Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02502465 2010-11-15
TITLE OF THE INVENTION
SEMI-CONDUCTIVE CORROSION AND FOULING
CONTROL APPARATUS, SYSTEM, AND METHOD
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a control apparatus, system, and method for
controlling
a semiconductor-based corrosion and fouling prevention system.
Discussion of the Background Art
The annual cost of metallic corrosion in the United States economy is
approximately
$300 billion, according to a report released by Battelle and the Specialty
Steel Industry of North
America entitled "Economic Effects of Metallic Corrosion in the United States"
dated 1995. The
report estimates that about one-third of the cost of corrosion ($100 billion)
is avoidable and
could be saved by broader application of corrosion-resistant materials and
application of best
anti-corrosive practice from design through maintenance. The estimates result
from a partial
update by Battelle scientists of the findings of a study conducted by Battelle
and the National
Institute of Standards and Technology titled "Economic Effects of Metallic
Corrosion in the
United States". The original work in 1978 included an estimate that, in 1975,
metallic corrosion
cost the US $82 billion (4.9 percent of the Gross National Product), and
approximately $33
billion was avoidable because best practices were not used at the time.
1
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
Regarding aviation, corrosion and the magnitude of its associated cost and
ettect on
safety is a leading concern of global aircraft manufacturers, airline
companies, and
passengers. In North America alone, aircraft industry corrosion costs exceed
$13 billion a
year. The impact is equally as great for government aircraft with, for
example, the U.S. Air
Force spending in excess of $800 million annually for aircraft corrosion
control and repair.
Corrosion, not design life, is the primary factor in the grounding and
retirement of aircraft.
The FAA has ranked preventing aircraft structural failure as a top priority
for improving
aircraft and passenger safety. Aircraft corrosion is linked to a significant
number of mishaps,
accidents, and plane crashes. The tragedy of the loss of human life aside, the
FAA has
calculated the monetary cost per passenger fatality at $2.7 million. Left
undetected and/or
untreated, corrosion undermines the integrity of an aircraft, increasing
maintenance costs, and
the risk to passenger safety.
Regarding marine vessels, interior and exterior hull corrosion and exterior
hull surface
fouling are major factors affecting ship operating costs and vessel life. Fuel
expenses
represent 35% to 50% of overall operating costs. Corrosion, fouling, and the
associated
exterior hull roughness and skin friction contribute up to an additional 50%
to these costs due
to the increased power requirement necessary to attain and maintain vessel
cruising speeds.
Corrosion damage to interior hull surfaces, its cumulative effects on
structural integrity, and
the cost of correction, not vessel age, are the major deciding factors in
vessel retirement and
can significantly shorten the useful life of a ship.
Regarding water towers, there are an estimated 150,000 to 200,000 municipal
water
towers in the United States. An average water tower has a surface area, inside
and outside,
and of 23,000 square feet and holds 310,000 gallons. These towers are
particularly corrosion
prone due to excessive condensation resulting from the storage of cool water.
To maintain
structurally sound water towers, municipalities refurbish tanks approximately
every six years
in coastal areas and every seven to nine years inland, with an average cost
per water tower in
excess of $100,000.
Regarding bridges, the National Bridge Inventory lists 575,413 highway bridges
in the
United States, with 199,277 of them described as structurally deficient or
obsolete as of 1992.
The Intermodal Surface Transportation Efficiency Act of 1991 authorized $16.1
billion over a
period of 6 years for the Highway Bridge Replacement and Rehabilitation
Program. The
Transportation Equity Act for the 215` Century, signed in 1998, continues the
program with
the authorization of $20.3 billion over the next 6 years for bridge
rehabilitation and
2
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
replacement. The Federal Highway Administration and the Transportation
Research Board
estimate that 100 million square feet of bridge surface is coated annually.
The square footage
painted per year has been restricted due to the costs and time required for
the removal and
containment of lead based paints. As a result, many states have delayed bridge
maintenance
painting and only an estimated 1,500 steel bridges are painted annually. With
current
coatings lasting only 10 to 12 years, the backlog of bridge recoating
continues to grow.
Regarding automotive concerns, corrosion issues affecting vehicle safety are a
major
problem for automobile manufacturers and consumers alike. According to the
National
Highway Transportation Safety Administration, between 1975 and 2001, over
25,000,000
vehicles have been officially recalled in the United States for corrosion
related safety
problems. In 1998 alone, Ford Motor Company recalled over 2,000,000 vehicles
for safety
related corrosion problems at a cost estimated to be in excess of $200
million.
A variety of methods for controlling corrosion have evolved over the past
several
centuries, with particular emphasis on methods to extend the life of metallic
structures in
corrosive environments. These methods typically include protective coatings,
which are used
principally to upgrade the corrosion resistance of ferrous metals, such as
steel, and some
nonferrous metals, such as aluminum, and to avoid the necessity for using more
costly alloys.
Thus, they both improve performance and reduce costs. However, such protective
coatings
typically have several pitfalls, including poor applicability to non-metallic
structures that
suffer from corrosion or fouling.
Protective coatings fall into two main categories. The largest of these
categories is the
topical coating such as a paint that acts as a physical barrier against the
environment. The
second category consists of sacrificial coatings, such as zinc or cadmium that
are designed to
preferentially corrode in order to save the base metal from attack.
Cathodic protection and coatings are both engineering disciplines with a
primary
purpose of mitigating and preventing corrosion. Each process is different:
cathodic protection
prevents corrosion by introducing an electrical current from external sources
to counteract the
normal electrical chemical corrosion reactions whereas coatings form a barrier
to prevent the
flow of corrosion current or electrons between the naturally occurring anodes
and cathodes or
within galvanic couples. Each of these processes provided limited success.
Coatings by far
represent the most wide-spread method of general corrosion prevention (see
Leon et al U.S.
Patent No. 3,562,124 and Hayashi et al U.S. Patent No. 4,219,358). Cathodic
protection,
3
CA 02502465 2010-11-15
however, has been used to protect hundreds of thousands of miles of pipe and
acres of steel
surfaces subject to buried or immersion conditions.
Cathodic protection is used to reduce the corrosion of the metal surface by
providing it
with enough cathodic current to make its anodic dissolution rate become
negligible (for
examples, see Prior, U. S. Patent No. 3,574, 801; Wasson U. S. Patent No.
3,864, 234; Maes
U. S. Patent No. 4, 381, 981; Wilson et al U. S. Patent No. 4,836, 768;
Webster U. S. Patent No.
4,863, 578; and Stewart et al U. S. Patent No. 4,957, 612). Cathodic
protection operates by
extinguishing the potential difference between the local anodic and cathodic
surfaces through
the application of sufficient current to polarize the cathodes to the
potential of the anodes. In
other words, the effect of applying cathodic currents is to reduce the area
that continues to act
as an anode, rather than reduce the rate of corrosion of such remaining
anodes. Complete
protection is achieved when all of the anodes have been extinguished. From an
electrochemical
standpoint, this indicates that sufficient electrons have been supplied to the
metal to be
protected, so that any tendency for the metal to ionize or go into solution
has been neutralized.
Recent work in the study of corrosion has found that electrochemical corrosion
processes appear to be associated with random fluctuations in the electrical
properties of
electrochemical systems, such as cell current and electrode potential. These
random
fluctuations are known in the art as "noise". About 20 years ago, scientists
found that all
conductive materials begin corroding as soon as they are produced due to
electrochemical
activity caused by impurities in the material. It was later found that this
activity could be
monitored using electronic instruments detecting the current generated, now
commonly referred
to as "corrosion noise". Essentially, the greater the magnitude of this
current, the "noisier" the
material and the faster the rate of corrosion. For example, steel is "noisier"
than bronze and
corrodes at a faster rate. Researchers have begun to apply noise analysis
techniques to study
the processes of corrosion in electrochemical systems.
Riffe U. S. 5,352, 342 and Riffe U. S. 5,009, 757 disclose a zinc/zinc oxide
based silicate
coating that is used in combination with electronics in a corrosion prevention
system. The
zinc/zinc oxide particles in the coating are disclosed as having semiconductor
properties,
primarily a p-n junction at the Zn-ZnO phase boundary. When reverse biased,
this p-n junction
is described as behaving as a diode and inhibiting electron transfer across
the boundary. This
restriction limits electron transfer from sites of Zn oxidation to the sites
of oxygen reduction on
the ZnO
4
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
surface. Effectively, there is increased resistance between the anode and
cathode of local
corrosion cells and corrosion is reduced.
On average, the Zn-ZnO based junction will be reversely biased due to the
potentials
associated with the oxidation of Zn at the Zn surface and the reduction of 02
at the ZnO
surface. However, significant stochastic voltage fluctuations occur. These
voltage
fluctuations cause the junction to episodically become forward biased. When
forward biased,
electron transfer across the junction increases and there is an acceleration
or "burst" of the
oxidation of Zn and reduction of 02. Effectively, there is a short circuit
between the anode
and cathode of local corrosion cells and corrosion is .enhanced.
The Riffe patents disclose attachment of a fixed value capacitor in the
electrochemical
circuit of the corrosion prevention system. However, as recognized by the
present inventors,
there is no recognition of the desirability of controlling the level of
capacitance nor any
method suggested for determining how to dynamically change the value of
capacitance
needed to effectively prevent corrosion in any given structure or an optimal
way to determine
the value of the capacitance needed.
Regarding anti-fouling, marine objects are degraded by barnacles, zebra
mussels, etc.
that, once attached, must be mechanically removed. Low-cost, non-mechanical,
and
environmentally friendly removal/prevention of marine fouling is desirable.
This has led to
research in anti-fouling toxicity. Toxicological studies have established the
fact that "poison"
must be operationally defined and in so doing, a compound's "toxicity" is
frequently defined
in terms of an amount or concentration. of a compound that produces' either
death or disease.
Accordingly, in an assessment of the relative toxicity of an element or
compound, the
concentration should be considered. Many metals are known to be "toxic."
However, a metal
labeled as toxic is, at the same time, "essential." Copper, a well-known anti-
fouling agent,
falls into this category. Without copper, life as we know it cannot exist.
Copper is an
essential part of certain enzymes that play critical roles in growth,
reproduction, and
metabolism. Unfortunately, at least for those wanting to use copper as an anti-
foulant, low
concentrations, measured in parts per million, are toxic, especially to
aquatic organisms
whose bodies are entirely bathed in their liquid environment. Tin is not an
essential metal in.
biology, but organic tin compounds are particularly good anti-fouling agents.
Unfortunately,
levels of these organotin compounds beginning at parts per billion levels are
toxic to non-
target species. In addition, the organotins accumulate in fatty tissues and
are "magnified" by
the food chain, having increasingly adverse affects on top of the chain
animals, like humans.
5
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
Zinc is an essential metal in biology but it does not, like copper, fall into
the category
of a heavy metal. Toxic levels of zinc are significantly higher than copper
and zinc finds its
way into many environmentally acceptable products and materials. Duke
University
scientists discovered that the anti-fouling properties associated with the
coatings of Riffe,
U.S. 5,352,342 and Riffe, U.S. 5,009,757 came from zinc toxicity of the
coating. These
scientists simultaneously determined that the levels of zinc release were of
such a low level
that they would not produce toxicity in the marine environment. They also
noted that zinc is
not a metal that is magnified in the food chain. Accordingly, it is possible
to use zinc ions as
a toxic, anti-marine fouling agent.
One drawback to previous corrosion preventive methods, such as that of Riffe
disclosed above, is the relative inflexibility of color selection available
for the silicate based'
coatings disclosed therein, with the only color readily available being gray.
While this is
acceptable in most marine and structural uses, there is a need for corrosion
preventive
coatings that are non-sacrificial and which can be provided in a range of
colors for use as
paint substitutes, particularly in the automotive and transportation
industries. These and other
drawbacks are largely overcome with the semiconductor coatings and related
systems of
Dowling's U.S. Patent Nos. 6,325,915, U.S. 6,402,933, and U.S. Application
Serial No.
09/887,024 filed on 25 June 2001, the entire contents of each hereby
incorporated by
reference. The semiconductive coating and system of the Dowling patents and
application
can be used with a variety of conductive substrates to provide an array of
interesting
properties. With the semiconductor always being a material less noble than the
substrate on
which it is applied, the coating stabilizes the potential of the protected
material. The
electrons produced by the electrochemical activity are transferred from the
protected substrate
to the semiconductor of the coating or, simply, the corrosion noise is
transferred from the
protected material to the coating.
Figure 1 is a representation of electrochemical noise present in untreated
metal 101
the randomly fluctuating voltage is measured and displayed as waveform 102
(shown as. a
sawtooth waveform, but an actual waveform would have broader band components
and
would be stochastic in nature).
Figure 2 shows the effect of applying a semiconductive protective coating on a
metal
surface so as to prevent corrosion and fouling where the coating 210 comprises
a material less
noble than the metal 201 it is protecting. Because the coating 210 is less
noble than the metal
201, it subsumes the electrochemical noise 211 that would be present in the
metal but for the
6
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
coating this result is displayed 202 as a flat waveform in the metal.
Individual semiconductor
particles within the coating 250 are responsible for the anti-corrosion
properties of the
coating.
Figure 3 is a representation of a layered semiconductor/metal composition.
When
doped with zinc, the anti-corrosion capabilities of the semiconductor material
for steel
(ferrous alloys) results from the establishment of a potential due to Zn
oxidation and oxygen
reduction, referred to as "corrosion potential." In this respect, the system
acts as a
conventional sacrificial anodic material with iron oxidation suppressed at the
potential
established by the Zn oxidation. However, Zn oxidation in a semiconductor is
significantly
reduced or passivated, with a reduction of the corrosion potential, resulting
in the extreme
long life of the coating. The passivation is a result of a combination of the
varistor-like
behavior of the Zn/ZnO boundary and an associated filter's ability to maintain
a potential
difference across the boundary, such that the boundary has a high electrical
resistance. A
semiconductor particle 250 is comprised of two regions: a P-type region 320
and an N-type
region 310 with a junction 330 that behaves as a varistor with electron flow
302 between the
two regions. When using zinc, the zinc particles are covered by a zinc oxide
layer with the
various oxide coated particles surrounded by a conductive binder. The boundary
of the P and
N semiconductors in the semiconductive coating acts as a varistor (back to
back diodes) that
controls the flow of electrons between them. Proper application of a current
to the
semiconductive coating, connected to the protected substrate, stabilizes the
potential at this
boundary. This slows the rate of electron transfer from the P to the N
semiconductor,
reducing its rate of corrosion by a factor of 103, yielding an extension in
the life of the
semiconductive coating that can exceed the design life of the treated object.
Varistors (variable resistors) have highly non-linear electrical
characteristics and are
functionally equivalent to back-to-back diodes. In a voltage limited region,
the "switch
region," they pass only a leakage current. When the voltage magnitude exceeds
the switch
voltage, for instance during a transient, the varistor becomes highly
conducting. Varistors are'
commonly based on ZnO. Figure 4 is a graph representing the current voltage
relationship for
varistor, within which an axis representing voltage 1101, an axis representing
current 1102,
and a curve representing current 1103 over a range of biasing voltage are
displayed. The
range between -Vb 1110 and Vb 1107 represents the voltage region 1104 in which
the varistor
behaves as a switch. The point along the curve labeled IL 1105 is the point
along the curve
that corresponds to leakage current-that is, the small level of current that
flows through the
7
CA 02502465 2010-11-15
varistor even when the varistor is biased to behave as an open switch. The
point labeled VN
1106 is the point along the curve that represents the switch voltage; in other
words, the highest
positive voltage value that corresponds to the switch region 1104 of the
varistor. The point
labeled VB 1107 represents the breakdown voltage of the varistor, where
biasing voltages
greater than VB cause the varistor to behave as a node. The point labeled
negative IL 1108
represents the point along the curve that represents the negative leakage
current. The point
labelled -VN 1109 represents the point along the curve that represents
negative switch voltage;
in other words, the most negative voltage of the range representing the switch
region 1104 of
the varistor. The point labelled -VB 1110 represents the negative breakdown
voltage.
The above-identified Dowling patents and application are at least directed to
systems
and devices for controlling corrosion comprising semi-conductive coatings and
a corrosive noise
controlling system that includes a filter. In the case of the pending Dowling
application, the
corrosive noise controlling system includes an adjustable filter which may be
adjusted based on
feedback signals corresponding to the corrosive noise present in the coating.
The performance of the corrosive noise reducing system of the Dowling patents
and
application varies in accordance with the system's internal filter, which in
its simplest form is
essentially a capacitor. The Dowling patents and application also disclose
combining the semi-
conductive coating with various passive and active filters. In the Dowling
patents and
application, the semiconductor coating acts somewhat as a resistor, which is
in parallel with the
system's internal filter. A summary of filter basics, such as how to implement
a high-pass or low-
pass filter, is found in Microelectronics Circuits, Fourth Edition, Sedra &
Smith, Oxford University
Press (1997).
Figure 5 is a graph of corrosion potential versus time with various filters.
The horizontal
axis 401 measures time in days while the vertical axis 402 represents
potential relative to the
semiconductor element measured in milli-volts. During an experiment directed
to determining
optimum filter characteristics for various corrosion environments,
measurements were taken for
seven systems at three points in time. The measured potential for each of
seven filter
configurations were recorded for those three samples and are indicated by
various symbols
listed in the legend. The graph displays the various results for each of the
seven filters at the
sampling points indicated from 410 through 430.
Electrochemical corrosion can be viewed schematically in terms of an
equivalent circuit.
Typically, the semi-conductive material is doped with zinc. Thus, the simple
8
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
equivalent circuit shown in Figure 6 relates to the case of Zn oxidation. The
anodic reaction
occurs on the Zn and the cathodic on the ZnO. Note the Zn/ZnO boundary
represents a
varistor in the circuit. If the potential difference generated by the Zn/02
redox couple falls
stably in the switch region, the Zn oxidation is inhibited (or passivated) by
the high resistance
of the boundary. However, over the past decade, it has been demonstrated that
there are self-
generated electrochemical potential fluctuations, "electrochemical noise"
associated with
corrosion. As a result, even though the Zn/02 potential may be in the switch
region, there are
likely to be fluctuations that drive the potential difference into the highly
conductive region
and allow electron flow and hence Zn oxidation.
The present inventors recognized that this is a way to passivate Zn so as to
remove or
filter the electrochemical noise. Removal of this electrochemical noise is via
the filter, which
in its simplest form, is a capacitor. The filtering effect maintains the
potential across the
Zn/ZnO boundary in the switch region and Zn oxidation is reduced and the life
of the coating
is increased. However, it is to be appreciated that the low pass filter may be
augmented with
passband (or notch) filters to selectively attenuate other frequency bands
depending on the
material being protected.
Figure 6 shows an equivalent circuit diagram for the system of the Dowling
patents
and application. This figure abstracts the behavior of the system into a
representative
electrical circuit based on the electro-chemical nature of metal corrosion
processes.
Specifically, corrosion can be modeled as a fluctuating voltage source, the
metal's inherent
resistance can be represented; the anti-corrosion coating can be modeled as a
varistor, and the
noise filter can be modeled as a capacitor. By placing these modeled elements
in a circuit
diagram, the noise and filter components of Dowling can be more clearly
conceptualized
using electrical circuit analysis.
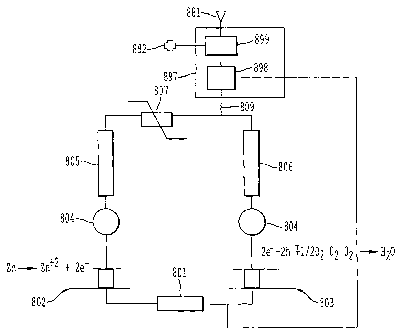
Within the representational circuit is a solution resistance 801 which
represents the
inherent resistance of the system in series with the galvanic electrode
potential, at the anode
802 which corresponds to the ionization process of zinc and the galvanic
electrode potential
at the cathode 803 which corresponds to the chemical process producing water.
Also present
and connected in series with the circuit are two noise sources 804, one of
which is interposed
between the galvanic electrode potential of the anode and the Faradaic
impedance of the
anode 805 and another interposed between the galvanic electrode potential at
the cathode 803
and the Faradaic impedance of the cathode 806 placed in series between the
Faradaic
impedances of the anode and cathode are the zinc oxide varistor 807 and the
noise filter 808.
9
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
The varistor and noise filter act to reduce the occurrence of voltage
fluctuations which can
induce corrosion. The noise filter 808 may be active, passive, or both and, by
selecting a
node in the circuit to be designated common potential 810, the filter 808 can
attenuate high
frequencies in the circuit due to the corrosion noise.
The substrate on which the semiconductive layer is placed may be conductive or
non-
conductive. Conductive substrates can be metallic or non-metallic. Non-
conductive
substrates can be any material that acts as an insulator, such as a silicon
wafer or other non-
metal substrate. The production of such non-conductive or conductive
substrates in the art of
semiconductor chip manufacture is well known to one of ordinary skill in the
art.
The corrosion noise reducing system of the Dowling patents and application
provides
a means for preventing corrosion of a conductive structure susceptible to
corrosion by coating
the conductive structure with a semiconductive coating and connecting the
resulting coated
structure to a passive or active electronic filter so as to minimize the
corrosive noise in the
coating. The electronic filter has a filter response such that it attenuates
the high frequency
spectral content of the corrosion noise. This is achieved by connecting a
filter, having an
impedance characteristic in the form of a low pass filter (possibly augmented
by notch filters)
across the material being protected. Furthermore, depending on the material
and the
application, possibly other frequency bands may selectively be attenuated so
as to reduce
corrosive and/or antifouling effects. The filter can be a passive filter or an
active filter. In
either case, the filter attenuates the higher frequency voltage fluctuations.
The junctions
present in the semiconductor coating then maintain a reverse bias. The time-
averaged
electron flow from the anodic to the cathodic domains in the semiconductive
coating is then
reduced and the coating is effectively passivated.
With the filter engaged to the circuit equivalent of the corrosion process,
the noise
signal can be dissipated as shown in Figure 7, where a metal surface 501 is
covered by a
protective coating 510 connected to a filter 508 so the metal has a
significantly attenuated
noise electrostatic 502. The filter 508 acts either as a standalone low pass
filter or possibly in
combination with filters having impedances in the form .of bandpass and/or
notch filters to
reduce the high frequency corrosive noise 522. Effectively, the filter
dissipates the energy
associated with the higher frequencies in the electrochemical noise signal.
Attenuation of the
high frequency spectral contents of the electrochemical noise will
significantly reduce the
corrosion process by inhibiting the voltage fluctuations across the varistor
outside the switch
voltage (Vn)
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
SUMMARY OF THE INVENTION
Accordingly, one object of the present invention is to address and resolve the
above-
identified and other deficiencies in conventional anti-fouling systems.
Another object of the invention is to provide a corrosion noise reducing
system having
an Electronic Control Unit (ECU), a controllable filter (optionally including
a fixed, passive
filter), and a semiconductive coating on a substrate so as to provide a low
resistance path to
ground for high frequency corrosion noise.
A further object of the present invention is to provide a system and method
for
optimizing a trade-off between extending the life of and depleting a
protective coating, for a
given structure, so as to balance anti-corrosion and the anti-fouling features
of a corrosion
noise reducing system employed on that structure.
These and other objects are achieved by the inventive system and method
described
herein. The present inventors recognized that a corrosion noise reducing
system having a
semiconductive coating on a substrate can be optimally operated with an
Electronic Control
Unit (ECU) and a controllable filter so as to control filter operations and
voltage fluctuations
in the conductive structure on which the semiconductive coating is placed.
These benefits are
achieved via a method for monitoring noise generated by said coating and
controlling a filter,
that optionally, although is not limited to, using adjustable filter
components and/or fixed
components based on a set of predetermined and/or measured parameters in
response to the
corrosion noise generated in the coating, thereby controlling the rate at
which corrosion
and/or providing anti-fouling protection components are expended. The set of
predetermined
and/or measured parameters include at least one of: temperature,
salinity/water purity,
humidity, age, short term duty cycle, long term duty cycle, immediate speed of
vessel, vessel
speed history, immediate geographic location, geographic location history, age
of coating,
coating deterioration, thickness of coating, surface area coated, and shape of
coated area.
The present invention is aimed at the prevention of corrosion in aviation
structures/craft; automotive structures/vehicles; bridges; marine
vessels/structures; pipelines;
rail cars/structures; steel structures; and storage tanks, although may be
used with other
objects as well. The present invention is also aimed at the prevention of
marine fouling in
marine vessels; marine structures; offshore platforms; power plants; and other
objects.
11
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
As determined by the present inventors, a controllable filter and controller
may be
used in a corrosive noise reducing system where the controller dynamically
adjusts the filter
characteristics of the corrosive noise reducing system by taking into account
various
parameters so as to balance the system's anti-corrosion and anti-fouling
characteristics. A
non-limiting list of examples of these parameters includes one or more of:
temperature,
salinity/water purity, humidity, age, short term duty cycle, long term duty
cycle, immediate
speed of vessel, vessel speed history, immediate geographic location,
geographic location
history, age of coating, thickness of coating, deterioration of the coating,
surface area coated,
and shape of coated area. In view of the discovery that it is possible to
strike this balance
between the system's anti-corrosion and anti-fouling characteristics, the
present inventors
identified, and describe herein, systems, devices, algorithms, methods, and
computer program
products for controlling filter operations associated with an anti-
corrosion/anti-fouling
semiconductive coating and a corrosive noise reducing system.
BRIEF DESCRIPTION OF THE FIGURES
A more complete appreciation of the invention and many of the attendant
advantages
thereof will be readily obtained as the same becomes better understood by
reference to the
following detailed description when considered in connection with the
accompanying Figures,
wherein:
Fig. 1 is a representation of corrosion noise in unprotected metal;
Fig. 2 is a representation of corrosion noise in protected metal and in a
semiconductor
coating; and
Fig. 3 is a representation of current flow between a metal and a semiconductor
protective coating;
Fig. 4 is a graph of varistor-like operations between a metal and a
semiconductor
protective coating;
Fig. 5 is a graph of corrosion noise vs. time for various filters;
Fig. 6 is a circuit diagram of a corrosion noise reducing system without an
Electronic
Control Unit (ECU);
Fig. 7 is a block diagram of a corrosion noise reducing system including
metal, a
semiconductor protective coating, a filter, and component noise
characteristics;
Fig. 8 is a circuit diagram of an ECU containing a controllable corrosion
noise filter
and ECU control circuit;
12
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
Fig. 9 is a block diagram of an ECU containing a controllable corrosion noise
filter
and ECU control circuit;
Figs. IOA and I OB are amplitude and phase response curves, respectively, for
a
corrosion noise bandpass filter of one embodiment of the present invention;
Figs. 11A and 11B are amplitude and phase response curves, respectively, for a
corrosion noise notch filter of one embodiment of the present invention;
Fig. 12 is a flow chart of method of reducing corrosion noise with an ECU;
Fig. 13 is a block diagram of a corrosion noise reducing system including
metal, a
semiconductor protective coating, a filter, an ECU, and component noise
characteristics;
Fig. 14 is a graph comparing the zinc release rate (micrograms/cm2) for a
corrosion
noise reducing system with and without an ECU;
Fig. 15 is a graph comparing the zinc release rate (%)- for a corrosion noise
reducing
system with and without an ECU; and
Fig. 16 is a block diagram of a computer system used in the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention provides a corrosion noise reducing system having an
Electronic Control Unit (ECU), a controllable filter, and a semiconductive
coating on a
substrate.
Figure 8 is a circuit diagram of one embodiment of the present invention where
components similar to those found in Figure 6 retain their previous indicia.
As shown, the
ECU 897 contains a controllable filter 898 and an ECU control circuit 899. The
ECU 897
may optionally be connected to one or more local sensors 882, and/or be
connected to, and/or
contain, an antenna (e.g., for use in wireless communication) 881 or other
mechanism for
achieving wireless communication, such as with optical transceivers. The ECU
may also
access data stored in a local data archive (not shown) or in a remote archive
accessible via the
antenna 881, other wireless communication mechanism or even wired connection,
such as a
network. The ECU control circuit 899 is configured to change a filter
characteristic of the
controllable filter 898, such that the frequency-dependent impedance of the
controllable filter
898 is changed depending on the mode of the operation of the ECU control
circuit 899. It is
also to be appreciated that the present invention is not limited to this
specific configuration, as
will be appreciated by one of ordinary skill in the control system art.
13
CA 02502465 2010-11-15
Figure 9 is a block diagram of an embodiment of the present invention and
includes an ECU
897 containing a controllable filter 898 and an ECU control circuit 899. While
a filter composed
of a single capacitor is shown, other circuit components may be used to
implement various
filters (e. g. , having impedances in the form of notch filters) augmenting a
low pass filter
impedance characteristic. Schematically, the combination of the controllable
filter 898 and an
ECU control circuit 899 is represented as a single system 897 connected to the
other elements
of the corrosion system by a conductive link 809. The controllable filter 898
may include any
configuration of various filters (e. g. , filters having impedances in the
form of low pass, notch
filters, bandpass, etc. ) configured to attenuate targeted high frequency
signals corresponding to
corrosion noise. The controllable filter 898 may optionally be disconnected
from the system
using an electronically controllable switch 905 that may be controlled by the
ECU control circuit
899 or by other means such as a manual toggle switch, patch panel or other
device that can
automatically or manually, electrically insert and/or remove components from a
circuit. The
controllable filter 898 may be controlled by the ECU control circuit 899 by
way of the control
lines 925, which open or close switches 923 and 924 connecting a plurality of
supplemental
filters 920 and 921 (this may optionally include a switchable filter bank,
which together can apply
different filter characteristics to corrosion noise). It is also a feature of
the invention that the ECU
control circuit 899 electronically controls/adjusts the filter characteristics
of the controllable filter
898 through adjustable circuit elements, which may optionally be voltage
controlled resistors or
switched variable capacitances. The ECU 899 may be connected to a wireless
receiver/transmitter 881 so as to receive and/or transmit one or more control
signals with a
remote ECU control location (optionally thru a wireless electromagnetic and/or
optical link). The
ECU control circuit 899 may be connected to one or more local sensors 882,
each configured to
monitor one or more parameters used by the ECU control circuit 899 such as
salinity,
temperature, local position, or another parameter.
Information received from the wireless receiver 881 and/or local sensors 882
may be
used by the ECU control circuit 899 to adjust the controllable filter 898 or
disconnect it entirely.
Additionally, the ECU control circuit 899 may interface with a local and/or
remote
database 912 so as to process the information received from the wireless
receiver/transmitter
881 and/or local sensors 882.
The effectiveness of the semi-conductive coating can be optimized through the
use of
filters with specific frequency response characteristics selected for the
needs of a particular
application, as well as the use of adaptive active filters, monitoring the
"electrochemical
14
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
noise" of the protected object and adjusting its response accordingly.
Specific filters are
configured and operated so as to excise corrosion noise thereby resulting in a
smaller
amplitude, low frequency voltage across the semiconductor coating. One or more
filters are
configured and attached to the coating in one or more places along protected
structure so as to
provide a low resistance path to ground for `high frequency' corrosion
currents formed in and
on the semiconductor coating. `High frequency' is a term used herein to
describe non-DC
components of corrosion noise. In practice for typical structures, the high
frequency
component of corrosion noise is in the 10's of Hertz and higher. High
frequency, as used
herein, may also include the transition band between DC and 10 Hz for example,
and thus
includes frequencies at 1-10 Hz for example. Thus, cut off (or 3 dB points) of
filter
characteristics for controllable filters employed by the present invention are
typically,
although need not be limited to, 1 to 10 Hz. Depending on the nature of the
corrosion noise,
the filter characteristics maybe adapted to suppress even lower frequencies,
such as 1/4, or 1/2
Hz and above, or even at one or more particular frequency bands (which may be
notched out
with one or more filters having impedances in the form of a=notch filter).
Figures IOA and I OB are amplitude and phase response curves, respectively,
for
impedance of an exemplary corrosion noise lowpass filter of one embodiment of
the present
invention. These Bode plots show a 3 dB point at about 10 Hz. Alternatively,
filters having
low pass impedance characteristic with 3dB points of 5 Hz, 15 Hz, 25 Hz, 100
Hz or other
values may be used depending on the protected material so long as significant
non-DC
components of spectral energy are removed from the protected structure so that
voltage
fluctuations outside the switch voltage range are significantly reduced. One
or more of such
filters having low pass impedance characteristic may be electrically connected
to the
protected structure at one or more locations to remove the unwanted corrosion
noise energy
while reducing or preventing any corrosion noise currents across the protected
structure. One
or more of these low pass filters maybe controlled by the Electronic Control
Unit in terms of
filter frequency response and/or physical connection. Alternatively, higher-
order filters may
be used to change the roll-off rate of the characteristic curve, thereby
further suppressing high
frequency energy at frequencies closer to the 3 dB point. This electronic
filter provides a path
to ground for the electrochemical noise signal that induces loss of electrons
and therefore
corrosion. To effectively reduce corrosive effects, smaller impedances at
lower frequencies
need to be achieved (i.e., by increasing the size of the capacitor, if the
system filter is purely a
capacitor).
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
Figures 1IA and 11B are amplitude and phase response curves, respectively, for
a
corrosion noise filter having low pass impedance characteristic augmented by
notch filters of
one embodiment of the present invention. As shown, multiple (or just one)
notches in the
impedance of the filter may be used in conjunction with the low pass impedance
characteristic
of Figures 10A and l OB to excise one or more corrosion noise spectral
content. One or more
such filters may be electrically connected to the protected structure at one
or more locations to
remove corrosion noise energy peaks while reducing or preventing any corrosion
noise
currents across the protected structure. One or more of these notch filters
may be controlled
by the Electronic Control Unit in terms of frequency response and/or physical
connection.
Alternatively, higher-order filters may be used.
The control of the one or more filters with low pass and/or notch impedance
characteristics, and higher-order filter exercised by the Electronic Control
Unit may be based
on one or more corrosion noise measurements provided by one or more corrosion
noise
sensors monitoring the protected structure.
For all combinations of filters and filter connections, the effectiveness of
the
semiconductive coating can be further optimized over the life of the object
being protected by
configuring the ECU to adjust its filter operations in response to a series of
measured and/or
predetermined parameters to include one or more of: measured corrosion noise,
temperature,
salinity, humidity, age of coating, surface area coated, thickness of coating,
deterioration of
coating, shape of coated area, location of vessel/object coated (e.g., North
Sea vs. South
China Sea), vessel moving or stationary, history of operation (e.g., ratio of
time stationary vs.
moving).
Figure 12 is a flowchart representing a non-limiting exemplary process used in
an
embodiment of the present invention. The process represented by this flowchart
may be used
in the ECU to control the behavior of the filter in order to optimize the
balance between anti-
corrosive effects and anti-fouling effects. In the process, the system
progresses from a start
step 1201 to a monitoring phase step 1202 in which inputs may be taken from
various
monitors and sensors, including salinity, position of the system, system
history or other
factors. The system then compares the monitor values and decides in step 1203
which of two
predetermined operating profiles the filter should adopt, steps 1204 and 1205,
respectively.
When this action is complete, the system returns to the monitoring phase step
1202 and
repeats the process. In this embodiment, two filter profiles are shown. In
other embodiments,
three or more profiles may be selected.
16
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
The control parameter measurement and exploitation aspects of the present
invention
are used to fine-tune the performance of the system for specific applications.
Based on the
control parameters, the requisite filter properties in the system can be
determined and can be
improved for consistent corrosion prevention over the entire surface of the
structure, even in
very large structures, such as aircraft carriers or large span bridges. In the
present invention,
the voltage fluctuations between the coated surface and a low-noise high
impedance reference
electrode are monitored for when the voltage peak exceeds a predetermined
threshold, a
predetermined number of times, per time interval (e.g., 3-tens per second),
and/or a
heightened noise environment is detected. This threshold detection technique
is one way to
measure the standard deviation of the noise, which in turn is a measure of
noise power.
Alternatively, an FFT, or other signal processing technique, could be used to
measure noise
power as a function. of frequency. The frequency content of the noise signal
and its power
content may be measured by such measuring devices such as a spectrum analyzer
or through
digitization of signal and performing various signal processing techniques in
a real-time
embedded processor in the ECU. In addition, other parameters may be used
(individually or
in combination) to manually or automatically adjust filter characteristics
and/or filter duty,
(i.e., on/off) cycle. These include, but are not limited to, the previously
identified parameters
of. measured corrosion noise, temperature, salinity, humidity, age of coating,
surface area
coated, thickness of coating, deterioration of coating, shape of coated area,
location of
vessel/object coated (e.g., North Sea vs. South China Sea), vessel moving or
stationary,
history of operation (e.g., ratio of time stationary vs. moving).
In another embodiment, the ECU is connected to a Global Positioning Satellite
subsystem through an industry standard or proprietary bus such as VMEbus or
through .a
wireless communication mechanism. By monitoring the geographic location of the
system,
the ECU adjusts the effective values of the corrosion noise filter
characteristics according to
predetermined criteria taking into account what is known about the effects of
salinity,
temperature, and other factors affecting corrosion that are associated with
the system's
geographic location.
Figure 13 is a representation of the effect of one embodiment of the present
invention
where components similar to those found in Figure 7 retain their previous
indicia. The ECU
599 is connected to and controls the filter 508. The ECU 599 maybe connected
to an antenna
581 (or other receptor of electromagnetic energy, such as infrared or optical)
and/or. one or
more local sensors 582 so as to receive data that affects ECU 599 control of
the filter 508. In
17
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
this embodiment of the present invention, the ECU controls the filter 508 so
that the filter has
an intermittent low-pass impedance characteristic 577 (alternating between an
open circuit
and a closed circuit so that the low pass filter is in and out of the circuit)
so as to
intermittently attenuate (at a controllable switching rate, or duty cycle)
high frequency
corrosive noise. When the filter is attenuating the high frequency component
of the corrosion
noise, the high frequency spectral content of the electrochemical noise across
the coating and
protected material 550 has been significantly reduced ; therefore, the noise
signal is
effectively been filtered so that it is a slowly changing voltage (i.e., not
"spiky"). When the
filter is not excising the corrosion noise, the noise characteristic of the
coating 550 is noisy
(spiky), indicating the zinc in the semiconductor layer is dissipating into
the environment. In
this situation, the ECU 599 controls the coating to act in an anti-foulant
mode of operation.
In other embodiments, the ECU 599 may control the filter 508 such that the
filter 508 has a
filter characteristic where the amplitude and/or frequency of predetermined
corrosion noise
frequencies are reduced and/or the filter 508 is intermittently connected. The
reason why the
filter is operated in a "pulsed" manner is to balance Zn depletion for anti-
fouling against Zn
preservation for anti-corrosion. Depletion rate can be controlled by setting
the pulsed on/off
cycles ranging from just above 0% (on) to always on (i.e., 100%). For example,
a 50% on/off
pulsed mode of operation, would have, over a predetermined period of time, the
filter
operating for 50% of the time, although not always at equal time intervals
(i.e., not always
with a 50% duty cycle). Furthermore, the pulsed operation may occur with
period or
aperiodic control waveforms.
Figure 14 is a graph comparing the zinc release rate measured over time for a
corrosion noise reducing system with and without an ECU. In this graph the
zinc release rates
of the two systems are displayed on a graph where the horizontal axis 601
measures elapsed
time and days and the vertical axis 602 measures the zinc release rate in
micrograms of zinc
per cm2. In the system where no ECU is used, the results are indicated by
squares 620. In the
other system, the zinc release rate was reduced by using a system with an ECU
and the results
are indicated by circles 610. The measurements were taken over a time period
of
approximately 300 days. A comparison of the two plots shows that the system
without an
ECU tended to release more zinc over the time period than did the system with
an ECU and,
then, had a shorter semiconductor coating lifespan.
Figure 15 is a renormalization of the results found in Figure 6, wherein the
horizontal
axis 701 represents time in days, and the vertical axis 702 represents the
release of zinc as a
18
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
percentage of total zinc released. In this graph, the results with an ECU
indicated by circles
710 correspond to results 610 and results indicated by squares 720 correspond
to results 620
in Figure 14, respectively.
Figure 16 shows a computer that can be used as an ECU control computer 899 in
an
embodiment of the present invention. The computer comprises a processor 1003,
a main
memory 1004, a ROM 1005, a system bus 1002, and is connected to various user
interface
devices 1010 through 1012 such as a monitor and keyboard. In. order to monitor
physical
conditions and other variables relevant to optimizing the operation of the
anti-corrosive and
anti-fouling measures of the present invention, the computer is connected to
sensors 882 such
as salinity and pressure gauges, geographic position sensors, etc.
A more detailed description of the ECU control computer 899 follows. The ECU
control computer 899 includes a bus 1002 or other communication mechanism for
communicating information (possibly in a wireless manner), and a processor
1003 coupled
with the bus 1002 for processing the information. The ECU control computer 899
also
includes a main memory 1004, such as a random access memory (RAM) or other
dynamic
storage device (e.g., dynamic RAM (DRAM), static RAM (SRAM), and synchronous
DRAM
(SDRAM)), coupled to the bus 1002 for storing information and instructions to
be executed
by processor 1003. In addition, the main memory 1004 may be used for storing
temporary
variables or other intermediate information during the execution of
instructions by the
processor 1003. The ECU control computer 899 further includes a read only
memory (ROM)
1005 or other static storage device (e.g., programmable ROM (PROM), erasable
PROM
(EPROM), and electrically erasable PROM (EEPROM)) coupled to the bus 1002 for
storing
static information and instructions for the processor 1003.
The ECU control computer 899 also includes a disk controller 1006 coupled to
the bus
1002 to control one or more storage devices for storing information and
instructions, such as
a magnetic hard disk 1007, and a removable media drive 1008 (e.g., floppy disk
drive, read-
only compact disc drive, read/write compact disc drive, compact disc jukebox,
tape drive, and
removable magneto-optical drive). The storage devices may be added to the
computer system
950 using an appropriate device interface (e.g., small computer system
interface (SCSI),
integrated device electronics (IDE), enhanced-IDE (E-IDE), direct memory
access (DMA), or
ultra-DMA).
The ECU control computer 899 may also include special purpose logic devices
(e.g.,
application specific integrated circuits (ASICs)) or configurable logic
devices (e.g., simple
19
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
programmable logic devices (SPLDs), complex programmable logic devices
(CPLDs), and
field programmable gate arrays (FPGAs)).
The ECU control computer 899 may also include a display controller 1009
coupled to
the bus 1002 to control a display 1010, such as a cathode ray tube (CRT), for
displaying
information to a computer user. The computer system includes input devices,
such as a
keyboard 1011 and a pointing device 1012, for interacting with a computer user
and providing
information to the processor 1003. The pointing device 1012, for example,
maybe a mouse,
a trackball, or a pointing stick for communicating direction information and
command
selections to the processor 1003 and for controlling cursor.movement on the
display 1010. In
addition, a printer may provide printed listings of data stored and/or
generated by the ECU
control computer 899.
The ECU control computer 899 performs a portion or all of the processing steps
of the
invention in response to the processor 1003 executing one or more sequences of
one or more
instructions contained in a memory, such as the main memory 1004. Such
instructions may
be read into the main memory 1004 from another computer readable medium, such
as a hard
disk 1007 or a removable media drive 1008. One or more processors in a multi-
processing
arrangement may also be employed to execute the sequences of instructions
contained in main
memory 1004. In alternative embodiments, hard-wired circuitry may be used in
place of or in
combination with software instructions. Thus, embodiments are not limited to
any specific
combination of hardware circuitry and software.
As stated above, the ECU control computer 899 includes at least one computer
readable medium or memory for holding instructions programmed according to the
teachings
of the invention and for containing data structures, tables, records, or other
data described
herein. Examples of computer readable media are compact discs, hard disks,
floppy disks,
tape, magneto-optical disks, PROMs (EPROM, EEPROM, flash EPROM), DRAM, SRAM,
SDRAM, or any other magnetic medium, compact discs (e.g., CD-ROM), or any
other optical
medium, punch cards, paper tape, or other physical medium with patterns of
holes, a carrier
wave (described below), or any other medium from which a computer can read.
Stored on any one or on a combination of computer readable media, the present
invention includes software for controlling the ECU control computer 899, for
driving a
device or devices for implementing the invention, and for enabling the ECU
control computer
899 to interact with a human user (e.g., print production personnel). Such
software may
include, but is not limited to, device drivers, operating systems, development
tools, and
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
applications software. Such computer readable media further includes the
computer program
product of the present invention for performing all or a portion (if
processing is distributed) of
the processing performed in implementing the invention.
The computer code devices of the present invention may be any interpretable or
executable code mechanism, including but not limited to scripts, interpretable
programs,
dynamic link libraries (DLLs), Java classes, and complete executable programs.
Moreover,
parts of the processing of the present invention may be distributed for better
performance,
reliability, and/or cost.
The term "computer readable medium" as used herein refers to any medium that
participates in providing instructions to the processor 1003 for execution. A
computer
readable medium may take many forms, including but not limited to, non-
volatile media,
volatile media, and transmission media. Non-volatile media-includes, for
example, optical,
magnetic disks, and magneto-optical disks, such as the hard disk 1007 or the
removable
media drive 1008. Volatile media includes dynamic memory, such as the main
memory 1004.
Transmission media includes coaxial cables, copper wire and fiber optics,
including the wires
that make up the bus 1002. Transmission media also may also take the form of
acoustic or
light waves, such as those generated during radio wave and infrared data
communications.
Various forms of computer readable media may be involved in carrying out one
or
more sequences of one or more instructions to processor 1003 for execution.
For example,
the instructions may initially be carried on a magnetic disk of a remote
computer. The remote
computer can load the instructions for implementing all or a portion of the
present invention
remotely into a dynamic memory and send the instructions over a telephone line
using a
modem. A modem local to the ECU control computer 899 may receive the data on
the
telephone line and use an infrared transmitter to convert the data to an
infrared signal. An
infrared detector coupled to the bus 1002 can receive the data carried in the
infrared signal
and place the data on the bus 1002. The bus 1002 carries the data to the main
memory 1004,
from which the processor 1003 retrieves and executes the instructions. The
instructions
received by the main memory 1004 may optionally be stored on storage device
1007 or 1008
either before or after execution by processor 1003.
The ECU control computer 899 also includes a communication interface 1013
coupled to the bus 1002. The communication interface 1013 provides a two-way
data
communication coupling to a network link 1014 that is connected to, for
example, a local area
network (LAN) 1015, or to another communications network 1016 such as the
Internet. For
21
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
example, the communication interface 1013 maybe a network interface card to
attach to any
packet switched LAN. As another example, the communication interface 1013
maybe an
asymmetrical digital subscriber line (ADSL) card, an integrated services
digital network
(ISDN) card or a modem to provide a data communication connection to a
corresponding type
of communications line. Wireless links may also be implemented. In any such
implementation, the communication interface 1013 sends and receives
electrical,
electromagnetic or optical signals that carry digital data streams
representing various types of
information.
The network link 1014 typically provides data communication through one or
more
networks to other data devices. For example, the network link 1014 may provide
a
connection to another computer through a local network 1015 (e.g., a LAN) or
through
equipment operated by a service provider, which provides communication
services through a
communications network 1016. The local network 1014 and the communications
network
1016 use, for example, electrical, electromagnetic, or optical signals that
carry digital data
streams, and the associated physical layer (e.g., CAT 5 cable, coaxial cable,
optical fiber, etc).
The signals through the various networks and the signals on the network link
1014 and
through the communication interface 1013, which carry the digital data to and
from the ECU
control computer 899 maybe implemented in baseband signals, or carrier wave
based signals.
The baseband signals convey the digital data as unmodulated electrical pulses
that are
descriptive of a stream of digital data bits, where the term "bits" is to be
construed broadly to
mean symbol, where each symbol conveys at least one or more information bits.
The digital
data may also be used to modulate a carrier wave, such as with amplitude,
phase and/or
frequency shift keyed signals that are propagated over a conductive media, or
transmitted as
electromagnetic waves through a propagation medium. Thus, the digital data may
be sent as.
unmodulated baseband data through a "wired" communication channel and/or sent
within a
predetermined frequency band, different than baseband, by modulating a carrier
wave. The
ECU control computer 899 can transmit and receive data, including program
code, through
the network(s) 1015 and 1016, the network link 1014 and the communication
interface 1013.
Moreover, the network link 1014 may provide a connection through a LAN 1015 to
a mobile
device 881 such as a personal digital assistant (PDA) laptop computer, or
cellular telephone.
The semiconductive coating of the present invention can be used in a variety
of end
uses. Chief among these end-uses is the prevention of corrosion of conductive
structures.
The present system for preventing corrosion of conductive substrates
comprises:
22
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
(a) a semiconductor coating in conductive contact with at least part of the
surface of
the conductive structure; and
(b) means for filtering corrosive noise, wherein the means comprise an
electron sink,
such as a battery or other power.supply, along with a filter (or bank of
filters), such as
a capacitor, connected to the coated conductive substrate.
The present system also includes corrosion prevention method comprising:
1) cleaning the external surface of a conductive structure;
2) coating the external surface with the semiconductive coating of the present
invention; and
3) using an electronic filter to minimize corrosive noise in the system.
One key to the anti-corrosion method and system of the present invention is
the
measurement of corrosive noise generated by the entire system (including, but
not limited to,
the substrate, coating and filter components) and minimizing that noise by
application of an
electronic filter.
Referring again to Figure 6, the effect of the ECU upon semiconductive coating
as well as overall performance was measured during the 249-day test period
(Figure 6).
In this test, the zinc release rates decreased over time in both conditions as
the coating
"aged." However, the use of ECUs showed significantly greater reductions in
zinc
release rates, the extent of which are dependent on the duty cycle used to
adjust or
alternatively switch the filter in and out of the circuit. It is to be
appreciated the duty
cycle for controlling the level of zinc release (and therefore toxicity)
depends on a
number of parameters (such as measured corrosion noise, temperature, salinity,
humidity, vessel speed, etc.) being dependent on the environmental conditions.
The
present invention addresses means of adjusting these rates through the ECU and
associated control algorithms. The zinc release rates were lowered by a factor
of 250,
or as low as 0.001 micrograms/cm2 per day, far below the U.S. Navy's maximum
allowable rate of 15 micrograms per cm2 per day (Office of Naval Research, S.
McElvany). These experiments indicate the life of the semiconductive coating,
with
respect to zinc loss (quantity of Zn/cm2 divided by the dissolution rate), can
be
significantly extended when used with the ECU. The results of the monitoring
of
potential, as shown in Figure 6, demonstrate that the test panels without the
ECU have a
significantly lower potential, approximately 150 to 250 mV, based on the ECU
value used.
With the zinc oxidation rate depending exponentially on the magnitude of the
potential, the
23
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
zinc oxide potential will increase and the zinc potential will decrease with
the electrical
resistance of the zinc/zinc oxide boundary. The exponential sensitivity is
indicated by the
Tafel constant, specified for zinc as approximately 30 mV. This Tafel constant
and the
magnitude of the measured voltage differences predict that the relative
passivation due to the
ECU is between a factor of 150 and 4,000. In summary, both the zinc
dissolution rate and
potential data are consistent with the theory of operation of semiconductive -
use of the ECU
leads to a reduction in oxidation rate of the zinc, and significantly extends
the life of the
semiconductive coating. These benefits will be further enhanced by the present
invention's
use of measured and/or predetermined parameters to include at least one of.
temperature,
salinity/water purity, humidity, age, short term duty cycle, long term duty
cycle, immediate
speed of vessel, vessel speed history, immediate geographic location,
geographic location
history, age of coating, thickness of coating, surface area coated, and shape
of coated area.
The present invention can be tailored for the prevention of corrosion of
conductive
materials and prevention of marine fouling to include, but are not limited to:
civilian and
military aircraft; petroleum storage tanks; government, including roads and
bridges, and
Navy, Coast Guard and Army Corps of Engineers projects; chemical industry;
pulp and paper
industries; power plants; railroad bridges and rail cars; manufactured steel
buildings, such as
farm silos and warehouses; water towers; marine vessels; offshore platforms;
and other
marine structures. The coating and ECU can also be adapted for devices and/or
vehicles
associated with nuclear power plants, deep space missions, volcanic
exploration and
monitoring, and deep underwater exploration of toxic seismic environments.
Regarding marine vessels, the present invention can be operated to greatly
reduce
costly hull degradations and to be a cost effective, durable, and
environmentally friendly
alternative to existing anti-fouling and anti-corrosion systems. The
semiconductive coating
can be applied on new vessels during construction and on existing vessels
during scheduled
dry-docking, occurring as frequently as every 2%2 years with traditional
coatings. With an
ECU, owners of vessels on which the semiconductive coating has been applied
can receive
the benefits of reduced fuel and maintenance costs, extended vessel hull life,
and greater
overall vessel usability from higher average operating speeds and reduced
annual dry-dock
time.
Regarding water tanks and towers, the ECU controlled corrosive noise reducing
system of the present invention is EPA approved for use inside potable water
containers.
With proper application and with use of the ECU, the coating is expected to
last for the
24
CA 02502465 2005-04-14
WO 2004/035865 PCT/US2003/029133
design life of the tank. As a result of this longevity, water tank owners will
not incur the
recoating expenses that can be expected with protective coatings.
Obviously, numerous modifications and variations of the present invention are
possible'in light of the above teachings. It is therefore to be understood
that within the scope
of the appended claims, the invention may be practiced otherwise than as
specifically
described herein.