Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02488861 2004-12-02
WO 03/103681 PCT/EP03/05990
Use of Orally Available Prostacyclin Derivatives for the Production of a
Pharmaceutical Agent for Treating Diseases That are Associated with Bone
Marrow
Edemas
The invention relates to the subject that is indicated in the claims, i.e.,
the use of
orally available prostacyclin derivatives for the production of a
pharmaceutical agent for
treating diseases that are associated with bone marrow edemas.
For osteonecrosis and recently also for osteoarthritis in the knee, a
connection
between the presence of a bone marrow edema and pain is assumed (Felson et
al., Ann
Intern Med, 2001, 134(7); 541-593).
Osteonecrosis (syn.: aseptic necrosis, avascular necrosis, ischemic necrosis)
is a
common disease. In this case, pain is the indicating sign. In about two thirds
of the
patients, this pain occurs at rest. Osteonecrosis is often described as a
transient process.
When the course proceeds spontaneously, in most cases what happens is a
complete
healing after 6 to 12 months. In this period, however, the patient is burdened
by intense
pain that is often not affected even by a commonly used pain therapy. The
incidence
rates of the progression of an early osteonecrosis up to the end stage with
pronounced
bone necroses are not known. Osteonecrosis is responsible for more than 10% of
all joint
replacement operations performed annually.
Osteoarthritis in the knee is a common disease that attacks about 11-15% of
those
over 65 years of age and is the most frequent cause of physical handicap in
older humans.
CA 02488861 2004-12-02
2
The main reason for physical limitations and visits to physicians is knee
pain.
Osteoarthritis is defined as primarily non-inflammatory joint destruction that
begins with
cartilage degeneration in adults, later also affects common joint structures
and progresses
irreversibly. The overall course is generally progressive, but alternating in
phases in
terms of clinical acuity. In the final stage, knee replacement is necessary.
Osteonecrosis and osteoarthritis are most often manifested on hip and knee
joints,
but also on shoulder joints, wrists and ankles.
While in the treatment of osteonecrosis, surgical bone marrow decompression
(perforation) with all its disadvantages such as in-patient stay and surgical
intervention is
performed as a means for alleviating pain, the treatment of osteoarthritis now
exists only
in symptomatic pain treatment and is inadequately effective. It usually
comprises the
permanent prescription of analgesics and/or antiphlogistic agents, combined
with
physiotherapy and weight reduction as well as optionally the intra-articular
injection of
anesthetics, corticoids or "cartilage-building/-replacing" substances. X-ray
excitation
radiation and acupuncture are also prescribed. Therapy of arthrosis is also
limited here to
date to a symptomatic treatment.
The established therapies are unsatisfactory both for osteonecrosis and for
osteoarthritis because of the limitation to symptomatic treatment. In
particular, the
continued existence of dolorogenic mechanisms requires a long-term treatment
that is
associated with additional risks and costs. A causal treatment of pain with
permanent
freedom from pain is therefore especially desirable for osteoarthritis.
It is known that alleviation of pain in osteonecrosis can be achieved by a
five-day
intravenous infusion therapy with Ilomedin (iloprost-trometamol) (Aigner et
al., J Bone
CA 02488861 2004-12-02
3
Joint Surg, 2001, 83(6): 855-858). 'The freedom from pain promptly reached
with the
reduction of bone marrow edema does not free the physician from close controls
over an
extended period, however, since the freedom from pain that is achieved masks a
possible
progression in a necrotic stage of the bone. Beyond the freedom from pain,
treatment of
necrotic lesions with the purpose of obtaining or restoring healthy bone
tissue is therefore
desirable.
The object was therefore to find a treatment of diseases that are associated
with
bone marrow edemas, especially osteonecrosis and osteoarthritis, that is not
limited to
only symptomatic treatment, i.e., combating pain, but rather also restores the
structural
integrity of the bone, and in addition to make available a pharmaceutical
agent that can be
taken over an extended period by the patient himself without the support of
the physician.
It has now been found, surprisingly enough, that orally available prostacyclin
derivatives, especially the cyclodextrin clathrates of the prostacyclin
derivatives of
general formula I
Y~"COOR'
Z
X
~A-W-D-E-Rz
R3
(I)
in which
RI means a hydrogen atom or a C~-Coo-alkyl radical,
A means a -CH2-CH2- group, a trans -CH=CH- group or a -C---C- group,
W means a free hydroxymethylene group, or a hydroxymethylene group that
CA 02488861 2004-12-02
4
is functionally modified to form a hydroxy group,
whereby the hydroxy group is in oc- or (3-position,
X, Y, independently of one another, mean a -CHZ- group or an oxygen atom,
Z means a hydrogen atom or a cyano group,
D means a straight-chain or branched, saturated C~-CS-alkylene group,
E means a -C=C- group or a direct bond,
Rz means a straight-chain or branched, saturated C,-C~-alkyl group,
R3 means a free or functionally modified hydroxy group,
and all optically active forms, racemates, diastereomers, diastereomer
mixtures,
clathrates thereof,
and if R' has the meaning of a hydrogen atom, the salts thereof with
physiologically compatible bases, achieve this object.
A 28-day treatment of patients with painful bone marrow edema by iloprost ~3-
cyclodextrin clathrate (iloprost oral) reduced not only the bone marrow edema
but also
restored the structural integrity, i.e., necrotic areas and lesions were
healed. The
alleviation of pain and the improved articulation could be observed even two
months
after the end of treatment. Treatment with orally available prostacyclin
derivatives,
especially with cyclodextrin clathrates of the prostacyclin derivatives of
formula I, thus
represents a novel therapy that not only suppresses the symptoms, i.e., the
pain, but
combats the causes of osteonecrosis and osteoarthritis and for the first time
can also be
clinical, studied independently.
This finding is all the more surprising in that earlier studies, such as,
e.g., the
placebo-controlled studies for treatment of severe ischemia of the legs, did
not show
CA 02488861 2004-12-02
iloprost (3-cyclodextrin clathrate to have any effectiveness (Eur J Vasc
Endovasc Surg.,
2000, 20(4): 358-362). The effectiveness of the intravenous treatment with
iloprost-
trometamol, which is approved for treatment of the serious ischemia of the
legs, thus
could not be reproduced.
It is consequently not possible to predict that iloprost [3-cyclodextrin
clathrate will
have an advantageous action in every case based on knowledge regarding
intravenous
iloprost-trometamol.
As alkyl group R', straight-chain or branched alkyl groups with 1-10 carbon
atoms can be considered, such as, for example, methyl, ethyl, propyl, 2-
methylethyl, n-
butyl, I-methylpropyl, 2-methylpropyl, I,1-dimethylethyl (tert-butyl), hexyl,
heptyl,
octyl, nonyl or decyl.
Alkyl group R' can also be substituted. As substituents, for example,
fluorine,
chlorine or bromine atoms, phenyl, dimethylamino, diethylamino, methoxy, and
ethoxy
can be mentioned.
As alkyl group R', alkyl groups with I-4 carbon atoms are preferred.
Alkyl group R2 is straight-chain or branched; by way of example, reference can
be
made to the chain length that corresponds to radicals that are mentioned for
R', and alkyl
group Rz has 1-7 carbon atoms, preferably I-3 carbon atoms, especially
preferably 1-2.
The hydroxy groups in R3 and W can be fianctionally modified. Thus, an
esterification or etherification is meant. The thus obtained ethers and/or
acyl radicals are
radicals that are known to one skilled in the art.
CA 02488861 2004-12-02
6
Ether radicals are, e.g., tetrahydropyranyl, tetrahydrofuranyl,
methoxyrnethyl,
methoxyethyl, ethoxyethyl, silyl ether, such as, e.g., trimethylsilyl,
dimethyl-tert-
butylsilyl, or triphenylsilyl.
Acyl radicals can be, for example, acetyl, propionyl, butyryl, or benzoyl.
X preferably has the meaning of a CHZ group.
Z preferably means a hydrogen atom.
As alkylene group D, straight-chain or branched saturated alkylene groups with
1-
carbon atoms are considered. For example, methylene, ethylene, propylene, l-
methylethylene, butylene, 1-methyl-propylene, 2-methylpropylene, pentylene, 1-
methylbutylene, and 1-ethylethylene can be mentioned. A branched alkylene
group with
3 carbon atoms is preferred.
If R1 means a hydrogen atom, the compounds of formula I can also be present as
salts of physiologically compatible bases.
Both inorganic and organic bases are suitable for salt formation. For example,
alkali hydroxides such as sodium or potassium hydroxide; alkaline-earth
hydroxides such
as calcium hydroxide, or ammonia; and amines such as ethanolamine,
diethanolamine,
triethanolamine, N-methylglucamine, morpholine, or tris-
(hydroxyrnethyl)methylamine
can be mentioned.
Prostacyclin derivatives are orally available as defined by the invention at
the
time when they can be used according to suitable formulation for oral
administration.
In addition, especially a.- or (3-cyclodextrin clathrates or hydroxylated
forms
thereof are suitable.
CA 02488861 2004-12-02
7
Especially suitable for the treatment of diseases that are associated with
bone
marrow edemas, especially for the treatment of osteonecroses and
osteoarthritis, are also
the following known prostacyclin derivatives of general formula I that are
formulated for
oral administration: cicaprost (5-{(E)-(1S,SS,6S,7R)-7-hydroxy-6-[(3S,4S)-3-
hydroxy-4-
methyl-1,6-nonadiinyl]-bicyclo[3.3.0]-oct-3-ylidene}-3-oxapentanoic acid),
epoprostenol
((SZ,9oc,13E,15S)-6,9-epoxy-11,15-dihydroxy-prosta-5,13-dienoic-1-acid),
beraprost (EP
0 084 856), ciprostene, taprostene, naxaprostene, CS 570, SC-39902, FCE-22509,
OP 41483 and RS 93427.
'The (3-cyclodextrin clathrate of iloprost, (5-{( 1 S,SS,6R,7R)-7-hydroxy-6-
[(E)-
(3R,4RS)-3-hydroxy-4-methyl-oct-1-en-6-inyl]-bicyclo[3.3.0]octan-3-
ylidene}pentanoic
acid)-(3-cyclodextrin clathrate, is especially suitable. Within a treatment of
28 days, a
significant improvement of the pain that is immediately noticeable to the
patients and that
is associated with a significant reduction of the bone marrow edema and a
healing of
necroses takes place.
'The use of iloprost-[3-cyclodextrin clathrate for the production of a
pharmaceutical agent for treating osteonecrosis and osteoarthritis is
therefore a preferred
subject of the invention.
IIoprost is a prostacyclin analog that is known from EP 11 591 and can be
produced by methods disclosed therein. The nomenclature name for iloprost is
(5-
{ ( 1 S, 5 S,6R,7R)-7-hydroxy-6-[(E)-(3R,4RS)-3-hydroxy-4-methyl-oct-1-en-6-
inyl]-
bicyclo[3.3.0]octan-3-ylidene} pentanoic. acid).
Iloprost-(3-cyclodextrin-clathrate is known from EP 259468 and can be produced
according to methods that are disclosed there.
CA 02488861 2004-12-02
8
It is known from EP 1 016 408 that prostanoids are C-C chemokine-production
inhibitors. For the bone marrow edema, the mechanisms that are disclosed there
are not
relevant according to present knowledge.
With respect to conventional methods for treating osteonecroses and
osteoarthritis, avoiding a cost-intensive in-patient stay, cutting down on
surgical
interventions (perforation, especially for osteonecroses), reducing the
necessity for
implantation of artificial joints, avoiding costs for treating side effects of
conventional
pain medications, reducing the morbidity, shortening the disability time,
improving the
quality of life and reducing the number of visits to the doctor are
significant advantages.
In particular, the oral administration makes possible a longer-term medication
with a causal therapy stock that the patients can take largely independently
of any
medical support.
The suitable dose range for the treatment of osteonecrosis and osteoarthritis
is
between 50 ~g/day and 350 ~g/day, preferably between 100 pglday and 300 pg/day
or
between 150 pg/day and 350 pg/day, whereby these amounts can be distributed
with
several individual dosages. A healing of the bone necroses can be achieved
within a few
weeks.
Description of the Figures
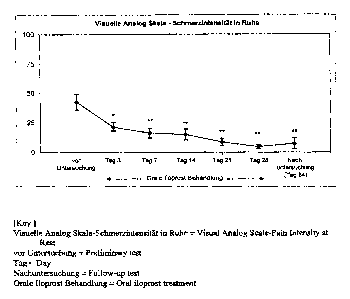
Figure 1: Significant abatement of the resting pain under oral iloprost
treatment
measured by means of a 100 mm visual analog scale with end points 0 = no pain
and
CA 02488861 2004-12-02
9
100 = intolerable pain. The alleviation of pain is already considerable after
3 days of
treatment. Almost complete freedom from pain is achieved after a 28-day
treatment and
persists over a period of 2 months without therapy.
Data as average values ~ SEM. p-Values as a comparison of the corresponding
times with the starting value, with *p < 0.05 and **p < 0.001 (ANOVA)
Figure 2: Significant abatement in pain after defined stress measured by means
of a 100
mm visual analog scale with end points 0 = no pain and 100 = intolerable pain.
The alleviation of pain is already considerable after 3 days of treatment.
Almost
complete freedom from pain is achieved after a 28-day treatment and persists
over a
period of 2 months without therapy.
Data as average values ~ SEM. p-Values as a comparison of the corresponding
times with the starting value, with *p < 0.05 and **p < 0.001 (ANOVA)
Figure 3: Significant increase of the knee activity score according to Larson
(maximum
score 100) after a 14-day treatment. The improvement persists over a period of
2 months
without therapy.
Data as average values ~ SEM. p-Values as a comparison of the corresponding
times with the starting value, with *p < 0.05 and **p < 0.001 (ANOVA)
Figure 4: Case example of a patient with complete degeneration of a diffused
bone
marrow edema in the early stages of osteonecrosis. The control-MRI study was
carned
out 2 months after the 28-day treatment with oral iloprost was completed.
CA 02488861 2004-12-02
Fire 5: Same pronounced abatement in resting pain when administered in a
double-
blind trial of oral iloprost treatment and Tramadol~ treatment measured by
means of a
100 mm visual analog scale with end points 0 = no pain and 100 = intolerable
pain.
Almost complete freedom from pain is achieved after 28 days of treatment and
persists
over a period of 2 months without therapy.
Data as average values ~ SEM. p-Values for comparing the two treatments
confirm their equivalence (Hodges-Lehmann-Schatzer and Wilcoxon Test).
Figure 6: Same pronounced abatement in activity-related pain under double-
blind
administration of oral iloprost treatment and Tramadol~ treatment measured by
means of
a 100 mm visual analog scale with the end points 0 = no pain and 100 =
intolerable pain.
A considerable reduction in pain is achieved after 28 days of treatment and
persists over a
period of 2 months without therapy.
Data as average values ~ SEM. p-Values for comparing the two treatments
confirm their equivalence (Hodges-Lehmann-Schatzer and Wilcoxon Test).
Figure 7: Same pronounced increase in the knee activity score according to
Larson
(maximum score 100) under double-blind administration of oral iloprost
treatment and
Tramadol~ treatment. The improvement persists over a period of 2 months
without
therapy.
Data as average values ~ SEM. p-Values for comparing the two treatments
confirm their equivalence (Hodges-Lehmann-Schatzer and Wilcoxon Test).
CA 02488861 2004-12-02
Figure $: Oral iloprost produced a complete degeneration of the bone marrow
edema in
at least one affected bone in 53% of patients, while this was the case in only
19% of the
patients under Tramadol~.
This difference was statistically significant (p = 0.0336, Fisher's Exact
Test)
CA 02488861 2004-12-02
12
Experiments
Pilot Studies
For the action of oral iloprost on the bone marrow edema and pain, an open
pilot
study with 19 patients with osteonecrosis of the knee was performed in the 1.
Orthopadische Abteilung des Orthopadischen Spital Wien-Speising [ 1 S'
Orthopedic
Department of the Orthopedic Hospital of Vienna-Speising], Prim. Dr. F.
Landsiedl/Dr.
N. Aigner, in the period from March to November 2001.
Before the beginning of the study, all patients gave their written consent to
participate in the study after they were informed extensively orally and in
writing on the
type, purpose and course of the study.
Patients of both sexes with resting pain because of an osteonecrosis were
included
in the study if the following criteria were met:
Age between 19 and 60 years,
Bone marrow edema found by means of MRT according to specified
criteria,
~ Normal x-ray finding without signs of osteoarthritis and no subchondral
attack of the bone.
The treatment was carried out with iloprost capsules that were packaged and
identified according to valid GLP rules. Each capsule contained 50 ~g of
iloprost.
During the first three days of treatment, iloprost was administered in a
dosage of
3x50 ~g of iloprost during the day, which starting on day 4 could be increased
individually up to a maximum dose of 3x 100 pg of iloprost during the day:
CA 02488861 2004-12-02
13
~ To increase the success of the therapy (alleviation of pain), this dosage
could be carned out both by increasing the individual dose (maximum:
3x 100 pg of iloprost during the day) and by shortening the intake-free
interval (minimum: 2 hours, i.e., 6x50 ~g of iloprost during the day).
~ In the case of poor tolerance, the dosage could always be reduced to a
minimum dose of 2x50 ~g of iloprost during the day.
The treatment period was one month. Previously administered pain-relieving
medications should be discontinued before the beginning of the study.
All medications for the treatment of accompanying diseases were able to be
maintained at the usual dosages.
The clinical action of the treatment was determined based on the following
parameters:
~ Evaluation of the intensity of the resting pain and the activity-related
pain
by the patient based on a 100 mm scale, whereby 0 means "no pain," and
100 means "intolerable pain." The stress was defined as climbing and
descending a specified set of stairs.
~ Evaluation of the functional condition of the knee by means of Larson
scores.
~ Evaluation of the bone marrow edema by means of MRT.
As safety parameters, undesirable events and side effects were detected. Known
side effects of iloprost are symptoms such as headache, reddening of the face,
nausea,
vomiting and diarrhea.
CA 02488861 2004-12-02
14
The course of the study was presented as follows:
Prelimi-Treatment Follow-
naryTest up
Test
Visit 1 2 3 4 5 6 7
D3y D~yDay D Da~ Da~ Day
4 ly 6 4
Declaration of consent
Inclusion and exclusion
criteria
Case history
Physical test
Pregnancy test
Polling for side effects
Action parameters:
Larson Score
inttens tyvaluation of
the pain
MRT
X-ra~~ image of the affected
knee
1) The study could take place over six weeks.
As a result of a 28-day treatment with oral iloprost, freedom from pain at
rest and
during activity was almost achieved in patients with osteonecrosis, the
majority of whom,
before the beginning of the study, had unsuccessfully taken a number of other
medication
combinations over several weeks. The action on the pain could already be
detected after
a 3-day treatment and persisted 2 months after.the end of the treatment. With
the
reduction of pain, a complete degeneration of bone marrow edema was noted in
MR
control images in 44% of the patients.
Double-Blind Comparison Studies
To confirm the action of oral iloprost on the bone marrow edema and the pain,
a
random, double-blind comparison study was performed on the opiate Tramadol~ in
41
CA 02488861 2004-12-02
patients with osteonecrosis or osteoarthritis of the knee in the period from
March 2001 to
November 2002 according to the GCP guidelines.
Before the beginning of the study, all patients gave their written consent to
participate in the study after they were informed extensively orally and in
writing on the
type, purpose and course of the study.
Patients of both sexes with resting pain because of an osteonecrosis or
osteoarthritis were included in the study if the following criteria were met:
~ Age between 19 and 60 years,
~ Bone marrow edema found by means of MRT according to specified
criteria.
The treatment was carried out either with iloprost capsules or with outwardly
indistinguishable Tramadol capsules that were packaged and characterized
according to
valid GLP rules. Each capsule contained either 50 ~g of iloprost or 50 mg of
Tramadol~.
During the first three days of treatment, the study medication was
administered in
a dosage of 3x1 capsules during the day, which starting on day 4 could be
increased
individually to increase the success of the therapy (alleviation of pain) up
to a maximum
dose of 3x2 capsules during the day. In the case of poor tolerance, the dosage
could
always be reduced to a minimum dose of 2x 1 capsules during the day.
The treatment period was one month, followed by a follow-up observation period
of two months during which no treatment was given. Previously administered
pain-
relieving medications should be discontinued before the beginning of the
study.
All medications for the treatment of accompanying diseases were able to be
maintained at the usual dosages.
CA 02488861 2004-12-02
16
The clinical action of the treatment was determined based on the following
parameters:
~ Evaluation of the intensity of the resting pain and the activity-related
pain
by the patient based on a 100 mm scale, whereby 0 means "no pain," and
100 means "intolerable pain."
Evaluation of the functional condition of the knee by means of Larson
scores.
~ Evaluation of the bone marrow edema by means of MRT.
As safety parameters, undesirable events and side effects were detected. Known
side effects of iloprost are symptoms such as headache, reddening of the face,
nausea,
vomiting and diarrhea.
CA 02488861 2004-12-02
17
The course of the study was presented as follows:
Preliminary Treatmentl) Alterna- Follow-
Test tive up
Therapy Testl)
Screen Base1 2 3 4 5 Da Da 6 ~
2~ a~ s~
-ing -line y y
1-4 5
Vlslt
Da Da Da Da Da Da Da
Y3 Y~ Y Y Y Y Y
14 21 28 56 84
Declaration of consent
Inclusion and exclusion,
criteria
Demography, general
and specific anamnesis
Em loyment status
Work disability
Physical test
Pregnancy test
6
Randomization
Dispensing of research
medication
Withdrawal of unused
medication
Dose/dosage adjustment,
if necessary
Poll_in for side
effects
Blood ressure, ulse
Blood samples
(hematology, clinical
chemis ), urinalysis
Preliminary and
~
accompanying treatment
Action parameters:
Larson score
Patient evaluation
of the
pain intensity
MRT
X-ray image of the
affected
knee
CA 02488861 2004-12-02
18
1 ) Visits outside of the planned time guide could be carried out both during
treatment and during the follow-up test.
2) End of research medication or early stopping of the research medication.
3) End of alternative therapy or early stopping of alternative therapy.
4) Visit 6 took place on day 56 or after 4 treatment-free weeks.
5) Visit 7 took place on day 84 or after 8 treatment-free weeks.
6) Urine-strip test.
7) The study can take place within 6 weeks of the baseline.
In patients with osteonecrosis or osteoarthritis, oral iloprost produced a
degeneration of the bone marrow edema in 53% of patients, while this was the
case in
only 19% of the patients under Tramadol~. This difference was statistically
significant (p
= 0.0336, Fisher's Exact Test). This effect coincided with a significant
alleviation of
pain as also shown in the pilot study. Moreover, only with iloprost therapy
was a
reduction in subchrondral lesions noted and thus a reference to the
restoration of
structural integrity was provided.