Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02493026 2005-01-19
WO 2004/013102 PCT/EP2003/007964
VEGFR-2 and VEGFR-3 Inhibitory Anthranilamide Pyridines
The invention relates to VEGFR-2 and VEGFR-3 inhibitory anthranilamide
pyridines, their production and use as pharmaceutical agents for treating
diseases that are
triggered by persistent angiogenesis as well as intermediate products for the
production of
compounds.
Persistent angiogenesis can be the cause or precondition of various diseases,
such
as tumor or metastasis growth, psoriasis; arthritis, such as rheumatoid
arthritis,
hemangioma, angiofibroma; eye diseases, such as diabetic retinopathy,
neovascular
glaucoma; renal diseases, such as glomerulonephritis, diabetic nephropathy,
malignant
nephrosclerosis, thrombic microangiopathic syndrome, transplant rejections and
glomerulopathy; fibrotic diseases, such as cirrhosis of the liver, mesangial
cell
proliferative diseases and arteriosclerosis, or can result in an aggravation
of these
diseases.
Persistent angiogenesis is induced by the factor VEGF via its receptor. So
that
VEGF can exert this action, it is necessary that VEGF bind to the receptor,
and a tyrosine
phosphorylation is induced.
Direct or indirect inhibition of the VEGF receptor (VEGF = vascular
endothelial
growth factor) can be used for treating such diseases and other VEGF-induced
pathological angiogenesis and vascular permeable conditions, such as tumor
CA 02493026 2005-01-19
2
vascularization. For example, it is known that the growth of tumors can be
inhibited by
soluble receptors and antibodies against VEGF.
Anthranilamide pyridones that are used as pharmaceutical agents for treating
psoriasis; arthritis, such as rheumatoid arthritis, hemangioma, angiofibroma;
eye diseases,
such as diabetic retinopathy, neovascular glaucoma; renal diseases, such as
glomerulonephritis, diabetic nephropathy, malignant nephrosclerosis, thrombic
microangiopathic syndrome, transplant rejections and glomerulopathy; fibrotic
diseases,
such as cirrhosis of the liver, mesangial cell proliferative diseases,
arteriosclerosis,
injuries to nerve tissue, and for inhibiting the reocclusion of vessels after
balloon catheter
treatment, in vascular prosthetics or after mechanical devices are used to
keep vessels open,
such as, e.g., stents, are known from WO 00/27820 (e.g., Example 38).
The compounds that are known from WO 00/27820 are generally effective in the
indications cited, but their effectiveness is not very pronounced.
Anthranilic acid amides that are are highly effective but also have a good
inhibition of the Cytochrome P 450 isoenzyme 3A4 are also known from WO
00/27819
(Example 2.54). The Cytochrome P 450 isoenzyme 3A4 is one of the essential
metabolic
enzymes via which pharmaceutical agents are degraded. An inhibition of this
isoenzyme
results in undesirable pharmaceutical agent interactions, especially in the
case of
multimorbid patients (patients with multiple disease conditions). There also
exists the
problem that in a combination therapy with other medications, increased
toxicity occurs,
which results from the inhibition of the degradation of the compounds and the
associated
excessive serum levels.
CA 02493026 2005-01-19
3
There is therefore the desire for active ingredients that on the one hand are
effective and on the other hand are more compatible or do not exhibit any
undesirable
side effects.
There is therefore a desire for, on the one hand, more effective, and, on the
other
hand, more compatible compounds.
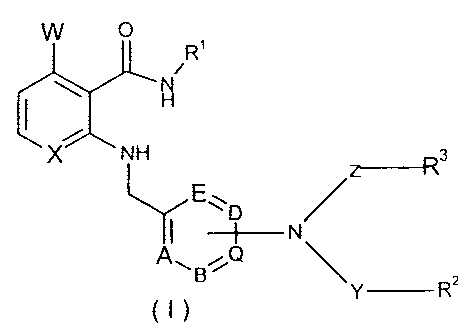
It has now been found that compounds of general formula I
W O
R1
X NH 3
E~ /
-}-- N
Y Rz
in which
X stands for CH or N,
W stands for hydrogen or fluorine,
A, B, D,
E and Q, in each case independently of one another, stand for a nitrogen or
carbon
atom, whereby only a maximum of two nitrogen atoms can be present in
the ring,
R1 stands for aryl or heteroaryl, which optionally can be substituted in one
or
more places in the same way or differently with halogen, hydroxy, C1-C12-
alkyl, C3-C6-cycloalkyl, C3-C6-alkenyl, C2-C6-alkinyl, aralkyloxy, C1-C12-
CA 02493026 2005-01-19
4
alkoxy, halo-C1-C6-alkyl, cyano-C1-C6-alkyl or with the group =O, -S02R 6
or -OR5, whereby the C1-C6-alkyl optionally also can be substituted with
the group -OR5 or -NR9R10,
Y and Z, in each case independently of one another, stand for a bond or for
the
group =CO, =CS or =SO2,
R2 and R3, independently of one another, stand for hydrogen or for the group
-CONR9R10, -S02R6, -COR", -COC,-C6-alkyl, -CO-C,-C6-alkyl-R",
-NR9R10 or for C,-C6-alkyl, C3-C,0-cycloalkyl, C3-C6-cycloalkenyl, aryl or
heteroaryl that is optionally substituted in one or more places in the same
way or differently with halogen, cyano, C,-C12-alkyl, C,-C12-alkoxy,
hydroxy-C,-C6-alkyl, halo-C1-C6-alkyl or with the group -NR 7R8, -ORS,
-C,-C6-alkyl-ORS, -SR4, -SOR4 or -S02R6, or
R2, R3, Y
and Z together with the nitrogen atom form a 3- to 8-membered saturated or
unsaturated ring, which optionally can contain additional heteroatoms in
the ring and optionally can be substituted in one or more places in the
same way or differently with halogen, cyano, C,-C12-alkyl, C,-C12-alkoxy,
halo-C,-C6-alkyl, hydroxy-C1-C6-alkyl, or with the group =O, -OR5 , -SR4
-SOR4 or -S02R6,
R4 stands for C,-C12-alkyl, aryl or heteroaryl,
R5 stands for hydrogen, C,-C12-alkyl, C3-C10-cycloalkyl, C,-C,2-alkoxy, halo-
C,-C12-alkyl, or halo-C3-C6-cycloalkyl,
R6 stands for hydrogen, C,-C12-alkyl, halo-C,-C,2-alkyl, aryl or heteroaryl,
or
CA 02493026 2005-01-19
for the group -NR9R10, whereby the aryl or heteroaryl itself optionally can
be substituted in one or more places in the same way or differently with
C1-C12-alkyl, C1-C6-alkoxy, halogen or halo-C1-C6-alkoxy,
R7 and R8, independently of one another, stand for hydrogen or CI-C12-alkyl,
and
R9 and R10, independently of one another, stand for hydrogen, C1-C6-alkyl,
C2-C6-alkenyl, aryl, C3-Cg-cycloalkyl or for the group -CONR7R8, or for
CI-C12-alkyl that is optionally substituted in one or more places in the
same way or differently with aryl, morpholino, hydroxy, halogen, C1-C,2-
alkoxy, or with the group -NR7R8, whereby the aryl itself optionally can
be substituted in one or more places in the same way or differently with
C1-C6-alkoxy or halo-C1-C6-alkyl, or
R9 and R'0 together form a 5- to 8-membered ring that can contain additional
heteroatoms, and
R" stands for C 1-C6-alkyl, C 1-C6-alkoxy, hydroxy-C 1-C6-alkyl, hydroxy-C 1-
C6-alkoxy, C3-C6-cycloalkyl, phenyl, pyridyl, biphenyl or naphthyl,
whereby the phenyl itself can be substituted in one or more places in the
same way or differently with C1-C6-alkyl, or halo-C1-C6-alkyl, as well as
isomers, diastereomers, tautomers and salts thereof,
exhibit improved properties, i.e., high effectiveness with simultaneously less
CYP450
3A4 inhibition.
The compounds according to the invention prevent a tyrosine phosphorylation or
stop persistent angiogenesis and thus the growth and propagation of tumors,
whereby
CA 02493026 2005-01-19
6
they are distinguished in particular by a slighter inhibition of isoforms of
Cytochrome P
450 (3A4).
Medication with the compounds according to the invention can therefore also be
done at no risk even without regard to pharmaceutical agents that are
administered at the
same time and that are degraded via these isoforms.
Alkyl is defined in each case as a straight-chain or branched alkyl radical,
such as,
for example, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl,
pentyl, isopentyl
or hexyl, heptyl, octyl, nonyl, decyl, undecyl, or dodecyl.
Alkoxy is defined in each case as a straight-chain or branched alkoxy radical,
such as, for example, methyloxy, ethyloxy, propyloxy, isopropyloxy, butyloxy,
isobutyloxy, sec-butyloxy, pentyloxy, isopentyloxy, hexyloxy, heptyloxy,
octyloxy,
nonyloxy, decyloxy, undecyloxy or dodecyloxy.
Cycloalkyls are defined as monocyclic alkyl rings, such as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, or cycloheptyl, cyclooctyl, cyclononyl or
cyclodecyl, but also bicyclic rings or tricyclic rings, such as, for example,
adamantanyl.
Cycloalkyl radicals can contain, instead of the carbon atoms, one or more
heteroatoms, such as oxygen, sulfur and/or nitrogen. Those heterocycloalkyls
with 3 to 8
ring atoms are preferred.
Cycloalkenyl is defined in each case as cyclobutenyl, cyclopentenyl,
cyclohexenyl, cycloheptenyl, cyclooctenyl, cyclononenyl or cyclodecenyl,
whereby the
linkage can be carried out both to the double bond and to the single bonds,.
Halogen is defined in each case as fluorine, chlorine, bromine or iodine.
CA 02493026 2005-01-19
7
Halo-alkyl, halo-alkoxy, etc., is defined in that the alkyl, alkoxy, etc., is
substituted in one or more places, in the same way or differently, with
halogen.
Alkenyl is defined in each case as a straight-chain or branched alkenyl
radical that
contains 2-6, preferably 4-6, C atoms. For example, the following radicals can
be
mentioned: vinyl, propen- l -yl, propen-2-yi, but- l -en- I -yl, but- l -en-2-
yl, but-2-en- I -yl,
but-2-en-2-yl, 2-methyl-prop-2-en- l -yl, 2-methyl-prop- l -en- I -yl, but- I -
en-3-yl, but-3-
en-1-yl, and ally].
The aryl radical in each case comprises 3-12 carbon atoms and can in each case
be benzocondensed.
For example, there can be mentioned: cyclopropenyl, cyclopentadienyl, phenyl,
tropyl, cyclooctadienyl, indenyl, naphthyl, azulenyl, biphenyl, fluorenyl,
anthracenyl, etc.
The heteroaryl radical in each case comprises 3-16 ring atoms, and instead of
the
carbon can contain one or more heteroatoms that are the same or different,
such as
oxygen, nitrogen or sulfur, in the ring, and can be monocyclic, bicyclic, or
tricyclic, and
in addition in each case can be benzocondensed.
For example, there can be mentioned:
Thienyl, furanyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, pyrazolyl,
isoxazolyl,
isothiazolyl, oxadiazolyl, triazolyl, thiadiazolyl, etc., and benzo
derivatives thereof, such
as, e.g., benzofuranyl, benzothienyl, benzoxazolyl, benzimidazolyl, indazolyl,
indolyl,
isoindolyl, etc.; or pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl,
etc., and benzo
derivatives thereof, such as, e.g., quinolyl, isoquinolyl, etc.; or azocinyl,
indolizinyl,
purinyl, etc., and benzo derivatives thereof; or quinolinyl, isoquinolinyl,
cinnolinyl,
CA 02493026 2005-01-19
8
phthalazinyl, quinazolinyl, quinoxalinyl, naphthyridinyl, pteridinyl,
carbazolyl, acridinyl,
phenazinyl, phenothiazinyl, phenoxazinyl, xanthenyl, or oxepinyl, etc.
The heteroaryl radical can be benzocondensed in each case. For example, there
can be mentioned as 5-ring heteroaromatic compounds: thiophene, furan,
oxazole,
thiazole, imidazole, pyrazole and benzo derivatives thereof, and as 6-ring
heteroaromatic
compounds: pyridine, pyrimidine, triazine, quinoline, isoquinoline and benzo
derivatives.
Heteroatoms are defined as oxygen, nitrogen or sulfur atoms.
A 3- to 8-membered ring in the meaning of R2, R3, Y and Z, which is formed
together with the nitrogen atom, is defined as C3-C8-cycloheteroalkyls and C3-
C8-
heteroaryls.
If an acid group is included, the physiologically compatible salts of organic
and
inorganic bases are suitable as salts, such as, for example, the readily
soluble alkali salts
and alkaline-earth salts as well as N-methyl-glucamine, dimethyl-glucamine,
ethyl-
glucamine, lysine, 1,6-hexadiamine, ethanolamine, glucosamine, sarcosine,
serinol, tris-
hydroxy-methyl-amino-methane, aminopropanediol, Sovak base, and 1-amino-2,3,4-
butanetriol.
If a basic group is included, the physiologically compatible salts of organic
and
inorganic acids are suitable, such as hydrochloric acid, sulfuric acid,
phosphoric acid,
citric acid, tartaric acid, fumaric acid, i.a.
The compounds of general formula I according to the invention also contain the
possible tautomeric forms and comprise the E-isomers or Z-isomers, or, if a
chiral center
is present, also the racemates and enantiomers.
CA 02493026 2005-01-19
9
Those compounds of general formula I in which
X stands for CH,
W stands for hydrogen,
A, B, D,
E and Q as a ring together stand for pyridyl,
R' stands for aryl or heteroaryl, which optionally can be substituted in one
or
more places in the same way or differently with halogen, hydroxy, C1-C6-
alkyl, C3-C6-cycloalkyl, C4-C6-alkenyl, C2-C6-alkinyl, aralkyloxy, C1-C6-
alkoxy, halo-C1-C6-alkyl, cyano-C I -C6-alkyl, or with the group =O,
-S02R 6 or -OR5, whereby Cj-C6-alkyl optionally also can be substituted
with the group -OR5 or -NR9R10,
Y and Z, in each case independently of one another, stand for a bond,
and R, independently of one another, stand for hydrogen or for the group
R2 3
-CONR9R10, -SO2R6, -COR", -COC1-C6-alkyl, -CO-Ci-C6-alkyl-R1 ,
-NR9R10 or for C1-C6-alkyl, C3-C6-cycloalkyl, C3-C6-cycloalkenyl, aryl or
heteroaryl that is optionally substituted in one or more places in the same
way or differently with halogen, cyano, CI-C6-alkyl, C I -C6-alkoxy,
hydroxy-C 1 -C6-alkyl, halo-C1-C6-alkyl or with the group --NR'R8, -ORS,
-C,-C6-alkyl-OR5, -SR4, -SOR4 or -S02R6, or
R2, R3, y
and Z together with the nitrogen atom form a 3- to 8-membered saturated or
unsaturated ring, which optionally can contain additional heteroatoms in
the ring and optionally can be substituted in one or more places in the
CA 02493026 2005-01-19
same way or differently with halogen, cyano, C1-C12-alkyl, C1-C12-alkoxy,
halo-C1-C6-alkyl, hydroxy-C1-C6-alkyl or with the group =O, -OR5 , -SR4,
-SOR4 or -S02R6,
R4 stands for C1-C6-alkyl, aryl or heteroaryl,
R5 stands for hydrogen, C1-C6-alkyl, halo-C1-C6-alkyl, C1-C12-alkoxy,
C3-C1 -cycloalkyl or halo-C3-C6-cycloalkyl,
R6 stands for hydrogen, C1-C6-alkyl, halo-C1-C6-alkyl, aryl or heteroaryl, or
for the group -NR9R10, whereby the aryl or heteroaryl itself optionally can
be substituted in one or more places in the same way or differently with
C1-C6-alkyl, C 1 -C6-alkoxy, halogen or halo-C1-C6-alkoxy,
R7 and R8, independently of one another, stand for hydrogen or C1-C6-alkyl,
R9 and R10, independently of one another, stand for hydrogen, C1-C6-alkyl, C2-
C6-
alkenyl, aryl, C3-CS-cycloalkyl, or for the group -CONR7R8, or for C1-C6-
alkyl that is optionally substituted in one or more places in the same way
or differently with aryl, morpholino, hydroxy, halogen or C1-C12-alkoxy,
or with the group -NR7R8, whereby the aryl itself optionally can be
substituted in one or more places in the same way or differently with C1-
C6-alkoxy or halo-C1-C6-alkyl, and
R' stands for C1-C6-alkyl, C1-C6-alkoxy, hydroxy-C1-C6-alkyl, hydroxy-C1-
C6-alkoxy, C3-C6-cycloalkyl, phenyl, pyridyl, biphenyl or naphthyl,
whereby the phenyl itself can be substituted in one or more places in the
same way or differently with C1-C6-alkyl, or halo-C1-C6-alkyl, as well as
isomers, diastereomers, tautomers and salts thereof,
CA 02493026 2005-01-19
11
have proven advantageous.
Those compounds of general formula I, in which
X stands for CH,
W stands for hydrogen,
A, B, D,
E, and Q as a ring together stand for pyridyl,
R1 stands for phenyl, quinolinyl, isoquinolinyl or indazolyl, which optionally
can be substituted in one or more places in the same way or differently
with halogen, hydroxy, C1-C6-alkyl, C2-C6-alkinyl, C1-C6-alkoxy, halo-C1-
C6-alkyl, or cyano-C1-C6-alkyl, whereby C1-C6-alkyl optionally also can
be substituted with the group -OR5 or -NR9R10,
Y and Z, in each case independently of one another, stand for a bond, or for
the
group =CO,
R2 and R3, independently of one another, stand for hydrogen or for the group
-CONR9R'0, -SO2R6, -COR", -COC1-C6-alkyl, -CO-C1-C6-alkyl-R",
-NR9R10 or for C1-C6-alkyl or phenyl that is optionally substituted in one
or more places in the same way or differently with the group -NR7R8 or
-ORS, or
R2, R3 , y
and Z together with the nitrogen atom form a 3- to 8-membered saturated or
unsaturated ring that optionally can contain additional heteroatoms in the
ring and optionally can be substituted in one or more places in the same
CA 02493026 2005-01-19
12
way or differently with halogen, cyano, C1-C12-alkyl, C1-C12-alkoxy, halo-
C,-C6-alkyl, hydroxy-C 1 -C6-alkyl or with the group =O, -ORS , -SR4,
-SOR4 or -S02R6,
R5 stands for hydrogen or C,-C6-alkyl,
R6 stands for hydrogen, C,-C6-alkyl, halo-C,-C6-alkyl, phenyl, benzyl,
thiophenyl, or pyridyl, whereby the phenyl, benzyl, thiophenyl and pyridyl
itself optionally can be substituted in one or more places in the same way
or differently with C1-C&-alkyl, C,-C6-alkoxy, halogen or halo-C1-C6-
alkoxy,
R7 and R8, independently of one another, stand for hydrogen or C,-C6-alkyl,
R9 and R'0, independently of one another, stand for hydrogen, C,-C6-alkyl, C2-
C6-
alkenyl, phenyl, biphenyl, C3-C8-cycloalkyl, naphthyl or for the group
-CONR7R8 or for C1-C6-alkyl that is optionally substituted in one or more
places in the same way or differently with phenyl, morpholino, hydroxy,
halogen, C1-C12-alkoxy, or with the group -NR7R8, whereby the phenyl
itself optionally can be substituted in one or more places in the same way
or differently with C,-C6-alkoxy or halo-CI-C6-alkyl, and
R" stands for C1-C6-alkyl, C,-C6-alkoxy, hydroxy-C1-C6-alkyl, hydroxy-C1-
C6-alkoxy, C3-C6-cycloalkyl, phenyl, pyridyl, biphenyl or naphthyl,
whereby the phenyl itself can be substituted in one or more places in the
same way or differently with C,-C6-alkyl, or halo-C, -C6-alkyl, as well as
isomers, diastereomers, tautomers and salts thereof,
are of special interest.
CA 02493026 2005-01-19
13
The compounds according to the invention as well as their physiologically
compatible salts prevent a tyrosine phosphorylation or stop persistent
angiogenesis and thus
the growth and propagation of tumors, whereby they are distinguished in
particular by a
slighter inhibition of isoforms of Cytochrome P 450 (3A4). Medication using
the
compounds according to the invention can therefore be done at no risk even
without regard
to pharmaceutical agents that are administered at the same time and that are
degraded via
these isoforms.
The compounds of formula I as well as their physiologically compatible salts
can
be used as pharmaceutical agents based on their inhibitory activity relative
to the
phosphorylation of the VEGF receptor. Based on their profile of action, the
compounds
according to the invention are suitable for treating diseases that are caused
or promoted
by persistent angiogenesis.
Since the compounds of formula I are identified as inhibitors of the tyrosine
kinases KDR and FLT, they are suitable in particular for treating those
diseases that are
caused or promoted by persistent angiogenesis that is triggered via the VEGF
receptor or
by an increase in vascular permeability.
The subject of this invention is also the use of the compounds according to
the
invention as inhibitors of the tyrosine kinases KDR and FLT.
Subjects of this invention are thus also pharmaceutical agents for treating
tumors
or use thereof.
The compounds according to the invention can be used either alone or in a
formulation as pharmaceutical agents for treating tumor or metastasis growth,
psoriasis,
Kaposi's sarcoma, restenosis, such as, e.g., stent-induced restenosis,
endometriosis,
CA 02493026 2005-01-19
14
Crohn's disease, Hodgkin's disease, leukemia; arthritis, such as rheumatoid
arthritis,
hemangioma, angiofibroma; eye diseases, such as diabetic retinopathy,
neovascular
glaucoma; renal diseases, such as glomerulonephritis, diabetic nephropathy,
malignant
nephrosclerosis, thrombic microangiopathic syndrome, transplant rejections and
glomerulopathy; fibrotic diseases, such as cirrhosis of the liver, mesangial
cell
proliferative diseases, arteriosclerosis, injuries to nerve tissue, and for
inhibiting the
reocclusion of vessels after balloon catheter treatment, in vascular
prosthetics or after
mechanical devices are used to keep vessels open, such as, e.g., stents, as
immunosuppressive agents, for supporting scar-free healing, in senile
keratosis and in
contact dermatitis.
In treating injuries to nerve tissue, quick scar formation on the injury sites
can be
prevented with the compounds according to the invention, i.e., scar formation
is prevented
from occurring before the axons reconnect. A reconstruction of the nerve
compounds was
thus facilitated.
The formation of ascites in patients can also be suppressed with the compounds
according to the invention. VEGF-induced edemas can also be suppressed.
Lymphangiogenesis plays an important role in lymphogenic metastasizing
(Karpanen, T. et al., Cancere Res. 2001 Mar 1, 61(5): 1786-90, Veikkola, T.,
et al., EMBO
J. 2001, Mar 15; 20 (6): 1223-31).
The compounds according to the invention now also show excellent action as
VEGFR kinase 3 inhibitors and are therefore also suitable as effective
inhibitors of
lymphangiogenesis.
CA 02493026 2005-01-19
By a treatment with the compounds according to the invention, not only a
reduction
of the size of metastases but also a reduction of the number of metastases is
achieved.
Such pharmaceutical agents, their formulations and uses are also subjects of
this
invention.
The invention thus also relates to the use of the compounds of general formula
I for
the production of a pharmaceutical agent for use as or for treatment of tumor
or metastasis
growth, psoriasis, Kaposi's sarcoma, restenosis, such as, e.g., stent-induced
restenosis,
endometriosis, Crohn's disease, Hodgkin's disease, leukemia; arthritis, such
as
rheumatoid arthritis, hemangioma, angiofibroma; eye diseases, such as diabetic
retinopathy, neovascular glaucoma; renal diseases, such as glomerulonephritis,
diabetic
nephropathy, malignant nephrosclerosis, thrombic microangiopathic syndrome,
transplant
rejections and glomerulopathy; fibrotic diseases, such as cirrhosis of the
liver, mesangial
cell proliferative diseases, arteriosclerosis, injuries to nerve tissue, and
for inhibiting the
reocclusion of vessels after balloon catheter treatment, in vascular
prosthetics or after
mechanical devices are used to keep vessels open, such as, e.g., stents, as
immunosuppressive agents, as a support in scar-free healing, in senile
keratosis and in
contact dermatitis.
The formation of ascites in patients can also be suppressed with the compounds
according to the invention. VEGF-induced edemas can also be suppressed.
To use the compounds of formula I as pharmaceutical agents, the latter are
brought into the form of a pharmaceutical preparation, which in addition to
the active
ingredient for enteral or parenteral administration contains suitable
pharmaceutical,
organic or inorganic inert carrier materials, such as, for example, water,
gelatin, gum
CA 02493026 2005-01-19
16
arabic, lactose, starch, magnesium stearate, talc, vegetable oils,
polyalkylene glycols, etc.
The pharmaceutical preparations can be present in solid form, for example as
tablets,
coated tablets, suppositories, or capsules, or in liquid form, for example as
solutions,
suspensions or emulsions. They optionally contain, moreover, adjuvants such as
preservatives, stabilizers, wetting agents or emulsifiers, salts for changing
osmotic
pressure or buffers.
For parenteral administration, especially injection solutions or suspensions,
especially aqueous solutions of the active compounds in polyhydroxyethoxylated
castor
oil, are suitable.
As carrier systems, surface-active adjuvants such as salts of bile acids or
animal
or plant phospholipids, but also mixtures thereof as well as liposomes or
components
thereof can also be used.
For oral administration, especially tablets, coated tablets or capsules with
talc
and/or hydrocarbon vehicles or binders, such as for example, lactose, corn
starch or
potato starch, are suitable. The administration can also be carried out in
liquid form, such
as, for example, as juice, to which optionally a sweetener or, if necessary,
one or more
flavoring substances, is added.
The dosage of the active ingredients can vary depending on the method of
administration, age and weight of the patient, type and severity of the
disease to be
treated and similar factors. The daily dose is 0.5-1000 mg, preferably 50-200
mg,
whereby the dose can be given as a single dose to be administered once or
divided into 2
or more daily doses.
CA 02493026 2005-01-19
17
The above-described formulations and forms for dispensing are also subjects of
this invention.
The production of the compounds according to the invention is carried out
according to methods that are known in the art. For example, compounds of
general
formula I are obtained in that a compound of general formula II
W O
R'
N
H
NH
E\~p
ri 1 M
A~~ Q
(II),
in which A, B, D, E, Q, W, X and R' have the meanings that are indicated in
general
formula I, and M stands for halogen, first is converted into an amine and then
is acylated,
or M is substituted by an NHCOR' group.
CA 02493026 2005-01-19
18
Compounds of general formula I are also obtained in that a compound of general
formula Ila,
W 0
RY
i
O
X NH
N FG
(IIa),
in which R'' stands for C1-C6-alkyl or hydrogen, and FG means a leaving group,
such as,
e.g., halogen, 0-triflate, 0-mesylate, 0-tosylate or sulfone, first is
converted into an
amide, and then the leaving group is substituted by an N(Y-R2)-R3 group, or a
compound
III
W 0
O
NH
R3
N
N
Y- R2
(III),
in which R2, R3, Y and Z have the meanings that are indicated in general
formula I and R3'
stands for Ci-C6-alkyl or hydrogen, first is saponified and then is converted
into the
amide.
CA 02493026 2005-01-19
19
The amide formation is carried out according to methods that are known in the
literature.
For amide formation, it is possible to start from a corresponding ester. The
ester
is reacted according to J. Org. Chem. 1995, 8414 with aluminum trimethyl and
the
corresponding amine in solvents such as toluene at temperatures of 0 C to the
boiling
point of the solvent. If the molecule contains two ester groups, both are
converted into
the same amide. Instead of aluminum trimethyl, sodium hexamethyidisilazide can
also
be used.
For amide formation, however, all processes that are known from peptide
chemistry are also available. For example, the corresponding acid can be
reacted with the
amine in aprotic polar solvents, such as, for example, dimethylformamide, via
an
activated acid derivative, that can be obtained, for example, with
hydroxybenzotriazole
and a carbodiimide, such as, for example, diisopropylcarbodiimide, at
temperatures of
between 0 C and the boiling point of the solvent, preferably at 80 C. The
reaction
between carboxylic acid and amine, however, can also be produced by activation
reagents, such as HATU (N-dimethylamino- IH-1,2,3-triazolo-[4,5-b]pyridin-I-
ylmethylene]-N-methylmethanaminium hexafluorophosphate-N-oxide), whereby polar
aprotic solvents, such as, for example, dimethylformamide, are suitable for
the reaction.
The addition of a base such as N-methylmorpholine is necessary. The reaction
proceeds
at temperatures of 0-100 C, whereby the procedure is preferably performed at
room
temperature, but in many cases heating is indispensable. For the amide
formation, the
process can also be used with the acid halide, the mixed acid anhydride,
imidazolide or
CA 02493026 2005-01-19
azide. A previous protection of an additional amino group, for example as an
amide, is
not necessary in all cases, but can advantageously influence the reaction.
In the case of bisacid chlorides, cyclic compounds can be produced. Also, in
the
case of halogen acid halides, cyclic compounds can be produced. The ring
closure is then
completed optionally by adding a strong base, such as, for example, sodium
alcoholates.
The same thing holds true for the sulfonic acid halides, whereby double
sulfonations can
also occur.
Ureas are produced from amino compounds by reaction with isocyanates. Inert
solvents such as methylene chloride or else dimethylformamide at temperatures
from
room temperature up to 100 C, preferably at 60 C. Pressure is advantageous for
the
reaction.
The reaction of halopyridines with amides is carried out under catalysis, for
example by palladium or copper catalysis. In the case of copper catalysis
(literature, see
Synlett. 2002, 427), solvents such as dioxane or dimethylformamide are used at
temperatures up to the boiling point of the solvent, preferably 120 C. As a
base,
potassium phosphate or else cesium carbonate is used. Ethylenediamine is
advantageous
for complexing the copper(I) iodide that is used as a catalyst. An application
of pressure
is not harmful. In the case of palladium catalysis, both palladium(II) salts,
such as
palladium(II) acetate, and palladium(O) complexes, such as
palladium(O)2dibenzylidene
acetone3 (literature, see JACS 2002, 6043, THL 1999, 2035, Org. Lett 2001,
2539, THL
2001, 4381 or THL 2001, 3681) can.... As a solvent, toluene, dioxane or
dimethylformamide is used at temperatures from room temperature up to the
boiling
point of the solvent, preferably around 100 C. As a co-ligand, BINAP, DPPF or
CA 02493026 2005-01-19
21
Xanthphos is used. A base is also necessary. To this end, cesium carbonate,
potassium
phosphate or else sodium-t-butylate is used. These components can be combined
in
various ways.
The production of the pyridinamines from the corresponding 2-halopyridines is
carried out in solvents such as pyridine or in protic polar solvents such as
ethylene glycol
at temperatures up to 200 C. Catalysis by copper(l) salts can be necessary for
the
reaction. The application of pressure is absolutely necessary in the case of
the reaction of
low-boiling amines, but can also be used advantageously in the conventional
amines.
The ether cleavage is accomplished according to known methods, for example by
reaction with boron tribromide in inert solvents, such as methylene chloride,
at
temperatures of -78 C up to room temperature, preferably at -78 C.
The compounds of general formulas II, Ila and 111,
W O
R'
H
X NH
E~
I I M
A~BiQ
(U),
CA 02493026 2005-01-19
22
w o
RY W o
RY
G \ O
NH /
X NH
/ Z Rs
FG
N N
N
(Ila) and (III), Y- R2
[and]
in which A, B, D, E, Q, W, X, Y, Z, R2, and R3 have the meanings that are
indicated in
general formula I and M stands for halogen, FG stands for a leaving group,
such as, e.g.,
halogen, 0-triflate, 0-mesylate, 0-tosylate or sulfone, and RY stands for C1-
C6-alkyl or
hydrogen, represent valuable intermediate products for the production of the
compounds
of general formula I according to the invention and are thus also subjects of
this
invention.
CA 02493026 2005-01-19
23
Production of the Compounds According to the Invention
The following examples explain the production of the compounds according to
the
invention without the scope of the claimed compounds being limited to these
examples.
Example 1.0
Production of 2-{ 12-(2-Dimethylamino-ethylamino)-pyridin-4-ylmethyl]-amino}-N-
(3-trifluoromethyl-phenyl)-benzamide
o i
OH N Me
N Me
90 mg (0.2 mmol) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide is dissolved in 3 ml of pyridine and mixed
with 1 ml
of N,N-dimethyl-aminoethylamine and heated in a pressure vessel for S hours to
a bath
temperature of 200 C. After cooling, it is concentrated by evaporation, and 90
mg of 2-
{ [2-(2-dmethylamino-ethylamino)-pyridin-4-ylmethyl]-amino} -N-(3-
trifluoromethyl-
phenyl)-benzamide is obtained.
Melting point: 100 C
CA 02493026 2005-01-19
24
Similarly produced are also the following compounds:
0 0 N~ R'
eN H ,R I / NH
Rz
N R3
'N ~N I R2
C
N
TypA TypB R
[Key: Typ =Type]
Exam- Type R R R MW Melting Point
ple No. [ C] or MS
Molar Peak
(m/e)
1.1 A -(CH2)2-OH H 430.5
, Q CF3
1.2 A -(CH2)2-OH H 413.5 130-132
1.3 A -(CH2)3OH H 444.5 148
, CF3
1.4 A -(CH2)40H H 458.5 124
, CF3
1.5 A -(CH2)50H H 472.5 70
CF3
1.6 A -(CH2)2OMe H ,a CF, ~ CF3
CA 02493026 2005-01-19
Exam- Type R R 111 MW Melting Point
ple No. [ C] or MS
Molar Peak
(m/e)
1.7 A H H 444.5 80
CF3
1.8 A H H 444.5 65
. ~_ aCF,
1.9 A \ ' OH H 444.5 81
aCF,
CA 02493026 2005-01-19
26
Exam- Type R 2 H R' MW Melting Point
ple No. I C] or MS
Molar Peak
(m/e)
1.10 A (CH2)3N H 471.5 68
CF3
Mee
1.11 B -(CH2)2- H 413.5 Resin
OH
1.12 A Phenyl H 462.5
CF3
1.13 A -(CH2)5- N 437.54
1.14 A -(CH2)2-0-(CH2)2- 439.52 174
N
1.15 A -(CH2)2-NMe- N 452.56 85
(CH2)2-
1.16 A -(CH2)2-S-(CH2)2- 455.58 158
1.17 A -(CH2)2-SO2- N 487.58
(CH2)2-
1.18 A -(CH2)4- 423.52 148
I
CA 02493026 2005-01-19
27
Example 2.0
Production of 2-I(2-Amino-pyridin-4-ylmethyl)-amino]-N-(3-trifluoromethyl-
phenyl)-benzamide
0
CF3
N "a
NH
NH2
8.747 g (19.4 mmol) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide is heated with 175 mg of copper(I) oxide in
150 ml of
ethanediol for 23 hours under 10 bar of ammonia pressure to 80 C in an
autoclave. After
the solvent is distilled off in a vacuum, the residue is purified on silica
gel with a gradient
of ethyl acetate:ethanol = 100:0 to 0:100 as an eluant. 4.15 g (51 % of
theory) of 2-[(2-
amino-pyridin-4-ylmethyl)-amino]-N-(3-trifluoromethyl-phenyl)-benzamide with a
melting point of 64 C is obtained.
Similarly produced are:
CA 02493026 2005-01-19
28
Example 2.1
2-[(2-Amino-pyridin-4-ylmethyl)-amino)-N-isoquinolin-3-yl-benzamide
O N;
ti
\ NH
NHZ
\ N
Melting point: 202 C
Example 2.2
2-[(2-Amin o-pyridin-4-ylmethyl)-aminol-N-(1H-indazol-5-yl)-benzamide
0 N
O11H
NH2
MS: m/e 358
Melting point: 200 C
CA 02493026 2005-01-19
29
Example 2.3
2-[(2-Amino-pyridin-4-ylmethyl)-amino]- N-(2-methyl-2H-indazol-6-yi)-benzamide
0 %\N-
'N N
i "
~~ ANN
N"z
YI
N
MW: 372.43
Example 3.0
Production of 2-{[2-(3-Benzyl-ureido)-pyridin-4-ylmethyl}-amino}-N-(3-
trifluoromethyl-phenyl)-benzamide
0
eNH CF3
T
1 N
100 mg (0.26 mmol) of 2-[(2-amino-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide is mixed in 2.5 ml of methylene chloride
with 37.9
mg (0.29 mmol) of benzyl isocyanate, and it is stirred overnight at room
temperature.
CA 02493026 2005-01-19
After concentration by evaporation, the residue is chromatographed. 66 mg (49%
of
theory) of 2-{[2-(3-benzyl-ureido)-pyridin-4-ylmethyl]-amino}-N-(3-
trifluoromethyl-
phenyl)-benzamide with a melting point of 153 C is obtained.
Similarly produced are also the following compounds:
0
o ,
/R, eNH N_R eNH
Y0\R'o
N O ~
Typ B ~=~~~Rio
TYp A O
[Key: Typ =Type]
Exam- Type R R19 MW Melting Point
ple No. [ Cl or MS
Molar Peak
(mle)
3.1 A Phenyl 505.5 185
"a CF,
3.2 A Ph (CH2)2- 533.5 76
CF3
3.3 A n-Butyl 485.5 84
Q CF3
CA 02493026 2005-01-19
31
Exam- Type R R MW Melting Point
ple No. j C] or MS
Molar Peak
(m/e)
3.4 A Me OMe 595.5 206
CF3
OMe
3.5 A 573.5 186
/ CF3 / CF3
CF3
3.6 A aCF,
573.5 211 3.7 A Ethyl 457.5 154
CF3
3.8 A / 581.6 195
aCF, \
3.9 A 555.5 180
CF3 II 6
3.10 A -CH3 443.4 159
/ CF3
3.11 A CH2CH2Cl 491.9 157
, / CF3
I n-Propyl 471.5 80
3.12 A /
CF3
CA 02493026 2005-01-19
32
Exam- Type R R10 MW Melting Point
ple No. [ C] or MS
Molar Peak
(m/e)
3.13 A i-Propyl 471.5 96
,a CF,
3.14 A I 497.5 103
CF3
3.15 A -CONH2 472.4 190
~ CF3
3.16 A IC We 578.6 213
OMe
'-0
.,60W
3.17 A I 556.5 203
,,a CF,
3.18 A 1CF3 556.5 165
3.19 A 488.5 198
3.20 A 538.6 213
3.21 A 502.6 185
CA 02493026 2005-01-19
33
Exam- Type R R MW Melting Point
ple No. [ CJ or MS
Molar Peak
(we)
3.22 A 516.6 171
3.23 A N -CH2CH2CI 474.9 195
3.24 A -CH3 426.5 225
3.25 A n-Propyl 454.5
3.26 A i-Propyl 454.5
3.27 A Ethyl 440.5
3.28 A 480.6 205
3.29 A -CONH2 455.5 129
Examples 3.15 and 3.29 are produced analogously to Example 3.0 with use of
trimethylsilyl isocyanate.
CA 02493026 2005-01-19
34
Example 3.30
Production of 2-{ [2-(3,3-Dimethyl-ureido)-pyridin-4-ylmethyl)-amino}-N-(3-
isoquinolinyl)-benzamide
o
N
H
NH H
OrNYNN O
0 mg (0.23 mmol) of 2-[(2-bromopyridin-4-ylmethyl)-amino]-N-(3-
isoquinolinyl)-benzamide is heated in 2 ml of dioxane with 89 mg (0.28 mmol)
of cesium
carbonate, 61 mg (0.69 mmol) of N,N-dimethylurea, 4.7 mg (0.0046 mmol) of
dipalladium-tribenzylidene acetone and 7.9 mg (0.014 mmol) of Xanthphos under
a cover
gas and in a moisture-free environment for 9 hours to a bath temperature of
100 C. It is
then mixed with 20 ml of methylene chloride, suctioned off and concentrated by
evaporation. The residue is chromatographed on silica gel with ethyl acetate
as an eluant.
24 mg (24% of theory) of 2- {[2 -(3,3 -dimethyl -ureido)-pyri din-4-ylmethyl] -
amino }-N-(3-
isoquinolinyl)-benzamide is obtained.
(MS (Cl): 441 (M++H))
CA 02493026 2005-01-19
Similarly produced are also the following compounds:
0
N
/R H
H e
NH RNH
s
N I N, R aN R
N O H
N\ /N~R,o
Typ A Typ B O
[Key: Typ =Type]
Exam- Type R R9 R10 MW Melting Point
ple No. 1 C) or MS
Molar Peak
(m/e)
3.31 A H H 412.5 222
3.32 A H H 429.4
/ CF,
3.33 A a Me H 415.46
N
3.34 A \ N Me H 415.46 110-113
3.35 A ~N Me H 429.48 230-232
Me
CA 02493026 2005-01-19
36
Exam- Type R R9 R10 MW Melting Point
ple No. [ C] or MS
Molar Peak
(m/e)
3.36 A N-Me Me H 429.48 130-133
N
3.37 A N/i-Prop Me H 457.54
N
3.38 A N * H 455.52
N-Me
3.39 A ~N H 455.52
Me
3.40 A
Me Me 443.51
QN\/
Me
3.41 A ~N, N-Me Me Me 443.51
3.42 A Me H 443.51
N
Et
3.43 A I \ Me H 457.54
N
\i-Prop
3.44 A / Me H 443.51
N-Et
3.45 A Me H 457.54
N-i-Prop
CA 02493026 2005-01-19
37
Exam- Type R R9 R MW Melting Point
ple No. [ C] or MS
Molar Peak
(m/e)
3.46 A " N-i-Prop Me H 457.54
3.47 A N/~OMe Me H 473.53 199.5
N
3.48 A N/E' Me H 443.51 208.8
N
3.49 A Me H 453.50 242
N
N
3.50 A ():N, Me H 429.48 m/e 429
N-Me
3.51 A N/Me Me H 429.48 205.1
N
3.52 A \N N cycl.Prop H 455.52 192
Me
3.53 A cycl.Prop H 455.52 216
N-Me
3.54 A Me H 473.53 247
N
OMe
CA 02493026 2005-01-19
38
Exam- Type R R 1119 MW Melting Point
ple No. [ C] or MS
Molar Peak
(m/e)
3.55 A H 499.57
JN-Me
N
3.56 A Me H 473.53
N
d _OMe
3.57 A " Me H 473.53
",-Lome
3.58 B Me H 429.48
N-Me
N
3.59 B I ~N Me H 429.48
Me
3.60 A Me H 454.48
CN
3.61 A [CN Me H 454.48
XILN
3.62 AMe Me H 486.53
Me
3.63 A N-Me -(CH2)2-O-CH3 H 473.53
3.64 A N-Me (CH2)2N(CH3)2 H 486.58
CA 02493026 2005-01-19
39
9 10
R MW Melting Point
Exam- Type R R
pie No. [ CJ or MS
Molar Peak
(m/e)
3.65 A '~/~N~ H 528.61
N-Me
3.66 A N -(CH2)2-O-CH3 H 473.53
Me
3.67 A N -(CH2)2N(CH3)2 H 486.58
3.68 A H 528.61
\ NN ~Io
" Me
3.69 A i t "N Me H 454.49
\
`CN
CA 02493026 2005-01-19
Example 4.0
Production of 2-[(2-Methanesulfonylamino-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide
O
F3
eN C
O
O -Me
90 mg (0.2 mmol) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide and 23 mg (0.24 mmol) of methanesulfonic
acid
amide are introduced into 5 ml of dioxane and mixed in succession with 4 mg
(0.02
mmol) of copper(I) iodide, 85 mg (0.4 mmol) of potassium phosphate and 2 mg
(0.02
mmol) of ethylenediamine. After I hour of stirring at a bath temperature of
120 C, it is
diluted with 20 ml of water and concentrated by evaporation. It is then made
alkaline
with ammonia and shaken out three times with 25 ml each of ethyl acetate. The
collected
organic phase is washed with water, dried, filtered and concentrated by
evaporation. The
residue is made crystalline with ethyl acetate and a little hexane, stirred
and suctioned off.
24 mg (26% of theory) of 2-[(2-methanesulfonyl-amino-pyridin-4-ylmethyl)-
amino]-N-
(3-trifluoromethyl-phenyl)-benzamide with a melting point of 214.5 C is
obtained.
CA 02493026 2005-01-19
41
Similarly produced are:
0
~ H/
e R
I
NH
0,11 R 2
N
Example R R MW Melting Point
No. [ C] or MS
Molar Peak
(m/e)
4.1 526.54 259.2
~SO\ /
CF3
o N/Me 541.55 >300
4.2 aCF, s
Z\
4.3 DSO OCF 610.53 248.6
CF3
4.4 4 -SO2CF3 518.44 238.9
CF3
4.5 rv S02CH3 447.52 m/e: 447
4.6 F 562.52 252.5
/ CF3 SOz F
2 O_
4.7 I SO OnBu 598.64 231
CF3
CA 02493026 2005-01-19
42
Example R 1 2 R 2 MW Melting Point
No. [ C] or MS
Molar Peak
(m/e)
4.8 ,/SO' IBS 567.01 255.2
CF, I /
4.9 N \ \ SO,irS\ 550.06 234.7
4.10 so CH 540.56 245.7
2-a ,
C F,
4.11 I \ / I 540.56 194.8
CF
3
4.12 498.57 256
N SO' /
512.59 219
4.13 so CH
N 2-a
CA 02493026 2005-01-19
43
Example 5.0
Production of 2-((2-Bismethanesulfonylamino-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide
0
NN aCF3
eNH Me
O=S=0
1 o
N~I
O -Me
193 mg (0.5 mmol) of 2 - [ (2-amino-pyri din-4-ylmethyl) -amino]-N-(3-
tri fluoromethyl-phenyl)-benzamide is mixed in 3 ml of dichloromethane with 69
mg (0.6
mmol) of methanesulfonic acid chloride and 61 mg (0.6 mmol) of triethylamine
and
stirred together for 1.5 hours at room temperature. Then, it is washed once
with dilute
sodium bicarbonate solution, dried, filtered and concentrated by evaporation.
The residue
is chromatographed via flash chromatography (5 g of ]solute) with a gradient
of
cyclohexane:ethyl acetate =100:0 to 50:50 as an eluant. 80 mg (30% of theory)
of 2-[(2-
bismethanesulfonylamino-pyridin-4-ylmethyl)-amino]-N-(3-trifluoromethyl-
phenyl)-
benzamide is obtained as a resin.
(MS: m/e 542)
CA 02493026 2005-01-19
44
Example 6.0
Production of 2-[(2-Butyrylamino-pyridin-4-ylmethyl)-amino]-N-(3-
isoquinolinyl)-
benzamide
N
O
aNH
N O
100 mg (0.23 mmol) of 2-[(2-bromopyridin-4-ylmethyl)-amino]-N-(3-
isoquinolinyl)-benzamide is heated in 1 ml of dioxane with 89 mg (0.28 mmol)
of cesium
carbonate, 24 mg (0.69 mmol) of butyramide, 4.7 mg (0.0046 mmol) of
dipalladium-
tribenzylidene acetone and 7.9 mg (0.014 mmol) of Xanthphos under a cover gas
and in a
moisture-free environment for 25 hours to a bath temperature of 90 C. It is
then mixed
with 20 ml of methylene chloride, suctioned off and concentrated by
evaporation. The
residue is chromatographed on silica gel first with hexane, then with
hexane:ethyl acetate
= 8:2 and then with hexane:ethyl acetate = 1:1 as an eluant. 45 mg (42% of
theory) of 2-
[(2-butyrylamino-pyridin-4-ylmethyl)-amino]-N-(3-isoquinolinyl)-benzamide with
a
melting point of 173 C is obtained.
CA 02493026 2005-01-19
Similarly produced are:
O RI
NH
R2
4 N
Example R R MW Melting Point
No. ( C] or MS
Molar Peak
(m/e)
6.1 I -COn-Prop 456.47 168
~ CF3
6.2 -COMe 411.46 220
6.3 N -COEt 425.49 183
6.4 -COn-Bu 453.54 167
6.5 ~CO--a 437.50 218
6.6 - 549.63 212
CA 02493026 2005-01-19
46
Example R R MW Melting Point
No. [ C] or MS
Molar Peak
(m/e)
6.7 N 453.54 112
/ ~co
6.8 i / 529.64 219
co /
6.9 N - 523.59 215
co /
6.10 -COt-Bu 453.54 91
6.11 aCF,
546.59 6.12 -000Et 441.50 185
6.13 COMe 428.41 185
CF3
6.14 N CO-cycl.Prop 426.48 210-212
6.15 a CO-cycl.Prop 426.48 127-128
N
6.16 CF' 558.48
CF3 /CO \
CA 02493026 2005-01-19
47
Example R R MW Melting Point
No. [ C) or MS
Molar Peak
(m/e)
6.17 COPh 490.48
CF3
6.18 - F 508.47
CF3 ,ico
6.19 N-Me CO-cycl.Prop 440.51 114-115
6.20 N CO-(CH2)4-OH 469.54 136
/
6.21 -COOEt 444.49 205-210
Me
6.22
N -COOEt 430.47
JXN1
6.23 \ N CO2CH2(i-Prop) 472.55 187
Me
6.24 N CO2(i-Prop) 458.52 204
Me
6.25 N CO-cycl.Prop 440.51 105-107
J:):::\/
Me
6.26 N 491.47
~co
CF3
CA 02493026 2005-01-19
48
Example R R MW Melting Point
No. [ Cl or MS
Molar Peak
(m/e)
6.27 COOEt 430.47 213
6.28 / COOEt 444.49 194-196
N-Me
~ N
6.29 CO-cycl-Prop 545.45 213
CF3
6.30 CO-t-Bu 470.49 155
CF3
6.31 -CO-CH2-O- 471.51 86
(CH2)20H
CA 02493026 2005-01-19
49
Example 6.32
Similarly produced is:
2- { [2-(Acetyl-methyl-amino)-pyridin-4-ylmethyl]-amino } -N-isoyuinolin-3-yl-
benzamide
N- /
O
)tl
NH
N O
Melting point 71 C
Example 7.0
Production of 2-{ [2-(2-Oxo-pyrrolidin-l-yl)-pyridin-4-ylmethyl]-amino}-N-(3-
trifluoromethyl-phenyl)-benzamide
0
CF,
NH ry
Q
}-
156 mg (0.5 mmol) of 2-{[2-(2-oxo-pyrrolidin-l-yl)-pyridin-4-ylmethyl]-amino
benzoic acid is mixed in 5 ml of dimethylformamide with 0.12 ml (1 mmol) of 3-
aminobenzotri fluoride, 228 mg (0.6 mmol) of HATU (N-dimethylamino- lH-1,2,3-
triazolo-[4,5-b]pyridin- l -ylmethylene]-N-
methylmethanammoniumhexafluorophosphate-
N-oxide) and 0.14 ml of N-methylmorpholine, and it is stirred overnight at
room
CA 02493026 2005-01-19
temperature. It is diluted with ethyl acetate and washed in succession with
saturated
sodium bicarbonate solution, water and saturated common salt solution. The
organic
phase is dried, filtered and concentrated by evaporation. The residue is
chromatographed
on Isolute as a mobile solvent. 95 mg (42% of theory) of 2-{[ 2-(2-oxo-
pyrrolidin- l-yl)-
pyridin-4-ylmethyl] -amino}-N-(3-trifluoromethyl-phenyl)-benzamide is
obtained.
(MS: m/e 454)
Similarly produced are:
0
R1
\ H/
NH
VN
G R2,R3,YandZ= G
Example R' G MW Melting Point [ C]
No. or MS Molar Peak
(m/e)
7.1 9 439.5 189 C
.-N 0
CA 02493026 2005-01-19
51
Example R G MW Melting Point [ C]
No. or MS Molar Peak
(m/e)
7.2 I ~ 455.4 m/e 455
a CF ~I H
3
7.3 I 469.5 209
CF .-N N_Me
3
7.4 I \ 438.5 154
-N(H
7.5 N 435.5 217.3
-N
7.6 0 ~ 426.48 195-200
= N -NV
7.7 0 426.48 105-110
N
CA 02493026 2005-01-19
52
Example 8.0
Produced similarly to Example 6.0 is:
2-1 [2-(2-Hydroxymethyl-5-oxo-pyrrolidin-1-yl)-pyridin-4-ylmethyll-amino}-N-
isoquinolin-3-yl-benzamide
N~
O
0
NH
N
OH
Similarly produced to this are:
O
RI
aNH
G
N
CA 02493026 2005-01-19
53
Example R G MW Melting Point [ C]
No. or MS Molar Peak
(m/e)
8.1 N \ \ 467.53 98 C
.-N
SOH
8.2 N \ \ _~ 467.53 76 C
0
8.3 N \ \ 467.53 95 C
.-N
OH
8.4 N \ \ 447.50 86 C
N~)
8.5 N \ \ 481.94 186 C
CI
8.6 0 440.51 196-198
N
Me
8.7 440.51 100 (Dec.)
N-Me
N . -N
8.8 " 440.51 159
N-Me
=-N
8.9 N Me 440.51
N --N~
CA 02493026 2005-01-19
54
Example R G MW Melting Point 1 C1
No. or MS Molar Peak
(m/e)
8.10 441.49
N-Me
_ \ N .-N H
8.11 " 441.49
N-Me
8.12 N,Me 0 441.49
/N =-N NH
8.13 I \ 441.49
/ N .-N H
Me
8.14 / ", 454.33
N-Me
\ ,-N
8.15 454.33
N
Me
8.16 454.33
N-Me
-N
8.17 N'Me 454.33
8.18 ", 468.56
N-i-Prop
\ ~ =-N
8.19 N `-Prop 0 468.56
N .-N
CA 02493026 2005-01-19
Example 9.0
Production of 2-{12-(2,5-Dioxo-pyrrolidin-l-yl)-pyridin-4-ylmethyl]-amino}-N-
(3-
triflu oromethyl-phenyl)-benzamide
O JaCF3
\ / O
NH
N
/N 0
193 mg (0.5 mmol) of 2-[(2-amino-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-phenyl)-benzamide is mixed in 20 ml of dichloromethane with
0.21 ml
(1.5 mmol) of triethylamine, and it is mixed at room temperature drop by drop
with a
solution of 93 mg (0.6 mmol) of succinic acid dichloride in 3 ml of methylene
chloride.
After stirring overnight at room temperature, it is diluted with methylene
chloride and
washed in succession with water, saturated sodium bicarbonate solution and
saturated
common salt solution. Then, the organic phase is dried, filtered and
concentrated by
evaporation. The residue is chromatographed on Isolute (Separtis Company) with
a
gradient of methylene chloride:ethanol = 100:0 to 95:5. 120 mg (51 % of
theory) of 2-
{ [2-(2,5-dioxo-pyrrolidin- l -yl)-pyridin-4-ylmethyl ]-amino } -N-(3-
trifluoromethyl-
phenyl)-benzamide is obtained.
(MS: m/e 468)
CA 02493026 2005-01-19
56
Similarly produced is:
Example 9.1
2- ([2-(3,5-Dioxo-morpholin-4-yl)-pyridin-4-ylmethyl]-amino) -N-(3-
trifluoromethyl-
phenyl)-benzamide
F F
F
O
N
H
O
eNH Y 0
N
N O
Melting point 201.9 C
CA 02493026 2005-01-19
57
Example 10.0
Production of 2-[(2-(3-Chloropropanesulfonylamino-pyridin-.4-ylmethyl)-amino]-
N-
(3-trifluoromethyl-phenyl)-benzamide
C I \
OCH
H 0
"C~Nr O
135 mg (0.35 mmol) of 2-[(2-amino-pyridin-4-ylmethyl)-amino]-N-(3-
tri fluoromethyl-phenyl)-benzamide is mixed in 10 ml of dichloromethane with
62 mg
(0.35 mmol) of 3-chloropropanesulfonic acid chloride and 49 l (0.35 mmol) of
triethylamine, and it is stirred for 2 hours at room temperature. Then, it is
washed once
with saturated sodium bicarbonate solution, filtered and concentrated by
evaporation.
The residue is chromatographed via flash chromatography (5 g of Isolute) with
a gradient
of dichloromethane:ethanol = 100:0 to 90:10 as an eluant. 67 mg (36% of
theory) of 2-
[(2-(3-chloropropanesulfonylamino-pyridin-4-ylmethyl)-amino]-N-(3-
trifluoromethyl-
phenyl)-benzamide is obtained.
(MS (Cl): 491 (100%, M++H-HC1))
CA 02493026 2005-01-19
58
Example 11.0
Production of 2-{ 12-(1,1-Dioxo-1 A6-isothiazolidin-2-yl)-pyridin-4-ylmethyl]-
amino}-
N-(3-trifluoromethyl-phenyl)-benzamide
0
N CF3
e H
H C)
N
N
58 mg (0.11 mmol) of 2-[(2-(3-chloropropanesulfonylamino-pyridin-4-ylmethyl)-
amino] -N -(3 -tri fluoromethyl-phenyl)-benzamide is suspended in 5 ml of
ethanol and
mixed with 5 mg of sodium hydride (55% in mineral oil). The mixture is
refluxed for I
hour, mixed with 10 ml of water and extracted with ethyl acetate. The aqueous
phase is
saturated with sodium sulfate and extracted repeatedly by stirring with ethyl
acetate
overnight. After the combined extracts are concentrated by evaporation, 50 mg
(93% of
theory) of 2-{[2-(1,l-dioxo-l? 6-isothiazolidin-2-yl)-pyridin-4-ylmethyl]-
amino}-N-(3-
trifluoromethyl-phenyl)-benzamide is obtained.
(MS (Cl): 491 (100%, M++H))
CA 02493026 2005-01-19
59
Example 12.0
N-[2-(2-Hydroxy-ethyl)-2H-indazol-5-yl]-2-{ [2-(3-methyl-ureido)-pyridin-4-
ylmethyl]-amino)-benzamide
0 N\
N
H OH
NNH HH HH
C Y N~1 Me
N O
50 mg (0.11 mmol) ofN-[2-(2-methoxy-ethyl)-2H-indazol-5-yl]-2-{[2-(3-methyl-
ureido)-pyridin-4-ylm ethyl] -amino }-benzamide is introduced into 5 ml of
methylene
chloride and mixed drop by drop under argon and in a moisture-free environment
at
-78 C with 0.56 ml of boron tribromide (I molar in methylene chloride). It is
stirred for
15 more minutes, the cold bath is removed, and it then is stirred for 2 more
hours. Then,
it is mixed with water, the methylene chloride is drawn off, made alkaline
with sodium
bicarbonate solution and extracted twice with 15 ml each of ethyl acetate. The
collected
organic phase is dried, filtered, and concentrated by evaporation. The residue
is
chromatographed on silica gel with a gradient of methylene chloride: ethanol =
100:0 to
90:10 as an eluant, and 27 mg ofN-[2-(2-hydroxy-ethyl)-2H-indazol-5-yl]-2-{[2-
(3-
methyl-ureido)-pyri din-4-ylm ethyl] -amino } -benzamide is obtained.
CA 02493026 2005-01-19
Similarly produced from the corresponding methoxy compounds are:
0
R1
e~N
R2
Example R R MW Melting Point [ C]
No. or MS Molar Peak
(m/e)
12.1 459.51 187
N-Me OH
12.2 459.51 228
N
OH
12.3 OH 459.51 229
N ~NN/
N
12.4 ^~ H 459.51
N
12.5 459.51 220
N N ~ ^/OH
me
CA 02493026 2005-01-19
61
Production of Intermediate Compounds
Example A
If the production of intermediate compounds is not described, the latter are
known
or can be produced analogously to known compounds or to processes that are
described
here.
Stage 1
a) Production of 2-Bromopyridine-5-carbaldehyde
0
-N
/ Br
is carried out according to F. J. Romero-Salguerra et al. THL 40,859 (1999).
b) Production of 2-Bromo-isonicotinic Acid
O OH
N Br
160 g (0.93 mol) of 2-bromo-4-methyl-pyridine is added in drops to 152 g (0.96
mol) of potassium permanganate in 4 1 of water. Then, it is stirred under
reflux for one
CA 02493026 2010-07-15
62
hour before 152 g (0.96 mol) of potassium permanganate is added once more.
After two
additional hours of stirring under reflux, it is suctioned off in a hot state
over CeliteTM and
washed with water. The aqueous phase is shaken out three times with
dichloromethane.
The aqueous phase is concentrated by evaporation to one-half its original
volume, and the
pH is set at 2 with concentrated hydrochloric acid. The precipitated solid is
suctioned off
and dried at 70 C in a vacuum. 56.5 g of white solid product accumulates.
Production of 2-Bromo-4-hydroxymethyl -pyri dine
OH
N Br
30.2 ml (295 mmol) of triethylamine is added to 56.5 g (280 mmol) of 2-bromo-
isonicotinic acid in 1.2 1 of THF. Then, it is cooled to -10 C and mixed drop
by drop
with 38.2 ml (295 mmol) of isobutyl chloroformate. After stirring has been
continued for
one hour at -10 C, it is cooled to -70 C and mixed drop by drop with 590 ml
(590 mmol)
of LiA1H4 solution (1 M in THF). After stirring is continued for one hour at -
70 C, it is
allowed to reach -40 C. 600 ml of 50% acetic acid is added. It is stirred
overnight at
room temperature. The insoluble components are suctioned off, and the filtrate
is
concentrated by evaporation. The residue is purified on silica gel with hexane
and
hexane/ethyl acetate 1:1. 28.0 g of white solidifying oil accumulates.
CA 02493026 2005-01-19
63
Production of 2-Bromo-4-formyl-pyri dine:
0
N Br
149 g (1714 mmol) of manganese dioxide is added in measured quantities to 28.0
g (148.9 mmol) of 2-bromo-4-hydroxymethyl-pyridine in 500 ml of dichlormethane
within 6 hours. Then, stirring is continued at room temperature for 48 hours.
It is
suctioned off over Celite and concentrated by evaporation. 16.4 g of
solidifying white oil
accumulates.
2-Bromo-4-formyl-pyridine can also be produced according to THL 42, 6815
(2001) from 2-bromo-4-picoline in two stages.
Stage 2
Production of 2-[(6-Bromo-pyridin-3-ylmethyl)-amino] -N-isoquinolin-3-yl-
benzamide
O N-:
Fi
eN H
--N
\ / Br
3.46 g (13.17 mmol) of 2-amino-N-isoquinolin-3-yl-benzamide is introduced into
50 ml of methanol, mixed with 1.5 ml of glacial acetic acid as well as 2.45 g
(13.17
mmol) of 2-bromopyridine-5-carbaldehyde and stirred for 24 hours under argon
and in a
CA 02493026 2005-01-19
64
moisture-free environment at room temperature. Then, it is mixed with 828 mg
(13.17
mmol) of sodium cyanoborohydride and stirred for another 24 hours at room
temperature.
After concentration by evaporation under vacuum, the residue is taken up in
dilute
sodium bicarbonate solution and suctioned off. The residue that is obtained is
absorptively precipitated in a little ethyl acetate and suctioned off
repeatedly. The
residue that is obtained in this case is chromatographed on silica gel with
hexane:ethyl
acetate = 1:1 as an eluant. 3.27 g (57% of theory) of 2-[(6-bromo-pyridin-3-
ylmethyl)-
amino] -N-isoquinolin-3-yl-benzamide is obtained.
CA 02493026 2005-01-19
Similarly produced are:
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-isoquinolin-3-yl-benzamide
O
NH Br
N
2-[ (2-Bromo-pyridin-4-ylmethyl)-amino]-N-(3-trifluoromethyl-phenyl)-benzamide
eN H \CF3
H Br
N
CA 02493026 2005-01-19
66
Example B
1St Stage
Production of 2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-benzoic Acid Methyl Ester
0
OMe
NH Br
zN
6.04 g (40 mmol) of anthranilic acid methyl ester in 600 ml of methanol is
mixed
with 3.2 ml of acetic acid and 7.4 g (40 mmol) of 2-bromopyridine-4-
carbaldehyde and
stirred overnight at 40 C. 3.8 g (60 mmol) of sodium cyanoborohydride is added
thereto
and stirred overnight at 40 C. 3.8 g (60 mmol) of sodium cyanoborohydride is
added
again and stirred over the weekend at 40 C. It is mixed with water and largely
concentrated by evaporation. The aqueous phase is extracted with ethyl
acetate, and the
combined organic phases are dried, filtered and concentrated by evaporation.
The crude
product is chromatographed on silica gel with a gradient that consists of
hexane and
hexane/ethyl acetate 1:3 and hexane/ethyl acetate 1:1 as an eluant. 10.0 g
(78% of
theory) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-benzoic acid methyl ester is
obtained
as a colorless oil.
CA 02493026 2005-01-19
67
Example C
1St Stage
Production of 2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-benzoic Acid
0
OH
eN H Br
N
10.0 g (31.2 mmol) of 2 - [(2-bromo-pyri din-4-ylmethyl) -amino] -benzoi c
acid
methyl ester is dissolved in 290 ml of ethanol and mixed with 31.2 ml of 2 M
sodium
hydroxide solution. After having been stirred overnight at room temperature,
the ethanol
is drawn off, and the aqueous phase is shaken out with ethyl acetate. The
aqueous phase
is acidified with concentrated hydrochloric acid. The precipitate that is
formed is
suctioned off and dried. 5.93 g (62%) of 2-[(2-bromo-pyridin-4-ylmethyl)-
amino]-
benzoic acid accumulates in the form of a white solid.
CA 02493026 2005-01-19
68
2nd Stage
Production of 2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-methyl-2H-indazol-6-
yl)-benzamide
/
0
N-Me
N N
NH g,
,N
0.500 g (1.6 mmol) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-benzoic acid,
0.471 g (3.2 mmol) of 2-methyl-2H-indazol-6-ylamine, 0.4 ml (3.68 mmol) of N-
methylmorpholine and 0.729 g (1.92 mmol) of O-(7-azabenzotriazol-l-yl)-1,1,3,3-
tetramethyluroniumhexafluorophosphate (HATU) in 25 ml of dimethylformamide are
stirred for 16 hours at room temperature. The dimethylformamide is drawn off
in an oil
pump vacuum. The remaining residue is drawn off in saturated sodium
bicarbonate
solution. It is extracted three times with ethyl acetate, and the combined
organic phases
are dried, filtered and concentrated by evaporation. The residue is
chromatographed on
silica gel with a gradient that consists of hexane:acetone = 100:0 to 50:50 as
an eluant.
0.669 g (96% of theory) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-N-(2-methyl-
2H-
indazol-6-yl)-benzamide is obtained in the form of a beige foam.
CA 02493026 2005-01-19
69
Similarly produced are also the following compounds:
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-methyl-1H-indazol-6-yl)-benzamide
O j:D[IQN
N H Me
NH Br
N
2-1(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1 H-indazol-6-yl)-benzamide
O \N
\ N \ H
H
NH
Br
N
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1H-indazol-5-yl)-benzamide
0 QJIJJN
NH Br
,N
CA 02493026 2005-01-19
Example D
Stage 1
Production of 2- { 12-(2-Oxo-pyrrolidin-1-yl)-pyridin-4-ylmethyl]-amino}-
benzoic
Acid Methyl Ester
0
ct rye(
`~ o
N
870 mg (2.78 mmol) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-benzoic acid
methyl ester, 53 mg (0.28 mmol) of copper (I) iodide, 1.126 g (5.5 mmol) of
potassium
phosphate and 0.26 ml (3.6 mmol) of pyrrolidin-2-one are refluxed in 15 ml of
dioxane
for 8 hours. After water is added, the dioxane is distilled off in a vacuum,
made alkaline
with about 12% ammonia solution and shaken out several times with ethyl
acetate. The
collected ethyl acetate phase is washed, dried, filtered and concentrated by
evaporation.
As a residue, 700 mg (77% of theory) of 2- { [2-(2-oxo-pyrrolidin- l -yl)-
pyridin-4-
ylmethyl]-amino}-benzoic acid methyl ester is obtained as a crude product,
which is used
without further purification in the next stage.
CA 02493026 2005-01-19
71
Similarly produced are:
0
OMe
NH
VN
G G MW Melting Point [ C]
or MS Molar Peak
(m/e)
327.3
. -N), 0
0 326.4
.-N ~NH
v
0 341.4
.-N ~N-Me
Stage 3
Production of 2-{ [2-(2-Oxo-pyrrolidin-1-yl)-pyridin-4-ylmethyl]-amino}-
benzoic
Acid
0
O(q
CA 02493026 2005-01-19
72
700 mg (2.15 mmol) of 2-{[ 2-(2-oxo-pyrrolidin- l-yl)-pyridin-4-ylmethyl]-
amino } -benzoic acid methyl ester is mixed in 15 ml of methanol with 2.7 ml
of IN
sodium hydroxide solution and refluxed for 1 hour. After the methanol is
distilled off in
a vacuum, it is diluted with water and shaken once with ethyl acetate. The
aqueous phase
is mixed with 5 ml of 1 mol citric acid solution and stirred overnight. The
solid
precipitation is suctioned off and very quickly dried. 600 mg of 2-{[2-(2-oxo-
pyrrolidin-
1-yl)-pyridin-4-ylmethyl]-amino}-benzoic acid, which is used as a crude
product in the
next stage, is obtained.
Similarly produced are:
0
OH
NH
VN
G RZ, R3, Y and Z = G
G MW Melting Point [ C]
or MS Molar Peak
(m/e)
313.3
. -N), O
CA 02493026 2005-01-19
73
G MW Melting Point [ C]
or MS Molar Peak
(mle)
312.3
. -Nlt~ NH
\J
326.4
-NVN_Me
\f
CA 02493026 2005-01-19
74
Example E
Production of 2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-methyl-IH-indazol-5-
yl)-benzamide and 2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-methyl-2H-
indazol-5-yl)-benzamide
Me
O / N\
N N
N-Me
eNNH Br
H Br
N
N
4.22 g (10 mmol) of 2 - [ (2 -bromo-pyri din-4-ylmethyl)-amino] -N-(IH-indazol-
5-
yl)-benzamide is mixed in 30 ml of dimethylformamide while being cooled with
ice with
3.6 g (1 1 mmol) of cesium carbonate and 0.68 ml (I 1 mmol) of methyl iodide,
and it is
stirred overnight at room temperature. It is then stirred into 250 ml of ice-
cold water,
stirring is continued for 15 minutes, and it is suctioned off. The filter cake
is very
quickly dried and chromatographed on silica gel with a gradient of ethyl
acetate:hexane =
1:1 to 100:0 as an eluant. 1.79 g (41 % of theory) of 2-[(2-bromo-pyridin-4-
ylmethyl)-
amino]-N-(1-methyl-IH-indazol-5-yl)-benzamide with a melting point of 173.8 C
as well
as 830 mg (19% of theory) of 2-[(2-bromo-pyridin-4-ylmethyl)-amino]-N-(2-
methyl-2H-
indazol-5-yl)-benzamide with a melting point of 183.8 C are obtained.
CA 02493026 2005-01-19
Similarly produced are:
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-isopropyl-1H-indazol-5-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(2-isopropyl-2H-indazol-5-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino)-N-(1-ethyl-1H-indazol-5-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-ethyl-2H-indazol-5-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(1-[2-methoxyethyll-lH-indazol-
5-yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(2-[2-methoxyethyll-2H-indazol-
5-yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(1-[cyanomethyll-1H-indazol-5-
yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(2-[cyanomethyll-2H-indazol-5-
yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(1-[2-dimethylaminoethyll-lH-
indazol-5-yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(2-[2-dimethylaminoethyll-2H-
indazol-5-yl)-benzamide,
CA 02493026 2005-01-19
76
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-methyl-1H-indazol-6-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-methyl-2H-indazol-6-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-isopropyl-1H-indazol-6-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-isopropyl-2H-indazol-6-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-ethyl-IH-indazol-6-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-ethyl-2H-indazol-6-yl)-
benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-[2-methoxyethyl]-1H-indazol-
6-yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-[2-methoxyethyl]-2H-indazol-
6-yl)-benzamide,
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-[cyanomethyl]-1H-indazol-6-
yl)-benzamide and
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(2-[cyanomethyl]-2H-indazol-6-
yl)-benzamide.
2-[(2-Bromo-pyridin-4-ylmethyl)-amino]-N-(1-[2-dimethylaminoethyl]-1H-
indazol-6-yl)-benzamide and
CA 02493026 2005-01-19
77
2-[(2-Bromo-pyridin-4-ylmethyl)-aminol-N-(2-(2-dimethylaminoethyll-2H-
indazol-6-yl)-benz amide.
CA 02493026 2005-01-19
78
The sample applications below explain the biological action and the use of the
compounds according to the invention without the latter being limited to the
examples.
Solutions Required for the Tests
Stock solutions
Stock solution A: 3 mmol of ATP in water, pH 7.0 (-70 C)
Stock solution B: g-33P-ATP 1 mCi/l00 l
Stock solution C: poly-(Glu4Tyr) 10 mg/ml in water
Solution for dilutions
Substrate solvent: 10 mmol of DTT, 10 mmol of manganese chloride, 100 mmol of
magnesium chloride
Enzyme solution: 120 mmol of tris/HCI, pH 7.5, 10 pM of sodium vanadium oxide
CA 02493026 2005-01-19
79
Sample Application I
Inhibition of the KDR- and FLT-1 Kinase Activity in the Presence of the
Compounds According to the Invention
In a microtiter plate (without protein binding) that tapers to a point, 10 pl
of
substrate mix (10 pl of volume of ATP stock solution A + 25 tCi of g-33P-ATP
(about 2.5
pl of stock solution B) + 30 pl of poly-(Glu4Tyr) stock solution C + 1.21 ml
of substrate
solvent), 10 pd of inhibitor solution (substances corresponding to the
dilutions, 3% DMSO in
substrate solvent as a control) and 10 pl of enzyme solution (11.25 pg of
enzyme stock
solution (KDR or FLT-1 kinase) are added at 4 C in 1.25 ml of enzyme solution
(dilute). It
is thoroughly mixed and incubated for 10 minutes at room temperature. Then, 10
pl of stop
solution (250 mmol of EDTA, pH 7.0) is added, mixed, and 10 PI of the solution
is
transferred to a P 81 phosphocellulose filter. Then, it is washed several
times in 0.1 M
phosphoric acid. The filter paper is dried, coated with Meltilex and measured
in a microbeta
counter.
The IC50 values are determined from the inhibitor concentration, which is
necessary
to inhibit the phosphate incorporation to 50% of the uninhibited incorporation
after removal
of the blank reading (EDTA-stopped reaction).
The results of the kinase inhibition IC50 in pM are presented in the table
below.
CA 02493026 2005-01-19
Sample Application 2
Cytochrome P450 Inhibition
The Cytochrome P450 inhibition was performed according to the publication of
Crespi et al. (Anal. Biochem., 248, 188-190 (1997)) with use of the
baculovirus/insect
cell-expressed, human Cytochrome P 450 isoenzyme (3A4).
The results are presented in the following table.
Example No. VEGFR II (KDR) Cytochrome P450
[nM] Isoenzyme 3A4
2.54 from 5 3.6
WO 00/27819
38 from 180 4.6
WO 00/27820
1.14 52 >30
3.24 12 14
3.30 10 5.5
6.2 41 >30
6.22 24 10
6.27 8 10
6.32 65 11
The superior action of the compounds according to the invention compared to
the
known compounds can be seen clearly from the result.