Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02486702 2007-02-08
-1-
PVDF-BASED PTC PAINTS AND THEIR APPLICATIONS
FOR SELF-REGULATED HEATING SYSTEMS
[Field of the invention]
The present invention relates to a conductive paint having a temperature-
wise self-regulating resistance. It relates more particularly to a paint based
on
PVDF (polyvinylidene fluoride), or PMMA and containing a conductor such
as, for example, carbon black or any other electrical conductor.
It is possible to make a polymer material conductive by incorporating
graphite. Application of a high enough voltage leads to the material heating
up
by the Joule effect. In the absence of a circuit breaker mechanism, the
temperature increases until the material is destroyed. The paint of the
present
invention shows an increase in resistance as a function of temperature
(Positive
Temperature Coefficient or PTC effect) so that the current stabilizes at an
equilibrium temperature. This PTC effect therefore allows the intensity of the
current to be thermally regulated - this has many advantages compared with
conventional resistors. Electrical heating systems are conventionally
regulated
by including a thermal cut-out in the circuit. Should the latter fail, the
circuit
or the safety fuse bums out. The PTC material self-regulates without it being
necessary to include either a cut-out or a fuse.
[Prior art and the technical problem]
Patent application EP 1 205 514 discloses a composite comprising, by
weight, the total being 100%:
40 to 90% of PVDF homopolymer or copolymer essentially crystallized
in the 0 form;
10 to 60% of a conductive filler;
CA 02486702 2004-11-02
_ 2 -
0 to 40% of a crystalline or semicrystalline
polymer;
0 to 40% of a filler different from the above
crystalline or semicrystalline polymer;
and such that the crystals are nucleated in the [3 form
on the surface of the conductive filler particles.
This material is applied as a coating deposited on
an insulating substrate such as one made of ceramic,
glass, wood, textile fibres, fabrics or any insulating
surface. To prepare the coating, all that is required
is to disperse the conductive filler in the PVDF, which
may be either in the melt or in solution in a suitable
solvent such as, for example, acetone or N-
methylpyrrolidone. The PVDF containing the conductive
filler and optionally the crystalline polymer and the
other filler, which is either in the melt or in a
solvent, is applied as a paint to the insulating
surface. The metal terminals for connection to the
electrical circuit may be placed at the ends of the
coating before or after application. After cooling the
molten polymer or after drying, in order to remove the
solvent, the heating element is ready.
As regards the filler other than the crystalline
or semicrystalline polymer, the description mentions
"....the usual fillers for fluoropolymers, such as
silica, PMMA, UV stabilizers, etc.".
It has now been found that. PMMA is a necessary
constituent and that this is not a simple filler in the
same sense as silica or UV stabilizers. It has also
been found that PVDF may be in any crystalline form.
[Brief description of the invention]
The present invention relates to a composition
comprising, by weight, the total being 100%:
A) 40 to 80% (advantageously 50 to 80%) of PVDF;
CA 02486702 2007-02-08
-3-
B) 10 to 40% of PMMA; and
C) 10 to 40% of a conductive filler.
This composition has a PTC effect and is advantageously used in the
form of a film (paint) covering a substrate.
The composition of the invention is dissolved in a solvent and then
spread over a substrate and the solvent evaporated. The metal terminals for
connection to the electrical circuit may be placed at the ends of the coating
before or after application.
The PVDF is responsible for the temperature self-regulation by the PTC
effect thanks to its semicrystalline morphology. The present composition
based on PVDF, on PMMA and on a conductive filler (graphite in the form of
flakes and/or carbon black) gives materials of higher performance and
operating with greater safety. The presence of the PMMA reduces the
crystallinity of the PVDF and makes it possible to achieve better cohesion of
the system and better adhesion of the paint.
The invention also relates to a composite, namely the substrate
completely or partly covered with the PTC composition described above.
[Brief description of the drawings]
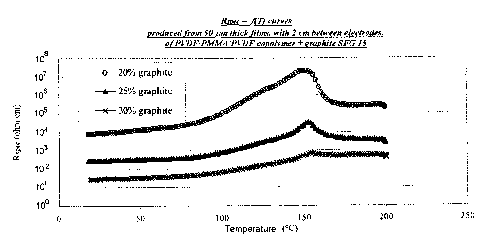
FIG. 1 is a plot from Example 1 illustrating RspeCvs. temperature for
different level of graphite filler.
FIG. 2 plots temperature and power of a graphite filled sample using a
thermal semi-block.
FIGS. 3.1 and 3.2 plot similar measurements to FIGS. 1 and 2, measured
in a cycling mode.
FIG. 4 is a plot from Example 4, showing power and temperature
measurement of a filled sample on a rear-view mirror support with thermal
semi-blocking.
FIG. 5.1 plots power and temperature data from cycling in Example 5.
FIG. 5.2 is a photograph of the honeycomb structure of Example 5.
CA 02486702 2007-02-08
- 3a -
[Detailed description of the invention]
As regards the PVDF, this term denotes both VDF (vinylidene fluoride,
sometimes also called VF2) homopolymers and VDF copolymers. The term
"VDF copolymers" denotes polymers based on VDF and on at least one other
fluorinated monomer. The fluorinated comonomer is advantageously selected
from compounds that contain a vinyl group capable of opening in order to be
polymerized and that contain, directly attached to this vinyl group, at least
one
fluorine atom, a fluoroalkyl group or a fluoroalkoxy group.
CA 02486702 2004-11-02
- 4 -
As examples of comonomers, mention may be made of
vinyl fluoride; trifluoroethylene (VF3);
chlorotrifluoroethylene (CTFE); 1,2-difluoroethylene;
tetra-fluoroethylene (TFE); hexafluoropropylene (HFP);
perfluoro(alkyl vinyl) ethers, such as perfluoro(methyl
vinyl) ether (PMVE), perfluoro(ethyl vinyl) ether
(PEVE) and perfluoro(propyl vinyl) ether (PPVE);
perfluoro(1,3-dioxole); perfluoro(2,2-dimethyl-1,3-
dioxole) (PDD); the product of formula
CF2=CFOCF2CF (CF3) OCF2CF2X in which X is S02F, C02H, CH2OH,
CHZOCN or CH2OPO3H; the product of formula
CF2=CFOCF2CF2SO2F; the product of formula
F(CF2)nCH2OCF=CF2 in which n is 1, 2, 3, 4 or 5; the
product of formula R1CH2OCF=CF2 in which R1 is hydrogen
or F(CF2)Z and z is 1, 2, 3 or 4; the product of formula
R30CF=CH2 in which R3 is F(CF2)Z- and z is 1, 2, 3 or 4;
perfluorobutylethylene (PFBE) ; 3,3,3-trifluoropropene
and 2-trifluoromethyl-3,3,3-trifluoro-l-propene.
Several comonomers may be used.
Mention may be made of VDF/VF3 copolymers
containing at least 60, advantageously at least 75 and
preferably at least 85 mol% VDF.
Mention may also be made of VDF/TFE/HFP copolymers
containing at least 15 mol% TFE units and
advantageously VDF/TFE/HFP copolymers having a
respective molar composition of 60 to 80/15 to 20/0 to
25 (the total being 100).
Mention may also be made of vinylidene fluoride
(VDF) copolymers preferably containing at least 60 wt%
VDF, the comonomer being selected from
chlorotrifluoroethylene (CTFE), hexafluoropropylene
(HFP), trifluoroethylene (VF3) and tetrafluoroethylene
(TFE). Advantageously, the proportion of VDF is at
least 75% and preferably at least 85%. Among these
comonomers, HFP is preferred.
CA 02486702 2004-11-02
- 5 -
Advantageously the PVDF (A) is a blend of (Al)
selected from PVDF homopolymers and VDF/HFP copolymers
containing at least 85 wt% VDF and of (A2) which are
VDF/TFE/HFP copolymers containing at least 15 mol% TFE
units and advantageously VDF/TFE/HFP copolymers of
respective molar composition 60 to 80/15 to 20/0 to 25
(the total being 100). The proportions of (Al) and (A2)
may be in the ratio (A1)/(A2) between 20/80 and 80/20
by weight.
The PVDF may be partly or completely modified,
that is to say functional groups may be introduced
thereinto the purpose of which is to promote bonding
between the paint and the substrate. Advantageously,
the modified PVDF is selected from:
- PVDFs grafted with an unsaturated monomer, the
grafting being carried out by irradiation of a blend in
the absence of oxygen;
- PVDFs irradiated in the presence of oxygen
(these also being referred to as oxidized PVDFs 1); and
- dehydrofluorinated and then oxidized PVDFs (also
referred to as oxidized PVDFs 2).
Advantageously, all that is required is to modify
one fraction of the PVDF (A) - this fraction may be
between 0.5 and 30% of (A) by weight. Preferably, it is
the PVDF (Al) that is completely or partly modified.
The modified PVDFs will now be described. They are
prepared from the PVDFs described above.
With regard to the grafted PVDFs, these may be
prepared by a method of grafting an unsaturated monomer
onto the PVDF, in which:
a) the fluoropolymer is melt-blended with the
unsaturated monomer;
b) the blend obtained in a) is formed into films,
sheets, granules or powder;
CA 02486702 2004-11-02
- 6 -
c) the products from step b) are subjected, in the
absence of air, to photon (y) or electron (R)
irradiation with a dose between 1 and 15 Mrad; and
d) the product obtained at c) is optionally
treated in order to remove all or some of the
unsaturated monomer that has not been grafted onto the
fluoropolymer. .
With regard to the unsaturated grafting monomer,
mention may be made, by way of examples, of carboxylic
acids and their derivatives, aci_d chlorides,
isocyanates, oxazolines, epoxides, ami_nes and
hydroxides. Examples of unsaturated carboxylic acids
are those having 2 to 20 carbon atoms, such as acrylic,
methacrylic, maleic, fumaric and itaconic acids. The
functional derivatives of these acids comprise, for
example, anhydrides, ester derivatives, amide
derivatives, imide derivatives and metal salts (such as
alkali metal salts) of unsaturated carboxylic acids.
Undecylenic acid may also be mentioned. Unsaturated
dicarboxylic acids having 4 to 10 carbon atoms and
their functional derivatives, particularly their
anhydrides, are particularly preferred grafting
monomers. These grafting monomers comprise, for
example, maleic, fumaric, itaconic, citraconic,
allylsuccinic, cyclohex-4-ene-1,2-dicarboxylic, 4-
methylcyclohex-4-ene-1,2-dicarboxylic,
bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic and x-
methylbicyclo-[2.2.1]hept-5-ene-2,3-dicarboxylic acids
and maleic, itaconic, citraconic, allylsuccinic,
cyclohex-4-ene-1,2-dicarboxylic, 4-methylenecyclohex-4-
ene-l,2-dicarboxylic, bicyclo-[2.2.1]hept-5-ene-2,3-
dicarboxylic and x-methyl-bicyclo[2.2.1]hept-5-ene-2,2-
dicarboxylic anhydrides.
Examples of other grafting monomers comprise C1-C8
alkyl esters or glycidyl ester derivatives of
CA 02486702 2004-11-02
- 7 -
unsaturated carboxylic acids, such as methyl acrylate,
methyl methacrylate, ethyl acrylate, ethyl
methacrylate, butyl acrylate, butyl methacrylate,
glycidyl acrylate, glycidyl methacrylate, monoethyl
maleate, diethyl maleate, monomethyl fumarate, dimethyl
fumarate, monomethyl itaconate and diethyl itaconate;
amide derivatives of unsaturated carboxylic acids, such
as acrylamide, methacrylamide, the monoamide of maleic
acid, the diamide of maleic acid, the N-monoethylamide
of maleic acid, the N,N-diethylamide of maleic acid,
the N-monobutylamide of maleic acid, the
N,N-dibutylamide of maleic acid, the monoamide of
fumaric acid, the diamide of fumaric acid, the
N-monoethylamide of fumaric acid, the N,N-diethylamide
of fumaric acid, the N-monobutylamide of fumaric acid
and the N,N-dibutylamide of fumaric acid; imide
derivatives of unsaturated carboxylic acids, such as
maleimide, N-butylmaleimide and N-phenylmaleimide; and
metal salts of unsaturated carboxylic acids, such as
sodium acrylate, sodium methacrylate, potassium
acrylate and potassium methacrylate.
Advantageously, maleic anhydride is used.
Step a) is carried out in any blending device,
such as extruders or mixers used in the thermoplastics
industry.
With regard to the proportions of the PVDF and of
the unsaturated monomer, the proportion of PVDF is
advantageously, by weight, from 90 to 99.9% per 0.1 to
10% of unsaturated monomer, respectively. Preferably,
the proportion of PVDF is from 95 to 99.9% per 0.1 to
5% of unsaturated monomer, respectively.
After step a), it is found that the blend of the
PVDF and the unsaturated monomer has lost about 10 to
50% of the unsaturated monomer that had been introduced
at the start ofstep a). This proportion depends on the
CA 02486702 2004-11-02
- 8 -
volatility and the nature of the unsaturated monomer.
In fact, the monomer was vented in the extruder or the
blender and it was recovered from the venting circuits.
As regards step c), the products recovered after
step b) are advantageously packaged in polyethylene
bags, the air is expelled and the bags then sealed. As
regards the method of irradiation, it is possible to
use, without distinction, electron irradiation, more
commonly known as (3 irradiation, and photon
irradiation, more commonly known as y irradiation.
Advantageously, the dose is between 2 and 6 Mrad and
preferably between 3 and 5 Mrad.
With regard to step d), the ungrafted monomer may be
removed by any means. The proportion of grafted monomer
relative to the amount of monomer present at the start
of step c) is between 50 and 100%. The product may be
washed with solvents that are inert to the PVDF and to
the grafted functional groups. For example, when
grafting with maleic anhydride, the product may be
washed with chlorobenzene. It is also possible, more
simply, to vacuum-degas the product recovered at step
c).
As regards the oxidized PVDF 1, these may be
prepared by a method of oxidizing the fluoropolymer, in
which:
a) the PVDF is formed into films, sheets, granules
or powder;
b) the products from step a) are subjected, in the
presence of oxygen, to photon (y) or electron ((3)
irradiation with a dose of between 1 and 15 Mrad; and
c) the product obtained at b) is optionally
treated in order to remove all or some of the by-
product impurities.
As regards the irradiation and, firstly, step a),
the products are advantageously packaged in
CA 02486702 2004-11-02
- 9 -
polyethylene bags and the bags are not inerted.
Advantageously, the PVDF is in the form of powder. The
bags may also include an aluminium layer in addition to
the polyethylene layer. It is unnecessary to irradiate
in the presence of pure oxygen - all that is required
is for oxygen to be present. The irradiation may be
carried out in the presence of an inert gas containing
oxygen. The term "inert gas" denotes a gas that is not
involved in the irradiation reaction or in the
modification of the fluoropolymer by oxygen.
Advantageously, the proportion of oxygen is between 1
and 20% by volume per 99 to 80% of inert gas,
respectively. Advantageously, the irradiation is
carried out in the presence of air. As regards the
irradiation method in step b), it will be possible to
use, without distinction, electron irradiation, more
commonly known as (3 irradiation, and photon
irradiation, more commonly known as y irradiation.
Advantageously, the dose is between 2 and 12 Mrad and
preferably between 2 and 8 Mrad.
As regards step c), the impurities may be removed
by any means. The product may be washed with solvents
inert to the oxidized PVDF. It is also possible, more
simply, to vacuum-degas the product recovered at step
b).
As regards the oxidized PVDFs 2, these may be
prepared by the process disclosed in Patent
EP 1 054 023. This discloses a process for chemically
modifying a fluoropolymer, consisting in partially
dehydrofluorinating it and then bringing it into
contact with an oxidizing agent, especially hydrogen
peroxide or a hypochlorite:
With regard to the PMMA, this denotes both methyl
methacrylate homopolymers and. copolymers containing at
least 50 wt% methyl methacrylate. As examples of
CA 02486702 2004-11-02
- 10 -
comonomers, mention may be made, for example, of alkyl
(meth)acrylates, acrylonitrile, butadiene, styrene and
isoprene. Examples of alkyl (meth)acrylates are
described in Kirk-Othmer, Encyclopedia of chemical
technology, 4th edition in Volume 1, pages 292-293 and
in Volume 16, pages 475-478. Advantageously, the PMMA
may contain, by weight, 0 to 20% and preferably 5 to
15% of at least one other alkyl (meth)acrylate such as,
for example methyl acrylate and/or ethyl acrylate. The
PMMA may be functionalized, that is to say it contains,
for example, acid, acid chloride, alcohol, anhydride or
ureido functional groups. These functional groups may
be introduced by grafting or by copolymerization. As
regards acid functional groups, these are
advantageously an acid functional group provided by the
acrylic or methacrylic acid comonomer. Two adjacent
acrylic acid functional groups may undergo dehydration
to form an anhydride.
The proportion of functional groups may be from 0
to 15% by weight of the PMMA, including the optional
functional groups.
The PMMA may contain an acrylic elastomer. There
are in fact commercially available grades of PMMA
called "impact grades" that contain acrylic impact
modifiers, usually of the core/shell type. These
acrylic impact modifiers may also be present in the
PMMA because they have been introduced during its
polymerization or prepared simultaneously with its
polymerization. This proportion of acrylic elastomer
may be, by weight, from 0 to 30 parts per 100 to 70
parts of PMMA respectively.
As regards the acrylic elastomer, this denotes
elastomers based on at least one moriomer selected from
acrylonitrile, alkyl (meth)acrylates and core/shell
copolymers. As regards the core/shell copolymer, this
CA 02486702 2004-11-02
- 11 -
is in the form of fine particles having an elastomer
core and at least one thermoplastic shell, the particle
size being generally less than 1pm and advantageously
between 50 and 300 nm. By way of example of the core,
mention may be made of isoprene homopolymers or
butadiene homopolymers, copolymers of isoprene with at
most 30 mol% of a vinyl monomer and copolymers of
butadiene with at most 30 mol% of a vinyl monomer. The
vinyl monomer may be styrene, an alkylstyrene,
acrylonitrile or an alkyl (meth)acrylate. Another core
family consists of the homopolymers of an alkyl
(meth)acrylate and the copolymers of an alkyl
(meth)acrylate with at most 30 mol% of a monomer
selected from another alkyl (meth) acrylate and a vinyl
monomer. The alkyl (meth)acrylate is advantageously
butyl acrylate. The vinyl monomer may be styrene, an
alkylstyrene, acrylonitrile, butadiene or isoprene. The
core of the core/shell copolymer may be completely or
partly crosslinked. All that is required is to add at
least difunctional monomers during the preparation of
the core; these monomers may be selected from
poly(meth)acrylic esters of polyols, such as butylene
di(meth)acrylate and trimethylolpropane
trimethacrylate. Other difunctional monomers are, for
example, divinylbenzene, trivinylbenzene, vinyl
acrylate and vinyl methacrylate. The core can also be
crosslinked by introducing into it, by grafting or as a
comonomer during the polymerization, unsaturated
functional monomers such as anhydrides of unsaturated
carboxylic acids, unsaturated carboxylic acids and
unsaturated epoxides. Mention may be made, by way of
example, of maleic anhydride, (meth)acrylic acid and
glycidyl methacrylate.
The shell(s) are styrene homopolymers,
alkylstyrene homopolymers or methyl methacrylate
CA 02486702 2004-11-02
- 12 -
homopolymers, or copolymers comprising at least 70 mol%
of one of the above monomers and at least one comonomer
selected from the other above monomers, another alkyl
(meth)acrylate, vinyl acetate and acrylonitrile. The
shell may be functionalized by introducing into it, by
grafting or as a comonomer during the polymerization,
unsaturated functional monomers such as anhydrides of
unsaturated carboxylic acids, unsaturated carboxylic
acids and unsaturated epoxides. Mention may be made,
for example, of maleic anhydride, (meth) acrylic acid
and glycidyl methacrylate.
By way of example, mention may be made of
core/shell copolymers having a polystyrene shell and
core-shell copolymers having a PMMA shell. There are
also core-shell copolymers having two shells, one made
of polystyrene and the other, on the outside, made of
PMMA. Examples of copolymers and their method of
preparation are described in the following patents:
US 4,180,494, US 3,808,180, US 4,096,202, US 4,260,693,
US 3,287,443, US 3,657,391, US 4,299,928, US 3,985,704
and US 5,773,520.
Advantageously, the core represents, by weight, 70
to 90% of the core/shell copolymer and the shell
represents 30 to 10%.
By way of example of a copolymer, mention may be
made of that consisting (i) of 75 to 80 parts of a core
comprising at least 93 mol% of butadiene, 5 molo of
styrene and 0.5 to 1 molo of divinylbenzene and (ii) of
25 to 20 parts of two shells essentially of the same
weight, the inner one made of polystyrene and the outer
one made of PMMA.
As another example, mention may be made of those
having a poly(butyl acrylate) or butyl
acrylate/butadiene copolymer core and a PMMA shell.
CA 02486702 2004-11-02
- 13 -
As regards the conductive filler (C), this may be
selected from powders of all electrically conducting
materials and advantageously powder metals, carbon
black, graphite and metal oxides. Advantageously, (C)
is selected from carbon black (preferably with a pH of
2 to 7) and graphite. Advantageously, the graphite
(whether natural or synthetic) is in the form of
flakes. Preferably, its particle size is between 5 and
50 pm.
The composite of the invention is prepared by
dissolving the various constituents (A), (B) and (C) in
a solvent until a thick dispersion is obtained, which
may be applied as a paint to the substrate. The solvent
may be selected from acetone, isophorone,
dimethylformamide (DMF), methyl ethyl ketone (MEK),
N,N-dimethylacetamide and N-methylpyrroli.done (NMP). A
person skilled in the art selects the solvent according
to the nature of the PVDF, i.e. depending on whether
there is a greater or lesser amount of homopolymer. The
amount of solvent needed may be from two to five parts
by weight per one part of the composition.
The substrate may be electrically conducting - and
in this case it serves as a current lead-in - and a
conducting material is placed on the paint, which
material covers the paint completely or partly and
allows the current to be returned.
Advantageously, the substrate is electrically
insulating; the composition of the invention is
deposited on the substrate and then at least one
current lead-in means and at least one current return
means are added. These means may already be present
before spreading the paint. These means may be a copper
or aluminium wire or strip, this wire or strip possibly
occupying a substantial portion of the surface of the
substrate in the manner of a printed circuit used in
CA 02486702 2004-11-02
- 14 -
the electronics industry. In this case, the substrate
is an insulating board on which the two - current lead-
in and return - electrodes are in the form of a printed
circuit and then the PTC composition is deposited on
this circuit, on the side with the electrodes. By
depositing a PTC paint film with a thickness of 20 to
30 pm on a PET support covered with a copper printed
circuit makes it possible to heat to 65 C under a
voltage of 13 V, and therefore inter alia to de-ice car
rear-view mirrors.
According to another embodiment, the insulating
substrate is a honeycomb that is covered with the
composition of the invention, for example by dipping it
into the solvent containing the said composition
(paint) and then by evaporating the solvent. The
honeycomb may be in the form of a sheet, a block, a
cube or a parallelepiped. Two opposed faces are
selected and a conductor is attached to each face, in
contact with the paint that covers the honeycomb. It is
also possible, instead of fixing a conductor, to cover
each face with a silver lacquer. These conductors or
this silver lacquer are (is) used for the current lead-
in and return. The present invention also relates to
the use of these honeycombs for heating a gas, for
example air. It is thus possible to heat the passenger
compartment of a motor vehicle or the cabin of an
aircraft.
According to yet another embodiment, the substrate
is a woven glass fabric (glass fibre meshes). The paint
is deposited by any means and then the current lead-in
and return wires are added, unless they are already
contained in the woven glass fabric. Instead of a woven
fabric made of glass, it is possible to use any
insulator. Meshes impregnated with PTC paints may be
used for the following applications: de-icing of
CA 02486702 2004-11-02
- 15 -
industrial floors, de-icing of ship decks, heating of
pipelines, heating of aircraft baggage holds, etc.
According to yet another embodiment, the
insulating substrate is the outer layer of a hose -
this outer layer may be made of rubber or of a
thermoplastic (for example a polyamide, polyolefin,
etc.). The current lead-in and return wires may be
deposited after painting, but preferably they are
deposited on the outer layer of the hose.
Advantageously, they are in the form of a braid (for
example made of polyester or polyamide) containing
current lead-in and return wires. The paint is then
deposited and this is advantageously followed by a
covering with a protective layer. This protective layer
may be made of a thermoplastic (provided that it
withstands the temperature) or made of rubber. Thus,
the invention is a hose comprising, going from the
inside to the outside:
- optionally, an inner layer in contact with the
fluid transported;
- an outer layer;
- a paint layer according to the invention and
electric current lead-in and return means; and
- optionally, a protective layer.
The paint layer can optionally be carried on a woven or
non-woven substrate, made for instance in polyamide or
in polyester.
The inside diameter of the hose may have any value
and is advantageously between 6 and 50 mm. The
thickness of these hoses, that is to say the sum of the
thickness of the optional inner layer(s) and the outer
layer may be between 1 and 20 mm. These hoses can
transport any type of fluid, but they are useful for
fluids that crystallize or form blockages at low
CA 02486702 2007-02-08
- 16-
temperature. Mention may be made, for example, of diesel fuel, for motor
vehicles with a diesel engine or for lorries, which often contains waxes that
are
deposited and cause blockages in winter or in cold countries.
The present invention also relates to heaters comprising the composite
described above.
[Examples]
PVDF copolymer: this denotes Kynar 9301, a VF2/TFE/HFP copolymer
having respective proportions of 72 / 18 / 10.
PVDF homopolymer: this denotes Kynar 500, a PVDF homopolymer having
an MFI of 4 g/10 min (at 230 C/5 kg).
Graphite SFG 15: this denotes a graphite of the TIMREX* SFG 15 type,
produced by Timcal Group. It is a synthetic graphite in the form of flakes and
its particle size distribution lies between 0 and 20 m.
PMMA: this denotes a PMMA copolymer containing MMA and ethyl acrylate,
having a Tg of 60 C and a molar mass of 140000.
Carbon black: this is of the RAVEN* 14 type, produced by Columbian
Chemicals Europa GmbH (Germany). It is of acid pH.
Maleic anhydride grafted PVDF: this denotes Kynar 720, which is a PVDF
homopolymer from Atofina and has an MVI (Melt Volume Index) of
10 cm3/10 min (230 C/5 kg) that is radiation-grafted with 1% maleic
anhydride.
Example 1. PVDF/PVDF copolymer/PMMA/graphite
20, 25 or 30 g of graphite SFG15 were added to 100 g of a PVDF
homopolymer/PVDF copolymer/PMMA composition having proportions of
40/30/30 by weight. The polymers were blended together at 70 C and at
2000 rpm in order to allow the NMP to be properly
* Trade-mark
CA 02486702 2004-11-02
- 17 -
dissolved. The fillers were then dispersed for
30 minutes at 3000 rpm.
The paint was applied to a Teflon
(polytetrafluoroethylene sold by Dupont) sheet, then
dried at 120 C and cured at 200 C in an oven. The film
thus obtained was stripped from the Teflon sheet and
then analyzed. The results are given in Figure 1. The
higher the filler content, the smaller the amplitude of
the PTC effect and the lower the initial specific
resistance. This therefore makes it possible to adjust
the formulation according to the power desired for an
application.
Example 2. PVDF/PVDF copolymer/PMMA/graphite;
application on a mesh
32 g of graphite SFG 15 were added to 100 g of a
PVDF homopolymer/PVDF copolymer/PMMA composition having
proportions of 40/30/30 by weight. This paint was
prepared in the same way as indicated in Example 1.
This paint was then applied to a glass fibre mesh
provided with electrodes (Cu wires placed every 5 cm in
the mesh), it was then dried at 120 C and then cured at
200 C. The stabilization temperature may be varied by
using a thermal semi-block means (polymer matrix, glass
wool, etc.), i.e. a glass wool or equivalent protection
means is placed above this paint-impregnated mesh. The
results are given in Figure 2.
Example 3: PVDF/PVDF copolymer/PMMA/graphite or
carbon black - application on a support covered with a
copper printed circuit
20 g of graphite SFG 15 or 18 g of carbon black
were added to 100 g of a PVDF homopolymer/PVDF
copolymer/PMMA composition having proportions of
40/30/30 by weight. This paint was prepared in the same
way as indicated in the case of Example 1.
CA 02486702 2004-11-02
- 18 -
Depositing a PTC paint film with a thickness of 20
to 30 pm on a PET support covered with a copper printed
circuit made it possible to heat to 65 C under a
voltage of 13 V and therefore inter alia, to de-ice car
rear-view mirrors. This paint, once applied, was oven-
dried at 1.20 C. The power and the temperature were
measured in cycling mode with a 13 volt supply (Figure
3-2).
This paint was also deposited, as in Example 1, as
a 50 pm film on a Teflon sheet and the specific
resistance measured (Figure 3-1) . Thus, the amplitude
of the PTC effect is better with carbon black than with
graphite. However, graphite allows better dispersion of
the heat.
. Example 4. PVDF/PVDF copolymer/PMNIA/graphi te -
application to a support covered with an aluminium
printed circuit
By dint of the intrinsic non-adhesive properties
of PVDF, good adhesion of the paint to a copper, and
especially an alunlinium, printed circuit is difficult.
The addition of maleic anhydride-grafted PVDF gives the
material good adhesive properties and allows the
dielectric properties to be maintained.
20 g of graphite SFG 15 were added to 100 g of a "PVDF
homopolymer"/PVDF copolymer/PMMA composition having
proportions of 40/30/30 by weight. The 40 g of PVDF
homopolymer consisted of 20 g of PVDF homopolymer and
20 g of maleic-anhydride-grafted PVDF. The preparation
of this paint was the same was that given in the case
of Example 1. This paint was deposited on a rear-view
mirror support consisting of a PET sheet covered with
an aluminium printed circuit. The results are given in
Figure 4.
It is possible to determine the resistance of such
a specimen covered with PTC paint when it is not being
CA 02486702 2004-11-02
- 19 -
used, by simply measuring it with an ohmmeter (called
Rcalculated) = It is also possible to determine, by
calculation, this same resistance when the rear-view
mirror is under voltage (called Rmeasured) = The formula R
= U/I is then used.
The ratio of Rmeasured to Rcalculated is denoted by "a".
Specimen 1 Specimen 2
easurerrment NO. 1;6easurea RcaZculated a1 Rmeasured Rcalculated a2
1 14.8 13.4 1.10 10.1 10.2 0.99
2 13.8 12.9 1.07 10.1 10.3 0.98
3 14.2 12.4 1.15 10.3 10.4 0.99
4 13.4 12.9 1.04 10.2 10.1 1.01
(where Specimen 1 = the composition of the example but
the PVDF homopolymer is entirely non-grafted PVDF
homopolymer; Specimen 2 the composition of the
example).
Thus, a PTC paint containing maleic-anhydride-
grafted PVDF allows better electrical contact with an
aluminium printed circuit. In addition, the resistance
is more constant and the return to a stable resistance
value after application of voltage is more rapid.
Example 5. PVDF/PVDF copolymer/PNIMA/graphite -
application to a three-dimensional support of the
honeycomb type
Applying the paint to a three-dimensional support
allows the surface area for heat transfer exchange to
be considerably increased.
32 g of graphite SFG 15 were added to 100 g of a
PVDF homopolymer/PVDF copolymer/PMMA composition having
proportions of 40/30/30 by weight. This paint was
prepared in the same way as that shown in Example 1.
. This paint, once applied to the honeycomb, was
oven-dried at 120 C and then at 180 C. A thin silver
lacquer was then deposited on the surface of each side
CA 02486702 2004-11-02
- 20 -
of the specimen so as to ensure electrical connection.
Two metal grids deposited on each side of the specimen
could also be used as electrical contacts. The results
are given in Figure 5-1. A photograph in Figure 5-2
shows this honeycomb.