Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
1
A material for structural components of an electrowinnin; cg ell for
praduction of metal
Field of Invention
The present invention relates to a material that can tie used for structural
components in
a cell for the electrolysis of alumina dissolved in a fluoride containing
molten salt bath
by the use of essentially inert electrodes.
Background Art
Conventionally, aluminium is produced by the electrolysis of alumina dissolved
in a
cryolite based molten salt bath by the more than hundred years old Hall-
Heroult process.
In this process carbon electrodes are used, where the carbon anode is taking
part in the
cell reaction resulting in the simultaneous production of CO2. The gross
consumption of
the anode is up to 550 kg/tonne of aluminium produced, causing emissions of
green-
house gases like fluorocarbon compounds in addition to COZ. For both cost and
environ-
mental reasons the replacement of carbon anodes with an effectively inert
material
would be highly advantageous. The electrolysis cell would then produce oxygen
and
aluminium.
An earlier, not yet disclosed Norwegian patent application No. 2001-0927
describes the
development and design of a novel electrowinning cell for aluminium
production. The
novel cell is based on vertical electrode technology and a two chamber
electrolysis cell
for separation of produced metal and evolved oxygen gas. The cell concept
requires that
certain structural elements are made of materials that must fulfil their
functional require-
ments at elevated temperatures in an environment of a molten fluoride-based
electrolyte.
In some regions of the cell an additional requirement is that the materials
must fulfil
their functional requirements in contact wrth liquid aluminium, while in other
regions
the materials must fulfil their functional requirements in contact with pure
oxygen gas at
a pressure of about one bar.
Object of the Invention
The object of the present invention is to identify a material that is stable
at an oxygen
partial pressure of 1 bar at temperatures above about 680°C and has a
sufficiently low
solubility in the electrolyte to be used as a material for structural cell
components in
SUBSTITUTE SHEET (RULE 26)
'''~y ~~,~~ ~~ !~~~', ~~ ~ CA 02496518 2005-02-22
~~ ~~e
~~ ~7a ~2~04 ~I~~O~ ~ Ia
a~:r.~ u~u~~.~'.aS'r~ I'h,~.~J.~ ~ ':,rs'~ ~'ij~ sa~'9~x ~ ~
15/07 ' 04 11: 34 FAg +4722532308 HYDRO PATEIV'r DEP -~ EPO M~NCHEN 1003
C ~C~a ~ Co~~
2a
oxidizing regions of an aluminium electrowinning cell based on substantially
inert
electrodes.
Summary of the Invention
The invention is the conclusion of an extensive search for materials capable
of
fulfilling the requirements for a material for structural cell components in
oxidizing
regions of an aluminium electrowinning cell based on substantially inert
electrodes.
The stability requirements of such a material are similar to those of an inert
anode in
said eleetrowinning cell. In the not yet published Norwegian Patent
Application No.
IO 2001-0928 the choice of possible element oxides for an inert anode is
narrowed to:
TiOz, Crz03, Fez03, MnzO3, CoO, NiO, CuO, ZnO, AlzO3, Gaz03, ZrOz, SnO~z and
I~fOz. The main requirements for a material intended for use in structural
cell
components ate stability at I bar oxygen pressure at temperatures above 680~C
and a
law solubility in the molten electrolyte. The electrical properkies are less
important,
but its electrical conductivity should be far less that the electrical
conductivity of the
electrodes and the electrolyte. The material should either itself fulfil the
requirements,
or it should upon contact with the molten electrolyte react to form a siuface
layer of
an aluminate that fulfils the said requirements. Based an solubility
considerations,
CuO, Gaz03, ZrOz and HfOz are eliminated from the list of possible element
oxides,
and we are left with: TiOz, Cr2O3, Fez03, MnzO3, CoO, NiO, ZnO, AlzO3, and
SnOz.
The evaluation leads to a family of materials that can be expressed by the
formula
/A11_uAnu~x~ ~l_yB y~Y~~-"1-W~,nw~z~t
in which A' and A" are elements from the group Co, Ni, or Zn, B' and B" are
elements
from the group Al, Cr, Mn, or Fe, and C' and C" are the elements Ti or Sn. O
is the
element oxygen. OSu<l, 0<_v<1, OSw<1 1<x<2, 0<_y<2, 0~1, and t is a number
that
renders the composition charge neutral.
Within this groug of oxides, materials most commonly crystallize in the
spinel,
ihnenite or rock salt structures. In materials of the present invention that
possess the
a ,~r, iy r.~
~~~~~u~ d ,r~'p , ~~ ~ ~~..~~.
Empf.zeit:15/0712004 12:30 ~~ ~ a,~.~~~ a ~.~,..~ ~",~~~~ :,..~,~',"~~.819
P.003 ..
w CA 02496518 2005-02-22 ~~,=.~~sr~,~~w, ,'~~~ '~~
f~' ' ~~gw ~i',~~1~' ~~
15%U7 ' 04. 11: 34 FAg +4722532308 HYDRO PATENT BEP -~ EPO ~i~NCHEN ~~~ 004
2b
spinel structure, x+y+z = 3, 2x + 3y + 4z = 8, and t = 4. 2n materials of the
present
invention that possess the iImenite structure, x + y + z = 2, 2x + 3y + 4z = 6
and t = 3.
In materials of the present invention that possess the rock salt structure, x
=1, y ~ z =
0, and t = 1.
Detailed Description of the Invention
A material suitable as an essentially inert material for structural components
in the
oxidizing regions of a cell for the electrolytic production of aluminium from
alumina
dissolved in an essentially fluoride based electrolyte where cryolite is an
important
ingredient, must be resistant to oxidation and dissolution in the electrolyte.
A
selection of the element oxides which a material for structural components can
consist of, was performed based on the following criteria:
-not a gas or having a high vapour pressure at process temperature
-not converted by cryolite or A1F3 in the cryolitic mixture, i.e, a large
positive
value of ~G° for the reaction between the element oxide and A1F3 to
form the
element fluoride and aluminium oxide (reaction 1).
MOx + 2x/3A1F3 = MFG+2x/6A1z03 , (I)
-not converted by alumina, i.e. riot a negative value of ~G° for the
reaction between
the element oxide aluminium oxide and sodium fluoride to fog a sodium element
oxide and aluminium fluoride (reaction 2)
MOx + 6yNaF + yAI2O3 = NasyMOx+3y + 2yA1F3 (2)
Of elements with the normal valence 2, the only possible elements are thus Co;
Ni;
Cu and Zn. Of elements with valence 3 one is Left with only;the elements Cr,
Mn, Fe,
Ga and A.l. Of elements with valence 4 one is 'left with only the elements Ti,
Zr, Hf,
Ge and Sn. Cu, Ga, Zr, Hf and Ge may be eliminated from the list based on
solubility
considerations, and we are left with the following list of elements: Co, Ni,
Zn, Al, Cr,
Mn, Fe, Ti and Sn. The possible materials for structural cell components in an
~' ~W~~'f'~
$19P00
Em~f .zei t:15107/2004 12:30 v~~'"'~ ~~~~u~~~~ i~I~'~ j~.~,~'~~':
r;n.."..~,I.:~E",,,-:~..c-,,E;u.~:w~, CA 02496518 2005-02-22 '~A'~'~~'a''
''E"~'.-~~"~~f.
.~ I!-
~a~q~n~~~~~ ~~ ~ a~u
~ ~ ~ ~ "~r'~~Q~~~~~ AF,..
~t~n~,i~3tf'~:~~~~:f~,",~F!.~r~,'.~i.~~ 'i~~~:~s~~.~!"i.xJ~r'~~'t?a~'src"~r !:
15/07 '.04 11:34 FAX +4722532308 aYDRO PATENT DEP j EPD ~tONCHEN I~j005
2c
aluminium electrowinning cell based on substantially inert electrodes are thus
limited
to the oxides of the listed elements, or combinations of these oxides in mixed
oxide
compounds.
The materials within this group can be expressed by the formula:
(.A~1 uA"u~(B~1 yB,y)x(C,~_wCnw)z~t (a)
in which A' and A" are divalent elements from the group Co, Ni, or Zn, B' and
B" are
trivalent elements from the group Al, Cr, Mn, or Fe, and C' and C" are the
tetravalent
elements Ti or Sn. O is the element oxygen. OSu<l, 0_<v<l, 0_<w<x 1~, 0_<yS2,
OSz<_l, and t is a number that renders the composition charge neutral.
Under favourable conditions the divalent oxides NiO, Co0 and 26°n~ all
react with
alumina tv form an essentially insoluble surface aluxninate layer (reaction
3).
A~(s) + A12~3 (disc) = AAlz04 (s) (3)
where A = Cv, Ni, Zn. Therefore, CoC, Ni0 and ~n~ and solid solutions of these
form one group of possible materials for structural cell components. These
compositions are expressed by formula (a) with x = l, y = z = 0, and t =1.
This is
further illustrated in Examples 1 and 2.
Compounds of di- and trivalent element oxides will in this case be of the
spinet
stricture. SpineIs like NiFez04., CoFez04, NiCrz04 and CoCrz04 have been
suggested
and extensively tested as candidates for inert anodes. In these materials, A1
from the
molten electrolyte has been observed to exchange with the trivalent canon to
form
, essentially insoluble, insulating solid solutions of the type
Ni(B'1_"Al")zU4, where
0<v<l, B' = Fe, Cr, Mn. This is further illusfrated in Examples 3, 4; and 6.
These
materials are thus possible matezials for structural cell. components. The
pure
aluminates NiA1z04, CoAi2O4 and 7nA_1204 are also possible materials for
stnzctural
cell components. '
~'t 4i ni lull t Btu ~ ~i~r ",
Em~f.zeit: : ~~~..~;..~~~.a.~l,~~~~~~,~!y.g~9 P.005
15/07'2004 12 3Q d~~~i~ r~ .~ ~~ .
.:~r~a'~Y~:.,~y,.,~e;.;:~:r,~.~~«<,,-s-~,' CA 02496518 2005-02-22
~"~'~=S~~'~x~I~'~~~~'.t"''~..'~
~ ~'~~, ixrs~~~ . r~'' '~ ~x
5n~~0~t'2~~()0 ~h~'(~~~~10~~'$
'~~'" ~ '~"~'"~w'~u'~'~ 11:35 FAg i-4722532308 HYDRO PATENT DEP -~ EPO
M~fNCHEN- "'c ~~ti
:J ~ ~tl J
2d
One compound of di- and tetravalent element oxides, Zn2SnOa, forms a spinet
oxide.
This material may in principle be used for structural cell components.
Other stable spinet compositions that are possible materials for structural
components
of an aluminium electrowinning cell are achieved by substituting a
divalent/trivalent
spinet with a tetravalent oxide, while simultaneously adjusting the contents
of the
divalent and trivalent oxides in order to maintain the site and charge balance
requirements of the spinet structure. This embodiment of the present invention
is
I0 exemplified in Example S.
Spinet type materials thus form another subset of materials for structural
components
of aluminium electrowinning cells. These compositions are expressed by formula
(a),
withx+y+z=3,2x+3y+4z=~,andt=~..
NiTiO3, CoTiO3 and solid solutions of these crystallize with the ilmenite
structure. A
cell material with the ilmenite structure may also be obtaizied by
substitution of a
trivalent element from the list of possible elements for equimoIar amounts of
divalent
and tetravalent elements. These compositions are expressed by formula (a) with
x + y
+z=3,Zx+3y+4z=6,andt=3. ,
s
i
I
~o r ' i i ~ m i a n,-;
Empf .ze i t :15/07!2004 12: 31 ~_~~ '~ :.~ ~ ~. pe'~ ~;,~sf ~~~~~, '
A ~~wy,~ ~ .~'v~ :.818 P.006
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
The invention shall in the following be further described by figures and
examples
where:
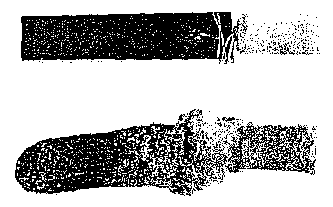
5 Figure 1: Shows a photograph of a sample of a material for structural
components in an
electrolysis cell before and after the stability test of Example 3.
Figure 2: Shows a backscatter SEM photograph of the reaction zone of a
Ni,.,Cr204
material after 50 hours of exposure to molten fluoride electrolyte under
anodic
polarization.
Figure 3: Shows a backscatter SEM photograph of a NiFeCr04 sample after 50
hours of
exposure to molten fluoride electrolyte under anodic polarization.
Figure 4: Shows a backscatter SEM photograph of a sample of Nil.stXFeTio.s-X04
after
the stability test of Example 5.
Figure 5: Shows a backscatter SEM photograph of a Ni,.o~FezOa sample after 30
hours
of exposure to molten fluoride electrolyte under anodic polarization.
Example 1:
Test of the stability of a Ni0 sample anodically polarized in a molten
fluoride
electrolyte.
A cermet with 75 wt% Ni0 and 25 wt% Ni was prepared using 1NC0 Ni powder type
210, and Ni0 from Merck, Darmstadt. The material was sintered in argon
atmosphere at
1400°C for 30 min.
The sample was exposed to a molten fluoride bath under anodic polarization in
order to
ensure a partial pressure of 1 bar oxygen on the sample surface. The
electrolyte was
contained in an alumina crucible with inner diameter 80 mm and height 150 mm.
An
outer alumina container with height 200 mm was used for safety, and the cell
was
covered with a lid made from high alumina cement. In the bottom of the
crucible a 5
mm thick TiB2 disc was placed, which made the liquid aluminium cathode stay
horizon-
tal. The electrical connection to the cathode was provided by a TiBz rod
supported by an
alumina tube to avoid oxidation. A platinum wire provided electrical
connection to the
TiB~ cathode rod. A Ni wire provided for the electrical connection to the
anode. The Ni
wire and the anode above the electrolyte bath was masked with an alumina tube
and
alumina cement to prevent oxidation.
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
6
340 g Al, (99.9% pure), from Hydro Aluminium was placed on the TiB~ disc at
the
bottom of the alumina crucible.
The electrolyte was made by adding to the alumina crucible a mixture of
532 g Na~AIF~ (Greenland cryolite)
105 g AIF3 (from Norzink, with about 10 % A1203 )
35 g A120; (annealed at 1200°C for some hours)
21 g CaFz (Fluka p.a.)
The sample of the material for structural cell components was suspended above
the
electrolyte during heating of the cell. The temperature was maintained at
970°C during
the whole experiment. The sample of the material for structural cell
components was
lowered into the molten electrolyte and polarized anodically with a current
density of
750 mAlcm2 based on the bottom end cross sectional area of the sample. The
real
current density was somewhat lower because the side surfaces of the anode were
also
dipped into in the electrolyte.
The experiment lasted for 8 hours. XRD (X-ray diffraction) analysis of the
anode after
the experiment showed that the Ni metal was oxidized to Ni0 and the anode
material
was covered by an dense, protective, insulating layer of NiA1204.
Example 2:
Test of the stability of a Zn0 sample anodically polarized in a molten
fluoride
electrolyte.
Zn0 was doped with 0.5 mol% AIO,.s. Two Pt wires were pressed into the
material in
the longitudinal axis of the Zn0 anode and acted as electrical conductors. The
material
was sintered at 1300°C for 1 hour.
The stability test was performed in the same manner as described in Example 1.
The
amounts of electrolyte and aluminium were the same. The temperature was
970°C. The
current density was set to 1000 mA/cm2 based on the bottom end cross sectional
area of
the sample The electrolysis experiment fasted for 24 hours. XRD (X-ray
diffraction)
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
7
analysis of the sample after the electrolysis experiment showed that Zn0 had
been
converted to ZnAlzOa during electrolysis.
Example 3:
Test of the stability of a Ni,,xCrz04 sample anodically polarized in a molten
fluoride electrolyte.
The starting powder was prepared by a soft chemistry route. The appropriate
amounts of
Ni(N03)z, and Cr(NO~)3 were complexed with citric acid in dilute nitric acid.
After
evaporation of excess water, the mixture was pyrolysed and calcined at
900°C for 10
hours. The sample was cold isostatically pressed at 200 MPa, then sintered at
1440°C
for 3 hours. The material was found by XRD to possess the spinel structure.
The stability test was performed in the same manner as described in Example l,
but a
platinum wire provided electrical connection to the sample. The platinum wire
to the
sample was protected by a 5 mm alumina tube. When the electrolysis started the
anode
was dipped approximately 1 cm into the electrolyte. A photograph of the sample
before
and after electrolysis is shown in Figure 1.
The electrolyte, temperature and current density were the same as described in
Example
2.
The stability test lasted for 50 hours. After the experiment the sample was
cut, polished
and examined in SEM (Scanning Electron Microscope). A reaction zone could be
seen
between the Ni~_~Crz04 - material and the electrolyte. Figure 2 shows the
backscatter
SEM photograph of the reaction zone. On the photograph one can see a reaction
zone
that has propagated along the grain boundaries of the Ni,.,CrzOa material. The
white
particles are NiO.
In the table below the relative EDS analysis results are reported. Ni, Cr, Al,
and O were
the only elements detected. The aluminium present in the interior of the
grains might be
due to the preparation of the sample for analysis.
Relative comparison between the elements Ni, Cr and Al;
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
8
Clement: Atom olo in the centre of the grains Atom °lo in the reaction
zone in
in Figure 2: grain boundaries in Figure 2:
Ni 33 47
Cr 66 8
Al 1 45
The SEM analysis shows that the reaction product consisted of a material where
the
chromium atoms were partly exchanged with aluminium atoms as described by the
formula NiCr2-XAlX04 where x varies from 0 to 2. The reaction product forms an
insulat-
ing coating.
Example 4:
Test of the stability of a NiFeCrOa sample anodically polarized in a molten
fluoride
electrolyte.
The starting powder was prepared by a soft chemistry route. The appropriate
amounts of
Ni(N03)z, Fe(N03)3 and Cr(N03)3 were complexed with citric acid in dilute
nitric acid.
After evaporation of excess water, the mixture was pyrolysed and calcined at
900°C for
10 hours. The sample was cold isostatically pressed at 200 MPa, then sintered
at 1600°C
1S for 3 hours. The material was found by XRD to possess the spinet structure.
The stability test was performed in the same manner as described in Example 3.
The
amounts of electrolyte and aluminium were the same. The current density was
set to
1000 mA/em2 based on the cross sectional area of the rectangular sample. The
experi-
ment lasted for 50 hours. Examinaton of the sample after exposure to molten
fluorides
under anodic polarization showed a several micron thick reaction layer where
Cr in the
material was partly exchanged with Al atoms. A backscatter SEM photograph of
the
reaction Iayer is shown in Figure 3. Light grey areas consist of original
NiFeCr04
material. Medium grey area contains almost no Cr atoms and a much lower
content of
Fe.
EDS analysis of the medium grey reaction layer shown in Fig. 3 compared to
original
NiFeCr04 material and the inner of the anode light grey area also shown in
Fig. 3 are
summarized in table below. The only elements detected were Ni, Cr, Fe, A1 and
O.
Comparison of the relative amounts of Cr, Fe, Ni and Al:
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
9
I:le~nent: Atom % in the original NireCrO, Atom % in the reaction layer after
material. Light grey area in rig. 3. the test. Medium grey area in Fig. 3.
Cr 33.3 0
Fe 33.3 16
Ni 33.3 35
A1 0 49
The conclusion of the stability test is that the NiFeCr04 material reacts with
alumina in
the electrolyte and forms a dense, essentially insoluble, insulating layer of
NiFe,_xAl,+xO4.
Example 5:
Test of the stability of a Ni,.s~xFeTio.s-xOa sample anodically polarized in a
molten
fluoride electrolyte.
The starting powder was prepared by a soft chemistry route. The appropriate
amounts of
Ni(N03)z, Fe(N03)3 and TiOsH,aC,o (titanyl acetylacetonate) were complexed
with citric
acid in dilute nitric acid. After evaporation of excess water, the mixture was
pyrolysed
and calcined at 900°C for 10 hours. The sample was cold isostatically
pressed at 200
MPa, then sintered at 1500°C for 3 hours. The material was found by XRD
to possess
the spinel structure.
The stability test was performed in the same manner as. described in Example
3. The
amounts of electrolyte and aluminium were the same. The current density was
set to
1000 mA/cmz based on the cross sectional area of the rectangular sample. The
experi-
ment lasted for 30 hours. After the experiment the sample was cut, polished
and
examined in SEM. The backscatter photo in Fig. 4 shows the end of the sample
facing
the cathode. In this experiment no reaction layer was detected on the
Ni,,s+xFeTio.s-XOa
anode after 30 hours.
Example G:
Test of the stability of a Ni,.o~FezOa sample anodically polarized in a molten
fluoride electrolyte.
The starting powder was prepared by a soft chemistry route. The appropriate
amounts of
Ni(N03)z, and Fe(N03)3 were complexed with citric acid in dilute nitric acid.
After
evaporation of excess water, the mixture was pyrolysed and calcined at
900°C for 10
hours. The sample was cold isostatically pressed at 200 MPa, then sintered at
1450°C
for 3 hours. The material was found by XRD to possess the spinet structure.
CA 02496518 2005-02-22
WO 2004/018738 PCT/N02003/000278
The stability test was performed in the same manner as described in Example 3.
The
amounts of electrolyte and aluminium were the same. The current density
wasw::~~t to
1000 mA/cmZ based on the cross sectional area of the rectangular anode. The
expeli-
5 ment was stopped after 30 hours. After the experiment the sample was cut,
polished and
examined in SEM. Figure 5 shows a backscatter photograph of the sample at the
end
facing the cathode. An approximately 10 micron thick reaction layer is seen.
A line scan EDS analysis was done to examine whether the layer was a reaction
layer or
10 electrolyte adhering to the surface. The line scan indicated a thin layer
of bath compo-
vents, and then a reaction layer of approximately 10 micron thickness. In the
interior of
the anode and in the reaction layer only oxygen was detected in addition to
Ni, Fe and
AI. The results are reported in the table below:
Comparison of the relative amounts of Ni, Fe and Al:
Element: Atom % of element in the interior of Atom % of element in the
reaction
the anode shown in h'igure 5 and layer as shown in h'igure 5 and
analysed with line scan EDS: analysed with line scan EDS:
Ni 33 30
Fe 67 30
Al 0 ~ 40
In the 10 micron thick reaction layer the iron atoms were partly exchanged
with alumin-
ium atoms to form an essentially insoluble, insulating layer of NiFe2_xAlx04.
1