Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02494177 2005-02-04
DESCRIPTION
AZULENE DERIVATIVE AND SALT THEREOF
FIELD OF THE INVENTION
The present invention relates to an azulene derivative
of a specific chemical formula and a salt thereof. More
particularly, the present invention relates to an azulene
derivative which effectively treats or prevents diabetes such
as insulin-dependent diabetes (type 1 diabetes), and
insulin-independent diabetes (type 2 diabetes), as well as
various diabetes-related diseases such as insulin-resistant
diseases and obesity, for example, as a pharmaceutical,
particularly as a Na+-glucose cotransporter inhibitor, and to
a salt thereof.
BACKGROUND OF THE INVENTION
In recent years, a pharmaceutical to inhibit a Na+-glucose
cotransporter (SGLT) in the intestinal tract and kidney to
reabsorb glucose (a Na+-glucose cotransporter inhibitor) has
been demanded as an antidiabetic agent to rapidly normalize
hyperglycemia and improve the energy balance in the body. Such
a Na+-glucose cotransporter inhibitor has been expected as an
excellent pharmaceutical for treating or preventing diabetes
such as insulin-dependent diabetes (type 1 diabetes) and
insulin-independent diabetes (type 2 diabetes), as well as
diabetes-related diseases such as insulin-resistant diseases
1
CA 02494177 2005-02-04
and obesity.
As compounds used as the Na+-glucose cotransporter
inhibitor, phloridzin describedin Welch, C.A.etal. (J. Natr.,
1989, 119(11) 1698) and a synthetic 0-glycoside described in
Hongu, M. et al. (Chem. Pharm. Bull., 1998, 46(1) 22) and
JP-A-11-21243 are known, for example. These compounds are
reported to discharge excess blood glucose into urine and reduce
the level of blood glucose by inhibiting a Na+-glucose
cotransporter in the kidney.
However, since any of these compounds are an 0-glycoside
comprising an 0-glucoside bond formed between glucose and an
aglycon moiety, it has a problem that the hypoglycemic effect
is disappeared due to hydrolysis of 0-glucoside bond by
glucosidase or the like in the small intestine when orally
absorbed.
Phloretin, an aglycon moiety of phloridzin, is known to
highly inhibit a facilitated diffusion-type glucose
transporter. For example, it is reported that the cerebral
glucose concentration decreases when phloretin is administered
to the vein of a rat (e.g. Stroke, 1983, 14, 388) . Phloretin
is also known to inhibit a vitamin C transporter (Wang, Y. et
al., Biochem. Biophys. Res. Commun., 2000, 267, 488-494).
Therefore, an attempt has been made to use a C-glycoside
prepared by converting oxygen in the glucoside bond of the
0-glycoside to carbon as the Na+-glucose cotransporter
inhibitor.
For example, JP-A-2001-288178 (hereinafter referred to
2
CA 02494177 2005-02-04
as Patent Document 1) describes that a compound of the following
formula has the effect of inhibiting a Na+-glucose cotransporter
and is useful as a treating agent or preventing agent for
diabetes and a hypoglycemic agent.
RS O Al
R O R {R~}n
OR2
wherein Rl represents H, OH, lower alkyl group, -0-lower alkyl
group, or the like, R2 represents H, -COO-lower alkyl group,
or the like, R5 represents -CHZOH, -CH2OC00-lower alkyl group,
or the like, Al represents pyridine, furan, thiophene, quinoline,
indole, or the like, n is 0, 1, 2, or 3, and m is 0 or 1 (See
Patent Document 1 for further details on the symbols of the above
formula).
In addition, the pamphlet of WO 01/27128 (hereinafter
referred to Patent Document 2) describes that a compound of the
following formula can be used as the Na+-glucose cotransporter
inhibitor to treat obesity or type 2 diabetes.
3
CA 02494177 2005-02-04
R2a R R4
O \ I ~
HO
2 A Rs
R
HO" "OH
OH
wherein Rl, R2, and R2a individually represent a hydrogen atom,
OH, OR5, alkyl, CF31 OCHFZ, OCHF3, or the like, R3 and R{
individually represent a hydrogen atom, OH, OR5a, -0-aryl,
-O-CHz-aryl, alkyl, cycloalkyl, CF31 or the like, A represents
0, S, NH, or (CH2),,, and n is 0, 1, 2, or 3(See Patent Document
2 for further details on the symbols of the above formula).
As explained above, the C-glycoside is useful to a certain
extent for treating diabetes due to the effect of inhibiting
a Na+-glucose cotransporter. However, due to the recent rise
in incidence of diabetes which is a lifestyle-related disease
and could even be called a national disease, a compound having
a chemical structure different from that of a known compound
and showing the effect of inhibiting a Na+-glucose cotransporter
more rapidly and more significantly has been increasingly
desired for the clinical practice of diabetes treatment or the
like.
DISCLOSURE OF THE INVENTION
The present inventors have conducted extensive studies
about a compound with a benzene ring directly bonded with a
4
CA 02494177 2005-02-04
glucose residue and having the effect of inhibiting a Na+-
glucose cotransporter. As a result, the inventors have found
that a compound (an azulene derivative) having an azulene ring
bonded to a benzene ring directly or via a lower alkylene (-A-)
which may be substituted with a halogen atom, with the benzene
ring being directly bonded to a glucose residue, shown by the
following formula (I) , has a significant effect of inhibiting
a Na+-glucose cotransporter. Specifically, the present
invention provides a compound of the following formula (I) and
a salt thereof (hereinafter both referred to as "compound of
the present invention") The compound of the present invention
can be suitably used as a Na+-glucose cotransporter inhibitor
using the compound as an active ingredient, particularly as a
therapeutic agent and/or preventive agent for diabetes.
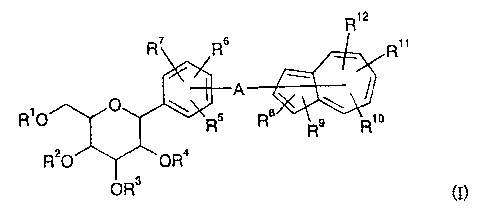
The chemical structure of the compound of the present
invention differs from those of Patent Documents 1 and 2 in that
the compound of the present invention has an azulene ring, for
example.
R12
R' R6 A R
~ p 8~
R O RS R 9 R10
R20 OR4
QR3 (I)
wherein R1 to R9 individually represent a hydrogen atom, an
5
CA 02494177 2005-02-04
optionally substituted lower alkyl, -C(=0)-optionally
substituted lower alkyl, or -optionally substituted lower
alkylene-optionally substituted aryl,
R5 to R1z individually represent a hydrogen atom, an
optionally substituted lower alkyl, halogen atom, -OH, -0-
optionally substituted lower alkyl, -optionally substituted
lower alkylene-OH, -optionally substituted lower alkylene-
0-optionally substituted lower alkyl, -0-optionally
substituted lower alkylene-0-optionally substituted lower
alkyl, -0-optionally substituted lower alkylene-optionally
substituted aryl, -optionally substituted lower alkylene-0-
C(=0) - optionally substituted lower alkyl, -COOH, nitro, cyano,
amino, substituted amino, or -C(=0)-0-optionally substituted
lower alkyl, and
A represents a bond or an optionally substituted lower
alkylene,
wherein -A- may be bonded to any one of the positions 1-8
of the azulene ring, and any two of R5, R6, and R' may form a
benzene ring together with the adjacent carbon atoms.
Theterm"optionallysubstituted"usedforthe definitions
of the groups represented by R' to R4, RS to R12, and A indicates
the groups in question may be either substituted or
unsubstituted with a halogen atom, -OH, -lower alkylene-OH,
-COOH, -C(=0)-0-lower alkyl, nitro, cyano, amino, or
substituted amino. A halogen atom, -OH, and -COOH are
preferable as a substituent.
The optionally substituted lower alkyl, -C(=0)-
6
CA 02494177 2005-02-04
optionally substituted lower alkyl, and -optionally
substituted lower alkylene-optionally substituted aryl
represented by R' to R9 in the above formula (I) are preferably
a lower alkyl, -C(=0)-lower alkyl, and -lower alkylene-aryl,
respectively. The optionally substituted lower alkyl, -0-
optionally substituted lower alkyl, -optionally substituted
lower alkylene-OH, -optionally substituted lower alkylene-
0-optionally substituted lower alkyl, -0-optionally
substituted lower alkylene-O-optionally substituted lower
alkyl, -0-optionally substituted lower alkylene-optionally
substituted aryl, -optionally substituted lower alkylene-0-
C(=0)-optionally substituted lower alkyl, and -C(=0)-0-
optionally substituted lower alkyl represented by R5 to R12 in
the formula (I) are preferably a lower alkyl, -0-lower alkyl,
-lower alkylene-OH, -lower alkylene-0-lower alkyl, -0-lower
alkylene-0-lower alkyl, -0-lower alkylene-aryl, -lower
alkylene-O-C(=0)-lower alkyl, and -C(=0)-0-lower alkyl,
respectively. The optionally substituted lower alkylene
represented by A in the above formula (I) is preferably a lower
alkylene or a halogen-substituted lower alkylene.
In the compound of the present invention, the group
represented by A of the above formula (I) is preferably a lower
alkylene, and particularly preferably a methylene.
The groups R1-R9 of the above formula (I) are preferably
a hydrogen atom.
The azulene derivative of the above formula (I) may be
preferably any one of compounds selected from the group
7
CA 02494177 2005-02-04
consisting of 1,5-anhydro-l-[3-(azulen-2-ylmethyl)phenyl]
hexytol, 1,5-anhydro-l-[5-(azulen-2-ylmethyl)-2-
methoxyphenyl]hexytol, 1,5-anhydro-l-[3-(azulen-2-
ylmethyl)-5-methoxyphenyl]hexytol, 1,5-anhydro-l-[3-
(azulen-2-ylmethyl)-4-methoxyphenyl]hexytol, 1,5-anhydro-l-
[5-(azulen-2-ylmethyl)-2-ethoxyphenyl]hexytol, 1,5-anhydro-
1-[5-(azulen-2-ylmethyl)-2-methylphenyl]hexytol, 1,5-
anhydro-l-[5-(azulen-2-ylmethyl)-2-hydroxyphenyl] hexytol,
1,S-anhydro-l-[5-(azulen-2-ylmethyl)-2-
fluorophenyl]hexytol, 1,5-anhydro-l-[5-(azulen-2-
ylmethyl)-2,4-dimethoxyphenyl]hexytol, and 1,5-anhydro- 1-
[4-(azulen-2-ylmethyl)-1-methoxy-2-naphthyl]hexytol.
The present invention also provides a pharmaceutical
composition containing the above azulene derivative or the salt
thereof as an active ingredient and pharmaceutically acceptable
adj uvant s .
The pharmaceutical composition of the present invention
is effectively used for a Na+-glucose cotransporter inhibitor
or a preventing agent or therapeutic agent for diabetes and
diabetic complications.
The present invention further provides use of the azulene
derivative or the salt thereof for producing a Na+-glucose
cotransporter inhibitor or a preventive agent and/or
therapeutic agent for diabetes and diabetic complications.
The present invention further provides a therapeutic
method for diabetes and diabetic complications comprising
administering an effective amount of the above azulene
8
CA 02494177 2005-02-04
derivative or the salt thereof to a patient.
In the definition of the formulas in this specification,
"lower" refers to a linear or branched carbon chain having 1-6
carbon atoms, unless otherwise specified. Accordingly,
examples of a "lower alkyl" include linear or branched alkyls
having 1-6 carbon atoms such as a methyl, ethyl, propyl,
isopropyl, butyl, isobutyl, sec-butyl, t-butyl, pentyl,
isopentyl, hexyl, and isohexyl. Of these, alkyls having 1-
3 carbon atoms are preferable, with a methyl and ethyl being
particularly preferable.
As a "lower alkylene, " in addition to a methylene, ethylene,
propylene, and butylene, a branched lower alkylene may be used.
Of these, a methylene and ethylene are preferable, with a
methylene being particularly preferable.
As a "halogen atom," a fluorine atom, chlorine atom,
bromine atom, or iodine atom can be given, with a chlorine atom
and bromine atom being preferable. As a"halogen- substituted
lower alkyl" or "halogen-substituted lower alkylene,"a lower
alkyl or lower alkylene substituted with the above halogen atom
can be given, with a lower alkyl or lower alkylene substituted
with one or more fluorine atoms being particularly preferable.
An "aryl" refers to a monocyclic to tricyclic aromatic
hydrocarbon group having 6-14 carbon atoms. Examples of the
aryl include a phenyl, naphthyl, anthranyl, and phenanthryl,
with a phenyl and naphthyl being particularly preferable. As
a"-lower alkylene-aryl," a benzyl and phenethyl are
preferable.
9
CA 02494177 2005-02-04
As a "substituted amino," an amino group of which one or
two hydrogen atoms are substituted with the lower alkyl, an acyl,
carbamoyl, or carbamate (NH2-C(=0)-0-) can be given.
As the "acyl," a formyl, acetyl, propionyl, butyryl, valeryl,
pivaloyl, or the like can be given, with an acetyl being
particularly preferable.
-A- in the above formula (I) may be bonded to any one of
the positions 1-8 of the azulene ring.
The compound of the present invention includes a mixture
or isolated product of various stereoisomers such as a tautomer
and an optical isomer.
The compound of the present invention may form an
acid-addition salt or, depending on the type of substituent,
a salt with a base. Specific examples of such a salt include
aid-addition salt with a mineral acid such as hydrochloric acid,
hydrobromic acid, hydroiodic acid, sulfuric acid, nitric acid,
and phosphoric acid; an organic acid such as formic acid, acetic
acid, propionic acid, oxalic acid, malonic acid, succinic acid,
fumaric acid, maleic acid, lactic acid, malic acid, tartaric
acid, citric acid, methanesulfonic acid, and ethanesulfonic
acid; an acidic amino acid such as aspartic acid and glutamic
acid; a salt of an inorganic base such as sodium, potassium,
magnesium, calcium, and aluminum; an organic base such as
methylamine, ethylamine, and ethanolamine; a basic amino acid
such as lysine and ornithine; and ammonium salts.
The compound of the present invention further includes
hydrates and various pharmaceutically acceptable solvates and
CA 02494177 2005-02-04
polymorphs.
The compound of the present invention should not be limited
to the compounds later described in examples, but includes all
the compounds of the above formula (I) (azulene derivatives)
and the pharmaceutically acceptable salts thereof.
Moreover, the compound of the present invention may
includes any prodrug which is converted to any one of compounds
of the above formula (I) or salts thereof in the body as a result
of the metabolism in the body. As a group for forming the
prodrug of the compound of the present invention, a group
described in Prog. Med. 5: 2157-2161 (1985) or a group described
in "Development of Pharmaceuticals," vol. 7, Molecular Design,
163-198 (Hirokawa Shoten, 1990) can be given. Therefore, the
whole contents of those literatures are incorporated herein by
reference.
The compound of the present invention or the
pharmaceutically acceptable salt thereof can be produced by
various known synthesizing methods utilizing characteristics
based on the type of its basic structure or substituent. In
this case, from the viewpoint of production technique, it may
be effective to replace the functional group with a suitable
protective group, specifically, a group which can be readily
converted to the functional group, at the stage of a starting
material or intermediate, depending on the type of functional
group. Following this, the protective group is optionally
removed to obtain the target compound. Examples of such a
functional group include a hydroxyl group and carboxyl group.
11
CA 02494177 2005-02-04
Examples of the protective group for these functional groups
include protective groups described in Greene and Wuts,
"Protective Groups in Organic Synthesis," Second Edition.
These groups may be suitably used according to the reaction
conditions.
Preparation Examples
Typical production processes of the compound of the
present invention will be described as follows.
Preparation Example 1
A process of Preparation Example 1 comprises subjecting
an azulene compound (1) to a Friedel-Crafts reaction followed
by reduction to prepare a compound (2), reacting the compound
(2) with a compound (3) by an addition reaction to prepare a
compound (4), reducing the compound (4) to prepare a compound
(I), and deprotecting the compound (I) to prepare a compound
(I'), as shown in the following formula.
R0 O O RS R6 R 0 R11
10 10 9
R11
RS R ga RRZO OR N R 1~
R Friedel Gafts rcaction (3) OR RIO 0 A RI2
~ ' R
i2 2) Redurtion Br R 7 A R12 R20 OR,R
R a {~) (2) R OR ~ (4)
5 r I0 RI I RIB Ri 1
R R R~ 5 6 R9
Reduct lon RIO O q e R12 tkp'otaotion O q Ra F{iz
R' HO R~
R20 OR HO OH
OR3 (~) OH (I')
wherein Rlto R12 and A are the same as above.
12
CA 02494177 2005-02-04
The Friedel-Crafts reaction is carried out in the presence
of an appropriate Lewis acid, in the absence of a solvent, or
in an appropriate solvent. Specific examples of the Lewis acid
include aluminum chloride, boron trichloride, zinc chloride,
vanadium chloride, ferric chloride, and stannic chloride.
Specific examples of the solvent include ethers such as diethyl
ether and tetrahydrofuran; haloalkyls such as chloroform,
dichloromethane, and l,2-dichloroethane; dimethylformamide;
dimethylsulfoxide; and a mixture of these solvents. The
solvent is appropriately selected according to the type of
reaction substrate or the reaction conditions. The reaction
temperature is about 20 C to 180 C, and preferably about 20 C
to 40 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
The subsequent reduction reaction is carried out in the
presence of an appropriate reducing agent and acid catalyst in
a suitable solvent. Specific examples of the reducing agent
include sodium borohydride, sodium cyanoborohydride, and
lithium aluminum hydride. Specific examples of the acid
include boron trifluoride-diethyl ether complex,
trifluoroacetic acid, and trifluoromethanesulfonic acid.
Specific examples of the solvent include ethers such as diethyl
ether, tetrahydrofuran, and diglyme; haloalkyls such as
chloroform, dichloromethane, and 1,2-dichloroethane; and a
mixture of these solvents. The solvent is appropriately
selected according to the type of reaction substrate or the
13
CA 02494177 2005-02-04
reaction conditions. The reaction temperature is about 0 C to
180 C, andpreferably about 0 C to 60 C, while it varies according
to the type of starting material compounds, the reaction
conditions, or the like within the above-mentioned range
though.
The subsequent addition reaction of the compound (2) to
the compound (3) is carried out in the presence of an alkyl
lithium reagent such as n-butyl lithium, sec-butyl lithium, or
t-butyl lithium in a suitable solvent. Specific examples of
the solvent include ethers such as diethyl ether,
tetrahydrofuran, and diglyme. The solvent is appropriately
selected according to the type of reaction substrate or the
reaction conditions. The reaction temperature is about -100 C
to 180 C, and preferably about -80 C to 30 C, while it varies
according to the type of starting material compounds, the
reaction conditions, or the like within the above-mentioned
range though. The compound (4) may also be prepared by reacting
the compound (2) with a Grignard reagent prepared using a metal
reagent such as magnesium in a suitable solvent. Specific
examples of the solvent include ethers such as diethyl ether,
tetrahydrofuran, and diglyme. The solvent is appropriately
selected according to the type of reaction substrate or the
reaction conditions. The reaction temperature is about 20 C
to 180 C, and preferably about 20 C to 80 C, while it varies
according to the type of starting material compounds, the
reaction conditions, or the like though.
The subsequent reduction reaction is carried out in the
14
CA 02494177 2005-02-04
presence of an appropriate reducing agent and acid catalyst in
a suitable solvent. Specific examples of the reducing agent
include triethylsilane, triisopropylsilane, and t-
butyldimethylsilane. Specific examples of the acid catalyst
include boron trifluoride-diethyl ether complex,
trifluoroacetic acid, and trimethylsilyl
trifluoromethanesulfonate. Specific examples of the solvent
include haloalkyls such as chloroform, dichloromethane, and
1,2-dichloroethane; ethers such as diethyl ether,
tetrahydrofuran, and diglyme; acetonitrile; and a mixture of
these solvents. The solvent is appropriately selected
according to the type of reaction substrate or the reaction
conditions. The reaction temperature is about -100 C to 180 C,
and preferably about -40 C to 20 C, while it varies according
to the type of starting material compounds, the reaction
conditions, or the like within the above-mentioned range
though.
The deprotection is carried out in the presence of a metal
catalyst such as palladium/carbon, palladium hydroxide, or
platinum/carbon in a suitable solvent in a hydrogen atmosphere,
or in the presence of a Lewis acid in a suitable solvent.
Specific examples of the Lewis acid include boron trichloride,
boron tribromide, and aluminumtrichloride. Specific examples
of the solvent include ethers such as tetrahydrofuran and
dioxane; esters such as ethyl acetate; alcohols such as methanol
and ethanol; acetonitrile; and a mixture of these solvents. The
solvent is appropriately selected according to the type of
CA 02494177 2005-02-04
reaction substrate or the reaction conditions. The reaction
temperature is about -100 C to 180 C, and preferably about -80 C
to 30 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
Preparation Example 2
A process of Preparation Example 2 comprises reacting a
compound (3) with a compound (5) to prepare a compound (6),
reducing the compound (6) to prepare a compound (7),
halogenating the compound (7) to prepare a compound (7'),
reacting the compound (7') with an azulene derivative (8) to
prepare a compound (I), and deprotecting the compound (I) to
prepare a compound (I'), as shown in the following scheme.
R,Q 0 O
R5 Rs R2OR4 S R R5 R6
~ {3) OR 3 - Ri0 f H CH3 Reeaotion 0 CH3
I~ '!~ -CH3 , r R O ,
Br~'~R '
7 RZO OR R R'O OR4R
(5) OR {6} OR (7)
Ria R9
RS R6 Rs R6 R9 Rs2
Rii Re ~ R
Ri Q 7 er 2 ( 8 ) R'0 0 I ~ A ~o
R20 OR' R20 OR R R" R
OR3 (~,) OR3 (~l
R R9 R12
S G
~
HO OH
Ucprotection 0
HO R~ A e Rio
OH
wherein X represents a halogen, B(OR") 3, wherein R13 is H or a
16
CA 02494177 2005-02-04
lower alkyl, or SnR143, wherein R14 is a lower alkyl.
The reaction of the compound (3) with the compound (5)
is carried out in the same manner as in the reaction of the
compound (2) with the compound (3) of Preparation Example 1.
The subsequent reduction reaction to prepare the compound
(7) is carried out in the same manner as in the reduction reaction
of the compound (4) of Preparation Example 1. The subsequent
halogenation of the compound (7) to prepare the compound (7')
is carried out in the presence of an appropriate halogenating
agent in a suitable solvent. Specific examples of the
halogenating agent include N-bromosuccinimide, bromine,
hydrogen bromide. Specific examples of the solvent include
haloalkyls such as methylene chloride, chloroform, and carbon
tetrachioride; esters such as ethyl acetate; ethers such as
tetrahydrofuran and dioxane; dimethylsulfoxide; acetic acid;
water; and a mixture of these solvents. The solvent is
appropriately selected according to the type of reaction
substrate or the reaction conditions. The reaction
temperature is about -100 C to 180 C, and preferably about 0 C
to 100 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
The subsequent reaction of the compound (7') with the
compound (8) is carried out in the presence of an appropriate
palladium catalyst or in the presence of an appropriate
palladium catalyst and an appropriate phosphine in a suitable
solvent. Specific examples of the catalyst include
17
CA 02494177 2005-02-04
tetrakistriphenylphosphine palladium (0), palladium acetate,
bistriphenylphosphine dichloropalladium (II), 1,2-
bis(diphenylphosphinoethane)dichloropalladium (II), 1,1'-
bis(diphenylphosphinoferrocene)dichloropalladium (II), and
tris(dibenzylideneacetone)dipalladium (0). Specific
examples of the phosphine include trifurylphosphine, 2-
(dicyclohexylphosphino)biphenyl, and tri(t-butyl)phosphine.
Specific examples of the solvent include ethers such as diethyl
ether, tetrahydrofuran, dioxane, and diglyme; alcohols such as
methanol, ethanol, and isopropanol; benzene; toluene; water;
and a mixture of these solvents. The solvent is appropriately
selected according to the type of reaction substrate or the
reaction conditions. The reaction temperature is about -100 C
to 180 C, and preferably about 0 C to 100 C, while it varies
according to the type of starting material compounds, the
reaction conditions, or the like within the above-mentioned
range though.
This reaction may be carried out by reacting the compound
(7' ) with a metal in a suitable solvent to prepare a metal reagent,
and then reacting the reagent with the compound (8) in the
presence of a palladium catalyst. Specific examples of the
metal include copper, zinc, iron, and magnesium. The palladium
catalyst, solvent, and reaction temperature are the same as
above.
The deprotection is carried out in the presence of an
appropriate base in a suitable solvent. Specific examples of
the base include sodium hydroxide, potassium hydroxide, sodium
18
CA 02494177 2005-02-04
methoxide, and sodium ethoxide. Specific examples of the
solvent include ethers such as tetrahydrofuran, dioxane, and
diglyme; alcohols such as methanol, ethanol, and isopropanol;
acetonitrile; water; and a mixture of these solvents. The
solvent is appropriately selected according to the type of
reaction substrate or the reaction conditions. The reaction
temperature is about -100 C to 180 C, and preferably about 0 C
to 100 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
The deprotection is also carried out in the presence of
an appropriate Lewis acid in a suitable solvent. Specific
examples of the Lewis acid include boron trichloride, boron
tribromide, and aluminum trichloride. Specific examples of
the solvent include ethers such as tetrahydrofuran and dioxane;
esters such as ethyl acetate; alcohols such as methanol and
ethanol; acetonitrile; and a mixture of these solvents. The
solvent is appropriately selected according to the type of
reaction substrate or the reaction conditions. The reaction
temperature is about -100 C to 180 C, and preferably about -80 C
to 60 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
Preparation Example 3
A process of Preparation Example 3 comprises protecting
an alcohol derivative (9), reacting the protected derivative
(9) with a compound (3) to prepare a compound (10), reducing
19
CA 02494177 2005-02-04
and deprotecting the compound (10) to prepare a compound (11) ,
halogenating the compound (11) to prepare a compound (7'),
reacting the compound (7') with an azulene derivative (8) to
prepare a compound (I), and deprotecting the compound (I) to
prepare a compound (I'), as shown in the following scheme.
R~0 0 O
R5 R6 Rz = R' R J P R5 R6 OH
OR' OH I I -
Protection (3) R'O - _To 7 Reduction Ocprotection R10 O
R R'
Br R7 ~ R20 OR~ Rz0 OR~
~g) OR 3 (10) OR3(1i)
Rio Rs
X R12 RS R6 R', / e RS R6 R/ Ri,
O
R~O 12 R ,
~ 2 R7 Br R(g~ R O R~ A RB R10
R 0 OR R20 OR'
OR' (7,)
Riz
RS R6 R9 R
/
Deprotection HO 0 R7 A a Rio
R
HO OH
OH
wherein P represents a protective group, and X represents a
halogen, B(0R13) 3, wherein R13 is H or a lower alkyl, or SnRI'3,
wherein R14 is a lower alkyl.
The alcohol derivative (9) is protected by a suitable
protective group such as a t-butyldimethylsilyl group, t-
butyldiphenylsilyl group, and tetrahydropyranyl group
according to a conventional method. The subsequent reaction
with the compound (3) is carried out in the same manner as in
the reaction of the compound (2) with the compound (3) of
CA 02494177 2005-02-04
Preparation Example 1.
The subsequent reduction reaction is carried out in the
same manner as in the reduction reaction of the compound (4)
of Preparation Example 1. The subsequent deprotection is
carried out in the presence of an appropriate catalyst in a
suitable solvent. Specific examples of the catalyst include
tetrabutylammonium fluoride, boron trifluoride-diethyl ether
complex, hydrogen fluoride, acetic acid, and p-toluenesulfonic
acid. Specific examples of the solvent include ethers such as
tetrahydrofuran and dioxane; alcohols such as methanol and
ethanol; water; and a mixture of these solvents. The solvent
is appropriately selected according to the type of reaction
substrate or the reaction conditions. The reaction
temperature is about -100 C to 180 C, and preferably about 20 C
to 80 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
The subsequent halogenation is carried out in the presence
of a halogenating agent and triphenyl phosphine in a suitable
solvent. Specific examples of the halogenating agent include
N-bromosuccinmide, bromine, carbon tetrabromide, and copper
(II) bromide. Specific examples of the solvent include
haloalkyls such as methylene chloride, chloroform, and carbon
tetrachloride; esters such as ethyl acetate; ethers such as
tetrahydrofuran and dioxane; benzene; toluene;
dimethylsulfoxide; acetic acid; water; and a mixture of these
solvents. The solvent is appropriately selected according to
21
CA 02494177 2005-02-04
the type of reaction substrate or the reaction conditions. The
reaction temperature is about -100 C to 180 C, and preferably
about 0 C to 100 C, while it varies according to the type of
starting material compounds, the reaction conditions, or the
like within the above-mentioned range though.
The subsequent reaction of the compound (7') with the
compound (8) and the deprotection are carried out in the same
manner as in Preparation Example 2.
Preparation Example 4
A process of Preparation Example 4 comprises reacting a
bromobenzene derivative (12) with a compound (3) to prepare a
compound (13 ), reducing the compound (13) to prepare a compound
(14), converting the compound (14) to a trialkyltin derivative
(15), reacting the derivative (15) with an azulene derivative
(16) to prepare a compound (I), and deprotecting the compound
(I) to prepare a compound (I' ), as shown in the following scheme.
22
CA 02494177 2005-02-04
R'O 0 0
RS R6 Rs RB
Rs Re R20 30R H
o Br (3) OR R'0 0 I 7Br Reduction R,0 0 1 7Br
Br R' R20 OR' R RZO OR R
(1 Z' OR' (13) OR3 (14)
R1a Ry
iz
Rs Re R1s A s s 9 R õ
! is Rii R
i p I Sn-R tz RB R R7 Rzs (16R0 O A Rio
RZO OR' RZO OR
OR (15~ OR3 (~)
Riz
RS R R9 R>>
*-~ Deprotecticn HO O q ,o
R' RR
HO OH
OH (~')
wherein Y represents a halogen and R15 represents a lower alkyl.
The reaction of the bromobenzene derivative (12) with the
compound (3) is carried out in the same manner as in the reaction
of the compound (2) with the compound (3) of Preparation Example
1.
The subsequent reduction reaction is carried out in the
same manner as in the reduction reaction of the compound (4)
of Preparation Example 1. The subsequent conversion to the
trialkyltin derivative is carried out in the presence of
hexaalkylditin and an appropriate palladium catalyst in a
suitable solvent. Specific examples of the palladium catalyst
include tetrakistriphenylphosphine palladium (0), palladium
acetate, bistriphenylphosphine dichloropalladium (II), 1,2-
bis(diphenylphosphinoethane)dichloropalladium (II), and
1,1'-bis(diphenylphosphinoferrocene)dichloropalladium (II).
23
CA 02494177 2005-02-04
Specific examples of the solvent include ethers such as diethyl
ether, tetrahydrofuran, dioxane, and diglyme; alcohols such as
methanol, ethanol, and isopropanol; benzene; toluene; water;
and a mixture of these solvents. The solvent is appropriately
selected according to the type of reaction substrate or the
reaction conditions. The reaction temperature is about -100 C
to 180 C, and preferably about 0 C to 100 C, while it varies
according to the type of starting material compounds, the
reaction conditions, or the like within the above-mentioned
range though.
The subsequent reaction with the azulene derivative (16)
is carried out in the presence of an appropriate palladium
catalyst or in the presence of an appropriate palladium catalyst
and an appropriate phosphine in a suitable solvent. Specific
examples of the catalyst include tetrakistriphenylphosphine
palladium (0), palladium acetate, bistriphenylphosphine
dichloropalladium (II), 1,2-bis(diphenylphosphinoethane)
dichloropalladium (II), 1,1'-bis(diphenylphosphinoferrocene)
dichloropalladium (II), and tris(dibenzylideneacetone)
dipalladium (0) . Specific examples of the phosphine include
trifurylphosphine, 2-(dicyclohexylphosphino)biphenyl, and
tri(t-butyl)phosphine. Specific examples of the solvent
include ethers such as diethyl ether, tetrahydrofuran, dioxane,
and diglyme; alcohols such as methanol, ethanol, and
isopropanol; benzene; toluene; water; and a mixture of these
solvents. The solvent is appropriately selected according to
the type of reaction substrate or the reaction conditions. The
24
CA 02494177 2005-02-04
reaction temperature is about -100 C to 180 C, and preferably
about 0 C to 100 C, while it varies according to the type of
starting material compounds, the reaction conditions, or the
like within the above-mentioned range though. The
deprotection is carried out in the same manner as in Preparation
Example 2.
Preparation Example 5
A process of Preparation Example 5 comprises brominating
a phenylacetic acid derivative (17) to prepare a compound (18),
converting the compound (18) to a phenylacetone derivative (19 ),
cyclizing the derivative (19) with a compound (20) to prepare
a compound (2), reacting the compound (2) with a compound (3)
to prepare a compound ( 4), reducing the compound (4) to prepare
a compound (I), and deprotecting the compound (I) to prepare
a compound (I'), as shown in the following scheme.
RIa Ra
O
t ,z
R5RfiRS R6 COOH RRRs Rs R9 R R~,
COOH ~ O (Za) y ,~j /
R~ Br..'j"'~CCCy~A ''( ~ ,o
Br R' Rs R
(17) (t 8) (19) (2)
R'p O O 9 R'2 9 ra
RzORa R5 Rs R Rõ Re
0"""R' R R R õ
111 O
(3) R'O O A ,o Reduction ' O
Re R,o
z ~H' ~' Ra n R O R~ A /
R O OR R0 OR
OR' (4) Riz OR' (0
RS Rs R9 RIt
Qeprotection HO O A ,o
R Rg
HO OH
OH (i)
CA 02494177 2005-02-04
The bromination of the phenylacetic acid derivative (17)
is carried out in the presence of an appropriate brominating
agent in a suitable solvent. Specific examples of the
brominating agent include N-bromosuccinimide, bromine,
hydrogen bromide. Specific examples of the solvent include
haloalkyls such as methylene chloride, chloroform, and carbon
tetrachloride; esters such as ethyl acetate; ethers such as
tetrahydrofuran and dioxane; dimethylsulfoxide; acetic acid;
water; and a mixture of these solvents. The solvent is
appropriately selected according to the type of reaction
substrate or the reaction conditions. The reaction
temperature is about -100 C to 180 C, and preferably about 0 C
to 100 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
The subsequent derivation to the phenylacetone derivative
(19) is carried out in the presence of an appropriate base in
a suitable solvent. Specific examples of the base include
sodium acetate, potassium acetate, and pyridine. Specific
examples of the solvent include acetic anhydride. The reaction
temperature is about -100 C to 180 C, and preferably about 30 C
to 150 C, while it varies according to the type of starting
material compounds, the reaction conditions, or the like within
the above-mentioned range though.
The subsequent cyclization reaction is carried out by
reacting the compound (19) with a suitable amine in the presence
of an appropriate dehydrating agent in a suitable solvent and
26
CA 02494177 2005-02-04
then reacting the mixture with the compound (20) in a suitable
solvent. Specific examples of the amine include morpholine,
pyrrolidine, N-methylpiperazine, diethylamine,
diisopropylamine. Specific examples of the dehydrating agent
include magnesium sulfate and sodium sulfate. Specific
examples of the solvent include ethers such as tetrahydrofuran,
dioxane, and diethyl ether; haloalkyls such as methylene
chloride, chloroform, and carbon tetrachloride; esters such as
ethyl acetate; alcohols such as methanol, ethanol, and
isopropanol; benzene; toluene; acetonitrile; water; and a
mixture of these solvents. The solvent is appropriately
selected according to the type of reaction substrate or the
reaction conditions. The reaction temperature is about -100 C
to 180 C, and preferably about 20 C to 120 C, while it varies
according to the type of starting material compounds, the
reaction conditions, or the like within the above-mentioned
range though.
The subsequent addition reaction to the compound (3) and
the reduction are carried out in the same manner as in the
addition reaction and the reduction of Preparation Example 1.
The deprotection is carried out in the same manner as in
Preparation Example 2.
BEST MODE FOR CARRYING OUT THE INVENTION
EXAMPLES
The compound of the present invention will now be described
in more detail by way of examples. Since starting material
27
CA 02494177 2005-02-04
compounds of the compound of the present invention include novel
compounds, the methods for preparing these compounds will also
be described in reference examples.
Reference Example 1
Aluminum chloride (1.87 g) was added to a solution of
1-methylazulene (2 g) in methylene chloride (20 ml) at 0 C and
the mixture was stirred for 15 minutes. Then, a solution of
3-bromobenzoyl chloride (1.86 ml) in methylene chloride (5 ml)
was added dropwise to the reaction mixture at 0 C and the mixture
was stirred for one hour. The reaction mixture was added to
10% aqueous solution of hydrochloric acid under cooling with
ice and was extracted with ethyl acetate. The organic layer
was washed with saturated brine and dried over anhydrous sodium
sulfate. After filtration, the filtrate was concentrated.
The residue was purified by silica gel column chromatography
(n-hexane-ethyl acetate) to obtain (3-bromophenyl)(3-
methylazulen-1-yl)methanone (1.2 g).
Reference Example 2
Boron trifluoride-diethyl ether complex (1.17 ml) was
added to a solution of (3-bromophenyl)(1-methylazulen-2-yl)
methanone (0.5 g) in diglyme-ether (ratio: 1:1, 2.0 ml) at 0 C
and the mixture was stirred for 20 minutes. Then, sodium
borohydride (0. 68 g) was added to the reaction mixture and the
mixture was stirred at room temperature for one hour. The
reaction mixture was added to ice-cooled water and extracted
with ethyl acetate. The organic layer was washed with saturated
brine and dried over anhydrous sodium sulfate. After
28
CA 02494177 2005-02-04
filtration, the filtrate was concentrated. The residue was
purified by silica gel column chromatography (n-hexane-ether)
to obtain 1-(3-bromobenzyl)-3-methylazulene (0.21 g).
Reference Example 3
A 1.6 M hexane solution of n-butyl lithium (2.44 ml) was
added dropwise to a solution of 2-(3-bromobenzyl)-1-
methylazulene (1.2 g) in THF (8.0 ml) at -78 C and the mixture
was stirred for one hour. Then, a solution of 2,3,4,6=
tetra-0-benzyl-D- (+) -glucono-l, 5-lactone (2. 08 g) in THF (8. 0
ml) was added dropwise to the reaction mixture and the mixture
was stirred for one hour. Saturated aqueous solution of
am.monium chloride was added to the reaction mixture and the
mixture was extracted with ethyl acetate. The organic layer
was washed with saturated brine and dried over anhydrous sodium
sulfate. After filtration, the filtrate was concentrated.
The residue was purified by silica gel column chromatography
(n-hexane-ethyl acetate) to obtain 2,3,4,6-tetra-O-benzyl-
1-C-[3-[(3-methylazulen-1-yl)methyl]phenyl]-D-glucopyranose
(1.74 g).
The compounds of Reference Examples 4, 5, and 6 were
obtained respectively in the same manner as in Reference
Examples 1, 2, and 3.
Reference Example 7
A 1.6 M hexane solution of n-butyl lithium (11 ml) was
added dropwise to a solution of 3-bromo-4-ethoxytoluene (3.6
g) in THF (50 ml) at -78 C and the mixture was stirred for 15
minutes. Then, a solution of 2,3,4,6-tetra-0-benzyl-D-(+)-
29
CA 02494177 2005-02-04
glucono-1,5-lactone (7.6 g) in THF (10 ml) was added dropwise
to the reaction mixture and the mixture was stirred for 2. 5 hours.
Saturated aqueous solution of ammonium chloride was added to
the reaction mixture and the mixture was extracted with ethyl
acetate. The organic layer was washed with saturated brine and
dried over anhydrous sodium sulfate. After filtration, the
filtrate was concentrated. The resulting precipitates were
collected by filtration to obtain 2,3,4,6-tetra- 0-benzyl-
1-C-(2-ethoxy-5-methylphenyl)-D-glucopyranose (3.53 g).
Reference Example 8
Boron trifluoride-diethyl ether complex (0.6 ml) and
triethylsilane (1.7 ml) were added dropwise to a solution of
2,3,4,6-tetra-0-benzyl-l-C-(2-ethoxy-5-methylphenyl)-D-
glucopyranose (3.5 g) at -50 C and the mixture was stirred for
two hours. Saturated aqueous solution of potassium carbonate
was added to the reaction mixture and the mixture was extracted
with chloroform. The organic layer was washed with saturated
brine and dried over anhydrous sodium sulfate. After
filtration, the filtrate was concentrated. The residue was
purified by silica gel column chromatography (hexane-ethyl
acetate) to obtain (1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-
1-(2-ethoxy-5-methylphenyl)-D-glucitol (3.4 g).
Reference Example 9
A 1.0 M methylene chloride solution (31.0 ml) of boron
trichloride was added dropwise to a solution of (1S)-1,5-
anhydro-2,3,4,6-tetra-0-benzyl-l-(2-ethoxy-5-methylphenyl)-
D-glucitol (3.4 g) in methylene chloride (50 ml) at -78 C and
CA 02494177 2005-02-04
the mixture was stirred for 30 minutes. Methanol (10 ml) was
added to the reaction mixture and the mixture was stirred for
minutes and concentrated. The residue was dissolved in
pyridine (20 ml) and acetic anhydride (10 ml) was added to the
5 solution, followed by stirring for 12 hours at room temperature.
The reaction mixture was diluted with ethyl acetate. The
diluted product was washed with 10% aqueous solution of
hydrochloric acid, saturated aqueous solution of sodium
hydrogencarbonate, and saturated brine in that order and dried
10 over anhydrous sodium sulfate. After filtration, the filtrate
was concentrated. The residue was purifiedby silica gel column
chromatography (n-hexane-ethyl acetate) to obtain (1S)-
2,3,4,6-tetra-0-acetyl-l,5-anhydro-l-(2-ethoxy-5-
methylphenyl)-D-glucitol (1.3 g).
Reference Example 10
N-bromosuccinimide (1.7 g) and benzoyl peroxide (0.1 g)
were added to a solution of (1S)-2,3,4,6-tetra-0-acetyl-
1,5-anhydro-l-(2-ethoxy-5-methylphenyl)-D-glucitol (3.7 g)
in carbon tetrachloride (30.0 ml) and the mixture was refluxed
with heating for one hour. The reaction mixture was diluted
with chloroform and the diluted product was washed with
saturated aqueous solution of sodium hydrogencarbonate,
saturated aqueous solution of sodiumthiosulfate, and saturated
brine in that order and dried over anhydrous sodium sulfate.
After filtration, the filtrate was concentrated. The
resulting precipitates were collected by filtration to obtain
(1S)-2,3,4,6-tetra-0-acetyl-1,5-anhydro-l-[5-(bromomethyl)-
31
CA 02494177 2005-02-04
2-ethoxyphenyl]-D-glucitol (1.4 g).
Reference Example 11
A catalytic amount of iodine was added to a suspension
of inetal magnesium (0.22 g) in THF (5. 0 ml) in an argon atmosphere.
Then, a solution of the compound in THF (5.0 ml) was added
dropwise to the mixture, followed by refluxing with heating for
one hour. The Grignard reagent thus prepared was added dropwise
to a solution of 3-bromo-4-methylbenzaldehyde dimethylacetal
(2.45 g) in THF (5.0 ml) at 0 C and the mixture was stirred for
one hour. An aqueous solution of ammonium chloride was added
to the reaction mixture and the mixture was extracted with ethyl
acetate. The organic layer was washed with saturated brine and
dried over anhydrous sodium sulfate. After filtration, the
filtrate was concentrated. The residue was purified by silica
gel column chromatography (n-hexane-ethyl acetate) to obtain
2,3,4,6-tetra-0-benzyl-l-C-[5- (dimethoxymethyl)-2-
methylphenyl]-D-glucopyranose (2.4 g).
Reference Example 12
Sulfamic acid (0.4 g) and sodium chlorite (0.4 g) were
added dropwise to a solution of 2,3,4,6-tetra-0-benzyl-1-C-
[5-(dimethoxymethyl)-2-methylphenyl]-D-glucopyranose (2.4 g)
in acetone-water (ratio: 5:1, 12 ml) at room temperature and
themixturewasstirredforthreehours. Acetone was evaporated
from the reaction mixture and water was added. The mixture was
extracted with ethyl acetate. The organic layer was washed with
water and saturated brine in that order and dried over anhydrous
sodium sulfate. After filtration, the filtrate was
32
CA 02494177 2005-02-04
concentrated to obtain 4-methyl-3- [(3R, 4S, 5R, 6R) -3, 4, 5-
tris(benzyloxy)-6-[(benzyloxy)methyl]-2-hydroxytetrahydro-
2H-pyran-2-yl]benzoic acid (1.5 g).
The compound of Reference Example 13 was obtained in the
same manner as in Reference Example 8.
Reference Example 14
Methyl iodide (0.17 ml) and potassium carbonate (0.4 g)
were added to a solution of 4-methyl-3-[(2S,3S,4R,5R,6R)-
3,4,5-tris(benzyloxy)-6-[(benzyloxy)methyl]-tetrahydro-2H-
pyran-2-yl]benzoic acid (1.5 g) in DMF (10 ml) at room
temperature and the mixture was stirred for three hours. The
insoluble matter was separated by filtration and the filtrate
was diluted with ethyl acetate. The diluted solution was washed
with water and saturated brine in that order and dried over
anhydrous sodium sulfate. After filtration, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (n-hexane-ethyl acetate) to obtain methyl 4-
methyl-3- [(2S, 3S, 4R, 5R, 6R) -3, 4, 5-tris (benzyloxy) -6-
[(benzyloxy)methyl]-tetrahydro-2H-pyran-2-yl]benzoate
(1.3 g).
Reference Example 15
Lithium aluminum hydride (73 mg) was added to a solution
of methyl 4-methyl-3- [(2S, 3S, 4R, 5R, 6R) -3, 4, 5-
tris(benzyloxy)-6-[(benzyloxy)methyl]tetrahydro-2H-pyran-2-
yl]benzoate (1.3 g) in THF (10 ml) at 0 C and the mixture was
stirred for one hour. The reaction mixture was poured into
ice-cooled water and the insoluble matter was separated by
33
CA 02494177 2005-02-04
filtration. The filtrate was extracted with ethyl acetate.
The organic layer was washed with saturated brine and dried over
anhydrous sodium sulfate. After filtration, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (n-hexane-ethyl acetate) to obtain (1S)-1,5-
anhydro-2,3,4,6-tetra-0-benzyl-l-[5- (hydroxymethyl)-2-
methylphenyl]-D-glucitol (1.0 g).
Reference Example 16
Carbon tetrabromide (0.62 g) andtriphenylphosphine (0.49
g) were added to a solution of (1S)-1,5-anhydro-2,3,4,6-
tetra-0-benzyl-l-[5-(hydroxymethyl)-2-methylphenyl]-D-
glucitol (1.0 g) in methylene chloride (10 ml) at room
temperature and the mixture was stirred for one hour. The
reaction mixture was concentrated and the residue was purified
by silica gel column chromatography (n-hexane-ethyl acetate)
to obtain (lS)-1,5-anhydro-2,3,4,6-tetra-0-benzyl- 1-[5-
(bromomethyl)-2-methylphenyl]-D-glucitol (0.8 g).
The compounds of Reference Examples 17 and 18 were obtained
respectively in the same manner as in Reference Examples 1 and
2.
Reference Example 19
A 1.6 M n-hexane solution of n-butyl lithium (14.0 ml)
was added dropwise to a solution of (1S) -1, 5-anhydro-2, 3, 4, 6-
tetra-0-benzyl-l-[3-bromo-5-(methoxymethyl)phenyl]-D-
glucitol (7.8 g) in THF (5.0 ml) at -78 C and the mixture was
stirred for 30 minutes. DMF (1. 0 ml) was added dropwise to the
reaction mixture and the mixture was stirred for four hours.
34
CA 02494177 2005-02-04
Saturated aqueous solution of ammonium chloride was added to
the reaction mixture and the mixture was extracted with ethyl
acetate. The organic layer was washed with saturated brine and
dried over anhydrous sodium sulfate. After filtration, the
filtrate was concentrated. The residue was purified by silica
gel column chromatography (n-hexane-ethyl acetate) to obtain
methyl 3-methoxymethyl-5-[(2S,3S,4R,5R,6R)-3,4,5-
tris(benzyloxy)-6-[(benzyloxy)methyl]-tetrahydro-2H-pyran-
2-yl]benzaldehyde (2.6 g).
Reference Example 20
Sodium borohydride (0.15 g) was added to a solution of
3-methoxymethyl-5-[(2S,3S,4R,5R,6R)-3,4,5-tris(benzyloxy)-
6-[(benzyloxy)methyl]tetrahydro-2H-pyran-2-yl]benzaldehyde
(2.6 g) in a 1:1 mixture of methanol and THF (10 ml) and the
mixture was stirred for one hour. Acetone (5.0 ml) was added
to the reaction mixture and the mixture was stirred for 10
minutes. Water was added to the reaction mixture and the
mixture was extracted with ethyl acetate. The organic layer
was washed with saturated brine and dried over anhydrous sodium
sulfate. After filtration, the filtrate was concentrated.
The residue was purified by silica gel column chromatography
(n-hexane-ethyl acetate) to obtain (1S)-1,5-anhydro-2,3,4,6-
tetra-0-benzyl-l-[3-(hydroxymethyl)-5-(methoxymethyl)
phenyl]-D-glucitol (2.1 g).
The compounds of Reference Examples 21, 22, and 23 were
obtained respectively in the same manner as in Reference
Examples 6, 11, and Example 1.
CA 02494177 2005-02-04
Reference Example 24
A 1.0 M THF solution of tetrabutylammonium fluoride (3.8
ml) was added to a solution of (1S)-1,5-anhydro-2,3,4,6-
tetra-0-benzyl-l-[3-([[t-butyl(diphenyl)silyl]oxy]methyl)
phenyl ]-D-glucitol (1. 7 g) in THF (10. 0 ml) at room temperature
and the mixture was stirred for two hours. 10% aqueous solution
of sodium hydroxide (3.0 ml) was further added to the reaction
mixture and the mixture was refluxed with stirring for one hour.
Water was added to the reaction mixture and the mixture was
extracted with ethyl acetate. The organic layer was washed with
saturated brine and dried over anhydrous sodium sulfate. After
filtration, the filtrate was concentrated. The residue was
purified by silica gel column chromatography (n-hexane-ethyl
acetate) to obtain (1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-
1-[3-(hydroxymethyl)phenyl]-D-glucitol (0.7 g).
The compound of Reference Example 25 was obtained in the
same manner as in Reference Example 16.
Reference Example 26
Imidazole (3.45 g) and t-butyldimethylchlorosilane (17.6
g) were added to a solution of 5-bromo-2-methoxybenzyl alcohol
(10.0 g) in DMF (100 ml) under cooling with ice and the mixture
was stirred for two hours. The reaction mixture was added to
ice-cooled water and extracted with ethyl acetate. The organic
layer was washed with saturated brine and dried over anhydrous
sodium sulfate. After filtration, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (n-hexane-ethyl acetate) to obtain [(5-
36
CA 02494177 2005-02-04
bromo-2-methoxybenzyl)oxy](t-butyl)dimethylsilane
(15.2 g).
The compounds of Reference Examples 27, 28, 29, 30, and
31 were obtained respectively in the same manner as in Reference
Example 3, Example 1, Reference Example 16, Reference Example
11, and Example 1.
Reference Example 32
Hexabutylditin (10.0 g) and tetrakistriphenylphosphine
palladium ( 0 ) ( 0 . 2 4 g) were added to a solution of ( 1 S ) -
1 0 1,5-anhydro-2,3,4,6-tetra-0-benzyl-l-(3-bromophenyl)-D-
glucitol ( 5. 0 g) in toluene (8. 0 ml) in an argon atmosphere and
the mixture was refluxed with stirring for 17 hours. The
reaction mixture was concentrated. The residue was purified
by silica gel column chromatography (n-hexane-ethyl acetate)
to obtain (1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-l- [3-
(tributylstanyl)phenyl]-D-glucitol (4.0 g).
Reference Example 33
Manganese dioxide (20.4 g) was added to a solution of
(1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-l-[3-
(hydroxymethyl)phenyl]-D-glucitol (6.8 g) obtained in
Reference Example 24 in chloroform (100 ml) and the mixture was
refluxed with stirring for 1.5 hours. After separating the
insoluble matter from the reaction mixture by filtration
through celite at room temperature, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (n-hexane-ethyl acetate) to obtain 3-
[(2S, 3S, 4R, 5R, 6R) -3, 4, 5- tris (benzyloxy) -6-
37
CA 02494177 2005-02-04
[(benzyloxy)methyl]tetrahydro-2H-pyran-2-yl]benzaldehyde
(6.8 g).
Reference Example 34
Potassium t-butoxide (3.6 g) was added to a solution of
methyltriphenylphosphonium bromide (11.6 g) in THF (100 ml) at
room temperature and the mixture was stirred for 10 minutes.
A solution of 3-[(2S,3S,4R,5R,6R)-3,4,5-tris(benzyloxy)- 6-
[(benzyloxy)methyl]tetrahydro-2H-pyran-2-yl] benzaldehyde
(6.8 g) in THF (10 ml) was added dropwise and the mixture was
stirred for one hour at room temperature. Saturated aqueous
solution of ammonium chloride was added to the reaction mixture
and the mixture was extracted with ethyl acetate. The organic
layer was washed with saturated brine and dried over anhydrous
sodium sulfate. After filtration, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (n-hexane-ethyl acetate) to obtain ( 1 S ) - 1 , 5 -
a n h y d r o - 2 , 3 , 4 , 6 - t e t r a - 0 - b e n z y l - l - ( 3 - v i n
y l p h e n y l ) - D-
glucitol ( 6 . 8 g).
The compounds of Reference Examples 35, 36, 37, 38, 39,
and 40 were obtained respectively in the same manner as in
Reference Example 26, Reference Example 3, Example 1, Reference
Example 16, Reference Example 3, and Example 1.
Reference Example 41
5% palladium/carbon (1.0 g) was added to a solution of
(1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-l-(3-bromo-5-
methoxyphenyl)-D-glucitol (10.Og) inTHF-methanol (ratio:1:1,
100 ml) Two drops of a 1 M aqueous solution of hydrochloric
38
CA 02494177 2005-02-04
acid was further added to the mixture, followed by stirring for
30 minutes in a hydrogen atmosphere. After filtrating the
reaction mixture, the filtrate was concentrated. The residue
was purified by silica gel column chromatography
(chloroform-methanol) to obtain (1S)-1,5-anhydro-l-(3-bromo-
5-methoxyphenyl)-D-glucitol (2.9 g).
The compounds of Reference Examples 42, 43, 44, 45, and
46 were obtained respectively in the same manner as in Example
37, Reference Example 32, Reference Example 41, Example 37, and
Reference Example 32.
Reference Example 47
1-bromo-2,4-dimethoxybenzene (9.1 ml) was added to a
solution of 2,3,4,6-tetra-O-benzyl-1-0-(trifluoroacetyl)-
a-D-glucopyranose (20.0 g) in methylene chloride (100 ml) and
the mixture was stirred for 10 minutes. Boron trifluoride-
diethyl ether complex (3. 9 ml) was added to the reaction mixture
and the mixture was stirred at room temperature for 12 hours.
Water was added to the reaction mixture and the mixture was
extracted with methylene chloride. The organic layer was
washed with saturated brine and dried over anhydrous sodium
sulfate. After filtration, the filtrate was concentrated.
The residue was purified by silica gel column chromatography
(n-hexane-ethyl acetate) to obtain (1S)-1,5-anhydro-
2,3,4,6-tetra-0-benzyl-l-(5-bromo-2,4- dimethoxyphenyl)-D-
glucitol (17.0 g).
The compound of Reference Example 48 was obtained in the
same manner as in Reference Example 41.
39
CA 02494177 2005-02-04
Reference Example 49
Diisopropylethylamine (2.98 g) and chloromethyl methyl
ether (1.3 ml) were added to a solution of (1S)-1,5-
anhydro-l-(5-bromo-2,4-dimethoxyphenyl)-D-glucitol (1.35 g)
in methylene chloride (15 ml) at 0 C and the mixture was stirred
at room temperature for 12 hours. The reaction mixture was
added to ice-cooled water and extracted with ethyl acetate. The
organic layer was washed with saturated brine and dried over
anhydrous sodium sulfate. After filtration, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (n-hexane-ethyl acetate) to obtain (1S)-1,5-
anhydro-l-(5-bromo-2,4- dimethoxyphenyl)-2,3,4,6-tetrakis-
0-(methoxymethyl)-D- glucitol (0.7 g).
The compound of Reference Example 50 was obtained in the
same manner as in Reference Example 32.
Reference Example 51
Sodium borohydride (0.26 g) was added to a solution of
6-isopropylazulene-2-carboaldehyde (1.4 g) in methanol (30 ml)
at 0 C and the mixture was stirred for one hour. Acetone was
added to the reaction mixture and the mixture was stirred for
15 minutes. The reaction mixture was concentrated. The
residue was purified by silica gel column chromatography
(n-hexane-ethyl acetate) to obtain (6-isopropylazulen-2-
yl)methanol (1.15 g).
Reference Example 52
Triphenylphosphine (0.66 g) was added to a solution of
(6-isopropylazulen-2-yl)methanol (0.5 g) in carbon
CA 02494177 2005-02-04
tetrachloride (10.0 ml) and the mixture was refluxed with
heating for 15 hours. The reaction mixture was concentrated.
The residue was purified by silica gel column chromatography
(n-hexane-diethyl ether) to obtain 2-(chloromethyl)-6-
isopropylazulene (0.38 g).
The compound of Reference Example 53 was obtained in the
same manner as in Reference Example 52.
Reference Example 54
[1,2-bis(diphenylphosphino)ethane]dichloropalladium
(II) (1.48 g) was added to a solution of methyl 2-
chloroazulene-l-carboxylate (11.38 g) and hexamethylditin
(35.9 g) in 1,4-dioxane (272 ml) at room temperature and the
mixture was heated to 60 C and stirred for 38 hours. After
evaporating the solvent under reduced pressure, the resulting
residue was purified by silica gel column chromatography
(hexane-ether acetate) to obtain methyl 2-
(tributylstanyl)azulene-l-carboxylate (12.36 g).
Reference Example 55
A 1.0 M dichloromethane solution of tin tetrachloride
(16.5 ml) was added to a suspension of 1,2,3,4,6- penta-O-
acetyl-(3-D-glucopyranose (6.41 g), 1,2-diethoxy- 4-
methylbenzene (2.47 g), and silver trifluoroacetate (3.63 g)
in 1, 2-dichloroethane (70 ml) at 0 C and the mixture was stirred
at the same temperature for one hour. After heating to room
temperature and stirring for 15 hours, saturated aqueous
solution of sodium bicarbonate was added to the mixture. The
reaction mixture was filtered through celite and the filtrate
41
CA 02494177 2005-02-04
was extracted with chloroform. The organic layer was washed
with water and saturated brine and dried over anhydrous sodium
sulfate. After filtration, the solvent was evaporated under
reduced pressure. Methanol (200 ml) and a catalytic amount of
sodium methoxide were added to the resulting residue and the
mixture was stirred at room temperature overnight. The solvent
was evaporated under reduced pressure and the resulting residue
was purified by silica gel column chromatography
(chloroform-methanol) . Pyridine (30 ml), acetic anhydride (5
ml), and a catalytic amount of 4-dimethylaminopyridine were
added to the resulting residue and the mixture was stirred at
room temperature for two days. Toluene was added to the
reaction mixture and the solvent was evaporated under reduced
pressure. The residue was purified by silica gel column
chromatography (ethyl acetate-n-hexane) to obtain (1S)-
2,3,4,6-tetra-0-acetyl-l,5-anhydro-l-(2,3- diethoxy-5-
methylphenyl)-D-glucitol (2.51 g).
Reference Example 56
A suspension of (1S)-2,3,4,6-tetra-0-acetyl-l,5-
anhydro-l-(2,3-diethoxy-5-methylphenyl)-D-glucitol (500 mg)
and N-bromosuccinimide (209 mg) in carbon tetrachloride (10 ml)
was refluxed with heating and 2,2'-azobis(isobutyronitrile)
(80 mg) was added. The mixture was stirred for 30 minutes under
refluxing and the reaction mixture was allowed to be cooled to
room temperature. After evaporating the solvent under reduced
pressure, the resulting residue was purified by silica gel
column chromatography (ethyl acetate-n-hexane) to obtain
42
CA 02494177 2005-02-04
(iS)-2,3,4,6-tetra-0-acetyl-1,5-anhydro-l-[5-(bromomethyl)-
2,3-diethoxyphenyl]-D-glucitol (464 mg).
The compounds of Reference Examples 57 and 58 were obtained
respectively in the same manner as in Reference Examples 55 and
56.
Reference Example 59
A 1.56 M n-hexane solution of n-butyl lithium (3.57 ml)
and 2-fluorotoluene (0.66 ml) were added in that order to a
suspension of potassium t-butoxide (625 mg) in THF (11 ml) at
-78 C and the mixture was stirred at the same temperature for
1.5 hours. A solution of 2,3,4,6-tetra-0-benzyl-D-(+)-
glucono-1, 5-lactone (3.00 g) in THF (10 ml) was added dropwise
to the reaction mixture and the mixture was stirred at the same
temperature for 30 minutes. After the addition of a 1 M aqueous
solution of hydrochloric acid, the mixture was heated to room
temperature. The reaction mixture was extracted with diethyl
ether. The organic layer was washed with saturated brine and
dried over anhydrous sodium sulfate. After filtration, the
solvent was evaporated under reduced pressure. The filtrate
was concentrated and dried to be solid. The resulting residue
was dissolved in dichloroethane (5 ml) and acetonitrile (25 ml)
Triisopropylsilane (2.27 ml) and boron trifluoride-diethyl
ether complex (0.85 ml) were added to the solution at -30 C.
After stirring at the same temperature for 30 minutes, saturated
aqueous solution of sodium bicarbonate was added to the reaction
mixture. The mixture was extracted with diethyl ether. The
organic layer was washed with saturated brine and dried over
43
CA 02494177 2005-02-04
anhydrous sodium sulfate. After filtration, the solvent was
evaporated under reduced pressure. The resulting residue was
purified by silica gel column chromatography (ethyl
acetate-n-hexane) to obtain (1S)-1,5-anhydro-2,3,4,6-tetra-
O-benzyl-l-(2-fluoro-3- methylphenyl)-D-glucitol (559 mg).
Reference Example 60
A suspension of (1S)-1,5-anhydro-2,3,4,6-tetra-0-
benzyl-l-(2-fluoro-3-methylphenyl)-D-glucitol (550 mg) and
20% palladium hydroxide/carbon (300 mg) in THF (10 ml) -methanol
(5 ml) was stirred in a hydrogen atmosphere (1 atm) for 2. 5 days.
The reaction mixture was filtered through celite and the
filtrate was concentrated. Pyridine (5 ml), acetic anhydride
(2 ml) , and a catalytic amount of 4-dimethylaminopyridine were
added to the resulting residue and the mixture was stirred at
room temperature for one hour. After evaporating the solvent
under reduced pressure, the residue was co-evaporated with
toluene and dissolved in diethyl ether. This solution was
washed with a 1 M aqueous solution of hydrochloric acid and
saturated brine and dried over anhydrous sodiumsulfate. After
filtration, the filtrate was concentrated under reduced
pressure to obtain (1S)-2,3,4,6-tetra-0-acetyl-l,5-anhydro-
1-(2-fluoro-3-methylphenyl)-D-glucitol (335 mg).
The compounds of Reference Examples 61, 62, 63, 64, and
65 were obtained respectively in the same manner as in Reference
Examples 56, 55, 56, 55, and 56.
Reference Example 66
Sodium acetate (50.5 g) was added to a solution of 3-
44
CA 02494177 2005-02-04
bromo-4-hydroxyphenylacetic acid (28.5 g) in acetic anhydride
(100 ml) and the mixture was refluxed with heating for 21 hours.
After cooling to room temperature, 20% aqueous solution of
sodium hydroxide was added to the reaction mixture to adjust
to pH 11. The mixture was refluxed with heating for one hour.
After cooling to room temperature, 10% aqueous solution of
hydrochloric acid was added to the reaction mixture to adjust
to pH 6. The mixture was extracted with ethyl acetate. The
organic layer was washed with water, saturated aqueous solution
of sodium bicarbonate, and saturated brine and dried over
anhydrous sodium sulfate. After filtration, the solvent was
evaporated under reduced pressure. The residue was purified
by silica gel column chromatography (ethyl acetate-n-hexane)
to obtain 1-(3-bromo-4-hydroxyphenyl) acetone (22.2 g).
Reference Example 67
Potassium carbonate (2.7 g) and benzyl bromide (2.3 ml)
were added to a solution of 1- (3-bromo-4-hydroxyphenyl) acetone
(4.0 g) in DMF (40 ml) and the mixture was stirred at room
temperature for six hours. The reaction mixture was poured into
water and the mixture was extracted with ethyl acetate. The
organic layer was washed with water and saturated brine and
dried over anhydrous sodium sulfate. After filtration, the
solvent was evaporated under reduced pressure. The resulting
residue was purified by silica gel column chromatography (ethyl
acetate-n-hexane) to obtain 1-[4-(benzyloxy)-3-bromophenyl]
acetone (3.65 g).
Reference Example 68
CA 02494177 2005-02-04
Pyrrolidine (1.9 ml) and magnesium sulfate (2.74 g) were
added to a solution of 1- [4- (benzyloxy) -3-bromophenyl] acetone
(3.65 g) in diethyl ether (30 ml) and the mixture was stirred
at room temperature for 12 hours. After filtration, the solvent
was evaporated under reduced pressure. The resulting residue
was dried under reduced pressure and dissolved in ethanol (30
ml). 2H-cyclohepta[b]furan-2-on (0.5 g) was added to the
solution and the mixture was refluxed with heating for eight
hours. The reaction mixture was concentrated. The resulting
residue was purified by silica gel column chromatography (ethyl
acetate-n-hexane) to obtain 2-[4-(benzyloxy)-3-bromobenzyl]
azulene (0.84 g).
Reference Example 69
A 1.6 M n-hexane solution of n-butyl lithium (0.32 ml)
was added dropwise to a solution of 2-[4-(benzyloxy)-3-
bromobenzyl ] azulene (0.17 g) in THF (3.0 ml) at -55 C and the
mixture was stirred at the same temperature for 10 minutes. A
solution of 2,3,4,6-tetra-0-benzyl-glucono-l,5-lactone (0.12
g) in THF (3.0 ml) was added dropwise to the reaction mixture
and the mixture was stirred at the same temperature for 30
minutes. Saturated aqueous solution of ammonium chloride was
added to the reaction mixture and the mixture was extracted with
ethyl acetate. The organic layer was washed with brine and
dried over anhydrous sodium sulfate. After filtration, the
solvent was evaporated under reduced pressure. The resulting
residue was purified by silica gel column chromatography (ethyl
acetate-n-hexane) to obtain 1-C-[5-(azulen-2-ylmethyl)- 2-
46
CA 02494177 2005-02-04
(benzyloxy)phenyl]-2,3,4,6-tetra-0-benzyl-D-glucopyranose
(0.9 g).
Example 1
Boron trifluoride-diethyl ether complex (0.39 ml) and
triisopropylsilane (1.23 ml) were added dropwise to a solution
of 2,3,4,6-tetra-0-benzyl-l-C-[3-[(3-methylazulen-l-yl)
methyl]phenyl] -D-glucopyranose (2.3 g) in acetonitrile (40 ml)
at -40 C and the mixture was stirred for two hours. Saturated
aqueous solution of potassium carbonate was added to the
reaction mixture and the mixture was extracted with ethyl
acetate. The organic layer was washed with saturated brine and
dried over anhydrous sodium sulfate. After filtration, the
filtrate was concentrated. The residue was purified by silica
gel column chromatography (n-hexane-ethyl acetate) to obtain
(1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-l-[3-[(3-methyl
azulen-1-yl)methyl]phenyl]-D-glucitol (1.47 g).
Example 2
A 1 M n-heptane solution of boron tribromide (20 ml) was
added dropwise to a solution of (1S)-1,5-anhydro-2,3,4,6-
tetra-0-benzyl-l-[3-[(3-methylazulen-1-yl)methyl]phenyl]-D-
glucitol (0.76 g) in methylene chloride (20 ml) at -78 C and
the mixture was stirred for 30 minutes. Methylene
chloride-toluene (ratio: 2:1, 60 ml) was added to the reaction
mixture and methanol (6 ml) was further added to the mixture.
The reaction mixture was cooled to room temperature and
concentrated to the half amount, followed by adding methanol
(25 ml) to the mixture and concentrating. This operation was
47
CA 02494177 2005-02-04
repeated three times. The residue prepared by the
concentration was purified by silica gel column chromatography
(chloroform-methanol) to obtain (1S)-1,5-anhydro-l-[3-[(3-
methylazulen-l-yl)methyl]phenyl]-D-glucitol (0.068 g).
The compounds of Examples 3 and 4 were obtained
respectively in the same manner as in Examples 1 and 2.
Example 5
Two drops of 1,2-dibromoethane was added to a suspension
of zinc powder (0.17 g) in THF (5.0 ml) in an argon atmosphere
and the mixture was refluxed with heating for five minutes.
After cooling to room temperature, two drops of
chlorotrimethylsilane was added to the reaction mixture. The
mixture was stirred for 15 minutes. Next, (1S) -2, 3, 4, 6-tetra-
0-acetyl-l,5-anhydro-l-[5-(bromomethyl)-2-ethoxyphenyl]-D-
glucitol (1.4 g) was added to the reaction mixture and the
mixture was refluxed with heating for one hour. After cooling
to room temperature, tetrakistriphenylphosphine palladium (0)
(0.27 g) and methyl2-chloroazulene-l-carboxylate (0.28 g) were
added to the reaction mixture. The mixture was refluxed with
heating for six hours. After cooling to room temperature, the
reaction mixture was poured into 10% aqueous solution of
hydrochloric acid under cooling with ice. The mixture was
extracted with ethyl acetate. The organic layer was washed with
saturated brine and dried over anhydrous sodium sulfate. After
filtration, the filtrate was concentrated. The residue was
purified by silica gel column chromatography (n-hexane-ethyl
acetate) to obtain methyl 2-(4-ethoxy-3-[(2S,3S,4R,5R,6R)-
48
CA 02494177 2005-02-04
3,4,5-tris(acetyloxy)-6-[(acetyloxy)methyl]tetrahydro-2H-
pyran-2-yl]benzyl)azulene-l-carboxylate (0.58 g).
Example 6
10% aqueous solution of sodium hydroxide ( 5. 0 ml) was added
dropwise to a solution of methyl 2-(4-ethoxy-3-
[(2S, 3S, 4R, 5R, 6R) -3, 4, 5-tris (acetyloxy) -6- [(acetyloxy)
methyl]tetrahydro-2H-pyran-2-yl]benzyl)azulene-l-
carboxylate (0.58 g) in methanol (5.0 ml) at room temperature.
The mixture was stirred for 30 minutes and refluxed with heating
for further six hours. After cooling with ice, the reaction
mixture was neutralized by adding 10% aqueous solution of
hydrochloric acid. The neutralized product was extracted with
chloroform. The organic layer was washed with saturated brine
and dried over anhydrous sodium sulfate. After filtration, the
filtrate was concentrated. The residue was purified by silica
gel column chromatography (chloroform-methanol) to obtain
2- (4-ethoxy-3- [(2S, 3R, 4R, 5S, 6R) -3, 4, 5-tris (acetyloxy) -6-
[(acetyloxy)methyl]tetrahydro-2H-pyran-2-yl]benzyl)azulene-
1-carboxylic acid (0.4 g).
Example 7
p-Toluenesulfonic acid monohydrate (40 mg) was added to
a suspension of 2-(4-ethoxy-3-[(2S,3R,4R,5S,6R)-3,4,5-
tris(acetyloxy)-6-[(acetyloxy)methyl]tetrahydro-2H-pyran-2-
yl]benzyl)azulene-l-carboxylic acid (0.36g) inbenzene (lOml)
and the mixture was refluxed with heating for 15 minutes. The
reaction mixture was concentrated. The residue was purified
by silica gel column chromatography (chloroform-methanol) to
49
CA 02494177 2005-02-04
obtain (1S)-1,5-anhydro-l-[5-(azulen-2-ylmethyl)-2-
ethoxyphenyl]-D-glucitol (203 mg).
The compound of Example 8 was obtained in the same manner
as in Example S.
Example 9
A 1.0 M methylene chloride solution of boron trichloride
(3.0 ml) was added dropwise to a solution of methyl 2-(4-
methyl-3-[(2S,3S,4R,5R,6R)-3,4,5-tris(benzyloxy)-6-
[(benzyloxy)methyl]tetrahydro-2H-pyran-2-yl]benzyl)azulene-
1-carboxylate (0.39 g) in methylene chloride (10 ml) at -78 C
and the mixture was stirred for 15 minutes. Methanol (10 ml)
was added to the reaction mixture and the mixture was stirred
for 10 minutes and concentrated. The residue was purified by
silica gel column chromatography (chloroform-methanol) to
obtain methyl 2- (4-methyl-3- [(2S, 3R, 4R, 5S, 6R) -3, 4, 5-
trihydroxy- 6-(hydroxymethyl)tetrahydro-2H-pyran-2-
yl]benzyl)azulene-l-carboxylate (0.08 g).
The compounds of Examples 10 and 11 were obtained
respectively in the same manner as in Examples 6 and 7.
Example 12
A 1.0 M methylene chloride solution of boron trichloride
(3.0 ml) was added dropwise to a solution of methyl 2-(4-
methyl-3-[(2S,3S,4R,5R,6R)-3,4,5-tris(benzyloxy)-6-
[(benzyloxy)methyl]tetrahydro-2H-pyran-2-yl]benzyl)azulene-
1-carboxylate (0.39 g) in methylene chloride (10 ml) at -78 C
and the mixture was stirred for 15 minutes. Methanol (10. 0 ml)
was added to the reaction mixture and the mixture was stirred
CA 02494177 2005-02-04
for 10 minutes and concentrated. The residue was purified by
silica gel column chromatography (chloroform-methanol) to
obtain methyl 3-benzyl-2-(4-methyl-3-[(2S,3R,4R,5S,6R)-
3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-
yl]benzyl)azulene-l-carboxylate (0.06 g).
The compounds of Examples 13, 14, 15, and 16 were obtained
respectively in the same manner as in Examples 6, 7, 5, and 9.
Example 17
28% methanol solution of sodium methoxide (0.5 ml) was
added to a solution of methyl 2-[3-(chloromethyl)-5-
[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)
tetrahydro-2H-pyran-2-yl]benzyl]azulene-l-carboxylate (0.06
g) in methanol (3.0 ml) at room temperature. The mixture was
stirred for one hour. 10oaqueous solution of sodium hydroxide
(3.0 ml) was further added to the reaction mixture and the
mixture was refluxed with heating for one hour. After cooling
to room temperature, the reaction mixture was neutralized by
adding 10% aqueous solution of hydrochloric acid, extracted
with chloroform. The organic layer was washed with saturated
brine and dried over anhydrous sodium sulfate. After
filtration, the filtrate was concentrated. The residue was
purified by silica gel column chromatography (chloroform-
methanol) to obtain (1S)-1,5- anhydro-l-[3-(azulen-2-
ylmethyl)-5-(methoxymethyl)phenyl]- D-glucitol (0.02 g).
The compounds of Examples 18, 19, 20, 21, and 22 were
obtained respectively in the same manner as in Examples 5, 9,
6, 7, and S.
51
CA 02494177 2005-02-04
The compounds of Examples 23, 24, 25, 26, and 27 were
obtained respectively in the same manner as in Examples 2, 6,
7, 5, and 6.
The compounds of Examples 28, 29, 30, 31, and 32 were
obtained respectively in the same manner as in Examples 7, 5,
9, 6, and 7.
Example 33
Potassium carbonate (0.15 g) and bis(triphenylphosphine)
dichloropalladium (II) (0.04 g) were added to a solution of
(1S) -l, 5-anhydro-2, 3, 4, 6-tetra-0-benzyl- 1- [3-
(tributylstanyl)phenyl]-D-glucitol (0.5 g) and methyl 2-
chloroazulene-l-carboxylate (0.1g) inl,4-dioxane (3.Oml) and
the mixture was refluxed with heating for 15 hours. The
reaction mixture was concentrated. The residue was purified
by silica gel column chromatography (n-hexane-ethyl acetate)
to obtain methyl 2- (3- [(2S, 3S, 4R, 5R, 6R) -3, 4, 5-
tris(benzyloxy)-6-[(benzyloxy)methyl]tetrahydro-2H-pyran-2-
yl]phenyl)azulene-l-carboxylate (0.25 g).
The compounds of Examples 34, 35, and 36 were obtained
respectively in the same manner as in Examples 2, 6, and 7.
Example 37
Acetic anhydride (0.3 ml) was added to a solution of
(iS)-1,5-anhydro-l-[3-(azulen-2-ylmethyl)phenyl]-D-
glucitol (0.24 g) in pyridine (5.0 ml) at room temperature and
the mixture was stirred for 15 hours. The reaction mixture was
diluted with ethyl acetate. The diluted solution was washed
with 10% aqueous solution of hydrochloric acid, saturated
52
CA 02494177 2005-02-04
aqueous solution of sodium hydrogencarbonate, and saturated
brine in that order and dried over anhydrous sodium sulfate.
After filtration, the filtrate was concentrated. The residue
was purified by silica gel column chromatography (n-hexane-
ethyl acetate) to obtain (1S)-2,3,4,6-tetra-0-acetyl-l,5-
anhydro-l-[3-(azulen-2-ylmethyl)phenyl]-D-glucitol (0.34 g)
Example 38
Aluminum chloride (0.24 g) was added to a solution of
(1S)-2,3,4,6-tetra-0-acetyl-1,5-anhydro-l-[3-(azulen-2-
ylmethyl)phenyl] -D-glucitol (0.20 g) in methylene chloride (20
ml) at 0 C and the mixture was stirred for 30 minutes. Next,
acetic anhydride (0.17 ml) was added dropwise to the reaction
mixture at 0 C and the mixture was refluxed with stirring for
30 minutes and further 16 hours. The reaction mixture was added
to 10% aqueous solution of hydrochloric acid under cooling with
ice and was extracted with ethyl acetate. The organic layer
was washed with saturated aqueous solution of sodium
hydrogencarbonate and saturated brine in that order and dried
over anhydrous sodium sulfate. After filtration, the filtrate
was concentrated. The residue was purifiedbysilica gel column
chromatography (n-hexane-ethyl acetate) to obtain
(2S,3S,4R,5R,6R)-2-[3-[(1-acetylazulen-2-yl)methyl]phenyl]-
6-[(acetyloxy)methyl]tetrahydro-2H-pyran-3,4,5-triyl
triacetate (0.09 g).
Example 39
Sodium methoxide (16 mg) was added to a solution of
(2S,3S,4R,5R,6R)-2-[3-[(1-acetylazulen-2-yl)methyl]phenyl]-
53
CA 02494177 2005-02-04
6-[(acetyloxy)methyl]tetrahydro-2H-pyran-3,4,5-triyl
triacetate (0.09 g) in THF-methanol (ratio: 1:1, 6.0 ml) at 0 C
and the mixture was stirred for two hours. After neutralizing
with a cation exchange resin, the reaction mixture was added
to 10% aqueous solution of hydrochloric acid under cooling with
ice. After filtration, the filtrate was concentrated. The
residue was purified by silica gel column chromatography
(chloroform-methanol) to obtain 1- (2- [3- [(2S, 3R, 4R, 5S, 6R) -
3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-
yl]benzyl]azulen-l-yl)ethanone (0.06 g).
The compounds of Examples 40 and 41 were obtained
respectively in the same manner as in Reference Example 2 and
Example 39.
Example 42
A THF solution (8. 0 ml) of 9-borabicyclo [3.3. 1] nonane was
added to (1S)-1,5-anhydro-2,3,4,6-tetra-0-benzyl-l- (3-
vinylphenyl)-D-glucitol (1.0 g) and the mixture was refluxed
with heating for four hours. Next, a 3 M aqueous solution of
potassium phosphate (1.3 ml) and DMF (12 ml) were added to the
reaction mixture. Methyl 2-chloroazulene-l-carboxylate (0.35
g) and1,1'-diphenylphosphinoferrocene dichloropalladium (II)
(0. 12 g) were further added to the mixture, followed by stirring
at 50 C for two hours. After cooling to room temperature, the
reaction mixture was poured into ice-cooled water and extracted
with ethyl acetate. The organic layer was washed with saturated
aqueous solution of sodium hydrogencarbonate and saturated
brine in that order and dried over anhydrous sodium sulfate.
54
CA 02494177 2005-02-04
The reaction mixture was concentrated. The residue was
purified by silica gel column chromatography (n-hexane-ethyl
acetate) to obtain methyl 2- [2 (3- [(2S, 3S, 4R, 5R, 6R) -3, 4, 5-
tris(benzyloxy)-6- [(benzyloxy)methyl]tetrahydro-2H-pyran-
2-yl]phenyl)ethyl] azulene-l-carboxylate (0.88 g).
The compounds of Examples 43, 44, 45, 46, 47, and 48 were
obtained respectively in the same manner as in Examples 2, 6,
7, 5, 2, and 6.
The compounds of Examples 49, 50, 51, 52, 53, and 54 were
obtained respectively in the same manner as in Examples 7, 33,
39, 2, 6, and 7.
The compounds of Examples 55, 56, 57, 58, and 59 were
obtained respectively in the same manner as in Examples 33, 39,
33, 39, and 33.
Example 60
10% aqueous solution of hydrochloric acid (0.5 ml) was
added to a solution of (1S)-1,5-anhydro-l-[5-(azulen-2-
ylmethyl)-2,4-dimethoxyphenyl]-2,3,4,6-tetrakis-0-
(methoxymethyl)-D-glucitol (0.09 g) in methanol (2.0 ml) and
the mixture was refluxed with heating for 30 minutes. The
reaction mixture was poured into ice-cooled water and extracted
with ethyl acetate. The organic layer was washed with saturated
brine and dried over anhydrous sodium sulfate. After
filtration, the filtrate was concentrated. The residue was
purified by silica gel column chromatography (chloroform-
methanol) to obtain (1S)-1,5-anhydro-l-[5-(azulen-2-
ylmethyl)-2,4-dimethoxyphenyl]-D-glucitol (0.02g).
CA 02494177 2005-02-04
Example 61
(1S)-2,3,4,6-tetra-0-acetyl-l,5-anhydro-l-[4-
(bromomethyl)-1-methoxy-2-naphthyl]-D-glucitol (0.62 g) was
added to a suspension of methyl 2- (tributylstanyl) azulene-l-
carboxylate (0.25 g), tris(dibenzylideneacetone)dipalladium
(0) (0.05 g), 2-(dicyclohexylphosphino)biphenyl (0.05 g),
potassium fluoride (0.09 g), and cesium carbonate (0.35 g) in
l, 4-dioxane (20.0 ml) . The mixture was stirred at 60 C for eight
hours and at 85 C for further 14 hours. Insoluble matters were
removed by filtration, the filtrate was evaporated to remove
the solvent. The residue was purified by silica gel column
chromatography to obtain methyl 2-[(4-methoxy-3-
[(2S, 3S, 4R, 5R, 6R) -3, 4, 5-tris (acetyloxy) -6- [(acetyloxy)
methyl]tetrahydro-2H-pyran-2-yl]-1-naphthyl)methyl]azulene-
1-carboxylate (0.34 g).
The compound of Example 62 was obtained in the same manner
as in Example 39.
Example 63
A 1 M aqueous solution of sodium hydroxide (12 ml) was
added dropwise to a solution of methyl 2- [3- [(1S) -1, 5-anhydro-
D-glucitol-l-yl]-4-methoxy-l-naphthyl]methylazulene-l-
carboxylate (0.23 g) in methanol (8.0 ml) and the mixture was
refluxed with heating for three hours. A 1 M aqueous solution
of hydrochloric acid (12 ml) was added to the reaction mixture
under cooling with ice and the solvent was evaporated. The
resulting residue was suspended in acetonitrile (15 ml) . A 4
M l, 4-dioxane solution of hydrochloric acid (0.4 ml) was added
56
CA 02494177 2005-02-04
dropwise to the suspension and the mixture was refluxed with
heating for 15 minutes. The insoluble matter was removed by
filtration and the solvent was evaporated. The resulting
residue was purified by silica gel column chromatography and
reverse phase column chromatography in that order to obtain
(1S)-1,5-anhydro-l-[4-(azulen-2-ylmethyl)-1-methoxy-2-
naphthyl]-D-glucitol (0.08 g).
The compounds of Examples 64, 65, and 66 were obtained
respectively in the same manner as in Examples 61, 39, and 63.
Example 67
Tris(dibenzylideneacetone)dipalladium (0) (17 mg), 2-
(dicyclohexylphosphino)biphenyl (22 mg), potassium fluoride
(44 mg) , and cesium carbonate (247 mg) were added to a solution
of (1S)-2,3,4,6-tetra-0-acetyl-1,5-anhydro-l-[5-
(bromomethyl)-2-ethoxy-3-methoxyphenyl]-D-glucitol (327 mg)
and methyl 2-(tributylstanyl)azulene-l-carboxylate (180 mg)
in 1, 4-dioxane (10 ml) . The mixture was vigorously stirred at
90 C for 14 hours. A 1 M aqueous solution of hydrochloric acid
was added to the reaction mixture and the mixture was extracted
with di ethyl ether. The organic layer was washed with saturated
brine and dried over anhydrous sodium sulfate. After
filtration, the filtrate was concentrated under reduced
pressure. The resulting residue was dissolved in THF (4 ml)
and MeOH (2 ml) , and a 1 M aqueous solution of sodium hydroxide
(0. 5 ml) was added to the reaction mixture at room temperature.
After stirring for 30 minutes, a 1 M aqueous solution of sodium
hydroxide (0.5 ml) was further added to the reaction mixture.
57
CA 02494177 2005-02-04
The mixture was stirred for 30 minutes. The solvent was
evaporated under reduced pressure and the resulting residue was
purified by silica gel column chromatography (chloroform-
methanol) . Methanol (2. 5 ml) and 10% aqueous solution of sodium
hydroxide (2.5 ml) were added to the resulting residue (133 mg)
and the mixture was refluxed with heating for one hour. After
evaporating the solvent under reduced pressure, ethanol was
added. The mixture was filtered. The filtrate was
concentrated under reduced pressure. The residue was purified
by silica gel column chromatography (chloroform-methanol) to
obtain 2-[4-ethoxy-3-methoxy-5-[(2S,3R,4R,5S,6R)-3,4,5-
trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]
benzyl]azulene-l-carboxylic acid (51 mg).
Example 68
Acetonitrile and a 4 M ethyl acetate solution (0.02 ml)
of hydrochloric acid (5 ml) were added to 2-[4-ethoxy-3-
methoxy-5- [(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-
(hydroxymethyl) tetrahydro-2H-pyran-2-yl]benzyl]azulene-l-
carboxylic acid (50 mg) and the mixture was refluxed with
heating for 10 minutes. A 4 M ethyl acetate solution (0. 02 ml)
of hydrochloric acid was further added to the reaction mixture
and the mixture was refluxed with heating for 30 minutes. The
reaction mixture was concentrated under reduced pressure. The
residue was purified by silica gel column chromatography
(chloroform- methanol) to obtain (1S)-1,5-anhydro-1-[5-
(azulen-2- ylmethyl)-2-ethoxy-3-methoxyphenyl]-D-glucitol
(42 mg).
58
CA 02494177 2005-02-04
The compounds of Examples 69, 70, and 71 were obtained
respectively in the same manner as in Examples 61, 39, and 63.
Example 72
Tris(dibenzylideneacetone)dipalladium (0) (17 mg), 2-
(dicyclohexylphosphino)biphenyl (22 mg), potassium fluoride
(44 mg) , and cesium carbonate (247 mg) were added to a solution
of (1S)-2,3,4,6-tetra-O-acetyl-1,5-anhydro-l-[3-
(bromomethyl)-2-fluorophenyl]-D-glucitol (198 mg) and methyl
2-(tributylstanyl)azulene-l-carboxylate (180 mg) in 1,4-
dioxane (10 ml) and the mixture was vigorously stirred at 90 C
for 15 hours. The reaction mixture was filtered through celite
and the filtrate was concentrated under reduced pressure. The
resulting residue was purified by silica gel column
chromatography (ethyl acetate-n-hexane) . The resulting
residue (71 ml) was dissolved in THF-methanol (ratio: 1:1, 6.0
ml). Sodium methoxide (30 mg) was added to the solution and
the resulting mixture was stirred at room temperature overnight.
The solvent was evaporated under reduced pressure. Theresidue
was purified by silica gel column chromatography
(chloroform-methanol) to obtain methyl 2-[2-fluoro-3-[(2S,
3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-
2H-pyran-2-yl]benzyl]azulene-l-carboxylate (19 mg).
Example 73
10% aqueous solution of sodium hydroxide (2 ml) was added
to a solution of methyl 2- [2-fluoro-3- [(2S, 3R, 4R, 5S, 6R) -
3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-
yl]benzyl]azulene-l-carboxylate (19 mg) in methanol (2 ml).
59
CA 02494177 2005-02-04
The mixture was refluxed with heating for 2 hours. The reaction
mixture was neutralized by adding a 4 M ethyl acetate solution
of hydrochloric acid and the solvent was evaporated under
reduced pressure. Acetonitrile (5 ml) and a 4 M ethyl acetate
solution of hydrochloric acid (1 ml ) were added to the resulting
residue and the mixture was refluxed with heating for 30 minutes.
The solvent was evaporated under reduced pressure. The residue
was purified by silica gel column chromatography
(chloroform-methanol) to obtain (1S)-1,5-anhydro-l-[3-
(azulen-2-ylmethyl)-2-fluorophenyl]-D-glucitol (10 mg).
The compound of Example 74 was obtained in the same manner
as in Reference Example 8.
Example 75
Aluminum chloride (0. 12 g) and anisole (4. 0 ml) were added
to a solution of (1S)-1,5-anhydro-l-[5-(azulen-2-ylmethyl)-
2-(benzyloxy)phenyl]-2,3,4,6-tetra-0-benzyl-D-glucitol
(0. 07 g) in methylene chloride (5 ml) and the mixture was stirred
at room temperature for two hours. The reaction mixture was
poured into ice-cooled water and extracted with ethyl acetate.
The organic layer was washed with saturated brine and dried over
anhydrous sodium sulfate. After filtration, the filtrate was
concentrated. The residue was purified by silica gel column
chromatography (chloroform-methanol) to obtain (1S)-1,5-
anhydro-l-[5-(azulen-2-ylmethyl)-2-hydroxyphenyl]-D-
glucitol (0.01 g).
The structural formulas and physicochemical properties
of the compounds of Reference Examples and Examples are
CA 02494177 2005-02-04
collectively shown by Tables 1-22 at the end of the present
specification.
The following abbreviations can be applied to the tables.
Rf refers to a number of Reference Example, Ex refers to
a number of Example, Structure refers to a structural formula,
Ac refers to an acetyl group, Bn refers to a benzyl group, Bu
refers to a butyl group, Data refers to a property data, NMR
refers to a nuclear magnetic resonance spectrum (TMS internal
standard), and MS refers to a mass analysis value.
Compounds listed in Table 23 can be easily prepared by
in the same manner as in Examples and Preparation Examples or
by a method with minor modifications which are obvious for
persons having an ordinary skill in the art. Table 23 is given
after Tables 1-22.
INDUSTRIAL APPLICABILITY
Since the azulene derivative and the salt thereof (the
compounds of the present invention) have the effects of
inhibiting a Na+-glucose cotransporter and reducing the level
of blood glucose, these compounds are useful for treating or
preventing insulin-dependent diabetes (type 1 diabetes),
insulin-independent diabetes (type 2 diabetes), insulin-
resistant diseases, and obesity, for example, as a
pharmaceutical, particularly as a Na+-glucose cotransporter
inhibitor.
The significant effects of inhibiting a Na+-glucose
cotransporter and reducing the blood glucose of the compound
61
CA 02494177 2005-02-04
of the present invention have been confirmed in the following
pharmacological tests (Test Examples 1 and 2).
Test Example 1
[Inhibition of human Na+-glucose cotransporter (human SGLT2)
activity]
(1) Preparation of human SGLT 2 expression vector
First, single-strand cDNA was reversely transcripted from
total RNA originating from the human kidney (manufactured by
BD Biosciences Clontech) using a Superscript II (manufactured
bylnvitrogen Corporation) and a random hexamer. Second, using
the cDNA as a template, a DNA fragment encoding a human SGLT2
(Wells, R.G. et al., Am. J. Physiol., 1992, 263(3) F459) was
amplified by the PCR reaction using Pyrobest DNA polymerase
(manufactured by Takara Bio Inc.). That is, a Hind III site
and an EcoRI site were inserted into the 5' side and the 3' side
of the DNA fragment, respectively by using primers.
The amplified fragment was cloned into a pCR2.1-Topo
vector using a Topo TA Cloning Kit (manufactured by Invitrogen
Corporation) and the cloned vector was transfected into a
competent cell of Escherichia coli JM109. Ampicillin-
resistant clones were cultured in a LB medium containing
ampicillin (100 mg/1) . Aplasmidwas purified from the cultured
Escherichia coli using the method of Hanahan (see Maniatis et
al., "Molecular Cloning") . A DNA fragment for encoding a human
SGLT2 was obtained by the Hind III/EcoRI digestion of the
plasmid and ligated and cloned to the same site of the expression
vector pcDNA3.1 (manufactured by Invitrogen Corporation) using
62
CA 02494177 2005-02-04
a DNA ligase (manufactured by Roche Diagnostics) The ligated
clone was transfected into a competent cell of Escherichia coli
JM109 in the same manner as described above and cultured in a
LB medium containing ampicillin, and a human SGLT2 expression
vector was obtained using the method of Hanahan.
(2) Preparation of human SGLT2 expressed cells
The human SGLT2 expression vector was transfected into
a CHO-Kl cell using Lipofectamine2000 (manufactured by
Invitrogen Corporation) . The cell was cultured in a Ham' s F12
medium (manufactured by Nissui Pharmaceutical Co., Ltd.)
containing penicillin (50 IU/ml, manufactured by Dainippon
Pharmaceutical Co., Ltd.), streptomycin (50 g/ml,
manufactured by Dainippon Pharmaceutical Co., Ltd.), Geneticin
(40 g/ml, manufactured by Invitrogen Corporation), and 10%
fetal bovine serum in the presence of 5% COZ at 37 C for two weeks,
and Geneticin-resistant clones were obtained. A cell which
stably expresses the human SGLT2, which exhibits sodium-
dependent intake of inethyl-a-D-glucopyranoside, was obtained
(See the following paragraphs for the method for measuring the
methyl-(x-D-glucopyranoside intake).
(3) Inhibition of inethyl-a-D-glucopyranoside intake
After removing the medium of a CHO cell which stably
expresses the human SGLT2, a pretreatment buffer solution
(buffer solution of pH 7.4 containing choline chloride (140mM),
potassium chloride (2 mM), calcium chloride (1 mM), magnesium
chloride (1 mM), 2-[4-(2-hydroxyethyl)1-piperazinyl]
ethanesulfonic acid (10 mM), and tris(hydroxymethyl)
63
CA 02494177 2005-02-04
aminomethane (5 mM) ) was added in the amount of 100 41 per well,
and incubated at 37 C for 20 minutes.
11 lof inethyl-a-D-(U-14C)glucopyranoside (manufactured
by Amersham Pharmacia Biotech) was mixed with 1,000 l of a
buffer solution for intake containing a test compound (buffer
solution of pH 7.4 containing sodium chloride (140 mM),
potassium chloride (2 mM), calcium chloride (1 mM), magnesium
chloride (1 mM), methyl-a-D-glucopyranoside (50 M), 2-[4-
(2-hydroxyethyl)1-piperazinyl]ethanesulfonic acid (10 mM),
and tris (hydroxymethyl ) aminomethane (5 mM) ) to prepare a buf fer
solution for intake. A buffer solution for intake without a
test compound was prepared for a control group. A buffer
solution for basal intake without a test compound containing
coline chloride (140 mM) instead of sodium chloride for
measuring the basal intake was prepared as well.
After removing the pretreatment buffer solution, the
buffer solution for intake was added (25 l per well)
andincubated at 37 C for two hours. After removing the buffer
solution for intake, a buffer solution for washing (buffer
solution of pH 7.4 containing choline chloride (140 mM),
potassium chloride (2 mM), calcium chloride (1 mM), magnesium
chloride (1 mM), methyl-a-D-glucopyranoside (10 mM), 2-[4-
(2-hydroxyethyl)1-piperazinyl]ethanesulfonic acid (10 mM),
and tris (hydroxymethyl) aminomethane (5 mM) ) was added (200 l
per one well) The mixture was immediately removed. This
washing operation was carried out once more. 0.5olaurylsodium
sulfate was added (25 l per well) to solubilize the cells. 75
64
CA 02494177 2005-02-04
l of Microscint 40 (manufactured by PerkinElmer, Inc.) was
added to the solubilized cell and the radiation activity was
measured using a microscintillation counter TopCount
(manufactured by PerkinElmer, Inc.). The value obtained by
subtracting the basal intake amount from the intake amount of
the control group was defined as 100%. The concentration for
50% inhibition of the above value (IC50 value) was calculated
from a concentration-inhibition curve using the least squares
method. As a result, the compound of the present invention
exhibited a strong effect of inhibiting a Na+-glucose
cotransporter. The IC50 values of typical compounds of the
present invention are shown in Table 24.
TABLE 24
Compound ICSO (nM) Compound IC50 (nM)
Example 7 16 Example 28 16
Example 11 29 Example 60 5.7
Example 21 99 Example 75 8.9
Example 25 22
Test Example 2
[Hypoglycemic activity confirmation test]
Fed KK-A'' mice (CLEA Japan, Inc., male) were used. The
test compound was suspended in 0.5% methylcellulose solution
to a concentration of 3 mg/10 ml. The weight of each mouse was
measured. The test compound suspension was orally
administered forcibly to the mice at a dose of 10 ml/kg. Only
0. 5% methylcellulose solution was administered to the mice of
a control group. Each group consisted of six mice. Blood was
CA 02494177 2005-02-04
collected from the tail vein immediately before administering
the compound and one, two, four, and eight hours after
administering the compound. The blood glucose value was
measured using a glucose CII Test Wako (manufactured by Wako
Pure Chemical Industries, Ltd.) . The intensity of
hypoglycemic activity was determined by calculating the area
under the blood glucose value-time curve (AUC) using a
trapezoidal method from the glucose value of 0-8 hours after
administering the compound and calculating the rate (%) of the
decrease in the AUC of the drug-administered group from that
of the control group.
As a result, the compound of the present invention
exhibited a strong hypoglycemic activity. The hypoglycemic
activity of typical compounds of the present invention are shown
in Table 25.
TABLE 25
Compound Hypoglycemic activity (%)
Example 28 46
Example 60 45
The pharmaceutical composition containing one or more of
the compounds of the present invention and the pharmaceutically
acceptable salts thereof is prepared as a tablet, powder, fine
granule, granule, capsule, pill, liquid, injection,
suppository, ointment, adhesive, or the like using a carrier,
vehicle, or other additives commonly used for preparation and
is orally or nonorally administered.
66
CA 02494177 2005-02-04
The amount of the compound of the present invention to
be clinically administered to the human body is appropriately
determined, taking the symptoms, weight, age, sex, and the like
of a patient to which the compound is administered into
consideration, in the range of 0.1-500 mg per day for oral
administration or in the range of 0. 01-100 mg per day for nonoral
administration, once or several times a day. Since the amount
to be administered varies according to various conditions, it
may be sufficient to administer the compound at a smaller amount
than the above-described amount.
As a solid composition for oral administration of the
compound of the present invention, a tablet, powder, granule,
or the like is used. In such a solid composition, one or more
active substances are mixed with at least one inert diluent such
as lactose, mannitol, glucose, hydroxypropylcellulose,
microcrystal cellulose, starch, polyvinylpyrrolidone, or
magnesium aluminometasilicate. The composition may contain
additives other than the inert diluent such as a lubricant such
as magnesium stearate, a disintegrator such as
carboxymethylcellulose calcium, and a solubilizer such as
glutamic acid and aspartic acid by a conventional method. The
tablet or pill may be optionally coated with a film of glucose
or a stomach-soluble or intestines-soluble substance such as
sucrose, gelatin, hydroxypropylcellulose, or hydroxypropyl-
methylcellulose phthalate.
The liquid composition for oral administration includes
pharmaceutically acceptable preparations such as an emulsion
67
CA 02494177 2005-02-04
preparation, solution preparation, suspension preparation,
syrup preparation, elixir preparation, and the like and
contains a commonly used inert diluent such as purified water
and ethyl alcohol. The composition may contain, in addition
to the diluent, adjuvants such as a solubilizer, humectant, and
suspending agent, sweetener, flavorer, perfume, and
preservative.
The injection for nonoral administration includes a
sterilized aqueous or nonaqueous solution, suspension, and
emulsion. Examples of the diluent for the aqueous solution or
suspension include distilled water and a physiological saline
solution for injection. Examples of the diluent for the
nonaqueous solution or suspension include propylene glycol,
polyethylene glycol, and vegetable oils such as olive oil;
alcohols such as ethyl alcohol; and Polysolvate 80 (trade name)
Such a composition may further contain additives such as an
isotonizing agent, preservative, humectant, emulsifier,
dispersant, stabilizer (e.g. lactose), and solubilizer. These
compounds are sterilized by filtering through a bacteria-
retaining filter and adding a disinfectant or irradiating, for
example. These compounds may be used by producing a sterilized
solid composition and dissolving the composition in a
sterilized water or injection solvent before using.
68
CA 02494177 2005-02-04
Table 1
Rf. Structure Data
0 'H-NMR(CDC13):2.63 (3H, s), 7.35-7.39 (1H,
Br m), 7.50 (1H, t), 7.59 (1H, t), 7.67-7.86 (4H,
1 I~ m), 7.96 (1H, s), 8.43 (1H, d), 9.67 (1H, d)
gr 'H-NMR(CDC13):2.63 (3H, s), 4.37 (2H, s),
6.95-7.53 (8H, m), 8.14-8.17 (2H, m)
2 \
'H-NMR(CDC13):2.56 (3H, s), 2.93 (1H, s),
3.54-4.36 (8H, m), 4.40 (2H, s), 4.50-4.91 (6H,
6.87-7.56 (28H, m), 8.13 (3H, dd)
3
OH
BnO
BnO ' 'OBn
OBn
0 'H-NMR(CDC13):1.35 (3H, t), 1.42 (6H, d),
Br 3.01 (2H, q), 3.22 (1H, q), 7.37 (1H, t), 7.46
4 (1H, t), 7.67 (1H, d), 7.74 (1H, d), 7.78 (1H, d),
7.88 (1H, s), 7.97 (1H, s), 8.35 (1H, d), 9.76
(1 H, d)
gr 'H-NMR(CDC13):1.30 (6H, d), 1.33 (3H, t),
2.95-3.05 (3H, q), 4.39 (2H, s), 6.96 (1H, t),
7.09-7.16 (2H, m), 7.28-7.31 (1H, m), 7.35-
~ 7.43 (2H, m), 7.59 (1H, s), 8.09-8.11 (2H, m)
'H-NMR(CDC13):1.24-1.29 (9H, t), 2.95-2.98
(3H, q), 3.55-5.01 (17H, m), 6.91-7.52 (26H,
m), 7.60 (1H, s), 8.07 (1H, d), 8.16 (1H, d)
6 O OH
BnO
Bn0"~ OBn
OBn
~O 'H-NMR(CDCI3):1.30 (3H, t), 2.27 (3H, s),
0 OH 3.73 (3H, dd), 3.84-4.01 (5H, m), 4.10 (1H, t),
7 Bn0 4.20 (IH, m), 4.52 (2H, m), 4.67 (2H, m), 4.91
Bn0 Bn 'OBn (3H, m), 6.77 (1H, d), 6.98 (2H, m), 7.01 (1H,
dd), 7.16-7.38 (19H, m)
O
69
CA 02494177 2005-02-04
Table 2
R.f. Structure Data
O 'H-NMR(CDC13):1.34 (3H, t), 2.28 (3H, s),
O 3.60 (1H, m), 3.79 (4H, m), 3.96 (3H, m), 4.40
8 Bn0 (IH, d), 4.53 (1H, d), 4.67 (2H, m), 4.63 (2H,
m), 4.89 (2H, m), 4.95 (1H, d), 6.76 (1H, d),
BnO~~~ OBn 6.90 (2H, m), 7.04 (1H, dd), 7.15-7.35 (19H,
OBn m)
O 'H-NMR(CDC13):1.43 (3H, t), 1.77 (3H, s),
O 2.01 (3H, s), 2.05 (3H, s), 2.08 (3H, s), 2.27
9 Ac0 (3H, s), 3.83 (1H, m), 4.01 (2H, q), 4.14 (1H,
m), 4.27 (1H, dd), 4.93 (1H, d), 5.22 (IH, t),
AcO'"""' ""OAc 5.35 (2H, m), 6.73 (1H, d), 7.03 (1H, d), 7.16
OAc (1H, s)
-,,,O 'H-NMR(CDC13):1.46 (3H t), 1.78 (3H, s),
O 1 Br 2.01 (3H, s), 2.06 (3H, s), 2.09 (3H, s), 3.84
Ac0 (1H, m), 4.05 (2H, q), 4.14 (1H, d), 4.27 (1H,
dd), 4.46 (1H, ABq), 4.48 (1H, ABq), 4.87 (1H,
AcO,''"' "'OAc m), 5.22 (1H, m), 5.35 (2H, m), 6.80 (1H, d),
OAc 7.29 (1H, dd), 7.37 (1H, s)
'H-NMR(CDC13):2.53 (3H, s), 3.28 (6H, s),
O H~ O 3.84-4.15 (6H, m), 4.34-4.90 (8H, m), 5.36
11 Bn0 ll, (1H, s), 6.95 (2H, d), 7.15-7.38 (20H, m), 7.80
BnO" OBn O~ (1H s)
OBn
ESI-MS (m/z):673 [M-H] -
O OH OH
12 Bn0
O
BnO" y" OBn
OBn
ESI-MS (m/z):657 [M-H] -
O OH
13 Bn0
BnO~~~ ""'OBn
OBn
'H-NMR(CDC13):2.41 (3H, s), 3.63 (2H, m),
O O 3.76-3.83 (5H, m), 3.90 (3H, s), 4.41 (1H, d),
14 Bn0 ll, 4.54-4.66 (4H, m), 4.86-4.94 (3H, m), 6.85
O (2H, m), 7.15-7.36 (19H, m), 7.89 (1H, dd),
BnO"~ ~~ OBn 8.17 (1H, d)
OBn
'H-NMR(CDC13):2.39 (3H, s), 3.45 (IH, s),
O OH 3.62 (2H, m), 3.76-3.83 (4H, m), 4.37 (1H, d),
Bn0 4.53-4.65 (5H, m), 4.86-4.94 (3H, m), 6.86
Bn0" 'OBn (2H, m), 7.15-7.34 (20H, m), 7.44 (1H, s)
OBn
CA 02494177 2005-02-04
Table 3
Rf. Structure Data
'H-NMR(CDC13):2.37 (3H, s), 3.61 (2H, m),
O 1 Br 3.80 (4H, m), 4.37 (1H, d), 4.48-4.66 (6H,
16 Bn0 m), 4.86-4.94 (3H, m), 6.88 (2H, m), 7.12-
B 7.35 (20H, m), 7.51 (1H, s)
nO~ OBn
OBn
Br 'H-NMR(CDC13):3.06 (1H, d), 3.36 (3H, s),
3.50 (1H, dd), 3.73 (1H, m), 3.82 (2H, m),
H 3.90(1H,d),4.05(1H,m),4.18(1H,m),
17 BnO O O, 4.39 (2H, s), 4.48-4.67 (4H, m), 4.86 (1H, d),
4.92 (2H, s), 6.98 (2H, m), 7.20-7.37 (18H,
Bn0"' Bn ~'OBn m), 7.48 (1H, d), 7.49 (1H, d), 7.70 (1H, t)
O
Br 'H-NMR(CDC13):3.36 (3H, s), 3.43 (1H, t),
3.57-3 p.97 (5H, m), 4.37-4.97 (10H, m),
18 O" 5.11 (1H, d), 6.94-7.81 (23H, m)
8 Bn0
BnO"' ~'OBn
OBn
O H 'H-NMR(CDC13):3.39 (31-, s), 3.49 (1H, m),
3.62 (1H, m), 3.74-3.83 (4H, m), 4.33 (1H,
d), 4.47-4.71 (7H, m), 4.87 (1H, d), 4.93 (21-L
19 O O, d), 6.88 (2H, m), 7.15-7.37 (18H, m), 7.67
Bn0
(1H, s), 7.82 (1H, s), 7.86 (1H, s), 9.97 (1H,
BnOBn s)
OBn
OH 'H-NMR(CDC13):3.37 (3H, s), 3.50 (1H, t),
3.62 (1H, m), 3.77 (4H, m), 4.26 (1H, d),
4.43 (1H, d), 4.46 (21-L s), 4.55-4.72 (6H, m),
20 O O, 4.87 (1H, d), 4.90 (1H, d), 4.95 (1H, d), 6.91
Bn0 (2H, m), 7.18-7.54 (21H, m)
Bn0" " OBn
OBn
Br 'H-NMR(CDC13):3.37 (31-, s), 3.50 (1H, t),
3.60 (1H, m), 3.79 (5H, m), 4.24 (1H, d),
4.41-4.66 (8H, m), 4.86 (1H, d), 4.90 (1H d),
21 Bn0 O 01-1 4.95 (1H, d), 6.92 (2H, m), 7.18-7.41 (21H,
m)
BnO'~~ OBn
OBn
'H-NMR(CDC13):1.47 (1H, t), 3.48-3.81 (6H,
/~ m), 4.25-4.97 (8H, m), 6.90-7.42 (34H, m)
OOH\ O ~
22 Bn0 ff
BnO"" "~'OBn OBn
71
CA 02494177 2005-02-04
T a b 1 e 4
Rf. Structure Data
'H-NMR(CDC13):1.09 (9H, s), 3.47-3.80 (7H,
m), 4.22-4.97 (8H, m), 4.88 (2H, s), 6.88-7.70
(34H, m) (1H, t)
23 Bn0 O O Si
BnO~~'OBn ~ ~
OBn
'H-NMR(CDC13):1.47 (1H, t), 3.48-3.82 (7H,
O OH m), 4.25-4.97 (10H, m), 6.90-7.42 (24H, m)
24 Bn0
BnO'~' "'OBn
OBn
'H-NMR(CDC13):3.48-3.82 (7H, m), 4.23-
0 Br 4.97 (l OH, m), 6.91-7.48 (24H, m)
25 Bn0
BnO'" "'OBn
OBn
O\ ~ 'H-NMR(CDC13):0.12 (6H, s), 0.96 (9H, s),
3.79 (3H, s), 4.70 (2H, s), 6.69 (1H, d), 7.31
26 (1H, dd), 7.56 (1H, d)
Br
~ 'H-NMR(CDC13):0.93 (9H, s), 2.97 (1H, s),
O 3.54-4.13 (9H, m), 4.36-4.93 (10H, m), 6.81
O H\ ~ O\ ~ (1H, d), 7.01-7.75 (22H, m)
27 Bn0 ~ i~
BnO'~~ ~~'OBn
OBn
~ 'H-NMR(CDC13):2.14 (1H, t), 3.46-3.84 (7H,
O m), 3.89 (3H, s), 4.19-4.97 (10H, m), 6.88
O OH (1H, d), 6.93-7.38 (22H, m)
28 Bn0 /---C
BnO"~ "OBn
OBn
~ 'H-NMR(CDC13):3.49-3.88 (7H, m), 3.91
O (3H, s), 4.17-4.96 (10H, m), 6.85-7.41 (23H,
29 Bn0 O Br m)
BnO~ OBn
OBn
72
CA 02494177 2005-02-04
Table 5
Rf. Structure Data
'H-NMR(CDC13):3.03 (1H, s), 3.49-4.92
O OH ~ I (14H, m), 6.97-7.79 (24H, m)
30 Bn0 Br
BnO"~ ~"'OBn
OBn
'H-NMR(CDC13):3.41-3,84 (7H, m), 4.19-
31 Bn0 O 4.97 (8H, m), 6.97-7.79 (24H, m)
Br
BnO"~ " OBn
OBn
'H-NMR(CDC13):0.84-1.55 (27H, m), 3.52-
0 Bu 3.83 (7H, m), 4.21-4.96 (8H, m), 6.88-7.54
32 Bn0 Sn-Bu (24H, m)
BnO~~~ OBn Bu
OBn
'H-NMR(CDC13):3.45-3.86 (7H, m), 4.32-
0 H 4.94 (8H, m), 6.86-7.93 (24H, m), 9.97 (1H,
33 Bn0 S)
BnO"'OBn O
OBn
'H-NMR(CDC13):3.48-3.83 (7H, m), 4.24-
0 4.98 (8H, m), 5.26 (1H, d), 5.75 (1H, d), 6.74
34 Bn0 (1H, m), 6.89-7.50 (24H, m)
BnO" OBn
OBn
O\ / 'H-NMR(CDC13):0.92 (9H, s), 7.05 (1H, t),
~S Br 7.18-7.24 (1H, m), 7.49 (1H, dd)
35 ~
F
F 'H-NMR(CDC13):0.l0 (6H, m), 0.94 (9H, s),
O OH O\ / 3.56-4.89 (17H, m), 6.92-7.60 (23H, m)
36 Bn0 S
BnO"~ "OBn
II
OBn
73
CA 02494177 2005-02-04
Table 6
Rf. Structure Data
F ESI-MS(m/z):666[M+NH4]+
O OH
37 Bn0
BnO"' "OBn OBn
F 'H-NMR(CDC13):3.60-4.00 (7H, m), 4.41-4.95
O Br (IOH, m), 6.90-7.51 (23H, m)
38 Bn0
BnO~~' "OBn
OBn
Br 'H-NMR(CDC13):3.05 (1H, s), 3.48-4.15 (6H,
m), 3.71 (3H, s), 4.50-4.92 (8H, m), 6.99-7.38
O OH (23H, m)
39 Bn0 O
BnO' "'OBn
OBn
Br 'H-NMR(CDC13):3.40-4.19 (1H, m), 4.46-4.97
(7H, m), 6.90-7.35 (23H, m)
40 BnO O O
BnO"' "OBn
OBn
Br 'H-NMR(CDC13):3.27-3.90 (6H, m), 3.79 (3H,
s), 4.09 (1H, d), 6.97-7.01 (2H, m), 7.19 (1H, t)
41 HO O O
HO~~' "'OH
OH
Br 'H-NMR(CDCl3):1.88 (3H, t), 2.00 (3H, s),
2.06 (3H, s), 2.10 (3H, s), 3.79 (3H, s), 3.80-
O 3.84 (1H, m), 4.15-5.33 (6H, m), 6.84 (1H, t),
42 Ac0 O 6.99 (1H, t), 7.06 (1H, t)
AcO '~ "'OAc
OAc
Bu 'H-NMR(CDC13):0.87-1.56 (27H, m), 1.80
Bu, Sn Bu (3H, s), 2.00 (3H, s), 2.06 (3H, s), 2.08 (3H, s),
3.80 (3H, s), 3.82-5.36 (7H, m), 6.83-6.95 (3H,
43 O m)
Ac0 O
AcO\'' ~ OAc
OAc
74
CA 02494177 2005-02-04
Table 7
Rf. Structure Data
ESI-MS(m/z):320[M+H]+
O Br
44 HO
HO"'OH
OH
'H-NMR(CDC13):1.86 (3H, s), 2.04 (3H, s),
O \ ~ 2.06 (3H, s), 2.10 (3H, s), 3.82-5.35 (7H, m),
45 Ac0 Br 7.20-7.28 (2H, m), 7.46 (1H, d), 7.49 (1H, s)
~''OAc
AcO""'
OAc
'H-NMR(CDC13):0.87-1.56 (27H, m), 1.78
O Bu (3H, s), 2.00 (3H, s), 2.06 (3H, s), 2.08 (3H, s),
46 Ac0 Sn~Bu 3.82-5.36 (7H, m), 7.30-7.41 (4H, m)
AcO%''~ ~~'OAc Bu
OAc
Br 'H-NMR(CDC13):3.57-3.83 (6H, m), 3.76 (3H,
0~1 s), 3.92 (3H, s), 4.03 (1H, d), 4.47-4.96 (8H,
m), 6.42 (1H, s), 6.90-7.34 (20H, m), 7.56 (1H,
47 Bn0 O s)
BnO%'" OBorni
OBn
Br 'H-NMR(CDC13):3.33-3.75 (6H, m), 3.85 (3H,
0~1 s), 3.89 (3K s), 4.16 (1H, t), 4.41 (1H, d), 4.56
(1H, d), 4.66 (1H, d), 4.74 (1H, d), 6.55 (1H, s),
48 HO O 7.47 (1H, s)
''OH
HO~~'
OH
Br 'H-NMR(CDC13):2.84 (3H, s), 3.34 (3H, s),
0~1 3.44 (3H, s), 3.45 (3H, s), 3.57-3.92 (7H, m),
O 3.83 (3H, s), 3.89 (3H, s), 4.24-4.92 (8H, m),
49 ~-O/--O 0 6.43 (1H, s), 7.54 (1H, s)
0 "1,.,0 ,,.~ ~
O~ O
O ~
CA 02494177 2005-02-04
Table 8
Rf. Structure Data
Bu 'H-NMR(CDC13):0.87 (9H, t), 0.96-1.50 (18H,
Bu-,,Sn--Bu m), 2.82 (3H, s), 3.33 (3H, s), 3.45 (3H, s),
O 3.46 (3H, s), 3.57-3.92 (7H, m), 3.75 (3H, s),
~ 3.83 (3H, s), 4.18-4.94 (8H, m), 6.36 (1H, s),
50 ~ ~O O I 7.25 (1H, s)
~OO,o~
-
O
'H-NMR(CDC13):1.34 (3H, s), 1.36 (3H, s),
51 I~ \ OH 1.76 (1H, t), 3.04-3.08 (1H, m), 5.09 (2H, d),
- 7.14 (2H, d), 8.23 (2H, d)
'H-NMR(CDC13):1.33 (3H, s), 1.35 (3H, s),
52 ~\ \ Ci 3.04-3.07 (1H, m), 4.95 (2H, s), 7.14 (2H, d),
7.28 (2H, s), 8.23 (2H, d)
'H-NMR(CDC13):4.98 (2H, s), 7.20 (2H, t),
53 1~\ C~ 7.37 (2H, s), 7.59 (1H, t), 8.30 (2H, d)
Bu 'H-NMR(CDCl3):0.87 (9H, t), 1.11-1.18 (6H,
~ m), 1.27-1.38 (6H, m), 1.51-1.58 (6H, m), 3.96
Sn-Bu (3H, s), 7.25 (1H, s), 7.42 (1H, t), 7.50 (1H, t),
54 Bu 7.74 (1H, t), 8.37 (1H, d), 9.58 (1H, d)
0 ~
/''- 'H-NMR(CDC13): 1.38-1.46 (6H, m), 1.80 (3H,
~ s), 2.01 (3H, s), 2.06 (3H, s), 2.07 (3H, s), 2.31
(3H, s), 3.83 (1H, ddd), 4.00-4.29 (6H, m),
55 AcO 0 4.55-4.65 (1H, m), 5.18-5.38 (3H, m), 6.64
(1H, s), 6.86 (1H, s)
AcOl''~~ ~~'OAc
OAc
,-,- 'H-NMR(CDC13):1.41-1.48 (6H, m), 1.82 (3H,
s), 2.02 (3H, s), 2.07 (3H, s), 2.17 (3H, s), 3.90
(1 H, ddd), 4.02-4.3 0(6H, m), 4.51 (1 H, d),
56 Br 4.62 (1H, d), 4.75-4.81 (1H, m), 5.20-5.42 (3H,
AcO m), 6.82 (1H, s), 6.90 (1H, s)
AcOl" "'OAc
OAc
76
CA 02494177 2005-02-04
Table 9
Rf. Structure Data
ll~ O 'H-NMR(CDC13):1.44 (3H, t), 1.81 (3H, s),
2.01 (3H, s), 2.04-2.08 (6H, m), 2.33 (3H, s),
O 3.78-4.28 (8H, m), 4.55-4.66 (IH, m), 5.17-
57 Ac0 O 5.38 (3H, m), 6.63 (1H, s), 6.85 (1H, s)
AcOl"' "'OAc
OAc
N, O 'H-NMR(CDC13):1.46 (3H, t), 1.82 (3H, s),
2.01 (3H, s), 2.07 (3H, s), 2.07 (3H, s), 3.88-
4.30 (8H, m), 4.52 (1H, d), 4.63 (1H, d), 4.76-
58 Br 4.82 (IH, m), 5.21-5.41 (3H, m), 6.81 (1H, s),
Ac0 O 6.90 (1H, s)
AcO"'~~ "OAc
OAc
'H-NMR(CDC13):2.27 (3H, d), 3.56-3.66 (2H,
O m), 3.71-3.94 (5H, m), 4.44-4.68 (5H, m), 4.84-
59 Bn0 4.98 (3H, m), 6.87-7.37 (23H, m)
BnO"'~~ "'OBn
OBn
FAB-MS(m/z):441 [M+H]+
O
60 Ac0
AcOl''~~ "'OAc
OAc
EI-MS:519[M]+
O Br
61 Ac0
AcO%'" ""'OAc
OAc
FAB-MS(m/z):503[M+H]+
62 O
Ac0
AcOl"" "'OAc
OAc
EI-MS:581[M]+
~
Br
O ~ ~
63
Ac0
AcO""" "'OAc
OAc
77
CA 02494177 2005-02-04
Table 10
Rf. Structure Data
O'll FAB-MS(m/z):483[M+H]+
O
64 Ac0
AcOl'""~ "'OAOF"'
OAc
Oll, FAB-MS(m/z):562[M+H]+
O Br
65 Ac0
AcOl''" ~~'OAOc~
OAc
66 HO FAB-MS(m/z):230[M+H]+
O
Br
'H-NMR(CDC13):2.16 (3H, s), 3.61 (2H, s),
O 5.14 (2H, s), 6.88-7.67 (2H, dd), 7.32-7.48
67 I ~ O (6H, m)
Br
FAB-MS (m/z) :404 [M+H]+
68 \ O \ I I / /
Br
'H-NMR(CDC13):3.70-4.98 (19H, m), 6.89
Bn0 ~ (2H, d), 3.44 (3H, s), 7.08-7.63 (31H, m),
OOH~ I 8.11 (2H, d)
69 Bn0
BnO'"~~ ~~'OBn
OBn
78
CA 02494177 2005-02-04
T a b 1 e 1 1
Ex. Structure Data
'H-NMR(CDC13):2.55 (3H, s), 3.47-3.81 (7H,
m), 4.11-4.32 (2H, m), 4.40 (2H, s), 4.51-4.93
0 ~ (6H, m),, 6.86-7.46 (28H, m), 7.49 (IH, s),
Bn0
8.13 (2H, dd,)
BnO" ''OBn ESI-MS(m/z):567[M+H]+
OBn
'H-NMR(CD3OD):2.57 (3H, s), 3.14-3.43 (5H,
m), 3.66-3.70 (1H, m),3.96 (IH, d), 4.35 (2H,
2 O s), 4.41-4.94 (4H, m),, 6.99-7.21 (6H, m), 7.54
HO (1H, t), 7.60 (1H, s), 8.20 (1H, d), 8.34 (IH, d)
HO" ~'~ OH EI-MS:393[M-H]-
OH
'H-NMR(CDC13):1.24-1.29 (9H, t), 2.92-3.01
(3H, q), 3.40-4.94 (17H, m), 6.85-8.08 (27H,
Bn0 O \ \/ ESI MS(m/z):5818[M+H]+ d)
\
3 BnO'~"~"'OBn
OBn
'H-NMR(CD3OD):1.30 (6H, d), 1.32 (3H, t),
2.99-3.04 (3H, q), 3.47-3.67 (4H, m), 3.82-3.92
I N (2H, ABq), 4.15 (1H, d), 4.43 (2H, s), 6.95
4 HO O (1H, t), 7.18-7.28 (3H, m), 7.20 (1H, s), 7.42
(1H, d), 7.56 (1H, d), 8.10 (1H, d), 8.15 (1H, s)
HO'' "OH EI-MS:450[M+]
OH
'H-NMR(CDC13):1.46 (3H, t), 1.75 (3H, s),
2.00 (3H, s), 2.03 (3H, s), 2.05 (3H, s), 3.84
-,,,0 (IH, m), 3.96 (3H, s), 4.03 (2H, q), 4.15 (1H,
m), 4.28 (IH, m), 4.54 (2H, s), 4.92 (1H, m),
Ac0 O 5.20 (1H, m), 5.35 (2H, m), 6.78 (IH, d), 6.89
O (1H, s), 7.12 (1H, dd), 7.31 (IH, s), 7.38 (1H,
AcO,''~~ "'OAc O \ t), 7.51 (IH, t), 7.70 (1H, t), 8.28 (IH, d), 9.52
OAc (1H, d)
ESI-MS(m/z):651 M+ +
'H-NMR(CD3OD):1,40 (3H, t), 3.39 (2H, m),
3.49 (1H, m), 3.60 (1H, t), 3.65 (1H, m), 3.85
(1H, m), 4.05 (2H, m), 4.58 (2H, s), 4.69 (IH,
6 O d), 6.88 (1H, d), 7.012 (1H, s), 7.15 (1H, dd),
HO OH 7.39 (IH, d), 7.43 (1H, t), 7.52 (1H, t), 7.75
HO"~ ""OH O
(1H,t),8.31(1H,d),9.50(1H d)
OH ESI-MS(m/z):469[M+H]+
79
CA 02494177 2005-02-04
Table 12
Ex. Structure Data
'H-NMR(CD30D):1.41 (3H, t), 2.13 (IH, brs),
2.54 (1H, brs), 2.79 (IH, brs), 2.97 (1H, brs),
3.55 (2H, m), 3.68 (2H, m), 3.81 (1H, m), 3.90
7 O (1H, m), 4.02 (1H, dd), 4.10 (1H, dd), 4.30 (2H,
HO s), 4.75 (1H, d), 6.84 (IH, d), 7.15 (5H, m),
HO "'OH 7.39 (1H, d), 7.51 (1H, t), 7.52 (1H, t), 8.19
OH (2H, d)
ESI-MS(m/z):425 M+ +
'H-NMR(CDC13):2.39 (3H, s), 3.62 (2H, m),
3.74-3.84 (6H, m), 3.93 (3H, s), 4.33 (IH, d),
4.47-4.64 (5H, m), 4.84-4.95 (3H, m), 6.85 (1H,
8 BnO O s), 6.91 (2H, d), 7.13-7.31 (21H, m), 7.45 (1H,
O O s), 7.49 (IH, t), 7.67 (1H, m), 8.00 (1H, d), 9.51
BnO%''~~ ~~'OBn (1H, d)
OBn ESI-MS(m/z):813 M+ +
'H-NMR(CD30D):2.33 (3H, s), 3.66-3.92 (6H,
m), 4.45 (1H, d), 4.53 (2H, s), 6.94 (1H, s), 7.05
(2H, m), 7.34 (2H, m), 7.46 (1H, t), 7.66 (1H,
9 HO O t), 8.18 (1H, d), 9.38 (1H, d)
HO"~ ~~'OH O 0 ESI-MS(m/z):453[M+H]+
OH
'H-NMR(CD30D):2.32 (3H, s), 3.57-3.88 (3H,
m), 4.35 (1H, d), 4.46 (2H, s), 6.88 (1H, s), 7.11
(2H, m), 7.27 (2H, m), 7.55 (1H, t), 7.68 (IH,
HO O t), 8.15 (IH, d), 9.33 (1H, d)
O OH ESI-MS(m/z):438[M+H]+
""OH
HO"~
OH
'H-NMR(CD30D):2.39 (3H, s), 3.41 (2H, m),
3.54 (21-L m), 3.68 (1H, m), 3.87 (1H, d), 4.29
O (2H, s), 4.45 (1H, d), 7.10 (6H, m), 7.41 (1H,
11 HO s), 7.50 (1H, s), 8.17 (2H, d)
HO~~l ~ "'OH ESI-MS(m/z):395[M+H]+
OH
'H-NMR(CD3OD):2.28 (3H, s), 3.45 (2H, m),
~~ 3.64 (2H, m), 3.81 (3H, s), 3.82 (2H, m), 4.39
(3H, s), 4.55 (2H, d), 6.83 (1H, d), 6.94 (31-I, d),
12 I/ 7.25 (4H, m), 7.33 (1H, t), 7.47 (1H, t), 7.68
O (1H, t), 8.27 (IH, d), 9.38 (IH, d)
HO O ESI-MS(m/z):543[M+H]+
HO"l ~~'OH O
OH
CA 02494177 2005-02-04
T a b 1 e 1 3
Ex. Structure Data
'H-NMR(CD30D):2.78 (3H, s), 3.33 (2H,
~~ m), 3.62 (2H, m), 3.81 (2H, m), 4.40 (3H,
~~ s), 4.55 (2H, d), 6.79 (1H, d), 6.85 (3H, d),
/ 7.11 (4H, m), 7.40 (1H, t), 7.47 (1H, t),
13 O 1/ 7.70 (1H, t), 8.22 (1H, d), 9.55 (1H, d)
HO OH ESI-MS(m/z):529[M+II]+
HO ""'OH O
OH
'H-NMR(CD30D):2.37 (3H, s), 3.31 (2H,
m), 3.50 (2H, m), 3.64 (1H, dd), 3.86 (1H,
m), 4.13 (2H, s), 4.42 (3H, s), 6.90 (1H,
14 HO O dd), 7.02-7.21 (9H, m), 7.27 (1H, s), 7.48
,,, -,, (1H, t), 8.12 (1H, d), 8.22 (1H, d)
HO OH OH ESI-MS(m/z):485[M+H]+
O, 'H-NMR(CDC13):2.32 (3H, s), 3.57-3.88
(3H, m), 4.35 (1H, d), 4.46 (2H, s), 6.88
(1H, s), 7.11 (2H, m), 7.27 (2H, m), 7.55
15 Bn0 O (1H, t), 7.68 (1H, t), 8.15 (1H, d), 9.33
O (1H, d)
BnO''''~ ~"'OBn O ~ ESI-MS(m/z):438[M+H]+
OBn
'H-NMR(CD30D):2.39 (4H, brs), 3.38
CI
(1H, m), 3.47 (1H, m), 3.60-3.80 (4H, m),
3.84 (3H, s), 4.10 (1H, d), 4.35 (2H, s),
4.53 (2H, s), 6.92 (1H, s), 7.16 (1H, s),
16 HO O O 7.21 (1H, s), 7.25 (1H, m), 7.30 (1H, t),
HO~~ "'OH O 7.44 (1H, t), 7.64 (1H, t), 8.17 (1H, d),
OH 9.39 (1H, d)
ESI-MS(m/z):532[M+I-H]+
O, 'H-NMR(CD30D):2.40 (2H, brs), 3.14
(3H, s), 3.22 (1H, m), 3.45-3.67 (5H, m),
4.02 (1H, d), 4.18 (2H, s), 4.21 (2H, s),
17 HO 'N~ O 4.97 (1H, brs), 5.21 (1H, brs), 6.99-7.06
HO~~ "'OH (5H, m), 7.20 (1H, s), 7.40 (1H, t), 7.30
(1H, t), 8.05 (2H, d)
OH ESI-MS(m/z):425 M+H]+
0 'H-NMR(CDC13):1.43 (6H, t), 3.46-3.80
(7H, m), 4.20-4.93 (14H, m), 6.85-7.39
(24H, m), 7.62 (2H, d), 8.77 (1H, s), 9.64
18 0 ~"~ (2H, d)
BnO 0 ESI-MS(m/z):885[M+H]+
Bn0'"~~ 'OBn
OBn
81
CA 02494177 2005-02-04
T a b 1 e 1 4
Ex. Structure Data
p O~ 'H-NMR(CD30D):1.36 (6H, t), 3.39-4.12
(7H, m), 4.13 (2H, s), 4.36 (4H, dd), 7.03-
7.28 (4H, m), 7.54 (2H, d), 8.66 (1H, s), 9.51
19 p o (2H, d)
HO p ESI-MS(m/z):523[M-H]-
HO" "dOH
OH
p OH 'H-NMR(CD30D):3.34-3.52 (4H, m), 3.71-
3.93 (2H, m), 4.16 (1H, d), 4.40 (2H, s),
7.29-7.45 (4H, m), 7.88 (2H, d), 8.76 (1H, s)
20 HO O OH ESI-MS(m/z):467[M-H]-
0
HO" "'OH
OH
'H-NMR(CD3OD):3.34-3.89 (6H, m), 4.12
(1H, d), 4.17 (2H, s), 7.18 (2H, d), 7.19-7.38
21 HO O (6H, m), 7.77 (1H, t), 8.25 (2H, d)
HO ESI-MS(m/z):381 [M+H]+
~" ~~'OH
OH
'H-NMR(CDC13):3.52-3.82 (7H, m), 3.93
(3H, s), 4.22-4.95 ( l OH, m), 6.86-7.68 (28H,
m), 8.04 (1H, d), 9.53 (1H, d)
22 BnO O ESI-MS(m/z):799[M+H]+
BnOl "~ "'OBn p
OBn
'H-NMR(CD30D):3.42-3.93 (6H, m), 3.93
(3H, s), 4.18 (1H, d), 4.63 (2H, s), 7.02 (1H,
O s), 7.02-7.33 (4H, m), 7.42 (1H, t), 7.53 (1H,
23 Hp t), 7.73 (1H, t), 8.30 (1H, d), 9.48 (1H, d)
HO~~~ ~''OH O 0 ESI-MS(m/z):423[M-H]-
OH
'H-NMR(CD30D):3.34-3.52 (4H, m), 3.70-
3.93 (2H, m), 4.15 (1H, d), 4.69 (2H, s), 7.06
O (1H, s), 7.24-7.84 (7H, m), 8.38 (1H, d),
24 HO 9.57 (1H, d)
O OH ESI-MS(m/z):437[M-H]-
HO~~ "'OH
OH
'H-NMR(CD30D):3.34-3.93 (6H, m), 4.16
(1H,d,J=9.3Hz),4.37(2H,s), 7.15-7.58
O (9H, m), 8.24 (21-, d)
25 Hp ESI-MS(m/z):381[M+H]+
HO"~ "'OH
OH
82
CA 02494177 2005-02-04
T a b 1 e 1 5
Ex. Structure Data
'H-NMR(CDC13):1.74-2.09 (12H, m), 3.82 (3H,
"lO s), 3.96 (3H, s), 4.11-4.27 (2H, m), 4.55 (2H, s),
O 4.91-5.36 (5H, m), 6.79-7.69 (7H, m), 4.92 (IH,
26 Ac0 O m), 8.29 (1H, d), 9.53 (IH, d)
AcOl"" OAc O ESI-MS(m/z):637[M+H]+
OAc
'H-NMR(CD30D):3.29-3.66 (5H, m), 3.78 (3H,
s), 3.84 (IH, m), 4.55 (2H, s), 4.70 (1H, d), 6.88
p (1H, d), 6.98 (1H, s), 7.15 (1H, dd), 7.37-7.42
27 HO (1H, m), 7.49 (1H, t), 7.72 (IH, t), 8.28 (1H, d),
HO~~~ ''OH O OH 9.48 (1H, d)
ESI-MS(m/z):435 [M-H]-
OH
'H-NMR(CD30D):3.36-3.90 (6H, m), 3.86 (3H,
s), 4.31 (2H, s), 4.74 (IH, d), 6.97 (1H, d), 7.15-
I 7.43 (6H, m), 7.54 (1H, t), 8.23 (2H, d)
28 HO O ESI-MS(m/z):411 [M+H]+
HO"l Cj~"'O
OH
1H-NMR(CDC13):3.49-3.89 (7H, m), 3.94 (3H,
O s), 4.09-4.95 (IOH, m), 6.74-7.68 (24H, m),
I 1 7.94 (IH, d), 9.52 (1H, d)
p ESI-MS(m/z): 85 1 [M+Na]+
29 BnO O
BnON''~ ~''OBn O \
OBn
1H-NMR(CD30D):3.29-4.56 (15H, m), 6.79-
~ O 7.18 (4H, m), 7.41 (1H, t), 7.52 (1H, t), 7.74
(1H, t), 8.28 (1H, d), 9.42 (1H, d)
30 HO O ESI-MS(m/z):469[M+H]+
HO~~~ ~''OH p O
OH
O 'H-NMR(CD30D):3.30-4.50 (7H, m), 3.80 (3H,
s), 4.28 (2H, s), 6.92-7.31 (6H, m), 7.48 (IH, t),
8.16 (2H, d)
31 HO O OH ESI-MS(m/z):477[M+Na]+
HO~~~ "'OH O
OH
'H-NMR(CD3OD):3.30-4.29 (7H, m), 3.83 (3H,
O s), 4.30 (2H, s), 6.94-7.29 (6H, m), 7.46 (1H, t),
I 8.16 (2H, d)
32 HO p ESI-MS(m/z):411 [M+H]+
HO~~~ "'OH
OH
83
CA 02494177 2005-02-04
Table 16
Ex. Structure Data
0 O 'H-NMR(CDC13):3.57-3.89 (7H, m), 3.69 (3H,
O s), 4.35 (IH, d), 4.45 (1H, d), 4.56-5.00 (6H,
33 Bn0 m), 6.95-7.76 (28H, m), 8.36 (IH, d)
ESI-MS(m/z):802[M+NH4]+
Bn0'"~' "'OBn
OBn
0 O 'H-NMR(CD30D):3.44-3.76 (5H, m), 3.77
O \( ~ (3H, s), 3.89-3.92 (1H, m), 4.23 (1H, d), 7.37-
34 HO 7.88 (8H, m), 8.47 (1H, d), 9.29 (1H, d)
ESI-MS(m/z):442[M+NH4]+
HO'~~ ~~'OH
OH
O OH 'H-NMR(CD30D):3.52-3.86 (5H, m), 3.89-
~ I 4.11 (1H, m), 4.17 (1H, d), 7.33-7.88 (8H, m),
35 HO 8.52 (1H, d), 9.25 (1H, d)
ESI-MS(m/z):409[M-H]-
HO~~'OH
OH
'H-NMR(CD30D):3.49-3.73 (2H, m), 4.14
O (1H, d), 4.50 (1H, t), 4.88 (IH, d), 4.98 (2H,
36 HO t), 7.23 (2H, t), 7.36 (1H, d), 7.44 (1H, t), 7.61
(1H, t), 7.82 (2H, s), 7.96 (1H, d), 8.00 (1H,
O"~ ~~'OH
H
s), 8.38 (21-L d)
OH ESI-MS(m/z):367 M+ +
'H-NMR(CDC13):1.63 (3H, s), 1.98 (3H, s),
2.05 (3H, s), 2.06 (3H, s), 3.80-4.38 (4H, m),
O 4.17 (2H, s), 5.12 (1H, t), 5.22 (1H, t), 5.31
37 Ac0 (1H, t), 7.11-7.32 (8H, m), 7.51 (1H, t), 8.20
Ac0''''~ ~~'OAc (2H, d)
OAc ESI-MS(m/z):549[M+H]+
'H-NMR(CDC13):1.71 (3H, s), 2.07 (3H, s),
2.08 (3H, s), 2.09 (3H, s), 2.70 (3H, s), 3.80-
4.3 8(4H, m), 4. 5 7(2H, s), 5.11 (1 H, t), 5.22
38 Ac0 O (1H, t), 5.30 (1H, t), 6.84 (1H, s), 7.17-7.55
Ac0''' " OAc O (6H, m), 7.72 (IH, t), 8.28 (1H, d), 9.35 (1H,
d)
OAc ESI-MS(m/z):589 M-H -
~ 'H-NMR(CD30D):2.69 (3H, s), 3.30-3.87
~ (6H, m), 4.10 (IH, d), 7.03 (1H, s), 7.12-7.46
O (4H, m), 7.49 (1H, t), 7.57 (1H, t), 7.79 (1H,
39 HO t), 8.37 (1H, d), 9.30 (1H, d)
HO'" ''OH O ESI-MS(m/z):423[M+H]+
OH
84
CA 02494177 2005-02-04
T a b 1 e 1 7
Ex. Structure Data
'H-NMR(CDC13):1.81 (3H, t), 1.67 (3H, s),
1.98 (3H, s), 2.05 (3H, s), 2.08 (3H, s), 3.04
O (2H, dd), 3.60-4.36 (6H, m), 5.08-5.31 (3H,
40 Ac0 m), 6.99-7.51 (8H, m), 8.09 (1H, d), 8.20
~~ -, (1 H, d)
Ac0 " OAc OAc ESI-MS(m/z):577[M+H]+
'H-NMR(CD30D):1.20 (3H, t), 3.07 (2H,
dd), 3.30-3.88 (6H, m), 4.10 (1H, d), 4.31
(2H, s), 7.00-7.45 (8H, m), 8.08 (1H, d), 8.22
41 HO O ~ (1H, d)
HO" "'OH ESI-MS(m/z):409[M+H]+
OH
0 O 'H-NMR(CDC13):3.08-3.12 (2H, m), 3.50-
0 3.82 (9H, m), 3.95 (3H, s), 4.13-4.97 (8H,
42 Bn0 m), 6.88-7.39 (26H, m), 7.50 (1H, t), 7.66
BnO%"~~ ~"'OBn (1H, t), 8.23 (1H, d), 9.53 (1H, d)
OBn ESI-MS(m/z):830[M+NH4]+
O 0 'H-NMR(CD30D):3.04 (2H, t), 3.30-3.89
(8H, m), 3.89 (3H, s), 4.11 (1H, d), 7.17-7.31
43 HO O (5H, m), 7.48 (1H, t), 7.55 (1H, t), 7.78 (1H,
,., -", t), 8.40 (1 H, d), 9.44 (1 H, d)
HO OH OH ESI-MS(m/z):453[M+H]+
~ 0 OH 'H-NMR(CD3OD):3.06-3.10 (2H, m), 3.30-
I 3.89 (8H, m), 4.12 (1H, d), 7.18-7.34 (5H,
44 HO O \ m), 7.46 (1H, t), 7.53 (1H, t), 7.76 (1H, t),
,,, ,,
~ 8.39 (1H, d), 9.50 (1H, d)
HO OH OH ESI-MS(m/z):439[M+H]+
'H-NMR(CD30D):3.11 (2H, t), 3.27-3.89
(8H, m), 4.11 (1H, d), 7.11-7.24 (7H, m),
45 HO O 7.33 (1H, s), 7.50 (1H, t), 8.20 (2H, d)
HO~~~ ~"'OH ESI-MS(m/z):395[M+H]+
OH
'H-NMR(CDC13):3.60-3.99 (7H, m), 3.91
F~ (3H, s), 4.43-4.93 (10H, m), 6.85-7.44 (25H,
O I m), 7.50 (1H, t), 7.68 (1H, t), 8.05 (1H, d),
46 Bn0 9.51 (1H, d)
BnO, O "'OBn O 0 ESI-MS(m/z):834[M+NH4]+
OBn
CA 02494177 2005-02-04
Table 18
Ex. Structure Data
'H-NMR(CD30D):3.38-3.88 (6H, m), 3.93
F~ (3H, s), 4.48-4.51 (1H, m), 4.57 (2H, s), 6.97
O (1H, dd), 7.06 (1H, s), 7.14-7.18 (1H, m),
47 Hp 7.44-7.51 (2H, m), 7.56 (1H, t), 7.80 (1H, m),
p 8.38(1H,d),9.46(1H,d)
''' ' O
HO OH ''OH ESI-MS(m/z):457[M+1-1]+
'H-NMR(CD30D):3.38-3.87 (6H, m), 4.49-
// 4.50 (1H, m), 4.61 (2H, s), 6.96-7.56 (6H, m),
O 7.77 (1H, t), 8.34 (1H, d), 9.52 (1H, d)
48 HO ESI-MS(m/z):441 [M-H]-
OH
HO"~ ""'OH O
OH
'H-NMR(CD30D):3.34-3.88 (6H, m), 4.31
F~ (2H, s), 4.49-4.83 (1H, m), 6.97-7.54 (8H, m),
O 8.20 (2H, d)
49 HO ESI-MS(m/z):399[M+H]+
HO~~~ "'OH
OH
O~ 'H-NMR(CDC13):1.63 (3H, s), 1.98 (3H, s),
2.04 (3H, s), 2.06 (3H, s), 3.78 (3H, s), 3.79-
3.86 (1H, m), 4.09-5.31 (6H, m), 6.80-6.81
50 Ac0 O (3H, m), 7.11-7.16 (4H, m), 7.51 (1H, t), 8.20
(2H, d)
Ac0'"" " " OAc ESI-MS(m/z):579[M+H]+
OAc
O~ 'H-NMR(CD30D):3.30-3.45 (4H, m), 3.69
(1H, dd), 3.75 (3H, s), 3.85-3.89 (1H, m), 4.08
(1H, d), 4.29 (2H, s), 6.78 (1H, t), 6.85 (1H, t),
51 HO O 6.97 (1H, t), 7.11-7.16 (4H, m), 7.50 (1H, t),
8.19 (2H, d)
HO'" "'OH ESI-MS(m/z):433[M+Na]+
OH
'H-NMR(CD30D):3.42-3.93 (6H, m), 3.93
(3H, s), 4.18 (1H, d), 4.33 (2H, s), 4.63 (2H,
s), 7.02 (1H, s), 7.02-7.33 (8H, m), 7.42 (1H,
52 0 t), 7.53 (1H, t), 7.73 (1H, t), 8.30 (1H, d), 9.48
HO
(1H, d)
HO"' "'OH 0 ESI-MS(m/z):527[M-H]-
OH
, 1 'H-NMR(CD3OD):3.31-3.87 (6H, m), 4.05
~ VO (1H, d), 4.17 (2H, s), 4.42 (2H, s), 6.92-7.19
(9H, m), 7.40 (1H, t), 7.51 (1H, t), 7.73 (1H,
53 HO O ~ I t), 8.40 (1H, d), 9.51 (1H, d)
ESI-MS(m/z):513[M-H]-
HO"' ""'OH H 0
OH
86
CA 02494177 2005-02-04
Table 19
Ex. Structure Data
'H-NMR(CD30D):3.31-3.88 (6H, m), 4.06
(1H, d), 4.17 (2H, s), 4.42 (2H, s), 7.02-7.26
O (12H, m), 7.48 (1 H, t), 8.14 (1 H, d), 8.24
54 HO (1H, d)
HO~~~ ~OOH ESI-MS(m/z):471[M+H]+
OH
'H-NMR(CDC13):1.32 (3H, s), 1.33 (3H, s),
1.63 (3H, s), 1.98 (3H, s), 2.05 (3H, s), 2.06
(3H, s), 3.01-3.05 (1H, m), 3.79-3.83 (1H,
55 O oj" m), 4.14-4.37 (5H, m), 5.11 (1H, t), 5.22
Ac0 (1H, t), 5.29 (1H, t), 7.05 (2H, s), 7.08 (2H,
,, d), 7.20-7.31 (4H, m), 8.13 (2H, d)
.~~
Ac0 OAc OAc ESI-MS(m/z):591 [M+I-1]+
'H-NMR(CD30D):1.32 (3H, s), 1.34 (3H,
s), 3.02-3.05 (1H, m), 3.37-3.88 (6H, m),
4.11 (1H, d), 4.29 (2H, s), 7.06 (2H, s), 7.10
56 O (2H, d), 7.21-7.27 (3H, m), 7.36 (1H, s),
HO 8.12 (2H, d)
HO'~~ 'OH ESI-MS(m/z):423[M+I-I]+
OH
'H-NMR(CDC13):1.32 (3H, s), 1.33 (3H, s),
0~ 1.64 (3H, s), 1.98 (3H, s), 2.05 (3H, s), 2.06
(3H, s), 3.01-3.05 (1H, m), 3.77 (3H, s),
57 O \ I/ / 3.78-3.81 (1H, m), 4.11-4.34 (3H, m), 4.26
AcO (2H, s), 5.11 (1H, t), 5.22 (1H, t), 5.28 (1H,
~~ . ,,, t), 6.78 (3H, m), 7.04 (2H, s), 7.06 (2H, d)
Ac0 ~ OAc OAc ESI-MS(m/z):621 [M+H]+
'H-NMR(CD30D):1.32 (3H, s), 1.34 (3H,
0~ s), 3.02-3.05 (1H, m), 3.36-3.43 (4H, m),
3.67 (IH, dd), 3.75 (3H, s), 3.78 (1H, d),
58 O 3.85 (1H, dd), 4.08 (1H, d), 4.25 (2H, s),
HO 6.77 (1H, d), 6.85 (1H, d), 6.96 (1H, s), 7.06
HO~~~ ~~'OH (2H, s), 7.11 (2H, d), 8.12 (2H, d)
OH ESI-MS(m/z):453 [M+H]'
'H-NMR(CDC13):2.81 (3H, s), 3.24 (3H, s),
O O 3.431 (3H, s), 3.435 (3H, s), 3.53-3.88 (6H,
O m), 4.18-4.91 (8H, m), 4.26 (1H, d), 6.44
59 O (1H, s), 7.10 (2H, t), 7.15 (2H, s), 7.23 (1H,
~,,= -,, brs), 7.47 (1H, t), 8.14 (2H, d)
O EI-MS:616[M+]
O O )
oll
87
CA 02494177 2005-02-04
Table 20
Ex. Structure Data
'H-NMR(CD30D):2.03 (1H, t), 2.12 (IH,
0 0 brs), 2.60 (1H, brs), 2.77 (3H, brs), 3.45-
3.80 (6H, m), 3.85 (3H, s), 3.87 (3H, s),
60 Hp
'N~r O 4.23-4.33 (2H, ABq), 4.69 (1H, d), 6.52
HO~~ ~"'OH (1H, s), 7.12 (2H, t), 7.15 (2H, s), 7.21 (1H,
s), 7.49 ( I I-I, t), 8.17 (2H, d)
OH EI-MS:440 M+
'H-NMR(CDC13):1.75 (3H, s), 2.00 (3H, s),
2.03 (3H, s), 2.08 (3H, s), 3.95 (1H, m),
4.04 (3H, s), 4.05 (3H, s), 4.16 (1H, dd),
O 4.28 (1H, dd), 4.90 (1H, d), 5.08-5.18 (2H,
61 Ac0 O m), 5.26 (1H, t), 5.40-5.54 (2H, m), 6.60
p (1H, s), 7.30 (1H, t), 7.38 (1H, t), 7.46-7.55
AcO"' "'OAc p (3H, m), 7.67 (1H, m), 7.88 (1H, d), 8.09
OAc (1H, d), 8.15 (1H, d), 9.56 (1H, d)
ESI-MS(m/z):423 M+H +
'H-NMR(CDCI3):3.85 (3H, s), 3.96 (3H, s),
p 4.83 (1H, d), 4.96 (2H, s), 6.63 (1H, s), 7.23
62 p (IH, t), 7.34-7.40 (2H, m), 7.43-7.50 (2H,
HO m), 7.62 (1H, t), 7.83 (1H, d), 8.02 (1H, d),
HO~" "'OH p O 8.12 (IH, d), 9.45 (1H, d)
OH ESI-MS(m/z) :453 [M+H]+
'H-NMR(CD3OD):3.41-3.52 (2H, m), 3.57
( /~ (IH, t), 3.65-3.76 (2H, m), 3.89 (1H, dd),
p 4.00-4.03 (4H, m), 7.07-7.14 (4H, m), 7.40
63 Hp O (1H, m), 7.44-7.51 (2H, m), 7.59 (1H, s),
8.01 (1H, d), 8.10-8.18 (3H, m)
HO" "'OH FAB-MS(m/z):461[M+H]+
OH
'H-NMR(CDCI,):1.78 (3H, s), 2.02 (3H, s),
2.04 (3H, s), 2.06 (3H, s), 3.64 (3H, s), 3.72
p (3H, s), 3.80-3.92 (2H, s), 4.04 (3H, s), 4.12
(1H, dd), 4.26 (1H, dd), 4.64 (2H, s), 4.82
64 Ac0 p (1H, d), 5.24 (1H, t), 5.35 (1H, t), 5.48 (1H,
AcOIN"' "'OAc~ O~ t), 6.59 (1H, s), 6.80 (1H, d), 7.25-7.40 (3H,
m), 7.50 (1H, t), 7.66 (1H, t), 8.11 (1H, d),
OAc 9.52 (1H, d)
FAB-MS(m/z):667 M+ +
'H-NMR(CDC13):3.53 (3H, s), 3.56 (3H, s),
O 3.94 (311, s), 4.52 (2H, s), 6.62 (1H, s), 6.72
p (1H, d), 7.18 (IH, t), 7.35-7.46 (2H, m),
65 Hp 7.57 (1H, t), 8.00 (1H, d), 9.45 (1H, d)
"'OH O 0\ FAB-MS(m/z):499[M+H]+
C)" 0F
H
OH
88
CA 02494177 2005-02-04
Table 21
Ex. Structure Data
'H-NMR(CD30D):3.37-3.42 (2H, m), 3.44 (1H,
O m), 3.62 (IH, m), 3.65 (3H, s), 3.69 (1H, m),
p 3.80 (3H, s), 3.84 (1H, m), 4.37 (2H, s), 4.53
66 Hp (1H, d), 6.88 (1H, d), 7.06-7.13 (4H, m), 7.37
HO':" "'0-o (1H, d), 7.46 (1H, m), 8.12 (2H, d)
OH FAB-MS(m/z):441 [M+H]+
.1o 'H-NMR(CD30D):1.37 (3H, t), 2.98-3.10 (1H,
m), 3.30-3.70 (8H, m), 4.05 (1H, d), 4.10 (1H,
~O d), 4.58 (1H, d), 6.74 (1H, s), 6.89 (1H, s), 7.10
67 HO 0 (1H, s), 7.35 (1H, dd), 7.47 (1H, dd), 7.69 (1H,
HO" "'OH p OH dd), 8.24 (1H, d), 9.46 (1H, d)
OH FAB-MS(m/z):497[M-H]-
0 ~ \ 'H-NMR(CD30D):1.40 (3H, t), 3.07-3.16 (1H,
m), 3.36-3.72 (5H, m), 3.74 (3H, s), 4.10 (2H,
ti
68 Hp p q), 4.30 (1H, d), 4.46 (1H, d), 4.59 (1H, d), 6.81
(1H, s), 7.09-7.18 (5H, m), 7.50 (1H, dd), 8.18
~~ ,~ (2H, d)
HO OH FAB-MS(m/z):455[M+H]+
OH
p~ \ 'H-NMR(CDCl3):1.39 (3H, t), 1.48 (3H, t),
1.78 (3H, s), 1.96-2.00 (9H, m), 3.42-4.33
69 p (11H, m), 4.51-5.45 (5H, m), 6.73 (1H, s), 6.76
Ac0 (1H, s), 7.00 (1H, s), 7.37 (1H, t), 7.55 (1H, t),
O 7.72 (1H, t), 8.12 (1H, d), 9.54 (1H, d)
AcO'"'* "'OAc O FAB-MS(m/z):695[M+H]+
OAc
'H-NMR(CD30D):1.21 (3H, t), 1.31 (3H, t),
3.18-3.23 (1H, m), 3.32-3.63 (6H, m), 3.78-
3.94 (5H, m), 4.02 (2H, q), 4.25 (1H, d), 4.44
70 Hp 0
(1H, d), 6.61 (1H, s), 6.84 (1H, s), 7.04 (1H, s),
HO~~ ''OH p O 7.33 (1H, t), 7.44 (1H, t), 7.66 (1H, t), 8.19
(1H, d), 9.35 (1H, d)
OH FAB-MS(m/z):527 M+ +
p~ \ 'H-NMR(CD30D):1.23 (3H, t), 1.30 (3H, t),
2.98-3.06 (1H, m), 3.25-3.64 (5H, m), 3.87
(2H, q), 4.00 (2H, q), 4.19 (1H, d), 4.36 (1H,
71 Hp p d), 4.48 (1H, d), 6.93 (1H, s), 6.98-7.07 (5H,
m), 7.40 (1H, t), 8.08 (1H, d)
HO"' "'OH
FAB-M S (m/z) :469 [M+H]+
OH
'H-NMR(CD3OD):3.19-3.50 (4H, m), 3.60 (1H,
dd), 3.75-3.85(4H, m), 4.45-4.55 (3H, m), 6.83-
72 O 7.04 (31-m), 7.26-7.52 (3H, m), 7.60-7.74
HO (1H, m), 8.14-8.28 (1H, m), 9.29-9.41 (1H, m)
HO"' ""'OH O FAB-MS(m/z):457[M+H]+
OH
89
CA 02494177 2005-02-04
Table 22
Ex. Structure Data
'H-NMR(CD30D):3.28-3.62 (5H, m), 3.77 (1H,
dd), 4.24 (2H, s), 4.47 (1H, d), 6.96-7.17 (6H,
I m), 7.25-7.34 (1H, m), 7.40 (1H, d), 8.08 (2H,
73 HO O d)
HO"' ~',p~ FAB-MS(m/z):399[M+H]+
OH
'H-NMR(CDC13):3.58-3.97 (7H, m), 4.29-4.99
BnO (12H, m), 6.85-7.49 (33H, m), 8.08 (2H, d)
p FAB-MS(m/z):848[M+H]+
74 Bn0
BnOl"" OBn
OBn
'H-NMR(CD30D):3.37-3.59 (4H, m), 3.70 (IH,
HO dd), 3.82 (1H, dd), 4.23 (2H, s), 4.56 (1H, d),
p 6.76 (1H, d), 7.02-7.16 (5H, m), 7.29 (1H, d),
75 Hp 7.49 (1H, dd), 8.17 (2H, d)
HO~" ~''OH FAB-MS(m/z):397[M+H]+
OH