), preferably up to about 30 I/min, more preferably from about 15 to
about 20
I/min and preferably supplies gas to the medical device at a rate of up to
about 30
I/min, preferably up to about 10 I/min and still more preferably up to about 5
I/min.
Each of the first and second fed gas supply flow control means may be, for
example, a valve or, preferably, a mass flow controller (MFC).
The concentration determining means measures the concentration of one
or more individual components of the gas mixture.
If required, the gas concentration determining means and/or the circuit
volume regulating means can provide a respective signal to alert an operator,
for
example, by way of an alarm, to the need to manually adjust the relevant
supply
flow control means.
Communication of the concentration determining means or circuit volume
regulating means with the supply flow control means may be via an analog
electrical circuit, on which the gain may be set as desired. For example, for
control of a supply of a gas, such as oxygen, which is rapidly consumed and/or
urgently required by the medical device, the analog circuit may have a high
gain.
Conversely, for control of a supply of a relatively slowly consumed gas, such
as
xenon, or inert, such as nitrogen, the analog circuit may have a low gain.
When the medical gas mixture is a binary gas mixture, typically the
3 0 concentration of only one component is measured and the corresponding
signal
used to control the feed of that component to the respective feed inlet with
the
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-6-
feed of the other component, or of a predetermined mixture of the two
components, being controlled by the circuit volume regulating means signal.
When the medical gas mixture is a tertiary gas mixture, it is possible to
operate in similar manner to a binary gas mixture using a separate feed for a
first
component and a mixed feed for the other two components optionally also with
an
amount of the first component. More usually, the three components at least
primarily will be provided by three separate feeds. The concentration of two
of the
components can be measured and the individual concentration measurement
1o signals used to control the corresponding respective feeds and the feed of
the
third component controlled by the circuit volume regulating means signal.
Alternatively, the concentration of two of the components can be measured, one
of the individual concentration measurement signals being used to control the
corresponding feed and both the other concentration measurement signal and the
circuit volume regulating means signal being used to control the feeds of the
other
two components.
For example, using a tertiary mixture-of 40% oxygen, 20% xenon and 40%,
inert gas (usually nitrogen), feed control to maintain the gas composition can
be
2o achieved by using an oxygen concentration measurement signal to control the
feed of oxygen and the circuit volume regulating means signal used to control
the
feed of a mixture of xenon, inactive gas and optionally oxygen. However, such
a
system does not allow full control in the presence of, for example air leaks
or
other events that affect only one of xenon and the inert gas and not the
other.
Accordingly, it is preferred to use three input gases, for example, (a)
oxygen, (b)
xenon or a mixture of xenon with a minor proportion of oxygen and (c) nitrogen
or
a mixture of nitrogen with a minor proportion of oxygen, and to control the
flow
rates of these using a tri-gas control system. A tri-gas control system can
compensate not only for oxygen uptake by the medical device, but can also
compensate, without error, for xenon and/or nitrogen uptake or emission
by/from
the device, dead volume filled with gas mixture or air, and leaks of air or
other
gases into or out of the system.
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-7-
The tri-gas control system can be implemented by a straightforward
proportional algorithm driven by two gas concentration signals and the system
volume signal. For example, the oxygen can be added in an amount dependant
on the difference between the measured and a predetermined oxygen
concentrations; the xenon (or xenon/oxygen mixture) added in an amount
dependant on the difference between the measured and a predetermined xenon
concentrations; and the nitrogen (or nitrogen/oxygen mixture) added in an
amount
dependant on the extent to which the volume in the main circuit differs from a
predetermined volume. However, in order to be assured of a stable control
system where the different gas additions to not interact in a deleterious way,
it is
preferred to use both the difference in measured and predetermined xenon
concentrations and between actual and predetermined circuit volumes to control
the addition of both the xenon- and nitrogen- containing feeds. In particular,
the
xenon-containing feed is determined by the function YF, where: F = M' x
(actual
circuit volume - predetermined circuit volume), Y = M" x (actual xenon
percentage
concentration - predetermined xenon percentage concentration) and M' and M"
are constant gain/multiplier factors, and the nitrogen-containing feed is
determined
by the function (A -Y)F, A is the maximum flow signal to the nitrogen-
containing
supply control means. Thus, if the gas supply control means are all MFCs
having
a 5V for I litre/min flow rate, the gain/multiplier factor for oxygen is 250,
M' is 50
and M" is 35, (a) a measured oxygen concentration 2% low relative to the datum
level, would result in the addition of 1 litre/min of oxygen; (b) a circuit
volume 10%
below the datum level would provide an F value of 5; and (c) a measured xenon
concentration 2% low relative to the datum level would provide a Y value of
0.7.
Thus under these conditions the YF signal would be 3.5, resulting in the
addition
of 0.7 litre/min of the xenon-containing gas, and the (A-Y)F signal would be
1.5 (A
= 5), resulting in the addition of 0.3 litre/min of the nitrogen containing
gas.
Preferably the medical device is an artificial ventilator or, especially, a
cardiopulmonary bypass oxygenator. The apparatus of the invention can
selectively supply an artificial ventilator or a cardiopulmonary bypass
oxygenator,
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-8-
whereby a patient can readily be ventilated immediately before and after
cardiopulmonary bypass.
In a third aspect of the invention, there is provided a method of providing a
medical device with a medical gas mixture comprising at least two components,
said method comprising:-
z
recirculating the medical gas mixture in a main circuit having a higher
pressure section maintained at constant pressure in series with a lower
pressure
section;
withdrawing a portion of the medical gas mixture from the higher pressure
section and feeding said portion to the medical device;
removing contaminant(s) from the spent gas mixture from the medical
device and returning the decontaminated spent gas to the lower pressure
section;
replenishing components in the medical gas mixture by addition of feed
gases to maintain the recirculating medical gas composition constant; and
varying the volume of the main gas circuit to maintain the gas flow therein.
Preferably, the method comprises operating a medical device system in
accordance with the second aspect of the present invention.
In a fourth aspect of the invention, there is provided a method for the
extracorporeal treatment of blood by contacting blood with a recirculating
medical
gas mixture in a device provided with the medical gas mixture using the method
of
the third aspect of the invention.
The gaseous composition for use in the present invention preferably
contains at least one high value gas, which it would be beneficial to recover
after
use in the process. Such gases include the noble gases, especially xenon,
krypton and neon or isotopes thereof, or stable isotopes of gases such as
oxygen
3o and carbon dioxide.
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-9-
In a preferred embodiment, the gaseous composition comprises xenon,
preferably in an amount of at least about 10% by volume, more'preferably at
least
about 30%, still more preferably at least about 50% and still more preferably
at
least about 70% by volume. Most preferably, the gaseous composition comprises
xenon in an amount of about 80%..by volume.
The gaseous composition preferably also comprises oxygen and more
preferably consists predominantly of xenon and oxygen. Most preferably, the
gaseous composition comprises xenon and oxygen in a ratio of about 80% to
1o about 20% by volume and usually will consist solely of xenon and oxygen.
The component gases may be replenished individually or in a mixture of
gases, preferably a binary mixture, of known relative proportions.
Optionally, the gaseous composition may also comprise, for example,
helium or nitrogen. Helium may be provide through a further supply flow
conduit
from, for example, a helium cylinder or a cylinder containing a helium/oxygen
mixture. Nitrogen may be provided, for example, by admitting air to the
circuit.
In a preferred embodiment of the invention, the medical device is a
cardiopulmonary bypass oxygenator and the gaseous composition is a mixture
predominantly of oxygen and xenon. Preferably the component gases are
supplied from a first gaseous supply comprising oxygen and a second gaseous
supply comprising xenon, which may be a xenon/oxygen mixture, for example in a
ratio of about 80% to about 20%. Preferably the first gaseous supply is oxygen
and the second gaseous supply is a xenon/oxygen mixture.
When oxygen is relatively, quickly consumed, by a patient connected to the
medical device, the oxygen concentration determining means, which may be, for
example, an oxygen fuel cell sensor, preferably is connected to the first
supply
flow control means by a high gain electronic circuit enabling relatively rapid
replenishment of oxygen to the circuit. For example, every I % difference
CA 02484066 2011-02-04
-10-
between the desired concentration and the detected concentration of oxygen may
correspond to a flow through the oxygen (first) supply conduit of I litre per
minute
(1/min). Conversely, for controlling the concentration of xenon, which is
relatively
slowly consumed by a patient connected to the medical device, a low gain
response may be more appropriate.
it is pref7rred that the concentration of xenon in a recirculating binary
mixture with oxygen is determined with an ultrasonic gas analyser. Preferably,
the
ultrasonic gas analyser has an ultrahigh frequency ultrasonic transmitter, for
1o example greater than 100 kHz. A suitable ultrasonic gas analyser is that
described in Canadian Patent Application 2,483,964 published November
13, 2003.
The ultrasonic gas analyser may be used in combination with monitoring
the recirculating volume to provide other information such as the
concentration of
contaminants In the circuit. .
Similarly, comparison of the measured concentration of oxygen and xenon,.
in the recirculating gas, may provide information on the concentration of
contaminants such as nitrogen or carbon dioxide.
When xenon or other high value gases are used, it is preferable to direct
spent or recirculating gas that may from time to time be vented into a gas
recovery space. Where the high value gas is provided from a supply in a fresh
gas space in a container having an ullage space, the ullage space may provide
the gas recovery space. Such a container can be as described in Canadian
Patent
Application 2,483,965 published November 13, 2003.
CA 02484066 2011-02-04
-11-
One or more of a carbon dioxide absorber, a carbon dioxide analyser and a
pressure relief device can be provided downstream from the medical device when
carbon dioxide is a waste product from that device.
The following is a description by way of example only and with reference to
the accompanying drawings of presently preferred embodiments of the invention.
In the drawings:
Figure 1 is a diagramatical representation of an apparatus according to one
embodiment of the present invention for providing a xenon/oxygen mixture to a
1o cardiopulmonary bypass oxygenator;
Figure 2 is a diagramatical representation of a ventilator circuit for
introduction into the apparatus of Figure 1 to replace the cardiopulmonary
bypass
oxygenator;
Figure 3 is a diagramatical representation of another ventilator circuit for
introduction into the apparatus of Figure 1 to replace the cardiopulmonary
bypass
oxygenator; and
Figure 4 is a diagramatical representation of an apparatus according-to
another embodiment of the present invention for selectively providing a
xenon/oxygen mixture to a cardiopulmonary bypass oxygenator and an artificial
ventilator.
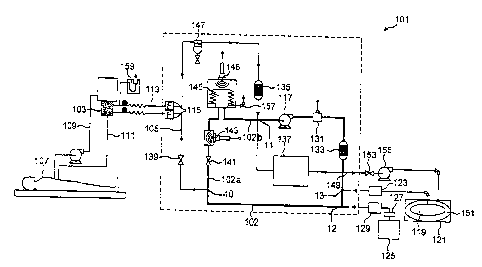
With reference to Figure 1, xenon/oxygen mixture in a ratio of 80% xenon to
20% oxygen is fed at inlet 13 into the main circuit 102 (a + b) of the
apparatus
(generally designated 101) from a xenon/oxygen supply in fresh gas space 119
of
container 121 via xenon mass flow controller (MFC) 123.
The oxygen content of main circuit 102 is topped up at inlet 12 from oxygen
cylinder 125 via regulator 127 and oxygen mass flow controller (MFC) 129.
One or more (preferably four) diaphragm pumps 117 pump the xenon/oxygen
mixture around the circuit 102 at a rate of up to 20 litres per minute (1/min)
at a
pressure of up to 150 millibar gauge (115 kPa).
CA 02484066 2011-02-04
-12-
The gaseous composition is fed from outlet 10 to cardiopulmonary bypass
(CPB) oxygenator 103 via medical device supply conduit 105, which is regulated
by
1 flow control valve 139, which may be set at a desired level by the operator.
CPB oxygenator 103, which is typically a membrane oxygenator, is fed ,
unoxygenated blood from a patient 107 via unoxygenated blood conduit 109 and
returned to the patient 107 via oxygenated blood conduit 111. Spent gas from
the
CPB oxygenator 103 is fed through spent gas return conduit 113 and then
through
lo water trap 147 and primary carbon dioxide absorber 135 to return to the
main
circuit section 102b upstream of pump(s) 117.
Gas passing through the spent gas return conduit 113 and medical device
supply conduit 105 pass through respective bacterial filters 115 to protect
the
patient 107 from contamination from the apparatus 101 and vice versa.
In order to ensure that a constant flow of gas at the set pressure is supplied
to the oxygenator 103 and thus available to the patient's blood, gas
circulates
through the main circuit 102 via pressure maintaining valve 141 downstream
from
the outlet to medical device supply conduit 105. Pressure maintaining valve
141
is a valve which allows gas flow only when the pressure exceeds a
predetermined
level, for example 30 mbarg (103 kPa) and accordingly maintains a constant
pressure between the pumps 117 and the valve 141.
Downstream from the pressure maintaining valve 141, the gaseous
composition is analysed for xenon content using ultrasonic xenon analyser 143
of
the kind described in Canadian Patent Application 2,483,964 published November
13, 2003. In an alternative arrangement (not shown) the xenon analyser is
located
upstream of the pressure maintaining valve 141.
CA 02484066 2011-02-04
-13-
The gas is then fed via bellows 145, which expand to take up any additional
volume of gas in the apparatus or contract to compensate for loss of volume in
the
apparatus, and receives the spent gas upstream of pump(s) 117.
The oxygen concentration in the main circuit 102 is monitored by an oxygen
fuel cell sensor 131 that is shown situated in the main circuit section 102a
downstream from pump(s) 117 but could be located downstream of the pressure
maintenance valve 141. The gas is then fed through backup carbon dioxide
absorber 133, which removes residual carbon dioxide from the recirculating
gas.
The carbon dioxide removed by absorbers 133 and 135 has entered via the
oxygenator 103 after being flushed from the patient's blood. At least absorber
135
should be replaced with each use of the system.
Downstream from the backup carbon dioxide absorber 133, a small sample
of gas is drawn from the main circuit 102 and fed to analyser unit 137 to be
analysed for carbon dioxide, via an infra red gas analyser, to ensure that the
carbon dioxide absorbers are working efficiently and for oxygen, via a
paramagnetic gas analyser, as a backup to the oxygen fuel cell sensor 131. The
sample is returned to the main circuit section 102b upstream from the pump(s)
117.
Recovery gas conduit 149 selectively feeds at least a portion of gas from
the main circuit 102 at a point downstream from the backup carbon dioxide
absorber 133 to the ullage space 151 of container 121, via recovery valve 153
and
compressor 155. This container 121 is of the kind described in Canadian
Patent Application 2,483,965 published November 13, 2003.
An atmospheric vent 157 from bellows 145 enables the gas within the
3o apparatus to be vented to atmosphere if desired.
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-14-
There is a U-tube relief device 159 on the spent gas return conduit 113 to
protect the oxygenator 103 and patient 107 in the event of any back pressure
from
the apparatus 101.
Addition of fresh gas to the apparatus is controlled by an analog electronic
circuit (not shown) between oxygen fuel cell sensor 131 and oxygen MFC 129 for
fresh oxygen addition and by an analog electronic circuit between an
ultrasonic
level sensor 146 measuring the position of the bellows and the xenon MFC 123
for fresh xenon/oxygen mixture addition.
As well as monitoring the concentration of oxygen in the main circuit 102,
oxygen fuel cell sensor 131 enables the oxygen concentration to be controlled.
The operator may choose a set point on the sensor 131 corresponding to the
desired oxygen concentration. When oxygen concentration measured by sensor
131 falls below the set point, oxygen MFC 129 is triggered to feed fresh
oxygen
into the main circuit 102 at a rate proportional to the difference between the
oxygen level set point and the oxygen sensor 131 measurement via a high gain
circuit connecting oxygen MFC 129 to sensor 131.
Typically, the high gain oxygen control circuit (not shown) will have a gain
of 1, corresponding to an oxygen flow rate through oxygen MFC 129 and into the
main circuit 102 of 1 I/min for every I% difference between the oxygen set
point
and the measured oxygen level.
The xenon concentration of the main circuit is controlled by ultrasonic
bellows level sensor 146. The operator may set the desired level on a
potentiometer (not shown) connected to sensor 146, which corresponds to an
expanded level of the bellows 145. This level corresponds to the volume in the
system and, given that the oxygen concentration is known, to a desired
concentration of xenon. When the sensor 146 detects that the bellows 145 has
fallen below the desired level, xenon MFC 123 is triggered to feed fresh
oxygen/xenon mixture into the main circuit 102 at a rate proportional to the
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-15-
difference between the potentiometer set point and the level measured by
bellows
sensor 146, via a low gain circuit (not shown) connecting sensor 146 to xenon
MFC 123.
Typically, the xenon low gain circuit will have a gain of 0.1, corresponding
to a flow of fresh xenon/oxygen mixture into the main circuit 102 of 0.1 I/min
for
every 1 % difference between the potentiometer setpoint and the level measured
by bellows sensor 146.
The various sensor readings and flow rates are displayed on a monitoring
unit (not shown).
In use, oxygen is consumed and replaced by carbon dioxide via the CPB
oxygenator 103. The operator may select the flow rate to the oxygenator 103 by
using flow control valve 139. This effectively controls the rate that carbon
dioxide
is flushed from patient's blood into the apparatus and hence provides some
control as to the relative acidity or alkalinity of the patient 107.
Carbon dioxide is absorbed by primary carbon dioxide absorber 135 and
2 0 the reduction in the oxygen level is detected by fuel cell sensor 131
triggering, via
the high gain circuit, replenishment of oxygen levels under the control of
oxygen
MFC 129.
Xenon sensor 143 measures the xenon concentration in the main circuit
102. This reading may be compared to other readings to reach various
conclusions. For example, if the oxygen concentration measured by oxygen fuel
cell sensor 131 does not equal 100 minus the xenon concentration measured by
xenon sensor 143, it is indicative of contamination, for example by carbon
dioxide
or nitrogen, and the operator may be alerted to vent the apparatus to
atmosphere
or recover the used gas. Alternatively, this may be done automatically at a
preset
level. The xenon sensor 143 is also used to monitor the xenon concentration
predicted from the level of the bellows. Similarly, if these two readings do
not
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-16-
agree, this may be indicative of too much carbon dioxide, nitrogen or oxygen.
As
a result, the operator may choose to vent to atmosphere or recover the used
gas.
If the gas volume in the apparatus is increased, the level of bellows 145
increases. If the level of bellows 145 exceeds a preset level, gas is vented
from
the apparatus, again either manually or automatically, via atmospheric vent
157
and/or xenon recovery valve 153. Optionally, the sensor 146 may be connected
to ultrasonic analyser 143 so that when the bellows 145 upper level is
exceeded,
vent 157 or valve 153 is selectively opened depending on the xenon content of
the
1o gas measured by analyser 143.
Referring now to Figure 2, a ventilator circuit generally designated 200 is
connected at the filters 115 of the apparatus of Figure. 1 to replace the CPB
circuit. Fresh gas passes through the outlet filter 115 (see Figure 1) into
the
ventilator circuit 200 via a check valve 213 to provide gas to the ventilator
thereby
maintaining the oxygen and xenon concentrations in the ventilator circuit 200
at
the required levels.
The ventilator circuit 200 includes a conventional ventilator 201 of the kind
providing a positive drive gas pressure (above atmospheric pressure) in pulses
for
a second or two, followed by a slightly longer period at atmospheric pressure.
The period, cycle time and power of the drive gas pressure is set, in
conventional
manner, to match the needs of the patient 205.
When the ventilator drive pressure is positive, it pushes gas out of the
bellows of a bellows assembly 202 via a control valve 203 and a check valve
204
into the lungs of the patient 205. Valve 203 is a pneumatically operated valve
that
is held closed by the positive ventilator drive pressure during the inflation
of the
patient's lungs. When the ventilator 201 proceeds to the atmospheric pressure
part of its cycle, which allows the patient's lung to relax and deflate,
exhaled gas
(oxygen removed, carbon dioxide added) flows from the lungs via a check valve
209 to a soda-lime absorber canister 210. The canister 210 absorbs carbon
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-17-
dioxide from the exhaled gas and then allows it to flow back to refill the
bellows of
the bellows assembly 202. This gas may then be pumped back to the patient's
lungs by the bellows during the next positive pressure pulse from the
ventilator
201. The level of carbon dioxide in the gas from the patient's lungs is
measured
continuously by a CO2 analyzer 207, which monitors both the end-tidal (peak)
CO2
level, which gives an indication of the patient's correct respiration, and the
minimum CO2 level, which gives an indication of exhaustion of the soda-lime
210.
When the ventilator 201 is in the atmospheric pressure part of its cycle, the
1o valve 203 is open and, if the bellows inside the bellows assembly 202 has
reached the top of its travel and the gas pressure becomes positive enough (a
few
millibar), gas may flow from the bellows into an optional bag 211a, past an
optional pressure relief valve 212a and back to the gas recycling circuit 102
(see
Figure 1) via an outlet 208 and filter 115 (see Figure 1). The bag 211 a and
optional pressure relief valve 212a are needed if the tubing connecting the
recycling circuit 102 to the ventilator circuit 200 are not large enough to
assure
correct operation of the bellows pressure relief via valve 203. In an
alternative
arrangement, the bag 211 b and relief valve 212b are located upstream of the
check valve 203.
Figure 3 shows an alternative ventilator circuit 300 for connection to gas
main circuit 102 of Figure 1 in corresponding manner to the ventilator circuit
200
of Figure 2. It is specially designed to ensure that the patient 308 receives
fresh
gas from the main circuit 102 of Figure 1 and that the exhaled gas is not
mixed
with fresh gas but is fed back to the main circuit 102.
Fresh gas from the outlet filter 115 (see Figure 1) is fed to the ventilator
circuit 300 at inlet 301. An optional feed bellows assembly 302 is connected
downstream of the inlet and has a weight 303 to ensure that it runs at a small
3 0 positive pressure, which is sufficient to feed gas through a check valve
304 to
raise the bellows in a ventilator bellows assembly 305 when the drive gas
pressure from ventilator 306 is atmospheric.
CA 02484066 2004-10-26
WO 03/092776 PCT/GB03/01875
-18-
The ventilator 306 and bellows assembly 305 function in a similar way to
that normally employed in prior art ventilator systems. Periodically,
ventilator 306
applies positive (above atmospheric) gas pressure to the outside of the
bellows in
the bellows assembly 305, collapsing the bellows and forcing gas from inside
the
bellows through a check valve 307 to the lungs of the patient 308. The
ventilator
drive gas to the bellows is also applied to a pneumatically operated valve 309
to
close it, so that all the gas from the bellows assembly 305 goes to the
patient 308.
When the gas pressure from the ventilator 306 is relaxed back to
atmospheric pressure, the bellows in bellows assembly 305 re-inflates with
fresh
gas from the inlet 301 and the feed bellows 302. The check valve 307 is biased
with a spring or weight so that it only opens at a few millibar, assuring that
100%
of fresh gas flows into the bellows assembly 305.
Simultaneously with the refill of the main bellows assembly 305 the
patient's lungs relax, exhaling gas containing less oxygen and more carbon
dioxide relative to fresh gas. The exhaled gas flows through pneumatically
operated valve 309, which is now open to the gas return circuit (since the
drive
gas pressure is atmospheric). The gas return circuit may optionally include a
variable gas volume comprising an additional bellows or flexible bag 310.
The embodiment of Figure 4 is similar to that of Figure 1 but provides for
the selective supply of the xenon/oxygen mixture to an artificial ventilator
and a
CPB oxygenator so that xenon can be administered to the patient before, during
and, if desired, after surgery. Many of the components of the embodiment of
Figure 4 correspond to those of Figure 1 and accordingly have been identified
by
reference numerals in the 400 series corresponding to those in the 100 series
used in Figure 1. Only the main differences between the two embodiments will
be
3 o described.
In the embodiment of Figure 4, the xenon/oxygen mixture is provided by a
CA 02484066 2011-02-04
-19-
conventional cylinder 419 instead of the ullage-space container 121 of Figure
1
and no provision is made for recovery of xenon. Further, the oxygen fuel cell
sensor 431 is provided downstream, instead of upstream, of the pressure
maintaining valve 441. A water adsorber 471 is provided immediately
downstream of the primary carbon dioxide absorber 435 and a carbon dioxide
analyzer 472 is provided to monitor the carbon dioxide content of the spent
oxygenator gas.
A ventilator supply conduit 460 regulated by flow control valve 461 connects
the main circuit section 402a downstream of the pumps 417 to an essentially
conventional artificial ventilator assembly via a bacterial filter 413. The
artificial
ventilator assembly comprises the ventilator 463, bellows 464, oxygen fuel
cell
sensor 465, carbon dioxide absorber 466, carbon dioxide analyzer 467 and
endotracheal tube 468 and operates generally as described with reference to
Figures 2 and 3. The spent gas from the artificial ventilator assembly is
returned
to the main circuit via ventilator spent gas return conduit 469. including a
bacterial
filter 470, connected to the primary carbon dioxide adsorber 435.
Although illustrated and described herein with reference to certain specific
embodiments, the present invention is nevertheless not intended to be limited
to
the details shown. Rather, various modifications may be made in the details
within the spirit and scope of the following claims.