Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
METHOD FOR RECONSTRUCTION OF LIMITED DATA IMAGES USING
FUSION-ALIGNED REPROJECTION AND NORMAL-ERROR-ALIGNED
REPROJECTION
Cross-Reference to Related Applications
This application is a continuation in part of U.S. Application No. 09/802,468,
filed March 9, 2001, entitled "System and Method for Fusion-Aligned
Reprojection of
Incomplete Data," the disclosure of which is incorporated herein by reference.
Sack~round of the Invention
The present invention relates generally to radiation therapy equipment for the
treatment of tumors, and more particularly to methods for reconstructing
incomplete
patient data for radiation therapy and treatment verification.
Medical equipment for radiation therapy treats tumorous tissue with high
energy
radiation. The amount of radiation and its placement must be accurately
controlled to
ensure both that the tumor receives sufficient radiation to be destroyed, and
that the
damage to the surrounding and adj acent non-tumorous tissue is minimized.
External source radiation therapy uses a radiation source that is external to
the
patient to treat internal tumors. The external source is normally collimated
to direct a
beam only to the tumorous site. Typically, the tumor will be treated from
multiple angles
with the intensity and shape of the beam adjusted appropriately. The source of
high
energy radiation may be x-rays or electrons from a linear accelerator in the
range of 2-25
MeV, or gamma rays from a highly focused radioisotope such as Co6°
source having an
energy of 1.25 MeV.
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
One form of external radiation therapy uses the precision of a computed
tomography (CT) scanner to irradiate cancerous tissue in addition to acquiring
CT images
immediately before, immediately after, and/or during radiation treatment
delivery. It is
particularly useful to have online CT imaging capability integrated into a
radiotherapy
delivery system since it helps identify changes in a patient's position and
anatomy
between the time of imaging and treatment. However, many current patient
imaging
systems, especially ones that are integrated into radiotherapy treatment
systems suffer
from a limited field-of view (LFOV) in that collected imaging data does not
encompass
the patient's complete cross-section. This LFOV can cause visibility problems
with the
images, images with artifacts, images with distorted values, and affect
applications that
use these images, including dose calculations, delivery verification,
deformable patient
registration, deformable dose registration, contouring (automatic, manual, or
template-
based).
Intensity modulated radiation therapy uses intensity modulated radiation beams
that enter the patient's body at a greater number of angles and positions than
conventional
therapies, thereby lessening the amount of radiation that healthy tissues are
subjected to
and concentrating the radiation where it is needed most, at the cancer
site(s). Essentially,
the radiation field is "sculpted" to match the shape of the cancerous tissue
and to keep the
dose of radiation to healthy tissue near the cancer low. This type of
radiotherapy greatly
benefits from visualization of a patient's internal anatomy and accurate
calculation of the
delivered radiation dose. A radiation treatment plan may be based on a CT
image of the
patient. As is known in the art, a CT image is produced by a mathematical
reconstruction
2
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
of many projection images obtained at different angles about the patient. In a
typical CT
image, the projections are one-dimensional line profiles indicating the
attenuation of the
beam by a "slice" of the patient. The actual CT data is held in sinogram space
as a matrix
wherein each row represents a gantry position, a gantry angle, a ray angle or
the like (a
first sinogram dimension); each column represents a detector number, a
detector distance,
a detector angle, a ray position, or the like (a second sinogram dimension). A
third
sinogram dimension is commonly used with multi-row or volumetric detectors,
representing each detector row. The matrix of data obtained in a CT image can
be
displayed as a sinogram 10 as shown in FIG. 1, or reconstructed into a two-
dimensional
image 12, as shown in FIG. 2.
In some radiotherapy systems, a physician views the cancerous areas on a CT
image and determines the beam angles and intensities (identified with respect
to the
tumor image) which will be used to treat the tmnor. In an automated system,
such as that
disclosed in U.S. Patent No. 5,661,773, the disclosure of which is hereby
incorporated by
reference, a computer program selects the beam angles and intensities after
the physician
identifies the tumorous region and upper and lower dose limits for the
treatment.
More specifically, planning CT images are used to create a three-dimensional
(3-
D) treatment plan of a region of interest. This region of interest is broken
down into units
called voxels, which are defined as volumetric pixels. Each voxel is then
assigned a
particular radiation dose depending on what type of tissue or other matter it
contains, e.g.
cancerous tissue, healthy tissue, air, water, etc.
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
Normally, the planning CT image of a patient is acquired substantially before
the
radiation treatment to allow time for the treatment plan to be prepared.
However, the
position of organs or other tissue to be treated can change from day-to-day
because of a
variety of factors. Further, patients move during treatment because of
breathing, muscle
twitching, or the like, and many patients are larger than the field-of view
(FOV) of the
online CT imaging system. Uncertainty in the positioning of the patient with
respect to
the planning CT image can undermine the conformality of the radiation
delivery.
Thus, it is highly preferable to verify the treatment plan based on data
obtained
just prior to the time of treatment. This verification process can be done by
techniques
that compare the planning image to an image of the patient at the time of
treatment.
Acquisition of an online tomographic image for the latter provides the
benefits of 3-D
tomographic imaging without requiring that the patient move between the
imaging and
treatment steps.
Unfortunately, the imaging data sets obtained on the day of treatment to be
used
for preparing the patient model are often incomplete or limited. These
limitations may be
caused by limited FOVs set by the field size of the multi-leaf collimator
(MLC) attached
to the linear accelerator and the detector size of the radiotherapy system.
The limitations
may also be caused by patients that are too large to fit within the FOV of the
CT imaging
system associated with the radiotherapy equipment applying the radiation dose,
yielding a
LFOV image as shown in FIG. 3, which shows only a portion of the image shown
in FIG.
2. The FOV or image data sets may also be intentionally limited by modulated
treatment
4
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
data or region-of interest tomography (ROIT) involving reconstruction of
treatment data,
intentionally only delivered to a specific region(s). For example, in FIG. 3,
not only is
there a LFOV, but the data around the edges contains significant artifacts so
that the
image has an irregular border and internal values that are distorted.
As mentioned above, the LFOV of radiotherapy images creates problems of
impaired visibility and degraded dose calculations. The most common reasons
for
impaired visibility are the limited field size of the MLC attached to the
linear accelerator
and the limited detector size. These limitations prevent the CT imaging system
from
collecting complete FOV data for all sizes of patients at all sites. The
problem of
degraded dose calculations is caused by distorted electron densities and the
loss of
peripheral information for attenuation and scatter from the LFOV images. This
distortion
of image values and loss of peripheral information can lilcewise affect other
applications
that utilize these images.
To resolve the problem of limited imaging data sets in which only a portion of
an
image is obtained, several scans of the patient may be made at various
detector or patient
positions, and then combined into a complete set. This has been done by adding
together
sinogram data, but requires that the imaging apparatus or patient position can
be reliably
modified accordingly. This is often not possible. Further, the problem of
artifacts is still
present due to the significant degree of mismatch between such data sets,
while the
additional handling of the patient is more costly, time intensive and can be
difficult for
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
frail patients. Moreover, patients receiving multiple scans receive higher
doses of
radiation than with a single scan.
Reconstruction of incomplete imaging data sets using available techniques
results
in images that do not show the complete extent of the patient's body, can have
artifacts
and incorrect voxel values, and thus, limit the extent to which the images can
be used for
applications including delivery verification, dose reconstruction, patient set-
up,
contouring, deformable patient registration and deformable dose registration.
Accordingly, a need exists for methods that can solve problems caused by
limited
imaging data sets.
Summary of the Invention
The present invention relates to methods by which an incomplete CT patient
data
set can be combined with an existing CT patient data set to create an image of
a patient
that is complete and with fewer artifacts. The present invention provides
methods for
utilizing complete planning CT data for reconstruction of incomplete CT data
with
particular regard for a patient's daily anatomical variations. The complete
planning CT
data is used as prior information to estimate the missing data for improving
and
reconstructing incomplete CT patient data.
In a first embodiment of the present invention, the method includes the steps
of
obtaiiung first and second sinogram data sets or images from a patient. Both
data sets are
converted to images, and aligned together so that statistically, there is
optimal registration
between the two images. The aligned or "fused" image is reprojected as a
sinogram.
6
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
This reprojected sinogram is compared to either the first or second sinogram
to determine
what data exists beyond the scope of the first or second sinogram. This
additional data is
added to the sinogram to which the reprojected sinogram was compared to obtain
an
augmented sinogram The augmented sinogram is then converted or reconstructed
to an
image, referred to as a fusion-aligned reproj ection (FAR) image.
The method of the first embodiment of the present invention is advantageous in
that the availability of only one limited data sinogram/image will not affect
the ability to
perform accurate delivery verification, dose reconstruction, patient setup or
the like. The
previously taken complete image or "second image" is fused, or aligned, to the
limited
data image or "first image." The sinogram representing the fused image is
compared to
the limited data sinogram, and the augmented limited data sinogram is prepared
therefrom. From the augmented limited data sinogram the FAR image is obtained.
The
FAR image is used to accurately apply radiation to the treatment area, which
may be
positioned differently or contain anatomical changes as compared to the
previously
obtained complete image.
FAR compensates for limited data radiotherapy images by enhancing the
conspicuity of structures in treatment images, improving electron density
values, and
estimating a complete representation of the patient. FAR combines the LFOV
data with
prior information about the patient including CT images used for planning the
radiotherapy. The method of the first embodiment includes aligning or "fusing"
the
LFOV image and the planning image, converting the images into "sinogram
space",
7
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
merging the images in sinogram space, and reconstructing the images from
sinograms
into normal images. A key step of the FAR method is "fusion" or alignment of
the
planning image with the LFOV image. However, if a patient's treatment position
is close
to the planning position, explicit fusion under the FAR method may not be
necessary.
Instead, an implicit fusion may be adequate if the normal setup error is
sufficiently small.
Under these circumstances when this implementation of FAR is not viable or
necessary, it is possible to replace the explicit fusion of FAR with an
implicit fusion,
referred to as normal-error-aligned reproj ection (NEAR). NEAR, another
embodiment of
the present invention, is a variation of FAR for situations where explicit
fusion is not
possible or does not yield good results. Specifically, NEAR is accomplished
when the
images are already sufficiently aligned, as often results from using common
radiotherapy
patient setup protocols. The patient is often positioned within a few
millimeters and a
few degrees of the intended position, creating a normal setup error which
constitutes the
implicit fusion of NEAR.
A benefit of NEAR is that it may enable an iterative (two or more) variation
of
FAR (NEAR2FAR). It is possible to iterate these methods using multiple
applications of
FAR, or going from NEAR to FAR (NEAR2FAR) for a two-iteration process. NEAR
can be followed by FAR iterations, or FAR can be tried multiple times with
different
registration results. After creating a NEAR image, the quantitatively improved
voxel
values in the FOV might enable an explicit fusion with the planning image, and
a FAR
image could be generated. NEAR and NEARZFAR may be particularly beneficial
when a
8
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
LFOV causes severe quantitative and qualitative degradation of the images,
whether
because of a large patient, a small detector or MLC, or because a ROIT
strategy is being
pursued. NEAR may also be quicker than FAR, as no time is required to do an
explicit
fusion.
S NEAR, FAR, and NBAR2FAR utilize planning CT data or other images as
imperfect prior information to reduce artifacts and quantitatively improve
images. These
benefits can also increase the accuracy of dose calculations and be used for
augmenting
CT images (e.g. megavoltage CT) acquired at different energies than planning
CT images.
FAR, NEAR and NEAR2FAR may also be used for mufti-modality imaging
(combining CT images with MRI images, etc.). While an MRI image may have
different
image values, they may be correctable, or they may show the patient boundary,
which
might be enough.
The methods of the present invention improve the data by aligning the LFOV and
planning images, and merging the data sets in sinogram space, or vice versa.
One
alignment option is explicit fusion, for producing FAR images. For cases where
explicit
fusion is not viable, FAR can be implemented using the implicit fusion of
NEAR. The
optional iterative use of NEAR and/or FAR is also possible, as are
applications of NEAR
and FAR to dose calculations and the compensation of LFOV online megavoltage
CT
images with kilovoltage CT planning images as mentioned above.
9
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
Various other features, objects, and advantages of the invention will be made
apparent to those skilled in the art from the following detailed description,
claims, and
accompanying drawings.
Brief Description of the Drawings
FIG. 1 an example of a sinogram obtained from the CT image of a patient;
FIG. 2 is an example of a planning image of a patient obtained from a sinogram
similar to that shown in FIG. l;
FIG. 3 is an example of a LFOV treatment image of a patient;
FIG. 4 is a flow diagram showing the steps involved in creating a FAR
treatment
image in accordance with a first embodiment of the present invention;
FIG. 5 is a schematic representation of a full image scan of a patient;
FIG. 6 is a schematic representation of FIG. 5 with illustrative "anatomical"
changes and a different alignment, a limited image portion is shown in the
center, and the
remaining portion, which was not fully scanned, is shown in phantom;
FIG. 7 demonstrates how the full image of FIG. 5 is aligned to the limited
image
of FIG. 6 as used to achieve the resulting FAR image;
FIG. 8 is a schematic representation of a FAR image;
FIG. 9 is a schematic representation of a full image corresponding to the
image of
FIG. 6;
FIG. 10 shows a schematic representation of the actual alignment or "fusion"
of
the images of FIGS. 5 and 6;
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
FIG. 11 is a reconstructed FAR image of FIGS. 2 and 3 aligned in accordance
with the method of the present invention;
FIG. 12 shows a comparison of a planning image, a LFOV treatment image, an
ideal treatment image, and a FAR treatment image;
FIG. 13 shows an example FAR sinogram obtained by merging a LFOV online
sinogram and an aligned planning CT sinogram;
FIG. 14 shows a comparison of radiotherapy dose calculations for a LFOV image
and a FAR image;
FIG. 15A is a flow diagram showing the steps involved in creating an aligned
reprojection image in accordance with the present invention;
FIG. 15B is a flow diagram showing the steps involved in creating an aligned
reprojection image in accordance with a different embodiment of the present
invention;
FIG. 15C is a flow diagram showing the steps involved in creating an aligned
reprojection image in accordance with another different embodiment of the
present
invention;
FIG. 16 shows examples of LFOV images, NEAR images, and FAR images for
field-of view sizes of 38.6, 29.3, and 19.9 cm based upon the online image;
FIG. 17 shows a LFOV reconstruction for a 10.5 cm FOV, a NEAR
reconstruction, and a two iteration NEAR2FAR reconstruction;
FIG. 18 shows a comparison of radiotherapy dose calculations for complete FOV
online images and a LFOV image, a NEAR image, and a NEAR2FAR image, for rectal
points, bladder points, and prostate points; and
11
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
FIG. 19 shows canine CT images from a kilovoltage CT scanner, a megavoltage
CT scanner, a LFOV version of the megavoltage image, and a FAR reconstruction
from
the LFOV data augmented with planning CT data.
Detailed Description of the Invention
Referring now to the drawings, FIG. 1 is an example of a sinogram 10 obtained
from the CT image of a patient. FIG. 2 is an example of a planning CT image
obtained
from a sinogram similar to that shown in FIG. 1, and FIG. 3 is an example of a
LFOV
image from an online CT scan of the patient just prior to radiotherapy
treatment.
A preferred method in accordance with a first embodiment of the present
invention is shown in the flow diagram of FIG. 4. FIG. 4 represents the first
embodiment
process involved in creating a fusion-aligned reprojection (FAR) image from a
limited
data image and a complete planning image. The process begins by obtaining a
limited
data sinogram 50 typically representing the treatment area from a patient. The
limited
data sinogram 50 is preferably obtained near the time that the patient is
receiving his or
her radiation treatment, but may be obtained at any time. The limited data
sinogram 50 is
reconstructed to a limited data image 52, as seen in the examples of FIGS. 1
and 3, and
represented schematically in FIG. 6 as limited object 156. FIG. 3 contains a
significant
amount of artifacts such as a white irregular border 53 around the image along
with some
image distortion of image values. By way of example, the treatment area
targeted in FIG.
3 is of a prostate. However, the methods of the present invention can be
applied to
12
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
images of any part of the body, or be used in other applications, such as
veterinary
medicine or extended to industrial uses.
A complete planning image 54 of the same patient and same treahnent area, as
shown by way of example in FIG. 2 as image 12, and represented schematically
in FIG. 5
as object 154, is typically obtained prior to obtaining the limited data image
52, image 14
of FIG. 3, for the purpose of treatment planning. Even if limited data image
52, image 14
of FIG. 3, were taken only minutes after the complete planning image 54, image
12 of
FIG. 2, there are often inherent differences between the location of certain
organs and/or
tissue due to motion caused by normal bodily functions as the patient travels
from the
planning CT system to the treatment system and is setup again. Additionally,
if enough
time has elapsed between images, weight loss or growth of certain tissue can
also occur.
Internal organ motion also causes some degradation relative to planned dose
distribution.
It is noted that complete planning image 54, image 12 of FIG. 2, or limited
data
image 52, image 14 of FIG. 3, need not be from a CT scanner or imager, and
that this
technique can be generally applied to matching images from different
projection imaging
or multi-modality imaging, such as magnetic resonance imaging (MRI), positron
emission
tomography (PET), or single photon emission tomography (SPECT). Where
different
imaging types are used, there may be misalignment or disagreement between
images
values due to the differing methods of data collection. In addition, cross-
energy
compensation of LFOV online megavoltage CT images with kilovoltage CT planning
images is also contemplated in the various embodiments of the present
invention.
13
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
The two images 12 and 14 shown in FIGS. 2 and 3 and represented schematically
in FIGS. 5 and 6 by objects 154 and 156, have differences between them. In the
actual
image examples of FIGS. 2 and 3, intestinal gas 16 is shown in FIG. 3, thereby
displacing
the treatment target. In the schematic example of FIGS. 5 and 6, object 154 is
composed
of diagonals 158a and 160a and an inclusion 161a, within a frame 162a. Limited
object
156 shows only corresponding diagonals 160b and 158b, and part of the
inclusion
designated as 161b. Thus, there is a change between diagonal 158a and 158b and
only
partial data for inclusion 161b.
As shown in FIG. 4, "fusion" or image registration techniques are used to
align
limited data image 52 and complete image 54. In the schematic example in FIG.
7,
limited object 156 is fused with complete object 154 so that statistically,
there is optimal
registration between the objects 154 and 156. FIG. 7 shows how the orientation
of object
154 is aligned to closely match that of object 156. FIG. 10 shows diagonal
160c as the
perfect registration between diagonals 160a and 160b. There is less than
perfect
registration between diagonals 158a and 158b. Both lines are superimposed only
by way
of example to show that fusion is not perfect as evidenced by the double edge
163. To
the contrary, a theoretically perfect fusion may not exist in the context of
anatomical
changes, and is not a requirement for these methods.
FAR is not specific to the registration technique. It could be through
automatic,
manual, or hybrid methods that are known in the art. Image registration or
fusion may be
achieved by several techniques. One such technique is known as mutual
information
14
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
(MI), for which a well-l~nown algorithm has been developed. One such example
of this
algoritlun being used to register multi-modal images is described in the
following
publication, incorporated herein by reference: Frederik Maes, Andre Collignon,
Dirlc
Vendermeulen, Guy Marchal, and Paul Suetens, Multimodality Image Registration
by
Maximization of Mutual Information, Vol. 16, No. 2, IEEE Transactions on
Medical
Imaging, 187 (April 1997).
Extracted Feature Fusion (EFF) is another registration technique providing
numerous advantages over prior art techniques. EFF is a voxel-based image
registration
method, wherein only extracted features of images are registered or fused. For
example, a
patient's bone structure usually stays the same even when a patient loses a
substantial
amount of weight. Therefore, the bones can in effect be extracted from each
image
subject to alignment, and then registered using statistical methods. In the
simple example
of FIG. 5, diagonal 160a and frame 162 may represent bone or tissue that
remains
relatively unchanged over time. Therefore, only these relatively static
features might be
selected for fusion, while other features that are more dynamic, perhaps
diagonals 158a,
158b and inclusion 161a, 161b, need not be included in the registration
calculations.
The benefits of registering only an extracted portion of an image are reduced
calculation times, improved accuracy, and more clearly defined goals for
alignment in
cases where the patient has significantly changed in shape. The speed benefits
arise from
the registration of fewer data points, which in this case are voxels. The
total processing
time is generally proportional to the number of points selected, so reducing
that number
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
from the size of the entire three-dimensional image set to a subset of points
meeting
certain criteria (e.g. voxels that represent bone or do not represent air)
will typically
reduce calculation times. This reduction of voxels can provide more accurate
results than
other methods of reducing the number of voxels for MI techniques, such as
regular down-
sampling.
Other image registration techniques include manual fusion, alignment using
geometric features (e.g., surfaces), gradient methods, and voxel-similarity
techniques.
Sinogram-based registration techniques could also be applied.
Any useful LFOV registration for FAR, whether automatic, manual or hybrid,
implies that there is some information in those images in spite of any
quantitative and
qualitative degradation. In these cases, the goal of FAR is to quantitatively
and
qualitatively improve upon the information present by incorporating additional
prior
information. Yet, as FOV's become more severely reduced, images may lose their
utility
for automatic fusion, manual fusion and visual inspection. There are also a
number of
other reasons why automatic fusion may not provide the desired result, such as
finding a
local minimum. Another problem with fusion is that in the presence of
anatomical
changes there may not be an unambiguous correct alignment, as some structures
may
align well at the expense of others, as demonstrated in FIG. 10. In these
cases, NEAR,
iterative application, and testing multiple registrations provide additional
opportunities.
Referring again to FIG. 4, the aligned or transformed complete image 56 is
reprojected as a sinogram 58. The data for sinogram 58 is once again in a
matrix wherein
16
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
each row represents an angle, and each column represents a distance. The data
matrix of
the reprojected sinogram 58 is compared to the data matrix for limited data
sinogram 50
to determine what data is missing from the limited data sinogram 50. This is
now
possible because the reprojected sinogram of the transformed complete image 58
is in
alignment with the limited data sinogram 50.
The approximation of the missing sinogram data from the reprojected sinogram
of
transformed complete image 58 is added to the limited data sinogram 50 to
create an
augmented limited data sinogram 60. The augmented limited data sinogram 60 is
reconstructed to a FAR image 62 that is an approximation of what the complete
image
would have looked like at the time the limited data image 52 was obtained. The
FAR
image 62 is represented schematically in FIG. 8. Frame 162a is the same as in
FIG. 5,
and diagonals 158c, 160c and inclusion 161c are now complete. This can
compared to
the object 168 in FIG. 9, which represents the image that would have been
taken at the
time of treatment if it were possible to obtain a complete image. The fact
that the outer
regions 170 of diagonal 158d are not the same as diagonal 158c is not critical
to the
invention.
FIG. 11 represents a reconstructed FAR image obtained by combining the
sinograms of the LFOV and the complete planning images shown in FIGS. 2 and 3
in
accordance with the method of a first embodiment of the present invention. It
can be seen
that slight artifacts such as the faint ring 180 as shown in FIG. 11 can still
result from this
method. However, such artifacts are insignificant because they do not impair
the
17
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
conspicuity of the important structures in the FOV, nor are they noticeably
detrimental to
dose calculations or other processes that utilize these images.
The reconstructed FAR image obtained from the method of the first embodiment
of the present invention can then be used for patient setup (positioning the
patient prior to
delivery), contouring (identifying target regions and sensitive structures,
either
automatically, manually, or with a template-based approach), dose registration
(changing
delivery patterns to compensate for patient position and/or tumor changes),
delivery
verification (using a signal measured at an exit detector to compute energy
fluence
directed toward a patient), deformable patient registration and deformable
dose
registration (using anatomical, biomechanical and region of interest data to
map changes
in the patient's anatomy between each fraction, a reconstructed dose is mapped
to a
reference image to obtain a cumulative dose).
FIG. 12 shows the comparison of a planning image 12', which is equivalent to
the
planning CT image 12 of FIG. 2, a LFOV treatment image 14', which is
equivalent to the
LFOV image 14 of FIG. 3, an ideal treatment image 20, and a FAR treatment
image 18',
which is equivalent to the FAR image 18 of FIG. 11. It should be noted that
the FAR
treatment image 18 and 18' is substantially similar to the ideal treatment
image 20, except
for the slight artifact rings 180 and 180' that do not impair the conspicuity
of the
important structures in the FOV, nor are they noticeably detrimental to dose
calculations.
The completion process of FIG. 4 can be seen in sinogram space in FIG. I3.
FIG.
13 shows an example FAR sinogram 26 obtained by merging a LFOV sinogram 22
with
18
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
an aligned planning sinogram 24. The truncated limited data sinogram 22 is
shown in
FIG. 13A. The missing data from the LFOV sinogram 22 is estimated from the
aligned
planning sinogram 24 shown in FIG. 13B. The resulting FAR sinogram 26 shown in
FIG.
13C estimates the missing data from the aligned planning sinogram 24 of FIG.
13B.
FIG. 14 shows a comparison of radiotherapy dose calculations for a LFOV image
28 and a FAR image 30. The LFOV image 28 results in substantial dose
calculation
errors, while the FAR image 30 yields near perfect dose calculations. The LFOV
dose
volume histogram 28 (DVH) shows both overestimation and underestimation
between the
calculated and delivered doses, while the FAR DVH 30 shows that the doses
calculated
and delivered for the FAR image are near perfect. The DVHs calculated with FAR
images are virtually identical to those for the complete images.
FIGS. 15A, 15B, and 15C represent different embodiments of methods involved
in creating an aligned-reprojection image from a limited data image or
sinogram and a
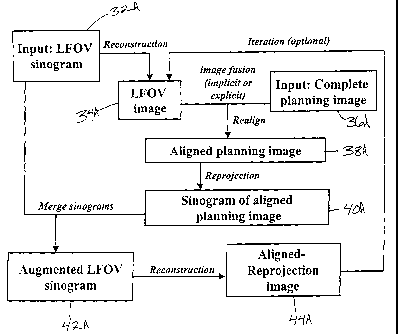
complete planning image or sinogram. Referring first to FIG. 15A, a FAR, NEAR,
or
NEAR2FAR image is created by obtaining a limited data sinogram 32A
representing the
treatment area from a patient. The limited data sinogram is reconstructed to a
limited
data image 34A. A complete planning image 36A of the same patient is typically
obtained prior to obtaining the limited data image 34A. Image fusion or image
registration techniques are used to align the complete planning image 36A with
the
limited data image 34A. The aligned complete planning image 38A is reprojected
as a
sinogram 40A. The reprojected sinogram of the aligned planning image 40A is
compared
19
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
to the limited data sinogram 32A. The missing sinogram data from the
reprojected
sinogram 40A is added or merged with the limited data sinogram 32A to create
an
augmented limited data sinogram 42A. The augmented limited data sinogram 42A
is
reconstructed to an aligned-reprojection image 44A that is an approximation of
what the
complete image would have looked like at the time the limited data image was
obtained.
The aligned-reprojection image may be fed back to the limited data image 34A
for a
multiple iteration method to possibly achieve better results. The above method
is flexible
with regard to which image (e.g., complete FOV planning image or limited
online FOV
image) is realigned to the other and reprojected. What matters is that the
complete
planning image is used to estimate the missing data from the limited data
image. For
example, the complete planning image could be realigned to the LFOV image
creating an
aligned planning image, reproject the aligned planning image to a sinogram,
augment or
merge the LFOV sinogram with the aligned planning sinogram to yield an
augmented
LFOV sinogram, and reconstruct the augmented LFOV sinogram to an aligned-
reprojection image as shown in FIG. 15A. Or alternatively, the LFOV image
could be
realigned to the complete planning image creating an aligned LFOV image,
reproj ect the
aligned LFOV image to a sinogram, augment that sinogram with the complete
planning
sinogram to yield an augmented LFOV sinogram, and reconstruct the augmented
LFOV
sinogram to an aligned-reprojection image.
The method of realigning the image and reprojecting it into a sinogram can be
mathematically streamlined as shown in FIGS. 15B and 15C. Generally, the
relative
alignment between the complete planning image and the limited data image is
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
determined. Then, instead of realigning the complete planning image to the
limited data
image and reprojecting the aligned planning image to a sinogram, one can
realign the
complete planning sinogram to the limited data sinogram (or vice versa), which
is an
alternate, but equivalent, method of achieving the same result; a realigned
sinogram of the
planning image. The aligned planning sinogram is then used to estimate the
missing data
from the limited data sinogram which is augmented into the limited data
sinogram. The
augmented limited data sinogram is then reconstructed to create an aligned-
reprojection
image.
This alternate embodiment allows an estimate of the missing data from a
limited
data sinogram with an aligned complete planning sinogram. It does not matter
conceptually how the sinogram is realigned, whether an image is realigned and
reprojected or if the sinogram is realigned directly.
FIG. 15B illustrates another embodiment of a method for creating an aligned-
reprojection image from a limited data sinogram or image and a complete
planning image
or sinogram. The inputs to the process are a complete planning image 36B or
complete
planning sinogram 10~B and a LFOV sinogram 32B. The LFOV sinogram 32B is
initially reconstructed into a LFOV image 34B and then fused (explicit (FAR)
or implicit
(NEAR)) with the complete planning image 36B. The complete planning image 36B
is
reprojected to a sinogram or the original planning sinogram 10~B is
transformed with the
fusion result to yield an aligned planning image 40B. The sinogram data of the
aligned
planning image 40B is used to estimate the data missing from the LFOV sinogram
32B.
21
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
The limited data sinogram 32B is merged with the aligned planning image
sinogram 40B,
resulting in an augmented limited data sinogram 42B. This augmented limited
data
sinogram 42B is reconstructed into an aligned-reprojection image 44B. The
aligned-
reprojection image may supersede the original limited data image 34B for a
multiple
iteration process (NEAR2FAR).
FIG. 15C illustrates yet another embodiment of the present invention for
creating
an aligned-reprojection image from a limited data sinogram and a complete
planning
image or sinogram. The inputs to the process are a limited data sinogram 32C
and either
an optional complete planning image 36C or most preferably a complete planning
sinogram 108C. If the process starts with a complete planning image 36C as one
of the
inputs, then that image is reprojected to sinogram space to yield a complete
planning
sinogram 1080. The limited sinogram 32C is fused in sinogram space (explicit
(FAR) or
implicit (NEAR)) with the complete planning sinogram 108C. The next step
involves
realigning the complete planning sinogram 1080, or realigning and reprojecting
the
complete planning image 36C using the same fusion result. The resulting
aligned
planning image sinogram 40C is merged with the limited data sinogram 32C to
create an
augmented limited data sinogram 42C. The augmented limited data sinogram 42C
is then
reconstructed into an aligned-reprojection image 44B.
To summarize the differences between the alternate embodiment methods of FIG.
15C, the fusions are performed in sinogram-space as the limited data sinogram
32C is
fused (implicit or explicit) to the complete data sinogram 108C, unlike the
embodiments
22
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
of FIGS. 15A and 15B that use image fusion. Based upon the sinogram fusion,
the
realigned planning sinogram 40C can be created by realigning sinogram 108C, or
by
realigning planing image 36C and reprojecting into sinogram space. The process
is then
the same for each case. The aligned planning sinogram 40C is merged with the
limited
data sinogram 32C to create an augmented limited data sinogram 42C. The
augmented
limited data sinogram 42C is then reconstructed into an aligned-reprojection
image 44B.
FIG. 16 shows representative images from a planning CT image 66 and the
corresponding online image 64. The contours 65 for the planning images are
shown in
blaclc, while the contours 67 for the online images are shown in white. Three
different
LFOV images 68, 70, 72, NEAR images 74, 76, 78, and FAR images 80, 82, 84 for
field-
of view sizes of 38.6, 29.3, and 19.9 cm are shown based upon the online image
64. As
the FOV decreases, the artifacts become more severe in the LFOV images 68, 70,
72,
while the NEAR 74, 76, 78 and FAR images 80, 82, 84 are less affected. These
images
are representative of how NEAR and FAR can utilize available information to
qualitatively improve the reconstructions for a range of FOV sizes. In this
particular case,
there is little visual difference between the NEAR and FAR images. The
similarity of
NEAR and FAR images can occur for several reasons. Where the normal setup
error is
small, the explicit fusion will generally not improve much upon the normal
error, or
because the anatomical differences between the planning CT image 66 and the
online
image 64 are a more significant factor than the alignment between those
images, there
will also be little improvement.
23
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
NEAR and FAR can utilize available information to qualitatively improve the
reconstructions for a range of FOV sizes. The explicit and implicit fusion
align the
planning data with the LFOV data. A LFOV online image augmented with NEAR or
FAR can produce images that are quantitatively closer to the complete FOV
online image
than the planning image alone. NEAR and FAR create quantitative improvements
and
artifact reductions, and also improve upon the accuracy of dose calculations.
FAR may
not be possible if the distortion of image values preclude a successful
fusion. In this case,
a NEAR image is created, and by fusing or aligning the NEAR image to the
planning CT
image, a NEAR2FAR image is generated, further reducing artifacts and improving
alignment. The results of an iterative application of NEAR and FAR are shown
in FIG.
17.
FIG. 17 shows a LFOV reconstruction 86 for a 10.5 cm FOV, a NEAR
reconstruction 88, and a two iteration NEAR2FAR reconstruction 90. In this
case, a FAR
reconstruction was not immediately possible because the distortion of image
values
precluded a successful fusion. A NEAR image was created, and by fusing the
interior
scan region to the planning CT image, a two iteration NEAR2FAR image could be
generated.
FIG. 18 shows a comparison of radiotherapy dose calculations for complete FOV
online images and a LFOV image 92, a NEAR image 94, and a NEAR2FAR image 96,
for prostate points, bladder points, and rectal points. The DVH's (Dose Volume
Histogram) are based upon the known contours from the complete FOV online
image.
24
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
The LFOV dose calculation overestimates the prostate dose by approximately
15%, and
the rectum and bladder doses have areas of both overestimation and
underestimation.
The dose distributions calculated using NEAR and NEAR2FAR produce DVH's
indistinguishable from the full FOV dose calculation.
FIG. 19 shows canine CT images from a kilovoltage CT scanner 98, a
megavoltage CT scanner 100, a LFOV version of the megavoltage image 102, and a
FAR
reconstruction 104 from the LFOV data augmented with planning CT data. Of
particular
interest is that these data sets were not only acquired on different CT
systems but at
different energies, requiring that FAR combine megavoltage and kilovoltage
data. The
resulting FAR image 104 includes slight artifacts 106 that can result from
this method.
However, such artifacts 106 are insignificant because they do not impair the
conspicuity
of the important structures in the FOV, nor are they noticeably detrimental to
dose
calculations or other processes that utilize these images.
As discussed above, the methods of the present invention may be used for
purposes beyond radiotherapy in cases where potentially imperfect prior
information is
available. While the present description has primarily disclosed use of prior
information
in the form of a planning CT, it is feasible to apply NEAR and FAR to multi-
modality
images, such as creating a FAR image by combining an online CT (megavoltage or
kilovoltage) data set with a planning MRI image. In such cases, the MRI or
other-
modality image needs to be converted to values compatible with the LFOV data
set. A
complex mapping of values will provide the best results, but even using the
alternate
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
modality image to describe the patient's outer contour and using a water-
equivalency
assumption will provide benefits. This is particularly true considering the
demonstrated
robustness of FAR with regard to anatomical changes, imperfect alignments, and
even
systematic differences in reconstructed values between megavoltage and
kilovoltage CT
images. As described above, FAR can also combine megavoltage and kilovoltage
CT
data. In FIG. 19, FAR was used to augment megavoltage CT data sets with
kilovoltage
planning CT data sets.
Other applications include using NEAR and FAR for dose calculations, iterative
application of NEAR and FAR for severely limited FOV's, FIG. 17, and using FAR
for a
combination of l~ilovoltage and megavoltage CT images, FIG. 19. Dose
calculations are
typically based upon CT images and require reconstructed values that can be
calibrated to
electron densities. The artifacts and quantitative distortions introduced by
FOV
truncations may degrade this calibration, while the lack of peripheral
information can
impair the scatter and attenuation calculations often performed when computing
dose.
The methods described above for the present invention can be applied
regardless
of the reasons) the image data set is limited. This includes hardware
constraints, such as
FOV's set by MLC size or detector size, etc. The methods may also be applied
to
intentionally limited data sets or FOV's. An example of this is called region-
of interest
tomography (ROIT), in which the scan FOV is intentionally limited to reduce
patient
dose, even though complete FOV data sets are available. A particular example
would be
reconstruction of treatment data, intentionally only delivered to a specific
regions) of the
26
CA 02489157 2004-12-13
WO 03/105069 PCT/US03/18229
body. This delivery would constitute a partial CT sinogram, and FAR or NEAR
could
estimate the missing data. More generally, the limited data is not necessarily
LFOV, but
can also be more complex patterns of missing data, such as modulated treatment
data.
NEAR and FAR may also be extensible to other types of limited data situations,
such as
limited slice or limited-proj ection images.
While the invention has been described with reference to preferred
embodiments,
it is to be understood that the invention is not intended to be limited to the
specific
embodiments set forth above. It is recognized that those spilled in the art
will appreciate
that certain substitutions, alterations, modifications, and omissions may be
made without
departing from the spirit or intent of the invention. Accordingly, the
foregoing
description is meant to be exemplary only, the invention is to be talcen as
including all
reasonable equivalents to the subject matter of the invention, and should not
limit the
scope of the invention set forth in the following claims.
27