Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02486894 2004-12-15
AEROSOL CONTAINER AND DISPENSER WITH DOSE COONTER
The present invention relates to a dispenser having an actuation indicator for
indicating the number of actuations thereof. In particular, the invention
relates to
metered dose inhalers by means of which medicaments contained in an aerosoY
container may be administered to a patient.
T'tris application is a division of Canadian Patent Application Serial No.
2,293,488,
filed June 8, 1998.
It is well known to treat patients with medicaments contained in an aerosol,
for
example, in bronchodilator therapy. It is also known to use for such therapy,
to rt~edicaments which are contained in an aerosol and are administered to a
patient by means of an inhalation device comprising a tubular housing or
sleeve
in which the aerosol container is~ located anc( an outlet tube leading out of
the
tubular housing. The aerosol containers used in such inhalation devices are
designed to deliver a predetermined dose of medicament upon each actuation
by means of an outlet valve merriber at one end which can be opened eitherby
depressing - the valve member while the container is held stationary or by
depressing the container while the valve member is held stationary. In the use
of such devices, the aerosol container is placed in the tubular housing with
the
outlet valve member of the container communicating via a support with the
outlet
tube, for example a nozzle or mouthpiece. When used for dispensing
medicaments, for example in bronchodilation therapy, the housing is then held
by the patient in a more or less upr~ht condition and the mouthpiece or nozzle
of the inhalation device is placed in the mouth or nose of the patient. The
aerosol container is pressed towards the support to dispense a dose of
medicament from the container which is then inhaled by the patient.
A disadvantage arising from use of such known devices is that the patient
cannot determine the amount of medicament in the contafier at any given time.
In an extreme case this could mean that the patient, possibly suffering from
severe bronchospasm and needing a dose of medicament, will find that the
container will not dispense a dose because its contents have already been
exhausted.
CA 02486894 1998-06-08
2
US Patent No. 4817822 describes an aerosol dispenser of the type described
above having a dose indicating device which, in a first embodiment is
removably
attached to the end of the protruding portion of the aerosol container. The
operating mechanism of the dose counter is located within a housing which
extends from the end of the aerosol container along the external surface of
the
tubular housing. !t is important with inhalation devices containing medicament
that the contents of the aerosol container are clearly marked to ensure that
the
patient knows exactly what medication is contained. One disadvantage
associated with this positioning of the dose indicating device is that the
device
obscures at least a part of the aerosol container and housing which creates
labelling difficulties.
A further disadvantage of the device described is that if the dose indicating
device is removably attached to the aerosol container, it is possible that the
dose indicating device may become separated from its aerosol container with
the result that the aerosol dispenser could be used without the dose
indicating
device, or the actuating mechanism of the indicating device could be tampered
with leading to a false reading when the indicating device is re-attached to
the
dispenser. With patients having several different inhalers, it could even
result in
the indicating device being re-attached to the wrong dispenser.
In a second embodiment described in US4817822 the operating mechanism of
the dose indicating device is located within a compartment in the housing and
is
actuated by means of an actuator member attached to the aerosol container. In
this embodiment, once the aerosol container is fitted into the housing it
cannot
be removed. This makes cleaning of the housing very difficult. Even if the
container were removable, the operating mechanism of the dose indicating
device would be vulnerable to damage when washing with water, soap,
disinfectant or antiseptic solutions. This is important because sprays of many
aerosol formulations leave residues which can entrap dust and dirt particles.
Some provide a media for the growth of undesired micro-organisms. If the
growth of these micro-organisms is unchecked, they can serve as a source of
infection for the patient and will often introduce pathogens into the
patient's
respiratory tract.
CA 02486894 1998-06-08
3
W096/16686 describes an aerosol dispenser wherein the operating mechanism
of the dose indicating device is electronic and wherein the actuating member
comprises a microswitch set into the wall of the housing. The electronic
counting mechanism and microswitch are contained within a hermetically sealed
enclosure. However, electronic assemblies of this type are relatively
expensive
compared to equivalent mechanical mechanisms, typically costing five or six
times as much to produce depending on quantities manufactured. Such
expense must ultimately be borne by the customer and may be prohibitive.
US5482030 describes an aerosol dispenser having a mechanical dose indicator
device located in and connected to the housing in the vicinity of the outlet
tube
of the aerosol container when fitted. Its mechanical construction makes it
dif6cuft to seal against moisture ingress and so this dispenser again is
difficult to
wash without damaging the operating mechanism of the dose indicating device.
Many different pharmaceutical products are sold in the form of aerosol
containers as discussed above, requiring different sized container bodies
andlor
valves according to the required specifications. it is therefore normal for
there to
be dimensional variations between different aerosol containers. Even between
the same products there can be dimensional variations due to manufacturing
tolerances. One problem which is common to all of the dose indicating devices
discussed above is that the indicator mechanism, which is actuated by means of
a switch which detects relative movement between the container body and
housing, lacks any means of compensating for dimensional variations between
different aerosol cantainers. Hence, the indicators described must be
dimensioned according to the product with which they are to be used, and so
will
not be interchangeable with other products. Furthermore, in order for the dose
indicators to work properly, the dimensions of the indicator, aerosol
container
and housing must be accurate.
It is an object to provide a dispenser having an actuation indicator which
overcomes at least some of the above described disadvantages. It is a further
object to provide such a dispenser which from the point of view of the patient
closely resembles currently marketed dispensers in both external appearance
and operation.
CA 02486894 1998-06-08
3a
The invention also seeks to provide an aerosol container assembly.
In accordance with the invention, there is provided an aerosol container
assembly
comprising an aerosol container containing a medicament and having an outlet
s member for dispensing metered doses of the medicament from the container,
the
outlet member dispensing a metered dose upon each actuation of the outlet
member, and a dose indicating device having a dose indicator adapted to
indicate
the number of metered doses of medicament dispensed from or remaining in the
container after each actuation of the outlet member, characterized in that the
dose
1 o indicating device tightly connects to the container in the vicinity of the
outlet
member.
CA 02486894 1998-06-08
4
According to one aspect of the present invention there is provided a dispenser
for dispensing medicament comprising a housing having a support; a container,
locatable within said housing, having an outlet member, wherein said container
is movable relative to the housing to enable dispensing therefrom and said
outlet
member is connectable with said support to prevent relative movement
therebetween; and an actuation indicator, locatable within said housing,
wherein
the container and dose indicator are reversably removable from the housing as
a single unit.
Suitably, the actuation indicator is engagable with the container in the
vicinity of
the outlet member. More preferably, the actuation indicator is engagable with
the
outlet member.
Suitably, the actuation indicator is provided with a grip member which is
engagable with a neck portion of the container. Preferably, the neck portion
is
adjacent to or on the outlet member.
Suitably, the container is an aerosol container.
Suitably, the housing is provided with an outlet, more preferably in the form
of a
mouthpiece. Preferably, the dispenser comprises a passage through which
dispensed doses may pass from the container to the outlet.
Suitably, the container provides measured doses.
Suitably, the actuation indicator indicates the number of doses dispensed from
or remaining in the container. -
Suitably, the actuation indicator comprises an indexing mechanism actuated by
a predetermined movement of the aerosol container relative to the housing.
Preferably, the indexing mechanism comprises a lost motion coupling to allow
and compensate for excess movement ('overtravel') of the aerosol container
relative to the housing.
CA 02486894 1998-06-08
By use of a lost motion coupling it is possible to create an actuation
indicator of
one size which can accomodate valves and actuators made within a wide range
of manufacturing tolerances and can even fit a range of dispensers made to
5 different dimensions.
Suitably, the indexing mechanism indexes the actuation indicator by means of a
predetermined rotary movement of a first member driven by movement relative
to a second member during actuation of the aerosol dispenser.
Suitably, the second member remains stationary relative to the housing during
actuation of the aerosol dispenser.
Suitably, the first member comprises a pinion carried by a shaft through the
lost
motion coupling and the second member comprises a rack. Alternatively, the
first member comprises a yoke and the second member comprises a post
engaged by the yoke through, the lost motion coupling.
Preferably, the lost motion coupling comprises a friction drive mechanism.
Suitably, the dispenser is a breath operated inhaler which is actuable in
response to the inward breath of a user.
According to a particularly preferred aspect of the present invention there is
provided an aerosol dispenser comprising a housing in which a container is
removably located, an outlet leading from the housing and a support in the
housing arranged to receive an outlet member of the container and having a
passage through which the contents of the container may pass to the outlet,
the
outlet member being held stationary in the housing support and the body of the
container being moveable relative to the outlet and housing to dispense its
contents in measured doses, and a dose indicating device having a dose
indicator for indicating the number of doses dispensed from or remaining in
the
container, characterised in that the dose indicating device is tightly
connected to
the container in the vicinity of the outlet member, such that the container
and
dose indicating device may be remaved from the housing as a single unit.
CA 02486894 1998-06-08
6
According to a further aspect of the present invention there is provided an
actuation indicating device for use with a dispenser comprising a housing and
a
container, locatable within said housing, having an outlet member, the
actuation
indicating device comprising attachment means to enable attachment to the
container.
Suitably, the attachment means comprises a grip member which firmly engages
a neck portion formed around the container. More preferably, the neck portion
is
located at the connection between the container and outlet member.
According to a particularly preferred aspect of the present invention the
actuation indicating device has an actuation indicator for indicating the
number
of doses dispensed from or remaining in the container, wherein the actuation
indicating device comprises attachment means to enable tight connection to the
container in the vicinity of the outlet member.
By fixing the actuation indicating device to the container in the vicinity of
the
outlet member may be possible to make use of the physical dimensions of the
crimped ferrule of standard containers to provide a tight snap fit between the
dose indicating device and aerosol container for easy assembly yet which once
assembled cannot be easily separated. This ensures that the actuation
indicator
presents accurate information concerning the container with which it is
assembled.
In a preferred aspect, the dispenser is a metered dose inhaler comprising a
housing in which the container is removably located, an outlet leading from
the
housing, a support in the housing arranged to receive the outlet member of the
container and having a passage through which the contents of the container
may pass to the outlet, the outlet member being held stationary in the housing
support and the body of the container being moveable relative to the outlet
and
housing to dispense its contents in measured doses, and a window through
which the dose indicator may be viewed.
CA 02486894 1998-06-08
7
The location of the dose indicator in the vicinity of the outlet member of the
container in a metered dose inhaler provides the advantage that visually and
operationally the device may appear very similar to current marketed metered
dose inhalers without dose indicating devices such that when switched to the
metered dose inhaler according to the invention, patients perceive little
change
over their conventional dispensers, so creating minimal impact upon patients
and their use of the device.
A dispenser according to the invention will now be described with reference to
the accompanying drawings in which:
Fig. 1 is a section through a standard inhalation device comprising an aerosol
dispenser;
Fig. 2 is a section through the dose indicating device as fitted to an aerosol
dispenser in an inhalation device;
Fig 2a is a sectional view of an aspect of a dose indicating device herein;
Fig. 3 is a perspective view of a counting mechanism used in the dose
indicating
device of Fig. 2;
Fig. 4 shows the sequence of operation of the counter mechanism of Fig. 3;
Fig. 5 shows a lateral and a longitudinal section through a second embodiment
of the dose indicating device as fitted into the housing of an inhalation
device;
Fig. 6 shows an exploded view of a dose indicating device according to a third
embodiment of the invention;
Fig. 7 shows another exploded view of the dose indicating device of figure 6
together with an aerosol container and housing;
Fig. 8 shows a schematic section through an inhalation device comprising the
dose indicating device of figure 6 in a rest position; and
CA 02486894 1998-06-08
Fig. 9 shows a schematic section through the inhalation device of figure 8 in
an
actuated position.
The standard metered dose inhaler shown in Fig 1 comprises a tubular housing
1 in which an aerosol container 2 can be located. The housing is open at one
end (which will hereinafter be considered to be the top of the device for
convenience of description) and is closed at the other. An outlet 3 leads
laterally
from the closed end of the housing 1. In the embodiment illustrated, the
outlet 3
is in the form of a mouthpiece intended for insertion into the mouth of the
patient
but it may, if desired, be designed as a nozzle for insertion into the
patient's
nostril.
The aerosol container 2 has an outlet valve stem 4 at one end. This valve
member can be depressed to release a measured dose from the aerosol
container or, alternatively, the valve stem 4 can be fixed and the main body
of
the container can be moved relative to the valve member to release the dose.
As shown clearly in Fig 1, the aerosol container 2 is located in the housing 1
so
that one end protrudes from its open top. Spacer ribs (not shown) may be
provided inside the housing to hold the external surface of the container 2
spaced from the internal surface of the housing 1. A support 5 is provided at
the
lower end of the housing 1 and has a passage fi in which the valve stem 4 of
the
aerosol container 2 can be located and supported. A second passage 7 is
provided in the support 5 and is directed towards the interior of the outlet
3.
Thus, when the parts are iri the positions shown in Fig 1, the protruding
portion
of the aerosol container 2 can be depressed to move the container relative to
the valve stem 4 to open the valve and a dose of medicament contained in the
aerosol wilt be discharged through the passage 7 and into the outlet 3 from
which it can be inhaled by a patient. One dose will be released from the
aerosol
container each time it is fully depressed.
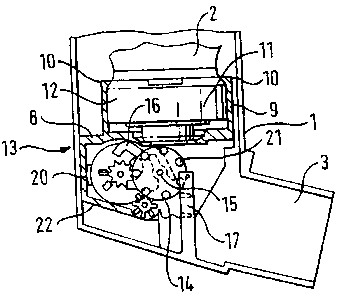
Fig 2 shows the lower part of a device similar to that of Fig 1 but
incorporating a
dose indicating device according to the invention. The dose indicating device
comprises a body 8 firmly attached to the aerosol container by means of
tubular
portion 9 formed with grips 10. Tubular portion 9 tightly engages the
periphery
CA 02486894 1998-06-08
9
of valve ferrule 11 while a grip in the form of lip 10 engages around neck 12
of
valve ferrule 11 which is formed during assembly when valve ferrule 11 is
crimped onto aerosol container 2. Thus the tubular portion 9 and lip 10 form a
tight connection to the aerosol container which once assembled by pushing the
tubular portion 9 over the valve ferrule 11 cannot easily be dissembled.
Below tubular portion 9, body 8 forms a cradle 22 for mounting counter
mechanism 13 and drive pinion 14. Drive pinion 14 is friction mounted on
counter mechanism drive shaft 15. Drive pinion 14 is formed with a number of
teeth or pegs 21 which can engage with a number of recesses or grooves
formed on post 17 in the form of a rack moulded inside housing 1 and extending
from the base of the housing 1 parallel to valve stem 4.
Whilst in the embodiment shown in Fig 2 the post 17 forms a moulded part of
the housing other variations can be envisaged in which the post 17 forms a
part
of the dose indicating device itself. For example, Fig 2a shows a simplified
representation of a dose indicating device in which the head of post 17 is
receivable within a recess provided in the body 8 of the device. Teeth formed
on
the post 17 engage corresponding teeth on drive pinion 14. Spring 28 acts such
as to urge the post from the recess. The protruding lip 24 of the post 17,
however, abuts stop 26, thereby retaining at least a portion of the post 17
within
the recess.
As shown in Figs 3 and 4, drive shaft 15 is connected to driver yoke 16 of
counter mechanism 13. Driver yoke 16 has two switching latches 18a and 18b
spaced either side of star wheel 19 such that driver yoke 16 may tilt about
the
axis of drive shaft 15 between a first position shown in Fig 4b in which
switching
latch 18a engages one side of star wheel 19, and a second position shown in
Fig 4d in which switching latch 18b engages the other side of star wheel 19.
Star
wheel 19 is connected through a mechanism, similar to that described with
reference to reference numerals 2 to 8 in Figs 1 to 3 of European Patent No.
0280104, to three digit wheels 33, which have numbers printed on their
circurnferential faces as described below. When located in the housing 1,
counter mechanism 13 is small enough to be located to the sides of and behind
CA 02486894 1998-06-08
support 5 so as not to interfere with the aerosol flume as it emerges from
passage 7.
The aerosol container 2 may be supplied to the patient with the dose
indicating
5 device ready assembled thereto. Altemativeiy, the housing 1 may be supplied
to
the patient with the dose indicating device located in the position shown in
Fig 2
and the aerosol container 2 supplied separately. In this case, the patient is
instructed to insert the aerosol container 2 into the housing 1 with the valve
stem
first. Upon first insertion of the container into the housing, the tubular
portion 9
10 and lip 10 of the dose indicating device ride over the periphery of valve
ferrule
11 of the aerosol container 2 until lip 10 snaps around neck 12. Thereafter,
the
dose indicating device is attached to the aerosol container 2.
Other means of attachment of the dose indicator to the container are envisaged
including adhesive attachment; use of welded shrink sleeves; heat forming;
crimping; ultra-sonic welding; and by the presence of an o-ring elastomer on
the
container which is fixedly piercabie by barbs on the attachment member of the
dose indicator. In one aspect, permanent means of attachment are preferred.
To actuate the device, the protruding portion of the aerosol container is
depressed as described above with reference to Fig. 1. As the aerosol
container carrying the dose indicating mechanism moves within housing 1, drive
pinion 14 starts to turn, through its engagement with post 17, causing
rotation of
drive shaft 15 and driver yoke 16. As driver yoke 16 tilts with rotation of
drive
shaft 15 switching latch 18a moves into engagement with star wheel 19 (Fig 4a)
causing an incremental anti-clockwise rotation of a half tooth pitch of the
star
wheel until the switching latch 18a can move no further in this direction, the
switching latch being positioned between two adjacent teeth of the star wheel
(Fig 4b). At this point, drive shaft 15 cannot rotate any further and any
further
movement of the aerosol container into housing 1 results in drive pinion 14
continuing to rotate through its engagement with post 17 by virtue of the
friction
coupling between pinion 14 and drive shaft 15.
When the valve stem 4 has reached its fully depressed position and a metered
dose of medicament has been discharged from the aerosol container, the
CA 02486894 1998-06-08
11
aerosol container is allowed to return to its original position. As the
aerosol
container and dose indicating mechanism return to their original position,
drive
pinion 14 starts to rotate in the opposite direction together with drive shaft
15
and driver yoke 16. Thus, driver yoke 16 tilts such that switching latch 18a
moves out of engagement with star wheel 19 while switching latch 18b moves
into engagement therewith (Fig 4c), causing further incremental anti-clockwise
rotation of a half tooth pitch of the star wheel until switching latch 18b can
move
no further in this direction (Fig 4d). Again, drive shaft 15 cannot rotate any
further at this point and any further movement of the aerosol container out of
housing 1 results in drive pinion 14 continuing to rotate through its
engagement
with post 17 by virtue of the friction coupling between pinion 14 and drive
shaft
15. In this way it can be seen that the friction coupling acts as a lost
motion
coupling which allows the dose indicating device to be used with aerosol
containers having valves with different lengths of travel of valve stem during
actuation.
Each time the aerosol dispenser is actuated the star wheel is made to rotate
through two incremental anti-clockwise movements as described above. These
movements are translated through the counter mechanism into appropriate
movements of the digit wheels 33, one number on each of the printed
circumferential faces of the digit wheels being clearly visible through the
widow
20 at the back of the housing 1 (as shown in Fig 2), to indicate that a
further
dose of medicament has been dispensed. By having three digit wheels 33 it is
possible for the dose counter to be used to count hundreds of doses. Clearly
if
fewer than one hundred doses are to be contained within the dispenser, the
dose counter could comprise fewer digit wheels. Alternatively, if a thousand
or
more doses are to be contained, then one or more additional digit wheels could
be added as appropriate.
To remove the aerosol container 2 from the housing for cleaning, the aerosol
container 2 may be withdrawn from the housing 1 in the usual manner. As the
container is withdrawn, the friction coupling between drive pinion 14 and
drive
shaft 15 allows such further movement as is required for the drive pinion to
come out of engagement with the post 17 without causing any further indexing
of the counter mechanism. Once removed, the housing 1 may be cleaned as
CA 02486894 1998-06-08
12
described without fear of interfering with or damaging the dose indicating
device,
which remains firmly connected to the aerosol container 2.
When the housing 1 is clean, the aerosol container 2 with dose indicating
device
may be re-inserted into the housing 1. During insertion, drive pinion 14 will
engage post 17 and start to rotate until the aerosol container reaches its
normal
rest position with the valve stem 4 located in support 5. As the drive pinion
14
rotates, the friction coupling will act as a lost motion mechanism as
described
above, allowing for any travel of the aerosol container as between first
engagement of drive pinion 14 anii post 17, and location of valve stem 14 in
support 5. In this way, the friction coupling automatically accommodates and
compensates for different lengths of valve stems protruding from the ferrule.
Fig 5 shows an alternative lost motion coupling mechanism which may be used
in an aerosol dispenser according to the invention. In this embodiment,
instead
of a pinion, driver yoke 16 is formed with two resilient arms 30 between which
post 17 is grippingly engaged (Fig 5a). Post 17 is formed with ribs on its
surface
(not shown) which provide a rough surface finish to create the level of
friction
required between arms 30 and post 17 such that arms 30 will grip post 17 until
the load applied overcomes the friction.
Upon actuation of the device, as the aerosol container and dose indicating
mechanism move, the friction engagement between arms 30 and post 17 cause
driver yoke 16 to tilt about the axis of shaft 15 (not shown in Fig 5), so
moving
switching latch 18a into engagement with star wheel 19 as discussed in
relation
to the first embodiment. As switching latch 18a reaches its limit of travel,
driver
yoke 16 can move no further, and any further movement of the aerosol container
into housing 1 results in arms 30 slipping down past 17 by virtue of the
friction
coupling. Upon return to its original position, driver yoke 16 tilts in the
other
direction until switching latch 18b moves into engagement with star wheel 19
and can move no further. Any further movement of the aerosol container out of
housing 1 results in arms 30 slipping up post 17.
Figures 6 to 9 show an inhalation device fitted with an electro-mechanical
dose
indicating device according to the invention. As with the mechanical
CA 02486894 1998-06-08
13
embodiments discussed above, the dose indicating device comprises a' body 40
firmly attached to the aerosol container by means of tubular portion 41 formed
with grips (not shown). Tubular portion 41 tightly engages the periphery of
valve
ferrule 11 while a grip in the form of a lip engages around neck 12 of valve
ferrule 11. Thus the tubular portion 41 and lip 'form a tight connection to
the
aerosol container which once assembled by pushing the tubular portion 41 over
the valve ferrule 11 cannot easily be disassembled.
Below tubular portion 41, body 40 forms a cradle for mounting counter
mechanism 43, and defines a chamber for accommodating switch slide 44.
Switch slide 44 is a cylindrical washer made of silicone rubber and having a
bore
of such a diameter that, with the can and dose indicating device mounted
within
the actuator housing, it provides a friction fit on pin 45, which is moulded
in the
housing and protrudes through a hole in body 40. The friction fit of switch
slide
44 on pin 45 ensures the switch slide will not move along the pin unless
pushed.
Two contact members 46, 47, both of which comprise a switch contact and a
circuit board contact, and one of which further comprises a battery contact,
are
mounted such that the battery and circuit board contacts are in constant
contact
with a first terminal of the battery 48 and printed circuit board (PCB) 49
respectively. The switch contacts do not contact each other but are positioned
either side of pin 45, and define the upper limit of movement of the switch
slide
44 within its chamber. Thus, when switch slide 44 is in its upper position as
shown in figure 9, it makes contact with both switch contacts, so closing the
circuit between them due to the electrical conductivity of the silicone rubber
of
the switch slide. Although in the embodiment described the switch slide is
made
of silicone rubber, it will be appreciated that it could alternatively be made
of a
non-conductive rubber having an insert at its upper face made of metal or some
other conductive material.
In addition to its connections with contact members 46, 47, PCB 49 also has
connections to the other terminal of the battery and to a three digit liquid
crystal
display (LCD) 50 in a conventional manner. The PCB comprises an application
specific integrated circuit (ASIC), which provides the logic by which the dose
indicator can be checked, programmed and made operational, as discussed in
more detail below. to keep a record of how many times the switch contact
circuit
CA 02486894 1998-06-08
14
is closed and drives the LCD to display the number of doses remaining in the
aerosol container. The ASlC is thus designed and programmed accordingly in a
known manner.
Instead of a digital display, the LCD could alternatively be formatted to
display
an analogue indication. When the aerosol container is mounted in the actuator
housing, LCD 50 is visible through window 20. In the embodiment depicted in
figure 7, the LCD and window are located at the back of the housing, but they
could equally be located at the front or some other part of the housing.
The Counter mechanism 43 is small enough to be located to the sides of and
behind the stem block (support 5) moulded in housing so as not to interfere
with
the aerosol flume as it emerges.
To actuate the device, the protruding portion of the aerosol container when
fitted
into the actuator housing is depressed as described above. As the aerosol
container carrying the dose indicating mechanism moves within the housing
from its rest position (shown in figure 8), the chamber accommodating switch
slide 44 moves down until the upper face of switch slide 44, which is mounted
on pin 45, meets switch contacts 46, 47 and the switch circuit is closed. This
causes the ASIC to decrement the number displayed by the LCD 50. As the
aerosol container continues to move, a metered dose of medicament is
discharged from the valve, while switch slide 44 is pushed down along pin 45
by
virtue of the friction fit of the switch slide on the pin until the valve stem
reaches
its limit of travel and the aerosol container moves no further (figure 9). In
this
way, it can be seen that the friction fit of the switch slide 44. on pin 45
allows for
over-travel of the valve stem after the switch circuit has been closed, so
acting
as a lost motion coupling. The aerosol container is then allowed to return to
its
original position within the housing, and as it returns, the chamber
accommodating switch slide 44 moves up breaking the sv~itch circuit as switch
contacts 46, 47 move away from switch slide 44. Body 40 then meets the lower
face of switch slide 44 and draws the switch slide up along pin 45 until the
valve
stem returns to its rest position (figure 8).
CA 02486894 1998-06-08
Because the dose indicating device is designed to be suitable for use in
connection with different sized aerosol containers containing different
numbers
of doses to be delivered, the ASIC is designed to be factory set in accordance
with the size of aerosol container with which the dose indicating device is
5 assembled. After assembly of the dose indicating device and first connection
of
the battery, the ASIC enters a self-test mode. After this, the programming
mode
may be entered by activating the switch, allowing it to be programmed to count
down from the appropriate number of doses (e.g. 200, 120, 80 or 60). This may
be done automatically on a packing line. After programming has taken place,
the
10 ASiC enters the counting mode, where the LCD decrements upon closing of the
switch contact circuit. When the count of zero is reached, the ASIC is
designed
to prevent the count from decrementing any further in a known manner. in order
to prevent spurious readings due to the effects of switch 'bounce', the ASIC
may
be designed to decrement only after the switch circuit has been closed for a
15 predetermined length of time in a known manner. In the event of the aerosol
container getting jammed in the actuated position after operation, or the
switch
circuit jamming closed due to mechanical damage or contamination, the ASIC
may be designed to blank the LCD to alert the user that there is a problem.
As with the other embodiments of the invention described above, the aerosol
container may be withdrawn from the actuator housing in the usual manner. As
the container is withdrawn, body 40 draws the switch slide up along pin 45
until
it clears the pin altogether. Once removed, the housing may be cleaned without
interfering with or damaging the dose indicating device, which remains firmly
connected to the aerosol container.
During re-insertion of the aerosol container, which can only occur when the
body
of the dose indicating device is correctly orientated with respect to the
housing
by virtue of their respective shapes, switch slide 44 engages and is pushed up
by pin 45 until the upper face meets the switch contacts. Further insertion of
the
aerosol container results in switch slide 44 being pushed down along pin 45
until
the valve stem is seated back within support 5.
It will be appreciated that by programming of the ASIC, one design of dose
indicating device could be used in conjunction with a range of aerosol
containers
CA 02486894 1998-06-08
16
of various capacities. By virtue of the switch mechanism, the same design of
dose indicating device can also be used in conjunction with a range of
different
valves having different lengths of valve stem and different stem travel
specifications.
Whilst the present invention has been described in detail in respect of a
metered
dose inhaler actuatable manually by the patient it will be appreciated that
other
actuation mechanisms can be substituted. In particular, the use of a breath
operated inhaler in which the actuation is assisted, arid is responsive to,
preferably triggered by, the inward breath of the patient, is also envisaged.
The dispenser of the invention is suitable for dispensing medicament,
particularly for the treatment of respiratory disorders. Appropriate
medicaments
may thus be selected from, for example, analgesics; e.g., codeine,
dihydromorphine, ergotamine, fentanyl or morphine; anginal preparations, e.g.,
diltiazem; antiallergics, e.g., cromoglycate, ketotifen or nedocromil;
antiinfectives
e.g., cephalosporins, penicillins, streptomycin, sulphonamides, tetracyclines
and
pentamidine; antihistamines, e.g., methapyrilene; anti- intiammatories, e.g.,
beclomethasone dipropionate, fluticasone propionate, flunisolide, budesonide,
rofleponide, mometasone furoate or triamcinolone acetonide; antitussives,
e.g.,
noscapine; bronchodilators, e.g., albuterol, salmeterol, ephedrine,
adrenaline.
fenoterol, formoterol, isoprenaline, metaproterenol, phenylephrine,
phenylpropanolamine, pirbuterol, reproterol, rimiterol, terbutaline,
isoetharine,
tulobuterol, or (-)-4-amino-3,5-dichloro-a-[[[6-[2-(2-pyridinyl)ethoxy)
hexyl]methyl]
benzenemethanol; diuretics, e.g., amiloride; anticholinergics, e.g.,
ipratropium,
tiotropium, atropine or oxitropium; hormones, e.g., cortisone, hydrocortisone
or
prednisolone; xanthines, e.g., aminophylline, choline theophyllinate, lysine
theophyllinate or theophylline; therapeutic proteins and peptides, e.g.,
insulin or
glucagon. It will be clear to a person skilled in the art that, where
appropriate,
the medicaments may be used in the form of salts, (e.g., as alkali metal or
amine
salts or as acid addition salts) or as esters (e.g., lower alkyl esters) or as
solvates (e.g., hydrates) to optimise the activity and/or stability of the
medicament.
CA 02486894 1998-06-08
17
Preferred medicaments are selected from albuterol, salmeterol, fluticasone
propionate and beclometasone dippropionate and salts or sovates thereof, e.g.,
the sulphate of albuterol and the xinafoate of salmeterol.
Medicaments can also be delivered in combinations. Preferred formulations
containing combinations of active ingredients contain salbutamol (e.g., as the
free base or the sulphate salt) or salmeterol (e.g., as the xinafoate salt) in
combination with an antiinflammatory steroid such as a beclomethasone ester
(e.g., the dipropionate) or a fluticasone ester (e.g., the propionate).
It will be understood that the present disclosure is for the purpose of
illustration
only and the invention extends to modifications, variations and improvements
thereto.
The application of which this description and claims form part may be used as
a
basis for priority in respect of any subsequent application. The claims of
such
subsequent application may be directed to any feature or combination of
features described therein. They may take the form of product, method or use
claims and may include, by way of example and without limitation, one or more
of the following claims: