Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02501474 1994-06-13
-1-
APPARATUS AND METHOD FOR
SUPPRESSING A FIRE
This is a division of Canadian Patent Application No. 2,165,320 filed June 13,
S 1994.
This invention relates to an apparatus and a method for suppressing a fire.
More particularly, a gas generator produces an elevated temperature first gas
which
interacts with a vaporizable liquid to generate a second gas having flame
suppressing
capabilities.
Fire involves a chemical reaction between oxygen and a fuel which is raised to
its ignition temperature by heat. Fire suppression systems operate by any one
or a
combination of the following: (i) removing oxygen, (ii) reducing the system
temperature, (iii) separating the fuel from oxygen, and (iv) interrupting the
chemical
reactions of combustion. Typical fire suppression agents include water, carbon
dioxide,
dry chemicals and the group of halocarbons collectively known as Halons.
The vaporization of water to steam removes heat from the fire. Water is an
electrical conductor and its use around electrical devices is hazardous.
However, in
non-electrical situations, when provided as a fine mist over a large area,
water is an
effective, environmentally friendly, fire suppression agent.
Carbon dioxide (COz) gas suppresses a fire by a combination of the
displacement of oxygen and absorption of heat. Carbon dioxide gas does not
conduct
electricity and may safely be used around electrical devices. The carbon
dioxide can be
stored as compressed gas, but requires high pressure cylinders for room
temperature
storage. The cylinders are heavy and the volume of compressed gas limited.
Larger
quantities of carbon dioxide are stored more economically as a liquid which
vaporizes
CA 02501474 1994-06-13
-2-
when exposed to room temperature and atmospheric
pressure.
When exposed to room temperature and atmospheric
pressure, the expansion characteristics of liquid COz
are such that approximately one third of the vessel
charge freezes during the blow down process. Only
about two thirds of the C02 is exhausted in a
reasonable time.' The remainder forms a dry ice mass
which remains in the storage vessel. While the dry
ice eventually sublimes and exits the vessel, the
sublimation period is measured in hours and is of
little use in fire suppression.
The problem with liquid carbon dioxide based
fire suppression systems is worse when low
temperature operation is required. At -55°C, the
vapor pressure of carbon dioxide is about 0.48 MPs
(70 psigj (compared to 4.8 MPs (700 psig) at 20°C)
which is totally inadequate for rapid expulsion.
The vessel freeze-up problem is worse. About 50% of
the liquid carbon dioxide solidifies when exposed to
-55°C and atmospheric pressure.
Improved carbon dioxide suppression systems add
pressurized nitrogen to facilitate the rapid
expulsion of carbon dioxide gas at room temperature.
The pressurized nitrogen does not resolve the
freezing problem at low temperatures and at upper
service extremes, about 70°C, the storage pressure
is extremely high, dictating the use of thick,
heavy, walled storage vessels.
Chemical systems extinguish a fire by separating
the fuel from oxygen. Typical dry chemical systems
include sodium bicarbonate, potassium bicarbonate,
ammonium phosphate and potassium chloride. Granular
CA 02501474 1994-06-13
-3-
graphite with organic phosphate added to improve
effectiveness, known as G-1 powder, is widely used
on metal fires. Other suitable dry compounds
include sodium chloride with tri-calcium phosphate
added to improve flow and metal stearates for water
repellency, dry sand, talc, asbestos powder,
powdered limestone, graphite powder and sodium
carbonate. Dry chemical systems are delivered to a
fire combined with a pressurized inert gas or
manually such as with a shovel. The distribution
system is inefficient for large fires and a
significant amount of time is required to deliver an
effective quantity of the dry powder to suppress a
large fire.
The most efficient fire suppression agents are
Halons. Halons are a class of brominated
fluorocarbons and are derived from saturated
hydrocarbons, such as methane or ethane, with their
hydrogen atoms replaced with atoms of the halogen
elements bromine, chlorine and/or fluorine. This
substitution changes the molecule from a flammable
substance to a fire extinguishing agent. Fluorine
increases inertness and stability, while bromine
increases fire extinguishing effectiveness. The
most widely used Halon is Halon 1301, CFjBr,
trifluorobromomethane. Halon 1301 extinguishes a
fire in concentrations far below the concentrations
required for carbon dioxide or nitrogen gas.
Typically, a Halon 1301 concentration above about
3.3% by volume will extinguish a fire.
Hslon fire suppression occurs through a
combination of effects, including decreasing the
available oxygen, isolation of fuel from atmospheric
CA 02501474 1994-06-13
-4-
oxygen, cooling and chemical interruption of the
combustion reactions, The superior fire suppression
efficiency of Halos 1301 is due to its ability to
terminate the runaway reaction associated with
combustion. The termination step is catalytic for
Halos 1301 due to the stability of bromine radicals
(Br~) formed when Halos 1301 is disposed on a
combustion source.
When unreacted Halos 1301 migrates into the
stratosphere, sunlight breaks down the Halos 1301
forming bromine radicals. Hr~ then reacts to
consume ozone in an irreversible manner.
Br~ + 03 ---> Bro~ + 02
In view of the current recognition that ozone
depletion is a serious environmental problem, a move
is on to identify: (i) fire suppression agents
having a less severe environmental impact than Halos
and (ii) devices to deliver these more
environmentally friendly agents.
Accordingly, it is an object of the invention to
provide a fire suppression apparatus for effectively
delivering a fire suppressant which is less
environmentally hazardous than Halos. It is a
feature of the invention that the apparatus
effectively delivers both liquid and solid fire
suppressants. It is an advantage of the invention
that the apparatus does not require significantly
more space than Halos fire suppression apparatus. A
further advantage of the invention is that both high
and low vapor pressure liquids are effectively
stored, vaporized and delivered in gaseous form.
CA 02501474 1994-06-13
According to one aspect of the invention, there is
provided an apparatus for suppressing a fire, comprising:
a) a gas generator comprising a housing supporting a
mixture of a nitrogen-rich fuel and an oxidizing agent as a
solid propellant that generates a high temperature gas
selected from the group consisting of nitrogen, carbon
dioxide, water vapor and mixtures thereof on ignition;
b) magnesium carbonate disposed between the housing and
the solid propellant;
c) a conduit directing the high temperature gas to a
chamber;
d) a vaporizable liquid contained within the chamber
that vaporizes on interaction with the high temperature gas;
and
e) a passageway between the chamber and the fire to
deliver the vaporized liquid to the fire.
According to another aspect of the invention, there is
provided an apparatus for suppressing a fire, comprising:
a gas generator containing a propellant and a fire
suppressant as a mixture of compacted powders with the
average diameter of a fire suppressant particle being larger
than the average diameter of a propellant particle; and
a passageway between the gas generator and the fire.
CA 02501474 1994-06-13
5a
According to a further aspect of the invention, there
is provided an apparatus for suppressing a fire, comprising
a gas generator containing a propellant that is a
mixture of a fuel, strontium nitrate as an oxidizer, and
magnesium carbonate, wherein the magnesium carbonate content
of the propellant is at least 35~ by weight that is
,sufficient to inhibit the generation of effluent by-products
that are corrosive to aluminum; and
a passageway between the gas generator and said fire.
According to still a further aspect of the invention,
there is provided an apparatus for suppressing a fire,
comprising:
a gas generator containing a propellant and a fire
suppressant;
a tank containing a mixture of water and ice;
a first conduit providing a passageway between the gas
generator and the tank; and
a second conduit providing a passageway between the
tank and the fire.
According to yet another aspect of the invention, there
is provided an apparatus for suppressing a fire, comprising:
a) a gas generator having a combustive propellant
effective to produce a gas yield in excess of 1.5 moles per
100 grams of propellant;
b) a packed powder contained within a chamber and
selected from the group consisting of magnesium
CA 02501474 1994-06-13
5b
hydroxide, calcium hydroxide, strontium hydroxide, barium
hydroxide, aluminum hydroxide, magnesium carbonate,
potassium sulfate, and mixtures thereof;
c) a first conduit providing a passageway between the
gas generator and the chamber; and
d) a second conduit providing a passageway between the
chamber and the fire.
The above stated objects, features and advantages will
become more apparent from the specification and drawings
which follow.
Figure 1 illustrates in cross-sectional representation
an apparatus for vaporizing a liquid to a flame suppressing
gas in accordance with a first embodiment of the invention.
Figure 2 illustrates in cross-sectional representation
an apparatus for vaporizing a liquid to a flame suppressing
gas in accordance with a second embodiment of the invention.
Figure 3 illustrates in cross-sectional representation
an apparatus for delivering a dry chemical flame suppressant
to a fire.
Figure 4 illustrates in cross-sectional representation
a carbon dioxide producing gas generator.
CA 02501474 1994-06-13
-6-
Figure 5 graphically illustrates increasing the
magnesium carbonate content in the gas generator
reduces the formation of corrosive effluent.
Figure 6 graphically illustrates the
relationship between pressure and density for ice
and water.
Figure ~ illustrates in cross sectional
representation a water based fire suppression system
in accordance with the invention.
Figure 1 shows in cross-sectional representation
a fire suppression apparatus 10 in accordance with a
first embodiment of the invention. A gas generator
12 containing a suitable solid propellant 14
delivers an elevated temperature first gas 16 to a
vaporizable liquid 18 contained in a chamber 20. A
first conduit 22 provides a passageway between the
gas generator 12 and the chamber 20. The first gas
16 interacts with the vaporizable liquid 18
converting the liquid to a second gas 24. By proper
selection of the vaporizable liquid 18, the second
gas has flame suppressing capabilities. A second
conduit 26 directs the second gas 24 to a fire. An
optional aspirator 28 uniformly distributes the
second gas 24 over a wide area.
The fire suppression apparatus 10 is permanently
mounted in a ceiling or wall of a building, aircraft
or other suitable structure or vehicle. A sensor 30
detects the presence of a fire. Typically, the
sensor 30 detects a rise in temperature or a change
in the ionization potential of air due to the
presence of smoke. On detecting a fire, the sensor
30 transmits an activating signal to a triggering
mechanism 32. The activating signal may be a radio
CA 02501474 1994-06-13
pulse, electric pulse transmitted by wires 34 or
other suitable means.
The triggering mechanism 32 is any device
capable of igniting the solid propellant 14. One
triggering mechanism is an electric squib. The
electric squib has two leads interconnected by a
bridge wire, typically o.076mm-O.lOmm (3-4 milj
diameter nichrome. When a current passes through
the leads, the bridge wire becomes red hot, igniting
l0 an adjacent squib mixture, typically, zirconium and
potassium perchlorate. The ignited squib mixture
then ignites an adjacent black powder charge,
creating a fire ball and pressure shock wave which
ignites the solid propellant I4 housed within the
gas generator 12.
The gas generator 12 contains a solid propellant
l4 which on ignition generates a large volume of a
high temperature gas containing fire suppressing
fluids such as carbon dioxide, nitrogen and water
vapor. Depending on the selection of the
vaporizable liquid and the type of fire anticipated
as requiring suppression, the gas is generated for a
period of time ranging from a few milliseconds to
several seconds. One particularly suitable gas
generator is the type used in automotive air bags as
described in U.S. Patent No. 3,904,221 to Shiki et
al. A housing 36 supports the solid propellant 14
and directs an explosive shock wave in the direction
of the vaporizable liquid 18. Typical materials for
the housing 36 include aluminum alloys and stainless
steel.
The preferred solid propellant 14 is a
combustible mixture which generates a copious amount
CA 02501474 1994-06-13
-8-
of high temperature gas. The chemical reactions
converting the propellant to the first gas generally
do not occur efficiently at temperatures below about
1093°C (2000°F). The gas yield in moles per 100
grams of propellant should be in excess of about 1.5
moles and preferably in excess of about 2.0 moles.
The propellants are generally a mixture of a
nitrogen rich fuel and an oxidizing agent in the
proper stoichiometric ratio to minimize the
l0 formation of hydrogen and oxygen. The preferred
fuels are guanidine compounds, azide compounds and
azole compounds.
Two preferred solid propellants are ~~RRC-3110'~
and "FS-01" (both available from Olin Aerospace
Company of Redmond, Washington, United States of
America). The compositions (in weight percent) of
these propellants are:
RRC-3110
5-Aminotetrazole 28.621
Strontium nitrate 57.38
Clay 8.001
Potassium 5-Aminotetrazole 6.00
When ignited, RRC-3110 generates H=0, NZ and COZ
as well as SrO, SrC03 and K~COj particulate.
FS-O1
5-Aminotetrazole 29.20
strontium nitrate 50.80
Magnesium carbonate 20.00
When ignited, FS-O1 generates HZO, NZ and C02 as
well as SrO, SrC03 and Mg0 particulate.
CA 02501474 1994-06-13
_g_
Another useful propellant composition is:
Guanidine nitrate 49.50%
Strontium nitrate 48.50%
Carbon 2.00%
When ignited, this composition releases a
mixture of HZO, Nz and COZ gases along with Sr0 and
SrC03 particulate solids.
Propellants which generate KCl salt are also
suitable. KC1 is effective in suppressing fires,
but the corrosive nature of the salt limits the
application of these propellants. Two such
propellants are:
5-Aminotetrazole 30.90%
Potassium perchlorate 44.10%
Magnesium carbonate 25.00%
When ignited, this propellant generates HZO, N2
and C02 gas as well as KC1 and Mg0 particulate.
Potassium chlorate 61.0%
Carbon 9.0%
Magnesium carbonate 30.0%
When ignited, this propellant generates COZ as
the only gas and KC1 and Mg0 particulate.
Another suitable propellant generates nitrogen
gas and solid slag which remains in the housing 36,
only the gas is delivered to the vaporizable liquid
eliminating contamination of the area by the solid
particulate.
Sodium azide 59.1%
Iron oxide 39.4%
CA 02501474 1994-06-13
~10~
Potassium nitrate 1.0%
Carbon 0.5%
When ignited, this propellant generates N2 gas
and slag which is not discharged from the housing.
The propellants useful in the apparatus of the
invention are not limited to the five specified
above. Any solid propellant capable of generating
similar gaseous products at high velocity and~high
temperature is suitable.
The most preferred propellants conta3,~h magnesium
carbonate as a suppressing agent. The m~gnesium
carbonate may be combined with a fuel, a~ in the FS~
01 propellant, combined with other suppressing
agents or utilized as a single component fire
suppressing propellant. The magnesium carbonate
endothermically decomposes to carbon dioxide (a good
oxygen displaces) and magnesium oxide (a good heat
sink and coolant).
Suitable propellants contain from that amount
effective to extinguish a fire up to about 95% by
weight magnesium carbonate and the balance being the
mixture of a fuel and an oxidizer. Preferably, the
propellant contains from about 20% to about 70% by
weight magnesium carbonate and most preferably, from
about 30% to about 60% by weight magnesium
carbonate.
When the magnesium carbonate content is low,
propellants containing strontium nitrate yield
effluent rich in strontium oxide. On exposure to
atmospheric moisture, this yields extremely basic
solutions that are corrosive to aluminum and other
materials utilized in aircraft manufacture. With
CA 02501474 1994-06-13
-il-
reference to Figure 5, applicant has determined a
minimum magnesium carbonate content of'about 35% is
desired to minimize the corrosion potential.
Propellant additives such as magnesium carbonate
act as endothermic heat sinks and carbon dioxide
generators. These effects decrease the corrosivity
of propellant effluent by minimizing the amount of
strontium oxide generated. Figure 5 graphically
illustrates the composition of the gaseous effluent
generated by igniting the FS-01 fuel with varying
amounts of magnesium carbonate present. The
strontium oxide content is identified by reference
line 80. Approximately 35 weight percent magnesium
carbonate is required to achieve an essentially
strontium oxide free effluent.
Strontium carbonate (reference line 82) and
magnesium oxide (reference line 84) form compounds
with a pH near 7 when exposed to atmospheric
moisture and generally do not cause significant
corrosion.
A preferred propellant contains a nitrogen rich
fuel, an oxidizer and magnesium carbonate. Suitable
propellants include modifications of FS-01
containing 5-aminotetrazole and an oxidizer, such as
strontium nitrate, potassium perchlorate or mixtures
thereof. The fuel to oxidizer ratio, by weight, is
from about 1:1 to about 1:2. Combined with the fuel
and oxidizer is from about 20% to about 70% by
weight magnesium carbonate (measured as a percentage
of the propellant/magnesium carbonate/additives
compacted mixture). The propellant may also contain
additives such as clay (to improve molding
CA 02501474 1994-06-13
-12-
characteristics) or graphite (to improve flow
characteristics).
The propellant is a mixture of compacted
powders. If all powder components are approximately
the same size, the burn rate is unacceptably low.
Preferably, the propellant is a mixture of
relatively large magnesium carbonate particles
having an average particle diameter of from about
150 microns to about 200 microns and relatively
to small fuel and oxidizer particles having an average
particle diameter of from about 50 microns to about
75 microns. The larger magnesium carbonate
particles form discrete coolant sites and do not
reduce the propellant burn rate as drastically as
when all components are approximately the same size,
The solid propellant may be required to generate
the gas over a time ranging from about 30
milliseconds to several seconds. Typically, a short
"burn time" is required in an explosive environment
while a longer burn time is required in a burning
environment. If a short burn time is desired, the
propellant is in the form of tablets, typically on
the order of 1 centimeter in diameter by about one
half centimeter thick. Increasing the pellet size
increases the burn time. For a burn time of several
seconds, the gas generator contains a single
propellant slug compression molded into the housing.
Referring back to Figure 1, to prevent the
housing 36 from melting during ignition of the solid
propellant 14, a cooling material 38 may be disposed
between the housing 36 and solid propellant 14. One
cooling material is granular magnesium carbonate
which generates carbon dioxide when heated above
CA 02501474 1994-06-13
-13-
150°C (300°F). One mole of MgC03 will produce one
mole of COz plus one mole of Mgo, which remains in
the housing 36 in the form of a slag. Small amounts
of Mg0 dust may be exhausted during ignition of the
solid propellant.
To prevent contamination of the chamber 20 by
the solid propellant 14 prior to ignition, a first
rupture diaphragm 40 isolates the vaporizable liquid
18. The isolation diaphragm 40 is ruptured by the
pressure of the shock wave. No active device such
as a disk rupturing detonator is required. To
prevent the generation of mechanical debris, the
isolation diaphragm 40 may have score lines and
hinge areas to open in a petal like fashion.
The first conduit 22 forms a passageway to
communicate the first gas 16 to the vaporizable
liquid 18. The first gas 16 is superheated and
traveling at high velocity. Interaction of the
first gas and the vaporizable liquid 18 vaporizes
the liquid, generating a second gas 24. The second
gas 24 ruptures the second isolation diaphragm 42
and is expelled as a fire suppressing gas,
preferably through aspirator 28.
The selection of the vaporizable liquid 18 is
based on a desire that the second gas 24 be less
reactive with atmospheric ozone than Halon. The
vaporizable liquid 18 contains no bromine, and
preferably also no chlorine. Preferred groups of
vaporizable liquids 18 include fluorocarbons,
molecules containing only a carbon-fluorine bond and
hydrogenated fluorocarbons, molecules containing
both carbon-hydrogen and carbon-fluorine bonds.
Table i identifies preferred fluorocarbons and
CA 02501474 1994-06-13
-14-
hydrogenated fluorocarbons and their vaporization
temperatures. For comparison, the data for Halon
1301 is also provided.
Vaporization Vaporization 8re~sure
Svete~n Fot'afula TemDeratu~g ( °C1 Room Temperature
(t~Pa) (psi)
HFC-32 C~'= -52 0.83 120
HFC-227 CF~CHFCH~ -15 0.41 59
HCFC-22 CHC1F= -41 0.96 139
HCFC-134A CF~CH=F -27 0.57 83
FC-116 CF~CF~ -78 3.2 465
HCFC-124 CHC1FCF~ -12 0.42 61
HFC-125 CF~CF'sti -48 1.3 195
FC31-10 C,F,o - 2
FC-C318 (CF=), - 4
HF-23 CF~H -82 4.8 700
HCFC-123 CF~CCl~i -28 0.09 13
FC-218 CF~CF=CF' -36 0.83 120
FC-614 C,F" +56 - -
HALON 1301 CF~Br -58 1.5 220
The most preferred fluorocarbons and
hydrogenated fluorocarbons are those with the higher
boiling points and lower vapor pressures. The
higher boiling point reduces the pressure required
to store the vaporizable liquid 18 as a liquid. The
lower vapor pressures increase the rate of
conversion of the vaporizable liquid to fire
suppressing gas on ignition. Particularly suitable
are HFC-227, FC-31-10, FC-318 and FC-218.
Unsaturated or alkene halocarbons have a low
vapor pressure and a relatively high boiling point.
CA 02501474 1994-06-13
-15-
These unsaturated molecules contain a carbon-carbon
double bond, together with a carbon-fluorine bond,
and in some cases, a carbon-hydrogen bond. The
unsaturation causes these compounds to be
considerably more photosensitive than a saturated
species, leading to significant photochemical
degradation in the lower atmosphere. The low
altitude photodegradation may lessen the
contribution of these compounds to stratospheric
ozone depletion. Through the use of an unsaturated
halocarbon in the fire suppression apparatus of the
invention, it is possible that bromine containing
compounds may be tolerated.
Representative haloalkenes have a boiling point
of from about 35°C to about 100°C and include
3-bromo-3,3-difluoro-propane,
3-bromo-1,1,3,3,tetrafluoropropene, 1-bromo-3,3,3-
trifluoro-1-propane, 4-bromo-3,3,4,4,tetra-
fluoro-1-butane and 4-bromo-3,4,4-trifluoro-3-
(trifluormethyl)-1-butane, as well as homologues,
analogs and related compounds.
One disadvantage with the fluorocarbons and
hydrogenated fluorocarbons, whether saturated or
unsaturated, is the generation of small amounts of
hydrogen fluoride when the vapor contacts a fire.
Hydrogen fluoride is corrosive to equipment and
hazardous to personnel.
The significant heat and pressure conducted by
the first gas 16 permits the use liquid carbon
dioxide or water as the vaporizable liquid 18. The
expansion problem identified above for
nonenergetically discharged liquid carbon dioxide is
eliminated by the superheating effect of the first
CA 02501474 1994-06-13
-16-
gas 16. Water is converted to a fine mist of steam
on interaction with the first gas and is highly
effective for flame suppression.
As water is such an effective fire suppression
media when delivered in the form of fine droplets, a
mist or as a superheated steam to a fire, it is one
of the most favored fluids for use in this gas
generation concept. However, because water freezes
at a temperature of 0°C (32°F), a means must be
1o incorporated to either suppress the freezing point
or the design of the gas generator must be such that
it can operate effectively with the water frozen
solid.
Most military and commercial applications
require that fire suppression equipment operate
effectively over a temperature range of -54°C to
+71°C (-65°F to +160°F). Many additives such as
ammonia, alcohol, glycols, and salts are capable of
suppressing the water freezing point to below
-54°C (-65°F), but a considerable portion of the
mixture becomes the additive. Most additives are
flammable or corrosive, degrading the effectiveness
and desirable features of a water system when
freezing point depressants are present in the water.
To maintain the desirable features of water as
the agent for the gas generator driven system, the
system can be designed to operate effectively over
the desired -54°C t0 +71°C (-65°F t0 +160°F)
temperature range even if the water has frozan
solid.
Figure 6 graphically illustrates the
relationship between density and temperature for
water and ice at atmospheric pressure, moderate
CA 02501474 1994-06-13
-17-
increased pressure and moderate vacuums. At
slightly over 0°C (+32°F), the density of liquid
water is 1.0 g/cm3 (62.40 lbm/ft') . If the
temperature of the water is reduced just below 0"C
(32°F), the water will freeze to ice and expand
considerably in voluune. The density of ice at o~C
(+32°F) is 0.92 g/cm3 (357.50 lbm/ft3).
Below 0°C, the density of ice increases as the
temperature is decreased as illustrated by reference
line 86. Above 0"C, the density of water decreases
as the temperature is increased as illustrated by
reference line 88.
Figure 7 shows in cross sectional
representation a water based fire suppression system
90 that accommodates the expansion of ice due to
freezing the water. The fire suppression system 90
includes a solid propellant gas generator 12
described above and previously illustrated in Figure
1. The gas generator 12 communicates with a tank 92
2o by a passageway formed by a first conduit 93. The
tank 92 contains a mixture of water 94 and ice 96.
The tank 92 has a volume larger than the volume of
ice that would be contained if all the water 94 was
frozen.
The gas generator 12 provides sufficient thermal
energy to heat the ice 96 to the freezing point and
melt the ice by directing a hot gas 98 produced by
the gas generator 12 in the direction of the ice 96.
Nozzle 100 may be provided to direct the flow of the
hot gas 98 to impinge the mixture of ice and water
inducing turbulence to assure good mixing and
vaporization of the water.
CA 02501474 1994-06-13
-18-
Heating of the ice 96 and water 94 is further
enhanced by the use of a propellant which exhausts a
significant percent of solids into the tank 92 along
with the hot gases 98. Preferably, at least about
20% by weight, and most preferably, at least about
40% by weight of the effluent is solid particles.
The-tank 92 is designed to facilitate
unrestricted expansion of ice 96. There are no
pockets or cavities to interfere with the ice
1o growth. Mechanical parts of the gas generator are
not in the path of ice growth to minimize breaking
of the mechanical parts.
The temperature of the generated gases is
preferably in excess of about 925°C (1700°F) and
typically exceeds 1093°C (2000°F). The gas
generator is preferably selected so that the exhaust
contains at least 20% and preferably in excess of
about 40% by weight hot solid particulate (i.e. MgO,
etc.). This exhaust stream provides a very
effective means for rapidly melting the ice.
Another feature of the water based fire
suppression system 90 is that the ullage space 102
above the water 94 and ice 96 is sufficiently large
to assure that the resulting pressure of the hot
gases 98 exhausting into the tank 92 do not produce
a pressure sufficient to rupture the outlet burst
disc 104, typically about 13.8 MPa (2000 psig). The
system is designed to reguire additional hot gases
98 from the gas generator 92 to be added to
superheat the vaporized water before the outlet disc
104 is ruptured and flow commences.
Once the outlet disc 104 has been ruptured, the
continuing flow of gases 98 from the gas generator
CA 02501474 1994-06-13
-19-
12 creates significant turbulence and mixing of the
water 94 within the tank 92 vaporizing the water to
produce steam 106. Depending upon the particular
fire suppressing application, it may be desirable to
design the unit to produce low quality steam at low
temperatures or superheated steam at higher
temperatures. Any temperature and steam quality can
be produced by the proper proportioning of the water
and solid propellant used in the system. The steam
l0 106 is directed at the fire through a second
passageway formed by a second conduit 107.
It is sometimes be desirable to incorporate an
additive 108 to the water 94 to reduce the heat of
fusion of the ice 96. Effective chemical additives
include polyvinyl alcohol and water soluble polymers
such as methyl cellulose, added to the water in
concentrations of less than about 15% by volume.
The additives 108 also tend to form a viscous gel
when properly added to the water. This higher
viscosity working fluid is much less prone to
leaking from the tank 92 than water.
Yn a second embodiment of the invention, the
fire suppression apparatus 50 is as illustrated in
cross-sectional representation in Figure 2. The
elements of the second fire suppression apparatus 50
are substantially the same as those illustrated in
Figure 1 and like elements are identified by like
Figure numerals. Typically the solid propellant 14
generates solid particulate along with the first
gas. Particulate may be also be generated by other
components of the fire suppression apparatus su:h as
the magnesium carbonate cooling layer 38. If the
environment in which the flame suppression apparatus
CA 02501474 1994-06-13
-20-
50 is located would be detrimentally effected by the
presence of solid particulate, a bladder 52 may be
disposed between the gas generator 12 and the
chamber 20. The energetic first gas 16 forcedly
deforms the flexible bladder 52, generating a shock
wave vaporizing the vaporizable liquid 18 and
generating the second gas 24. The bladder 52 may be
any suitable material such as a high temperature
elastomer.
This second embodiment does not superheat the
vaporizable liquid 18 as effectively as the first
embodiment. The transfer of heat through the
elastomeric material 52 is limited. Accordingly,
lower boiling point vaporizable liquids such as
HFC-32, FC-116 and HF-23 are preferred.
In a third embodiment of the invention, a solid
flame suppressant may be utilized as illustrated by
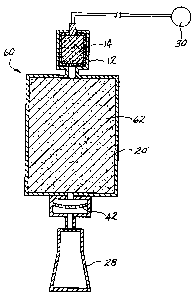
the flame suppression apparatus 60 of Figure 3. The
flame suppression apparatus 6o illustrated in
cross-sectional representation is similar to the
earlier embodiments and like elements are identified
by like reference numerals, while elements
performing a similar function are identified by
primed reference numerals. The chamber 20' is
packed with small diameter, on the order of from
about 5 to about 100 micron, and preferably from
about 10 to about 50 micron, particles 62 of any
effective flame suppressing material. Suitable
materials include potassium bicarbonate, sodium
bicarbonate, ammonium phosphate, potassium chloride,
granular graphite, sodium chloride, m;:~nesium
hydroxide, calcium hydroxide, strontium hydroxide,
barium hydroxide, aluminum hydroxide, magnesium
CA 02501474 1994-06-13
-21-
carbonate, potassium sulfate, sand, talc, powdered
limestone, graphite powder, sodium carbonate,
strontium carbonate, calcium carbonate and magnesium
carbonate. These and other suitable materials may
be mixed with boron oxide as disclosed in U.S.
Patent No. 4,915,853 to Yamaguchi.
In the preceding embodiments of the invention,
the flame suppression apparatus has been described
in terms of a superheated gas interacting with a
vaporizable liquid. The superheated gas is
predominantly nitrogen, carbon dioxide and water
vapor, all effective fire suppressants. In certain
applications, it is preferred to omit the
vaporizable liquid and discharge the flame
suppressing gases generated by the solid propellant
directly onto the fire. A carbon dioxide producing
gas generator 70 is illustrated in cross-sectional
representation in Figure 4.
The carbon dioxide producing gas generator 70 is
2o similar to the gas generators described above. An
electric squib 32 activates an energetic mixture of
a solid propellant 14. On ignition, the solid
propellant 14 ignites a magnesium carbonate
containing propellant 72 generating MgO, COi, Nz, and
water vapor. A perforated screen 74 separates the
propellants from the housing 12. A magnesium
carbonate cooling bed 76 is disposed between the
housing 12 and propellants and on heating generates
additional COZ. The propellant 72 may contain other
fire suppressing agents, in addition to magnesium
carbonate, either alone or in combination. Suitable
fire suppressing agents include magnesium hydroxide,
CA 02501474 1994-06-13
-22-
calcium hydroxide, strontium hydroxide, barium
hydroxide and aluminum hydroxide.
The following examples illustrates the
effectiveness of the flame suppressing apparatus of
the invention.
Examgle 1
The gas generator 70 is an efficient apparatus
for delivering a low molecular weight inerting
l0 agent, such as CO2, Ni, or water vapor, to a fire.
The weight of the apparatus and propellant compares
favorably to the weight of a halon based fire
suppression system.
Gas Generator Characteristics
Length - 42.24 centimeters (16.63 inches)
Diameter - 13.97 centimeters (5.50 inches)
Displaced external volume - 0.0065 meters (395 inch3)
FS-O1 propellant load - 2.01 kilograms (4.437
pounds), generates 1.41 kilograms (3.10 pounds) of
COZ, N2, and water vapor
MgC03 coolant load - 6.00 kilograms (13.21 pounds),
generates 3.13 kilograms (6.894 pounds) of
COZ )
Total inerting gas produced - 4.54 kilograms (10.00
pounds)
Estimated weight of total system - 11.8 kilograms
(26.10 pounds)
Gas Generator Materials
Housing 12 - Aluminum alloy 6061-T6
Solid propellant 14 - BKNOj
CA 02501474 1994-06-13
-23-
FS-Ol propellant 72 - in pellet form, size of
pellets based
on desired burn time, about 1 centimeter
diameter by 0.5 centimeter thick tablets provide
a 30 millisecond burn.
MgCO~ coolant bed 76 - granular
Perforated retaining screen 74 has 1.27 millimeter
(0.050 inch) perforations.
This system will produce about 4.54 kilograms
(10 pounds) of COZ, N2, and water vapor, weigh about
11.8 kilograms (26.10 pounds) and occupy 0.0065
meter3 (395 inch') of space. By comparison, a Halon
1301 system containing 4.54 kilograms (10 pounds) of
fire suppressant weighs about 8.6 kilograms (19
pounds) and occupies 0.0065 meter3 (365 inchs) of
space. While the system of the invention is only
sightly larger and heavier than the Halon system,
other Halon replacement systems are predicted to
increase the weight by a factor of 2 or 3.
Example 2
The corrosive action of saturated solutions of
the effluent components on materials commonly
utilized in aircraft was evaluated. An aqueous
solution saturated With the effluent was prepared
and the pH measured. Various materials were then
exposed to a 50% relative humidity atmosphere of
each saturated solution. After a 30 day exposure,
the coupons were analyzed for corrosion pits. Table
2 illustrates the benefit of removing strontium
oxide from the effluent.
It is apparent that there has been provided in
accordance with this invention an apparatus and
CA 02501474 1994-06-13
-24-
method for suppressing a fire which fully satisfies
the objects, means and advantages set forth
hereinbefore. While the invention has been
described in combination with specific embodiments
thereof, it is evident that many alternatives,
modifications and variations will be apparent to
those skilled in the art in light of the foregoing
description. Accordingly, it is intended to embrace
all such alternatives, modifications and variations
as fall within the spirit and broad scope of the
claims.
CA 02501474 1994-06-13
-25-
w
K : ~ o O
~
0
ef . i~
1
a .~..,
o
a w~
w
i ri .:ni~ ~' .:~~ O o
a ai
a
t a .r a o ~
n o .~ .~
aw Dw
p,.N w~
p
'~: w O
. y m O
.
., .., ~r
v a
a
e
b
a N
O O O 1v
O '1
mr w O nt O ..1
a ~
m ~ ..~ a~
o ~ a
m
i.N~ ..r p.l O
a
p a
Or
.H
1 ~ O O O O O O
0
~
r. o~
a
s
O Ir
O O O O O
~ ~
m o' p %
w
'1f
0
N
h
Q ~ % O Q C C
1 ii
a
0 0~
no o ~od
a ~:: ~;
oo .o a
u ~
..o ~oe~n ~n.~oo a
~ ~ a
v a
a
~ ~ ~ 0
~ ~ ~
hr ~ ~
o ao ~
o 0
~o .r n
~ .r a
..~ H v
o .~ n
..~ ..~ ..~
p . . . p
. . .
w . .
. .
p 4
OOOOIp lna.rOCI er.~OOl1 ~
f4
~
sl4VV Na N '
! Vt ~
'
i VA ~C
Z ~H
~.
a
'~!
~1
00
N10
s
t1 Zf
o i a ~ a v
~ a in o v u,
w ~
a
9 0 w .a y n .t e~
1 .a a a
a
~ 1 o ~ ,1 n
H w N
i f
i b ~ P 0 s ~
w~ W i~ o N -.,aooea
u aa
~ e iC N ~C
s o J~ .0
w a ~
CA 02501474 1994-06-13
~26~
a o 0
m a
0
rc
a o 0
N
N
b
0
N
O I"~ O O
ri ~l
ca
e~ p
'~
0
N
'1
O ri O O
O
I il
1r b
U
NO 0
G'~ a
wNx a'e
o O O O
V
r
NO
yJ i~.
a
x
O O O O
U
w
tA
E~
O O O
O
x
I
b
o
~
a
a
o
w
.i
a
~
a o
I
.a
o a
o
..~
wu
a
~
aii
-a:a
.~
w
e' i
i
w
.
w
~
b
x
a
0
o v
o .~w
a
~n a a
a
0 p,..a
o I
v.
n a >
<A
>a
o w o
a
a
it o tG
.G
a